INTRODUCTION
Multipotent stromal cells and various progenitor cells derived from adipose tissue have raised interest for regenerative medicine applications, especially because adipose tissue can be harvested in large quantities (several hundred mL) by a minimally invasive liposuction procedure. This was the topic of several recent reviews[1-3]. In humans and mice, the stromal vascular fraction (SVF) is a heterogeneous mixture of cells isolated by enzymatic dissociation of adipose tissue followed by gradient centrifugation in order to remove the differentiated adipocytes, which float over the aqueous layer. The pellet of SVF cells contains multipotent mesenchymal cells, which are typically referred to as adipose derived stem/stromal/progenitor cells (ASC). Human ASC (hASC) have biological capacities highly comparable to bone marrow-derived mesenchymal stem/stromal cells (BMSC) and therefore are considered to be a promising alternative source of cells for clinical use in pathological contexts as diverse as cardiovascular disorders, pulmonary diseases, musculoskeletal disorders, soft tissue reconstruction/augmentation, liver dysfunction, gastrointestinal, urogenital or neuronal disorders, skin and wound healing, bone regeneration, corneal diseases and immuno-modulation (reviewed in[1,4]).
In addition to ASC, the SVF also contains blood-derived cells, in particular erythrocytes and leukocytes, characterized by expression of the pan-hematopoietic marker CD45 and/or the monocytic marker CD14. Interestingly, SVF was shown to also include vascular endothelial and mural/pericytic cells, harboring vasculogenic properties in vitro and in vivo ([5,6] and recently reviewed in[7]). The majority of SVF cells (60%-80%[8,9]) express CD34, which was first used for the identification and isolation of hematopoietic progenitors cells. Very little is known about its possible function in adipose-derived cells, but given the pivotal role played by CD34 in the biology of several stem/progenitor cells, including hematopoietic stem cells[10], skeletal muscle satellite cells[11], keratinocyte stem cells[12], hair follicle stem cells[13,14] or adipogenic precursors[15], a function of CD34 also in the biology of adipose-derived stem/progenitor cells can reasonably be hypothesized. The present editorial provides a concise overview of the CD34 family of sialomucins and compiles the data from the literature concerning expression and function of these proteins in SVF cells and their in vitro expanded progeny.
The CD34 family of sialomucins: Structure and functions
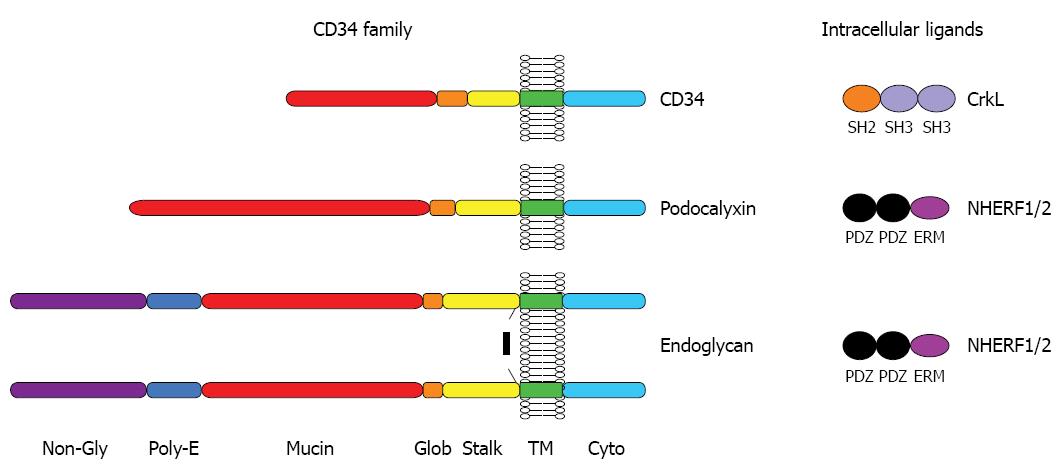
The CD34 family of cell surface proteins is a discrete subset of the large family of transmembrane sialomucins and comprises 3 members: CD34, podocalyxin (Podxl) and endoglycan (Endgl). A schematic overview of the biochemical features of CD34 family of proteins is shown in Figure 1. Although CD34 family members exhibit relatively limited linear protein sequence identity, they each contain a very similar array of biochemical domains and motifs that distinguishes them as a distinct subfamily from the much larger family of transmembrane mucins. All three contain an N-terminal signal peptide followed by a serine-, threonine- and proline-rich mucin domain that becomes highly decorated with O-linked and, to a lesser extent, N-linked glycosylation (Endgl is somewhat unique in that it also contains an intervening N-terminal domain that lacks potential glycosylation sites but contains a site for chondroitin sulfate attachment and a poly-glutamic acid motif)[16-18]. The mucin domain is followed by a disulfide-bonded globular domain, a juxta-membrane “stalk” domain, a transmembrane region and a charged intracellular domain of about 75 amino acids containing consensus phosphorylation sites for PKC or CKII.
Figure 1 Protein structure of the CD34 family.
CD34, podocalixin and endoglycan are transmembrane proteins which display O-glycosylated and sialylated serine-,threonine- and proline-rich extracellular mucin domain, putative sites of N-glycosylation, a cysteine-containing globular domain and a juxtamembrane stalk region. NHERF1 and 2 bind to the C-terminal tail of podocalyxin and endoglycan but not CD34. Conversely the CrkL selectively binds to a distinct juxtamembrane sequence in CD34.
At their C-termini, all three proteins contain short motifs (DTHL or DTEL) that resemble binding sites for PDZ-domain scaffolding proteins, which are known to play a role in targeting bound proteins to discrete subcellular localizations and facilitating signal transduction[19,20]. The DTHL motifs of Podxl and Endgl have been shown to bind the PDZ-domain proteins, NHERF-1 and NHERF-2, which are well known for their ability to bind a large array of G-protein coupled receptors, tyrosine kinases, transcription factors, etc.[17,21]. There is data suggesting that Podxl directs the recruitment of NHERF1, and presumably all of its signaling ligands, to discrete membrane domains within the cell[22]. Somewhat surprisingly, CD34 does not bind NHERF1 or NHERF2 and it is therefore likely that there is a distinct, yet-to-be-discovered, PDZ domain protein that binds this family member[21].
Relatively little is known of the intracellular ligands for CD34. In an unbiased phosphoproteomic survey of mast cells, CD34 was identified as one of the most rapidly (10 s) and highly (> 50-fold) tyrosine phosphorylated proteins in response FcεRI crosslinking[23] providing evidence that CD34 can be modified dynamically in response to extracellular stimuli and could provide a docking site for phosphopeptide binding proteins. In addition, the membrane proximal region of CD34 has been shown to bind the SH2-SH3-SH3 containing adapter protein, CrkL[24] through a motif that is not present in Podxl or Endgl. CrkL is known to interact with Abl, Bcr-Abl, C3G, Sos, EPS15, and DOCK180 through its N-terminal SH3 domain whereas CD34 is the first protein known to bind through its C-terminal SH3 domain[24]. Thus, like its relatives, Podxl and Endgl, there is reason to believe that CD34 can target signal transduction complexes to discrete cell membrane domains, possibly in response to phosphorylation.
Although the biological function of CD34 has not yet been fully clarified, several roles have been attributed to the proteins of the CD34 family. Due to its restricted expression on cycling hematopoietic progenitors, CD34 has been proposed to both promote proliferation and block differentiation (reviewed in[25]). Indeed, ectopic expression of CD34 in cell lines[26] and evaluation of progenitors derived from knockout mice[27] tend to support this view. A similar study showing resistance of CD34 knockout mice to formation of skin tumors posited a similar role for CD34 in the proliferation of precursors[14]. More recent studies have also linked Podxl and CD34 expression to enhanced trafficking and migration of hematopoietic cells[10,28,29] (reviewed in[17]). These studies suggest that the strong negative charge conferred by the highly glycosylated extracellular domain of CD34 family proteins serves an anti-adhesive function and enhance the mobility of cells. This may be further facilitated by a role in aiding chemotactic signaling responses as was recently suggested for CD34+ dendritic cell precursors[30]. The fact that expression of a human transgene was able to rescue the disease phenotype in these mice suggests that the human gene functions in a similar fashion[30]. While, at face value, it is difficult to reconcile the different roles for CD34 (and Podxl) observed in proliferation and cell migration, it should be noted that these may not be mutually exclusive; it is possible that CD34 plays subtle roles in both pathways. Alternatively, it is possible that by altering cell adhesion, CD34 could alter the downstream sensitivity of cells to entering cell cycle or differentiation. This would be quite compatible with a known role for integrins and other adhesion molecules in regulating cell cycle progression and differentiation (reviewed in[17]). Finally, through their potent ability to traffic to the apical domains of cells and interact with cytoskeletal signaling proteins, CD34 and its relatives have been speculated to play a role in regulating the recruitment of cell differentiation factors to discrete cellular membranes and thereby to regulate asymmetric cell division of undifferentiated precursors (reviewed in[17]).
Expression of CD34 in different cell types
CD34 family proteins have both unique and overlapping expression patterns. All three proteins (CD34, Podxl and Endgl) have been described as markers of hematopoietic precursors and vascular tissue (reviewed in[17]) giving them ample opportunity to serve redundant functions as evidenced in knock-out mice. However, in addition to their overlapping expression on these cell types, these molecules are also uniquely expressed on other cells. In the case of CD34, it is uniquely expressed by inflammatory cell precursors (mast cells, eosinophils and dendritic cells) and has been shown to be important for facilitating cell trafficking and the development of mucosal inflammatory disease, while in its absence, mice are rendered remarkably resistant to a range of disease including allergic asthma, hypersensitivity pneumonitis, colitis, Salmonella induced inflammation, and colon cancer[30-34]. As already mentioned in the introduction, CD34 is expressed by various stem/progenitor cells such as muscle satellite cells, adipogenic precursors and by hair follicle and keratinocyte stem cells. Podxl in contrast is expressed selectively by anemic erythroid lineage cells[28,35], kidney podocytes[36], a subset of developing neurons[37], and a variety of embryonic tissues including mesothelial precursors and epithelial precursors[16,38] (and Hughes et al, In Tech - Advances in Cancer Management, in press). Accordingly, deletion of the Podxl gene leads to perinatal lethality due to defective morphogenesis in a number of these cell types[16,38,39]. In BMSC, the expression and the role of CD34-related sialomucins remain unclear. However, Podxl was shown, together with α6-integrin (CD49f, VLA-6), to identify early progenitor BMSC with increased clonogenicity and differentiation potential in vitro and highly efficient migration to infracted heart in mice[40]. In addition, Podxl was shown to be strikingly upregulated in the most life-threatening epithelial tumors and appears to play a role in enhancing the mobility and invasiveness of tumors[22,38,41] (and Hughes et al, In Tech - Advances in Cancer Management, in press).
Expression of CD34 by hASC
Human adipose tissue was shown to turn over[42] and adipocytic progenitor cells, i.e., ASC in mice have been shown to reside in the adipose vasculature[43]. However, the precise origin of the native hASC still remains a debated question. Indeed, although it was recently proposed that ASC originate from a pericyte population lacking CD34 expression[44], Traktuev et al[6] suggested a CD34+ pericytic origin for ASC, which have been previously characterized as Lin-/CD29+/CD34+/Sca-1+/CD24+ cells[45]. To clarify this question, the expression of CD34 by hASC in native adipose tissue was addressed by characterizing expression of CD34 in subpopulations of cells from the SVF[46,47]. Initially, two CD34+ populations with a difference in the intensity of antigen expression were identified and a majority of the cells expressed CD34 at low intensity. ASC freshly isolated from human SVF were characterized as CD31-/CD34+/CD45-/CD90+/CD105-/CD146- cells, but were shown to become CD105+ when plated[48]. Upon adhesion to tissue culture plastic, cell expansion and passaging, CD34+/CD45- cells from SVF, were shown to gradually loose CD34 expression in monolayer culture, although the kinetics of decrease in CD34 expression seemed to vary strongly with culture conditions, such as plating density or culture medium used[48,49]. As a consequence, hASC, which are typically expanded as a monolayer on tissue culture plastic for several passages prior to their use, have been reported to be CD34- in most publications so far. This is in accordance with similar reports made on freshly cultured primary human vascular endothelial cells, where endothelial cells initially express CD34, which is later down-regulated with proliferation in continuous culture and is predominantly absent after only nine population doublings[50]. This was confirmed by the fact that CD34 seems to be especially expressed by endothelial “tip cells”, which are endothelial cells actively participating in the angiogenic process but which do not proliferate[51]. A follow up study on adipose-derived cells from the group of Yoshimura correlated CD34 expression by freshly isolated hASCs in SVF with a higher clonogenicity and proliferation capacity, reduced differentiation potentials into mesenchymal lineages, namely adipocytic and osteoblastic, increased expression of angiogenesis-related genes[52]. One limitation of the latter study resides in the fact that CD34+ cells were considered as a homogenous population. Our group investigated which cell population in SVF gives rise to hASC in monolayer culture[5] by sorting SVF cells according to the expression of CD34 and the endothelial marker CD31. We showed that the CD34+/CD31- fraction contains progenitors able to differentiate into the osteogenic lineage and colony-forming cells. This indicates that hASC are CD34+/CD31- cells in the SVF, which upon adhesion give rise to expanded hASC in culture. A study by another group concomitantly compared freshly isolated hASC and hASC expanded as monolayers on tissue culture plastic with serial passaging and also evaluated hASC morphology in their niche to define their tissue localization within intact human adipose samples[53]. This study showed that native human ASC (1) are CD34 positive and that its expression is dramatically decreased with in vitro proliferation; (2) display both stromal and perivascular positions but (3) do not express in situ pericytic markers such as NG2 and CD140b; and (4) exhibit a peculiar cell morphology with long protrusions in situ[53]. In conclusion, hASC are CD34+ in the native adipose tissue and in SVF, but rapidly change their phenotype and lose CD34 expression when expanded as monolayer (Figure 2).
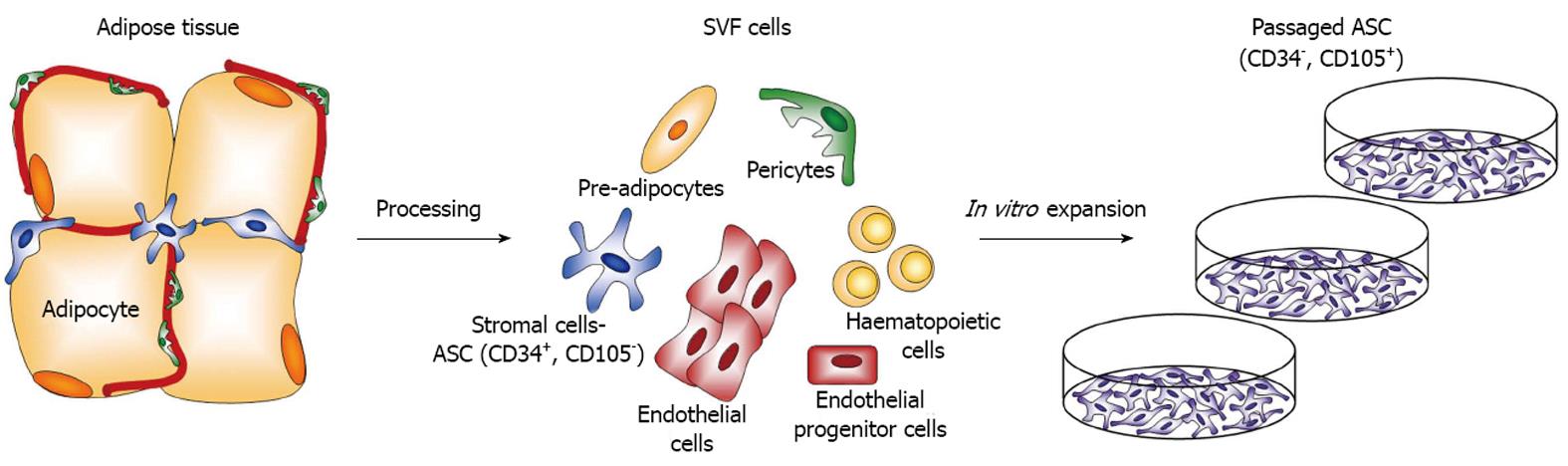
Figure 2 In the adipose tissue, adipose mesenchymal stem/stromal cells are localized perivascularly and are characterized by the expression of CD34.
Adipose mesenchymal stem/stromal cells (ASC) are isolated upon collagenase digestion together with other cell types including endothelial and endothelial progenitor cells. Upon adhesion to plastic and active in vitro proliferation, ASC change their phenotype, lose CD34 expression and begin to express CD105.
Expression of CD34 by vascular cells in human SVF
The presence of vasculogenic cells within the SVF was first proposed in 2004[54] and thereafter confirmed by different groups[8,55,56]. The CD34+/CD31- cell population from adipose tissue differentiates into endothelial cells and exhibits the capacity to rescue hindlimb ischemia in animal models[8,57]. Also, both CD34+ and CD34- primitive mesodermal progenitors within the SVF of human adipose tissue were shown to exhibit hematopoietic and hemangioblastic activities in vitro[58]. In a recent study, our group investigated the vasculogenic potential of different subpopulations of SVF cells. We showed that neither CD34+CD31- nor CD34+CD31+ from SVF were able to form vascular structures alone, but that a combination of the two resulted in robust vascular structure formation in vitro[5], extending a preliminary report showing tubule formation on Matrigel® by CD90+/CD34+ cells[59]. We thereby showed that CD34+CD31- cells not only include hASC but also cells with a pericytic phenotype necessary for the stabilization of endothelial capillaries formed by CD34+CD31+ adipose endothelial cells (hAEC, Figure 1), as previously suggested[6]. However, despite a clear potential of these vasculogenic cells derived from adipose tissue to initiate vascularization in regenerative medicine applications (reviewed in[2] or to treat ischemic disorders[8], their safety for clinical applications should still be considered with caution. For example, the adipose tissue used for autologous lipotransfer procedures, e.g., for breast reconstruction, in some cancer patients (which is rich in CD34+ progenitors) was shown to promote tumor growth, angiogenesis and metastases in several orthotopic models of human breast cancer[60]. This possibly reflects a side-effect due to mast cells, which are present in the adipose transplants, release VEGF and thereby induce tumor angiogenesis[61].
In summary, it is important to make a distinction between the CD34+CD31+ hAEC constitutively present in the native adipose tissue and in the SVF and CD34+CD31+ endothelial-like cells obtained by differentiation of CD34-/CD31- expanded hASC[62,63]. Interestingly, this indicates that, like primary vascular endothelial cells[50,51], cells derived from adipose tissue, that lose the expression of CD34 in monolayer culture retain the ability to re-express this marker if stimulated appropriately.
Function of CD34 in SVF cells and hASC
Despite the loss of CD34 expression during in vitro culture, expanded hASC retain proliferative capacity and multipotency even after several passages. However, little is known about how CD34 affects the biological features and the functionality of SVF cells, i.e., hASC or endothelial/vasculogenic cells.
With regard to the role of CD34 in the biology of endothelial cells from SVF, it is likely to involve mechanisms mostly similar to the ones demonstrated for lumen formation in the developing mouse aorta[64], as suggested by our preliminary data showing similar tubes as well as lumen formation by SVF cells in vitro[5]. Moreover, the endothelial CD34+/CD31+ fraction within SVF cells exhibit the ability to form functional blood vessels in vivo and connect to the vasculature of the recipient mice, both after perfusion culture[9] or if implanted directly after isolation from adipose tissue[65]. We also find that vasculogenic cells from the SVF can generate highly organized vascular structures in vitro, which by anastomosis with the vasculature of the host greatly enhance both the amount and depth of bone tissue formation inside tissue engineered osteogenic constructs in vivo[5]. The functional role of CD34 in vasculogenic cells from SVF, and the mechanisms involved will be further investigated by us in the future.
With regard to CD34 expression in ASC, few studies have addressed the role of CD34 in hASC function, in vitro. Suga et al[52] used SVF cells after a short initial expansion phase on tissue culture plastic and showed that, in sorted SVF-derived cells, both CD34+ and CD34- cells are clonogenic and that the impact of CD34 expression on hASC function was limited. Indeed, CD34+ cells showed a slightly decreased proliferation and a limited increase in differentiation capacity towards mesodermal lineages. More recently, in 8-d expanded hASC, Maumus et al[53] demonstrated that CD34+ hASC are the only subpopulation of hASC containing clonogenic cells, and the only one able to differentiate into adipogenic and osteogenic lineages. We simultaneously confirmed and extended this finding to unexpanded, SVF cells by showing that it is more specifically the CD34+/CD31- cell population which contains the cells with osteoblastic differentiation capacity and also clonogenic, colony-forming cells[5].
In the context of bone tissue engineering, several reports suggest that, despite the loss of CD34 upon expansion of hASC, they can maintain an actual capacity to form bone tissue in vivo, if cultured adequately (reviewed in[2]). SVF cells were used by our group to generate osteogenic grafts with intrinsic vasculogenic capacity, both if implanted immediately[65] or when SVF cells were seeded and cultured for 5 d within hydroxyapatite porous scaffolds inside a perfusion-based bioreactor system[9]. In both cases, it is important to mention that more than 65% of the cells, including hASC, still expressed CD34 in the experimental conditions used. These studies critically established that human SVF cells, similarly to expanded hASC, are capable of generating frank bone tissue in vivo. This was possible even in the absence of exogenous osteoinductive signals, when SVF cells were cultured under perfusion[9], whereas direct implantation of SVF cells required an osteogenic trigger such as bone morphogenetic protein 2 to support ectopic osteogenesis in vivo (Mehrkens et al, In press). The potential importance of CD34 in bone formation by hASC is currently being investigated by our group by ectopic implantation of the CD34+ and CD34- fractions.
Conclusion and perspectives
The use of CD34 as an adipose-derived cell marker, and the numerous reports about expression of CD34 by hASC and its progressive loss upon in vitro cell culture can not compensate for a major lack of references in the literature about the precise role and function of CD34 in hASC and other cells derived from the SVF. This fact should prompt the increasing number of research teams using hASC and SVF cells in regenerative medicine applications worldwide to design new research projects aimed at addressing this question more specifically. In particular, a better understanding of the membrane localization and trafficking of CD34, of the signaling pathways induced by CD34 and of the promoters controlling the expression of CD34 in SVF-derived cells would help in better investigating its role on functions such as adhesion, migration, proliferation or differentiation, and consequently the tissue formation capacities of these cells in vivo.
Furthermore, alternative culture methods should be developed to avoid the loss of CD34 expression and to preserve a physiological phenotype. Our group recently investigated the possibility of expanding hASC while maintaining their expression of CD34. This study is based on the hypothesis that cell-extracellular matrix and cell-cell interactions should be favored to mimic the physiological situation. The final aim would be to reconstitute a three-dimensional physiological environment in a controlled setting as it was proposed for the bone marrow niche in one of our recent reports[66]. The system aimed at recapitulating the complex microenvironment of the niche and establishing the chemico-physical cues required for a physiological stem cell function regulation. By applying this principle to SVF cells, it was possible to maintain CD34+/CD31-/CD105- hASC, i.e., a phenotype much more similar to native hASC, for up to 6 wk in culture (our preliminary, unpublished data). SVF cells from the same donors, cultured in parallel as standard monolayer with serial, weekly passaging, generated hASC with minimal expression of CD34. A deeper characterization is needed to better understand the biological features and functionality of these cells and is currently ongoing.