Published online Aug 26, 2025. doi: 10.4252/wjsc.v17.i8.109106
Revised: June 10, 2025
Accepted: July 14, 2025
Published online: August 26, 2025
Processing time: 114 Days and 4.8 Hours
This study by Zhang et al elucidates the role of exosome miR-137-3p targeting ubiquitin protein ligase E3C to activate signal transducer and activator of transcription 3 under hypoxia conditions, thereby promoting the migration and differentiation of human umbilical cord mesenchymal stem cells to endometrial epithelial cells. It emphasizes that exosomal miR-137-3p/ubiquitin protein ligase E3C/signal transducer and activator of transcription 3 axis is a promising pathway for endometrial regeneration. This article introduced the therapeutic potential of exosomal microRNAs in regenerative medicine while underscoring the need for standardized protocols in optimizing exosome delivery and vali
Core Tip: Zhang et al employed integrated bioinformatics analysis and functional validation to demonstrate that hypoxia-induced exosomal miR-137-3p promotes human umbilical cord mesenchymal stem cell migration and differentiation into endometrial epithelial cells by targeting ubiquitin protein ligase E3C and activating the signal transducer and activator of transcription 3. These findings provide novel insights into exosome-mediated stem cell therapy for thin endometrium. This article underscored the paramount importance of elucidating underlying mechanisms and rigorously assessing translational potential. Further investigation focusing on mechanism depth and trans
- Citation: Han LF, Fu GK, Wen YQ, Bian XL. Advancing mechanistic insights and clinical translation of exosomal miR-137-3p in endometrial regeneration. World J Stem Cells 2025; 17(8): 109106
- URL: https://www.wjgnet.com/1948-0210/full/v17/i8/109106.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v17.i8.109106
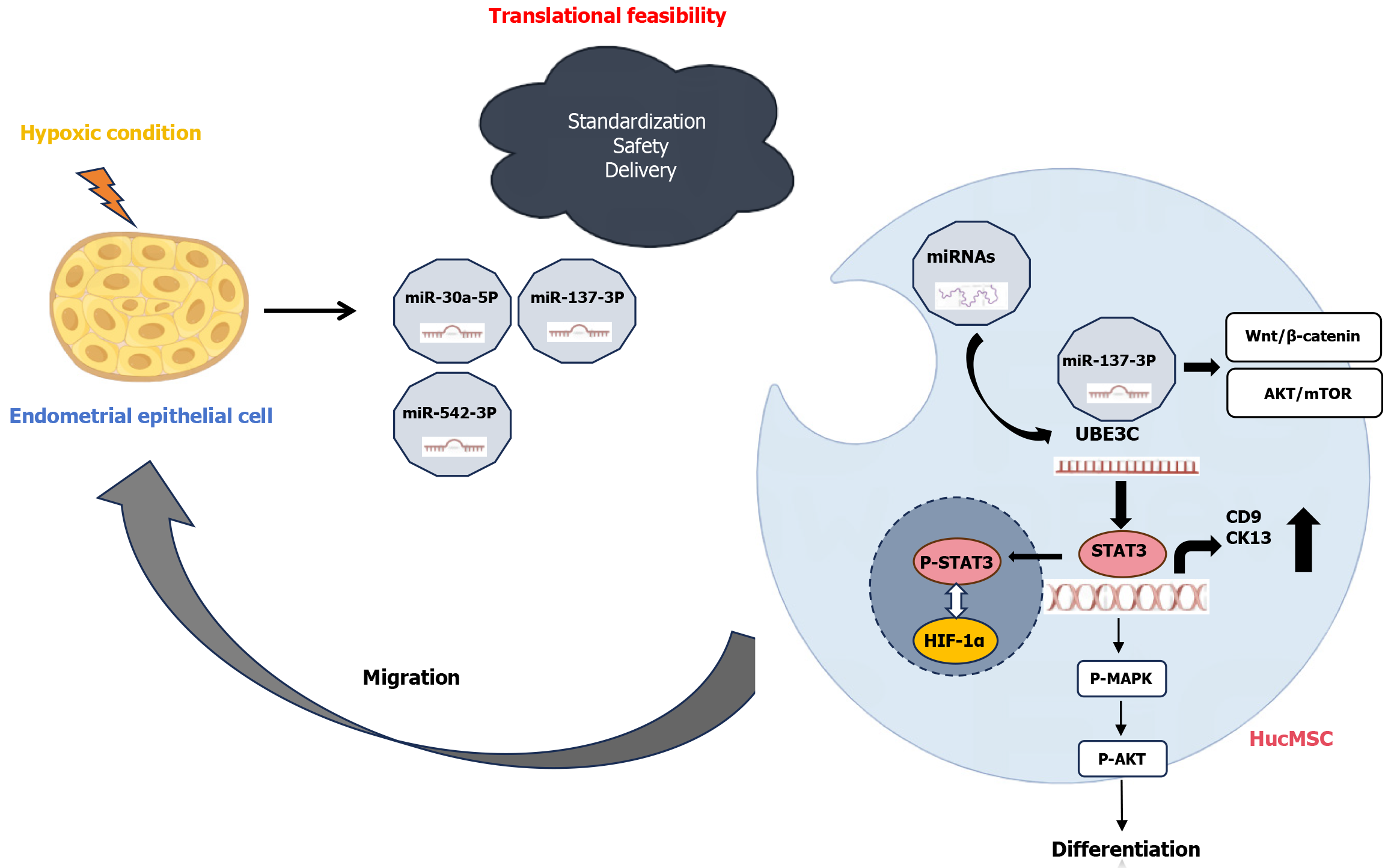
We read with great interest the article titled “Exosomal miR-137-3p targets UBE3C to activate STAT3, promoting migration and differentiation into endometrial epithelial cell of human umbilical cord mesenchymal stem cells under hypoxia”[1]. The authors elegantly demonstrated that hypoxia-damaged endometrial epithelial cell-derived exosomes deliver miR-137-3p to human umbilical cord mesenchymal stem cells, thereby suppressing ubiquitin protein ligase E3C (UBE3C) expression and activating signal transducer and activator of transcription 3 (STAT3) signaling. This cascade enhances human umbilical cord mesenchymal stem cell (hucMSC) migration and differentiation into endometrial epithelial cells, offering novel insights into therapies for thin endometrium. While this work significantly advances our understanding of exosome-mediated endometrial repair, we wish to highlight two critical areas for further exploration to strengthen clinical translation: Mechanistic depth and translational feasibility.
First, the identification of miR-137-3p as a key regulator of STAT3 by UBE3C suppression is convincing. However, microRNA (miRNA) networks are pleiotropic in nature, and miR-137-3p may interact with additional targets other than UBE3C. For example, miR-137 is known to regulate pathways such as wingless/integrated-β-catenin and protein kinase B/mechanistic target of rapamycin in other stem cell systems[2]. Methods such as proteomics or RNA sequencing can clarify whether these pathways contribute to the observed phenotypic changes. Furthermore, while STAT3 activation is central to epithelial differentiation, its crosstalk with hypoxia-inducible factor 1α (HIF-1α), a master regulator of hypoxic responses, remains unexplored[3]. Co-immunoprecipitation or chromatin immunoprecipitation assays can elucidate the interaction of STAT3-HIF-1α under hypoxia, providing a more comprehensive mechanism framework.
Second, the authors noted that STAT3 mRNA levels remained unchanged despite increased phosphorylation of STAT3 protein. This is related to post-translational modification. Since UBE3C is an E3 ubiquitin ligase, its downregulation by miR-137-3p might reduce STAT3 ubiquitination, stabilizing the active phosphorylated form. Future studies on STAT3 ubiquitination status and nuclear translocation in miR-137-3p-overexpressing hucMSCs may elucidate this mechanism.
Third, while miR-137-3p was identified as a key regulator, other differentially expressed miRNAs (e.g., 53 miRNAs identified in sequencing) may synergize or antagonize its effects. For example, miR-30a-5p and miR-542-3p, known to target UBE3C in cancers, might share functional redundancy with miR-137-3p in this context[4,5].
Fourth, the study’s in vitro findings underscore the therapeutic potential of miR-137-3p-enriched exosomes[1]. However, the hypoxic microenvironment in vivo involves dynamic interactions among immune cells, cytokines, and stromal components, which may modulate STAT3 activation or exosome uptake efficiency. Validating these results in animal models of thin endometrium would strengthen the translational relevance. For instance, tracking exosome biodistribution and assessing endometrial thickness post-therapy could clarify dose-response relationships and long-term efficacy.
Lastly, the therapeutic use of exosomes derived from hypoxia-preconditioned hucMSCs is promising but requires rigorous validation. For example, standardization: Exosome yield and miRNA cargo composition can be significantly influenced by variables such as hypoxia duration, oxygen tension, and donor heterogeneity[6]. To improve reproducibility, it is crucial to optimize hypoxic culture conditions, for example, comparing 1% O2vs 3% O2 - and to establish standardized quality control metrics[7]. These should include nanoparticle tracking analysis for quantifying exosome concentration, size distribution, and integrity, as well as quantitative polymerase chain reaction and miRNA sequencing for precise measurement of exosomal miR-137-3p levels[8]. Such standardization is essential for ensuring consistency and facilitating clinical translation. Safety: Although activation of STAT3 signaling has been shown to facilitate tissue regeneration, its dysregulation is associated with pathological fibrosis, oncogenesis, and systemic side effects[9]. Beyond tumorigenic potential, other safety concerns include immune modulation, off-target effects, and risks of abnormal angiogenesis or chronic inflammation[10]. Comprehensive safety evaluation for clinical translation must encompass long-term in vivo studies assessing not only endometrial remodeling efficacy but also systemic toxicity, off-target organ effects, and immunogenicity. Additionally, thorough genetic and epigenetic assessments are important to detect any undesired cellular transformations or persistent inflammatory responses resulting from exosome therapy. Delivery: Achieving efficient, targeted delivery of exosomes to the endometrium remains a major challenge. Engineering exosomes with tissue-specific ligands - such as endometrial integrin-binding peptides - may improve homing efficiency and therapeutic precision[11]. Developing such targeted delivery systems is key to enhancing outcomes and minimizing off-target effects in future clinical applications.
In summary, Zhang et al[1] presented compelling evidence that the miR-137-3p/UBE3C/STAT3 axis plays a crucial role in promoting endometrial repair through exosome-mediated regulation of hucMSC differentiation. To advance these findings toward clinical translation, future studies should: (1) Verify potential off-target effects and explore miRNA crosstalk to ensure pathway specificity; (2) Validate therapeutic efficacy in physiologically relevant in vivo models; and (3) Optimize exosome production protocols while addressing safety concerns. In addition, in order to substantiate the molecular mechanisms and reinforce the mechanistic framework, it is necessary to incorporate comprehensive transcriptomic profiling data of umbilical cord-derived mesenchymal stem cells post-miRNA transfection, including analysis of differentially expressed genes and involved pathways. Furthermore, a comparative RNA sequencing analysis between the differentiated cells and native endometrial epithelial cells isolated in vivo is essential to evaluate the fidelity of cellular differentiation and validate the functional relevance of the induced phenotype. These systematic investigations will be critical for developing a clinically viable exosome-based therapy to restore endometrial function in infertility patients.
Zhang et al[1] revealed that hypoxia-induced exosomal miR-137-3p promoted hucMSC migration and differentiation via UBE3C/STAT3, highlighting therapeutic potential for thin endometrium. However, mechanistic and translational gaps persist. Future studies should explore miRNA networks, STAT3-HIF-1α crosstalk, and preclinical validation, while optimizing exosome protocols, safety, and tissue-targeting strategies to advance clinical translation for endometrial regeneration (Figure 1).
| 1. | Zhang WY, Liu SM, Wang HB, Deng CY. Exosomal miR-137-3p targets UBE3C to activate STAT3, promoting migration and differentiation into endometrial epithelial cell of human umbilical cord mesenchymal stem cells under hypoxia. World J Stem Cells. 2025;17:100359. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 2. | Wang Y, Chen R, Zhou X, Guo R, Yin J, Li Y, Ma G. miR-137: A Novel Therapeutic Target for Human Glioma. Mol Ther Nucleic Acids. 2020;21:614-622. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 3. | Zhang F, Zhang Y, Zhou J, Cai Y, Li Z, Sun J, Xie Z, Hao G. Metabolic effects of quercetin on inflammatory and autoimmune responses in rheumatoid arthritis are mediated through the inhibition of JAK1/STAT3/HIF-1α signaling. Mol Med. 2024;30:170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 4. | Xiong J, Wei B, Ye Q, Liu W. MiR-30a-5p/UBE3C axis regulates breast cancer cell proliferation and migration. Biochem Biophys Res Commun. 2019;516:1013-1018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 38] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 5. | Tao J, Liu Z, Wang Y, Wang L, Yao B, Li Q, Wang C, Tu K, Liu Q. MiR-542-3p inhibits metastasis and epithelial-mesenchymal transition of hepatocellular carcinoma by targeting UBE3C. Biomed Pharmacother. 2017;93:420-428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 41] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 6. | Mathieu M, Névo N, Jouve M, Valenzuela JI, Maurin M, Verweij FJ, Palmulli R, Lankar D, Dingli F, Loew D, Rubinstein E, Boncompain G, Perez F, Théry C. Specificities of exosome versus small ectosome secretion revealed by live intracellular tracking of CD63 and CD9. Nat Commun. 2021;12:4389. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 112] [Cited by in RCA: 497] [Article Influence: 124.3] [Reference Citation Analysis (0)] |
| 7. | Rhatomy S, Utomo DN, Prakoeswa CRS, Suroto H, Tinduh D, Notobroto HB, Arfian N, Rantam FA, Mahyudin F. The optimum oxygen level in hypoxic culture conditions of ligament derived stem cells: experimental research. Ann Med Surg (Lond). 2023;85:2689-2694. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 8. | Yu D, Li Y, Wang M, Gu J, Xu W, Cai H, Fang X, Zhang X. Exosomes as a new frontier of cancer liquid biopsy. Mol Cancer. 2022;21:56. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 335] [Cited by in RCA: 488] [Article Influence: 162.7] [Reference Citation Analysis (0)] |
| 9. | Samad MA, Ahmad I, Hasan A, Alhashmi MH, Ayub A, Al-Abbasi FA, Kumer A, Tabrez S. STAT3 Signaling Pathway in Health and Disease. MedComm (2020). 2025;6:e70152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 10. | Xiao Q, Tan M, Yan G, Peng L. Revolutionizing lung cancer treatment: harnessing exosomes as early diagnostic biomarkers, therapeutics and nano-delivery platforms. J Nanobiotechnology. 2025;23:232. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 11. | Tang K, Tang Z, Niu M, Kuang Z, Xue W, Wang X, Liu X, Yu Y, Jeong S, Ma Y, Wu A, Kim BYS, Jiang W, Yang Z, Li C. Allosteric targeted drug delivery for enhanced blood-brain barrier penetration via mimicking transmembrane domain interactions. Nat Commun. 2025;16:3410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |