Published online Apr 26, 2023. doi: 10.4252/wjsc.v15.i4.268
Peer-review started: December 13, 2022
First decision: February 11, 2023
Revised: February 24, 2023
Accepted: March 21, 2023
Article in press: March 21, 2023
Published online: April 26, 2023
Processing time: 134 Days and 5.2 Hours
There is no established treatment to impede the progression or restore kidney function in human chronic kidney disease (CKD).
To examine the efficacy of cultured human CD34+ cells with enhanced proliferating potential in kidney injury in mice.
Human umbilical cord blood (UCB)-derived CD34+ cells were incubated for one week in vasculogenic conditioning medium. Vasculogenic culture significantly increased the number of CD34+ cells and their ability to form endothelial progenitor cell colony-forming units. Adenine-induced tubulointerstitial injury of the kidney was induced in immunodeficient non-obese diabetic/severe combined immunodeficiency mice, and cultured human UCB-CD34+ cells were administered at a dose of 1 × 106/mouse on days 7, 14, and 21 after the start of adenine diet.
Repetitive administration of cultured UCB-CD34+ cells significantly improved the time-course of kidney dysfunction in the cell therapy group compared with that in the control group. Both interstitial fibrosis and tubular damage were significantly reduced in the cell therapy group compared with those in the control group (P < 0.01). Microvasculature integrity was significantly preserved (P < 0.01) and macrophage infiltration into kidney tissue was dramatically decreased in the cell therapy group compared with those in the control group (P < 0.001).
Early intervention using human cultured CD34+ cells significantly improved the progression of tubulointerstitial kidney injury. Repetitive administration of cultured human UCB-CD34+ cells significantly improved tubulointerstitial damage in adenine-induced kidney injury in mice via vasculoprotective and anti-inflammatory effects.
Core Tip: There is no established treatment for retarding the progression or improving advanced chronic kidney disease (CKD). Here, we provided effectiveness of cord blood derived cultured human CD34+ cell on progressive CKD model in mice. Kidney function and pathological damage were improved by repetitive cell therapy along with the improvement of microvasculature and inhibition of inflammatory cell infiltration in the kidney. Enhanced cell potential (proliferation and increase of endothelial progenitor cell colony-forming unit) by culture might be a key factor for improving progressive kidney injury.
- Citation: Ohtake T, Itaba S, Salybekov AA, Sheng Y, Sato T, Yanai M, Imagawa M, Fujii S, Kumagai H, Harata M, Asahara T, Kobayashi S. Repetitive administration of cultured human CD34+ cells improve adenine-induced kidney injury in mice. World J Stem Cells 2023; 15(4): 268-280
- URL: https://www.wjgnet.com/1948-0210/full/v15/i4/268.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v15.i4.268
Chronic kidney disease (CKD) is a progressive disease that leads to end-stage renal disease (ESRD) with increasing incidence and mortality[1]. Incomplete recovery from acute kidney injury (AKI) can also lead to CKD (AKI-CKD transition) through tubular atrophy and failed tubular recovery[2]. CKD affected nearly 750 million people globally in 2016 and constitutes a serious public health burden worldwide[3]. Several treatment strategies, including pharmaceutical therapies, such as renin-angiotensin-aldosterone blockers, and diet control, including salt and protein restriction, have been applied to impede CKD progression. However, satisfactory outcomes have not been achieved. Considering the progressive nature of CKD, which is associated with high mortality rates, especially in late-stage CKD patients, including those undergoing dialysis, there is a pressing need to develop novel therapeutic strategies to treat CKD.
The efficacy of cell-based regenerative therapy in animal models of CKD has been tested, and preclinical studies have provided favorable results[4]. Cell-based therapy has been shown to improve functional and histological parameters of kidney injury and delay the development of CKD in animal models[4]. However, clinical trials of cell-based therapy for CKD in humans are still limited, with only three clinical trials published[5-7]. These trials used single administration of autologous bone marrow-derived mesenchymal stem cells[5,6] or granulocyte colony-stimulating factor-mobilized autologous peripheral blood-derived CD34+ cells[7]. Although the safety and tolerability of cell therapy were confirmed, the efficacy in improving or stabilizing renal dysfunction was not established by these early phase trials.
Adenine-induced renal injury in mouse is used as a model of chronic kidney injury caused by 2,8-dihydroxyadenine (DHA), a metabolic product of adenine[8,9]. The primary site of adenine-induced renal injury is the tubulointerstitial region. Accumulation of DHA crystals in the renal tubules and interstitial tissue causes progressive degeneration of tubular epithelial cells and induces interstitial fibrosis. Moreover, adenine-containing diet induces chronic and progressive renal injury in mice in a dose-dependent manner. This animal model is suitable for evaluating the efficacy of cell-based therapy for progressive tubulointerstitial injuries. However, to evaluate the efficacy of xenotransplantation of human cells, the animal’s immune response has to be avoided. Therefore, we stablished an adenine-induced renal injury model in non-obese diabetic/severe combined immunodeficiency (NOD/SCID) mice, which lack mature T, B, and natural killer cells.
Human CD34+ endothelial progenitor cells (EPC) have high angiogenic and anti-inflammatory potential, and thus used for this study[10,11]. However, because EPCs from patients with several diseases have impaired function and are insufficient, allogeneic sources are considered as good alternatives. We therefore used umbilical cord blood (UCB)-derived CD34+ cells. However, in our preliminary study, uncultured human UCB-derived CD34+ cell administration could not effectively retard the progression of chronic kidney injury in this model. Hence, we cultured UCB-CD34+ cells to enhance their vasculogenic potential using the quality and quantity culture (QQc) method, which was established for EPCs[12], and used in our previous report[13]. Once or twice administration of human UCB-QQc-CD34+ cells could not improve the time-course of kidney injury. However, administration of human UCB-QQc-CD34+ cells once a week for 3 consecutive weeks demonstrated a significant improvement of kidney function and reduction of renal histological damages. This is the first report of the application of human UCB-QQc-CD34+ cells as an effective regenerative therapy for CKD.
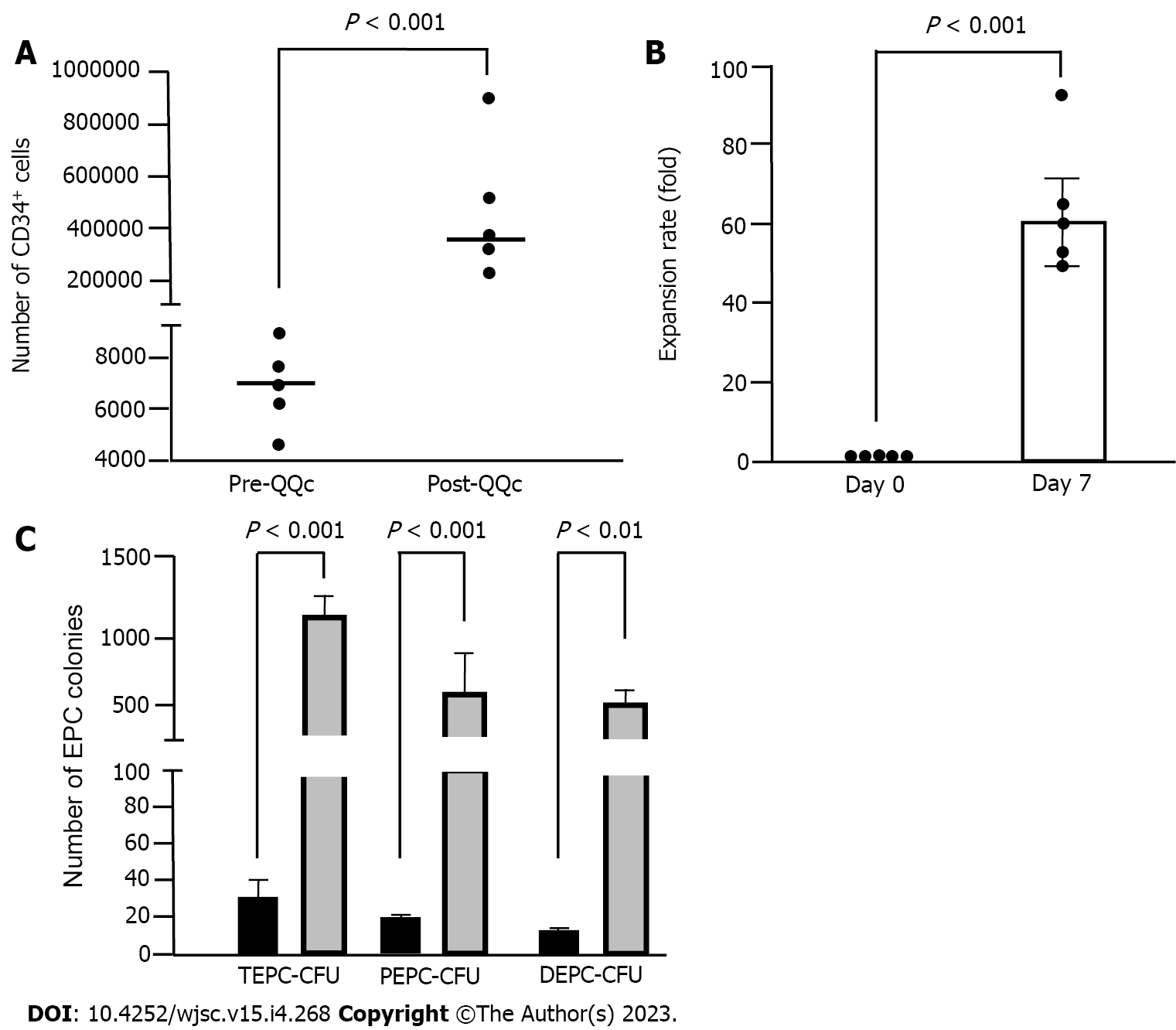
Human UCB-CD34+ cells were purchased from the RIKEN Bioresource Center (Tsukuba, Japan), plated at a density of 1 × 104 cells/well in a 24-well tissue culture plate (BD Falcon, Bedford), and cultured in QQc media for 7 d as previously described[12,13]. QQc uses a serum-free StemSpan SFEM media (Stem Cell Technologies, Vancouver, BC, Canada) with an optimized combination of growth factors as well as cytokines (20 ng/mL thyroid peroxidase, 20 ng/mL interleukine-6, 100 ng/mL SCF, 100 ng/mL Flt-3 Ligand, and 50 ng/mL vascular endothelial growth factor). All reagents were purchased from PeproTech Inc. (Rocky Hill, NJ, United States). This culture method significantly enhanced the vasculogenesis, proliferation, and colony-forming units of CD34+ cells[10]. UCB-CD34+ cells from five separate samples were cultured, and cell populations of pre- and post-QQc human UCB-CD34+ EPCs were evaluated using FACS analysis (FACSVerseTM, BD, United States). FlowJoTM (version 10.6, BD, United States) was used for data analysis.
EPC colony-forming units (EPC-CFUs) of pre-QQc and post-QQc human UCB-CD34+ cells were assessed using a colony-forming assay as previously described[12,13]. The EPC colony-forming assay was designed to evaluate the vasculogenic potential of EPCs and categorize total EPC-CFUs (TEPC-CFUs) into two different types of EPC-CFUs: Primitive (small cell-sized) and definitive (large cell-sized). Primitive EPC-CFUs (PEPC-CFUs) have greater potential for proliferation, whereas definitive EPC-CFUs (DEPC-CFUs) are predominantly vasculogenic populations with a greater differentiation potential. Briefly, 500 human UCB-CD34+ cells per dish were seeded into a 35-mm hydrophilic tissue culture dish. Ten to fourteen days later, total EPC colony-forming unit (TEPC-CFUs), PEPC-CFUs, and DEPC-CFUs were counted under light microscopy by two investigators who were blinded to the experimental conditions. Five samples of UCB-CD34+ cells were used for EPC colony-forming assay. All experiments were performed in triplicate.
Six-week-old male NOD/SCID mice were purchased from Charles River Laboratories Inc. (Tsukuba, Japan) and used for all experiments. All animal use was in accordance with institutional and ARRIVE guidelines (https://arriveguidelines.org). The experimental protocol was approved by the Administration Committee of Experimental Animals at Kamakura Techno-science, Inc. (No. 20-034, 20-079, Kanagawa, Japan).
The CKD animal model (adenine-induced chronic tubulointerstitial kidney injury) using NOD/SCID mouse was developed in collaboration with Kamakura Techno-science, Inc. First, several doses of adenine were used to optimize and induce progressive tubulointerstitial kidney injury. Kidney damage showed a dose-dependent pattern, and the optimized dose and period of adenine diet ingestion were finally determined as 0.14% and 3 wk, respectively.
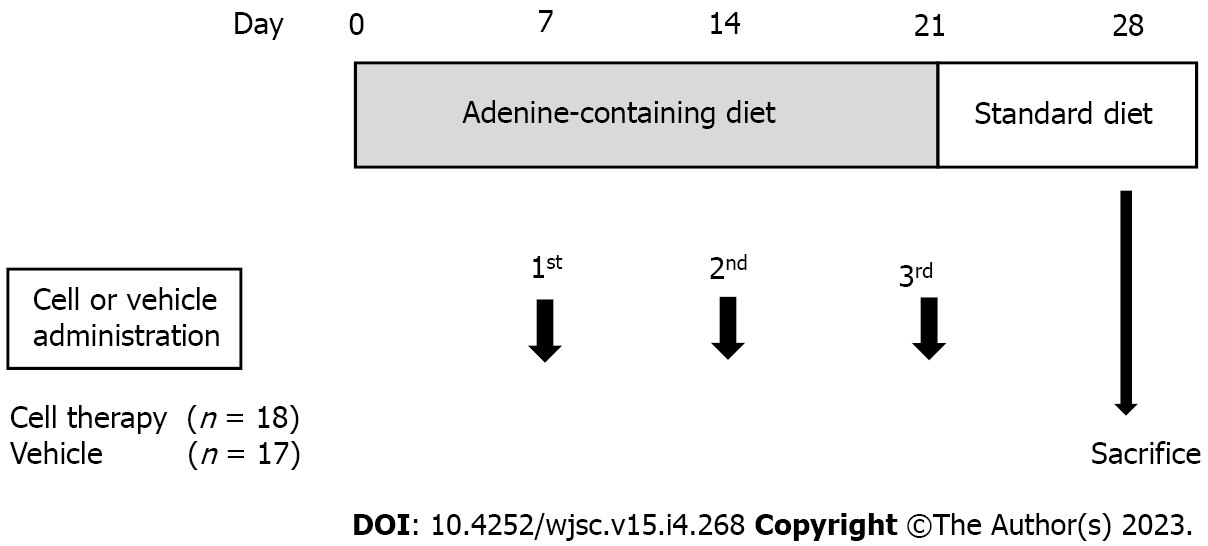
The experimental design is shown in Figure 1. Thirty-five male NOD/SCID mice were used in this experiment. All mice had free access to tap water, and were fed a 0.14% (w/w) adenine-containing diet from day 0 to day 21. The adenine-containing diet was stopped on day 21 and changed to standard laboratory chow diet (CRF-1 powder diet, Oriental Yeast Co., Ltd., Tokyo, Japan) from days 22 to 28 in all mice. Body weight and food intake were measured weekly. Blood sampling from the tail vein was performed on days 0, 7, 14, 21, and 28. After evaluating the serum creatinine levels on day 7, the mice were divided into two groups, i.e., control group (n = 17) and the cell therapy group (n = 18). After blood sampling on day 28, mice were anesthetized using isoflurane, and kidney tissues were collected for histological analysis. All animal experiments were performed at Kamakura Techno-science, Inc. (Kanagawa, Japan).
Mice in the control group were injected with 100 µL of vehicle (Iscove’s modified Dulbecco’s medium: IMDM), and those in the cell therapy group were injected with 1 × 106 UCB-QQc-CD34+ cells in 100 µL IMDM via the tail vein. UCB-QQc-CD34+ cells were administered three times on days 7, 14, and 21. Cell purity and viability were evaluated just before cell therapy.
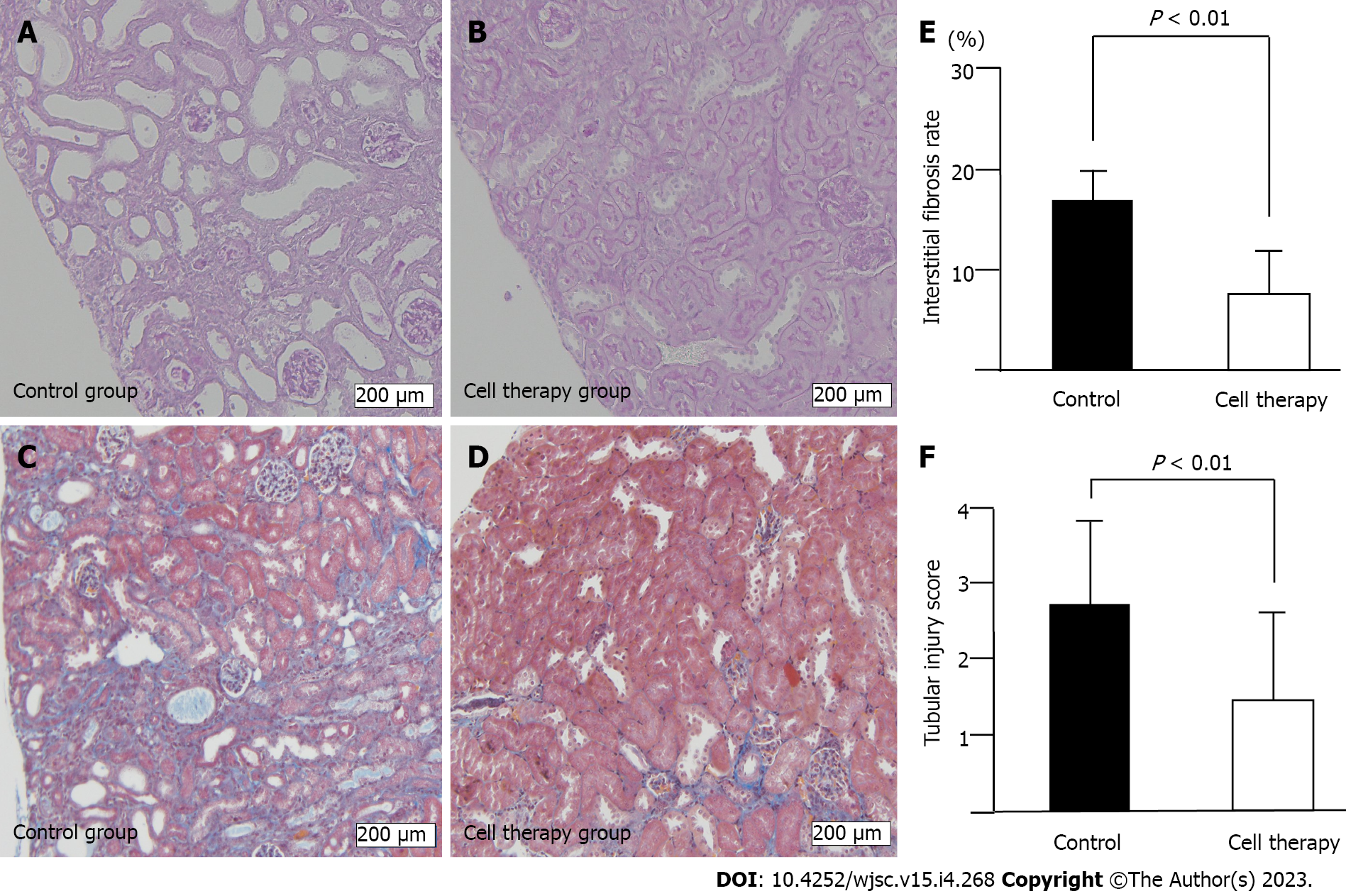
Serial blood samples were drawn from the tail vein on days 0, 7, 14, 21, and 28, and serum creatinine levels were immediately measured using DRI-CHEM CRE-P III (FUJIFILM Co., Ltd., Tokyo, Japan). Kidney tissues were harvested and fixed in 4% paraformaldehyde, transferred to 70% ethanol, and embedded in paraffin. From these paraffin blocks, 2 μm-thick sections were prepared and stained with periodic acid-Schiff (PAS) and Masson’s trichrome (MT) stain for analysis of tubular damage and interstitial fibrosis, respectively. To examine the extent of tubulointerstitial injury, 10 non-overlapping fields from the entire cortical and outer medulla areas in PAS-stained specimens and MT-stained specimens were captured under 100 × magnification. Tubulointerstitial injury in PAS-stained sections were categorized as tubular dilatation with epithelial and tubular atrophy. The degree of injury was scored on a scale of 0 to 4 according to the percentage of the damaged area; 0: 0%, normal; 1, 0%-25%; 2, 25%-50%;3, 50%-75%; and 4, 75% or more. Interstitial fibrosis in MT-stained sections was quantified as fibrotic area per total area (%) and measured using an automatic image analyzer (cellSens, Olympus, Japan). Tubulointerstitial injury score and interstitial fibrosis area were evaluated in each mouse (n = 17 in the control group and n = 18 in the cell therapy group), and mean values in each mouse were used to calculate the group mean value for further comparison between the control group and cell therapy group.
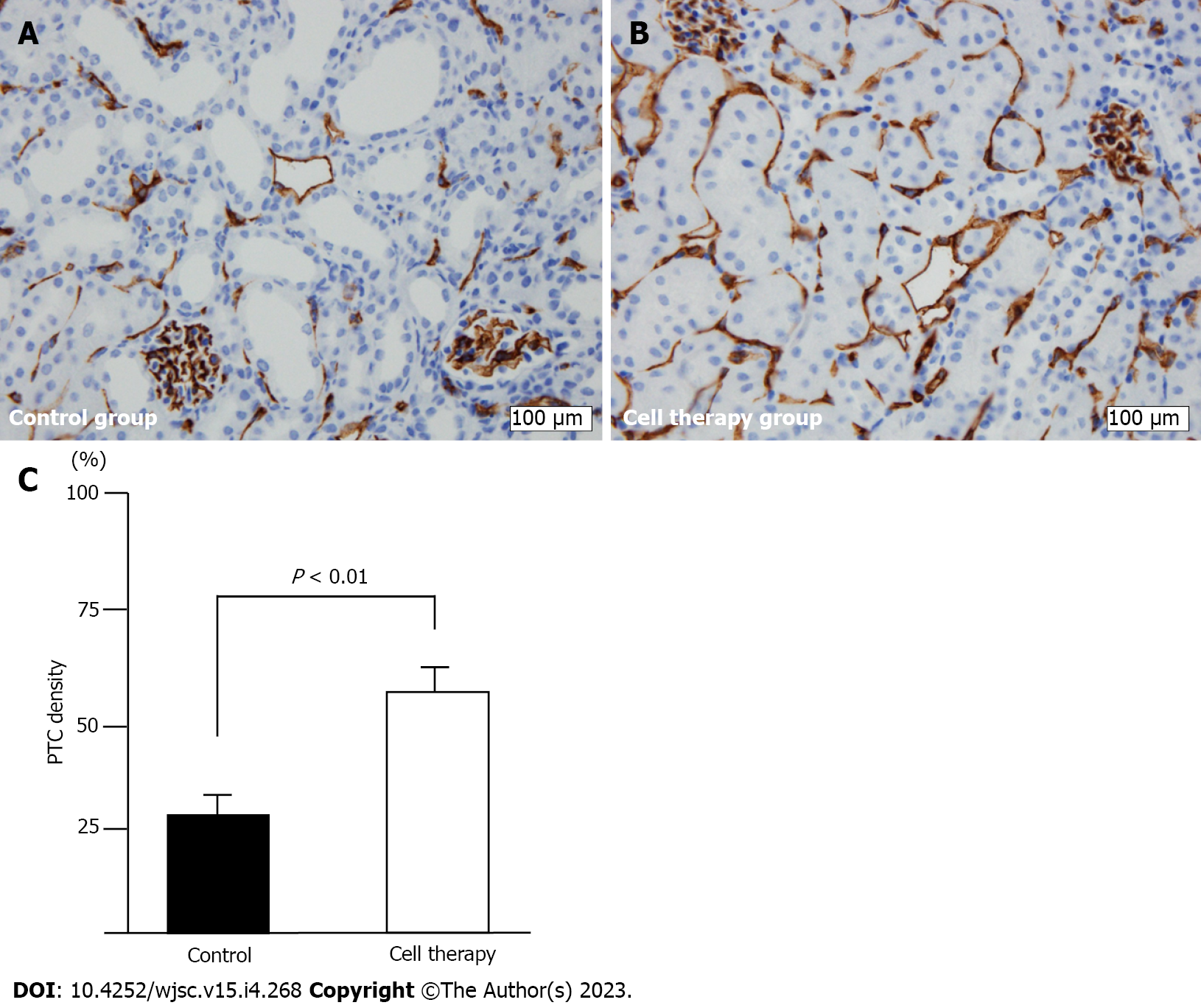
Since CD31 antigen is constitutively expressed on the endothelial cell surface, the loss of CD31-positive staining most likely reflects capillary loss. We performed CD31 immunostaining to evaluate peritubular capillary (PTC) loss using 3 μm-thick paraffin sections. PTCs were identified by immunostaining with a rabbit monoclonal anti-CD31 (PECAM-1) antibody (1:100; #77699, Cell Signaling Technology, Danvers, MA, United States). Immunostaining was performed using a BOND-III autostainer (Leica Biosystems, Wetzlar, Germany) according to the manufacturer’s protocol. Briefly, after deparaffinization of paraffin sections, heat-induced epitope retrieval was performed using BOND Epitope Retrieval Solution 2 (prediluted, pH 9.0; Leica Biosystems, Wetzlar, Germany) for 20 min at 100 °C. Sections were incubated sequentially with endogenous peroxidase block for 5 min, primary antibody for 30 min, secondary detection polymer for 10 min, diaminobenzidine for 10 min, and hematoxylin for 5 min.
PTC density was evaluated according to a previously published method with some modifications[11]. Twenty randomly selected fields encompassing the renal cortex and outer medulla were randomly captured by digital imaging (× 400). Each image was divided into 270 squares using a grid, and the number of squares with CD31-positive staining was counted. The PTC density was represented as a percentage of CD31 positive squares per total number of squares.
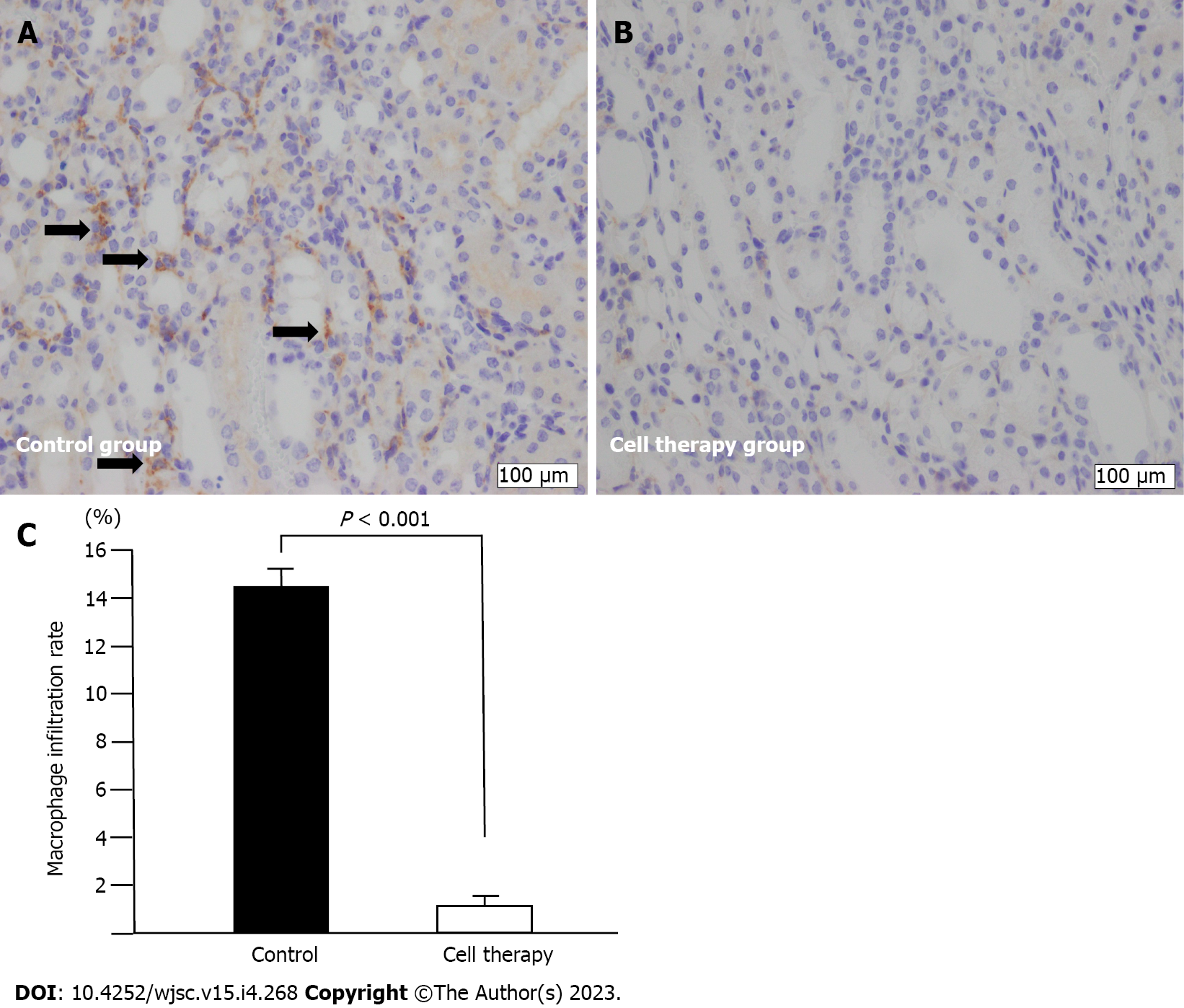
Macrophages were identified using a rabbit polyclonal anti-F4/80 antibody (1:100; ab100790, Abcam, Cambridge, United Kingdom). Ten non-overlapping fields in the cortical and outer medulla areas were randomly selected and captured by digital imaging (× 100). The degree of macrophage infiltration was calculated using the 270-square grid method, as described above. The rate of macrophage infiltration was represented as a percentage of F4/80- positive squares relative to the entire cortical and outer medulla areas. Macrophage infiltration rate and PTC density were assessed in the control group and cell therapy group.
All data are expressed as the mean ± SE, unless otherwise specified. Comparison between two groups were performed using the Mann-Whitney U test. Differences in time-course of serum creatinine levels during the experimental period between the control group and the cell therapy group were analyzed using repeated measures analysis of variance (ANOVA). Statistical analysis was performed using SPSS version 11.0 software (SPSS Inc., Chicago, IL, United States) and a P value < 0.05 was considered statistically significant.
The QQc significantly increased the number of CD34+ cells. CD34+ positivity per the 1 × 104 initially seeded cells was significantly increased in post-QQc-CD34+ cells compared with that in pre-QQc-CD34+ cells (353821 ± 122429 vs 7341 ± 947, P < 0.001) (Figure 2A). Cell viability of QQc-CD34+ cells was higher than that of pre-QQc CD34+ cells (93.6% ± 1.3% vs 87.0% ± 0.8%, P < 0.01). After seven days of culturing using the QQc method, the number of CD34+ cells showed almost a 60-fold increase on day 7 compared with that on day 0 (59.0 ± 10.4 vs 1-fold, P < 0.001) (Figure 2B). Post-QQc CD34+ cells formed more TEPC-CFUs than did pre-QQc CD34+ cells (1138.0 ± 124.0 vs 27.0 ± 4.5, P < 0.001) (Figure 2C). Moreover, both PEPC-CFUs and DEPC-CFUs showed a significant increase after QQc compared with pre-QQc (PEPC-CFUs: 17.4 ± 3.0 vs 666.0 ± 127.5, P < 0.001; DEPC-CFUs: 9.4 ± 2.5 vs 472.0 ± 112.0, P < 0.01).
The body weight was not significantly different between the cell therapy and control groups on days 0, 7, 14, 21, and 28. Diet consumption was also comparable between the two groups throughout the experimental period (data not shown).
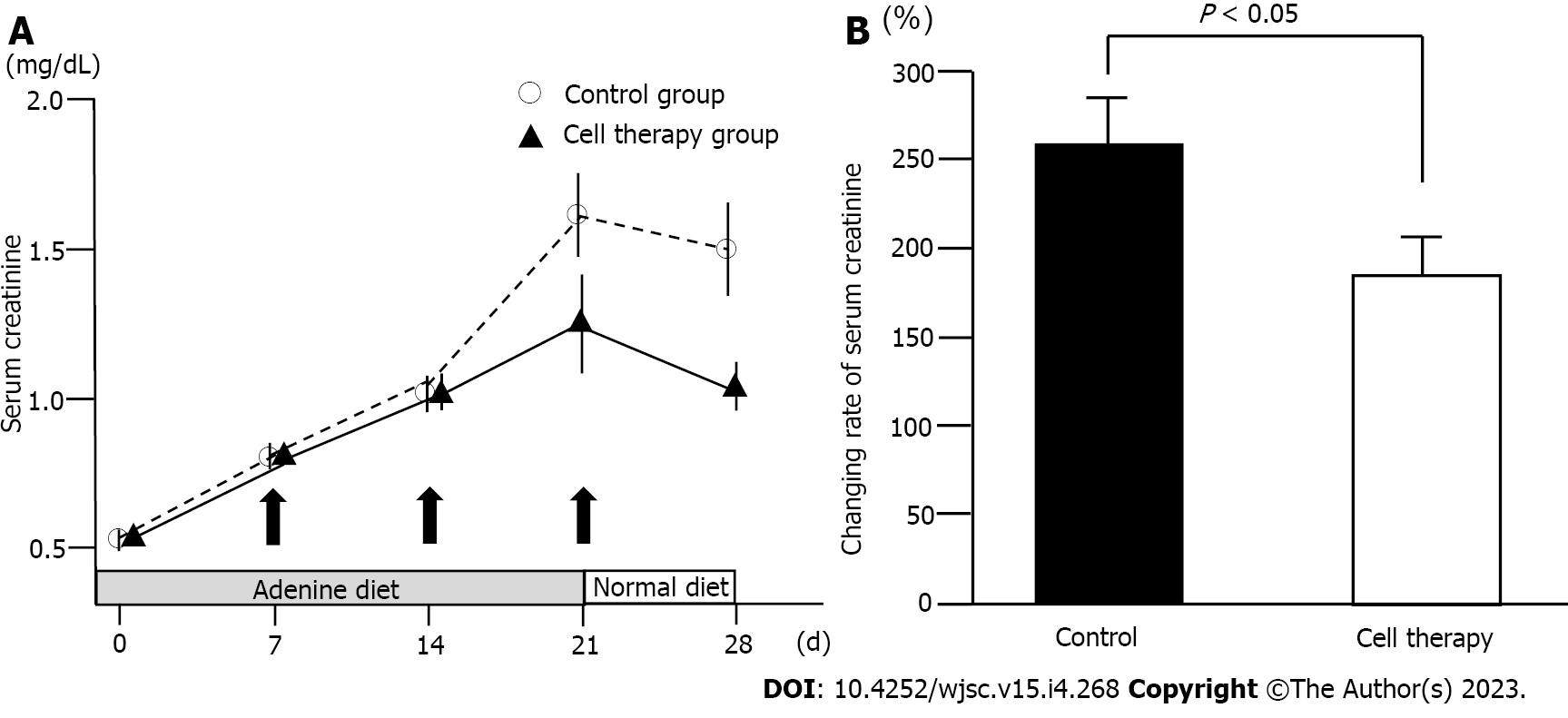
To evaluate the role of UCB-QQc-CD34+ cell therapy in renal injury, serum creatinine levels were determined in the cell therapy and control groups. The time-course in serum creatinine levels is shown in Figure 3A. Serum creatinine levels were not statistically different between the cell therapy and control groups (0.56 mg/ dL ± 0.02 mg/dL vs 0.61 mg/dL ± 0.03 mg/dL) on day 0. During adenine-containing diet feeding, serum creatinine levels showed a gradual and constant increase in the control group (1.51 mg/dL ± 0.14 mg/dL on day 21) demonstrating a state of renal dysfunction. After switching to the standard diet on day 22, serum creatinine levels in the control group decreased to 1.41 mg/dL ± 0.14 mg/dL on day 28. In the cell therapy group, serum creatinine levels increased until day 14 and then showed a gradual increasing trend on day 21 (1.29 mg/dL ± 0.09 mg/dL). After switching to the standard diet, serum creatinine levels decreased to 1.09 mg/dL ± 0.09 mg/dL on day 28 in the cell therapy group. Time-course of serum creatinine levels between the control and the cell therapy groups showed borderline significance (P = 0.06). However, the relative ratio of changes in serum creatinine levels from day 0 to day 28 showed a statistically significant difference between the control and the cell therapy groups (255.1% ± 25.9% vs 185.9% ± 18.5%, P = 0.035) (Figure 3B). Thus, repetitive administration of UCB-QQc-CD34+ cells significantly improved the time-course of adenine-induced chronic kidney injury.
The adenine-containing diet induced prominent tubular damage, including tubular dilatation and tubular atrophy accompanied by interstitial fibrosis (Figure 4A-D). In line with the reduced time-course of renal injury in cell therapy mice, the interstitial fibrosis area was significantly reduced in the cell therapy group compared with that in the control group (8.1% ± 5.4% vs 16.3% ± 7.0%, P < 0.01) (Figure 4E). The tubular damage score was also significantly decreased in the cell therapy group compared with that in the control group (1.48 ± 1.27 vs 2.83 ± 1.00, P < 0.01) (Figure 4F).
Representative images of PTC density as evaluated by CD31 immunohistochemical staining are shown in Figure 5. CD31+ staining was significantly decreased, especially around the dilated and degenerated tubules, in this model (Figure 5A). The cell therapy group showed significantly higher PTC density than did the control group (57.3% ± 6.4% vs 31.0% ± 6.1%, P < 0.01) (Figure 5B and C).
Macrophage infiltration is shown in Figure 6. Infiltration of F4/80-positive macrophages was mainly observed in the area with interstitial fibrotic changes and dilated and degenerated tubules in the control group (Figure 6A). However, macrophage infiltration was dramatically reduced in the cell therapy group compared with that in the control group (1.3% ± 0.3% vs 14.4% ± 1.1%, P < 0.001) (Figure 6B and C).
We demonstrated that repetitive administration of human UCB-QQc-CD34+ cells significantly improved the time-course of adenine-induced tubulointerstitial injury in mice. The relative changes in serum creatinine levels from baseline to day 28 were significantly improved in the cell therapy group compared with those in the control group. Interstitial fibrosis area and tubulointerstitial injury score were also significantly improved by cell therapy. The PTC network, visualized by CD31+ staining, was found to be severely damaged in adenine-induced kidney injury. However, PTC rarefaction was significantly improved by cell therapy. Furthermore, macrophage infiltration was dramatically reduced in the cell therapy group. These results might imply the significant potential of CD34+ cell-based therapy for clinical application in CKD.
CKD progression is caused by multiple factors, including diabetes, hypertension, and glomerulonephritis. Regardless of the diverse causes of CKD, tubulointerstitial damage is the final common pathway that results in its transition to ESRD[14]. Therefore, one major target of cell-based regenerative therapy for CKD is the arrest of chronic tubular and interstitial damage. Loss of integrity of the intrarenal microvasculature is also a major contributor to the progression of renal diseases[15-17]. Tubular damage, interstitial fibrosis, and microvasculature impairment are associates with each other and form a vicious cycle to promote CKD[18-24]. A close association between PTC rarefaction and tubulointerstitial fibrosis has been proven in the human kidney[20]. Hypoxia due to PTC rarefaction induces the production of local inflammatory cytokines[25], which in turn, worsen hypoxia in inflamed tissues. Thus, hypoxia also creates a vicious cycle of inflammation[26].
CD34+ cells have been used in regenerative therapy for several organ damages, including critical limb ischemia[27,28], myocardial ischemia[29], stroke[30], and acute kidney injury[31]. CD34+ cells possess two important properties that are considered necessary for regenerative therapy: Enhanced angiogenic and anti-inflammatory potential[10,11]. Externally administered CD34+ EPCs improved kidney injury such as endothelial cell damage in several animal models[13,15,19,32-35]. In cases of acute and severe renal endothelial cell injury, intrinsic repair may not function sufficiently[36]. Furthermore, endothelial disturbances related to ischemia induce permanent damage to PTCs[37]. Therefore, a strategy for vascular protection or angiogenesis might be important for the long-term preservation of kidney function. Recently, horizontal transfer of regenerative information via extracellular vesicles (EVs) between regeneration associated stem/progenitor cells and injured cells in the damaged organ was shown to be a major regenerative mechanism. Exosomes from human CD34+ cells, by themselves, have demonstrated angiogenic activity in in vivo and in vitro experiments[38,39]. Hence, CD34+ based cell therapy could be an excellent strategy to induce vasculogenesis and repair kidney function.
Regarding cell administration, frequency of transplantation as well as the number and regenerative potential of transplanted cells is thought to be very important. Multiple rather than single administration might show more advantages to improve organ damage. Lv et al[40] demonstrated that multiple administration of UCB-mesenchymal stem cells showed a superior therapeutic effect compared with that of single administration in an animal model of chemotherapy-induced premature ovarian failure. We also demonstrated that repetitive administration (3 times, once in a week) of cultured UCB-CD34+ cells significantly improved the time-course of adenine-induced chronic kidney injury. The causative signals for CKD, including oxidative stress, tissue hypoxia, and inflammatory cell infiltration with the release of inflammatory cytokines continuously injure the kidney. Therefore, multiple administration of regeneration-associated CD34+ cells at fixed time intervals might be a better treatment strategy for CKD. For the number of administered cells, 1 × 106 cells/20 g mouse is equivalent to about 50 × 106/kg in humans, an amount that is probably not achievable clinically. It is known that in cases of cell administration via the tail vein in mice, many cells are trapped in the lung, spleen, and bone marrow (extra-renal trap)[13]. Extra-renal trap might necessitate more cells to be administered in mice to deliver sufficient number of cells into the kidney. Therefore, future clinical trials should consider the route of cell administration. To get better effects in future clinical trials, cells should be designed for direct arterial administration.
Herein, we used human UCB-derived CD34+ cells. UCB-CD34+ cells can be used as an off-the-shelf treatment. Because EPCs from patients with several diseases have impaired function and are insufficient, allogeneic source is considered as a good alternative. Immune reaction against allogeneic cells might limit their use. However, a recent study reported the immunosuppressive effect of cultured human EPCs. The proliferation and activation of mouse T cells were significantly suppressed by co-culture with allogeneic human UCB-EPCs[41]. Therefore, cultured UCB-EPCs with enhanced potential might have a chance of emerging as a possible tool for allogeneic cell therapy. One major limitation to using UCB-CD34+ cells is the limited number obtained from one UCB donor. Regarding this, QQc of UCB-CD34+ cells significantly increased the number of CD34+ cells (almost 60 folds) in one week, and EPC-CFUs (total, primitive, and definite EPC-CFUs) were significantly increased. The increased UCB-CD34+ cell number enabled multiple administration. Furthermore, the enhanced vasculogenic and anti-inflammatory potential of UCB-CD34+ cells might be critical for preserving PTC integrity and reducing inflammatory cell infiltration.
There are several limitations to this study. First, the effect of tissue hypoxia was not evaluated to establish the relationship among tubular damage, interstitial fibrosis, PTC integrity, macrophage infiltration, and hypoxia in the kidney tissue of control and cell therapy-treated mice. Second, the cellular behavior of UCB-CD34+ cells (homing and staying in injured kidney) remain undetermined. Third, we could not compare the contents (mRNA, miRNA, and proteins, including growth factors and cytokines) of EVs of pre-QQc and post-QQc UCB-CD34+ cells. Therefore, the EV-mediated mechanism by which UCB-QQc-CD34+ cells showed an effective response in repairing kidney injury remains to be elucidated. We evaluated the effect of cell therapy during the progressive phase, and not the advanced ESRD stage. Therefore, it is unclear whether cell therapy might be effective at the ESRD stage. Stopping adenine diet induced recovery from dysfunction. However, it remains unknown whether stopping adenine diet without cell therapy brings kidney function completely to normal, because we did not conduct a longer follow-up after switching adenine diet to normal chow. At the same time, we could not elucidate whether the effects of cell therapy were long-lasting since the animals were sacrificed only one week after the last dose of cells. These limitations should be addressed in future research.
In conclusion, although the more precise mechanisms should be clarified, this study provides a new insight in the field of regenerative medicine. Repetitive administration of human UCB-QQc-CD34+ cells improved kidney function and reduced histological damage in adenine-induced tubulointerstitial injury. Tubular damage and interstitial fibrosis were significantly improved by cell therapy in accordance with improved PTC integrity and reduced inflammatory cell infiltration. Hence, clinical trials using repetitive administration of regenerative CD34+ cells with enhanced proliferative and angiogenic potential might be expected for patients with progressive CKD.
Chronic kidney Disease (CKD) constitutes a serious public health burden worldwide, and there is no established treatment to impede the progression or restore kidney function in human CKD.
To discover and establish a new effective treatment for CKD is urgently needed. Cell-based therapy has a potential as a new effective treatment for progressive CKD.
To evaluate the efficacy of cultured human cord-blood-derived CD34+ cells.
Progressive tubulointerstitial injury with kidney dysfunction was made in mice. Human umbilical cord blood (UCB)-derived CD34+ cells were incubated in vasculogenic conditioned medium for 1 wk to increase the number of CD34+ cells and ability to form endothelial progenitor cell (EPC)-colony-forming units. These cultured human CD34+ cells were administered on days 7, 14, and 21 after the start of adenine diet.
Cell therapy significantly improved the time-course of progressive kidney dysfunction. Pathological injuries including tubular injury and interstitial fibrosis were significantly reduced in the cell therapy group compared with those in the control group. Preserved microvasculature integrity and decreased macrophage infiltration were shown in the cell therapy group.
Early intervention using human cultured CD34+ cells significantly improved the progression of tubulointerstitial kidney injury.
In future research, the efficacy of cultured human CD34+ cells for more advanced CKD such as end-stage renal disease (ESRD), cell behavior (homing and staying in injured kidney) after cell administration, differences in extracellular vesicles from pre- and post-incubated UCB-derived CD34+ cells, should be evaluated for further analysis of the efficacy of cell therapy. A future clinical trial of cell therapy for progressive CKD might be expected.
We gratefully thank Mr. Hirata (Center for Clinical and Translational Science) and Mr. Okamura (sRIIM) in Shonan Kamakura General Hospital for their support of cell preparation. We would like to thank Editage (www.editage.com) for English language editing.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Research and experimental medicine
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Goebel WS, United States; Zhang LS, China S-Editor: Chen YL L-Editor: A P-Editor: Chen YL
| 1. | Fraser SDS, Roderick PJ. Kidney disease in the Global Burden of Disease Study 2017. Nat Rev Nephrol. 2019;15:193-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 58] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 2. | Venkatachalam MA, Weinberg JM, Kriz W, Bidani AK. Failed Tubule Recovery, AKI-CKD Transition, and Kidney Disease Progression. J Am Soc Nephrol. 2015;26:1765-1776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 483] [Cited by in RCA: 555] [Article Influence: 55.5] [Reference Citation Analysis (0)] |
| 3. | Bikbov B, Perico N, Remuzzi G; on behalf of the GBD Genitourinary Diseases Expert Group. Disparities in Chronic Kidney Disease Prevalence among Males and Females in 195 Countries: Analysis of the Global Burden of Disease 2016 Study. Nephron. 2018;139:313-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 146] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 4. | Papazova DA, Oosterhuis NR, Gremmels H, van Koppen A, Joles JA, Verhaar MC. Cell-based therapies for experimental chronic kidney disease: a systematic review and meta-analysis. Dis Model Mech. 2015;8:281-293. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 72] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 5. | Makhlough A, Shekarchian S, Moghadasali R, Einollahi B, Hosseini SE, Jaroughi N, Bolurieh T, Baharvand H, Aghdami N. Safety and tolerability of autologous bone marrow mesenchymal stromal cells in ADPKD patients. Stem Cell Res Ther. 2017;8:116. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 53] [Article Influence: 6.6] [Reference Citation Analysis (1)] |
| 6. | Makhlough A, Shekarchian S, Moghadasali R, Einollahi B, Dastgheib M, Janbabaee G, Hosseini SE, Falah N, Abbasi F, Baharvand H, Aghdami N. Bone marrow-mesenchymal stromal cell infusion in patients with chronic kidney disease: A safety study with 18 months of follow-up. Cytotherapy. 2018;20:660-669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 53] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 7. | Yang CC, Sung PH, Cheng BC, Li YC, Chen YL, Lee MS, Yip HK. Safety and efficacy of intrarenal arterial autologous CD34+ cell transfusion in patients with chronic kidney disease: A randomized, open-label, controlled phase II clinical trial. Stem Cells Transl Med. 2020;9:827-838. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 8. | Kamijo-Ikemori A, Sugaya T, Hibi C, Nakamura T, Murase T, Oikawa T, Hoshino S, Hisamichi M, Hirata K, Kimura K, Shibagaki Y. Renoprotective effect of the xanthine oxidoreductase inhibitor topiroxostat on adenine-induced renal injury. Am J Physiol Renal Physiol. 2016;310:F1366-F1376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 9. | Rivera-Valdés JJ, García-Bañuelos J, Salazar-Montes A, García-Benavides L, Dominguez-Rosales A, Armendáriz-Borunda J, Sandoval-Rodríguez A. Human adipose derived stem cells regress fibrosis in a chronic renal fibrotic model induced by adenine. PLoS One. 2017;12:e0187907. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 10. | Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964-967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6624] [Cited by in RCA: 6349] [Article Influence: 226.8] [Reference Citation Analysis (1)] |
| 11. | Abd-Allah SH, Shalaby SM, Abd-Elbary E, Saleh AA, El-Magd MA. Human peripheral blood CD34+ cells attenuate oleic acid-induced acute lung injury in rats. Cytotherapy. 2015;17:443-453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 12. | Masuda H, Iwasaki H, Kawamoto A, Akimaru H, Ishikawa M, Ii M, Shizuno T, Sato A, Ito R, Horii M, Ishida H, Kato S, Asahara T. Development of serum-free quality and quantity control culture of colony-forming endothelial progenitor cell for vasculogenesis. Stem Cells Transl Med. 2012;1:160-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 58] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 13. | Ohtake T, Kobayashi S, Slavin S, Mochida Y, Ishioka K, Moriya H, Hidaka S, Matsuura R, Sumida M, Katagiri D, Noiri E, Okada K, Mizuno H, Tanaka R. Human Peripheral Blood Mononuclear Cells Incubated in Vasculogenic Conditioning Medium Dramatically Improve Ischemia/Reperfusion Acute Kidney Injury in Mice. Cell Transplant. 2018;27:520-530. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 14. | Nath KA. Tubulointerstitial changes as a major determinant in the progression of renal damage. Am J Kidney Dis. 1992;20:1-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 714] [Cited by in RCA: 775] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 15. | Kang DH, Hughes J, Mazzali M, Schreiner GF, Johnson RJ. Impaired angiogenesis in the remnant kidney model: II. Vascular endothelial growth factor administration reduces renal fibrosis and stabilizes renal function. J Am Soc Nephrol. 2001;12:1448-1457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 303] [Cited by in RCA: 323] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 16. | Bohle A, Mackensen-Haen S, Wehrmann M. Significance of postglomerular capillaries in the pathogenesis of chronic renal failure. Kidney Blood Press Res. 1996;19:191-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 154] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 17. | Fine LG, Orphanides C, Norman JT. Progressive renal disease: the chronic hypoxia hypothesis. Kidney Int Suppl. 1998;65:S74-S78. [PubMed] |
| 18. | Norman JT, Clark IM, Garcia PL. Hypoxia promotes fibrogenesis in human renal fibroblasts. Kidney Int. 2000;58:2351-2366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 269] [Cited by in RCA: 282] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 19. | Kang DH, Joly AH, Oh SW, Hugo C, Kerjaschki D, Gordon KL, Mazzali M, Jefferson JA, Hughes J, Madsen KM, Schreiner GF, Johnson RJ. Impaired angiogenesis in the remnant kidney model: I. Potential role of vascular endothelial growth factor and thrombospondin-1. J Am Soc Nephrol. 2001;12:1434-1447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 256] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 20. | Choi YJ, Chakraborty S, Nguyen V, Nguyen C, Kim BK, Shim SI, Suki WN, Truong LD. Peritubular capillary loss is associated with chronic tubulointerstitial injury in human kidney: altered expression of vascular endothelial growth factor. Hum Pathol. 2000;31:1491-1497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 157] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 21. | Nangaku M. Chronic hypoxia and tubulointerstitial injury: a final common pathway to end-stage renal failure. J Am Soc Nephrol. 2006;17:17-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 784] [Cited by in RCA: 867] [Article Influence: 43.4] [Reference Citation Analysis (0)] |
| 22. | Tanaka T. A mechanistic link between renal ischemia and fibrosis. Med Mol Morphol. 2017;50:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 23. | Mimura I, Nangaku M. The suffocating kidney: tubulointerstitial hypoxia in end-stage renal disease. Nat Rev Nephrol. 2010;6:667-678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 239] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 24. | Grgic I, Campanholle G, Bijol V, Wang C, Sabbisetti VS, Ichimura T, Humphreys BD, Bonventre JV. Targeted proximal tubule injury triggers interstitial fibrosis and glomerulosclerosis. Kidney Int. 2012;82:172-183. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 316] [Cited by in RCA: 387] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 25. | Snodgrass RG, Boß M, Zezina E, Weigert A, Dehne N, Fleming I, Brüne B, Namgaladze D. Hypoxia Potentiates Palmitate-induced Pro-inflammatory Activation of Primary Human Macrophages. J Biol Chem. 2016;291:413-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 70] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 26. | Koeppen M, Eckle T, Eltzschig HK. The hypoxia-inflammation link and potential drug targets. Curr Opin Anaesthesiol. 2011;24:363-369. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 42] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 27. | Ohtake T, Mochida Y, Ishioka K, Oka M, Maesato K, Moriya H, Hidaka S, Higashide S, Ioji T, Fujita Y, Kawamoto A, Fukushima M, Kobayashi S. Autologous Granulocyte Colony-Stimulating Factor-Mobilized Peripheral Blood CD34 Positive Cell Transplantation for Hemodialysis Patients with Critical Limb Ischemia: A Prospective Phase II Clinical Trial. Stem Cells Transl Med. 2018;7:774-782. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 28. | Kawamoto A, Katayama M, Handa N, Kinoshita M, Takano H, Horii M, Sadamoto K, Yokoyama A, Yamanaka T, Onodera R, Kuroda A, Baba R, Kaneko Y, Tsukie T, Kurimoto Y, Okada Y, Kihara Y, Morioka S, Fukushima M, Asahara T. Intramuscular transplantation of G-CSF-mobilized CD34(+) cells in patients with critical limb ischemia: a phase I/IIa, multicenter, single-blinded, dose-escalation clinical trial. Stem Cells. 2009;27:2857-2864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 170] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 29. | Losordo DW, Schatz RA, White CJ, Udelson JE, Veereshwarayya V, Durgin M, Poh KK, Weinstein R, Kearney M, Chaudhry M, Burg A, Eaton L, Heyd L, Thorne T, Shturman L, Hoffmeister P, Story K, Zak V, Dowling D, Traverse JH, Olson RE, Flanagan J, Sodano D, Murayama T, Kawamoto A, Kusano KF, Wollins J, Welt F, Shah P, Soukas P, Asahara T, Henry TD. Intramyocardial transplantation of autologous CD34+ stem cells for intractable angina: a phase I/IIa double-blind, randomized controlled trial. Circulation. 2007;115:3165-3172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 427] [Cited by in RCA: 386] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 30. | Banerjee S, Bentley P, Hamady M, Marley S, Davis J, Shlebak A, Nicholls J, Williamson DA, Jensen SL, Gordon M, Habib N, Chataway J. Intra-Arterial Immunoselected CD34+ Stem Cells for Acute Ischemic Stroke. Stem Cells Transl Med. 2014;3:1322-1330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 122] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 31. | Suzuki H, Ohtake T, Tsukiyama T, Morota M, Ishioka K, Moriya H, Mochida Y, Hidaka S, Sato T, Asahara T, Kobayashi S. Acute kidney injury successfully treated with autologous granulocyte colony-stimulating factor-mobilized peripheral blood CD34-positive cell transplantation: A first-in-human report. Stem Cells Transl Med. 2021;10:1253-1257. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 32. | Patschan D, Krupincza K, Patschan S, Zhang Z, Hamby C, Goligorsky MS. Dynamics of mobilization and homing of endothelial progenitor cells after acute renal ischemia: modulation by ischemic preconditioning. Am J Physiol Renal Physiol. 2006;291:F176-F185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 107] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 33. | Uchimura H, Marumo T, Takase O, Kawachi H, Shimizu F, Hayashi M, Saruta T, Hishikawa K, Fujita T. Intrarenal injection of bone marrow-derived angiogenic cells reduces endothelial injury and mesangial cell activation in experimental glomerulonephritis. J Am Soc Nephrol. 2005;16:997-1004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 74] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 34. | Huang TH, Chen YT, Sung PH, Chiang HJ, Chen YL, Chai HT, Chung SY, Tsai TH, Yang CC, Chen CH, Chang HW, Sun CK, Yip HK. Peripheral blood-derived endothelial progenitor cell therapy prevented deterioration of chronic kidney disease in rats. Am J Transl Res. 2015;7:804-824. [PubMed] |
| 35. | Li B, Cohen A, Hudson TE, Motlagh D, Amrani DL, Duffield JS. Mobilized human hematopoietic stem/progenitor cells promote kidney repair after ischemia/reperfusion injury. Circulation. 2010;121:2211-2220. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 136] [Cited by in RCA: 133] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 36. | Basile DP, Yoder MC. Renal endothelial dysfunction in acute kidney ischemia reperfusion injury. Cardiovasc Hematol Disord Drug Targets. 2014;14:3-14. [PubMed] |
| 37. | Basile DP, Donohoe D, Roethe K, Osborn JL. Renal ischemic injury results in permanent damage to peritubular capillaries and influences long-term function. Am J Physiol Renal Physiol. 2001;281:F887-F899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 266] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 38. | Sahoo S, Klychko E, Thorne T, Misener S, Schultz KM, Millay M, Ito A, Liu T, Kamide C, Agrawal H, Perlman H, Qin G, Kishore R, Losordo DW. Exosomes from human CD34(+) stem cells mediate their proangiogenic paracrine activity. Circ Res. 2011;109:724-728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 451] [Cited by in RCA: 497] [Article Influence: 35.5] [Reference Citation Analysis (0)] |
| 39. | Salybekov AA, Kunikeyev AD, Kobayashi S, Asahara T. Latest Advances in Endothelial Progenitor Cell-Derived Extracellular Vesicles Translation to the Clinic. Front Cardiovasc Med. 2021;8:734562. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 40. | Lv X, Guan C, Li Y, Su X, Zhang L, Wang X, Xia HF, Ma X. Effects of single and multiple transplantations of human umbilical cord mesenchymal stem cells on the recovery of ovarian function in the treatment of premature ovarian failure in mice. J Ovarian Res. 2021;14:119. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 41. | Naserian S, Abdelgawad ME, Afshar Bakshloo M, Ha G, Arouche N, Cohen JL, Salomon BL, Uzan G. The TNF/TNFR2 signaling pathway is a key regulatory factor in endothelial progenitor cell immunosuppressive effect. Cell Commun Signal. 2020;18:94. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 76] [Article Influence: 15.2] [Reference Citation Analysis (0)] |