Published online Nov 26, 2014. doi: 10.4252/wjsc.v6.i5.571
Revised: September 11, 2014
Accepted: September 16, 2014
Published online: November 26, 2014
Processing time: 46 Days and 2.2 Hours
Intercellular communication via gap junctions allows cells within multicellular organisms to share small molecules. The effect of such interactions has been elucidated using mouse gene knockout strategies. Although several mutations in human gap junction-encoding connexin (Cx) have been described, Cx mutants in mice do not always recapitulate the human disease. Among the 20 mouse Cxs, Cx26, Cx43, and Cx45 play roles in early cardiac or placental development, and disruption of the genes results in lethality that hampers further analyses. Embryonic stem cells (ESCs) that lack Cx43 or Cx45 have made analysis feasible in both in vitro differentiated cell cultures and in vivo chimeric tissues. The success of mouse ESCs studies is leading to the use of induced pluripotent stem cells to learn more about the pathogenesis of human Cx diseases. This review summarizes the current status of mouse Cx disruption models and ESC differentiation studies, and discusses their implication for understanding human Cx diseases.
Core tip: Numerous gap junction-encoding connexin (Cx) mutant mice have been established as models of human diseases. Although these analyses have facilitated current understanding of native Cx functions and the pathogenesis of related diseases, care must be taken when extrapolating findings from mice to humans, and vice versa, because there can be striking diversity in tissue organization and Cx expression patterns between these species. Recently, the use of human induced pluripotent stem cells (iPSCs) allowed further direct approaches for studying human diseases. According to the studies using mutant mouse embryonic stem cells, Cx mutant human iPSCs may become a useful model.
- Citation: Nishii K, Shibata Y, Kobayashi Y. Connexin mutant embryonic stem cells and human diseases. World J Stem Cells 2014; 6(5): 571-578
- URL: https://www.wjgnet.com/1948-0210/full/v6/i5/571.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v6.i5.571
Gap junctions consist of arrays of intercellular channels between adjacent cells. The channels are formed by the head-to-head docking of hexameric hemichannels called connexons, whose subunit proteins are encoded by the connexin (Cx) gene family in mammals (Table 1)[1,2]. Most cell types communicate with each other via gap junctions, which require cell-cell contacts, to maintain their homeostasis. This is likely a critical mode of communication in multicellular animals because Cx expression is highly conserved. In contrast, intercellular communication is performed via membrane-lined channels called plasmodesmata in plants and fungi[3]. Unique combinations of Cx isoforms are expressed in each animal tissue, thereby regulating cell-type specific homeostasis[4]. A classical experiment revealed that trophoblasts in the blastocyst are linked by gap junctions to other trophoblasts, as well as to cells in the inner cell mass, in preimplantation embryos; those cells that are linked by gap junctions to both trophoblasts and cells in the inner cell mass cells probably form the polar trophectoderm[5,6]. However, shortly after implantation the intercellular communication between trophoblasts and inner cell mass cells is lost[7]. Another typical example occurs in the cardiac conduction system. In ventricular cardiac myocytes (CM), Cx43 is the main gap junction protein, whereas Cx40 expression predominates within the core conduction system. Although Cx43 and Cx40 both have high conductance, Cx45 forms low conductance and voltage-sensitive gap junctions between the ventricular CM and the core conduction system[1,8,9]. It is believed that this expression pattern effectively insulates the conduction system while also maintaining proper contacts between the conductive and ventricular CM.
| Mouse gene[1] | Mouse KO phenotypes | Human disease | Ref. |
| Cx231 | |||
| Cx26 | Embryonic lethality due to defective transplacental glucose uptake | Deafness; Skin disease | [14,68-70] |
| Cx29 | No phenotype | [71] | |
| Cx30 | Hearing impairment; accelerated heart rate | Deafness; Skin disease | [31,68,72] |
| Cx30.2 | Accelerated atrioventricular nodal conduction | [73] | |
| Cx30.3 | Difference in behavioral reactivity to vanilla scent | Skin disease | [69,74] |
| Cx312 | Partial embryonic lethality due to a defect in early placental development | Deafness; Skin disease | [15,68,69,75] |
| Cx31.12 | Partial embryonic lethality due to impaired placental development; Changed gene expression in the central nervous system | [16,76] | |
| Cx32 | Liver dysfunction; High incidence of liver tumors; Peripheral neuropathy | Charcot-Marie-Tooth disease | [36-38,40,77,78] |
| Cx331 | |||
| Cx36 | Loss of electrical coupling in interneurons of the neocortex; Disrupted rod pathways; Altered spontaneous firing patterns in the retina; Alterations in insulin secretion | Juvenile myoclonic epilepsy | [25,79-84] |
| Cx37 | Female infertility; High bone mass | [11,85] | |
| Cx39 | Accelerated myogenesis and regeneration of skeletal muscle | [86] | |
| Cx40 | Cardiac conduction abnormalities; High incidence of cardiac malformations | Atrial standstill; Atrial fibrillation | [23,42,43,45,87,88] |
| Cx43 | Early postnatal lethality due to cardiac malformation; Osteoblast dysfunction | Oculodentodigital dysplasia; Visceroatrial heterotaxia; Hypoplastic left heart syndrome; Atrial fibrillation | [10,52,55,67,89-94] |
| Cx45 | Embryonic lethality due to cardiovascular defects; Altered spontaneous firing patterns in the retina | [12,25,44,53] | |
| Cx46 | Cataracts; Reduced heart rate and aberrant conduction along the His bundle branches | Cataract | [17,33,69] |
| Cx47 | Myelin abnormalities | Pelizaeus–Merzbacher-like disease | [24,95-98] |
| Cx50 | Microphthalmia and cataract | Cataract | [18,19,69] |
| Cx57 | Reduction in horizontal cell receptive fields | [99,100] |
Approximately 20 Cx isoforms have been reported in mice and humans[1]. They are expressed in most tissues at varying levels and stoichiometry. One gap junction is composed of two hexameric connexons: 12 Cxs form a single channel. Many types of Cxs can be assembled into one connexon[4]. Because a single cell usually expresses multiple Cx isoforms, theoretically there can be a plethora of different gap junction channels between cells, each with unique properties. Recent in vivo studies elucidated how the expression of a multitude of Cxs results in specific biological functions using mouse mutagenesis, as well as the molecular cloning of Cx mutations related to human diseases.
Cx gene knockout (KO) strategies in mice were first applied to Cx43 by Reaume et al[10] in 1995. Subsequently, mouse mutants have been reported for all of the Cxs, except for Cx23 and Cx33 (Table 1). Some Cx-KO strains show specific abnormalities. For example, Cx37 forms a unique gap junction between oocytes and granulosa cells in mice. Accordingly, Cx37-KO mice show impaired oocyte development and female infertility[11]. Cx45 is thought to confer unique characteristics on peristaltic contractions in the early developing heart. Therefore, Cx45-KO embryos show lethality that is caused by a conduction block in early cardiogenesis[12,13]. The placenta is dependent on Cx26, Cx31, and Cx31.1, and each KO strain shows placental dysmorphogenesis[14-16]. Similarly, the lens epithelium co-expresses Cx46 and Cx50, and both Cx46-KO and Cx50-KO mice experience cataracts[17-19]. Cx46 and Cx50 have a redundant role in lens development, but individual roles in overall growth. Specifically, the targeted replacement of Cx50 with Cx46 prevented cataracts, but did not restore microphthalmia, which was apparent only in the Cx50-KO mice[20]. Thus, a specific individual Cx does not seem to possess one-to-one association with a unique cell type in vivo. Instead, most cells express multiple Cxs to maintain intercellular communication. This might partly explain why the lack of two Cxs results in phenotypes that were not present in each individual KO[21-29]. In the heart, CMs express Cx30, Cx30.2, Cx40, Cx43, Cx45, and Cx46, and their expression is regulated both temporally and regionally[30-33]. Each Cx-KO strain exhibits developmental and electrophysiological abnormalities that are closely related to their expression patterns and channel properties (Table 1). As a result, three Cx-KO strains are shown to be lethal: Cx26-KO mice with defective transplacental glucose uptake, Cx43-KO mice with cardiac malformation, and Cx45-KO mice with blocked conduction in early cardiogenesis. Because these constitutive KO mice are embryonically lethal, other approaches are required to obtain insights into the role of these Cxs in adult tissues.
Mouse Cx mutants do not always exhibit the same phenotype as would be expected from human Cx diseases (Table 1). The most divergent one is probably that of the placenta, whose structure is highly variable among mammalian species. The mouse feto-maternal barrier consists of three trophoblast layers (two syncytiotrophoblastic and one cytotrophoblastic layers), whereas that of humans has two (one syncytiotrophoblastic and one cytotrophoblastic layer). Moreover, the Cx isoforms expressed in the placenta differ among species[34]. These structural and expression differences are probably a reason why placental defects are prevalent in Cx mutant mice. Accordingly, KO of the human deafness and skin disease-associated genes Cx26 and Cx31, together with Cx31.1, which is not a known human disease-related gene, causes placental dysfunction. Because of the striking diversity in Cx expression in placental structures, care must be taken when extrapolating findings from one species to another. The lethality of Cx26-KO mice was overcome using Cre/loxP technology to create tissue-specific Cx26-KO mice. For example, knocking out Cx26 in the mouse inner ear epithelium caused cell death in the cochlear epithelial network and sensory hair cells, which greatly enhanced our understanding of the pathogenesis of deafness[35].
Cx37-KO mice show complete female infertility[11]. Although this finding provides an important insight into oogenesis, no human diseases that cause female infertility have been linked to Cx37. Cx32 is the causative gene of human X-linked Charcot-Marie-Tooth disease[36,37]. Although Cx32-KO mice exhibit peripheral neuropathy similar to that observed with the abovementioned disease, they also show liver dysfunction, which has not been described in humans[38-40]. Generally, interspecies differences in Cx expression and organogenesis make loss-of-function phenotypes somewhat divergent. In addition, minor phenotypes in Cx-KO mice might not yet have been described as symptoms of human diseases.
In contrast, the major spatio-temporal expression patterns of Cxs in the heart appear to be relatively conserved among mammalian species[9]. A detailed comparison of the expression of Cx40, Cx43, and Cx45 in developing mouse and human hearts indicated that their expression paralleled one another[41]. Although no null mutations have been reported in human Cx40, Cx43, and Cx45, the loss of Cx40 blocked atrioventricular conduction and caused a high incidence of cardiac malformations in mice. Cx43-KO mice exhibited neonatal lethality due to cardiac malformation; Cx45-KO mice experienced a lethal conduction block in early cardiogenesis[10,12,23,42-45]. It is possible that null mutations in human Cx40, Cx43, and Cx45 exist, but that the development of the fetus could be aborted. However, several missense mutations in Cx40 and Cx43 have been described in human heart diseases, and attempts have been made to create mice with the Cx43 missense mutations related to oculodentodigital dysplasia in humans (Table 1)[46,47]. In addition to CM with missense mutations, adult mice with Cx-KOs are required to understand why or how Cx30, Cx30.2, Cx40, Cx43, Cx45, and Cx46 are expressed differentially in the heart and also to extrapolate human Cx functions from mouse studies. Adult CM cannot be obtained from lethal Cx43-KO and Cx45-KO mice. Therefore, attempts have been made to mutate a unique Cx isoform in a tissue-specific manner.
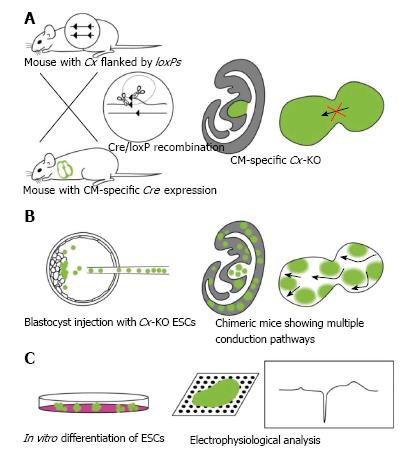
A widely accepted approach to circumvent the lethality of constitutive KOs is the tissue-specific deletion of a gene using Cre/loxP technology (Figure 1). In this method, the target gene is flanked by loxP sequences, and the tissue-specific expression of Cre recombinase deletes the gene of interest. The embryonic lethal genes Cx26, Cx43, and Cx45 have all been analyzed using this method. They were all deleted specifically in adult tissues, for example in the inner ear epithelium, CM, and neurons[13,35,48-51].
The use of ESCs lacking Cx43 or Cx45 has advantages in addition to those afforded by Cre/loxP technology (Figure 1)[52,53]. The CM-specific deletion of Cx43 slowed conduction and caused sudden arrhythmic death[49]. Similarly, the CM-specific deletion of Cx45 was embryonic lethal, similar to constitutive Cx45-KO mice[13]. In both these examples, Cre recombinase was used to delete the genes in most of the CM. Because Cx is a gap junction protein, understanding what happens at the borders between Cx-positive and -negative cells has been of great interest. Chimeric mice, which are formed from mutant ESCs and recipient blastocysts, allow these experiments to be performed. Mouse ESCs express Cx31, Cx43, and Cx45 proteins[54]. Cx43-KO ESCs were used to form chimeric tissues with wild-type cells, and the chimeric heart showed conduction defects and diminished cardiac performance[52]. This study supports the concept that tissue mosaicism in different Cx isoforms might be responsible for reentrant arrhythmias. Indeed, in humans, atrial tissue genetic mosaicism in a loss-of-function Cx43 mutation was reported to be associated with sporadic lone atrial fibrillation[55]. Cx43 chimeric mice form a model of atrial fibrillation, which might facilitate the development of therapeutic approaches for modifying the function of cardiac gap junctions.
Research using ESCs that lack Cx45 developed very differently from those lacking Cx43. Cx45-KO ESCs cannot be integrated into chimeras, because they never mix with the inner cell mass of the recipient[53]. Innate Cx45 is expressed abundantly in early embryos, suggesting that it might play a role in cell adhesiveness during early development. Irrespective of their incompatibility with chimera production, Cx45-KO ESCs differentiate into the three germ layers in vitro. CMs induced from Cx45-KO ESCs showed conduction abnormalities[53]. Constitutive Cx45-KO mice were reported initially by two laboratories independently[12,44]. One group reported heart abnormalities, whereas the other focused on vascular abnormalities. Later, as described above, the CM-specific Cx45-KO mice were shown to be similar to the constitutive Cx45-KO mice[13]. Taken together, the heart abnormalities are expected to be the primary defect associated with the loss of Cx45 in developing embryos.
Induced pluripotent stem cells (iPSCs) have similar potential to ESCs, and can differentiate into many cell types including germ cells[56,57]. Importantly, iPSCs can be derived from adult somatic cells, including from individuals with genetic diseases[58]. Human iPSCs from patients might provide unlimited supplies of specific tissues, and the use of human cells is more important than creating mouse genetic models for the understanding of human diseases[59]. Theoretically, chimeric human tissue formed from diseased and normal iPSCs could be generated in vitro. As studies performed using mouse ESCs indicate, this approach might be particularly useful for studying human junction proteins including Cxs. Even minor tissues such as endocrine cells can be supplied in unlimited amounts in rare diseases, and biological specimens of uniform quality will improve reproducibility greatly, which is often problematic in human studies. The future of iPSC technology also seems very promising in mouse studies because iPSCs can be derived from many mouse genetic models. For example, attempts have been made to improve disease conditions by the transplantation of tissues differentiated in vitro. The transplanted tissues were derived from autologous iPSCs in which the specific genetic disorder had been corrected[60]. Although establishing iPSCs with multiple targeted mutations might require breeding different mutant mice, this is likely far easier than performing multiple gene targeting using ESCs. Therefore, the use of iPSCs might allow the unique and redundant contributions of Cxs in intercellular communication to be elucidated further.
Cx mutant mouse strategies have revealed detailed in vivo functions of intercellular communication carried out by individual Cx species. The use of Cx mutant ESCs and iPSCs has additional advantages. Especially, iPSCs can be obtained from individuals with genetic diseases. Analysis of chimeric and in vitro differentiated tissues is useful for understanding the molecular target in human Cx diseases. To date, some reagents are known to modulate gap junctional intercellular communication and are used in clinical trials for the treatment of wound, arrhythmia, migraine, and cancer[61-66]. Reproducibility in the stem cell-based experimental systems will be a great advantage for the development of such therapeutic drugs.
P- Reviewer: Guo ZK, Tanaka T, Zaminy A S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ
| 1. | Söhl G, Willecke K. Gap junctions and the connexin protein family. Cardiovasc Res. 2004;62:228-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 675] [Cited by in RCA: 710] [Article Influence: 33.8] [Reference Citation Analysis (0)] |
| 2. | Goodenough DA, Paul DL. Gap junctions. Cold Spring Harb Perspect Biol. 2009;1:a002576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 379] [Cited by in RCA: 454] [Article Influence: 30.3] [Reference Citation Analysis (0)] |
| 3. | Panchin YV. Evolution of gap junction proteins--the pannexin alternative. J Exp Biol. 2005;208:1415-1419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 148] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 4. | White TW, Paul DL. Genetic diseases and gene knockouts reveal diverse connexin functions. Annu Rev Physiol. 1999;61:283-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 305] [Cited by in RCA: 282] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 5. | Gardner RL. Contributions of blastocyst micromanipulation to the study of mammalian development. Bioessays. 1998;20:168-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 6. | Lo CW, Gilula NB. Gap junctional communication in the preimplantation mouse embryo. Cell. 1979;18:399-409. [PubMed] |
| 7. | Lo CW, Gilula NB. Gap junctional communication in the post-implantation mouse embryo. Cell. 1979;18:411-422. [PubMed] |
| 8. | Coppen SR, Severs NJ, Gourdie RG. Connexin45 (alpha 6) expression delineates an extended conduction system in the embryonic and mature rodent heart. Dev Genet. 1999;24:82-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 9. | Giovannone S, Remo BF, Fishman GI. Channeling diversity: gap junction expression in the heart. Heart Rhythm. 2012;9:1159-1162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 10. | Reaume AG, de Sousa PA, Kulkarni S, Langille BL, Zhu D, Davies TC, Juneja SC, Kidder GM, Rossant J. Cardiac malformation in neonatal mice lacking connexin43. Science. 1995;267:1831-1834. [PubMed] |
| 11. | Simon AM, Goodenough DA, Li E, Paul DL. Female infertility in mice lacking connexin 37. Nature. 1997;385:525-529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 528] [Cited by in RCA: 484] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 12. | Kumai M, Nishii K, Nakamura K, Takeda N, Suzuki M, Shibata Y. Loss of connexin45 causes a cushion defect in early cardiogenesis. Development. 2000;127:3501-3512. [PubMed] |
| 13. | Nishii K, Kumai M, Egashira K, Miwa T, Hashizume K, Miyano Y, Shibata Y. Mice lacking connexin45 conditionally in cardiac myocytes display embryonic lethality similar to that of germline knockout mice without endocardial cushion defect. Cell Commun Adhes. 2003;10:365-369. [PubMed] |
| 14. | Gabriel HD, Jung D, Bützler C, Temme A, Traub O, Winterhager E, Willecke K. Transplacental uptake of glucose is decreased in embryonic lethal connexin26-deficient mice. J Cell Biol. 1998;140:1453-1461. [PubMed] |
| 15. | Plum A, Winterhager E, Pesch J, Lautermann J, Hallas G, Rosentreter B, Traub O, Herberhold C, Willecke K. Connexin31-deficiency in mice causes transient placental dysmorphogenesis but does not impair hearing and skin differentiation. Dev Biol. 2001;231:334-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 99] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 16. | Zheng-Fischhöfer Q, Kibschull M, Schnichels M, Kretz M, Petrasch-Parwez E, Strotmann J, Reucher H, Lynn BD, Nagy JI, Lye SJ. Characterization of connexin31.1-deficient mice reveals impaired placental development. Dev Biol. 2007;312:258-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 39] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 17. | Gong X, Li E, Klier G, Huang Q, Wu Y, Lei H, Kumar NM, Horwitz J, Gilula NB. Disruption of alpha3 connexin gene leads to proteolysis and cataractogenesis in mice. Cell. 1997;91:833-843. [PubMed] |
| 18. | Rong P, Wang X, Niesman I, Wu Y, Benedetti LE, Dunia I, Levy E, Gong X. Disruption of Gja8 (alpha8 connexin) in mice leads to microphthalmia associated with retardation of lens growth and lens fiber maturation. Development. 2002;129:167-174. [PubMed] |
| 19. | White TW, Goodenough DA, Paul DL. Targeted ablation of connexin50 in mice results in microphthalmia and zonular pulverulent cataracts. J Cell Biol. 1998;143:815-825. [PubMed] |
| 20. | White TW. Unique and redundant connexin contributions to lens development. Science. 2002;295:319-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 163] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 21. | Schrickel JW, Kreuzberg MM, Ghanem A, Kim JS, Linhart M, Andrié R, Tiemann K, Nickenig G, Lewalter T, Willecke K. Normal impulse propagation in the atrioventricular conduction system of Cx30.2/Cx40 double deficient mice. J Mol Cell Cardiol. 2009;46:644-652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 22. | Simon AM, McWhorter AR, Dones JA, Jackson CL, Chen H. Heart and head defects in mice lacking pairs of connexins. Dev Biol. 2004;265:369-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 23. | Simon AM, Goodenough DA, Paul DL. Mice lacking connexin40 have cardiac conduction abnormalities characteristic of atrioventricular block and bundle branch block. Curr Biol. 1998;8:295-298. [PubMed] |
| 24. | Menichella DM, Goodenough DA, Sirkowski E, Scherer SS, Paul DL. Connexins are critical for normal myelination in the CNS. J Neurosci. 2003;23:5963-5973. [PubMed] |
| 25. | Blankenship AG, Hamby AM, Firl A, Vyas S, Maxeiner S, Willecke K, Feller MB. The role of neuronal connexins 36 and 45 in shaping spontaneous firing patterns in the developing retina. J Neurosci. 2011;31:9998-10008. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 47] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 26. | Xia CH, Cheng C, Huang Q, Cheung D, Li L, Dunia I, Benedetti LE, Horwitz J, Gong X. Absence of alpha3 (Cx46) and alpha8 (Cx50) connexins leads to cataracts by affecting lens inner fiber cells. Exp Eye Res. 2006;83:688-696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 47] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 27. | Kanady JD, Dellinger MT, Munger SJ, Witte MH, Simon AM. Connexin37 and Connexin43 deficiencies in mice disrupt lymphatic valve development and result in lymphatic disorders including lymphedema and chylothorax. Dev Biol. 2011;354:253-266. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 155] [Cited by in RCA: 146] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 28. | Koval M, Billaud M, Straub AC, Johnstone SR, Zarbock A, Duling BR, Isakson BE. Spontaneous lung dysfunction and fibrosis in mice lacking connexin 40 and endothelial cell connexin 43. Am J Pathol. 2011;178:2536-2546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 39] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 29. | Simon AM, McWhorter AR. Vascular abnormalities in mice lacking the endothelial gap junction proteins connexin37 and connexin40. Dev Biol. 2002;251:206-220. [PubMed] |
| 30. | Nishii K, Kumai M, Shibata Y. Regulation of the epithelial-mesenchymal transformation through gap junction channels in heart development. Trends Cardiovasc Med. 2001;11:213-218. [PubMed] [DOI] [Full Text] |
| 31. | Gros D, Théveniau-Ruissy M, Bernard M, Calmels T, Kober F, Söhl G, Willecke K, Nargeot J, Jongsma HJ, Mangoni ME. Connexin 30 is expressed in the mouse sino-atrial node and modulates heart rate. Cardiovasc Res. 2010;85:45-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 27] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 32. | Kreuzberg MM, Söhl G, Kim JS, Verselis VK, Willecke K, Bukauskas FF. Functional properties of mouse connexin30.2 expressed in the conduction system of the heart. Circ Res. 2005;96:1169-1177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 99] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 33. | Chi NC, Bussen M, Brand-Arzamendi K, Ding C, Olgin JE, Shaw RM, Martin GR, Stainier DY. Cardiac conduction is required to preserve cardiac chamber morphology. Proc Natl Acad Sci USA. 2010;107:14662-14667. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 88] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 34. | Malassiné A, Cronier L. Involvement of gap junctions in placental functions and development. Biochim Biophys Acta. 2005;1719:117-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 42] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 35. | Cohen-Salmon M, Ott T, Michel V, Hardelin JP, Perfettini I, Eybalin M, Wu T, Marcus DC, Wangemann P, Willecke K. Targeted ablation of connexin26 in the inner ear epithelial gap junction network causes hearing impairment and cell death. Curr Biol. 2002;12:1106-1111. [PubMed] |
| 36. | Bruzzone R, White TW, Scherer SS, Fischbeck KH, Paul DL. Null mutations of connexin32 in patients with X-linked Charcot-Marie-Tooth disease. Neuron. 1994;13:1253-1260. [PubMed] |
| 37. | Fairweather N, Bell C, Cochrane S, Chelly J, Wang S, Mostacciuolo ML, Monaco AP, Haites NE. Mutations in the connexin 32 gene in X-linked dominant Charcot-Marie-Tooth disease (CMTX1). Hum Mol Genet. 1994;3:29-34. [PubMed] |
| 38. | Anzini P, Neuberg DH, Schachner M, Nelles E, Willecke K, Zielasek J, Toyka KV, Suter U, Martini R. Structural abnormalities and deficient maintenance of peripheral nerve myelin in mice lacking the gap junction protein connexin 32. J Neurosci. 1997;17:4545-4551. [PubMed] |
| 39. | Scherer SS, Xu YT, Nelles E, Fischbeck K, Willecke K, Bone LJ. Connexin32-null mice develop demyelinating peripheral neuropathy. Glia. 1998;24:8-20. [PubMed] |
| 40. | Willecke K, Temme A, Teubner B, Ott T. Characterization of targeted connexin32-deficient mice: a model for the human Charcot-Marie-Tooth (X-type) inherited disease. Ann N Y Acad Sci. 1999;883:302-309. [PubMed] |
| 41. | Coppen SR, Kaba RA, Halliday D, Dupont E, Skepper JN, Elneil S, Severs NJ. Comparison of connexin expression patterns in the developing mouse heart and human foetal heart. Mol Cell Biochem. 2003;242:121-127. [PubMed] |
| 42. | Gu H, Smith FC, Taffet SM, Delmar M. High incidence of cardiac malformations in connexin40-deficient mice. Circ Res. 2003;93:201-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 94] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 43. | Kirchhoff S, Nelles E, Hagendorff A, Krüger O, Traub O, Willecke K. Reduced cardiac conduction velocity and predisposition to arrhythmias in connexin40-deficient mice. Curr Biol. 1998;8:299-302. [PubMed] |
| 44. | Krüger O, Plum A, Kim JS, Winterhager E, Maxeiner S, Hallas G, Kirchhoff S, Traub O, Lamers WH, Willecke K. Defective vascular development in connexin 45-deficient mice. Development. 2000;127:4179-4193. [PubMed] |
| 45. | Sankova B, Benes J, Krejci E, Dupays L, Theveniau-Ruissy M, Miquerol L, Sedmera D. The effect of connexin40 deficiency on ventricular conduction system function during development. Cardiovasc Res. 2012;95:469-479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 46. | Dobrowolski R, Sasse P, Schrickel JW, Watkins M, Kim JS, Rackauskas M, Troatz C, Ghanem A, Tiemann K, Degen J. The conditional connexin43G138R mouse mutant represents a new model of hereditary oculodentodigital dysplasia in humans. Hum Mol Genet. 2008;17:539-554. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 146] [Cited by in RCA: 139] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 47. | Flenniken AM, Osborne LR, Anderson N, Ciliberti N, Fleming C, Gittens JE, Gong XQ, Kelsey LB, Lounsbury C, Moreno L. A Gja1 missense mutation in a mouse model of oculodentodigital dysplasia. Development. 2005;132:4375-4386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 187] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 48. | Eckardt D, Theis M, Degen J, Ott T, van Rijen HV, Kirchhoff S, Kim JS, de Bakker JM, Willecke K. Functional role of connexin43 gap junction channels in adult mouse heart assessed by inducible gene deletion. J Mol Cell Cardiol. 2004;36:101-110. [PubMed] |
| 49. | Gutstein DE, Morley GE, Tamaddon H, Vaidya D, Schneider MD, Chen J, Chien KR, Stuhlmann H, Fishman GI. Conduction slowing and sudden arrhythmic death in mice with cardiac-restricted inactivation of connexin43. Circ Res. 2001;88:333-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 436] [Cited by in RCA: 466] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 50. | Maxeiner S, Dedek K, Janssen-Bienhold U, Ammermüller J, Brune H, Kirsch T, Pieper M, Degen J, Krüger O, Willecke K. Deletion of connexin45 in mouse retinal neurons disrupts the rod/cone signaling pathway between AII amacrine and ON cone bipolar cells and leads to impaired visual transmission. J Neurosci. 2005;25:566-576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 124] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 51. | van Rijen HV, Eckardt D, Degen J, Theis M, Ott T, Willecke K, Jongsma HJ, Opthof T, de Bakker JM. Slow conduction and enhanced anisotropy increase the propensity for ventricular tachyarrhythmias in adult mice with induced deletion of connexin43. Circulation. 2004;109:1048-1055. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 228] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 52. | Gutstein DE, Morley GE, Vaidya D, Liu F, Chen FL, Stuhlmann H, Fishman GI. Heterogeneous expression of Gap junction channels in the heart leads to conduction defects and ventricular dysfunction. Circulation. 2001;104:1194-1199. [PubMed] |
| 53. | Egashira K, Nishii K, Nakamura K, Kumai M, Morimoto S, Shibata Y. Conduction abnormality in gap junction protein connexin45-deficient embryonic stem cell-derived cardiac myocytes. Anat Rec A Discov Mol Cell Evol Biol. 2004;280:973-979. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 54. | Wörsdörfer P, Maxeiner S, Markopoulos C, Kirfel G, Wulf V, Auth T, Urschel S, von Maltzahn J, Willecke K. Connexin expression and functional analysis of gap junctional communication in mouse embryonic stem cells. Stem Cells. 2008;26:431-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 59] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 55. | Thibodeau IL, Xu J, Li Q, Liu G, Lam K, Veinot JP, Birnie DH, Jones DL, Krahn AD, Lemery R. Paradigm of genetic mosaicism and lone atrial fibrillation: physiological characterization of a connexin 43-deletion mutant identified from atrial tissue. Circulation. 2010;122:236-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 124] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 56. | Okita K, Ichisaka T, Yamanaka S. Generation of germline-competent induced pluripotent stem cells. Nature. 2007;448:313-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3332] [Cited by in RCA: 3078] [Article Influence: 171.0] [Reference Citation Analysis (0)] |
| 57. | Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17989] [Cited by in RCA: 18142] [Article Influence: 954.8] [Reference Citation Analysis (0)] |
| 58. | Nagata N, Yamanaka S. Perspectives for induced pluripotent stem cell technology: new insights into human physiology involved in somatic mosaicism. Circ Res. 2014;114:505-510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 59. | Yamanaka S. Induced pluripotent stem cells: past, present, and future. Cell Stem Cell. 2012;10:678-684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 543] [Cited by in RCA: 527] [Article Influence: 40.5] [Reference Citation Analysis (0)] |
| 60. | Hanna J, Wernig M, Markoulaki S, Sun CW, Meissner A, Cassady JP, Beard C, Brambrink T, Wu LC, Townes TM. Treatment of sickle cell anemia mouse model with iPS cells generated from autologous skin. Science. 2007;318:1920-1923. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1104] [Cited by in RCA: 995] [Article Influence: 55.3] [Reference Citation Analysis (0)] |
| 61. | Chin KY. Connexins, a new target in wound treatment. J Wound Care. 2011;20:386-390. [PubMed] |
| 62. | De Vuyst E, Boengler K, Antoons G, Sipido KR, Schulz R, Leybaert L. Pharmacological modulation of connexin-formed channels in cardiac pathophysiology. Br J Pharmacol. 2011;163:469-483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 66] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 63. | Goadsby PJ. Emerging therapies for migraine. Nat Clin Pract Neurol. 2007;3:610-619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 40] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 64. | Grek CL, Rhett JM, Ghatnekar GS. Cardiac to cancer: connecting connexins to clinical opportunity. FEBS Lett. 2014;588:1349-1364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 39] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 65. | Knollmann BC, Roden DM. A genetic framework for improving arrhythmia therapy. Nature. 2008;451:929-936. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 59] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 66. | Murray KT, Mace LC, Yang Z. Nonantiarrhythmic drug therapy for atrial fibrillation. Heart Rhythm. 2007;4:S88-S90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 37] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 67. | Fahrenbach JP, Ai X, Banach K. Decreased intercellular coupling improves the function of cardiac pacemakers derived from mouse embryonic stem cells. J Mol Cell Cardiol. 2008;45:642-649. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 68. | Leibovici M, Safieddine S, Petit C. Mouse models for human hereditary deafness. Curr Top Dev Biol. 2008;84:385-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 46] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 69. | Gerido DA, White TW. Connexin disorders of the ear, skin, and lens. Biochim Biophys Acta. 2004;1662:159-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 113] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 70. | Xu J, Nicholson BJ. The role of connexins in ear and skin physiology - functional insights from disease-associated mutations. Biochim Biophys Acta. 2013;1828:167-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 101] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 71. | Eiberger J, Kibschull M, Strenzke N, Schober A, Büssow H, Wessig C, Djahed S, Reucher H, Koch DA, Lautermann J. Expression pattern and functional characterization of connexin29 in transgenic mice. Glia. 2006;53:601-611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 46] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 72. | Teubner B, Michel V, Pesch J, Lautermann J, Cohen-Salmon M, Söhl G, Jahnke K, Winterhager E, Herberhold C, Hardelin JP. Connexin30 (Gjb6)-deficiency causes severe hearing impairment and lack of endocochlear potential. Hum Mol Genet. 2003;12:13-21. [PubMed] |
| 73. | Kreuzberg MM, Schrickel JW, Ghanem A, Kim JS, Degen J, Janssen-Bienhold U, Lewalter T, Tiemann K, Willecke K. Connexin30.2 containing gap junction channels decelerate impulse propagation through the atrioventricular node. Proc Natl Acad Sci USA. 2006;103:5959-5964. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 87] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 74. | Zheng-Fischhöfer Q, Schnichels M, Dere E, Strotmann J, Loscher N, McCulloch F, Kretz M, Degen J, Reucher H, Nagy JI. Characterization of connexin30.3-deficient mice suggests a possible role of connexin30.3 in olfaction. Eur J Cell Biol. 2007;86:683-700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 23] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 75. | Koch Y, van Fürden B, Kaiser S, Klein D, Kibschull M, Schorle H, Carpinteiro A, Gellhaus A, Winterhager E. Connexin 31 (GJB3) deficiency in mouse trophoblast stem cells alters giant cell differentiation and leads to loss of oxygen sensing. Biol Reprod. 2012;87:37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 76. | Dere E, Zheng-Fischhöfer Q, Viggiano D, Gironi Carnevale UA, Ruocco LA, Zlomuzica A, Schnichels M, Willecke K, Huston JP, Sadile AG. Connexin31.1 deficiency in the mouse impairs object memory and modulates open-field exploration, acetylcholine esterase levels in the striatum, and cAMP response element-binding protein levels in the striatum and piriform cortex. Neuroscience. 2008;153:396-405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 77. | Nelles E, Bützler C, Jung D, Temme A, Gabriel HD, Dahl U, Traub O, Stümpel F, Jungermann K, Zielasek J. Defective propagation of signals generated by sympathetic nerve stimulation in the liver of connexin32-deficient mice. Proc Natl Acad Sci USA. 1996;93:9565-9570. [PubMed] |
| 78. | Temme A, Buchmann A, Gabriel HD, Nelles E, Schwarz M, Willecke K. High incidence of spontaneous and chemically induced liver tumors in mice deficient for connexin32. Curr Biol. 1997;7:713-716. [PubMed] |
| 79. | Deans MR, Gibson JR, Sellitto C, Connors BW, Paul DL. Synchronous activity of inhibitory networks in neocortex requires electrical synapses containing connexin36. Neuron. 2001;31:477-485. [PubMed] |
| 80. | Deans MR, Volgyi B, Goodenough DA, Bloomfield SA, Paul DL. Connexin36 is essential for transmission of rod-mediated visual signals in the mammalian retina. Neuron. 2002;36:703-712. [PubMed] |
| 81. | Demb JB, Pugh EN. Connexin36 forms synapses essential for night vision. Neuron. 2002;36:551-553. [PubMed] |
| 82. | Hempelmann A, Heils A, Sander T. Confirmatory evidence for an association of the connexin-36 gene with juvenile myoclonic epilepsy. Epilepsy Res. 2006;71:223-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 35] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 83. | Mas C, Taske N, Deutsch S, Guipponi M, Thomas P, Covanis A, Friis M, Kjeldsen MJ, Pizzolato GP, Villemure JG. Association of the connexin36 gene with juvenile myoclonic epilepsy. J Med Genet. 2004;41:e93. [PubMed] |
| 84. | Nlend RN, Michon L, Bavamian S, Boucard N, Caille D, Cancela J, Charollais A, Charpantier E, Klee P, Peyrou M. Connexin36 and pancreatic beta-cell functions. Arch Physiol Biochem. 2006;112:74-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 26] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 85. | Pacheco-Costa R, Hassan I, Reginato RD, Davis HM, Bruzzaniti A, Allen MR, Plotkin LI. High bone mass in mice lacking Cx37 because of defective osteoclast differentiation. J Biol Chem. 2014;289:8508-8520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 54] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 86. | von Maltzahn J, Wulf V, Matern G, Willecke K. Connexin39 deficient mice display accelerated myogenesis and regeneration of skeletal muscle. Exp Cell Res. 2011;317:1169-1178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 87. | Makita N, Sasaki K, Groenewegen WA, Yokota T, Yokoshiki H, Murakami T, Tsutsui H. Congenital atrial standstill associated with coinheritance of a novel SCN5A mutation and connexin 40 polymorphisms. Heart Rhythm. 2005;2:1128-1134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 59] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 88. | Tsai CT, Lai LP, Hwang JJ, Lin JL, Chiang FT. Molecular genetics of atrial fibrillation. J Am Coll Cardiol. 2008;52:241-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 68] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 89. | Lecanda F, Warlow PM, Sheikh S, Furlan F, Steinberg TH, Civitelli R. Connexin43 deficiency causes delayed ossification, craniofacial abnormalities, and osteoblast dysfunction. J Cell Biol. 2000;151:931-944. [PubMed] |
| 90. | Plotkin LI, Bellido T. Beyond gap junctions: Connexin43 and bone cell signaling. Bone. 2013;52:157-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 110] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 91. | Paznekas WA, Karczeski B, Vermeer S, Lowry RB, Delatycki M, Laurence F, Koivisto PA, Van Maldergem L, Boyadjiev SA, Bodurtha JN. GJA1 mutations, variants, and connexin 43 dysfunction as it relates to the oculodentodigital dysplasia phenotype. Hum Mutat. 2009;30:724-733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 201] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 92. | Britz-Cunningham SH, Shah MM, Zuppan CW, Fletcher WH. Mutations of the Connexin43 gap-junction gene in patients with heart malformations and defects of laterality. N Engl J Med. 1995;332:1323-1329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 289] [Cited by in RCA: 260] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 93. | Dasgupta C, Martinez AM, Zuppan CW, Shah MM, Bailey LL, Fletcher WH. Identification of connexin43 (alpha1) gap junction gene mutations in patients with hypoplastic left heart syndrome by denaturing gradient gel electrophoresis (DGGE). Mutat Res. 2001;479:173-186. [PubMed] |
| 94. | Gutstein DE, Danik SB, Lewitton S, France D, Liu F, Chen FL, Zhang J, Ghodsi N, Morley GE, Fishman GI. Focal gap junction uncoupling and spontaneous ventricular ectopy. Am J Physiol Heart Circ Physiol. 2005;289:H1091-H1098. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 95. | Odermatt B, Wellershaus K, Wallraff A, Seifert G, Degen J, Euwens C, Fuss B, Büssow H, Schilling K, Steinhäuser C. Connexin 47 (Cx47)-deficient mice with enhanced green fluorescent protein reporter gene reveal predominant oligodendrocytic expression of Cx47 and display vacuolized myelin in the CNS. J Neurosci. 2003;23:4549-4559. [PubMed] |
| 96. | Orthmann-Murphy JL, Enriquez AD, Abrams CK, Scherer SS. Loss-of-function GJA12/Connexin47 mutations cause Pelizaeus-Merzbacher-like disease. Mol Cell Neurosci. 2007;34:629-641. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 92] [Cited by in RCA: 85] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 97. | Bugiani M, Al Shahwan S, Lamantea E, Bizzi A, Bakhsh E, Moroni I, Balestrini MR, Uziel G, Zeviani M. GJA12 mutations in children with recessive hypomyelinating leukoencephalopathy. Neurology. 2006;67:273-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 83] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 98. | Uhlenberg B, Schuelke M, Rüschendorf F, Ruf N, Kaindl AM, Henneke M, Thiele H, Stoltenburg-Didinger G, Aksu F, Topaloğlu H. Mutations in the gene encoding gap junction protein alpha 12 (connexin 46.6) cause Pelizaeus-Merzbacher-like disease. Am J Hum Genet. 2004;75:251-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 226] [Cited by in RCA: 210] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 99. | Hombach S, Janssen-Bienhold U, Söhl G, Schubert T, Büssow H, Ott T, Weiler R, Willecke K. Functional expression of connexin57 in horizontal cells of the mouse retina. Eur J Neurosci. 2004;19:2633-2640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 134] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 100. | Shelley J, Dedek K, Schubert T, Feigenspan A, Schultz K, Hombach S, Willecke K, Weiler R. Horizontal cell receptive fields are reduced in connexin57-deficient mice. Eur J Neurosci. 2006;23:3176-3186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 65] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 101. | Puk O, Löster J, Dalke C, Soewarto D, Fuchs H, Budde B, Nürnberg P, Wolf E, de Angelis MH, Graw J. Mutation in a novel connexin-like gene (Gjf1) in the mouse affects early lens development and causes a variable small-eye phenotype. Invest Ophthalmol Vis Sci. 2008;49:1525-1532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |