Published online Jan 26, 2015. doi: 10.4252/wjsc.v7.i1.149
Peer-review started: July 22, 2014
First decision: August 14, 2014
Revised: August 30, 2014
Accepted: September 16, 2014
Article in press: December 16, 2014
Published online: January 26, 2015
Processing time: 175 Days and 12.9 Hours
In facing the mounting clinical challenge and suboptimal techniques of craniofacial bone defects resulting from various conditions, such as congenital malformations, osteomyelitis, trauma and tumor resection, the ongoing research of regenerative medicine using stem cells and concurrent advancement in biotechnology have shifted the focus from surgical reconstruction to a novel stem cell-based tissue engineering strategy for customized and functional craniofacial bone regeneration. Given the unique ontogenetical and cell biological properties of perinatal stem cells, emerging evidence has suggested these extraembryonic tissue-derived stem cells to be a promising cell source for extensive use in regenerative medicine and tissue engineering. In this review, we summarize the current achievements and obstacles in stem cell-based craniofacial bone regeneration and subsequently we address the characteristics of various types of perinatal stem cells and their novel application in tissue engineering of craniofacial bone. We propose the promising feasibility and scope of perinatal stem cell-based craniofacial bone tissue engineering for future clinical application.
Core tip: Given the unique ontogenetical and cell biological properties of perinatal stem cells, emerging evidence has suggested these extraembryonic tissue-derived stem cells to be a promising cell source for extensive use in regenerative medicine and tissue engineering. In this review, we summarize the current achievements and obstacles in stem cell-based craniofacial bone regeneration and subsequently we address the characteristics of various types of perinatal stem cells and their novel application in tissue engineering of craniofacial bone. We propose the promising feasibility and scope of perinatal stem cell-based craniofacial bone tissue engineering for future clinical application.
- Citation: Si JW, Wang XD, Shen SG. Perinatal stem cells: A promising cell resource for tissue engineering of craniofacial bone. World J Stem Cells 2015; 7(1): 149-159
- URL: https://www.wjgnet.com/1948-0210/full/v7/i1/149.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v7.i1.149
The craniofacial bone has an essential role in supporting the adjacent soft tissues, providing anchorage for dental structures, maintaining structural stability for many physical functions and composing the esthetics of the human body. Craniofacial bone defects resulting from various conditions, such as congenital malformation, trauma, osteomyelitis and tumor resection, often lead to large psychomedical burdens as well as difficult reconstructive challenges for both patients and craniofacial surgeons[1-5]. Current treatments for such skeletal defects often require invasive and technically challenging operations such as alloplastic reconstruction, bone grafting from various anatomic areas like the iliac crest and fibula and microvascular free-flap techniques[3,4,6-8]. While these procedures have been proven to be reliable and effective, they also result in secondary donor site defects, associated intra- and post-operative complications and extended hospitalization. Furthermore, these techniques were largely limited by the scarcity of donor bone graft volume and patient physical conditions and struggled to restore pre-operative form and function. Suggested by recent clinical studies, stem cell-based bone tissue engineering has been recognized as a promising strategy for both aesthetic and functional reconstruction of craniofacial bone defects[9-14].
To date, several stem cell sources, such as adult mesenchymal stem cells or mesenchymal stromal cells (MSCs), embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs), have displayed promising osteogenic potential in vivo and in vitro and thus have been proposed as a potential cell source for bone tissue engineering[9,11-13,15,16]. However, the limitations associated with these stem cell sources are also significant[13,17-20]. In the last decade, the list of putative human stem cell sources was amended to include human perinatal extraembryonic tissues, such as amniotic fluid, fetal membranes (amnion and chorion) and umbilical cord[21-23]. Due to their unique ontogenetic relationship to fetal development, the extraembryonic perinatal stem cells represent an intermediate cell type which has recently been described to combine qualities of both their adult stem cell counterparts and ESCs and possess immunoprivileged characteristics, as well as a broad multipotent plasticity[24-29]. Most importantly, these cells, simply isolated from extraembryonic tissues which are normally discarded after birth, effectively avoid ethical issue involvement. All these attractive characteristics make perinatal stem cells a promising and noncontroversial source of stem cells for extensive use in craniofacial bone tissue engineering (CBTE).
In the present review, we summarize the current research progress on stem cell-based craniofacial bone regeneration and subsequently we address the characteristics of various perinatal stem cells and their novel application in CBTE.
As an alternative to surgical reconstruction, multidisciplinary developments in cell and molecular biology, developmental biology, materials science and bioengineering promoted the tissue engineering approach, which was composed of stem/progenitor cells, biocompatible scaffolds and biochemical signals, to regenerate large tissue defects[30]. The ongoing tissue engineering strategy offers several potential benefits, including the avoidance of secondary donor site defect, reduction of hospitalization and medical burdens and most importantly, the ability to closely restore the normal anatomic structure and function. Among the variety of current tissue regenerative indications based on the tissue engineering strategy, craniofacial bone defect is particularly suited to be tissue engineered. In fact, stem cell-based bone tissue engineering has already entered many preclinical or clinical applications in the craniofacial region.
Currently, one of the most well-defined and utilized stem cell types in CBTE is the adult MSCs. These stem cells have been isolated and identified from various tissues such as bone marrow, adipose, muscle, dental pulp and periodontal ligament[9,11,12,14,31,32]. In 2001, Shang et al[33] showed augmented healing of sheep cranial defects with calcium alginate gel containing autologous bone marrow-derived MSCs (BMSCs). In 2004, Cowan et al[34] first demonstrated that hydroxyapatite-coated polylactic-co-glycolic acid scaffolds seeded with adipose-derive MSCs (AMSCs) promoted bone regeneration of critical-size calvarial defects using a rodent model[34]. More recently, a stem cell-based temporomandibular joint (TMJ) condylar bone graft using a tissue engineering strategy was reported, which suggested the possibility of generating an entire articular condyle in the same shape and dimensions of a human TMJ in vivo, with both cartilage and bone layers from a single population of MSCs[35]. These observations were constantly supported in various craniofacial defect models of mouse, rabbit and canine, with the utilization of different MSC and biomaterial scaffolds[9,11,12,14,36-42]. In clinical experiments, autologous BMSCs were seeded onto bioscaffolds and transplanted to repair the mandible or alveolar defect in patients and showed satisfied ossification at the regenerated site in terms of both esthetic and functional outcome[10,43-45]. In 2009, Mesimäki et al[46] reported the first use of a microvascular custom-made ectopic bone flap developed from a preformed titanium cage filled with autologous AMSCs, β-tricalcium phosphate (β-TCP) granules to reconstruct a maxillary defect. Most recently, Thesleff et al[47] described a novel method to reconstruct critical-sized calvarial defects of adult patients using autologous AMSC and β-TCP granules, providing evidence that AMSCs combined with β-TCP can be used to regenerate calvarial bone, with favorable clinical results. Although there have been many reports of preliminary success using adult MSCs both in the laboratory and clinic for craniofacial bone regeneration, these stem cell sources are still significantly limited by their scarcity, invasive cell collection and cell aging[15,16,19,48].
After the revolutionary discovery and characterization of ESCs and iPSCs, further advances have been made towards applying bone tissue engineering methods with these promising pluripotent stem cells[15,16,19,20,49]. Harkness et al[50] reported a subpopulation of fibroblast-like ESCs which showed up-regulation of osteogenic specific markers and production of extracellular mineralization when cultured in osteogenic induction medium. The implantation of these cells in a critical-sized calvarial defect of immune deficient mice resulted in promoted new bone formation and partial repair of the calvarial defect[50]. More recently, Ye et al[13] demonstrated de novo osteogenesis of iPSCs within a critical-sized calvarial bone defect[13]. However, the application of ESCs and iPSCs in craniofacial regeneration are still at a preliminary stage and significantly limited by controversial political and ethical concerns, as well as genomic instability, tumorigenesis and immune rejection[16-18]. The drawbacks of these stem cell types may restrict their utility and performance in further clinical practice, prompting increasing interest in alternative stem cell sources for bone tissue engineering.
Perinatal stem cell research has been attracting mounting interest worldwide in recent years. Various populations of stem cells, most of which are comprised within the category of mesenchymal stem cells, have been isolated from different extraembryonic-associated tissues.
Amniotic fluid (AF) is the nourishing and protective liquid located within the fetal sac throughout the gestational stage which contains heterogeneous cells originating from embryonic and extraembryonic tissues[51,52]. Previously, cells obtained from AF were mostly used for diagnostic purposes, such as sex determination, detection of fetal infections and genetic diseases. During the last decades, different groups of researchers have provided evidence that human amniotic fluid could be a pool of stem cells. In 2003, Prusa et al demonstrated that a distinct subpopulation of cells expressing OCT4, a key transcript factor for pluripotent human stem cells and preventing differentiation of stem cells, can be found in human amniotic fluid. In the same year, In’t Anker et al[53] demonstrated that human amniotic fluid contained a fibroblast-shaped cell population positive for mesenchymal markers such as CD90, CD105, CD73 and CD166 but negative for the hematopoietic markers such as CD45, CD34 and CD14[53]. Notably, De Coppi et al[54] successfully isolated a multipotential subpopulation of stem cells in the amniotic fluid by fluorescence-activated cell sorting for c-kit positive cells. These amniotic fluid-derived stem cells (AFSCs) maintain a round shape for 1 wk after isolation and begin to change to a fibroblastoid morphology from the second week. Long term culture shows a high self-renewal capacity of these cells with > 300 population doublings, far exceeding Hayflick’s limit. The doubling time of the undifferentiated cells is noted to be 36 h, with little variation during passaging. Analysis of stem cell markers shows that the AFSCs express pluripotent markers SSEA4 and OCT4, as well as typical mesenchymal markers, but they did not express the full complement of pluripotent markers, such as SSEA1, SSEA3, TRA1-60 or TRA1-81, indicating that AFSCs are not as primitive as ESCs and yet maintain greater potential than most adult MSCs[54-56]. Later, the existence and multi-differentiation capabilities of AFSCs into cells and tissues from all three embryonic germ layers was widely confirmed by various independent reports[54,57-63]. Promising results in terms of structural and functional outcomes highlight the true clinical potential of AFSCs in cell-based therapies and tissue engineering perspectives.
The amniotic membrane is the innermost fetal layer of the placenta which lines the amniotic cavity and assures the normal growth and development of the fetus during gestation. Normally, two types of stem cells can be isolated from the amniotic membrane through mechanical separation and the subsequent enzymatic digestion, namely amniotic epithelial cells (AECs) and the amniotic mesenchymal stem cells (AMMSCs)[64]. What makes these cells especially attractive is that large amounts can be isolated from an uncontroversial material that is usually discarded after birth. AECs, developing from the central region of the epiblast, build a single layer adjacent to the amniotic fluid and display a cobblestone-like epithelial morphology[64,65]. While AECs reside in the innermost layer of the amnion, AMMSCs differentiated from somatopleuric mesodermal cells reside in the stromal matrix of the amniotic membrane and exhibit a fibroblastoid morphology[66-68]. Given their different ontological and cell biological characteristics, subpopulations from both cell types have been confirmed to express a wide range of pluripotency markers, such as OCT4, SOX2, SSEA4, SSEA3, as well as typical mesenchymal markers[65-70]. Further in vitro and in vivo studies confirmed their multilineage differentiation potential into cells derived from all three germ layers, such as neuron-like cells, cardiomyocytes, chondrocytes, osteocytes and hepatocytes[69,71-81]. Most importantly, the immunomodulatory capacity and immunoprivileged status of amnion-derived stem cells make them a promising candidate for allogeneic transplantation and stem cell based therapies[2,25-27,82-91]. However, standardized collection, quality control and further preclinical and clinical research are still needed before safe amnion-derived stem cell banking products can be achieved[92].
The chorionic membrane of human term placenta is a rich source of stem cells from which mesenchymal stem cells from chorionic villi and chorionic plate can be isolated[24,93]. Notwithstanding the potential contamination of decidual maternal stem cells suggested by many studies of term chorionic cells with both fetal and maternal origin, or even pure maternal origin cells only, previous studies of these chorion-derived mesenchymal stem cells (CMSCs) have been focused on primary isolation and limited cell characterization[24,66]. Like their stem cell counterparts from the placenta, CMSCs participate in placental tissue generation, maintenance, repair and possess a intermediate phenotype of adult MSCs and pluripotent stem cells[93-95]. In vitro, the CMSCs have been reported to be more primitive than adult MSCs with evidence of greater self-renewal and potential to differentiate beyond mesenchymal cell lineages to other lineages such as hepatocyte-like cells and neuron-like cells[24,93,95-98]. In vivo, the CMSCs have recently been reported to have a therapeutic effect on liver injury and osteogenesis imperfecta through not just direct hepatocyte and osteocyte differentiation, but also secretion of cytokines and inhibition of donor cell apoptosis[97,98]. Very recently, Jones et al[99] reported that CMSCs isolated from first trimester but not term human placenta accelerated tissue repair in dermal excision skin wounds and improved bone quality and plasticity in osteogenesis imperfecta of mice. Interestingly, the first trimester CMSCs showed an earlier state of stemness, such as smaller size, faster kinetics, unique formation of embryoid bodies and higher expression levels of NANOG, SOX2, c-MYC and KLF4, than its term isolated counterparts. However, collection of CMSCs in the first trimester is technically challenging and usually requires early termination of pregnancy, an ethical obstacle to clinical applications.
The umbilical cord (UC) is an elastic cord that connects the fetus to the placenta during pregnancy. Anatomically, the UC consists of two umbilical arteries (UA) and one umbilical vein (UV) embedded in a mucous connective proteoglycan-rich matrix called Wharton’s jelly (WJ). Since more than a decade ago when two independent teams reported their successful isolation of mesenchymal like cells from porcine and human umbilical cord respectively[100,101], there has been increasing interest in studying the potential of umbilical cord-derived mesenchymal stem cells (UCMSCs) for regenerative medicine. Generally, UCMSCs have been reported to be isolated from different parts of the UC such as UA, UV, WJ, umbilical-lining membrane and umbilical cord blood (UCB)[102]. Based on the optimized methods to isolate, culture and cryopreserve human UCMSCs, a human UC-MSC bank complying with good manufacturing practices has been established[103]. Given the controversy that exists on whether UCB or umbilical vessel-derived MSCs can be isolated, MSCs isolated from these various parts of UC have been shown to resemble adult BMSCs while retaining some specific characteristics. In vitro, UCMSCs appear with a fibroblastoid morphology and display a greater expansion capacity and faster doubling time than adult BMSCs. Their morphology and proliferative characteristics did not change even after 30 passages[100,101]. These cells consistently express typical mesenchymal markers as well as α-smooth muscle actin and low levels of pluripotency markers, such as Oct4, Sox2 and Nanog[100,104,105]. Moreover, UCMSCs express a lower level of HLA-ABC than BMSCs and were negative for CD31, CD34, CD45, HLA-DR, CD80 and CD86[106]. In vitro and in vivo studies confirmed their multi-differentiation potential of mesodermal lineages, such as osteocytes, chondrocytes and cardiomyocytes, as well as other lineages such as neuron-like cell and hepatocytes[100,107-111]. Most importantly, UCMSCs possess immunomodulatory effects in vitro and in vivo and have been shown to be well tolerated in allogeneic transplantation experiments[28,106,112]. Promising results of preclinical and clinical studies using UCMSCs for treatment of liver fibrosis, systemic lupus erythematosus, Sjogren’s syndrome and GVHD have been reported which will further promote the therapeutic application of UCMSCs in cell-based regenerative medicine[112-115].
Due to their same ontogenetic origin, perinatal stem cells isolated from different extraembryonic tissues possess similar phenotypic and functional properties. In fact, AFSCs, AECs, AMMSCs, CMSCs and UCMSCs have been shown to exhibit osteogenic differentiation capacity both in vitro and in vivo[77,93,97,116-119]. In limited comparative studies, perinatal stem cells such as AFSCs and UCMSCs have been shown to display lower basal levels of bone-related genes, higher proliferation rate and much later senescence than BMSCs, resulting in a delayed but robust osteogenic differentiation capacity, which render them a promising candidate for cell banking and stem cell-based bone tissue engineering[120-122].
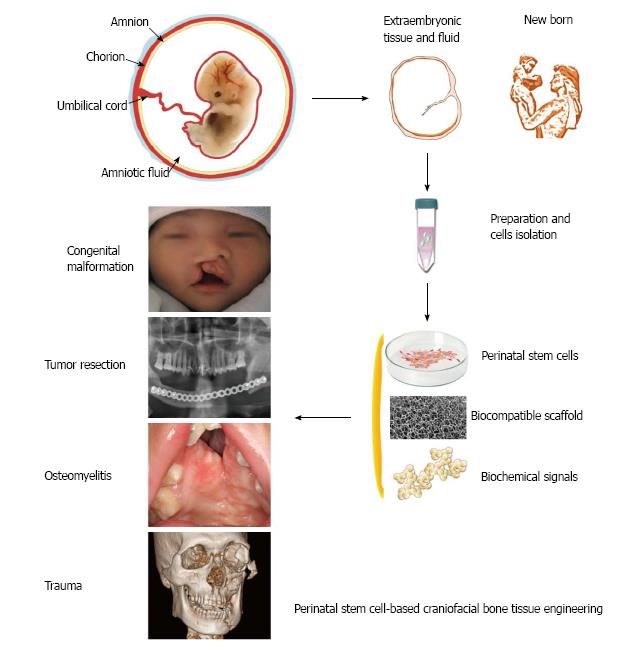
In face of the mounting clinical demand and suboptimal techniques for craniofacial skeleton reconstruction, perinatal stem cells may be particularly useful for autologous or allogeneic CBTE for newborns and children and may also be used for adult patients after banking at later stages of life (Figure 1). Although preclinical applications of perinatal stem cells in craniofacial bone regeneration are still limited, recent research progress of these cells revealed multiple possibilities for their potential applications in CBTE.
One of the most advanced applications of perinatal stem cells in CBTE was demonstrated by the use of UCMSCs in restoring rat cranial defects. Chen and colleagues investigated the in vitro and in vivo bone regenerative performance of human UCMSCs and BMSCs with RGD-modified macroporous calcium phosphate cements (CPC), using a critical-sized athymic rat parietal bone defect (8 mm in diameter) model[123]. They observed similarly high expressions of osteogenic specific genes and high percentages of live cells and cell density on CPC for UCMSCs and BMSCs. After implantation for 24 wk, the new bone tissue of UCMSC-CPC and BMSC-CPC groups in vivo exhibited similarly higher bone mineral density, new bone amount and vessel density compared to the CPC control group. Notably, this observation was in agreement with a former study by Liu et al[124] using human UCMSCs and partially demineralized bone matrix for reconstruction of critical-sized parietal bone defects (5 mm in diameter) in athymic rats. Interestingly, Barboni et al[125] reported AEC-enhanced bone regeneration using an ovine maxillary sinus augmentation model. Calcium-phosphate synthetic bone substitutes, alone or engineered with ovine AECs, were grafted bilaterally into maxillary sinuses of six adult sheep. After implantation, the scaffold integration and bone deposition are positively influenced by allotransplanted AECs. Sinus explants derived from an AEC-scaffold group displayed a reduced fibrotic reaction, a limited inflammatory response and an accelerated process of angiogenesis compared with the control group. In addition, a direct osteogenic differentiation of ovine AECs in vivo was observed at 45 d post operation, suggested by the presence of AECs-derived osteocalcin-expressing osteocytes entrapped within the newly deposited bone matrix. The general concept of perinatal stem cell-based bone tissue engineering has also been validated using ASCs in several craniofacial bone defect models. A commercial magnesium-enriched hydroxyapatite (MgHA)/collagen-based scaffold seeded with or without ovine AFMCs was implanted in the bilateral maxillary sinus of sheep models for up to 90 d. Results showed that application of AFMCs increased new bone deposition and stimulated a more rapid angiogenic reaction[126]. In another study, researchers developed two combined constructs by seeding dental pulp stem cells (DPSCs) and AFSCs onto silk fibroin scaffolds respectively. The AFSCs-scaffold construct as well as DPSC-scaffold construct promoted early bone regeneration in the critical-sized rat parietal bone defects compared with scaffold alone[127]. However, the relative long-term outcome of AFSCs in craniofacial bone repair was still in question and the optimal scaffold composition for this particular application remains to be defined[128,129]. In addition, suggested by most of the discussed studies, perinatal stem cells may play an essential role in new vessel formation in the regeneration site, which indirectly promotes the restoration of craniofacial bone defects[123,125,126,128].
The ontological and anatomical origins of stem cells have profound influences on their biological properties and performance in regenerative medicine. Promoted by the developments in cell and molecular biology, developmental biology and tissue engineering, the unique and advanced properties of perinatal stem cells have been largely unveiled within the last decade: (1) the collection, preparation and application of perinatal stem cells are scarcely involved with ethical issues; (2) most perinatal stem cells are easily harvested and manipulated with no harm to the baby or mother; (3) compared to adult MSCs which decreased in cell number, proliferation ability and differentiation capacities with age, perinatal stem cells are initially available in substantial numbers and exhibit greater proliferative activity than BMSCs, indicating the advantage for rapid expansion and consequent downstream application; (4) with an intermediate multipotency between pluripotent stem cells and adult MSCs, perinatal stem cells do not form a teratoma while maintaining a broad multi-differentiation capacity in vitro and in vivo; and (5) in terms of their involvement in materno-fetal immunotolerance, perinatal stem cells exhibit low immunogenicity and potentially high immunomodulatory capacity, indicating a clinical prospective of allogeneic cell transplantation. However, the mechanisms underlying the immunoprivileged and immunosuppressive effects of perinatal stem cells have not yet been clearly defined.
Despite the problems researchers still face in translating scientific concepts from the bench to the bedside, these exciting experimental and preclinical achievements from intense research efforts suggest perinatal stem cells may offer a most promising scope and will continue to produce new and exciting progress for the novel cell-based CBTE in the future.
We would like to thank Professor Lihe Guo from Shanghai Institute of Biochemistry and Cell Biology, Chinese Academy of Sciences, for his professional advice on this article.
P- Reviewer: Hiroyuki K, Louboutin JP, Yin H S- Editor: Tian YL L- Editor: Roemmele A E- Editor: Lu YJ
| 1. | Ward BB, Brown SE, Krebsbach PH. Bioengineering strategies for regeneration of craniofacial bone: a review of emerging technologies. Oral Dis. 2010;16:709-716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 56] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 2. | Yuan J, Cao Y, Liu W. Biomimetic scaffolds: implications for craniofacial regeneration. J Craniofac Surg. 2012;23:294-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 3. | Genden EM. Reconstruction of the mandible and the maxilla: the evolution of surgical technique. Arch Facial Plast Surg. 2010;12:87-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 21] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 4. | Mendonça JJ, Juiz-Lopez P. Regenerative facial reconstruction of terminal stage osteoradionecrosis and other advanced craniofacial diseases with adult cultured stem and progenitor cells. Plast Reconstr Surg. 2010;126:1699-1709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 5. | Scheller EL, Krebsbach PH, Kohn DH. Tissue engineering: state of the art in oral rehabilitation. J Oral Rehabil. 2009;36:368-389. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 124] [Cited by in RCA: 99] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 6. | Smith DM, Cray JJ, Weiss LE, Dai Fei EK, Shakir S, Rottgers SA, Losee JE, Campbell PG, Cooper GM. Precise control of osteogenesis for craniofacial defect repair: the role of direct osteoprogenitor contact in BMP-2-based bioprinting. Ann Plast Surg. 2012;69:485-488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 7. | Horswell BB, Henderson JM. Secondary osteoplasty of the alveolar cleft defect. J Oral Maxillofac Surg. 2003;61:1082-1090. [PubMed] |
| 8. | Tessier P, Kawamoto H, Matthews D, Posnick J, Raulo Y, Tulasne JF, Wolfe SA. Taking long rib grafts for facial reconstruction--tools and techniques: III. A 2900-case experience in maxillofacial and craniofacial surgery. Plast Reconstr Surg. 2005;116:38S-46S; discussion 92S-94S. [PubMed] |
| 9. | Bueno DF, Kerkis I, Costa AM, Martins MT, Kobayashi GS, Zucconi E, Fanganiello RD, Salles FT, Almeida AB, do Amaral CE. New source of muscle-derived stem cells with potential for alveolar bone reconstruction in cleft lip and/or palate patients. Tissue Eng Part A. 2009;15:427-435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 55] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 10. | Behnia H, Khojasteh A, Soleimani M, Tehranchi A, Atashi A. Repair of alveolar cleft defect with mesenchymal stem cells and platelet derived growth factors: a preliminary report. J Craniomaxillofac Surg. 2012;40:2-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 116] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 11. | Lin CY, Chang YH, Kao CY, Lu CH, Sung LY, Yen TC, Lin KJ, Hu YC. Augmented healing of critical-size calvarial defects by baculovirus-engineered MSCs that persistently express growth factors. Biomaterials. 2012;33:3682-3692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 67] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 12. | Pieri F, Lucarelli E, Corinaldesi G, Aldini NN, Fini M, Parrilli A, Dozza B, Donati D, Marchetti C. Dose-dependent effect of adipose-derived adult stem cells on vertical bone regeneration in rabbit calvarium. Biomaterials. 2010;31:3527-3535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 51] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 13. | Ye JH, Xu YJ, Gao J, Yan SG, Zhao J, Tu Q, Zhang J, Duan XJ, Sommer CA, Mostoslavsky G. Critical-size calvarial bone defects healing in a mouse model with silk scaffolds and SATB2-modified iPSCs. Biomaterials. 2011;32:5065-5076. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 140] [Cited by in RCA: 124] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 14. | Zou D, Zhang Z, Ye D, Tang A, Deng L, Han W, Zhao J, Wang S, Zhang W, Zhu C. Repair of critical-sized rat calvarial defects using genetically engineered bone marrow-derived mesenchymal stem cells overexpressing hypoxia-inducible factor-1α. Stem Cells. 2011;29:1380-1390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 84] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 15. | Bigdeli N, de Peppo GM, Lennerås M, Sjövall P, Lindahl A, Hyllner J, Karlsson C. Superior osteogenic capacity of human embryonic stem cells adapted to matrix-free growth compared to human mesenchymal stem cells. Tissue Eng Part A. 2010;16:3427-3440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 16. | de Peppo GM, Marcos-Campos I, Kahler DJ, Alsalman D, Shang L, Vunjak-Novakovic G, Marolt D. Engineering bone tissue substitutes from human induced pluripotent stem cells. Proc Natl Acad Sci USA. 2013;110:8680-8685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 157] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 17. | Bayart E, Cohen-Haguenauer O. Technological overview of iPS induction from human adult somatic cells. Curr Gene Ther. 2013;13:73-92. [PubMed] |
| 18. | Miura K, Okada Y, Aoi T, Okada A, Takahashi K, Okita K, Nakagawa M, Koyanagi M, Tanabe K, Ohnuki M. Variation in the safety of induced pluripotent stem cell lines. Nat Biotechnol. 2009;27:743-745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 669] [Cited by in RCA: 640] [Article Influence: 40.0] [Reference Citation Analysis (0)] |
| 19. | Amit M, Carpenter MK, Inokuma MS, Chiu CP, Harris CP, Waknitz MA, Itskovitz-Eldor J, Thomson JA. Clonally derived human embryonic stem cell lines maintain pluripotency and proliferative potential for prolonged periods of culture. Dev Biol. 2000;227:271-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1117] [Cited by in RCA: 1014] [Article Influence: 40.6] [Reference Citation Analysis (0)] |
| 20. | Hanna J, Cheng AW, Saha K, Kim J, Lengner CJ, Soldner F, Cassady JP, Muffat J, Carey BW, Jaenisch R. Human embryonic stem cells with biological and epigenetic characteristics similar to those of mouse ESCs. Proc Natl Acad Sci USA. 2010;107:9222-9227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 649] [Cited by in RCA: 645] [Article Influence: 43.0] [Reference Citation Analysis (0)] |
| 21. | Witkowska-Zimny M, Wrobel E. Perinatal sources of mesenchymal stem cells: Wharton’s jelly, amnion and chorion. Cell Mol Biol Lett. 2011;16:493-514. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 62] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 22. | Parolini O, Soncini M, Evangelista M, Schmidt D. Amniotic membrane and amniotic fluid-derived cells: potential tools for regenerative medicine? Regen Med. 2009;4:275-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 111] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 23. | Rennie K, Gruslin A, Hengstschläger M, Pei D, Cai J, Nikaido T, Bani-Yaghoub M. Applications of amniotic membrane and fluid in stem cell biology and regenerative medicine. Stem Cells Int. 2012;2012:721538. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 47] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 24. | Kim MJ, Shin KS, Jeon JH, Lee DR, Shim SH, Kim JK, Cha DH, Yoon TK, Kim GJ. Human chorionic-plate-derived mesenchymal stem cells and Wharton’s jelly-derived mesenchymal stem cells: a comparative analysis of their potential as placenta-derived stem cells. Cell Tissue Res. 2011;346:53-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 109] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 25. | Manuelpillai U, Moodley Y, Borlongan CV, Parolini O. Amniotic membrane and amniotic cells: potential therapeutic tools to combat tissue inflammation and fibrosis? Placenta. 2011;32 Suppl 4:S320-S325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 111] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 26. | Wolbank S, Peterbauer A, Fahrner M, Hennerbichler S, van Griensven M, Stadler G, Redl H, Gabriel C. Dose-dependent immunomodulatory effect of human stem cells from amniotic membrane: a comparison with human mesenchymal stem cells from adipose tissue. Tissue Eng. 2007;13:1173-1183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 299] [Cited by in RCA: 286] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 27. | Banas RA, Trumpower C, Bentlejewski C, Marshall V, Sing G, Zeevi A. Immunogenicity and immunomodulatory effects of amnion-derived multipotent progenitor cells. Hum Immunol. 2008;69:321-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 116] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 28. | Zhou C, Yang B, Tian Y, Jiao H, Zheng W, Wang J, Guan F. Immunomodulatory effect of human umbilical cord Wharton’s jelly-derived mesenchymal stem cells on lymphocytes. Cell Immunol. 2011;272:33-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 120] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 29. | Rameshwar P, Moorefield EC, McKee EE, Solchaga L, Orlando G, Yoo JJ, Walker S, Furth ME, Bishop CE. Cloned, cd117 selected human amniotic fluid stem cells are capable of modulating the immune response. PloS one. 2011;6:e26535. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 73] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 30. | Vacanti JP, Langer R. Tissue engineering: the design and fabrication of living replacement devices for surgical reconstruction and transplantation. Lancet. 1999;354 Suppl 1:SI32-SI34. [PubMed] |
| 31. | Liu HC, E LL, Wang DS, Su F, Wu X, Shi ZP, Lv Y, Wang JZ. Reconstruction of alveolar bone defects using bone morphogenetic protein 2 mediated rabbit dental pulp stem cells seeded on nano-hydroxyapatite/collagen/poly(L-lactide). Tissue Eng Part A. 2011;17:2417-2433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 94] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 32. | Tour G, Wendel M, Moll G, Tcacencu I. Bone repair using periodontal ligament progenitor cell-seeded constructs. J Dent Res. 2012;91:789-794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 33. | Shang Q, Wang Z, Liu W, Shi Y, Cui L, Cao Y. Tissue-engineered bone repair of sheep cranial defects with autologous bone marrow stromal cells. J Craniofac Surg. 2001;12:586-593; discussion 594-595. [PubMed] |
| 34. | Cowan CM, Shi YY, Aalami OO, Chou YF, Mari C, Thomas R, Quarto N, Contag CH, Wu B, Longaker MT. Adipose-derived adult stromal cells heal critical-size mouse calvarial defects. Nat Biotechnol. 2004;22:560-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 725] [Cited by in RCA: 686] [Article Influence: 32.7] [Reference Citation Analysis (0)] |
| 35. | Grayson WL, Fröhlich M, Yeager K, Bhumiratana S, Chan ME, Cannizzaro C, Wan LQ, Liu XS, Guo XE, Vunjak-Novakovic G. Engineering anatomically shaped human bone grafts. Proc Natl Acad Sci USA. 2010;107:3299-3304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 312] [Cited by in RCA: 282] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 36. | Baba S, Inoue T, Hashimoto Y, Kimura D, Ueda M, Sakai K, Matsumoto N, Hiwa C, Adachi T, Hojo M. Effectiveness of scaffolds with pre-seeded mesenchymal stem cells in bone regeneration--assessment of osteogenic ability of scaffolds implanted under the periosteum of the cranial bone of rats. Dent Mater J. 2010;29:673-681. [PubMed] |
| 37. | Castro-Govea Y, Cervantes-Kardasch VH, Borrego-Soto G, Martínez-Rodríguez HG, Espinoza-Juarez M, Romero-Díaz V, Marino-Martínez IA, Robles-Zamora A, Álvarez-Lozano E, Padilla-Rivas GR. Human bone morphogenetic protein 2-transduced mesenchymal stem cells improve bone regeneration in a model of mandible distraction surgery. J Craniofac Surg. 2012;23:392-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 38] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 38. | Jiang X, Zou S, Ye B, Zhu S, Liu Y, Hu J. bFGF-Modified BMMSCs enhance bone regeneration following distraction osteogenesis in rabbits. Bone. 2010;46:1156-1161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 58] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 39. | Liao HT, Chen CT, Chen CH, Chen JP, Tsai JC. Combination of guided osteogenesis with autologous platelet-rich fibrin glue and mesenchymal stem cell for mandibular reconstruction. J Trauma. 2011;70:228-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 40. | Zhang L, Wang P, Mei S, Li C, Cai C, Ding Y. In vivo alveolar bone regeneration by bone marrow stem cells/fibrin glue composition. Arch Oral Biol. 2012;57:238-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 41. | Zhang WB, Zheng LW, Chua DT, Cheung LK. Treatment of irradiated mandibles with mesenchymal stem cells transfected with bone morphogenetic protein 2/7. J Oral Maxillofac Surg. 2012;70:1711-1716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 42. | Wang F, Yu M, Yan X, Wen Y, Zeng Q, Yue W, Yang P, Pei X. Gingiva-derived mesenchymal stem cell-mediated therapeutic approach for bone tissue regeneration. Stem Cells Dev. 2011;20:2093-2102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 110] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 43. | Gimbel M, Ashley RK, Sisodia M, Gabbay JS, Wasson KL, Heller J, Wilson L, Kawamoto HK, Bradley JP. Repair of alveolar cleft defects: reduced morbidity with bone marrow stem cells in a resorbable matrix. J Craniofac Surg. 2007;18:895-901. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 91] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 44. | Kaigler D, Pagni G, Park CH, Tarle SA, Bartel RL, Giannobile WV. Angiogenic and osteogenic potential of bone repair cells for craniofacial regeneration. Tissue Eng Part A. 2010;16:2809-2820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 46] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 45. | Pradel W, Tausche E, Gollogly J, Lauer G. Spontaneous tooth eruption after alveolar cleft osteoplasty using tissue-engineered bone: a case report. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;105:440-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 29] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 46. | Mesimäki K, Lindroos B, Törnwall J, Mauno J, Lindqvist C, Kontio R, Miettinen S, Suuronen R. Novel maxillary reconstruction with ectopic bone formation by GMP adipose stem cells. Int J Oral Maxillofac Surg. 2009;38:201-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 338] [Cited by in RCA: 302] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 47. | Thesleff T, Lehtimäki K, Niskakangas T, Mannerström B, Miettinen S, Suuronen R, Öhman J. Cranioplasty with adipose-derived stem cells and biomaterial: a novel method for cranial reconstruction. Neurosurgery. 2011;68:1535-1540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 134] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 48. | Arvidson K, Abdallah BM, Applegate LA, Baldini N, Cenni E, Gomez-Barrena E, Granchi D, Kassem M, Konttinen YT, Mustafa K. Bone regeneration and stem cells. J Cell Mol Med. 2011;15:718-746. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 275] [Cited by in RCA: 252] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 49. | Kuznetsov SA, Cherman N, Robey PG. In vivo bone formation by progeny of human embryonic stem cells. Stem Cells Dev. 2011;20:269-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 52] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 50. | Harkness L, Mahmood A, Ditzel N, Abdallah BM, Nygaard JV, Kassem M. Selective isolation and differentiation of a stromal population of human embryonic stem cells with osteogenic potential. Bone. 2011;48:231-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 39] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 51. | Antonucci I, Pantalone A, Tete S, Salini V, Borlongan CV, Hess D, Stuppia L. Amniotic fluid stem cells: a promising therapeutic resource for cell-based regenerative therapy. Curr Pharm Des. 2012;18:1846-1863. [PubMed] |
| 52. | Roubelakis MG, Bitsika V, Zagoura D, Trohatou O, Pappa KI, Makridakis M, Antsaklis A, Vlahou A, Anagnou NP. In vitro and in vivo properties of distinct populations of amniotic fluid mesenchymal progenitor cells. J Cell Mol Med. 2011;15:1896-1913. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 74] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 53. | In ‘t Anker PS, Scherjon SA, Kleijburg-van der Keur C, Noort WA, Claas FH, Willemze R, Fibbe WE, Kanhai HH. Amniotic fluid as a novel source of mesenchymal stem cells for therapeutic transplantation. Blood. 2003;102:1548-1549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 580] [Cited by in RCA: 524] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 54. | De Coppi P, Bartsch G, Siddiqui MM, Xu T, Santos CC, Perin L, Mostoslavsky G, Serre AC, Snyder EY, Yoo JJ. Isolation of amniotic stem cell lines with potential for therapy. Nat Biotechnol. 2007;25:100-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1406] [Cited by in RCA: 1238] [Article Influence: 68.8] [Reference Citation Analysis (0)] |
| 55. | Arnhold S, Glüer S, Hartmann K, Raabe O, Addicks K, Wenisch S, Hoopmann M. Amniotic-Fluid Stem Cells: Growth Dynamics and Differentiation Potential after a CD-117-Based Selection Procedure. Stem Cells Int. 2011;2011:715341. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 54] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 56. | Chen J, Lu Z, Cheng D, Peng S, Wang H. Isolation and characterization of porcine amniotic fluid-derived multipotent stem cells. PLoS One. 2011;6:e19964. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 52] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 57. | Jezierski A, Gruslin A, Tremblay R, Ly D, Smith C, Turksen K, Sikorska M, Bani-Yaghoub M. Probing stemness and neural commitment in human amniotic fluid cells. Stem Cell Rev. 2010;6:199-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 40] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 58. | Park JS, Shim MS, Shim SH, Yang HN, Jeon SY, Woo DG, Lee DR, Yoon TK, Park KH. Chondrogenic potential of stem cells derived from amniotic fluid, adipose tissue, or bone marrow encapsulated in fibrin gels containing TGF-β3. Biomaterials. 2011;32:8139-8149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 54] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 59. | Sun H, Feng K, Hu J, Soker S, Atala A, Ma PX. Osteogenic differentiation of human amniotic fluid-derived stem cells induced by bone morphogenetic protein-7 and enhanced by nanofibrous scaffolds. Biomaterials. 2010;31:1133-1139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 96] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 60. | Bollini S, Pozzobon M, Nobles M, Riegler J, Dong X, Piccoli M, Chiavegato A, Price AN, Ghionzoli M, Cheung KK. In vitro and in vivo cardiomyogenic differentiation of amniotic fluid stem cells. Stem Cell Rev. 2011;7:364-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 66] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 61. | Carraro G, Perin L, Sedrakyan S, Giuliani S, Tiozzo C, Lee J, Turcatel G, De Langhe SP, Driscoll B, Bellusci S. Human amniotic fluid stem cells can integrate and differentiate into epithelial lung lineages. Stem Cells. 2008;26:2902-2911. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 183] [Cited by in RCA: 164] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 62. | Zhang P, Baxter J, Vinod K, Tulenko TN, Di Muzio PJ. Endothelial differentiation of amniotic fluid-derived stem cells: synergism of biochemical and shear force stimuli. Stem Cells Dev. 2009;18:1299-1308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 63] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 63. | Decembrini S, Cananzi M, Gualdoni S, Battersby A, Allen N, Pearson RA, Ali RR, De Coppi P, Sowden JC. Comparative analysis of the retinal potential of embryonic stem cells and amniotic fluid-derived stem cells. Stem Cells Dev. 2011;20:851-863. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 64. | Barbati A, Grazia Mameli M, Sidoni A, Di Renzo GC. Amniotic membrane: separation of amniotic mesoderm from amniotic epithelium and isolation of their respective mesenchymal stromal and epithelial cells. Curr Protoc Stem Cell Biol. 2012;Chapter 1:Unit 1E.8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 65. | Pratama G, Vaghjiani V, Tee JY, Liu YH, Chan J, Tan C, Murthi P, Gargett C, Manuelpillai U. Changes in culture expanded human amniotic epithelial cells: implications for potential therapeutic applications. PLoS One. 2011;6:e26136. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 95] [Cited by in RCA: 103] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 66. | In ‘t Anker PS, Scherjon SA, Kleijburg-van der Keur C, de Groot-Swings GM, Claas FH, Fibbe WE, Kanhai HH. Isolation of mesenchymal stem cells of fetal or maternal origin from human placenta. Stem Cells. 2004;22:1338-1345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 824] [Cited by in RCA: 818] [Article Influence: 40.9] [Reference Citation Analysis (0)] |
| 67. | Diaz-Prado S, Muinos-Lopez E, Hermida-Gomez T, Rendal-Vazquez ME, Fuentes-Boquete I, de Toro FJ, Blanco FJ. Isolation and Characterization of Mesenchymal Stem Cells from Human Amniotic Membrane. Tissue Eng Part C Methods. 2010;17:49-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 43] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 68. | Bačenková D, Rosocha J, Tóthová T, Rosocha L, Šarisský M. Isolation and basic characterization of human term amnion and chorion mesenchymal stromal cells. Cytotherapy. 2011;13:1047-1056. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 45] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 69. | Miki T, Lehmann T, Cai H, Stolz DB, Strom SC. Stem cell characteristics of amniotic epithelial cells. Stem Cells. 2005;23:1549-1559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 576] [Cited by in RCA: 557] [Article Influence: 27.9] [Reference Citation Analysis (0)] |
| 70. | Dobreva MP, Pereira PN, Deprest J, Zwijsen A. On the origin of amniotic stem cells: of mice and men. Int J Dev Biol. 2010;54:761-777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 112] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 71. | Marcus AJ, Coyne TM, Rauch J, Woodbury D, Black IB. Isolation, characterization, and differentiation of stem cells derived from the rat amniotic membrane. Differentiation. 2008;76:130-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 66] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 72. | Toda A, Okabe M, Yoshida T, Nikaido T. The potential of amniotic membrane/amnion-derived cells for regeneration of various tissues. J Pharmacol Sci. 2007;105:215-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 252] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 73. | Huang GL, Zhang NN, Wang JS, Yao L, Zhao YJ, Wang YY. Transdifferentiation of human amniotic epithelial cells into acinar cells using a double-chamber system. Cell Reprogram. 2012;14:377-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 74. | Marongiu F, Gramignoli R, Dorko K, Miki T, Ranade AR, Paola Serra M, Doratiotto S, Sini M, Sharma S, Mitamura K. Hepatic differentiation of amniotic epithelial cells. Hepatology. 2011;53:1719-1729. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 124] [Cited by in RCA: 106] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 75. | Wei JP, Nawata M, Wakitani S, Kametani K, Ota M, Toda A, Konishi I, Ebara S, Nikaido T. Human amniotic mesenchymal cells differentiate into chondrocytes. Cloning Stem Cells. 2009;11:19-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 50] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 76. | Zhou J, Yu G, Cao C, Pang J, Chen X. Bone morphogenetic protein-7 promotes chondrogenesis in human amniotic epithelial cells. Int Orthop. 2011;35:941-948. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 77. | Mattioli M, Gloria A, Turriani M, Mauro A, Curini V, Russo V, Tetè S, Marchisio M, Pierdomenico L, Berardinelli P. Stemness characteristics and osteogenic potential of sheep amniotic epithelial cells. Cell Biol Int. 2012;36:7-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 78. | Zhong ZN, Zhu SF, Yuan AD, Lu GH, He ZY, Fa ZQ, Li WH. Potential of placenta-derived mesenchymal stem cells as seed cells for bone tissue engineering: preliminary study of osteoblastic differentiation and immunogenicity. Orthopedics. 2012;35:779-788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 79. | Fang CH, Jin J, Joe JH, Song YS, So BI, Lim SM, Cheon GJ, Woo SK, Ra JC, Lee YY. In vivo differentiation of human amniotic epithelial cells into cardiomyocyte-like cells and cell transplantation effect on myocardial infarction in rats: comparison with cord blood and adipose tissue-derived mesenchymal stem cells. Cell Transplant. 2012;21:1687-1696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 63] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 80. | Zeng G, Wang G, Guan F, Chang K, Jiao H, Gao W, Xi S, Yang B. Human amniotic membrane-derived mesenchymal stem cells labeled with superparamagnetic iron oxide nanoparticles: the effect on neuron-like differentiation in vitro. Mol Cell Biochem. 2011;357:331-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 81. | Tamagawa T, Oi S, Ishiwata I, Ishikawa H, Nakamura Y. Differentiation of mesenchymal cells derived from human amniotic membranes into hepatocyte-like cells in vitro. Hum Cell. 2007;20:77-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 74] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 82. | Tsuji H, Miyoshi S, Ikegami Y, Hida N, Asada H, Togashi I, Suzuki J, Satake M, Nakamizo H, Tanaka M. Xenografted human amniotic membrane-derived mesenchymal stem cells are immunologically tolerated and transdifferentiated into cardiomyocytes. Circ Res. 2010;106:1613-1623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 139] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 83. | Sun CC, Su Pang JH, Cheng CY, Cheng HF, Lee YS, Ku WC, Hsiao CH, Chen JK, Yang CM. Interleukin-1 receptor antagonist (IL-1RA) prevents apoptosis in ex vivo expansion of human limbal epithelial cells cultivated on human amniotic membrane. Stem Cells. 2006;24:2130-2139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 31] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 84. | Li H, Niederkorn JY, Neelam S, Mayhew E, Word RA, McCulley JP, Alizadeh H. Immunosuppressive factors secreted by human amniotic epithelial cells. Invest Ophthalmol Vis Sci. 2005;46:900-907. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 220] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 85. | Kubo M, Sonoda Y, Muramatsu R, Usui M. Immunogenicity of human amniotic membrane in experimental xenotransplantation. Invest Ophthalmol Vis Sci. 2001;42:1539-1546. [PubMed] |
| 86. | Kronsteiner B, Wolbank S, Peterbauer A, Hackl C, Redl H, van Griensven M, Gabriel C. Human mesenchymal stem cells from adipose tissue and amnion influence T-cells depending on stimulation method and presence of other immune cells. Stem Cells Dev. 2011;20:2115-2126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 124] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 87. | Qureshi KM, Oliver RJ, Paget MB, Murray HE, Bailey CJ, Downing R. Human amniotic epithelial cells induce localized cell-mediated immune privilege in vitro: implications for pancreatic islet transplantation. Cell Transplant. 2011;20:523-534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 88. | Bailo M, Soncini M, Vertua E, Signoroni PB, Sanzone S, Lombardi G, Arienti D, Calamani F, Zatti D, Paul P. Engraftment potential of human amnion and chorion cells derived from term placenta. Transplantation. 2004;78:1439-1448. [RCA] [DOI] [Full Text] [Cited by in Crossref: 275] [Cited by in RCA: 253] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 89. | Rossi D, Pianta S, Magatti M, Sedlmayr P, Parolini O. Characterization of the conditioned medium from amniotic membrane cells: prostaglandins as key effectors of its immunomodulatory activity. PLoS One. 2012;7:e46956. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 92] [Cited by in RCA: 107] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 90. | Insausti CL, Blanquer M, García-Hernández AM, Castellanos G, Moraleda JM. Amniotic membrane-derived stem cells: immunomodulatory properties and potential clinical application. Stem Cells Cloning. 2014;7:53-63. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 64] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 91. | Garfias Y, Zaga-Clavellina V, Vadillo-Ortega F, Osorio M, Jimenez-Martinez MC. Amniotic membrane is an immunosuppressor of peripheral blood mononuclear cells. Immunol Invest. 2011;40:183-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 92. | Wolbank S, van Griensven M, Grillari-Voglauer R, Peterbauer-Scherb A. Alternative sources of adult stem cells: human amniotic membrane. Adv Biochem Eng Biotechnol. 2010;123:1-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 93. | Abumaree MH, Al Jumah MA, Kalionis B, Jawdat D, Al Khaldi A, AlTalabani AA, Knawy BA. Phenotypic and functional characterization of mesenchymal stem cells from chorionic villi of human term placenta. Stem Cell Rev. 2013;9:16-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 115] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 94. | Castrechini NM, Murthi P, Gude NM, Erwich JJ, Gronthos S, Zannettino A, Brennecke SP, Kalionis B. Mesenchymal stem cells in human placental chorionic villi reside in a vascular Niche. Placenta. 2010;31:203-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 112] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 95. | Wang J, Zhu Z, Huang Y, Wang P, Luo Y, Gao Y, Du Z. The subtype CD200-positive, chorionic mesenchymal stem cells from the placenta promote regeneration of human hepatocytes. Biotechnol Lett. 2014;36:1335-1341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 96. | Calzarossa C, Bossolasco P, Besana A, Manca MP, De Grada L, De Coppi P, Giardino D, Silani V, Cova L. Neurorescue effects and stem properties of chorionic villi and amniotic progenitor cells. Neuroscience. 2013;234:158-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 97. | Jones GN, Moschidou D, Abdulrazzak H, Kalirai BS, Vanleene M, Osatis S, Shefelbine SJ, Horwood NJ, Marenzana M, De Coppi P. Potential of human fetal chorionic stem cells for the treatment of osteogenesis imperfecta. Stem Cells Dev. 2014;23:262-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 98. | Lee MJ, Jung J, Na KH, Moon JS, Lee HJ, Kim JH, Kim GI, Kwon SW, Hwang SG, Kim GJ. Anti-fibrotic effect of chorionic plate-derived mesenchymal stem cells isolated from human placenta in a rat model of CCl(4)-injured liver: potential application to the treatment of hepatic diseases. J Cell Biochem. 2010;111:1453-1463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 104] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 99. | Jones GN, Moschidou D, Puga-Iglesias TI, Kuleszewicz K, Vanleene M, Shefelbine SJ, Bou-Gharios G, Fisk NM, David AL, De Coppi P. Ontological differences in first compared to third trimester human fetal placental chorionic stem cells. PLoS One. 2012;7:e43395. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 54] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 100. | Mitchell KE, Weiss ML, Mitchell BM, Martin P, Davis D, Morales L, Helwig B, Beerenstrauch M, Abou-Easa K, Hildreth T. Matrix cells from Wharton’s jelly form neurons and glia. Stem Cells. 2003;21:50-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 457] [Cited by in RCA: 445] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 101. | Romanov YA, Svintsitskaya VA, Smirnov VN. Searching for alternative sources of postnatal human mesenchymal stem cells: candidate MSC-like cells from umbilical cord. Stem Cells. 2003;21:105-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 641] [Cited by in RCA: 613] [Article Influence: 27.9] [Reference Citation Analysis (0)] |
| 102. | Dalous J, Larghero J, Baud O. Transplantation of umbilical cord-derived mesenchymal stem cells as a novel strategy to protect the central nervous system: technical aspects, preclinical studies, and clinical perspectives. Pediatr Res. 2012;71:482-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 61] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 103. | Secco M, Zucconi E, Vieira NM, Fogaça LL, Cerqueira A, Carvalho MD, Jazedje T, Okamoto OK, Muotri AR, Zatz M. Multipotent stem cells from umbilical cord: cord is richer than blood! Stem Cells. 2008;26:146-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 265] [Cited by in RCA: 259] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 104. | Hatlapatka T, Moretti P, Lavrentieva A, Hass R, Marquardt N, Jacobs R, Kasper C. Optimization of culture conditions for the expansion of umbilical cord-derived mesenchymal stem or stromal cell-like cells using xeno-free culture conditions. Tissue Eng Part C Methods. 2011;17:485-493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 54] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 105. | Tong CK, Vellasamy S, Tan BC, Abdullah M, Vidyadaran S, Seow HF, Ramasamy R. Generation of mesenchymal stem cell from human umbilical cord tissue using a combination enzymatic and mechanical disassociation method. Cell Biol Int. 2011;35:221-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 63] [Article Influence: 4.5] [Reference Citation Analysis (1)] |
| 106. | Weiss ML, Anderson C, Medicetty S, Seshareddy KB, Weiss RJ, VanderWerff I, Troyer D, McIntosh KR. Immune properties of human umbilical cord Wharton’s jelly-derived cells. Stem Cells. 2008;26:2865-2874. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 420] [Cited by in RCA: 426] [Article Influence: 25.1] [Reference Citation Analysis (1)] |
| 107. | Campard D, Lysy PA, Najimi M, Sokal EM. Native umbilical cord matrix stem cells express hepatic markers and differentiate into hepatocyte-like cells. Gastroenterology. 2008;134:833-848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 197] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 108. | Schneider RK, Puellen A, Kramann R, Raupach K, Bornemann J, Knuechel R, Pérez-Bouza A, Neuss S. The osteogenic differentiation of adult bone marrow and perinatal umbilical mesenchymal stem cells and matrix remodelling in three-dimensional collagen scaffolds. Biomaterials. 2010;31:467-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 163] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 109. | Wang L, Tran I, Seshareddy K, Weiss ML, Detamore MS. A comparison of human bone marrow-derived mesenchymal stem cells and human umbilical cord-derived mesenchymal stromal cells for cartilage tissue engineering. Tissue Eng Part A. 2009;15:2259-2266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 133] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 110. | Yang CC, Shih YH, Ko MH, Hsu SY, Cheng H, Fu YS. Transplantation of human umbilical mesenchymal stem cells from Wharton’s jelly after complete transection of the rat spinal cord. PLoS One. 2008;3:e3336. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 154] [Cited by in RCA: 170] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 111. | Pereira WC, Khushnooma I, Madkaikar M, Ghosh K. Reproducible methodology for the isolation of mesenchymal stem cells from human umbilical cord and its potential for cardiomyocyte generation. J Tissue Eng Regen Med. 2008;2:394-399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 58] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 112. | Wu KH, Chan CK, Tsai C, Chang YH, Sieber M, Chiu TH, Ho M, Peng CT, Wu HP, Huang JL. Effective treatment of severe steroid-resistant acute graft-versus-host disease with umbilical cord-derived mesenchymal stem cells. Transplantation. 2011;91:1412-1416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 101] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 113. | Tsai PC, Fu TW, Chen YM, Ko TL, Chen TH, Shih YH, Hung SC, Fu YS. The therapeutic potential of human umbilical mesenchymal stem cells from Wharton’s jelly in the treatment of rat liver fibrosis. Liver Transpl. 2009;15:484-495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 149] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 114. | Xu J, Wang D, Liu D, Fan Z, Zhang H, Liu O, Ding G, Gao R, Zhang C, Ding Y. Allogeneic mesenchymal stem cell treatment alleviates experimental and clinical Sjögren syndrome. Blood. 2012;120:3142-3151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 222] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 115. | Wang D, Li J, Zhang Y, Zhang M, Chen J, Li X, Hu X, Jiang S, Shi S, Sun L. Umbilical cord mesenchymal stem cell transplantation in active and refractory systemic lupus erythematosus: a multicenter clinical study. Arthritis Res Ther. 2014;16:R79. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 193] [Cited by in RCA: 233] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 116. | Chen M, Wang X, Ye Z, Zhang Y, Zhou Y, Tan WS. A modular approach to the engineering of a centimeter-sized bone tissue construct with human amniotic mesenchymal stem cells-laden microcarriers. Biomaterials. 2011;32:7532-7542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 83] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 117. | Diao Y, Ma Q, Cui F, Zhong Y. Human umbilical cord mesenchymal stem cells: osteogenesis in vivo as seed cells for bone tissue engineering. J Biomed Mater Res A. 2009;91:123-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 56] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 118. | Peister A, Deutsch ER, Kolambkar Y, Hutmacher DW, Guldberg RE. Amniotic fluid stem cells produce robust mineral deposits on biodegradable scaffolds. Tissue Eng Part A. 2009;15:3129-3138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 51] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 119. | Steigman SA, Ahmed A, Shanti RM, Tuan RS, Valim C, Fauza DO. Sternal repair with bone grafts engineered from amniotic mesenchymal stem cells. J Pediatr Surg. 2009;44:1120-1126; discussion 1126. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 47] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 120. | Kouroupis D, Churchman SM, English A, Emery P, Giannoudis PV, McGonagle D, Jones EA. Assessment of umbilical cord tissue as a source of mesenchymal stem cell/endothelial cell mixtures for bone regeneration. Regen Med. 2013;8:569-581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 121. | Peister A, Woodruff MA, Prince JJ, Gray DP, Hutmacher DW, Guldberg RE. Cell sourcing for bone tissue engineering: amniotic fluid stem cells have a delayed, robust differentiation compared to mesenchymal stem cells. Stem Cell Res. 2011;7:17-27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 34] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 122. | Vidal MA, Walker NJ, Napoli E, Borjesson DL. Evaluation of senescence in mesenchymal stem cells isolated from equine bone marrow, adipose tissue, and umbilical cord tissue. Stem Cells Dev. 2012;21:273-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 122] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 123. | Chen W, Liu J, Manuchehrabadi N, Weir MD, Zhu Z, Xu HH. Umbilical cord and bone marrow mesenchymal stem cell seeding on macroporous calcium phosphate for bone regeneration in rat cranial defects. Biomaterials. 2013;34:9917-9925. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 121] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 124. | Liu G, Li Y, Sun J, Zhou H, Zhang W, Cui L, Cao Y. In vitro and in vivo evaluation of osteogenesis of human umbilical cord blood-derived mesenchymal stem cells on partially demineralized bone matrix. Tissue Eng Part A. 2010;16:971-982. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 44] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 125. | Barboni B, Mangano C, Valbonetti L, Marruchella G, Berardinelli P, Martelli A, Muttini A, Mauro A, Bedini R, Turriani M. Synthetic bone substitute engineered with amniotic epithelial cells enhances bone regeneration after maxillary sinus augmentation. PLoS One. 2013;8:e63256. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 52] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 126. | Berardinelli P, Valbonetti L, Muttini A, Martelli A, Peli R, Zizzari V, Nardinocchi D, Vulpiani MP, Tetè S, Barboni B. Role of amniotic fluid mesenchymal cells engineered on MgHA/collagen-based scaffold allotransplanted on an experimental animal study of sinus augmentation. Clin Oral Investig. 2013;17:1661-1675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 127. | Riccio M, Maraldi T, Pisciotta A, La Sala GB, Ferrari A, Bruzzesi G, Motta A, Migliaresi C, De Pol A. Fibroin scaffold repairs critical-size bone defects in vivo supported by human amniotic fluid and dental pulp stem cells. Tissue Eng Part A. 2012;18:1006-1013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 83] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 128. | Maraldi T, Riccio M, Pisciotta A, Zavatti M, Carnevale G, Beretti F, La Sala GB, Motta A, De Pol A. Human amniotic fluid-derived and dental pulp-derived stem cells seeded into collagen scaffold repair critical-size bone defects promoting vascularization. Stem Cell Res Ther. 2013;4:53. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 70] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 129. | Turner CG, Klein JD, Gray FL, Ahmed A, Zurakowski D, Fauza DO. Craniofacial repair with fetal bone grafts engineered from amniotic mesenchymal stem cells. J Surg Res. 2012;178:785-790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |