Published online Sep 26, 2014. doi: 10.4252/wjsc.v6.i4.448
Revised: August 27, 2014
Accepted: August 30, 2014
Published online: September 26, 2014
Processing time: 65 Days and 9.7 Hours
The tissue kallikrein-kinin system exerts a wide spectrum of biological activities in the cardiovascular, renal and central nervous systems. Tissue kallikrein-kinin modulates the proliferation, viability, mobility and functional activity of certain stem cell populations, namely mesenchymal stem cells (MSCs), endothelial progenitor cells (EPCs), mononuclear cell subsets and neural stem cells. Stimulation of these stem cells by tissue kallikrein-kinin may lead to protection against renal, cardiovascular and neural damage by inhibiting apoptosis, inflammation, fibrosis and oxidative stress and promoting neovascularization. Moreover, MSCs and EPCs genetically modified with tissue kallikrein are resistant to hypoxia- and oxidative stress-induced apoptosis, and offer enhanced protective actions in animal models of heart and kidney injury and hindlimb ischemia. In addition, activation of the plasma kallikrein-kinin system promotes EPC recruitment to the inflamed synovium of arthritic rats. Conversely, cleaved high molecular weight kininogen, a product of plasma kallikrein, reduces the viability and vasculogenic activity of EPCs. Therefore, kallikrein-kinin provides a new approach in enhancing the efficacy of stem cell therapy for human diseases.
Core tip: Tissue kallikrein-kinin exerts beneficial actions in the cardiovascular, renal and central nervous systems. Recent studies demonstrated that genetic modification of mesenchymal stem cells (MSCs) and endothelial progenitor cells (EPCs) by tissue kallikrein provides enhanced protection against renal ischemia/reperfusion, lupus nephritis, myocardial infarction and hindlimb ischemia. Tissue kallikrein stimulates the proliferation, viability, migration and functional activity of cultured MSCs, EPCs and neural stem cells. Moreover, plasma kallikrein-kinin augments EPC mobility and function in arthritis, whereas the cleaved kininogen product of plasma kallikrein inhibits EPC viability and tube formation. Thus, kallikrein-kinin may enhance the efficacy of stem cell therapy for human diseases.
- Citation: Chao J, Bledsoe G, Chao L. Kallikrein-kinin in stem cell therapy. World J Stem Cells 2014; 6(4): 448-457
- URL: https://www.wjgnet.com/1948-0210/full/v6/i4/448.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v6.i4.448
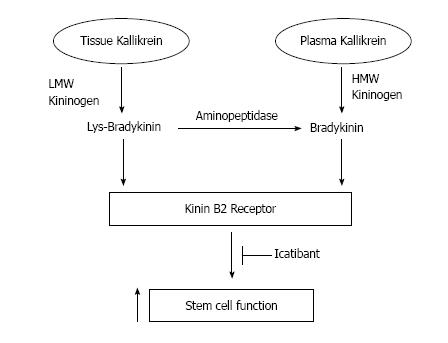
Tissue kallikrein (KLK1) and plasma kallikrein (KLKB1) are serine proteinases encoded by distinct genes, and thus differ in molecular weight, amino acid sequence and immunogenicity[1-3]. Human tissue kallikrein cleaves low molecular weight (LMW) kininogen to produce Lys-bradykinin (Lys-BK), which is subsequently converted to BK by aminopeptidase[2]. Plasma kallikrein processes high molecular weight (HMW) kininogen substrate to form BK[2]. Both kinin peptides bind to the kinin B2 receptor to elicit a diverse array of biological effects[2-5], including enhancing stem cell function (Figure 1). The kinin B2 receptor is constitutively expressed with a wide tissue distribution, but can be blocked by the specific antagonist icatibant (Hoe140)[5]. Kinin metabolites of kininase I, such as des-Arg9-BK and des-Arg10-Lys-BK, bind to the kinin B1 receptor, which is expressed at very low levels under normal conditions but is induced by inflammation[5]. The tissue kallikrein-kinin system triggers a broad spectrum of biological activities, including stimulation of angiogenesis and reduction of hypertension, cardiac and renal damage, ischemic stroke, restenosis, diabetes and skin wound injury[6]. Plasma kallikrein circulates in the blood as a proenzyme and, upon its activation, functions to produce BK to increase vascular permeability and stimulate vasodilation and inflammation[7,8]. Activated plasma kallikrein also initiates the intrinsic pathway of coagulation and the fibrinolytic system[7,8]. In this review, we discuss the involvement of tissue kallikrein, plasma kallikrein and kinin peptides in promoting the mobility and functional capacity of stem cells, which may lead to enhanced protection against organ injury in human diseases.
Tissue kallikrein was first discovered in human urine as a hypotensive substance[9]. Urinary (tissue) kallikrein excretion is significantly reduced in patients with mild kidney disease and severe renal failure[10,11]. Tissue kallikrein gene transfer or protein infusion in hypertensive Dahl salt-sensitive (DSS) rats has been observed to decrease kidney injury, improve renal function, and stimulate nitric oxide (NO) generation via the kinin B2 receptor[12-15]. Moreover, tissue kallikrein or kinin administration not only attenuated but also reversed renal inflammation, apoptosis and fibrosis in conjunction with reduced oxidative stress and increased NO production in hypertensive DSS and deoxycorticosterone acetate (DOCA)-salt rats[15-18]. The renal protective effects of tissue kallikrein in DSS rats were abolished by icatibant, indicating a kinin B2 receptor-mediated event[15]. Conversely, endogenous tissue kallikrein depletion in DOCA-salt rats augmented renal injury, inflammation and fibrosis in association with increased expression of pro-inflammatory and pro-fibrotic genes, oxidative stress, and reduced NO levels[19]. Moreover, double knockout of the kinin B1 and B2 receptors in mice demonstrated that these receptors protect against ischemia/reperfusion (I/R)-induced renal damage, apoptosis and mortality[20]. In a unilateral ureteral obstruction model, interstitial collagen content in the kidney was increased in kinin B2 receptor deficient mice, whereas transgenic rats expressing human tissue kallikrein displayed reduced renal fibrosis[21]. Therefore, endogenous tissue kallikrein-kinin via kinin B2 receptor signaling can prevent and reverse renal injury by inhibiting oxidative stress, apoptosis, inflammation and fibrosis.
Tissue kallikrein-kinin components have been localized in the heart and blood vessels, indicating their involvement in cardiovascular function[22-24]. Indeed, both tissue kallikrein and kinin B2 receptor knockout mice develop dilated cardiomyopathy, and mice with kinin B2 receptor genetic ablation exhibit cardiac fibrosis[25,26]. However, expression of tissue kallikrein in transgenic rats reduces isoproterenol-induced cardiac hypertrophy and fibrosis[27]. Likewise, tissue kallikrein gene delivery protects against cardiac remodeling as well as neovascularization in spontaneously hypertensive rats (SHR) and salt- and pressure-induced hypertensive rats[28-31]. Tissue kallikrein infusion or gene transfer also improved impaired cardiac function and reduced heart remodeling, apoptosis and inflammation in animal models of myocardial infarction (MI), myocardial I/R and streptozotocin-induced diabetes[32-36]. The cardioprotective effects of tissue kallikrein on apoptosis and inflammation were blocked by icatibant and a NO synthase (NOS) inhibitor, indicating a kinin B2 receptor-NO-mediated event[35,36]. Furthermore, tissue kallikrein gene delivery to the peri-infarct myocardium increased cardiac progenitor cell (CPC) levels and promoted cardiac neovascularization and function in rats with post-MI heart failure[37]. Although tissue kallikrein increased CPC density, their levels were low compared to other cardiac cells. Thus, the regenerative capacity of CPCs in the adult heart appears to be limited and requires further investigation. Taken together, tissue kallikrein-kinin elicits cardiac protection by inhibiting apoptosis, inflammation and myocardial remodeling, and increasing angiogenesis through kinin B2 receptor-NO signaling.
Endothelial cell loss leads to vascular dysfunction and vascular-related diseases. Tissue kallikrein levels in the circulation are significantly higher in patients with coronary artery disease (CAD) compared to non-CAD patients, and increase with disease severity, from moderate CAD to multi-vessel CAD with acute obstruction[38]. This suggests that circulating tissue kallikrein levels may be used as a predictive tool to assess the presence and extent of CAD. Tissue kallikrein gene transfer into rat left common carotid artery after balloon angioplasty was shown to cause a marked reduction in neointima formation at the injured vessel, and this effect was mediated by a kinin B2 receptor-NO pathway[39,40]. In addition, endothelium-dependent relaxation was improved in tissue kallikrein transgenic rats with diabetic cardiomyopathy, but significantly reduced in kinin B1 and B2 receptor knockout mice in association with a decrease in NO production[41,42]. Moreover, kinin B2 receptor-deficient mice exhibit myocardial capillary rarefaction[43]. Conversely, tissue kallikrein gene delivery promoted neovascularization and attenuated cardiac remodeling in animal models of hypertension and MI[31,32]. Tissue kallikrein is capable of accelerating spontaneous angiogenesis in a mouse model of hindlimb ischemia by activating Akt and endothelial NOS (eNOS) signaling pathways[44,45]. Tissue kallikrein also enhanced the migration and tube formation of cultured endothelial cells, but these effects were blocked by icatibant, constitutively active glycogen synthase kinase (GSK)-3β, vascular endothelial growth factor (VEGF) antibody and VEGF receptor inhibitor[46]. Furthermore, kinin stimulated the proliferation and capillary tube formation of endothelial cells via transactivation of VEGF receptor-2 through the kinin B2 receptor[47,48]. These findings indicate that tissue kallikrein-kinin attenuates vascular injury by preventing neointima formation and promoting angiogenesis through Akt-eNOS and Akt-GSK-3β-VEGF mediated signaling pathways.
The time window for treatment of stroke patients is limited, as the clinically accepted treatment regimen with tissue plasminogen activator (tPA) requires initiation within 3 h of symptom onset[49]. Tissue kallikrein has a superior advantage over tPA with a wide time window after stroke. In a double-blinded clinical trial, human tissue kallikrein was shown to be effective in the treatment of patients with acute brain infarction when infused within 48 h of established stroke[50]. These findings indicate that tissue kallikrein therapy is a promising regimen in the treatment of ischemic stroke in humans. Moreover, tissue kallikrein-kinin therapy has been shown to be an effective approach in the treatment of stroke-induced brain injury in animal models[51,52]. Neuroprotective effects were observed upon local injection of the human tissue kallikrein gene into rat brain immediately after cerebral I/R injury, or by systemic delivery of the tissue kallikrein gene at 8 h after ischemic stroke onset[51,52]. Tissue kallikrein administration reduced I/R-induced cerebral infarction and promoted the survival and migration of glial cells from penumbra to the ischemic core up to two weeks[51,52]. Tissue kallikrein also decreased I/R-induced apoptosis of neuronal cells and inhibited inflammatory cell accumulation in the ischemic brain, but these effects were blocked by icatibant[52]. Furthermore, tissue kallikrein gene transfer enhanced neurogenesis and angiogenesis in rats after cerebral I/R[52]. Tissue kallikrein’s effects occurred in association with increased NO levels and reduced oxidative stress via activation of the kinin B2 receptor[51,52]. In contrast, ischemic brain injury is exacerbated in kinin B2 receptor knockout mice[53]. Thus, tissue kallikrein-kinin therapy may serve as a valuable approach in the treatment of stroke-induced brain injury, especially if treatment is delayed.
Mesenchymal stem cells (MSCs) are heterogeneous, multi-potent stromal cells that possess non-immunogenic and immunosuppressive properties[54]. MSCs have been documented to reside in bone marrow, adipose tissue, umbilical cord blood, placenta, amniotic fluid and amniotic membrane[55]. MSCs can be characterized by three main criteria: (1) adherent to plastic culture dishes; (2) expression of the cell surface markers CD73, CD90, CD105, and CD271; and (3) differentiation into lineages of osteoblasts, adipocytes, and chondroblasts in vitro[55]. MSCs have the ability to migrate to sites of organ injury and participate in tissue repair by exerting paracrine actions to produce therapeutic effects, such as neovascularization and organ regeneration[54,56-59]. Clinical trials using human bone narrow-derived MSCs are currently underway to treat diseases such as renal, cardiovascular, and cerebrovascular disorders (http://clinicaltrials.gov). Efficacy can be maximized by pre-treatment of MSCs with drugs, cytokines, and growth factors, and by genetically modifying MSCs[60]. Indeed, enhancing stem cell therapy by genetic modification has been shown provide advanced benefits in the treatment of various diseases[61]. For example, MSCs genetically modified with hepatocyte growth factor or VEGF ameliorated I/R- or cisplatin-induced renal damage, inflammation and apoptosis[62,63]. Moreover, modification of MSCs with the anti-apoptotic Akt gene or the anti-oxidant heme oxygenase-1 gene was observed to augment ischemic cardiac function and stem cell viability, and decrease ventricular remodeling and apoptosis compared to control MSCs[64,65]. Thus, modification of MSCs with a gene that suppresses inflammation, apoptosis and oxidative stress would be highly desirable in the treatment of renal and cardiovascular dysfunction. Tissue kallikrein fits this profile, and MSCs modified with tissue kallikrein have been shown to exert enhanced protective actions in the heart and kidney as well as in vitro[66,67].
Bone marrow-derived rat MSCs transduced with adenovirus harboring the human tissue kallikrein gene (TK-MSCs) secrete tissue kallikrein along with elevated VEGF levels in culture medium[66,67]. TK-MSCs were also found to be more resistant to hypoxia- and H2O2-induced apoptosis, and exhibited less caspase-3 activity compared to control MSCs. In addition, TK-MSC conditioned medium stimulated the proliferation, migration and tube formation of cultured human endothelial cells, most likely via VEGF[67]. In cultured cardiomyocytes, conditioned medium from TK-MSCs suppressed hypoxia-induced apoptosis and caspase-3 activity, and increased Akt phosphorylation[67]. Moreover, human MSCs possess kinin B2 receptors, as kinin stimulation increased intracellular calcium levels in MSCs, but this effect was blocked by icatibant[68]. This suggests that TK-MSCs exert their effects via autocrine and paracrine mechanisms. Furthermore, these results demonstrate that culture medium of MSCs genetically modified with the tissue kallikrein gene promotes the function, migration and viability of cultured endothelial and cardiac cells.
Acute renal failure is a common disease with high morbidity and mortality[69]. In kidney transplants, ischemia can lead to long-term renal dysfunction[69,70]. However, implantation of bone marrow-derived MSCs after acute I/R resulted in renal function and morphological recovery, implicating the high therapeutic potential of MSCs in healing damaged kidney[56,71]. Indeed, TK-MSC administration in rats subjected to I/R injury was shown to be protective against kidney damage[66]. After systemic injection of TK-MSCs, human tissue kallikrein expression was identified in rat glomeruli. Rats receiving TK-MSCs exhibited an improvement in renal function after I/R. TK-MSC implantation in the kidney also markedly reduced tubular injury, renal cell apoptosis, and interstitial inflammatory cell accumulation. The protective effects of TK-MSCs occurred in conjunction with decreased myeloperoxidase activity, superoxide formation, and pro-inflammatory gene expression. Therefore, MSCs incorporating the human tissue kallikrein gene have advanced benefits in protection against ischemia-induced renal injury by suppression of oxidative stress, apoptosis and inflammation.
Tissue kallikrein has been identified as a lupus nephritis-susceptibility gene and is associated with anti-glomerular basement membrane (GBM) antibody-induced nephritis[72,73]. TK-MSCs were shown to exert beneficial effects in mice receiving anti-GBM antibody injection and in a murine model of lupus nephritis by suppressing inflammation and oxidative stress[74]. TK-MSC administration to mice subjected to anti-GBM antibody injection resulted in the expression of human tissue kallikrein in the kidney as well as a significant reduction in proteinuria, blood urea nitrogen levels and renal pathology, compared to mice injected with control MSCs. Similarly, TK-MSC implantation in lupus-prone bicongenic mice improved kidney function and attenuated renal inflammatory cell infiltration and apoptosis in conjunction with reduced expression of numerous inflammatory cytokines and apoptotic factors in both kidney and serum. These novel findings indicate that tissue kallikrein-modified MSCs may serve as a targeted therapeutic agent in lupus nephritis.
Chronic heart failure induced by MI leads to a loss of cardiac tissue and impairs left ventricular function[58]. MSCs are a promising strategy for the repair and regeneration of heart cells as well as the restoration of cardiac function after an ischemic insult. However, a major limitation to the efficacy of stem cell therapy is the poor viability of implanted cells. Thus, genetic modification of MSCs to promote their viability may further aid in the treatment of cardiac damage. Cell culture studies showed that TK-MSCs display decreased apoptosis induced by hypoxia or oxidative stress[66,67]. In rats with acute and chronic MI, myocardial injection of TK-MSCs resulted in enhanced cardiac protection compared to control MSC treatment[67]. One day after MI, rats receiving TK-MSC administration were shown to have improved cardiac function and decreased apoptosis, inflammatory cell accumulation, and expression of pro-inflammatory genes. At two weeks after MI, TK-MSC implantation enhanced cardiac function, decreased infarct size, and attenuated cardiac hypertrophy and fibrosis. Furthermore, TK-MSC injection increased capillary and arteriole density in the peri-infarct area. These results indicate that TK-MSC treatment after acute and chronic MI provides significant protection against heart damage by promoting neovascularization and preventing apoptosis and inflammation.
Endothelial injury is a critical factor for complications associated with cardiovascular disease[75]. Endothelial progenitor cells (EPCs) are a continuous endogenous source of replenishment for damaged vessels, and thus serve to maintain vascular integrity in response to endothelial injury[75,76]. Bone marrow-derived EPCs are considered to be adult stem cells due to their participation in postnatal angiogenesis[77]. EPCs contribute to vasculogenesis by incorporating into the vasculature, thereby implicating their therapeutic potential in endothelial repair[78]. Decreased numbers of circulating EPCs have been observed in patients with hypertension, chronic renal failure, CAD, and rheumatoid arthritis[78-81]. Moreover, EPCs isolated from patients with hypertension and CAD displayed an impaired migratory response[79]. However, the correlation of circulating EPC number and outcome of stroke patients is inconsistent. Lower EPC numbers were found to be associated with acute ischemic stroke[82], whereas higher EPC levels were reported in hemorrhagic stroke patients[83]. Reduced EPC numbers may be attributed not only to defective mobility and proliferation, but also to accelerated apoptosis or senescence. Therefore, augmented viability and mobilization of EPCs from bone marrow may be an alternative means to promote vascular repair. Furthermore, EPCs may serve as a vehicle for gene transfer approaches in the treatment of cardiovascular diseases. The tissue kallikrein-kinin system has been shown to be involved in cardiovascular remodeling, vascular function and angiogenesis[6], making tissue kallikrein an ideal candidate for EPC genetic modification.
Healthy human subjects express high levels of kinin B2 receptor in CD133+CD34+ peripheral blood-mononuclear cell (PB-MNC) subsets and EPCs; kinin B1 receptor expression, however, is barely detectable in these cells[84]. kinin administration exerted a potent chemoattractant activity on EPCs via a kinin B2 receptor-phosphoinositide 3-kinase (PI3K)-eNOS-mediated mechanism. The role of the kinin B2 receptor in kinin-induced migration was verified using EPCs derived from kinin B2 receptor knockout mice. Kinin-responsive human PB-MNCs exhibited a pronounced pro-angiogenic activity, whereas EPCs from kinin B2 receptor-deficient mice were unable to sufficiently stimulate neovascularization in a mouse model of hindlimb ischemia. In addition, circulating CD133+CD34+ progenitor cells from patients with acute MI or stable angina expressed low levels of kinin B2 receptor, which corresponded to diminished migratory capacity toward kinin. Moreover, human circulating CD34+CXCR4+ MNCs expressing high levels of kinin B2 receptor adhered to cultured endothelial cells upon kinin treatment, and these kinin-stimulated mononuclear subsets were recruited to injured arterial wall in vivo via the kinin B2 receptor[85]. Conversely, CD34+CXCR4+ MNCs from CAD patients exhibited low kinin B2 receptor expression levels. Furthermore, kinin administration had no effect on cellular recruitment upon icatibant treatment or in monocytes with low kinin B2 receptor expression. These studies indicate a novel mechanism of kinin B2 receptor activation in endothelial repair through recruitment of circulating EPCs and MNC subsets.
Tissue kallikrein was recently demonstrated to promote vasculogenesis and improve cardiac function after MI by enhancing peripheral EPC functional capacity[86,87]. Human tissue kallikrein gene delivery significantly increased the number of circulating CD34+Flk-1+ EPCs as well as the growth of capillaries and arterioles in the peri-infarct myocardium in a mouse model of MI[86]. In cultured EPCs, tissue kallikrein treatment stimulated cell migration and tube formation, and decreased hypoxia-induced apoptosis[86]. Tissue kallikrein’s effects were blocked by icatibant and a PI3K inhibitor, indicating a kinin B2 receptor-Akt signaling event. Moreover, adenovirus-mediated transduction of cultured EPCs with tissue kallikrein (TK-EPCs) resulted in the secretion of tissue kallikrein and VEGF into culture medium[86,87]. TK-EPCs were also resistant to oxidative stress- and hypoxia-induced apoptosis in association with increased Akt phosphorylation and decreased caspase activity. Furthermore, mice receiving intra-myocardial injection of TK-EPCs after MI exhibited advanced protection against ischemic damage, as indicated by improved cardiac function and reduced infarct size[87]. TK-EPC engraftment significantly decreased cardiomyocyte apoptosis and increased the retention of transplanted EPCs in the myocardium. The effects of TK-EPC administration were accompanied by increased capillary and arteriole density in the infarct border zone. These results show that implantation of tissue kallikrein-modified EPCs in the heart augments protection against cardiac injury by reducing apoptosis and promoting angiogenesis.
Tissue kallikrein’s pro-angiogenic activity has been clearly established[6,44,45,86], and genetic modification of EPCs with tissue kallikrein was shown to promote neovascularization and cardiac function in an MI mouse model[87]. Moreover, the effect of TK-EPC administration on spontaneous angiogenesis was identified in a rat model of hindlimb ischemia[88]. Compared to control EPCs, TK-EPC injection via the caudal vein markedly increased muscular capillary density, blood flow and myofiber number at 7, 14 and 21 d after femoral artery ligation. The angiogenic effect of TK-EPCs correlated with elevated expression of eNOS and integrin v 3 on the surface of EPCs. Moreover, cultured TK-EPCs exhibited higher proliferative, migratory and adhesive activity than control EPCs[88]. Inhibition of integrin v 3 blocked TK-EPC migration and adhesion, but had no effect on the proliferative activity of TK-EPCs. This suggests that EPCs genetically modified with tissue kallikrein enhance neovascularization and blood perfusion recovery after hindlimb ischemia.
Tissue kallikrein-kinin treatment has been shown to be effective in preventing stroke-induced ischemic brain injury by promoting neurogenesis and angiogenesis in animal models and cultured cells[51,52]. In addition, tissue kallikrein was observed to stimulate the growth of rat neural stem cells independent of kinin formation, as icatibant had no effect on tissue kallikrein’s actions[89,90]. However, tissue kallikrein did not induce the differentiation of neural stem cells to neurons or glial cells[90]. The proliferation of neural stem cells by tissue kallikrein is quite specific, with no detectable effect on other cell types, such as glial, pheochromocytoma, pituitary tumor, and cervical cancer cells[90]. Thus, stimulation of neural stem cell proliferation by tissue kallikrein administration may lead to the generation of new neurons in the ischemic brain. Importantly, this stimulating effect of tissue kallikrein on neural stem cells may have significant value in the treatment of ischemic stroke.
Plasma kallikrein has been demonstrated to play a role in the pathogenesis of arthritis[91,92]. As kinins are known to promote EPC mobilization and functional activity[84,85], the involvement of the plasma kallikrein-kinin system in EPC mobilization was examined in a Lewis rat model of arthritis[93]. The Lewis rat strain possesses a mutation in HMW kininogen (HK), resulting in accelerated HK cleavage and increased susceptibility to chronic inflammation[94]. In arthritic Lewis rats, EPCs were recruited to the synovium at the acute phase of arthritis, and then differentiated into endothelial cells to form new blood vessels[93]. Inhibition of plasma kallikrein by a specific inhibitor or anti-plasma kallikrein antibody dramatically suppressed synovial recruitment of EPCs and the proliferation of synovial cells. Moreover, EPCs isolated from bone marrow of Lewis rats were observed to have higher expression levels of kinin B2 receptor compared to control rat lung microvessel endothelial cells[93]. In addition, kinin stimulated EPC migration and up-regulated expression of the homing receptor CXCR4 in vitro via the kinin B2 receptor. These results demonstrate a potential role of plasma kallikrein-kinin, via a kinin B2 receptor-dependent mechanism, in the recruitment of EPCs to inflamed synovium in arthritis.
Cleaved HMW kininogen (HKa), a product of plasma kallikrein, has been shown to reduce the angiogenic function of endothelial cells as well as to stimulate their apoptosis[95,96]. In cultured EPCs, HKa significantly inhibited VEGF-mediated tube formation and cellular differentiation into capillary-like networks[97]. VEGF stimulated the secretion and activation of matrix metalloproteinase-2 (MMP-2), but not MMP-9, in the conditioned medium of EPCs. Inhibition or gene knockdown of MMP-2 indicated that this enzyme is required for EPC vasculogenesis. Although HKa prevented the conversion of pro-MMP-2 to MMP-2, it had no effect on MMP-2 activity. Furthermore, HKa was demonstrated to accelerate EPC senescence by increasing oxidative stress, leading to activation of the p38MAPK-p16INK4a signaling cascade[98]. These results indicate that HKa inhibits the vasculogenic capacity of EPCs by suppressing MMP-2 activation and promoting EPC senescence via oxidative stress-p38MAPK signaling, thus providing a link between the plasma kallikrein product HKa and EPC function.
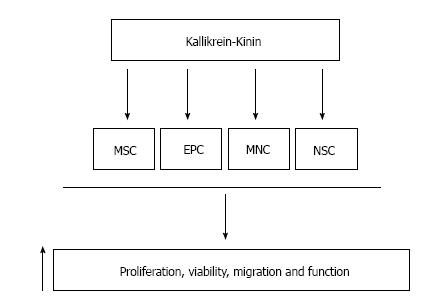
The tissue kallikrein-kinin system plays an important role in the cardiovascular, renal and central nervous systems by inhibiting apoptosis, inflammation, fibrosis and oxidative stress. Tissue kallikrein-kinin may also enhance stem cell number and function. Indeed, tissue kallikrein-kinin increases the mobility, viability and functional capacity of stem cells, such as MSCs, EPCs, and MNC subsets, leading to protection against multi-organ injury and stimulating neovascularization. Tissue kallikrein may also exert a protective effect against cerebral ischemic damage in stroke patients by promoting neural stem cell growth. Moreover, studies showed that tissue kallikrein-modified MSC or EPC engraftment into injured tissues provided advanced protection against vascular and organ damage (Table 1). Thus, transplantation of tissue kallikrein-modified stem cells may be used for the treatment of patients with renal, cardiovascular, and cerebrovascular diseases. Furthermore, plasma kallikrein-kinin was observed to enhance EPC mobility and functional capacity in arthritis, while the cleaved kininogen product HKa inhibited EPC tube formation and viability. Collectively, these studies show that kallikrein-kinin stimulates the proliferation, viability, migration and function of various types of stem cells (Figure 2), and implicate the potential role of kallikrein-kinin in stem cell-based therapy for numerous human diseases.
P- Reviewer: Miloso M, Pimentel-Coelho PM, Pochynyuk O, Yu J S- Editor: Tian YL L- Editor: A E- Editor: Lu YJ
| 1. | Schachter M. Kallikreins (kininogenases) – a group of serine proteases with bioregulatory actions. Pharmacol Rev. 1979;31:1-17. [DOI] [Full Text] |
| 2. | Bhoola KD, Figueroa CD, Worthy K. Bioregulation of kinins: kallikreins, kininogens, and kininases. Pharmacol Rev. 1992;44:1-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 3. | Clements JA. The human kallikrein gene family: a diversity of expression and function. Mol Cell Endocrinol. 1994;99:C1-C6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 71] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 4. | Moreau ME, Garbacki N, Molinaro G, Brown NJ, Marceau F, Adam A. The kallikrein-kinin system: current and future pharmacological targets. J Pharmacol Sci. 2005;99:6-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 317] [Cited by in RCA: 334] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 5. | Regoli D, Rhaleb NE, Drapeau G, Dion S. Kinin receptor subtypes. J Cardiovasc Pharmacol. 1990;15 Suppl 6:S30-S38. [PubMed] |
| 6. | Chao J, Shen B, Gao L, Xia CF, Bledsoe G, Chao L. Tissue kallikrein in cardiovascular, cerebrovascular and renal diseases and skin wound healing. Biol Chem. 2010;391:345-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 84] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 7. | Colman RW, Schmaier AH. Contact system: a vascular biology modulator with anticoagulant, profibrinolytic, antiadhesive, and proinflammatory attributes. Blood. 1997;90:3819-3843. [PubMed] |
| 8. | Björkqvist J, Jämsä A, Renné T. Plasma kallikrein: the bradykinin-producing enzyme. Thromb Haemost. 2013;110:399-407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 112] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 9. | Abelous JE, Bardier E. Les substances hypertensives de l’urine humaine normale. C R Soc Biol. 1909;66:511-512. |
| 10. | Price RG. Urinary enzymes, nephrotoxicity and renal disease. Toxicology. 1982;23:99-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 313] [Cited by in RCA: 277] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 11. | Naicker S, Naidoo S, Ramsaroop R, Moodley D, Bhoola K. Tissue kallikrein and kinins in renal disease. Immunopharmacology. 1999;44:183-192. [PubMed] |
| 12. | Uehara Y, Hirawa N, Kawabata Y, Suzuki T, Ohshima N, Oka K, Ikeda T, Goto A, Toyo-oka T, Kizuki K. Long-term infusion of kallikrein attenuates renal injury in Dahl salt-sensitive rats. Hypertension. 1994;24:770-778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 51] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 13. | Chao J, Zhang JJ, Lin KF, Chao L. Adenovirus-mediated kallikrein gene delivery reverses salt-induced renal injury in Dahl salt-sensitive rats. Kidney Int. 1998;54:1250-1260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 43] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 14. | Hirawa N, Uehara Y, Suzuki T, Kawabata Y, Numabe A, Gomi T, lkeda T, Kizuki K, Omata M. Regression of glomerular injury by kallikrein infusion in Dahl salt-sensitive rats is a bradykinin B2-receptor-mediated event. Nephron. 1999;81:183-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 31] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 15. | Bledsoe G, Shen B, Yao Y, Zhang JJ, Chao L, Chao J. Reversal of renal fibrosis, inflammation, and glomerular hypertrophy by kallikrein gene delivery. Hum Gene Ther. 2006;17:545-555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 47] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 16. | Zhang JJ, Bledsoe G, Kato K, Chao L, Chao J. Tissue kallikrein attenuates salt-induced renal fibrosis by inhibition of oxidative stress. Kidney Int. 2004;66:722-732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 50] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 17. | Xia CF, Bledsoe G, Chao L, Chao J. Kallikrein gene transfer reduces renal fibrosis, hypertrophy, and proliferation in DOCA-salt hypertensive rats. Am J Physiol Renal Physiol. 2005;289:F622-F631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 18. | Chao J, Li HJ, Yao YY, Shen B, Gao L, Bledsoe G, Chao L. Kinin infusion prevents renal inflammation, apoptosis, and fibrosis via inhibition of oxidative stress and mitogen-activated protein kinase activity. Hypertension. 2007;49:490-497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 52] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 19. | Liu Y, Bledsoe G, Hagiwara M, Yang ZR, Shen B, Chao L, Chao J. Blockade of endogenous tissue kallikrein aggravates renal injury by enhancing oxidative stress and inhibiting matrix degradation. Am J Physiol Renal Physiol. 2010;298:F1033-F1040. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 20. | Kakoki M, McGarrah RW, Kim HS, Smithies O. Bradykinin B1 and B2 receptors both have protective roles in renal ischemia/reperfusion injury. Proc Natl Acad Sci USA. 2007;104:7576-7581. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 110] [Cited by in RCA: 102] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 21. | Schanstra JP, Neau E, Drogoz P, Arevalo Gomez MA, Lopez Novoa JM, Calise D, Pecher C, Bader M, Girolami JP, Bascands JL. In vivo bradykinin B2 receptor activation reduces renal fibrosis. J Clin Invest. 2002;110:371-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 32] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 22. | Xiong W, Chen LM, Woodley-Miller C, Simson JA, Chao J. Identification, purification, and localization of tissue kallikrein in rat heart. Biochem J. 1990;267:639-646. [PubMed] |
| 23. | Nolly H, Carbini LA, Scicli G, Carretero OA, Scicli AG. A local kallikrein-kinin system is present in rat hearts. Hypertension. 1994;23:919-923. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 107] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 24. | Wolf WC, Harley RA, Sluce D, Chao L, Chao J. Localization and expression of tissue kallikrein and kallistatin in human blood vessels. J Histochem Cytochem. 1999;47:221-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 64] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 25. | Emanueli C, Maestri R, Corradi D, Marchione R, Minasi A, Tozzi MG, Salis MB, Straino S, Capogrossi MC, Olivetti G. Dilated and failing cardiomyopathy in bradykinin B(2) receptor knockout mice. Circulation. 1999;100:2359-2365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 107] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 26. | Meneton P, Bloch-Faure M, Hagege AA, Ruetten H, Huang W, Bergaya S, Ceiler D, Gehring D, Martins I, Salmon G. Cardiovascular abnormalities with normal blood pressure in tissue kallikrein-deficient mice. Proc Natl Acad Sci USA. 2001;98:2634-2639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 122] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 27. | Silva JA, Araujo RC, Baltatu O, Oliveira SM, Tschöpe C, Fink E, Hoffmann S, Plehm R, Chai KX, Chao L. Reduced cardiac hypertrophy and altered blood pressure control in transgenic rats with the human tissue kallikrein gene. FASEB J. 2000;14:1858-1860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 88] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 28. | Chao J, Zhang JJ, Lin KF, Chao L. Human kallikrein gene delivery attenuates hypertension, cardiac hypertrophy, and renal injury in Dahl salt-sensitive rats. Hum Gene Ther. 1998;9:21-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 81] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 29. | Yayama K, Wang C, Chao L, Chao J. Kallikrein gene delivery attenuates hypertension and cardiac hypertrophy and enhances renal function in Goldblatt hypertensive rats. Hypertension. 1998;31:1104-1110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 60] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 30. | Wolf WC, Yoshida H, Agata J, Chao L, Chao J. Human tissue kallikrein gene delivery attenuates hypertension, renal injury, and cardiac remodeling in chronic renal failure. Kidney Int. 2000;58:730-739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 64] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 31. | Bledsoe G, Chao L, Chao J. Kallikrein gene delivery attenuates cardiac remodeling and promotes neovascularization in spontaneously hypertensive rats. Am J Physiol Heart Circ Physiol. 2003;285:H1479-H1488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 34] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 32. | Agata J, Chao L, Chao J. Kallikrein gene delivery improves cardiac reserve and attenuates remodeling after myocardial infarction. Hypertension. 2002;40:653-659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 66] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 33. | Montanari D, Yin H, Dobrzynski E, Agata J, Yoshida H, Chao J, Chao L. Kallikrein gene delivery improves serum glucose and lipid profiles and cardiac function in streptozotocin-induced diabetic rats. Diabetes. 2005;54:1573-1580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 59] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 34. | Yin H, Chao L, Chao J. Kallikrein/kinin protects against myocardial apoptosis after ischemia/reperfusion via Akt-glycogen synthase kinase-3 and Akt-Bad.14-3-3 signaling pathways. J Biol Chem. 2005;280:8022-8030. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 88] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 35. | Yao YY, Yin H, Shen B, Chao L, Chao J. Tissue kallikrein infusion prevents cardiomyocyte apoptosis, inflammation and ventricular remodeling after myocardial infarction. Regul Pept. 2007;140:12-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 43] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 36. | Yin H, Chao L, Chao J. Nitric oxide mediates cardiac protection of tissue kallikrein by reducing inflammation and ventricular remodeling after myocardial ischemia/reperfusion. Life Sci. 2008;82:156-165. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 37] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 37. | Spillmann F, Graiani G, Van Linthout S, Meloni M, Campesi I, Lagrasta C, Westermann D, Tschöpe C, Quaini F, Emanueli C. Regional and global protective effects of tissue kallikrein gene delivery to the peri-infarct myocardium. Regen Med. 2006;1:235-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 38. | Yao YY, Fu C, Ma GS, Feng Y, Shen CX, Wu GQ, Zhang XG, Ding JD, Tang CC, Chen Z. Tissue kallikrein is related to the severity of coronary artery disease. Clin Chim Acta. 2013;423:90-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 39. | Murakami H, Yayama K, Miao RQ, Wang C, Chao L, Chao J. Kallikrein gene delivery inhibits vascular smooth muscle cell growth and neointima formation in the rat artery after balloon angioplasty. Hypertension. 1999;34:164-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 31] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 40. | Murakami H, Miao RQ, Chao L, Chao J. Adenovirus-mediated kallikrein gene transfer inhibits neointima formation via increased production of nitric oxide in rat artery. Immunopharmacology. 1999;44:137-143. [PubMed] |
| 41. | Tschöpe C, Walther T, Escher F, Spillmann F, Du J, Altmann C, Schimke I, Bader M, Sanchez-Ferrer CF, Schultheiss HP. Transgenic activation of the kallikrein-kinin system inhibits intramyocardial inflammation, endothelial dysfunction and oxidative stress in experimental diabetic cardiomyopathy. FASEB J. 2005;19:2057-2059. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 99] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 42. | Loiola RA, Reis FC, Kawamoto EM, Scavone C, Abdalla DS, Fernandes L, Pesquero JB. Role of vascular Kinin B1 and B2 receptors in endothelial nitric oxide metabolism. Peptides. 2011;32:1700-1705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 43. | Maestri R, Milia AF, Salis MB, Graiani G, Lagrasta C, Monica M, Corradi D, Emanueli C, Madeddu P. Cardiac hypertrophy and microvascular deficit in kinin B2 receptor knockout mice. Hypertension. 2003;41:1151-1155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 56] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 44. | Emanueli C, Minasi A, Zacheo A, Chao J, Chao L, Salis MB, Straino S, Tozzi MG, Smith R, Gaspa L. Local delivery of human tissue kallikrein gene accelerates spontaneous angiogenesis in mouse model of hindlimb ischemia. Circulation. 2001;103:125-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 122] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 45. | Emanueli C, Madeddu P. Angiogenesis therapy with human tissue kallikrein for the treatment of ischemic diseases. Arch Mal Coeur Vaiss. 2004;97:679-687. [PubMed] |
| 46. | Yao YY, Yin H, Shen B, Smith RS, Liu Y, Gao L, Chao L, Chao J. Tissue kallikrein promotes neovascularization and improves cardiac function by the Akt-glycogen synthase kinase-3beta pathway. Cardiovasc Res. 2008;80:354-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 47] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 47. | Thuringer D, Maulon L, Frelin C. Rapid transactivation of the vascular endothelial growth factor receptor KDR/Flk-1 by the bradykinin B2 receptor contributes to endothelial nitric-oxide synthase activation in cardiac capillary endothelial cells. J Biol Chem. 2002;277:2028-2032. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 95] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 48. | Miura S, Matsuo Y, Saku K. Transactivation of KDR/Flk-1 by the B2 receptor induces tube formation in human coronary endothelial cells. Hypertension. 2003;41:1118-1123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 62] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 50. | Ding DY, Lu CZ, Ding MP, Su BH, Chen FA. Multicenter, randomized, double-blinded and placebo-controlled study of acute brain infarction treated by human urinary kallidinogenase. Chin J Neurol. 2007;40:306-310. |
| 51. | Xia CF, Yin H, Borlongan CV, Chao L, Chao J. Kallikrein gene transfer protects against ischemic stroke by promoting glial cell migration and inhibiting apoptosis. Hypertension. 2004;43:452-459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 94] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 52. | Xia CF, Yin H, Yao YY, Borlongan CV, Chao L, Chao J. Kallikrein protects against ischemic stroke by inhibiting apoptosis and inflammation and promoting angiogenesis and neurogenesis. Hum Gene Ther. 2006;17:206-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 99] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 53. | Xia CF, Smith RS, Shen B, Yang ZR, Borlongan CV, Chao L, Chao J. Postischemic brain injury is exacerbated in mice lacking the kinin B2 receptor. Hypertension. 2006;47:752-761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 82] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 54. | Yokoo T, Sakurai K, Ohashi T, Kawamura T. Stem cell gene therapy for chronic renal failure. Curr Gene Ther. 2003;3:387-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 55. | Lee KD. Applications of mesenchymal stem cells: an updated review. Chang Gung Med J. 2008;31:228-236. [PubMed] |
| 56. | Tögel F, Hu Z, Weiss K, Isaac J, Lange C, Westenfelder C. Administered mesenchymal stem cells protect against ischemic acute renal failure through differentiation-independent mechanisms. Am J Physiol Renal Physiol. 2005;289:F31-F42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 894] [Cited by in RCA: 878] [Article Influence: 43.9] [Reference Citation Analysis (0)] |
| 57. | Gnecchi M, He H, Liang OD, Melo LG, Morello F, Mu H, Noiseux N, Zhang L, Pratt RE, Ingwall JS. Paracrine action accounts for marked protection of ischemic heart by Akt-modified mesenchymal stem cells. Nat Med. 2005;11:367-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1249] [Cited by in RCA: 1263] [Article Influence: 63.2] [Reference Citation Analysis (0)] |
| 58. | Choi SH, Jung SY, Kwon SM, Baek SH. Perspectives on stem cell therapy for cardiac regeneration. Advances and challenges. Circ J. 2012;76:1307-1312. [PubMed] [DOI] [Full Text] |
| 59. | Shin L, Peterson DA. Human mesenchymal stem cell grafts enhance normal and impaired wound healing by recruiting existing endogenous tissue stem/progenitor cells. Stem Cells Transl Med. 2013;2:33-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 99] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 60. | Mastri M, Lin H, Lee T. Enhancing the efficacy of mesenchymal stem cell therapy. World J Stem Cells. 2014;6:82-93. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 78] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 61. | Dzau VJ, Gnecchi M, Pachori AS. Enhancing stem cell therapy through genetic modification. J Am Coll Cardiol. 2005;46:1351-1353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 40] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 62. | Chen Y, Qian H, Zhu W, Zhang X, Yan Y, Ye S, Peng X, Li W, Xu W. Hepatocyte growth factor modification promotes the amelioration effects of human umbilical cord mesenchymal stem cells on rat acute kidney injury. Stem Cells Dev. 2011;20:103-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 76] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 63. | Yuan L, Wu MJ, Sun HY, Xiong J, Zhang Y, Liu CY, Fu LL, Liu DM, Liu HQ, Mei CL. VEGF-modified human embryonic mesenchymal stem cell implantation enhances protection against cisplatin-induced acute kidney injury. Am J Physiol Renal Physiol. 2011;300:F207-F218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 80] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 64. | Mangi AA, Noiseux N, Kong D, He H, Rezvani M, Ingwall JS, Dzau VJ. Mesenchymal stem cells modified with Akt prevent remodeling and restore performance of infarcted hearts. Nat Med. 2003;9:1195-1201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1200] [Cited by in RCA: 1145] [Article Influence: 52.0] [Reference Citation Analysis (0)] |
| 65. | Tang YL, Tang Y, Zhang YC, Qian K, Shen L, Phillips MI. Improved graft mesenchymal stem cell survival in ischemic heart with a hypoxia-regulated heme oxygenase-1 vector. J Am Coll Cardiol. 2005;46:1339-1350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 321] [Cited by in RCA: 313] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 66. | Hagiwara M, Shen B, Chao L, Chao J. Kallikrein-modified mesenchymal stem cell implantation provides enhanced protection against acute ischemic kidney injury by inhibiting apoptosis and inflammation. Hum Gene Ther. 2008;19:807-819. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 67. | Gao L, Bledsoe G, Yin H, Shen B, Chao L, Chao J. Tissue kallikrein-modified mesenchymal stem cells provide enhanced protection against ischemic cardiac injury after myocardial infarction. Circ J. 2013;77:2134-2144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 68. | Kim YM, Jeon ES, Kim MR, Lee JS, Kim JH. Bradykinin-induced expression of alpha-smooth muscle actin in human mesenchymal stem cells. Cell Signal. 2008;20:1882-1889. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 69. | Chertow GM, Burdick E, Honour M, Bonventre JV, Bates DW. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol. 2005;16:3365-3370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2256] [Cited by in RCA: 2366] [Article Influence: 118.3] [Reference Citation Analysis (0)] |
| 70. | Hertig A, Verine J, Mougenot B, Jouanneau C, Ouali N, Sebe P, Glotz D, Ancel PY, Rondeau E, Xu-Dubois YC. Risk factors for early epithelial to mesenchymal transition in renal grafts. Am J Transplant. 2006;6:2937-2946. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 86] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 71. | Lange C, Tögel F, Ittrich H, Clayton F, Nolte-Ernsting C, Zander AR, Westenfelder C. Administered mesenchymal stem cells enhance recovery from ischemia/reperfusion-induced acute renal failure in rats. Kidney Int. 2005;68:1613-1617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 321] [Cited by in RCA: 301] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 72. | Li QZ, Zhou J, Yang R, Yan M, Ye Q, Liu K, Liu S, Shao X, Li L, Zhou XJ. The lupus-susceptibility gene kallikrein downmodulates antibody-mediated glomerulonephritis. Genes Immun. 2009;10:503-508. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 34] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 73. | Liu K, Li QZ, Delgado-Vega AM, Abelson AK, Sánchez E, Kelly JA, Li L, Liu Y, Zhou J, Yan M. Kallikrein genes are associated with lupus and glomerular basement membrane-specific antibody-induced nephritis in mice and humans. J Clin Invest. 2009;119:911-923. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 95] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 74. | Li Y, Raman I, Du Y, Yan M, Min S, Yang J, Fang X, Li W, Lu J, Zhou XJ. Kallikrein transduced mesenchymal stem cells protect against anti-GBM disease and lupus nephritis by ameliorating inflammation and oxidative stress. PLoS One. 2013;8:e67790. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 75. | Besler C, Doerries C, Giannotti G, Lüscher TF, Landmesser U. Pharmacological approaches to improve endothelial repair mechanisms. Expert Rev Cardiovasc Ther. 2008;6:1071-1082. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 57] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 76. | Mikirova NA, Jackson JA, Hunninghake R, Kenyon J, Chan KW, Swindlehurst CA, Minev B, Patel AN, Murphy MP, Smith L. Circulating endothelial progenitor cells: a new approach to anti-aging medicine? J Transl Med. 2009;7:106. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 77. | Kopp HG, Ramos CA, Rafii S. Contribution of endothelial progenitors and proangiogenic hematopoietic cells to vascularization of tumor and ischemic tissue. Curr Opin Hematol. 2006;13:175-181. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 172] [Cited by in RCA: 159] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 78. | Umemura T, Higashi Y. Endothelial progenitor cells: therapeutic target for cardiovascular diseases. J Pharmacol Sci. 2008;108:1-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 48] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 79. | Vasa M, Fichtlscherer S, Aicher A, Adler K, Urbich C, Martin H, Zeiher AM, Dimmeler S. Number and migratory activity of circulating endothelial progenitor cells inversely correlate with risk factors for coronary artery disease. Circ Res. 2001;89:E1-E7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1571] [Cited by in RCA: 1600] [Article Influence: 66.7] [Reference Citation Analysis (0)] |
| 80. | Choi JH, Kim KL, Huh W, Kim B, Byun J, Suh W, Sung J, Jeon ES, Oh HY, Kim DK. Decreased number and impaired angiogenic function of endothelial progenitor cells in patients with chronic renal failure. Arterioscler Thromb Vasc Biol. 2004;24:1246-1252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 250] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 81. | Grisar J, Aletaha D, Steiner CW, Kapral T, Steiner S, Seidinger D, Weigel G, Schwarzinger I, Wolozcszuk W, Steiner G. Depletion of endothelial progenitor cells in the peripheral blood of patients with rheumatoid arthritis. Circulation. 2005;111:204-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 200] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 82. | Tsai NW, Hung SH, Huang CR, Chang HW, Chang WN, Lee LH, Wang HC, Lin YJ, Lin WC, Cheng BC. The association between circulating endothelial progenitor cells and outcome in different subtypes of acute ischemic stroke. Clin Chim Acta. 2014;427:6-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 83. | Paczkowska E, Gołąb-Janowska M, Bajer-Czajkowska A, Machalińska A, Ustianowski P, Rybicka M, Kłos P, Dziedziejko V, Safranow K, Nowacki P. Increased circulating endothelial progenitor cells in patients with haemorrhagic and ischaemic stroke: the role of endothelin-1. J Neurol Sci. 2013;325:90-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 40] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 84. | Kränkel N, Katare RG, Siragusa M, Barcelos LS, Campagnolo P, Mangialardi G, Fortunato O, Spinetti G, Tran N, Zacharowski K. Role of kinin B2 receptor signaling in the recruitment of circulating progenitor cells with neovascularization potential. Circ Res. 2008;103:1335-1343. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 90] [Cited by in RCA: 83] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 85. | Kränkel N, Kuschnerus K, Müller M, Speer T, Mocharla P, Madeddu P, Bader M, Lüscher TF, Landmesser U. Novel insights into the critical role of bradykinin and the kinin B2 receptor for vascular recruitment of circulating endothelial repair-promoting mononuclear cell subsets: alterations in patients with coronary disease. Circulation. 2013;127:594-603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 86. | Yao Y, Sheng Z, Li Y, Yan F, Fu C, Li Y, Ma G, Liu N, Chao J, Chao L. Tissue kallikrein promotes cardiac neovascularization by enhancing endothelial progenitor cell functional capacity. Hum Gene Ther. 2012;23:859-870. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 87. | Yao Y, Sheng Z, Li Y, Fu C, Ma G, Liu N, Chao J, Chao L. Tissue kallikrein-modified human endothelial progenitor cell implantation improves cardiac function via enhanced activation of akt and increased angiogenesis. Lab Invest. 2013;93:577-591. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 88. | Fu SS, Li FJ, Wang YY, You AB, Qie YL, Meng X, Li JR, Li BC, Zhang Y, Da Li Q. Kallikrein gene-modified EPCs induce angiogenesis in rats with ischemic hindlimb and correlate with integrin αvβ3 expression. PLoS One. 2013;8:e73035. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 89. | Kizuki K, Ookubo R, Iwadate H, Sada K. [Growth-stimulating effect of kallikrein on rat neural stem cells]. Yakugaku Zasshi. 2006;126:997-1001. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 90. | Kizuki K, Iwadate H, Ookubo R. Growth-stimulating effect of kallikrein on rat neural stem cells--II. Immunocytochemical analysis and specificity of the enzyme for neural stem cells. Yakugaku Zasshi. 2007;127:919-922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 91. | Isordia-Salas I, Pixley RA, Sáinz IM, Martínez-Murillo C, Colman RW. The role of plasma high molecular weight kininogen in experimental intestinal and systemic inflammation. Arch Med Res. 2005;36:87-95. [PubMed] |
| 92. | Colman RW. Regulation of angiogenesis by the kallikrein-kinin system. Curr Pharm Des. 2006;12:2599-2607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 50] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 93. | Dai J, Agelan A, Yang A, Zuluaga V, Sexton D, Colman RW, Wu Y. Role of plasma kallikrein-kinin system activation in synovial recruitment of endothelial progenitor cells in experimental arthritis. Arthritis Rheum. 2012;64:3574-3582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 94. | DeLa Cadena RA, Laskin KJ, Pixley RA, Sartor RB, Schwab JH, Back N, Bedi GS, Fisher RS, Colman RW. Role of kallikrein-kinin system in pathogenesis of bacterial cell wall-induced inflammation. Am J Physiol. 1991;260:G213-G219. [PubMed] |
| 95. | Colman RW, Jameson BA, Lin Y, Johnson D, Mousa SA. Domain 5 of high molecular weight kininogen (kininostatin) down-regulates endothelial cell proliferation and migration and inhibits angiogenesis. Blood. 2000;95:543-550. [PubMed] |
| 96. | Zhang JC, Claffey K, Sakthivel R, Darzynkiewicz Z, Shaw DE, Leal J, Wang YC, Lu FM, McCrae KR. Two-chain high molecular weight kininogen induces endothelial cell apoptosis and inhibits angiogenesis: partial activity within domain 5. FASEB J. 2000;14:2589-2600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 96] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 97. | Wu Y, Dai J, Schmuckler NG, Bakdash N, Yoder MC, Overall CM, Colman RW. Cleaved high molecular weight kininogen inhibits tube formation of endothelial progenitor cells via suppression of matrix metalloproteinase 2. J Thromb Haemost. 2010;8:185-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 98. | Dai J, Zhu X, Yoder MC, Wu Y, Colman RW. Cleaved high-molecular-weight kininogen accelerates the onset of endothelial progenitor cell senescence by induction of reactive oxygen species. Arterioscler Thromb Vasc Biol. 2011;31:883-889. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |