Published online Nov 26, 2015. doi: 10.4252/wjsc.v7.i10.1202
Peer-review started: January 22, 2015
First decision: March 6, 2015
Revised: September 22, 2015
Accepted: October 12, 2015
Article in press: October 13, 2015
Published online: November 26, 2015
Processing time: 309 Days and 9.6 Hours
The identification of an ideal cell source for tissue regeneration remains a challenge in the stem cell field. The ability of progeny cells to differentiate into other cell types is important for the processes of tissue reconstruction and tissue engineering and has clinical, biochemical or molecular implications. The adaptation of stem cells from adipose tissue for use in regenerative medicine has created a new role for adipocytes. Mature adipocytes can easily be isolated from adipose cell suspensions and allowed to dedifferentiate into lipid-free multipotent cells, referred to as dedifferentiated fat (DFAT) cells. Compared to other adult stem cells, the DFAT cells have unique advantages in their abundance, ease of isolation and homogeneity. Under proper condition in vitro and in vivo, the DFAT cells have exhibited adipogenic, osteogenic, chondrogenic, cardiomyogenc, angiogenic, myogenic, and neurogenic potentials. In this review, we first discuss the phenomena of dedifferentiation and transdifferentiation of cells, and then dedifferentiation of adipocytes in particular. Understanding the dedifferentiation process itself may contribute to our knowledge of normal growth processes, as well as mechanisms of disease. Second, we highlight new developments in DFAT cell culture and summarize the current understanding of DFAT cell properties. The unique features of DFAT cells are promising for clinical applications such as tissue regeneration.
Core tip: Multipotent dedifferentiated fat (DFAT) cells provide evidence of plasticity in adipocytes. The newly established DFAT cells exhibit vigorous proliferation and multipotent abilities with advantages over other adult stem cells. Modified culture methods reduce the risk of contamination by cells from the stromal vascular fraction to a minimum. In in vitro and/or in vivo experiments have revealed adipogenic, osteogenic, chondrogenic, myogenic, angiogenic and neourogenic potentials in DFAT cells. Moreover, the DFAT cells express embryonic stem cell markers and are similar to induced pluripotent stem cells in certain physiological aspects. Based on the abundance, ease of preparation, homogeneity, and multi-lineage potential, the DFAT cells are uniquely suited for regenerative medicine.
- Citation: Jumabay M, Boström KI. Dedifferentiated fat cells: A cell source for regenerative medicine. World J Stem Cells 2015; 7(10): 1202-1214
- URL: https://www.wjgnet.com/1948-0210/full/v7/i10/1202.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v7.i10.1202
Adipose tissue is well known to house the largest energy reserve in the body. However, adipose tissue is more than just a simple storage depot. Adipocytes secrete hormones, growth factors and cytokines, such as leptin and tumor necrosis factor-alpha, as well as proteins related to immunological and vascular functions[1-3]. Through this network of endocrine, paracrine, and autocrine signals, adipose tissue participate in energy homeostasis and is a global regulator of energy metabolism. Normal adipocyte function is also important for host defense and reproduction, and dysfunction may contribute to the development of pathological states such as insulin resistance[4,5]. Interestingly, recent research indicates that mature adipocytes can be eliminated by dedifferentiation[6-9]. With the advancement of tissue culture techniques it has been shown that mature adipocytes are able to dedifferentiate into progenitor cells, referred to as dedifferentiated fat (DFAT) cells. The DFAT cells are multipotent, and are able to redifferentiate into a variety of cell lineages[10,11]. The DFAT cells may serve as an alternative source of adult multipotent cells, with significant potential for use in tissue engineering and regenerative medicine. In this review, we focus on the recent literature addressing dedifferentiation of mature adipocytes as well as the isolation, characterization, and multipotency of DFAT cells.
The ultimate goal of regenerative medicine is to restore structure and function of damaged tissues and organs. To successfully support new tissue development, three regenerative processes are essential: dedifferentiation, transdifferentiation, and reprogramming of cells. These are all involved in the transition of adipocytes to DFAT cells, which may be an appropriate model for enhancing our understanding of these phenomena.
Cellular dedifferentiation is considered a regression of a cell from a highly specialized state to a simpler state that confers pluripotency, giving rise to undifferentiated progenitor cells. Transdifferentiation implies a process where one mature somatic cell transitions to another mature somatic cell. The induction of pluripotency in somatic cells is referred to as reprogramming. Stem cells are by definition able to self-renew, remain in an undifferentiated state, and differentiate along multiple cell lineages. Progenitor cells, on the other hand, exhibit less capacity for self-renewal and differentiate along one or a few lineages.
Dedifferentiation is the basis of tissue regeneration. The first evidence of dedifferentiation during regeneration was found in plants[12,13], where it is a common process during secondary growth and wound healing. However, in mammals, the capacity to regenerate subsequent to dedifferentiation is limited. For example, myotubes in newts are able to dedifferentiate and proliferate in vivo[14], but this has not been shown for mouse myotubes. The MYOD and MYOG (myogenin) genes have been shown to be essential for myotube dedifferentiation. When mouse myotubes were treated with extracts from regenerating limbs of newts, MYOD and MYOG were downregulated, which allowed the myotubes to dedifferentiate and proliferate[15]. Fortunately, recent studies have demonstrated that dedifferentiation can also occur in defined situations in many cell types in human tissue[16,17].
Dedifferentiation and cell division are important intermediate processes in the process of switching phenotype, although they do not appear to be obligatory in all cases. Studies on the role of retinoblastoma protein (RB) and RB-like 2 showed that dedifferentiation of mature cardiomyocytes facilitated cardiomyocyte proliferation in cardiac hypertrophy[18]. Furthermore, inhibition of the p38 mitogen-activated protein kinase induced mammalian cardiomyocytes to dedifferentiate, which may be essential for cardiomyocyte regeneration[19,20]. However, other experimental data suggest that dedifferentiation may not be required for cardiomyocyte proliferation[21]. It has also been observed that proliferation promoted by neuregulin, an essential extracellular ligand of the epidermal growth factor receptor during cardiomyocyte development, causes cardiomyocytes to reenter the cell cycle[22,23]. Alternatively, dedifferentiation may cause cellular plasticity to emerge and allow rerouting of cells into different cell lineages. This could lead to intermediary transdifferentiation of the cell, and result in a progressive conversion into another terminally differentiated cell. Furthermore, dedifferentiation occurs during rare pathological events, which have been found in osteosarcoma, chondrosarcoma, and epithelial-myoepithelial carcinoma in humans[24-26]. A population-based study and a 20-year survey on soft tissue sarcoma also included cases of dedifferentiated liposarcoma[27].
During normal cellular development, cellular dedifferentiation has been shown to relate to the regenerating cells entering the cell cycle[28]. Prolonged stress, injury, or activation of oncogenic pathways may trigger conversion of a “dedifferentiated” cell into a diseased cell, thereby opening the door for pathological changes. Hypoxia is believed to be the main factor driving the emergence of DFAT cells from adipocytes, as well as dedifferentiation of chondrocytes and smooth muscle cells[29,30]. However, more studies are needed to fully evaluate the connection between hypoxia and dedifferentiation.
Specific injuries or manipulations stimulate cellular dedifferentiation[31]. Cells lose their maturity and become susceptible to lineage modification. Dedifferentiation might occur, resulting in loss of cell function while the cells remain as undefined or resting cells as long as the insult persists. Thus, dedifferentiation would be a transition step prior to adopting a new identity, which would be distinguished by the reemergence of factors that redirect cell fate. Alternatively, dedifferentiation may be seen as an adaption to stressful stimuli, causing the cell to cease normal activity, thereby prohibiting progression toward cellular dysfunction, which in extreme cases could end in cell death. Thus, it is possible that specific changes in the intrinsic, environmental, or hormonal milieu might stimulate cells such as adipocytes to withdraw from the cell cycle, undergo dedifferentiation and acquire stem cell characteristics. Understanding the details of the dedifferentiation process would enhance our knowledge of normal regeneration.
Mature cells acquire stem cell features by undergoing dedifferentiation prior to the acquisition of a new cell fate. The newly undifferentiated stem cells are primed to respond to specific cues and differentiate into a variety of cell lineages. Reprogramming can be attained through cell fusion with embryonic stem cells (ESC), somatic cell nuclear transfer, exposure to stem cell extracts, or induction of pluripotency by defined factors generating what are referred to as induced pluripotent stem cells (iPSCs). During the reprogramming, an erasure and remodeling of epigenetic marks occur including DNA methylation and modification of histone and chromatin structures[32]. A major challenge in the field of iPSCs is to convert mature cells into pluripotent cells resembling ESC for use in transplantation therapies. The reprogramming of the somatic cells is induced by the transfer of pluripotent factors, many of which are oncogenes. It has also been suggested that iPSCs might be the product of dedifferentiation of somatic cells following oncogenic insult[33,34]. To address the fear of tumorgenecity in iPSCs, a study recently showed that overexpression of core transcription factor genes or its activators support the maintenance of the cell type-specific transcriptional profile, thus inhibiting alterations in the expression of genes required for iPSC induction[35]. The stable nature of gene signatures limits dedifferentiation and promotes the cell type-specific transcriptional profiles.
A normal, fully differentiated cell can either change its identity to a new cell type, referred to as transdifferentiation, or lose its functionality and revert to an immature state referred to as dedifferentiattion. The transition to a new phenotype may occur directly or through dedifferentiation triggered by genetic factors or environmental cues. Intermediary transdifferentiation has been shown to occur when acinar cells undergo dedifferentiation into duct-like cells with exocrine as well as endocrine potentials[36]. Another type of scenario is the conversion of phenotype without a detectable intermediate step, which is referred to as direct reprogramming. It is possible that dedifferentiated cells retrace normal development toward a different, sometimes closely related, cell lineage after reaching the progenitor state.
Different interventions, including naturally occurring events and experimental manipulations, might result in transdifferentiation of cells in unfamiliar compartments. Dedifferentiation is described as the entrance to such transdifferentiation. A recent study demonstrated that cultures of purified hepatic oval stem cells are capable of transdifferentiation into functional endocrine cells[37]. It has also been reported that increased expression of CAAT/enhancer-binding protein (C/EBP)α and C/EBPβ in differentiated islet β cells leads to reprogramming into macrophages without DNA methylation[38]. Another example is fibroblasts that have transdifferentiated into cardiomyocytes after transfer of the transcription factors GATA4, MEF2C, and TBX5[39].
The concept that terminally differentiated cells retain intrinsic plasticity increases the number of cell sources that could be used for tissue regeneration in cases of injury or disease. The generation of DFAT cells is an example of reemergence of plasticity in adipocytes, resulting in the ability to transdifferentiate into alternate cell types and to serve as a model for dedifferentiation and transdifferentiation.
The goal of tissue engineering is to repair and regenerate damaged organs with the help of stem cells, biomaterials, and cytokines. However, the limited availability of human stem cells that are able to differentiate along multiple lineages has hampered the progress and slowed the development of these treatments. While ESCs inherently exhibit nearly unlimited differentiation potential in vitro and in vivo, their use is constrained by scientific concerns regarding safety and efficacy, as well as by ethical, legal, and political concerns. An alternative approach is to use stem cells derived from adult tissues, which would circumvent most of these concerns.
Multipotent stem cells have been defined as a special kind of cells with a unique capacity to self-renew indefinitely, which implies that the stem cells can be extensively expanded ex vivo. Human mesenchymal stem cells (MSCs) were initially derived from bone marrow, but have now been isolated from most types of tissue[40], including the brain, dermis, periosteum, skeletal muscle, synovium, trabecular bone, vasculature, and adipose tissue, which is the most abundant and accessible source of adult stem cells[41-43]. MSCs express cell surface markers like cluster of differentiation (CD)10, CD29, CD44, CD73, CD90, CD105, CD117 and STRO-1, but are negative for the hematopoietic lineage markers CD14, CD34, CD45 and HLA-DR. Identification of adipose-derived stem cells (ASCs) suggests that a pool of stem cells exists within the adult adipose tissue. The ASCs are derived from the adipose stroma vascular fraction (SVF), which includes all cells in adipose tissue except the white adipocytes. The ASCs are similar to MSCs in their expression of MSC markers, but lack expression of hematopoietic lineage markers and the endothelial markers CD31 and von Willebrand factor (vWF)[43,44]. Studies have also identified a periendothelial pericyte-like subpopulation of ASCs, possibly due to the inclusion of vascular elements in the SVF. These cells express CD34, as well as mesenchymal, pericytic, and smooth muscle markers, including chondroitin sulfate proteoglycan (NG2), CD140a, and CD140b[45], but are negative for CD31, CD45 and CD144. However CD34 and CD104b did not co-localize in these cells, suggesting that CD34+/CD31- cells in the adipose vasculature are not pericytes[46].
The DFAT cells initially lack expression of CD34, CD31, CD146, CD45, and pericyte markers, distinguishing them from ASCs derived from the SVF[6-9] (see below for further characterization of DFAT cells). Interestingly, lineage tracing in mice suggest that part of the stromal cells may be derived from adipocytes in vivo[47], suggesting that ASCs and DFAT cells in part have the same precursor cells. The DFAT cells, however, constitute a more homogeneous cell population than the ASCs, further supporting a role of the mature adipocyte fraction as a source of stem cells[48-50].
Three major types of pluripotent stem cells have so far been identified, ESCs, iPSCs from reprogrammed adult somatic cells[51], and multilineage-differentiating stress-enduring cells, referred to as Muse cells, isolated from mesenchymal human tissues[52]. The Muse cells are considered MSCs, capable of forming cell clusters and expressing a set of genes associated with pluripotency.
All three cell types have factors that limit their use. Ethical concerns make the ESCs controversial, and heterologous transplantation of ESCs may produce immune rejection in the recipient. Transplantation of both ESCs and iPSCs run the risk of producing teratomas in the recipient because of their uncontrolled capacity of proliferation and differentiation. The paucity of Muse cells has so far been a limitation for their widespread use. Other limitations to the use of iPSCs are derived from the fact that reprogramming genes have been introduced and remain with low efficiency expression in the host cells. One of the ways to overcome this problem is to achieve efficient transgene-free reprogramming using Sendai virus[53]. Sendai virus remain in the cytoplasm and do not have the ability to integrate into the host genome. Most commonly, virus clearance is achieved by clonal propagation of primary colonies leading to isolation of sub-clones free of the viral genome. However, about 10% of the cells still have the virus after 10 passages[54]. Although human is not the natural host for Sendai virus, and the virus is non-pathogenic to humans, the potential mucosal exposure to the virus remains a concern. Therefore, identification and development of new sources of pluripotent adult stem cells remain important.
DFAT cells have been shown to express ESC markers including the POU homeodomain protein Oct4, sex determining region Y-box 2 (SOX2), myelocytomatosis oncogene (c-Myc), and the homeobox protein Nanog, which are key factors in maintaining pluripotency[55]. In addition, high alkaline phosphatase and telomerase activity further support similarities between DFAT cells and undifferentiated pluripotent stem cells. However, the expression of pluripotency markers decrease significantly in DFAT cells that have been cultured for longer than 2 wk. It is possible that the early expression of pluripotency markers in DFAT cells was missed in previous investigations where specific lineages were studied, causing the pluripotency in DFAT cells to be overlooked. Several investigators have also reported low levels of expression of pluripotency markers in human ASCs[56-58], and other studies have revealed similar degrees of pluripotency in DFAT cells and iPSCs[59,60].
The DFAT cells are unlike the iPSCs, in which simultaneous overexpression of the transcription factors Oct4, SOX2, c-Myc, and the Kruppel-like factor 4 leads to the generation of a pluripotent, ESC-like state in fibroblasts. After derivation from adipocytes, pluripotency emerges transiently of DFAT cells with expression of the same transcription factors as in iPSCs, as well as low levels of Nanog, stage-specific embryonic antigen (SSEA)-3, and CD105[59]. The DFAT cells are able to differentiate into cells representative of the three germ layers, with no evidence of teratoma after injection of human DFAT cells in immunodeficient mice[59]. It was recently shown that mature porcine adipocytes, in response to dedifferentiation, downregulate many genes important for lipid metabolism and upregulate genes involved in cell proliferation, cell morphology and regulation of cell differentiation[53]. By this process, the dedifferentiated adipocytes achieved the appropriate DNA methylation status, underwent gene-reprogramming, and gained stem cell properties. Thus, the available data support that dedifferentiated adipocytes have the molecular signature of a reprogrammed cell similar to pluripotent stem cells.
Large lipid accumulations make the white adipocytes naturally buoyant and therefore difficult to culture. This technical difficulty has in part made their in vitro characteristics inaccessible for study. Additionally, they have been considered to be in the terminal stages of differentiation and lacking proliferative activity, further decreasing the interest in their in vitro and in vivo behavior on a cellular level. It is more than five decades since Rodbell[61] first succeeded in separating unilocular adipocytes from mature white fat tissue using collagenase treatment. Since then, most biochemical studies of adipocytes have made use of such dispersed cells. However, mature adipocytes do not attach to the bottom of tissue culture plates, but rather float to the surface of any given culture medium. To overcome this issue, Sugihara et al[9,62] proposed to use using ceiling culture as a way of culturing adipocytes. Most adipocytes lose their intracellular lipid and buoyancy with time in vitro. Ultimately, the cells have lost all lipids, appear fibroblastic, and proliferate to confluence. In vivo, tissue expanders were placed within the inguinal fat pad of rats[63]. Expanded fat pads were then autotransplanted to a distant location. Histologic analysis demonstrated that the tissue-expanded fat pads had lost over half their original volume, and the adipocytes had become elongated, fibroblast-like cells. These changes were attributed to the mechanical forces of the expander but may represent adipocyte dedifferentiation. Interestingly, after these same “atrophied” fat pads were transplanted as autografts, they regained their previous volume, suggesting adipocyte redifferentiation. This supports the concept of an adipocyte equilibrium in which dedifferentiated adipocytes may withstand ischemic insult better than differentiated adipocytes.
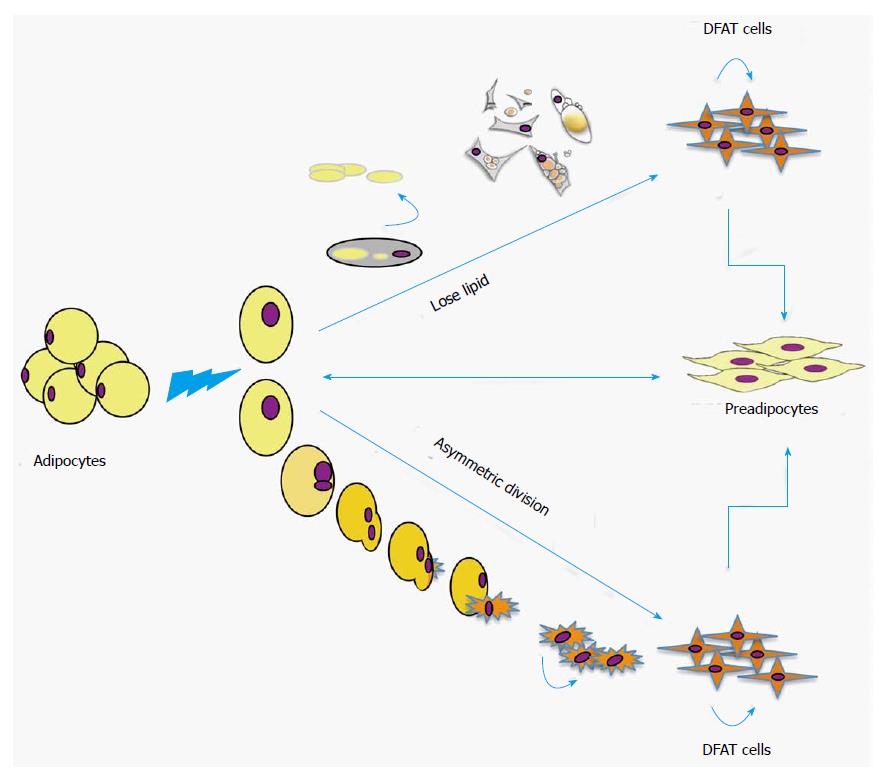
In the last decade, several studies have explored the plasticity of mature adipocytes and introduced to them to the stem cell field. Adipocyte dedifferentiation is readily seen in vitro. Matsumoto et al[6] showed that adipocytes containing two nuclei were occasionally detected in adipocytes before they were placed in ceiling culture, but were frequently seen after 3 d of culture. Such bi-nuclear adipocytes were always positive for BrdU in both nuclei, suggesting that the cells had entered S-phase and the nuclei had divided. The authors also performed time-lapse fluorescence microscopy, which revealed that fibroblast-like cells were indeed generated from lipid-filled adipocytes with single nuclei through asymmetric division[6,64]. Another similar study demonstrated isolation of stem cells with characteristics of immature neural crest cells through asymmetric cell division in cultured human hair follicles in vitro[65]. Asymmetric cell division permits a single mother cell to generate daughter cells that are distinct in size, shape, function and fate. The generation of two progeny with different fates requires a highly regulated molecular program. In general, disruption of asymmetric cell division leads to the creation of two progeny that retain stem cell characteristics, but with reduced ability to achieve full differentiation[66-69]. As far as we have observed, the emergence of DFAT cells from mature adipocytes occurs via two phenomena; mature adipocytes lose their lipid content and acquire a fibroblast-like shape, and asymmetric cell division of mature adipocytes into one lipid-filled adipocyte and another small daughter cells without lipid (Figure 1). Subsequently, the cells undergo dedifferentiation without the use of inducing agents, resulting in proliferative DFAT cells.
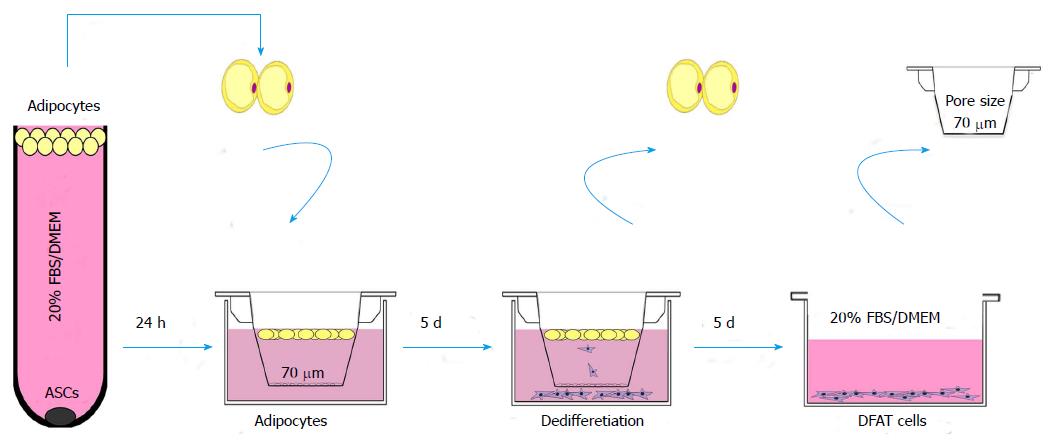
Recent studies exploring DFAT cells describe specific purification steps to ensure, to the extent possible, the purity of the initial preparation of mature adipocytes. Indeed, the purity of the primary adipocytes is essential in the preparation of DFAT cells, as to avoid the possibility of floating adipocytes “dragging” contaminating cells with them[70]. Recently, we proposed a new method to prepare DFAT cells, using insert culture (Figure 2)[59]. In this method, DFAT cells are generated from lipid-filled mature adipocytes isolated from small pieces of subcutaneous adipose tissue or human fresh lipoaspirate, washed repeatedly with phosphate-buffered saline until the washes are clear[6,59,62]. Approximately 1 g of adipose tissue is minced and digested in 0.1% (w/v) collagenase solution (Collagenase type I) at 37 °C for 1 h with gentle agitation. After filtration through nylon filters (core size 100 μm) and centrifugation at 135 g for 3 min, the floating top layer of adipocytes is collected. The adipocytes are then washed repeatedly (usually three times) in Dulbecco’s Modified Eagle Medium supplemented with 20% fetal bovine serum before further use. Adipocytes intended for DFAT cell generation are floated on top of medium in culture dishes or plastic tubes to let remaining non-adipocytes detach and sink to the bottom and be discarded after centrifugation. Adipocytes from the top creamy layer (30-50 μL) are subsequently transferred to 6-well plates fitted with 70 μm-filters and incubated for 5 d in culture medium. DFAT cells derived from the adipocytes sink through the filters and attach to the bottom of the dishes. The filters with remains of the adipocytes are removed after 5 d (Figure 2). This method of preparing DFAT cells does not include attachment of the adipocytes to plastic surfaces or ceiling culture, as previously described[6-8,62,71]. In addition, this method allows the separation of the DFAT cells from the adipocytes as soon as they sink through the filter and attach to the bottom of the dish. This reduces the influence of adipocyte remnants on the surface of the medium on the characteristics of the nascent DFAT cells. We regularly collect up to 10000 DFAT cells during 5 d of collection. The inclusion of these additional steps not only enhances the purity of DFAT cells, but significantly increases the early expression of pluripotency markers previously described[59].
Most studies on the DFAT cells have concluded that they are a largely homogeneous cell population with an immunophenotype similar to those of ASCs and other MSCs[72,73]. Human ASCs are relatively heterogeneous and carry hematopoietic-associated markers such as CD11a, CD14, CD45, CD86 and HLA-DR, and low levels of the MSCs-associated markers CD13, CD29, CD34, CD44, CD63, CD73, CD90 and CD166[73,74]. On the other hand, the DFAT cells are positive for CD13, CD29, CD44, CD90, CD105, CD9, CD166 and CD54, and negative for CD14, CD31, CD34, CD45, CD66b, CD106, CD117, CD133, CD146, CD271, CD309, HLA-DR and alpha-smooth muscle cell actin[1,50,75]. Both ASCS and DFAT cells express HLA-A, -B and -C, which suggests that both cell types have allogenic transplantation potential. In addition, we detected that 7.1% of the human DFAT cells expressed SSEA-3, and that most of the human DFAT cells expressed CD105, whereas mouse DFAT cells expressed Sca-1[59]. This expression was maintained for multiple passages. However, the expression of stem cell markers in human DFAT cells varies with the donor’s age, culture conditions, and the degree of differentiation.
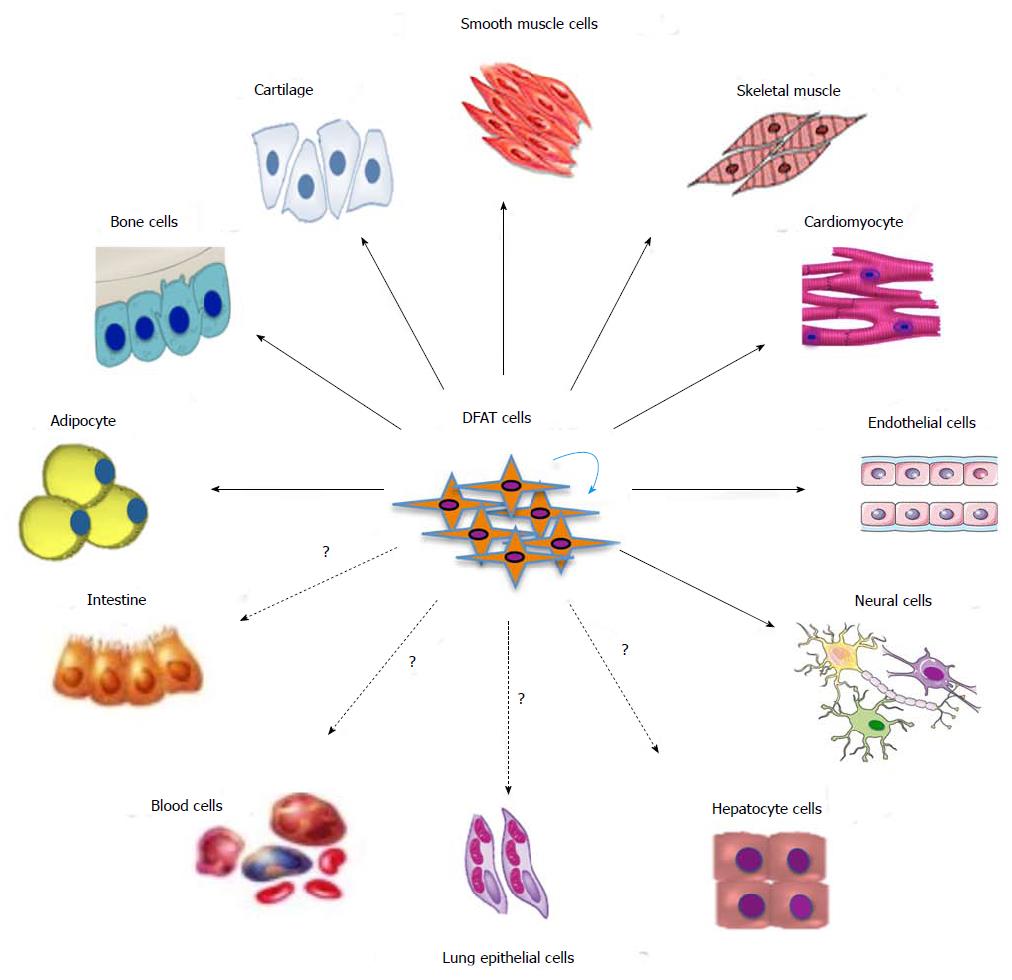
DFAT cells have emerged as a potential cell source for regenerative medicine because of their transdifferentiation capability and similarity to ASCs and bone marrow MSCs[75-77]. Multiple studies have demonstrated differentiation of DFAT cells into multiple lineages including adipogenic, osteogenic, chondrogenic, myogenic, angiogenic and neurogenic lineages (Figure 3). To monitor the fate of DFAT cells, investigators have used DFAT cells prepared from the adipose tissue of GFP-transgenic mice for transplantation into wild type mice[64,78], or adipocyte protein 2-Cre+/+; LacZ ROSA (R26R)+/+ double transgenic mice[79]. Human DFAT cells used for injection of immunodeficient mice were traced by anti-human mitochondria staining[59]. However, the potential need to maintain a specific cell identity once the DFAT cells have achieved a desired phenotype, or the methodology to do so, has not been assessed thus far.
Soft tissue reconstruction is an important aspect of tissue engineering, and adipose grafts are needed for minimally invasive injectable therapies in order to restore soft tissue volume. The most important features of adipose tissue as a cell source might be its relative expendability and the ease with which large quantities can be obtained with minimal risk. Correctly prepared adipocytes could therefore be a useful alternative for tissue augmentation, such as breast surgery with allogeneic material or tissue flap surgery.
Previous studies have confirmed that mature adipocytes easily redifferentiate into adipocytes[6,7,11]. We demonstrated that mouse DFAT cells spontaneously underwent adipogenic differentiation without special treatment, whereas human DFAT cells required adipogenic induction[51]. Another study showed that although DFAT cells expressed lower levels of lipoprotein lipase, leptin, and glucose transporter 4 compared to mature adipocytes, they still expressed important adipogenic markers such as peroxisome proliferator-activated receptor gamma (PPARγ), C/EBPα, C/EBPβ, C/EBPδ, and sterol regulatory element-binding protein-1c[6,80]. In addition, the DFAT cells have been shown to have adipogenic capacity in vivo. Direct injection of DFAT cell into the subcutaneous portion over sternum of mice resulted in fat pad formation after 3 wk without the use of chemical induction[7,81]. Furthermore, it was found that that PPARγ and C/EBPα mRNA levels were higher in DFAT cells derived from intramuscular adipose tissue rather than visceral adipose tissue in pigs, suggesting a more active adipogenesis in intramuscular DFAT cells, as compared to visceral DFAT cells[82]. It implies that DFAT cells from the same donor may differ in rates of redifferentiation and expression of molecular markers depending on the depot of origin.
DFAT cells can be derived from small amounts of subcutaneous adipose tissue regardless of the age of the donors, and may be useful in cell-based therapies for a variety of diseases commonly affecting elderly subjects, including metabolic bone disorders and osteoporosis. An earlier study found that the transcription factors RUNX2 and SP7, secreted phosphoprotein 1, bone Gla protein, parathyroid hormone 1 receptor and SOX9 were expressed in DFAT cells, suggesting osteogenic and chondrogenic potentials[6]. Osteogenic differentiation was stimulated by the addition of dexamethasone, β-glycerophosphate and L-ascorbic acid-2-phosphate to the culture medium. It was also stimulated by the addition of retinoic acid, an analogue of retinol that interacts with bone morphogenetic proteins (BMPs) to limit adipogenesis and promote osteogenesis[83]. Chondrogenic induction, however, was facilitated by the addition of L-ascorbic acid-2-phosphate, proline, pyruvate, and transforming growth factor β3. Appropriate mineralization of the cells was confirmed by alkaline phosphate, Alizarin Red S and von Kossa staining, whereas chondrocyte differentiation was confirmed by Alcian Blue staining. An experiment using implantation of DFAT cells in combination with collagen-based scaffolds further showed the ability of the cells to undergo osteochondrogenesis in vivo[84]. Another study proposed DFAT cells as a cell source for periodontal regeneration, after the cells promoted osteogenic differentiation in co-cultures with periodontal ligament stem cells[85]. Furthermore, the ability of human DFAT cells from the buccal fat pad to undergo osteoblastic differentiation appears to be higher than that of ASCs from the same fat depot[86]. Thus, the DFAT cells may be attractive as a cell source for tissue engineering in bone disorders such as nonunion fractures and osteoporosis.
Myocytes are generally divided into three categories: skeletal, cardiac and smooth muscle cells, which differ in their cellular characteristics and behaviors. Myogenesis is a multi-stage process resulting in the formation of muscular cells and tissue. It involves myoblast proliferation, secretion of fibronectin into the extracellular matrix, alignment of the myoblasts into multi-nucleated myotubes, and cell fusion[87]. The two muscle-specific transcription factors Myf5 and MyoD are known to actively regulate these processes. Treatment with 5-azacytidine (Aza-C), a demethylating agent, led to induction of MyoD and Myogenin in DFAT cells and the formation of multinucleated cells with expression of myosin heavy chain, even though Myf5 was not expressed after induction[88]. Another study found that DFAT cells underwent smooth muscle cell differentiation and contributed to the regeneration of bladder smooth muscle[78]. It also was shown that DFAT cell transplantation promoted the recovery of the sphincter muscle and improved the urethral sphincter contractility in a rat vaginal distension model[89]. Our previous study demonstrated that DFAT cells were able to differentiate into cardiomyocytes in vitro, and injection of DFAT cells into ischemic rat hearts induced neovascularization and supported rehabilitation of the cardiac tissue[64]. Although pluripotent stem cells have exhibited spontaneous cardiomyocyte differentiation, both in vitro and in vivo, none of the adult stem cell models studied thus far have spontaneously undergone cardiomyocyte differentiation in vitro, except for the DFAT cells[8,90]. Other adult stem cell models have required either co-culture with isolated cardiomyocytes or treatment with methylation inhibitors and histone deacetylase inhibitors. However, mouse DFAT cells spontaneously differentiated into functional cardiomyocytes without specific induction or genetic treatment[59]. Human DFAT cells, on the other hand, did not undergo spontaneous cardiomyocyte differentiation, although they expressed cardiomyocyte markers after cardiogenic induction[59]. Thus, cardiomyogenic differentiation differs between mice and human DFAT cells, a difference that could be explored to uncover pathways active in spontaneous cardiomyogenesis.
Blood vessels consist of an interior lining of endothelial cells surrounded by perivascular support provided by smooth muscle cells or pericytes. Human DFAT cells without induction have been shown to express low levels of endothelial cell or progenitor markers in culture such as the vWF, CD31 or CD34[6,10,11], and to be stimulated to undergo endothelial differentiation by treatment with BMP4 and BMP9[10]. They also differentiated into endothelial cells in established in vitro and in vivo models of angiogenesis[91-93]. DFAT cells cultured in Matrigel® form tube-like structures that are stable for weeks and stain for endothelial markers, including CD31 and VE-cadherin[10]. Some researchers have proposed the tube-like structures to be a result of the perivascular nature of DFAT cells, as suggested by the expression of the pericyte-related markers CD140b and NG2[74]. Similarly, MSCs within adult mesenchymal tissues may differentiate into pericytes without induction by growth factors[94,95]. In our experiments we initially did not detect pericyte markers in DFAT cells even though they appeared later during culture. Moreover, in Matrigel®, the human DFAT cells differentiated into cells that expressed either endothelial markers or α-smooth muscle actin, which suggests that the cells undergo differentiation into multiple cell types (unpublished observations). It is possible that interactions between endothelial cells and support cells, also derived from the DFAT cells, strengthen the tube structures and promote cell maturity. Thus, the DFAT cells are able to differentiate into endothelial cells in vitro, and participate in neovascularization in vivo.
Treatments of peripheral or central nerve injuries are still suboptimal despite significant advances in neuroscience and microsurgery. The standard treatment of autologous nerve grafting is unsatisfactory because of morbidity and loss of function at the donor site. Neural progenitor cells such as ESCs and neural stem cells derived from the adult central nervous system may provide new prospects although logistical, ethical, and immunological factors are likely to limit potential applications. However, recent studies have revealed differentiation of ASCs into early neural progenitors, which has generated interest in the field of regenerative medicine. Hsueh et al[96] observed that, when seeded on a chitosan-coated surface, human ASCs form spheres containing up to 19.5% nestin positive cells. Ahmadi et al[97] further reported that, after culture in serum-free medium, 51% nestin-positive cells could be generated from human ASCs. However, Zuk et al[43] found that nestin was expressed in multiple cell types, including myogenic, endothelial, and hepatic cells, indicating that nestin expression alone is not suitable for the identification of neural cells, especially without functional analysis. Some studies suggest that adipose tissue stem cells are able to induce neurite outgrowth in vitro, as well as tissue-specific commitment to neural cell lineages and expression of the tropomyosin receptor kinase A neurotrophin receptor in vivo[98,99]. Most recently, a study demonstrated that ASCs differentiated into astrocytes, oligodendrocytes, and functional neurons, which were able to generate tetrodotoxin-sensitive sodium currents[100]. DFAT cells, prepared in parallel with ASCs, also had neurogenic potential and expressed neurotrophic factors such as brain-derived neurotrophic factor and glial cell line-derived neurotrophic factor. After transplantation, the DFAT cells supported functional recovery from spinal cord injury (SCI)-induced motor dysfunction in rats[101]. Furthermore, the DFAT cells promoted remyelination and inhibited glial scar formation in SCI mice, possibly through cell-autonomous as well as cell-non-autonomous effects[102]. It is possible that the neurotrophic factors secreted from the grafted DFAT cells contribute to the functional recovery. Further characterization of the capacity of DFAT cells to undergo neurogenesis, with particular focus on neurophysiologic and neurochemical signal transduction properties, is needed.
Even though hematopoietic linage differentiation has not yet been reported in DFAT cells, studies comparing porcine-derive mature adipocytes and DFAT cells suggest that such potential exists[60,103]. Upregulation of genes related to multiple lineages occurred during the dedifferentiation, including those related to hematopoietic cell differentiation.
Overall, DFAT cells appear to constitute an excellent source of cells for tissue engineering. However, the mechanisms of dedifferentiation, and whether it occurs under physiological or pathological conditions in vivo, need further exploration. In addition, culture conditions for the maintenance of stem cell characteristics, as well as induction of specific linages, need development. DFAT cells can also be prepared to match the individual patient, thus allowing for replacement therapies using autologous transplantation. Although no human trial has ever been reported on DFAT cells, it should not deter us from anticipating their clinical application, with focus on tissue regeneration.
Multipotent DFAT cells provide evidence of plasticity in adipocytes. The recently characterized DFAT cell model exhibit robust proliferation and multipotency potential giving them an advantage over other adult stem cell models. Compared to bone marrow derived stem cells, white adipose tissue is mostly located subcutaneously and their abundance is generally guaranteed. Furthermore, access to mature adipocytes is obtained though less invasive methods such as liposuction, which have less physical and psychological effects on the donor. Compared to ASCs, DFAT cells comprise a more homogeneous population of cells. Modified culture methods reduce the risk of contamination by cells from the stromal vascular fraction to a minimum. In vitro and/or in vivo experiments have revealed adipogenic, osteogenic, chondrogenic, myogenic, angiogenic and neourogenic differentiation potentials in DFAT cells. Moreover, the DFAT cells express ESC markers and are similar to iPS cells in certain physiological aspects. Based on the abundance, ease of preparation, homogeneity, and multi-lineage potential, the DFAT cells are uniquely suited for regenerative medicine.
P- Reviewer: Holan V, Wakao H, Zaminy A S- Editor: Gong XM L- Editor: A E- Editor: Jiao XK
| 1. | Fonseca-Alaniz MH, Takada J, Alonso-Vale MI, Lima FB. Adipose tissue as an endocrine organ: from theory to practice. J Pediatr (Rio J). 2007;83:S192-S203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 73] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 2. | Cao Y. Angiogenesis and vascular functions in modulation of obesity, adipose metabolism, and insulin sensitivity. Cell Metab. 2013;18:478-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 245] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 3. | Rosen ED, Spiegelman BM. What we talk about when we talk about fat. Cell. 2014;156:20-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1414] [Cited by in RCA: 1772] [Article Influence: 161.1] [Reference Citation Analysis (0)] |
| 4. | Nadal A, Alonso-Magdalena P, Soriano S, Ropero AB, Quesada I. The role of oestrogens in the adaptation of islets to insulin resistance. J Physiol. 2009;587:5031-5037. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 101] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 5. | Al-Goblan AS, Al-Alfi MA, Khan MZ. Mechanism linking diabetes mellitus and obesity. Diabetes Metab Syndr Obes. 2014;7:587-591. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 459] [Cited by in RCA: 541] [Article Influence: 49.2] [Reference Citation Analysis (0)] |
| 6. | Matsumoto T, Kano K, Kondo D, Fukuda N, Iribe Y, Tanaka N, Matsubara Y, Sakuma T, Satomi A, Otaki M. Mature adipocyte-derived dedifferentiated fat cells exhibit multilineage potential. J Cell Physiol. 2008;215:210-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 274] [Cited by in RCA: 291] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 7. | Nobusue H, Endo T, Kano K. Establishment of a preadipocyte cell line derived from mature adipocytes of GFP transgenic mice and formation of adipose tissue. Cell Tissue Res. 2008;332:435-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 72] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 8. | Jumabay M, Zhang R, Yao Y, Goldhaber JI, Boström KI. Spontaneously beating cardiomyocytes derived from white mature adipocytes. Cardiovasc Res. 2010;85:17-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 57] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 9. | Sugihara H, Yonemitsu N, Miyabara S, Yun K. Primary cultures of unilocular fat cells: characteristics of growth in vitro and changes in differentiation properties. Differentiation. 1986;31:42-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 194] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 10. | Jumabay M, Abdmaulen R, Urs S, Heydarkhan-Hagvall S, Chazenbalk GD, Jordan MC, Roos KP, Yao Y, Boström KI. Endothelial differentiation in multipotent cells derived from mouse and human white mature adipocytes. J Mol Cell Cardiol. 2012;53:790-800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 48] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 11. | Fernyhough ME, Hausman GJ, Guan LL, Okine E, Moore SS, Dodson MV. Mature adipocytes may be a source of stem cells for tissue engineering. Biochem Biophys Res Commun. 2008;368:455-457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 40] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 12. | Eguizabal C, Montserrat N, Veiga A, Izpisua Belmonte JC. Dedifferentiation, transdifferentiation, and reprogramming: future directions in regenerative medicine. Semin Reprod Med. 2013;31:82-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 57] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 13. | Grafi G, Barak S. Stress induces cell dedifferentiation in plants. Biochim Biophys Acta. 2015;1849:378-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 14. | Sandoval-Guzmán T, Wang H, Khattak S, Schuez M, Roensch K, Nacu E, Tazaki A, Joven A, Tanaka EM, Simon A. Fundamental differences in dedifferentiation and stem cell recruitment during skeletal muscle regeneration in two salamander species. Cell Stem Cell. 2014;14:174-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 237] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 15. | McGann CJ, Odelberg SJ, Keating MT. Mammalian myotube dedifferentiation induced by newt regeneration extract. Proc Natl Acad Sci USA. 2001;98:13699-13704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 156] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 16. | Suzuki K, Mitsutake N, Saenko V, Suzuki M, Matsuse M, Ohtsuru A, Kumagai A, Uga T, Yano H, Nagayama Y. Dedifferentiation of human primary thyrocytes into multilineage progenitor cells without gene introduction. PLoS One. 2011;6:e19354. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 17. | Sun X, Fu X, Han W, Zhao Y, Liu H, Sheng Z. Dedifferentiation of human terminally differentiating keratinocytes into their precursor cells induced by basic fibroblast growth factor. Biol Pharm Bull. 2011;34:1037-1045. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 18. | Hanley SC, Assouline-Thomas B, Makhlin J, Rosenberg L. Epidermal growth factor induces adult human islet cell dedifferentiation. J Endocrinol. 2011;211:231-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 19. | Lee J, Hong F, Kwon S, Kim SS, Kim DO, Kang HS, Lee SJ, Ha J, Kim SS. Activation of p38 MAPK induces cell cycle arrest via inhibition of Raf/ERK pathway during muscle differentiation. Biochem Biophys Res Commun. 2002;298:765-771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 64] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 20. | Engel FB, Schebesta M, Duong MT, Lu G, Ren S, Madwed JB, Jiang H, Wang Y, Keating MT. p38 MAP kinase inhibition enables proliferation of adult mammalian cardiomyocytes. Genes Dev. 2005;19:1175-1187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 439] [Cited by in RCA: 440] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 21. | Rumyantsev PP. Interrelations of the proliferation and differentiation processes during cardiact myogenesis and regeneration. Int Rev Cytol. 1977;51:186-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 102] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 22. | Gassmann M, Casagranda F, Orioli D, Simon H, Lai C, Klein R, Lemke G. Aberrant neural and cardiac development in mice lacking the ErbB4 neuregulin receptor. Nature. 1995;378:390-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 838] [Cited by in RCA: 820] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 23. | Lee KF, Simon H, Chen H, Bates B, Hung MC, Hauser C. Requirement for neuregulin receptor erbB2 in neural and cardiac development. Nature. 1995;378:394-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 937] [Cited by in RCA: 933] [Article Influence: 31.1] [Reference Citation Analysis (0)] |
| 24. | Yoshida A, Ushiku T, Motoi T, Shibata T, Fukayama M, Tsuda H. Well-differentiated liposarcoma with low-grade osteosarcomatous component: an underrecognized variant. Am J Surg Pathol. 2010;34:1361-1366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 26] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 25. | Kumta SM, Griffith JF, Chow LT, Leung PC. Primary juxtacortical chondrosarcoma dedifferentiating after 20 years. Skeletal Radiol. 1998;27:569-573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 17] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 26. | Kusafuka K, Takizawa Y, Ueno T, Ishiki H, Asano R, Kamijo T, Iida Y, Ebihara M, Ota Y, Onitsuka T. Dedifferentiated epithelial-myoepithelial carcinoma of the parotid gland: a rare case report of immunohistochemical analysis and review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;106:85-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 46] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 27. | Wibmer C, Leithner A, Zielonke N, Sperl M, Windhager R. Increasing incidence rates of soft tissue sarcomas? A population-based epidemiologic study and literature review. Ann Oncol. 2010;21:1106-1111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 162] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 28. | Jopling C, Sleep E, Raya M, Martí M, Raya A, Izpisúa Belmonte JC. Zebrafish heart regeneration occurs by cardiomyocyte dedifferentiation and proliferation. Nature. 2010;464:606-609. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1141] [Cited by in RCA: 1035] [Article Influence: 69.0] [Reference Citation Analysis (0)] |
| 29. | Lafont JE. Lack of oxygen in articular cartilage: consequences for chondrocyte biology. Int J Exp Pathol. 2010;91:99-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 82] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 30. | Aitken KJ, Tolg C, Panchal T, Leslie B, Yu J, Elkelini M, Sabha N, Tse DJ, Lorenzo AJ, Hassouna M. Mammalian target of rapamycin (mTOR) induces proliferation and de-differentiation responses to three coordinate pathophysiologic stimuli (mechanical strain, hypoxia, and extracellular matrix remodeling) in rat bladder smooth muscle. Am J Pathol. 2010;176:304-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 31. | Puri S, Folias AE, Hebrok M. Plasticity and dedifferentiation within the pancreas: development, homeostasis, and disease. Cell Stem Cell. 2015;16:18-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 124] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 32. | Hochedlinger K, Jaenisch R. Nuclear reprogramming and pluripotency. Nature. 2006;441:1061-1067. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 338] [Cited by in RCA: 317] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 33. | Grafi G. Stress cycles in stem cells/iPSCs development: implications for tissue repair. Biogerontology. 2013;14:603-608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 34. | Friedmann-Morvinski D, Verma IM. Dedifferentiation and reprogramming: origins of cancer stem cells. EMBO Rep. 2014;15:244-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 291] [Cited by in RCA: 373] [Article Influence: 33.9] [Reference Citation Analysis (0)] |
| 35. | Hikichi T, Matoba R, Ikeda T, Watanabe A, Yamamoto T, Yoshitake S, Tamura-Nakano M, Kimura T, Kamon M, Shimura M. Transcription factors interfering with dedifferentiation induce cell type-specific transcriptional profiles. Proc Natl Acad Sci USA. 2013;110:6412-6417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 36. | Talchai C, Xuan S, Lin HV, Sussel L, Accili D. Pancreatic β cell dedifferentiation as a mechanism of diabetic β cell failure. Cell. 2012;150:1223-1234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 939] [Cited by in RCA: 1112] [Article Influence: 85.5] [Reference Citation Analysis (0)] |
| 37. | Yang L, Li S, Hatch H, Ahrens K, Cornelius JG, Petersen BE, Peck AB. In vitro trans-differentiation of adult hepatic stem cells into pancreatic endocrine hormone-producing cells. Proc Natl Acad Sci USA. 2002;99:8078-8083. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 374] [Cited by in RCA: 363] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 38. | Xie H, Ye M, Feng R, Graf T. Stepwise reprogramming of B cells into macrophages. Cell. 2004;117:663-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 716] [Cited by in RCA: 723] [Article Influence: 34.4] [Reference Citation Analysis (0)] |
| 39. | Rodríguez-Ubreva J, Ciudad L, Gómez-Cabrero D, Parra M, Bussmann LH, di Tullio A, Kallin EM, Tegnér J, Graf T, Ballestar E. Pre-B cell to macrophage transdifferentiation without significant promoter DNA methylation changes. Nucleic Acids Res. 2012;40:1954-1968. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 40. | Crisan M, Yap S, Casteilla L, Chen CW, Corselli M, Park TS, Andriolo G, Sun B, Zheng B, Zhang L. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3:301-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2900] [Cited by in RCA: 2865] [Article Influence: 168.5] [Reference Citation Analysis (0)] |
| 41. | Ieda M, Fu JD, Delgado-Olguin P, Vedantham V, Hayashi Y, Bruneau BG, Srivastava D. Direct reprogramming of fibroblasts into functional cardiomyocytes by defined factors. Cell. 2010;142:375-386. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2088] [Cited by in RCA: 1855] [Article Influence: 123.7] [Reference Citation Analysis (0)] |
| 42. | Weissman IL, Anderson DJ, Gage F. Stem and progenitor cells: origins, phenotypes, lineage commitments, and transdifferentiations. Annu Rev Cell Dev Biol. 2001;17:387-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 659] [Cited by in RCA: 611] [Article Influence: 26.6] [Reference Citation Analysis (0)] |
| 43. | Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, Alfonso ZC, Fraser JK, Benhaim P, Hedrick MH. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13:4279-4295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4817] [Cited by in RCA: 5012] [Article Influence: 217.9] [Reference Citation Analysis (0)] |
| 44. | Musina RA, Bekchanova ES, Sukhikh GT. Comparison of mesenchymal stem cells obtained from different human tissues. Bull Exp Biol Med. 2005;139:504-509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 102] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 45. | Traktuev DO, Merfeld-Clauss S, Li J, Kolonin M, Arap W, Pasqualini R, Johnstone BH, March KL. A population of multipotent CD34-positive adipose stromal cells share pericyte and mesenchymal surface markers, reside in a periendothelial location, and stabilize endothelial networks. Circ Res. 2008;102:77-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 638] [Cited by in RCA: 633] [Article Influence: 35.2] [Reference Citation Analysis (0)] |
| 46. | Lin G, Garcia M, Ning H, Banie L, Guo YL, Lue TF, Lin CS. Defining stem and progenitor cells within adipose tissue. Stem Cells Dev. 2008;17:1053-1063. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 323] [Cited by in RCA: 306] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 47. | Gimble J, Guilak F. Adipose-derived adult stem cells: isolation, characterization, and differentiation potential. Cytotherapy. 2003;5:362-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 810] [Cited by in RCA: 1003] [Article Influence: 47.8] [Reference Citation Analysis (0)] |
| 48. | Tholpady SS, Aojanepong C, Llull R, Jeong JH, Mason AC, Futrell JW, Ogle RC, Katz AJ. The cellular plasticity of human adipocytes. Ann Plast Surg. 2005;54:651-656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 70] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 49. | Poloni A, Maurizi G, Leoni P, Serrani F, Mancini S, Frontini A, Zingaretti MC, Siquini W, Sarzani R, Cinti S. Human dedifferentiated adipocytes show similar properties to bone marrow-derived mesenchymal stem cells. Stem Cells. 2012;30:965-974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 111] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 50. | Wei S, Duarte MS, Zan L, Du M, Jiang Z, Guan L, Chen J, Hausman GJ, Dodson MV. Cellular and molecular implications of mature adipocyte dedifferentiation. J Genomics. 2013;1:5-12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 51. | Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17989] [Cited by in RCA: 18138] [Article Influence: 954.6] [Reference Citation Analysis (0)] |
| 52. | Kuroda Y, Kitada M, Wakao S, Nishikawa K, Tanimura Y, Makinoshima H, Goda M, Akashi H, Inutsuka A, Niwa A. Unique multipotent cells in adult human mesenchymal cell populations. Proc Natl Acad Sci USA. 2010;107:8639-8643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 379] [Cited by in RCA: 374] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 53. | Fusaki N, Ban H, Nishiyama A, Saeki K, Hasegawa M. Efficient induction of transgene-free human pluripotent stem cells using a vector based on Sendai virus, an RNA virus that does not integrate into the host genome. Proc Jpn Acad Ser B Phys Biol Sci. 2009;85:348-362. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1024] [Cited by in RCA: 964] [Article Influence: 60.3] [Reference Citation Analysis (0)] |
| 54. | Choi IY, Lim H, Lee G. Efficient generation human induced pluripotent stem cells from human somatic cells with Sendai-virus. J Vis Exp. 2014;(86). [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 55. | Gao Q, Zhao L, Song Z, Yang G. Expression pattern of embryonic stem cell markers in DFAT cells and ADSCs. Mol Biol Rep. 2012;39:5791-5804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 56. | Zuk PA. The intracellular distribution of the ES cell totipotent markers OCT4 and Sox2 in adult stem cells differs dramatically according to commercial antibody used. J Cell Biochem. 2009;106:867-877. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 57. | Sun N, Panetta NJ, Gupta DM, Wilson KD, Lee A, Jia F, Hu S, Cherry AM, Robbins RC, Longaker MT. Feeder-free derivation of induced pluripotent stem cells from adult human adipose stem cells. Proc Natl Acad Sci USA. 2009;106:15720-15725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 384] [Cited by in RCA: 369] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 58. | Sachs PC, Francis MP, Zhao M, Brumelle J, Rao RR, Elmore LW, Holt SE. Defining essential stem cell characteristics in adipose-derived stromal cells extracted from distinct anatomical sites. Cell Tissue Res. 2012;349:505-515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 50] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 59. | Jumabay M, Abdmaulen R, Ly A, Cubberly MR, Shahmirian LJ, Heydarkhan-Hagvall S, Dumesic DA, Yao Y, Boström KI. Pluripotent stem cells derived from mouse and human white mature adipocytes. Stem Cells Transl Med. 2014;3:161-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 49] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 60. | Ono H, Oki Y, Bono H, Kano K. Gene expression profiling in multipotent DFAT cells derived from mature adipocytes. Biochem Biophys Res Commun. 2011;407:562-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 61. | Rodbell M. Metabolism of isolated fat cells. I. Effects of hormones on glucose metabolism and lipolysis. J Biol Chem. 1964;239:375-380. [PubMed] |
| 62. | Sugihara H, Funatsumaru S, Yonemitsu N, Miyabara S, Toda S, Hikichi Y. A simple culture method of fat cells from mature fat tissue fragments. J Lipid Res. 1989;30:1987-1995. [PubMed] |
| 63. | von Heimburg D, Lemperle G, Dippe B, Krüger S. Free transplantation of fat autografts expanded by tissue expanders in rats. Br J Plast Surg. 1994;47:470-476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 33] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 64. | Jumabay M, Matsumoto T, Yokoyama S, Kano K, Kusumi Y, Masuko T, Mitsumata M, Saito S, Hirayama A, Mugishima H. Dedifferentiated fat cells convert to cardiomyocyte phenotype and repair infarcted cardiac tissue in rats. J Mol Cell Cardiol. 2009;47:565-575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 90] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 65. | Yu H, Kumar SM, Kossenkov AV, Showe L, Xu X. Stem cells with neural crest characteristics derived from the bulge region of cultured human hair follicles. J Invest Dermatol. 2010;130:1227-1236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 80] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 66. | Knoblich JA. Asymmetric cell division: recent developments and their implications for tumour biology. Nat Rev Mol Cell Biol. 2010;11:849-860. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 513] [Cited by in RCA: 446] [Article Influence: 29.7] [Reference Citation Analysis (0)] |
| 67. | Conklin EG. The Mutation Theory From the Standpoint of Cytology. Science. 1905;21:525-529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 68. | Yamashita YM, Mahowald AP, Perlin JR, Fuller MT. Asymmetric inheritance of mother versus daughter centrosome in stem cell division. Science. 2007;315:518-521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 417] [Cited by in RCA: 431] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 69. | Bultje RS, Castaneda-Castellanos DR, Jan LY, Jan YN, Kriegstein AR, Shi SH. Mammalian Par3 regulates progenitor cell asymmetric division via notch signaling in the developing neocortex. Neuron. 2009;63:189-202. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 298] [Cited by in RCA: 271] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 70. | Wei S, Bergen WG, Hausman GJ, Zan L, Dodson MV. Cell culture purity issues and DFAT cells. Biochem Biophys Res Commun. 2013;433:273-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 71. | Zhang HH, Kumar S, Barnett AH, Eggo MC. Ceiling culture of mature human adipocytes: use in studies of adipocyte functions. J Endocrinol. 2000;164:119-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 108] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 72. | Kou L, Lu XW, Wu MK, Wang H, Zhang YJ, Sato S, Shen JF. The phenotype and tissue-specific nature of multipotent cells derived from human mature adipocytes. Biochem Biophys Res Commun. 2014;444:543-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 73. | Miyazaki T, Kitagawa Y, Toriyama K, Kobori M, Torii S. Isolation of two human fibroblastic cell populations with multiple but distinct potential of mesenchymal differentiation by ceiling culture of mature fat cells from subcutaneous adipose tissue. Differentiation. 2005;73:69-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 55] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 74. | Shen JF, Sugawara A, Yamashita J, Ogura H, Sato S. Dedifferentiated fat cells: an alternative source of adult multipotent cells from the adipose tissues. Int J Oral Sci. 2011;3:117-124. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 74] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 75. | Watson JE, Patel NA, Carter G, Moor A, Patel R, Ghansah T, Mathur A, Murr MM, Bickford P, Gould LJ. Comparison of Markers and Functional Attributes of Human Adipose-Derived Stem Cells and Dedifferentiated Adipocyte Cells from Subcutaneous Fat of an Obese Diabetic Donor. Adv Wound Care (New Rochelle). 2014;3:219-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 76. | Sugawara A, Sato S. Application of dedifferentiated fat cells for periodontal tissue regeneration. Hum Cell. 2014;27:12-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 77. | Poulos SP, Dodson MV, Hausman GJ. Cell line models for differentiation: preadipocytes and adipocytes. Exp Biol Med (Maywood). 2010;235:1185-1193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 220] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 78. | Sakuma T, Matsumoto T, Kano K, Fukuda N, Obinata D, Yamaguchi K, Yoshida T, Takahashi S, Mugishima H. Mature, adipocyte derived, dedifferentiated fat cells can differentiate into smooth muscle-like cells and contribute to bladder tissue regeneration. J Urol. 2009;182:355-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 67] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 79. | Urs S, Harrington A, Liaw L, Small D. Selective expression of an aP2/Fatty Acid Binding Protein 4-Cre transgene in non-adipogenic tissues during embryonic development. Transgenic Res. 2006;15:647-653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 93] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 80. | Fernyhough ME, Hausman GJ, Dodson MV. Progeny from dedifferentiated bovine adipocytes display protracted adipogenesis. Cells Tissues Organs. 2008;188:359-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 25] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 81. | Yagi K, Kondo D, Okazaki Y, Kano K. A novel preadipocyte cell line established from mouse adult mature adipocytes. Biochem Biophys Res Commun. 2004;321:967-974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 132] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 82. | Chen J, Dodson MV, Jiang Z. Cellular and molecular comparison of redifferentiation of intramuscular- and visceral-adipocyte derived progeny cells. Int J Biol Sci. 2010;6:80-88. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 35] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 83. | Oki Y, Watanabe S, Endo T, Kano K. Mature adipocyte-derived dedifferentiated fat cells can trans-differentiate into osteoblasts in vitro and in vivo only by all-trans retinoic acid. Cell Struct Funct. 2008;33:211-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 67] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 84. | Kikuta S, Tanaka N, Kazama T, Kazama M, Kano K, Ryu J, Tokuhashi Y, Matsumoto T. Osteogenic effects of dedifferentiated fat cell transplantation in rabbit models of bone defect and ovariectomy-induced osteoporosis. Tissue Eng Part A. 2013;19:1792-1802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 47] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 85. | Nakamura T, Shinohara Y, Momozaki S, Yoshimoto T, Noguchi K. Co-stimulation with bone morphogenetic protein-9 and FK506 induces remarkable osteoblastic differentiation in rat dedifferentiated fat cells. Biochem Biophys Res Commun. 2013;440:289-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 86. | Kishimoto N, Momota Y, Hashimoto Y, Tatsumi S, Ando K, Omasa T, Kotani J. The osteoblastic differentiation ability of human dedifferentiated fat cells is higher than that of adipose stem cells from the buccal fat pad. Clin Oral Investig. 2014;18:1893-1901. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 41] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 87. | Vlahopoulos S, Zimmer WE, Jenster G, Belaguli NS, Balk SP, Brinkmann AO, Lanz RB, Zoumpourlis VC, Schwartz RJ. Recruitment of the androgen receptor via serum response factor facilitates expression of a myogenic gene. J Biol Chem. 2005;280:7786-7792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 42] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 88. | Kazama T, Fujie M, Endo T, Kano K. Mature adipocyte-derived dedifferentiated fat cells can transdifferentiate into skeletal myocytes in vitro. Biochem Biophys Res Commun. 2008;377:780-785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 80] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 89. | Obinata D, Matsumoto T, Ikado Y, Sakuma T, Kano K, Fukuda N, Yamaguchi K, Mugishima H, Takahashi S. Transplantation of mature adipocyte-derived dedifferentiated fat (DFAT) cells improves urethral sphincter contractility in a rat model. Int J Urol. 2011;18:827-834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 45] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 90. | Barbuti A. The ‘hearty’ fat: adipocytes as a source of functional cardiomyocytes. Cardiovasc Res. 2010;85:1-2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 91. | Soejima K, Kashimura T, Asami T, Kazama T, Matsumoto T, Nakazawa H. Effects of mature adipocyte-derived dedifferentiated fat (DFAT) cells on generation and vascularisation of dermis-like tissue after artificial dermis grafting. J Plast Surg Hand Surg. 2015;49:25-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 92. | Poloni A, Maurizi G, Anastasi S, Mondini E, Mattiucci D, Discepoli G, Tiberi F, Mancini S, Partelli S, Maurizi A. Plasticity of human dedifferentiated adipocytes toward endothelial cells. Exp Hematol. 2015;43:137-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 93. | Planat-Benard V, Silvestre JS, Cousin B, André M, Nibbelink M, Tamarat R, Clergue M, Manneville C, Saillan-Barreau C, Duriez M. Plasticity of human adipose lineage cells toward endothelial cells: physiological and therapeutic perspectives. Circulation. 2004;109:656-663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1053] [Cited by in RCA: 1264] [Article Influence: 60.2] [Reference Citation Analysis (0)] |
| 94. | Natesan S, Zhang G, Baer DG, Walters TJ, Christy RJ, Suggs LJ. A bilayer construct controls adipose-derived stem cell differentiation into endothelial cells and pericytes without growth factor stimulation. Tissue Eng Part A. 2011;17:941-953. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 56] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 95. | Bexell D, Gunnarsson S, Tormin A, Darabi A, Gisselsson D, Roybon L, Scheding S, Bengzon J. Bone marrow multipotent mesenchymal stroma cells act as pericyte-like migratory vehicles in experimental gliomas. Mol Ther. 2009;17:183-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 182] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 96. | Hsueh YY, Chiang YL, Wu CC, Lin SC. Spheroid formation and neural induction in human adipose-derived stem cells on a chitosan-coated surface. Cells Tissues Organs. 2012;196:117-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 97. | Ahmadi N, Razavi S, Kazemi M, Oryan S. Stability of neural differentiation in human adipose derived stem cells by two induction protocols. Tissue Cell. 2012;44:87-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 50] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 98. | Dhar S, Yoon ES, Kachgal S, Evans GR. Long-term maintenance of neuronally differentiated human adipose tissue-derived stem cells. Tissue Eng. 2007;13:2625-2632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 44] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 99. | Lattanzi W, Geloso MC, Saulnier N, Giannetti S, Puglisi MA, Corvino V, Gasbarrini A, Michetti F. Neurotrophic features of human adipose tissue-derived stromal cells: in vitro and in vivo studies. J Biomed Biotechnol. 2011;2011:468705. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 39] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 100. | Feng N, Han Q, Li J, Wang S, Li H, Yao X, Zhao RC. Generation of highly purified neural stem cells from human adipose-derived mesenchymal stem cells by Sox1 activation. Stem Cells Dev. 2014;23:515-529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 45] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 101. | Ohta Y, Takenaga M, Tokura Y, Hamaguchi A, Matsumoto T, Kano K, Mugishima H, Okano H, Igarashi R. Mature adipocyte-derived cells, dedifferentiated fat cells (DFAT), promoted functional recovery from spinal cord injury-induced motor dysfunction in rats. Cell Transplant. 2008;17:877-886. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 61] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 102. | Yamada H, Ito D, Oki Y, Kitagawa M, Matsumoto T, Watari T, Kano K. Transplantation of mature adipocyte-derived dedifferentiated fat cells promotes locomotor functional recovery by remyelination and glial scar reduction after spinal cord injury in mice. Biochem Biophys Res Commun. 2014;454:341-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 103. | Kono S, Kazama T, Kano K, Harada K, Uechi M, Matsumoto T. Phenotypic and functional properties of feline dedifferentiated fat cells and adipose-derived stem cells. Vet J. 2014;199:88-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 45] [Article Influence: 3.8] [Reference Citation Analysis (0)] |