Published online Apr 26, 2015. doi: 10.4252/wjsc.v7.i3.596
Peer-review started: October 1, 2014
First decision: October 28, 2014
Revised: November 13, 2014
Accepted: December 29, 2014
Article in press: December 31, 2014
Published online: April 26, 2015
Processing time: 205 Days and 14.1 Hours
Human cell types affected by retinal diseases (such as age-related macular degeneration or retinitis pimentosa) are limited in cell number and of reduced accessibility. As a consequence, their isolation for in vitro studies of disease mechanisms or for drug screening efforts is fastidious. Human pluripotent stem cells (hPSCs), either of embryonic origin or through reprogramming of adult somatic cells, represent a new promising way to generate models of human retinopathies, explore the physiopathological mechanisms and develop novel therapeutic strategies. Disease-specific human embryonic stem cells were the first source of material to be used to study certain disease states. The recent demonstration that human somatic cells, such as fibroblasts or blood cells, can be genetically converted to induce pluripotent stem cells together with the continuous improvement of methods to differentiate these cells into disease-affected cellular subtypes opens new perspectives to model and understand a large number of human pathologies, including retinopathies. This review focuses on the added value of hPSCs for the disease modeling of human retinopathies and the study of their molecular pathological mechanisms. We also discuss the recent use of these cells for establishing the validation studies for therapeutic intervention and for the screening of large compound libraries to identify candidate drugs.
Core tip: Human pluripotent stem cells (hPSCs) are usually evoked for their potential for regenerative medicine. However, beside these aspects, novel interests raise from the potential of hPSCs to model human diseases including retinopathies. In this review, we describe how these cells allow the study of the molecular mechanisms leading to some form of retinopathies through the development of retinal specific cell type derived from the differentiation of hPSCs. We also discuss the use of hPSCs cellular models for the validation of gene therapy and for drug screening purpose.
- Citation: Ben M’Barek K, Regent F, Monville C. Use of human pluripotent stem cells to study and treat retinopathies. World J Stem Cells 2015; 7(3): 596-604
- URL: https://www.wjgnet.com/1948-0210/full/v7/i3/596.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v7.i3.596
Retinal diseases, such as age-related macular degeneration (AMD) and retinitis pigmentosa (RP) are the leading causes of blindness in the developed world, collectively affecting to different degree as many as one-third of all people over the age of 75[1-3]. This burden is predicted to increase with the ageing of the population in Western countries.
RP is one of the most common inherited diseases of the retina (retinopathies). It is estimated to affect 1 in 3500 to 1 in 4000 people in the United States and Europe. Genes involved in RP are numerous (https://sph.uth.edu/retnet/disease.htm) and can affect specifically retinal pigment epithelium (RPE) or photoreceptors or both. Forms of RP and related diseases include Usher syndrome, Leber’s congenital amaurosis, rod-cone disease, Bardet-Biedl syndrome, and Refsum disease, among others[4]. RP is traduced clinically with a gradual decline in vision as a consequence of photoreceptor cells (rods and cones) dying either from cell autonomous origin or secondary from a defect of RPE cells, which support their survival.
Up to now, there is no curative treatment for these pathologies. Among others (for review, see[5]), the more promising areas of research for RP therapies include gene therapy aimed at restoring defective genes, and cell transplantation to replace defective or dead cells.
Gene therapy has been shown to improve visual function in inherited retinal diseases[6,7]. Most gene therapies involved integration of vector DNA into the specific cells of retina[8,9] and even combine RNA interference (RNAi)-based gene silencing with gene replacement in RP[10,11]. While promising, gene therapy aiming at restoring gene defects in the RPE (like RPE65) shows limitations. Indeed, the prerequisite to this type of gene therapy is to know precisely the defective gene (need the genotyping of each patient) and a second limitation is to develop a gene delivery candidate drug for each one of the defective genes (cost-intensive). Moreover, recent data from preclinical and clinical studies show that, despite stabilization of vision during the follow-up period, patients treated by RPE65 gene therapy still undergo photoreceptor degeneration[6].
The other strategy consists on the replacement of died or defective cells by transplantation procedures. The eye has numerous advantages for developing cell therapies as all tissues of the eye are surgically accessible, transplanted cells can be monitored by microscopic analysis and the inherent amplification of the visual system means that relatively small number of rescued cells or transplanted cells could have a detectable effect on vision. Moreover the ocular immune privilege might greatly simplify immunosuppressive treatment after transplantation. Initial subretinal transplant studies employed tissue-specific stem cells obtained from human fetuses, known as retinal progenitors[12]. Extension of this work revealed that post-mitotic photoreceptor precursor cells are optimal for retinal integration and restoration of retinal structures[13-16]. Unfortunately, in human embryonic development, both retinal progenitor and photoreceptor are generated relatively late (i.e., 14 and 24 wk of gestation respectively[17-20]). The serious ethical implication of this fact, coupled with the practical reality of the relatively small number of photoreceptor precursor cells that can be obtained from a pair of fetal donor eye make it quite unlikely that this cell will be used to any meaningful extent in the treatment of human retinal disease.
In that context, scientists developed new strategies to overcome these constraints. Moreover, a better comprehension of the different molecular mechanisms leading ultimately to RP or AMD is crucial in order to find new treatments. Current animal models of RP are not available for the hundreds of causal mutations. That’s why human pluripotent stem cells (hPSCs) lines are rapidly changing the strategies scientists can implement to understand pathological mechanisms and cure genetic diseases.
In this review, we focus on the different opportunities concerning the use of human pluripotent stem cells for the disease modeling as well as the future challenges of such research. We also develop the recent improvements in the genetic manipulation of human pluripotent stem cells and the consequences of these on disease modeling and drug screening for retinal diseases. Finally the availability of such human cellular models allowing the validation of therapeutical strategies as unexpected as gene therapy will be discussed.
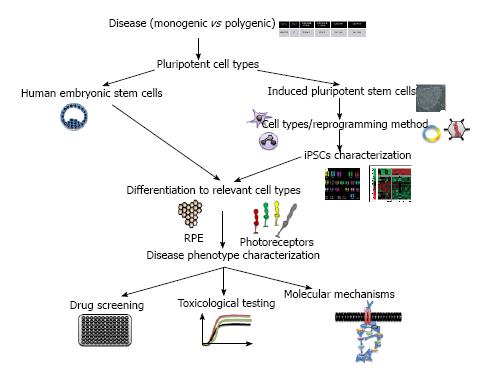
Two main sources of hPSCs are described: human embryonic stem cells (hESCs) and human induced pluripotent stem cells (hiPSCs). hESCs are pluripotent cells harvested from the inner cell mass of day-5 human blastocysts that are leftover from in vitro fertilizations[21]. hiPSCs are reprogrammed somatic cells that share many similarities with hESCs[22,23]. Pluripotency is key to the derivation of cell phenotypes that are relevant for disease, including those that are essentially inaccessible in any other way (i.e., post-mitotic retinal cells). Unlimited self-renewal provides easy access to the biological resource of interest[24].
iPSCs have been generated from a range of primary cell types, including fibroblasts, keratinocytes, lymphoblasts, chord blood cells, mesenchymal stem cells and even RPE cells (Figure 1). Each cell type has its own characteristics in terms of the number of factors needed for reprogramming and the dynamics of derivation (for review[25,26]). The generation of iPSCs from older individuals is more difficult and less efficient than the generation of iPSCs from young individuals[27]. To help overcome this limitation, a variety of different reprogramming factors, reprogramming enhancers, and cell types have been evaluated[27-39] (Figure 1). Of the accessible cell types used for iPSC generation, the reprogramming of keratinocytes has been shown to be as much as 100-fold more efficient and at least twofold faster than the reprogramming of dermal fibroblasts[40]. Moreover, the keratinocytes-derived iPSCs are more similar to embryonic stem cells than those generated from fibroblasts[41]. Finally, for disease modeling, non-integrative methods should be preferable to integrative ones even if less mandatory than for cell therapy programs.
hPSC-derived retinal cells are valuable new tools for investigators seeking to understand and treat degenerative retinal diseases. These cells will allow scientists to explore the pathophysiology of human diseases in ways that were previously possible only in some animal models. The need for better models, given our poor understanding of the pathogenesis of complex diseases and the dismal predictive record of animal models and tumor-derived cell lines push towards the use of hPSCs for modeling diseases in a dish.
Any disorder confirmed or suspected to have genetic basis can be modeled, but the choice of which disease to model depends on a number of considerations[42]. Not all diseases will be equally easy to model. For example, one should consider modeling monogenic and early-onset diseases than polygenic and late-onset diseases, as it would reproduce better aspects of the diseases (Figure 1). Monogenic diseases modeling will allow for a more carefully controlled comparison with genetically corrected cells and demonstration that the disease phenotypes observed are really caused by the original mutation. Commercial and academic repositories offer primary cell lines or immortalized cell lines for use in research. Most, if not all, disease-causing mutations will be specific to the cell context and affect some cell types more than others. For certain purposes, working with cell lines could be difficult even impossible. This is the case for most retinopathies affecting cells that are not available for biopsies. Genetic disorders of the eye are so numerous that multiple examples exist of conditions that primarily affect photoreceptor cells, ganglion cells, RPE cells, retinal vasculature, choroidal vasculature and eye development. hPSCs systems can offer the possibility to model disease that show a non-cell-autonomous component as both component (i.e., RPE and photoreceptors) can be differentiated from hPSCs and then eventually co-cultured.
Several groups attempted to reconstitute retinal tissue in vitro using mice or human cells. A variety of different protocols, utilizing both two- and three-dimensional culture systems, have succeeded in deriving photoreceptor precursor cells from less differentiated precursors[43-60]. In vitro generated organs from hPSCs constitute powerful systems to model human diseases and perform large-scale drug screening.
Within the spectrum of primary retinal disorders, genetic diseases of the RPE are perhaps the most promising candidates for hiPSCs and re-seeded onto a variety of substrates[61]. RPE cultured from multiple sources, including hPSCs, has been shown to adopt a mature phenotype, and exhibit key physiological functions in vitro[47,62-64].
Several studies have been published describing disease modeling using hPSCs. Singh and collaborators recently developed a hiPSC-RPE “model in a dish” of Best vitelliform macular dystrophy (BVMD)[65]. BVDM is caused by a defect in the RPE gene BEST1, which results in the subretinal accumulation of photoreceptor waste products, i.e., lipofuscin, and fluid, leading to secondary photoreceptor death and central vision loss. Using physiological stressors, they reproduced some of the cellular defects observed in the disease and suggested a role of intracellular calcium regulation and oxidative stress in the mechanism of disease[65]. Similar conclusions were made with other RP models[66] involving endoplasmic reticulum stress. In this study, several hiPSCs lines were created from 5 patients carrying mutations in different genes involved in RP (RP1, PRPH2, RHO, RP9). After 120-150 d of culture, mature photoreceptors (Rhodopsin positive) derived from all cell lines died, reproducing features observed in the diseases. One line, RP9, showed a much lower level of rhodopsin expression and has higher levels of oxidative stress. Using a slightly different differentiation protocol and using integration-free hiPSC, this research team and others confirm that patient-specific rod cells may recapitulate RP features in vitro and could be appropriate model to study retinal degenerative diseases[67,68]. hiPSC-derived retinal precursors were also used to identify a likely disease-causing homozygous mutation in a gene that had not been previously reported to be associated with disease[50]. In a more recent study, the same group combined Next-Generation and Sanger sequencing to identify disease-causing USH2A (responsible for Usher syndrome type I) mutations in an adult patient with autosomal recessive RP[51]. Moreover, by grafting the mutated photoreceptors into mice model of the disease, they showed that the mutation might act via post-developmental photoreceptor degeneration rather than during development.
Finally, hPSCs could model systems to study retinal development and implication of specific transcription factors. Phillips et al[69] (2014) generate hiPSC from a patient with a mutation in the transcription factor visual systems homeobox 2 (VSX2 also known as CHX10) homeodomain. They showed that, using differentiation protocols in order to obtain optical vesicles (OV), mutated hiPSC-OV failed to produce bipolar cells and demonstrated delayed photoreceptor maturation[69].
All these studies highlight the potential of retinal cells derived from hPSCs to help to identify pathophysiological pathway for targeted development of therapies and decipher key factors in retinal development.
The identification and functional validation of sequence variants affecting diverse human traits, including disease susceptibility, is essential for the understanding of human biology and disease mechanisms. Considering the limited number of individual lines often used to model disease mechanisms and the high incidence of line-to-line variations, the possibility of wrongly attributing to a disease-causing mutation a phenotype that is in fact the result of the uneven distribution of such alterations in control and diseased groups needs to be considered seriously. When disease-specific hPSCs are used to identify novel aspects of disease mechanisms, rather than simply to replicate already known pathological features, internal (isogenic) control experiments such as genetic correction or more classical gain- and loss-of-function experiments are extremely valuable[24]. While classical gene-targeting technology via homologous recombination in mouse embryonic stem cells has proven a powerful tool to dissect gene function[70-72], this approach has been quite inefficient when applied to hPSCs[73]. The development of site-specific TALEN- or CRISPR-based genome editing approaches is proving to have great utility for endogenous gene correction in many cell lines[74-76], including hPSCs[77-79]. A major advantage of these approaches is that unlike exogenous gene addition in which promoter strength and multiplicity of infection may require patient-specific adjustment, TALEN- and CRISPR-mediated corrections have the advantage that the gene remains under the control of the endogenous promoter. Acting as “DNA scissors”, they induce double strand breaks (DSB) at desired genomic loci, triggering the endogenous DNA repair machinery. Processing of DSB by the error-prone nonhomologous end-joining pathway leads to small insertions and deletions (Indels) useful for generating loss-of-function mutations, whereas error-free homology directed repair enables targeted integration of exogenously provided DNA sequences for introducing precise nucleotide alterations or knocking reporters.
Unlike other editing tools, the guide RNAs used for CRISPR/Cas9-based genome editing can be generated relatively easily. The guide RNAs used in this system requires the presence of an adjacent motif, downstream of a 17-20-nucleotide DNA target, termed the protospacer[80-84]. Complementary guide oligonucleotides can be synthetized, annealed and subsequently ligated into a bicistronic vector-expressing scaffold RNA and a modified Cas9 nuclease optimized for efficient targeting of human cells[85,86]. Recent study has shown that by combining these gene editing tools (i.e., TALEN and CRISPR) they have developed a genome-engineering platform, named iCRISPR, that enable rapid and highly efficient biallelic knockout hPSCs for loss-of-function studies, as well as homozygous knocking hPSCs with specific nucleotide alterations[87].
Another strategy could be to use adenovirus in order to replace the mutated gene in hiPSC derived from patients. This was done by Yoshida et al[68], who generated hiPSC from somatic cell of an RP patient carrying a heterozygous mutation in the rhodopsin gene[88]. They observed that rod cells had reduced survival rate. To confirm that the phenotype was due to the expression of mutant rhodopsin and not by genetic polymorphism, they used helper-dependent adenoviral vector to replace the mutated gene and reverted the phenotype observed. Moreover, replacing the wild-type gene reconstructed the pathological condition[68].
A number of potential therapeutic interventions are strictly specific to human cells or tissues, such as biotherapies based upon targeting human gene/mutation with RNA interference, exon skipping, and therapeutic antibodies. With these approaches, the uses of classic animal models of RP for experimental or preclinical validation are often not enough informative. Obtaining sufficient cellular material to conduct a wide range of tests, from the initial assessment of therapeutic claim to the final quality of assurance process before clinical batch release, can be challenging.
Some diseases are so rare that it is not economically possible to bring a treatment through the regulatory gauntlet and into clinical availability. One possibility to overcome this issue would be to use cells or tissues created from a patient’s own cells to test the molecular efficacy of a viral-mediated gene therapy, including the optimal level of expression, and then deliver that therapy to one eye in a compassionate use manner. Moreover, some genes have a very narrow range of therapeutic dose such that overexpression could be as harmful as under expression[89]. The first human gene therapy trial for a retinal disease was performed in 2008 in patients with Leber congenital amaurosis (LCA), a congenital blindness resulting from the breakdown of an important metabolic pathway in the RPE but with long-lasting preservation of the photoreceptors. LCA arises due to mutations in the RPE-specific gene, RPE65[90]. Prior to clinical trials, preclinical trials are usually performed on animal models. Many mouse models have been generated that are defective for a gene causing a specific retinal dystrophy, however, certain of these models have proven asymptomatic[91] or lethal[92]. Moreover, in general, the mouse eye poorly mimics the situation in the human eye due to its small size and structure. Larger animal models are more appropriate for testing the biodistribution of a gene therapy vector and for ascertaining long-term efficiency and safety. The dog eye is particularly suitable as it resembles the human eye, except for the absence of a macula and foveal pit, in size, and many retinal diseases in man have canine counterparts[93]. However, for certain diseases, the identification of a corresponding canine model has proven elusive. Thus, taken together, for a growing number of diseases (and this holds true for disorders affecting other tissues), human cell culture is becoming an essential complement to animal disease research[94]. The results obtained from gene therapy studies in a human system, coupled to in vivo studies using animal models, could lead more rapidly to preclinical gene therapy studies indeed a phase I clinical trial.
For pigmentary retinopathies for which an appropriate animal model does not exist, like choroideremia[95] evaluating the biochemical restoration of a normal cellular phenotype after gene therapy could provide complementary approaches to in vivo animal models that investigate systemic effects that are essentially beyond the reach of in vitro studies[96].
Many drugs that work in animal models have not performed well in human clinical trials[97,98]. This discrepancy could be attributable among other proposed reasons to species variation or incomplete phenotype recapitulation. Numerous studies have now showed that retinal cells derived from hPSCs are closely enough representatives of their in vivo counterparts[60,99] that they could be used for identifying and evaluate pharmacological agents capable of mitigating degenerative retinal diseases. High-throughput drug screens of differentiated hPSCs-derived cells have been suggested as a means for drug discovery and personalized medicine[100-102]. To date, there have been few publications looking at using hPSCs for drug discovery in retinal diseases. Meyer et al[47] reported the restoration of OAT (Ornithine Amino Transferase) enzyme activity in gyrate atrophy hiPSC-RPE following vitamin B6 treatment. In their study, Jin et al[66] showed that treatment of the rhodopsin-positive cells (photoreceptors) with α-tocopherol led to a significant preservation of these cells in RP9 cell line. This study provides proof of principle that hPSC technology is useful in screening for drug responses across numerous related, yet genetically distinct retinal diseases. Even if, major advances have been made to differentiate retinal progenitors from hPSCs, the differentiation effect could vary from various hPSC lines. In a very elegant study, Ferrer et al[103] developed highly efficient methods to differentiate, expand and authenticated iPSC-RPE in parallel to their use in high-throughput assay. The RPE cells were derived from iPSCs and grown in 96- and 384-well plates. They demonstrate differential expression of eight genes in iPSCs, iPSC-derived RPE at two differentiation stages, and primary human RPE using this multiplex assay. This assay provides the basis to screen for compounds that improve RPE function and/or maturation and target disease pathways[103].
Regenerative medicine based on stem cells is likely to make important contributions to the cure of human blindness. This is the main focus of the public creating both fears and hopes. However, it remains only the visible part of the iceberg. Indeed, as described in this review, hPSCs are essential to model disease specific conditions closed to the patient context. They contribute to the development of pharmaceutical compounds that will be identified for efficacy in disease specific cellular of hPSCs derivatives and for which toxicity will be tested in the same cells. Moreover, hPSCs provides valuable complement to animal models by either highlighting species-specific differences or further validating the observations already made in rodents or larger animals.
P- Reviewer: Danisovic L, Jun Y S- Editor: Song XX L- Editor: A E- Editor: Lu YJ
| 1. | de Jong PT. Age-related macular degeneration. N Engl J Med. 2006;355:1474-1485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 585] [Cited by in RCA: 600] [Article Influence: 31.6] [Reference Citation Analysis (0)] |
| 2. | Jager RD, Mieler WF, Miller JW. Age-related macular degeneration. N Engl J Med. 2008;358:2606-2617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1061] [Cited by in RCA: 1071] [Article Influence: 63.0] [Reference Citation Analysis (0)] |
| 3. | Zarbin MA. Current concepts in the pathogenesis of age-related macular degeneration. Arch Ophthalmol. 2004;122:598-614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 642] [Cited by in RCA: 688] [Article Influence: 32.8] [Reference Citation Analysis (0)] |
| 4. | Sparrow JR, Hicks D, Hamel CP. The retinal pigment epithelium in health and disease. Curr Mol Med. 2010;10:802-823. [PubMed] |
| 5. | He Y, Zhang Y, Liu X, Ghazaryan E, Li Y, Xie J, Su G. Recent advances of stem cell therapy for retinitis pigmentosa. Int J Mol Sci. 2014;15:14456-14474. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 6. | Cideciyan AV, Jacobson SG, Beltran WA, Sumaroka A, Swider M, Iwabe S, Roman AJ, Olivares MB, Schwartz SB, Komáromy AM. Human retinal gene therapy for Leber congenital amaurosis shows advancing retinal degeneration despite enduring visual improvement. Proc Natl Acad Sci USA. 2013;110:E517-E525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 303] [Cited by in RCA: 352] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 7. | Wert KJ, Davis RJ, Sancho-Pelluz J, Nishina PM, Tsang SH. Gene therapy provides long-term visual function in a pre-clinical model of retinitis pigmentosa. Hum Mol Genet. 2013;22:558-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 47] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 8. | Conlon TJ, Deng WT, Erger K, Cossette T, Pang JJ, Ryals R, Clément N, Cleaver B, McDoom I, Boye SE. Preclinical potency and safety studies of an AAV2-mediated gene therapy vector for the treatment of MERTK associated retinitis pigmentosa. Hum Gene Ther Clin Dev. 2013;24:23-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 76] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 9. | Dinculescu A, Min SH, Deng WT, Li Q, Hauswirth WW. Gene therapy in the rd6 mouse model of retinal degeneration. Adv Exp Med Biol. 2014;801:711-718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 10. | Millington-Ward S, Chadderton N, O’Reilly M, Palfi A, Goldmann T, Kilty C, Humphries M, Wolfrum U, Bennett J, Humphries P. Suppression and replacement gene therapy for autosomal dominant disease in a murine model of dominant retinitis pigmentosa. Mol Ther. 2011;19:642-649. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 122] [Cited by in RCA: 115] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 11. | Mao H, Gorbatyuk MS, Hauswirth WW, Lewin AS. Gene delivery of wild-type rhodopsin rescues retinal function in an autosomal dominant retinitis pigmentosa mouse model. Adv Exp Med Biol. 2012;723:199-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 12. | Carter-Dawson LD, LaVail MM. Rods and cones in the mouse retina. II. Autoradiographic analysis of cell generation using tritiated thymidine. J Comp Neurol. 1979;188:263-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 289] [Cited by in RCA: 307] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 13. | MacLaren RE, Pearson RA, MacNeil A, Douglas RH, Salt TE, Akimoto M, Swaroop A, Sowden JC, Ali RR. Retinal repair by transplantation of photoreceptor precursors. Nature. 2006;444:203-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 826] [Cited by in RCA: 763] [Article Influence: 40.2] [Reference Citation Analysis (0)] |
| 14. | Bartsch U, Oriyakhel W, Kenna PF, Linke S, Richard G, Petrowitz B, Humphries P, Farrar GJ, Ader M. Retinal cells integrate into the outer nuclear layer and differentiate into mature photoreceptors after subretinal transplantation into adult mice. Exp Eye Res. 2008;86:691-700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 122] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 15. | Pearson RA, Barber AC, Rizzi M, Hippert C, Xue T, West EL, Duran Y, Smith AJ, Chuang JZ, Azam SA. Restoration of vision after transplantation of photoreceptors. Nature. 2012;485:99-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 383] [Cited by in RCA: 382] [Article Influence: 29.4] [Reference Citation Analysis (0)] |
| 16. | Warre-Cornish K, Barber AC, Sowden JC, Ali RR, Pearson RA. Migration, integration and maturation of photoreceptor precursors following transplantation in the mouse retina. Stem Cells Dev. 2014;23:941-954. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 57] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 17. | Schmitt S, Aftab U, Jiang C, Redenti S, Klassen H, Miljan E, Sinden J, Young M. Molecular characterization of human retinal progenitor cells. Invest Ophthalmol Vis Sci. 2009;50:5901-5908. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 45] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 18. | Luo J, Baranov P, Patel S, Ouyang H, Quach J, Wu F, Qiu A, Luo H, Hicks C, Zeng J. Human retinal progenitor cell transplantation preserves vision. J Biol Chem. 2014;289:6362-6371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 83] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 19. | Baranov PY, Tucker BA, Young MJ. Low-oxygen culture conditions extend the multipotent properties of human retinal progenitor cells. Tissue Eng Part A. 2014;20:1465-1475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 20. | Aftab U, Jiang C, Tucker B, Kim JY, Klassen H, Miljan E, Sinden J, Young M. Growth kinetics and transplantation of human retinal progenitor cells. Exp Eye Res. 2009;89:301-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 51] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 21. | Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145-1147. [PubMed] |
| 22. | Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861-872. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14327] [Cited by in RCA: 14315] [Article Influence: 842.1] [Reference Citation Analysis (0)] |
| 23. | Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917-1920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7589] [Cited by in RCA: 7244] [Article Influence: 402.4] [Reference Citation Analysis (0)] |
| 24. | Perrier A, Peschanski M. How can human pluripotent stem cells help decipher and cure Huntington’s disease? Cell Stem Cell. 2012;11:153-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 25. | Tiscornia GC, Moretta R, Argenziano MA, Amorena CE, Garcia Gras EA. Inhibition of connexin 43 in cardiac muscle during intense physical exercise. Scand J Med Sci Sports. 2014;24:336-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 26. | Hu BY, Weick JP, Yu J, Ma LX, Zhang XQ, Thomson JA, Zhang SC. Neural differentiation of human induced pluripotent stem cells follows developmental principles but with variable potency. Proc Natl Acad Sci USA. 2010;107:4335-4340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 778] [Cited by in RCA: 757] [Article Influence: 50.5] [Reference Citation Analysis (0)] |
| 27. | Mahmoudi S, Brunet A. Aging and reprogramming: a two-way street. Curr Opin Cell Biol. 2012;24:744-756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 124] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 28. | Liao J, Wu Z, Wang Y, Cheng L, Cui C, Gao Y, Chen T, Rao L, Chen S, Jia N. Enhanced efficiency of generating induced pluripotent stem (iPS) cells from human somatic cells by a combination of six transcription factors. Cell Res. 2008;18:600-603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 126] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 29. | Judson RL, Babiarz JE, Venere M, Blelloch R. Embryonic stem cell-specific microRNAs promote induced pluripotency. Nat Biotechnol. 2009;27:459-461. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 593] [Cited by in RCA: 530] [Article Influence: 33.1] [Reference Citation Analysis (0)] |
| 30. | Mali P, Ye Z, Chou BK, Yen J, Cheng L. An improved method for generating and identifying human induced pluripotent stem cells. Methods Mol Biol. 2010;636:191-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 31. | Cheng Z, Ito S, Nishio N, Xiao H, Zhang R, Suzuki H, Okawa Y, Murohara T, Isobe K. Establishment of induced pluripotent stem cells from aged mice using bone marrow-derived myeloid cells. J Mol Cell Biol. 2011;3:91-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 46] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 32. | Niibe K, Kawamura Y, Araki D, Morikawa S, Miura K, Suzuki S, Shimmura S, Sunabori T, Mabuchi Y, Nagai Y. Purified mesenchymal stem cells are an efficient source for iPS cell induction. PLoS One. 2011;6:e17610. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 44] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 33. | Szablowska-Gadomska I, Zayat V, Buzanska L. Influence of low oxygen tensions on expression of pluripotency genes in stem cells. Acta Neurobiol Exp (Wars). 2011;71:86-93. [PubMed] |
| 34. | Zhang F, Citra F, Wang DA. Prospects of induced pluripotent stem cell technology in regenerative medicine. Tissue Eng Part B Rev. 2011;17:115-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 35. | Li Z, Rana TM. A kinase inhibitor screen identifies small-molecule enhancers of reprogramming and iPS cell generation. Nat Commun. 2012;3:1085. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 77] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 36. | Lin Y, Cheng Z, Yang Z, Zheng J, Lin T. DNp73 improves generation efficiency of human induced pluripotent stem cells. BMC Cell Biol. 2012;13:9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 37. | Liu Y, Cheng D, Li Z, Gao X, Wang H. The gene expression profiles of induced pluripotent stem cells (iPSCs) generated by a non-integrating method are more similar to embryonic stem cells than those of iPSCs generated by an integrating method. Genet Mol Biol. 2012;35:693-700. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 38. | Okita K, Yamakawa T, Matsumura Y, Sato Y, Amano N, Watanabe A, Goshima N, Yamanaka S. An efficient nonviral method to generate integration-free human-induced pluripotent stem cells from cord blood and peripheral blood cells. Stem Cells. 2013;31:458-466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 483] [Cited by in RCA: 561] [Article Influence: 46.8] [Reference Citation Analysis (0)] |
| 39. | Zhang Z, Wu WS. Sodium butyrate promotes generation of human induced pluripotent stem cells through induction of the miR302/367 cluster. Stem Cells Dev. 2013;22:2268-2277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 46] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 40. | Aasen T, Raya A, Barrero MJ, Garreta E, Consiglio A, Gonzalez F, Vassena R, Bilić J, Pekarik V, Tiscornia G. Efficient and rapid generation of induced pluripotent stem cells from human keratinocytes. Nat Biotechnol. 2008;26:1276-1284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1042] [Cited by in RCA: 972] [Article Influence: 57.2] [Reference Citation Analysis (0)] |
| 41. | Barrero MJ, Berdasco M, Paramonov I, Bilic J, Vitaloni M, Esteller M, Izpisua Belmonte JC. DNA hypermethylation in somatic cells correlates with higher reprogramming efficiency. Stem Cells. 2012;30:1696-1702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 42. | Tiscornia G, Vivas EL, Izpisúa Belmonte JC. Diseases in a dish: modeling human genetic disorders using induced pluripotent cells. Nat Med. 2011;17:1570-1576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 163] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 43. | Osakada F, Ikeda H, Mandai M, Wataya T, Watanabe K, Yoshimura N, Akaike A, Sasai Y, Takahashi M. Toward the generation of rod and cone photoreceptors from mouse, monkey and human embryonic stem cells. Nat Biotechnol. 2008;26:215-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 453] [Cited by in RCA: 453] [Article Influence: 26.6] [Reference Citation Analysis (0)] |
| 44. | Osakada F, Ikeda H, Sasai Y, Takahashi M. Stepwise differentiation of pluripotent stem cells into retinal cells. Nat Protoc. 2009;4:811-824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 208] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 45. | Hirami Y, Osakada F, Takahashi K, Okita K, Yamanaka S, Ikeda H, Yoshimura N, Takahashi M. Generation of retinal cells from mouse and human induced pluripotent stem cells. Neurosci Lett. 2009;458:126-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 302] [Cited by in RCA: 300] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 46. | Meyer JS, Shearer RL, Capowski EE, Wright LS, Wallace KA, McMillan EL, Zhang SC, Gamm DM. Modeling early retinal development with human embryonic and induced pluripotent stem cells. Proc Natl Acad Sci USA. 2009;106:16698-16703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 468] [Cited by in RCA: 472] [Article Influence: 29.5] [Reference Citation Analysis (0)] |
| 47. | Meyer JS, Howden SE, Wallace KA, Verhoeven AD, Wright LS, Capowski EE, Pinilla I, Martin JM, Tian S, Stewart R. Optic vesicle-like structures derived from human pluripotent stem cells facilitate a customized approach to retinal disease treatment. Stem Cells. 2011;29:1206-1218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 370] [Cited by in RCA: 362] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 48. | Lamba DA, Karl MO, Ware CB, Reh TA. Efficient generation of retinal progenitor cells from human embryonic stem cells. Proc Natl Acad Sci USA. 2006;103:12769-12774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 508] [Cited by in RCA: 507] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 49. | Lamba DA, McUsic A, Hirata RK, Wang PR, Russell D, Reh TA. Generation, purification and transplantation of photoreceptors derived from human induced pluripotent stem cells. PLoS One. 2010;5:e8763. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 295] [Cited by in RCA: 303] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 50. | Tucker BA, Scheetz TE, Mullins RF, DeLuca AP, Hoffmann JM, Johnston RM, Jacobson SG, Sheffield VC, Stone EM. Exome sequencing and analysis of induced pluripotent stem cells identify the cilia-related gene male germ cell-associated kinase (MAK) as a cause of retinitis pigmentosa. Proc Natl Acad Sci USA. 2011;108:E569-E576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 173] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 51. | Tucker BA, Anfinson KR, Mullins RF, Stone EM, Young MJ. Use of a synthetic xeno-free culture substrate for induced pluripotent stem cell induction and retinal differentiation. Stem Cells Transl Med. 2013;2:16-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 84] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 52. | Nakano T, Ando S, Takata N, Kawada M, Muguruma K, Sekiguchi K, Saito K, Yonemura S, Eiraku M, Sasai Y. Self-formation of optic cups and storable stratified neural retina from human ESCs. Cell Stem Cell. 2012;10:771-785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 955] [Cited by in RCA: 1045] [Article Influence: 80.4] [Reference Citation Analysis (0)] |
| 53. | Phillips MJ, Wallace KA, Dickerson SJ, Miller MJ, Verhoeven AD, Martin JM, Wright LS, Shen W, Capowski EE, Percin EF. Blood-derived human iPS cells generate optic vesicle-like structures with the capacity to form retinal laminae and develop synapses. Invest Ophthalmol Vis Sci. 2012;53:2007-2019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 159] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 54. | Sasai Y, Eiraku M, Suga H. In vitro organogenesis in three dimensions: self-organising stem cells. Development. 2012;139:4111-4121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 142] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 55. | Mekala SR, Vauhini V, Nagarajan U, Maddileti S, Gaddipati S, Mariappan I. Derivation, characterization and retinal differentiation of induced pluripotent stem cells. J Biosci. 2013;38:123-134. [PubMed] |
| 56. | Mellough CB, Sernagor E, Moreno-Gimeno I, Steel DH, Lako M. Efficient stage-specific differentiation of human pluripotent stem cells toward retinal photoreceptor cells. Stem Cells. 2012;30:673-686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 129] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 57. | Boucherie C, Mukherjee S, Henckaerts E, Thrasher AJ, Sowden JC, Ali RR. Brief report: self-organizing neuroepithelium from human pluripotent stem cells facilitates derivation of photoreceptors. Stem Cells. 2013;31:408-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 66] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 58. | Sridhar A, Steward MM, Meyer JS. Nonxenogeneic growth and retinal differentiation of human induced pluripotent stem cells. Stem Cells Transl Med. 2013;2:255-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 50] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 59. | Decembrini S, Koch U, Radtke F, Moulin A, Arsenijevic Y. Derivation of traceable and transplantable photoreceptors from mouse embryonic stem cells. Stem Cell Reports. 2014;2:853-865. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 83] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 60. | Reichman S, Terray A, Slembrouck A, Nanteau C, Orieux G, Habeler W, Nandrot EF, Sahel JA, Monville C, Goureau O. From confluent human iPS cells to self-forming neural retina and retinal pigmented epithelium. Proc Natl Acad Sci USA. 2014;111:8518-8523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 223] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 61. | Rowland TJ, Buchholz DE, Clegg DO. Pluripotent human stem cells for the treatment of retinal disease. J Cell Physiol. 2012;227:457-466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 65] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 62. | Carr AJ, Vugler A, Lawrence J, Chen LL, Ahmado A, Chen FK, Semo M, Gias C, da Cruz L, Moore HD. Molecular characterization and functional analysis of phagocytosis by human embryonic stem cell-derived RPE cells using a novel human retinal assay. Mol Vis. 2009;15:283-295. [PubMed] |
| 63. | Liao JL, Yu J, Huang K, Hu J, Diemer T, Ma Z, Dvash T, Yang XJ, Travis GH, Williams DS. Molecular signature of primary retinal pigment epithelium and stem-cell-derived RPE cells. Hum Mol Genet. 2010;19:4229-4238. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 158] [Cited by in RCA: 152] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 64. | Maminishkis A, Chen S, Jalickee S, Banzon T, Shi G, Wang FE, Ehalt T, Hammer JA, Miller SS. Confluent monolayers of cultured human fetal retinal pigment epithelium exhibit morphology and physiology of native tissue. Invest Ophthalmol Vis Sci. 2006;47:3612-3624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 323] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 65. | Singh R, Phillips MJ, Kuai D, Meyer J, Martin JM, Smith MA, Perez ET, Shen W, Wallace KA, Capowski EE. Functional analysis of serially expanded human iPS cell-derived RPE cultures. Invest Ophthalmol Vis Sci. 2013;54:6767-6778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 101] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 66. | Jin ZB, Okamoto S, Osakada F, Homma K, Assawachananont J, Hirami Y, Iwata T, Takahashi M. Modeling retinal degeneration using patient-specific induced pluripotent stem cells. PLoS One. 2011;6:e17084. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 165] [Cited by in RCA: 168] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 67. | Jin ZB, Takahashi M. Generation of retinal cells from pluripotent stem cells. Prog Brain Res. 2012;201:171-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 68. | Yoshida T, Ozawa Y, Suzuki K, Yuki K, Ohyama M, Akamatsu W, Matsuzaki Y, Shimmura S, Mitani K, Tsubota K. The use of induced pluripotent stem cells to reveal pathogenic gene mutations and explore treatments for retinitis pigmentosa. Mol Brain. 2014;7:45. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 82] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 69. | Phillips MJ, Perez ET, Martin JM, Reshel ST, Wallace KA, Capowski EE, Singh R, Wright LS, Clark EM, Barney PM. Modeling human retinal development with patient-specific induced pluripotent stem cells reveals multiple roles for visual system homeobox 2. Stem Cells. 2014;32:1480-1492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 95] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 70. | Capecchi MR. Gene targeting in mice: functional analysis of the mammalian genome for the twenty-first century. Nat Rev Genet. 2005;6:507-512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 513] [Cited by in RCA: 470] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 71. | Thomas KR, Capecchi MR. Targeting of genes to specific sites in the mammalian genome. Cold Spring Harb Symp Quant Biol. 1986;51 Pt 2:1101-1113. [PubMed] |
| 72. | Thomas KR, Folger KR, Capecchi MR. High frequency targeting of genes to specific sites in the mammalian genome. Cell. 1986;44:419-428. [PubMed] |
| 73. | Hockemeyer D, Jaenisch R. Gene targeting in human pluripotent cells. Cold Spring Harb Symp Quant Biol. 2010;75:201-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 42] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 74. | Hockemeyer D, Wang H, Kiani S, Lai CS, Gao Q, Cassady JP, Cost GJ, Zhang L, Santiago Y, Miller JC. Genetic engineering of human pluripotent cells using TALE nucleases. Nat Biotechnol. 2011;29:731-734. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 996] [Cited by in RCA: 958] [Article Influence: 68.4] [Reference Citation Analysis (0)] |
| 75. | Ding Q, Lee YK, Schaefer EA, Peters DT, Veres A, Kim K, Kuperwasser N, Motola DL, Meissner TB, Hendriks WT. A TALEN genome-editing system for generating human stem cell-based disease models. Cell Stem Cell. 2013;12:238-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 401] [Cited by in RCA: 387] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 76. | Wang H, Hu YC, Markoulaki S, Welstead GG, Cheng AW, Shivalila CS, Pyntikova T, Dadon DB, Voytas DF, Bogdanove AJ. TALEN-mediated editing of the mouse Y chromosome. Nat Biotechnol. 2013;31:530-532. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 93] [Cited by in RCA: 87] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 77. | Joung JK, Sander JD. TALENs: a widely applicable technology for targeted genome editing. Nat Rev Mol Cell Biol. 2013;14:49-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1038] [Cited by in RCA: 1078] [Article Influence: 82.9] [Reference Citation Analysis (0)] |
| 78. | Ran D, Shia WJ, Lo MC, Fan JB, Knorr DA, Ferrell PI, Ye Z, Yan M, Cheng L, Kaufman DS. RUNX1a enhances hematopoietic lineage commitment from human embryonic stem cells and inducible pluripotent stem cells. Blood. 2013;121:2882-2890. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 109] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 79. | Urnov FD, Rebar EJ, Holmes MC, Zhang HS, Gregory PD. Genome editing with engineered zinc finger nucleases. Nat Rev Genet. 2010;11:636-646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1482] [Cited by in RCA: 1495] [Article Influence: 99.7] [Reference Citation Analysis (0)] |
| 80. | Cong L, Ran FA, Cox D, Lin S, Barretto R, Habib N, Hsu PD, Wu X, Jiang W, Marraffini LA. Multiplex genome engineering using CRISPR/Cas systems. Science. 2013;339:819-823. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10336] [Cited by in RCA: 11165] [Article Influence: 930.4] [Reference Citation Analysis (0)] |
| 81. | Mali P, Yang L, Esvelt KM, Aach J, Guell M, DiCarlo JE, Norville JE, Church GM. RNA-guided human genome engineering via Cas9. Science. 2013;339:823-826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6555] [Cited by in RCA: 7005] [Article Influence: 583.8] [Reference Citation Analysis (0)] |
| 82. | Cho SW, Kim S, Kim JM, Kim JS. Targeted genome engineering in human cells with the Cas9 RNA-guided endonuclease. Nat Biotechnol. 2013;31:230-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1480] [Cited by in RCA: 1461] [Article Influence: 121.8] [Reference Citation Analysis (0)] |
| 83. | Fu Y, Sander JD, Reyon D, Cascio VM, Joung JK. Improving CRISPR-Cas nuclease specificity using truncated guide RNAs. Nat Biotechnol. 2014;32:279-284. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1554] [Cited by in RCA: 1503] [Article Influence: 136.6] [Reference Citation Analysis (0)] |
| 84. | Maeder ML, Linder SJ, Cascio VM, Fu Y, Ho QH, Joung JK. CRISPR RNA-guided activation of endogenous human genes. Nat Methods. 2013;10:977-979. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 909] [Cited by in RCA: 927] [Article Influence: 77.3] [Reference Citation Analysis (0)] |
| 85. | Xie K, Zhang J, Yang Y. Genome-wide prediction of highly specific guide RNA spacers for CRISPR-Cas9-mediated genome editing in model plants and major crops. Mol Plant. 2014;7:923-926. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 211] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 86. | Yang L, Mali P, Kim-Kiselak C, Church G. CRISPR-Cas-mediated targeted genome editing in human cells. Methods Mol Biol. 2014;1114:245-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 40] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 87. | González F, Zhu Z, Shi ZD, Lelli K, Verma N, Li QV, Huangfu D. An iCRISPR platform for rapid, multiplexable, and inducible genome editing in human pluripotent stem cells. Cell Stem Cell. 2014;15:215-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 338] [Cited by in RCA: 371] [Article Influence: 33.7] [Reference Citation Analysis (0)] |
| 88. | Saga M, Mashima Y, Akeo K, Oguchi Y, Kudoh J, Shimizu N. Autosomal dominant retinitis pigmentosa. A mutation in codon 181 (Glu--& gt; Lys) of the rhodopsin gene in a Japanese family. Ophthalmic Genet. 1994;15:61-67. [PubMed] |
| 89. | Seo S, Mullins RF, Dumitrescu AV, Bhattarai S, Gratie D, Wang K, Stone EM, Sheffield V, Drack AV. Subretinal gene therapy of mice with Bardet-Biedl syndrome type 1. Invest Ophthalmol Vis Sci. 2013;54:6118-6132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 67] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 90. | Marlhens F, Bareil C, Griffoin JM, Zrenner E, Amalric P, Eliaou C, Liu SY, Harris E, Redmond TM, Arnaud B. Mutations in RPE65 cause Leber’s congenital amaurosis. Nat Genet. 1997;17:139-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 388] [Cited by in RCA: 398] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 91. | Williams DS. Usher syndrome: animal models, retinal function of Usher proteins, and prospects for gene therapy. Vision Res. 2008;48:433-441. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 130] [Cited by in RCA: 132] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 92. | van den Hurk JA, Hendriks W, van de Pol DJ, Oerlemans F, Jaissle G, Rüther K, Kohler K, Hartmann J, Zrenner E, van Bokhoven H. Mouse choroideremia gene mutation causes photoreceptor cell degeneration and is not transmitted through the female germline. Hum Mol Genet. 1997;6:851-858. [PubMed] |
| 93. | Tsai KL, Clark LA, Murphy KE. Understanding hereditary diseases using the dog and human as companion model systems. Mamm Genome. 2007;18:444-451. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 78] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 94. | Seymour LW, Fisher KD. Preclinical screening of gene therapy in human tissues. Hum Gene Ther. 2009;20:291-292. [PubMed] |
| 95. | Kalatzis V, Hamel CP, MacDonald IM; First International Choroideremia Research Symposium. Choroideremia: towards a therapy. Am J Ophthalmol. 2013;156:433-437.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 96. | Hippert C, Ibanes S, Serratrice N, Court F, Malecaze F, Kremer EJ, Kalatzis V. Corneal transduction by intra-stromal injection of AAV vectors in vivo in the mouse and ex vivo in human explants. PLoS One. 2012;7:e35318. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 97. | Perel P, Roberts I, Sena E, Wheble P, Briscoe C, Sandercock P, Macleod M, Mignini LE, Jayaram P, Khan KS. Comparison of treatment effects between animal experiments and clinical trials: systematic review. BMJ. 2007;334:197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 555] [Cited by in RCA: 532] [Article Influence: 29.6] [Reference Citation Analysis (0)] |
| 98. | Savitz SI. A critical appraisal of the NXY-059 neuroprotection studies for acute stroke: a need for more rigorous testing of neuroprotective agents in animal models of stroke. Exp Neurol. 2007;205:20-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 101] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 99. | Lustremant C, Habeler W, Plancheron A, Goureau O, Grenot L, de la Grange P, Audo I, Nandrot EF, Monville C. Human induced pluripotent stem cells as a tool to model a form of Leber congenital amaurosis. Cell Reprogram. 2013;15:233-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 100. | Inoue H, Yamanaka S. The use of induced pluripotent stem cells in drug development. Clin Pharmacol Ther. 2011;89:655-661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 163] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 101. | Maury Y, Gauthier M, Peschanski M, Martinat C. Human pluripotent stem cells for disease modelling and drug screening. Bioessays. 2012;34:61-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 55] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 102. | Charbord J, Poydenot P, Bonnefond C, Feyeux M, Casagrande F, Brinon B, Francelle L, Aurégan G, Guillermier M, Cailleret M. High throughput screening for inhibitors of REST in neural derivatives of human embryonic stem cells reveals a chemical compound that promotes expression of neuronal genes. Stem Cells. 2013;31:1816-1828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 63] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 103. | Ferrer M, Corneo B, Davis J, Wan Q, Miyagishima KJ, King R, Maminishkis A, Marugan J, Sharma R, Shure M. A multiplex high-throughput gene expression assay to simultaneously detect disease and functional markers in induced pluripotent stem cell-derived retinal pigment epithelium. Stem Cells Transl Med. 2014;3:911-922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 43] [Article Influence: 3.9] [Reference Citation Analysis (0)] |