Published online May 26, 2015. doi: 10.4252/wjsc.v7.i4.669
Peer-review started: January 31, 2015
First decision: February 7, 2015
Revised: March 13, 2015
Accepted: April 10, 2015
Article in press: April 14, 2015
Published online: May 26, 2015
Spermatogonial stem cells (SSCs) are the germ stem cells of the seminiferous epithelium in the testis. Through the process of spermatogenesis, they produce sperm while concomitantly keeping their cellular pool constant through self-renewal. SSC biology offers important applications for animal reproduction and overcoming human disease through regenerative therapies. To this end, several techniques involving SSCs have been developed and will be covered in this article. SSCs convey genetic information to the next generation, a property that can be exploited for gene targeting. Additionally, SSCs can be induced to become embryonic stem cell-like pluripotent cells in vitro. Updates on SSC transplantation techniques with related applications, such as fertility restoration and preservation of endangered species, are also covered on this article. SSC suspensions can be transplanted to the testis of an animal and this has given the basis for SSC functional assays. This procedure has proven technically demanding in large animals and men. In parallel, testis tissue xenografting, another transplantation technique, was developed and resulted in sperm production in testis explants grafted into ectopical locations in foreign species. Since SSC culture holds a pivotal role in SSC biotechnologies, current advances are overviewed. Finally, spermatogenesis in vitro, already demonstrated in mice, offers great promises to cope with reproductive issues in the farm animal industry and human clinical applications.
Core tip: This article reviews the current body of knowledge on biotechnological applications of spermatogonial stem cells (SSCs). SSCs are the founding adult germ stem cells of the sperm producing process spermatogenesis. SSCs belong to the male germline and can be expanded in vitro in several species. Through mechanisms not fully understood they can derive pluripotent stem cells in vitro. Thus, they can be genetically modified with some advantages over embryonic stem cell-based technologies. SSCs can be transplanted to homotopical or ectopical locations, offering great potentials in fertility related issues and regenerative clinical applications in domestic or wild animals and men.
- Citation: Aponte PM. Spermatogonial stem cells: Current biotechnological advances in reproduction and regenerative medicine. World J Stem Cells 2015; 7(4): 669-680
- URL: https://www.wjgnet.com/1948-0210/full/v7/i4/669.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v7.i4.669
Spermatogonial stem cells (SSCs), the germ stem cells of the seminiferous epithelium in the testis are the founder cells of a sperm producing stem cell system called spermatogenesis. SSCs were initially regarded as unipotential cells. Nowadays, however, current knowledge about SSCs has expanded to new limits and the general concept of SSCs as cells just supporting the production of the terminally differentiated sperm has rather radically changed. As a result of continuous and elaborate SSC biology research, the manipulation of this special cell type offers novel biotechnological solutions to contemporary human problems. SSCs are the only adult stem cells capable of transmitting the genome of a given species from one generation to the next, while at the same time having the capacity to convert into pluripotent stem cells[1-3]. They can also give rise to cells from the basic three embryonic layers in vitro, which opened new opportunities for regenerative medicine[1]. Since SSCs are adult cells, they do not bring about ethical concerns as occurs with the use of embryonic stem (ES) cells. However, the use of SSCs is not without risk, since they show some issues regarding epigenetic inheritance, as such a topic will be covered later on this article.
The faculty of SSCs to deliver genes over generations makes them a valuable vehicle for transgenesis techniques. This is particularly important for gene targeting in domestic animals, in which, contrary to laboratory rodent species, the techniques involving SSCs are still not feasible. In this process, SSC culture plays a central role in the exploitation of the unique properties of SSCs for biotechnological applications. Historically, the characterization of the SSC niche in vivo gave the basis for SSC culture conditions, especially SSC population expansion via self-renewal in several species, included men. A stem cell niche consists of a microenvironment with supporting cells and a corresponding signal exchange that regulates self-renewal and differentiation of the stem cells. Accordingly, SSCs are located in the seminiferous epithelium, near the basement membrane and the niche environment normally contains factors produced by neighboring somatic cells (sertoli cells, leydig cells, and peritubular myoid cells), the basement membrane and in particular the vascular network, near where SSCs are preferentially located[4]. This microenvironment in the testis provides signals to control stem cell self-renewal, differentiation, or survival, guaranteeing a normal cell kinetics in spermatogenesis[5,6].
SSC behavior in vitro could be further investigated thanks to the development of germ cell transplantation assays, which allow functional verification of the presence of SSCs (see the topic Germ cell transplantation). The addition of certain growth factors to the culture media, in particular glial cell line-derived neurotrophic factor (GDNF), and the availability of a functional SSC assay, allows SSC populations to be expanded during long term culture, an advance first made in rodent models.
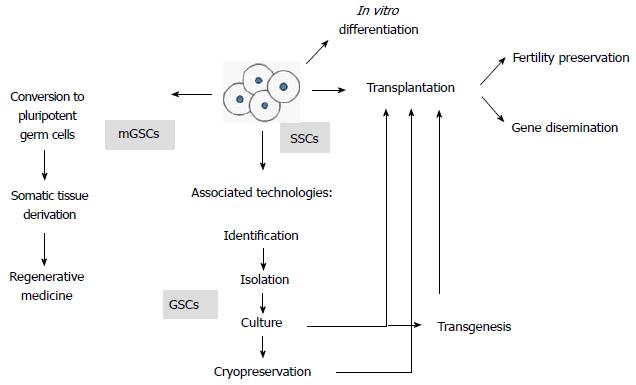
SSC biotechnologies are being aimed to solve fertility related issues (i.e., fertility restoration in oncological patients, infertility treatments and reproduction of endangered species). They also provide important developments in germplasm preservation and the emerging technology of spermatogenesis in vitro. Finally, several of these applications require species-specific knowledge of SSC culture conditions as well as transplantation techniques, two aspects that will be covered in this article (for a summary of the biotechnological approaches that involve the use of SSCs, see Figure 1).
SSC isolation procedures have been described for several domestic species and humans[7-14]. SSCs are isolated by enzymatic digestion, usually involving two steps of various combinations of proteolytic enzymes (commonly, collagenases I or IV, trypsin, hyaluronidase and DNAse I). From this enzymatic digestion, a germ cell suspension is obtained that now has to go through at least one type of purification methods, but more often a combination of them (Zheng et al[15], 2014 summarize the current enriching methods for SSC isolation). There are two groups of purification methods, one group based on the SSC phenotype, like fluorescence activated cell sorting (FACS) and magnetic activated cell sorting (MACS), and the other utilizes physical methods, like differential plating, extracellular matrix selection and density gradient sedimentation[15]. Simple models for SSC isolation include the use of young animals[16], cryptorchid animals[17], vitamin A deficient animals[18], but SSC isolation becomes more complex when their source is the adult testis with full spermatogenesis. In this scenario, all differentiating germ cell types are present and SSCs represent a smaller proportion (see the topic SSC markers). Most recent articles in which SSC isolation is carried on, include one phenotype-based method and one or more physical methods. Most researchers do not use a functional assay (germ cell transplantation) to unambiguously identify the isolated germ cells as SSCs. They instead characterize the germ cell colonies arising during culture by means of immunohistochemistry or RT-PCR, aiming to identify gene products or RNA expression respectively (see the topic SSC markers). MACS is the most widely used technique nowadays. It is simple and inexpensive, but relies on specific SSC markers usually present in subsets of the SSCs, therefore sub-sampling the general SSC population. Interestingly, one group, in a recent work, produced an enriched human SSC suspension by using MACS and the marker ITGA6 (alpha 6 integrin) together with a proper authentication of SSCs through the germ cell transplantation assay[11]. A recent novel approach for SSC isolation involved the combination of traditional isolation methods with testicular tissue grafting (see the topic Germ cell transplantation). In this work, adult mouse testis tissue was grafted into the dorsal skin of immunodeficient mice and after 2-4 wk the researchers observed the depletion of advanced differentiating germ cells of the seminiferous epithelium. Afterwards, the tissues left only with undifferentiated type A spermatogonia (a population including the SSCs) were recovered. The SSCs were isolated from the tissues, which were not only able to proliferate in vitro but also to recapitulate spermatogenesis in receptor testes devoid of endogenous spermatogenesis and furthermore to produce offspring through the original donor derived sperm[19]. This method, although perhaps not as practical as MACS, results in a larger true SSC population since the whole array of markers confirm the phenotypic profiles of SSCs.
Culture conditions for SSC propagation in vitro were first developed in mice[16,20]. This was the base for the development of domestic animals SSC culture systems[7-10,21-25]. Most of these systems have in common the use of GDNF and serum. Common growth factors, besides GDNF, include LIF, FGF2 and EGF[26], however important responses of SSCs regarding maintenance, survival and growth in vitro are dependent on age, species and even the strain of the donor animals[27]. Very recently, a feeder/serum-free culture was established for mice SSCs[28] and this method was improved for maintenance of SSCs[29]. This represents a big advance because serum, when present, introduces unknown variable factors into the culture systems[27] besides promoting the growing of contaminating somatic cells[2]. Current efforts are being made to set up functional human SSC culture systems[11,30,31].
Normally, SSCs self-renew during the steady state cell kinetics of spermatogenesis. They do so to maintain the undifferentiated pool while at the same time producing daughter cells that enter the differentiation path, ultimately generating sperm[32]. Thus, the production of sperm in vitro requires culture conditions favoring the pathways of sperm production and not self-renewal. Going through the meiosis step of germ cell differentiation in vitro was one of the most difficult steps for many research groups. This fact hindered the progress in this particular research area for many years. An spermatogenesis in vitro system going beyond that developmental stage and traversing spermiogenesis was achieved and mouse sperm could be generated when testicular tissue was set on the liquid/gas interphase[33]. This system included a very simple culture medium and testicular tissues came from young animals[34]. One of the main features of this culture system is the preservation of the cytoarchitecture of the testis, that is, maintaining the structure of SSC niches. The reconstitution of spermatogenesis in vitro from SSC suspensions has proven more challenging. Some groups have obtained sperm in vitro by using testicular cell suspensions from prepubertal animals but three dimensional support matrices are necessary[35,36]. Although these successful systems have been developed for sperm production in vitro with rodent species, it is not yet foreseeable that these techniques can be translated to a clinical setting in the near future. Human SSC differentiation in culture systems is still under investigation at the present time.
The exciting possibility of producing sperm in vitro has large implications for farm animal industries, human fertility restoration, preservation of endangered species and many other associated technologies related to mammalian reproduction. For instance, in the cattle industry, keeping animals in large facilities would be a thing of the past when renewable SSC pools from elite bulls produce high numbers of sperm in Petri dishes at small biotechnological facilities. In many cases of human infertility it would just be enough to have one healthy SSC derived (and possibly engineered) sperm to produce offspring through assisted reproduction technologies (ARTs). Autologous SSC culture systems could be set for individuals before oncological treatments and sperm generated in vitro could be cryopreserved for later use to fertilize an egg and produce offspring from these patients. This way, the danger of reintroducing malignant cells back into the patient could be avoided. SSC banking could then become possible, as another mean for germplasm preservation in many species. Moreover, the cumbersome step of transplanting genetically modified SSCs to a receptor male in order to recapitulate spermatogenesis and thus obtain transgenic sperm could then be omitted. In addition, these techniques could also provide means for preserving endangered species, for instance, animal populations with very few individuals that at the moment of dying could have their SSCs collected for sperm to be produced in the laboratory.
The characterization of SSCs is an important tool to study their presence on culture systems and during other techniques. SSCs, representing only the 0.03% of germ cells in the testis[37], and the more numerous type A spermatogonia committed to differentiate, are morphologically undistinguishable. Furthermore, there is no general consensus on the phenotypic profile of SSC as this may vary among different species[38] and there seems to be no unique SSC marker. In general, some phenotypic characteristics of SSCs have been advanced thanks to the molecular dissection of the SSC niche in the testis. Within the testis, Sertoli cells are the main cell type involved in the SSC niche[39]. The key factor GDNF, secreted by Sertoli cells, promotes SSC proliferation[40,41]. Any receptors expressed in SSCs that bind self-renewal promoting ligand molecules from the niche define their stem cell phenotype and constitute potential SSC markers[42]. That is the case of the widely accepted marker GFRα1, the receptor for GDNF in SSCs[43]. In domestic animals, besides GFRα1, another important marker for both gonocytes (perinatal germline stem cells) and SSCs is THY1[44-46]. In monkeys, SSCs express the phenotype (CD90+ DC49f+ CD117-), SSEA-4+ PLZF+[47]. Apparently, SSEA-4 seems to be conserved in humans[48]. SSCs from humans also express GPR125[49], besides GFRα1[50]. Another human SSC marker that adds to the known panel is ITGA6[51]. Recently, a new SSC gene product has been reported: PAX7, that might represent a definite common SSC marker for several species including mice, cats, dogs, pigs, bulls, monkeys and humans[52].
Pluripotent stem cells so far considered for regenerative medicine strategies include inner cell mass derived ES cells[53,54], somatic derived induced pluripotent stem cells (iPS cells)[55], fetal derived embryonic germ (EG) cells[56] and SSC derived multipotent germ stem (mGS) cells[57,58]. All of these cells form teratomas when injected to seminiferous tubules or subcutaneous tissue of immunocompromised mice and contribute to chimera formation when injected to a blastocyst. When SSCs are placed in culture they proliferate in the presence of GDNF. This growth factor is produced by Sertoli cells in the testis, stimulating SSCs to self-renew[41]. SSCs proliferating in culture are designated germ stem cells (GS) cells[16]. They are unipotent, genetically and epigenetically stable, do not produce teratomas and do not contribute to chimera[59]. Extraordinarily, while in culture, they can spontaneously derive multipotent germ stem cells (designated mGS cells) at a low frequency (1 out of 30 testes)[58]. They represent SSCs with pluripotency, sharing not only ES cell traits such as self-renewal properties, tumorogenicity and chimera production but also genomic and epigenetic abnormalities[2]. Pluripotent germ cells from the testis can circumvent some of the typical objections or technical limitations of other pluripotent cells (i.e., ES cells and iPS cells) for their use in therapeutic purposes. These limitations include issues in cell proliferation, cell differentiation, genetic stability, allogenicity and ethical/religious issues (reviewed in[60]). Remarkably and unlike other pluripotent cells, the conversion of GS cells into mGS cells occurs on a dedifferentiation process not dependent on genetic manipulations, which opens important theoretical possibilities for these technologies to be translated into clinical applications in regenerative medicine. Nevertheless, despite all the promising traits of mGS cells, caution is required on the grounds of their genetic and epigenetic behavior. Although GS cells maintain a normal karyotype, genomic imprinting, and SSC activity during long-term culture[59], mGS cells are unstable and tend to accumulate genomic and epigenomic abnormalities[2,61]. Also, GS-like cells derived from mouse fetal germ cells had SSC activity but some of the produced offspring had abnormal genomic imprinting patterns, which were also transmitted to future generations[56]. SSC-derived pluripotent cells are able to generate very diverse tissues while in cell culture[1,3]. For instance, differentiated tissues appear in culture from a specific subpopulation of mouse mGS cells that show a pluripotent cell phenotype (POU5F1+) while at the same time expressing SSC proteins (c-Kit+)[62].
