Published online Sep 26, 2014. doi: 10.4252/wjsc.v6.i4.497
Revised: August 20, 2014
Accepted: August 30, 2014
Published online: September 26, 2014
Processing time: 128 Days and 6.6 Hours
AIM: To compare seven commercially available bone graft substitutes (BGS) in terms of these properties and without using any additional biological growth factors.
METHODS: Porcine osteoprogenitor cells were loaded on seven commercially available BGS and allowed to proliferate for one week followed by osteogenic induction. Staining for live/dead cells as well as scanning electron microscopy (SEM) was carried out to determine viability and cellular binding. Further outcome measures included alkaline phosphatase (ALP) assays with normalisation for DNA content to quantify osteogenic potential. Negative and positive control experiments were carried out in parallel to validate the results.
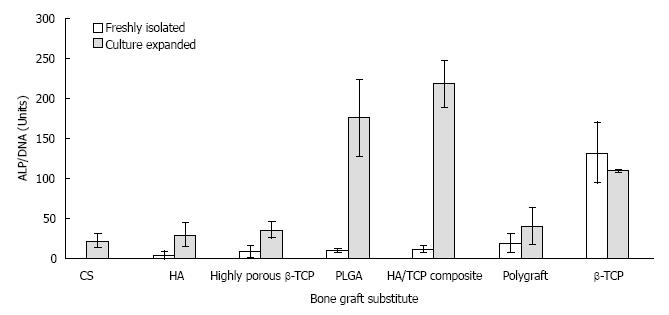
RESULTS: Live/dead and SEM imaging showed higher viability and attachment with β-tricalcium phosphate (β-TCP) than with other BGS (P < 0.05). The average ALP activity in nmol/mL (normalised value for DNA content in nmol/μg DNA) per sample was 657.58 (132.03) for β-TCP, 36.22 (unable to normalise) for calcium sulphate, 19.93 (11.39) for the Hydroxyapatite/Tricalcium Phosphate composite, 14.79 (18.53) for polygraft, 13.98 (8.15) for the highly porous β-Tricalcium Phosphate, 5.56 (10.0) for polymers, and 3.82 (3.8) for Hydroxyapatite.
CONCLUSION: Under the above experimental conditions, β-TCP was able to maintain better the viability of osteoprogenitor cells and allow proliferation and differentiation (P < 0.05).
Core tip: Various commercially available bone graft substitutes (BGS) exist today and are used for the restoration of bone defects resulting from traumatic injury, tumor resection and congenital or degenerative diseases. Such BGS should pose osteoinductive and osteoconductive properties and support cell response to the osteogenic signalling. This study evaluated seven commercially available BGS in terms of osteoprogenitor cell adherence, proliferation and osteogenic differentiation. β-tricalcium phosphate was found to have the most favourable effect on cell viability and allow for their subsequent proliferation and differentiation.
-
Citation: Dahabreh Z, Panteli M, Pountos I, Howard M, Campbell P, Giannoudis PV. Ability of bone graft substitutes to support the osteoprogenitor cells: An
in-vitro study. World J Stem Cells 2014; 6(4): 497-504 - URL: https://www.wjgnet.com/1948-0210/full/v6/i4/497.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v6.i4.497
Bone graft materials are routinely used to fill bony defects and provide structural support stimulating the bone healing process[1,2]. Autologous bone grafting is currently the gold standard graft material which contains all the requisite osteogenic, osteoinductive and osteoconductive properties[3,4]. However, the available size, shape and quantity of autologous bone graft is limited and considerable morbidity is associated with its harvest[5-7]. Due to these limitations, the development of alternative approaches resulted in several allogeneic and synthetic materials to be commercially available for clinical use. Concerns suggesting unfavourable osteogenic properties, host immune reactions and risk of pathogen transmission have been raised[8,9]. However, synthetic bone graft substitutes (BGS) offer potentially limitless supply, have no risk of disease transmission or an immunogenic response, and offers optimum osteoconductive properties[1,10,11]. The ideal BGS should provide a suitable environment for tissue development. It should favour cell attachment, growth and differentiation, bone growth, in vivo vascularisation, osteointegration with host bone, and the gradual replacement of the scaffold by newly formed bone[12].
Combining a biological element such as mesenchymal stem cells or osteoblasts with BGS is believed to enhance some of these characteristics and may improve the bone healing process[12-17]. Therefore, experimental models where such bone graft materials were loaded with either bone marrow aspirates or even culture expanded osteoprogenitor cells have been previously explored by several authors[18,19]. Osteoprogenitor cells are relatively rare in bone marrow aspirates, estimated at 0.01%-0.001% of nucleated cells in the bone marrow, hence it is of paramount importance the graft material to allow the adherence, proliferation and differentiation of these cells[12,15-17,20,21]. Therefore, the aim of this study was to compare seven commercially available BGS in terms of these properties and without using any additional biological growth factors. An observation of macroscopic properties of the BGS was also carried out.
Seven commercially available BGS were included in the study (Table 1). Polyglycolic acid (PGA), poly (Lactide-co-Glycolide) [PLGA], β-tricalcium phosphate (β-TCP), calcium sulphate (CS) and bovine DBM granules were supplied by Smith and Nephew, Inc. (Memphis, TN, United States). Highly porous (90%) β-TCP morsels were supplied by Orthovita® (Malvern, PA, United States), hydroxyapatite/tricalcium phosphate (HA/TCP) composite by Zimmer® (Swindon, United Kingdom), hydroxyapatite (HA) by Interpore (Irvine, CA, United States) and Polygraft® (PG) by Osteobiologics, Inc. (San Antonio, TX, United States).
| BGS | Micropore size | Macropore size | Resorption |
| β-TCP | < 5 μm | 55% inter-granular | 9-12 mo |
| CS | NA | 55% inter-granular | 1-3 mo |
| Highly porous (90% porosity) β-TCP | 1-100 μm | 100-1000 μm | 4 mo |
| HA 60%/β-TCP 40% (HA/TCP) | < 5 μm | 400-600 μm | |
| HA | - | 280-770 μm | yr |
| PG1 | 250 μm | 75% | 4-8 mo |
| PLGA | - | - | 1-6 mo |
Cell isolation and culture expansion was performed according to previously described methods[22-25]. Nucleated cells were isolated from the cancellous bone of the greater trochanter of fresh porcine bone obtained from the local abattoir on the day of sampling. Using porcine bone enabled us to obtain a fresh population of cells thus simulating a clinical situation where an autologous population of cells would be harvested and concentrated intra-operatively before being added to BGS. Using porcine cells also avoids all the limitations of human tissue handling. The femur was detached from the hip joint and all the muscle, cartilage, tendons and any other tissue that could contaminate the sample was removed. The proximal femur was dissected using a coping saw to expose cancellous bone, which was removed with a borer under sterile conditions and collected into phosphate buffered saline (PBS) solution. This was further crushed into smaller chips using scissors. The sample was then agitated on an orbital plate shaker at 37 °C in a 5% CO2 humidified atmosphere for 30 min. After agitation samples were filtered through a 70 μ cell strainer to remove debris. Samples were then centrifuged at 1000 rpm for 5 min at room temperature and the cellular pellet re-suspended in minimum essential medium eagle with alpha modification, known as alpha-MEM (Sigma Aldrich®) containing 15% (v/v) foetal calf serum, 50 IU/mL Penicillin, 50 IU/mL Streptomycin, 2 mmol/L L-glutamine, 1% non-essential amino acids (Sigma Aldrich®) to obtain a concentration of 2.5 × 105 nucleated cells/mL.
Mesenchymal osteoprogenitor cells for the positive control were initially isolated from porcine femora using the same technique and then were culture expanded in T-175 flasks containing alpha-MEM. Cells were released from their culture using a 0.05% trypsin-EDTA. The cells were then centrifuged at 1000 rpm for 5 min at room temperature and then re-suspended in media to obtain concentrations of 2.5 × 105 cells/mL.
Sterile, non-tissue culture, 24-well plates (Becton Dickenson Labware Europe, France) were used. Each well was filled with 0.5 cm3 of BGS utilising a standardised measuring beaker which has a maximum volume of 0.5 cm3. A fixed volume of BGS (0.5 cm3) rather than a fixed weight was used in order to simulate the clinical situation where the volume of BGS required would be dependent on the size of defect to be filled, irrespective of such parameters as surface area, surface geometry or weight of the BGS.
β-TCP, Calcium Sulphate, DBM, Highly porous β-TCP, HA/TCP composite, HA and Polygraft were used in granular form. Polyglycolic acid (PGA) and poly (Lactide-co-Glycolide) [PLGA] were used in soft solid form.
Experimental wells (n = 6) received 1.5 mL (2.5 × 105 cells/mL) of freshly isolated cells (freshly isolated group). Positive control wells (n = 6) received 1.5 mL (2.5 × 105 cells/mL) of culture expanded cells (culture expanded group). The third group of wells (n = 6) received 1.5 mL of media only (negative control group). Plates were continuously agitated for 24 h to enhance uniform exposure of BGS to cells. The medium was changed after the first 48 h to remove non-adherent cells. Subsequently, the medium was replaced three times a week. During the first week, the medium used was alpha-MEM. For the two weeks that followed, the medium was replaced with an osteogenic medium containing 3 mmol/L beta-glycerophosphate, 1 × 10-8 M dexamethasone, and 50 µg/mL ascorbic acid (Sigma Aldrich®) in addition to the same constituents of alpha-MEM. Cells and BGS were left in culture for a total of 21 d at 37 °C in a 5% CO2 humidified atmosphere. Outcome measures were performed after the 21st day.
Equal amounts of BGS were collected from all 6 wells of each group to constitute a total volume of 0.5 cm3 for each of the groups. One half (0.25 cm3) was utilised for analysis by staining for live/dead cells. The other half was utilised for analysis by SEM. Further outcome measures included ALP (an early marker for the osteoblastic differentiation of osteoprogenitor cells[26,27]) assays with normalisation for DNA content. Similar analysis was performed on the negative and the positive (culture expanded) control groups. The prevalence of potential osteoblastic progenitors in cancellous bone may be estimated by counting colony-forming units (CFU), which express ALP[28]. Previous CFU assays using similar porcine cells in our laboratories have shown that 2.5 × 105 cells/mL was the optimum working concentration[29]. Furthermore in-vitro analysis in our institution of similarly isolated populations of mononuclear cells showed evidence that they do possess osteogenic properties (alizarin red staining to confirm mineralisation)[29]. However, such analysis was not performed on the specimens used in this study in order to avoid compromising the quantitative analyses of ALP activity and DNA content. Furthermore, alizarin red staining would have strongly stained the mineral in most BGSs such that cellular contribution towards ALP activity would be masked.
Staining for viable cells was carried out using a live/dead assay according to manufacturer’s instructions (20 μmol/L ethidium bromide and 5 μmol/L calcein in 10 mL of PBS, prepared fresh from stock (Molecular Probes). Samples were wrapped in foil and were incubated for at least 30 min at 37 °C. After removal of the live-dead stain, 1 mL of PBS was added to each sample and images were captured by fluorescent microscopy, (Excitation wavelength of 488 nm for Green and 568 nm for Red).
Alkaline phosphatase activity was assessed by detecting the conversion of p-nitrophenyl phosphate to p-nitrophenol (Sigma Aldrich®, Dorset, United Kingdom, N3129-5G, 124K5371). Deoxyribonucleic acid (DNA) content was estimated using the Quant-iT™ Picogreen® dsDNA reagent (Molecular Probes®, Invitrogen®, Oregon, USA). Cell lysis was achieved by washing the BGS with 100 μL of 0.1% Triton X-100/0.2 mol/L carbonate buffer followed by three cycles of freeze-thawing. Each cycle involved immersion of the plates in liquid nitrogen followed by incubation at 37 °C. Samples were then loaded into a 96-well plates in duplicates (50 μL per well). The plates were incubated at 37 °C for one hour. The absorbance was then measured at 405 nm after 20, 40 and 60 min. For DNA assays, the fluorescence was measured at excitation 485 nm and emission 538 nm. The ALP activity of cultures was normalised with respect to DNA content (nmol/μg).
Pertinent observations of handling properties and stability of the BGS in medium were recorded.
Statistical analysis was performed using SPSS version 18.0 for Windows. Normality was confirmed using the Shapiro-Wilk test and equality of variance between groups was confirmed using Levene’s test. Paired t-test was used to test the significance of the difference between freshly isolated and culture expanded groups of the same BGS. ANOVA analysis was carried out between the various groups of BGS. Normalised ALP activity/DNA content for each BGS was represented on a bar chart in descending order from right to left for the freshly isolated groups. Significance was assumed at the P < 0.05 level.
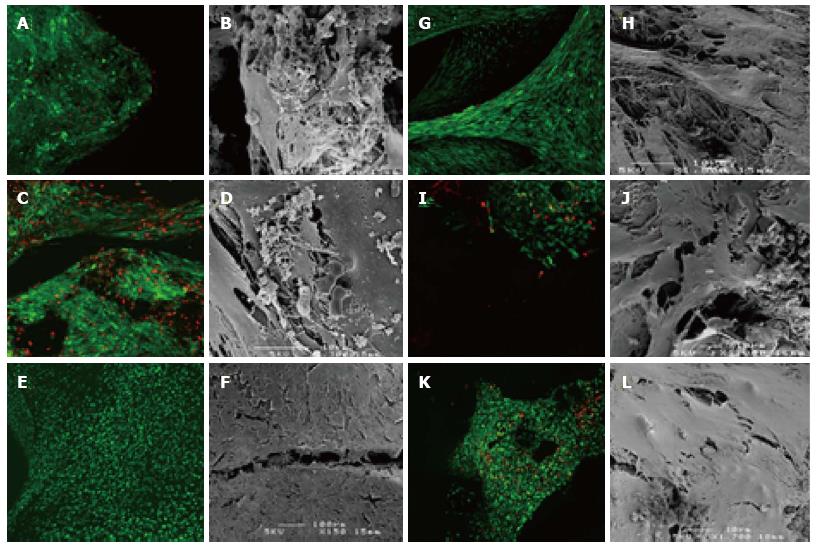
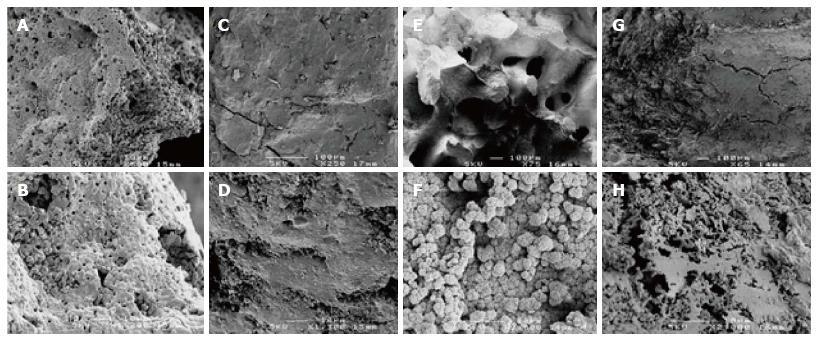
The assay for cell viability (Figure 1) revealed that most BGS support the viability and proliferation of mesenchymal cells. SEM showed cellular attachment to the BGS and in some cases matrix deposition. Typically, both live/dead staining as well as SEM showed more cells and more matrix deposition in the culture expanded group as compared to the freshly isolated group (Figures 1 and 2). In some samples it was easier to see the cells on live/dead staining than on SEM’s. Compared to the amount of green staining, there appears to be a small to moderate (Figure 1C and K) proportion of red staining (non-viable cells) in all BGS in both the freshly isolated and the culture expanded groups. SEM was more difficult to perform on BGS that had a patchy colonisation of cells (e.g., HA).
A gradual increase in absorbance with time was recognised in samples exhibiting active ALP enzymatic activity. Measured ALP levels at 60 min were compared for samples and plotted in ascending order for the freshly isolated group after subtracting the values obtained from the negative control group. Significantly highest levels were recorded for β-TCP (P < 0.05). It was noticed that in the culture expanded group, three BGS’s had very high ALP activity (PLGA, HA/TCP composite, and β-TCP). Overall, ALP activity was higher in the culture expanded group when compared to the freshly isolated group for the same BGS (P < 0.05). Complete dissolution of PGA occurred at the end of the three-week culture period and no ALP activity was recorded. The fluid phase obtained after cell lysis of the DBM samples was very turbid and neither ALP absorbance nor DNA content analysis using the fluorescent plate reader could be performed using the above method. Therefore, PGA and DBM were excluded from final analysis. After subtracting the values obtained from the negative control group, the DNA content in BGS ranged from zero to 4.98 µg/mL and from 1.46 to 9.98 µg/mL in the freshly isolated and the culture expanded groups respectively. For each BGS, DNA content in the culture group was higher than that in the freshly isolated group. In the freshly isolated group, highest levels (P < 0.05) were seen for β-TCP. In the culture expanded group, highest levels of DNA content (µg/mL) were detected with β-TCP (9.98), PLGA (5.32), HA/TCP (4.75), highly porous TCP (4.61), and CS (2.53). When the ALP activity was normalised for DNA content, β-TCP was again the BGS with the highest ALP activity (132.03 Units). As would be expected, the culture expanded group for all samples (except β-TCP) showed higher values for normalised ALP activity than their freshly isolated counterparts (Figure 3).
Polymer BGS (PGA, PLGA) were too friable in a wet environment. PGA (which has been excluded from final analysis) completely disintegrated while PLGA lost its strength towards the end of the culture period in the freshly isolated group. Calcium sulphate started to disintegrate towards the end of the culturing process. Inter-particulate bonds were formed in some samples mainly in the culture expanded group (β-TCP > HA/TCP composite > polygraft).
This study demonstrates that a fresh population of cells extracted from porcine cancellous bone has the ability to attach to commercially available BGS with simple seeding techniques and after a period of incubation to proliferate and undergo osteogenic differentiation. Adding a concentrated or enriched population of bone marrow derived osteoprogenitor cells to an osteoconductive BGS is believed to enhance bone healing[12,14-16,30,31]. The cells in this study were concentrated by filtration and centrifugation. In a clinical situation, the volume of BGS required would depend on the bony defect size irrespective of such parameters as BGS surface area, surface geometry or weight. Therefore, our standard for comparison of BGS was a fixed volume rather than a fixed weight. Modified seeding techniques were not used thus simulating simple mixing of cells with BGS that would occur in the clinical situation.
Despite the small starting number of osteoprogenitor cells in the population of nucleated cells in the freshly isolated group, we believe this was compensated for by allowing the cells to proliferate for one week in medium before the environment became osteogenic. An assumption supported by DNA content analysis on the freshly isolated and the culture expanded groups.
Collectively, ALP activity was higher in the culture expanded group either due to higher cell numbers; or due to removal of dead cells during cell culture and passage leaving a healthy population prior to seeding. The ability of β-TCP to attach fresh cells that produced the highest ALP activity indicates that it has the ability to either retain a high proportion of cells initially, or to enable attached cells to proliferate into considerable numbers before differentiation. Qualitative imaging demonstrated high cellularity with β-TCP as well as other BGS (Figure 1). Other BGS that supported cellular proliferation in the culture expanded group (HA/TCP composite, PLGA, highly porous β-TCP) did not show correlating high levels of ALP in the freshly isolated group. Furthermore, even when DNA content suggested a high amount of freshly isolated cells attached to BGS (e.g., HA/TCP composite, highly porous β-TCP, HA), ALP activity was not impressive. Experiments using culture expanded cells may not be representative of how freshly isolated cells behave towards a certain BGS. Normalisation of ALP activity for DNA content may simplify comparison, but it may not reflect the magnitude of ALP activity or the amount of cells investigated. For instance, β-TCP showed higher ratios of ALP activity for DNA content in the freshly isolated group than in the culture expanded group, although the absolute DNA content and ALP activity were both higher in the culture expanded group. In contrast, Polygraft showed a reasonable ALP to DNA ratio although fewer cells seemed to attach to the BGS. Absolute ALP activity and DNA content values remain useful in conjunction with normalised values.
The chosen BGS were commercially available, biocompatible, non- immunogenic products with no pathogen transmission risk (excluding DBM). BGS that are used in cement form[32] were excluded as they lack the ability to support the viability of cells. Other factors influencing the performance of a cellular-BGS composite include the mechanical properties, surface area, surface chemistry, surface texture, pore size, pore geometry, three-dimensional architecture, and in-vivo degradation properties of the BGS. Finally, the number and concentration of cells transplanted into a given site will have a profound influence on the biologic microenvironment. The biologic environment will have a critical balance between the local metabolic demand of transplanted cells and the capacity for nutrients and oxygen to diffuse into or out of the site through the BGS. This relationship between cells and matrix or BGS structure presents a wide range of variables that need consideration when trying to optimise cellular-BGS composites for bone grafting applications[16]. However, little is known regarding the efficacy of different BGS as means for selective attachment and delivery of osteoprogenitor cells.
We can only speculate about the factors responsible for the performance of β-TCP. Its mechanical properties would not have influenced the outcome since our system was not subjected to mechanical stresses. Macroporosity provides a space in which bone in growth occurs by osteoconduction. Small pores (1-100 µm) are less available for bone ingrowth but may enhance fluid flow and diffusion, thus improving the metabolic environment within the matrix. It has been suggested that optimal macroporosity for cell infiltration has a range of 150-500 µm[33-35]. Larger pores support deeper penetration of new tissues, but optimal pore size for ingrowth deeper than 3-4 mm into the scaffold has not been studied systematically. This is relevant to current clinical practice of filling large bone defects with granular BGS, since the spaces between packed particles are generally significantly larger than the stated microstructure or pore size of most BGS granules [36]. The β-TCP granulesused in our study are six-armed granules which interlock to provide 55% porosity, allowing for cell and nutrient infiltration. They are clinically indicated for filling non load-bearing defects of 4-5 cm. This unique geometry and inter-granular porosity may enhance cellular attachment, proliferation and extracellular matrix deposition. Tissue connections were indeed observed between individual granules of β-TCP [Figure 1G].
Other characteristics of BGS, such as, surface chemistry, topography, roughness, wettability and surface energy were not compared in this study. Proteins and lipids can coat BGS and act as bio-mediators of the cellular responses to BGS. Surface characteristics of BGS may play a role in preferential interaction with certain proteins or adhesion molecules leading to better cell adhesion and subsequent proliferation[10,36,37]. We do not have data regarding the surface characteristics of the β-TCP granules and can only assume that they have been favoured by the osteoprogenitor cells for adhesion and proliferation.
A limitation of the study was the disintegration of polymer BGS (PGA, PLGA), and calcium sulphate. Adding an earlier time point to the analysis such as at day 14 would have affected the study of other BGS and would have been too early to show significant cellular proliferation and differentiation as previous experiments in our institution have shown. This may explain the un-recordable reading of DNA content in the freshly isolated CS group, which then precluded normalising ALP activity to DNA content. It may be that such BGS are not appropriate for this type of study or even for use in a clinical situation where some structural integrity is required. Despite the fact that the BGSs group was rather heterogenous in terms of composition and porosity this study highlights their efficacy in terms of cellular attachment and viability and differentiation within the graft. Therefore, it could be hypothesized that such structural differences could contribute to the results presented in the herein study. Future research to identify a property responsible for these results together with additional assays to demonstrate specific osteoblast functions like osteocalcin, bone sialoprotein or osteopontinas well as in-vivo data would shed more light in this the area.
We believe that under the in-vitro conditions described in this paper, β-TCP was able to favourably maintain the viability of osteoprogenitor cells and allow for their subsequent proliferation and differentiation. Further work needs to be carried out to understand the effect that BGS have on osteoprogenitor cells and to assess the optimum ratio of cell number/concentration to BGS volume. The introduction of biomaterial technologies enhanced with growth factors, genes and cells are certain to have far-reaching effects on the way that musculoskeletal conditions are managed in the future.
Commercially available bone graft substitutes (BGS) are routinely used in the clinical practice. The ideal BGS should provide a suitable environment for tissue development. It should favour cell attachment, growth and differentiation, bone growth, in vivo vascularisation, osteointegration with host bone, and the gradual replacement of the scaffold by newly formed bone.
The authors of this study have analysed the efficacy of seven commercially available BGS to support the adherence, proliferation and differentiation of osteoprogenitor cells loaded within the graft material in-vitro.
Under the in-vitro conditions described in this paper, ß-tricalcium phosphate (ß-TCP) was able to favourably maintain the viability of osteoprogenitor cells and allow for their subsequent proliferation and differentiation.
ß-TCP showed the most favourable results in terms of MSCs adhesion, proliferation and osteogenic differentiation. These results warrant further animal studies to determine the grafts behaviour in in-vivo.
BGS consist of several types and encompass various materials, material sources, and origins (natural or synthetic). Osteoprogenitor stromal cells are undifferentiated cells found in bone but also in a variety of other tissues. Under specific signals (example: trauma) they give rise to those cells that form mesenchymal tissues, including bone and cartilage.
This manuscript describes, in a clearly written style, a series of experiments in vivo attempting to define which, out of a panel of commercially available materials used as bone graft substitutes, performs best with respect to colonization by osteogenic cells and expression of a differentiation marker (alkaline phosphatase). The study was carried out in a system which minimizes the relevance of mechanical factors on the outcome, and care was taken to normalize alkaline phosphatase activity relative to the number of cells in the same, as estimated from the DNA content.
P- Reviewer: Kan L, Pedro XE, Yao CL S- Editor: Song XX L- Editor: A E- Editor: Lu YJ
| 1. | Bucholz RW. Nonallograft osteoconductive bone graft substitutes. Clin Orthop Relat Res. 2002;395:44-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 268] [Cited by in RCA: 232] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 2. | Larsson S, Hannink G. Injectable bone-graft substitutes: current products, their characteristics and indications, and new developments. Injury. 2011;42 Suppl 2:S30-S34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 83] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 3. | Hennig J, Schieker M, Seitz H. Cell seeding chamber for bone graft substitutes. Biomed Tech (Berl). 2012;7. |
| 4. | Calori GM, Mazza E, Colombo M, Ripamonti C. The use of bone-graft substitutes in large bone defects: any specific needs? Injury. 2011;42 Suppl 2:S56-S63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 295] [Cited by in RCA: 281] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 5. | St John TA, Vaccaro AR, Sah AP, Schaefer M, Berta SC, Albert T, Hilibrand A. Physical and monetary costs associated with autogenous bone graft harvesting. Am J Orthop (Belle Mead NJ). 2003;32:18-23. [PubMed] |
| 6. | Lichte P, Pape HC, Pufe T, Kobbe P, Fischer H. Scaffolds for bone healing: concepts, materials and evidence. Injury. 2011;42:569-573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 172] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 7. | Gazdag AR, Lane JM, Glaser D, Forster RA. Alternatives to Autogenous Bone Graft: Efficacy and Indications. J Am Acad Orthop Surg. 1995;3:1-8. [PubMed] |
| 8. | Friedlaender GE, Strong DM, Sell KW. Studies on the antigenicity of bone. II. Donor-specific anti-HLA antibodies in human recipients of freeze-dried allografts. J Bone Joint Surg Am. 1984;66:107-112. [PubMed] |
| 9. | Boyce T, Edwards J, Scarborough N. Allograft bone. The influence of processing on safety and performance. Orthop Clin North Am. 1999;30:571-581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 239] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 10. | Burg KJ, Porter S, Kellam JF. Biomaterial developments for bone tissue engineering. Biomaterials. 2000;21:2347-2359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1241] [Cited by in RCA: 903] [Article Influence: 36.1] [Reference Citation Analysis (0)] |
| 11. | Greenwald AS, Boden SD, Goldberg VM, Khan Y, Laurencin CT, Rosier RN. Bone-graft substitutes: facts, fictions, and applications. J Bone Joint Surg Am. 2001;83-A Suppl 2 Pt 2:98-103. [PubMed] |
| 12. | Connolly JF. Injectable bone marrow preparations to stimulate osteogenic repair. Clin Orthop Relat Res. 1995;313:8-18. [PubMed] |
| 13. | Attawia MA, Herbert KM, Uhrich KE, Langer R, Laurencin CT. Proliferation, morphology, and protein expression by osteoblasts cultured on poly(anhydride-co-imides). J Biomed Mater Res. 1999;48:322-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 14. | Connolly JF, Guse R, Tiedeman J, Dehne R. Autologous marrow injection as a substitute for operative grafting of tibial nonunions. Clin Orthop Relat Res. 1991;259-270. [PubMed] |
| 15. | Fleming JE, Cornell CN, Muschler GF. Bone cells and matrices in orthopedic tissue engineering. Orthop Clin North Am. 2000;31:357-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 145] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 16. | Muschler GF, Midura RJ. Connective tissue progenitors: practical concepts for clinical applications. Clin Orthop Relat Res. 2002;395:66-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 148] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 17. | Pountos I, Georgouli T, Kontakis G, Giannoudis PV. Efficacy of minimally invasive techniques for enhancement of fracture healing: evidence today. Int Orthop. 2010;34:3-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 18. | Chapman MW, Bucholz R, Cornell C. Treatment of acute fractures with a collagen-calcium phosphate graft material. A randomized clinical trial. J Bone Joint Surg Am. 1997;79:495-502. [PubMed] |
| 19. | Muschler GF, Matsukura Y, Nitto H, Boehm CA, Valdevit AD, Kambic HE, Davros WJ, Easley KA, Powell KA. Selective retention of bone marrow-derived cells to enhance spinal fusion. Clin Orthop Relat Res. 2005;432:242-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 89] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 20. | Bruder SP, Jaiswal N, Haynesworth SE. Growth kinetics, self-renewal, and the osteogenic potential of purified human mesenchymal stem cells during extensive subcultivation and following cryopreservation. J Cell Biochem. 1997;64:278-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 21. | McLain RF, Fleming JE, Boehm CA, Muschler GF. Aspiration of osteoprogenitor cells for augmenting spinal fusion: comparison of progenitor cell concentrations from the vertebral body and iliac crest. J Bone Joint Surg Am. 2005;87:2655-2661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 75] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 22. | Maniatopoulos C, Sodek J, Melcher AH. Bone formation in vitro by stromal cells obtained from bone marrow of young adult rats. Cell Tissue Res. 1988;254:317-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 961] [Cited by in RCA: 889] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 23. | Pountos I, Georgouli T, Henshaw K, Bird H, Jones E, Giannoudis PV. The effect of bone morphogenetic protein-2, bone morphogenetic protein-7, parathyroid hormone, and platelet-derived growth factor on the proliferation and osteogenic differentiation of mesenchymal stem cells derived from osteoporotic bone. J Orthop Trauma. 2010;24:552-556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 97] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 24. | Pountos I, Giannoudis PV, Jones E, English A, Churchman S, Field S, Ponchel F, Bird H, Emery P, McGonagle D. NSAIDS inhibit in vitro MSC chondrogenesis but not osteogenesis: implications for mechanism of bone formation inhibition in man. J Cell Mol Med. 2011;15:525-534. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 53] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 25. | Pountos I, Corscadden D, Emery P, Giannoudis PV. Mesenchymal stem cell tissue engineering: techniques for isolation, expansion and application. Injury. 2007;38 Suppl 4:S23-S33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 128] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 26. | Bianco P, Riminucci M, Bonucci E, Termine JD, Robey PG. Bone sialoprotein (BSP) secretion and osteoblast differentiation: relationship to bromodeoxyuridine incorporation, alkaline phosphatase, and matrix deposition. J Histochem Cytochem. 1993;41:183-191. [PubMed] |
| 27. | Turksen K, Aubin JE. Positive and negative immunoselection for enrichment of two classes of osteoprogenitor cells. J Cell Biol. 1991;114:373-384. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 124] [Cited by in RCA: 115] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 28. | Majors AK, Boehm CA, Nitto H, Midura RJ, Muschler GF. Characterization of human bone marrow stromal cells with respect to osteoblastic differentiation. J Orthop Res. 1997;15:546-557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 256] [Cited by in RCA: 236] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 29. | Zhao DM, Wang HB, Yang JF, Wu SQ, Liu JL, Xu FY, Qiu LP, Cai JL. Effect of vascular endothelial growth factor 165 gene transfection on bone defects and its mRNA expression in rabbits. Chin Med J (Engl). 2007;120:1187-1191. [PubMed] |
| 30. | Healey JH, Zimmerman PA, McDonnell JM, Lane JM. Percutaneous bone marrow grafting of delayed union and nonunion in cancer patients. Clin Orthop Relat Res. 1990;256:280-285. [PubMed] |
| 31. | Muschler GF, Midura RJ, Nakamoto C. Practical Modeling Concepts for Connective Tissue Stem Cell and Progenitor Compartment Kinetics. J Biomed Biotechnol. 2003;2003:170-193. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 60] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 32. | Gosain AK, Song L, Riordan P, Amarante MT, Nagy PG, Wilson CR, Toth JM, Ricci JL. A 1-year study of osteoinduction in hydroxyapatite-derived biomaterials in an adult sheep model: part I. Plast Reconstr Surg. 2002;109:619-630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 123] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 33. | Holmes RE. Bone regeneration within a coralline hydroxyapatite implant. Plast Reconstr Surg. 1979;63:626-633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 349] [Cited by in RCA: 269] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 34. | Tsuruga E, Takita H, Itoh H, Wakisaka Y, Kuboki Y. Pore size of porous hydroxyapatite as the cell-substratum controls BMP-induced osteogenesis. J Biochem. 1997;121:317-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 522] [Cited by in RCA: 393] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 35. | Vaccaro AR. The role of the osteoconductive scaffold in synthetic bone graft. Orthopedics. 2002;25:s571-s578. [PubMed] |
| 36. | Muschler GF, Nakamoto C, Griffith LG. Engineering principles of clinical cell-based tissue engineering. J Bone Joint Surg Am. 2004;86-A:1541-1558. [PubMed] |
| 37. | Liao H, Andersson AS, Sutherland D, Petronis S, Kasemo B, Thomsen P. Response of rat osteoblast-like cells to microstructured model surfaces in vitro. Biomaterials. 2003;24:649-654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 99] [Article Influence: 4.5] [Reference Citation Analysis (0)] |