Published online Apr 26, 2015. doi: 10.4252/wjsc.v7.i3.535
Peer-review started: December 4, 2014
First decision: January 8, 2015
Revised: January 20, 2015
Accepted: February 9, 2015
Article in press: February 11, 2015
Published online: April 26, 2015
Processing time: 143 Days and 1.4 Hours
All cells are derived from one cell, and the origin of different cell types is a subject of curiosity. Cells construct life through appropriately timed networks at each stage of development. Communication among cells and intracellular signaling are essential for cell differentiation and for life processes. Cellular molecular networks establish cell diversity and life. The investigation of the regulation of each gene in the genome within the cellular network is therefore of interest. Stem cells produce various cells that are suitable for specific purposes. The dynamics of the information in the cellular network changes as the status of cells is altered. The components of each cell are subject to investigation.
Core tip: The cells in the body orchestrate the unique roles of each organ through a cellular network. It is important to investigate alterations in cellular phenotypes and the regulation of genes, the genome and molecules in order to understand the origin of the cells. Insights into the changes in cellular features, including epithelial-mesenchymal transition, and recent database advances are described in this editorial.
- Citation: Tanabe S. Origin of cells and network information. World J Stem Cells 2015; 7(3): 535-540
- URL: https://www.wjgnet.com/1948-0210/full/v7/i3/535.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v7.i3.535
Recently, pluripotent stem cells have played an increasing role in disease and developmental models, including the challenge of generating novel organs such as intestines[1]. Stem cell differentiation is one of the mechanisms by which regenerative tissues are produced. In each cell, the genome encodes the plan for the life of the cell and the path for organizing each tissue. The gene segments travel through the genome to settle at the gene loci[2]. Variations within the genome produce individual differences. Dramatic transitions of cellular phenotypes, such as the Warburg effect, occur in disease states such as cancer[3,4]. Epigenetic alterations provide cellular identity and phenotypic diversity. RNA transcription is altered in cancer; this alteration is caused by somatic DNA translocation or mutation[5]. Variants of genes such as BRCA2 and CHEK2 increase the risk of lung cancer[6]. Genome sequencing of normal cells has revealed the accumulation of mutations and differences in each cell lineage and tissue[7]. Genome editing has recently been developed. Additionally, gene therapy using clustered regularly interspaced short palindromic repeats/Cas9 is an emerging technique[8]. The construction and architecture of the genome are important for understanding the cell.
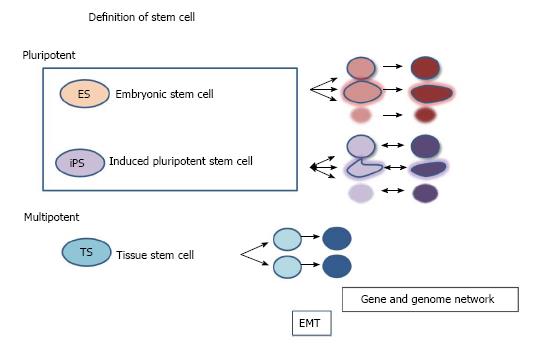
Emerging roles for stem cells as sources for cell-based therapy remind us of the importance of the definition of stem cells[9]. Stem cells are generally defined as cells with self-renewal and differentiation potential[10]. Accumulating knowledge and insights have shown that stem cells are able to differentiate into several cell types in the body. However, a paradigm shift occurred after the discovery of induced pluripotent stem (iPS) cells that can be created by reprogramming differentiated cells with several factors[11]. This finding may allow for a shift in the cell type of stem cells derived from differentiated cells in the body. Thus, the range of stem cells needs to be defined. Stem cells can be classified into two categories (Figure 1): (1) pluripotent stem cells, such as embryonic stem cells or iPS cells[12-15]; or (2) tissue multipotent stem cells such as neural stem cells, hematopoietic stem cells or mesenchymal stem cells[16]. Recently, SNAI1 (SNAIL) has been reported to localize to the nucleus and to play a role in epithelial-mesenchymal transition (EMT) during the early stage of reprogramming of differentiated cells[17]. EMT and mesenchymal-epithelial transition processes may promote the reprogramming of differentiated cells toward stem cells[17]. Altered phenotypes and gene networks of stem cells have been reported, suggesting that the cells themselves have various gene dynamics during culture[18]. Cancer stem cells may be included as stem cells in cancer states. In some cases, engineered differentiated cells with gene modification or genome editing may also be included as stem cells if the cells are reprogrammed.
The cell phenotype transition has been observed in cancer stem cells (CSCs)[19]. SOX2, which is a reprogramming factor, is a CSC biomarker in embryonal carcinoma cells and is related to stem-like cancer cells[20]. Genome analysis of SOX2-silenced human embryonal carcinoma cell lines has revealed that the cellular networks of these cells are enriched for microRNAs that are regulated by SOX2 and that are associated with EMT markers[20]. In contrast, an epidermal growth factor receptor exon 19-deleted lung cancer cell line was induced to exhibit CSC-like phenotypes and EMT by DDX3X transfection[21]. Moreover, DDX3X overexpression was reported to induce Sox2 up-regulation[21].
CSCs are related to chemotherapy and radiation resistance in squamous cell carcinomas (SCCs)[22]. The CSC population is diverse in SCCs; this diversity contributes to difficulty in cancer treatment[22]. Understanding the mechanisms of CSCs and EMT are important for the development of novel therapeutics.
Cellular networks characterize both cells and the body, and gene combinations are critical for the presentation of phenotypes[23]. EMT is one of the mechanisms by which the cell phenotype transitions; dihydropyrimidine has been reported to induce EMT[24]. EMT is associated with metastasis in tumor progression and is induced by Notch activation and p53 deletion in mice[25]. Erythropoietin-producing hepatoma (EPH) receptors, which are receptor tyrosine kinases related to cancer, may be related to EMT signaling[26]. EPH receptor A2 induces EMT viaβ-catenin activation, followed by Snail expression and cadherin 1, type 1, E-cadherin (epithelial) (CDH1) suppression[26]. Wnt/β-catenin signaling is inhibited by SOX10, leading to the inhibition of the growth and metastasis of digestive cancers[27]. SRY (sex determining region Y)-box 10 (SOX10) inhibits EMT, which may be one of the possible mechanisms of cancer inhibition[27]. Frizzled2, the Wnt receptor, induces EMT and cell migration through the noncanonical pathway[28]. EMT is monitored by cell rigidity, and human equilibrative nucleoside transporter-1 suppression induces EMT in pancreatic cancer cells[29]. EMT characterization is needed for further understanding cell type transition and cancer progression.
EMT can be characterized by the following three features: (1) changes in cellular morphology; (2) increases in cellular motility; and (3) alterations in the expression of E-cadherin and N-cadherin[29]. Cellular morphological changes are typically observed in the transition from connective-like cells to mesenchymal-like cells[29]. The expression of CDH1 is usually up-regulated in connective- or epithelial-like cells, whereas the expression of N-cadherin (CDH2) is up-regulated in mesenchymal-like cells[29,30]. EMT is associated with tumor metastasis[30]. The metastasis potential or invasiveness of cancer can be measured by the mechanical rigidity of the cells[31,32]. Several genes are involved in EMT, including BMI1 proto-oncogene, polycomb ring finger (BMI1), hypoxia inducible factor 1, alpha subunit (HIF1A, HIF-1α) and twist family bHLH transcription factor 1 (TWIST1, Twist)[33]. HIF-1α, which is a key transcription factor, is up-regulated in gastric cancer. Additionally, network pathway genes, such as NFκB1, BRCA1, STAT3 and STAT1, and network hub genes, such as MMP1, TIMP1, TLR2, FCGR3A, IRF1, FAS and TFF3, have been identified[34].
An abundant number of genes are regulated in cancer. Genes are regulated not only by transcription factors but also by microRNAs (miRNAs). miRNA-9 is up-regulated in esophageal squamous cell carcinoma, which may induce EMT and metastasis in cancer[35]. CD151, which is a regulator of laminin-binding integrin function and signaling, represses EMT and canonical Wnt signaling, leading to the inhibition of ovarian tumor growth[36]. Wnt/β-catenin signaling is involved in EMT induction by the parathyroid hormone in human renal proximal tubular cells[37]. Endothelin-1 and endothelin A receptor signaling, together with Wnt signaling, regulate EMT in epithelial ovarian cancer[38]. Endothelin/β-arrestin signaling and Wnt/β-catenin signaling may be involved in chemotherapy resistance in cancer[38]. Hypoxia-inducible factors (HIFs) play roles in Wnt signaling in human colon cancer cells[39]. HIF-1α depletion induces the reversal of EMT, and HIF-2α silencing affects the expression of stem cell markers and increases β-catenin transcriptional activity under hypoxic conditions[39]. The roles of HIFs in Wnt/β-catenin signaling and in the surrounding networks are essential for understanding cancer cell phenotypes. The silencing of β-catenin via promoter methylation is also involved in the enhancement of non-small cell lung cancer invasiveness[40].
Notch1, which is one of the important molecules in cancer signaling, is involved in Ras/phosphoinositide 3 kinase (PI3K)/Akt signaling in T-cell acute lymphoblastic leukemia (T-ALL)[41], and PI3K and Notch1 may be targets for drug resistance in T-ALL[41]. Sox2, which is one of the reprogramming factors used to produce iPS cells, may be a regulator of EMT during neural crest development[42]. The Wnt pathway induces the EMT pathway, and the inhibition of the Wnt pathway may be involved in the re-differentiation of human islet β-cells[43]. Thus, the investigation of the molecules associated with EMT and disease is of interest[44].
Several megaprojects have been established in response to the genome projects, one of which is called the ENCyclopedia Of DNA Elements (ENCODE) Project, which aims to translate the human genome sequence into biological and health mechanisms[45]. The ENCODE Project has identified functional elements in the genome (http://www.genome.gov/ENCODE/)[46,47].
The cross-cancer alteration of genes and their networks can be examined in cBioPortal, which is a cancer genomics database (http://www.cbioportal.org/public-portal/)[48,49]. The cBioPortal includes network analysis for the visualization of networks that are altered in cancer[49]. The precise information obtained through network analysis has been reported in several studies[50-53]. The sources of the networks are derived from pathways and interactions from the Human Reference Protein Database[53], Reactome[51], the Pathway Interaction Database created by the National Cancer Institute in collaboration with Nature Publishing Group (http://pid.nci.nih.gov/)[52], and the Memorial Sloan-Kettering Cancer Center Cancer Cell Map, which are all included as source information in the Pathway Commons Project (http://www.pathwaycommons.org)[50]. Pathway Commons is an open pathway that includes interaction information for multiple species, such as humans and model organisms[50].
The web interface called Gene Expression Commons is an interesting tool for gene expression analysis and microarray data that can be analyzed with reference data to model biological relationships (https://gexc.stanford.edu/)[54]. The amount of data available in these databases is increasing and includes data from microarrays, next-generation sequencing, and clinical data.
The cell is the fundamental unit of life. The investigation of gene and genome regulation is critical for a deep understanding of phenotypic alterations and of the origin of cells. The transition of cell characteristics, including differentiation, reprogramming and EMT, and cell-to-cell communications requires further investigation to reveal the cell of origin.
P- Reviewer: Mandic R S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ
| 1. | Watson CL, Mahe MM, Múnera J, Howell JC, Sundaram N, Poling HM, Schweitzer JI, Vallance JE, Mayhew CN, Sun Y. An in vivo model of human small intestine using pluripotent stem cells. Nat Med. 2014;20:1310-1314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 400] [Cited by in RCA: 426] [Article Influence: 38.7] [Reference Citation Analysis (0)] |
| 2. | Chen X, Bracht JR, Goldman AD, Dolzhenko E, Clay DM, Swart EC, Perlman DH, Doak TG, Stuart A, Amemiya CT. The architecture of a scrambled genome reveals massive levels of genomic rearrangement during development. Cell. 2014;158:1187-1198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 118] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 3. | Warburg O. On the origin of cancer cells. Science. 1956;123:309-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9117] [Cited by in RCA: 9838] [Article Influence: 142.6] [Reference Citation Analysis (0)] |
| 4. | Yizhak K, Le Dévédec SE, Rogkoti VM, Baenke F, de Boer VC, Frezza C, Schulze A, van de Water B, Ruppin E. A computational study of the Warburg effect identifies metabolic targets inhibiting cancer migration. Mol Syst Biol. 2014;10:744. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 102] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 5. | Cancer Genome Atlas Research Network. Comprehensive molecular profiling of lung adenocarcinoma. Nature. 2014;511:543-550. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3395] [Cited by in RCA: 4247] [Article Influence: 386.1] [Reference Citation Analysis (0)] |
| 6. | Wang Y, McKay JD, Rafnar T, Wang Z, Timofeeva MN, Broderick P, Zong X, Laplana M, Wei Y, Han Y. Rare variants of large effect in BRCA2 and CHEK2 affect risk of lung cancer. Nat Genet. 2014;46:736-741. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 339] [Cited by in RCA: 341] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 7. | Behjati S, Huch M, van Boxtel R, Karthaus W, Wedge DC, Tamuri AU, Martincorena I, Petljak M, Alexandrov LB, Gundem G. Genome sequencing of normal cells reveals developmental lineages and mutational processes. Nature. 2014;513:422-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 300] [Cited by in RCA: 263] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 8. | Long C, McAnally JR, Shelton JM, Mireault AA, Bassel-Duby R, Olson EN. Prevention of muscular dystrophy in mice by CRISPR/Cas9-mediated editing of germline DNA. Science. 2014;345:1184-1188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 487] [Cited by in RCA: 519] [Article Influence: 47.2] [Reference Citation Analysis (0)] |
| 9. | Buzhor E, Leshansky L, Blumenthal J, Barash H, Warshawsky D, Mazor Y, Shtrichman R. Cell-based therapy approaches: the hope for incurable diseases. Regen Med. 2014;9:649-672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 123] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 10. | Blau HM, Brazelton TR, Weimann JM. The evolving concept of a stem cell: entity or function? Cell. 2001;105:829-841. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 773] [Cited by in RCA: 663] [Article Influence: 27.6] [Reference Citation Analysis (0)] |
| 11. | Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17989] [Cited by in RCA: 18009] [Article Influence: 947.8] [Reference Citation Analysis (0)] |
| 12. | Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861-872. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14327] [Cited by in RCA: 14206] [Article Influence: 835.6] [Reference Citation Analysis (0)] |
| 13. | Mournetas V, Nunes QM, Murray PA, Sanderson CM, Fernig DG. Network based meta-analysis prediction of microenvironmental relays involved in stemness of human embryonic stem cells. PeerJ. 2014;2:e618. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 14. | Pera MF, Reubinoff B, Trounson A. Human embryonic stem cells. J Cell Sci. 2000;113:5-10. [PubMed] |
| 15. | Courtot AM, Magniez A, Oudrhiri N, Féraud O, Bacci J, Gobbo E, Proust S, Turhan AG, Bennaceur-Griscelli A. Morphological analysis of human induced pluripotent stem cells during induced differentiation and reverse programming. Biores Open Access. 2014;3:206-216. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 16. | Tanabe S. Role of mesenchymal stem cells in cell life and their signaling. World J Stem Cells. 2014;6:24-32. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 17. | Unternaehrer JJ, Zhao R, Kim K, Cesana M, Powers JT, Ratanasirintrawoot S, Onder T, Shibue T, Weinberg RA, Daley GQ. The epithelial-mesenchymal transition factor SNAIL paradoxically enhances reprogramming. Stem Cell Reports. 2014;3:691-698. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 67] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 18. | Tanabe S, Sato Y, Suzuki T, Suzuki K, Nagao T, Yamaguchi T. Gene expression profiling of human mesenchymal stem cells for identification of novel markers in early- and late-stage cell culture. J Biochem. 2008;144:399-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 42] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 19. | Kemper K, de Goeje PL, Peeper DS, van Amerongen R. Phenotype switching: tumor cell plasticity as a resistance mechanism and target for therapy. Cancer Res. 2014;74:5937-5941. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 154] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 20. | Vencken SF, Sethupathy P, Blackshields G, Spillane C, Elbaruni S, Sheils O, Gallagher MF, O’Leary JJ. An integrated analysis of the SOX2 microRNA response program in human pluripotent and nullipotent stem cell lines. BMC Genomics. 2014;15:711. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 21. | Nozaki K, Kagamu H, Shoji S, Igarashi N, Ohtsubo A, Okajima M, Miura S, Watanabe S, Yoshizawa H, Narita I. DDX3X induces primary EGFR-TKI resistance based on intratumor heterogeneity in lung cancer cells harboring EGFR-activating mutations. PLoS One. 2014;9:e111019. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 22. | Facompre N, Nakagawa H, Herlyn M, Basu D. Stem-like cells and therapy resistance in squamous cell carcinomas. Adv Pharmacol. 2012;65:235-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 23. | Tanabe S. Perspectives of gene combinations in phenotype presentation. World J Stem Cells. 2013;5:61-67. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 24. | Shaul YD, Freinkman E, Comb WC, Cantor JR, Tam WL, Thiru P, Kim D, Kanarek N, Pacold ME, Chen WW. Dihydropyrimidine accumulation is required for the epithelial-mesenchymal transition. Cell. 2014;158:1094-1109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 168] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 25. | Chanrion M, Kuperstein I, Barrière C, El Marjou F, Cohen D, Vignjevic D, Stimmer L, Paul-Gilloteaux P, Bièche I, Tavares Sdos R. Concomitant Notch activation and p53 deletion trigger epithelial-to-mesenchymal transition and metastasis in mouse gut. Nat Commun. 2014;5:5005. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 110] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 26. | Li RX, Chen ZH, Chen ZK. The role of EPH receptors in cancer-related epithelial-mesenchymal transition. Chin J Cancer. 2014;33:231-240. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 27. | Tong X, Li L, Li X, Heng L, Zhong L, Su X, Rong R, Hu S, Liu W, Jia B. SOX10, a novel HMG-box-containing tumor suppressor, inhibits growth and metastasis of digestive cancers by suppressing the Wnt/β-catenin pathway. Oncotarget. 2014;5:10571-10583. [PubMed] |
| 28. | Gujral TS, Chan M, Peshkin L, Sorger PK, Kirschner MW, MacBeath G. A noncanonical Frizzled2 pathway regulates epithelial-mesenchymal transition and metastasis. Cell. 2014;159:844-856. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 273] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 29. | Lee Y, Koay EJ, Zhang W, Qin L, Kirui DK, Hussain F, Shen H, Ferrari M. Human equilibrative nucleoside transporter-1 knockdown tunes cellular mechanics through epithelial-mesenchymal transition in pancreatic cancer cells. PLoS One. 2014;9:e107973. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 30. | Grant CM, Kyprianou N. Epithelial mesenchymal transition (EMT) in prostate growth and tumor progression. Transl Androl Urol. 2013;2:202-211. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 56] [Reference Citation Analysis (0)] |
| 31. | Xu W, Mezencev R, Kim B, Wang L, McDonald J, Sulchek T. Cell stiffness is a biomarker of the metastatic potential of ovarian cancer cells. PLoS One. 2012;7:e46609. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 557] [Cited by in RCA: 511] [Article Influence: 39.3] [Reference Citation Analysis (0)] |
| 32. | Swaminathan V, Mythreye K, O’Brien ET, Berchuck A, Blobe GC, Superfine R. Mechanical stiffness grades metastatic potential in patient tumor cells and in cancer cell lines. Cancer Res. 2011;71:5075-5080. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 493] [Cited by in RCA: 532] [Article Influence: 38.0] [Reference Citation Analysis (0)] |
| 33. | Du R, Xia L, Ning X, Liu L, Sun W, Huang C, Wang H, Sun S. Hypoxia-induced Bmi1 promotes renal tubular epithelial cell-mesenchymal transition and renal fibrosis via PI3K/Akt signal. Mol Biol Cell. 2014;25:2650-2659. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 83] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 34. | Wang J, Ni Z, Duan Z, Wang G, Li F. Altered expression of hypoxia-inducible factor-1α (HIF-1α) and its regulatory genes in gastric cancer tissues. PLoS One. 2014;9:e99835. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 44] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 35. | Song Y, Li J, Zhu Y, Dai Y, Zeng T, Liu L, Li J, Wang H, Qin Y, Zeng M. MicroRNA-9 promotes tumor metastasis via repressing E-cadherin in esophageal squamous cell carcinoma. Oncotarget. 2014;5:11669-11680. [PubMed] |
| 36. | Baldwin LA, Hoff JT, Lefringhouse J, Zhang M, Jia C, Liu Z, Erfani S, Jin H, Xu M, She QB. CD151-α3β1 integrin complexes suppress ovarian tumor growth by repressing slug-mediated EMT and canonical Wnt signaling. Oncotarget. 2014;5:12203-12217. [PubMed] |
| 37. | Guo Y, Li Z, Ding R, Li H, Zhang L, Yuan W, Wang Y. Parathyroid hormone induces epithelial-to-mesenchymal transition via the Wnt/β-catenin signaling pathway in human renal proximal tubular cells. Int J Clin Exp Pathol. 2014;7:5978-5987. [PubMed] |
| 38. | Rosanò L, Cianfrocca R, Tocci P, Spinella F, Di Castro V, Caprara V, Semprucci E, Ferrandina G, Natali PG, Bagnato A. Endothelin A Receptor/β-Arrestin Signaling to the Wnt Pathway Renders Ovarian Cancer Cells Resistant to Chemotherapy. Cancer Res. 2014;74:7453-7464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 79] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 39. | Santoyo-Ramos P, Likhatcheva M, García-Zepeda EA, Castañeda-Patlán MC, Robles-Flores M. Hypoxia-inducible factors modulate the stemness and malignancy of colon cancer cells by playing opposite roles in canonical Wnt signaling. PLoS One. 2014;9:e112580. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 54] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 40. | Miao Y, Wang L, Zhang X, Xu X, Jiang G, Fan C, Liu Y, Lin X, Yu J, Zhang Y. Promoter methylation-mediated silencing of β-catenin enhances invasiveness of non-small cell lung cancer and predicts adverse prognosis. PLoS One. 2014;9:e112258. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 41. | Dail M, Wong J, Lawrence J, O’Connor D, Nakitandwe J, Chen SC, Xu J, Lee LB, Akagi K, Li Q. Loss of oncogenic Notch1 with resistance to a PI3K inhibitor in T-cell leukaemia. Nature. 2014;513:512-516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 52] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 42. | Mandalos N, Rhinn M, Granchi Z, Karampelas I, Mitsiadis T, Economides AN, Dollé P, Remboutsika E. Sox2 acts as a rheostat of epithelial to mesenchymal transition during neural crest development. Front Physiol. 2014;5:345. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 43. | Lenz A, Toren-Haritan G, Efrat S. Redifferentiation of adult human β cells expanded in vitro by inhibition of the WNT pathway. PLoS One. 2014;9:e112914. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 44. | Tanabe S, Aoyagi K, Yokozaki H, Sasaki H. Gene expression signatures for identifying diffuse-type gastric cancer associated with epithelial-mesenchymal transition. Int J Oncol. 2014;44:1955-1970. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 45. | The ENCODE Project Consortium. A user’s guide to the encyclopedia of DNA elements (ENCODE). PLoS Biol. 2011;9:e1001046. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1052] [Cited by in RCA: 1093] [Article Influence: 78.1] [Reference Citation Analysis (0)] |
| 46. | The ENCODE Project Consortium. The ENCODE (ENCyclopedia Of DNA Elements) Project. Science. 2004;306:636-640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1646] [Cited by in RCA: 1697] [Article Influence: 80.8] [Reference Citation Analysis (0)] |
| 47. | Birney E, Stamatoyannopoulos JA, Dutta A, Guigó R, Gingeras TR, Margulies EH, Weng Z, Snyder M, Dermitzakis ET, Thurman RE. Identification and analysis of functional elements in 1% of the human genome by the ENCODE pilot project. Nature. 2007;447:799-816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4030] [Cited by in RCA: 3865] [Article Influence: 214.7] [Reference Citation Analysis (0)] |
| 48. | Cerami E, Gao J, Dogrusoz U, Gross BE, Sumer SO, Aksoy BA, Jacobsen A, Byrne CJ, Heuer ML, Larsson E. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012;2:401-404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9144] [Cited by in RCA: 12539] [Article Influence: 964.5] [Reference Citation Analysis (0)] |
| 49. | Gao J, Aksoy BA, Dogrusoz U, Dresdner G, Gross B, Sumer SO, Sun Y, Jacobsen A, Sinha R, Larsson E. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal. 2013;6:pl1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8187] [Cited by in RCA: 11064] [Article Influence: 922.0] [Reference Citation Analysis (0)] |
| 50. | Cerami EG, Gross BE, Demir E, Rodchenkov I, Babur O, Anwar N, Schultz N, Bader GD, Sander C. Pathway Commons, a web resource for biological pathway data. Nucleic Acids Res. 2011;39:D685-D690. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 805] [Cited by in RCA: 779] [Article Influence: 51.9] [Reference Citation Analysis (0)] |
| 51. | Matthews L, Gopinath G, Gillespie M, Caudy M, Croft D, de Bono B, Garapati P, Hemish J, Hermjakob H, Jassal B. Reactome knowledgebase of human biological pathways and processes. Nucleic Acids Res. 2009;37:D619-D622. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 639] [Cited by in RCA: 602] [Article Influence: 35.4] [Reference Citation Analysis (0)] |
| 52. | Schaefer CF, Anthony K, Krupa S, Buchoff J, Day M, Hannay T, Buetow KH. PID: the Pathway Interaction Database. Nucleic Acids Res. 2009;37:D674-D679. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1235] [Cited by in RCA: 1155] [Article Influence: 72.2] [Reference Citation Analysis (0)] |
| 53. | Keshava Prasad TS, Goel R, Kandasamy K, Keerthikumar S, Kumar S, Mathivanan S, Telikicherla D, Raju R, Shafreen B, Venugopal A. Human Protein Reference Database--2009 update. Nucleic Acids Res. 2009;37:D767-D772. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2600] [Cited by in RCA: 2516] [Article Influence: 157.3] [Reference Citation Analysis (0)] |
| 54. | Seita J, Sahoo D, Rossi DJ, Bhattacharya D, Serwold T, Inlay MA, Ehrlich LI, Fathman JW, Dill DL, Weissman IL. Gene Expression Commons: an open platform for absolute gene expression profiling. PLoS One. 2012;7:e40321. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 190] [Cited by in RCA: 204] [Article Influence: 15.7] [Reference Citation Analysis (0)] |