All in all, SSCs show excellent perspectives for applications in regenerative medicine. According to the “organ niche” theory, developmental failure in organogenesis caused by defective genes, can be compensated by xenogeneic pluripotent stem cell transplantation into genetically affected blastocysts[63]. The pluripotential cells would then integrate into the embryo, resulting in chimera formation with colonization of stem cells of xenogeneic origin in the organ forming niches during development. This has been demonstrated through animal models such as mouse and pigs to successfully generate functional kidneys[64] and pancreas[63,65]. SSC-derived pluripotent stem cells, with all their inherent advantages, could be used for these purposes.
Although the current advances in the generation of testicular pluripotent cells in rodents are unquestionable, similar results in the humans have been controversial. Although one work demonstrated teratoma formation and pluripotency markers expression[66] on these cells, tumors showed a relatively small volume[67]. Several other groups have produced allegedly human mGS cells as well, but these cells, although expressing several pluripotency markers, do not produce teratomas when transplanted into the skin of immunocompromised mice[68-70]. In fact, one group recently found that human testicular embryonic-like cells (allegedly mGS pluripotent cells) do not possess a transcriptome similar to that of human ES cells but rather have the phenotype of mesenchymal stem cells (MSCs), therefore, concluding that these cells are not pluripotent and most likely not of germ cell origin but instead mesenchymal[71]. Current knowledge of human SSC culture conditions probably is insufficient and do not emulate the mouse SSC culture environment that has allowed the generation of mGS cells in this species. For further progress to be made, the exact mechanisms of SSC reprogramming need to be addressed. For instance, we know that primordial germ cells from the fetal period convert to ES-like pluripotent cells[72] via the AKT signaling pathway[73]. A very recent study found that spontaneous SSC reprogramming would be caused by unstable DNA methylation with involvement of the Dmrt1-Sox2 signaling pathway[74]. However, more research is required to successfully obtain human mGS cells. This will require very specific culture conditions and the molecular dissection of SSC pluripotency, all of which should continue to be investigated.
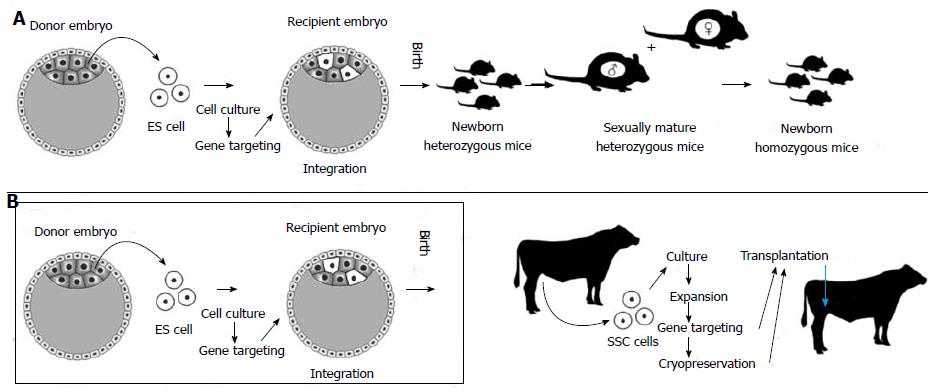
ES cell-based transgenesis technologies are well developed and routinely used in the context of mouse species. However, SSCs can be used for a more straightforward route to transgenesis. ES cells, after genetically intervened, are injected into blastocysts with the hope of successful integration and contribution to the germline, which not always can be guaranteed. By contrast, SSCs are already constituents of the germline. Some other important differences with ES cells make SSCs best suited for mutagenesis (Table 1), especially in species for which ES technologies are not feasible or physiological limitations exist like in farm animals. Additionally, unlike mice, some farm animals, primates and humans are uniparous species as a consequence of not producing large numbers of oocytes under normal physiological conditions. In addition, these species go through lengthy periods of time to attain puberty and sexual maturity. SSC genetic and epigenetic stability keep these cells committed to the germline phenotype so that they do not tend to differentiate into other lineages[59]. This trait makes their potential role for gene therapies clinically safer than other pluripotent stem cells. The use of SSCs for transgenesis in farm animals in comparison to ES cell-based technologies in mice is depicted in Figure 2.
| Trait | Embryonic stem cells | SSCs |
| Mutagenesis technical status | Available for mice. Many genes mutated | Experimental level in mouse[75], rats[78], goats[81], pigs[82] |
| Source | Embryo | Adult testis |
| Age of derivation | Embryonic period | Postnatal period |
| Ethical/legal concerns | Yes | No |
| Efficiency | 5 × to 10 × higher than with ES cells | |
| Cell differentiation state | Undifferentiated - pluripotential | Differentiated up to germ-line |
| Tumorogenesis | Produce teratomas | Do not produce teratomas |
| Chimera formation | (+) | (-) |
| Germline gene transmission | Do not transmit genes from one generation to the next | Transmit genes from one generation to the next |
| In vitro phenotype | Colonies with tightly attached cells | GS cells loosely attached (easily dissociated with trypsin)[2] |
| Speed of growth | Faster | Slower |
| Requires other non-transfected SSCs during culture | No | Yes2 |
| Karyotype | Variable - unstable1 | Normal - stable |
| Epigenetics | Variable - unstable DNA methylation pattern | Normal - stable DNA methylation pattern |
| Offspring | Abnormal | Normal |
Another alternative for knockout (KO) animal production is nuclear transfer of genetically modified somatic cells, but these cells have limited proliferation capacities, the technique shows inefficient offspring production and postnatal degenerative problems. For these reasons, cloning does not efficiently compete with ES cells or SSC technologies for gene targeting.
Due to their potentials, SSCs have been used to generate KO animals in several species. Transgenic mice generation by using SSCs was first accomplished with the use of retroviral vectors, however with low efficiency[75]. Later on, with the advent of GS cells (SSCs that proliferate in vitro) this cell type could be expanded in vitro and genetically modified through vectors (viruses or plasmids) with drug resistant genes to allow for the detection of SSCs carrying the transgene. These SSCs were transplanted to recipient mice and took over the spermatogenetic functions in the new environment to ultimately produce transgenic sperm. Transgenic heterozygous males with transgene carrying sperm were mated with females and progeny was produced, near 50% of which carried the transgene[76]. This illustrates the importance of an optimal culture system in order to expand SSC populations and thus increase the efficiency of the transgene incorporation. Soon after, sophisticated techniques added to the gene function modification toolbox such as the CRE/LoxP system for conditional gene targeting, was successfully adapted to the use with SSCs[77]. Interestingly, SSC mediated rat transgenesis was achieved in 2007[78] while parallel efforts with ES cells on this species were only successful until 2010[79]. Just recently, ES cells from domestic animals could be obtained for gene targeting goals[80]. Advances with farm animal SSCs continued in parallel. For instance, goat transgenic sperm for human growth hormone was derived from male goats that had been transplanted with SSCs previously manipulated in vitro[81]. In a report from the same group, pig embryos, transgenic for a reporter gene (enhanced green fluorescent protein), could be obtained after oocytes were fertilized with transgenic sperm from culture transduced and later transplanted SSCs[82]. Very recently, bovine gonocytes were transduced through a lentivirus vector and the reporter transgene product proved the success of the procedure[83]. In dogs, a species with a strong potential to provide new models for human genetic diseases, SSCs have been characterized, cultured, genetically modified and allogeneically transplanted with eventual collection of transgenic sperm[84].
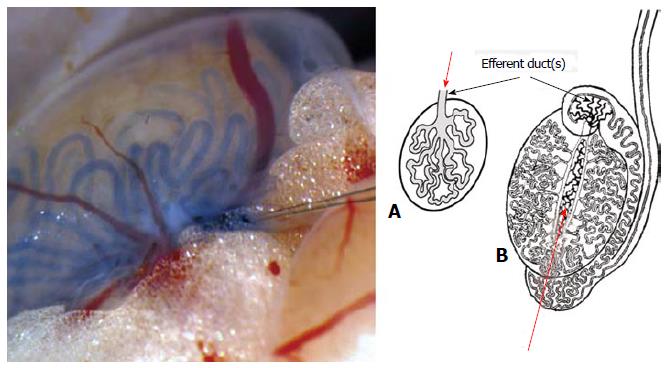
SSC transplantation is an important tool for several other biotechnological applications such as gene therapy or fertility restoration. The first SSC successful transplantation was reported in 1994 in the pioneering work of the Brinster’s group[85]. Inspired on original hemopoietic fundamental transplantation work[86], this research group isolated germ cells from a donor mice that were later unequivocally identified as SSCs on a recipient mouse testis after injection into the seminiferous tubule system, based on their property to regenerate spermatogenesis in the foreign testicular niche (for a comprehensive review on this technique see[87]). This allogeneic type of transplantation is presently the basis for the functional assay to detect true SSCs in experimental models. The technique could be developed due to the presence of a single, easy to cannulate efferent duct in mouse species and to the fact that the mice strain used was immunocompromised. Unfortunately, this SSC transplantation procedure is technically demanding in species with several efferent ducts because, in these species they tend to be short, thin and highly convoluted (Figure 3). This anatomical condition corresponds to most farm animals[88], primates[89] and humans[90].
Xenogeneic transplantation of SSC cell suspensions into the mouse testis has been attempted by several groups[91]. For the first time, SSCs from a foreign species (rat) not only were able to colonize mouse seminiferous tubules but also to differentiate and produce normal rat sperm[92]. However, attempts with other SSC donor species showed that the farther apart in phylogenetical distance is the donor species from the mouse (recipient species), the lower level of progress of differentiation in spermatogenesis from the transplanted SSCs will be achieved within the foreign seminiferous epithelium environment (for details on SSC transplantation into the mouse testis, involving different donor species, see[91]). Accordingly, domestic animals and human beings as SSC donors are among those species in which this technique is not yet successful. Thus, on these and many other species, functional assays to unequivocally detect the presence of their SSCs are very necessary.
Allogeneic SSC transplantation (SSCs transplanted from a donor animal to a recipient testis of another animal, both from the same species) has proven difficult in species other than rodents, particularly in large animals. Besides anatomical difficulties, each species seems to require specific technical protocols. Allogeneic transplantation has been attempted in several farm animals, including pigs, goats and bulls[93-95]. For instance, transgenic sperm from a donor goat could be unequivocally identified in a recipient buck semen, meaning that transplanted SSCs could colonize and regenerate the spermatogenetic process in the unrelated testis[96]. When the limitations of these techniques are overcome they will facilitate several of the biotechnological applications described on this article. One aspect that still needs to be addressed is the method for depletion of endogenous spermatogenesis in recipient animals. This is typically performed by using either chemical compounds like the alkylating agent busulfan or alternatively testis irradiation. However, protocols need to be species optimized, which requires further research[97,98]. SSC transplantation could also be used for gene delivering purposes in order to increase the frequency of certain genes in animal populations. This would be particularly useful in extensive systems of livestock production, in which animals reproduce through natural service. Surrogate males could be transplanted with SSCs that over-express certain genes linked to increased production traits or from elite bulls in order to disseminate the desired genes in a population. Endangered species could also be benefited with such technologies. For instance, SSCs from the wild species to be preserved could be transferred to a phylogenetically related domestic animal embryo and this way become incorporated into the germline to eventually produce exotic sperm[99,100], this way saving the endangered germplasm.
As an alternative to SSC transplantation into testicular environments, a new technique was developed in which whole testicular tissue explants (containing SSCs attached to their niche) from the desired species are placed in ectopical places such as the back skin (subcutaneous tissue) of immunocompromised mice. This approach was first successfully tried with mouse testis tissue (donor) grafted into mouse skin (recipient)[101,102]. This technique was designated testis tissue xenografting. When xenotransplanting testicular tissues from prepubertal animals (with only undifferentiated germ cells, including SSCs), complete spermatogenesis developed within the transplanted testicular tissues under the sponsoring of the host (mouse) vascular environment and intrinsic steroidogenesis of the donor tissue. This technique has been so far successful with a wide range of donor species including hamsters, rabbits, dogs, cats, sheep, goats, horses, bovines, alpacas and monkeys[103]. Work has also shown that testicular tissue pieces can be cryopreserved and later used with this technique in various species[104,105].
Tissue xenografting has represented a step forward in SSC biology that circumvents many of the current limitations the transplantation of germ cell suspensions into the seminiferous tubules of foreign recipient animals. This technique has been privileged by researchers during the recent years because of the positive outcomes in a range of very dissimilar species. Recently, a related technique was developed in which no whole tissue pieces are transplanted into the mouse skin but instead an assorted cell suspension composed of germ cells and testicular somatic cells, including Sertoli cells. Interestingly, all these cells reassemble under the mouse skin and testicular tissue becomes organized in a process that has been called de novo testicular morphogenesis[106,107]. This novel technique has been tested with testicular cell suspensions from rats[108], mice[109], pigs[110], sheep[111] and recently from a wild pig, the peccary[112]. Testis xenografts have produced so far fertile sperm in pigs and the related peccaries[112-114] demonstrating that SSCs present in the testicular tissues show a correct functioning that leads to the production of viable normal sperm within the body of a foreign species.
Infertility is one of the critical side-effects of oncologycal treatments in humans and particularly in children, an effect which is caused either by chemotherapeutic agents or irradiation. A proposed solution for this problem is the restoration of fertility via SSC transplantation. Since many of the patients in this situation are prepubertal (i.e., do not yet produce sperm), a current approach consists on banking testicular tissue or cell suspensions prior to the oncological treatment and submitting the patient to an autologous transplantation of the preserved material if fertility problems arise later in life[115] (for recent advances in the methods for preservation of fertility on young male human oncological patients see the comprehensive review from Goossens et al[116], 2013). A word of caution is valid regarding safety issues when malignant diseases are managed with transplantation procedures. Invariably, cells suspensions including SSCs to be transplanted should be devoid of cancer cells. In experiments with rats, only as few as 20 leukemia cancer cells were enough to produce the disease when transplanted into healthy rat testis[117]. It is unknown to what extent this is reproducible in humans, but as an obvious measure, cell suspensions should be screened and positively selected for SSCs and/or negatively selected for cancer cells, through approaches such as FACS or special culture conditions[116]. An alternative approach would be to use testis xenografting or allografting with the hope to restore the spermatogenic process ectopically to a degree just enough to produce a few sperm to be later used with ARTs. Nevertheless, although already developed for several animal species (see the topic on Germ cell transplantation above) testicular xenografting requires further investigations to be implemented in humans. In general, more research in monkeys and humans is still needed, particularly on safety issues.
The tremendous progress in SSC research during the last decade is undeniable. Appropriate conditions for SSC long-term culture are already widely known for the most important experimental model: the mouse, but research efforts are still being made to develop culture systems suitable for humans and other species, altogether with the study of the very specific SSC nutrient requirements. Advances such as SSC in vitro mutagenesis are already possible in the mouse. The imminent unraveling of the molecular mechanisms of SSC pluripotency will bring about many other interesting biotechnological possibilities. The long-awaited accomplishment of SSC transplantation techniques for farm animals and humans is under current development, despite the widespread use of testis xenografting in many species with high rates of success. Finally, spermatogenesis in vitro is already possible for laboratory species, so important applications in regenerative medicine and animal reproduction are expected.
The author would like to thank Project Prometeo, SENESCYT and AGROCALIDAD, Ecuador for financial support.
P- Reviewer: Kerkis I, Roelen B, Saeki K S- Editor: Ji FF L- Editor: A E- Editor: Wu HL
| 1. | Guan K, Nayernia K, Maier LS, Wagner S, Dressel R, Lee JH, Nolte J, Wolf F, Li M, Engel W. Pluripotency of spermatogonial stem cells from adult mouse testis. Nature. 2006;440:1199-1203. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 650] [Cited by in F6Publishing: 596] [Article Influence: 33.1] [Reference Citation Analysis (0)] |
| 2. | Kanatsu-Shinohara M, Takehashi M, Shinohara T. Brief history, pitfalls, and prospects of mammalian spermatogonial stem cell research. Cold Spring Harb Symp Quant Biol. 2008;73:17-23. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 3. | Seandel M, James D, Shmelkov SV, Falciatori I, Kim J, Chavala S, Scherr DS, Zhang F, Torres R, Gale NW. Generation of functional multipotent adult stem cells from GPR125+ germline progenitors. Nature. 2007;449:346-350. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 313] [Cited by in F6Publishing: 337] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 4. | Yoshida S, Sukeno M, Nabeshima Y. A vasculature-associated niche for undifferentiated spermatogonia in the mouse testis. Science. 2007;317:1722-1726. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 366] [Cited by in F6Publishing: 377] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 5. | Campos-Junior PH, Costa GM, de Avelar GF, Segatelli TM, Lacerda SM, Aponte PM, de França LR. Morphometric evaluation of the spermatogonial stem cell distribution and niche in vertebrates. Methods Mol Biol. 2013;1035:35-42. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 6. | Hofmann MC. Gdnf signaling pathways within the mammalian spermatogonial stem cell niche. Mol Cell Endocrinol. 2008;288:95-103. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 165] [Cited by in F6Publishing: 168] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 7. | Tiptanavattana N, Thongkittidilok C, Techakumphu M, Tharasanit T. Characterization and in vitro culture of putative spermatogonial stem cells derived from feline testicular tissue. J Reprod Dev. 2013;59:189-195. [PubMed] [Cited in This Article: ] |
| 8. | Lee KH, Lee R, Lee WY, Kim DH, Chung HJ, Kim JH, Kim NH, Choi SH, Kim JH, Song H. Identification and in vitro derivation of spermatogonia in beagle testis. PLoS One. 2014;9:e109963. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 9. | Heidari B, Rahmati-Ahmadabadi M, Akhondi MM, Zarnani AH, Jeddi-Tehrani M, Shirazi A, Naderi MM, Behzadi B. Isolation, identification, and culture of goat spermatogonial stem cells using c-kit and PGP9.5 markers. J Assist Reprod Genet. 2012;29:1029-1038. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 50] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 10. | Izadyar F, Den Ouden K, Creemers LB, Posthuma G, Parvinen M, De Rooij DG. Proliferation and differentiation of bovine type A spermatogonia during long-term culture. Biol Reprod. 2003;68:272-281. [PubMed] [Cited in This Article: ] |
| 11. | Nickkholgh B, Mizrak SC, Korver CM, van Daalen SK, Meissner A, Repping S, van Pelt AM. Enrichment of spermatogonial stem cells from long-term cultured human testicular cells. Fertil Steril. 2014;102:558-565.e5. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 12. | Shinohara T, Avarbock MR, Brinster RL. beta1- and alpha6-integrin are surface markers on mouse spermatogonial stem cells. Proc Natl Acad Sci USA. 1999;96:5504-5509. [PubMed] [Cited in This Article: ] |
| 13. | Shinohara T, Avarbock MR, Brinster RL. Functional analysis of spermatogonial stem cells in Steel and cryptorchid infertile mouse models. Dev Biol. 2000;220:401-411. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 128] [Cited by in F6Publishing: 125] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 14. | Orwig KE, Shinohara T, Avarbock MR, Brinster RL. Functional analysis of stem cells in the adult rat testis. Biol Reprod. 2002;66:944-949. [PubMed] [Cited in This Article: ] |
| 15. | Zheng Y, Zhang Y, Qu R, He Y, Tian X, Zeng W. Spermatogonial stem cells from domestic animals: progress and prospects. Reproduction. 2014;147:R65-R74. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 56] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 16. | Kanatsu-Shinohara M, Ogonuki N, Inoue K, Miki H, Ogura A, Toyokuni S, Shinohara T. Long-term proliferation in culture and germline transmission of mouse male germline stem cells. Biol Reprod. 2003;69:612-616. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 764] [Cited by in F6Publishing: 731] [Article Influence: 34.8] [Reference Citation Analysis (0)] |
| 17. | Shinohara T, Orwig KE, Avarbock MR, Brinster RL. Spermatogonial stem cell enrichment by multiparameter selection of mouse testis cells. Proc Natl Acad Sci U S A. 2000;97:8346-8351. [PubMed] [Cited in This Article: ] |
| 18. | van Pelt AM, Morena AR, van Dissel-Emiliani FM, Boitani C, Gaemers IC, de Rooij DG, Stefanini M. Isolation of the synchronized A spermatogonia from adult vitamin A-deficient rat testes. Biol Reprod. 1996;55:439-444. [PubMed] [Cited in This Article: ] |
| 19. | Lim JJ, Seol DW, Choi KH, Shin DH, Kim HJ, Song SH, Lee DR. Spermatogonial stem cell enrichment using simple grafting of testis and in vitro cultivation. Sci Rep. 2014;4:5923. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 20. | Nagano M, Avarbock MR, Leonida EB, Brinster CJ, Brinster RL. Culture of mouse spermatogonial stem cells. Tissue Cell. 1998;30:389-397. [PubMed] [Cited in This Article: ] |
| 21. | Aponte PM, Soda T, van de Kant HJ, de Rooij DG. Basic features of bovine spermatogonial culture and effects of glial cell line-derived neurotrophic factor. Theriogenology. 2006;65:1828-1847. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 64] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 22. | Aponte PM, Soda T, Teerds KJ, Mizrak SC, van de Kant HJ, de Rooij DG. Propagation of bovine spermatogonial stem cells in vitro. Reproduction. 2008;136:543-557. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 107] [Cited by in F6Publishing: 112] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 23. | Kuijk EW, Colenbrander B, Roelen BA. The effects of growth factors on in vitro-cultured porcine testicular cells. Reproduction. 2009;138:721-731. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 58] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 24. | Zheng Y, Tian X, Zhang Y, Qin J, An J, Zeng W. In vitro propagation of male germline stem cells from piglets. J Assist Reprod Genet. 2013;30:945-952. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 25. | Zhu H, Liu C, Li M, Sun J, Song W, Hua J. Optimization of the conditions of isolation and culture of dairy goat male germline stem cells (mGSC). Anim Reprod Sci. 2013;137:45-52. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 26. | Aponte PM, van Bragt MP, de Rooij DG, van Pelt AM. Spermatogonial stem cells: characteristics and experimental possibilities. APMIS. 2005;113:727-742. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 99] [Cited by in F6Publishing: 102] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 27. | Nagano MC. Techniques for culturing spermatogonial stem cells continue to improve. Biol Reprod. 2011;84:5-6. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 28. | Kanatsu-Shinohara M, Inoue K, Ogonuki N, Morimoto H, Ogura A, Shinohara T. Serum- and feeder-free culture of mouse germline stem cells. Biol Reprod. 2011;84:97-105. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 96] [Cited by in F6Publishing: 99] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 29. | Kanatsu-Shinohara M, Ogonuki N, Matoba S, Morimoto H, Ogura A, Shinohara T. Improved serum- and feeder-free culture of mouse germline stem cells. Biol Reprod. 2014;91:88. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 56] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 30. | Kokkinaki M, Djourabtchi A, Golestaneh N. Long-term Culture of Human SSEA-4 Positive Spermatogonial Stem Cells (SSCs). J Stem Cell Res Ther. 2011;2:pii: 2488. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 31. | Akhondi MM, Mohazzab A, Jeddi-Tehrani M, Sadeghi MR, Eidi A, Khodadadi A, Piravar Z. Propagation of human germ stem cells in long-term culture. Iran J Reprod Med. 2013;11:551-558. [PubMed] [Cited in This Article: ] |
| 32. | de Rooij DG, Griswold MD. Questions about spermatogonia posed and answered since 2000. J Androl. 2012;33:1085-1095. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 79] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 33. | Sato T, Katagiri K, Kubota Y, Ogawa T. In vitro sperm production from mouse spermatogonial stem cell lines using an organ culture method. Nat Protoc. 2013;8:2098-2104. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 96] [Cited by in F6Publishing: 102] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 34. | Yokonishi T, Sato T, Katagiri K, Ogawa T. In vitro spermatogenesis using an organ culture technique. Methods Mol Biol. 2013;927:479-488. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 49] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 35. | Stukenborg JB, Schlatt S, Simoni M, Yeung CH, Elhija MA, Luetjens CM, Huleihel M, Wistuba J. New horizons for in vitro spermatogenesis? An update on novel three-dimensional culture systems as tools for meiotic and post-meiotic differentiation of testicular germ cells. Mol Hum Reprod. 2009;15:521-529. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 142] [Cited by in F6Publishing: 146] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 36. | Abu Elhija M, Lunenfeld E, Schlatt S, Huleihel M. Differentiation of murine male germ cells to spermatozoa in a soft agar culture system. Asian J Androl. 2012;14:285-293. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 105] [Cited by in F6Publishing: 113] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 37. | Tegelenbosch RA, de Rooij DG. A quantitative study of spermatogonial multiplication and stem cell renewal in the C3H/101 F1 hybrid mouse. Mutat Res. 1993;290:193-200. [PubMed] [Cited in This Article: ] |
| 38. | Kossack N, Terwort N, Wistuba J, Ehmcke J, Schlatt S, Schöler H, Kliesch S, Gromoll J. A combined approach facilitates the reliable detection of human spermatogonia in vitro. Hum Reprod. 2013;28:3012-3025. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 48] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 39. | Oatley JM, Brinster RL. The germline stem cell niche unit in mammalian testes. Physiol Rev. 2012;92:577-595. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 328] [Cited by in F6Publishing: 343] [Article Influence: 28.6] [Reference Citation Analysis (0)] |
| 40. | Johnston DS, Olivas E, DiCandeloro P, Wright WW. Stage-specific changes in GDNF expression by rat Sertoli cells: a possible regulator of the replication and differentiation of stem spermatogonia. Biol Reprod. 2011;85:763-769. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 65] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 41. | Meng X, Lindahl M, Hyvönen ME, Parvinen M, de Rooij DG, Hess MW, Raatikainen-Ahokas A, Sainio K, Rauvala H, Lakso M. Regulation of cell fate decision of undifferentiated spermatogonia by GDNF. Science. 2000;287:1489-1493. [PubMed] [Cited in This Article: ] |
| 42. | Kubota H, Avarbock MR, Brinster RL. Growth factors essential for self-renewal and expansion of mouse spermatogonial stem cells. Proc Natl Acad Sci USA. 2004;101:16489-16494. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 685] [Cited by in F6Publishing: 691] [Article Influence: 34.6] [Reference Citation Analysis (0)] |
| 43. | Buageaw A, Sukhwani M, Ben-Yehudah A, Ehmcke J, Rawe VY, Pholpramool C, Orwig KE, Schlatt S. GDNF family receptor alpha1 phenotype of spermatogonial stem cells in immature mouse testes. Biol Reprod. 2005;73:1011-1016. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 152] [Cited by in F6Publishing: 145] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 44. | Reding SC, Stepnoski AL, Cloninger EW, Oatley JM. THY1 is a conserved marker of undifferentiated spermatogonia in the pre-pubertal bull testis. Reproduction. 2010;139:893-903. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 79] [Cited by in F6Publishing: 81] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 45. | Abbasi H, Tahmoorespur M, Hosseini SM, Nasiri Z, Bahadorani M, Hajian M, Nasiri MR, Nasr-Esfahani MH. THY1 as a reliable marker for enrichment of undifferentiated spermatogonia in the goat. Theriogenology. 2013;80:923-932. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 43] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 46. | Zheng Y, He Y, An J, Qin J, Wang Y, Zhang Y, Tian X, Zeng W. THY1 is a surface marker of porcine gonocytes. Reprod Fertil Dev. 2014;26:533-539. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 28] [Article Influence: 2.8] [Reference Citation Analysis (1)] |
| 47. | Maki CB, Pacchiarotti J, Ramos T, Pascual M, Pham J, Kinjo J, Anorve S, Izadyar F. Phenotypic and molecular characterization of spermatogonial stem cells in adult primate testes. Hum Reprod. 2009;24:1480-1491. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 59] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 48. | Altman E, Yango P, Moustafa R, Smith JF, Klatsky PC, Tran ND. Characterization of human spermatogonial stem cell markers in fetal, pediatric, and adult testicular tissues. Reproduction. 2014;148:417-427. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 49. | Sachs C, Robinson BD, Andres Martin L, Webster T, Gilbert M, Lo HY, Rafii S, Ng CK, Seandel M. Evaluation of candidate spermatogonial markers ID4 and GPR125 in testes of adult human cadaveric organ donors. Andrology. 2014;2:607-614. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 50. | He Z, Kokkinaki M, Jiang J, Dobrinski I, Dym M. Isolation, characterization, and culture of human spermatogonia. Biol Reprod. 2010;82:363-372. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 226] [Cited by in F6Publishing: 223] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 51. | Valli H, Sukhwani M, Dovey SL, Peters KA, Donohue J, Castro CA, Chu T, Marshall GR, Orwig KE. Fluorescence- and magnetic-activated cell sorting strategies to isolate and enrich human spermatogonial stem cells. Fertil Steril. 2014;102:566-580.e7. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 116] [Cited by in F6Publishing: 122] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 52. | Aloisio GM, Nakada Y, Saatcioglu HD, Peña CG, Baker MD, Tarnawa ED, Mukherjee J, Manjunath H, Bugde A, Sengupta AL. PAX7 expression defines germline stem cells in the adult testis. J Clin Invest. 2014;124:3929-3944. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 114] [Cited by in F6Publishing: 121] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 53. | Doetschman T, Williams P, Maeda N. Establishment of hamster blastocyst-derived embryonic stem (ES) cells. Dev Biol. 1988;127:224-227. [PubMed] [Cited in This Article: ] |
| 54. | Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145-1147. [PubMed] [Cited in This Article: ] |
| 55. | Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663-676. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17989] [Cited by in F6Publishing: 17055] [Article Influence: 947.5] [Reference Citation Analysis (0)] |
| 56. | Lee J, Kanatsu-Shinohara M, Ogonuki N, Miki H, Inoue K, Morimoto T, Morimoto H, Ogura A, Shinohara T. Heritable imprinting defect caused by epigenetic abnormalities in mouse spermatogonial stem cells. Biol Reprod. 2009;80:518-527. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 33] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 57. | de Rooij DG, Mizrak SC. Deriving multipotent stem cells from mouse spermatogonial stem cells: a new tool for developmental and clinical research. Development. 2008;135:2207-2213. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 46] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 58. | Kanatsu-Shinohara M, Inoue K, Lee J, Yoshimoto M, Ogonuki N, Miki H, Baba S, Kato T, Kazuki Y, Toyokuni S. Generation of pluripotent stem cells from neonatal mouse testis. Cell. 2004;119:1001-1012. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 618] [Cited by in F6Publishing: 561] [Article Influence: 29.5] [Reference Citation Analysis (0)] |
| 59. | Kanatsu-Shinohara M, Ogonuki N, Iwano T, Lee J, Kazuki Y, Inoue K, Miki H, Takehashi M, Toyokuni S, Shinkai Y. Genetic and epigenetic properties of mouse male germline stem cells during long-term culture. Development. 2005;132:4155-4163. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 175] [Cited by in F6Publishing: 165] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 60. | Ramirez JM, Bai Q, Dijon-Grinand M, Assou S, Gerbal-Chaloin S, Hamamah S, De Vos J. Human pluripotent stem cells: from biology to cell therapy. World J Stem Cells. 2010;2:24-33. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 61. | Lee J, Shinohara T. Epigenetic modifications and self-renewal regulation of mouse germline stem cells. Cell Res. 2011;21:1164-1171. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 62. | Izadyar F, Pau F, Marh J, Slepko N, Wang T, Gonzalez R, Ramos T, Howerton K, Sayre C, Silva F. Generation of multipotent cell lines from a distinct population of male germ line stem cells. Reproduction. 2008;135:771-784. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 96] [Cited by in F6Publishing: 103] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 63. | Kobayashi T, Yamaguchi T, Hamanaka S, Kato-Itoh M, Yamazaki Y, Ibata M, Sato H, Lee YS, Usui J, Knisely AS. Generation of rat pancreas in mouse by interspecific blastocyst injection of pluripotent stem cells. Cell. 2010;142:787-799. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 402] [Cited by in F6Publishing: 385] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 64. | Usui J, Kobayashi T, Yamaguchi T, Knisely AS, Nishinakamura R, Nakauchi H. Generation of kidney from pluripotent stem cells via blastocyst complementation. Am J Pathol. 2012;180:2417-2426. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 152] [Cited by in F6Publishing: 143] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 65. | Matsunari H, Nagashima H, Watanabe M, Umeyama K, Nakano K, Nagaya M, Kobayashi T, Yamaguchi T, Sumazaki R, Herzenberg LA. Blastocyst complementation generates exogenic pancreas in vivo in apancreatic cloned pigs. Proc Natl Acad Sci USA. 2013;110:4557-4562. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 192] [Cited by in F6Publishing: 184] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 66. | Conrad S, Renninger M, Hennenlotter J, Wiesner T, Just L, Bonin M, Aicher W, Bühring HJ, Mattheus U, Mack A. Generation of pluripotent stem cells from adult human testis. Nature. 2008;456:344-349. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 374] [Cited by in F6Publishing: 396] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 67. | Tapia N, Araúzo-Bravo MJ, Ko K, Schöler HR. Concise review: challenging the pluripotency of human testis-derived ESC-like cells. Stem Cells. 2011;29:1165-1169. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 68. | Golestaneh N, Kokkinaki M, Pant D, Jiang J, DeStefano D, Fernandez-Bueno C, Rone JD, Haddad BR, Gallicano GI, Dym M. Pluripotent stem cells derived from adult human testes. Stem Cells Dev. 2009;18:1115-1126. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 144] [Cited by in F6Publishing: 155] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 69. | Kossack N, Meneses J, Shefi S, Nguyen HN, Chavez S, Nicholas C, Gromoll J, Turek PJ, Reijo-Pera RA. Isolation and characterization of pluripotent human spermatogonial stem cell-derived cells. Stem Cells. 2009;27:138-149. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 208] [Cited by in F6Publishing: 222] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 70. | Mizrak SC, Chikhovskaya JV, Sadri-Ardekani H, van Daalen S, Korver CM, Hovingh SE, Roepers-Gajadien HL, Raya A, Fluiter K, de Reijke TM. Embryonic stem cell-like cells derived from adult human testis. Hum Reprod. 2010;25:158-167. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 92] [Cited by in F6Publishing: 102] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 71. | Chikhovskaya JV, Jonker MJ, Meissner A, Breit TM, Repping S, van Pelt AM. Human testis-derived embryonic stem cell-like cells are not pluripotent, but possess potential of mesenchymal progenitors. Hum Reprod. 2012;27:210-221. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 42] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 72. | Resnick JL, Bixler LS, Cheng L, Donovan PJ. Long-term proliferation of mouse primordial germ cells in culture. Nature. 1992;359:550-551. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 645] [Cited by in F6Publishing: 662] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 73. | Kimura T, Tomooka M, Yamano N, Murayama K, Matoba S, Umehara H, Kanai Y, Nakano T. AKT signaling promotes derivation of embryonic germ cells from primordial germ cells. Development. 2008;135:869-879. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 71] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 74. | Takashima S, Hirose M, Ogonuki N, Ebisuya M, Inoue K, Kanatsu-Shinohara M, Tanaka T, Nishida E, Ogura A, Shinohara T. Regulation of pluripotency in male germline stem cells by Dmrt1. Genes Dev. 2013;27:1949-1958. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 49] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 75. | Nagano M, Brinster CJ, Orwig KE, Ryu BY, Avarbock MR, Brinster RL. Transgenic mice produced by retroviral transduction of male germ-line stem cells. Proc Natl Acad Sci USA. 2001;98:13090-13095. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 229] [Cited by in F6Publishing: 238] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 76. | Kanatsu-Shinohara M, Toyokuni S, Shinohara T. Genetic selection of mouse male germline stem cells in vitro: offspring from single stem cells. Biol Reprod. 2005;72:236-240. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 88] [Cited by in F6Publishing: 89] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 77. | Takehashi M, Kanatsu-Shinohara M, Inoue K, Ogonuki N, Miki H, Toyokuni S, Ogura A, Shinohara T. Adenovirus-mediated gene delivery into mouse spermatogonial stem cells. Proc Natl Acad Sci USA. 2007;104:2596-2601. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 47] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 78. | Ryu BY, Orwig KE, Oatley JM, Lin CC, Chang LJ, Avarbock MR, Brinster RL. Efficient generation of transgenic rats through the male germline using lentiviral transduction and transplantation of spermatogonial stem cells. J Androl. 2007;28:353-360. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 70] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 79. | Tong C, Li P, Wu NL, Yan Y, Ying QL. Production of p53 gene knockout rats by homologous recombination in embryonic stem cells. Nature. 2010;467:211-213. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 220] [Cited by in F6Publishing: 209] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 80. | Miao X. Recent advances in the development of new transgenic animal technology. Cell Mol Life Sci. 2013;70:815-828. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 81. | Zeng W, Tang L, Bondareva A, Luo J, Megee SO, Modelski M, Blash S, Melican DT, Destrempes MM, Overton SA. Non-viral transfection of goat germline stem cells by nucleofection results in production of transgenic sperm after germ cell transplantation. Mol Reprod Dev. 2012;79:255-261. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 82. | Zeng W, Tang L, Bondareva A, Honaramooz A, Tanco V, Dores C, Megee S, Modelski M, Rodriguez-Sosa JR, Paczkowski M. Viral transduction of male germline stem cells results in transgene transmission after germ cell transplantation in pigs. Biol Reprod. 2013;88:27. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 46] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 83. | Kim KJ, Cho CM, Kim BG, Lee YA, Kim BJ, Kim YH, Kim CG, Schmidt JA, Ryu BY. Lentiviral modification of enriched populations of bovine male gonocytes. J Anim Sci. 2014;92:106-118. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 84. | Harkey MA, Asano A, Zoulas ME, Torok-Storb B, Nagashima J, Travis A. Isolation, genetic manipulation, and transplantation of canine spermatogonial stem cells: progress toward transgenesis through the male germ-line. Reproduction. 2013;146:75-90. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 85. | Brinster RL, Zimmermann JW. Spermatogenesis following male germ-cell transplantation. Proc Natl Acad Sci USA. 1994;91:11298-11302. [PubMed] [Cited in This Article: ] |
| 86. | Till JE, Mcculloch EA. A direct measurement of the radiation sensitivity of normal mouse bone marrow cells. Radiat Res. 1961;14:213-222. [PubMed] [Cited in This Article: ] |
| 87. | Tang L, Rodriguez-Sosa JR, Dobrinski I. Germ cell transplantation and testis tissue xenografting in mice. J Vis Exp. 2012;pii: 3545. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 88. | Dobrinski I. Germ cell transplantation and testis tissue xenografting in domestic animals. Anim Reprod Sci. 2005;89:137-145. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 50] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 89. | Schlatt S, Rosiepen G, Weinbauer GF, Rolf C, Brook PF, Nieschlag E. Germ cell transfer into rat, bovine, monkey and human testes. Hum Reprod. 1999;14:144-150. [PubMed] [Cited in This Article: ] |
| 90. | Faes K, Tournaye H, Goethals L, Lahoutte T, Hoorens A, Goossens E. Testicular cell transplantation into the human testes. Fertil Steril. 2013;100:981-988. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 91. | Honaramooz A, Yang Y. Recent advances in application of male germ cell transplantation in farm animals. Vet Med Int. 2010;2011:pii: 657860. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 92. | Clouthier DE, Avarbock MR, Maika SD, Hammer RE, Brinster RL. Rat spermatogenesis in mouse testis. Nature. 1996;381:418-421. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 276] [Cited by in F6Publishing: 267] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 93. | Honaramooz A, Megee SO, Dobrinski I. Germ cell transplantation in pigs. Biol Reprod. 2002;66:21-28. [PubMed] [Cited in This Article: ] |
| 94. | Honaramooz A, Behboodi E, Blash S, Megee SO, Dobrinski I. Germ cell transplantation in goats. Mol Reprod Dev. 2003;64:422-428. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 135] [Cited by in F6Publishing: 140] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 95. | Izadyar F, Den Ouden K, Stout TA, Stout J, Coret J, Lankveld DP, Spoormakers TJ, Colenbrander B, Oldenbroek JK, Van der Ploeg KD. Autologous and homologous transplantation of bovine spermatogonial stem cells. Reproduction. 2003;126:765-774. [PubMed] [Cited in This Article: ] |
| 96. | Honaramooz A, Behboodi E, Megee SO, Overton SA, Galantino-Homer H, Echelard Y, Dobrinski I. Fertility and germline transmission of donor haplotype following germ cell transplantation in immunocompetent goats. Biol Reprod. 2003;69:1260-1264. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 178] [Cited by in F6Publishing: 183] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 97. | Honaramooz A, Behboodi E, Hausler CL, Blash S, Ayres S, Azuma C, Echelard Y, Dobrinski I. Depletion of endogenous germ cells in male pigs and goats in preparation for germ cell transplantation. J Androl. 2005;26:698-705. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 66] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 98. | Wang DZ, Zhou XH, Yuan YL, Zheng XM. Optimal dose of busulfan for depleting testicular germ cells of recipient mice before spermatogonial transplantation. Asian J Androl. 2010;12:263-270. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 33] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 99. | Lacerda SM, Costa GM, Campos-Junior PH, Segatelli TM, Yazawa R, Takeuchi Y, Morita T, Yoshizaki G, França LR. Germ cell transplantation as a potential biotechnological approach to fish reproduction. Fish Physiol Biochem. 2013;39:3-11. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 100. | Roe M, McDonald N, Durrant B, Jensen T. Xenogeneic transfer of adult quail (Coturnix coturnix) spermatogonial stem cells to embryonic chicken (Gallus gallus) hosts: a model for avian conservation. Biol Reprod. 2013;88:129. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 101. | Honaramooz A, Snedaker A, Boiani M, Schöler H, Dobrinski I, Schlatt S. Sperm from neonatal mammalian testes grafted in mice. Nature. 2002;418:778-781. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 343] [Cited by in F6Publishing: 350] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 102. | Schlatt S, Honaramooz A, Boiani M, Schöler HR, Dobrinski I. Progeny from sperm obtained after ectopic grafting of neonatal mouse testes. Biol Reprod. 2003;68:2331-2335. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 176] [Cited by in F6Publishing: 152] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 103. | Arregui L, Dobrinski I. Xenografting of testicular tissue pieces: 12 years of an in vivo spermatogenesis system. Reproduction. 2014;148:R71-R84. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 42] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 104. | Jahnukainen K, Ehmcke J, Hergenrother SD, Schlatt S. Effect of cold storage and cryopreservation of immature non-human primate testicular tissue on spermatogonial stem cell potential in xenografts. Hum Reprod. 2007;22:1060-1067. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 100] [Cited by in F6Publishing: 105] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 105. | Zeng W, Snedaker AK, Megee S, Rathi R, Chen F, Honaramooz A, Dobrinski I. Preservation and transplantation of porcine testis tissue. Reprod Fertil Dev. 2009;21:489-497. [PubMed] [Cited in This Article: ] |
| 106. | Dobrinski I. De novo morphogenesis of functional testis tissue after ectopic transplantation of isolated cells. Organogenesis. 2007;3:79-82. [PubMed] [Cited in This Article: ] |
| 107. | Dores C, Dobrinski I. De novo morphogenesis of testis tissue: an improved bioassay to investigate the role of VEGF165 during testis formation. Reproduction. 2014;148:109-117. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 108. | Gassei K, Schlatt S, Ehmcke J. De novo morphogenesis of seminiferous tubules from dissociated immature rat testicular cells in xenografts. J Androl. 2006;27:611-618. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 77] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 109. | Kita K, Watanabe T, Ohsaka K, Hayashi H, Kubota Y, Nagashima Y, Aoki I, Taniguchi H, Noce T, Inoue K. Production of functional spermatids from mouse germline stem cells in ectopically reconstituted seminiferous tubules. Biol Reprod. 2007;76:211-217. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 77] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 110. | Honaramooz A, Megee SO, Rathi R, Dobrinski I. Building a testis: formation of functional testis tissue after transplantation of isolated porcine (Sus scrofa) testis cells. Biol Reprod. 2007;76:43-47. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 77] [Cited by in F6Publishing: 79] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 111. | Arregui L, Rathi R, Megee SO, Honaramooz A, Gomendio M, Roldan ER, Dobrinski I. Xenografting of sheep testis tissue and isolated cells as a model for preservation of genetic material from endangered ungulates. Reproduction. 2008;136:85-93. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 64] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 112. | Campos-Junior PH, Costa GM, Avelar GF, Lacerda SM, da Costa NN, Ohashi OM, Miranda Mdos S, Barcelos LS, Jorge EC, Guimarães DA. Derivation of sperm from xenografted testis cells and tissues of the peccary (Tayassu tajacu). Reproduction. 2014;147:291-299. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 113. | Honaramooz A, Cui XS, Kim NH, Dobrinski I. Porcine embryos produced after intracytoplasmic sperm injection using xenogeneic pig sperm from neonatal testis tissue grafted in mice. Reprod Fertil Dev. 2008;20:802-807. [PubMed] [Cited in This Article: ] |
| 114. | Nakai M, Kaneko H, Somfai T, Maedomari N, Ozawa M, Noguchi J, Ito J, Kashiwazaki N, Kikuchi K. Production of viable piglets for the first time using sperm derived from ectopic testicular xenografts. Reproduction. 2010;139:331-335. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 61] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 115. | Struijk RB, Mulder CL, van der Veen F, van Pelt AM, Repping S. Restoring fertility in sterile childhood cancer survivors by autotransplanting spermatogonial stem cells: are we there yet? Biomed Res Int. 2013;2013:903142. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 46] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 116. | Goossens E, Van Saen D, Tournaye H. Spermatogonial stem cell preservation and transplantation: from research to clinic. Hum Reprod. 2013;28:897-907. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 104] [Cited by in F6Publishing: 98] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 117. | Jahnukainen K, Hou M, Petersen C, Setchell B, Söder O. Intratesticular transplantation of testicular cells from leukemic rats causes transmission of leukemia. Cancer Res. 2001;61:706-710. [PubMed] [Cited in This Article: ] |
| 118. | Takehashi M, Kanatsu-Shinohara M, Shinohara T. Generation of genetically modified animals using spermatogonial stem cells. Dev Growth Differ. 2010;52:303-310. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |