Published online Sep 26, 2014. doi: 10.4252/wjsc.v6.i4.505
Revised: June 5, 2014
Accepted: July 25, 2014
Published online: September 26, 2014
Processing time: 169 Days and 5.2 Hours
AIM: To investigate the effect of stem cells from human exfoliated deciduous teeth (SHED) transplanted for bone regeneration in the dog mandibular defect.
METHODS: In this prospective comparative study, SHEDs had been isolated 5 years ago from human exfoliated deciduous teeth. The undifferentiated stem cells were seeded into mandibular bone through-and-through defects of 4 dogs. Similar defects in control group were filled with cell-free collagen scaffold. After 12 wk, biopsies were taken and morphometric analysis was performed. The percentage of new bone formation and foreign body reaction were measured in each case. The data were subject to statistical analysis using the Mann-Whitney U and Kruskalwalis statistical tests. Differences at P < 0.05 was considered as significant level.
RESULTS: There were no significant differences between control and SHED-seeded groups in connective tissue (P = 0.248), woven bone (P = 0.248) and compact bone (P = 0.082). There were not any side effects in transplanted SHED group such as teratoma or malignancy and abnormalities in this period.
CONCLUSION: SHEDs which had been isolated and characterized 5 years ago and stored with cryopreservation banking were capable of proliferation and osteogenesis after 5 years, and no immune response was observed after three months of seeded SHEDs.
Core tip: Stem cells from human exfoliated deciduous teeth (SHED) exist in the living pulp remnants of exfoliated deciduous teeth. The aim of this study was to investigate the effect of SHED transplanted for bone regeneration in the dog mandibular defect.In this study we found that SHEDs which had been isolated and characterized 5 years ago and stored with cryopreservation banking were capable of proliferation and osteogenesis after 5 years, and no immune response was observed after three months of seeded SHEDs.
- Citation: Behnia A, Haghighat A, Talebi A, Nourbakhsh N, Heidari F. Transplantation of stem cells from human exfoliated deciduous teeth for bone regeneration in the dog mandibular defect. World J Stem Cells 2014; 6(4): 505-510
- URL: https://www.wjgnet.com/1948-0210/full/v6/i4/505.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v6.i4.505
Most of the head and neck lesions are caused by maxillofacial tumor surgeries, infection, trauma, and congenital skeletal deformities. Reconstruction of these defects is one of the most difficult and complex parts in maxillofacial surgeries. Autogenous tissue and alloplastic materials have been used for the treatment of these defects, each of which has its own disadvantages such as bleeding, nerve injuries, esthetic problems, pain, infection and loss of tissue function[1,2].
Tissue engineering is a fundamental science that can be used as a solution for autograft and allograft tissue in reconstruction surgeries. To the best of our knowledge, the tissue engineering approach requires three key elements: stem cell, scaffold and growth factor. Using this method the individual cell will be isolated and cultured and then can be implanted or injected directly to the damaged tissue, and surgeons can consider tissue engineering to reconstruct the tissue defect without any severe complications[3,4].
Stem cells have been isolated and characterized from a variety of sources such as bone marrow[5], adipose tissue[6], hair follicles[7], synovial membrane[8], skeletal muscle[9], dental pulp[10], etc. The types of isolated stem cells are very important due to the capacity of proliferation and differentiation rate of each tissue. It has been demonstrated that stem cells from human exfoliated deciduous teeth (SHED) can be easily isolated and expanded in the culture medium[11]. Moreover, Miura et al[12] showed that SHED is capable of extensive proliferation and multi-potential differentiation. They also discussed that deciduous teeth can be considered as an ideal source of stem cells to induce bone regeneration.
In-vitro studies have shown that there is no immune reaction and tissue rejection of SHED, which lead to non-immunosuppressive therapy[13-15]. de Mendonça Costa et al[16] studied the capacity of human dental pulp stem cells isolated to reconstruct large-sized cranial bone defects in non-immunosuppressed rats. The results of their study showed that using these cells can induce osteogenesis without any graft rejection.
Recently in-vivo studies have shown that using human dental pulp stem cells will not lead to any tissue rejection[16,17]. The aim of the present study was to investigate the effect of transplantation of SHED for bone regeneration in the dog mandibular defect.
This study was approved by the Regional Bioethics Committee of Isfahan Province (191086) and was conducted in accordance with the ethical principles and standards for the conduct of human and animal biological rhythm[18].
Four male dogs (mixed breed, Iranian) between 15-25 kg were included in this prospective experimental study. The animals were accommodated in the animal house at 22˚C-24˚C with 55%-70% humidity, light cycle of 12 h, air renewal 15 times/h with the same diet. Also, during the study the animals were monitored for general appearance, activity, exertion, and weight. Exclusion criteria included undesirable changes in vital signs, physical examination and any visible swelling of lymph nodes in the head and neck area that disqualify subjects from inclusion in the study.
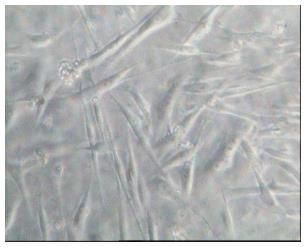
Normal exfoliated human deciduous teeth were collected from 6- to 9-year-old children under approved guidelines which were set by Nourbakhsh et al[19]. Based on this protocol, we used SHED which had been isolated and characterized their markers 5 years ago and stored with cryopreservation banking in which cells were preserved in liquid nitrogen vapor (Royan institute, Isfahan, Iran.) at a temperature of less than -150 °C. After defrosting the isolated cells, they were transferred to the flask containing medium consisting of Dulbecco modified eagle medium (sigma, St.Louis, United States) enriched with fetal bovine serum 10% (FBS; Dainippon pharmaceutical, Osaka, Japan) and penicillin-streptomycin 0.5% (Gibco-BRL, Life Technologies, MD, United States) (Figure 1).
The SHEDs were expanded through 3 passages (Figure 2), for each passage the medium was removed, irrigation was performed with phosphate buffered saline (Gibco, Grand Island, NY, United States) Trypsin-EDTA (Gibco, Grand Island, NY, United States) was added for 3 min and then neutralized with medium consisting of Dulbecco modified eagle medium enriched with fetal bovine serum 10% and penicillin-streptomycin 0.5%. After centrifuging (1400 rpm, 10 min), the fluid on top was discarded and the remainder of the suspension was transferred to a new flask.
Non-adherent cells were removed from the culture by washing with PBS. The adherent cells were expanded as monolayer culture in the medium consisting of Dulbecco modified eagle medium enriched with fetal bovine serum 10% and penicillin-streptomycin 0.5% at 5% Co2 and at the temperature of 37 °C.
The obtained stem cells were counted and trypsinized with Trypsin-EDTA (Gibco,Grand Island, NY, United States). About 106 cells were suspended in a little amount of medium (100 μL)[14,16]. The suspension was transferred to a 9 mm × 5 mm cylindrical collatamp (syntacoll, GmbH, Germany) scaffold with a sampler and incubated for 2 h. After that the medium consisting of Dulbecco modified eagle medium enriched with fetal bovine serum 10% and penicillin-streptomycin 0.5% was added and incubated again for 48 h before implanting in mandibular bone defects.
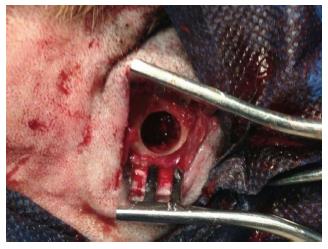
Fiest each dog was firstly sedated by 0.02 mg/kg Acepromazine (Aveco Co; Inc, Fort Dodge, LA, United States) and sub-cutaneous 0.05 mg/kg Atropine sulfate (Darou Pakhsh pharmaceutical, Tehran, Iran). Then, each dog was anesthetized with an intramuscular injection of ketamine 10% (Alfasan, Woerden, Holland). Animals were also intubated by anesthesiologist. The mandible was shaved and the skin surface was disinfected with povidone iodine solution (Aida chemie co, Mashad, Iran) before the operation. The mandible bone was exposed through a skin incision of approximately 5 cm. Layered dissection was performed through the mandibular bone and full thickness through-and-through bony defects were created on each side of the inferior mandibular border by trephine bur (Meisinger, Dusseldorf, Germany) that were 9 mm in diameter (Figure 3).
One defect was filled with scaffold plus SHED and the other one was only filled with scaffold to serve as a control group. The periousteum was closed with resorbable 4/0 suture (Vicryl, Johnson Somerville. NJ, United States) and non-resorbable 4/0 suture (SURG1PRO Polypropylene Monofilament, Richmond, VA, United States) for the skin. All animals recovered from anesthesia without complications. Postoperative medications included tramadol (Darou Pakhsh pharmaceutical, Tehran, Iran) 2 mg/d for 3 d and antibiotic Penicillin 6.3.3 (Jaber Ebne Hayyan Pharmaceutical Co., Tehran, Iran) for 6 d, intramuscularly.
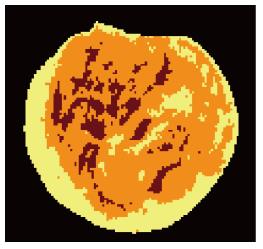
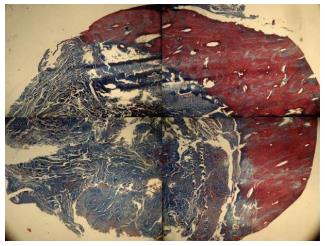
After 12 wk, biopsies were taken by a larger trephine bur (Meisinger, Dusseldorf, Germany). The animals were not sacrificed at the end of the experiments and that only biopsy specimens were taken. Samples were cut horizontally from the middle by surgical saw (Stryker Instruments, Kalamazoo, MI, United States), the specimens were fixed in formaldehyde 10% (Sina chemical industrial, Tehran, Iran) buffer solution at pH 7.0 and were treated with 10% formic acid decalcifying solution (Kimia Tehran Acid, Tehran, Iran) for two weeks. Samples were dehydrated with alcohols and embedded in paraffin. For macroscopic evaluation, the features were analyzed by Image Analysis software (IHMMA, Ver. 1, Sbmu. Iran) which is able to segment the input image into predefined regions (three regions were chosen) based on the closeness of colors in each region. First, images were taken from samples cross section and bone formation area and original bone defects were defined using color codes with Image Analysis Software. In order to determine the area of bone formation we used the image segmentation in our analysis, which is the process of partitioning a digital image into multiple segments (super pixels). The goal of segmentation is to simplify and/or change the representation of an image into something that is more meaningful and easier to analyze. Image segmentation is typically used to locate objects and boundaries (lines, curves, etc.) in images. More precisely, image segmentation is the process of assigning a label to every pixel in an image such that pixels with the same label share certain visual characteristics. Then, percentage of each area was provided by the software (Figure 4). Light intensity was constant for all samples. The software automatically calculates the percent of different tissues and reports the area ratio of each region to the total, quantitatively. The specimens were stained with hematoxylin and eosin (sigma) for the new bone tissue regeneration and Masson’s trichrome (sigma) for detecting tumors arising from fibroblasts (Figure 5).
The histological evaluation was performed at 40 × magnified optical microscope (Olympus BX 51-Olympus co, Tokyo, Japan).
In histological evaluation, the presence of macrophages, monocytes, and giant cells was considered as foreign body reaction. The presence of neutrophils was considered as acute inflammation and presence of mononuclear cells like lymphocytes and monocytes was considered as chronic inflammation[20]. Pathologists were blinded to the graft material for each sample.
All data were expressed as Median (Range) and were analyzed by SPSS version 16 (SPSS Inc., Chicago, United States). Data were analyzed using non parametric test such as Mann-whitney U and Kruskalwalis statistical tests. P < 0.05 was considered as a significant test.
The histological results did not show any foreign body reaction or severe inflammation in each of the specimens. Bone tissue type in both control and SHED-seeded group was similar. There were not any side effects owing to transplanted SHED such as teratoma or malignancy. In control and SHED-seeded groups the new bone formation was observed in both lingual and floor parts of the defect, that was compact bone. The middle part revealed newly formed lamellar and woven bones with limited connective tissue and in lateral cortex of mandible the defect site was restored with the connective tissue (Figure 6).
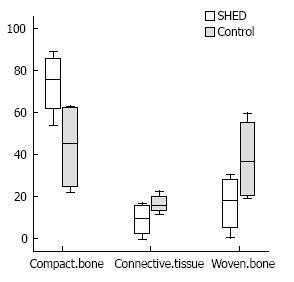
The median (range) of osteogenesis rate of the area 1 (connective tissue), area 2 (woven bone) and area 3 (compact bone) in the SHED-seeded group were 16.1 (10.66), 36.59 (40.36) and 45.23 (41.07), respectively (Figure 7). There were no significant differences between control and SHED-seeded groups in area1 (P = 0.248) area 2 (P = 0.248) and area 3 (P = 0.082) There were not any side effects in transplanted SHED group such as teratoma or malignancy and abnormalities in this period.
The results of the present study showed that bone regeneration in the segmental defects of the dog mandible can not be enhanced by the presence of cultivated SHEDs. In the study by Schliephake et al[21] human bone cells isolated and seeded in three different types of scaffolds and stem cell seeded scaffolds were implanted in rat mandibular defects and after 6 wk the presence of human cells was assessed. The results of their study showed no increase in the amount of early bone formation.
de Mendonça Costa et al[16] conducted a research in which the stem cells from human deciduous teeth were isolated and implanted in the cranial bone defect of rat. They reported bone regeneration without any immune reaction. Kerkis et al[22] transplanted the human immature dental pulp stem cells to dog’s retriever muscular dystrophy locally and systemically, reporting no immune reaction. It has been assumed that SHEDs transplantation have immunosuppressive activity which is an important item in the field of regenerative medicine[13,14]. Also, Yamaza et al[15] investigated the immunomodulatory properties of SHEDs and reported that SHEDs has significant effects on inhibiting T helper cells in vitro. Also they reported that SHED transplantation is capable of effectively reversing Systemic Lupus Erythematosus- associated disorders in mice.
The results of the present study showed no immune reaction after three months of implanted cell in the dog mandibular bony defect, so the results of our study are in agreement with the de Mendonça Costa et al[16] and Kerkis et al[22] studies. Therefore it can be concluded that the time of isolation had no effect on the immunomodulatory properties of SHEDs.
The bone regeneration was evaluated after three months[23], because after three months we were able to evaluate the osteogenesis rate without missing any variation, immune reaction and inflammatory process. After this period, histological results did not show any foreign body reaction or severe inflammation in each of the specimens.
Miura et al[12] and Seo et al[17] studies showed that SHEDs have the ability to produce lamellar bone. Therefore, the results of this study are similar to Miura et al[12] and Seo et al[17] studies.
SHEDs have extensive proliferation and differentiation, which make them a critical source of stem cells for the regeneration and repair of craniofacial defects, tooth loss and bone regeneration[24]. SHED cells may also be beneficial for the treatment of neurodegenerative diseases and the repair of motoneurons following stroke or injury[12]. There are many advantages for SHEDs banking; such as no immune reaction and tissue rejection of the cells, and no immunosuppressive therapy[24].
These cells can be best utilized for the patients including children. The cost and technical simplicity of this procedure makes it an ideal source. In the present study we used human exfoliated deciduous teeth, which had been isolated 5 years ago. We found that SHEDs were still capable of proliferation and differentiation.
In this study the defect created was small, but results were promising. Hence, further studies should be done with larger defects using the same methods for more confirmation. The use of stem cells to repair bone defects is not perfect. The biggest disadvantage of using this method is the possibility of uncontrolled cell growth and tumor formation. Therefore further studies should be done to evaluate the risk and prognosis of this therapeutic method. Furthermore more studies with longer follow up should be done to evaluate the efficacy of this method. The advantage of this study compared to other studies was the use of fewer stem cells in the repair of bone defects that showed good results.
SHEDs which had been isolated and characterized 5 years ago and stored with cryopreservation banking were capable of proliferation and osteogenesis after 5 years, and no immune response was observed after three months of seeded SHEDs.
The authors would like to thank the staff of Torabinejad Dental Research Center for their cooperation in all stages of this research.
Stem cells from human exfoliated deciduous teeth (SHED) exist in the living pulp remnants of exfoliated deciduous teeth. They are a heterogeneous population with a fibroblastic morphology that has clonogenic capacity and the ability to form adherent colony clusters with extensive proliferating capacity. The aim of this study was to investigate the effect of SHED transplanted for bone regeneration in the dog mandibular defect.
Tissue engineering is a fundamental science that can be used as a solution for autograft and allograft tissue in reconstruction surgeries. Using this method the individual cell will be isolated and cultured and then can be implanted or injected directly to the damaged tissue, and surgeons can consider tissue engineering to reconstruct the tissue defect without any severe complications. SHED can be easily isolated and expanded, SHEDs are capable of extensive proliferation and multi-potential differentiation, also deciduous teeth can be considered as an ideal source of stem cells to induce bone regeneration.
In-vitro studies have shown that there is no immune reaction and tissue rejection of SHED, which lead to non-immunosuppressive therapy. Researchers studied the capacity of human dental pulp stem cells isolated to reconstruct large-sized cranial bone defects in non-immunosuppressed rats. The results of their study showed that using these cells can induce osteogenesis without any graft rejection. Recently in-vivo studies have shown that using human dental pulp stem cells will not lead to any tissue rejection.
The present study enhanced the reconstruction of facial skeleton using direct bone regeneration to avoid the need for autogenous bone grafts. The results of the present study showed that bone regeneration in the segmental defects of the dog mandible can be enhanced by the presence of cultivated SHEDs, These cells can be best utilized for the patients including children. The cost and technical simplicity of this procedure makes it an ideal source. In the present study we used human exfoliated deciduous teeth, which had been isolated 5 years ago. The authors found that SHEDs were still capable of proliferation and differentiation.
Stem cell: Cell that upon division replaces its own numbers and also gives rise to cells that differentiate further into one or more specialized types; SHED: Stem cells from human exfoliated deciduous teeth exist in the living pulp remnants of exfoliated deciduous teeth; Tissue engineering: Use of a combination of cells, engineering and materials methods, and suitable biochemical and physio-chemical factors to improve or replace biological functions; Scaffold: A temporary structure for holding materials during the repair, Regrowing bone requires a scaffold that is stiff, long-lasting and safe.
This is an interesting and outstanding article. Methods are appropriate. Results are clearly presented. Discussion is interesting.
P- Reviewer: Boffano P, Kita K, Louboutin JP, Niyibizi C S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ
| 1. | Shanti RM, Li WJ, Nesti LJ, Wang X, Tuan RS. Adult mesenchymal stem cells: biological properties, characteristics, and applications in maxillofacial surgery. J Oral Maxillofac Surg. 2007;65:1640-1647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 52] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 2. | Mankani MH, Kuznetsov SA, Shannon B, Nalla RK, Ritchie RO, Qin Y, Robey PG. Canine cranial reconstruction using autologous bone marrow stromal cells. Am J Pathol. 2006;168:542-550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 59] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 3. | Paganelli C, Fontana P, Porta F, Majorana A, Pazzaglia UE, Sapelli PL. Indications on suitable scaffold as carrier of stem cells in the alveoloplasty of cleft palate. J Oral Rehabil. 2006;33:625-629. [PubMed] |
| 4. | Rodríguez-Lozano FJ, Insausti CL, Meseguer L, Ramírez MC, Martínez S, Moraleda JM. Tissue engineering with dental pulp stem cells: isolation, characterization, and osteogenic differentiation. J Craniofac Surg. 2012;23:e571-e575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 5. | Li S, Guo YW, Wang ZY, Ning ZR, Fang DJ. [The isolation and identification of bone marrow mesenchymal stem cells derived from rabbit mandible]. Shanghai Kou Qiang Yi Xue. 2013;22:19-24. [PubMed] |
| 6. | Vindigni V, Michelotto L, Lancerotto L, Puppa AD, D’Avella D, Abatangelo G, Cortivo R, Zavan B. Isolation method for a stem cell population with neural potential from skin and adipose tissue. Neurol Res. 2009;Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 7. | Li L, Fukunaga-Kalabis M, Herlyn M. Isolation, characterization, and differentiation of human multipotent dermal stem cells. Methods Mol Biol. 2013;989:235-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 8. | Futami I, Ishijima M, Kaneko H, Tsuji K, Ichikawa-Tomikawa N, Sadatsuki R, Muneta T, Arikawa-Hirasawa E, Sekiya I, Kaneko K. Isolation and characterization of multipotential mesenchymal cells from the mouse synovium. PLoS One. 2012;7:e45517. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 70] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 9. | Pasut A, Jones AE, Rudnicki MA. Isolation and culture of individual myofibers and their satellite cells from adult skeletal muscle. J Vis Exp. 2013;e50074. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 99] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 10. | Ranganathan K, Lakshminarayanan V. Stem cells of the dental pulp. Indian J Dent Res. 2013;23:558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 11. | Volponi AA, Pang Y, Sharpe PT. Stem cell-based biological tooth repair and regeneration. Trends Cell Biol. 2010;20:715-722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 191] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 12. | Miura M, Gronthos S, Zhao M, Lu B, Fisher LW, Robey PG, Shi S. SHED: stem cells from human exfoliated deciduous teeth. Proc Natl Acad Sci USA. 2003;100:5807-5812. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1922] [Cited by in RCA: 1982] [Article Influence: 90.1] [Reference Citation Analysis (0)] |
| 13. | Pierdomenico L, Bonsi L, Calvitti M, Rondelli D, Arpinati M, Chirumbolo G, Becchetti E, Marchionni C, Alviano F, Fossati V. Multipotent mesenchymal stem cells with immunosuppressive activity can be easily isolated from dental pulp. Transplantation. 2005;80:836-842. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 368] [Cited by in RCA: 383] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 14. | Yalvac ME, Rizvanov AA, Kilic E, Sahin F, Mukhamedyarov MA, Islamov RR, Palotás A. Potential role of dental stem cells in the cellular therapy of cerebral ischemia. Curr Pharm Des. 2009;15:3908-3916. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 36] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 15. | Yamaza T, Kentaro A, Chen C, Liu Y, Shi Y, Gronthos S, Wang S, Shi S. Immunomodulatory properties of stem cells from human exfoliated deciduous teeth. Stem Cell Res Ther. 2010;1:5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 251] [Cited by in RCA: 251] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 16. | de Mendonça Costa A, Bueno DF, Martins MT, Kerkis I, Kerkis A, Fanganiello RD, Cerruti H, Alonso N, Passos-Bueno MR. Reconstruction of large cranial defects in nonimmunosuppressed experimental design with human dental pulp stem cells. J Craniofac Surg. 2008;19:204-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 150] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 17. | Seo BM, Sonoyama W, Yamaza T, Coppe C, Kikuiri T, Akiyama K, Lee JS, Shi S. SHED repair critical-size calvarial defects in mice. Oral Dis. 2008;14:428-434. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 204] [Cited by in RCA: 180] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 18. | Touitou Y, Portaluppi F, Smolensky MH, Rensing L. Ethical principles and standards for the conduct of human and animal biological rhythm research. Chronobiol Int. 2004;21:161-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 283] [Cited by in RCA: 286] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 19. | Nourbakhsh N, Soleimani M, Taghipour Z, Karbalaie K, Mousavi SB, Talebi A, Nadali F, Tanhaei S, Kiyani GA, Nematollahi M. Induced in vitro differentiation of neural-like cells from human exfoliated deciduous teeth-derived stem cells. Int J Dev Biol. 2011;55:189-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 81] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 20. | Anderson JM, Rodriguez A, Chang DT. Foreign body reaction to biomaterials. Semin Immunol. 2008;20:86-100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4004] [Cited by in RCA: 3387] [Article Influence: 199.2] [Reference Citation Analysis (0)] |
| 21. | Schliephake H, Zghoul N, Jäger V, van Griensven M, Zeichen J, Gelinsky M, Szubtarsky N. Bone formation in trabecular bone cell seeded scaffolds used for reconstruction of the rat mandible. Int J Oral Maxillofac Surg. 2009;38:166-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 22. | Kerkis I, Ambrosio CE, Kerkis A, Martins DS, Zucconi E, Fonseca SA, Cabral RM, Maranduba CM, Gaiad TP, Morini AC. Early transplantation of human immature dental pulp stem cells from baby teeth to golden retriever muscular dystrophy (GRMD) dogs: Local or systemic? J Transl Med. 2008;6:35. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 116] [Cited by in RCA: 111] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 23. | Petite H, Viateau V, Bensaïd W, Meunier A, de Pollak C, Bourguignon M, Oudina K, Sedel L, Guillemin G. Tissue-engineered bone regeneration. Nat Biotechnol. 2000;18:959-963. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1136] [Cited by in RCA: 1018] [Article Influence: 40.7] [Reference Citation Analysis (0)] |
| 24. | Arora V, Arora P, Munshi AK. Banking stem cells from human exfoliated deciduous teeth (SHED): saving for the future. J Clin Pediatr Dent. 2009;33:289-294. [PubMed] |