Published online Jul 26, 2016. doi: 10.4252/wjsc.v8.i7.223
Peer-review started: March 23, 2016
First decision: April 15, 2016
Revised: May 6, 2016
Accepted: May 31, 2016
Article in press: June 2, 2016
Published online: July 26, 2016
Processing time: 118 Days and 13.9 Hours
AIM: To evaluate the safety and efficacy of human embryonic stem cells (hESCs) for the management of type 2 diabetes mellitus (T2DM).
METHODS: Patients with a previous history of diabetes and its associated complications were enrolled and injected with hESC lines as per the defined protocol. The patients were assessed using Nutech functional score (NFS), a numeric scoring scale to evaluate the patients for 11 diagnostic parameters. Patients were evaluated at baseline and at the end of treatment period 1 (T1). All the parameters were graded on the NFS scale from 1 to 5. Highest possible grade (HPG) of 5 was considered as the grade of best improvement.
RESULTS: Overall, 94.8% of the patients showed improvement by at least one grade of NFS at the end of T1. For all the 11 parameters evaluated, 54% of patients achieved HPG after treatment. The four essential parameters (improvement in glycated hemoglobin (HbA1c) and insulin level, and fall in number of other oral hypoglycemic drugs with and without insulin) are presented in detail. For HbA1c, 72.6% of patients at the end of T1 met the World Health Organization cut off value, i.e., 6.5% of HbA1c. For insulin level, 65.9% of patients at the end of T1 were able to achieve HPG. After treatment, the improvement was seen in 16.3% of patients who required no more than two medications along with insulin. Similarly, 21.5% of patients were improved as their dosage regimen for using oral drugs was reduced to 1-2 from 5.
CONCLUSION: hESC therapy is beneficial in patients with diabetes and helps in reducing their dependence on insulin and other medicines.
Core tip: We began research on human embryonic stem cell (hESC) therapy in 1999. Today, we have used it in > 1400 patients, and have got patent for our technology in 65 countries including the United States. This study focused on the safety and efficacy of hESCs in patients who were chronically affected with type 2 diabetes mellitus (T2DM). Patients with a previous history of diabetes and its associated complications were enrolled and injected with hESC lines as per the defined protocol. The patients were assessed using Nutech functional score (NFS) (another invention of Nutech Mediworld), a numeric scoring scale to evaluate the patients for 11 diagnostic parameters. All the parameters were graded on the NFS scale from 1 to 5. Highest possible grade (HPG) of 5 was considered as the grade of best improvement. Patients were evaluated at baseline and at the end of treatment period 1 (T1). Overall, 94.8% of the patients showed improvement by at least one grade of NFS at the end of T1. For all the 11 parameters evaluated, 54% of patients achieved HPG after treatment. Important parameters like glycated hemoglobin, insulin and use of oral hypoglycemic drugs with and without use of insulin were measured and their results have been presented in detail. It has been concluded from the study that hESC therapy is beneficial in patients with diabetes and helps in reducing their dependence on insulin and other conventional medicines. Their remarkable properties of reducing risk of immune mediated rejections and absence of hypoglycemic episodes while treating T2DM patients favor their use in management of T2DM.
- Citation: Shroff G. Therapeutic potential of human embryonic stem cells in type 2 diabetes mellitus. World J Stem Cells 2016; 8(7): 223-230
- URL: https://www.wjgnet.com/1948-0210/full/v8/i7/223.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v8.i7.223
Diabetes mellitus (DM) is an endocrine disorder caused by absolute (type 1) or relative (type 2) insulin deficiency leading to hyperglycemia[1]. Individuals with type 2 diabetes mellitus (T2DM), which is also known as non-insulin dependent diabetes mellitus (NIDDM), present with a combination of varying degrees of insulin resistance and relative insulin deficiency. Insulin resistance caused by impaired β cell functioning is usually associated with abnormal insulin secretion or action. Thus, even after insulin administration, the cells can no longer use it to keep blood sugar levels in control and insulin deficiency occurs due to loss of insulin producing cells (IPCs)[2].
The pathogenesis of insulin resistance is complex. Various cellular and molecular mechanisms have been established to explain the cause of insulin resistance. Hyperglycemia itself can impair pancreatic β cell function and lead to insulin resistance, causing a worsening metabolic state. Besides β cell impairment, immune cells like lymphocytes and myeloid cells (monocyte/macrophages) also play important roles in the pathogenesis of DM. Myeloid cells secrete cytokines and regulate the adipose tissue remodeling which accompanies hyper-nutrition, thus are critical players in metabolic homeostasis. Any type of alterations such as pro-inflammatory changes in lymphocyte function causes insulin resistance in T2DM patients[3-5].
At present, there is no cure for diabetes and β cell failure is progressive; once there is an onset of disease, the impairment can never be fully restored. Patients with T2DM need to control their hyperglycemic condition through various means, including diet and exercise, oral anti-hyperglycemic (blood glucose-lowering) drugs, and/or daily insulin shots. Most people who live with T2DM for a period of time eventually require insulin to survive. However, administering insulin alone via conventional techniques does not prevent the long-term complications of the disease, as the optimal insulin dosage is difficult to adjust and also simply re-growing the missing IPCs which could secrete or produce insulin is not enough to solve the problem[4,5]. Studies for pancreatic transplantation and islet cell replacement at the global level are ongoing; however, they were found to be often associated with complications of donor availability, graft survival, transplant rejection and other complications with long-term use of immunosuppressants[6-8].
Clinical management of T2DM is complex and is planned as per the severity of the condition. As per the United Kingdom Prospective Diabetes Study (UKPDS) and Action to Control Cardiovascular Risk in Diabetes (ACCORD), the management for diabetes is decided with an objective for intensive glycemic control where it is tried to maintain blood glucose concentrations close to the normal range using either insulin alone or in combination with few oral hypoglycemic drugs followed by conventional diet plans. The dosage regimen is designed in such a manner, so as to overcome the associated risks and complications of the disease. These all have shown successful results in achieving normal glucose levels but have the most common limitation of influencing long-term glycemic control like increased body weight, higher risk of hypoglycemic episodes and insulin resistance[9-11].
While diabetes can be managed, at present it cannot be cured. Recent advances in stem cell therapy, where it is possible to replace the IPCs of the pancreas that are destroyed by a patient’s own immune system, have given a new hope for the management of DM[12]. Various studies in animal models using stem cells for managing diabetes have been conducted and ongoing[5,13-16]. Hayek et al[17] have reported that grafts of human fetal islet like cell clusters when transplanted to immune deficient and streptozocin induced diabetic mice, successfully matured into glucose-responsive IPCs (β cells).
Human embryonic stem cells (hESCs) are useful as they can produce an unlimited number of pancreatic islet cells. The cells are easy to be transplanted and when they were directly tissue-cultured to the endoderm, they differentiated into pancreatic progenitor cells which further lead to the formation of mature pancreatic endocrine cells in vivo[15,18]. We have earlier reported the safe and effective use of hESCs in diabetes in one of our published case studies of patients with diabetes. A reduction in secondary complications associated with high blood sugar such as affection of the heart, kidneys, vision and polyneuropathy, was observed without any adverse events (AEs) or teratoma formation[19].
Potential of hESCs prepared at our facility has been reported in patients with various terminal/incurable conditions[20] and disorders like chronic obstructive pulmonary disease (COPD)[21], cerebral palsy[22], cortico-visual impairment[23] and Friedrich ataxia[19].
Besides appropriate treatment, it is also very important to correctly diagnose the condition. Diagnostic methods available for diabetes do not provide the complete evaluation of patients with diabetes[24]. Commonly used diagnostic methods are less reliable and have been found to possess higher sensitivity but low specificity[25]. Currently, there is no scoring system for assessing patients with DM. In the present study, we evaluated the safety and efficacy of hESC therapy in patients with T2DM using an 11-point numeric, Nutech functional scoring scale (NFS). It is composed of 11 diagnostic tests or parameters that are further categorized into five grades. These five ordinal grades (1, 2, 3, 4, and 5) run in the direction 1 → 5, i.e., BAD → GOOD which represent bad, not so bad, medium, not so good and good, respectively, with reference to the World Health Organization (WHO) cut off point[26].
hESCs were prepared from a single, spare, expendable, pre-implantation stage fertilized ovum taken during natural in vitro fertilization (IVF) process with due consent of the donor. The cells thus obtained are very small (50 nm-2.5 μm), procured 24 h after fertilization. The cells were cultured and maintained as per our patented technology (United States Granted Patent No. US 8592, 208, 52) in a good manufacturing practice (GMP), good laboratory practice (GLP), and good tissue practice (GTP) compliant laboratory. The cell lines obtained are free of animal product and are chromosomally stable. They harbor all the properties of hESCs and blastocysts and express pluripotent stem cells markers like octamer-binding transcription factor 4, sex determining region Y-box 2, Nanog, stage-specific embryonic antigen-4, trophoectoderm marker, keratin 18, β-human chorionic gonadotropin (negative), immune-regulatory marker, human leukocyte antigen G (negative), gene activating marker 5-methylcytosin, and other markers like telomerase and α fetoprotein. The detailed procedure of cell culture and differentiation was elaborated previously[21]. The safety and efficacy of these cells have been established in patients with incurable conditions[27].
Patients diagnosed with T2DM who were on conventional therapies of insulin and other commonly used medications were included in the study. We also included those patients who visited our facility and were diagnosed with the disease at the institute by regular diagnostic procedures. A verbal, written and video consent form was obtained from all the patients included in the study. The doctors and the rehabilitation team performed a detailed examination of the patients before, during and after each treatment cycle.
The study protocol was approved by an Institutional Independent Ethics Committee (IEC) for stem cell research and therapy of our institute. The treatment regimen followed a defined protocol. An initial dose of 0.25 mL (≤ 4 million cells) of hESCs was administered via an intramuscular (i.m.) route twice daily so as to induce immune tolerance against hESCs, subsequently another dose of 1 mL hESCs (≤ 16 million cells) was administered twice weekly via an intravenous (i.v) route to inoculate the required area and 5 mL of hESCs were also administered intravenously thereafter every 7 d. This gap was kept in order to allow the injected hESCs to develop into mature cells and regenerate the affected part.
All the patients enrolled were evaluated at baseline and at the end of treatment period 1 (T1) for 11 different diagnostic parameters by NFS. The findings were recorded for those who scored as highest possible grade (HPG), i.e., grade 5 of NFS scale and who showed change in condition by at least one grade of NFS at the end of T1. The results from essential parameters, i.e., glycated hemoglobin (HbA1c) level, insulin level, number of oral hypoglycemics used without insulin, number of oral hypoglycemics used with insulin were reported for each grade of NFS scale.
Overall 95 patients including 36 (37.9%) women and 59 (62.1%) men were enrolled. The mean age of the patients was 57.1 years.
Table 1 shows the number and percentage of patients evaluated for all the 11 parameters who scored less than the HPG at baseline and reached HPG at the end of T1. Table 2 represents patients who showed improvement by at least one grade of NFS at baseline to the end of T1.
| Parameter | < HPG (n) at baseline | HPG n (%) at T1 |
| FBS | 95 | 61 (64.2) |
| HbA1c | 93 | 44 (47.3) |
| Insulin level | 91 | 60 (65.9) |
| Insulin with medication | 92 | 24 (26.1) |
| Medication level | 93 | 25 (26.9) |
| Post-prandial blood insulin 60 min | 95 | 58 (61.1) |
| Post-prandial blood sugar | 95 | 58 (61.1) |
| Pre-dinner | 95 | 55 (57.9) |
| Secondary complication | 84 | 27 (32.1) |
| Serum insulin | 94 | 74 (78.7) |
| Serum peptide | 90 | 65 (72.2) |
| Parameter | Patients (n) affected at baseline | Improved n (%) |
| FBS | 95 | 93 (97.9) |
| HbA1c | 93 | 90 (96.8) |
| Insulin level | 91 | 88 (96.7) |
| Insulin with medication | 92 | 88 (95.7) |
| Medication level | 93 | 82 (88.2) |
| Post-prandial blood insulin 60 min | 95 | 92 (96.8) |
| Post-prandial blood sugar | 95 | 92 (96.8) |
| Pre-dinner | 95 | 93 (97.9) |
| Secondary complication | 84 | 79 (94.0) |
| Serum insulin | 94 | 80 (85.1) |
| Serum peptide | 90 | 87 (96.7) |
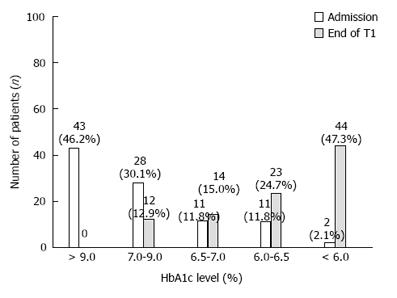
Figure 1 shows the data of patients being evaluated for HbA1c level at the time of admission, and at the end of T1. Of the 95 patients included, 67 (70.5%) had HbA1c levels ≤ 6.5% (normal level as per WHO criteria) at the end of T1.
Before starting the treatment, 93 patients had a score that was less than HPG or had HbA1c levels < 6.0%. After the hESC therapy, 44 (47.3%) patients scored HPG. Overall, 96.8% of patients showed an improvement by at least one grade of NFS at the end of T1.
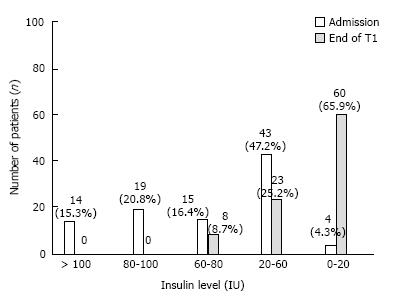
Figure 2 shows the data of patients being studied for insulin level at the time of admission, and at the end of T1. As per WHO, the cut-off range for fasting insulin level is ≥ 20 μU/mL or 0-20 IU. Considering grade 5 of NFS as HPG, of 95 patients enrolled, 60 (65.9%) at the end of T1 were able to achieve HPG.
Before starting the treatment, 91 (95.7%) patients were qualifying the positive test of fasting insulin for diabetes. After getting treatment, the improvement was seen among 96.7% of patients who scored differently by at least one grade of NFS scale at the end of T1.
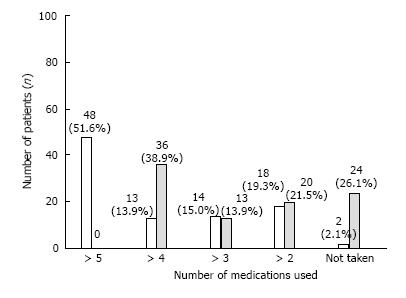
Of the 95 patients, more than 50% were prescribed to take at least 5 or more than 5 oral hypoglycemics at the time of admission. After hESC therapy, the patients showed remarkable improvement, i.e., the dosage regimen was reduced to 1-2 medicines (grade 4). There were 20 (21.5%) patients at this grade at the end of T1.
HPG grade (grade 5) where no intake of medicine is required was achieved in 24 (26.1%) patients at the end of T1. Overall, 88.2% of patients showed an improvement by at least one grade of NFS scale at the end of T1 (Figure 3).
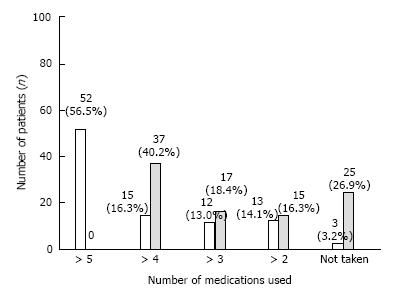
Before the treatment, 92 (97%) patients were taking both insulin and oral therapy to manage their glycemic levels. Depending on the severity of their condition, their dosing regimen was varying with the number of medications being combined with insulin therapy. After hESC therapy, 15 (16.3%) patients were at grade 4 where they required only 2 medications along with insulin (Figure 4).
Overall, HPG was achieved in 25 (26.9%) patients and 88 (95.7%) patients showed an improvement by at least one grade of NFS scale at the end of T1.
T2DM is a complex disorder characterized by hyperglycemia, insulin resistance, and variable degrees of insulin deficiency. To manage T2DM, there are four major factors to be considered which can monitor drug’s glycemic and non-glycemic effects: Insulin resistance, decreased insulin secretion, increased hepatic glucose production, and reduced glucagon-like peptide-1 levels[3]. The majority of treatments available focus on intensive glucose control by giving intensive insulin therapy. Intensive glucose control should be done in a stepwise procedure considering the severity of disease and the risk factors experienced with earlier treatment[28]. American Diabetes Association (ADA) stated that “it is reasonable to recommend tight glucose control in diabetic patients” after the publication of Diabetes Control and Complications Trial (DCCT) results. However, ADA also pointed out that intensive insulin therapy may result in weight gain and unfavorable changes in cardiovascular risk factors among obese and insulin-resistant patients[29]. The UKPDS (1998) study also concluded that all intensive treatments (either sulphonylureas or insulin) increase the cardiovascular risks in diabetes. It also shows that prolonged use of insulin treatment for T2DM might increase the risk of weight gain and hypoglycaemia and may lead to any cerebrovascular event like stroke due to low glycemic level[10,11]. It has been observed that insulin injection does not precisely mimic the dynamic regulation of β cells on glucose homeostasis, which increase the risks of renal failure or blindness. It also causes diabetic foot syndrome, the severity of which may force the patient to undergo limb amputation[1].
The ADA and the European Association for the Study of Diabetes (EASD) have recommended various research based guidelines for the first line and second line management of T2DM which includes use of other drugs like sulphonylureas, thiazolidines, DPP-IV inhibitors either alone or in combination of 2-3 drugs or along with insulin therapy depending on the severity of disease. However, all have other associated risks like cardiovascular events, resistance or hypoglycemia with their long term use[28].
Stem cell therapy is an emerging area of research for diabetes. Sources for stem cell therapies in DM are multiple, including embryonic stem cells (ESCs), cord blood stem cells, induced pluripotent stem cells (iPSCs), and mesenchymal stem cells which have shown their benefits in the long term treatment of diabetes. hESCs are pleuripotent and derived from human fertilized eggs, therefore they have a much lower risk of tumorigenesis than iPSCs[16,30,31]. Li et al[32] have also reported that hESCs have unique immune-privileged characteristics and therefore there are lesser chances of immune-mediated rejection in their transplantation. A similar study conducted by Drukker et al[33] also favors that hESCs and their differentiated derivatives are less susceptible to immune rejection than adult cells.
Hypoglycemia is also one of the major drawbacks accompanied with management of T2DM. Few more recent studies favor that most of the treatment approaches for diabetes focus on intensive glycemic control, which might increase the risk of hypoglycemia[34]. hESC therapy overcomes the limitation of hypoglycemic episodes which is usually associated with other conventional regimens to manage T2DM[35,36].
Preclinical studies conducted by Soria et al[37] have shown that ES-derived insulin-containing cells are able to normalize blood glucose in streptozotocin-induced diabetic mice. The studies reported that glycemic control was found to be not only normal but stabilized as well[37].
Stem cells are able to differentiate into islet-like cells and have immunomodulatory abilities; they cause paracrine secretion that involves production of growth factors and cytokines. These secreted factors promote differentiation of progenitor cells into endogenous progenitor cells (EPCs) and mobilization and homing of EPCs, resulting in angiogenesis and tissue regeneration in diabetic wounds[1,4,38]. Khorsandi et al[4] in their studies using advanced genetic techniques showed that cultured adipose-derived mesenchymal stem cells can be differentiated into IPCs. The induced IPCs were morphologically similar to pancreatic islet like cells and were able to produce as well as secrete insulin in response to different concentrations of glucose stimulation in a regulated manner[4].
Stem cells have the capability to migrate to the damaged cells and repair/regenerate the impaired cells, which also helps in reducing the autoimmune process by producing a new functional immune system and hence shows faster recovery[39,40]. Various clinical trials registered on ct.gov using stem cells for T2DM also have the same hypothesis that stem cell transplantation in human pancreas results in increased angiogenesis, secretion of various cytokines and up-regulation of pancreatic transcription factors and vascular endothelial growth factor, which creates a microenvironment to support β cell/resident stem cell activation and survival[41,42].
hESCs used in our study might have shown their therapeutic effect by following the same mechanism. These cells might “home in” to the damaged cells and repair/regenerate the cells and might reduce the autoimmune process. They are pleuripotent in nature and their competency to that with other drug therapies is well proven by the findings obtained; the patients enrolled in the study had chronic diabetic condition and were all kept on the similar pattern of diet and exercises although they had different intake of medicines and insulin therapy as per the severity of disease before starting hESC treatment. After the therapy, most of the patients benefitted as they showed a reduction in medicines and insulin intake to manage hyperglycemia. We did not observe any incidence of hypoglycemia in our patients during hESC treatment. The patients also showed a reduction in HbA1c levels. None of the patients experienced severe AEs or SAEs during the study as a result of hESC therapy. The embryonic stem cell transplantation also reduces the risk of other secondary complications of eyes, heart, kidney and nerves associated with diabetes[1,2,40,43].
Besides management of diabetes with new therapies, an important area of concern is the precise diagnosis of severity of disease using reliable biochemical parameters so that an appropriate therapeutic strategy can be applied. Factors such as extreme variability in patient populations (i.e., pregnancy, elderly and non-Hispanic Blacks, Mexican white people), lifestyle changes and insulin resistance affect the diagnosis and management of DM[44-46]. These factors create hindrance for most of the commonly used diagnostic parameters like fasting blood sugar, oral glucose tolerance test, HbA1c, serological examination and post-prandial blood sugar in producing the accurate results[46], which raises the question on their reliability. No single test can be considered as a perfect method to diagnose diabetes precisely. Many studies in the past few decades have been conducted where combination of diagnostic tests was carried out, but most of the studies could not completely explore the severity of disease accurately. False positive and negative results were also reported during the study[25,47]. To overcome these limitations of variability, we developed a novel numeric scoring system, NFS which comprises a battery of tests. It has 11 different diagnostic parameters of diabetes on a single scale. The cutoff values of these parameters are as per the WHO criteria.
After hESC therapy, the majority of the patients with diabetes showed an improvement for all the 11 parameters evaluated in NFS. Overall, 94.8% of patients showed improvement by at least one grade of NFS scale at the end of T1. Important parameters like HbA1c and insulin level showed improvement and use of medicines alone and use of medicines with insulin at different levels of treatment phases were reduced after the therapy, suggesting that the hESC therapy helped the patients in being independent of intensive insulin therapy and other conventional drugs.
hESCs might have a good therapeutic potential in the treatment of patients with diabetes. However, well designed studies are needed to prove the long term efficacy and safety of hESCs in the treatment of patients with diabetes.
The author acknowledges all the doctors, staff and patients of the Nutech Mediworld. The author also acknowledges Knowledge Isotopes Pvt. Ltd. (http://www.knowledgeisotopes.com) for the medical writing assistance.
Type 2 diabetes mellitus (T2DM) is the most common lifestyle disorder nowadays. At present, there is no cure for diabetes. Human embryonic stem cells (hESCs) therapy is a new approach to treat it. hESCs can produce unlimited number of pancreatic islet cells and are easy to be transplanted. When they were directly tissue-cultured to the endoderm, they differentiated into pancreatic progenitor cells which further lead to the formation of mature pancreatic endocrine cells in vivo. They have less chances of immune mediated rejection.
This study focuses on treating the patients who were chronically affected by T2DM with latest stem cell therapy. hESC cell lines produced in their lab are safe, effective and are easily transplantable. The in vitro fertilization technique used to prepare hESC is unique. The improvement in diabetic patients is measured by a novel scale, Nutech functional score, which is able to measure 11 parameters simultaneously. Hence the diagnosis is more reliable.
Undoubtedly hESCs have better potential than other treatments. Being pleuripotent and derived from human fertilized eggs, they have a much lower risk of tumorigenesis than induced pluripotent stem cells. They are easily transplantable.
The patients were treated with hESC therapy have shown remarkable improvement. This innovative treatment approach has overcome the life long insulin dependence of patients and have also reduced the intake of oral hypoglycemic and other conventional drugs.
This research article is excellently written. This work has been performed as an advance step for the previous case studies by Dr. Shroff G (J Diabetes Mellitus 2015; 5: 313-318). The current report will make a great contribution to the promotion of the hESC-based regenerative medicine in the field of diabetes.
Manuscript source: Unsolicited manuscript
P- Reviewer: Saeki K, Tanabe S, Valenti MT S- Editor: Ji FF L- Editor: Wang TQ E- Editor: Lu YJ
| 1. | Czubak P, Bojarska-Junak A, Tabarkiewicz J, Putowski L. A modified method of insulin producing cells’ generation from bone marrow-derived mesenchymal stem cells. J Diabetes Res. 2014;2014:628591. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 2. | Assady S, Maor G, Amit M, Itskovitz-Eldor J, Skorecki KL, Tzukerman M. Insulin production by human embryonic stem cells. Diabetes. 2001;50:1691-1697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 676] [Cited by in RCA: 577] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 3. | Nikolajczyk BS, Jagannathan-Bogdan M, Shin H, Gyurko R. State of the union between metabolism and the immune system in type 2 diabetes. Genes Immun. 2011;12:239-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 117] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 4. | Khorsandi L, Khodadadi A, Nejad-Dehbashi F, Saremy S. Three-dimensional differentiation of adipose-derived mesenchymal stem cells into insulin-producing cells. Cell Tissue Res. 2015;361:745-753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 40] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 5. | Barclay RS. Type 2 diabetes is common and hard to treat. Now, a cure may be on the horizon. Healthline News. 2015; Available from: http://www.healthline.com/health-news/combination-of-stem-cell-drug-therapy-could-reverse-type 2-diabetes-051915. |
| 6. | National Institutes of Health. Stem cells and diabetes. Bethesda, USA, 2001. [accessed Oct 15]. 2015; Available from: http://stemcells.Nih.Gov/info/scireport/pages/chapter7.Aspx. |
| 7. | Bromberg JS, Kaplan B, Halloran PF, Robertson RP. The islet transplant experiment: time for a reassessment. Am J Transplant. 2007;7:2217-2218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 8. | Gruessner RW, Gruessner AC. The current state of pancreas transplantation. Nat Rev Endocrinol. 2013;9:555-562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 147] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 9. | Russell SJ, El-Khatib FH, Sinha M, Magyar KL, McKeon K, Goergen LG, Balliro C, Hillard MA, Nathan DM, Damiano ER. Outpatient glycemic control with a bionic pancreas in type 1 diabetes. N Engl J Med. 2014;371:313-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 483] [Cited by in RCA: 401] [Article Influence: 36.5] [Reference Citation Analysis (0)] |
| 10. | Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352:837-853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14327] [Cited by in RCA: 12670] [Article Influence: 469.3] [Reference Citation Analysis (0)] |
| 11. | King P, Peacock I, Donnelly R. The UK prospective diabetes study (UKPDS): clinical and therapeutic implications for type 2 diabetes. Br J Clin Pharmacol. 1999;48:643-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 375] [Cited by in RCA: 383] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 12. | Mazzola N. Review of current and emerging therapies in type 2 diabetes mellitus. Am J Manag Care. 2012;18:S17-S26. [PubMed] |
| 13. | Zhou Y, Hu Q, Chen F, Zhang J, Guo J, Wang H, Gu J, Ma L, Ho G. Human umbilical cord matrix-derived stem cells exert trophic effects on β-cell survival in diabetic rats and isolated islets. Dis Model Mech. 2015;8:1625-1633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 14. | Liang D, Zhang Y, Han J, Wang W, Liu Y, Li J, Jiang X. Embryonic stem cell-derived pancreatic endoderm transplant with MCT1-suppressing miR-495 attenuates type II diabetes in mice. Endocr J. 2015;62:907-920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 15. | Rezania A, Bruin JE, Riedel MJ, Mojibian M, Asadi A, Xu J, Gauvin R, Narayan K, Karanu F, O’Neil JJ. Maturation of human embryonic stem cell-derived pancreatic progenitors into functional islets capable of treating pre-existing diabetes in mice. Diabetes. 2012;61:2016-2029. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 408] [Cited by in RCA: 428] [Article Influence: 32.9] [Reference Citation Analysis (0)] |
| 16. | Lee SH, Hao E, Savinov AY, Geron I, Strongin AY, Itkin-Ansari P. Human beta-cell precursors mature into functional insulin-producing cells in an immunoisolation device: implications for diabetes cell therapies. Transplantation. 2009;87:983-991. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 78] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 17. | Hayek A, Beattie GM. Experimental transplantation of human fetal and adult pancreatic islets. J Clin Endocrinol Metab. 1997;82:2471-2475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 24] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 18. | Hua XF, Wang YW, Tang YX, Yu SQ, Jin SH, Meng XM, Li HF, Liu FJ, Sun Q, Wang HY. Pancreatic insulin-producing cells differentiated from human embryonic stem cells correct hyperglycemia in SCID/NOD mice, an animal model of diabetes. PLoS One. 2014;9:e102198. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 19. | Shroff G. A novel approach of human embryonic stem cells therapy in treatment of friedrich’s ataxia. Int J Case Rep Images. 2015;6:261-266. [RCA] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 20. | Shroff G, Gupta R. Human embryonic stem cells in the treatment of patients with spinal cord injury. Ann Neurosci. 2015;22:208-216. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 57] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 21. | Shroff G. Establishment and characterization of neuronal cell line derived from a 2-cell stage human embryo. Int J Recent Sci Res. 2015;6:3730-3738. |
| 22. | Shroff G, Gupta A, Barthakur JK. Therapeutic potential of human embryonic stem cell transplantation in patients with cerebral palsy. J Transl Med. 2014;12:318. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 23. | Shroff G, Das L. Human embryonic stem cell therapy in cerebral palsy children with cortical visual impairment: A case series of 40 patients. J Cell Sci Ther. 2014;5:189. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 24. | Givens CR, Schriock ED, Dandekar PV, Martin MC. Elevated serum progesterone levels on the day of human chorionic gonadotropin administration do not predict outcome in assisted reproduction cycles. Fertil Steril. 1994;62:1011-1017. [PubMed] |
| 25. | Mo M, Zhong W, Zhao G, Ruan Y, Zhang H, Shi L, Lu D, Yang Q, Li Y, Jiang Q. Combining glycosylated hemoglobin A1c and fasting plasma glucose for diagnosis of type 2 diabetes in Chinese adults. BMC Endocr Disord. 2013;13:44. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 26. | Shroff G. A scoring system to assess patients with diabetes: Nutech functional score. J Diabetes Mellitus. 2015;5:245-251. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 27. | Chavakis E, Urbich C, Dimmeler S. Homing and engraftment of progenitor cells: A prerequisite for cell therapy. J Mol Cell Cardiol. 2008;45:514-522. [RCA] [DOI] [Full Text] [Cited by in Crossref: 243] [Cited by in RCA: 243] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 28. | Stolar MW, Hoogwerf BJ, Gorshow SM, Boyle PJ, Wales DO. Managing type 2 diabetes: going beyond glycemic control. J Manag Care Pharm. 2008;14:s2-19. [PubMed] |
| 29. | Cerveny JD, Leder RD, Weart CW. Issues surrounding tight glycemic control in people with type 2 diabetes mellitus. Ann Pharmacother. 1998;32:896-905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 30. | Weir G. Do stem cells hold the key to a future cure for diabetes? Diabetes Voice. 2008; Available from: http://www.idf.org/diabetesvoice/articles/do-stem-cells-hold-the-key-to-a-future-cure-for-diabetes. |
| 31. | Pagliuca FW, Millman JR, Gürtler M, Segel M, Van Dervort A, Ryu JH, Peterson QP, Greiner D, Melton DA. Generation of functional human pancreatic β cells in vitro. Cell. 2014;159:428-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1321] [Cited by in RCA: 1526] [Article Influence: 152.6] [Reference Citation Analysis (1)] |
| 32. | Li L, Baroja ML, Majumdar A, Chadwick K, Rouleau A, Gallacher L, Ferber I, Lebkowski J, Martin T, Madrenas J. Human embryonic stem cells possess immune-privileged properties. Stem Cells. 2004;22:448-456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 271] [Cited by in RCA: 245] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 33. | Drukker M, Katchman H, Katz G, Even-Tov Friedman S, Shezen E, Hornstein E, Mandelboim O, Reisner Y, Benvenisty N. Human embryonic stem cells and their differentiated derivatives are less susceptible to immune rejection than adult cells. Stem Cells. 2006;24:221-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 312] [Cited by in RCA: 279] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 34. | Chatterjee S, Sharma A, Lichstein E, Mukherjee D. Intensive glucose control in diabetics with an acute myocardial infarction does not improve mortality and increases risk of hypoglycemia-a meta-regression analysis. Curr Vasc Pharmacol. 2013;11:100-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 35. | Mishra PK, Singh SR, Joshua IG, Tyagi SC. Stem cells as a therapeutic target for diabetes. Front Biosci (Landmark Ed). 2010;15:461-477. [PubMed] |
| 36. | Leung PS, Ng KY. Current progress in stem cell research and its potential for islet cell transplantation. Curr Mol Med. 2013;13:109-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 37. | Soria B, Roche E, Berná G, León-Quinto T, Reig JA, Martín F. Insulin-secreting cells derived from embryonic stem cells normalize glycemia in streptozotocin-induced diabetic mice. Diabetes. 2000;49:157-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 635] [Cited by in RCA: 571] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 38. | Gu C, Huang S, Gao D, Wu Y, Li J, Ma K, Wu X, Fu X. Angiogenic Effect of Mesenchymal Stem Cells as a Therapeutic Target for Enhancing Diabetic Wound Healing. Int J Low Extrem Wounds. 2014;13:88-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 39. | Rosa SB, Voltarelli JC, Chies JAB, Pranke P. The use of stem cells for the treatment of autoimmune diseases. Braz J Med Biol Res. 2007;40:1579-1597. [RCA] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 40. | El-Badri N, Ghoneim MA. Mesenchymal stem cell therapy in diabetes mellitus: progress and challenges. J Nucleic Acids. 2013;2013:194858. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 41. | Bhansali A, Upreti V, Khandelwal N, Marwaha N, Gupta V, Sachdeva N, Sharma RR, Saluja K, Dutta P, Walia R. Efficacy of autologous bone marrow-derived stem cell transplantation in patients with type 2 diabetes mellitus. Stem Cells Dev. 2009;18:1407-1416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 93] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 42. | Stem cell therapy for type 2 diabetes mellitus. 2010. ClinicalTrials.gov Identifier: NCT01142050. Available from: https://clinicaltrials.gov/ct2/show/NCT01142050?rank=6&term=stem%20cells%20and%20diabetes%20type%202. |
| 43. | Davey GC, Patil SB, O’Loughlin A, O’Brien T. Mesenchymal stem cell-based treatment for microvascular and secondary complications of diabetes mellitus. Front Endocrinol (Lausanne). 2014;5:86. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 65] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 44. | Lippi G, Targher G. Glycated hemoglobin (HbA1c): old dogmas, a new perspective? Clin Chem Lab Med. 2010;48:609-614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 53] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 45. | Mack R, Skurnick B, Sterling-Jean Y, Pedra-Nobre M, Bigg D. Fasting insulin levels as a measure of insulin resistance in American blacks. J Med. 2003;34:31-38. [PubMed] |
| 46. | Snehalatha C, Ramachandran A, Satyavani K, Vijay V. Limitations of glycosylated haemoglobin as an index of glucose intolerance. Diabetes Res Clin Pract. 2000;47:129-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 47. | Cox ME, Edelman D. Tests for screening and diagnosis of type 2 diabetes. Clinical Diabetes. 2009;27:132-138. [DOI] [Full Text] |