Published online Mar 26, 2015. doi: 10.4252/wjsc.v7.i2.235
Peer-review started: November 2, 2014
First decision: November 27, 2014
Revised: December 31, 2014
Accepted: January 15, 2015
Article in press: January 15, 2015
Published online: March 26, 2015
Processing time: 138 Days and 9.4 Hours
Cardiovascular disease, nervous system disorders, and cancer in association with other diseases such as diabetes mellitus result in greater than sixty percent of the global annual deaths. These noncommunicable diseases also affect at least one-third of the population in low and middle-income countries and lead to hypertension, elevated cholesterol, malignancy, and neurodegenerative disorders such as Alzheimer’s disease and stroke. With the climbing lifespan of the world’s population, increased prevalence of these disorders is expected requiring the development of new therapeutic strategies against these disabling disease entities. Targeting stem cell proliferation for cardiac disease, vascular disorders, cancer, and neurodegenerative disorders is receiving great enthusiasm, especially those that focus upon SIRT1, a mammalian homologue of the yeast silent information regulator-2. Modulation of the cellular activity of SIRT1 can involve oversight by nicotinamide/nicotinic acid mononucleotide adenylyltransferase, mammalian forkhead transcription factors, mechanistic of rapamycin pathways, and cysteine-rich protein 61, connective tissue growth factor, and nephroblastoma over-expressed gene family members that can impact cytoprotective outcomes. Ultimately, the ability of SIRT1 to control the programmed cell death pathways of apoptosis and autophagy can determine not only cardiac, vascular, and neuronal stem cell development and longevity, but also the onset of tumorigenesis and the resistance against chemotherapy. SIRT1 therefore has a critical role and holds exciting prospects for new therapeutic strategies that can offer reparative processes for cardiac, vascular, and nervous system degenerative disorders as well as targeted control of aberrant cell growth during cancer.
Core tip: SIRT1, a mammalian homologue of the yeast silent information regulator-2, holds exciting prospects for new therapeutic strategies that can offer reparative processes for cardiac, vascular, and nervous system degenerative disorders as well as targeted control of unchecked cell growth during cancer.
- Citation: Maiese K. SIRT1 and stem cells: In the forefront with cardiovascular disease, neurodegeneration and cancer. World J Stem Cells 2015; 7(2): 235-242
- URL: https://www.wjgnet.com/1948-0210/full/v7/i2/235.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v7.i2.235
Life expectancy is increasing in developed countries such as the United States and has been accompanied by a one percent decrease in the age-adjusted death rate from the years 2000 through 2011[1]. Yet, a number of disorders on a global scale continue to plague the population with increased morbidity and mortality from cardiovascular disease, disorders of the nervous system, and cancer. The World Health Organization reports that in 2008, greater than 60% of 57 million global deaths were primarily due to cardiovascular diseases, diabetes, cancer, and respiratory disorders[2]. Almost 80% of these noncommunicable diseases (NCDs) occur in low and middle-income countries. These NCDs affect approximately 30% of the population under 60 in low and middle-income countries. In contrast, in high-income countries, 13% of the population under 60 is affected. Hypertension and elevated cholesterol are significant risk factors for cardiovascular disease with hypertension alone contributing to approximately 13% of all deaths[3]. Disorders such as hypertension and elevated cholesterol also contribute to acute neurodegenerative disease such as stroke, the fourth leading cause of death[4,5]. With the increasing lifespan of the world’s population and advancing age, it is expected that the incidence of neurodegenerative disorders also will grow. As an example, ten percent of the global population over the age of 65 is now affected with the sporadic form of Alzheimer’s disease, but this is expected to increase significantly[6-8]. Continued development of new therapeutic strategies directed against the NCDs of cardiovascular disease, neurodegeneration, and cancer are necessary to increase our armamentarium against the complexity of these disease entities.
In this arsenal directed against cardiovascular disease, neurodegenerative disorders, and cancer, multiple therapeutic strategies are being advanced that involve novel stem cell applications. Targeting stem cell proliferation is being considered for cardiac disease[9], vascular disorders[10,11], cancer[12,13], and neurodegenerative disease[14-16]. However, it is the investigation of stem cells that focus upon sirtuins, mammalian homologues of the yeast silent information regulator-2 (Sir2), that are proving to be extremely exciting.
Sirtuins are histone deacetylases that transfer acetyl groups from ε-N-acetyl lysine amino acids on the histones of DNA to regulate transcription[17-19]. This family of histone deacetylases also mediates post-translational changes of proteins involved with cellular proliferation, survival, and senescence[20-23]. There are seven identified mammalian homologues of Sir2 that include SIRT1 through SIRT7. Of these, SIRT1 has been tied to the modulation of multiple cellular functions that include protection against oxidative stress[24-28], development of atherosclerosis[29,30], modulation of vascular survival and senescence[17,20,21,31,32], proliferation of cancer cells[33-36], changes in diabetic cellular metabolism[33-36], control of vascular tone through the transient receptor potential cation channel A1[37], promotion of neuronal survival and cognitive function[21,38-41], and the extension of lifespan[25,42,43]. Furthermore, SIRT1 appears to be necessary for efficient post-reprogramming of telomere elongation, the maintenance of pluripotency, and the modulation of differentiation in induced pluripotent stem cells[44]. In differentiated cells, SIRT1 also controls telomere length and maintenance[45].
SIRT1 is dependent upon NAD+ as substrate[17,38,46,47]. Through the salvage pathway of NAD+ synthesis, nicotinamide phosphoribosyltransferase (NAMPT) catalyzes the conversion of nicotinamide to nicotinamide mononucleotide[48]. Nicotinamide mononucleotide is subsequently converted to NAD+ by enzymes in the nicotinamide/nicotinic acid mononucleotide adenylyltransferase (NMNAT) family. NAMPT is a rate-limiting enzyme in mammalian NAD+ biosynthesis pathway. Elevated levels of NAMPT activity increase cellular NAD levels as well as the activity of SIRT1 transcription.
The level of SIRT1 activity and its modulation in these cellular processes is considered to be an important factor in determining cell survival and protection against toxic insults. Insufficient SIRT1 activity can have a detrimental affect upon vascular cell survival[22,23,49], protection against cardiovascular disease[31], and prevention of neuronal injury[28,50,51]. Yet, a reduction in SIRT1 activity also may be required to promote cellular survival in systems involving trophic factors such as as insulin growth factor-1[52].
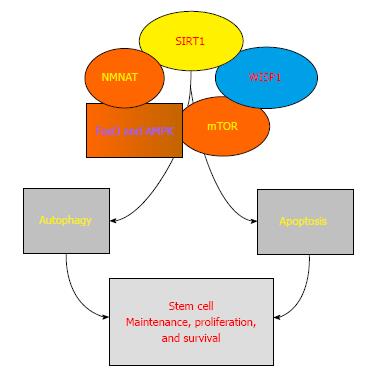
Several biological systems can control the activity of SIRT1 (Figure 1). For example, NMNAT can modulate the deacetylating activity of SIRT1. In addition, mammalian forkhead transcription factors[53] can bind to the SIRT1 promoter region that contains a cluster of five putative core binding repeat motifs (IRS-1) and a forkhead-like consensus-binding site (FKHD-L). As a result, forkhead transcription factors such as FoxO1 can govern SIRT1 transcription and increase SIRT1 expression[54]. AMP activated protein kinase (AMPK) represents another pathway for the control of SIRT1 activity. AMPK is a member of the mechanistic of rapamycin (mTOR) pathway that phosphorylates tuberous sclerosis protein 2 and inhibits the activity of mTORC1[55,56]. AMPK can increase the cellular NAD+/NADH ratio leading to the deacetylation of downstream SIRT1 targets that include the peroxisome proliferator-activated receptor-gamma coactivator 1 α, FoxO1, and FoxO3a[57]. AMPK also can increase NAMPT during glucose restriction that results in increased NAD+ and decreased levels of nicotinamide[58], an inhibitor of SIRT1[59]. Resveratrol, a SIRT1 activator, also has been shown to activate AMPK through SIRT1 dependent or independent mechanisms[57,60].
The impact of SIRT1 on cellular function is intimately associated with programmed cell death pathways that involve apoptosis and autophagy[61-63]. Apoptosis leads to DNA degradation and caspase activation through an early phase that involves the loss of plasma membrane lipid phosphatidylserine (PS) asymmetry and a later phase that results in genomic DNA degradation[64]. During the early phase of apoptosis, prevention of membrane PS externalization in injured cells is necessary to block the loss of functional cells that may be removed by activated inflammatory cells[56]. SIRT1 activation limits external membrane PS exposure during the early phases of apoptosis in mature cells[22,23,65,66]. In endothelial progenitor cells, SIRT1 activity can counteract the “pro-apoptotic” effects of tumor necrosis factor-α (TNF-α)[67]. During exposure to TNF-α, SIRT1 also has been shown to protect skeletal myoblast survival[68]. Loss of SIRT1 activity in human mesenchymal stem cells yields a reduction of proliferation rate with increased apoptosis[69]. During aging in the mouse auditory system, loss of SIRT1 in cochlear neurons and in the auditory cortex is associated with hearing loss[70]. In addition, endothelial progenitor cell dysfunction with apoptotic cell death that can occur in smokers and chronic obstructive disease patients has been associated with the loss of SIRT1 expression[71].
Stem cell survival with SIRT1 can be reliant upon forkhead transcription factors and mTOR (Figure 1). Although several studies involving differentiated cells support the premise that down-regulation of forkhead transcription factors by SIRT1 activation can protect against apoptotic cell death especially during oxidant stress[22,23,65,72,73], other studies in embryonic stem cells suggest that SIRT1 down-regulation can lead to the acetylation/phosphorylation of forkhead transcription factor pathways such as FoxO1, and in association with phosphatase and tensin homolog (PTEN) and c-Jun N-terminal kinase (JNK), block oxidant stress induced apoptosis[74]. However, in embryonic stem cells, the presence of SIRT1 also can be protective and appears to have an inverse relationship with mTOR[35]. SIRT1 can depress mTOR mediated pathways as well as promote autophagy to preserve the integrity of embryonic stem cells during oxidant stress[75]. SIRT1 can foster inhibition of mTOR signaling to promote neuronal growth[76]. In addition, during high glucose exposure to mesangial cells, the loss of SIRT1 activity is necessary for mTOR to arrest mesangial cell senescence[77].
It is important to note that during apoptotic cell injury with the induction of caspase activity, SIRT1 is susceptible to degradation by caspases. Although SIRT1 degradation also may be mediated by apoptotic pathways associated with p38[78] and JNK1[79], loss of SIRT1 activity can be the result of caspase degradation of the SIRT1 protein[80] that can then accelerate further activation of caspases[80,81]. In some systems that involve the cysteine-rich protein 61, connective tissue growth factor, and nephroblastoma over-expressed gene (CCN) family (defined by the first three members of the family that include cysteine-rich protein 61, connective tissue growth factor, and nephroblastoma over-expressed gene)[12], the CCN member WISP1 increases SIRT1 activity to protect cells from oxidative stress and apoptotic injury[28] (Figure 1). WISP1 also prevents SIRT1 degradation and oversees forkhead transcription activity with SIRT1 similar to other cytoprotective pathways[20,73,82] to block FoxO3a activity and prevent caspase activation that would otherwise lead to the degradation of SIRT1[28,83-85].
In contrast to apoptosis, autophagy promotes tissue remodeling by recycling cytoplasmic components and eliminating no longer useful organelles[62]. Macroautophagy is the classification of autophagy most commonly described[86]. It involves the sequestration of cytoplasmic proteins into autophagosomes that fuse with lysosomes for degradation and are eventually recycled. In most cases, SIRT1 activation with the induction of autophagy appears to be vital to promote cell survival in mature cells. In differentiated chondrocytes during oxidant stress, knockdown of the forkhead transcription factors FoxO1 and FoxO3 result in cell death with decreased SIRT1 activity and reduced autophagic related proteins, suggesting that SIRT1 with the activation of autophagy is necessary for cellular protection[24]. SIRT1 also plays a role in autophagic flux and promoting autophagy in mitochondria[87] that may be required to maintain a healthy pool of mitochondria[88]. In endothelial cells exposed to oxidized low density lipoproteins that can lead to atherosclerosis, SIRT1 up-regulation in conjunction with AMPK results in autophagy that is necessary for cellular protection[89]. In models of cognitive loss with chronic intermittent hypoxia hypercapnia exposure, SIRT1 activation is able to block apoptotic cell injury, up-regulate autophagy, and improve cognitive performance[90]. However, in pulmonary models of oxidant stress such as the exposure to cigarette smoke in bronchial epithelial cells, SIRT1 has been shown to prevent cell injury through the inhibition of of autophagy[91,92]. In regards to stem cells and the autophagic pathway, stem cells rely upon SIRT1 to modulate autophagic flux[93]. In muscle stem cells, SIRT1 is necessary to initiate autophagy and transition muscle stem cells from a quiescence state to an active state[94]. In endothelial progenitor cells, SIRT1 blocks apoptotic cell injury during oxidative stress through the induction of autophagy[95].
In the cardiovascular system, SIRT1 expression can affect not only the survival of stem cells, but also the ability of stem cells to differentiate and the efficacy of these cells for therapeutic applications. Increased SIRT1 expression can improve the survival of cardiomyoblasts[96] and prevent senescence and impaired differentiation in endothelial progenitor cells[97]. In regards to treatment efficacy, mesenchymal stem cells that are subjected to SIRT1 over-expression exhibit increased blood vessel density in the area of cardiac infarcts, reduced cardiac remodeling, and improved cardiac performance in rodent models[98], factors that may be associated with cardiac stem migration that is vital to tissue repair[99]. SIRT1 also can limit expression of aged mesenchymal stem cell phenotypes[98]. Loss of SIRT1 in circulating endothelial progenitor cells that can occur during tobacco exposure or chronic obstructive pulmonary disease may lead to increased senescence and apoptotic cell death that presents increased risk for vascular disease or cardiac disease[71]. SIRT1 also may improve the function of aged stem cells that are senescent. Aged mesenchymal stem cells that were exposed to pre-conditioning with glucose depletion exhibited increased expression of SIRT1 in addition to other proliferative entities such as growth factors and resulted in increased cardiac performance[100].
In the nervous system, SIRT1 has been tied to the differentiation, maturation, and maintenance of neurons. Loss of SIRT1 expression with the concurrent induction of heat shock protein-70 promotes neural differentiation, maturation of embryonic cortical neurons[101], and the differentiation of human embryonic stem cells into motor neurons[102]. SIRT1 also is considered a negative regulator of subventricular zone and hippocampal neural precursors in murine animal models. Knockdown of SIRT1 does not eliminate neural precursor numbers or proliferation but increases the production of neurons in the subventricular zone and the hippocampus[103]. In the mouse cerebral cortex, repression of SIRT1 by the oncogene BCL6 leads to the conversion of neural stem cell/progenitor cells to become neurons[104]. Neural stem cell differentiation also can be controlled through alternate pathways that involve SIRT1. In mouse neural stem cells, neuronal differentiation can be driven through the microRNA miR-34a that leads to decreased SIRT1 expression and DNA-binding of p53[105]. Interestingly, in these studies, increased expression of SIRT1 enhanced the astrocytic subpopulation of cells[105].
The cellular proliferative effects of SIRT1 also play a critical role in tumorigenesis. For example, SIRT1 activity can maintain acute myeloid leukemia stem cells and confer resistance against chemotherapy[106], stimulate endometrial cell tumor growth through lipogenesis[107], maintain neural stem cells and promote oncogenic transformation[108], and foster hepatocellular carcinoma[109]. As a result, SIRT1 and agents that modulate SIRT1 activity may represent new therapeutic strategies against tumorigenesis. For example, down-regulation of endoglin, a protein over-expressed in tumor associated endothelial cells, leads to apoptotic cell death, DNA damage, inhibition of several DNA repair genes including SIRT1, and enhanced chemotherapy sensitivity[110]. In addition, pathways linked to SIRT1 also may provide new strategies against cancer. Activation of p53 through SIRT1 inhibition can result in apoptotic cell death for quiescent leukemia stem cells in chronic myelogenous leukemia[111]. In breast cancer, estrogen receptor-α can lead to SIRT1 expression that activates pro-survival genes in breast cancer cells, such as catalase and glutathione peroxidase, and inhibits tumor suppressor genes, such as cyclin G2 (CCNG2) and p53. In these breast cancer cells, if SIRT1 is inhibited, estrogen receptor-induced breast cell growth is blocked through apoptotic cell death[112].
Cardiac disease, vascular disorders, neurodegenerative disease, and cancer lead to significant disability and death in the global population. Development of stem cell strategies for these disorders and the targeting of SIRT1 to drive stem cell viability and function holds great promise for the future. In the cardiovascular system, SIRT1 through stem cell proliferation can drive angiogenesis, improve cardiac performance following ischemic injury, limit cell senescence, and enhance the function of aged stem cells. In the nervous system, SIRT1 can be a negative modulator of neural precursors with the loss of SIRT1 leading to differentiation and maturation of embryonic stem cells in the nervous system. During tumorigenesis, SIRT1 foster the development of acute myeloid leukemia stem cells, promote oncogenic transformation of neural stem cells, and lead to hepatocellular cancer. Vital to the clinical outcomes controlled by SIRT1 is its level of activity overseen by pathways that include NMNAT, mammalian forkhead transcription factors, mTOR, and CCN family members such as WISP1 that determine cell survival through apoptosis and autophagy. Future work that can target SIRT1 and navigate stem cell proliferation under required conditions to either cellular proliferation or cellular death can open new avenues for the treatment of cardiovascular disorders, neurodegenerative disease, and cancer.
P- Reviewer: Aponte PM, de la Serna IL, Liu SH, Scarfi S S- Editor: Gong XM L- Editor: A E- Editor: Lu YJ
| 1. | Miniño AM. Death in the United States, 2011. NCHS Data Brief. 2013;1-8. [PubMed] |
| 2. | World Health Organization. Description of the global burden of ncds, their risk factors and determinants. USA: Global status report on noncommunicable diseases, 2010 2011; 1-176. |
| 3. | Sivaraman V, Yellon DM. Pharmacologic therapy that simulates conditioning for cardiac ischemic/reperfusion injury. J Cardiovasc Pharmacol Ther. 2014;19:83-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 60] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 4. | Maiese K. Cutting through the complexities of mTOR for the treatment of stroke. Curr Neurovasc Res. 2014;11:177-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 43] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 5. | Pergola PE, White CL, Szychowski JM, Talbert R, Brutto OD, Castellanos M, Graves JW, Matamala G, Pretell EJ, Yee J. Achieved blood pressures in the secondary prevention of small subcortical strokes (SPS3) study: challenges and lessons learned. Am J Hypertens. 2014;27:1052-1060. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 6. | Maiese K. Taking aim at Alzheimer’s disease through the mammalian target of rapamycin. Ann Med. 2014;46:587-596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 64] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 7. | Maiese K. Driving neural regeneration through the mammalian target of rapamycin. Neural Regen Res. 2014;9:1413-1417. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 62] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 8. | Schluesener JK, Zhu X, Schluesener HJ, Wang GW, Ao P. Key network approach reveals new insight into Alzheimer’s disease. IET Syst Biol. 2014;8:169-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 9. | Jung DW, Kim WH, Williams DR. Reprogram or reboot: small molecule approaches for the production of induced pluripotent stem cells and direct cell reprogramming. ACS Chem Biol. 2014;9:80-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 48] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 10. | Fraineau S, Pal CG 2nd, Allan DS, Brand M. Epigenetic Regulation of Endothelial Cell-mediated Vascular Repair. FEBS J. 2014;Dec 24; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 61] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 11. | Puthanveetil P, Wan A, Rodrigues B. FoxO1 is crucial for sustaining cardiomyocyte metabolism and cell survival. Cardiovasc Res. 2013;97:393-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 110] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 12. | Maiese K. WISP1: Clinical insights for a proliferative and restorative member of the CCN family. Curr Neurovasc Res. 2014;11:378-389. [PubMed] |
| 13. | Teschendorff AE, West J, Beck S. Age-associated epigenetic drift: implications, and a case of epigenetic thrift? Hum Mol Genet. 2013;22:R7-R15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 215] [Cited by in RCA: 221] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 14. | Maiese K. The challenges for drug development: cytokines, genes, and stem cells. Curr Neurovasc Res. 2012;9:231-232. [PubMed] |
| 15. | Mittal D, Ali A, Md S, Baboota S, Sahni JK, Ali J. Insights into direct nose to brain delivery: current status and future perspective. Drug Deliv. 2014;21:75-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 222] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 16. | Yi BR, Kim SU, Choi KC. Development and application of neural stem cells for treating various human neurological diseases in animal models. Lab Anim Res. 2013;29:131-137. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 17. | Chong ZZ, Shang YC, Wang S, Maiese K. SIRT1: new avenues of discovery for disorders of oxidative stress. Expert Opin Ther Targets. 2012;16:167-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 133] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 18. | Oblong JE. The evolving role of the NAD+/nicotinamide metabolome in skin homeostasis, cellular bioenergetics, and aging. DNA Repair (Amst). 2014;23:59-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 19. | Patel SA, Velingkaar NS, Kondratov RV. Transcriptional control of antioxidant defense by the circadian clock. Antioxid Redox Signal. 2014;20:2997-3006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 63] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 20. | Arunachalam G, Samuel SM, Marei I, Ding H, Triggle CR. Metformin modulates hyperglycaemia-induced endothelial senescence and apoptosis through SIRT1. Br J Pharmacol. 2014;171:523-535. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 142] [Cited by in RCA: 191] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 21. | Gong H, Pang J, Han Y, Dai Y, Dai D, Cai J, Zhang TM. Age-dependent tissue expression patterns of Sirt1 in senescence-accelerated mice. Mol Med Rep. 2014;10:3296-3302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 57] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 22. | Hou J, Chong ZZ, Shang YC, Maiese K. Early apoptotic vascular signaling is determined by Sirt1 through nuclear shuttling, forkhead trafficking, bad, and mitochondrial caspase activation. Curr Neurovasc Res. 2010;7:95-112. [PubMed] |
| 23. | Hou J, Wang S, Shang YC, Chong ZZ, Maiese K. Erythropoietin employs cell longevity pathways of SIRT1 to foster endothelial vascular integrity during oxidant stress. Curr Neurovasc Res. 2011;8:220-235. [PubMed] |
| 24. | Akasaki Y, Alvarez-Garcia O, Saito M, Caramés B, Iwamoto Y, Lotz MK. FoxO Transcription Factors Support Oxidative Stress Resistance in Human Chondrocytes. Arthritis Rheumatol. 2014;66:3349-3358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 173] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 25. | Balan V, Miller GS, Kaplun L, Balan K, Chong ZZ, Li F, Kaplun A, VanBerkum MF, Arking R, Freeman DC. Life span extension and neuronal cell protection by Drosophila nicotinamidase. J Biol Chem. 2008;283:27810-27819. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 120] [Cited by in RCA: 141] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 26. | Chong ZZ, Maiese K. Enhanced tolerance against early and late apoptotic oxidative stress in mammalian neurons through nicotinamidase and sirtuin mediated pathways. Curr Neurovasc Res. 2008;5:159-170. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 67] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 27. | Li J, Feng L, Xing Y, Wang Y, Du L, Xu C, Cao J, Wang Q, Fan S, Liu Q. Radioprotective and antioxidant effect of resveratrol in hippocampus by activating Sirt1. Int J Mol Sci. 2014;15:5928-5939. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 78] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 28. | Wang S, Chong ZZ, Shang YC, Maiese K. WISP1 neuroprotection requires FoxO3a post-translational modulation with autoregulatory control of SIRT1. Curr Neurovasc Res. 2013;10:54-69. [PubMed] |
| 29. | Kedenko L, Lamina C, Kedenko I, Kollerits B, Kiesslich T, Iglseder B, Kronenberg F, Paulweber B. Genetic polymorphisms at SIRT1 and FOXO1 are associated with carotid atherosclerosis in the SAPHIR cohort. BMC Med Genet. 2014;15:112. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 48] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 30. | Stein S, Matter CM. Protective roles of SIRT1 in atherosclerosis. Cell Cycle. 2011;10:640-647. [PubMed] |
| 31. | Kilic U, Gok O, Bacaksiz A, Izmirli M, Elibol-Can B, Uysal O. SIRT1 gene polymorphisms affect the protein expression in cardiovascular diseases. PLoS One. 2014;9:e90428. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 71] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 32. | Liu J, Wu X, Wang X, Zhang Y, Bu P, Zhang Q, Jiang F. Global Gene Expression Profiling Reveals Functional Importance of Sirt2 in Endothelial Cells under Oxidative Stress. Int J Mol Sci. 2013;14:5633-5649. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 33. | Audrito V, Vaisitti T, Rossi D, Gottardi D, D’Arena G, Laurenti L, Gaidano G, Malavasi F, Deaglio S. Nicotinamide blocks proliferation and induces apoptosis of chronic lymphocytic leukemia cells through activation of the p53/miR-34a/SIRT1 tumor suppressor network. Cancer Res. 2011;71:4473-4483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 131] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 34. | Knight JR, Allison SJ, Milner J. Active regulator of SIRT1 is required for cancer cell survival but not for SIRT1 activity. Open Biol. 2013;3:130130. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 35. | Maiese K, Chong ZZ, Shang YC, Wang S. Novel directions for diabetes mellitus drug discovery. Expert Opin Drug Discov. 2013;8:35-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 53] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 36. | Zhang JG, Zhao G, Qin Q, Wang B, Liu L, Liu Y, Deng SC, Tian K, Wang CY. Nicotinamide prohibits proliferation and enhances chemosensitivity of pancreatic cancer cells through deregulating SIRT1 and Ras/Akt pathways. Pancreatology. 2013;13:140-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 34] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 37. | Pazienza V, Pomara C, Cappello F, Calogero R, Carrara M, Mazzoccoli G, Vinciguerra M. The TRPA1 channel is a cardiac target of mIGF-1/SIRT1 signaling. Am J Physiol Heart Circ Physiol. 2014;307:H939-H944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 38. | Maiese K, Chong ZZ, Shang YC, Wang S. Translating cell survival and cell longevity into treatment strategies with SIRT1. Rom J Morphol Embryol. 2011;52:1173-1185. [PubMed] |
| 39. | Martin A, Tegla CA, Cudrici CD, Kruszewski AM, Azimzadeh P, Boodhoo D, Mekala AP, Rus V, Rus H. Role of SIRT1 in autoimmune demyelination and neurodegeneration. Immunol Res. 2015;61:187-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 52] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 40. | Paraíso AF, Mendes KL, Santos SH. Brain activation of SIRT1: role in neuropathology. Mol Neurobiol. 2013;48:681-689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 74] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 41. | Zhao Y, Luo P, Guo Q, Li S, Zhang L, Zhao M, Xu H, Yang Y, Poon W, Fei Z. Interactions between SIRT1 and MAPK/ERK regulate neuronal apoptosis induced by traumatic brain injury in vitro and in vivo. Exp Neurol. 2012;237:489-498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 108] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 42. | Moroz N, Carmona JJ, Anderson E, Hart AC, Sinclair DA, Blackwell TK. Dietary restriction involves NAD(+) -dependent mechanisms and a shift toward oxidative metabolism. Aging Cell. 2014;13:1075-1085. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 70] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 43. | Wang Y, Liang Y, Vanhoutte PM. SIRT1 and AMPK in regulating mammalian senescence: a critical review and a working model. FEBS Lett. 2011;585:986-994. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 136] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 44. | De Bonis ML, Ortega S, Blasco MA. SIRT1 is necessary for proficient telomere elongation and genomic stability of induced pluripotent stem cells. Stem Cell Reports. 2014;2:690-706. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 57] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 45. | Palacios JA, Herranz D, De Bonis ML, Velasco S, Serrano M, Blasco MA. SIRT1 contributes to telomere maintenance and augments global homologous recombination. J Cell Biol. 2010;191:1299-1313. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 210] [Cited by in RCA: 206] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 46. | Duan W. Sirtuins: from metabolic regulation to brain aging. Front Aging Neurosci. 2013;5:36. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 68] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 47. | Srivastava S, Haigis MC. Role of sirtuins and calorie restriction in neuroprotection: implications in Alzheimer’s and Parkinson’s diseases. Curr Pharm Des. 2011;17:3418-3433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 58] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 48. | Maiese K, Chong ZZ, Hou J, Shang YC. The vitamin nicotinamide: translating nutrition into clinical care. Molecules. 2009;14:3446-3485. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 167] [Cited by in RCA: 182] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 49. | Marampon F, Gravina GL, Scarsella L, Festuccia C, Lovat F, Ciccarelli C, Zani BM, Polidoro L, Grassi D, Desideri G. Angiotensin-converting-enzyme inhibition counteracts angiotensin II-mediated endothelial cell dysfunction by modulating the p38/SirT1 axis. J Hypertens. 2013;31:1972-1983. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 50. | Kim DW, Kim YM, Kang SD, Han YM, Pae HO. Effects of Resveratrol and trans-3,5,4’-Trimethoxystilbene on Glutamate-Induced Cytotoxicity, Heme Oxygenase-1, and Sirtuin 1 in HT22 Neuronal Cells. Biomol Ther (Seoul). 2012;20:306-312. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 51. | Zhang J, Feng X, Wu J, Xu H, Li G, Zhu D, Yue Q, Liu H, Zhang Y, Sun D. Neuroprotective effects of resveratrol on damages of mouse cortical neurons induced by β-amyloid through activation of SIRT1/Akt1 pathway. Biofactors. 2013;40:258-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 52. | Sansone L, Reali V, Pellegrini L, Villanova L, Aventaggiato M, Marfe G, Rosa R, Nebbioso M, Tafani M, Fini M. SIRT1 silencing confers neuroprotection through IGF-1 pathway activation. J Cell Physiol. 2013;228:1754-1761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 42] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 53. | Maiese K, Chong ZZ, Shang YC. OutFOXOing disease and disability: the therapeutic potential of targeting FoxO proteins. Trends Mol Med. 2008;14:219-227. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 119] [Cited by in RCA: 144] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 54. | Xiong S, Salazar G, Patrushev N, Alexander RW. FoxO1 mediates an autofeedback loop regulating SIRT1 expression. J Biol Chem. 2011;286:5289-5299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 168] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 55. | Maiese K, Chong ZZ, Shang YC, Wang S. mTOR: on target for novel therapeutic strategies in the nervous system. Trends Mol Med. 2013;19:51-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 173] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 56. | Maiese K, Chong ZZ, Wang S, Shang YC. Oxidant stress and signal transduction in the nervous system with the PI 3-K, Akt, and mTOR cascade. Int J Mol Sci. 2012;13:13830-13866. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 86] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 57. | Cantó C, Auwerx J. Caloric restriction, SIRT1 and longevity. Trends Endocrinol Metab. 2009;20:325-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 271] [Cited by in RCA: 302] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 58. | Fulco M, Cen Y, Zhao P, Hoffman EP, McBurney MW, Sauve AA, Sartorelli V. Glucose restriction inhibits skeletal myoblast differentiation by activating SIRT1 through AMPK-mediated regulation of Nampt. Dev Cell. 2008;14:661-673. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 658] [Cited by in RCA: 635] [Article Influence: 37.4] [Reference Citation Analysis (0)] |
| 59. | Maiese K, Chong ZZ, Shang YC, Hou J. Novel avenues of drug discovery and biomarkers for diabetes mellitus. J Clin Pharmacol. 2011;51:128-152. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 60. | Herranz D, Serrano M. SIRT1: recent lessons from mouse models. Nat Rev Cancer. 2010;10:819-823. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 208] [Cited by in RCA: 209] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 61. | Chong ZZ, Shang YC, Wang S, Maiese K. Shedding new light on neurodegenerative diseases through the mammalian target of rapamycin. Prog Neurobiol. 2012;99:128-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 93] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 62. | Maiese K, Chong ZZ, Shang YC, Wang S. Targeting disease through novel pathways of apoptosis and autophagy. Expert Opin Ther Targets. 2012;16:1203-1214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 124] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 63. | Shah N, Morsi Y, Manasseh R. From mechanical stimulation to biological pathways in the regulation of stem cell fate. Cell Biochem Funct. 2014;32:309-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 48] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 64. | Chong ZZ, Li F, Maiese K. Oxidative stress in the brain: novel cellular targets that govern survival during neurodegenerative disease. Prog Neurobiol. 2005;75:207-246. [PubMed] |
| 65. | Fong Y, Lin YC, Wu CY, Wang HM, Lin LL, Chou HL, Teng YN, Yuan SS, Chiu CC. The antiproliferative and apoptotic effects of sirtinol, a sirtuin inhibitor on human lung cancer cells by modulating Akt/β-catenin-Foxo3a axis. ScientificWorldJournal. 2014;2014:937051. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 66. | Wang T, Cui H, Ma N, Jiang Y. Nicotinamide-mediated inhibition of SIRT1 deacetylase is associated with the viability of cancer cells exposed to antitumor agents and apoptosis. Oncol Lett. 2013;6:600-604. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 67. | Du G, Song Y, Zhang T, Ma L, Bian N, Chen X, Feng J, Chang Q, Li Z. Simvastatin attenuates TNF-α-induced apoptosis in endothelial progenitor cells via the upregulation of SIRT1. Int J Mol Med. 2014;34:177-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 54] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 68. | Saini A, Al-Shanti N, Sharples AP, Stewart CE. Sirtuin 1 regulates skeletal myoblast survival and enhances differentiation in the presence of resveratrol. Exp Physiol. 2012;97:400-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 69. | Chiara B, Ilaria C, Antonietta C, Francesca C, Marco M, Lucia A, Gilda C. SIRT1 inhibition affects angiogenic properties of human MSCs. Biomed Res Int. 2014;2014:783459. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 70. | Xiong H, Dai M, Ou Y, Pang J, Yang H, Huang Q, Chen S, Zhang Z, Xu Y, Cai Y. SIRT1 expression in the cochlea and auditory cortex of a mouse model of age-related hearing loss. Exp Gerontol. 2014;51:8-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 51] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 71. | Paschalaki KE, Starke RD, Hu Y, Mercado N, Margariti A, Gorgoulis VG, Randi AM, Barnes PJ. Dysfunction of endothelial progenitor cells from smokers and chronic obstructive pulmonary disease patients due to increased DNA damage and senescence. Stem Cells. 2013;31:2813-2826. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 106] [Cited by in RCA: 131] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 72. | Chen CJ, Yu W, Fu YC, Wang X, Li JL, Wang W. Resveratrol protects cardiomyocytes from hypoxia-induced apoptosis through the SIRT1-FoxO1 pathway. Biochem Biophys Res Commun. 2009;378:389-393. [PubMed] |
| 73. | Wang W, Yan C, Zhang J, Lin R, Lin Q, Yang L, Ren F, Zhang J, Ji M, Li Y. SIRT1 inhibits TNF-α-induced apoptosis of vascular adventitial fibroblasts partly through the deacetylation of FoxO1. Apoptosis. 2013;18:689-701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 46] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 74. | Chae HD, Broxmeyer HE. SIRT1 deficiency downregulates PTEN/JNK/FOXO1 pathway to block reactive oxygen species-induced apoptosis in mouse embryonic stem cells. Stem Cells Dev. 2011;20:1277-1285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 75] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 75. | Ou X, Lee MR, Huang X, Messina-Graham S, Broxmeyer HE. SIRT1 positively regulates autophagy and mitochondria function in embryonic stem cells under oxidative stress. Stem Cells. 2014;32:1183-1194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 271] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 76. | Guo W, Qian L, Zhang J, Zhang W, Morrison A, Hayes P, Wilson S, Chen T, Zhao J. Sirt1 overexpression in neurons promotes neurite outgrowth and cell survival through inhibition of the mTOR signaling. J Neurosci Res. 2011;89:1723-1736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 131] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 77. | Zhang S, Cai G, Fu B, Feng Z, Ding R, Bai X, Liu W, Zhuo L, Sun L, Liu F. SIRT1 is required for the effects of rapamycin on high glucose-inducing mesangial cells senescence. Mech Ageing Dev. 2012;133:387-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 42] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 78. | Hong EH, Lee SJ, Kim JS, Lee KH, Um HD, Kim JH, Kim SJ, Kim JI, Hwang SG. Ionizing radiation induces cellular senescence of articular chondrocytes via negative regulation of SIRT1 by p38 kinase. J Biol Chem. 2010;285:1283-1295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 133] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 79. | Gao Z, Zhang J, Kheterpal I, Kennedy N, Davis RJ, Ye J. Sirtuin 1 (SIRT1) protein degradation in response to persistent c-Jun N-terminal kinase 1 (JNK1) activation contributes to hepatic steatosis in obesity. J Biol Chem. 2011;286:22227-22234. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 149] [Cited by in RCA: 146] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 80. | Kozako T, Aikawa A, Shoji T, Fujimoto T, Yoshimitsu M, Shirasawa S, Tanaka H, Honda S, Shimeno H, Arima N. High expression of the longevity gene product SIRT1 and apoptosis induction by sirtinol in adult T-cell leukemia cells. Int J Cancer. 2012;131:2044-2055. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 69] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 81. | Balaiya S, Ferguson LR, Chalam KV. Evaluation of sirtuin role in neuroprotection of retinal ganglion cells in hypoxia. Invest Ophthalmol Vis Sci. 2012;53:4315-4322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 32] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 82. | Lai CS, Tsai ML, Badmaev V, Jimenez M, Ho CT, Pan MH. Xanthigen suppresses preadipocyte differentiation and adipogenesis through down-regulation of PPARγ and C/EBPs and modulation of SIRT-1, AMPK, and FoxO pathways. J Agric Food Chem. 2012;60:1094-1101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 87] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 83. | Dong L, Zhou S, Yang X, Chen Q, He Y, Huang W. Magnolol protects against oxidative stress-mediated neural cell damage by modulating mitochondrial dysfunction and PI3K/Akt signaling. J Mol Neurosci. 2013;50:469-481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 45] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 84. | Qi XF, Li YJ, Chen ZY, Kim SK, Lee KJ, Cai DQ. Involvement of the FoxO3a pathway in the ischemia/reperfusion injury of cardiac microvascular endothelial cells. Exp Mol Pathol. 2013;95:242-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 85. | Yang Y, Su Y, Wang D, Chen Y, Wu T, Li G, Sun X, Cui L. Tanshinol attenuates the deleterious effects of oxidative stress on osteoblastic differentiation via Wnt/FoxO3a signaling. Oxid Med Cell Longev. 2013;2013:351895. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 60] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 86. | Maiese K. Therapeutic targets for cancer: current concepts with PI 3-K, Akt, & amp; mTOR. Indian J Med Res. 2013;137:243-246. [PubMed] |
| 87. | Jang SY, Kang HT, Hwang ES. Nicotinamide-induced mitophagy: event mediated by high NAD+/NADH ratio and SIRT1 protein activation. J Biol Chem. 2012;287:19304-19314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 206] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 88. | Fang EF, Scheibye-Knudsen M, Brace LE, Kassahun H, SenGupta T, Nilsen H, Mitchell JR, Croteau DL, Bohr VA. Defective mitophagy in XPA via PARP-1 hyperactivation and NAD(+)/SIRT1 reduction. Cell. 2014;157:882-896. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 440] [Cited by in RCA: 549] [Article Influence: 49.9] [Reference Citation Analysis (0)] |
| 89. | Jin X, Chen M, Yi L, Chang H, Zhang T, Wang L, Ma W, Peng X, Zhou Y, Mi M. Delphinidin-3-glucoside protects human umbilical vein endothelial cells against oxidized low-density lipoprotein-induced injury by autophagy upregulation via the AMPK/SIRT1 signaling pathway. Mol Nutr Food Res. 2014;58:1941-1951. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 80] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 90. | Min JJ, Huo XL, Xiang LY, Qin YQ, Chai KQ, Wu B, Jin L, Wang XT. Protective effect of Dl-3n-butylphthalide on learning and memory impairment induced by chronic intermittent hypoxia-hypercapnia exposure. Sci Rep. 2014;4:5555. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 57] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 91. | Chun P. Role of sirtuins in chronic obstructive pulmonary disease. Arch Pharm Res. 2015;38:1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 92. | Shi J, Yin N, Xuan LL, Yao CS, Meng AM, Hou Q. Vam3, a derivative of resveratrol, attenuates cigarette smoke-induced autophagy. Acta Pharmacol Sin. 2012;33:888-896. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 38] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 93. | Mazzoccoli G, Tevy MF, Borghesan M, Delle Vergini MR, Vinciguerra M. Caloric restriction and aging stem cells: the stick and the carrot? Exp Gerontol. 2014;50:137-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 94. | Tang AH, Rando TA. Induction of autophagy supports the bioenergetic demands of quiescent muscle stem cell activation. EMBO J. 2014;33:2782-2797. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 225] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 95. | Chen J, Xavier S, Moskowitz-Kassai E, Chen R, Lu CY, Sanduski K, Špes A, Turk B, Goligorsky MS. Cathepsin cleavage of sirtuin 1 in endothelial progenitor cells mediates stress-induced premature senescence. Am J Pathol. 2012;180:973-983. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 93] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 96. | Passariello CL, Zini M, Nassi PA, Pignatti C, Stefanelli C. Upregulation of SIRT1 deacetylase in phenylephrine-treated cardiomyoblasts. Biochem Biophys Res Commun. 2011;407:512-516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 97. | Lemarié CA, Shbat L, Marchesi C, Angulo OJ, Deschênes ME, Blostein MD, Paradis P, Schiffrin EL. Mthfr deficiency induces endothelial progenitor cell senescence via uncoupling of eNOS and downregulation of SIRT1. Am J Physiol Heart Circ Physiol. 2011;300:H745-H753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 44] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 98. | Liu X, Chen H, Zhu W, Chen H, Hu X, Jiang Z, Xu Y, Zhou Y, Wang K, Wang L. Transplantation of SIRT1-engineered aged mesenchymal stem cells improves cardiac function in a rat myocardial infarction model. J Heart Lung Transplant. 2014;33:1083-1092. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 99. | Liang SX, Phillips WD. Migration of resident cardiac stem cells in myocardial infarction. Anat Rec (Hoboken). 2013;296:184-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 100. | Choudhery MS, Khan M, Mahmood R, Mohsin S, Akhtar S, Ali F, Khan SN, Riazuddin S. Mesenchymal stem cells conditioned with glucose depletion augments their ability to repair-infarcted myocardium. J Cell Mol Med. 2012;16:2518-2529. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 48] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 101. | Liu DJ, Hammer D, Komlos D, Chen KY, Firestein BL, Liu AY. SIRT1 knockdown promotes neural differentiation and attenuates the heat shock response. J Cell Physiol. 2014;229:1224-1235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 102. | Zhang Y, Wang J, Chen G, Fan D, Deng M. Inhibition of Sirt1 promotes neural progenitors toward motoneuron differentiation from human embryonic stem cells. Biochem Biophys Res Commun. 2011;404:610-614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 42] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 103. | Saharan S, Jhaveri DJ, Bartlett PF. SIRT1 regulates the neurogenic potential of neural precursors in the adult subventricular zone and hippocampus. J Neurosci Res. 2013;91:642-659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 71] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 104. | Tiberi L, van den Ameele J, Dimidschstein J, Piccirilli J, Gall D, Herpoel A, Bilheu A, Bonnefont J, Iacovino M, Kyba M. BCL6 controls neurogenesis through Sirt1-dependent epigenetic repression of selective Notch targets. Nat Neurosci. 2012;15:1627-1635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 101] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 105. | Aranha MM, Santos DM, Solá S, Steer CJ, Rodrigues CM. miR-34a regulates mouse neural stem cell differentiation. PLoS One. 2011;6:e21396. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 138] [Cited by in RCA: 157] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 106. | Li L, Osdal T, Ho Y, Chun S, McDonald T, Agarwal P, Lin A, Chu S, Qi J, Li L. SIRT1 activation by a c-MYC oncogenic network promotes the maintenance and drug resistance of human FLT3-ITD acute Myeloid Leukemia stem cells. Cell Stem Cell. 2014;15:431-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 177] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 107. | Lin L, Zheng X, Qiu C, Dongol S, Lv Q, Jiang J, Kong B, Wang C. SIRT1 promotes endometrial tumor growth by targeting SREBP1 and lipogenesis. Oncol Rep. 2014;32:2831-2835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 48] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 108. | Lee JS, Park JR, Kwon OS, Lee TH, Nakano I, Miyoshi H, Chun KH, Park MJ, Lee HJ, Kim SU. SIRT1 is required for oncogenic transformation of neural stem cells and for the survival of “cancer cells with neural stemness” in a p53-dependent manner. Neuro Oncol. 2015;17:95-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 109. | Mao B, Hu F, Cheng J, Wang P, Xu M, Yuan F, Meng S, Wang Y, Yuan Z, Bi W. SIRT1 regulates YAP2-mediated cell proliferation and chemoresistance in hepatocellular carcinoma. Oncogene. 2014;33:1468-1474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 129] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 110. | Ziebarth AJ, Nowsheen S, Steg AD, Shah MM, Katre AA, Dobbin ZC, Han HD, Lopez-Berestein G, Sood AK, Conner M. Endoglin (CD105) contributes to platinum resistance and is a target for tumor-specific therapy in epithelial ovarian cancer. Clin Cancer Res. 2013;19:170-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 51] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 111. | Li L, Wang L, Li L, Wang Z, Ho Y, McDonald T, Holyoake TL, Chen W, Bhatia R. Activation of p53 by SIRT1 inhibition enhances elimination of CML leukemia stem cells in combination with imatinib. Cancer Cell. 2012;21:266-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 304] [Cited by in RCA: 333] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 112. | Elangovan S, Ramachandran S, Venkatesan N, Ananth S, Gnana-Prakasam JP, Martin PM, Browning DD, Schoenlein PV, Prasad PD, Ganapathy V. SIRT1 is essential for oncogenic signaling by estrogen/estrogen receptor α in breast cancer. Cancer Res. 2011;71:6654-6664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 115] [Article Influence: 8.2] [Reference Citation Analysis (0)] |