Published online Aug 26, 2025. doi: 10.4252/wjsc.v17.i8.109006
Revised: June 6, 2025
Accepted: July 28, 2025
Published online: August 26, 2025
Processing time: 115 Days and 16.6 Hours
Alzheimer’s disease (AD) is a multifaceted neurodegenerative disease for which effective disease-modifying therapies are lacking. Mesenchymal stem cell-derived exosomes (MSC-Exos) have emerged as a promising therapeutic approach due to their unique biological functions and favorable biocompatibility. This review systematically explores the mechanism of action of MSC-Exos in AD therapy, including the removal of β-amyloid via the delivery of degradative enzymes, modulation of neuroinflammation, and promotion of neural regeneration. Mean
Core Tip: Mesenchymal stem cell-derived exosomes represent a promising therapeutic modality for Alzheimer’s disease, owing to their biological functions and biocompatibility. Mesenchymal stem cell-derived exosomes facilitate the clearance of β-amyloid by carrying degradative enzymes, modulate neuroinflammation, and promote neurorestoration. While prec
- Citation: Shan XQ, He MH, Gao WL, Li YJ, Liu SZ, Liu Y, Wang CL, Zhao L, Xu SX. Mesenchymal stem cell-derived exosomes: Shaping the next era of Alzheimer’s disease treatment. World J Stem Cells 2025; 17(8): 109006
- URL: https://www.wjgnet.com/1948-0210/full/v17/i8/109006.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v17.i8.109006
Alzheimer’s disease (AD) is a prevalent and progressive neurodegenerative disease characterized by significant memory impairment, cognitive dysfunction, and behavioral disturbances. With the acceleration of global population aging, the prevalence of AD is on a significant rise, and the number of patients is projected to exceed 150 million by 2050, posing a critical global public health challenge[1]. Currently available first-line treatments, such as acetylcholinesterase inhibitors and N-methyl-D-aspartate receptor antagonists, provide temporary symptomatic relief and modest improvements in cognitive function, without altering the pathological process of the disease[2]. In particular, under the concept of “symptomatic relief”, therapeutic efficacy exhibits substantial interindividual variability, influenced by factors such as drug mechanism, genetic background, and disease progression[2,3]. Compounding the challenge, the pathology of AD involves multiple interacting pathways, including β-amyloid (Aβ) plaques deposition, highly phosphorylated tau protein-mediated neurofibrillary tangles (NFTs), and neuroinflammation driven by microglia activation, all of which synergistically cause loss of synaptic plasticity, neuronal degeneration, and brain parenchymal atrophy, ultimately leading to irreversible neurological damage[4,5]. However, existing drugs target only a single pathological component and lack the ability to intervene in a holistic manner[6,7]. Therefore, there is an urgent need for novel therapeutic strategies that can target the multifactorial nature of AD and promote repair of damaged neural functions. Developing interventions that fundamentally address the pathogenesis of AD has become a central focus of current research.
Mesenchymal stem cells (MSC) and their derived exosomes have garnered significant attention in recent years as an innovative therapeutic strategy. MSC are considered promising candidates for the treatment of neurodegenerative diseases due to their potent immunomodulatory, neuroprotective, and regenerative abilities[8]. However, MSC therapy is associated with several limitations, including immune rejection after cell transplantation, low cell survival, tumor risk, and unstable therapeutic effects[9]. To overcome these challenges, MSC derived exosomes (MSC-Exos), as a natural carrier for intercellular communication, offer new ideas for the treatment of AD[8-10]. Compared to MSCs, MSC-Exos has superior biosafety, stronger blood-brain barrier penetration efficiency and lower immunogenicity[11]. Existing preclinical studies have confirmed that MSC-Exos can demonstrate the pathological process of AD at multiple levels, particularly by clearing Aβ plaques, attenuating neuroinflammation, modulating tau protein pathology and promoting neural repair, thereby contributing to functional improvement[7,12-14]. In addition, the ability of MSC-Exos to strongly penetrate the blood-brain barrier expands their potential for clinical application[11]. This review summarizes current evidence supporting the use of MSC-Exos in AD therapy. In addition, this review highlights the challenges to the clinical translation of exosome therapies and discusses strategies to enhance their function and targeting.
MSC-Exos are extracellular vesicles with a single-membrane structure, typically ranging from 30 to 150 nm in diameter. They naturally carry bioactive components, such as proteins, nucleic acids [mRNAs, microRNAs (miRNAs), long noncoding RNAs, circular RNAs] and lipids of matricellular origin. These molecules are involved in intercellular communication and play an important role in neurological disorders therapy[15]. For example, MSC secretes neurotrophic factors such as brain-derived neurotrophic factor (BDNF) and vascular endothelial growth factor, which support neuronal survival, neurogenesis, and synaptic plasticity[16,17]. In addition, specific miRNAs such as miR-21, miR-22, and others have been widely discussed in AD, and they show therapeutic potential for treating AD by targeting a great variety of multiple pathologic mechanisms in AD, including Aβ clearance, immunoinflammation, and neuroprotection[18-21].
The biogenesis of MSC-Exos mainly involves endosomal sorting complex required for transport (ESCRT) machinery mechanisms and lipid-mediated pathways[8,22,23]. Its biogenesis begins with the cell internalizing external material through endocytosis, forming early sorting endosomes. As the early sorting endosomes matures, the endosomal boundary membrane buds inward to generate intraluminal vesicles (ILV). ILV formation is mainly regulated by the ESCRT. This machinery complex is mainly composed of complexes ESCRT-0, ESCRT-I, ESCRT-II and ESCRT-III and ILV-forming proteins such as vacuolar protein sorting 4, ALG-2-interacting protein X, and tumor susceptibility 101, each of which performs distinct roles in cargo recognition, membrane deformation, and vesicle segregation[22]. ESCRT-0 is mainly responsible for recognizing ubiquitinated cargoes, whereas ESCRT-I/II/III mediates membrane bending and invagination. The vacuolar protein sorting 4 complex ensures vesicle cleavage and ESCRT component recycling[22,23]. Another pathway for exosome biogenesis is a lipid based ESCRT-independent mechanism responsible, which involves lipids such as sphingolipids (SLs), phosphatidic acid, and cholesterol, ceramides (Cers), as well as the family of four-transmembrane structural domain proteins[22,24,25]. The lipid mediation mechanism was first described by Trajkovic et al[25], who emphasized that exosome biosynthesis occurs in the membrane domains enriched in SLs. To be specific, SLs are hydrolyzed to Cers by neutral sphingomyelinase, a key enzyme in lipid metabolism, leading to the formation of Cer-rich microdomains. These microdomains coalesce into larger membrane platforms, promoting inward vesicle outgrowth[26,27]. In addition, lipid components such as Cers, SL and cholesterol also mediate the sorting of specific cargoes (RNA and other molecules) into vesicles[25,28]. Specific cytoplasmic molecules are encapsulated into the lumen and accumulate in the late endosomes, resulting in the formation of multivesicular bodies. Some multivesicular bodies fuse with the plasma membrane and release their contents as exosomes into the extracellular space, while others fuse with autophagosomes or are translocated to lysosomes[8,29,30]. The released exosomes carry various biomolecules which can interact with recipient cells locally or at a distance, thereby influencing their physiological activities[22]. In this regard, MSC-Exos are considered to be the best cell-free candidate to promote repair processes by activating positive responses in the brain microenvironment through intercellular communication, showing promising prospects especially in the treatment of neurodegenerative diseases.
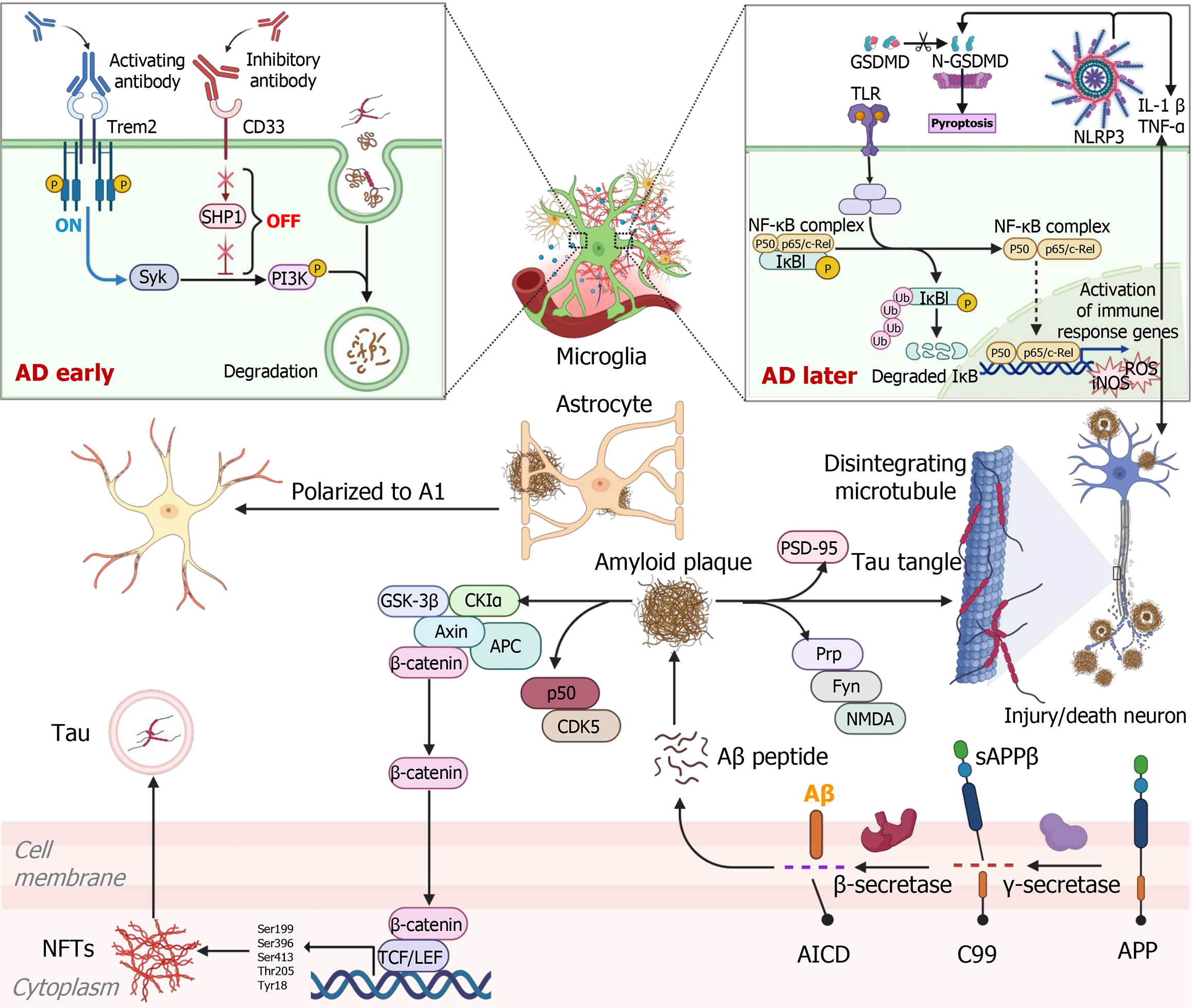
Typical pathologic features of AD include Aβ deposition, abnormal phosphorylation of tau proteins to form NFTs, loss of synaptic plasticity, and widespread neuroinflammation. Although Aβ has long been recognized as a main causative factor of AD, recent evidence suggests that disease onset and progression result from the interplay of multiple biological processes, involving protein aggregation imbalances, immune dysfunction, and metabolic disorders, among others (Figure 1)[31-33]. These findings underscore the necessity of approaching AD from a systems biology perspective, focusing on the integration of complex molecular networks involved in disease pathogenesis. Exosomes, as carriers of intercellular communication, are increasingly being recognized for their significant roles in the pathological progression and treatment of AD. MSC-Exos are enriched with a variety of bioactive molecules that can modulate AD-related processes through cell-to-cell messaging. These include Aβ clearance, modulation of neuroinflammation, promotion of neuroregeneration, and targeted delivery across the blood-brain barrier (Table 1). Thus, MSC-Exos are expected to serve as an innovative therapeutic tool that provides integrated interventions for multiple pathologies of AD.
| Source | Cargoes | Model | Injection/culture | Machine | Character | Ref. |
| Wharton’s jelly MSCs | Exosomes | FAD human neural cell | Matrigel culture model | NEP | Aβ degradation | [7] |
| Bone marrow MSCs | MiR-29c-3p | Aβ1-42 injected rat | Lateral ventricle injection | Wnt/β-catenin | Aβ degradation | [13] |
| Bone marrow MSCs | GDF-15 | Aβ42 incubated SH-SY5Y | Gibco | Akt/GSK-3β/β-catenin | Aβ degradation | [17] |
| Human umbilical cord MSCs | Exosomes | APP/PS1 mice | Caudal vein injection | Glial cell polarisation | Aβ degradation, immune regulation | [61] |
| Induced pluripotent stem cell derived MSCs | MiR-223-3p | STZ mice | Intracisternal injection | NLRP3/GSDMD | Immune regulation | [75] |
| Bone marrow MSCs | Exosomes | STZ mice | Lateral ventricle/caudal vein injection | BDNF | Neuroregeneration, immune regulation | [12] |
| Human bone marrow MSCs | Exosomes | 3xTg mice | Intranasal route | Glial cell polarisation | Immune regulation | [70] |
| Wharton’s jelly MSCs | Exosomes | J20 (JAX-006293) | IV | Glial cell polarisation | Immune regulation | [7] |
| Adipose derived MSCs | MiR-22 | APP/PS1 mice | Caudal vein injection | GSDMD | Immune regulation | [19] |
| MSCs | MiR-223 | Aβ1-40 incubated SH-SY5Y | - | PTEN | Neuroreprotection | [18] |
| Bone marrow MSCs | Exosomes | Aβ1-42 injected mice | Lateral ventricle injection | - | Neuroregeneration | [14] |
| Bone marrow MSCs | MiR-21, miR-155, miR-17-5p, miR-126-3p | AlCl3 incubated rat | Intraperitoneal injection | PI3K/Akt/mTOR | Neuroregeneration | [20] |
| Bone marrow MSCs | MiR-146a | APP/PS1 mice, astrocyte | Intracerebroventricular injection/transwell | NF-κB | Neuroregeneration | [81] |
| Bone marrow MSCs | MiR-21 | APP/PS1 mice | Caudal vein injection | STAT3 NF-κB | Neuroregeneration | [21] |
| Human amniotic fluid | Exosomes | Aβ incubated SH-SY5Y, LSP incubated BV-2 | Transwells | Oxidative stress | Neuroreprotection | [82] |
| Bone marrow MSCs | Exosomes | Hippocampal neuronal exposed AβO | Transwells | Oxidative stress | Neuroreprotection Aβ degradation | [83] |
| Olfactory mucosa MSCs | Exosomes | Aβ1-42 injected mice | Caudal vein injection | LRP1 | Neuroreprotection | [84] |
AD is a progressively worsening neurodegenerative disease marked by a time-dependent, multistep pathological cascade. The disease course involves a gradual deterioration from early molecular imbalances to late neural network breakdown[34,35]. The different pathological factors in the course of AD reinforce each other in a vicious cycle, ultimately leading to widespread degeneration of the brain and loss of cognitive function[35-37].
The initial stages of AD are triggered by abnormal shearing of Aβ precursor protein (APP), which generates Aβ1-42 oligomers via the β/γ-secretase pathway[38,39]. Gradually, the accumulation of Aβ exceeds the clearance threshold in the brain. Aβ activates localized prion protein receptors on neuronal membranes, which further initiates the Fyn kinase signaling pathway. This activation leads to overactivation of N-methyl-D-aspartate receptors and impairs synaptic plasticity, one of the core pathological features of the early stages of AD[40,41]. With the accumulation of Aβ and the exacerbation of early synaptic damage, AD progresses to a more advanced stage, where the abnormal phosphorylation of tau protein becomes a key feature of this stage[35,42]. Aβ not only exerts direct neurotoxic effects but also promotes tau hyperphosphorylation at specific localization sites (e.g., Ser199, Ser396, Ser413, Thr205, Tyr18) by activating the pathways, such as CDK5/p25 and glycogen synthase kinase-3β, and the formation of NFTs[17,43-45]. The abnormal phos
Aβ degradation: Aβ plaques are composed of Aβ peptides, which undergo an aggregation process that results in the formation of toxic soluble oligomers and insoluble fibrous material, eventually being deposited as plaques[4,39,42]. In healthy brains, Aβ production and clearance remain balanced; however, impaired clearance disrupts this equilibrium, leading to Aβ accumulation, synaptic dysfunction, neuronal damage, and neurodegeneration. Enhancing the clearance of pathogenic proteins has been shown to be beneficial in treating AD[57]. In the degradative clearance system, extracellular Aβ can be degraded by proteases such as neprilysin (NEP), matrix metalloproteinases and glutamate carboxypeptidase II, insulin degrading enzymes (IDE) and zinc metallopeptidases[58,59]. NEPs expression and function are significantly reduced in AD patients. In 2000, researchers observed that brain-derived NEP could degrade disease-causing Aβ1-42 peptides in the hippocampus of rats injected with radiolabeled synthetic Aβ1-42[60]. Interestingly, Chen et al[7] observed the expression of NEP-specific enzymatic activity on the membrane of MSC-Exos. They further co-cultured familial AD neuronal cells with MSC-Exos and found that they could reduce Aβ levels, along with restoration of BDNF exon IV and Homer genes (related to neuronal memory and synaptic plasticity)[7]. Bone marrow MSC-Exos (BMMSC-Exos) miR-29c-3p treatment effectively inhibited Aβ formation and deposition, increased the expression of NEP and IDE, and promoted soluble Aβ1-42 degradation, thereby significantly improving cognitive behavior in AD rats[13]. Further, the downstream mechanism by which miR-29c-3p alleviates AD is associated with targeting APP cleaving enzyme 1 (BACE1), an APP cleavage enzyme. BMMSC-Exos inhibit BACE1 expression and activate the Wnt/β-catenin pathway by carrying miR-29c-3p into neurons, reducing Aβ production and deposition, ultimately leading to reduced inflammation and neuronal apoptosis[13]. Additionally, in vitro experiments revealed that BMMSC-Exos containing growth differentiation factor-15 also upregulated NEP and IDE through activation of the protein kinase B (Akt)/glycogen synthase kinase-3β/β-catenin pathway, which degraded the Aβ42 protein to attenuate SH-SY5Y cell injury. In vivo, M2 microglia have likewise been noted to increase IDE and NEP expression[17]. One study found that human umbilical cord MSC-Exos treatment significantly enhanced the expression of IDE and NEP in AD mice by alternatively activating microglia, thereby reducing Aβ deposition and soluble Aβ levels[61]. In conclusion, the critical role of MSC-Exos in Aβ degradation highlights their potential in the treatment of AD.
Immune regulation: Neuroinflammation is a major contributor to the pathogenesis and progression of AD, primarily mediated by glial cells, including microglia and astrocytes[62]. These cells play two roles in the entire pathological process of AD, by acting as the “protector” in the early stage of AD to the “accomplice” later[63,64]. The immunomodulatory role of MSC in disease treatment has been widely discussed, owing to their ability to limit the tissue inflammatory microenvironment through the release of immunomodulatory factors such as prostaglandin E2 (PGE2), growth factors, ILs and nitric oxide[64-66]. It has been proposed that MSC regulation of the immune response is at least partially dependent on the activation of two negative feedback loops, PGE2 and TNF-α stimulating gene 6[67,68]. According to the “loop hypothesis”, MSC suppress inflammation by activating one of these two loops depending on the brain environment. Only when the inflammatory response is activated will microglia be driven toward an M2 anti-inflammatory phenotype[68]. MSC-Exos inherit a wide range of immunomodulatory molecules from their parental MSCs, thereby exerting comparable immunosuppressive effects[69]. Losurdo et al[70] demonstrated that MSC-Exos induced the upregulation of cyclooxygenase 2 (associated with increased PGE2) and indoleamine 2,3-dioxygenase when microglia were subjected to pro-inflammatory stimulation with TNFα and interferon γ, thereby driving microglia polarization towards the M2 phenotype in vitro. Moreover, they delivered MSC-Exos to 3xTg mice for the first time via the nasalroute and found that MSC-Exos inhibited microglia activation and increased dendritic spine density, exerting neuroprotective effects in the early stages of AD[70]. Ex vivo and in vivo studies of human umbilical cord MSC-Exos have demonstrated their capacity to regulate microglial activation in the brain, evidenced by increased transforming growth factor-β and IL-10 expression and decreased IL-1β and TNF-α levels in both the brain and peripheral blood, thereby attenuating neuroinflammation[61]. Also, hyperactivation of microglia and astrocytes in the hippocampus was suppressed after BMMSC-Exos injection in the lateral ventricle of AD mice, accompanied by a decrease in the expression of IL-1β, IL-6, and TNF-α[12].
In addition, pyroptosis has become a focus in cellular inflammatory responses research in recent years. In response to noxious stimuli, intracellular and extracellular signals induce the inflammasome in the cytoplasm via the caspase pathway, activating caspase-1 to promote the release of inflammatory factors (IL-18, IL-1β), thereby inducing inflammatory cascade response[71]. Adipose derived MSC-Exos loaded with miR-22 have been shown to enhance neurological function and locomotor ability in AD mice by regulating gasdermin D (GSDMD), down-regulating the expression of cellular focal death-related protein caspase-1 and inflammatory factor NLRP3[19]. Notably, NLRP3 is also an important pathway for the secretion of inflammatory cytokines by reactive microglia. Once NLRP3 is activated, it cleaves caspase-1 and subsequently induces the release of GSDMD (the pyroptosis regulatory protein), which triggers pyroptosis[72]. A previous study found that GSDMD was expressed in Aβ plaque-associated microglia, further confirming the role of pyroptosis in AD[73]. In AD, in addition to Aβ-driven M1 polarization of microglia to secrete inflammatory factors, microglial cell pyroptosis also affects their normal clearance function, ultimately contributing to a vicious cycle of Aβ pathology[73,74]. MSC-Exos have been reported to inhibit cellular pyroptosis and neuroinflammation, reduce Aβ accumulation, and mitigate neurodegeneration in AD mice via miR-223-3p-mediatedinhibition of the NLRP3/GSDMD pathway[75].
Neuro restoration: Brain dysfunction in AD arises from reduced synaptic plasticity, altered homeostatic scaling, and disrupted neuronal connectivity[76,77]. The neuroprotective and neuro regenerative effects of MSC-Exo in AD have been well-documented[14]. Wei et al[18] found that the establishment of an AD cell model was accompanied by elevated hypoxia-inducible factor-1 alpha expression and apoptosis, impaired cell migration, and decreased miR-223. When MSC-Exos were introduced into the AD cell co-culture model, internalization occurred in a time-dependent manner, reversing these pathological changes. The increased MSC-Exos miR-223 binds to phosphatase and tensin homolog and initiates its downstream phosphatidylinositol-3-kinase (PI3K)/Akt/mammalian target of rapamycin (mTOR) signaling pathway that controls axonal remodeling, cell proliferation and differentiation[18]. These findings suggest that MSC-Exos miR-223 can reduce neuronal apoptosis in AD by interfering with signaling pathways related to neuronal cell cycle. Ebrahim et al[20] also demonstrated that BMMSC-Exos enhances synaptic function, reduces astrocyte scarring, and promotes neurogenesis. These beneficial effects are associated with modulation of the PI3K/Akt/mTOR signaling pathway, autophagy, and inhibition of neuroinflammation. Additionally, BMMSC-Exos regulate specific miRNAs to support their therapeutic potential, including miR-17-5p, miR-21, miR-155 and miR-126-3p[20]. Interestingly, enhanced activation of the PI3K/Akt/mTOR signaling cascade has been closely associated with the pathological signaling from Aβ to tau in AD, which underlies cognitive decline[78]. MSC-Exos regulates miRNAs to improve the pathological process of AD. In this context, MSC-Exos represent a promising option for the treatment of AD.
Recent studies have reported that exosomal administration increases the number of newborn neurons in the neurogenesis microenvironment (subventricular zone and dentate gyrus)[79]. One of the mechanisms by which exosomes interact with the neurogenic microenvironment is the transfer of miRNAs to precursor neural precursor cells, which triggers neural remodeling events, neurogenesis, angiogenesis, and synapse formation[80]. One study used bilateral dentate gyrus injections of Aβ1-42 aggregates to establish an AD mouse model followed by in situ administration of MSC-Exos, and found that MSC-Exos were able to stimulate neurogenesis in the subventricular zone and attenuate Aβ1-42-inducedcognitive deficits, and that these effects were similar to those of MSCs[14]. Astrocytes are key cells in synapse formation, and restoring their function may help correct neurogenesis and improve cognitive deficits. BMMSC-Exos therapy promotes synaptogenesis by down-regulating nuclear factor-kappa B expression in astrocytes after transferring miR-146a to astrocytes[81]. The expression level of synaptic proteins can reflect the function of synapses to some extent. Hypoxia preconditioning of BMMSC-Exos miR-21 significantly enhances the expression of synaptic proteins (synapsin 1 and postsynaptic density protein-95), thereby rescuing synaptic dysfunction[21]. In addition, human amniotic fluid endometrial stromal cell derived exosomes blocked the negative effects of elevated oxidative stress induced by microglial and neuronal cell interactions and significantly restored neurotoxicity by efficiently reducing inducible nitric oxide synthase and ROS activity[82]. Another study using a transwell co-culture system of rat hippocampal neurons and BMMSCs found that BMMSCs reduce the expression of ROS in neurons by releasing exosomes containing antioxidant enzymes. This effect may help maintain synaptic integrity in neurons exposed to soluble amyloid-β oligomers[83]. Additionally, olfactory mucosa MSC-Exos exerts neuroprotective effects by regulating glial cell activation and influencing the endoplasmic reticulum stress response potentially via the low-density lipoprotein receptor-related protein 1[84].
Notably, it has been reported that a limited number of exosomes reach the brain through the peripheral circulation compared to the liver, kidneys, and other peripheral organs of the body[85]. It has been demonstrated that AD mice treated by injection of BMMSC-Exos through the lateral ventricle, rather than tail vein injection, exhibit significant modulation of hippocampal plasticity by up-regulating BDNF to promote neuronal regeneration, reversing the AD - like behavior induced by streptozotocin injection[12]. Furthermore, Losurdo et al[70] achieved the observed effects on microglia activation and neuronal recovery by only two temporally close nasal injections of MSC-Exos. This suggests that intranasal delivery may achieve higher brain concentrations compared to those administered via other routes. Controversy still exists regarding the ability of exosomes to reach the brain. Therefore, further studies are needed to optimize delivery strategies and investigate the precise biodistribution of exosomes under different administration routes.
In summary, the cascade of pathological responses triggered by Aβ and tua, primarily centered on neurons, ultimately leads to dementia[86]. Neuronal networks, astrocytes, microglia, oligodendrocytes, and the vascular system contribute all to a complex cellular phase of AD evolving over decades[86]. MSC-Exos act pleiotropically on Aβ, inflammation, and regeneration, making them a promising approach for the treatment of AD.
Although results from cellular and murine models of AD suggest that MSC therapies are promising in AD, only one clinical trial to date has investigated the safety and efficacy of MSC-Exos in patients with mild to moderate dementia (http://www.clinicaltrials.gov, NCT04388982, accessed April 27, 2025). This phase I/II clinical trial used nasal drops to give three doses of dipose derived MSC-Exos (5, 10, and 20 μg) twice weekly to AD patients, with treatment lasting 12 weeks and follow-up lasting 9 months[87]. No serious adverse events occurred during the 12-week treatment period, and the drug did not cause any significant toxicity to vital organs such as the liver and kidneys, indicating that the MSC-Exos treatment was safe and well-tolerated. Specifically, patients receiving the intermediate dose demonstrated significant improvement in cognitive function after 12 weeks of treatment and the effect was maintained for up to 6 months, suggesting potential long-term benefits. Although no significant changes in Aβ and tau deposition were observed, hippocampal volume reduction was less observed in the mid-dose group, which was neuroprotective for brain tissue.
While MSC-Exos hold substantial therapeutic potential for AD, several major challenges must be addressed before widespread clinical application is feasible[10,87-89].
Production standardization: One of the major challenges to the clinical application of MSC-Exos is the lack of standardized protocols for production, isolation and purification. Although a variety of methods including ultracentrifugation, size-exclusion chromatography, and immunoaffinity capture are widely used, there are limitations inherent in these techniques[10,90]. For example, ultracentrifugation is typically time-consuming with low yields, and contamination with non-exosomal particles such as apoptotic bodies or cellular debris is common[90]. In addition, variation in exosome yield is influenced by numerous factors, including MSC source, culture conditions, and passage number, leading to variability and poor reproducibility[88]. In the future, there is a need to further refine and standardize protocols for the isolation, purification and characterization of MSC-Exos. These protocols must be scalable, reproducible, and adaptable to clinical applications to ensure that exosome production is stable, efficient, and meets regulatory standards. In addition, determining the optimal method for large-scale production is critical, as regulatory agencies demand stringent control over product quality and batch consistency.
Safety assessment: While MSC-Exos generally demonstrate excellent biocompatibility and low immunogenicity, comprehensive safety evaluations are essential. Harmful paracrine factors may interfere with the desired therapeutic outcome or even lead to adverse reactions[10,91]. Consequently, rigorous evaluations are necessary to assess off-target effects, long-term toxicity, and the potential for triggering an immune response. In addition to short-term safety, long-term studies are needed to evaluate cumulative effects on organ systems, immune modulation, and systemic health[88,92].
Long-term efficacy verification: While MSC-Exos have shown great potential in preclinical models of neurodegenerative diseases and in short-term efficacy in clinical trials, their long-term efficacy in the clinical setting remains underexplored[39,57,75,87]. Future clinical trials should evaluate whether MSC-Exos can halt or slow disease progression, restore lost cognitive function and prevent further neuronaldegeneration over months or years. In addition, the mode of exosome administration, optimal dosage, targeting and functionality can also influence clinical effectiveness[93-95].
Despite their therapeutic promise, MSC-Exos face several practical limitations, including low targeting efficiency, limited intrinsic functionality, and suboptimal yield[89]. These problems can be addressed by various bioengineering techniques[96,97].
Preprocessing: Many studies have indicated that altering the culture conditions of MSC and pretreating these cells can affect exosome production, activity and metabolic regulatory capacity. Compared with two-dimensional, three-dimensional-cultured human umbilical cord MSC-Exos exhibited significantly higher levels of 195 miRNAs and Aβ hydrolases, which exerted enhanced therapeutic effects on improving memory and cognitive deficits in AD mice[98]. Compared with the group administered normoxic MSC-Exos, MSC-Exos pretreated in hypoxia effectively increased the level of miR-21 in the brains of AD mice, producing greater improvements in synaptic function[21]. In addition to physical factors, biochemical stimuli such as melatonin, interferon γ, and TNF-α memory exogenous genes can also be introduced into the culture environment to modify and optimize their functions[99-102].
Drug loading: Due to its nanoscale structure and biocompatibility, MSC-Exos are ideal candidates for efficient drug loading[96,103]. Two strategies for drug loading include collection of drug-loaded exosomes after pretreatment of parental cells by using various methods and loading of exogenous drugs into isolated exosomes[104]. The Fe65-engineered HT22 hippocampus neuron cell-derived exosomes loaded with corynoxine-B (autophagy inducer) hijacked signaling and blocked the natural interaction between Fe65 and APP for targeted delivery of exosomes and drug[105]. Jahangard et al[106] loaded miR-29 into MSC-Exos to target BACE1 and Bcl-2-interacting mediator of cell death, thereby restoring hippocampal learning function in AD rats.
Surface modification: The surface modification of exosomes by gene manipulation targeting peptides or chemical modification sites can enhance their specific functions. To improve the brain targeting ability of intravenously injected exosomes, the surface of the exosomes can be modified by linking peptides. For example, MSC-Exos conjugated with central nervous system-specific rabies virus glycoprotein peptides demonstrated improved targeting of the cortex and hippocampus in AD mice[94]. Furthermore, MSC-Exos with high tyrosine phosphatase-2 expression improved blood-brain barrier penetration in AD mice[107]. Han et al[108] designed engineered exosomes with a light-induced protein delivery system that enables CRISPR-Cas-based epigenome editing in AD.
Artificial exosomes: In recent years, artificial exosomes, based on nanobiotechnology, have emerged to overcome the limitations of natural exosomes. These artificial exosomes are usually obtained by top-down, top-down, bottom-up and biohybridization strategies including extrusion, microfluidics, nitrogen cavitation and sonication to break down cell membranes, yielding higher amounts of exosomes. These biohybrid strategies are proving to be a promising alternative for drug delivery systems[109]. However, artificial exosomes are not yet ready for translation due to challenges in preparation protocols, characterization and biocompatibility. In AD therapy, natural exosomes are still in the initial clinical trial stage, while the emerging field of artificial exosomes remains largely unexplored.
In conclusion, MSC-Exos have demonstrated great potential as a novel therapeutic strategy in the treatment of AD. Advances in MSC-Exos in the areas of protein disorders, neuroinflammation, and neurogenesis undoubtedly herald the dawn of a new era in the treatment of neurodegenerative disorders, and may pave the way for clinical applications in AD in the future. While early-stage clinical trials have produced encouraging results, several key challenges remain. These include addressing the heterogeneity of MSC-Exos production and mining, optimizing safety assessments, confirming the efficacy, determining the optimal delivery and dosage, improving their targeting capabilities and functions before clinical translation. Recent advances in pretreatment strategies, drug loading techniques, surface engineering, and the development of synthetic exosomes have improved the targeting specificity and therapeutic potential of MSC-Exos, addressing several key limitations in their clinical translation. Future investigations will focus on optimizing the treatment regimen and exploring the underlying mechanisms.
| 1. | GBD 2019 Dementia Forecasting Collaborators. Estimation of the global prevalence of dementia in 2019 and forecasted prevalence in 2050: an analysis for the Global Burden of Disease Study 2019. Lancet Public Health. 2022;7:e105-e125. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2320] [Cited by in RCA: 2197] [Article Influence: 732.3] [Reference Citation Analysis (1)] |
| 2. | Jia L, Quan M, Fu Y, Zhao T, Li Y, Wei C, Tang Y, Qin Q, Wang F, Qiao Y, Shi S, Wang YJ, Du Y, Zhang J, Zhang J, Luo B, Qu Q, Zhou C, Gauthier S, Jia J; Group for the Project of Dementia Situation in China. Dementia in China: epidemiology, clinical management, and research advances. Lancet Neurol. 2020;19:81-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 505] [Article Influence: 84.2] [Reference Citation Analysis (0)] |
| 3. | Vitali F, Branigan GL, Brinton RD. Preventing Alzheimer's disease within reach by 2025: Targeted-risk-AD-prevention (TRAP) strategy. Alzheimers Dement (N Y). 2021;7:e12190. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 4. | Scheltens P, De Strooper B, Kivipelto M, Holstege H, Chételat G, Teunissen CE, Cummings J, van der Flier WM. Alzheimer's disease. Lancet. 2021;397:1577-1590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1527] [Cited by in RCA: 2686] [Article Influence: 671.5] [Reference Citation Analysis (0)] |
| 5. | Smirnov D, Galasko D. Dynamics of neuroinflammation in Alzheimer's disease. Lancet Neurol. 2022;21:297-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 15] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 6. | The Lancet. Lecanemab for Alzheimer's disease: tempering hype and hope. Lancet. 2022;400:1899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 57] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 7. | Chen YA, Lu CH, Ke CC, Chiu SJ, Jeng FS, Chang CW, Yang BH, Liu RS. Mesenchymal Stem Cell-Derived Exosomes Ameliorate Alzheimer's Disease Pathology and Improve Cognitive Deficits. Biomedicines. 2021;9:594. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 96] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 8. | Guo M, Yin Z, Chen F, Lei P. Mesenchymal stem cell-derived exosome: a promising alternative in the therapy of Alzheimer's disease. Alzheimers Res Ther. 2020;12:109. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 92] [Cited by in RCA: 115] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 9. | Zou Y, Mu D, Ma X, Wang D, Zhong J, Gao J, Yu S, Qiu L. Review on the roles of specific cell-derived exosomes in Alzheimer's disease. Front Neurosci. 2022;16:936760. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 10. | Ma YN, Hu X, Karako K, Song P, Tang W, Xia Y. Exploring the multiple therapeutic mechanisms and challenges of mesenchymal stem cell-derived exosomes in Alzheimer's disease. Biosci Trends. 2024;18:413-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 11. | Tian M, Yi X. The Role of Mesenchymal Stem Cell Exosomes in the Onset and Progression of Alzheimer’s Disease. Biomed Sci. 2024;10:6-13. [DOI] [Full Text] |
| 12. | Liu S, Fan M, Xu JX, Yang LJ, Qi CC, Xia QR, Ge JF. Exosomes derived from bone-marrow mesenchymal stem cells alleviate cognitive decline in AD-like mice by improving BDNF-related neuropathology. J Neuroinflammation. 2022;19:35. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 156] [Article Influence: 52.0] [Reference Citation Analysis (0)] |
| 13. | Sha S, Shen X, Cao Y, Qu L. Mesenchymal stem cells-derived extracellular vesicles ameliorate Alzheimer's disease in rat models via the microRNA-29c-3p/BACE1 axis and the Wnt/β-catenin pathway. Aging (Albany NY). 2021;13:15285-15306. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 69] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 14. | Reza-Zaldivar EE, Hernández-Sapiéns MA, Gutiérrez-Mercado YK, Sandoval-Ávila S, Gomez-Pinedo U, Márquez-Aguirre AL, Vázquez-Méndez E, Padilla-Camberos E, Canales-Aguirre AA. Mesenchymal stem cell-derived exosomes promote neurogenesis and cognitive function recovery in a mouse model of Alzheimer's disease. Neural Regen Res. 2019;14:1626-1634. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 161] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 15. | Nakano M, Fujimiya M. Potential effects of mesenchymal stem cell derived extracellular vesicles and exosomal miRNAs in neurological disorders. Neural Regen Res. 2021;16:2359-2366. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 46] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 16. | Shah S, Mansour HM, Aguilar TM, Lucke-Wold B. Mesenchymal Stem Cell-Derived Exosomes as a Neuroregeneration Treatment for Alzheimer's Disease. Biomedicines. 2024;12:2113. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 17. | Xiong WP, Yao WQ, Wang B, Liu K. BMSCs-exosomes containing GDF-15 alleviated SH-SY5Y cell injury model of Alzheimer's disease via AKT/GSK-3β/β-catenin. Brain Res Bull. 2021;177:92-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 35] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 18. | Wei H, Xu Y, Chen Q, Chen H, Zhu X, Li Y. Mesenchymal stem cell-derived exosomal miR-223 regulates neuronal cell apoptosis. Cell Death Dis. 2020;11:290. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 101] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 19. | Zhai L, Shen H, Sheng Y, Guan Q. ADMSC Exo-MicroRNA-22 improve neurological function and neuroinflammation in mice with Alzheimer's disease. J Cell Mol Med. 2021;25:7513-7523. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 81] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 20. | Ebrahim N, Al Saihati HA, Alali Z, Aleniz FQ, Mahmoud SYM, Badr OA, Dessouky AA, Mostafa O, Hussien NI, Farid AS, El-Sherbiny M, Salim RF, Forsyth NR, Ali FEM, Alsabeelah NF. Exploring the molecular mechanisms of MSC-derived exosomes in Alzheimer's disease: Autophagy, insulin and the PI3K/Akt/mTOR signaling pathway. Biomed Pharmacother. 2024;176:116836. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 29] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 21. | Cui GH, Wu J, Mou FF, Xie WH, Wang FB, Wang QL, Fang J, Xu YW, Dong YR, Liu JR, Guo HD. Exosomes derived from hypoxia-preconditioned mesenchymal stromal cells ameliorate cognitive decline by rescuing synaptic dysfunction and regulating inflammatory responses in APP/PS1 mice. FASEB J. 2018;32:654-668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 291] [Article Influence: 41.6] [Reference Citation Analysis (0)] |
| 22. | Yang K, Fu W, Deng M, Li X, Wu M, Wang Y. The sphingolipids change in exosomes from cancer patients and association between exosome release and sphingolipids level based on a pseudotargeted lipidomics method. Anal Chim Acta. 2024;1305:342527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 23. | Hessvik NP, Llorente A. Current knowledge on exosome biogenesis and release. Cell Mol Life Sci. 2018;75:193-208. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1613] [Cited by in RCA: 1748] [Article Influence: 249.7] [Reference Citation Analysis (0)] |
| 24. | Mashouri L, Yousefi H, Aref AR, Ahadi AM, Molaei F, Alahari SK. Exosomes: composition, biogenesis, and mechanisms in cancer metastasis and drug resistance. Mol Cancer. 2019;18:75. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 893] [Cited by in RCA: 1033] [Article Influence: 172.2] [Reference Citation Analysis (0)] |
| 25. | Trajkovic K, Hsu C, Chiantia S, Rajendran L, Wenzel D, Wieland F, Schwille P, Brügger B, Simons M. Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science. 2008;319:1244-1247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2186] [Cited by in RCA: 2686] [Article Influence: 158.0] [Reference Citation Analysis (0)] |
| 26. | Arya SB, Chen S, Jordan-Javed F, Parent CA. Ceramide-rich microdomains facilitate nuclear envelope budding for non-conventional exosome formation. Nat Cell Biol. 2022;24:1019-1028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 47] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 27. | Verderio C, Gabrielli M, Giussani P. Role of sphingolipids in the biogenesis and biological activity of extracellular vesicles. J Lipid Res. 2018;59:1325-1340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 172] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 28. | Horbay R, Hamraghani A, Ermini L, Holcik S, Beug ST, Yeganeh B. Role of Ceramides and Lysosomes in Extracellular Vesicle Biogenesis, Cargo Sorting and Release. Int J Mol Sci. 2022;23:15317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 50] [Reference Citation Analysis (0)] |
| 29. | Matsui T, Sakamaki Y, Hiragi S, Fukuda M. VAMP5 and distinct sets of cognate Q-SNAREs mediate exosome release. Cell Struct Funct. 2023;48:187-198. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 30. | Mahmood A, Otruba Z, Weisgerber AW, Palay MD, Nguyen MT, Bills BL, Knowles MK. Exosome secretion kinetics are controlled by temperature. Biophys J. 2023;122:1301-1314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 31. | Ainani H, Bouchmaa N, Ben Mrid R, El Fatimy R. Liquid-liquid phase separation of protein tau: An emerging process in Alzheimer's disease pathogenesis. Neurobiol Dis. 2023;178:106011. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 25] [Reference Citation Analysis (0)] |
| 32. | Yan H, Wang W, Cui T, Shao Y, Li M, Fang L, Feng L. Advances in the Understanding of the Correlation Between Neuroinflammation and Microglia in Alzheimer's Disease. Immunotargets Ther. 2024;13:287-304. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 33. | Doroszkiewicz J, Mroczko J, Winkel I, Mroczko B. Metabolic and Immune System Dysregulation: Unraveling the Connections between Alzheimer's Disease, Diabetes, Inflammatory Bowel Diseases, and Rheumatoid Arthritis. J Clin Med. 2024;13:5057. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 34. | Jack CR Jr, Knopman DS, Jagust WJ, Petersen RC, Weiner MW, Aisen PS, Shaw LM, Vemuri P, Wiste HJ, Weigand SD, Lesnick TG, Pankratz VS, Donohue MC, Trojanowski JQ. Tracking pathophysiological processes in Alzheimer's disease: an updated hypothetical model of dynamic biomarkers. Lancet Neurol. 2013;12:207-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2590] [Cited by in RCA: 3169] [Article Influence: 264.1] [Reference Citation Analysis (0)] |
| 35. | Han P, Shi J. A Theoretical Analysis of the Synergy of Amyloid and Tau in Alzheimer's Disease. J Alzheimers Dis. 2016;52:1461-1470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 36. | Arnsten AFT, Datta D, Leslie S, Yang ST, Wang M, Nairn AC. Alzheimer's-like pathology in aging rhesus macaques: Unique opportunity to study the etiology and treatment of Alzheimer's disease. Proc Natl Acad Sci U S A. 2019;116:26230-26238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 48] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 37. | Ho YS, Yang X, Lau JC, Hung CH, Wuwongse S, Zhang Q, Wang J, Baum L, So KF, Chang RC. Endoplasmic reticulum stress induces tau pathology and forms a vicious cycle: implication in Alzheimer's disease pathogenesis. J Alzheimers Dis. 2012;28:839-854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 112] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 38. | Chiarini A, Armato U, Liu D, Dal Prà I. Calcium-Sensing Receptors of Human Neural Cells Play Crucial Roles in Alzheimer's Disease. Front Physiol. 2016;7:134. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 39. | Antonino M, Marmo P, Freites CL, Quassollo GE, Sánchez MF, Lorenzo A, Bignante EA. Aβ Assemblies Promote Amyloidogenic Processing of APP and Intracellular Accumulation of Aβ42 Through Go/Gβγ Signaling. Front Cell Dev Biol. 2022;10:852738. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 40. | Um JW, Nygaard HB, Heiss JK, Kostylev MA, Stagi M, Vortmeyer A, Wisniewski T, Gunther EC, Strittmatter SM. Alzheimer amyloid-β oligomer bound to postsynaptic prion protein activates Fyn to impair neurons. Nat Neurosci. 2012;15:1227-1235. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 463] [Cited by in RCA: 526] [Article Influence: 40.5] [Reference Citation Analysis (0)] |
| 41. | Laurén J, Gimbel DA, Nygaard HB, Gilbert JW, Strittmatter SM. Cellular prion protein mediates impairment of synaptic plasticity by amyloid-beta oligomers. Nature. 2009;457:1128-1132. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1307] [Cited by in RCA: 1247] [Article Influence: 77.9] [Reference Citation Analysis (0)] |
| 42. | Bloom GS. Amyloid-β and tau: the trigger and bullet in Alzheimer disease pathogenesis. JAMA Neurol. 2014;71:505-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 965] [Cited by in RCA: 1517] [Article Influence: 137.9] [Reference Citation Analysis (0)] |
| 43. | Demuro S, Sauvey C, Tripathi SK, Di Martino RMC, Shi D, Ortega JA, Russo D, Balboni B, Giabbai B, Storici P, Girotto S, Abagyan R, Cavalli A. ARN25068, a versatile starting point towards triple GSK-3β/FYN/DYRK1A inhibitors to tackle tau-related neurological disorders. Eur J Med Chem. 2022;229:114054. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 44. | Noble W, Olm V, Takata K, Casey E, Mary O, Meyerson J, Gaynor K, LaFrancois J, Wang L, Kondo T, Davies P, Burns M, Veeranna, Nixon R, Dickson D, Matsuoka Y, Ahlijanian M, Lau LF, Duff K. Cdk5 is a key factor in tau aggregation and tangle formation in vivo. Neuron. 2003;38:555-565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 365] [Cited by in RCA: 383] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 45. | Yang W, Xu QQ, Yuan Q, Xian YF, Lin ZX. Sulforaphene, a CDK5 Inhibitor, attenuates cognitive deficits in a transgenic mouse model of Alzheimer's disease via reducing Aβ Deposition, tau hyperphosphorylation and synaptic dysfunction. Int Immunopharmacol. 2023;114:109504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 46. | Venkatramani A, Panda D. Regulation of neuronal microtubule dynamics by tau: Implications for tauopathies. Int J Biol Macromol. 2019;133:473-483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 54] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 47. | Aisen PS, Cummings J, Jack CR Jr, Morris JC, Sperling R, Frölich L, Jones RW, Dowsett SA, Matthews BR, Raskin J, Scheltens P, Dubois B. On the path to 2025: understanding the Alzheimer's disease continuum. Alzheimers Res Ther. 2017;9:60. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 269] [Cited by in RCA: 349] [Article Influence: 43.6] [Reference Citation Analysis (0)] |
| 48. | Wu L, Xian X, Xu G, Tan Z, Dong F, Zhang M, Zhang F. Toll-Like Receptor 4: A Promising Therapeutic Target for Alzheimer's Disease. Mediators Inflamm. 2022;2022:7924199. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 43] [Reference Citation Analysis (0)] |
| 49. | He Z, Li X, Han S, Ren B, Hu X, Li N, Du X, Ni J, Yang X, Liu Q. Bis(ethylmaltolato)oxidovanadium (IV) attenuates amyloid-beta-mediated neuroinflammation by inhibiting NF-κB signaling pathway via a PPARγ-dependent mechanism. Metallomics. 2021;13:mfab036. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 50. | de Brito Toscano EC, Rocha NP, Lopes BNA, Suemoto CK, Teixeira AL. Neuroinflammation in Alzheimer's Disease: Focus on NLRP1 and NLRP3 Inflammasomes. Curr Protein Pept Sci. 2021;22:584-598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 51. | Kent SA, Spires-Jones TL, Durrant CS. The physiological roles of tau and Aβ: implications for Alzheimer's disease pathology and therapeutics. Acta Neuropathol. 2020;140:417-447. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 225] [Cited by in RCA: 269] [Article Influence: 53.8] [Reference Citation Analysis (0)] |
| 52. | Tsai ST, Chen SY, Lin SZ, Tseng GF. Rostral intralaminar thalamic deep brain stimulation ameliorates memory deficits and dendritic regression in β-amyloid-infused rats. Brain Struct Funct. 2020;225:751-761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 53. | Lee SH, Son HJ. Second Wave, Late-Stage Neuroinflammation in Cleared Brains of Aged 5xFAD Alzheimer's Mice Detected by Macrolaser Light Sheet Microscopy Imaging. Int J Mol Sci. 2023;24:17058. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 54. | Li W, Yong-Yan X, Jia-Xin M, Shu-Chao G, Li-Ping H. Senescent microglia: The hidden culprits accelerating Alzheimer's disease. Brain Res. 2025;1851:149480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 55. | Fakhoury M. Microglia and Astrocytes in Alzheimer's Disease: Implications for Therapy. Curr Neuropharmacol. 2018;16:508-518. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 304] [Cited by in RCA: 344] [Article Influence: 49.1] [Reference Citation Analysis (0)] |
| 56. | Ahmad MH, Fatima M, Mondal AC. Influence of microglia and astrocyte activation in the neuroinflammatory pathogenesis of Alzheimer's disease: Rational insights for the therapeutic approaches. J Clin Neurosci. 2019;59:6-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 117] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 57. | Lian Y, Jia YJ, Wong J, Zhou XF, Song W, Guo J, Masters CL, Wang YJ. Clarity on the blazing trail: clearing the way for amyloid-removing therapies for Alzheimer's disease. Mol Psychiatry. 2024;29:297-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 58. | Tarasoff-Conway JM, Carare RO, Osorio RS, Glodzik L, Butler T, Fieremans E, Axel L, Rusinek H, Nicholson C, Zlokovic BV, Frangione B, Blennow K, Ménard J, Zetterberg H, Wisniewski T, de Leon MJ. Clearance systems in the brain-implications for Alzheimer disease. Nat Rev Neurol. 2015;11:457-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1122] [Cited by in RCA: 1172] [Article Influence: 117.2] [Reference Citation Analysis (0)] |
| 59. | de Dios C, Bartolessis I, Roca-Agujetas V, Barbero-Camps E, Mari M, Morales A, Colell A. Oxidative inactivation of amyloid beta-degrading proteases by cholesterol-enhanced mitochondrial stress. Redox Biol. 2019;26:101283. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 60. | Iwata N, Tsubuki S, Takaki Y, Watanabe K, Sekiguchi M, Hosoki E, Kawashima-Morishima M, Lee HJ, Hama E, Sekine-Aizawa Y, Saido TC. Identification of the major Abeta1-42-degrading catabolic pathway in brain parenchyma: suppression leads to biochemical and pathological deposition. Nat Med. 2000;6:143-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 415] [Cited by in RCA: 509] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 61. | Ding M, Shen Y, Wang P, Xie Z, Xu S, Zhu Z, Wang Y, Lyu Y, Wang D, Xu L, Bi J, Yang H. Exosomes Isolated From Human Umbilical Cord Mesenchymal Stem Cells Alleviate Neuroinflammation and Reduce Amyloid-Beta Deposition by Modulating Microglial Activation in Alzheimer's Disease. Neurochem Res. 2018;43:2165-2177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 195] [Article Influence: 27.9] [Reference Citation Analysis (0)] |
| 62. | Chen X, Holtzman DM. Emerging roles of innate and adaptive immunity in Alzheimer's disease. Immunity. 2022;55:2236-2254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 155] [Article Influence: 51.7] [Reference Citation Analysis (0)] |
| 63. | Kwon HS, Koh SH. Neuroinflammation in neurodegenerative disorders: the roles of microglia and astrocytes. Transl Neurodegener. 2020;9:42. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1085] [Cited by in RCA: 1482] [Article Influence: 296.4] [Reference Citation Analysis (0)] |
| 64. | Ye Y, Gao M, Shi W, Gao Y, Li Y, Yang W, Zheng X, Lu X. The immunomodulatory effects of mesenchymal stem cell-derived extracellular vesicles in Alzheimer's disease. Front Immunol. 2023;14:1325530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 65. | Chen YA, Lu CH, Ke CC, Liu RS. Mesenchymal Stem Cell-Derived Extracellular Vesicle-Based Therapy for Alzheimer's Disease: Progress and Opportunity. Membranes (Basel). 2021;11:796. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 66. | Mendiratta M, Mendiratta M, Ganguly S, Rai S, Gupta R, Kumar L, Bakhshi S, Dadhwal V, Pushpam D, Malik PS, Pramanik R, Aggarwal M, Gupta AK, Dhawan R, Seth T, Mahapatra M, Nayak B, Singh TD, Kumar S, Mir RA, Kaur G, GuruRao H, Singh M, Prasad CP, Prakash H, Mohanty S, Sahoo RK. Concurrent hypoxia and apoptosis imparts immune programming potential in mesenchymal stem cells: Lesson from acute graft-versus-host-disease model. Stem Cell Res Ther. 2024;15:381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 67. | Maggini J, Mirkin G, Bognanni I, Holmberg J, Piazzón IM, Nepomnaschy I, Costa H, Cañones C, Raiden S, Vermeulen M, Geffner JR. Mouse bone marrow-derived mesenchymal stromal cells turn activated macrophages into a regulatory-like profile. PLoS One. 2010;5:e9252. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 430] [Cited by in RCA: 460] [Article Influence: 30.7] [Reference Citation Analysis (0)] |
| 68. | Prockop DJ. Concise review: two negative feedback loops place mesenchymal stem/stromal cells at the center of early regulators of inflammation. Stem Cells. 2013;31:2042-2046. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 174] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 69. | Shan XQ, Luo YY, Chang J, Song JJ, Hao N, Zhao L. Immunomodulation: The next target of mesenchymal stem cell-derived exosomes in the context of ischemic stroke. World J Stem Cells. 2023;15:52-70. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 6.5] [Reference Citation Analysis (1)] |
| 70. | Losurdo M, Pedrazzoli M, D'Agostino C, Elia CA, Massenzio F, Lonati E, Mauri M, Rizzi L, Molteni L, Bresciani E, Dander E, D'Amico G, Bulbarelli A, Torsello A, Matteoli M, Buffelli M, Coco S. Intranasal delivery of mesenchymal stem cell-derived extracellular vesicles exerts immunomodulatory and neuroprotective effects in a 3xTg model of Alzheimer's disease. Stem Cells Transl Med. 2020;9:1068-1084. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 123] [Cited by in RCA: 170] [Article Influence: 34.0] [Reference Citation Analysis (0)] |
| 71. | Rogers C, Fernandes-Alnemri T, Mayes L, Alnemri D, Cingolani G, Alnemri ES. Cleavage of DFNA5 by caspase-3 during apoptosis mediates progression to secondary necrotic/pyroptotic cell death. Nat Commun. 2017;8:14128. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 578] [Cited by in RCA: 1098] [Article Influence: 137.3] [Reference Citation Analysis (0)] |
| 72. | Pandey A, Shen C, Feng S, Man SM. Cell biology of inflammasome activation. Trends Cell Biol. 2021;31:924-939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 113] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 73. | Moonen S, Koper MJ, Van Schoor E, Schaeverbeke JM, Vandenberghe R, von Arnim CAF, Tousseyn T, De Strooper B, Thal DR. Pyroptosis in Alzheimer's disease: cell type-specific activation in microglia, astrocytes and neurons. Acta Neuropathol. 2023;145:175-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 117] [Article Influence: 58.5] [Reference Citation Analysis (0)] |
| 74. | Friker LL, Scheiblich H, Hochheiser IV, Brinkschulte R, Riedel D, Latz E, Geyer M, Heneka MT. β-Amyloid Clustering around ASC Fibrils Boosts Its Toxicity in Microglia. Cell Rep. 2020;30:3743-3754.e6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 132] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 75. | Lin L, Huang L, Huang S, Chen W, Huang H, Chi L, Su F, Liu X, Yuan K, Jiang Q, Li C, Smith WW, Fu Q, Pei Z. MSC-Derived Extracellular Vesicles Alleviate NLRP3/GSDMD-Mediated Neuroinflammation in Mouse Model of Sporadic Alzheimer's Disease. Mol Neurobiol. 2024;61:5494-5509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 76. | Kotredes KP, Pandey RS, Persohn S, Elderidge K, Burton CP, Miner EW, Haynes KA, Santos DFS, Williams SP, Heaton N, Ingraham CM, Lloyd C, Garceau D, O'Rourke R, Herrick S, Rangel-Barajas C, Maharjan S, Wang N, Sasner M, Lamb BT, Territo PR, Sukoff Rizzo SJ, Carter GW, Howell GR, Oblak AL. Characterizing molecular and synaptic signatures in mouse models of late-onset Alzheimer's disease independent of amyloid and tau pathology. Alzheimers Dement. 2024;20:4126-4146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 77. | Gowda P, Reddy PH, Kumar S. Deregulated mitochondrial microRNAs in Alzheimer's disease: Focus on synapse and mitochondria. Ageing Res Rev. 2022;73:101529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 97] [Article Influence: 32.3] [Reference Citation Analysis (0)] |
| 78. | O' Neill C. PI3-kinase/Akt/mTOR signaling: impaired on/off switches in aging, cognitive decline and Alzheimer's disease. Exp Gerontol. 2013;48:647-653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 228] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 79. | Han Y, Seyfried D, Meng Y, Yang D, Schultz L, Chopp M, Seyfried D. Multipotent mesenchymal stromal cell-derived exosomes improve functional recovery after experimental intracerebral hemorrhage in the rat. J Neurosurg. 2019;131:290-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 84] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 80. | Cheng X, Zhang G, Zhang L, Hu Y, Zhang K, Sun X, Zhao C, Li H, Li YM, Zhao J. Mesenchymal stem cells deliver exogenous miR-21 via exosomes to inhibit nucleus pulposus cell apoptosis and reduce intervertebral disc degeneration. J Cell Mol Med. 2018;22:261-276. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 156] [Cited by in RCA: 250] [Article Influence: 31.3] [Reference Citation Analysis (0)] |
| 81. | Nakano M, Kubota K, Kobayashi E, Chikenji TS, Saito Y, Konari N, Fujimiya M. Bone marrow-derived mesenchymal stem cells improve cognitive impairment in an Alzheimer's disease model by increasing the expression of microRNA-146a in hippocampus. Sci Rep. 2020;10:10772. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 149] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 82. | Zavatti M, Gatti M, Beretti F, Palumbo C, Maraldi T. Exosomes Derived from Human Amniotic Fluid Mesenchymal Stem Cells Preserve Microglia and Neuron Cells from Aβ. Int J Mol Sci. 2022;23:4967. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 26] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 83. | de Godoy MA, Saraiva LM, de Carvalho LRP, Vasconcelos-Dos-Santos A, Beiral HJV, Ramos AB, Silva LRP, Leal RB, Monteiro VHS, Braga CV, de Araujo-Silva CA, Sinis LC, Bodart-Santos V, Kasai-Brunswick TH, Alcantara CL, Lima APCA, da Cunha-E Silva NL, Galina A, Vieyra A, De Felice FG, Mendez-Otero R, Ferreira ST. Mesenchymal stem cells and cell-derived extracellular vesicles protect hippocampal neurons from oxidative stress and synapse damage induced by amyloid-β oligomers. J Biol Chem. 2018;293:1957-1975. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 167] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 84. | Hu X, Ma YN, Peng J, Wang Z, Liang Y, Xia Y. Exosomes derived from olfactory mucosa mesenchymal stem cells attenuate cognitive impairment in a mouse model of Alzheimer's disease. Biosci Trends. 2025;19:189-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 85. | Wiklander OP, Nordin JZ, O'Loughlin A, Gustafsson Y, Corso G, Mäger I, Vader P, Lee Y, Sork H, Seow Y, Heldring N, Alvarez-Erviti L, Smith CI, Le Blanc K, Macchiarini P, Jungebluth P, Wood MJ, Andaloussi SE. Extracellular vesicle in vivo biodistribution is determined by cell source, route of administration and targeting. J Extracell Vesicles. 2015;4:26316. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 723] [Cited by in RCA: 1197] [Article Influence: 119.7] [Reference Citation Analysis (0)] |
| 86. | De Strooper B, Karran E. The Cellular Phase of Alzheimer's Disease. Cell. 2016;164:603-615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 948] [Cited by in RCA: 1280] [Article Influence: 142.2] [Reference Citation Analysis (0)] |
| 87. | Xie X, Song Q, Dai C, Cui S, Tang R, Li S, Chang J, Li P, Wang J, Li J, Gao C, Chen H, Chen S, Ren R, Gao X, Wang G. Clinical safety and efficacy of allogenic human adipose mesenchymal stromal cells-derived exosomes in patients with mild to moderate Alzheimer's disease: a phase I/II clinical trial. Gen Psychiatr. 2023;36:e101143. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 54] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 88. | Teixeira FG, Salgado AJ. Mesenchymal stem cells secretome: current trends and future challenges. Neural Regen Res. 2020;15:75-77. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 88] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 89. | Kim HY, Kwon S, Um W, Shin S, Kim CH, Park JH, Kim BS. Functional Extracellular Vesicles for Regenerative Medicine. Small. 2022;18:e2106569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 46] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 90. | Xu WM, Li A, Chen JJ, Sun EJ. Research Development on Exosome Separation Technology. J Membr Biol. 2023;256:25-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 38] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 91. | Al-Dhalimy AMB, Salim HM, Shather AH, Naser IH, Hizam MM, Alshujery MK. The pathological and therapeutically role of mesenchymal stem cell (MSC)-derived exosome in degenerative diseases; Particular focus on LncRNA and microRNA. Pathol Res Pract. 2023;250:154778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 92. | Farhan SH, Jasim SA, Bansal P, Kaur H, Abed Jawad M, Qasim MT, Jabbar AM, Deorari M, Alawadi A, Hadi A. Exosomal Non-coding RNA Derived from Mesenchymal Stem Cells (MSCs) in Autoimmune Diseases Progression and Therapy; an Updated Review. Cell Biochem Biophys. 2024;82:3091-3108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 93. | Kawikova I, Askenase PW. Diagnostic and therapeutic potentials of exosomes in CNS diseases. Brain Res. 2015;1617:63-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 103] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 94. | Cui GH, Guo HD, Li H, Zhai Y, Gong ZB, Wu J, Liu JS, Dong YR, Hou SX, Liu JR. RVG-modified exosomes derived from mesenchymal stem cells rescue memory deficits by regulating inflammatory responses in a mouse model of Alzheimer's disease. Immun Ageing. 2019;16:10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 228] [Article Influence: 38.0] [Reference Citation Analysis (0)] |
| 95. | Pardridge WM. Drug transport across the blood-brain barrier. J Cereb Blood Flow Metab. 2012;32:1959-1972. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1049] [Cited by in RCA: 1282] [Article Influence: 98.6] [Reference Citation Analysis (0)] |
| 96. | Yin T, Liu Y, Ji W, Zhuang J, Chen X, Gong B, Chu J, Liang W, Gao J, Yin Y. Engineered mesenchymal stem cell-derived extracellular vesicles: A state-of-the-art multifunctional weapon against Alzheimer's disease. Theranostics. 2023;13:1264-1285. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 56] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 97. | Zhang W, Uyemura R, Zhong K, Guo R, Zhong L. Current Advances and Future Perspectives on Mesenchymal Stem Cell-Derived Extracellular Vesicles in Alzheimer's Disease. Aging Dis. 2024;15:2015-2027. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 98. | Yang L, Zhai Y, Hao Y, Zhu Z, Cheng G. The Regulatory Functionality of Exosomes Derived from hUMSCs in 3D Culture for Alzheimer's Disease Therapy. Small. 2020;16:e1906273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 97] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 99. | Noronha NC, Mizukami A, Caliári-Oliveira C, Cominal JG, Rocha JLM, Covas DT, Swiech K, Malmegrim KCR. Priming approaches to improve the efficacy of mesenchymal stromal cell-based therapies. Stem Cell Res Ther. 2019;10:131. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 211] [Cited by in RCA: 402] [Article Influence: 67.0] [Reference Citation Analysis (0)] |
| 100. | Kim JY, Rhim WK, Woo J, Cha SG, Park CG, Han DK. The Upregulation of Regenerative Activity for Extracellular Vesicles with Melatonin Modulation in Chemically Defined Media. Int J Mol Sci. 2022;23:15089. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 101. | Yea JH, Yoon YM, Lee JH, Yun CW, Lee SH. Exosomes isolated from melatonin-stimulated mesenchymal stem cells improve kidney function by regulating inflammation and fibrosis in a chronic kidney disease mouse model. J Tissue Eng. 2021;12:20417314211059624. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 41] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 102. | Liu X, Li X, Zhu W, Zhang Y, Hong Y, Liang X, Fan B, Zhao H, He H, Zhang F. Exosomes from mesenchymal stem cells overexpressing MIF enhance myocardial repair. J Cell Physiol. 2020;235:8010-8022. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 94] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 103. | Sun Y, Liu G, Zhang K, Cao Q, Liu T, Li J. Mesenchymal stem cells-derived exosomes for drug delivery. Stem Cell Res Ther. 2021;12:561. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 114] [Article Influence: 28.5] [Reference Citation Analysis (0)] |
| 104. | Xu M, Yang Q, Sun X, Wang Y. Recent Advancements in the Loading and Modification of Therapeutic Exosomes. Front Bioeng Biotechnol. 2020;8:586130. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 99] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 105. | Iyaswamy A, Thakur A, Guan XJ, Krishnamoorthi S, Fung TY, Lu K, Gaurav I, Yang Z, Su CF, Lau KF, Zhang K, Ng RC, Lian Q, Cheung KH, Ye K, Chen HJ, Li M. Fe65-engineered neuronal exosomes encapsulating corynoxine-B ameliorate cognition and pathology of Alzheimer's disease. Signal Transduct Target Ther. 2023;8:404. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 53] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 106. | Jahangard Y, Monfared H, Moradi A, Zare M, Mirnajafi-Zadeh J, Mowla SJ. Therapeutic Effects of Transplanted Exosomes Containing miR-29b to a Rat Model of Alzheimer's Disease. Front Neurosci. 2020;14:564. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 109] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 107. | Xu F, Wu Y, Yang Q, Cheng Y, Xu J, Zhang Y, Dai H, Wang B, Ma Q, Chen Y, Lin F, Wang C. Engineered Extracellular Vesicles with SHP2 High Expression Promote Mitophagy for Alzheimer's Disease Treatment. Adv Mater. 2022;34:e2207107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 73] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 108. | Han J, Sul JH, Lee J, Kim E, Kim HK, Chae M, Lim J, Kim J, Kim C, Kim JS, Cho Y, Park JH, Cho YW, Jo DG. Engineered exosomes with a photoinducible protein delivery system enable CRISPR-Cas-based epigenome editing in Alzheimer's disease. Sci Transl Med. 2024;16:eadi4830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 15] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 109. | Li YJ, Wu JY, Liu J, Xu W, Qiu X, Huang S, Hu XB, Xiang DX. Artificial exosomes for translational nanomedicine. J Nanobiotechnology. 2021;19:242. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 190] [Article Influence: 47.5] [Reference Citation Analysis (0)] |