BODY OF THE TEXT
Complex adaptive systems are self-organizing systems involved everywhere in all disciplines[1]. They have in common the emergence of self-organization on the macro-scale from micro-scale interactions of the agents contributing to the system. Complex adaptive systems share common traits of which: (1) simple rules of interaction potentially leading to self-organization when a group of individuals achieve a certain size; (2) the complexity is only at a macro level, individuals are ignorant of the overall organization since simple rules regulate local interactions between individuals and their environment; (3) local self-organization fails to emerge; and (4) interactions between agents or agents and their environment form negative feedback loops leading to adapted responses, maintaining the complexity of the system. Although hematopoiesis is classically regarded as a linear process, stem cell processes and cell lineages that arise from them, are working within the framework of an adaptive complex network[2,3]. Bone marrow cells share many of the above defining characteristics. They only respond to local signals and interactions through feedback loops, have no action on the entire organism and can work independently of the whole hematopoietic system.
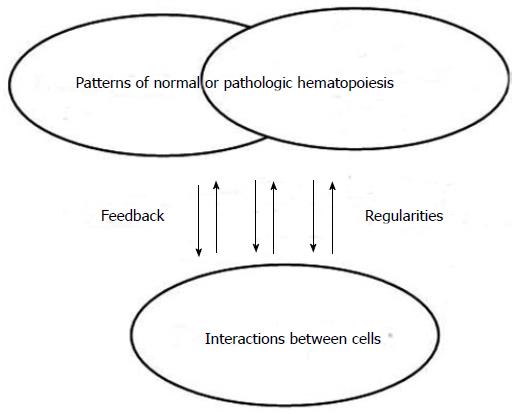
Despite intensive studies and analyses to complete the picture, malignant hemopathies did not always behave as expected. It is apparent that pathologic hematopoiesis (like normal hematopoiesis) is working according to a very different set of rules to cause and effect than those commonly admitted. Complexity theory, which has emerged in all disciplines and can be also considered for hematopoiesis, is based on relationships, emergence, patterns and iterations (Figure 1). All cells present in the hematopoietic system should be considered as involved in that system. All interactions and connections between those cells are not predictable and not planned. A pattern will emerge from cell interactions, will feedback on the hematopoietic system and will inform the interactions of the various agents leading to the establishment of a new balance.
Figure 1 Normal and pathologic hematopoiesis as complex adaptive system.
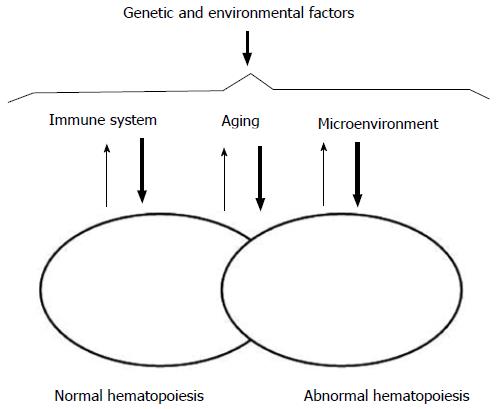
Our understanding of the pathogenesis of acute leukemia has evolved over the years. Leukemic hematopoiesis appears to retain some semblance of its normal counterpart, and also works as a complex adaptive system. Actually, the first one derives from the second one, and both normal and abnormal systems can coexist at variable functional, qualitative, and quantitative intensities under the influence of inner and outer factors (Figure 2). Most systems are not unique, but are within other systems and participate to those bigger systems. In the standard leukemia stem cell theory, rare stem cells with self-renewal capacities can give rise to partially differentiated progenies which will constitute the bulk of leukemia[4,5]. However, this view of leukemia hematopoiesis reveals only dominant pathways and do not take into account the alternate pathways representative of a complex adaptive system. It is based on linear relationships which imply precise solutions. Actually, a hierarchy of command and control does not exist. Nothing is planned or managed. The hematopoietic system is continually self-organizing to find the best fit with the microenvironment. Cells interact through the process of emergence and feedback apparently at random with non-linear relationships and only approximate solutions. Patterns emerge from these interactions and will influence the behaviour of these cells within the system and the hematopoietic system itself.
Figure 2 Factors influencing interactions between normal and abnormal hematopoiesis.
The ways in which the cells interact to one another is critical to the survival of hematopoietic cell lineages. Patterns are formed from these connections as well as the feedback. Connections between the cells appear generally more important than the cells themselves. The bulk leukemia cells are supposed to possess limited proliferative potential and to have few capacities for initiating or maintaining the disease. Eradicating these cells is therefore not enough to cure the disease, while drug-resistant leukemia stem cells will re-populate the bone marrow and cause leukemia relapse[6,7]. The potential emerging patterns are numerous, even if the rules governing the overall function of the system remain quite simple. The total number of mutations potentially found in leukemia cells is quite large. However, few mutations commonly occur in coding regions and are relevant to disease pathogenesis. Any leukemic cell has relatively few of these recurring mutations[8,9]. Small changes in an initial condition can have significant effects. In a hematopoietic stem cell, clonal expansion starts with the occurrence of an “initiating” mutation. Such a mutation might be a translocation or a coding sequence mutation. Within the expanding clone, new mutations can occur, completing the transformation process. Different sub-clones may develop within this founding clone. The situation may also evolve under the influence of disease evolution. New mutations may randomly occur as a matter of time. They can also occur as a result of the treatment mutagenic potential. Clonal evolution is common at the time of relapse and occurs through the acquisition of new mutations, either in the dominant primitive clone or in a subclone of the founder clone[10]. Therapy itself can contribute to the acquisition of therapy-resistant mutations. A dominant sub-clone may evolve, possibly because of varying sensitivities to therapy or the influence of therapy on the microenvironment and on residual normal hematopoiesis[10].
Other factors may influence the balance between normal and abnormal hematopoiesis.
Hematopoietic cells are part of their microenvironment. Cell populations present in the bone marrow niche are able to regulate stem cells. Leukemia stem cells depend on the same signals as their normal counterpart[11]. In response to infection or bone marrow stress, the cellular composition of the microenvironment and therefore the production of cytokines are able to change fundamentally[12]. Hematopoietic cells will have to adapt and to respond to all changes susceptible to occur in this environment to ensure the best fit. They will also modify their microenvironment by causing inflammation and by inducing an adaptive and innate immune response in the bone marrow. Furthermore, as the environment has already changed they will need to change again as a constant process. Leukemia-specific effector T cells infiltrating bone marrow may potentially target leukemia stem cells and participate to their eradication. However, mechanisms that evolved to protect normal hematopoietic stem cells and to regulate demand-adapted responses can protect leukemia stem cells against an attack from the immune system and favor their progression. Quiescence warrants the genetic integrity of stem cells as frequent chromosomal replications may introduce oncogenic DNA mutations and also protect stem cells from uncontrolled proliferation. Hematopoietic stem cells can undergo asymmetrical division. This non-exhaustive proliferation capacity can be initiated by all stress situations, of which infections and cytotoxic chemotherapy, and respond to signals from immune cells[13]. Self-renewing hematopoietic stem cells persist for long periods of time, allowing the accumulation of genetic damage and malignant transformation. They can therefore serve as the cell-of-origin for leukemia stem cells[14]. However, more mature progenitor cell types can serve as leukemia-initiating cells in some cases[15]. Although not excluding a hierarchical model, this sustains the stochastic model hypothesis. Here, every malignant cell, given that it enters a permissive situation, has the ability to self-renew and to recapitulate the disease phenotype[16].
During leukemogenesis, leukemia stem cells can “hijack” the niche and the signaling molecules from normal hematopoietic cells. Molecular changes in the bone marrow niche contribute to leukemia development. In the niche, leukemia stem cells may be more therapy resistant and may therefore potentially provide important cellular markers of clinically relevant minimal residual disease[17]. Immune cells are involved in the regulation of hematopoietic stem cell homeostasis and emergency hematopoiesis[18]. CD4+ T cells are particularly important in maintaining hematopoietic stem cell function[19]. T regulators represent one-third of all CD4+ T cells in the bone marrow. They provide an immune-privileged niche, protecting hematopoietic stem cells from immune destruction[20]. Monocytes and macrophages are also important cells serving as regulators of hematopoietic stem cell egress from the bone marrow[21]. The immune system interacts with hematopoietic stem cells via direct cell-cell interactions and via soluble factors. Leukemic cells express can interact with T cells through the molecular repertoire of major histocompatibility molecules and co-stimulatory ligands. Cytotoxic T lymphocytes directed against leukemia antigens have been detected[22]. However, an activated immune system can also contribute to the leukemia progression by CD27 signaling on leukemia stem cells.
Hematopoietic stem cells are not spared by the aging process, in which DNA damage, telomerase shortening, oxidative stress, and poor homing efficiency have been reported. This could explain some of the differences between childhood and adult leukemias. Such genetic and epigenetic damage together with decreased immune system efficacy, vulnerability of older patients with a decreased function of various organs, and environmental factors can result in the development of malignant hemopathies in elderly patients[23,24]. Furthermore, two obvious contributory factors are the dominant determinant of the country-to-country variability of leukemia incidence: heredity and environment.
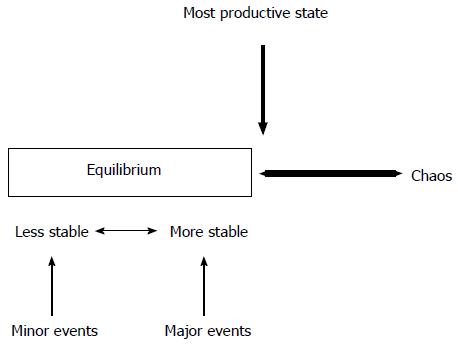
Hematopoiesis permanently fluctuates between equilibrium and chaos. In a state of equilibrium, the hematopoietic system will slowly fail because its internal dynamics is not able to allow enough responses to its microenvironment. Similarly, a system in chaos will also fail, but that is because it will cease to function as a system. The most productive state always remains at the edge of chaos. This situation is the one that generates a maximum of variety and creativity, and a maximum of new possibilities for the evolution of the system. Leukemia is a disease of chaos, a breakdown of the existing biological order within the bone marrow, more than only the presence of abnormal cells. The disorder derives directly from malfunctioning of the controls that are normally present. We are still far from being able to understand with any precision how any single human leukemia arises. The process of leukemia formation is a complex one of multiple steps involving multiple alterations of cells and their physiologic control mechanisms. These steps may occur rapidly and therefore may not be “rate-limiting”, while others may require more time to complete. Multistep leukemia progression can be depicted as a form of Darwinian evolution. Darwinian evolution involves expansions of cells that are endowed with advantageous genotypes and thus phenotypes. Recent whole genome and exome sequencing studies have begun to illustrate the extent of molecular heterogeneity in acute leukemia. The biologic behavior of leukemia cells is the sum of all the acquired genetic changes. Leukemia stem cells, rather than the bulk leukemia cell population, may be the objects of genetic alteration and clonal selection. The clonal descendants of the “favourable” mutated cell dominate by the cells that lack this mutation, resulting in a clonal expansion.
The development and the behavior of hematopoietic cell lineages appear therefore as a balance between normal and abnormal hematopoiesis in the setting of a functioning or malfunctioning microenvironment under the control of the immune system and the influence of hereditary and environmental events. Multiple equilibrium states may exist. Having leukemia cells does not mean having leukemia. All states seem possible, either stable or transitory, going from normal and functional hematopoiesis to the presence of abnormal cells easily controled by the immune system, or to pre-leukemic stages without any consequences on normal hematopoiesis, or pre-leukemic stages with inhibition of normal cell lineages without major expansion of abnormal cells, or to the development of an abnormal clone without major infiltrative and proliferative abilities, or to the development of an aggressive leukemia clone taking absolute control of the bone marrow leading to an overt disease. The number of events necessary to affect the equilibrium depends on its stability. In this setting, massive events are required to modify a stable state, while a less stable equilibrium will be changed by minor events. The system, that will emerge, will be the one that will be better than its competitors. Then, the system will trade off increased efficiency every time in favor of greater effectiveness. The strength of a system is proportional to the variety within the system. Contradictions are used by the hematopoietic cells, as by all complex adaptive systems. They are required to create new possibilities to co-evolve with their microenvironment (Figure 3).
Figure 3 Hematopoiesis between equilibrium and chaos.
Better understanding pathologic hematopoiesis as a complex adaptive system should have consequences in a therapeutic point of view and should modify our vision to consider disease control. Current therapeutic successes using aggressive treatment approaches, such as intensive chemotherapy or stem cell transplantation, have been conceived based upon the hierarchical concept of hematopoiesis with a “black or white” initial response to therapy and long-term survival defining “cure”, relying on a definitively fixed organization of hematopoiesis. Instead of this traditional reductionist approach, a moving status with a permanent adapting condition of hematopoietic cells according to inner and outer factors as suggested in a complex adaptive system is probably more realistic and could represent the common rule. Indeed, in real life, the concept of morphologic complete remission appears not anymore as an absolute requisite for therapeutic decision-making. The development of more accurate techniques to evaluate residual disease and a better knowledge of intra-cellular mechanisms leading to the introduction of targeted therapies should help the change our way to conceive leukemia therapy. Novel therapeutics should authorize more individualized therapeutic approaches. As it has already been the case in chronic leukemias since the introduction of tyrosine kinase inhibitors, a concept of acute leukemia therapy moving to an effective control of the disease compatible with a normal life instead of a supposed “complete” eradication of malignant cells seems a more accurate and more realistic goal.