Published online Aug 26, 2024. doi: 10.4252/wjsc.v16.i8.773
Revised: July 4, 2024
Accepted: July 26, 2024
Published online: August 26, 2024
Processing time: 104 Days and 14.9 Hours
In this editorial, we delved into the article titled “Cellular preconditioning and mesenchymal stem cell ferroptosis.” This groundbreaking study underscores a pivotal discovery: Ferroptosis, a type of programmed cell death, drastically reduces the viability of donor mesenchymal stem cells (MSCs) after engraftment, thereby undermining the therapeutic value of cell-based therapies. Furthermore, the article proposes that by manipulating ferroptosis mechanisms through preconditioning, we can potentially enhance the survival rate and functionality of MSCs, ultimately amplifying their therapeutic potential. Given the crucial role ferroptosis plays in shaping the therapeutic outcomes of MSCs, we deem it im
Core Tip: As ferroptosis, a distinct form of programmed cell death, significantly impairs the post-engraftment viability of donor mesenchymal stem cells (MSCs), it assumes paramount importance in dictating the therapeutic efficacy of these cells. Recognizing the pivotal role ferroptosis exerts in shaping the outcome of MSC-based therapies, we underscore the urgency to delve deeper into the intricate relationship between this mode of cell death and the therapeutic potency of MSCs. In this context, we presented our viewpoint on the recently published editorial entitled “Cellular preconditioning and mesenchymal stem cell ferroptosis,” offering insights into this intricate interplay.
- Citation: Wan XX, Hu XM, Zhang Q, Xiong K. Pretreatment can alleviate programmed cell death in mesenchymal stem cells. World J Stem Cells 2024; 16(8): 773-779
- URL: https://www.wjgnet.com/1948-0210/full/v16/i8/773.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v16.i8.773
Mesenchymal stem cells (MSCs), derived from a diverse array of sources, exhibit remarkable ease in in vitro culturing, along with pluripotent differentiation capabilities and robust self-renewal potential[1]. Their therapeutic applications have been demonstrated to effectively alleviate various conditions, including diabetes and recalcitrant wounds, while offering promising immunosuppressive treatment strategies for a spectrum of inflammatory diseases[2,3]. Despite the ability of MSCs to modulate the organ microenvironment and enhance cellular differentiation and regeneration, their clinical success is still constrained by limited understanding of the underlying mechanisms in vivo and the absence of standardized criteria for patient selection[4,5]. Further research is urgently needed to address these challenges.
One of the significant challenges in MSC therapy is the decreased survival and increased cell death of MSCs following transplantation into recipients. To enhance the survival rate of grafted MSCs, it is crucial to minimize MSC death. As a potential solution, preconditioning strategies, such as cytokine preconditioning, can be employed to bolster the vitality of MSCs[6]. For instance, our laboratory has explored the transfection of c-Jun plasmid into human umbilical cord MSCs (HUCMSCs) to boost their wound healing capabilities by enhancing their survival rates[7]. Furthermore, we have utilized hydrocolloid dressings to cover MSCs, which facilitates vascular reconstruction by increasing the vitality of these cells[8]. These studies underscore the critical role of reducing MSC death in clinical MSC treatments.
In general, MSC death occurs in two distinct manners. Firstly, there is a passive and unregulated process triggered by tissue damage, known as accidental cell death or necrosis. This occurs when MSCs are exposed to severe stress conditions, such as exposure to highly toxic compounds, starvation, DNA damage, and other factors. Under such conditions, MSCs are unable to maintain intracellular homeostasis, leading to rapid cellular swelling and rupture of the plasma membrane. This, in turn, results in the passive release of intracellular material into the microenvironment[9,10].
The second manner of MSC death involves highly regulated mechanisms that involve various signaling cascades to elicit different effector functions. This type of death is referred to as programmed cell death (PCD)[11,12]. PCD is a genetically determined and actively orderly process and is ubiquitous in the development of living organisms. It represents the suicide protection measures triggered by gene regulation in response to stimuli from both internal and external environmental factors[13]. This process involves the activation of specific molecular mechanisms and genetic programming, leading to the elimination of non-essential cells or cells destined for specialization from the body.
The various pathways of PCD, including apoptosis, necroptosis, autophagy, ferroptosis, pyroptosis, PANoptosis, etc. exhibit distinct morphological and biochemical characteristics[14-18]. By understanding these mechanisms, we can gain insights into the complex cellular processes that underlie organismal development and homeostasis. Unlike accidental cell death, PCD is a controlled process that is essential for maintaining tissue homeostasis and regulating cellular responses to various stimuli.
Both forms of MSC death have important implications for MSC therapy. Understanding the mechanisms underlying these processes is crucial for developing effective strategies to enhance the survival and functionality of MSCs and improve the outcomes of cell-based therapies.
Apoptosis, a fundamental biological process of the cell, serves as a crucial mechanism for eliminating unwanted or abnormal cells in multicellular organisms. It plays a pivotal role in the evolution of organisms, the maintenance of internal environmental stability, and the development of various systems. Beyond being a specific form of cell death, apoptosis holds significant biological importance and is governed by intricate molecular biological mechanisms. This process is tightly regulated by multiple genes that exhibit remarkable conservation across species, including the Bcl-2 family, the caspase family, oncogenes like c-myc, and the tumor suppressor gene P53[19,20]. These genes work in concert to orchestrate the orderly and controlled dismantling of cells, ensuring the precise removal of nonfunctional or potentially harmful cells from the body[21].
Necroptosis, also known as necrotic apoptosis, is a cellular self-destruction process triggered by either extracellular signals or intracellular signals[22,23]. During necroptosis, distinct morphological changes occur, including organelle swelling, cell membrane rupture, and the breakdown of the cytoplasm and nucleus[24,25]. Notably, necroptosis differs from apoptosis and other forms of PCD in its independence from caspase activity. Instead, it relies on receptor-interacting protein kinase 3-regulated phosphorylation of mixed lineage kinase domain-like protein. This phosphorylation triggers mixed lineage kinase domain-like protein to form a pore complex at the plasma membrane, resulting in the secretion of damage-associated molecular patterns, cell swelling, and ultimately, membrane rupture[26].
Whether autophagy is a PCD type is currently controversial. Although autophagy is sometimes called autophagic cell death, there are also many researchers who report autophagy as a type of PCD[27,28]. Cell death mediated by autophagy is characterized by cytoplasmic vacuolization, the formation of autophagic vesicles, and the lysosomal degradation of cellular material[29]. Autophagy can be classified into three types: Macroautophagy; microautophagy; and selective autophagy[30]. In macroautophagy, autophagosomes, which are double-membraned vesicles, envelope a portion of the cell. These autophagosomes then fuse with lysosomes to create autolysosome, where the contents are degraded by proteases. Microautophagy involves the direct interaction and fusion of vesicles containing organelles or inclusions with lysosomes. This process is more specific than macroautophagy and can be triggered by signaling molecules on the surface of damaged organelles. Selective autophagy, also known as chaperone-mediated autophagy, involves the fusion of cytoplasmic proteins with lysosomes via cytoplasmic chaperones[31]. These chaperones interact with pentapeptides within the amino acid sequence of the substrate proteins.
Pyroptosis is triggered by intracellular infections of bacteria, viruses, fungi, and protozoa in the presence of either pathogen-associated molecular patterns or cell-derived damage-associated molecular patterns[32]. It typically serves as the primary mode of cell death in response to pathogen infection and is commonly observed in cells of the innate immune system, such as monocytes, macrophages, and dendritic cells[33]. The classical pathway of pyroptosis unfolds through a two-step process. Initially, nuclear factor- kappaB is activated, initiating the expression of multiple proteins. Then, caspase-1 is activated to cleave gasdermin D and cytokines, pro-interleukin (IL)-1β and pro-IL-18. These cytokines are then proteolytically hydrolyzed into their proinflammatory forms, IL-1β and IL-18[34,35]. Additionally, caspase-1 cleaves the amino-terminal fragment of the key protein, gasdermin D, leading to its oligomerization and the formation of a pore in the cell membrane[36]. This pore formation results in cytokine secretion, water influx, and ultimately, cell rupture.
Ferroptosis exhibits no chromatin condensation or loss of plasma membrane integrity, yet it is characterized by mitochondrial condensation, reduction of mitochondrial cristae, and an increase in membrane density[37-39]. The process of iron death is primarily governed by iron homeostasis and oxidative stress pathways. Iron homeostasis is partially controlled by ferritin, and the levels of ferritin are modulated through “ferritin autophagy,” a process facilitated by nuclear receptor coactivator 4 (NCOA4), which serves as a selective cargo receptor for ferritin[40]. Ferritin autophagy is a meticulously orchestrated process, which is distinctly marked by the liberation of iron. In this intricate pathway, the pivotal NCOA4 plays a crucial role by directly recognizing and engaging with ferritin heavy chain 1, the ferritin subtype harboring iron. Subsequently, NCOA4 facilitates the targeted delivery of iron-laden ferritin to autophagosomes, which then undergo lysosomal degradation, ultimately releasing the sequestered iron. Experimental findings indicate that iron death can be suppressed through the use of common iron chelators, such as desferrioxamine.
Multiple forms of PCD such as pyroptosis, apoptosis, and necroptosis occur extensively in complex crosstalk and coordination, which cause a newly emerging concept known as PANoptosis[41,42]. PANoptosis is usually triggered by infectious and inflammatory factors[43,44]. Like other PCD types, PANoptosis has a multimeric protein complex (e.g., ZBP1-NLRP3), which was named the PANoptosome. However, the exact role of PANoptosis remains unclear.
The programmed death of MSCs results in a significant reduction in their transplantation efficiency, and a variety of factors can lead to PCD of MSCs. Most reported cell death in PCD is apoptosis, while necroptosis, PANoptosis and pyroptosis are rarely reported. To address this problem, various pretreatment has been used to enhance the survival of MSCs.
Cytotoxicity, stimuli, virus infection, and radiation can all trigger apoptosis in MSCs. It has been demonstrated that pretreatment with drugs reduces the apoptosis of MSCs and improves the efficacy of MSCs. Recently, preconditioning with prostaglandin E1 (PGE1) has been shown to enhance the protein expression of hypoxia-inducible factor-1 alpha (HIF-1α) in MSCs[45]. This upregulation of HIF-1α not only mitigates MSCs apoptosis but also boosts the protein levels of C-X-C chemokine receptor type 4, thus enhancing MSCs migration and promoting the secretion of vascular endothelial growth factor. These findings suggest that PGE1 modulates the properties of MSCs through the regulation of the HIF pathway. This understanding provides valuable insights into how PGE1 preconditioning can potentially enhance the therapeutic benefits of MSCs in the treatment of pulmonary arterial hypertension.
Myricetin could alleviate hydrogen peroxide-induced senescence and apoptosis in rat nucleus pulposus-derived MSCs through the silent information regulator 1/proliferator-activated receptor gamma coactivator-1 alpha pathway[46]. Preconditioning MSCs with 1.5% oxygen significantly enhances their activities, effectively boosting proliferation, migration, angiogenesis, antioxidant, antiapoptotic, and antifibrotic properties[47]. Preconditioning MSCs with 2% oxygen optimizes the expression of prion protein, activating prion protein-dependent Janus kinase 2 and signal transducer and activator of transcription 3 signaling pathways. This, in turn, upregulates the activity of superoxide dismutase and catalase, effectively inhibiting oxidative stress-induced apoptosis of MSCs and promoting the recovery of ischemic tissue[48].
The coordination of autophagy and apoptosis is the key to maintaining homeostasis in bone marrow MSCs (BM-MSCs). Hypoxic stress increases autophagy and apoptosis in BM-MSCs time dependently, and the increased autophagy and apoptosis in BM-MSCs induced by hypoxia are abolished by AMP-activated protein kinase (AMPK) inhibitor compound C[49]. Hypoxia pretreatment could promote BM-MSC survival by inducing autophagy through HIF-1α[50]. Besides, iron overload (IO) could significantly induce cell apoptosis and reduce cell viability in MSCs. This process is accompanied by extensive mitochondrial fragmentation and an enhancement of autophagy, both of which were dependent on reactive oxygen species (ROS). Notably, MSCs exposed to IO exhibited a marked decrease in ATP concentration, attributed to elevated ROS levels and a reduction in electron respiratory chain complex II/III activity. This depletion of ATP led to the phosphorylation of AMPK, which in turn triggered mitochondrial fission. Furthermore, genetic ablation of AMPK using CRISPR/Cas9 technology effectively mitigated cell apoptosis, enhanced cell viability and attenuated the mitochondrial fragmentation and autophagy triggered by IO in MSCs. These findings provide a deeper understanding of the cellular mechanisms underlying the deleterious effects of IO on MSCs and suggest potential therapeutic targets for mitigating these effects[51].
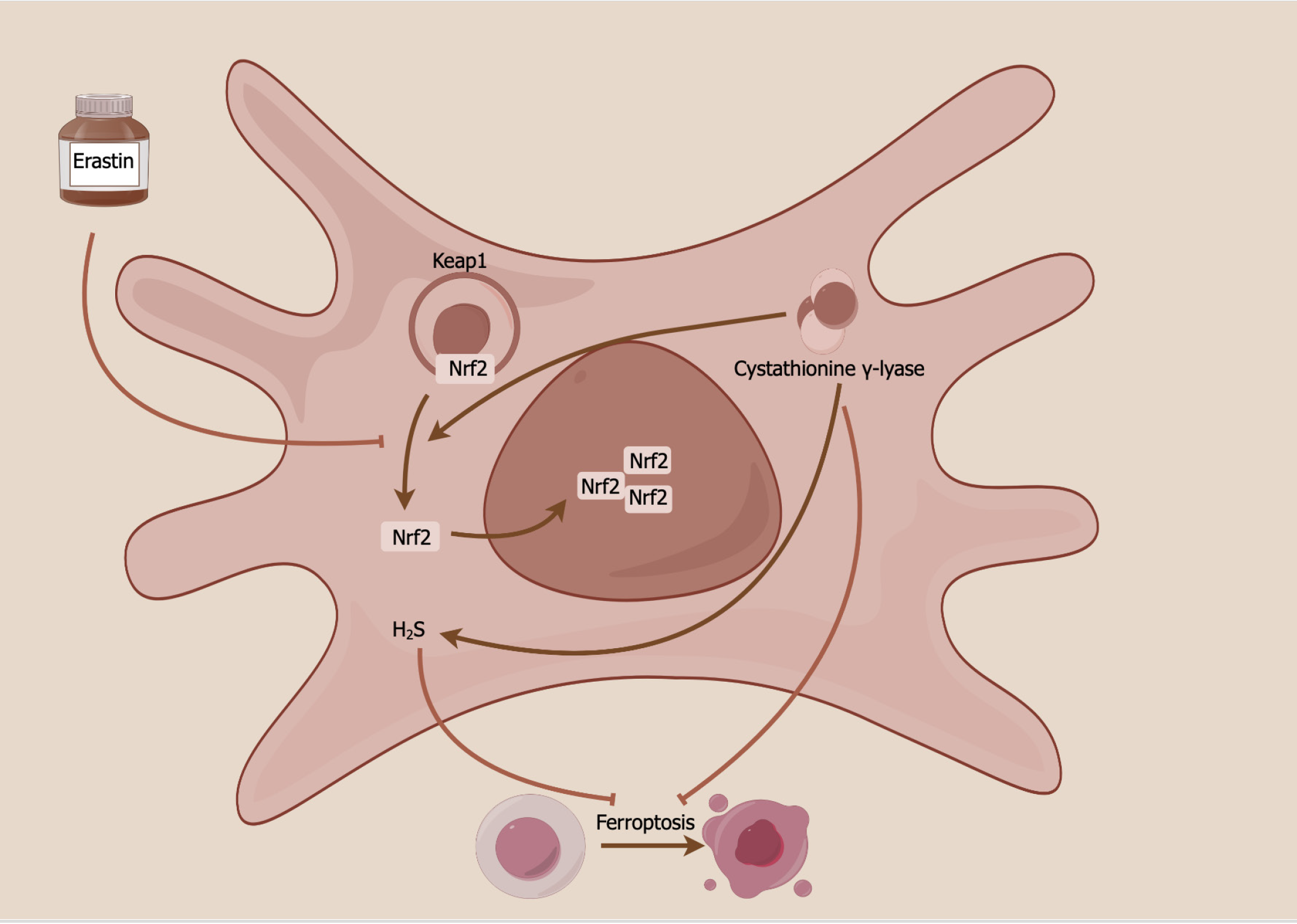
There is little research focused on ferroptosis in pretreated MSCs. A recently published article in the World Journal of Stem Cells showed a novel ferroptosis mechanism of MSCs through the cystathionine γ-lyase (CSE)/hydrogen sulfide (H2S) pathway (Figure 1)[52]. MSC overexpression of CSE has been shown to enhance H2S production and various ferroptosis-related indices, encompassing cell viability, iron concentration, ROS generation, cystine absorption, lipid peroxidation, mitochondrial membrane density, and ferroptosis-related protein expression, in erastin-treated HUCMSCs. By modulating the CSE/H2S pathway, ferroptosis in HUCMSCs was mitigated, subsequently leading to improved vascular remodeling in mice with hypoxia-induced pulmonary arterial hypertension. This occurs by maintaining a delicate balance between stem cell maintenance and differentiation. The CSE/H2S pathway exerts its protective effects against ferroptosis in HUCMSCs through the mediation of S-sulfhydrated Keap1/nuclear factor erythroid 2-related factor 2 signaling[52,53].
Enhancing the transplantation efficacy of MSCs by decreasing programmed death of MSCs can significantly increase their survival rate, boost their differentiation potential, and modulate the immune microenvironment of the body. Although research on PCD types such as necroptosis, PANoptosis, and pyroptosis is still limited, MSC transplants can prevent various kinds of cells from necroptosis, PANoptosis and pyroptosis. As investigations progress, more types of PCD will be reported in MSCs. To improve the efficacy of MSCs, it is very important to inhibit PCD of MSCs, which can increase the survival of MSCs after transplants. Currently, the majority of preconditioning drugs aim to modulate apoptosis. However, with an enhanced comprehension of PCD, we can now target the prevention of various forms of cell death in MSCs. For example, the recently reported ferroptosis of MSCs suggests that we can target CSE to enhance the survival of MSCs. This represents a pivotal guideline in our efforts to enhance the therapeutic effectiveness of MSCs.
| 1. | Xiao Y, Peng J, Liu Q, Chen L, Shi K, Han R, Yang Q, Zhong L, Zha R, Qu Y, Qian Z. Ultrasmall CuS@BSA nanoparticles with mild photothermal conversion synergistically induce MSCs-differentiated fibroblast and improve skin regeneration. Theranostics. 2020;10:1500-1513. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 67] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 2. | Kraskiewicz H, Paprocka M, Bielawska-Pohl A, Krawczenko A, Panek K, Kaczyńska J, Szyposzyńska A, Psurski M, Kuropka P, Klimczak A. Can supernatant from immortalized adipose tissue MSC replace cell therapy? An in vitro study in chronic wounds model. Stem Cell Res Ther. 2020;11:29. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 43] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 3. | Lu B, Lerman LO. MSC therapy for diabetic kidney disease and nephrotic syndrome. Nat Rev Nephrol. 2023;19:754-755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 4. | Zanetti SR, Romecin PA, Vinyoles M, Juan M, Fuster JL, Cámos M, Querol S, Delgado M, Menendez P. Bone marrow MSC from pediatric patients with B-ALL highly immunosuppress T-cell responses but do not compromise CD19-CAR T-cell activity. J Immunother Cancer. 2020;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 5. | Wan XX, Zhang DY, Khan MA, Zheng SY, Hu XM, Zhang Q, Yang RH, Xiong K. Stem Cell Transplantation in the Treatment of Type 1 Diabetes Mellitus: From Insulin Replacement to Beta-Cell Replacement. Front Endocrinol (Lausanne). 2022;13:859638. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 35] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 6. | Liu C, Zhang YS, Chen F, Wu XY, Zhang BB, Wu ZD, Lei JX. Immunopathology in schistosomiasis is regulated by TLR2,4- and IFN-γ-activated MSC through modulating Th1/Th2 responses. Stem Cell Res Ther. 2020;11:217. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 7. | Yue C, Guo Z, Luo Y, Yuan J, Wan X, Mo Z. c-Jun Overexpression Accelerates Wound Healing in Diabetic Rats by Human Umbilical Cord-Derived Mesenchymal Stem Cells. Stem Cells Int. 2020;2020:7430968. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 38] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 8. | Luo Y, Liang F, Wan X, Liu S, Fu L, Mo J, Meng X, Mo Z. Hyaluronic Acid Facilitates Angiogenesis of Endothelial Colony Forming Cell Combining With Mesenchymal Stem Cell via CD44/ MicroRNA-139-5p Pathway. Front Bioeng Biotechnol. 2022;10:794037. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 14] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 9. | Pasternak-Mnich K, Szwed-Georgiou A, Ziemba B, Pieszyński I, Bryszewska M, Kujawa J. Effect of photobiomodulation therapy on the morphology, intracellular calcium concentration, free radical generation, apoptosis and necrosis of human mesenchymal stem cells-an in vitro study. Lasers Med Sci. 2024;39:75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 10. | Ni D, Lei C, Liu M, Peng J, Yi G, Mo Z. Cell death in atherosclerosis. Cell Cycle. 2024;23:495-518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Reference Citation Analysis (0)] |
| 11. | Ban XX, Wan H, Wan XX, Tan YT, Hu XM, Ban HX, Chen XY, Huang K, Zhang Q, Xiong K. Copper Metabolism and Cuproptosis: Molecular Mechanisms and Therapeutic Perspectives in Neurodegenerative Diseases. Curr Med Sci. 2024;44:28-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 22] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 12. | Cai H, Meng Z, Yu F. The involvement of ROS-regulated programmed cell death in hepatocellular carcinoma. Crit Rev Oncol Hematol. 2024;197:104361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 20] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 13. | Xuan X, Zhang S. Targeting the programmed cell death (PCD) signaling mechanism with natural substances for the treatment of diabetic cardiomyopathy (DCM). Phytother Res. 2023;37:5495-5508. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 14. | Xiao Z, Liu M, Yang F, Liu G, Liu J, Zhao W, Ma S, Duan Z. Programmed cell death and lipid metabolism of macrophages in NAFLD. Front Immunol. 2023;14:1118449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 15. | Erekat NS. Programmed Cell Death in Diabetic Nephropathy: A Review of Apoptosis, Autophagy, and Necroptosis. Med Sci Monit. 2022;28:e937766. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 47] [Reference Citation Analysis (0)] |
| 16. | Sun J, Huang L, Wang J, Hu Y, Wang W, Zhu H. Programmed cell death in autoimmune diseases: ferroptosis. Ann Biol Clin (Paris). 2024;82:33-42. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 17. | Zhu M, Liu D, Liu G, Zhang M, Pan F. Programmed Cell Death in Prostate Cancer: From Apoptosis, Necroptosis, and Pyroptosis to PANoptosis. Biomolecules. 2023;13:1715. |
| 18. | Liu X, Miao M, Sun J, Wu J, Qin X. PANoptosis: a potential new target for programmed cell death in breast cancer treatment and prognosis. Apoptosis. 2024;29:277-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 19. | Martinou JC, Youle RJ. Mitochondria in apoptosis: Bcl-2 family members and mitochondrial dynamics. Dev Cell. 2011;21:92-101. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1103] [Cited by in RCA: 1117] [Article Influence: 79.8] [Reference Citation Analysis (0)] |
| 20. | Saxena K, Konopleva M. An expert overview of emerging therapies for acute myeloid leukemia: novel small molecules targeting apoptosis, p53, transcriptional regulation and metabolism. Expert Opin Investig Drugs. 2020;29:973-988. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 21. | Kim G, Lim S, Kim KD. N-myc Downstream-Regulated Gene 2 (NDRG2) Function as a Positive Regulator of Apoptosis: A New Insight into NDRG2 as a Tumor Suppressor. Cells. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 22. | Wendlocha D, Kubina R, Krzykawski K, Mielczarek-Palacz A. Selected Flavonols Targeting Cell Death Pathways in Cancer Therapy: The Latest Achievements in Research on Apoptosis, Autophagy, Necroptosis, Pyroptosis, Ferroptosis, and Cuproptosis. Nutrients. 2024;16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 23. | Lee B, Kim YY, Jeong S, Lee SW, Lee SJ, Rho MC, Kim SH, Lee S. Oleanolic Acid Acetate Alleviates Cisplatin-Induced Nephrotoxicity via Inhibition of Apoptosis and Necroptosis In Vitro and In Vivo. Toxics. 2024;12. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 24. | Ning J, Chen L, Zeng Y, Xiao G, Tian W, Wu Q, Tang J, He S, Tanzhu G, Zhou R. The scheme, and regulative mechanism of pyroptosis, ferroptosis, and necroptosis in radiation injury. Int J Biol Sci. 2024;20:1871-1883. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 11] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 25. | Wan H, Yang YD, Zhang Q, Chen YH, Hu XM, Huang YX, Shang L, Xiong K. VDAC1, as a downstream molecule of MLKL, participates in OGD/R-induced necroptosis by inducing mitochondrial damage. Heliyon. 2024;10:e23426. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 26. | Zhang Q, Hu XM, Zhao WJ, Ban XX, Li Y, Huang YX, Wan H, He Y, Liao LS, Shang L, Jiang B, Qing GP, Xiong K. Targeting Necroptosis: A Novel Therapeutic Option for Retinal Degenerative Diseases. Int J Biol Sci. 2023;19:658-674. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 32] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 27. | Zhang Q, Fan X, Zhang X, Ju S. Ferroptosis in tumors and its relationship to other programmed cell death: role of non-coding RNAs. J Transl Med. 2023;21:514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 28. | Khan AQ, Agha MV, Ahmad F, Anver R, Sheikhan KSAM, Mateo J, Alam M, Buddenkotte J, Uddin S, Steinhoff M. Metabolomics analyses reveal the crucial role of ERK in regulating metabolic pathways associated with the proliferation of human cutaneous T-cell lymphoma cells treated with Glabridin. Cell Prolif. 2024;e13701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 29. | Chen C, Liu J, Lin X, Xiang A, Ye Q, Guo J, Rui T, Xu J, Hu S. Crosstalk between cancer-associated fibroblasts and regulated cell death in tumors: insights into apoptosis, autophagy, ferroptosis, and pyroptosis. Cell Death Discov. 2024;10:189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 20] [Reference Citation Analysis (0)] |
| 30. | Xi H, Wang S, Wang B, Hong X, Liu X, Li M, Shen R, Dong Q. The role of interaction between autophagy and apoptosis in tumorigenesis (Review). Oncol Rep. 2022;48. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 68] [Reference Citation Analysis (0)] |
| 31. | Chen X, Tsvetkov AS, Shen HM, Isidoro C, Ktistakis NT, Linkermann A, Koopman WJH, Simon HU, Galluzzi L, Luo S, Xu D, Gu W, Peulen O, Cai Q, Rubinsztein DC, Chi JT, Zhang DD, Li C, Toyokuni S, Liu J, Roh JL, Dai E, Juhasz G, Liu W, Zhang J, Yang M, Liu J, Zhu LQ, Zou W, Piacentini M, Ding WX, Yue Z, Xie Y, Petersen M, Gewirtz DA, Mandell MA, Chu CT, Sinha D, Eftekharpour E, Zhivotovsky B, Besteiro S, Gabrilovich DI, Kim DH, Kagan VE, Bayir H, Chen GC, Ayton S, Lünemann JD, Komatsu M, Krautwald S, Loos B, Baehrecke EH, Wang J, Lane JD, Sadoshima J, Yang WS, Gao M, Münz C, Thumm M, Kampmann M, Yu D, Lipinski MM, Jones JW, Jiang X, Zeh HJ, Kang R, Klionsky DJ, Kroemer G, Tang D. International consensus guidelines for the definition, detection, and interpretation of autophagy-dependent ferroptosis. Autophagy. 2024;20:1213-1246. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 66] [Article Influence: 66.0] [Reference Citation Analysis (0)] |
| 32. | Burdette BE, Esparza AN, Zhu H, Wang S. Gasdermin D in pyroptosis. Acta Pharm Sin B. 2021;11:2768-2782. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 199] [Cited by in RCA: 421] [Article Influence: 105.3] [Reference Citation Analysis (0)] |
| 33. | Chen Y, Long T, Chen J, Wei H, Meng J, Kang M, Wang J, Zhang X, Xu Q, Zhang C, Xiong K. WTAP participates in neuronal damage by protein translation of NLRP3 in an m6A-YTHDF1-dependent manner after traumatic brain injury. Int J Surg. 2024;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 34. | Rabolli V, Lison D, Huaux F. The complex cascade of cellular events governing inflammasome activation and IL-1β processing in response to inhaled particles. Part Fibre Toxicol. 2016;13:40. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 58] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 35. | He YF, Hu XM, Khan MA, Yu BY, Sheng YC, Xiao XZ, Wan XX, Tan SP, Xiong K. HSF1 Alleviates Brain Injury by Inhibiting NLRP3-Induced Pyroptosis in a Sepsis Model. Mediators Inflamm. 2023;2023:2252255. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 9] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 36. | Wang W, Chen J, Yu X, Lan HY. Signaling mechanisms of SARS-CoV-2 Nucleocapsid protein in viral infection, cell death and inflammation. Int J Biol Sci. 2022;18:4704-4713. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 40] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 37. | Hou J, Wang B, Li J, Liu W. Ferroptosis and its role in gastric and colorectal cancers. Korean J Physiol Pharmacol. 2024;28:183-196. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 38. | Chen J, Wang Y, Li M, Zhu X, Liu Z, Chen Q, Xiong K. Netrin-1 Alleviates Early Brain Injury by Regulating Ferroptosis via the PPARγ/Nrf2/GPX4 Signaling Pathway Following Subarachnoid Hemorrhage. Transl Stroke Res. 2024;15:219-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 40] [Article Influence: 40.0] [Reference Citation Analysis (0)] |
| 39. | Chen J, Shi Z, Zhang C, Xiong K, Zhao W, Wang Y. Oroxin A alleviates early brain injury after subarachnoid hemorrhage by regulating ferroptosis and neuroinflammation. J Neuroinflammation. 2024;21:116. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Reference Citation Analysis (0)] |
| 40. | Wang J, Wu N, Peng M, Oyang L, Jiang X, Peng Q, Zhou Y, He Z, Liao Q. Ferritinophagy: research advance and clinical significance in cancers. Cell Death Discov. 2023;9:463. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 34] [Reference Citation Analysis (0)] |
| 41. | Arrázola MS, Court FA. Commentary on "PANoptosis-like cell death in ischemia/reperfusion injury of retinal neurons". Neural Regen Res. 2023;18:341. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 42. | Hu XM, Zheng S, Zhang Q, Wan X, Li J, Mao R, Yang R, Xiong K. PANoptosis signaling enables broad immune response in psoriasis: From pathogenesis to new therapeutic strategies. Comput Struct Biotechnol J. 2024;23:64-76. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 43. | Malireddi RKS, Kesavardhana S, Kanneganti TD. ZBP1 and TAK1: Master Regulators of NLRP3 Inflammasome/Pyroptosis, Apoptosis, and Necroptosis (PAN-optosis). Front Cell Infect Microbiol. 2019;9:406. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 459] [Cited by in RCA: 420] [Article Influence: 70.0] [Reference Citation Analysis (0)] |
| 44. | Kuriakose T, Kanneganti TD. Pyroptosis in Antiviral Immunity. Curr Top Microbiol Immunol. 2023;442:65-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 27] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 45. | Jiang DT, Tuo L, Bai X, Bing WD, Qu QX, Zhao X, Song GM, Bi YW, Sun WY. Prostaglandin E1 reduces apoptosis and improves the homing of mesenchymal stem cells in pulmonary arterial hypertension by regulating hypoxia-inducible factor 1 alpha. Stem Cell Res Ther. 2022;13:316. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 21] [Reference Citation Analysis (0)] |
| 46. | Xie T, Pan R, Huang W, Dong S, Wu S, Ye Y. Myricetin alleviates H2O2-induced senescence and apoptosis in rat nucleus pulposus-derived mesenchymal stem cells. Folia Histochem Cytobiol. 2023;61:98-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 47. | Cruz FF, Rocco PR. Hypoxic preconditioning enhances mesenchymal stromal cell lung repair capacity. Stem Cell Res Ther. 2015;6:130. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 48. | Han YS, Lee JH, Yoon YM, Yun CW, Noh H, Lee SH. Hypoxia-induced expression of cellular prion protein improves the therapeutic potential of mesenchymal stem cells. Cell Death Dis. 2016;7:e2395. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 56] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 49. | Zhang Z, Yang M, Wang Y, Wang L, Jin Z, Ding L, Zhang L, Zhang L, Jiang W, Gao G, Yang J, Lu B, Cao F, Hu T. Autophagy regulates the apoptosis of bone marrow-derived mesenchymal stem cells under hypoxic condition via AMP-activated protein kinase/mammalian target of rapamycin pathway. Cell Biol Int. 2016;40:671-685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 41] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 50. | Liu J, Hao H, Huang H, Tong C, Ti D, Dong L, Chen D, Zhao Y, Liu H, Han W, Fu X. Hypoxia regulates the therapeutic potential of mesenchymal stem cells through enhanced autophagy. Int J Low Extrem Wounds. 2015;14:63-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 46] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 51. | Zheng Q, Zhao Y, Guo J, Zhao S, Fei C, Xiao C, Wu D, Wu L, Li X, Chang C. Iron overload promotes mitochondrial fragmentation in mesenchymal stromal cells from myelodysplastic syndrome patients through activation of the AMPK/MFF/Drp1 pathway. Cell Death Dis. 2018;9:515. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 88] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 52. | Hu B, Zhang XX, Zhang T, Yu WC. Dissecting molecular mechanisms underlying ferroptosis in human umbilical cord mesenchymal stem cells: Role of cystathionine γ-lyase/hydrogen sulfide pathway. World J Stem Cells. 2023;15:1017-1034. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 53. | Zineldeen DH, Mushtaq M, Haider KH. Cellular preconditioning and mesenchymal stem cell ferroptosis. World J Stem Cells. 2024;16:64-69. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Reference Citation Analysis (9)] |