Published online Sep 26, 2012. doi: 10.4252/wjsc.v4.i9.94
Revised: March 1, 2012
Accepted: March 25, 2012
Published online: September 26, 2012
AIM: To establish the potential of poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) (PHBHHx) as a material for tendon repair.
METHODS: The biocompatibility of PHBHHx with both rat tenocytes (rT) and human mesenchymal stem cells (hMSC) was explored by monitoring adhesive characteristics on films of varying weight/volume ratios coupled to a culture atmosphere of either 21% O2 (air) or 2% O2 (physiological normoxia). The diameter and stiffness of PHBHHx films was established using optical coherence tomography and mechanical testing, respectively.
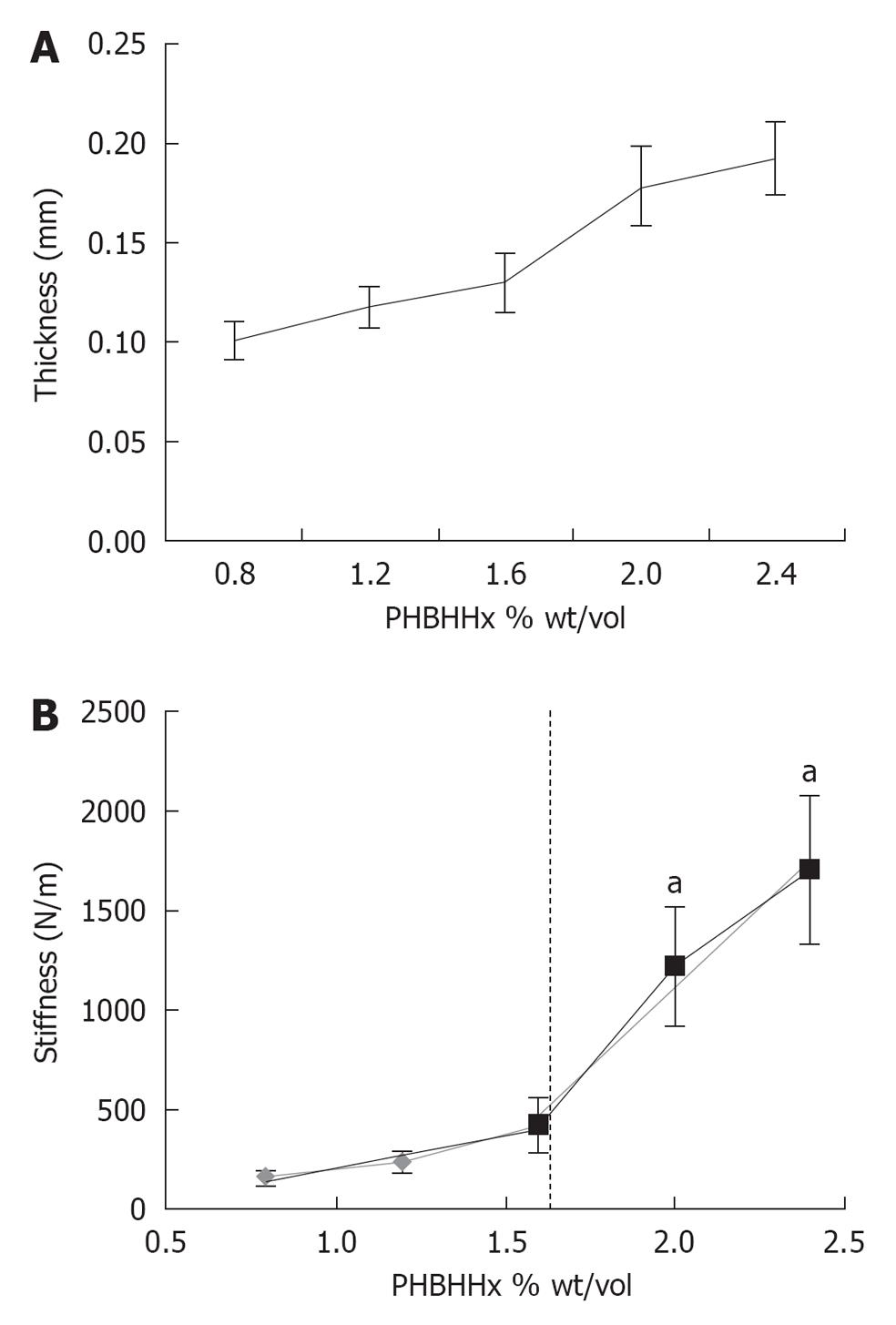
RESULTS: Film thickness correlated directly with weight/volume PHBHHx (r2 = 0.9473) ranging from 0.1 mm (0.8% weight/volume) to 0.19 mm (2.4% weight/volume). Film stiffness on the other hand displayed a biphasic response which increased rapidly at values > 1.6% weight/volume. Optimal cell attachment of rT required films of ≥ 1.6% and ≥ 2.0% weight/volume PHBHHx in 2% O2 and 21% O2 respectively. A qualitative adhesion increase was noted for hMSC in films ≥ 1.2% weight/volume, becoming significant at 2% weight/volume in 2% O2. An increase in cell adhesion was also noted with ≥ 2% weight/volume PHBHHx in 21% O2. Cell migration into films was not observed.
CONCLUSION: This evaluation demonstrates that PHBHHx is a suitable polymer for future cell/polymer replacement strategies in tendon repair.
- Citation: Lomas AJ, Chen GG, El Haj AJ, Forsyth NR. Poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) supports adhesion and migration of mesenchymal stem cells and tenocytes. World J Stem Cells 2012; 4(9): 94-100
- URL: https://www.wjgnet.com/1948-0210/full/v4/i9/94.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v4.i9.94
Polyhydroxyalkanoates (PHA) are a family of biopolymers consisting of polyesters of many different hydroxycarboxylic acid molecules. Micro-organisms produce PHAs as an energy storage molecule when exposed to unbalanced growth conditions during culture; for instance, excess lauric acid, limited nitrogen and limited phosphorous supply[1]. Originally viewed as replacements for traditional petrochemical-derived polymers, PHAs are now largely redundant as everyday materials due to the prohibitive cost of large quantity production[1]. There is now increased interest in these polymers from the medical device sector where the earlier prohibitive costs are reduced due to the reduced scale of operations. In addition, PHAs display relatively high immunotolerance, low toxicity and biodegradability which are all crucial for the medical device sector[2].
PHBHHx is the designation of molecules consisting of random co polymers of 3-Hydroxybutyrate and 3-Hydroxyhexanoate[3]. It is one of the few PHA molecules that can currently be produced on a large enough scale for use in both scientific research and medical device construction[4]. PHBHHx has a melting temperature of 111.7 °C, a glass transition temperature of -0.67 °C, a tensile strength of 4.1 MPa, an elongation at break of 103.8%, and a Young’s modulus of 130.4 MPa, making it potentially useful for widespread biomaterial applications and different cell types[5,6].
Tendons form the bridge between muscle and bone. They are typically slow to repair after injury or disease, have a poor blood supply and are relatively acellular when compared to other tissues[7]. Tendon is composed mainly of collagen type I fibrils arranged in a hierarchical structure surrounded by a layer of endotenon[8]. These fascicles come together to form larger and larger subunits, eventually forming the complete tendon. The arrangement of collagen I fibrils give the tendon its strength in tension. Tenocytes are the major cell group present in tendons, making up around 95% of the cellular mass[9]. They are a highly specialized form of fibroblast and are responsible for the maintenance of tendon extracellular matrix (ECM) [collagen I (the major component of tendon), collagen III, collagen V, glycosaminoglycans, elastin and fibronectin] and for the repair of tendon tissue, either after injury or as part of normal physiological process[10,11]. Under normal physiological conditions, tenocytes are found in small numbers spread between the collagen fibrils[10].
Human mesenchymal stem cells (hMSCs) are viewed as a candidate cell source for tendon tissue engineering as, unlike tenocytes, they can be readily sourced, isolated and expanded in vitro. The exposure of hMSCs to external tensile forces and/or supplementation with additional growth factors can induce differentiation into cells that resemble tenocytes in physiological activity and marker expression profile have been produced[12,13].
The objective of this investigation was to monitor and quantify the interaction (attachment) and monitor the migration of tenocytes and hMSCs with PHBHHx polymer films of a variety of weight:volume ratios to characterize the optimal ratios for use in tissue engineering application. Here we show that both cell types adhere to PHBHHx films, with tenocytes preferring a thicker, stiffer scaffold, whereas MSCs adhered to all PHBHHx films tested, with greater adhesion noted in physiological oxygen conditions. Migration across, but not into, PHBHHx films was also apparent for both rat tenocytes (rT) and hMSC. Taken together, this demonstrates that PHBHHx is a suitable biomaterial for tendon tissue engineering.
Tenocytes were isolated from the Achilles tendon of 8 wk old male Wistar rats. The tendon was dissected, minced into 1 mm sections, and placed onto a dry Petri dish. The tendon was allowed to adhere for 1 h before careful addition of 5 mL pre-warmed media [DMEM (4.5 g/L glucose) (Lonza, UK), 10% Fetal bovine serum (FBS) (Lonza, UK), 1% L-Glutamine (L-Glut) (Lonza, UK), 1% non essential amino acids (NEAA) (Lonza, UK)], taking care not to dislodge tissue pieces. These were then incubated under standard conditions for 7 d, during which time cells migrated from the tendon tissue onto the petri dish. Cells were then expanded in T-75 flasks in either 21% O2 or 2% O2 using previously described methodologies[14,15].
hMSC were isolated from human bone marrow via an adhesion method described elsewhere[16]. hMSC were maintained on 10 ng/mL Fibronectin (Lonza, UK) coated flasks in DMEM, 5% FBS, NEAA, and L-Glut and incubated at 37 °C, 7% CO2 and either 21% O2 or 2% O2. Nitrogen gas was supplied using an N2 generator supplied by Peak Scientific. Culture media was changed twice weekly. hMSCs were passaged at 90%-95% confluency using Trypsin/EDTA. During experimentation, passage numbers of 4 or less were used.
PHBHHx [87.9% Hydroxybutyrate (HB), 12.1% Hydroxyhexanoate (HHx)] was dissolved in 10 mL chloroform (Sigma Alrich, UK), at varying weights (0.08-0.24 g/10 mL) at room temperature in a sealed, clean glass tube. Once dissolved, 3.2 mL was poured into an open 60 mm glass Petri dish and left overnight to ensure complete evaporation. Films were then transferred into non-adherent 60 mm Petri dishes (Sterilin, UK), one film/dish. Films were then washed in 3 mL 70% Ethanol/ 30% distilled H2O for 3 h, before washing with sterile PBS (Lonza, UK) 3 times. The films were dried for 1 h before use.
Polymer thickness was measured using a home built Optical Coherence Tomography (OCT) system according to a previously published method[17]. OCT generated images (laser wavelength interference patterns) were taken at random areas of 3 different films, with 3 images taken of each film. Image J analysis software was then used to determine thickness.
Stiffness was measured using a BOSE ElectroForce 3200 system. Samples were cut to 22 mm × 5 mm ribbons and placed into the grips, with 10 mm of the polymer in each grip, leaving a 2 mm initial sample length. This was then deformed by 0.5 mm in a uniaxial direction and the force required measured. Stiffness was calculated with the equation: k = F/δ, where k = stiffness, F = force and δ = displacement in single direction of freedom (i.e., direction the force acts in).
Following preparation, PHBHHx films were immersed in 3 mL media containing 3 × 104 cells/mL in non-adherent, 6 well plates (Costar, UK). After 24 h incubation in either 21% O2 or 2% O2, films were removed from the dishes, gently washed in PBS, placed in a 15 mL centrifuge tube and immersed in pre-warmed Trypsin/EDTA (Lonza, UK) for 5 min, before quenching with excess media and removing the film. The surface of the non-adherent dish was also washed once with PBS and exposed to 1 mL Trypsin/EDTA for 5 min, before quenching with excess media. After centrifugation, cell pellets were re-suspended and cell counts established from both film and non-adherent well by hemocytometer counts of Trypan Blue (Sigma Alrich, UK) positive cells only. A control group where cells were seeded into wells containing no polymer film was also performed. The combined film and well cell counts were treated as 100% and used to establish percentage attachment.
Cell migration was measured by labeling cells with DiO (Vybrant Multicolor Cell Labeling Kit, Invitrogen, UK) and inoculating them as described earlier onto 2% PHBHHx films. These were then incubated in a 21% O2 or 2% O2 incubator for 24 or 72 h, after which time the media was removed, the well washed with PBS, fixed with 4% Paraformaldehyde (Sigma Alrich, UK) for 5 min, and then re-immersed in PBS. Confocal microscopy (Olympus Fluoview, Olympus IX71) was performed to determine if cells had migrated into polymer films, by creating a “z-stack” representation of the polymer cross section, giving a fluorescent signal where cells are located and allowing for a plane of reference to be made from the images.
Results were deemed to be significant if P≤ 0.05, or as indicated in figure legends using a 2-tailed, paired, Students T-test.
Films were first characterized by determining both thickness and stiffness. Thickness was determined using OCT and Image J analysis software. The thickness of polymer films correlated directly with the initial polymer input (r2 = 0.947). The 0.8% weight/volume films had an average thickness 0.10 ± 0.009 mm, while the 2.4% weight/volume films had a measured thickness of 0.19 ± 0.018 mm (Figure 1A). We next sought to determine the stiffness of the polymer films examined above. Stiffness was determined with mechanical testing with the Bose ElectroForce 3200 system as described in Materials and Methods. The calculated stiffness (resistance to elongation) values ranged from 153 ± 42 N/m (0.8% weight/volume PHBHHx) to 1706 ± 371 N/m (2.4% weight/volume PHBHHx). A biphasic increase in stiffness was observed between ≤ 1.6% weight/volume (N/m = 135.24 * weight/volume, r2 = 0.9605) and ≤ 1.6% weight/volume (N/m = 570 * weight/volume, r2 = 0.9657) A significant increase in stiffness was observed between films ≥ 2% weight/volume when compared to ≤ 1.6% weight/volume (P≤ 0.024) (Figure 1B).
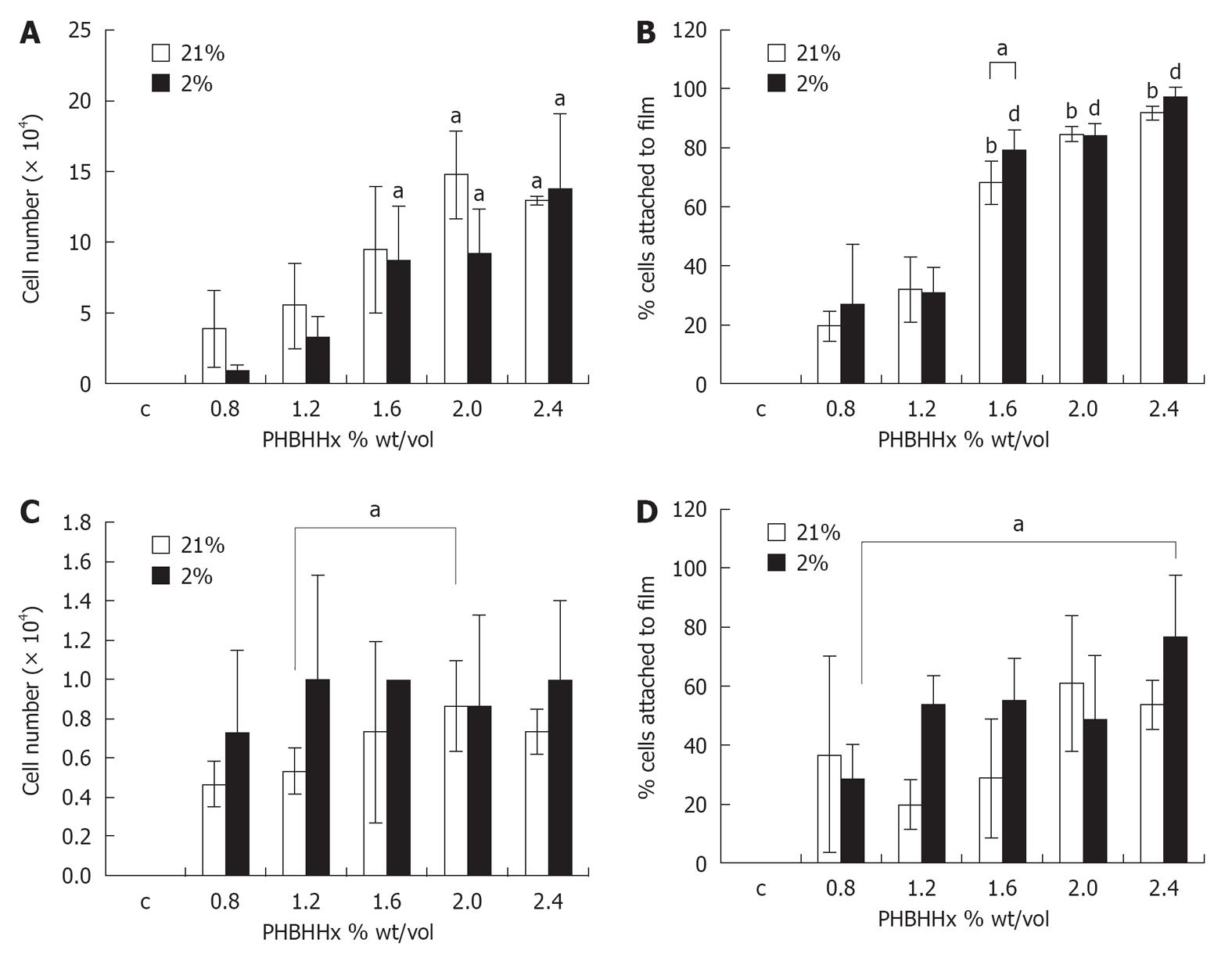
rT seeded onto PHBHHx films in 21% O2 displayed a significant increase in film-adherence between 0.8% weight/volume (3.87 × 104± 2.73 × 104 cells/film) and ≤ 2.0% weight/volume (≤ 9.47 ± 4.46 cells/film, P≤ 0.02). Similarly, the percentage of cells attached to the film in relationship to the overall number of cells in each demonstrated a significant increase between 0.8% weight/volume (19.36% ± 4.98%) and ≤ 1.6% weight/volume (≤ 68.38% ± 7.31%, P≤ 0.02) (Figure 2B). The use of physiological oxygen (2% O2) in rT PHBBHx film adherence and percentage attachment studies yielded similar results to above. Significant increases in adherence were noted between films of 0.8% weight/volume and 1.6% weight/volume (0.87 ± 0.42 × 104vs 8.67 ± 3.84 × 104 - 13.73 ± 5.36 × 104, P≤ 0.05) (Figure 2A), demonstrating that rT cells adhere better to substrates with a stiffness ≥ 420 N/m. Significant increases in percentage cell attachment were also noted between films of 0.8% weight/volume (34.37% ± 8.27%) and ≤ 1.6% weight/volume (≤ 84.36% ± 3.98%, P≤ 0.01) (Figure 2B). Direct comparison of attachment profiles in 21% O2 and 2% O2 revealed a significant increase in cell attachment to 1.6% weight/volume films in 2% O2vs 21% O2 (P = 0.05) (Figure 2B).
hMSC adherence to PHBHHx films with varying weight/volume ratios was relatively consistent in 21% O2 across all films tested although a significant increase was noted between films of 1.2% weight/volume (0.53 × 104± 0.12 × 104 cells/film) and 2% weight/volume (0.88 × 104± 0.23 × 104 cells/film) (P = 0.04) (Figure 2C). When expressed as a percentage of total cells present in the dish (film and well), considerable variability was noted. Qualitative increases in cell attachment were noted between 1.2% weight/volume (19.7% ± 8.4%) and 2% weight/volume (61.1% ± 22.9%) (P = 0.059) (Figure 2D), suggesting that hMSCs require a stiffer substrate than rT cells for optimal attachment. Reducing atmospheric oxygen to 2% O2 created a qualitative rise in cellular attachment between 0.8% weight/volume (0.73 × 104± 0.41 × 104) and 1.2% weight/volume (1.0 × 104± 0.53 × 104) (P = 0.63) (Figure 2C). Little variation was seen between films where weight/volume ≥ 1.2%. When expressing values as a percentage of total cells present, non significant increases were seen between 0.8% weight/volume (28.7% ± 11.8%) and 1.2% weight/volume (53.8% ± 9.7%); however, a significant increase is observed when 0.8% weight/volume (28.7% ± 11.8%) and 2.4% weight/volume (77.1% ± 20.6%) (P = 0.03) (Figure 2D) are compared. Taken together, this indicated that hMSC cultured in physiological oxygen display an adherence preference for PHBHHx films with stiffness of 240 N/m (vs 1220 N/m in 21% O2).
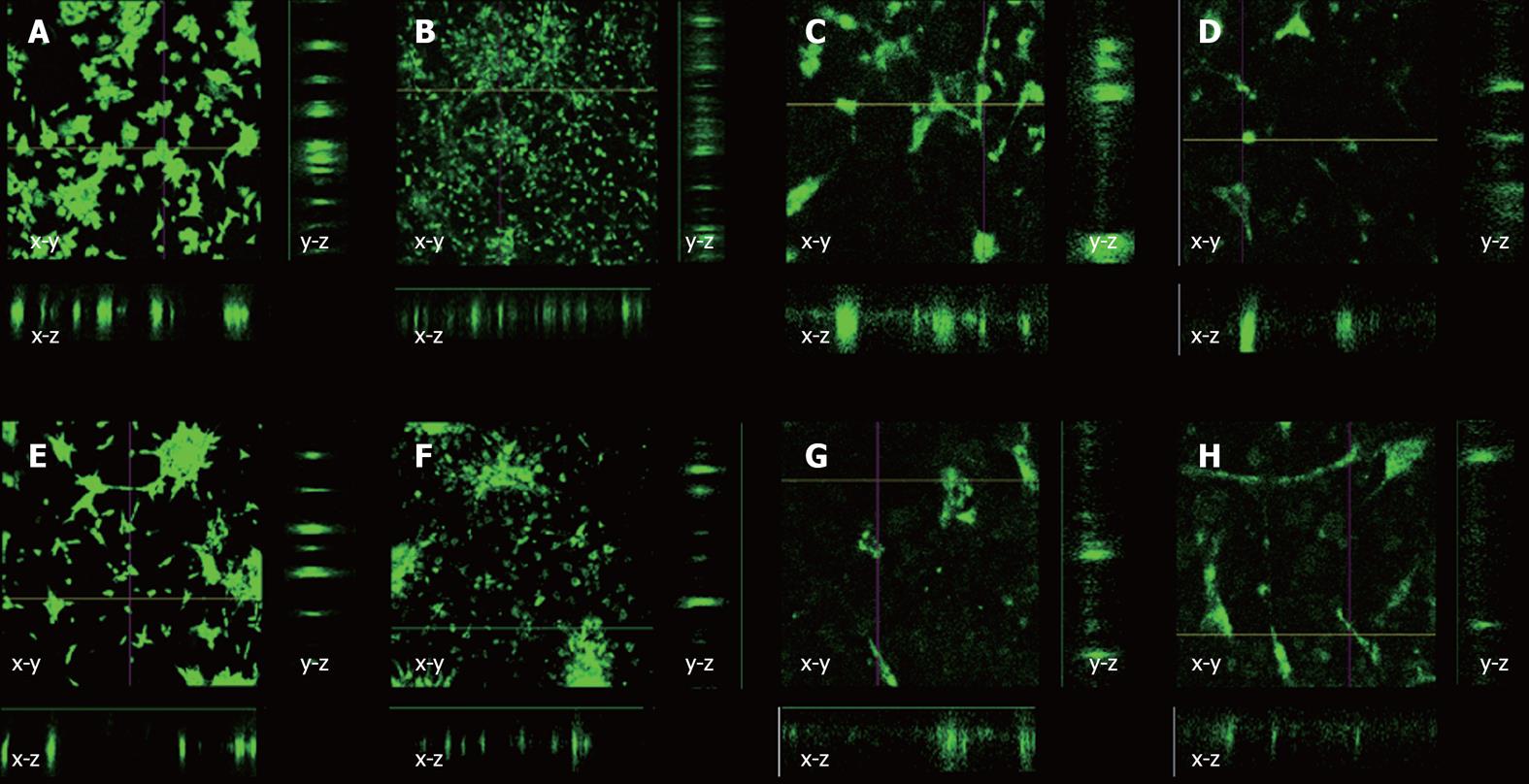
Our final investigation was intended to determine if cells rapidly migrated into PHBHHx films. Based on our previous observations, we used a 2% PHBHHx film throughout. No tenocyte or MSC migration was observed into the polymer film after either 24 or 72 h in either O2 concentration in either x-z or y-z directions (Figure 3). Substantial spreading across the surface of the PHBHHx was apparent after 72 h, indicating its high compatibility with tenocytes and hMSCs (Figure 3F).
This study demonstrates for the first time that tenocytes will adhere to and spread across PHBHHx polymer films with a preferred rigidity of > 420 N/m. This supports the assertion of PHBHHx as a candidate material for tendon tissue engineering and adds to the body of literature supporting the biocompatibility of PHBHHx.
PHBHHx has been previously used to culture many different cell types. A previous report demonstrated that hMSC adherence was greatly improved on PHBHHx films when compared to both tissue culture plastic and other PHA molecules[6]. Adipose derived MSCs were also successfully cultured in 3D PHBHHx scaffolds before being stimulated into chondrogenic differentiation[18]. The PHBHHx scaffold provided the cells with a suitable matrix for support of growth and differentiation in vitro and when implanted in vivo as evidence by the production of cartilaginous ECM, a key requirement of cartilage tissue engineered constructs. ECM is also the key component in tendon as it is this, rather than the cellular component, that sustains the mechanical load[8]. Therefore, PHBHHx can be used as a 3D scaffold to support cells while they express and develop an ECM. This support capacity is not limited to chondrocytes, as demonstrated by the osteogenic differentiation of rabbit bone marrow derived stem cells on PHBHHx providing additional demonstration of the polymers applicability for orthopedic application use[3].
The proportion of HHx in the polymer has been proposed as an important modulator of cell behavior. Proliferative capacity of rat smooth muscle cells was enhanced by 20% HHx although attachment at seeding was optimal with 12% HHx[19]. In our study we also found robust attachment of hMSC and rT to 12% HHx polymer films when used at the optimal weight/volume ratio.
Investigation into rT attachment to PHBHHx films as a total of all cells present in the well demonstrates that when weight/volume ratios of ≥ 1.6% were used, virtually all cells adhered to the film in preference to the untreated plastic surface. This can in some part be explained by the increase in stiffness of the polymer film between 1.6% and 2.0% weight/volume. In other words, increased polymer rigidity promoted increased tenocyte adhesion. This reinforces a number of previous studies which have demonstrated that material stiffness affects cellular behavior in many ways, including adhesion[20-22]. hMSCs have previously been shown to adhere to PHBHHx and many other different surfaces with differing mechanical properties[3,23,24], explaining why little difference was found between polymer concentrations. As cell fate was not investigated in this study, it is not known what, if any, effect on differentiation potency this had. Ongoing 3-D tissue engineering experimentation will address these questions.
The tendon is poorly vascularized and has a low mean oxygen concentration[25]. We therefore performed our investigation in both room oxygen (21% O2) and tendon tissue normoxia (2% O2). Previous studies into the effects of different oxygen concentrations on cells have also demonstrated enhanced proliferation, enhanced clonogenicity, reduced karyotypic abnormalities, reduced spontaneous differentiation, altered transcriptional profiles, and altered FTIR profiles across numerous cell types, including hMSCs[14,15,26-28]. When comparing 2% O2 with 21% O2, only small differences were found between cell number or percentage attachment at the same PHBHHx concentration for either cell type. A qualitative increase was observed in tenocytes (≥ 1.6% weight/volume) in 21% O2 over 2% O2; however, this was not significant. For reasons we do not fully understand, we observed large standard deviations in a number of 2% O2 sample groups, which could be contributing to this. It should be noted that little difference in the percentage of cells attached to the polymer were observed between the differing oxygen conditions, demonstrating that oxygen tension was not effecting cellular attachment to the films per se but was rather reducing the population of cells available for attachment. hMSCs were generally noted to adhere better in hypoxic conditions to all polymer film compositions; however, no significant rises were found, possibly due to large inter-group deviations. To our knowledge, this is the first study looking into the in vitro effects of oxygen tension on the interaction of primary mammalian cells with polyhydroxyalkanoate scaffolds.
Cell spreading was monitored across polymer surfaces in the absence of mechanical stimuli or directional forces over a period of 72 h by marking cells with fluorescent tracker dye (DiO). This method was essential due to polymer opacity. After 24 h, the cells were clumped together on the surface of the polymer. This behavior is not uncommon in cell culture and can be explained by the cells not being separated sufficiently when re-suspending after centrifugation. After 72 h, the cells were seen to move apart from each other, filling the available space on the polymer. Yang et al[29] found that mouse islet cells showed increased metabolic activity when cells were cultured on PHBHHx when compared to tissue culture plastic and Poly Lactic Acid. This investigation also looked into cell migration across a PHBHHx film surface, finding that cells were moving from an even distribution to clump together to start to form functional units. These findings agree with this work that cell locomotion is possible across PHBHHx surfaces.
DiO and other similar dyes used for tracking cells can be expelled by the ABCG2 multi-drug transporter pathway[30]. hMSC retained dye more efficiently than tenocytes although both had undergone reductions in intensity after 72 h, suggesting that the dye had been exocytosed. Migration into the polymer surface was measured with confocal microscope generated z-stacks, allowing for cross sectional views to be created in both the x-z and y-z directions. Cells were always found to be in one plane of view, with no further fluorescent signatures being seen above or below the single plane. This indicated that the cells remained on the surface of the polymer as opposed to migrating into it, indicating that localized polymer degradation had not occurred over the time period tested. This observation is reinforced by previous reports which state that PHBHHx is broken down in vivo[31] and in vitro[32] at very slow rates via hydrolysis. Further investigation of cell migration into and across PHBHHx surfaces could potentially form the basis of a mathematical modeling study; however, this is beyond the scope of this investigation.
This investigation demonstrates that tenocytes and hMSCs can adhere to and spread across PHBHHx films over 24 and 72 h time periods. Film scaffolds fabricated with ≥ 1.6% weight/volume polymer/solvent, with a stiffness ≥ 420 N/m are the most effective in supporting this activity with rT cells; however, hMSCs displayed a capacity for adhesion to all polymer films of stiffness ≥ 240 N/m. Physiological normoxia increased hMSC adhesion to most PHBHHx films; however, no significant differences were seen due to large intergroup variation and little effect was observed on rT cell adhesion. PHBHHx can now be considered to be a potential material for use in future tendon tissue engineering application.
We are grateful to Miss Samantha Wilson and Dr. Ying Yang for their assistance in OCT operation.
Tendon injury is an increasing problem in medical science due to the very slow rate at which damaged tendon repairs. Current surgical repair techniques can be ineffective in some cases. As a result, tissue engineering is seen as a viable option in tendon repair. Previous studies into the effectiveness of alternative replacement materials to tendon are starting to show good results; however, a perfect solution is yet to be found. Poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) (PHBHHx) has been shown to support several cell types, but as yet tendon cells have not been investigated.
Current research into tendon tissue engineering focuses on finding materials that can support cellular adhesion while at the same time being able to withstand the high mechanical forces transmitted through tendons, restoring function at a faster rate than would otherwise be possible.
This investigation has for the first time looked into the interaction of PHBHHx with rat tenocytes (rT) and the effect of PHBHHx scaffold stiffness on human mesenchymal stem cells (hMSCs). It has also looked at how atmospheric oxygen effects PHBHHx/cell interaction.
This research provides the basis for further investigation of Polyhydroxyalkanoate polymer molecules in the field of tendon tissue engineering. This is an exciting development, as PHA molecules, specifically PHBHHx, are renowned for their long term mechanical integrity and biocompatibility in vivo.
PHBHHx: is a natural polymer produced as an intracellular energy storage molecule by bacteria in certain conditions. hMSC: are pre curser cells to many different tissue types in the body, including bone, cartilage, skeletal muscle and tendon. rT: Rat Tendon cell (tenocyte) are cells isolated from tendon from adult rats. OCT: Optical Coherence Tomography is a laser based system used for investigating thin sections of materials or the upper layers of block materials.
The manuscript is a brief research communication reporting mainly attachment of MSC and tenocyte to poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) (or PHBHHx) films. The adhesive properties of both cell types were examined in relationship to varying weight/volume ratios of PHBHHx and O2 tension. The manuscript by Lomas et al demonstrates that rT and human MSCs adhere to and migrate on PHBHHx. The work is noteworthy due to the fact that PHBHHx is one of the few polymers that can be produced on a large scale and also has properties suitable for medical use.
Peer reviewer: Techung Lee, PhD, Associate Professor, Department of Biochemistry, State University of New York, 3435 main street, Buffalo, NY 14214, United States
S- Editor Wang JL L- Editor Roemmele A E- Editor Zheng XM
| 1. | Chen GQ. A microbial polyhydroxyalkanoates (PHA) based bio- and materials industry. Chem Soc Rev. 2009;38:2434-2446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 878] [Cited by in RCA: 747] [Article Influence: 46.7] [Reference Citation Analysis (0)] |
| 2. | Chen GQ, Wu Q. The application of polyhydroxyalkanoates as tissue engineering materials. Biomaterials. 2005;26:6565-6578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1085] [Cited by in RCA: 837] [Article Influence: 41.9] [Reference Citation Analysis (0)] |
| 3. | Wang YW, Wu Q, Chen GQ. Attachment, proliferation and differentiation of osteoblasts on random biopolyester poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) scaffolds. Biomaterials. 2004;25:669-675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 170] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 4. | Chen GQ, Zhang G, Park SJ, Lee SY. Industrial scale production of poly(3-hydroxybutyrate-co-3-hydroxyhexanoate). Appl Microbiol Biotechnol. 2001;57:50-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 170] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 5. | Bian YZ, Wang Y, Aibaidoula G, Chen GQ, Wu Q. Evaluation of poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) conduits for peripheral nerve regeneration. Biomaterials. 2009;30:217-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 116] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 6. | Hu YJ, Wei X, Zhao W, Liu YS, Chen GQ. Biocompatibility of poly(3-hydroxybutyrate-co-3-hydroxyvalerate-co-3-hydroxyhexanoate) with bone marrow mesenchymal stem cells. Acta Biomater. 2009;5:1115-1125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 51] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 7. | Bullough R, Finnigan T, Kay A, Maffulli N, Forsyth NR. Tendon repair through stem cell intervention: cellular and molecular approaches. Disabil Rehabil. 2008;30:1746-1751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 42] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 8. | Kannus P. Structure of the tendon connective tissue. Scand J Med Sci Sports. 2000;10:312-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 684] [Cited by in RCA: 654] [Article Influence: 26.2] [Reference Citation Analysis (1)] |
| 9. | Schulze-Tanzil G, Mobasheri A, Clegg PD, Sendzik J, John T, Shakibaei M. Cultivation of human tenocytes in high-density culture. Histochem Cell Biol. 2004;122:219-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 101] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 10. | Richardson LE, Dudhia J, Clegg PD, Smith R. Stem cells in veterinary medicine--attempts at regenerating equine tendon after injury. Trends Biotechnol. 2007;25:409-416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 109] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 11. | Bernard-Beaubois K, Hecquet C, Houcine O, Hayem G, Adolphe M. Culture and characterization of juvenile rabbit tenocytes. Cell Biol Toxicol. 1997;13:103-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 12. | Wang QW, Chen ZL, Piao YJ. Mesenchymal stem cells differentiate into tenocytes by bone morphogenetic protein (BMP) 12 gene transfer. J Biosci Bioeng. 2005;100:418-422. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 124] [Cited by in RCA: 143] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 13. | Krampera M, Pizzolo G, Aprili G, Franchini M. Mesenchymal stem cells for bone, cartilage, tendon and skeletal muscle repair. Bone. 2006;39:678-683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 215] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 14. | Forsyth NR, Musio A, Vezzoni P, Simpson AH, Noble BS, McWhir J. Physiologic oxygen enhances human embryonic stem cell clonal recovery and reduces chromosomal abnormalities. Cloning Stem Cells. 2006;8:16-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 139] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 15. | Forsyth NR, Kay A, Hampson K, Downing A, Talbot R, McWhir J. Transcriptome alterations due to physiological normoxic (2% O2) culture of human embryonic stem cells. Regen Med. 2008;3:817-833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 42] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 16. | Wimpenny I, Hampson K, Yang Y, Ashammakhi N, Forsyth NR. One-step recovery of marrow stromal cells on nanofibers. Tissue Eng Part C Methods. 2010;16:503-509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 17. | Ahearne M, Wilson SL, Liu KK, Rauz S, El Haj AJ, Yang Y. Influence of cell and collagen concentration on the cell-matrix mechanical relationship in a corneal stroma wound healing model. Exp Eye Res. 2010;91:584-591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 38] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 18. | Ye C, Hu P, Ma MX, Xiang Y, Liu RG, Shang XW. PHB/PHBHHx scaffolds and human adipose-derived stem cells for cartilage tissue engineering. Biomaterials. 2009;30:4401-4406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 81] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 19. | Qu XH, Wu Q, Liang J, Zou B, Chen GQ. Effect of 3-hydroxyhexanoate content in poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) on in vitro growth and differentiation of smooth muscle cells. Biomaterials. 2006;27:2944-2950. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 71] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 20. | Sharma RI, Snedeker JG. Biochemical and biomechanical gradients for directed bone marrow stromal cell differentiation toward tendon and bone. Biomaterials. 2010;31:7695-7704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 109] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 21. | Chatterjee K, Lin-Gibson S, Wallace WE, Parekh SH, Lee YJ, Cicerone MT, Young MF, Simon CG. The effect of 3D hydrogel scaffold modulus on osteoblast differentiation and mineralization revealed by combinatorial screening. Biomaterials. 2010;31:5051-5062. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 253] [Cited by in RCA: 224] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 22. | Georges PC, Janmey PA. Cell type-specific response to growth on soft materials. J Appl Physiol. 2005;98:1547-1553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 385] [Cited by in RCA: 347] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 23. | Chen JL, Yin Z, Shen WL, Chen X, Heng BC, Zou XH, Ouyang HW. Efficacy of hESC-MSCs in knitted silk-collagen scaffold for tendon tissue engineering and their roles. Biomaterials. 2010;31:9438-9451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 159] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 24. | Lyons FG, Al-Munajjed AA, Kieran SM, Toner ME, Murphy CM, Duffy GP, O'Brien FJ. The healing of bony defects by cell-free collagen-based scaffolds compared to stem cell-seeded tissue engineered constructs. Biomaterials. 2010;31:9232-9243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 163] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 25. | Doral MN, Alam M, Bozkurt M, Turhan E, Atay OA, Dönmez G, Maffulli N. Functional anatomy of the Achilles tendon. Knee Surg Sports Traumatol Arthrosc. 2010;18:638-643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 151] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 26. | Pijanka JK, Kumar D, Dale T, Yousef I, Parkes G, Untereiner V, Yang Y, Dumas P, Collins D, Manfait M. Vibrational spectroscopy differentiates between multipotent and pluripotent stem cells. Analyst. 2010;135:3126-3132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 47] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 27. | Holzwarth C, Vaegler M, Gieseke F, Pfister SM, Handgretinger R, Kerst G, Müller I. Low physiologic oxygen tensions reduce proliferation and differentiation of human multipotent mesenchymal stromal cells. BMC Cell Biol. 2010;11:11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 221] [Cited by in RCA: 240] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 28. | Ezashi T, Das P, Roberts RM. Low O2 tensions and the prevention of differentiation of hES cells. Proc Natl Acad Sci USA. 2005;102:4783-4788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 625] [Cited by in RCA: 628] [Article Influence: 31.4] [Reference Citation Analysis (0)] |
| 29. | Yang XD, Li HM, Chen M, Zou XH, Zhu LY, Wei CJ, Chen GQ. Enhanced insulin production from murine islet beta cells incubated on poly(3-hydroxybutyrate-co-3-hydroxyhexanoate). J Biomed Mater Res A. 2010;92:548-555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 30. | Sales-Pardo I, Avendaño A, Barquinero J, Domingo JC, Marin P, Petriz J. The Hoechst low-fluorescent profile of the side population: clonogenicity versus dye retention. Blood. 2006;108:1774; author reply 1774-1775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 97] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 31. | Qu XH, Wu Q, Zhang KY, Chen GQ. In vivo studies of poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) based polymers: biodegradation and tissue reactions. Biomaterials. 2006;27:3540-3548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 30] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 32. | Liu Q, Chen GQ. In vitro biocompatibility and degradation of terpolyester 3HB-co-4HB-co-3HHx, consisting of 3-hydroxybutyrate, 4-hydroxybutyrate and 3-hydroxyhexanoate. J Biomater Sci Polym Ed. 2008;19:1521-1533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |