Published online May 26, 2024. doi: 10.4252/wjsc.v16.i5.486
Revised: March 1, 2024
Accepted: April 7, 2024
Published online: May 26, 2024
Processing time: 185 Days and 16 Hours
A decreased autophagic capacity of bone marrow mesenchymal stromal cells (BMSCs) has been suggested to be an important cause of decreased osteogenic differentiation. A pharmacological increase in autophagy of BMSCs is a potential therapeutic option to increase osteoblast viability and ameliorate osteoporosis.
To explore the effects of sinomenine (SIN) on the osteogenic differentiation of BMSCs and the underlying mechanisms.
For in vitro experiments, BMSCs were extracted from sham-treated mice and ovariectomized mice, and the levels of autophagy markers and osteogenic differentiation were examined after treatment with the appropriate concentrations of SIN and the autophagy inhibitor 3-methyladenine. In vivo, the therapeutic effect of SIN was verified by establishing an ovariectomy-induced mouse model and by morphological and histological assays of the mouse femur.
SIN reduced the levels of AKT and mammalian target of the rapamycin (mTOR) phosphorylation in the phosphatidylinositol 3-kinase (PI3K)/AKT/mTOR signaling pathway, inhibited mTOR activity, and increased autophagy ability of BMSCs, thereby promoting the osteogenic differentiation of BMSCs and effectively alleviating bone loss in ovariectomized mice in vivo.
The Chinese medicine SIN has potential for the treatment of various types of osteoporosis, bone homeostasis disorders, and autophagy-related diseases.
Core Tip: Sinomenine (SIN) reduces the levels of AKT and mammalian target of the rapamycin (mTOR) phosphorylation in the phosphatidylinositol 3-kinase/AKT/mTOR signaling pathway, inhibits mTOR activity, and increases autophagy of bone marrow mesenchymal stromal cells (BMSCs), thereby promoting the osteogenic differentiation of BMSCs and effectively alleviating bone loss in ovariectomized mice when used in vivo, thus providing support for the use of the traditional Chinese medicine SIN in the treatment of various types of osteoporosis, imbalances in bone homeostasis, and autophagy-related diseases.
- Citation: Xiao HX, Yu L, Xia Y, Chen K, Li WM, Ge GR, Zhang W, Zhang Q, Zhang HT, Geng DC. Sinomenine increases osteogenesis in mice with ovariectomy-induced bone loss by modulating autophagy. World J Stem Cells 2024; 16(5): 486-498
- URL: https://www.wjgnet.com/1948-0210/full/v16/i5/486.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v16.i5.486
Human bone homeostasis is a sophisticated metabolic process that is maintained by osteogenic differentiation and bone mineralization of osteoblasts, and bone resorption by osteoclasts[1]. Decreased osteogenic differentiation of osteoblasts or overactivation of osteoclasts may lead to greater bone loss than bone formation, resulting in the development of osteoporosis[2] due to the destruction of bone microstructure and a reduction in bone mass; these changes lead to clinical susceptibility to bone fracture[3,4]. Multiple etiologies contribute to the development of osteoporosis, with postmenopausal estrogen withdrawal being one of the most common causes[5]. A previous report showed that after the withdrawal of estrogen, the decreased osteogenic differentiation capacity of osteoblasts and insufficient mineralization, as well as osteoclast overactivity, are important causes of postmenopausal osteoporosis.
Bone marrow-derived mesenchymal stem cells (BMSCs) are mesenchymal-derived pluripotent stem cells with multidirectional differentiation potential[6] that can differentiate into pre-osteoblasts and osteoblasts under certain conditions to repair damaged bone tissue[7]. Previous studies have shown that the autophagic capacity of BMSCs is necessary to maintain their osteogenic differentiation[8], and autophagy, a self-digestive process in which protein aggregates, damaged organelles, and lipid vesicles are detached and encapsulated in an autophagosome, which then fuses with a lysosome to degrade its contents, is a self-regulatory process through which the cell adapts to its envi
In recent years, traditional Chinese medicine has gained attention in the prevention and treatment of osteoporosis due to its easy availability and minimal side effects. Sinomenine (SIN, Supplementary Figure 1A) is an alkaloid extracted from the vine stems of Sinomenium acutum (S. acutum) and S. acutum var. cinerum[11] that has analgesic, sedative, anti-inflammatory and antioxidant effects, as well as few adverse effects on the digestive system, and is clinically used to treat various conditions such as acute and chronic arthritis, rheumatoid arthritis, osteoarthritis, synovitis, and neuropathic pain[12]. In addition, SIN was reported to affect bone metabolism through multiple mechanisms[13,14]. Zhou et al[15] reported that cytarabine could inhibit osteoclast differentiation through the RANKL signaling pathway. Liao et al[16] concluded that SIN significantly alleviated arthritis symptoms in mice with collagen-induced arthritis by inhibiting proinflammatory cytokine secretion and inflammatory mediator production by synovial cells in arthritic mice and could decrease the formation, activity and survival of osteoclasts. SIN exerted beneficial therapeutic effects on periodontal erosive diseases and loose teeth both in vitro and in vivo by promoting the osteogenic differentiation of periodontal ligament stem cells (PDLSCs)[17]. However, there have been no reports on the effects of SIN in the treatment of osteoporosis, especially the specific mechanism by which SIN regulates the osteogenic differentiation of BMSCs.
On the basis of previous studies, the present study demonstrated that SIN plays an important role in increasing the osteogenic differentiation ability of BMSCs in vitro and reducing bone loss in ovariectomized (OVX) mice in vivo. Mechanistically, SIN affects the osteogenic differentiation of BMSCs by altering the autophagic ability of BMSCs. The findings of this research will provide a valuable reference for the clinical effectiveness of SIN in the treatment of osteoporosis.
Alpha modified Eagle’s medium (α-DMEM; 12571063, Gibco) and 10% foetal bovine serum (FBS, 16140071, Gibco) used for cell extraction and culture were purchased from Thermo Fisher. Drugs for in vivo and in vitro interventions, including SIN (115-53-7, HPLC ≥ 98%, MCE) and 3-methyladenine (3-MA, 5142-23-4, HPLC ≥ 98%, MCE), were purchased from MedChemExpress. SIN was dissolved in DMSO, diluted to the intervention concentration in medium and stored at -20 °C. Water-soluble 3-MA was added directly to the medium to reach the appropriate concentration before the intervention. The names, concentrations, and manufacturers of the antibodies used in this study are listed in Supplementary Table 1.
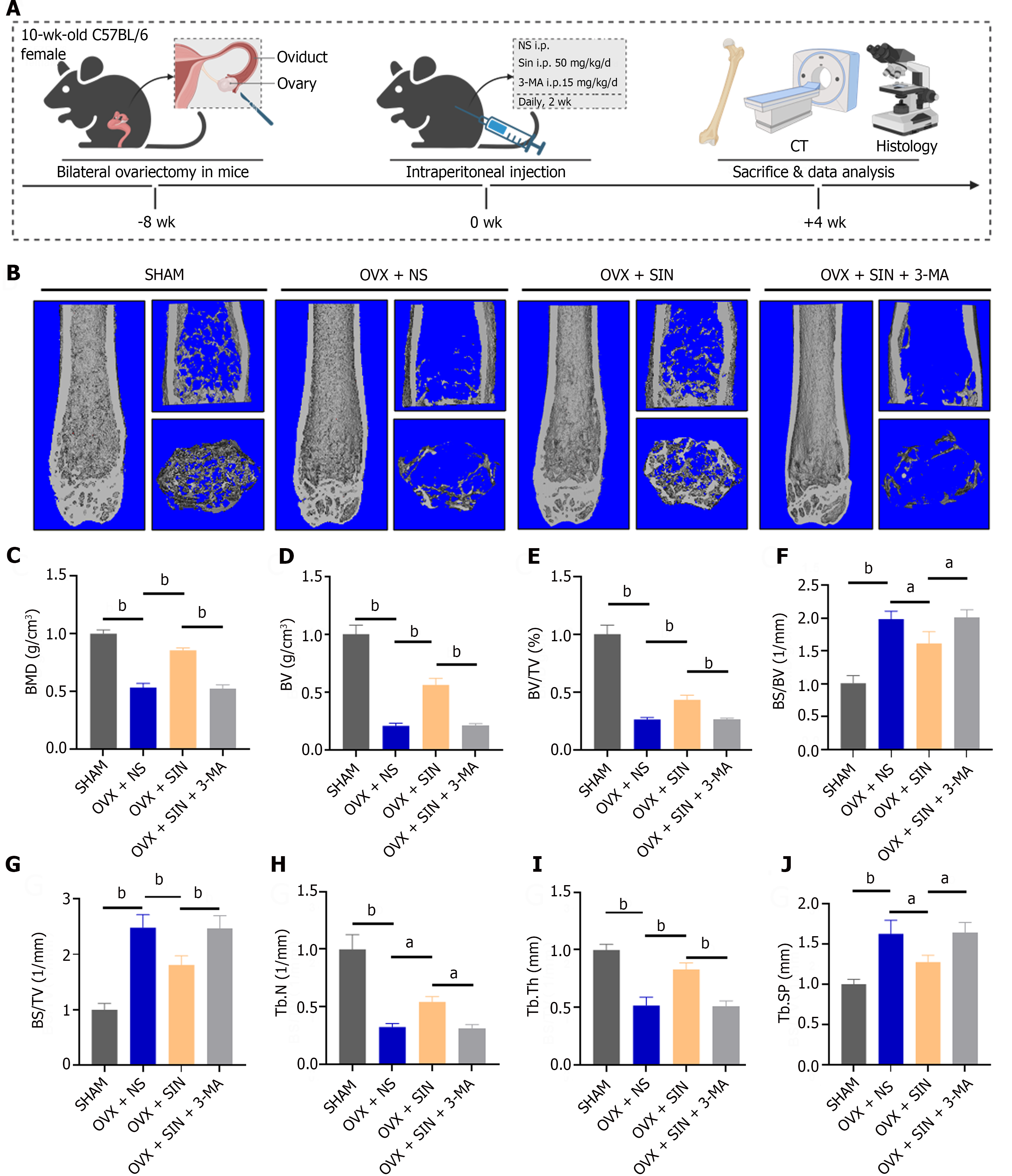
All animal experiments in this study were carried out with the approval of the Ethics Committee of the First Affiliated Hospital of Soochow University (ethics approval number: SUDA20221229A02). Forty female C57BL/6 mice, aged ten weeks, were randomly allocated to four groups, each consisting of 10 mice: The sham group (with an equal amount of adipose tissue adjacent to the ovaries removed), the OVX + NS group (with bilateral OVX + NS i.p. daily, 2 wk), the OVX + SIN group (with OVX + 50 mg/kg/d SIN i.p., daily, 2 wk), and the OVX + SIN + 3-MA group (with OVX + 50 mg/kg/d SIN i.p., daily, 2 wk + 15 mg/kg/d 3-MA i.p., daily, 2 wk). The intervention concentrations and doses of the drugs used were selected according to previous literature reports[16,18-20]. The experimental animals were anaesthetized with sodium pentobarbital and subjected to sham or ovariectomy surgery, and pharmacological interventions were performed 8 wk after modelling. Mice were kept in a controlled environment maintained at 20-25 °C with a relative humidity range of 40%-70%. At 12 wk after the surgery, all the mice were sacrificed. Left femur samples were obtained for micron-scale computed tomography (micro-CT) and histological assessments; right femur samples were obtained for western blotting; and heart, liver, spleen, lung, and kidney tissues were collected for drug toxicity analysis.
Mouse femur samples were scanned by micro-CT (Aartselaar, Belgium) after fixation in 10% neutral formalin for 24 h. Morphological analysis and 3D reconstruction were performed using NRecon software (SkyScan micro-CT, Aartselaar, Belgium). The following morphometric parameters were evaluated: Bone mineral density (BMD, g/cm3), bone volume (BV, mm3), percent bone volume (BV/TV, %), bone surface/BV ratio (BS/BV, 1/mm), bone surface density (BS/TV, 1/mm), trabecular number (Tb.N, 1/mm), trabecular thickness (Tb.Th, mm), and trabecular separation (Tb.Sp, mm).
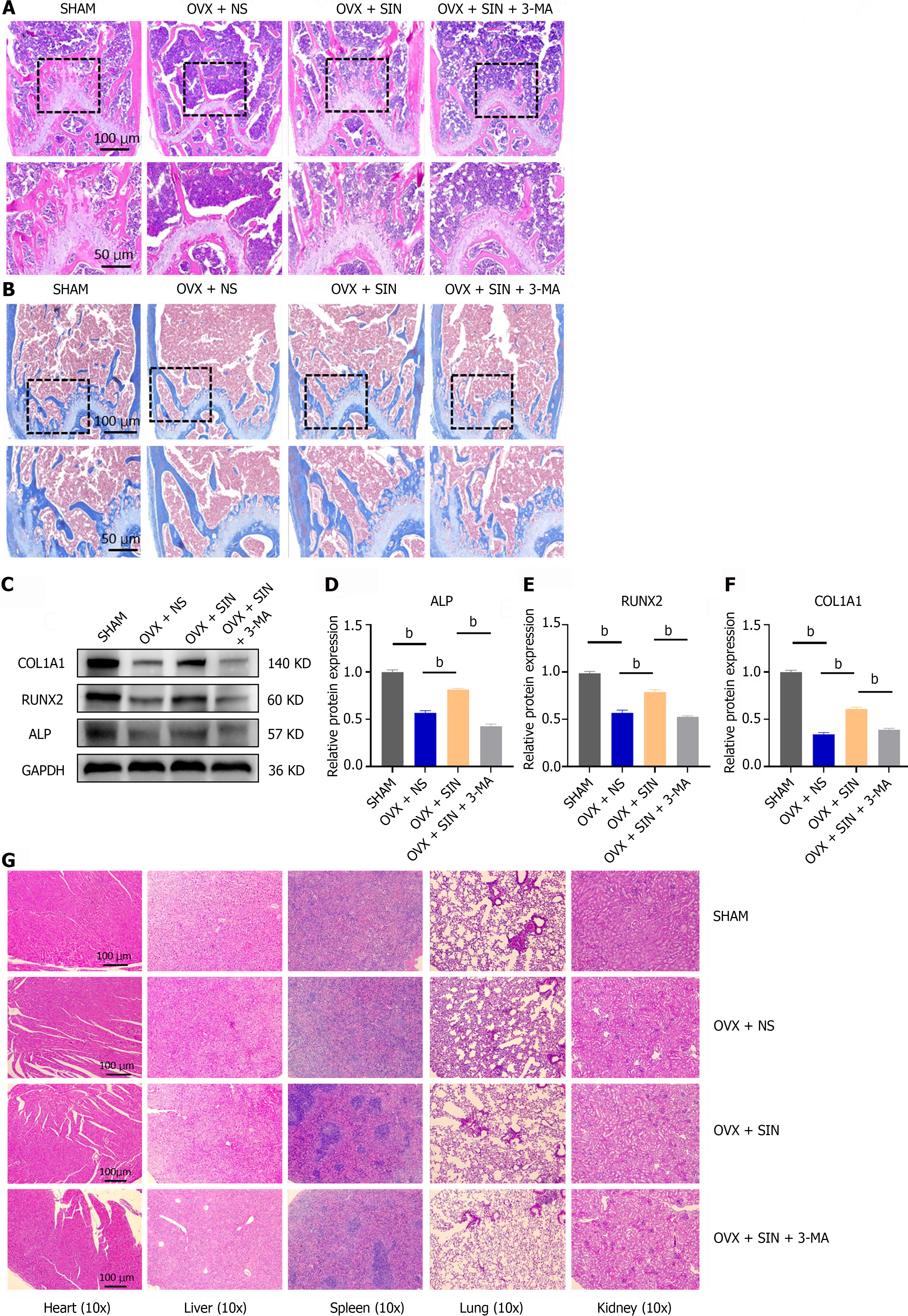
Femoral samples for hematoxylin and eosin (H&E) staining and Masson staining were prefixed in 10% neutral formalin for 24 h and vibrationally decalcified in at least five times as much 10% ethylenediaminetetraacetic acid (EDTA, Sigma-Aldrich, V900081-500 G) as bone tissue for 2 wk at room temperature. The samples were embedded in paraffin, sectioned, and subjected to H&E staining and Masson staining to observe morphological changes.
Femoral and tibial BMSCs were extracted from C57BL/6 mice that underwent sham surgery and ovariectomy 6 wk after surgery, according to our method reported in previous literature[21]. Briefly, the femurs and tibiae of mice were removed under aseptic conditions, the bone marrow was flushed with culture medium three to five times, and the extracted bone marrow suspension was centrifuged and lysed for erythrocytes with erythrocyte lysis buffer (Beyotime, C3702). After centrifugation, the cells at the bottom of the centrifuge tube were resuspended in α-MEM supplemented with 10% FBS and plated in cell culture dishes. When the confluence of BMSCs in the cell culture dish reached 80%, cell passaging was performed, and the P3 generation of cells was used for autophagic detection or osteogenic induction. The α-MEM used for osteogenic induction was composed of 10% FBS, 10 mmol/L β-glycerophosphate (Sigma, G9422), 0.5 mmol/L vitamin C (Sigma, V047) and 0.1 μM dexamethasone (Sigma, D4902).
The effect of SIN on the proliferation and viability of BMSCs was assessed by Cell Counting Kit-8 (CCK-8, C0037, Beyotime) assay according to the manufacturer’s protocol. BMSCs were initially cultured in 96-well plates at a density of 5000 cells per well, and SIN at various concentrations (0, 0.125, 0.25, 0.5, 1, and 2 mmol/L) was then added. The cells were cultivated for 12 h, 24 h, 48 h and 72 h. After this incubation period, the cells were washed and then incubated with 100 μL of α-MEM containing 10 μL of CCK-8 solution for a minimum of 1 h at 37 °C. Finally, absorbance measurements were taken at a wavelength of 450 nm.
Proteins were obtained from cellular or tissue sources after lysis with radioimmunoprecipitation assay buffer at 4 °C for 20 min, followed by centrifugation at 17700 rpm for 20 min (Sorvall Legend Micro 21R, Thermo Scientific, Germany), yielding the supernatant fraction. After the quantification and standardization of protein concentrations within each experimental group, 20 μg of protein was resolved via sodium-dodecyl sulfate gel electrophoresis (NCM Biotechnology, Inc.) and subsequently transferred to a polyvinylidene fluoride membrane (Bio-Rad Laboratories). The membranes were subsequently blocked with 5% skim milk powder and incubated with primary antibodies, which included antibodies targeting ALP, Runx2, COL1A1, BECN1, SQSTM, LC3B, AKT, p-AKT, mammalian target of the rapamycin (mTOR), p-mTOR, and phosphatidylinositol 3-kinase (PI3K), overnight at 4 °C. After three washes with western blot wash buffer, a secondary antibody was applied at room temperature for one hour. The protein bands were visualized through enhanced chemiluminescence (ECL, GE Healthcare), and their grayscale intensities were analysed using Image Lab 3.0 software.
Total RNA was isolated from the samples using TRIzol reagent (Beyotime, R0011), and the RNA concentration was assessed with a NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific). Subsequently, 2 μg of RNA was utilized for reverse transcription, generating complementary DNA (cDNA) based on the specific RNA concentration for each experimental group. cDNA amplification was performed in a 96-well plate with a 10 μL reaction volume, comprising 5 μL of qPCR MasterMix (Vazyme, Q712-02), 0.2 μL of forward primers ,0.2 μL of reverse primers, 2.6 μL of enzyme-free water, and 2 μL of cDNA per well. Amplification protocols were established in a CFX96™ thermocycler (Bio-Rad Laboratories) following the manufacturer’s guidelines. Four replicates were analysed for each sample, and the fold change in mRNA expression was determined using the 2-ΔΔCt method. The primers used in this study are detailed in Supplementary Table 2. Relative mRNA expression levels were quantified using the 2-ΔΔCt method with normalization to the positive control expression.
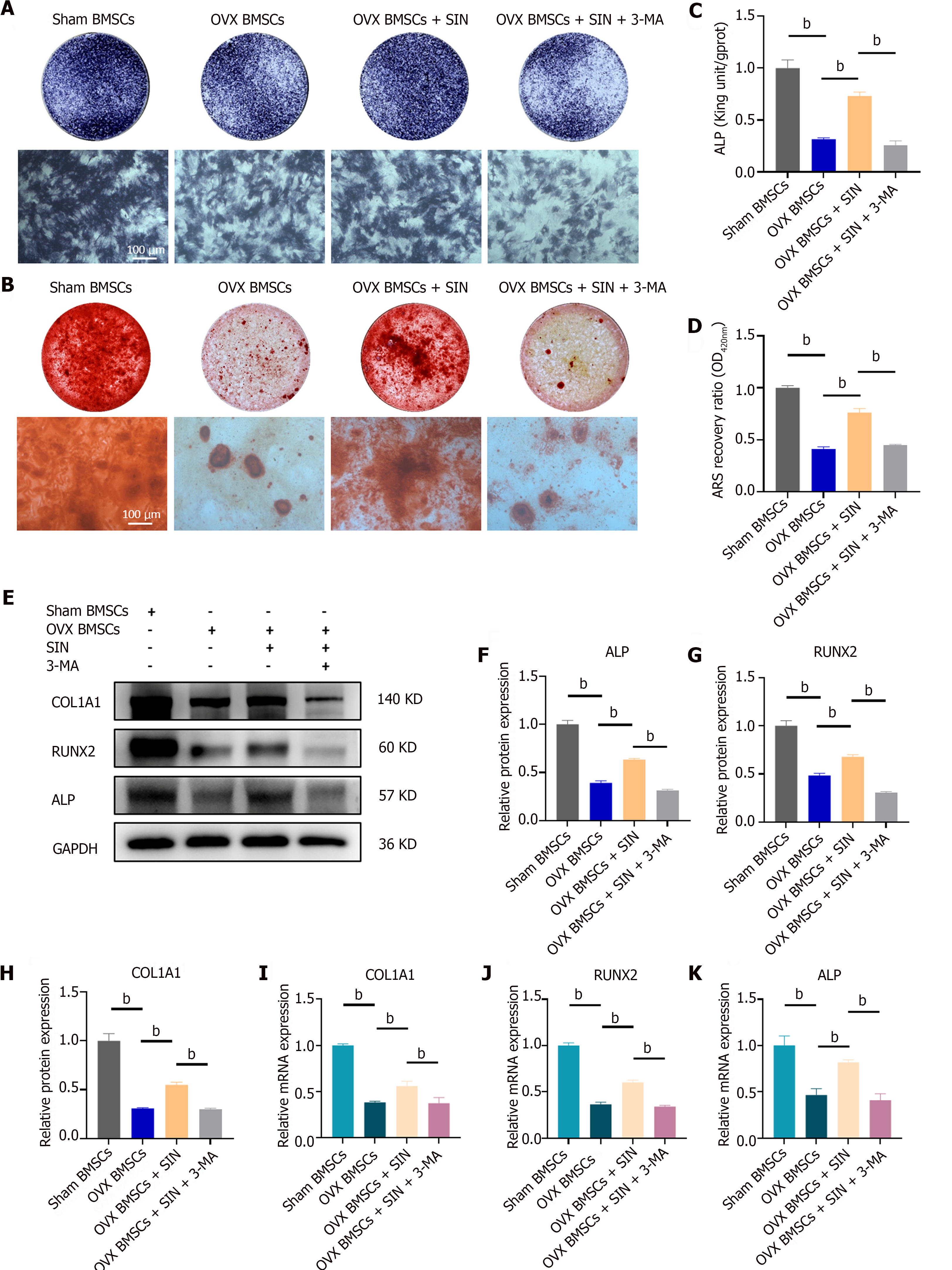
The cells were cultivated in 24-well plates and subjected to 7 d of incubation in osteogenic medium. ALP staining was performed in accordance with the guidelines provided by the ALP staining kit (Beyotime, P0321S). Initial fixation involved treatment with 4% paraformaldehyde at 4 °C for 30 min. Subsequently, 300 μL of BCIP/NBT working solution was added to each well, followed by a 1-h incubation at 37 °C in the dark. Finally, ALP activity was assessed using an alkaline phosphatase quantification kit (Jiancheng Bioengineering Institute, Nanjing, China).
BMSCs for Alizarin red S (ARS) staining were cultured for 21 d in osteogenic medium. The cells were rinsed with phosphate buffered saline and fixed for 20 min with 4% paraformaldehyde. Then, the cells were incubated in the dark with 0.1% ARS staining solution (pH = 4.1) for 20 min. Before imaging, the cells were rinsed with ddH2O until the excess staining solution was removed. ARS staining was quantified as follows: Calcium nodules were lysed in a 5% perchloric acid solution for half an hour at 37 °C, and the optical density was determined using an enzyme meter set at 420 nm (BioTek Instruments, lnc.).
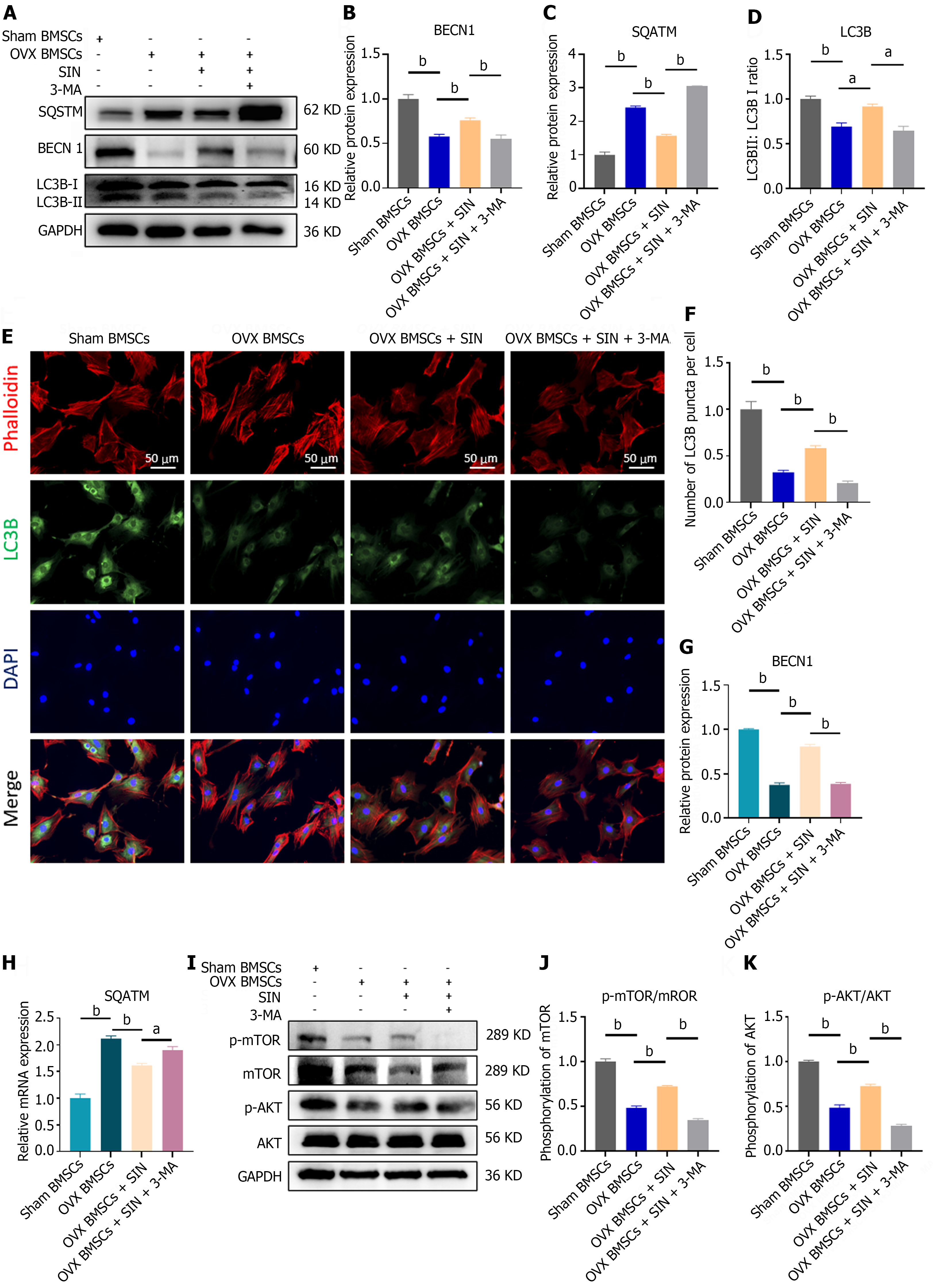
BMSCs were seeded on coverslips in a 24-well plate (5000 cells per well), and after intervention, the cells were fixed with 4% paraformaldehyde and permeabilized with 0.2% Triton X-100 (Beyotime) for 15 min. Then, the coverslips were sealed with goat serum blocking buffer for 60 min on ice. Next, a primary antibody, LC3B (1:200), was added to each well and incubated at 4 °C overnight. Then, the cells were rinsed and incubated for 60 min in the dark with the appropriate fluorescent secondary antibody [Alexa Fluor® 488 (Abcam, ab150077)] and phalloidin-iFluor 680 (Abcam, ab176760). The nuclei were subsequently redyed with DAPI for 10 min. Then, 10 μL of anti-fluorescence quencher (Beyotime, P0126) was added to the microscope slides, and the cell slides were inverted and placed under an inverted fluorescence microscope (Zeiss, Germany) for observation. Quantification of fluorescence intensity was performed using ImageJ software.
Values are represented as the means ± SD and were assessed via one-way ANOVA of groups by SPSS Version 20. Statistical significance was defined as P < 0.05.
To investigate the ability of SIN to ameliorate ovariectomy-induced bone loss and the ability to promote osteogenesis in vivo, we intraperitoneally injected mice with SIN and 3-MA for two weeks starting eight weeks of ovariectomy (Figure 1A). Quantitative analysis of bone parameters by micro-CT revealed a decrease in BMD, BV, BV/TV, Tb.N, and Tb.Th in the OVX mice compared to the sham-treated mice, while BS/BV, BS/TV and Tb.Sp increased. The changes in these parameters were reversed after SIN treatment; however, blockade of autophagy with 3-MA prevented the amelioration of bone loss in mice (Figure 1B-J).
Histological staining of mouse femur tissue sections was performed by H&E staining and Masson staining to further assess the microstructure of the bone tissues. Compared with the mice in the OVX group, the SIN-treated OVX mice showed a significant amelioration in the osteoporotic phenotype, as demonstrated by both H&E staining and Masson staining, whereas intervention with an autophagy inhibitor abrogated this improvement in bone turnover by SIN (Figure 2A and B). These results were further validated by western blot analysis of osteogenic markers in bone tissues (Figure 2C-F). In addition, H&E staining of visceral sections showed no notable organ toxicity for either SIN or 3-MA (Figure 2G). Overall, these results indicate that in OVX mice, SIN at a safe dose significantly ameliorates bone loss induced by ovariectomy and, in particular, significantly increases the formation of neoplastic bone trabeculae, suggesting that this increase in bone formation is mainly due to an increase in the osteogenic differentiation capacity of osteoblasts.
The chemical structure of cycloheximide is shown in Supplementary Figure 1A. According to previous reports, the effects of different concentrations of SIN on the proliferative activity of BMSCs at 12, 24, 48, and 72 h were determined by CCK-8 assays, and concentrations of SIN lower than 1.0 mmol/L did not have a significant effect on the proliferative activity of BMSCs. However, 0.5 mmol/L SIN slightly promoted the proliferation of BMSCs (Supplementary Figure 1B-E). Therefore, to investigate the effect of SIN on the osteogenic differentiation of BMSCs in vitro, we selected 0.5 mmol/L SIN as the intervention concentration.
We assessed the ALP activity and bone mineralization capacity of BMSCs under SIN and 3-MA intervention by alkaline phosphatase staining (day 7) and alizarin red staining (day 21). The results showed that there was a significant decrease in the ALP activity and bone mineralization capacity of the BMSCs in the OVX group compared to the BMSCs in the sham group and that SIN partially reversed the decrease in ALP activity and the reduction in of the bone mineralization capacity of BMSCs caused by ovariectomy, however, the osteogenesis promoting effect was reversed by the addition of the autophagy blocker 3-MA to the osteogenic medium (Figure 3A-D). Subsequently, we examined the effect of SIN on the osteogenic differentiation ability of BMSCs at the molecular level. Western blot results showed that five days after osteogenic induction, the levels of the nuclear transcription factor Runx2 and the osteogenic markers COL1A1 and ALP were significantly decreased in BMSCs from the OVX group compared with BMSCs of the sham group, whereas SIN treatment significantly upregulated the expression of these osteogenic markers; similarly, the addition of 3-MA reversed the changes in the expression of these osteogenic markers (Figure 3E-H). In addition, the quantitative real-time polymerase chain reaction results were consistent with the western blot results showing that SIN intervention increased the expression of the osteogenic marker genes COL1A1, ALP, and Runx2 and this effect was also reversed by the autophagy blocker (Figure 3I-K). All these results verified that 0.5 mmol/L SIN partially reversed the decrease in the osteogenic differentiation ability of BMSCs caused by ovariectomy; however, this osteogenesis-promoting effect was reversed by 3-MA.
The present research revealed that BMSCs extracted from the bone marrow of OVX mice showed significantly decreased expression of both LC3 II and BECN1, which are typical markers of autophagic flux, while the expression of SQSTM, which has an overall negative correlation with autophagic activity in the cell, increased (Figure 4A-D, G and H). Moreover, immunofluorescence assays revealed a decrease in the intensity of LC3B puncta (green) in the BMSCs from the OVX group (Figure 4E and F). These results indicated that the autophagic activity of BMSCs was inhibited by ovariectomy. Interestingly, the expression levels of the autophagy markers LC3 II and BECN1 both increased after the addition of SIN in the OVX-BMSC group, and SQSTM appeared to decrease, accompanied by an increase in the intensity of LC3B green dotted fluorescence, suggesting that SIN may increase autophagic flux in OVX-BMSCs through an unknown pathway. To further validate the autophagy-promoting effect of SIN on BMSCs, we assessed the autophagic flux in BMSCs and found that it was significantly reduced by the pre-addition of 3-MA to the SIN- treated OVX-BMSCs (Figure 4A-H).
To explore the specific mechanisms of SIN-induced autophagy, we performed further western blot assays, which revealed that SIN significantly inhibited the phosphorylation of AKT and mTOR, key signaling molecules in the classic autophagy pathway PI3K/AKT/mTOR, whereas the reduction in the phosphorylation level and activity of mTOR increased the downstream autophagic flux; in contrast, the phosphorylation levels of AKT and mTOR were increased after 3-MA pretreatment (Figure 4I-K). These results further validate that SIN is important in decreasing the phosphorylation levels of AKT and mTOR and increasing the autophagic activity of BMSCs and that this promotion of autophagy can be reversed by the autophagy inhibitor 3-MA.
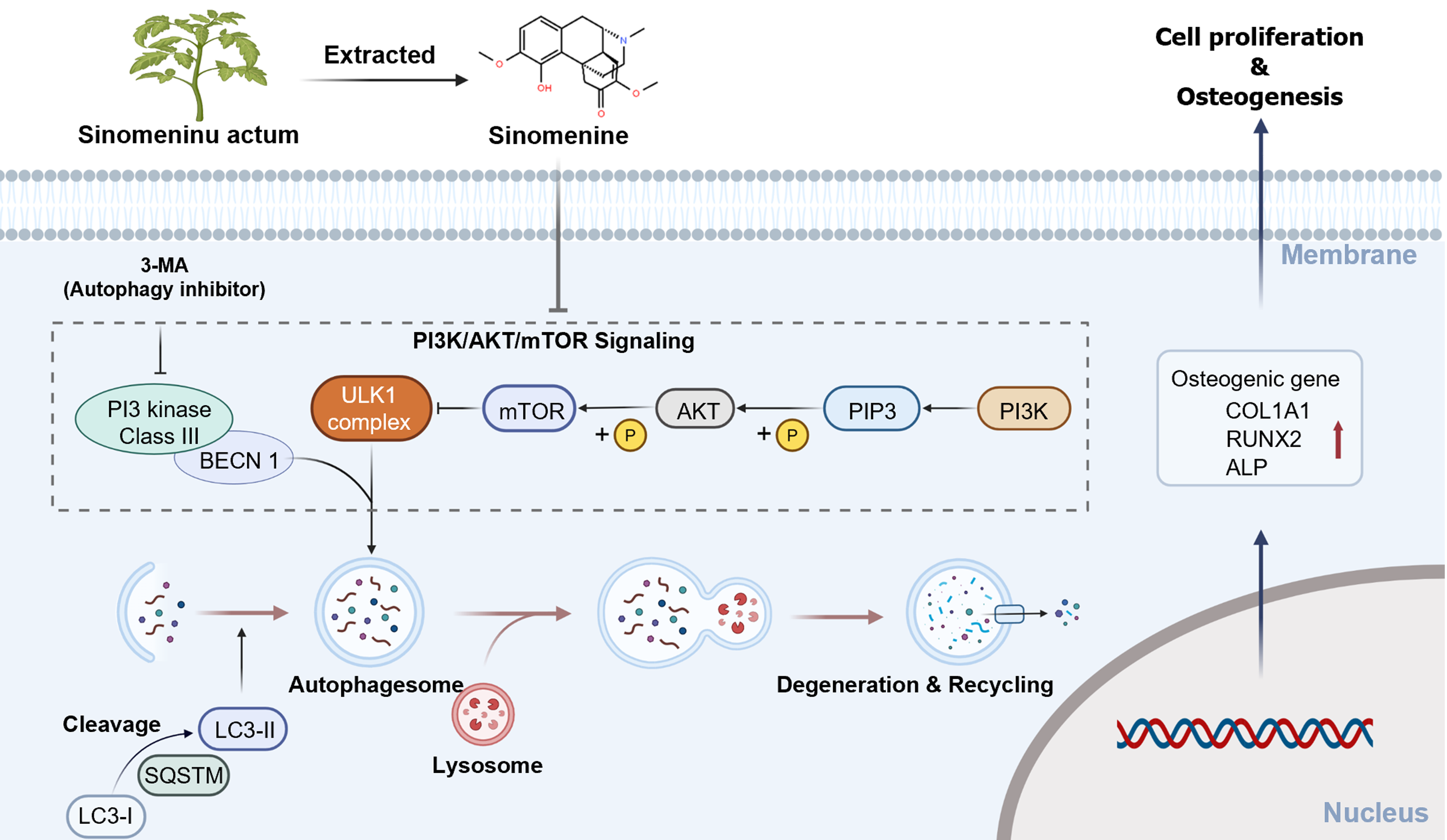
The present study demonstrated that SIN reduces the levels of AKT and mTOR phosphorylation in the PI3K/AKT/mTOR signaling pathway, inhibits mTOR activity, and increases the autophagic ability of BMSCs, thereby promoting the osteogenic differentiation of BMSCs and effectively alleviating bone loss in OVX mice in vivo (Figure 5), This study provides a reference for the use of the traditional Chinese medicine SIN in the treatment of various types of osteoporosis, imbalances in bone homeostasis, and autophagy-related diseases.
Osteoporosis is one of the most common metabolic diseases caused by an imbalance of bone homeostasis in the field of orthopedics[2], and its harmful effects are mainly fragility fractures due to bone loss and destruction of the bone microstructure, which have a high rate of disability and lethality[4]. Disruption of the balance between the low differentiation of osteoblasts and overactive osteoclasts is the intrinsic mechanism leading to the loss of bone[22], so increases in the osteogenic differentiation of bone stem cells and pre-osteoblast cell lineages or the suppression of overactive osteoclast activities are regarded as the two main strategies for the treatment of osteoporosis[3]. Currently, the most representative drug for the treatment of osteoporosis, bisphosphonates, exert anti-osteoporotic effects by inhibiting the differentiation and activity of osteoclasts; however, these drugs have pronounced adverse effects, such as digestive reactions and an increased risk of atypical proximal femur fracture[23]. These complications limit the therapeutic value of bisphosphonates in osteoporosis treatment. Hence, in recent years, osteoporosis treatment strategies that target osteoblasts and promote osteogenic differentiation have become a hot research topic[24].
BMSCs are considered to be important cells in the field of bone science research and in the field of tissue engineering research due to their easy accessibility and multiple differentiation potential. In this study, mouse BMSCs were selected as seed cells and cultured for osteogenic induction by extracting mouse BMSCs from the sham group and the OVX group. Under SIN intervention conditions, osteogenic markers were assessed by an ALP activity assay, ARS staining, western blotting and real-time polymerase chain reaction, and SIN was shown to partially alleviate the low osteogenic differentiation capacity of OVX-BMSCs.
SIN, the active ingredient in the traditional Chinese medicine S. acutum, has been used in China for thousands of years to treat rheumatic diseases. Its advantages include easy availability, low cost, and few side effects on the digestive system. In recent years, because of its pharmacological anti-inflammatory, antioxidant, anti-rheumatic, anti-angiogenic, antitumor, and immunosuppressive properties, SIN has been used to treat osteoarthritis[16], rheumatoid arthritis[25], central nervous system disorders[26], and tumors[27,28]. However, very few studies have been reported on SIN in the field of bone metabolic disease treatment. Zhang et al[29] reported that SIN decreased osteoclast differentiation or activity and reduced bone destruction in breast cancer patients. In a study on the treatment of orthodontic tooth movement and root resorption, Li et al[17] reported that SIN promoted the osteogenic differentiation of PDLSCs in vitro, but the study failed to further elucidate the specific mechanism by which SIN promotes the osteogenic differentiation of PDLSCs. However, these studies provide useful insight into SIN as a therapeutic option for osteoporosis.
The present study revealed that the SIN-induced increase in autophagy plays an important role in the osteogenic differentiation of BMSCs. As previously reported in the literature, the levels of autophagy and osteogenesis are correlated in BMSCs. Mechanistically, autophagy can eliminate unwanted and harmful components of the cell, such as some lipid components and peroxide components, and reduce lipotoxicity and oxidative stress damage to the cell[30,31]. However, the formation of a large number of autophagosomes results in a sudden increase in energy demand during the osteogenic differentiation of the cell[32], as cellular energy metabolism is known to be closely related to autophagy. In fact, we found that changes in the ADP/ATP ratio were negatively correlated with the strength of autophagy through the detection of the cellular metabolic state after the OVX-BMSC line was treated with SIN and 3-MA (Supplementary Figure 1F), and this evidence is consistent with our findings.
The PI3K/AKT/mTOR, a typical signaling pathway regulating upstream autophagy, has been reported to be involved in the regulation of autophagy in melanoma[33], renal carcinoma[34], and central nervous system tumors[28] upon SIN intervention and to influence the biological behaviors of tumors, but the effects of SIN on the PI3K/AKT/mTOR signaling pathway and autophagy in the field of bone metabolism have not been reported. As a regulator of the autophagy axis, mTOR affects the formation of downstream autophagosomes[35]. In our study, we found that SIN significantly decreased the phosphorylation of AKT and mTOR, the key molecules in the PI3K/AKT/mTOR signaling pathway, leading to a reduction in the active form of mTOR, which reversed the inhibitory effect on the formation of downstream autophagosomes. The levels of BECN1 and the ratio of LC3B II/LC3B I, which are indicators of autophagic flux[9], were significantly increased by SIN intervention. Interestingly, pretreatment of BMSCs with the typical autophagy inhibitor 3-MA prior to SIN intervention resulted in a partial increase in the levels of phosphorylated AKT and mTOR, and a concomitant decrease in autophagic flux. More importantly, the level of osteogenic differentiation in BMSCs was consistent with the level of autophagy, i.e., osteogenic differentiation was promoted when autophagy was increased and inhibited when autophagy was decreased. These results suggest that SIN increases autophagy and promotes the osteogenic differentiation of BMSCs through the classic autophagic regulatory axis PI3K/AKT/mTOR.
The in vivo results of this study revealed that SIN was effective at alleviating ovariectomy-induced bone loss in mice; notably, the autophagy inhibitor 3-MA limited the ameliorative effect of SIN on bone loss, and these results are consistent with the in vitro findings. As a classic model for studying osteoporosis, bilateral ovariectomy is unequivocally effective, easy to perform, and reproducible and is able to better simulate the physiological phenomenon of postmenopausal estrogen withdrawal and bone loss[36]. In the present study, 8 wk after the mice received the ovariectomy surgery and successful osteoporosis modelling, a considerable degree of bone loss was detected by examination of the lower femur in the model group; in contrast, bone loss was significantly ameliorated with SIN treatment. To the best of our knowledge, our study is the first attempt to utilize SIN for the in vivo treatment of osteoporosis. Liao et al[16] applied SIN to treat of arthritis in mice and reported that SIN significantly alleviated arthritis-induced bone destruction in mice, mechanistically, the activation of the Keap1-Nrf2 signaling pathway by the phosphorylation of p62, an important substrate of SIN-induced autophagy, is a key part of this process. Liao et al[16] did not explicitly indicate the role that autophagy plays in this process, but these researchers did find that SIN improved localized bone structure in arthritic mice, and these findings are consistent with our results. Our study focused on the role of SIN in increasing autophagy and promoting osteogenesis and revealed a significant increase in autophagic and osteogenic indices after SIN intervention. In addition, Masson staining showed increased formation of neoplastic bone trabeculae. Together, these results suggest that the ameliorative effect on bone loss in OVX mice is mainly due to increased osteoblast activity.
The limitations of our study are as follows: Our in vitro study revealed that the activation of autophagy and the maintenance of autophagic capacity are critical for BMSCs to maintain their activity and increase osteogenic differentiation; however, the extent to which autophagy is carried out to sustain beneficial cellular autophagy cannot be defined at present. In fact, whether cellular autophagy is beneficial or harmful has been controversial, depending on the particular situation. In the field of oncology, the activation of autophagy and autophagy-induced apoptosis seems to be a potential means of tumor therapy, and based on the results of our study, we believe that, to a certain extent or to a certain extent limited to a specific cell type, the increase in autophagic capacity is beneficial to the promotion of cellular viability and maintain the ability to differentiate into osteoblasts. Furthermore, in vivo, although we detected enhanced osteoclast activity, based on the present findings, we could not determine to what extent the restriction of osteoclasts by SIN played a role in the amelioration of bone loss. An in vitro study by Zhou et al[15] demonstrated that SIN was able to have a degree of limiting effect on osteoclast differentiation; however, the current reports, including the study by Zhou et al[15], did not determine osteoclast activity after SIN intervention in an osteoporosis model. Based on our current findings, in the future, we may further clarify the relationship between the effects of SIN on osteoclasts and bone loss through in vitro coculture of osteoblasts and osteoclasts and in vivo assays of osteoclast indicators.
SIN ameliorates adaptive autophagy and promotes the osteogenic differentiation of BMSCs, which is a potential option for the treatment of postmenopausal osteoporosis, as well as other types of osteoporosis and autophagy-related diseases.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Cell and tissue engineering
Country/Territory of origin: China
Peer-review report’s classification
Scientific Quality: Grade B, Grade B, Grade B
Novelty: Grade B
Creativity or Innovation: Grade B
Scientific Significance: Grade B
P-Reviewer: Li SC, United States; Lin SR, Taiwan S-Editor: Wang JJ L-Editor: A P-Editor: Cai YX
| 1. | Tuckermann J, Adams RH. The endothelium-bone axis in development, homeostasis and bone and joint disease. Nat Rev Rheumatol. 2021;17:608-620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 127] [Article Influence: 31.8] [Reference Citation Analysis (0)] |
| 2. | Compston JE, McClung MR, Leslie WD. Osteoporosis. Lancet. 2019;393:364-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 866] [Cited by in RCA: 1472] [Article Influence: 245.3] [Reference Citation Analysis (0)] |
| 3. | Reid IR, Billington EO. Drug therapy for osteoporosis in older adults. Lancet. 2022;399:1080-1092. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 375] [Article Influence: 125.0] [Reference Citation Analysis (2)] |
| 4. | Black DM, Rosen CJ. Clinical Practice. Postmenopausal Osteoporosis. N Engl J Med. 2016;374:254-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 818] [Cited by in RCA: 1029] [Article Influence: 114.3] [Reference Citation Analysis (0)] |
| 5. | Lane NE. Epidemiology, etiology, and diagnosis of osteoporosis. Am J Obstet Gynecol. 2006;194:S3-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 501] [Cited by in RCA: 779] [Article Influence: 41.0] [Reference Citation Analysis (0)] |
| 6. | Miura M, Miura Y, Sonoyama W, Yamaza T, Gronthos S, Shi S. Bone marrow-derived mesenchymal stem cells for regenerative medicine in craniofacial region. Oral Dis. 2006;12:514-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 57] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 7. | Xu X, Zhao L, Terry PD, Chen J. Reciprocal Effect of Environmental Stimuli to Regulate the Adipogenesis and Osteogenesis Fate Decision in Bone Marrow-Derived Mesenchymal Stem Cells (BM-MSCs). Cells. 2023;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 13] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 8. | Ma Y, Qi M, An Y, Zhang L, Yang R, Doro DH, Liu W, Jin Y. Autophagy controls mesenchymal stem cell properties and senescence during bone aging. Aging Cell. 2018;17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 162] [Cited by in RCA: 257] [Article Influence: 36.7] [Reference Citation Analysis (0)] |
| 9. | Kumariya S, Ubba V, Jha RK, Gayen JR. Autophagy in ovary and polycystic ovary syndrome: role, dispute and future perspective. Autophagy. 2021;17:2706-2733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 173] [Article Influence: 43.3] [Reference Citation Analysis (0)] |
| 10. | Qi M, Zhang L, Ma Y, Shuai Y, Li L, Luo K, Liu W, Jin Y. Autophagy Maintains the Function of Bone Marrow Mesenchymal Stem Cells to Prevent Estrogen Deficiency-Induced Osteoporosis. Theranostics. 2017;7:4498-4516. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 103] [Cited by in RCA: 139] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 11. | Yamasaki H. Pharmacology of sinomenine, an anti-rheumatic alkaloid from Sinomenium acutum. Acta Med Okayama. 1976;30:1-20. [PubMed] |
| 12. | Wang S, Zhang L, Zhou Y, Liu Z, Zhou Z, Huang J. A review on pharmacokinetics of sinomenine and its anti-inflammatory and immunomodulatory effects. Int Immunopharmacol. 2023;119:110227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 13. | Li X, He L, Hu Y, Duan H, Li X, Tan S, Zou M, Gu C, Zeng X, Yu L, Xu J, Liu S. Sinomenine suppresses osteoclast formation and Mycobacterium tuberculosis H37Ra-induced bone loss by modulating RANKL signaling pathways. PLoS One. 2013;8:e74274. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 14. | Lin Y, Yi O, Hu M, Hu S, Su Z, Liao J, Wang W, Wang S, Liu L, Liu B, Cai X. Multifunctional nanoparticles of sinomenine hydrochloride for treat-to-target therapy of rheumatoid arthritis via modulation of proinflammatory cytokines. J Control Release. 2022;348:42-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 48] [Reference Citation Analysis (0)] |
| 15. | Zhou B, Lu X, Tang Z, Liu D, Zhou Y, Zeng P, Xiong H. Influence of sinomenine upon mesenchymal stem cells in osteoclastogenesis. Biomed Pharmacother. 2017;90:835-841. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 16. | Liao K, Su X, Lei K, Liu Z, Lu L, Wu Q, Pan H, Huang Q, Zhao Y, Wang M, Cai J, Liu L, Li T. Sinomenine protects bone from destruction to ameliorate arthritis via activating p62(Thr269/Ser272)-Keap1-Nrf2 feedback loop. Biomed Pharmacother. 2021;135:111195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 17. | Li H, Li Y, Zou J, Yang Y, Han R, Zhang J. Sinomenine Inhibits Orthodontic Tooth Movement and Root Resorption in Rats and Enhances Osteogenic Differentiation of PDLSCs. Drug Des Devel Ther. 2022;16:2949-2965. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 18. | Tong B, Yu J, Wang T, Dou Y, Wu X, Kong L, Dai Y, Xia Y. Sinomenine suppresses collagen-induced arthritis by reciprocal modulation of regulatory T cells and Th17 cells in gut-associated lymphoid tissues. Mol Immunol. 2015;65:94-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 57] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 19. | Feng ZT, Yang T, Hou XQ, Wu HY, Feng JT, Ou BJ, Cai SJ, Li J, Mei ZG. Sinomenine mitigates collagen-induced arthritis mice by inhibiting angiogenesis. Biomed Pharmacother. 2019;113:108759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 66] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 20. | Jiang H, Lu Q, Xu J, Huo G, Cai Y, Geng S, Xu H, Zhang J, Li H, Yuan K, Huang G. Sinomenine ameliorates adjuvant-induced arthritis by inhibiting the autophagy/NETosis/inflammation axis. Sci Rep. 2023;13:3933. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 21. | Yang C, Tao H, Zhang H, Xia Y, Bai J, Ge G, Li W, Zhang W, Xiao L, Xu Y, Wang Z, Gu Y, Yang H, Liu Y, Geng D. TET2 regulates osteoclastogenesis by modulating autophagy in OVX-induced bone loss. Autophagy. 2022;18:2817-2829. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 55] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 22. | Rachner TD, Khosla S, Hofbauer LC. Osteoporosis: now and the future. Lancet. 2011;377:1276-1287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1770] [Cited by in RCA: 1905] [Article Influence: 136.1] [Reference Citation Analysis (0)] |
| 23. | Ensrud KE. Bisphosphonates for Postmenopausal Osteoporosis. JAMA. 2021;325:96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 51] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 24. | Hu L, Yin C, Zhao F, Ali A, Ma J, Qian A. Mesenchymal Stem Cells: Cell Fate Decision to Osteoblast or Adipocyte and Application in Osteoporosis Treatment. Int J Mol Sci. 2018;19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 159] [Cited by in RCA: 301] [Article Influence: 43.0] [Reference Citation Analysis (0)] |
| 25. | Liu W, Zhang Y, Zhu W, Ma C, Ruan J, Long H, Wang Y. Sinomenine Inhibits the Progression of Rheumatoid Arthritis by Regulating the Secretion of Inflammatory Cytokines and Monocyte/Macrophage Subsets. Front Immunol. 2018;9:2228. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 178] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 26. | Hong H, Lu X, Lu Q, Huang C, Cui Z. Potential therapeutic effects and pharmacological evidence of sinomenine in central nervous system disorders. Front Pharmacol. 2022;13:1015035. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 28] [Reference Citation Analysis (0)] |
| 27. | Jiang Y, Jiao Y, Liu Y, Zhang M, Wang Z, Li Y, Li T, Zhao X, Wang D. Sinomenine Hydrochloride Inhibits the Metastasis of Human Glioblastoma Cells by Suppressing the Expression of Matrix Metalloproteinase-2/-9 and Reversing the Endogenous and Exogenous Epithelial-Mesenchymal Transition. Int J Mol Sci. 2018;19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 42] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 28. | Zheng X, Li W, Xu H, Liu J, Ren L, Yang Y, Li S, Wang J, Ji T, Du G. Sinomenine ester derivative inhibits glioblastoma by inducing mitochondria-dependent apoptosis and autophagy by PI3K/AKT/mTOR and AMPK/mTOR pathway. Acta Pharm Sin B. 2021;11:3465-3480. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 82] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 29. | Zhang Y, Zou B, Tan Y, Su J, Wang Y, Xu J, Tao L, Zhou H, Liu L, Li X. Sinomenine inhibits osteolysis in breast cancer by reducing IL-8/CXCR1 and c-Fos/NFATc1 signaling. Pharmacol Res. 2019;142:140-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 30. | Liu F, Yuan Y, Bai L, Yuan L, Li L, Liu J, Chen Y, Lu Y, Cheng J, Zhang J. LRRc17 controls BMSC senescence via mitophagy and inhibits the therapeutic effect of BMSCs on ovariectomy-induced bone loss. Redox Biol. 2021;43:101963. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 97] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 31. | Zhu C, Shen S, Zhang S, Huang M, Zhang L, Chen X. Autophagy in Bone Remodeling: A Regulator of Oxidative Stress. Front Endocrinol (Lausanne). 2022;13:898634. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 59] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 32. | Wang J, Zhang Y, Cao J, Wang Y, Anwar N, Zhang Z, Zhang D, Ma Y, Xiao Y, Xiao L, Wang X. The role of autophagy in bone metabolism and clinical significance. Autophagy. 2023;19:2409-2427. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 143] [Reference Citation Analysis (0)] |
| 33. | Sun Z, Zheng L, Liu X, Xing W. Sinomenine inhibits the growth of melanoma by enhancement of autophagy via PI3K/AKT/mTOR inhibition. Drug Des Devel Ther. 2018;12:2413-2421. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 34. | Deng F, Ma YX, Liang L, Zhang P, Feng J. The pro-apoptosis effect of sinomenine in renal carcinoma via inducing autophagy through inactivating PI3K/AKT/mTOR pathway. Biomed Pharmacother. 2018;97:1269-1274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 51] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 35. | Kim YC, Guan KL. mTOR: a pharmacologic target for autophagy regulation. J Clin Invest. 2015;125:25-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1352] [Cited by in RCA: 1575] [Article Influence: 157.5] [Reference Citation Analysis (0)] |
| 36. | Wang Y, Ma Z, Zheng Y, Liu B, Bao P, Wu X, Yu C, Wen Z, Ma T, Liu J, Liu C, Ma D, Wu H, Li J, Yuan Y, Lu N, Zhao H, Li Y, Yang S, Zhang R, Dai J, Hu M. Establishment of an osteoporosis model in tree shrews by bilateral ovariectomy and comprehensive evaluation. Exp Ther Med. 2019;17:3644-3654. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |