Published online Jan 26, 2024. doi: 10.4252/wjsc.v16.i1.19
Peer-review started: October 23, 2023
First decision: November 13, 2023
Revised: November 30, 2023
Accepted: January 5, 2024
Article in press: January 5, 2024
Published online: January 26, 2024
Processing time: 90 Days and 12.5 Hours
Peripheral nerve injury can result in significant clinical complications that have uncertain prognoses. Currently, there is a lack of effective pharmacological interventions for nerve damage, despite the existence of several small compounds, peptides, hormones, and growth factors that have been suggested as potential enhancers of neuron regeneration. Despite the objective of achieving full functional restoration by surgical intervention, the persistent challenge of inadequate functional recovery remains a significant concern in the context of peripheral nerve injuries.
To examine the impact of exosomes on the process of functional recovery following a complete radial nerve damage.
A male individual, aged 24, who is right-hand dominant and an immigrant, arrived with an injury caused by a knife assault. The cut is located on the left arm, specifically below the elbow. The neurological examination and electrodiagnostic testing reveal evidence of left radial nerve damage. The sural autograft was utilized for repair, followed by the application of 1 mL of mesenchymal stem cell-derived exosome, comprising 5 billion microvesicles. This exosome was split into four equal volumes of 0.25 mL each and delivered microsurgically to both the proximal and distal stumps using the subepineural pathway. The patient was subjected to a period of 180 d during which they had neurological examination and electrodiagnostic testing.
The duration of the patient’s follow-up period was 180 d. An increasing Tinel’s sign and sensory-motor recovery were detected even at the 10th wk following nerve grafting. Upon the conclusion of the 6-mo post-treatment period, an evaluation was conducted to measure the extent of improvement in motor and sensory functions of the nerve. This assessment was based on the British Medical Research Council scale and the Mackinnon-Dellon scale. The results indicated that the level of improvement in motor function was classified as M5, denoting an excellent outcome. Additionally, the level of improvement in sensory function was classified as S3+, indicating a good outcome. It is noteworthy that these assessments were conducted in the absence of physical therapy. At the 10th wk post-injury, despite the persistence of substantial axonal damage, the nerve exhibited indications of nerve re-innervation as evidenced by control electromyography (EMG). In contrast to the preceding. EMG analysis revealed a significant electrophysiological enhancement in the EMG conducted at the 6th-mo follow-up, indicating ongoing regeneration.
Enhanced comprehension of the neurobiological ramifications associated with peripheral nerve damage, as well as the experimental and therapy approaches delineated in this investigation, holds the potential to catalyze future clinical progress.
Core Tip: Peripheral nerve damage can manifest in several contexts, including civil, military, or iatrogenic circumstances. Despite the advancements in microsurgical techniques in recent times, the treatment outcomes for peripheral nerve damage have not yet reached a desirable level. This study investigates the functional recovery of a patient who received a sural nerve transplant and exosome application to treat a whole radial nerve lesion caused by a knife assault. Stem cell-derived treatments, such as the use of exosomes, have the potential to provide a novel and promising outlook for the treatment of peripheral nerve injury.
- Citation: Civelek E, Kabatas S, Savrunlu EC, Diren F, Kaplan N, Ofluoğlu D, Karaöz E. Effects of exosomes from mesenchymal stem cells on functional recovery of a patient with total radial nerve injury: A pilot study. World J Stem Cells 2024; 16(1): 19-32
- URL: https://www.wjgnet.com/1948-0210/full/v16/i1/19.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v16.i1.19
Peripheral nerve injury (PNI) can manifest in several contexts, including both civilian and military settings, as well as iatrogenic harm resulting from surgical interventions. The hand plays a crucial role in several everyday tasks, hence any impairment in its functionality might lead to significant challenges in one’s daily life. Inadequate treatment modalities often lead to functional limitations, hence exerting adverse consequences on both familial units and broader societal structures. Sensory and motor dysfunction might potentially result in the full paralysis of a limb or the onset of unmanageable neuropathic pain.
According to a study, a significant proportion of nerve injury, namely up to 73.5%, is attributed to the upper extremities[1]. The radial nerve, an important peripheral nerve of the upper limb, plays a critical role in the motor function of the forearm, wrist, and fingers. The radial nerve is commonly categorized into four sections when discussing injuries: Infraclavicular, humeral shaft, from the lateral arm to the antebrachial fossa, and posterior interosseous nerve[2]. It was determined that, after an average follow-up duration of 21.5 mo, the results for injuries treated within five months of occurrence were more favorable in the distal subgroup compared to the proximal segment of the nerve. Furthermore, it has been asserted by Roganovic and Petkovic[3] that proximal radial nerve injuries provide more unfavorable results compared to intermediate and distal lesions.
The primary aim of nerve repair is to achieve reinnervation of the target organs through the guidance of regenerated sensory, motor, and autonomic axons, while minimizing the loss of fibers at the suture line. Currently, the ideal treatment strategy involves the utilization of tensionless epineurial sutures for end-to-end microsurgical repair. Autologous nerve transplantation is the recommended approach for reconstruction in cases when a nerve gap exists and direct end-to-end suturing is not feasible. Despite the presence of notable limitations, such as the morbidity associated with donor site and the limited length of graft material, other approaches including the use of natural or artificial conduits for tubulization operations are viable options for addressing small nerve deficits. Nevertheless, it is worth noting that nerve autografting remains the prevailing and most esteemed method for bridging nerve gaps at present.
The utilization of intraoperative nerve stimulation, the enhancement of motor nerve recovery, and the successful attainment of nerve exposure and mobilization are all advantageous elements of acute repair, often performed within a three-day timeframe. According to existing literature in the field of biochemistry, it has been documented that within a time frame of 72 h following an injury, nerve endings retain the presence of neurotransmitters[4]. From a histopathological standpoint, it can be observed that nerve endings, upon rapid transection, initially exhibit symmetrically aligned bundles of nerve fibers. However, as time progresses, the task of aligning these nerve ends becomes progressively more difficult due to the occurrence of Schwann cell (SC) proliferation, fibrosis, and angiogenesis at each respective end. One significant limitation associated with early nerve healing is the inability to accurately ascertain the specific site and extent of the lesion. The process of restoring nerve function following extended periods of time is sometimes referred to as delayed repair.
In their study, Shergill et al[5] observed that the outcomes were least favorable, with an overall failure rate of 42%, when dealing with uneven wounds and significant gaps in a cohort of 220 radial nerve gaps that underwent sural nerve grafting. Terzis and Konofaos[6] conducted a study in which they found that younger patients, those with denervation duration of three months or less, lesions in continuity, no accompanying nerve injuries, distal lesions, neurolysis, and nerve grafts of five centimeters or less in length, had improved functional results.
The presence of SCs represents a significant benefit in the context of autograft procedures. The generation of an optimal environment for axonal development is facilitated by the presence of live SCs and trophic substances within the graft. The aforementioned components, including SC basal laminae, neurotrophic factors, and adhesion molecules, together form a crucial scaffold. One advantage of an autogenous nerve transplant is its ability to avoid any immunoreaction. This is due to the graft’s absorbable and permeable nature, allowing it to directly interact with its surrounding environment. Additional options for treatment involve the utilization of synthetic nerve guiding conduits. However, due to their deficiency in biological and cellular assistance, it is more advantageous to prioritize the preservation of a working nerve. One potential strategy to overcome these limitations is to introduce SCs or exosomes into the conduits, since they have shown the ability to facilitate axon regeneration[7]. Although the use of tissue engineering techniques to generate artificial conduits has been demonstrated to be advantageous for PNI, the results are still far from ideal. Numerous natural (such as vein grafts) and synthetic (artificial) materials have been subjected to testing in both clinical and experimental settings[7].
In contrast to autografts, nerve allografts do not need a further incision and have the advantage of an unrestricted supply of nerve tissue for transplantation. Moreover, the injured nerve of the receiver might potentially be substituted by a nerve of the same kind obtained from the donor. An enhanced motor recovery can be achieved by replacing a mixed sensory-motor ulnar nerve with a comparable mixed-type ulnar nerve procured from a donor, as opposed to utilizing a sensory-only sural nerve transplant. Although allogenic nerve grafts possess a restricted ability to provoke an immune response, the utilization of immunosuppressive medication is necessary to avert graft rejection. In contrast to the central nervous system (CNS), the peripheral nervous system (PNS) has the capacity for regeneration following injury. In the PNS, SCs are responsible for the release of growth factors and the removal of debris.
The activation of macrophages and subsequent formation of a new medullary sheath is initiated by the presence of myelin and axonal debris. Nevertheless, achieving good outcomes poses a challenge due to factors such as sluggish neuron regeneration, Wallerian degeneration, tissue adhesion, and muscle atrophy. PNI results in the occurrence of Wallerian degeneration, a process characterized by the infiltration of macrophages into the damaged nerve on the third day post-trauma. These macrophages secrete substantial quantities of variables, including C-C motif ligand 2, tumor necrosis factor-α, interleukin (IL)-1α, and IL-1β[8].
The growth rate of regenerating axons is often limited to around 1 millimeter every day. The process of regeneration is facilitated by various mechanisms, which encompass mechanical components like Büngner’s cell bands, pathway-markers that are localized at the axons and SCs, chemical factors such as cytokines that have a more localized action, and growth factors like ciliary neurotrophic factor, epidermal growth factor, platelet-derived growth factor, transforming growth factor, vascular endothelial growth factor, and nerve growth factor (NGF) that have a more distant effect.
Autologous nerve transplantation remains the established benchmark in the treatment of peripheral nerve abnormalities; nonetheless, it is imperative to explore other approaches. The local administration of stem cells or exosomes has been shown to have the potential to augment axonal regeneration and promote the creation of myelin sheaths in the treatment of PNI. Several factors, such as fibroblast growth factor, NGF, ciliary neurotrophic factor, brain derived neurotrophic factor, and glial cell line-derived neurotropic factor, have been identified as potentially advantageous for promoting the survival of neural cells and facilitating nerve regeneration. These factors are released by stem cells during the process of tissue repair[9]. While stem cell-based therapies have shown beneficial effects on tissue regeneration, it has been noted that the fundamental mechanism responsible for stem cell-mediated tissue healing is paracrine signaling rather than stem cell differentiation[10]. There exists a considerable amount of empirical data indicating that exosomes, with a notable capacity to serve as an innovative alternative to whole cell treatment, are capable of facilitating the paracrine activity of stem cells[11]. Moreover, it has been shown that the utilization of exosomes is comparatively safer in comparison to stem cell therapy. The administration of some interventions has the potential to overcome cellular immune rejection and carcinogenic mutations[12].
In a recent study, it was shown that SCs have the ability to produce exosomes that can promote the regeneration of axons. This effect was observed both in laboratory settings (in vitro) and in living organisms (in vivo)[13]. The internalization of SC exosomes by peripheral nerve axons suggests a probable specificity of their payload in relation to the development, protection, or regeneration of the PNS. According to a study conducted by Kingham et al[14], it was shown that adipose-derived stem cells produce exosomes that have resemblance to SCs. These exosomes contain identical cargo and have the ability to promote the rebuilding of axons.
Mesenchymal stem cells (MSCs) are a type of stem cell that possess multipotent capabilities and are obtained from various mesenchymal tissues such as bone marrow, adipose tissue, dental pulp, umbilical cord blood, and others. Previous studies have demonstrated that multipotent MSCs have the potential to significantly improve functional recovery following nerve damage[15]. A recent study has provided evidence that MSCs release exosomes, which play a significant role in intercellular communication and the maintenance of dynamic and balanced microenvironments necessary for tissue repair[16]. Exosomes, which measure between 40 and 100 nm, are the most diminutive membranous vesicles. Different types of cells, including neurons, tumor cells, and kidney cells, secrete nanovesicles. These nanovesicles may be detected in a range of bodily fluids, such as urine, amniotic fluid, malignant ascites, bronchoalveolar lavage fluid, synovial fluid, breast milk, saliva, blood, and cerebrospinal fluid. Exosomes exhibit variations in their protein, lipid, noncoding RNA, mRNA, and microRNA (miRNA) composition, together referred to as “cargo” contents, depending on their parental origin. These cargo contents are then transported to adjacent cells or sent to cells located at a distance. Remarkably, recent research has shown that a multitude of cells inside the nervous system have the ability to produce exosomes, which are extracellular membrane vesicles. This observation indicates their active participation in the operation, growth, and disorders of this particular system. Recent studies have provided evidence for the importance of miRNAs in exosomes as mediators of intracellular communication between donor and recipient cells[17]. The ability of these entities to traverse the blood-brain barrier has several prospects in the field of neuroprotection. This phenomenon is evidenced by their active participation in the process of neuronal repair and the restoration of peripheral nerves[18].
Exosomes generated by MSCs have the capacity to activate phosphatidylinositol 3-kinase/protein kinase B, extra
Previous studies have demonstrated the indispensability of heat shock protein 70 in providing metabolic support and safeguarding neurons[24]. The protein galectin-3, which is associated with the phagocytosis of myelin, has been detected in exosomes and has been shown to be increased by SCs following nerve injury[13]. In their study, Krämer-Albers et al[25] documented the presence of myelin proteins, such as myelin-associated glycoprotein and proteolipid protein, within exosomes. The authors emphasized the significant contribution of these proteins in the process of nerve remyelination.
The denervation of SCs leads to alterations in the synthesis of several substances, resulting in both an increase and reduction in their production. This procedure facilitates the shift of SCs from a phenotype characterized by proliferation and myelinization to a regenerative phenotype within the initial 24-h period. A delicate equilibrium exists between degenerative and regenerative mechanisms. The presence of exosomes harboring diverse compounds is likely to expedite the process of regeneration.
According to research findings, exosomes have been observed to possess significant amounts of IL-6, IL-8, and several other cytokines[10]. The aforementioned results together indicate that exosomes include a diverse array of components that play a vital role in the regeneration and remodeling of the nervous system. Exosomes are also shown to possess DNA, however its specific function is yet to be determined[26]. Exosomes participate in cellular communication, contribute to the presentation of antigens by immune cells, and exhibit either pro-inflammatory or anti-inflammatory properties[27].
Maintaining vascular integrity is crucial for the maintenance of the milieu of the nervous system, ensuring its homeostasis and facilitating the processes of nerve system healing, development, and optimal functioning. The use of exosomes originating from MSCs has been shown to have a positive impact on the restoration of function following nerve injury in rats. This is achieved through the activation of the body’s own processes of angiogenesis and neurogenesis, as supported by previous research[28]. Additionally, these entities may possess clinical therapeutic potential and function as paracrine agents that stimulate the growth of new blood vessels[29]. This study proposes that exosome-mediated intercellular communication within the nervous system facilitates the activation of angiogenesis by neurons and MSCs, therefore establishing exosomes as a crucial instrument in the process of peripheral nerve regeneration.
The complexity of cellular and molecular processes involved in peripheral nerve regeneration has become apparent, indicating that microsurgery alone is insufficient for effective nerve repair. There are several reasons that contribute to the unfavorable results observed in nerve repair. These include the sluggish, inadequate, and misdirected regeneration of axons, the atrophy of end-organs and the failure of reinnervation, as well as the fast and long-lasting reconfiguration of the cortical regions involved[30].
Despite the accumulation of substantial information regarding neuropathophysiology throughout the last three decades, the fundamental principles governing clinical interventions for nerve damage have remained unaltered. Consequently, the clinical results associated with such interventions have failed to meet expectations. Potential future techniques to the repair of peripheral nerves include cell-based supportive treatment and the bioengineering of nerve conduits. Current research highlights the increasing importance of cell-based treatments that provide assistance in the process of nerve regeneration[31]. The initial pilot investigation presented in this paper describes the treatment of a clinical case using sural autograft and perioperative implantation of exosomes produced from Wharton’s jelly-derived MSCs (WJ-MSCs).
The current investigation received approval from the medical ethics committee of the authors’ institution, with the assigned protocol number 56733164-203-E.5863. Upon entering the trial, the patient provided written informed permission. patient provided informed consent for their use in the study. The patient’s explicit endorsement, as demonstrated by their written informed permission, established their willingness to participate in future clinical investigations.
Various approaches have been employed for the extraction of exosomes, including differential ultracentrifugation, density gradient separation, ultrafiltration, size exclusion chromatography, immunoisolation, and flow cytometry. Differential ultracentrifugation is now the prevailing technique employed for exosome purification, since it has been widely acknowledged as the benchmark approach for separating exosome subpopulations characterized by generally consistent sizes (Supplementary Table 1, Supplementary Figures 1 and 2).
The WJ-MSC cells were cultivated until they reached 90% confluency. Subsequently, the cell medium was replaced with serum-free MSC NutriStem® XF Medium, and the cells were cultured in a humidified atmosphere for a minimum of 48 h. After the incubation period, the medium was collected and subjected to centrifugation at 300 × g for 5 min, followed by centrifugation at 1000 × g for 10 min in order to eliminate the cells and cellular debris, respectively. Subsequently, a centrifugation phase was conducted at a force of 5000 times the acceleration due to gravity (5000 × g) for a duration of 20 min in order to eliminate nuclei and cellular debris. Subsequently, the clarified supernatant underwent ultracentrifugation at a force of 100000 times the acceleration due to gravity for a duration of 70 min in order to achieve the concentration of exosomes (OPTIMA MAX-XP ultracentrifuge, Beckman Coulter, United States). The protein content of 100 mL vesicles was determined using the BCA protein assay method to confirm the production of MSC-derived exosomes. The BCA protein Assay Kit method was used in order to show the presence of protein in the exosomes. After the exosomes had been isolated, 20 μL of the resulting pellet was prepared by adding 200 μL of working solution (50:1 Reagent A:B) following the kit protocol and incubated for one hour at 37 °C. After incubation, the amount of protein was determined by spectrophotometer at 562 nm according to the BSA standard (Pierce BCA Protein Assay Kit, Thermo Fisher Scientific, United States). The pellet was resuspended in 500 μL of Dulbecco’s phosphate buffered saline, with a pH of 7.4, and stored at a temperature of -80 °C until it was utilized. To examine the characteristics of exosomes, the researchers employed a method where the isolated exosomes were tagged with well-established tetraspanin markers (CD81; 97%, CD9; 79%, and CD63; 95%) (Supplementary Figure 3). Subsequently, flow cytometry was utilized to study these labeled exosomes (BD Facs Canto, United States). In addition, the morphology and size of isolated exosomes were evaluated via transmission electron microscopy. Dynamic light scattering was also used to determine the size distribution of MSC-derived exosomes.
An immigrant man of 24 years’ age, who was right-hand dominant, appeared with a stab wound to his left arm below the elbow. There was a strong indication of radial nerve palsy since the patient could barely extend their wrist, fingers, or thumb upon first presentation. This is because the nerve branch that supplies the extensor carpi radialis longus muscle was not severed. The extensor carpi radialis brevis (ECRB) muscle was assumed to be entirely impaired, along with the other extensors of the fingers (Video). After being sent to the cardiovascular surgery unit from the emergency room on the off chance that the patient had sustained a vascular damage, it was determined that no such injury had occurred. The patient’s muscular strength in the neurological examination was measured at 1/5 in wrist and finger extension and 1/5 in forearm supination on the patient’s left side. The dorsal surface of the left hand save for the dorsal side of the little finger was in anesthetic state. After evaluating the patient’s nervous system, it was decided to contemplate amputating the radial nerve completely below the elbow (before the superficial and deep branches). Electrodiagnostic tests performed before to surgery (on the third day after the accident) revealed a lack of motor response in the left radial nerve segment below the elbow.
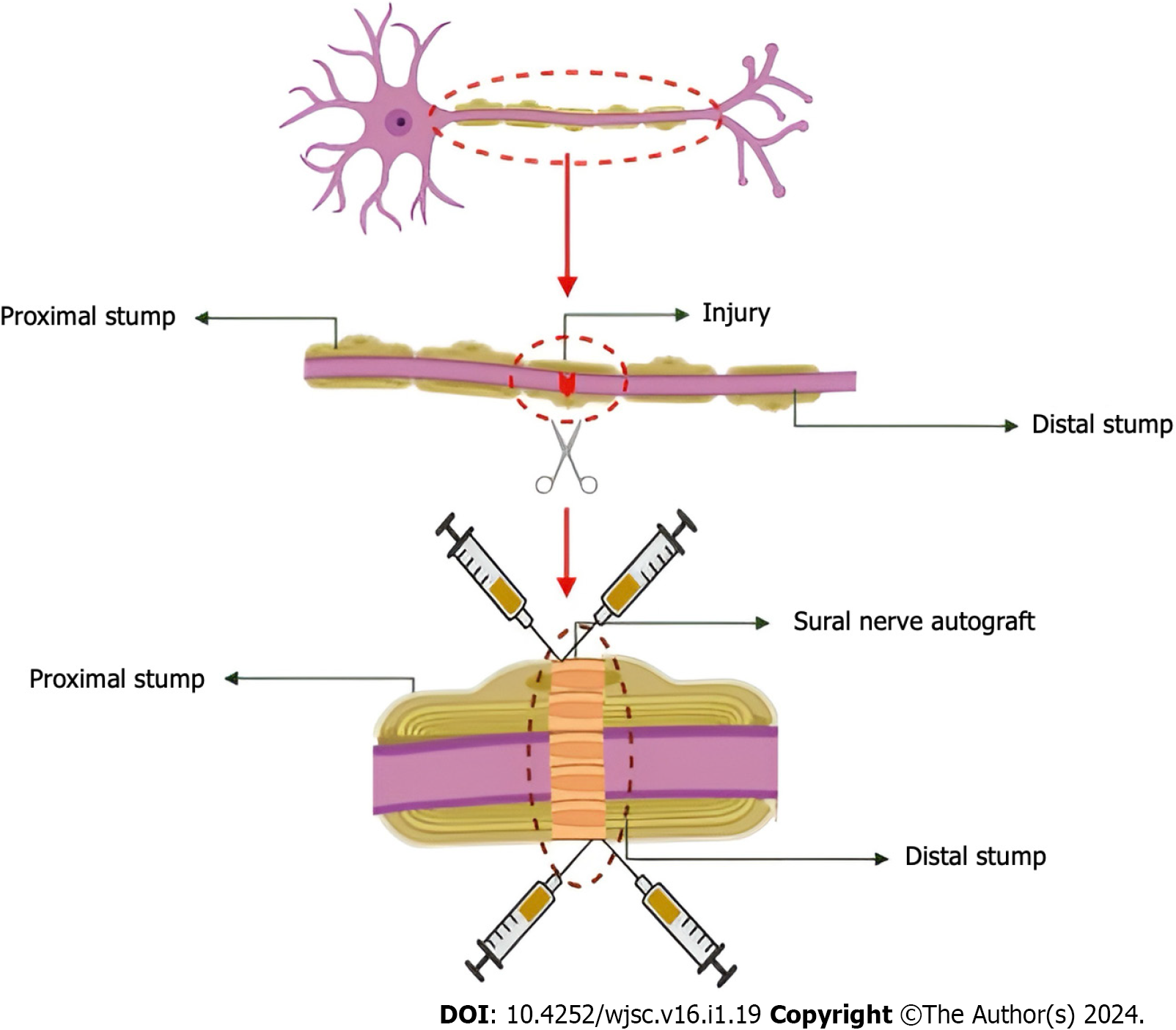
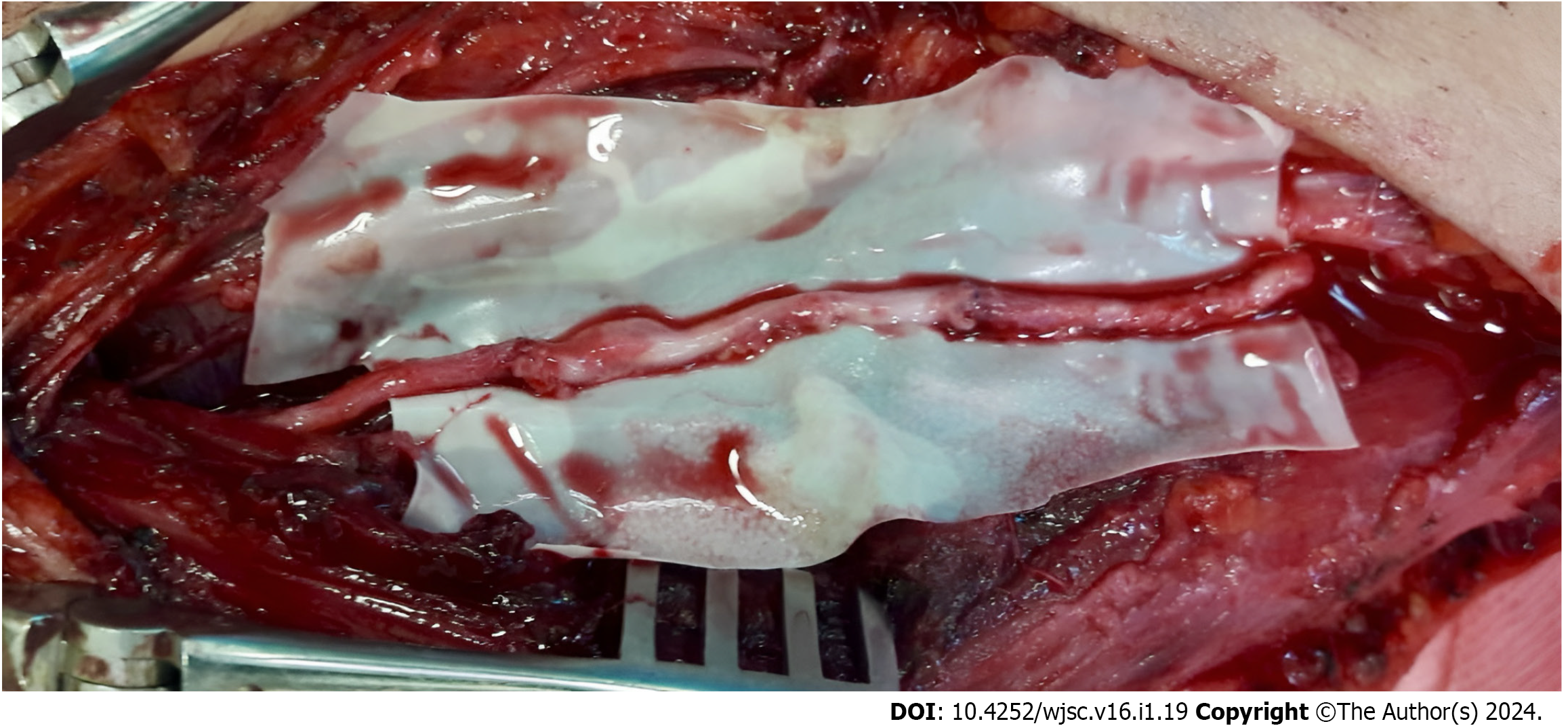
Subsequently, a surgical procedure to investigate the radial nerve in the left forearm and the subsequent implantation of exosomes were scheduled for the patient. The patient was placed in a supine posture with the forearm extended on a hand table while under general anesthesia. Additionally, the surgical incision site was appropriately demarcated. In cases where primary nerve repair is not feasible, the localization of the left sural nerve was duly noted and prepared under sterile conditions. This was done to facilitate the potential acquisition of a sural autograft, if required, and to enable subsequent nerve repair utilizing the autograft. A curvilinear incision was made, starting at the lateral antecubital fossa and extending down the medial side of the brachioradialis muscle to the midway of the forearm, including the area associated with stabbing. The brachioradialis muscle was detected and subsequently retracted in a lateral direction. Following this, the superficial branch of the radial nerve and the radial arteries were located underneath it. The radial blood arteries exhibited no signs of damage or disruption. In order to locate the primary branch of the radial nerve, the brachioradialis muscle was laterally retracted, and the radial superficial nerve was traced proximally until the radial nerve was successfully located. The radial nerve was dissected in a distal manner, leading to the identification of the branches associated with the posterior interosseous nerve and ECRB. A full avulsed injury (neurotemesis, Sunderland categories grade 5) was detected in the nerve just prior to the branching of the posterior interosseous nerve and ECRB. Once the nerve had been resected to restore its healthy proximal and distal extremities, the resulting segments were joined and the distance between them was determined. Approximately 7 centimeters of space was present, and an 8.5 centimeter sural autograft was obtained. Approximately sixty percent of the cross-sectional area of the damaged nerve was covered by the autograft. Grafting was accomplished using 8-0 prolene to create four epineural sutures on each side while observing through an operative microscope at a magnification of × 12. Following that, a subepineural route was utilized to microsurgically apply 0.25 mL of 1 mL of exosome derived from MSCs (containing 5 billion microvesicles) to both sides of the proximal and distal stumps (Figure 1). Each portion contained 5 billion microvesicles. A photograph taken intraoperatively is illustrated in Figure 2. To mitigate the unintended dissemination of the exosome during injection into the adjacent tissue, fibrin adhesive was administered to the affected areas. In order to facilitate neovascularization in the adjacent tissues, a minute quantity of exosomes administered via ubepineural route was permitted to traverse into the surrounding tissue. Technical difficulties prevented intraoperative neurophysiological monitoring from being conducted throughout the operation; therefore, tourniquets were not utilized. Two weeks after the operation, the hand was immobilized in the most functionally advantageous position conceivable. With early motor reeducation, we intended to initiate intensive and protracted physical therapy of the hand two weeks postoperatively; at one month, we initiated light strengthening and moderate range of motion exercises to alleviate edema. Opportunistic behavior on the part of the patient rendered the physical therapy and rehabilitation program unfeasible to incorporate. Ten weeks subsequent to the injury, he demonstrated active wrist extension with a muscle strength rating of 3/5 (Video). Electrodiagnostic testing revealed that although the extension of the finger and hand had commenced, rehabilitation and reinnervation were still in the early stages, characterized by severe axonal injury. During sensory evaluation, it was noted that the senses of contact and pain had been restored without any 14-excessive reactions. During the control examination conducted six months postoperatively, the strength of the muscles involved in extending the wrist, fingers, and thumb was assessed to be +4-5/5 (Video). Additionally, the patient’s sensory examination exhibited a near-complete improvement.
The efficacy evaluation encompassed assessments of both motor and sensory functions. An assessment of the motor and sensory nerves’ recovery was conducted in adherence to global benchmarks. Strength and range of motion assessments are components of the motor function evaluation. In order to evaluate sensory functions, static and dynamic two-point discrimination was conducted. Pain, the patient’s or physician’s assessment of the improvement in function, and electromyography (EMG) were additional examinations. Each assessment was conducted bilaterally on the upper limb.
Motor and sensory functions were assessed using the Mackinnon-Dellon scale (Table 1) and the British Medical Research Council (BRMC) scale (Table 2), the two most widely used methodologies for evaluating outcomes following repair of PNI, respectively[32]. The improvement in sensitivity was evaluated using a four-point scale, whereas the enhancement in motor functions was assessed using a five-point scale. M0-M2 and S0-S1 outcomes were considered to be inadequate. Improvements of M4 and S3 or greater were deemed “excellent” and “very good”, respectively. In conclusion, both M3 and S2 were classified as “good”. This study utilized the most commonly applied criteria: adequate motor recovery (grade M4 or M5), and satisfactory sensory recovery (grade S3+ or S4).
| Grade | Recovery of sensibility |
| S0 | Poor: No recovery of sensibility |
| S1 | Poor: Recovery of deep cutaneous pain sensibility |
| S1+ | Poor: Recovery of superficial pain sensibility |
| S2 | Poor: Recovery of superficial pain and some touch sensibility |
| S2+ | Poor: As in S2, but with overresponse |
| S3 | Poor: Recovery of pain and touch sense with no overresponse (> 15 mm s2PD, > 7 mm m2PD) |
| S3+ | Good: As S3, but localization of stimulus is good and imperfect recovery of 2PD (7-15 mm s2PD, 4-7 mm m2PD) |
| S4 | Excellent: Complete recovery (2-6 mm s2PD, 2-3 mm m2PD) |
| Grade | Recovery level | Muscle strength |
| M0 | Failure | No contraction |
| M1 | Poor | Return of perceptible contraction in the proximal muscle group |
| M2 | Fair | Return of perceptible contraction in both proximal and distal muscles; the extensor carpi radialis muscles may contract against force, but absence or trace of wrist extension |
| M3 | Moderate | Wrist extension against gravity to the neutral position; absence or trace of finger or thumb extension |
| M4 | Good | Wrist extension against force; trace or better finger and thumb extension |
| M5 | Excellent | Wrist, finger, and thumb extensors restored close to normal |
The duration of the patient’s follow-up was 180 d. An improvement in Tinel’s sign and sensory-motor recovery was observed as early as the tenth week following nerve transplantation. Upon the conclusion of the 6-mo follow-up phase, the nerve’s motor and sensory functions (as measured by the Mackinnon-Dellon scale and the BRMC scale) returned to M5 (outstanding) and S3+ (good), respectively, without physical therapy.
Electrophysiology was utilized in order to gauge the electrical conduction of the nerve. The thickness of the myelin sheath and the quantity of myelinated nerve fibers are both factors that influence electrical conduction. EMG exhibits greater accuracy in detecting early re-innervation compared to physical examination. Consequently, upon needle examination of the muscle closest to the site of injury, the recovery of motor unit action potentials is frequently the initial indication of re-innervation. Neurophysiological indicators of axonal regeneration often manifest weeks to months following PNI, prior to the manifestation of voluntary contraction. Preoperative electrodiagnostic testing conducted on the third day following the injury revealed the absence of any motor response (total denervation) in the left radial nerve segment below the elbow. The patient demonstrated significant improvement as indicated by BMRC scores prior to the scheduled date of the control EMG. Notwithstanding the persistent extensive axonal damage, indications of nerve re-innervation were detected on the control EMG of the nerve (at the tenth week after the injury). Physical therapy and rehabilitation were additional treatment modalities that the patient was incompatible with. In contrast, the sixth-month control EMG revealed a notable electrophysiological improvement in comparison to the previous EMG; furthermore, the regeneration process persisted.
Despite advancements in microsurgical techniques and comprehension of the pathophysiology underlying PNS injury and regeneration, PNIs continue to pose a substantial obstacle. The PNS possesses the ability to restore and regenerate by nature. PNIs elicit a substantial cellular and molecular reaction that involves not only the damaged neurons but also the supporting SCs. Antidromic electrical activity, which initiates kinase cascades and activates calcium channels, is the initial signal received by the neuronal cell body following axonal injury. This results in a substantial response in both protein and gene expression; the equilibrium of protein and gene expression determines whether the neuron survives and attempts to regenerate or undergoes apoptotic death.
The degeneration of the axon and myelin in the distal stump occurs within a very short timeframe, typically within a few hours. Subsequently, macrophages gather at the site of injury, playing a crucial role in the clearance of cellular debris. During the initial 24-h period, SCs undergo proliferation and transition from a myelinating state to a regenerative one. This transformation is accompanied by an increase in the expression of several molecules that play a role in both the degenerative and regenerative processes occurring simultaneously[33]. Following the clearance of debris by SCs and macrophages, SCs initiate the formation of Büngner bands, which create a trophic-rich environment that facilitates directed axonal regeneration. Similarly, the target organ that has been denervated experiences a depletion of trophic factors, resulting in the atrophy of muscle fibers and the death of satellite cells.
The regenerative capacity is contingent upon several factors, including the patient’s age, the specific type of damage, and notably, the proximity of the injury to the nerve cell soma. Regeneration following nerve damage involves several crucial factors from a pathophysiological perspective, including the activities of macrophages and SCs, the inflammatory response, and vascular regeneration. The degree of patient participation during the therapy process has a notable influence on the probability of obtaining recovery, particularly among patients with a moderate to high socio-cultural status who tend to exhibit the most favorable outcomes. In contrast to analogous lesions observed in older individuals or those with a generally compromised state of health, adolescents and teens consistently exhibit a more favorable trajectory and result. Matejcík[34] conducted a study which revealed that those under the age of 20 had the most favorable results. In contrast to injuries of a more complex nature, such as lacerations and contusions, pure severance injuries have been found to provide more favorable circumstances for effective autotransplantation. According to Matejcík[34], it was shown that injuries in close proximity to a certain anatomical region had the most unfavorable results in relation to the severity of the damage. The most favorable results were observed in injuries located further out from the center, specifically around the wrist, with a success rate of 87.6%[34].
Following an injury, nerves undergo a process of progressive regeneration, wherein they must successfully extend, identify, and reestablish connections with the anatomical structures under their control. In order to prevent degeneration, it is imperative that endoneurial tubes establish contact with regenerated axons within a timeframe ranging from 18 to 24 mo subsequent to the damage. Following a period of denervation lasting between 12 and 18 mo, the atrophy of the target muscle reaches a point where it becomes permanent, hence imposing limitations on the functional efficacy of the healing process. Although sensory function has the potential to be restored at a later stage, even several years following the initial injury, it is worth noting that sensory receptors persist for a significantly extended duration.
Nerve damage of varied degrees can be a consequence of an injury, necessitating the potential requirement for reconstructive surgery. The primary objectives of this surgical procedure are to provide optimal enhancements in both motor and sensory functions within the denervated region located distally. However, despite the use of meticulous surgical techniques and a range of corrective interventions, achieving a complete restoration of functionality, especially in terms of motor function, is seldom attainable. Primary nerve repair is considered the most effective approach for restoring functionality in instances of acute nerve transections characterized by abrupt injuries, minimal or absent compression, sufficient blood circulation, and uncontaminated wounds. In order to optimize nerve regeneration following the healing process, it is important to ensure that nerve stumps are aligned in a precise manner, devoid of any strain. Moreover, the restoration procedure should be conducted atraumatically, minimizing tissue damage and the number of sutures employed. Autologous nerve grafting is now considered the most effective method for repairing nerve gaps that cannot be brought together or joined without strain. In contrast to direct repairs conducted under conditions of high strain leading to nerve ischemia, nerve grafts had much superior results.
Artificial nerve guidance scaffolds have been developed with the aim of facilitating nerve regeneration by the restriction of myofibroblast infiltration, reduction of scar formation, and the concentration of neurotrophic substances. The regeneration capabilities of commercially available technologies, often consisting of hollow tubes composed of biodegradable polymer or collagen, have not been able to attain the same levels as autologous nerve grafting. These technologies are limited to treating tiny lesions (less than 2 cm) and demonstrate inadequate functional recovery[35]. A novel methodology integrates stem cells into biomaterial scaffolds, therefore amalgamating neuroprotective interventions with nerve restoration and enhanced axonal regeneration. Successful regeneration has not been achieved only via the use of nerve guides, making it particularly crucial for addressing big gaps.
Several variables, such as surgical delay, patient age, injury type, autograft length, injury location, and nerve damage type, might potentially influence the success of peripheral nerve repair using autografts. The results of nerve injury repair in the upper limbs were shown to be more favorable compared to those in the lower limbs. The temporal interval between the occurrence of the damage and the subsequent reconstructive surgical intervention had a crucial role in achieving favorable outcomes, particularly among individuals in younger age groups.
The determination of the anatomic nerve with the most favorable prognosis recovery has been extensively studied, yielding a multitude of conflicting findings. Several studies have shown contrasting findings on the optimal healing of the median nerve and radial nerve in the upper extremities[36]. Furthermore, additional research conducted on the anatomical peripheral nerves pertaining to motor-sensory recovery failed to demonstrate any statistically significant disparities[37]. The occurrence of “crossing over” inside the regenerated nerve has been shown to result in a reduced likelihood of complete recovery for mixed nerves, such as the proximal section of the ulnar or median, compared to pure nerves, such as the motor branch of the ulnar or median[36].
The study conducted by Renner et al[38] shown a significant improvement in motor function for around 75% of patients with radial nerve injuries who underwent nerve grafting. Nevertheless, the present study lacks data regarding the administration of postoperative physical therapy to the patients as well as the duration required for complete recuperation. A suggested minimum follow-up time of one to two years is typically advised for the repair of the median and ulnar nerves. Additionally, the final functional evaluation should be conducted two to three years after the repair in children and adolescents, and five years after the repair in adults[39]. The existing research does not provide any information on the optimal period for evaluating outcomes in radial nerve repair. Furthermore, the existing research does not provide conclusive evidence about the specific timeframe for the onset of electrophysiological or clinical impro
In the present case study, it is noteworthy that the patient did not undergo post-operative physical therapy. However, it is important to highlight that both clinical and electrophysiological recovery started at the 10th wk, with nearly full restoration of motor and sensory functions documented during the 6th mo follow-up assessment. In the present study, the duration of follow-up was limited to a maximum of six months subsequent to the surgical procedure and administration of exosomes. This constraint was imposed due to the patient’s non-adherence to the prescribed treatment regimen, hence preventing a more extensive follow-up period that would have encompassed physical therapy and rehabilitation interventions.
The proximal part of the nerve tract is often composed of mixed nerve bundles. As a result, there exists significant potential for interplay and development between sensory and motor nerve fibers. The technique of perineural suturing involves the joining of the motor and sensory tracts’ respective ends, hence promoting a favorable functional recovery. This approach is effective due to the preexisting separation of the nerve into distinct sensory and motor tracts at the distal end. Consequently, the process of regeneration is prolonged in injuries that occur closer to the point of origin.
The sural nerve is commonly used as the donor nerve. When the sural nerve proper is harvested alone, a graft material exceeding 20 cm can be obtained. Conversely, when it is harvested in conjunction with the medial sural cutaneous nerve, a maximum of 50 cm can be achieved. According to a study, it was shown that sural nerve autografts had the most unfavorable motor and sensory results[37]. Furthermore, it has been established that there is a negative correlation between graft length and clinical outcomes. Matejcík[34] found that the duration of the transplant procedure exerts a detrimental influence on the overall success rate of transplantation. According to the study conducted by[34], the success rate of grafts measuring up to 5.0 cm was found to be 80.6%. However, when the length of the grafts surpassed 10 cm, the success rate dropped significantly to 16.7%. In the presented case study, it was necessary to utilize a sural autograft of considerable length (8.5 cm) in response to the significant distance separating the nerve ends. Nevertheless, a rapid and nearly full clinical recovery was attained.
The temporal interval between the occurrence of the injury and the subsequent undertaking of reconstructive surgery plays a pivotal role in influencing the results of surgical interventions aimed at restoring the functionality of peripheral nerves. The influence of this ingredient is more pronounced in younger patients[34]. The surgical procedure for the patient was scheduled to take place on the third day following the occurrence of the injury, aligning with the anticipated arrival time of the exosome sourced from the laboratory accredited under Good Manufacturing Practice standards.
The two primary factors that significantly impact axonal outgrowth via the transplanted nerve are the diameter of the grafted nerve fragment and the vascularity of the surrounding tissue bed. The process of nerve revascularization in nonvascularized autografts relies on the crucial mechanism of diffusion from the surrounding tissues. The clinical observations indicate that grafts with smaller calibers have more favorable outcomes. Nerve grafting entails the manipulation of regenerating fibers to traverse two coaptation sites, hence heightening the risk of axonal loss due to the development of scar tissue and the potential diversion of fibers into the perifascicular and epineurial connective tissue at each suture line. Similarly, a tissue bed that lacks sufficient vascularization hinders functional results, retards nerve regeneration, and enhances scar formation.
Inflammation has a big effect on peripheral nerve regrowth, and more and more research shows that cytokines and inflammatory reactions are key factors in this process[40]. Inflammation is needed to get rid of waste so that nerves can grow again, but it can also lead to problems like neuropathic pain and slow down nerve growth. So, the right amount of inflammation is important for nerves to heal properly. Exosomes from MSCs are known to help new blood vessels grow in nearby tissues[41]. Along with their ability to change the immune system, exosomes may also stop scars from forming in and around the restored peripheral nerve and at two places where they connect. The application of subepineural exosomes after surgery to repair a peripheral nerve may have sped up nerve healing by controlling the growth of new blood vessels in the nearby tissue.
Cell-to-cell contact is very important for maintaining balance in the body, especially in the nervous system. New research shows that exosomes can carry information between cells, which is important for the health and growth of brain systems[42]. Exosomes can help cells talk to each other in a number of different ways. One way is that miRNAs are sent from exosomes to target cells. These miRNAs can control how genes are expressed and how signals are sent in the receiver cells. Exosomes can also move proteins and lipids to other cells, which can change how cells work by changing their roles like growth, development, and death. Exosomes can also work with immune cells and parts of the extracellular matrix to control inflammation and metabolic balance[43]. These results make it seem like there are good ways to keep looking into new ways to help peripheral nerves grow back. When a peripheral nerve is damaged or dying, SCs around it move vesicles with polyribosomes into the axon, where the contents are released[44]. As a result, exosomes help get mRNA and ribosomes to damaged nerves, where they start the protein production that is needed for healing.
In the context of regenerative medicine for nerve repair, the utilization of cell-based treatments, namely stem cell therapy, holds significant promise in harnessing the regenerative capabilities of cells. Numerous in vitro and in vivo studies have been conducted to evaluate the potential of neural stem cells, SCs, olfactory ensheathing cells, induced pluripotent stem cells, and adult MSCs derived from different sources, for the purpose of nerve repair[45]. In relation to the origin of the cells, the potential for teratoma development, and the likelihood of unintended cellular differentiation, the utilization of MSCs in the field of regenerative medicine presents a reduced number of ethical concerns. These entities have the potential to facilitate the process of remyelination and provide trophic assistance to neurons undergoing regeneration.
Patient-specific stem cell exosomes might potentially be utilized as a strategy to enhance nerve regeneration. Nevertheless, in order to acquire stem cells, it is necessary to sacrifice a nerve that is in good health. The available evidence indicates that exosomes derived from matured MSCs that contain miRNAs have the potential to enhance axonal regeneration. Additionally, these exosomes may also indirectly facilitate the process of nerve repair by modulating the inflammatory response, hence promoting recovery[46].
Multiple studies have demonstrated the efficacy of MSCs in enhancing the process of peripheral nerve regeneration. Nevertheless, some notable limitations, including as immunogenicity, retention, and neoplasticity, have also been documented in the literature[46]. Exosomes, which are a form of acellular treatment, has a reduced immunogenicity that allows them to alleviate the limitations associated with MSC transplantation while maintaining their biological functionality. Hence, exosomes has the potential to be utilized in the development of groundbreaking therapeutic interventions for the restoration and regeneration of peripheral nerves.
As previously indicated, optimal outcomes are achieved when regenerated axons are able to traverse a single coaptation site, hence facilitating tension-free end-to-end nerve repair. On the other hand, in the case of utilizing a nerve graft, the regenerating axons are required to traverse two healing sites, each potentially undergoing an independent inflammatory process that might result in further axonal degeneration. It is believed that the utilization of a subepineural exosome on the coaptation regions demonstrates efficacy in the treatment of the inflammatory process that arises inside these areas.
In the future, the combination of appropriate surgical intervention and postoperative rehabilitation programs has the potential to yield ideal outcomes in the treatment of peripheral nerve abnormalities. Considering the CNS’s pivotal role in determining the functional result of peripheral nerve regeneration, it is imperative to prioritize the stimulation of cortical and subcortical remodeling as part of CNS-level rehabilitation. Hence, it is advisable to prescribe physical therapy and rehabilitation regimens for individuals having surgical procedures.
The primary constraint of our study resides in the utilization of a solitary example, which may engender erroneous deductions. Furthermore, the limited number of participants hindered the possibility of conducting a distinct examination of various nerve clusters. Hence, in order to evaluate our findings, it is imperative to conduct controlled, prospective, and randomized studies including bigger case series and other nerve groups.
Furthermore, the utilization of intraoperative electrophysiologic evaluation has gained recognition as an essential technique in the management of lesions in continuity. Assessing the whole amount of internal nerve damage only by macroscopic examination is a significant challenge, especially in cases of PNI accompanied by neuroma formation. The utilization of electrical stimulation for confirmation purposes plays a pivotal role in optimizing surgical efficiency. Electrophysiological monitoring was not conducted during the surgery. In the present study, the utilization of intraoperative EMG was deemed unnecessary due to the prompt surgical intervention conducted on the third day following the occurrence of the injury, hence resulting in a minimal likelihood of neuroma development. The utilization of intraoperative electrophysiological monitoring is recommended in surgical procedures to assess the functional integrity of vulnerable brain components.
In our perspective, the utilization of perioperative subepineural exosome application does not entail a significant expenditure of time. Despite the potential for increased expenses, this approach has the advantage of facilitating a prompt resumption of regular daily activities and professional obligations. Given the promising results observed thus far with autogenous nerve grafts, it is imperative to do a cost-benefit analysis of this novel approach. The findings of our study indicate that the utilization of exosomes formed from WJ-MSCs, in conjunction with microsurgical repair, holds significant potential as a new approach for nerve repair and regeneration. Additionally, our findings are anticipated to provide valuable insights for future investigations on the reparative methodologies for PNI.
The potential enhancements in nerve repair results by modifications to microsurgical techniques are unlikely to be substantial[47]. Therefore, it is imperative to conduct clinical trials to examine the therapeutic benefits of exosomes, since they have previously been explored in experimental studies. Currently, there is a lack of prospective randomized double-blind research pertaining to this particular issue in the existing literature. Hence, case-based research, such as our work, which represents the first clinical investigation on this topic within the existing body of literature, hold significant academic value.
In peripheral nerve injury, there are some cellular and molecular changes in damaged axon, neuron and also in end-organ. For this reason, factors that will accelerate regeneration are needed in addition to surgical treatment. Exosomes may be used to enhance angiogenesis around the damaged axon, to inhibit scar formation, to regulate immune system by immunomodulatory action and to show trophic effects. In this pilot study, we observed that there was a rapid recovery in a short time without any physical therapy after total radial nerve injury. In the next stage, prospective, randomized and controlled clinical studies on this subject will be needed. Potential future investigations might include the integration of exosomes sourced from stem cells, MSCs, or macrophages with nerve conduit technology or their direct injection into nerve stumps.
Poor functional outcome after surgery is a great challenge in peripheral nerve injury (PNI). Surgical treatments fail to address the complexity of the events that occur following a PNI. There is a clear clinical need to find new approaches. Exosomas from Wharton’s jelly-derived mesenchymal stem cells (WJ-MSCs). have the ability to accelerate the improvement of nerve regeneration.
The therapeutic results of exosome application in PNI require investigation.
This study aimed to determine the effects of treatment of peripheral nerve damage with sural autograft and perioperative exosome application.
The patient, with total radial nerve injury, underwent a sural autograft repair followed by the per-operative subepineural application of MSC-derived exosome (WJ-MSCs). The patient was monitored for 180 d with neurological examination and electrodiagnostic testing.
Although physical therapy was not applied after the procedure, improvement in motor and sensory functions occurred in an unusually short time. The nerve exhibited re-innervation signs as evidenced by control electromyography (EMG) at the 10th wk post-injury, and a significant electrophysiological enhancement was observed in the EMG conducted at the 6th-mo follow-up, indicating ongoing regeneration.
Exosomes (WJ-MSCs) can accelerate the recovery in the treatment of peripheral nerve damage and lead to the impro
New strategies to improve the functional outcome after PNI treatment should be the focus of future studies which can help in increasing the chances of using exosomes clinically for the treatment of PNI.
The authors would like to express their gratitude to Gizem Demirturk for her invaluable contribution in conducting the illustrations.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Cell and tissue engineering
Country/Territory of origin: Turkey
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Li SC, United States; Muzes G, Hungary; Qin Y, China S-Editor: Wang JJ L-Editor: A P-Editor: Zhang XD
| 1. | Kouyoumdjian JA. Peripheral nerve injuries: a retrospective survey of 456 cases. Muscle Nerve. 2006;34:785-788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 162] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 2. | Pan CH, Chuang DC, Rodríguez-Lorenzo A. Outcomes of nerve reconstruction for radial nerve injuries based on the level of injury in 244 operative cases. J Hand Surg Eur Vol. 2010;35:385-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 33] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 3. | Roganovic Z, Petkovic S. Missile severances of the radial nerve. Results of 131 repairs. Acta Neurochir (Wien). 2004;146:1185-1192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 41] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 4. | Moore AM, Wagner IJ, Fox IK. Principles of nerve repair in complex wounds of the upper extremity. Semin Plast Surg. 2015;29:40-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 67] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 5. | Shergill G, Bonney G, Munshi P, Birch R. The radial and posterior interosseous nerves. Results fo 260 repairs. J Bone Joint Surg Br. 2001;83:646-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 35] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 6. | Terzis JK, Konofaos P. Radial nerve injuries and outcomes: our experience. Plast Reconstr Surg. 2011;127:739-751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 7. | di Summa PG, Kalbermatten DF, Pralong E, Raffoul W, Kingham PJ, Terenghi G. Long-term in vivo regeneration of peripheral nerves through bioengineered nerve grafts. Neuroscience. 2011;181:278-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 156] [Article Influence: 11.1] [Reference Citation Analysis (1)] |
| 8. | Chen P, Piao X, Bonaldo P. Role of macrophages in Wallerian degeneration and axonal regeneration after peripheral nerve injury. Acta Neuropathol. 2015;130:605-618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 363] [Article Influence: 36.3] [Reference Citation Analysis (0)] |
| 9. | Du J, Zhen G, Chen H, Zhang S, Qing L, Yang X, Lee G, Mao HQ, Jia X. Optimal electrical stimulation boosts stem cell therapy in nerve regeneration. Biomaterials. 2018;181:347-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 101] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 10. | Zhang B, Wu X, Zhang X, Sun Y, Yan Y, Shi H, Zhu Y, Wu L, Pan Z, Zhu W, Qian H, Xu W. Human umbilical cord mesenchymal stem cell exosomes enhance angiogenesis through the Wnt4/β-catenin pathway. Stem Cells Transl Med. 2015;4:513-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 376] [Cited by in RCA: 359] [Article Influence: 35.9] [Reference Citation Analysis (0)] |
| 11. | Phinney DG, Pittenger MF. Concise Review: MSC-Derived Exosomes for Cell-Free Therapy. Stem Cells. 2017;35:851-858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 831] [Cited by in RCA: 1224] [Article Influence: 153.0] [Reference Citation Analysis (0)] |
| 12. | Rani S, Ryan AE, Griffin MD, Ritter T. Mesenchymal Stem Cell-derived Extracellular Vesicles: Toward Cell-free Therapeutic Applications. Mol Ther. 2015;23:812-823. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 625] [Cited by in RCA: 879] [Article Influence: 87.9] [Reference Citation Analysis (0)] |
| 13. | Lopez-Verrilli MA, Court FA. Transfer of vesicles from schwann cells to axons: a novel mechanism of communication in the peripheral nervous system. Front Physiol. 2012;3:205. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 69] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 14. | Kingham PJ, Kalbermatten DF, Mahay D, Armstrong SJ, Wiberg M, Terenghi G. Adipose-derived stem cells differentiate into a Schwann cell phenotype and promote neurite outgrowth in vitro. Exp Neurol. 2007;207:267-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 453] [Cited by in RCA: 481] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 15. | Marote A, Teixeira FG, Mendes-Pinheiro B, Salgado AJ. MSCs-Derived Exosomes: Cell-Secreted Nanovesicles with Regenerative Potential. Front Pharmacol. 2016;7:231. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 154] [Cited by in RCA: 210] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 16. | Wen D, Peng Y, Liu D, Weizmann Y, Mahato RI. Mesenchymal stem cell and derived exosome as small RNA carrier and Immunomodulator to improve islet transplantation. J Control Release. 2016;238:166-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 140] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 17. | Santonocito M, Vento M, Guglielmino MR, Battaglia R, Wahlgren J, Ragusa M, Barbagallo D, Borzì P, Rizzari S, Maugeri M, Scollo P, Tatone C, Valadi H, Purrello M, Di Pietro C. Molecular characterization of exosomes and their microRNA cargo in human follicular fluid: bioinformatic analysis reveals that exosomal microRNAs control pathways involved in follicular maturation. Fertil Steril. 2014;102:1751-61.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 195] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 18. | Kalani A, Tyagi A, Tyagi N. Exosomes: mediators of neurodegeneration, neuroprotection and therapeutics. Mol Neurobiol. 2014;49:590-600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 260] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 19. | Shabbir A, Cox A, Rodriguez-Menocal L, Salgado M, Van Badiavas E. Mesenchymal Stem Cell Exosomes Induce Proliferation and Migration of Normal and Chronic Wound Fibroblasts, and Enhance Angiogenesis In Vitro. Stem Cells Dev. 2015;24:1635-1647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 371] [Cited by in RCA: 505] [Article Influence: 50.5] [Reference Citation Analysis (0)] |
| 20. | Xu JF, Yang GH, Pan XH, Zhang SJ, Zhao C, Qiu BS, Gu HF, Hong JF, Cao L, Chen Y, Xia B, Bi Q, Wang YP. Altered microRNA expression profile in exosomes during osteogenic differentiation of human bone marrow-derived mesenchymal stem cells. PLoS One. 2014;9:e114627. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 157] [Cited by in RCA: 193] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 21. | Xin H, Li Y, Buller B, Katakowski M, Zhang Y, Wang X, Shang X, Zhang ZG, Chopp M. Exosome-mediated transfer of miR-133b from multipotent mesenchymal stromal cells to neural cells contributes to neurite outgrowth. Stem Cells. 2012;30:1556-1564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 613] [Cited by in RCA: 700] [Article Influence: 53.8] [Reference Citation Analysis (0)] |
| 22. | Johnstone RM. Exosomes biological significance: A concise review. Blood Cells Mol Dis. 2006;36:315-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 246] [Cited by in RCA: 270] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 23. | Baglio SR, Rooijers K, Koppers-Lalic D, Verweij FJ, Pérez Lanzón M, Zini N, Naaijkens B, Perut F, Niessen HW, Baldini N, Pegtel DM. Human bone marrow- and adipose-mesenchymal stem cells secrete exosomes enriched in distinctive miRNA and tRNA species. Stem Cell Res Ther. 2015;6:127. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 443] [Cited by in RCA: 598] [Article Influence: 59.8] [Reference Citation Analysis (0)] |
| 24. | Hessvik NP, Llorente A. Current knowledge on exosome biogenesis and release. Cell Mol Life Sci. 2018;75:193-208. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1613] [Cited by in RCA: 1740] [Article Influence: 248.6] [Reference Citation Analysis (0)] |
| 25. | Krämer-Albers EM, Bretz N, Tenzer S, Winterstein C, Möbius W, Berger H, Nave KA, Schild H, Trotter J. Oligodendrocytes secrete exosomes containing major myelin and stress-protective proteins: Trophic support for axons? Proteomics Clin Appl. 2007;1:1446-1461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 324] [Cited by in RCA: 404] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 26. | Waldenström A, Gennebäck N, Hellman U, Ronquist G. Cardiomyocyte microvesicles contain DNA/RNA and convey biological messages to target cells. PLoS One. 2012;7:e34653. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 271] [Cited by in RCA: 337] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 27. | Théry C, Ostrowski M, Segura E. Membrane vesicles as conveyors of immune responses. Nat Rev Immunol. 2009;9:581-593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2703] [Cited by in RCA: 3103] [Article Influence: 193.9] [Reference Citation Analysis (0)] |
| 28. | Zhang Y, Chopp M, Meng Y, Katakowski M, Xin H, Mahmood A, Xiong Y. Effect of exosomes derived from multipluripotent mesenchymal stromal cells on functional recovery and neurovascular plasticity in rats after traumatic brain injury. J Neurosurg. 2015;122:856-867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 419] [Cited by in RCA: 531] [Article Influence: 53.1] [Reference Citation Analysis (0)] |
| 29. | Merino-González C, Zuñiga FA, Escudero C, Ormazabal V, Reyes C, Nova-Lamperti E, Salomón C, Aguayo C. Mesenchymal Stem Cell-Derived Extracellular Vesicles Promote Angiogenesis: Potencial Clinical Application. Front Physiol. 2016;7:24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 134] [Cited by in RCA: 168] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 30. | Dahlin LB. The biology of nerve injury and repair. J Am Soc Surg Hand. 2004;4:143-155. [RCA] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 30] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 31. | Funakoshi H, Frisén J, Barbany G, Timmusk T, Zachrisson O, Verge VM, Persson H. Differential expression of mRNAs for neurotrophins and their receptors after axotomy of the sciatic nerve. J Cell Biol. 1993;123:455-465. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 488] [Cited by in RCA: 569] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 32. | Mackinnon SE, Dellon AL. Results of nerve repair and grafting. In: Lee A, Mackinnon SE. Surgery of the Peripheral Nerves. New York: Thieme Medical, 1988: 115. |
| 33. | Burnett MG, Zager EL. Pathophysiology of peripheral nerve injury: a brief review. Neurosurg Focus. 2004;16:E1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 372] [Cited by in RCA: 413] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 34. | Matejcík V. Peripheral nerve reconstruction by autograft. Injury. 2002;33:627-631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 35. | Pabari A, Lloyd-Hughes H, Seifalian AM, Mosahebi A. Nerve conduits for peripheral nerve surgery. Plast Reconstr Surg. 2014;133:1420-1430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 86] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 36. | Barrios C, de Pablos J. Surgical management of nerve injuries of the upper extremity in children: a 15-year survey. J Pediatr Orthop. 1991;11:641-645. [PubMed] |
| 37. | Wang E, Inaba K, Byerly S, Escamilla D, Cho J, Carey J, Stevanovic M, Ghiassi A, Demetriades D. Optimal timing for repair of peripheral nerve injuries. J Trauma Acute Care Surg. 2017;83:875-881. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 41] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 38. | Renner A, Cserkuti F, Hankiss J. [Late results after nerve transplantation on the upper extremities]. Handchir Mikrochir Plast Chir. 2004;36:13-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 39. | Ruijs AC, Jaquet JB, Kalmijn S, Giele H, Hovius SE. Median and ulnar nerve injuries: a meta-analysis of predictors of motor and sensory recovery after modern microsurgical nerve repair. Plast Reconstr Surg. 2005;116:484-94; discussion 495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 260] [Cited by in RCA: 289] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 40. | Zhang S, Chuah SJ, Lai RC, Hui JHP, Lim SK, Toh WS. MSC exosomes mediate cartilage repair by enhancing proliferation, attenuating apoptosis and modulating immune reactivity. Biomaterials. 2018;156:16-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 391] [Cited by in RCA: 648] [Article Influence: 92.6] [Reference Citation Analysis (0)] |
| 41. | Burrello J, Monticone S, Gai C, Gomez Y, Kholia S, Camussi G. Stem Cell-Derived Extracellular Vesicles and Immune-Modulation. Front Cell Dev Biol. 2016;4:83. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 162] [Cited by in RCA: 211] [Article Influence: 23.4] [Reference Citation Analysis (1)] |
| 42. | Rajendran L, Bali J, Barr MM, Court FA, Krämer-Albers EM, Picou F, Raposo G, van der Vos KE, van Niel G, Wang J, Breakefield XO. Emerging roles of extracellular vesicles in the nervous system. J Neurosci. 2014;34:15482-15489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 220] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 43. | Isaac R, Reis FCG, Ying W, Olefsky JM. Exosomes as mediators of intercellular crosstalk in metabolism. Cell Metab. 2021;33:1744-1762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 453] [Article Influence: 113.3] [Reference Citation Analysis (0)] |
| 44. | Court FA, Hendriks WT, MacGillavry HD, Alvarez J, van Minnen J. Schwann cell to axon transfer of ribosomes: toward a novel understanding of the role of glia in the nervous system. J Neurosci. 2008;28:11024-11029. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 182] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 45. | Kalbermatten DF, Erba P, Mahay D, Wiberg M, Pierer G, Terenghi G. Schwann cell strip for peripheral nerve repair. J Hand Surg Eur Vol. 2008;33:587-594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 46. | Sun L, Xu R, Sun X, Duan Y, Han Y, Zhao Y, Qian H, Zhu W, Xu W. Safety evaluation of exosomes derived from human umbilical cord mesenchymal stromal cell. Cytotherapy. 2016;18:413-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 134] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 47. | Guerra WK, Baldauf J, Schroeder HW. Long-term results after microsurgical repair of traumatic nerve lesions of the upper extremities. Zentralbl Neurochir. 2007;68:195-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |