Published online Apr 26, 2014. doi: 10.4252/wjsc.v6.i2.203
Revised: December 24, 2013
Accepted: January 17, 2014
Published online: April 26, 2014
Processing time: 177 Days and 11.9 Hours
Hormone replacement therapy is necessary for patients with adrenal and gonadal failure. Steroid hormone treatment is also employed in aging people for sex hormone deficiency. These patients undergo such therapies, which have associated risks, for their entire life. Stem cells represent an innovative tool for tissue regeneration and the possibility of solving these problems. Among various stem cell types, mesenchymal stem cells have the potential to differentiate into steroidogenic cells both in vivo and in vitro. In particular, they can effectively be differentiated into steroidogenic cells by expressing nuclear receptor 5A subfamily proteins (steroidogenic factor-1 and liver receptor homolog-1) with the aid of cAMP. This approach will provide a source of cells for future regenerative medicine for the treatment of diseases caused by steroidogenesis deficiencies. It can also represent a useful tool for studying the molecular mechanisms of steroidogenesis and its related diseases.
Core tip: Stem cells can be a potential source of cells for regenerative medicine for diseases caused by steroidogenesis deficiency. Among various stem cell types, mesenchymal stem cells have the potential to differentiate into steroidogenic cells both in vivo and in vitro. This system can also provide a powerful tool for studying the molecular mechanisms of steroidogenesis and its related diseases.
- Citation: Yazawa T, Imamichi Y, Miyamoto K, Umezawa A, Taniguchi T. Differentiation of mesenchymal stem cells into gonad and adrenal steroidogenic cells. World J Stem Cells 2014; 6(2): 203-212
- URL: https://www.wjgnet.com/1948-0210/full/v6/i2/203.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v6.i2.203
In mammals, steroid hormones are produced from cholesterol mainly in adrenal glands and gonads. Steroid hormones are essential for glucose metabolism, the stress response, fluid and electrolyte balance, sex differentiation and reproduction via binding to cognate receptors in target tissues. Therefore, a steroidogenesis abnormality can often be life threatening. Congenital adrenal hyperplasia (CAH) is one of the most common disorders caused by deficiency of any enzyme involved in steroidogenesis in adrenal glands[1,2]. Impaired cortisol and aldosterone production increases adrenocorticotropic hormone (ACTH) secretion from the pituitary gland, leading to adrenal hyperplasia and accumulation of adrenal androgens. Female patients are prenatally virilized because of excess androgen and neonates of both genders may suffer from a life-threatening Addisonian crisis. Steroid hormone deficiency also occurs in aging people by hypogonadism. In males, testosterone concentrations decline with age, causing various clinical symptoms such as obesity and hypertension[3-6]. Postmenopausal women often suffer from osteoporosis caused by estrogen deficiency[7,8]. Hormone replacement therapy has been well established for the treatment of such patients, although they require hormone replacement for their entire lifetime. In addition, these patients suffer from various side effects (liver and kidney damage, immune system dysfunction) and risks associated with long-term replacement therapy (cancer). Therefore, another therapy is needed to resolve these problems. Stem cells represent an innovative tool for tissue regeneration and gene therapy, which could possibly solve these problems. In this review, we provide an overview of differentiation and regeneration of steroidogenic cells using mesenchymal stem cells (MSCs), preceded by a description of the development of steroidogenic organs. We also describe molecular events, such as coactivator function and epigenetic modifications, which occur during differentiation.
Steroidogenesis begins with conversion of cholesterol into pregnenolone in mitochondria by the P450 side chain cleavage enzyme (P450scc/CYP11A1/Cyp11a1), a rate-limiting enzyme in the synthesis of all steroid hormones. Thereafter, various hormones are synthesized by tissue-specific P450 hydroxylases and hydroxysteroid dehydrogenases[9,10]. Although adrenal glands and gonads produce various steroid hormones in adult life, they have a common developmental origin, a so-called adrenogonadal primordium (AGP) that mainly originates from the intermediate mesoderm and is localized on the coelomic epithelia of the developing urogenital ridge[11-13]. As development proceeds, AGP separates into two distinct populations, adrenocortical and gonadal primordia, characterized by the existence of chromaffin cell precursors and primordial germ cells, respectively, which originate and migrate from other germ layers. During differentiation, adrenal glands and gonads synthesize tissue-specific steroid hormones by specific expression patterns of steroidogenic enzymes.
Steroidogenic factor-1 (SF-1, also known as Ad4BP) is one of the earliest markers of the appearance of AGP[11,14]. Because SF-1 knockout mice fail to develop adrenal glands and gonads, SF-1 represents a master regulator of the development of these organs[15-17]. SF-1/Ad4BP is also important for steroidogenesis by regulating the transcription of steroidogenic genes. SF-1/Ad4BP was originally discovered by Keith Parker and Ken Morohashi as a transcription factor that binds to the Ad4 sequence in promoter regions of all cytochrome P450 steroid hydroxylase genes for transactivation[18,19]. They concluded from the expression of SF-1 in steroidogenic cells and its regulation of all steroid hydroxylase genes that SF-1 is a determinant factor in cell-specific expression of steroidogenic enzymes. In addition to steroidogenic enzymes, diverse groups of SF-1 target genes, such as other steroidogenic genes, pituitary hormones and cognate receptors, and sex differentiation-related genes have been identified thus far[17,20,21]. SF-1 belongs to the nuclear receptor (NR) superfamily. NRs are lipophilic ligand-dependent and independent transcription factors and essential for various physiological phenomena[22,23]. A large number of family members have been identified from invertebrate to mammals. There are a total of 48 family members on the human genome. They share a common structural organization: zinc finger DNA-binding domain and a carboxyl-terminal ligand-binding domain. The NR superfamily can be broadly divided into four classes based on their characteristics (steroid hormone receptors, RXR heterodimers, dimeric orphan receptors and monomeric orphan receptors). SF-1 is categorized into monomeric orphan receptors, although Ingraham and colleague argued the possibility that phosphatidylinositols are ligands for SF-1[24]. SF-1 is very similar to liver receptor homolog-1 (LRH-1). LRH-1 was originally identified in the liver[25] and is known to function in metabolism, cholesterol and bile acid homeostasis by regulating the transcription of a number of genes[26-29]. In addition to the liver, LRH-1 is highly expressed in tissues of endodermal origin. It is also expressed in gonads and involved in steroidogenesis; in particular, its ovarian expression levels are the most abundant among tissues[30]. These factors constitute one of the NR subfamilies and are designated as NR5A proteins (Table 1, SF-1 is NR5A1 and LRH-1 is NR5A2). SF-1 and LRH-1 have various common characteristics, such as binding sequences, target genes and cofactors[24,31-38].
| Nuclear receptor | Expressing tissues | Function | Phenotypes of knockout mice |
| SF-1/Ad4BP/NR5A1 | Testis, ovary, adrenal, | Steroidogenesis Sex differentiation Energy homeostasis | Adrenal and gonadal agenesis |
| Sex reversal in external genitalia | |||
| Impaired expression of pituitary gonadotropins | |||
| Abnormality of ventromedial hypothalamic nucleus | |||
| LRH-1/NR5A2 | Ovary, testis, liver, pancreas, intestine, early embryo | Steroidogenesis Ovulation Bile acid synthesis Glucose metabolism | Embryonic lethal around E6.5-7.5 d |
Consistent with its role in steroidogenesis, SF-1 expression is detected in adults in three layers of the adrenal cortex (zona reticularis, zona fasciculata and zona glomerulosa), testicular Leydig and Sertoli cells, ovarian theca, granulosa cells and, to a lesser extent, in the corpus lutea[39,40]. In the corpus lutea, LRH-1 rather than SF-1 is highly expressed and is important for progesterone production[36,41,42]. LRH-1 is also expressed in testicular Leydig cells[12,43,44].
SF-1 knockout mice die shortly after birth because of adrenal insufficiency and exhibit male-to-female sex reversal in external genitalia[15]. These phenotypes are caused by the complete loss of adrenal glands and gonads. Although the initial stages of adrenal and gonadal development occur in the absence of SF-1, they regress and disappear during the following developmental stage. Because gonads disappear prior to male sexual differentiation, the internal and external urogenital tracts of SF-1 knockout mice are of the female type, irrespective of genetic sex. Heterozygous SF-1 knockout mice show decreased adrenal volume associated with impaired corticosterone production in response to stress[45-47], whereas transgenic overexpression of SF-1/Ad4BP increases adrenal size and ectopic adrenal tissue in the thorax[48,49]. Total SF-1 disruption in mice demonstrated that SF-1 is crucial for the determination of steroidogenic cell fate in vivo. It has also been shown in Leydig cell and granulosa cell-specific knockout (LCKO and GCKO, respectively) models that SF-1 plays important roles in steroidogenesis following the development of steroidogenic organs. In LCKO mice, testicular steroidogenic acute regulatory protein (StAR) and Cyp11a1 expression is impaired, indicating a defect in androgen production[50]. Consistent with this hypothesis, the testes fail to descend (an androgen-dependent developmental process) and are hypoplastic. In GCKO mice, the ovaries are hypoplastic, adults are sterile and ovaries show reduced numbers of oocytes and lack corpora lutea[51]. Gonadotropin-induced steroid hormone production are also markedly reduced in this model.
LRH-1 knockout mouse embryos die around E6.5-7.5 d[52,53]. Moreover, heterozygous and GCKO models revealed the importance of LRH-1 in steroidogenesis[41,54,55]. In heterozygous Lrh-1-deficient male mice, testicular testosterone production is decreased along with the expression of steroidogenic enzymes and the development of sexual characteristics[54]. In addition, GCKO mice are infertile because of anovulation with impaired progesterone production[41]. It has also been demonstrated that LRH-1 has a broader role beyond steroidogenesis in these cells as they fail to luteinize.
Although SF-1 and LRH-1-deficient models revealed a common function in gonadal steroidogenesis, both factors cannot compensate for the deficiency of the other factor, even in cells expressing both factors. These facts indicate that even although SF-1 and LRH-1 control transcription by binding to the same response sequences, each has selective actions on the pattern of gene expression in the development of steroidogenic cells and steroidogenesis.
In an early study, forced expression of SF-1 has been shown to direct differentiation of murine embryonic stem cells (ESCs) toward the steroidogenic lineage and then Cyp11a1 mRNA was expressed after the addition of cAMP and retinoic acid[56]. However, the steroidogenic capacity of these cells is very limited and they do not undergo de novo synthesis because progesterone is the only steroid hormone produced in the presence of the exogenous substrate, 20α-hydroxycholesterol. In addition, major differences between these differentiated cells and natural steroidogenic cells have been shown in cholesterol delivery and the steroidogenic pathway, including deficiencies of StAR (cholesterol delivery protein from the outer to inner mitochondrial membrane in steroidogenic cells) and steroidogenic enzymes, except for Cyp11a1 and Hsd3b1[56-58]. It is also very difficult to isolate clones expressing SF-1 from ESCs and induced pluripotent stem cells[37,57,59] because SF-1 (and LRH-1) overexpression is cytotoxic to these cells. These studies clearly indicate that SF-1 initiates the fate-determination program of the steroidogenic lineage in stem cells, although it is not completed in pluripotent stem cells.
Based on these results, we focused on MSCs[57], multipotent adult stem cells that have been shown to differentiate into mesodermal lineages, such as adipocytes, chondrocytes, osteoblasts and hematopoietic-supporting stroma, both in vivo and ex vivo[60-63]. Furthermore, MSCs are able to generate cells of all three germ layers, at least in vitro. Although MSCs were originally discovered in bone marrow (BM-MSCs)[60,64-66], they have also been isolated from various origins, such as fat, placenta, umbilical cord blood and other tissues[62,63,67-69]. In addition to their multipotency, MSCs have attracted considerable interest for use in cell and gene therapies because they can be obtained from adult tissues and suppress immune responses[70,71]. Indeed, their therapeutic applicability has been assessed in some cases and particularly in bone tissue engineering[72,73].
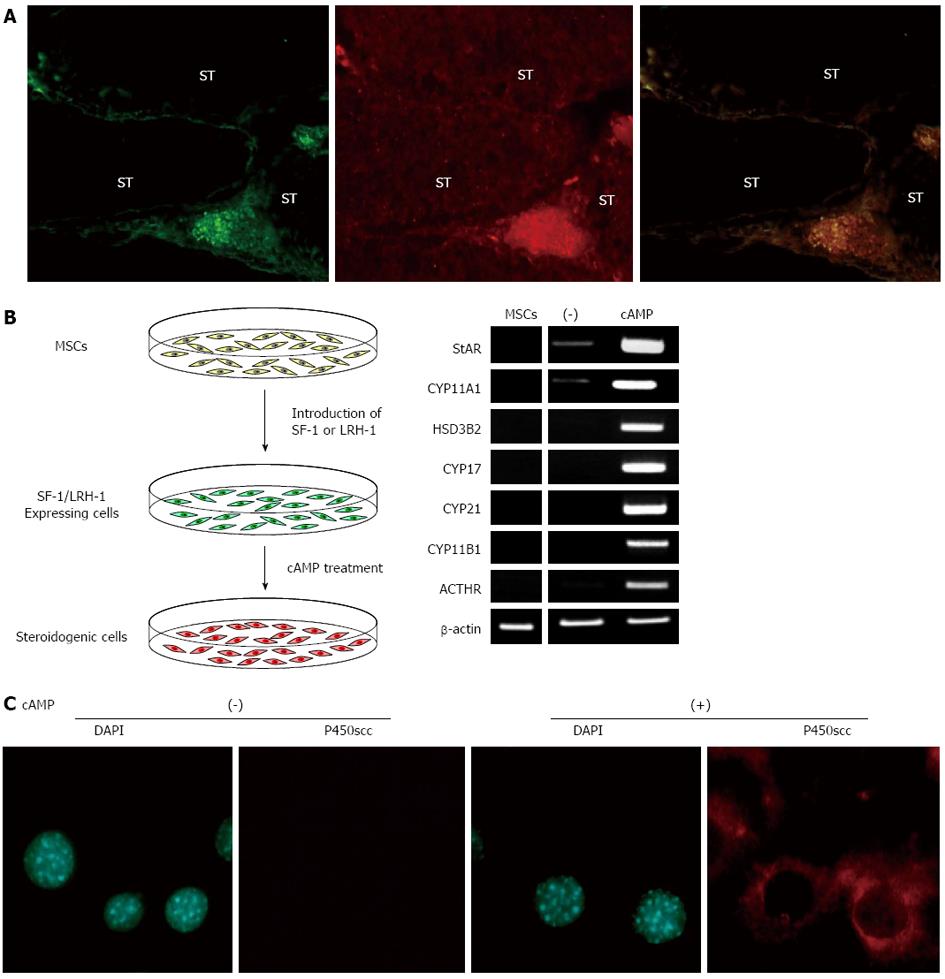
To investigate the potential of MSCs to differentiate into steroidogenic cells, BM-MSCs from GFP-transgenic rats were transplanted into prepubertal testes (Figure 1A)[57]. In testes, there are two different steroidogenic populations, fetal and adult Leydig cells[74-76]. Even although the cells in these two populations share a common characteristic of producing androgen, they are different in their origin, ultrastructure, lifespan, steroidogenic pathway and its regulation. Fetal Leydig cells have multiple origins and appear in the interstitial space to induce sex differentiation just after the formation of the testis cord. Adult Leydig cells, which originate from mesenchymal precursor cells present in the testicular interstitium, appear to induce puberty. During the postnatal period, fetal Leydig cells are replaced by adult Leydig cells in prepubertal testis. Therefore, it should be possible to use transplanted BM-MSCs in such conditions in vivo. Indeed, after 3 wk, transplanted GFP-positive cells were located in the interstitium and expressed various steroidogenic enzymes for androgen production (P450scc/Cyp11a1, 3β-HSD I and Cyp17). These results indicate that MSCs have the capacity to differentiate into steroidogenic Leydig cells in vivo.
Although these data suggest that the injected stem cells differentiated into Leydig cells, the apparent stem cell plasticity may also be explained by possible cell-nuclear fusion between donor and recipient cells. However, purified murine BM-MSC lines spontaneously differentiate into steroidogenic cells in vitro[57]. A human CYP11A1 promoter-driven GFP reporter, which consisted of a 2.3-kb fragment that drives reporter gene expression selectively in adrenal and gonadal steroidogenic cells[77], has been transfected into BM-MSCs to detect cell populations committed to the steroidogenic lineage. In some transfected cell lines, GFP fluorescence was detected in very small populations that were also positive for Cyp11a1. Further analysis showed that these cells expressed several Leydig cell markers, including 3β-HSD type I and VI and luteinizing hormone (LH) receptor. These observations further support the in vivo findings that MSCs have the capacity to differentiate into steroidogenic cells, even under the isolated condition. Therefore, part of population of MSCs can spontaneously differentiate into steroidogenic cells in vitro. Interestingly, SF-1 expression was also detected in the GFP-positive cells.
The above mentioned results strongly suggest that SF-1 can effectively direct the differentiation of MSCs into the steroidogenic lineage. Indeed, MSCs completely differentiate into steroidogenic cells and show their phenotype after stable expression of SF-1 (using plasmids or retroviruses) and cAMP treatment (Figure 1B)[36,37,44,57,78,79]. SF-1 by itself induces morphological changes in BM-MSCs, such as the accumulation of numerous lipid droplets, although these cells hardly express steroidogenic enzyme genes or produce steroid hormones at detectable levels. However, SF-1 expressing cells strongly become positive for CYP11A1/Cyp11a1 after cAMP treatment (Figure 1C). These cells express many other steroidogenesis-related genes (SR-BI, StAR, 3β-HSD and other P450 steroid hydroxylases) and autonomously produce steroid hormones, including androgen, estrogen, progestin, glucocorticoid and aldosterone. Notably, this approach differentiates human BM-MSCs into high cortisol-producing cells in response to ACTH, which are very similar to fasciculata cells in the adrenal cortex (Figure 1B). Adenovirus-mediated transient expression of SF-1 also differentiates BM-MSCs into steroidogenic cells with the capacity of de novo synthesis of various steroid hormones[80-84]. After transplantation into animal models, these MSC-derived steroidogenic cells can improve symptoms of steroid hormone deficiencies caused by adrenalectomy. However, as mentioned above, these methods are not applicable to ESCs, embryonal carcinoma cells and terminally differentiated cells, such as fibroblasts and adipocytes[37,57,81]. These results indicate that MSCs are suitable stem cells for differentiation of steroidogenic cells. This hypothesis is supported by the fact that after pre-differentiation into MSCs, ESCs can also be subsequently differentiated into steroidogenic cells using SF-1[37].
As in the case of SF-1, introduction of LRH-1 (using retroviruses) into BM-MSCs with the aid of cAMP induced the expression of steroidogenic enzymes and differentiation into steroid hormone-producing cells[44]. Expression of SF-1 was never induced in LRH-1-transduced cells and vice versa. Therefore, LRH-1 could act as another master regulator for determining the MSC fate to the steroidogenic lineage. This phenomenon is likely to represent a situation of active progesterone production in human corpus luteum; LRH-1 is highly expressed, whereas SF-1 is expressed at very low levels[36,42].
In addition to BM-MSCs, various MSC types have been differentiated into steroidogenic cells by the above mentioned methods. However, their steroidogenic properties markedly vary and depend on the derivation tissues and species (Table 2)[36,42,57,83,84]. For example, hBM-MSCs differentiated into cortisol-producing adrenocortical-like cells and umbilical cord blood (UCB)-derived MSCs differentiated into granulosa luteal-like cells, which produced high levels of progesterone[36,57]. Gondo et al[83] also reported that steroidogenic profiles of adipose tissue-derived MSCs were markedly different from those of BM-MSCs prepared from the same mouse. However, the cell differentiation fate was consistent in each MSC. These findings suggest that the steroidogenic properties of the differentiated cells depend on the characteristics of the originating MSCs.
| Cells | Origin | SF-1/LRH-1 | Produced | Properties of differentiated cells |
| KUM9 | Mouse | Plasmid | Testosterone | Testicular leydig cells |
| Bone marrow | ||||
| hMSC-TERT-E6/7 | Human | Plasmid | Cortisol | Adrenal fasciculata cells |
| Bone marrow | Retrovirus | Cortisol | ||
| UE7T-13 | HumanBone marrow | Retrovirus | Testosterone, cortisol | Fetal adrenal-like cells |
| UE6E7T-12 | Retrovirus | Testosterone, cortisol | ||
| UE6E7T-11 | Retrovirus | Testosterone, cortisol | ||
| UCB408E6E7T-33 | Human | Retrovirus | Progesterone cells | Ovarian granulosa-luteal cells |
| Umbilical cord blood |
To determine the difference between BM-MSCs and UBC-MSCs, the fluctuations in gene expression were investigated by a DNA microarray[36,85]. Among the identified genes, peroxisome proliferator-activated receptor γ coactivator-1α (PGC-1α) was expressed only in UBC-MSCs at relatively high levels. Consistent with these results, the expression of PGC-1α was observed in ovarian granulosa cells. Overexpression of PGC-1α in granulosa cells induced the genes essential for progesterone synthesis, whereas knockdown of PGC-1α in granulosa cells attenuated the expression of these genes. These results demonstrate that PGC-1α represents one of the important factors for progesterone production in luteinized granulosa cells.
Differentiation of stem cells into specialized cells can be viewed as a process in which epigenetic changes result in alterations in genes expressed by the cell as it becomes more specialized[86,87]. Thus, stem cell differentiation is a process that involves a series of epigenetic changes in the genome: histone and DNA modifications cause chromatin structural changes and affect the profiles of gene expression. In fact, such epigenetic modifications contribute to the induction of steroidogenesis-related genes when MSCs differentiate into steroidogenic cells[44,88-90].
The histone code hypothesis predicts that post-translational modifications of histone tails, alone or in combination, function to direct specific and distinct DNA-templated programs[91]. Histone acetylation is a positive marker of transcription, while histone methylation correlates with transcriptional activation (H3K4, H3K36) and repression (H3K9, H3K27) that are dependent on their amino acid residues[92]. In hMSCs-derived steroidogenic cells, H3K27 acetylation and H3K4 dimethylation (active enhancer markers) increased in the regulatory regions of some steroidogenesis-related genes (glutathione S-transferase A and ferrodoxin reductase) after the introduction of SF-1[89,90]. Conversely, histone eviction, which has been reported in actively transcribed genes[93], took place on the promoter and the enhancer regions of the StAR gene[88]. Because these modifications occurred around the SF-1 binding sites, recruitment of SF-1 to the regulatory regions is likely to induce recruitment of various transcriptional regulators and histone modifiers, which in turn alter chromatin structure and lead to the expression of steroidogenesis-related genes.
In addition to histone modifications, DNA methylation at cytosine residues of the dinucleotide sequence CpG, which induces gene silencing, is essential for differentiation and development[94,95]. In MSC-derived steroidogenic cells, the DNA methylation status changes in the promoter regions of some steroidogenic genes during differentiation[44]. In undifferentiated hBM-MSCs, the CYP11A1 promoter region is hypomethylated, whereas the CYP17A1 promoter region is highly methylated. In SF-1/LRH-1-introduced MSCs during cAMP treatment, this condition was almost completely unchanged in the CYP11A1 promoter region, whereas the CYP17A1 promoter region was progressively demethylated. These methylation patterns of the CYP11A1 and CYP17A1 promoters closely paralleled the induction patterns of both genes by cAMP. There is a time lag associated with the induction of steroidogenic enzymes by cAMP treatment in SF-1/LRH-1-introduced MSCs[44,57]. The order of induction of the enzymes is similar to the sequential order of the steroid hormone synthesis pathway; upstream enzymes (CYP11A1 and 3β-HSD) were rapidly induced at earlier time points (6-12 h), whereas downstream enzymes (CYP17A1 and CYP11B1) were induced at later time points (24-48 h). Because this time lag disappeared by treatment with a demethylating agent, the status of DNA methylation in the promoter regions could be important for regulating the expression of steroidogenic enzymes in MSCs.
It is clear that SF-1 represents a master regulator, not only for the development of steroidogenic organs, but also for steroidogenesis following organogenesis. LRH-1 is also important for steroidogenesis in gonads. In addition, SF-1 and LRH-1 direct differentiation of non-steroidogenic stem cells into steroidogenic cells. Among the various stem cell types, MSCs are suitable stem cells for the differentiation of steroidogenic cells. After pre-differentiation into MSCs, pluripotent stem cells can also be subsequently differentiated into steroidogenic cells using SF-1. These cells may provide a source for regenerative and gene therapies, although various problems should be resolved in future studies. It is essential to delineate the conditions that allow the directed differentiation into specific steroidogenic lineages with the characteristics of testicular Leydig cells, ovarian granulosa and theca cells, as well as various types of adrenocortical cells (reticularis, fasciculata and glomerulosa). In addition, it is necessary to establish methods for inducing SF-1 and LRH-1 expression in stem cells without gene transfer. Further studies are required for the realization of regeneration of steroidogenic tissues.
MSC-derived steroidogenic cells also provide opportunities for investigating various phenomena involved in differentiation of steroidogenic cells and steroidogenesis. In addition to the molecular mechanisms of differentiation described herein, the conservation and evolution of the androgen metabolic pathway (11-ketotestosterone production) between teleost fish and mammals has been revealed[78,96]. Genome-wide analyses of differentiated cells identified novel target genes regulated by SF-1 and LRH-1[89,90,97,98]. In addition, they contributed to the elucidation of one of the causes of steroidogenesis disorders[99-101]. Thus, progression of these studies is also important for the understanding of steroidogenesis and its related disorders.
P- Reviewers: Holan V, Lalli E, Pixley JS S- Editor: Qi Y L- Editor: Roemmele A E- Editor: Zhang DN
| 1. | Claahsen-van der Grinten HL, Stikkelbroeck NM, Otten BJ, Hermus AR. Congenital adrenal hyperplasia--pharmacologic interventions from the prenatal phase to adulthood. Pharmacol Ther. 2011;132:1-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 36] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 2. | White PC, Bachega TA. Congenital adrenal hyperplasia due to 21 hydroxylase deficiency: from birth to adulthood. Semin Reprod Med. 2012;30:400-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 3. | Haring R. Perspectives for metabolomics in testosterone replacement therapy. J Endocrinol. 2012;215:3-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 4. | Morris PD, Channer KS. Testosterone and cardiovascular disease in men. Asian J Androl. 2012;14:428-435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 54] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 5. | Shelton JB, Rajfer J. Androgen deficiency in aging and metabolically challenged men. Urol Clin North Am. 2012;39:63-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 6. | Staerman F, Léon P. Andropause (androgen deficiency of the aging male): diagnosis and management. Minerva Med. 2012;103:333-342. [PubMed] |
| 7. | Marjoribanks J, Farquhar C, Roberts H, Lethaby A. Long term hormone therapy for perimenopausal and postmenopausal women. Cochrane Database Syst Rev. 2012;7:CD004143. [PubMed] |
| 8. | Rozenberg S, Vandromme J, Antoine C. Postmenopausal hormone therapy: risks and benefits. Nat Rev Endocrinol. 2013;9:216-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 88] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 9. | Miller WL. Molecular biology of steroid hormone synthesis. Endocr Rev. 1988;9:295-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 961] [Cited by in RCA: 880] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 10. | Miller WL, Auchus RJ. The molecular biology, biochemistry, and physiology of human steroidogenesis and its disorders. Endocr Rev. 2011;32:81-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1539] [Cited by in RCA: 1572] [Article Influence: 112.3] [Reference Citation Analysis (0)] |
| 11. | Morohashi K. The ontogenesis of the steroidogenic tissues. Genes Cells. 1997;2:95-106. [PubMed] |
| 12. | Val P, Martinez-Barbera JP, Swain A. Adrenal development is initiated by Cited2 and Wt1 through modulation of Sf-1 dosage. Development. 2007;134:2349-2358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 103] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 13. | Bandiera R, Vidal VP, Motamedi FJ, Clarkson M, Sahut-Barnola I, von Gise A, Pu WT, Hohenstein P, Martinez A, Schedl A. WT1 maintains adrenal-gonadal primordium identity and marks a population of AGP-like progenitors within the adrenal gland. Dev Cell. 2013;27:5-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 90] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 14. | Hatano O, Takakusu A, Nomura M, Morohashi K. Identical origin of adrenal cortex and gonad revealed by expression profiles of Ad4BP/SF-1. Genes Cells. 1996;1:663-671. [PubMed] |
| 15. | Luo X, Ikeda Y, Parker KL. A cell-specific nuclear receptor is essential for adrenal and gonadal development and sexual differentiation. Cell. 1994;77:481-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1163] [Cited by in RCA: 1090] [Article Influence: 35.2] [Reference Citation Analysis (0)] |
| 16. | Sadovsky Y, Crawford PA, Woodson KG, Polish JA, Clements MA, Tourtellotte LM, Simburger K, Milbrandt J. Mice deficient in the orphan receptor steroidogenic factor 1 lack adrenal glands and gonads but express P450 side-chain-cleavage enzyme in the placenta and have normal embryonic serum levels of corticosteroids. Proc Natl Acad Sci USA. 1995;92:10939-10943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 324] [Cited by in RCA: 327] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 17. | Parker KL, Schimmer BP. Steroidogenic factor 1: a key determinant of endocrine development and function. Endocr Rev. 1997;18:361-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 116] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 18. | Lala DS, Rice DA, Parker KL. Steroidogenic factor I, a key regulator of steroidogenic enzyme expression, is the mouse homolog of fushi tarazu-factor I. Mol Endocrinol. 1992;6:1249-1258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 161] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 19. | Morohashi K, Honda S, Inomata Y, Handa H, Omura T. A common trans-acting factor, Ad4-binding protein, to the promoters of steroidogenic P-450s. J Biol Chem. 1992;267:17913-17919. [PubMed] |
| 20. | Parker KL, Rice DA, Lala DS, Ikeda Y, Luo X, Wong M, Bakke M, Zhao L, Frigeri C, Hanley NA. Steroidogenic factor 1: an essential mediator of endocrine development. Recent Prog Horm Res. 2002;57:19-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 272] [Cited by in RCA: 262] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 21. | Schimmer BP, White PC. Minireview: steroidogenic factor 1: its roles in differentiation, development, and disease. Mol Endocrinol. 2010;24:1322-1337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 195] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 22. | Mangelsdorf DJ, Thummel C, Beato M, Herrlich P, Schütz G, Umesono K, Blumberg B, Kastner P, Mark M, Chambon P. The nuclear receptor superfamily: the second decade. Cell. 1995;83:835-839. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5272] [Cited by in RCA: 5179] [Article Influence: 172.6] [Reference Citation Analysis (0)] |
| 23. | Robinson-Rechavi M, Escriva Garcia H, Laudet V. The nuclear receptor superfamily. J Cell Sci. 2003;116:585-586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 327] [Cited by in RCA: 327] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 24. | Krylova IN, Sablin EP, Moore J, Xu RX, Waitt GM, MacKay JA, Juzumiene D, Bynum JM, Madauss K, Montana V. Structural analyses reveal phosphatidyl inositols as ligands for the NR5 orphan receptors SF-1 and LRH-1. Cell. 2005;120:343-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 310] [Cited by in RCA: 324] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 25. | Galarneau L, Paré JF, Allard D, Hamel D, Levesque L, Tugwood JD, Green S, Bélanger L. The alpha1-fetoprotein locus is activated by a nuclear receptor of the Drosophila FTZ-F1 family. Mol Cell Biol. 1996;16:3853-3865. [PubMed] |
| 26. | Fayard E, Auwerx J, Schoonjans K. LRH-1: an orphan nuclear receptor involved in development, metabolism and steroidogenesis. Trends Cell Biol. 2004;14:250-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 327] [Cited by in RCA: 332] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 27. | Lee YK, Moore DD. Liver receptor homolog-1, an emerging metabolic modulator. Front Biosci. 2008;13:5950-5958. [PubMed] |
| 28. | Lazarus KA, Wijayakumara D, Chand AL, Simpson ER, Clyne CD. Therapeutic potential of Liver Receptor Homolog-1 modulators. J Steroid Biochem Mol Biol. 2012;130:138-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 53] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 29. | Wilson C. Metabolism: LRH-1 is a transcriptional regulator of glucokinase in the liver. Nat Rev Endocrinol. 2012;8:566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 30. | Bookout AL, Jeong Y, Downes M, Yu RT, Evans RM, Mangelsdorf DJ. Anatomical profiling of nuclear receptor expression reveals a hierarchical transcriptional network. Cell. 2006;126:789-799. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 801] [Cited by in RCA: 790] [Article Influence: 41.6] [Reference Citation Analysis (0)] |
| 31. | Wang ZN, Bassett M, Rainey WE. Liver receptor homologue-1 is expressed in the adrenal and can regulate transcription of 11 beta-hydroxylase. J Mol Endocrinol. 2001;27:255-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 43] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 32. | Suzuki T, Kasahara M, Yoshioka H, Morohashi K, Umesono K. LXXLL-related motifs in Dax-1 have target specificity for the orphan nuclear receptors Ad4BP/SF-1 and LRH-1. Mol Cell Biol. 2003;23:238-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 118] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 33. | Wang W, Zhang C, Marimuthu A, Krupka HI, Tabrizizad M, Shelloe R, Mehra U, Eng K, Nguyen H, Settachatgul C. The crystal structures of human steroidogenic factor-1 and liver receptor homologue-1. Proc Natl Acad Sci USA. 2005;102:7505-7510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 120] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 34. | Saxena D, Escamilla-Hernandez R, Little-Ihrig L, Zeleznik AJ. Liver receptor homolog-1 and steroidogenic factor-1 have similar actions on rat granulosa cell steroidogenesis. Endocrinology. 2007;148:726-734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 61] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 35. | Heng JC, Feng B, Han J, Jiang J, Kraus P, Ng JH, Orlov YL, Huss M, Yang L, Lufkin T. The nuclear receptor Nr5a2 can replace Oct4 in the reprogramming of murine somatic cells to pluripotent cells. Cell Stem Cell. 2010;6:167-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 351] [Cited by in RCA: 371] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 36. | Yazawa T, Inaoka Y, Okada R, Mizutani T, Yamazaki Y, Usami Y, Kuribayashi M, Orisaka M, Umezawa A, Miyamoto K. PPAR-gamma coactivator-1alpha regulates progesterone production in ovarian granulosa cells with SF-1 and LRH-1. Mol Endocrinol. 2010;24:485-496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 81] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 37. | Yazawa T, Kawabe S, Inaoka Y, Okada R, Mizutani T, Imamichi Y, Ju Y, Yamazaki Y, Usami Y, Kuribayashi M. Differentiation of mesenchymal stem cells and embryonic stem cells into steroidogenic cells using steroidogenic factor-1 and liver receptor homolog-1. Mol Cell Endocrinol. 2011;336:127-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 39] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 38. | Yazawa T, Mizutani T, Yamada K, Kawata H, Sekiguchi T, Yoshino M, Kajitani T, Shou Z, Miyamoto K. Involvement of cyclic adenosine 5’-monophosphate response element-binding protein, steroidogenic factor 1, and Dax-1 in the regulation of gonadotropin-inducible ovarian transcription factor 1 gene expression by follicle-stimulating hormone in ovarian granulosa cells. Endocrinology. 2003;144:1920-1930. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 46] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 39. | Ikeda Y, Lala DS, Luo X, Kim E, Moisan MP, Parker KL. Characterization of the mouse FTZ-F1 gene, which encodes a key regulator of steroid hydroxylase gene expression. Mol Endocrinol. 1993;7:852-860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 79] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 40. | Kawabe K, Shikayama T, Tsuboi H, Oka S, Oba K, Yanase T, Nawata H, Morohashi K. Dax-1 as one of the target genes of Ad4BP/SF-1. Mol Endocrinol. 1999;13:1267-1284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 14] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 41. | Duggavathi R, Volle DH, Mataki C, Antal MC, Messaddeq N, Auwerx J, Murphy BD, Schoonjans K. Liver receptor homolog 1 is essential for ovulation. Genes Dev. 2008;22:1871-1876. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 166] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 42. | Peng N, Kim JW, Rainey WE, Carr BR, Attia GR. The role of the orphan nuclear receptor, liver receptor homologue-1, in the regulation of human corpus luteum 3beta-hydroxysteroid dehydrogenase type II. J Clin Endocrinol Metab. 2003;88:6020-6028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 60] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 43. | Pezzi V, Sirianni R, Chimento A, Maggiolini M, Bourguiba S, Delalande C, Carreau S, Andò S, Simpson ER, Clyne CD. Differential expression of steroidogenic factor-1/adrenal 4 binding protein and liver receptor homolog-1 (LRH-1)/fetoprotein transcription factor in the rat testis: LRH-1 as a potential regulator of testicular aromatase expression. Endocrinology. 2004;145:2186-2196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 82] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 44. | Yazawa T, Inanoka Y, Mizutani T, Kuribayashi M, Umezawa A, Miyamoto K. Liver receptor homolog-1 regulates the transcription of steroidogenic enzymes and induces the differentiation of mesenchymal stem cells into steroidogenic cells. Endocrinology. 2009;150:3885-3893. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 62] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 45. | Bland ML, Jamieson CA, Akana SF, Bornstein SR, Eisenhofer G, Dallman MF, Ingraham HA. Haploinsufficiency of steroidogenic factor-1 in mice disrupts adrenal development leading to an impaired stress response. Proc Natl Acad Sci USA. 2000;97:14488-14493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 144] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 46. | Bland ML, Fowkes RC, Ingraham HA. Differential requirement for steroidogenic factor-1 gene dosage in adrenal development versus endocrine function. Mol Endocrinol. 2004;18:941-952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 77] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 47. | Fatchiyah M, Shima Y, Oka S, Ishihara S, Fukui-Katoh Y, Morohashi K. Differential gene dosage effects of Ad4BP/SF-1 on target tissue development. Biochem Biophys Res Commun. 2006;341:1036-1045. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 30] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 48. | Zubair M, Oka S, Parker KL, Morohashi K. Transgenic expression of Ad4BP/SF-1 in fetal adrenal progenitor cells leads to ectopic adrenal formation. Mol Endocrinol. 2009;23:1657-1667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 47] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 49. | Morohashi K, Zubair M. The fetal and adult adrenal cortex. Mol Cell Endocrinol. 2011;336:193-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 28] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 50. | Jeyasuria P, Ikeda Y, Jamin SP, Zhao L, De Rooij DG, Themmen AP, Behringer RR, Parker KL. Cell-specific knockout of steroidogenic factor 1 reveals its essential roles in gonadal function. Mol Endocrinol. 2004;18:1610-1619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 197] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 51. | Pelusi C, Ikeda Y, Zubair M, Parker KL. Impaired follicle development and infertility in female mice lacking steroidogenic factor 1 in ovarian granulosa cells. Biol Reprod. 2008;79:1074-1083. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 80] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 52. | Paré JF, Malenfant D, Courtemanche C, Jacob-Wagner M, Roy S, Allard D, Bélanger L. The fetoprotein transcription factor (FTF) gene is essential to embryogenesis and cholesterol homeostasis and is regulated by a DR4 element. J Biol Chem. 2004;279:21206-21216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 127] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 53. | Gu P, Goodwin B, Chung AC, Xu X, Wheeler DA, Price RR, Galardi C, Peng L, Latour AM, Koller BH. Orphan nuclear receptor LRH-1 is required to maintain Oct4 expression at the epiblast stage of embryonic development. Mol Cell Biol. 2005;25:3492-3505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 238] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 54. | Volle DH, Duggavathi R, Magnier BC, Houten SM, Cummins CL, Lobaccaro JM, Verhoeven G, Schoonjans K, Auwerx J. The small heterodimer partner is a gonadal gatekeeper of sexual maturation in male mice. Genes Dev. 2007;21:303-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 81] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 55. | Labelle-Dumais C, Paré JF, Bélanger L, Farookhi R, Dufort D. Impaired progesterone production in Nr5a2+/- mice leads to a reduction in female reproductive function. Biol Reprod. 2007;77:217-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 56. | Crawford PA, Sadovsky Y, Milbrandt J. Nuclear receptor steroidogenic factor 1 directs embryonic stem cells toward the steroidogenic lineage. Mol Cell Biol. 1997;17:3997-4006. [PubMed] |
| 57. | Yazawa T, Mizutani T, Yamada K, Kawata H, Sekiguchi T, Yoshino M, Kajitani T, Shou Z, Umezawa A, Miyamoto K. Differentiation of adult stem cells derived from bone marrow stroma into Leydig or adrenocortical cells. Endocrinology. 2006;147:4104-4111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 115] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 58. | Jadhav U, Jameson JL. Steroidogenic factor-1 (SF-1)-driven differentiation of murine embryonic stem (ES) cells into a gonadal lineage. Endocrinology. 2011;152:2870-2882. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 35] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 59. | Sonoyama T, Sone M, Honda K, Taura D, Kojima K, Inuzuka M, Kanamoto N, Tamura N, Nakao K. Differentiation of human embryonic stem cells and human induced pluripotent stem cells into steroid-producing cells. Endocrinology. 2012;153:4336-4345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 50] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 60. | Prockop DJ. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science. 1997;276:71-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3486] [Cited by in RCA: 3347] [Article Influence: 119.5] [Reference Citation Analysis (0)] |
| 61. | Gojo S, Umezawa A. Plasticity of mesenchymal stem cells--regenerative medicine for diseased hearts. Hum Cell. 2003;16:23-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 62. | Le Blanc K, Ringdén O. Mesenchymal stem cells: properties and role in clinical bone marrow transplantation. Curr Opin Immunol. 2006;18:586-591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 164] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 63. | Bernardo ME, Locatelli F, Fibbe WE. Mesenchymal stromal cells. Ann N Y Acad Sci. 2009;1176:101-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 228] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 64. | Friedenstein AJ, Gorskaja JF, Kulagina NN. Fibroblast precursors in normal and irradiated mouse hematopoietic organs. Exp Hematol. 1976;4:267-274. [PubMed] |
| 65. | Caplan AI. Mesenchymal stem cells. J Orthop Res. 1991;9:641-650. |
| 66. | Umezawa A, Maruyama T, Segawa K, Shadduck RK, Waheed A, Hata J. Multipotent marrow stromal cell line is able to induce hematopoiesis in vivo. J Cell Physiol. 1992;151:197-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 93] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 67. | Terai M, Uyama T, Sugiki T, Li XK, Umezawa A, Kiyono T. Immortalization of human fetal cells: the life span of umbilical cord blood-derived cells can be prolonged without manipulating p16INK4a/RB braking pathway. Mol Biol Cell. 2005;16:1491-1499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 73] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 68. | Okamoto K, Miyoshi S, Toyoda M, Hida N, Ikegami Y, Makino H, Nishiyama N, Tsuji H, Cui CH, Segawa K. ‘Working’ cardiomyocytes exhibiting plateau action potentials from human placenta-derived extraembryonic mesodermal cells. Exp Cell Res. 2007;313:2550-2562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 42] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 69. | Bassi G, Pacelli L, Carusone R, Zanoncello J, Krampera M. Adipose-derived stromal cells (ASCs). Transfus Apher Sci. 2012;47:193-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 70. | Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15372] [Cited by in RCA: 15201] [Article Influence: 584.7] [Reference Citation Analysis (0)] |
| 71. | Ding Y, Xu D, Feng G, Bushell A, Muschel RJ, Wood KJ. Mesenchymal stem cells prevent the rejection of fully allogenic islet grafts by the immunosuppressive activity of matrix metalloproteinase-2 and -9. Diabetes. 2009;58:1797-1806. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 225] [Cited by in RCA: 211] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 72. | Amini AR, Laurencin CT, Nukavarapu SP. Bone tissue engineering: recent advances and challenges. Crit Rev Biomed Eng. 2012;40:363-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1365] [Cited by in RCA: 1443] [Article Influence: 120.3] [Reference Citation Analysis (0)] |
| 73. | Wang X, Wang Y, Gou W, Lu Q, Peng J, Lu S. Role of mesenchymal stem cells in bone regeneration and fracture repair: a review. Int Orthop. 2013;37:2491-2498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 238] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 74. | Haider SG. Cell biology of Leydig cells in the testis. Int Rev Cytol. 2004;233:181-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 241] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 75. | Dong L, Jelinsky SA, Finger JN, Johnston DS, Kopf GS, Sottas CM, Hardy MP, Ge RS. Gene expression during development of fetal and adult Leydig cells. Ann N Y Acad Sci. 2007;1120:16-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 37] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 76. | Svechnikov K, Landreh L, Weisser J, Izzo G, Colón E, Svechnikova I, Söder O. Origin, development and regulation of human Leydig cells. Horm Res Paediatr. 2010;73:93-101. [PubMed] |
| 77. | Hu MC, Chou SJ, Huang YY, Hsu NC, Li H, Chung BC. Tissue-specific, hormonal, and developmental regulation of SCC-LacZ expression in transgenic mice leads to adrenocortical zone characterization. Endocrinology. 1999;140:5609-5618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 78. | Yazawa T, Uesaka M, Inaoka Y, Mizutani T, Sekiguchi T, Kajitani T, Kitano T, Umezawa A, Miyamoto K. Cyp11b1 is induced in the murine gonad by luteinizing hormone/human chorionic gonadotropin and involved in the production of 11-ketotestosterone, a major fish androgen: conservation and evolution of the androgen metabolic pathway. Endocrinology. 2008;149:1786-1792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 75] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 79. | Miyamoto K, Yazawa T, Mizutani T, Imamichi Y, Kawabe SY, Kanno M, Matsumura T, Ju Y, Umezawa A. Stem cell differentiation into steroidogenic cell lineages by NR5A family. Mol Cell Endocrinol. 2011;336:123-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 80. | Gondo S, Yanase T, Okabe T, Tanaka T, Morinaga H, Nomura M, Goto K, Nawata H. SF-1/Ad4BP transforms primary long-term cultured bone marrow cells into ACTH-responsive steroidogenic cells. Genes Cells. 2004;9:1239-1247. [PubMed] |
| 81. | Yanase T, Gondo S, Okabe T, Tanaka T, Shirohzu H, Fan W, Oba K, Morinaga H, Nomura M, Ohe K. Differentiation and regeneration of adrenal tissues: An initial step toward regeneration therapy for steroid insufficiency. Endocr J. 2006;53:449-459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 82. | Tanaka T, Gondo S, Okabe T, Ohe K, Shirohzu H, Morinaga H, Nomura M, Tani K, Takayanagi R, Nawata H. Steroidogenic factor 1/adrenal 4 binding protein transforms human bone marrow mesenchymal cells into steroidogenic cells. J Mol Endocrinol. 2007;39:343-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 48] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 83. | Gondo S, Okabe T, Tanaka T, Morinaga H, Nomura M, Takayanagi R, Nawata H, Yanase T. Adipose tissue-derived and bone marrow-derived mesenchymal cells develop into different lineage of steroidogenic cells by forced expression of steroidogenic factor 1. Endocrinology. 2008;149:4717-4725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 59] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 84. | Wei X, Peng G, Zheng S, Wu X. Differentiation of umbilical cord mesenchymal stem cells into steroidogenic cells in comparison to bone marrow mesenchymal stem cells. Cell Prolif. 2012;45:101-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 47] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 85. | Kawabe S, Yazawa T, Kanno M, Usami Y, Mizutani T, Imamichi Y, Ju Y, Matsumura T, Orisaka M, Miyamoto K. A novel isoform of liver receptor homolog-1 is regulated by steroidogenic factor-1 and the specificity protein family in ovarian granulosa cells. Endocrinology. 2013;154:1648-1660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 86. | Collas P. Programming differentiation potential in mesenchymal stem cells. Epigenetics. 2010;5:476-482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 37] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 87. | Tollervey JR, Lunyak VV. Epigenetics: judge, jury and executioner of stem cell fate. Epigenetics. 2012;7:823-840. [PubMed] |
| 88. | Mizutani T, Yazawa T, Ju Y, Imamichi Y, Uesaka M, Inaoka Y, Matsuura K, Kamiki Y, Oki M, Umezawa A. Identification of a novel distal control region upstream of the human steroidogenic acute regulatory protein (StAR) gene that participates in SF-1-dependent chromatin architecture. J Biol Chem. 2010;285:28240-28251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 89. | Matsumura T, Imamichi Y, Mizutani T, Ju Y, Yazawa T, Kawabe S, Kanno M, Ayabe T, Katsumata N, Fukami M. Human glutathione S-transferase A (GSTA) family genes are regulated by steroidogenic factor 1 (SF-1) and are involved in steroidogenesis. FASEB J. 2013;27:3198-3208. [PubMed] |
| 90. | Imamichi Y, Mizutani T, Ju Y, Matsumura T, Kawabe S, Kanno M, Yazawa T, Miyamoto K. Transcriptional regulation of human ferredoxin reductase through an intronic enhancer in steroidogenic cells. Biochim Biophys Acta. 2014;1839:33-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 91. | Strahl BD, Allis CD. The language of covalent histone modifications. Nature. 2000;403:41-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6156] [Cited by in RCA: 6152] [Article Influence: 246.1] [Reference Citation Analysis (0)] |
| 92. | Fischle W, Wang Y, Allis CD. Histone and chromatin cross-talk. Curr Opin Cell Biol. 2003;15:172-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 893] [Cited by in RCA: 868] [Article Influence: 39.5] [Reference Citation Analysis (0)] |
| 93. | Pokholok DK, Harbison CT, Levine S, Cole M, Hannett NM, Lee TI, Bell GW, Walker K, Rolfe PA, Herbolsheimer E. Genome-wide map of nucleosome acetylation and methylation in yeast. Cell. 2005;122:517-527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1048] [Cited by in RCA: 1079] [Article Influence: 54.0] [Reference Citation Analysis (0)] |
| 94. | Ehrlich M. Expression of various genes is controlled by DNA methylation during mammalian development. J Cell Biochem. 2003;88:899-910. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 167] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 95. | Ng RK, Gurdon JB. Epigenetic inheritance of cell differentiation status. Cell Cycle. 2008;7:1173-1177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 112] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 96. | Yazawa T, Kawabe S, Kanno M, Mizutani T, Imamichi Y, Ju Y, Matsumura T, Yamazaki Y, Usami Y, Kuribayashi M. Androgen/androgen receptor pathway regulates expression of the genes for cyclooxygenase-2 and amphiregulin in periovulatory granulosa cells. Mol Cell Endocrinol. 2013;369:42-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 97. | Ju Y, Mizutani T, Imamichi Y, Yazawa T, Matsumura T, Kawabe S, Kanno M, Umezawa A, Kangawa K, Miyamoto K. Nuclear receptor 5A (NR5A) family regulates 5-aminolevulinic acid synthase 1 (ALAS1) gene expression in steroidogenic cells. Endocrinology. 2012;153:5522-5534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 98. | Imamichi Y, Mizutani T, Ju Y, Matsumura T, Kawabe S, Kanno M, Yazawa T, Miyamoto K. Transcriptional regulation of human ferredoxin 1 in ovarian granulosa cells. Mol Cell Endocrinol. 2013;370:1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 99. | Inaoka Y, Yazawa T, Mizutani T, Kokame K, Kangawa K, Uesaka M, Umezawa A, Miyamoto K. Regulation of P450 oxidoreductase by gonadotropins in rat ovary and its effect on estrogen production. Reprod Biol Endocrinol. 2008;6:62. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 100. | Fukami M, Nishimura G, Homma K, Nagai T, Hanaki K, Uematsu A, Ishii T, Numakura C, Sawada H, Nakacho M. Cytochrome P450 oxidoreductase deficiency: identification and characterization of biallelic mutations and genotype-phenotype correlations in 35 Japanese patients. J Clin Endocrinol Metab. 2009;94:1723-1731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 68] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 101. | Soneda S, Yazawa T, Fukami M, Adachi M, Mizota M, Fujieda K, Miyamoto K, Ogata T. Proximal promoter of the cytochrome P450 oxidoreductase gene: identification of microdeletions involving the untranslated exon 1 and critical function of the SP1 binding sites. J Clin Endocrinol Metab. 2011;96:E1881-E1887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |