Published online Mar 26, 2015. doi: 10.4252/wjsc.v7.i2.512
Peer-review started: August 25, 2014
Revised: October 2, 2014
Accepted: November 17, 2014
Article in press: November 19, 2014
Published online: March 26, 2015
Processing time: 206 Days and 18.2 Hours
Scientific evidence suggests that stem cells possess the anti-aging ability to self-renew and maintain differentiation potentials, and quiescent state. The objective of this review is to discuss the micro-environment where stem cells reside in vivo, the secreted factors to which stem cells are exposed, the hypoxic environment, and intracellular factors including genome stability, mitochondria integrity, epigenetic regulators, calorie restrictions, nutrients, and vitamin D. Secreted tumor growth factor-β and fibroblast growth factor-2 are reported to play a role in stem cell quiescence. Extracellular matrices may interact with caveolin-1, the lipid raft on cell membrane to regulate quiescence. N-cadherin, the adhesive protein on niche cells provides support for stem cells. The hypoxic micro-environment turns on hypoxia-inducible factor-1 to prevent mesenchymal stem cells aging through p16 and p21 down-regulation. Mitochondria express glucosephosphate isomerase to undergo glycolysis and prevent cellular aging. Epigenetic regulators such as p300, protein inhibitors of activated Stats and H19 help maintain stem cell quiescence. In addition, calorie restriction may lead to secretion of paracrines cyclic ADP-ribose by intestinal niche cells, which help maintain intestinal stem cells. In conclusion, it is crucial to understand the anti-aging phenomena of stem cells at the molecular level so that the key to solving the aging mystery may be unlocked.
Core tip: This review approaches anti-aging from aspect of stem cells. Stem cells may interact directly with their extracellular surroundings through caveolin-1, a lipid raft protein, to carry out and maintain stem cell functions. Mechanisms through hypoxia-inducible factor-1, glycolysis, and epigenetic regulators such as p300, protein inhibitors of activated Stats and H19 play crucial role in stem cell quiescence, and anti-aging regulations. Conversely, genomic instability such as DNA double-strand-breaks modulate cellular aging through the mammalian target of rapamycin pathway, which may lead to decreased lifespan.
- Citation: Wong TY, Solis MA, Chen YH, Huang LLH. Molecular mechanism of extrinsic factors affecting anti-aging of stem cells. World J Stem Cells 2015; 7(2): 512-520
- URL: https://www.wjgnet.com/1948-0210/full/v7/i2/512.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v7.i2.512
In 1881, Weismann et al[1] discovered that organ performance was influenced by the finite cell division of tissues. Followed by Hayflick et al[2] in 1961, who discovered that normal human cells have finite cell replication and lifetime which represents human aging. When cells reach maximal replicative capacity, it is a phenomenon termed cellular senescence, or cellular aging. Recent evidence supports the model that stem cells in vivo are retained in a quiescent state, which can be reactivated into cell cycle progression in response to extracellular stimuli even after prolonged period of quiescence. Upon stimulation, stem cells divide to yield undifferentiated progeny and also differentiated cells through subsequent rounds of proliferation[3]. With self-renewal capability, stem cells pool assures a constant supply of stem cells, and also differentiated cells throughout an organism lifespan. However, loss of stem cells number or functionality with age can lead to profound consequences on tissue viability[4]. Recent data suggests that stem cells aging is partly due to heritable intrinsic event such as DNA damage, changes in their niches and micro-environment[3]. Through systemic influences, old tissues might be rejuvenated to a young state[5]. The significant sign of aging in stem cell culture is a diminishing replicative capacity, in which the maximal population doublings of mesenchymal stem cells (MSC) are reported as 30-40[6]. In vitro, MSC cellular aging is associated with the age of donor[7]. Conversely, in vitro culture of embryonic stem cells (ESC) showed no loss of proliferative potency[8]. Human MSC cultured in vitro have spindle-shaped fibroblastic morphology and usually cease to proliferate no more than 40 population doublings, with the cells becoming enlarged and more flattened[9]. The increase in cell size is associated with aging in vitro[10]. Unravelling these distinctive features of aging stem cell phenotype is critical to the success of therapeutic application of stem cells in the field of regenerative medicine with respect to tissue injury, degenerative diseases or organ declines that accompany aging[11].
The involvement of MSC in cell replenishment and lifespan regulations[12] is evident since aging is defined as the sum of primary restrictions in regenerative mechanisms of multicellular organisms[13]. Recently, the best strategy suggested to “cure” aging due to DNA damage is a rapid and effective elimination of the damaged stem cells by apoptosis[14]. This theory implies that stem cells may conceive ability to limit and repair intracellular damage. For example, stem cells possess robust cell surface transporters that exclude toxins[15], the machinery for double-stranded DNA breaks repair[16], and the production of telomerase to ameliorate telomere shortening[17]. Also, stem cells appear to have evolved multiple mechanisms such as senescence and apoptosis to sense damaged stem cell genome with malignant potential in order to limit replicative expansion[3,18]. These tumor-suppressor mechanisms may contribute to stem-cell irreversible growth arrest[3]. It is worthy to understand the impacts of aging on stem cells to propose valuable strategies, gather information for potential medical anti-aging interventions.
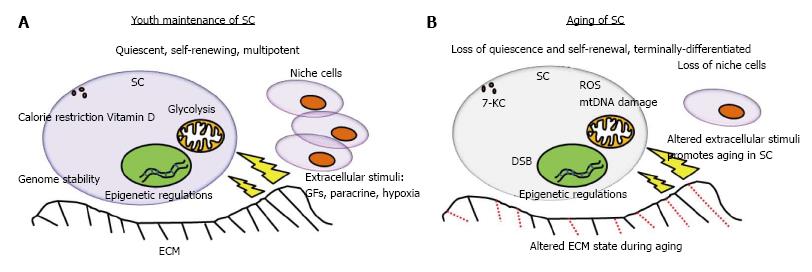
Aging can lead to changes of not only properties and number of stem cells, but also the niche cells, which gradually lead to decline in homeostasis and tissue regeneration. Aging has both quantitative and qualitative effects on stem cells. The qualitative changes affect self-renewal, developmental potential, and interactions with extrinsic signals[19]. Changes in the niche or micro-environment include amount and composition of extracellular matrix (ECM), altered expression of membrane proteins and lipids in niche cells that are in direct contact with stem cells, and changes in secretion of soluble paracrine or endocrine factors (Figure 1B). In the case of tissue injury or disease, factors released from damaged cells are altered, leading to inflammatory response[11]. Micro-environment in vivo is comprised of a stem cell compartment, niche cells that support stem cells, and complex ECM including fibronectin, collagen (I and IV), heparin sulphate, chondroitin sulphate, and hyaluronan[20]. The three different micro-environments: environmental enrichment, physical exercise and calorie restriction increased the number of newly regenerated neural cells in the dentate gyrus[21]. Hyaluronan preserved differentiation potentials of MSC derived from mouse[22]. Hyaluronan also maintained MSC derived from human placenta in a slow-cycling mode, which was similar to quiescent state[23]. Besides, hyaluronan is widely reviewed to regulate stem cells behavior via cluster determinant 44, receptor for hyaluronan-mediated motility, lymphatic vessel endothelial hyaluronan receptor, hyaluronan receptor for endocytosis, liver endothelial cell clearance receptor, or toll-like receptor 4[24]. A report implied that the activation of cells for productive tissue regeneration required a systemic micro-environment that maintains stem cells[5].
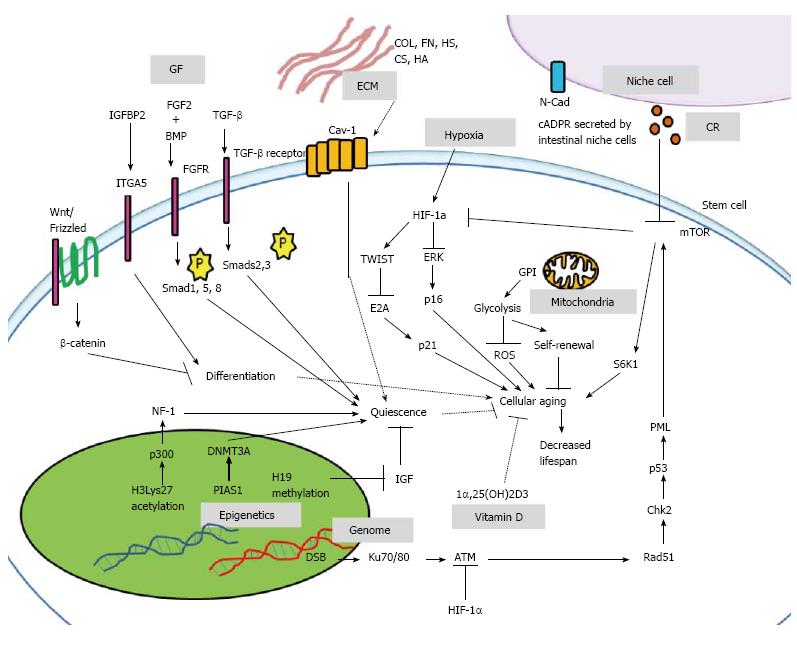
The micro-environment where stem cells reside in vivo plays important role in maintaining stem cells properties, and in regulating stem cells aging. In vivo, the stem cells are tightly linked to their micro-environment such as ECM, and surrounding niche cells which promote self-renewal, maintain differentiation potential, maintain quiescence, regulate cell survival, homeostasis and also cellular lifespan (Figure 1). The adhesive protein N-cadherin on niche cells support stem cells; whereas integrins on stem cells and extrinsic regulators such as angiopoietin-1 (Ang-1), tumor growth factor-β (TGF-β), bone morphogenetic protein (BMP), thrombopoietin (TPO), wingless/integrated (Wnt), β-catenin (CTNNB) and osteopontin (OPN) play a role in stem cells quiescence[25]. Hematopoiesis of hematopoietic stem cells (HSC) required the secreted factors, including stem cell factor, Ang-1, TPO, Wnt, NOTCH, OPN, chemokine stromal cell-derived factor-1α (SDF-1α), and direct interaction with integrin[26]. Using genome-wide chromatin and transcriptional profiling of hair follicle stem cells in vivo, the hair follicle micro-environment triggered LIM homeobox-2 (LHX2) expression, which trans-activated genes that orchestrated cytoskeletal dynamics and adhesion[27]. The removal of LHX2 led to disastrous cellular disorganization and polarization, loss of quiescence for hair follicle stem cells, loss of normal hair anchoring, as well as progressive transformation of the niche into a sebaceous gland[27]. Since the extrinsic micro-environmental factors are required for maintaining the youth of stem cells, their interactions with stem cell membrane may also play important roles. Recently, a membrane lipid raft protein known as caveolin-1 (Cav-1), was reported to be involved in HSC quiescence and self-renewal, wherein Cav-1-deficient mice had impaired self-renewal and altered quiescent state[28].
Furthermore, stem cells require growth factors, cytokines and mitogens for maintenance. The activation of the Wnt-Frizzled receptor with co-receptor LRP5/6 which target cytosolic CTNNB, and activation of the TGF-β receptor kinases 1/2 that phosphorylate Smads 2/3 helped maintain differentiation potential[29]. TGF-β is a potent inhibitor of stem cell growth and cell cycle in vitro, and is suggested to be a regulator of stem cell quiescence in vivo[30]. Another micro-environmental factor, insulin-like growth factor-binding protein-2 (IGFBP2), used its C-terminus domain to support HSC survival and cell cycle[31]. In addition, IGFBP2 promoted hMSC osteogenesis via interaction with integrin alpha-5[32]. The BMP also interacted with fibroblast growth factor-2 (FGF2) through FGF receptor, which in turn activated Smad1, Smad5 and Smad8 to inhibit differentiation, and maintain quiescence in rat neural stem cells[33]. Meanwhile, FGF2 signaling alone suppressed terminal astrocytic differentiation, and maintained rat neural stem cell potency during quiescence[33].
Stem cells are maintained in a low oxygen environment or hypoxia in their native state. In general, 5% of pO2 is the physiologically-relevant O2 concentration in stem cells micro-environment. When 5% of pO2 was exposed to the micro-environment, Wharton Jelly derived-MSC culture rejuvenated toward less differentiated, more primitive phenotypes[34]. It also promoted and stabilized expression of OCT4A, NANOG, SOX2, and REX1 in induced pluripotent stem cells (iPSC)[35]. Because 2% O2 decreased mitochondrial DNA content[36], this suggests that hypoxia-induced stem cell rejuvenation may be linked to energy metabolism via the mitochondria. Under condition of 1% O2, hypoxia-inducible factor-1 (HIF-1) activation led to decreased extracellular signal regulated kinase, followed by decreased p16 expression[37]. The decreased expression of the aging marker p16 helped MSC escaped cellular aging in vitro[37]. A recent study further confirmed that 1% O2 down-regulated DNA damage responsive molecules, including ATM/ATR, Chk1, and Chk2, and also cellular aging markers, including senescence-associated-β-galactosidase, H3K9me3, heterochromatin protein 1-γ (HP1γ), p53, p21, and p16[38]. Since cellular aging is often accompanied with telomere length loss, it was observed that bone marrow MSC expanded under hypoxia (3% pO2) for 15 d demonstrated telomere length maintenance; however, telomere length decreased over time under normoxia[39]. Recently reported a major pathway that inhibits MSC senescence due to hypoxia is the HIF-1α-TWIST pathway which down-regulated E2A-p21[40]. In addition, hypoxia induced an immediate and concerted down-regulation of genes involved in DNA repair and damage response pathways (including MLH1, RAD51, BRCA1, and Ku80 genes)[36], which suggests that hypoxia plays a role in genome stability. Furthermore, p53 may suppressed HIF-1 translation through targeting pro-myelocytic leukemia (PML) protein, which activated the mammalian target of rapamycin (mTOR) pathway to induce aging (Figure 2)[41,42]. Conversely, HIF-1 interacted with the aging marker p53 that led to p53 stabilization[43], resulting to a pro-aging phenomena. Taken together, hypoxia provides stem cells with an anti-aging micro-environment and is required for healthy aging progression.
The genome is like an internal turbine engine inside a cell that works perpetually throughout the life of cell. Without it, cell survival is impossible, and youth of cells cannot be maintained. In the 1950s, researchers Mortimer and Johnston stated that aging in yeast is accompanied by genome instability[44]. In principle, there are three different modes of genome instability: mutations, mismatch repair deficiency and chromosomal instability[45]. Mechanisms of genome instability linked to mammalian aging are stress-induced reactive oxygen species (ROS), telomere loss, and germ-line genetic variations for DNA repair[46,47]. In addition, DNA damage caused by ROS was stated to elicit transient growth arrest, apoptosis and cellular senescence[48]. In adult hematopoietic stem and progenitor cells (HSPCs), the histone deacetylase Sirt1 is linked to longevity[49], DNA damage accumulation, and loss of progenitor cells. Under stress of Sirt1 ablation, HSPCs increased Hoxa-9 expression, leading to increased DNA damage[50]. Another sirtuin family protein, SIRT6, also regulates aging and genome stability. During DNA double-strand break repair, SIRT6 promoted DNA end resection in order to maintain genome integrity[51]. Interestingly, a family member of p53, TAp63, prevented premature aging in skin cells as observed in TAp63-/- mice by maintaining properties of epidermal and dermal precursors[52]. The most prominent evidence that links genome stability with aging is the human disease known as progeroid syndrome. Defects in the DNA repair system known as transcription-coupled excision repair (TCER) leads to progeroid syndrome[53], wherein lesions in transcribed strand of active genes are repaired with defective TCER[54]. In contrast to what is known about quiescence, the stem cells are prone to DNA damage accumulation while in quiescence. A previous study stated that following DNA damage, stem cells utilized non-homologous end-joining (NHEJ) repair system during quiescence; however, the NHEJ response is normally associated with genome rearrangement and abnormalities in HSC[55]. In brief, genome stability is crucial for stem cells maintenance.
Mitochondria play a significant role in the aging-induced signaling pathway since aging is generally characterized as a progressive decline of tissue and organ function accompanied by increased oxidative damage and mitochondrial dysfunction. Stem cells in the quiescent state appear to be less metabolically active and may be subjected to lower levels of cellular metabolism byproducts, mainly ROS. Since mitochondria are the sites for oxidative metabolism, ROS may inflict damage to its DNA and the organelle itself. Previously, functional analysis demonstrated altered mitochondrial morphology, decreased antioxidant capacities and elevated ROS levels in long-term cultivated young MSC as well as aged MSC[56]. Another previous study stated that mice expressing a mutant form of mitochondrial DNA polymerase-γ led to HSC with compromised functions, indicating that mitochondrial integrity is crucial for stem cell maintenance[57]. In addition, long-lived post-mitotic cells with acquired stem cell damage may be inherited by progeny cells since the mitochondrial DNA damage is passed down the generations. Recent evidence indicates that loss of a negative regulator of the Ink4a/Arf locus, also known as Cdkn2a or BMI1, is associated with decreased mitochondrial function, decreased electron transport flux, increased ROS concentrations, and loss of stem cell functions[58]. Furthermore, hypoxic stimulation of glycolytic flux as well as inhibition of the p53 pathway[59] partly stimulated glycolysis to improve stem cell pluripotency[60] and also resulted in a younger cell phenotype. Interestingly, direct pharmacological modulation of energy metabolism or supplementation with glycolytic intermediates augmented reprogramming efficiency through glycolysis[61]. Over-expression of the glycolytic enzyme, glucosephosphate isomerase (GPI), showed enhanced glycolysis and bypassed senescence with an increased resistance to oxidative DNA damage. In addition, depletion of GPI shortened cellular lifespan. Coincidently, mouse embryonic stem cells, which are stem cells highly resistant to oxidative stress demonstrated a high level of glycolysis[62]. Overall, these findings suggest that stem cells can utilize mitochondria to escape restrictions of cellular lifespan via an anti-aging mechanism (Figure 2).
The process of aging is accompanied by nuclear architectural instability in the cell[63,64]; however, there is a lack of evidence to show that DNA damage reduction can extend lifespan of cells in vitro. As a result, scientists have suggested that aging may also be caused by epigenetic regulations[65]. Recently, protein inhibitors of activated Stats (PIAS1), a SUMO E3 ligase was shown to regulate HSCs self-renewal via GATA1 suppression by binding to GATA1 promoter region to recruit DNMT3A[66]. Using genome-wide mapping at an enhancer associated histone mark H3Lys27 acetylation and p300 binding at promoter site, the nuclear factor-1 (NF1) protein family seemed to be required for neural stem cell (NSC) entry into quiescence[67]. In addition, integrin alpha-6 was down-regulated when the NSC entered quiescence[67]. Interestingly, the imprinting alleles H19-Igf2 helped maintain HSC quiescence, wherein the maternal H19 region is differentially methylated (H19-DMR). Deletion of the H19-DMR led to Igf-Igfr1 activation and loss of quiescence[68]. Previous findings suggested that Mysm1 modulated histone modification of the Gfi1 promoter region in order to recruit Gata2 and Runx1 while maintaining HSC quiescence[69]. Moreover, the rejuvenation of muscle cells, skin cells and bone marrow cells of mice were observed through systemic connection of one old mouse to another young mouse and were suggested to be modulated by epigenetics[65].
Apart from these, caloric restriction due to limited calorie intake is also responsible for extrinsic signaling in stem cell maintenance and aging. In general, mechanisms involved during the stem cell quiescent state are different from those during active proliferative state. Calorie restriction led to up-regulation of cyclic ADP-ribose signaling in the intestinal niche cells known as Paneth cells, and induced proliferation of leucine rich repeat-containing G protein-coupled receptor 5-positive intestinal stem cells by inhibiting mTOR signaling[70]. From the perspective of energy metabolism for various calorie consumptions, stem cells usually undergo glycolysis rather than oxidative phosphorylation. Under normoxia, MSCs switched to oxidative phosphorylation that led to three- to fourfold increase in senescence; however, hypoxia induced glycolysis in order to prevent oxidative stress-induced senescence and preserve MSC long-term self-renewal[71]. Surprisingly, study stated that over-expression of phosphoinositide-dependent kinase may restore glycolytic metabolism in glycolytic-defective HSC[72]. In general, native stem cell metabolisms are linked to increased glycolysis, limited oxidative metabolism, and resistance to oxidative damage[73].
Nutrient-sensitive signaling pathways that are known to regulate organismal aging include the insulin-PI3K, Akt-FOXO, mTOR and AMPK pathways, which regulate the balance between quiescence and proliferation of stem cells during aging[74-78]. Decreased activity of the mTOR and its target S6K1 homologs increased life span in yeast, nematodes, and fruit flies[79]. The S6K1 is a ribosomal S6 protein kinase targeted by mTOR, and is a determinant of mammalian aging in the nutrient-sensitive signaling pathways[80]. For the AMPK pathway, deletion of the AMPK regulator LKB1 (serine/threonine protein kinase 11) may lead to loss of mouse LT-HSC quiescence[81]. In human ESCs and iPSCs, methionine deprivation resulted in rapid decrease of intracellular S-adenosylmethionine, which triggered stem cell differentiation, p53 and p38 activation, and reduced NANOG expression[82]. With regard to lipid metabolism, various lipids and fatty acids regulated proliferation and differentiation of pluripotent stem cells and adult progenitors[83], and albumin-associated lipids promoted self-renewal of hESCs[84]. Biosynthesis of lipid such as cholesterol may influence progenitor cell differentiation, and inhibitors of cholesterol biosynthesis enhanced differentiation of mouse C2C12 myoblasts[85]. Cholesterol is easily oxidized and may be converted to various oxidation products known as oxysterols. One of the major oxysterols is 7-ketocholesterol (7-KC), wherein increased 7-KC led to gradual changes in morphology of human adipocyte MSC, beginning with the loss of unidirectional alignment at lower concentrations, and leading towards loss of actin organization and loss of intracellular contact at higher concentrations[86].
Vitamins are important part of our diets and play some role in anti-aging of stem cells. It was reported that 1α,25-dihydroxyvitamin D3 (1α, 25(OH)2D3) delayed replicative senescence in primary hMSC; however, its pro-aging effects due to elevated systemic phosphate levels were seen in mouse models[87]. Other findings suggested that vitamin D metabolism played a role in human osteoblastogenesis in hMSCs, in which both 25(OH)D3 and 1α,25(OH)2D3 mediated osteoblast differentiation in hMSCs through up-regulation of insulin-like growth factor-1[88].
The direct connection between stem cell aging and organismal aging, and lifespan is yet to be defined. The onset of aging process is often believed to influence cellular lifespan based on lowered in vitro replicative capability of old MSC from older compared to MSC from younger individual. Whether the influence on replicative capability is caused by systemic organismal aging, or the altered state of MSC during aging process, remains a question. Stem cells are empowered with self-renewal and differentiation potentials, and stay quiescent for long period of time while preserving their functions. The dormant state, or quiescence, seems to preserve the youth of stem cells despite the passing of time. Nevertheless, evidence suggests that stem cell population and functions may decrease in old individuals. It is likely that the youthful phenotypes of stem cells are preserved as long as it takes in order to allow healthy aging process. In times of trauma, injury, environmental stress such as ultra-violet over-exposure, and psychological stress, the process of aging may be affected, and lifespan may be shortened. Through understanding the prospect of stem cell anti-aging phenomena, the therapeutic application of stem cells can be broadened, and perhaps, a direct link between stem cell anti-aging and longevity may be established.
P- Reviewer: Faienza MF, Li YZ, Zhu F S- Editor: Tian YL L- Editor: A E- Editor: Lu YJ
Accepted: November 17, 2014
| 1. | Weismann A. Poulton EB, editor. Collected Essays upon Heredity and Kindred Biological Problems. Oxford, UK: Clarendon Press 1889; . |
| 2. | Hayflick L, Moorhead PS. The serial cultivation of human diploid cell strains. Exp Cell Res. 1961;25:585-621. [PubMed] |
| 3. | Sharpless NE, DePinho RA. How stem cells age and why this makes us grow old. Nat Rev Mol Cell Biol. 2007;8:703-713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 654] [Cited by in RCA: 624] [Article Influence: 34.7] [Reference Citation Analysis (1)] |
| 4. | Pelicci PG. Do tumor-suppressive mechanisms contribute to organism aging by inducing stem cell senescence? J Clin Invest. 2004;113:4-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 26] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 5. | Conboy IM, Conboy MJ, Wagers AJ, Girma ER, Weissman IL, Rando TA. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature. 2005;433:760-764. [PubMed] |
| 6. | Baxter MA, Wynn RF, Jowitt SN, Wraith JE, Fairbairn LJ, Bellantuono I. Study of telomere length reveals rapid aging of human marrow stromal cells following in vitro expansion. Stem Cells. 2004;22:675-682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 571] [Cited by in RCA: 589] [Article Influence: 29.5] [Reference Citation Analysis (0)] |
| 7. | Rubin H. Promise and problems in relating cellular senescence in vitro to aging in vivo. Arch Gerontol Geriatr. 2002;34:275-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 26] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 8. | Rosenberger RF. The initiation of senescence and its relationship to embryonic cell differentiation. Bioessays. 1995;17:257-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 9. | Bruder SP, Jaiswal N, Haynesworth SE. Growth kinetics, self-renewal, and the osteogenic potential of purified human mesenchymal stem cells during extensive subcultivation and following cryopreservation. J Cell Biochem. 1997;64:278-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 10. | Hayflick L. The limited in vitro lifetime of human diploid cell strains. Exp Cell Res. 1965;37:614-636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4095] [Cited by in RCA: 4035] [Article Influence: 67.3] [Reference Citation Analysis (0)] |
| 11. | Rando TA. Stem cells, ageing and the quest for immortality. Nature. 2006;441:1080-1086. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 521] [Cited by in RCA: 501] [Article Influence: 26.4] [Reference Citation Analysis (0)] |
| 12. | Sethe S, Scutt A, Stolzing A. Aging of mesenchymal stem cells. Ageing Res Rev. 2006;5:91-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 450] [Cited by in RCA: 466] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 13. | Sames K, Sebastian S, Alexandra S, editors . Extending the Lifespan: Biotechnical, Gerontological, and Social Problems. Hamburg: LIT Verlag Münster 2005; . |
| 14. | Cairns J. Somatic stem cells and the kinetics of mutagenesis and carcinogenesis. Proc Natl Acad Sci USA. 2002;99:10567-10570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 161] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 15. | Gottesman MM, Fojo T, Bates SE. Multidrug resistance in cancer: role of ATP-dependent transporters. Nat Rev Cancer. 2002;2:48-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4043] [Cited by in RCA: 4140] [Article Influence: 180.0] [Reference Citation Analysis (0)] |
| 16. | Park IK, He Y, Lin F, Laerum OD, Tian Q, Bumgarner R, Klug CA, Li K, Kuhr C, Doyle MJ. Differential gene expression profiling of adult murine hematopoietic stem cells. Blood. 2002;99:488-498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 115] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 17. | Morrison SJ, Prowse KR, Ho P, Weissman IL. Telomerase activity in hematopoietic cells is associated with self-renewal potential. Immunity. 1996;5:207-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 292] [Cited by in RCA: 278] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 18. | Sharpless NE, DePinho RA. Telomeres, stem cells, senescence, and cancer. J Clin Invest. 2004;113:160-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 340] [Cited by in RCA: 309] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 19. | Roobrouck VD, Ulloa-Montoya F, Verfaillie CM. Self-renewal and differentiation capacity of young and aged stem cells. Exp Cell Res. 2008;314:1937-1944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 193] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 20. | Dorshkind K. Regulation of hemopoiesis by bone marrow stromal cells and their products. Annu Rev Immunol. 1990;8:111-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 357] [Cited by in RCA: 369] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 21. | Rao MS, Mattson MP. Stem cells and aging: expanding the possibilities. Mech Ageing Dev. 2001;122:713-734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 242] [Cited by in RCA: 241] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 22. | Chen PY, Huang LL, Hsieh HJ. Hyaluronan preserves the proliferation and differentiation potentials of long-term cultured murine adipose-derived stromal cells. Biochem Biophys Res Commun. 2007;360:1-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 44] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 23. | Liu CM, Yu CH, Chang CH, Hsu CC, Huang LL. Hyaluronan substratum holds mesenchymal stem cells in slow-cycling mode by prolonging G1 phase. Cell Tissue Res. 2008;334:435-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 24. | Solis MA, Chen YH, Wong TY, Bittencourt VZ, Lin YC, Huang LL. Hyaluronan regulates cell behavior: a potential niche matrix for stem cells. Biochem Res Int. 2012;2012:346972. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 138] [Cited by in RCA: 160] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 25. | Li L, Bhatia R. Molecular Pathways: Stem Cell Quiescence. Clin Cancer Res. 2011;17:4936-4941. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 233] [Cited by in RCA: 221] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 26. | Shiozawa Y, Havens AM, Pienta KJ, Taichman RS. The bone marrow niche: habitat to hematopoietic and mesenchymal stem cells, and unwitting host to molecular parasites. Leukemia. 2008;22:941-950. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 155] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 27. | Folgueras AR, Guo X, Pasolli HA, Stokes N, Polak L, Zheng D, Fuchs E. Architectural niche organization by LHX2 is linked to hair follicle stem cell function. Cell Stem Cell. 2013;13:314-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 77] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 28. | Bai L, Shi G, Zhang L, Guan F, Ma Y, Li Q, Cong Y-S, Zhang L. Cav-1 deletion impaired hematopoietic stem cell function. Cell Death Dis. 2014;5:e1140. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 29. | Pekovic V, Hutchison CJ. Adult stem cell maintenance and tissue regeneration in the ageing context: the role for A-type lamins as intrinsic modulators of ageing in adult stem cells and their niches. J Anat. 2008;213:5-25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 60] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 30. | Blank U, Karlsson G, Karlsson S. Signaling pathways governing stem-cell fate. Blood. 2008;111:492-503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 245] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 31. | Huynh H, Zheng J, Umikawa M, Zhang C, Silvany R, Iizuka S, Holzenberger M, Zhang W, Zhang CC. IGF binding protein 2 supports the survival and cycling of hematopoietic stem cells. Blood. 2011;118:3236-3243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 70] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 32. | Hamidouche Z, Fromigué O, Ringe J, Häupl T, Marie PJ. Crosstalks between integrin alpha 5 and IGF2/IGFBP2 signalling trigger human bone marrow-derived mesenchymal stromal osteogenic differentiation. BMC Cell Biol. 2010;11:44. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 68] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 33. | Sun Y, Hu J, Zhou L, Pollard SM, Smith A. Interplay between FGF2 and BMP controls the self-renewal, dormancy and differentiation of rat neural stem cells. J Cell Sci. 2011;124:1867-1877. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 58] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 34. | Drela K, Sarnowska A, Siedlecka P, Szablowska-Gadomska I, Wielgos M, Jurga M, Lukomska B, Domanska-Janik K. Low oxygen atmosphere facilitates proliferation and maintains undifferentiated state of umbilical cord mesenchymal stem cells in an hypoxia inducible factor-dependent manner. Cytotherapy. 2014;16:881-892. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 70] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 35. | Yamanaka S. Induced pluripotent stem cells: past, present, and future. Cell Stem Cell. 2012;10:678-684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 543] [Cited by in RCA: 527] [Article Influence: 40.5] [Reference Citation Analysis (0)] |
| 36. | Oliveira PH, Boura JS, Abecasis MM, Gimble JM, da Silva CL, Cabral JM. Impact of hypoxia and long-term cultivation on the genomic stability and mitochondrial performance of ex vivo expanded human stem/stromal cells. Stem Cell Res. 2012;9:225-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 37. | Jin Y, Kato T, Furu M, Nasu A, Kajita Y, Mitsui H, Ueda M, Aoyama T, Nakayama T, Nakamura T. Mesenchymal stem cells cultured under hypoxia escape from senescence via down-regulation of p16 and extracellular signal regulated kinase. Biochem Biophys Res Commun. 2010;391:1471-1476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 90] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 38. | Kilic Eren M, Tabor V. The Role of Hypoxia Inducible Factor-1 Alpha in Bypassing Oncogene-Induced Senescence. PLoS One. 2014;9:e101064. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 39. | D'Ippolito G, Diabira S, Howard GA, Roos BA, Schiller PC. Low oxygen tension inhibits osteogenic differentiation and enhances stemness of human MIAMI cells. Bone. 2006;39:513-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 287] [Cited by in RCA: 280] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 40. | Tsai CC, Chen YJ, Yew TL, Chen LL, Wang JY, Chiu CH, Hung SC. Hypoxia inhibits senescence and maintains mesenchymal stem cell properties through down-regulation of E2A-p21 by HIF-TWIST. Blood. 2011;117:459-469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 267] [Cited by in RCA: 298] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 41. | Ferbeyre G, de Stanchina E, Querido E, Baptiste N, Prives C, Lowe SW. PML is induced by oncogenic ras and promotes premature senescence. Genes Dev. 2000;14:2015-2027. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 242] [Cited by in RCA: 254] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 42. | Bernardi R, Guernah I, Jin D, Grisendi S, Alimonti A, Teruya-Feldstein J, Cordon-Cardo C, Simon MC, Rafii S, Pandolfi PP. PML inhibits HIF-1alpha translation and neoangiogenesis through repression of mTOR. Nature. 2006;442:779-785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 293] [Cited by in RCA: 308] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 43. | An WG, Kanekal M, Simon MC, Maltepe E, Blagosklonny MV, Neckers LM. Stabilization of wild-type p53 by hypoxia-inducible factor 1alpha. Nature. 1998;392:405-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 611] [Cited by in RCA: 632] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 44. | Mortimer RK, Johnston JR. Life span of individual yeast cells. Nature. 1959;183:1751-1752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 591] [Cited by in RCA: 572] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 45. | Steinemann D, Göhring G, Schlegelberger B. Genetic instability of modified stem cells - a first step towards malignant transformation? Am J Stem Cells. 2013;2:39-51. [PubMed] |
| 46. | Kenyon C. The plasticity of aging: insights from long-lived mutants. Cell. 2005;120:449-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 978] [Cited by in RCA: 965] [Article Influence: 48.3] [Reference Citation Analysis (0)] |
| 47. | Lombard DB. Sirtuins at the breaking point: SIRT6 in DNA repair. Aging (Albany NY). 2009;1:12-16. [PubMed] |
| 48. | Hasty P, Campisi J, Hoeijmakers J, van Steeg H, Vijg J. Aging and genome maintenance: lessons from the mouse? Science. 2003;299:1355-1359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 425] [Cited by in RCA: 409] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 49. | Haigis MC, Sinclair DA. Mammalian sirtuins: biological insights and disease relevance. Annu Rev Pathol. 2010;5:253-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1428] [Cited by in RCA: 1631] [Article Influence: 108.7] [Reference Citation Analysis (0)] |
| 50. | Singh SK, Williams CA, Klarmann K, Burkett SS, Keller JR, Oberdoerffer P. Sirt1 ablation promotes stress-induced loss of epigenetic and genomic hematopoietic stem and progenitor cell maintenance. J Exp Med. 2013;210:987-1001. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 89] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 51. | Kaidi A, Weinert BT, Choudhary C, Jackson SP. Human SIRT6 promotes DNA end resection through CtIP deacetylation. Science. 2010;329:1348-1353. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 294] [Cited by in RCA: 280] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 52. | Su X, Paris M, Gi YJ, Tsai KY, Cho MS, Lin YL, Biernaskie JA, Sinha S, Prives C, Pevny LH. TAp63 prevents premature aging by promoting adult stem cell maintenance. Cell Stem Cell. 2009;5:64-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 200] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 53. | Lehmann AR. DNA repair-deficient diseases, xeroderma pigmentosum, Cockayne syndrome and trichothiodystrophy. Biochimie. 2003;85:1101-1111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 357] [Cited by in RCA: 354] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 54. | Schumacher B, Garinis GA, Hoeijmakers JH. Age to survive: DNA damage and aging. Trends Genet. 2008;24:77-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 185] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 55. | Mohrin M, Bourke E, Alexander D, Warr MR, Barry-Holson K, Le Beau MM, Morrison CG, Passegué E. Hematopoietic stem cell quiescence promotes error-prone DNA repair and mutagenesis. Cell Stem Cell. 2010;7:174-185. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 510] [Cited by in RCA: 483] [Article Influence: 32.2] [Reference Citation Analysis (0)] |
| 56. | Geißler S, Textor M, Kühnisch J, Könnig D, Klein O, Ode A, Pfitzner T, Adjaye J, Kasper G, Duda GN. Functional comparison of chronological and in vitro aging: differential role of the cytoskeleton and mitochondria in mesenchymal stromal cells. PLoS One. 2012;7:e52700. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 117] [Cited by in RCA: 140] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 57. | Chen ML, Logan TD, Hochberg ML, Shelat SG, Yu X, Wilding GE, Tan W, Kujoth GC, Prolla TA, Selak MA. Erythroid dysplasia, megaloblastic anemia, and impaired lymphopoiesis arising from mitochondrial dysfunction. Blood. 2009;114:4045-4053. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 94] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 58. | Liu J, Cao L, Chen J, Song S, Lee IH, Quijano C, Liu H, Keyvanfar K, Chen H, Cao LY. Bmi1 regulates mitochondrial function and the DNA damage response pathway. Nature. 2009;459:387-392. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 391] [Cited by in RCA: 388] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 59. | Hong H, Takahashi K, Ichisaka T, Aoi T, Kanagawa O, Nakagawa M, Okita K, Yamanaka S. Suppression of induced pluripotent stem cell generation by the p53-p21 pathway. Nature. 2009;460:1132-1135. [PubMed] |
| 60. | Mohyeldin A, Garzón-Muvdi T, Quiñones-Hinojosa A. Oxygen in stem cell biology: a critical component of the stem cell niche. Cell Stem Cell. 2010;7:150-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1249] [Cited by in RCA: 1143] [Article Influence: 76.2] [Reference Citation Analysis (0)] |
| 61. | Folmes CD, Nelson TJ, Martinez-Fernandez A, Arrell DK, Lindor JZ, Dzeja PP, Ikeda Y, Perez-Terzic C, Terzic A. Somatic oxidative bioenergetics transitions into pluripotency-dependent glycolysis to facilitate nuclear reprogramming. Cell Metab. 2011;14:264-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 752] [Cited by in RCA: 792] [Article Influence: 56.6] [Reference Citation Analysis (0)] |
| 62. | Kondoh H, Lleonart ME, Gil J, Wang J, Degan P, Peters G, Martinez D, Carnero A, Beach D. Glycolytic enzymes can modulate cellular life span. Cancer Res. 2005;65:177-185. [PubMed] |
| 63. | Oberdoerffer P, Sinclair DA. The role of nuclear architecture in genomic instability and ageing. Nat Rev Mol Cell Biol. 2007;8:692-702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 208] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 64. | Campisi J, Vijg J. Does damage to DNA and other macromolecules play a role in aging? If so, how? J Gerontol A Biol Sci Med Sci. 2009;64:175-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 70] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 65. | Rando TA, Chang HY. Aging, rejuvenation, and epigenetic reprogramming: resetting the aging clock. Cell. 2012;148:46-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 344] [Cited by in RCA: 373] [Article Influence: 28.7] [Reference Citation Analysis (0)] |
| 66. | Liu B, Yee KM, Tahk S, Mackie R, Hsu C, Shuai K. PIAS1 SUMO ligase regulates the self-renewal and differentiation of hematopoietic stem cells. EMBO J. 2014;33:101-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 67. | Martynoga B, Mateo JL, Zhou B, Andersen J, Achimastou A, Urbán N, van den Berg D, Georgopoulou D, Hadjur S, Wittbrodt J. Epigenomic enhancer annotation reveals a key role for NFIX in neural stem cell quiescence. Genes Dev. 2013;27:1769-1786. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 162] [Cited by in RCA: 161] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 68. | Venkatraman A, He XC, Thorvaldsen JL, Sugimura R, Perry JM, Tao F, Zhao M, Christenson MK, Sanchez R, Yu JY. Maternal imprinting at the H19-Igf2 locus maintains adult haematopoietic stem cell quiescence. Nature. 2013;500:345-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 237] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 69. | Wang T, Nandakumar V, Jiang XX, Jones L, Yang AG, Huang XF, Chen SY. The control of hematopoietic stem cell maintenance, self-renewal, and differentiation by Mysm1-mediated epigenetic regulation. Blood. 2013;122:2812-2822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 74] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 70. | Yilmaz ÖH, Katajisto P, Lamming DW, Gültekin Y, Bauer-Rowe KE, Sengupta S, Birsoy K, Dursun A, Yilmaz VO, Selig M. mTORC1 in the Paneth cell niche couples intestinal stem-cell function to calorie intake. Nature. 2012;486:490-495. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 498] [Cited by in RCA: 585] [Article Influence: 45.0] [Reference Citation Analysis (0)] |
| 71. | Pattappa G, Thorpe SD, Jegard NC, Heywood HK, de Bruijn JD, Lee DA. Continuous and uninterrupted oxygen tension influences the colony formation and oxidative metabolism of human mesenchymal stem cells. Tissue Eng Part C Methods. 2013;19:68-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 98] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 72. | Takubo K, Nagamatsu G, Kobayashi CI, Nakamura-Ishizu A, Kobayashi H, Ikeda E, Goda N, Rahimi Y, Johnson RS, Soga T. Regulation of glycolysis by Pdk functions as a metabolic checkpoint for cell cycle quiescence in hematopoietic stem cells. Cell Stem Cell. 2013;12:49-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 514] [Cited by in RCA: 619] [Article Influence: 51.6] [Reference Citation Analysis (0)] |
| 73. | Vacanti NM, Metallo CM. Exploring metabolic pathways that contribute to the stem cell phenotype. Biochim Biophys Acta. 2013;1830:2361-2369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 74. | Chen C, Liu Y, Liu Y, Zheng P. mTOR regulation and therapeutic rejuvenation of aging hematopoietic stem cells. Sci Signal. 2009;2:ra75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 400] [Cited by in RCA: 511] [Article Influence: 31.9] [Reference Citation Analysis (0)] |
| 75. | Jasper H, Jones DL. Metabolic regulation of stem cell behavior and implications for aging. Cell Metab. 2010;12:561-565. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 46] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 76. | Kharas MG, Okabe R, Ganis JJ, Gozo M, Khandan T, Paktinat M, Gilliland DG, Gritsman K. Constitutively active AKT depletes hematopoietic stem cells and induces leukemia in mice. Blood. 2010;115:1406-1415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 223] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 77. | Kalaitzidis D, Sykes SM, Wang Z, Punt N, Tang Y, Ragu C, Sinha AU, Lane SW, Souza AL, Clish CB. mTOR complex 1 plays critical roles in hematopoiesis and Pten-loss-evoked leukemogenesis. Cell Stem Cell. 2012;11:429-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 162] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 78. | Magee JA, Ikenoue T, Nakada D, Lee JY, Guan KL, Morrison SJ. Temporal changes in PTEN and mTORC2 regulation of hematopoietic stem cell self-renewal and leukemia suppression. Cell Stem Cell. 2012;11:415-428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 173] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 79. | Stanfel MN, Shamieh LS, Kaeberlein M, Kennedy BK. The TOR pathway comes of age. Biochim Biophys Acta. 2009;1790:1067-1074. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 266] [Cited by in RCA: 263] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 80. | Selman C, Tullet JM, Wieser D, Irvine E, Lingard SJ, Choudhury AI, Claret M, Al-Qassab H, Carmignac D, Ramadani F. Ribosomal protein S6 kinase 1 signaling regulates mammalian life span. Science. 2009;326:140-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 878] [Cited by in RCA: 880] [Article Influence: 55.0] [Reference Citation Analysis (0)] |
| 81. | Gan B, Hu J, Jiang S, Liu Y, Sahin E, Zhuang L, Fletcher-Sananikone E, Colla S, Wang YA, Chin L. Lkb1 regulates quiescence and metabolic homeostasis of haematopoietic stem cells. Nature. 2010;468:701-704. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 362] [Cited by in RCA: 344] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 82. | Shiraki N, Shiraki Y, Tsuyama T, Obata F, Miura M, Nagae G, Aburatani H, Kume K, Endo F, Kume S. Methionine metabolism regulates maintenance and differentiation of human pluripotent stem cells. Cell Metab. 2014;19:780-794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 354] [Cited by in RCA: 399] [Article Influence: 36.3] [Reference Citation Analysis (0)] |
| 83. | Yun DH, Song HY, Lee MJ, Kim MR, Kim MY, Lee JS, Kim JH. Thromboxane A(2) modulates migration, proliferation, and differentiation of adipose tissue-derived mesenchymal stem cells. Exp Mol Med. 2009;41:17-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 43] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 84. | Garcia-Gonzalo FR, Izpisúa Belmonte JC. Albumin-associated lipids regulate human embryonic stem cell self-renewal. PLoS One. 2008;3:e1384. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 126] [Cited by in RCA: 135] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 85. | Bracha AL, Ramanathan A, Huang S, Ingber DE, Schreiber SL. Carbon metabolism-mediated myogenic differentiation. Nat Chem Biol. 2010;6:202-204. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 69] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 86. | Levy D, Ruiz JL, Celestino AT, Silva SF, Ferreira AK, Isaac C, Bydlowski SP. Short-term effects of 7-ketocholesterol on human adipose tissue mesenchymal stem cells in vitro. Biochem Biophys Res Commun. 2014;446:720-725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 87. | Klotz B, Mentrup B, Regensburger M, Zeck S, Schneidereit J, Schupp N, Linden C, Merz C, Ebert R, Jakob F. 1,25-dihydroxyvitamin D3 treatment delays cellular aging in human mesenchymal stem cells while maintaining their multipotent capacity. PLoS One. 2012;7:e29959. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 48] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 88. | Geng S, Zhou S, Bi Z, Glowacki J. Vitamin D metabolism in human bone marrow stromal (mesenchymal stem) cells. Metabolism. 2013;62:768-777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |