Published online Jun 26, 2025. doi: 10.4252/wjsc.v17.i6.109283
Revised: May 21, 2025
Accepted: June 12, 2025
Published online: June 26, 2025
Processing time: 50 Days and 1.7 Hours
This article comments on the study by Zhang et al, which proposed that exosomes derived from hypoxia-injured endometrial epithelial cells promote human umbilical cord mesenchymal stem cell migration and differentiation into endo
Core Tip: This commentary examines the study by Zhang et al on exosomal miR-137-3p-mediated endometrial regeneration. While the proposed mechanism involving ubiquitin protein ligase E3C inhibition and signal transducer and activator of transcription 3 activation is compelling, several issues remain unresolved, including the unclear source of miR-137-3p, lack of exosome characterization, and absence of in vivo validation. We highlight the need for direct evidence of exosomal microRNA transfer, mechanistic exploration of the ubiquitin protein ligase E3C-signal transducer and activator of transcription 3 ubiquitination axis, and functional evaluation in animal models to substantiate the therapeutic relevance of this approach.
- Citation: Lin F, Ding Y, Ma KX, Liang XT. Clarifying the role of exosomal miR-137-3p in endometrial regeneration: Mechanistic gaps and future directions. World J Stem Cells 2025; 17(6): 109283
- URL: https://www.wjgnet.com/1948-0210/full/v17/i6/109283.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v17.i6.109283
We read with interest the recent article by Zhang et al[1], which reports that exosomal miR-137-3p derived from hypoxia-injured endometrial epithelial cells (EECD) enhances human umbilical cord mesenchymal stem cell (hUCMSC) migration and epithelial differentiation by targeting ubiquitin protein ligase E3C (UBE3C) and activating the signal transducer and activator of transcription 3 (STAT3) pathway. This work provides important insights into microRNA (miRNA)-mediated communication under hypoxic stress and highlights a potentially novel molecular axis (miR-137-3p/UBE3C/STAT3) in endometrial regeneration. The findings contribute to the expanding field of exosome-based regenerative strategies; however, several scientific and conceptual ambiguities merit deeper discussion.
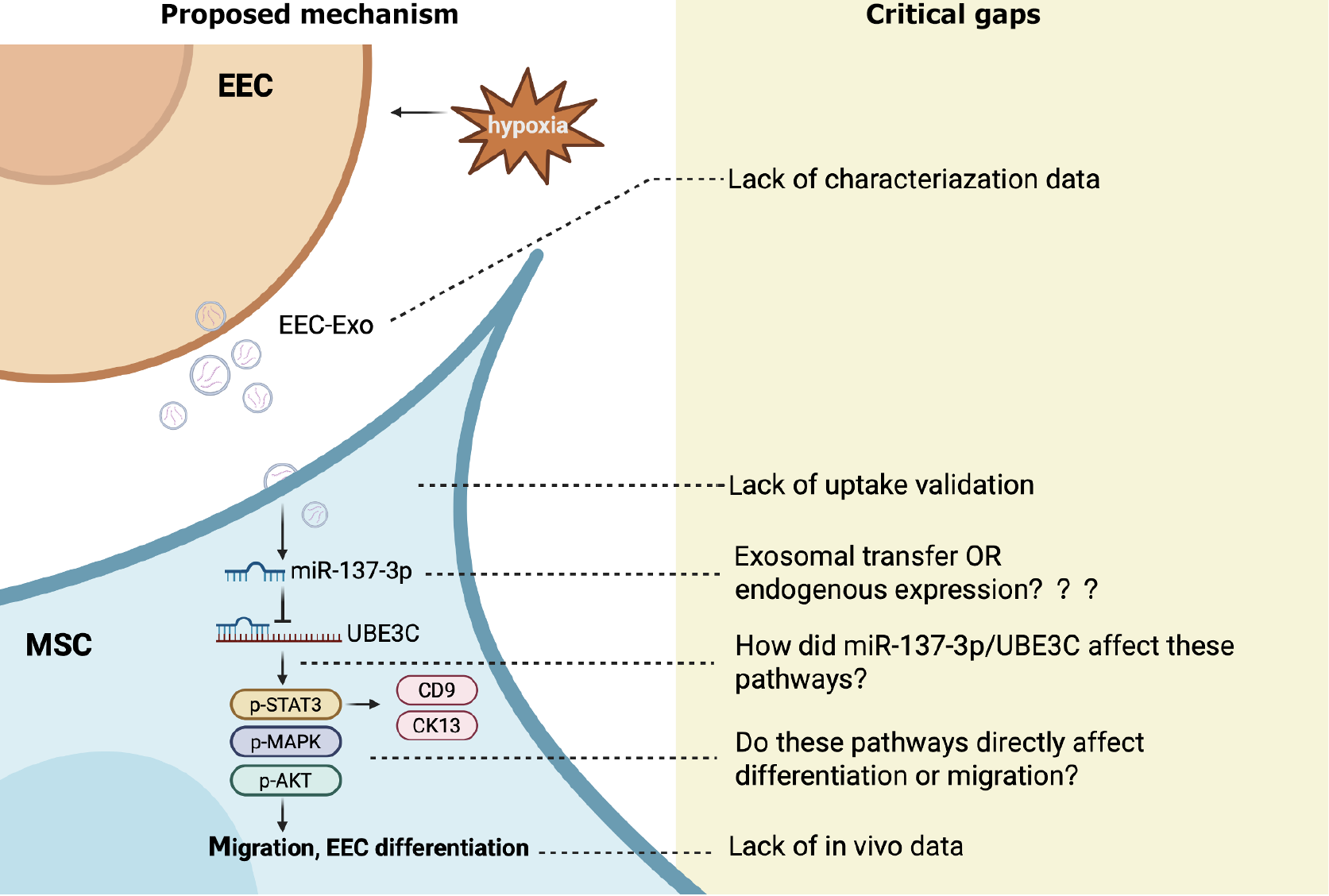
A central claim of the study is that miR-137-3p from EECD-exosomes acts as a key effector. However, the miRNA sequencing was conducted on hUCMSCs treated with exosomes, not on the exosomes themselves. Therefore, it remains unclear whether the observed upregulation of miR-137-3p originates from direct exosomal transfer or is a secondary response of hUCMSCs to other EECD-exosomal signals. This distinction is critical because if miR-137-3p is endogenously induced rather than exogenously delivered, the mechanism of action shifts from cargo-mediated signaling to cellular adaptation. The schematic in Figure 7 also suggests direct transfer of miR-137-3p from EECD-exosomes, which lacks direct experimental support and might therefore misrepresent the actual biological mechanism(s). Furthermore, while the authors identified miR-137-3p as a differentially expressed miRNA in mesenchymal stem cells (MSCs) after EECD-exosome exposure, all subsequent functional validations were performed using synthetic mimics or inhibitors of miR-137-3p in MSCs. These experiments confirmed that miR-137-3p modulated MSC migration and differentiation, but they did not establish that miR-137-3p is a major functional component of the EECD-exosomes themselves.
Stress signals can reshape the miRNA landscape of MSCs and their exosomes. For instance, exosomes derived from lipopolysaccharide-preconditioned MSCs were shown to have enhanced anti-inflammatory effects in sepsis models by enriching miR-150-5p, which targeted insulin receptor substrate 1 and suppressed the phosphatidylinositol 3-kinase/protein kinase B/mammalian target of the rapamycin (PI3K/Akt/mTOR) pathway to promote M2 macrophage polarization and improve survival outcomes[2]. Hypoxia-preconditioned hair follicle MSC-derived exosomes exhibit enhanced anti-inflammatory effects in ulcerative colitis models by upregulating miR-214-3p, which mediates suppression of the PI3K/AKT/mTOR pathway and promotes mitophagy and mitochondrial homeostasis[3]. Exosomes derived from MSCs exposed to combined hypoxic and inflammatory stress alleviated intervertebral disc degeneration by delivering miR-221-3p to suppress DNA damage induced transcript 4 and inhibit nuclear factor kappa B activation, thus reducing the senescence of nucleus pulposus cells through epigenetic modification[4]. Similarly, a study using equine adipose-derived MSCs found that various stimuli, including interleukin-1β, hypoxia, shockwave stimulation, and senescence, significantly altered the miRNA composition of secreted extracellular vesicles, with several anti-inflammatory miRNAs
One major regulatory mechanism underlying these stress-induced miRNA changes involves hypoxia-inducible factors (HIFs), particularly HIF-1α, which orchestrate transcriptional responses to low oxygen conditions. A subset of miRNAs, termed hypoxia-associated miRNAs, are transcriptionally regulated by HIFs and mediate key adaptive responses under hypoxic stress[6]. These include well-characterized miRNAs such as miR-210, miR-21, and miR-214, which are upregulated in MSCs and their exosomes under hypoxia and contribute to inflammation resolution, mitochondrial protection, or metabolic reprogramming[7-10]. Based on the data presented by Zhang et al[1], miR-137-3p may also represent a hypoxia-responsive miRNA, potentially expanding the repertoire of known hypoxia-associated miRNAs. However, this proposed protective role appears to contrast with previously published studies, in which miR-137-3p was upregulated in ischemia/reperfusion-injured cardiomyocytes, and its inhibition attenuated hypoxia/reoxygenation-induced apoptosis, oxidative stress, and pyroptosis in H9c2 and HL-1 cells[11,12]. This discrepancy could be attributed to several context-dependent factors. First, cell type specificity may play a critical role. The behavior of miR-137-3p in cardiomyocytes under ischemic stress may differ markedly from its function in mesenchymal or epithelial cells under hypoxia. Second, whether miR-137-3p is upregulated endogenously in stressed cells or introduced through exosomal transfer may influence its intracellular concentration, subcellular localization, and ability to access target genes. Third, the functional outcome of miR-137-3p may depend on the surrounding microenvironment, including factors such as inflammation, oxidative stress levels, and mitochondrial status, which can collectively influence whether its effect is harmful or beneficial. Despite these uncertainties, the identification of miR-137-3p as a potentially functional exosomal cargo involved in endometrial regeneration represents a novel and valuable contribution by Zhang et al[1]. These insights reinforce the concept that stress-conditioned exosomes can trigger complex adaptive responses in recipient cells, rather than acting as passive carriers. Further studies involving direct quantification of miR-137-3p in EECD-exosomes, along with loss-of-function experiments, are essential to substantiate the proposed mechanism and clarify whether the observed effects truly result from exosomal transfer or reflect an endogenous hypoxic response.
Another concern lies in the insufficient characterization of the EECD-exosomes. No data were provided regarding their size distribution (e.g., via nanoparticle tracking analysis), morphology (e.g., transmission electron microscopy), or surface marker expression (e.g., CD9, CD63, and CD81), which are considered standard for exosome identification. This lack of definition undermines the interpretability of the proposed exosome-mediated signaling.
Furthermore, the study does not include any direct evidence showing that hUCMSCs internalized the EECD-derived exosomes. As highlighted in the MISEV2018 guidelines, uptake validation is a necessary step in functional extracellular vesicle studies, particularly when biological effects are attributed to specific RNA cargos[13]. This is particularly critical given that the central mechanism of the current study involves the transfer of miR-137-3p via exosomes to recipient MSCs.
Among the 53 differentially expressed miRNAs identified, the selection of miR-137-3p as the study’s focus is not well substantiated. The authors did not provide comparative expression levels, fold changes, or literature-based rationale supporting its prioritization over other candidates. A deeper analysis of miRNA-mRNA regulatory networks or competing endogenous RNA interactions may have yielded a more robust mechanistic foundation. Additionally, no loss-of-function experiments (e.g., miR-137-3p inhibitors) were conducted, which would be necessary to confirm its necessity in driving the observed effects.
The study establishes that miR-137-3p downregulates UBE3C and upregulates phosphorylated STAT3, suggesting a novel post-translational regulatory axis. However, the exact molecular mechanism by which UBE3C modulates STAT3 phosphorylation remains speculative. The authors hypothesize that inhibiting UBE3C may prevent STAT3 ubiquitination, thereby stabilizing or enhancing its phosphorylation status. This intriguing possibility deserves further validation via co-immunoprecipitation or ubiquitination-specific assays. Moreover, while total STAT3 protein was elevated, STAT3 mRNA remained unchanged, reinforcing the need to investigate ubiquitin-proteasome system dynamics.
While Zhang et al’s study provides meaningful in vitro data, its therapeutic potential remains to be fully understood[1]. In vivo studies using animal models of thin endometrium would be a valuable next step to determine whether the observed effects can be replicated in a physiologically relevant context. Such studies would also help address the complexity of endometrial regeneration under hypoxia, which cannot be fully modeled in vitro.
The study is commendable for identifying a novel miRNA target (UBE3C) in the context of hUCMSC differentiation and for proposing a link to STAT3 signaling. Functional assays (e.g., wound healing, transwell, Western blot) are methodologically sound and reproducible. However, the most critical limitation lies in the unverified claim that miR-137-3p is transferred via exosomes rather than endogenously expressed in recipient cells. This key mechanistic assertion is not supported by direct experimental evidence and significantly undermines the strength of the proposed pathway. In addition, the study has other omissions, including insufficient exosome characterization, lack of in vivo validation, and a speculative explanation regarding ubiquitination, all of which reduce the overall impact and translational relevance of the findings. We summarize the proposed mechanism and critical evidential gaps in Figure 1.
To fully clarify the role of EECD-exosomal miR-137-3p in endometrial regeneration, several follow-up studies are warranted: (1) Direct quantification of miR-137-3p in EECD-exosomes (e.g., via exosomal RNA isolation and reverse transcription-quantitative polymerase chain reaction); (2) Loss-of-function studies using miR-137-3p inhibitors and CRISPR-based knockout of UBE3C in hUCMSCs; (3) Ubiquitination assays to confirm UBE3C-mediated regulation of STAT3 stability; (4) Animal models of Asherman syndrome or thin endometrium to evaluate functional repair; and (5) Exosome biodistribution and safety studies to assess translational potential.
Zhang et al[1] present an interesting molecular framework linking EECD-derived miR-137-3p to enhanced epithelial differentiation of hUCMSCs via UBE3C and STAT3. However, uncertainties surrounding the actual source of miR-137-3p, the definition of the exosome preparation, and the speculative nature of the ubiquitination mechanism weaken the translational value of the findings. Future studies with direct exosome cargo analysis, robust mechanistic dissection, and in vivo validation will be essential to elevate this work from a conceptual hypothesis to a clinically relevant therapeutic strategy.
| 1. | Zhang WY, Liu SM, Wang HB, Deng CY. Exosomal miR-137-3p targets UBE3C to activate STAT3, promoting migration and differentiation into endometrial epithelial cell of human umbilical cord mesenchymal stem cells under hypoxia. World J Stem Cells. 2025;17:100359. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 2. | Zheng T, Li S, Zhang T, Fu W, Liu S, He Y, Wang X, Ma T. Exosome-shuttled miR-150-5p from LPS-preconditioned mesenchymal stem cells down-regulate PI3K/Akt/mTOR pathway via Irs1 to enhance M2 macrophage polarization and confer protection against sepsis. Front Immunol. 2024;15:1397722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 3. | Li N, Zhao L, Geng X, Liu J, Zhang X, Hu Y, Qi J, Chen H, Qiu J, Zhang X, Jin S. Stimulation by exosomes from hypoxia-preconditioned hair follicle mesenchymal stem cells facilitates mitophagy by inhibiting the PI3K/AKT/mTOR signaling pathway to alleviate ulcerative colitis. Theranostics. 2024;14:4278-4296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 23] [Reference Citation Analysis (0)] |
| 4. | Zhao Y, Chen L, Jiang S, Wu Z, Xiang Q, Lin J, Tian S, Sun Z, Sun C, Li W. Exosomes derived from MSCs exposed to hypoxic and inflammatory environments slow intervertebral disc degeneration by alleviating the senescence of nucleus pulposus cells through epigenetic modifications. Bioact Mater. 2025;49:515-530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 5. | Klymiuk MC, Speer J, Marco I, Elashry MI, Heimann M, Wenisch S, Arnhold S. Determination of the miRNA profile of extracellular vesicles from equine mesenchymal stem cells after different treatments. Stem Cell Res Ther. 2025;16:162. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 6. | Azzouzi HE, Leptidis S, Doevendans PA, De Windt LJ. HypoxamiRs: regulators of cardiac hypoxia and energy metabolism. Trends Endocrinol Metab. 2015;26:502-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 63] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 7. | Zaccagnini G, Greco S, Voellenkle C, Gaetano C, Martelli F. miR-210 hypoxamiR in Angiogenesis and Diabetes. Antioxid Redox Signal. 2022;36:685-706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 23] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 8. | Zhuang Y, Cheng M, Li M, Cui J, Huang J, Zhang C, Si J, Lin K, Yu H. Small extracellular vesicles derived from hypoxic mesenchymal stem cells promote vascularized bone regeneration through the miR-210-3p/EFNA3/PI3K pathway. Acta Biomater. 2022;150:413-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 55] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 9. | Yang Z, Liang Z, Rao J, Xie H, Zhou M, Xu X, Lin Y, Lin F, Wang C, Chen C. Hypoxic-preconditioned mesenchymal stem cell-derived small extracellular vesicles promote the recovery of spinal cord injury by affecting the phenotype of astrocytes through the miR-21/JAK2/STAT3 pathway. CNS Neurosci Ther. 2024;30:e14428. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 23] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 10. | Mallis P. Hypoxic endometrial epithelial cell-derived microRNAs effectively regulate the regenerative properties of mesenchymal stromal cells. World J Stem Cells. 2025;17:102482. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | Zhao T, Qiu Z, Gao Y. MiR-137-3p exacerbates the ischemia-reperfusion injured cardiomyocyte apoptosis by targeting KLF15. Naunyn Schmiedebergs Arch Pharmacol. 2020;393:1013-1024. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 12. | Tang RF, Li WJ, Lu Y, Wang XX, Gao SY. LncRNA SNHG1 alleviates myocardial ischaemia-reperfusion injury by regulating the miR-137-3p/KLF4/TRPV1 axis. ESC Heart Fail. 2024;11:1009-1021. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 13. | Théry C, Witwer KW, Aikawa E, Alcaraz MJ, Anderson JD, Andriantsitohaina R, Antoniou A, Arab T, Archer F, Atkin-Smith GK, Ayre DC, Bach JM, Bachurski D, Baharvand H, Balaj L, Baldacchino S, Bauer NN, Baxter AA, Bebawy M, Beckham C, Bedina Zavec A, Benmoussa A, Berardi AC, Bergese P, Bielska E, Blenkiron C, Bobis-Wozowicz S, Boilard E, Boireau W, Bongiovanni A, Borràs FE, Bosch S, Boulanger CM, Breakefield X, Breglio AM, Brennan MÁ, Brigstock DR, Brisson A, Broekman ML, Bromberg JF, Bryl-Górecka P, Buch S, Buck AH, Burger D, Busatto S, Buschmann D, Bussolati B, Buzás EI, Byrd JB, Camussi G, Carter DR, Caruso S, Chamley LW, Chang YT, Chen C, Chen S, Cheng L, Chin AR, Clayton A, Clerici SP, Cocks A, Cocucci E, Coffey RJ, Cordeiro-da-Silva A, Couch Y, Coumans FA, Coyle B, Crescitelli R, Criado MF, D'Souza-Schorey C, Das S, Datta Chaudhuri A, de Candia P, De Santana EF, De Wever O, Del Portillo HA, Demaret T, Deville S, Devitt A, Dhondt B, Di Vizio D, Dieterich LC, Dolo V, Dominguez Rubio AP, Dominici M, Dourado MR, Driedonks TA, Duarte FV, Duncan HM, Eichenberger RM, Ekström K, El Andaloussi S, Elie-Caille C, Erdbrügger U, Falcón-Pérez JM, Fatima F, Fish JE, Flores-Bellver M, Försönits A, Frelet-Barrand A, Fricke F, Fuhrmann G, Gabrielsson S, Gámez-Valero A, Gardiner C, Gärtner K, Gaudin R, Gho YS, Giebel B, Gilbert C, Gimona M, Giusti I, Goberdhan DC, Görgens A, Gorski SM, Greening DW, Gross JC, Gualerzi A, Gupta GN, Gustafson D, Handberg A, Haraszti RA, Harrison P, Hegyesi H, Hendrix A, Hill AF, Hochberg FH, Hoffmann KF, Holder B, Holthofer H, Hosseinkhani B, Hu G, Huang Y, Huber V, Hunt S, Ibrahim AG, Ikezu T, Inal JM, Isin M, Ivanova A, Jackson HK, Jacobsen S, Jay SM, Jayachandran M, Jenster G, Jiang L, Johnson SM, Jones JC, Jong A, Jovanovic-Talisman T, Jung S, Kalluri R, Kano SI, Kaur S, Kawamura Y, Keller ET, Khamari D, Khomyakova E, Khvorova A, Kierulf P, Kim KP, Kislinger T, Klingeborn M, Klinke DJ 2nd, Kornek M, Kosanović MM, Kovács ÁF, Krämer-Albers EM, Krasemann S, Krause M, Kurochkin IV, Kusuma GD, Kuypers S, Laitinen S, Langevin SM, Languino LR, Lannigan J, Lässer C, Laurent LC, Lavieu G, Lázaro-Ibáñez E, Le Lay S, Lee MS, Lee YXF, Lemos DS, Lenassi M, Leszczynska A, Li IT, Liao K, Libregts SF, Ligeti E, Lim R, Lim SK, Linē A, Linnemannstöns K, Llorente A, Lombard CA, Lorenowicz MJ, Lörincz ÁM, Lötvall J, Lovett J, Lowry MC, Loyer X, Lu Q, Lukomska B, Lunavat TR, Maas SL, Malhi H, Marcilla A, Mariani J, Mariscal J, Martens-Uzunova ES, Martin-Jaular L, Martinez MC, Martins VR, Mathieu M, Mathivanan S, Maugeri M, McGinnis LK, McVey MJ, Meckes DG Jr, Meehan KL, Mertens I, Minciacchi VR, Möller A, Møller Jørgensen M, Morales-Kastresana A, Morhayim J, Mullier F, Muraca M, Musante L, Mussack V, Muth DC, Myburgh KH, Najrana T, Nawaz M, Nazarenko I, Nejsum P, Neri C, Neri T, Nieuwland R, Nimrichter L, Nolan JP, Nolte-'t Hoen EN, Noren Hooten N, O'Driscoll L, O'Grady T, O'Loghlen A, Ochiya T, Olivier M, Ortiz A, Ortiz LA, Osteikoetxea X, Østergaard O, Ostrowski M, Park J, Pegtel DM, Peinado H, Perut F, Pfaffl MW, Phinney DG, Pieters BC, Pink RC, Pisetsky DS, Pogge von Strandmann E, Polakovicova I, Poon IK, Powell BH, Prada I, Pulliam L, Quesenberry P, Radeghieri A, Raffai RL, Raimondo S, Rak J, Ramirez MI, Raposo G, Rayyan MS, Regev-Rudzki N, Ricklefs FL, Robbins PD, Roberts DD, Rodrigues SC, Rohde E, Rome S, Rouschop KM, Rughetti A, Russell AE, Saá P, Sahoo S, Salas-Huenuleo E, Sánchez C, Saugstad JA, Saul MJ, Schiffelers RM, Schneider R, Schøyen TH, Scott A, Shahaj E, Sharma S, Shatnyeva O, Shekari F, Shelke GV, Shetty AK, Shiba K, Siljander PR, Silva AM, Skowronek A, Snyder OL 2nd, Soares RP, Sódar BW, Soekmadji C, Sotillo J, Stahl PD, Stoorvogel W, Stott SL, Strasser EF, Swift S, Tahara H, Tewari M, Timms K, Tiwari S, Tixeira R, Tkach M, Toh WS, Tomasini R, Torrecilhas AC, Tosar JP, Toxavidis V, Urbanelli L, Vader P, van Balkom BW, van der Grein SG, Van Deun J, van Herwijnen MJ, Van Keuren-Jensen K, van Niel G, van Royen ME, van Wijnen AJ, Vasconcelos MH, Vechetti IJ Jr, Veit TD, Vella LJ, Velot É, Verweij FJ, Vestad B, Viñas JL, Visnovitz T, Vukman KV, Wahlgren J, Watson DC, Wauben MH, Weaver A, Webber JP, Weber V, Wehman AM, Weiss DJ, Welsh JA, Wendt S, Wheelock AM, Wiener Z, Witte L, Wolfram J, Xagorari A, Xander P, Xu J, Yan X, Yáñez-Mó M, Yin H, Yuana Y, Zappulli V, Zarubova J, Žėkas V, Zhang JY, Zhao Z, Zheng L, Zheutlin AR, Zickler AM, Zimmermann P, Zivkovic AM, Zocco D, Zuba-Surma EK. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles. 2018;7:1535750. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6453] [Cited by in RCA: 7665] [Article Influence: 1095.0] [Reference Citation Analysis (1)] |