Published online Apr 26, 2023. doi: 10.4252/wjsc.v15.i4.150
Peer-review started: December 20, 2022
First decision: January 6, 2023
Revised: January 20, 2023
Accepted: March 20, 2023
Article in press: March 20, 2023
Published online: April 26, 2023
Processing time: 127 Days and 0.1 Hours
Acute respiratory distress syndrome (ARDS) is a common and clinically devastating disease that causes respiratory failure. Morbidity and mortality of patients in intensive care units are stubbornly high, and various complications severely affect the quality of life of survivors. The pathophysiology of ARDS includes increased alveolar–capillary membrane permeability, an influx of protein-rich pulmonary edema fluid, and surfactant dysfunction leading to severe hypoxemia. At present, the main treatment for ARDS is mechanical treatment combined with diuretics to reduce pulmonary edema, which primarily improves symptoms, but the prognosis of patients with ARDS is still very poor. Mesen
Core Tip: Acute respiratory disease syndrome (ARDS) is a common disease with high morbidity and mortality. ARDS is characterized by increased alveolar-capillary membrane permeability, influx of protein-rich pulmonary edema fluid, and surfactant dysfunction, resulting in severe hypoxemia. Mesenchymal stem cells (MSCs) have the self-renewal and multilineage differentiation properties, and their immunomodulatory abilities have been implicated in the treatment of disease. Herein, we discuss the pathophysiology of ARDS and recent research surrounding the clinical application of MSCs in the treatment of ARDS.
- Citation: Liang TY, Lu LH, Tang SY, Zheng ZH, Shi K, Liu JQ. Current status and prospects of basic research and clinical application of mesenchymal stem cells in acute respiratory distress syndrome. World J Stem Cells 2023; 15(4): 150-164
- URL: https://www.wjgnet.com/1948-0210/full/v15/i4/150.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v15.i4.150
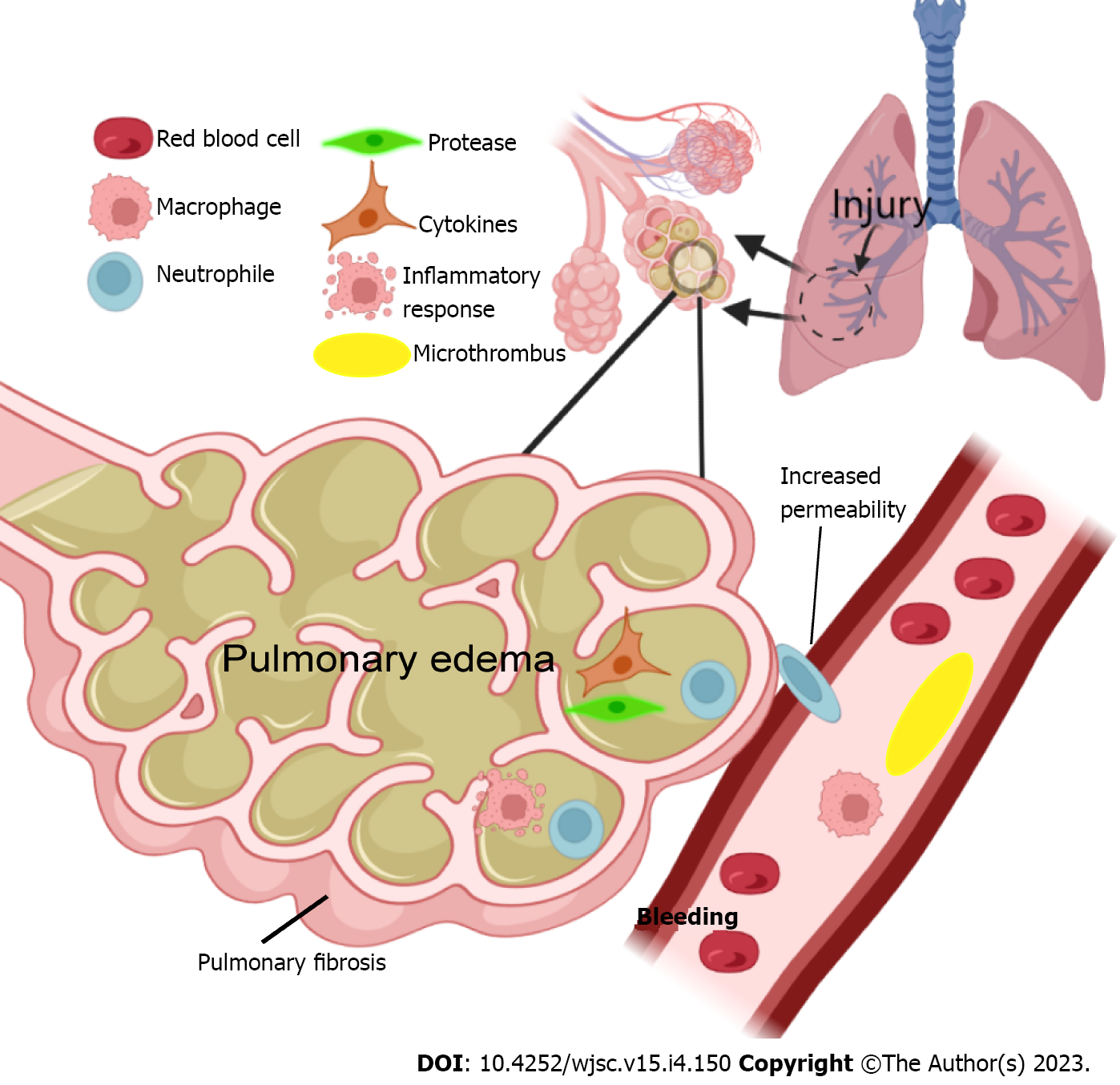
Acute respiratory distress syndrome (ARDS) is a clinicopathological condition characterized by increased lung fluid, decreased lung compliance, and severe hypoxemia[1,2]. ARDS was defined in 1994 by the American-European Consensus Conference[3]. After several decades of research and discussions, the current internationally recognized definition of ARDS is the Berlin definition which proposes three categories of ARDS based on the severity of hypoxemia: Mild [200 mmHg < arterial oxygen pressure (PaO2)/fraction of inspired oxygen (FiO2) < 300 mmHg], moderate (100 mmHg < PaO2/FiO2 < 200 mmHg), and severe (PaO2/FiO2 < 100 mmHg), along with explicit criteria related to the timing of the syndrome’s onset, the origin of edema, and chest radiograph findings[4-6]. The pathogenesis of ARDS is characterized by an unregulated inflammatory cascade with increased pulmonary endothelial and epithelial permeability[7]. Endogenous chemicals and microbial products linked to cell injury are hypothesized to attach to receptors on epithelial cells and alveolar macrophages, triggering an immunological response. The unrestricted synthesis of reactive oxygen species, leukocyte proteases, chemokines, and inflammatory substances that results to gradual lung damage. The immune-mediated reaction is known as a "cytokine storm"[8,9]. The pathophysiological changes that occur during the development of ARDS are shown in Figure 1. Currently, the clinical treatment of ARDS is rather limited and is mainly based on organ function support, such as lung protective ventilation, liver and kidney function protection, gastrointestinal function protection, venous thrombosis prevention, and nutritional support[10]. Despite the profound understanding of the molecular mechanism of ARDS, improvement of pulmonary ventilation strategies, and strengthening of supportive care for critically ill patients, the prognosis of patients with ARDS is still unsatisfactory. Currently, the global mortality rate of ARDS exceeds 40%, whilst 6%–10% of patients with respiratory failure may develop ARDS in the emergency room[11]. The long-term sequelae of ARDS include long-term cognitive impairment, psychological disease, neuromuscular weakness, pulmonary dysfunction, and decline in quality of life because of long-term medical expenses[12]. Therefore, new and safer therapies are urgently needed for ARDS treatment.
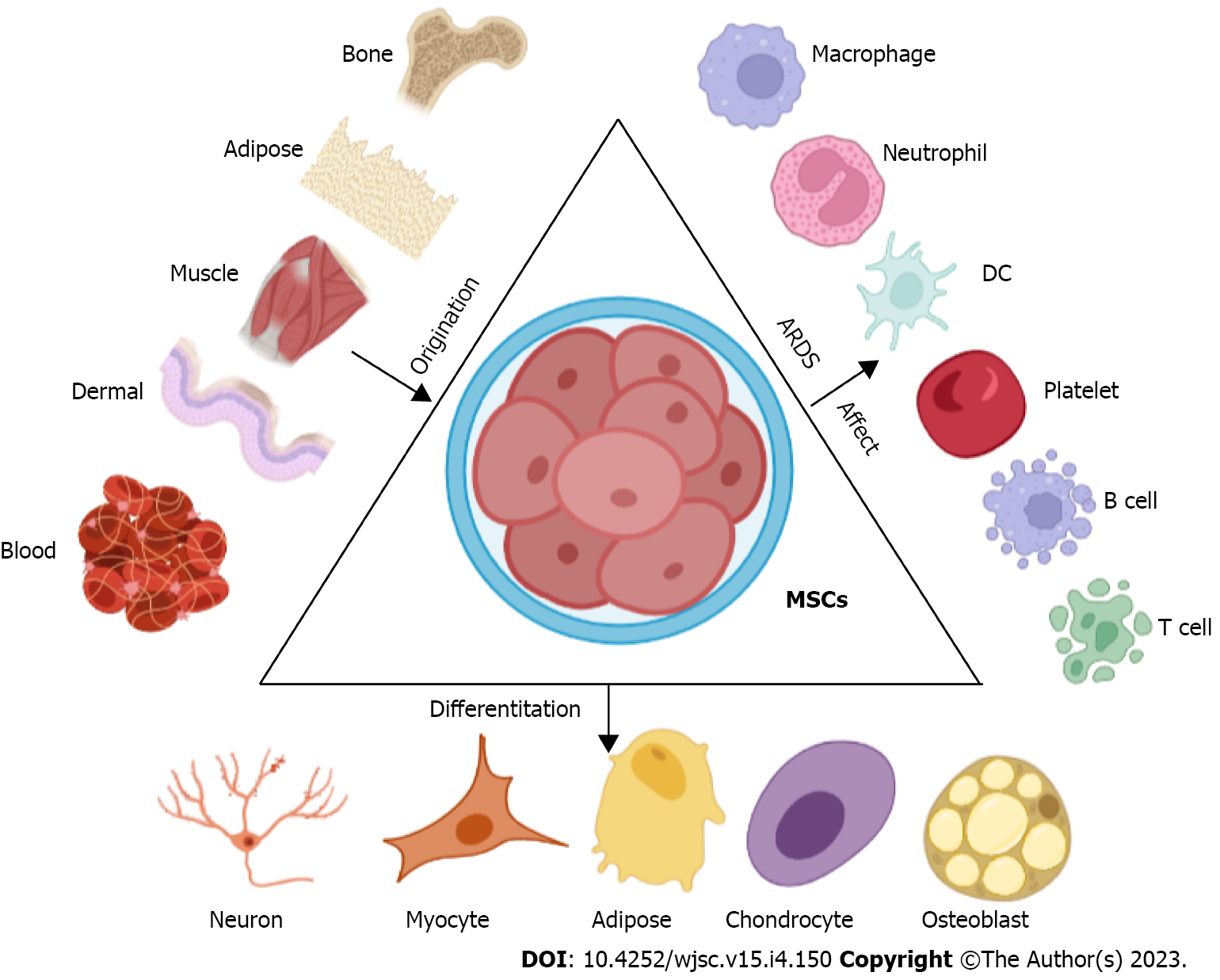
Mesenchymal stem cells (MSCs) were first described by Friedenstein et al[13], as an adherent, fibroblast-like cell population in the bone marrow (BM) that could regenerate rudiments of bone in vivo[13,14]. After decades of research, it has been found that MSCs are present in a variety of tissues and organs and can also differentiate into a variety of cells to play related roles (Figure 2)[15,16]. However, the understanding of MSCs is still inadequate. MSCs were officially defined by the International Society of Cell Therapy in 2006 as follows: (1) MSCs must display plastic-adherent capacities; (2) A simultaneous expression of stromal markers, an absence of hematopoietic or endothelial markers and human leukocyte antigen-DR surface molecules; and (3) An in vitro differentiation potential for osteoblasts, adipocytes, and chondroblasts[17,18]. The method of obtaining and culturing MSCs is simpler than other stem cells, and MSCs have broad application prospects in a variety of inflammatory-related diseases because of their unique immunomodulatory properties[19].
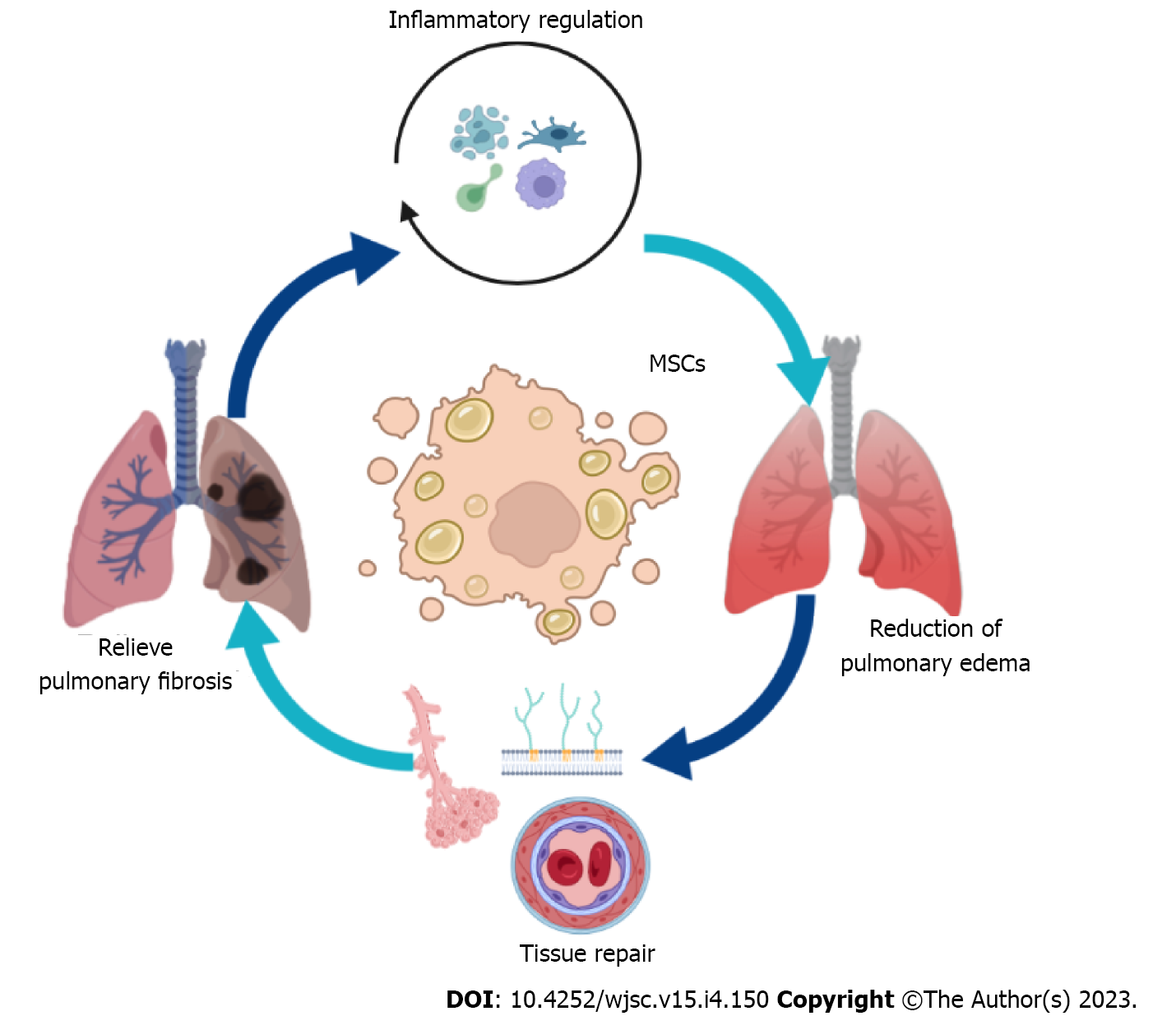
MSCs are considered a new approach for the treatment of ADRS[20]. The mechanism of MSCs in the treatment of ARDS is multifaceted, and the immunomodulatory effect of MSCs is a crucial aspect of it (Figure 3). At present, the collective view of the immune regulation ability of MSCs is based on the secretion of cytokines, such as tumor growth factor (TGF)-β and tumor necrosis factor-stimulated gene-6[21]. By releasing a variety of cytokines and extracellular vesicles (EVs), MSCs play anti-inflammatory and anti-cell death roles and promote the generation of microcirculation, thereby promoting the clearance of bacteria and alveolar fluid, alleviating organ damage, thus alleviating ARDS-related symptoms[22-24].
The hallmark of ARDS is a series of inflammatory responses. Uncontrollable inflammatory responses are known to cause catastrophic damage to various organs[25]. MSCs are pluripotent stem cells with immune properties that can secrete a variety of cytokines, such as anti-inflammatory factors, antiapoptotic factors, and antimicrobial peptides[26-28]. MSCs regulate immune activity via three different mechanisms: (1) Direct contact with tissue cells; (2) Production of a series of cytokines to regulate cell activities; and (3) Exerting immune effects by regulating the activity of T cells[29-31]. Currently, the MSC treatment of ARDS is mediated by controlling inflammatory responses. Therefore, the related mechanisms have become a hot research topic, and new research findings are constantly emerging, which introduce new views.
MSCs regulate the immune activity of the dendritic cells: Conventional dendritic cells (cDCs) are unique antigen-presenting cells that bridge antigen immunity and innate immunity and can be activated by MSCs as regulatory DCs[32,33]. An existing study has shown that after lung injury induced by lipopolysaccharide, a large number of DCs accumulate in the lungs, which in turn aggravates lung inflammation and lung injury[34]. The underlying mechanism may involve the polarization of the T-helper cell (Th) 1 response and regulating neutrophil infiltration[35,36]. The aggregation of cDCs can lead to the activation of the Th1 pathway and aggravate the inflammatory response. At the same time, cDCs can also recruit neutrophils, prolong the life of neutrophils, upregulate innate immunity, and further intensify the inflammatory response[37,38]. MSCs also abolish the capacity of mDCs to migrate to chemokine (C–C motif) ligand 19, for DCs to display major histocompatibility complex class II peptide complexes recognized by specific antibodies, and for ovalbumin-pulsed DCs to support antigen-specific CD4+ T-cell proliferation[39].
Additionally, many studies have shown that MSC–EVs play key roles in the pathogenesis and progression of acute lung injury (ALI)/ARDS[40]. One of the underlying mechanisms may involve the potential impairment of antigen uptake, which may halt DC maturation[41]. MSC–EVs from the human BM may regulate the levels of maturation and activation markers (CD83, CD38, and CD80) and inflammatory cytokines [interleukin (IL)-6, IL-12p70, and TGF-β] in vitro via regulating the CCR7 gene by carrying miR-21-5p[42]. Additionally, the emerging role of MSC–EVs in facilitating pulmonary epithelium repair, rescuing mitochondrial dysfunction, and restoring pulmonary vascular leakage has been shown[43,44]. Therefore, regulating the maturation of DC cells in the early stage of lung injury can effectively alleviate secondary lung injury[45] (Table 1).
| Ref. | Time | Animal/cell line | Interference | Pathway | Conclusion/main effect |
| Zhang et al[112] | 2022 | C-mice | Human dermal fibroblasts or MSCs were intravenously | CAP | MSC treatment significantly protects mice against bacterial pneumonia or LPS-induced lung injury via the CAP pathway. When the CAP was inhibited through vagotomy (VGX) and pharmacological and genetic ablation experiments, the anti-inflammatory effects of MSCs were markedly reduced in lung injury models |
| Kakabadze et al[113] | 2022 | Wistar rats | HPMSCs | - | HPMSCs have the ability to migrate and attach to damaged lung tissue, contributing to the resolution of pathology, restoration of function, and tissue repair in the alveolar space |
| Wang et al[114] | 2022 | C-mice | Human placental MSCs | Macrophage polarization pathway | Human PMSC treatment preferentially rescued resident M2 AMΦs over recruited M1 BMMΦs with overall M2 polarization to improve KP-related ARDS survival |
| Wang et al[115] | 2022 | SD rats | LRMSC/HMSC-C/HMSC-BM | - | Three kinds of LRMSC, HMSC-C and HMSC-BM are protective against LPS-induced lung injury, HMSC-C was more effective than LRMSC and HMSC-BM to treat LPS-induced lung injury |
| Zhang et al[116] | 2022 | C-mice | MSC derived microvesicles | KEGG pathway and GO function | MSV microvesicles treatment was involved in alleviated lung injury and promoting lung tissue repair by dysregulated miRNAs |
| Xu et al[60] | 2022 | BALB/c mice | Umbilical cord-derived MSCs | - | Transplantation of UC-MSCs transfected with SP-B could potentiate M2 macrophage polarization and further relieve LPS-stimulated lung injury |
| Xue et al[117] | 2022 | C-mice | Bone marrow-derived MSC | - | TGF-β1 from MSCs restored skewed Treg/Th17 levels induced by hypoxic- and LPS-stimulated conditions and reduced inflammation |
| He et al[118] | 2022 | Hnsclc cell line A549 (ATCC, CCL-185) | MSCs | CXCL12/CXCR4 signal axis | In vivo transplantation of MSCs significantly attenuated lung injury in ARDS, inhibited serum pro-inflammatory factors in mice, and down-regulated expression of apoptotic and focal factors in lung tissues |
| Zhang et al[119] | 2022 | C-mice | Mouse bone marrow-derived MSCs | Wnt/β-catenin transition signaling | MVs released from MSCs exerted protective effects on early fibrosis by suppressing EMT in LPS-induced ARDS |
| Meng et al[120] | 2021 | - | MSCs derived from normal mouse bone marrow | Akt/Mtor signaling | MTORC2 like mTORC1 as an important signaling of regulation of MSC-secreted HGF protective against LPS-induced lung endothelial dysfunction |
| Ishii et al[121] | 2021 | Adult male Fischer 344 rats | Adipose-derived MSCs | - | AD-MSCs enhanced the barrier function between lung epithelial cells, suggesting that both direct adhesion and indirect paracrine effects strengthened the barrier function of lung alveolar epithelium in vitro |
| Wang et al[122] | 2021 | C-mice | Bone MSCs | Vimentin-Rab7a pathway | MSCs can reach the damaged lung tissue through migration, reduce inflammatory responses and alleviate lung injury |
| Liu et al[123] | 2021 | SD rats | Bone marrow mesenchymal stem cell | Beclin-1 | BMSC-derived exosomes were taken up by the alveolar macrophages and attenuated LPS-induced alveolar macrophage viability loss and apoptosis. Exosomes effectively improved the survival rate of ALI rats, which was associated with alleviating lung pathological changes pulmonary vascular permeability and attenuating inflammatory response |
MSCs induce the activation of macrophages: Alveolar macrophages are guardians of the alveoli and airways, and interstitial macrophages are guardians of blood vessels and the lung interstitium[46]. After lung injury, tissue monocyte-derived macrophages accumulate and have increased viability in the lungs, and persist at the lesion for a long time after lung injury[47]. Macrophages respond in a variety of ways, including modulation of function (activation), the release of inflammatory chemical mediators that control immune cell recruitment, and the modulation of epithelial responses[48]. Macrophages can be divided into two phenotypes based on their functions, the M1 type in resting states and the M2 type in the activated state[49]. M1-type macrophages can secrete a variety of cytokines and participate in many processes including pro-inflammatory, pro-apoptosis, free radical formation, and matrix degradation pathways, while M2-type macrophages play a role in anti-inflammatory and anti-cell death processes during the inflammatory response, and promote angiogenesis and tissue repair[50-52]. The transformation of the M1/M2 phenotype helps subside the inflammatory response and alleviate tissue damage. In ARDS, this balance can effectively remove harmful substances and pro-inflammatory factors from the body and promote lung tissue repair. Conversely, the destruction of this balance aggravates the pathological development of ARDS. Studies have shown that MSCs can control the pathological development of ARDS by regulating the polarization of macrophages and effectively promoting the repair process in ARDS[53]. Basic studies have shown that MSC treatment reduces the expression of CD86 on macrophages in the ALI models, indicating that MSCs can inhibit the transformation of macrophages to the M1 phenotype[54]. Several mechanisms of MSCs have been described, such as: (1) MSCS can promote the phenotypic transformation of macrophages through paracrine secretion of soluble cytokines[55]; (2) MSCS promotes macrophage polarization through exosomes[51]; (3) Metabolic Reprogramming[56]; (4) MSCS regulate mitochondrial transfer[57]; and (5) Apoptotic and efferocytosis effects[58]. The involved signaling pathways are as follows: (1) The nuclear factor erythroid 2-related factor 2/heme oxygenase-1 signaling pathway[59]; (2) The Notch signaling pathway; (3) The Janus kinase-signal transducer and activator of transcription signaling pathway[60]; and (4) The nuclear factor-kappa B signaling pathway[61].
MSCs regulate the T-cell balance: Imbalances between regulatory T cells (Tregs) and IL-17-producing Th17 are a sign of the development of inflammatory response in ARDS[62,63]. The main function of Th17 cells is to promote inflammation, release inflammatory factors, and play an important role in autoimmune diseases. Fortunately, they are precisely regulated by regulatory cells[64]. However, Treg cells can release anti-inflammatory factors (IL-4 and IL-10), control the inflammatory reaction process, and induce tissue damage repair[65,66]. Previous studies have shown that Tregs transferred into ALI animals can reduce the level of alveolar pro-inflammatory cytokines and inhibit neutrophil apoptosis and fibroblast recruitment[67,68]. A recent study also showed that a proportion of Th17 cells and Tregs > 0.79 was an independent predictor of 28-d mortality in patients with ARDS[62]. Therefore, maintaining the balance of Tregs and Th17 cells is crucial for patients with ARDS.
Existing studies indicated that, in vitro, MSCs repress the Th17 molecular program through the programmed cell death protein 1 pathway, prevent the differentiation of naive CD4+ T cells into Th17 cells, inhibit the production of inflammatory cytokines by Th17 cells, and induce Treg phenotype[69-71]. A study has shown that TGF-β1, as the main paracrine cytokine of MSCs, can significantly regulate the transformation of T cells into Tregs, disturb the Th17/Treg balance, and significantly contribute to the control of inflammatory response in ARDS[72]. It is also reported that MSCs can prevent the initial differentiation of CD4+ T cells into Th17 cells, inhibit the generation of inflammation, and induce the generation of Tregs in vitro[73]. Several experiments have proved that controlling the level of Th17/Treg is the key to MSC-mediated control of the inflammatory response in ARDS. Therefore, it is extremely critical to find a method to regulate this balance, which may be the basis for a revolutionary breakthrough in the treatment of ARDS.
Lung epithelial cell and endothelial cell damage and the exudation of highly concentrated protein fluid are the basic pathological changes in ARDS. Therefore, the treatment of ARDS requires a combination of a lung-protective ventilation strategy and fluid manipulation[74]. The immediate effect of these strategies is that MSCs can directly participate in the reconstruction of lung injury by migrating to the site of lung injury, but this aspect has less impact on ARDS injury repair[75]. Evidence shows that MSCs can be directly transformed into type II alveolar epithelial cells to support the role of injured cells[74]. Meanwhile, cell-to-cell contact also provides a prerequisite for the control of inflammatory responses[76]. The formation and tissue damage of pulmonary edema is also related to the dysfunction of the pulmonary vascular system. The increased permeability of pulmonary capillaries leads to a series of serious consequences, such as the exudation of a variety of cells and cytokines and the formation of intravascular microthrombosis. Studies have shown that MSCs can also enhance the barrier system of the pulmonary vascular system, which is beneficial for promoting the repair of lung tissue[77]. Hepatocyte growth factor, angiopoietin-1, and keratinocyte growth factor secreted by MSCs can improve vascular endothelial barrier function[78-82]. Genetic engineering in situ has shown that MSCs could promote the potential of pulmonary angiogenesis[83]. Therefore, various results have shown that MSCs could inhibit pulmonary edema and also provide the basis for the regeneration of lung tissue.
The cellular basis of the lung is composed of alveoli, various types of parenchymal cells, and BM-derived cells[84]. The interaction between various cells is crucial for maintaining the basic functions of the lungs[85]. Although lung protective ventilation strategies have been applied in clinical practice, ARDS survivors still have related health problems, and some patients develop fibroproliferative responses characterized by fibroblast accumulation and deposition of collagen and other extracellular matrix components in the lungs[86]. After ALI, vascular permeability increases, plasma exudates, and protein fluid aggregates, leading to pulmonary edema. Inflammatory factors from the coagulation/anticoagulation system and inflammatory system enter the lungs and damage the alveolar–capillary membrane barrier[87]. In the early inflammatory phase of ARDS, various immune cells continuously release a variety of harmful substances, including reactive oxygen species and nitrogen, as well as proteolytic enzymes such as elastase and matrix metalloproteinases, leading to lung endothelial and epithelial cell damage[86,88]. Persistent damage and failure to quickly repair this damage are the main factors that induce a pathological fibroproliferative response[89]. Late in the inflammatory response, massive and persistent accumulation of macrophages, fibroblasts, fibroblasts, and myofibroblasts in the alveolar space results in excessive deposition of fibronectin, collagen types I and III, and other components of the extracellular matrix[90,91]. The pro-fibrotic/anti-fibrotic balance is disrupted, and the fibrogenic effect increases dramatically, leading to irreversible pulmonary fibrosis. MSCs have shown gratifying advantages in anti-fibrotic effects. In preclinical models of lung fibrosis produced by bleomycin, silica, paraquat, and radiation, MSCs obviously show the ability to prolong life time[92-95]. However, MSC control of pulmonary fibrosis is also a double-edged sword. Studies have shown that MSCS can differentiate into ATII cells in vitro, inhibit the production of degradation enzymes, and thereby inhibit the secretion of pro-fibrotic factors by various immune cells[96]. There is also evidence that abnormally activated Wnt/β-catenin and TGF-β signaling pathways can induce the differentiation of pulmonary intrinsic MSCs into myofibroblasts and promote the development of pulmonary fibrosis[97].
To date, experience with the application of MSCs in patients is limited. The data of the available clinical evidence is summarized in Table 2. Wilson and his colleagues reported the results of the phase I stem cell research for ARDS treatment (START) in 2015[98]. Patients with moderate to severe ARDS received a single intravenous dose of low [1 × 106 MSCs/kg predicted body weight (PBW)], medium (5 × 106 MSCs/kg PBW), or high (1 × 107 MSCs/kg PBW) (n = 3/dose). All patients tolerated MSC infusion without prespecified infusion-related adverse events. High-dose MSCs improved daily SOFA scores compared with low-dose MSCs. Based on these promising results, the knowledge of the safety of administering MSCs to critically ill patients with ARDS is improving. A phase 2a clinical trial to evaluate the safety of BM-MSCs administered to patients with moderate to severe ARDS has also been conducted[99]. The primary outcome was safety, and secondary outcomes included respiratory, systemic, and serum biomarker endpoints. The study included 60 patients with ARDS, and intravenous Adipose-MSCs, 1 × 106/kg predicted body weight, vs the placebo was administered; however, there was no difference in the outcome in patients treated with Adipose-MSCs vs the placebo. In another phase I study, nine consecutive patients were enrolled, between December 2017 and August 2019, the first three patients got low-dose human umbilical cord-derived MSCs, the following three patients received an intermediate dosage, and the last three patients received a high dose[100]. The results of the first phase of clinical trials demonstrated that a single dose of human umbilical cord-derived MSCs was safe and showed good results in all nine patients with ARDS. Swedish researchers tested the systemic administration of allogeneic BM-derived MSCs (2 × 106 cells/kg) in two patients with severe refractory ARDS, both of whom recovered from multiple organ failure and showed reduced markers of systemic and pulmonary inflammation[101]. In summary, clinical studies report that MSC administration is safe for patients with ARDS, with few adverse reactions. However, due to the relatively small number of patients in these studies, further research is needed to test the curative effect.
| Ref. | Cell type | Patient number | Outcome | Study design/evidence level | Publish time |
| Wilson et al[98] | MSC | 9 | No serious adverse events | Phase 1 clinical trial: A multicenter, open-label phase | 2015 |
| Matthay et al[99] | BM-MSCs | 60 | (1) No patients had any adverse events; (2) Mortality at 28 and 60 d was not significantly increased; and (3) ↑Oxygenation index | Phase 2a safety trial: Prospective, double-blind, multicenter, randomized trial | 2019 |
| Yip et al[100] | UC-MSCs | 9 | (1) In-hospital mortality was 33.3% (3/9); (2) No serious prespecified cell infusion-associated or treatment-related adverse events; (3) ↓Circulating inflammatory biomarkers; (4) ↓Mesenchymal stem cell markers; and (5) ↑Immune cell markers | Phase I clinical trial: Prospective | 2020 |
| Lanzoni et al[107] | UC-MSCs | 24 | (1) No serious adverse events; (2) ↑Survival; and (3) ↓Inflammatory cytokines at day 6 | Phase 1/2a clinical trial: A double-blind, randomized controlled trial | 2021 |
| Dilogo et al[108] | UC-MSCs | 20 | (1) ↑Survival; and (2) ↓Interleukin 6 | Clinical trial: A multicentered, double-blind, randomized clinical trial | 2021 |
| Monsel et al[109] | UC-MSCs | 45 | (1) PaO2/FiO2 changes between D0 and D7 did not differ significantly; and (2) Clinical improvement | Clinical trial: A multicentered, double-blind, randomized clinical trial | 2022 |
| Grégoire et al[110] | BM-MSCs | 8 | (1) ↑Survival; (2) Clinical improvement; and (3) ↓Day-7 D-dimer value | A phase I/II Clinical Trial | 2022 |
| Kaffash Farkhad et al[111] | UC-MSCs | 10 | (1) ↑PaO2/FiO2; (2) ↓Serum CRP; (3) ↓IL-6, IFN-γ, TNF-α and IL-17 A; and (4) ↑TGF-β, IL-1B and IL-10 | Phase 1 clinical trial: A single-center, open-label | 2022 |
Coronavirus disease 2019 (COVID-19) is an infectious disease responsible for the COVID-19 pandemic, caused by a novel coronavirus called severe acute respiratory syndrome-coronavirus 2[102,103]. COVID-19 has various respiratory and non-respiratory clinical manifestations, including mild or severe influenza-like syndrome, pneumonia, or respiratory failure, which may eventually lead to sepsis with multiple organ failure. The most common reason for being admitted to intensive care units is a respiratory failure caused by ARDS[104,105]. In a case series, Hashemian et al[106] found that multiple infusions of high-dose allogeneic prenatal MSCs are safe and can relieve the respiratory distress of severe patients with COVID-19 and inhibit the inflammatory response[106]. 24 participants were randomly assigned to either the umbilical cord-derived mesenchymal stem cell (UC-MSC) therapy or the control group in a double-blind, phase ½a randomized controlled trial. The UC-MSC treatment group got two intravenous infusions of 100 ± 20 × 106 UC-MSCs, while the control group received two infusions of vehicle solution[107]. The primary endpoint was safety (adverse events) after 6 h; cardiac arrest or death within 24 h post-infusion) and secondary endpoints included patient survival at 31 d after the first infusion and time to recovery. Serious adverse events related to UC-MSC infusion were not observed. Thus, UC-MSC is safe to inject into patients with COVID-19-induced ARDS. In subjects who received UC-MSC treatment, inflammatory cytokines decreased significantly on the sixth day. In a recent clinical research, 40 COVID-19 patients who were critically unwell got either saline or intravenous UC-MSCs[108]. The findings revealed that the survival rate of patients in the UC-MSCs group was 2.5 times greater than that of the control group. Among patients with complications, the UC-MSCs group had a fourfold greater survival rate than the control group. A multicenter, double-blind, randomized, placebo-controlled trial (STROMA–CoV-2) in France, with 45 enrolled patients, has also been conducted[109]. Patients were randomly assigned to receive three intravenous infusions of 1 × 106 UC-MSCs/kg or placebo (0.9% NaCl) over 5 d after recruitment. PaO2/FiO2 changes between D0 and D7 did not differ significantly between the UC-MSCs and placebo groups. Six (28.6%) of the 21 UC-MSCs patients and six (25%) of the 24 (25%) placebo patients had serious adverse events not related to UC-MSCs treatment. A phase I/II clinical study was also done in patients with severe COVID-19 to assess the safety and effectiveness of three intravenous infusions of BM-derived MSCs at 3-d intervals[110]. Eight intensive care unit patients requiring supplemental oxygen were treated with BM-MSCs. Survival was significantly higher in the MSC group at 28 and 60 d, but there was no significant difference in the number of invasive ventilation-free days, high flow nasal oxygenation-free days, oxygen support-free days, or intensive care unit-free days. MSC infusion was well tolerated, and no adverse effects associated with MSC infusion were reported. Furthermore, a single-center, open-label, phase 1 clinical trial enrolled 20 confirmed COVID-19 patients with mild-to-moderate degree ARDS, who were divided into two groups: The control and the intervention group (UC-MSCs)[111]. The patients received three intravenous infusions of UC-MSCs (1 × 106 cells/kg BW per injection) every other day. There were no adverse effects to cell infusion throughout the clinical study, oxygenation was greatly enhanced, anti-inflammatory factor levels were significantly increased, and pro-inflammatory factor levels were dramatically lowered. This intervention may reduce cytokine storms and restore respiratory function.
To summarize, MSCs from different tissues, such as BM, adipose, UC, and placental tissues, have entered the clinical trial stage. Some studies have used MSCs to treat COVID-19-induced ARDS.
With the progress of scientific research, our understanding of the physiological and pathological processes of ARDS has gradually deepened, and the relevant treatment methods are also improving year by year. However, the final prognosis of patients has not improved much; therefore, it is particularly important to find a method to treat ARDS. MSCs have a variety of characteristics that are striking. At present, as a potential therapeutic method, MSCs have gradually entered the international arena of research and have been unanimously recognized by scientists worldwide. Their application has achieved some effect in improving the survival rate of patients with ARDS. However, because of various reasons, only a few clinical trials are conducted. Although the achievements of basic research are emerging endlessly, there is a theoretical basis for MSC use to enter clinical treatment, and the side effects of MSCs are not clear. Moreover, their clinical application involves ethical issues. As a cell therapy, its safety needs a lot of control experiments to be proven. This has hindered the successful application of MSCs. Fortunately, the basic experimental research on their mechanism of action is becoming more and more in-depth, and the application value of MSC therapy is also much clearer. Their successful application for the treatment of ARDS is expected to improve the quality of life of patients.
Due to the impasse that has been reached in the treatment of ARDS, MSC therapy has gained increasing attention. MSCs are known for their anti-inflammatory, differentiation, paracrine, and microvesicle transport abilities, which could perfectly target the pathological mechanisms of ARDS, providing a theoretical basis for treatment and precision treatment. Despite the current evaluation of MSC treatment of ARDS, further research is needed to observe the specific response to MSC treatment in the long term.
We thank the authors of the primary studies for their timely and helpful responses to our information requests.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Cell and tissue engineering
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Prasetyo EP, Indonesia; Scuteri A, Italy; Ventura C, Italy S-Editor: Fan JR L-Editor: A P-Editor: Fan JR
| 1. | Nanchal RS, Truwit JD. Recent advances in understanding and treating acute respiratory distress syndrome. F1000Res. 2018;7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 332] [Article Influence: 66.4] [Reference Citation Analysis (0)] |
| 2. | Bernard GR, Artigas A, Brigham KL, Carlet J, Falke K, Hudson L, Lamy M, Legall JR, Morris A, Spragg R. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med. 1994;149:818-824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4145] [Cited by in RCA: 4157] [Article Influence: 134.1] [Reference Citation Analysis (0)] |
| 3. | ARDS Definition Task Force, Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E, Camporota L, Slutsky AS. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307:2526-2533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1812] [Cited by in RCA: 4296] [Article Influence: 330.5] [Reference Citation Analysis (0)] |
| 4. | Fan E, Brodie D, Slutsky AS. Acute Respiratory Distress Syndrome: Advances in Diagnosis and Treatment. JAMA. 2018;319:698-710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 733] [Cited by in RCA: 997] [Article Influence: 142.4] [Reference Citation Analysis (0)] |
| 5. | Hanley C, Giacomini C, Brennan A, McNicholas B, Laffey JG. Insights Regarding the Berlin Definition of ARDS from Prospective Observational Studies. Semin Respir Crit Care Med. 2022;43:379-389. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 6. | Gorman EA, O'Kane CM, McAuley DF. Acute respiratory distress syndrome in adults: diagnosis, outcomes, long-term sequelae, and management. Lancet. 2022;400:1157-1170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 183] [Article Influence: 61.0] [Reference Citation Analysis (0)] |
| 7. | Bos LDJ, Ware LB. Acute respiratory distress syndrome: causes, pathophysiology, and phenotypes. Lancet. 2022;400:1145-1156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 382] [Article Influence: 127.3] [Reference Citation Analysis (0)] |
| 8. | Cao C, Zhang L, Liu F, Shen J. Therapeutic Benefits of Mesenchymal Stem Cells in Acute Respiratory Distress Syndrome: Potential Mechanisms and Challenges. J Inflamm Res. 2022;15:5235-5246. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 9. | Kaku S, Nguyen CD, Htet NN, Tutera D, Barr J, Paintal HS, Kuschner WG. Acute Respiratory Distress Syndrome: Etiology, Pathogenesis, and Summary on Management. J Intensive Care Med. 2020;35:723-737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 10. | Saguil A, Fargo MV. Acute Respiratory Distress Syndrome: Diagnosis and Management. Am Fam Physician. 2020;101:730-738. [PubMed] |
| 11. | Bellani G, Laffey JG, Pham T, Fan E, Brochard L, Esteban A, Gattinoni L, van Haren F, Larsson A, McAuley DF, Ranieri M, Rubenfeld G, Thompson BT, Wrigge H, Slutsky AS, Pesenti A; LUNG SAFE Investigators; ESICM Trials Group. Epidemiology, Patterns of Care, and Mortality for Patients With Acute Respiratory Distress Syndrome in Intensive Care Units in 50 Countries. JAMA. 2016;315:788-800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2627] [Cited by in RCA: 3599] [Article Influence: 399.9] [Reference Citation Analysis (0)] |
| 12. | Mart MF, Ware LB. The long-lasting effects of the acute respiratory distress syndrome. Expert Rev Respir Med. 2020;14:577-586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 47] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 13. | Friedenstein AJ, Petrakova KV, Kurolesova AI, Frolova GP. Heterotopic of bone marrow. Analysis of precursor cells for osteogenic and hematopoietic tissues. Transplantation. 1968;6:230-247. [PubMed] |
| 14. | Chen Y, Shao JZ, Xiang LX, Dong XJ, Zhang GR. Mesenchymal stem cells: a promising candidate in regenerative medicine. Int J Biochem Cell Biol. 2008;40:815-820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 162] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 15. | Vizoso FJ, Eiro N, Cid S, Schneider J, Perez-Fernandez R. Mesenchymal Stem Cell Secretome: Toward Cell-Free Therapeutic Strategies in Regenerative Medicine. Int J Mol Sci. 2017;18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 548] [Cited by in RCA: 880] [Article Influence: 110.0] [Reference Citation Analysis (0)] |
| 16. | Assis-Ribas T, Forni MF, Winnischofer SMB, Sogayar MC, Trombetta-Lima M. Extracellular matrix dynamics during mesenchymal stem cells differentiation. Dev Biol. 2018;437:63-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 83] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 17. | Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop Dj, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11055] [Cited by in RCA: 12686] [Article Influence: 704.8] [Reference Citation Analysis (2)] |
| 18. | Roberts I. Mesenchymal stem cells. Vox Sang. 2004;87 Suppl 2:38-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 19. | Yao P, Zhou L, Zhu L, Zhou B, Yu Q. Mesenchymal Stem Cells: A Potential Therapeutic Strategy for Neurodegenerative Diseases. Eur Neurol. 2020;83:235-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 48] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 20. | Xiao K, Hou F, Huang X, Li B, Qian ZR, Xie L. Mesenchymal stem cells: current clinical progress in ARDS and COVID-19. Stem Cell Res Ther. 2020;11:305. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 67] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 21. | Zhang S, Danchuk SD, Bonvillain RW, Xu B, Scruggs BA, Strong AL, Semon JA, Gimble JM, Betancourt AM, Sullivan DE, Bunnell BA. Interleukin 6 mediates the therapeutic effects of adipose-derived stromal/stem cells in lipopolysaccharide-induced acute lung injury. Stem Cells. 2014;32:1616-1628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 38] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 22. | Qin H, Zhao A. Mesenchymal stem cell therapy for acute respiratory distress syndrome: from basic to clinics. Protein Cell. 2020;11:707-722. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 91] [Cited by in RCA: 97] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 23. | Walter J, Ware LB, Matthay MA. Mesenchymal stem cells: mechanisms of potential therapeutic benefit in ARDS and sepsis. Lancet Respir Med. 2014;2:1016-1026. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 210] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 24. | Gorman E, Millar J, McAuley D, O'Kane C. Mesenchymal stromal cells for acute respiratory distress syndrome (ARDS), sepsis, and COVID-19 infection: optimizing the therapeutic potential. Expert Rev Respir Med. 2021;15:301-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 39] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 25. | Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med. 2000;342:1334-1349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3976] [Cited by in RCA: 3860] [Article Influence: 154.4] [Reference Citation Analysis (0)] |
| 26. | Lee JW, Fang X, Gupta N, Serikov V, Matthay MA. Allogeneic human mesenchymal stem cells for treatment of E. coli endotoxin-induced acute lung injury in the ex vivo perfused human lung. Proc Natl Acad Sci U S A. 2009;106:16357-16362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 534] [Cited by in RCA: 558] [Article Influence: 34.9] [Reference Citation Analysis (0)] |
| 27. | Lee JW, Krasnodembskaya A, McKenna DH, Song Y, Abbott J, Matthay MA. Therapeutic effects of human mesenchymal stem cells in ex vivo human lungs injured with live bacteria. Am J Respir Crit Care Med. 2013;187:751-760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 246] [Cited by in RCA: 286] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 28. | Petrou P, Gothelf Y, Argov Z, Gotkine M, Levy YS, Kassis I, Vaknin-Dembinsky A, Ben-Hur T, Offen D, Abramsky O, Melamed E, Karussis D. Safety and Clinical Effects of Mesenchymal Stem Cells Secreting Neurotrophic Factor Transplantation in Patients With Amyotrophic Lateral Sclerosis: Results of Phase 1/2 and 2a Clinical Trials. JAMA Neurol. 2016;73:337-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 232] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 29. | Beyth S, Borovsky Z, Mevorach D, Liebergall M, Gazit Z, Aslan H, Galun E, Rachmilewitz J. Human mesenchymal stem cells alter antigen-presenting cell maturation and induce T-cell unresponsiveness. Blood. 2005;105:2214-2219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 771] [Cited by in RCA: 748] [Article Influence: 35.6] [Reference Citation Analysis (0)] |
| 30. | Glennie S, Soeiro I, Dyson PJ, Lam EW, Dazzi F. Bone marrow mesenchymal stem cells induce division arrest anergy of activated T cells. Blood. 2005;105:2821-2827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 825] [Cited by in RCA: 834] [Article Influence: 41.7] [Reference Citation Analysis (0)] |
| 31. | Di Nicola M, Carlo-Stella C, Magni M, Milanesi M, Longoni PD, Matteucci P, Grisanti S, Gianni AM. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood. 2002;99:3838-3843. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2455] [Cited by in RCA: 2363] [Article Influence: 102.7] [Reference Citation Analysis (0)] |
| 32. | Waisman A, Lukas D, Clausen BE, Yogev N. Dendritic cells as gatekeepers of tolerance. Semin Immunopathol. 2017;39:153-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 185] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 33. | Yin X, Chen S, Eisenbarth SC. Dendritic Cell Regulation of T Helper Cells. Annu Rev Immunol. 2021;39:759-790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 239] [Article Influence: 59.8] [Reference Citation Analysis (0)] |
| 34. | Li L, Dong L, Zhao D, Gao F, Yan J. Classical dendritic cells regulate acute lung inflammation and injury in mice with lipopolysaccharideinduced acute respiratory distress syndrome. Int J Mol Med. 2019;44:617-629. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 35. | Thompson BT, Chambers RC, Liu KD. Acute Respiratory Distress Syndrome. N Engl J Med. 2017;377:562-572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 921] [Cited by in RCA: 1180] [Article Influence: 147.5] [Reference Citation Analysis (0)] |
| 36. | Whartenby KA, Calabresi PA, McCadden E, Nguyen B, Kardian D, Wang T, Mosse C, Pardoll DM, Small D. Inhibition of FLT3 signaling targets DCs to ameliorate autoimmune disease. Proc Natl Acad Sci U S A. 2005;102:16741-16746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 87] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 37. | Shao Z, Bharadwaj AS, McGee HS, Makinde TO, Agrawal DK. Fms-like tyrosine kinase 3 ligand increases a lung DC subset with regulatory properties in allergic airway inflammation. J Allergy Clin Immunol. 2009;123:917-924.e2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 28] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 38. | Ludwig IS, Geijtenbeek TB, van Kooyk Y. Two way communication between neutrophils and dendritic cells. Curr Opin Pharmacol. 2006;6:408-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 46] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 39. | Lu Z, Meng S, Chang W, Fan S, Xie J, Guo F, Yang Y, Qiu H, Liu L. Mesenchymal stem cells activate Notch signaling to induce regulatory dendritic cells in LPS-induced acute lung injury. J Transl Med. 2020;18:241. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 38] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 40. | Hu Q, Zhang S, Yang Y, Yao JQ, Tang WF, Lyon CJ, Hu TY, Wan MH. Extracellular vesicles in the pathogenesis and treatment of acute lung injury. Mil Med Res. 2022;9:61. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 69] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 41. | Reis M, Mavin E, Nicholson L, Green K, Dickinson AM, Wang XN. Mesenchymal Stromal Cell-Derived Extracellular Vesicles Attenuate Dendritic Cell Maturation and Function. Front Immunol. 2018;9:2538. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 114] [Cited by in RCA: 205] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 42. | Zhou L, Luo H, Lee JW. Role of extracellular vesicles in lung diseases. Chin Med J (Engl). 2022;135:1765-1780. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 43. | Dutra Silva J, Su Y, Calfee CS, Delucchi KL, Weiss D, McAuley DF, O'Kane C, Krasnodembskaya AD. Mesenchymal stromal cell extracellular vesicles rescue mitochondrial dysfunction and improve barrier integrity in clinically relevant models of ARDS. Eur Respir J. 2021;58. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 153] [Article Influence: 38.3] [Reference Citation Analysis (0)] |
| 44. | Sun H, Zhang T, Gao J. Extracellular Vesicles Derived from Mesenchymal Stem Cells: A Potential Biodrug for Acute Respiratory Distress Syndrome Treatment. BioDrugs. 2022;36:701-715. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 45. | Lu Z, Chang W, Meng S, Xu X, Xie J, Guo F, Yang Y, Qiu H, Liu L. Mesenchymal stem cells induce dendritic cell immune tolerance via paracrine hepatocyte growth factor to alleviate acute lung injury. Stem Cell Res Ther. 2019;10:372. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 109] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 46. | Aegerter H, Lambrecht BN, Jakubzick CV. Biology of lung macrophages in health and disease. Immunity. 2022;55:1564-1580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 300] [Article Influence: 100.0] [Reference Citation Analysis (0)] |
| 47. | Morales-Nebreda L, Misharin AV, Perlman H, Budinger GR. The heterogeneity of lung macrophages in the susceptibility to disease. Eur Respir Rev. 2015;24:505-509. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 117] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 48. | Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8:958-969. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7233] [Cited by in RCA: 6857] [Article Influence: 403.4] [Reference Citation Analysis (0)] |
| 49. | Dang W, Tao Y, Xu X, Zhao H, Zou L, Li Y. The role of lung macrophages in acute respiratory distress syndrome. Inflamm Res. 2022;71:1417-1432. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 45] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 50. | Chen X, Tang J, Shuai W, Meng J, Feng J, Han Z. Macrophage polarization and its role in the pathogenesis of acute lung injury/acute respiratory distress syndrome. Inflamm Res. 2020;69:883-895. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 255] [Article Influence: 51.0] [Reference Citation Analysis (0)] |
| 51. | Li JW, Wei L, Han Z, Chen Z. Mesenchymal stromal cells-derived exosomes alleviate ischemia/reperfusion injury in mouse lung by transporting anti-apoptotic miR-21-5p. Eur J Pharmacol. 2019;852:68-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 149] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 52. | Murray PJ, Allen JE, Biswas SK, Fisher EA, Gilroy DW, Goerdt S, Gordon S, Hamilton JA, Ivashkiv LB, Lawrence T, Locati M, Mantovani A, Martinez FO, Mege JL, Mosser DM, Natoli G, Saeij JP, Schultze JL, Shirey KA, Sica A, Suttles J, Udalova I, van Ginderachter JA, Vogel SN, Wynn TA. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity. 2014;41:14-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4289] [Cited by in RCA: 4532] [Article Influence: 412.0] [Reference Citation Analysis (0)] |
| 53. | Liu C, Xiao K, Xie L. Advances in the Regulation of Macrophage Polarization by Mesenchymal Stem Cells and Implications for ALI/ARDS Treatment. Front Immunol. 2022;13:928134. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 44] [Reference Citation Analysis (0)] |
| 54. | Sadeghi S, Mosaffa N, Hashemi SM, Mehdi Naghizadeh M, Ghazanfari T. The immunomodulatory effects of mesenchymal stem cells on long term pulmonary complications in an animal model exposed to a sulfur mustard analog. Int Immunopharmacol. 2020;80:105879. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 55. | Wakayama H, Hashimoto N, Matsushita Y, Matsubara K, Yamamoto N, Hasegawa Y, Ueda M, Yamamoto A. Factors secreted from dental pulp stem cells show multifaceted benefits for treating acute lung injury in mice. Cytotherapy. 2015;17:1119-1129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 73] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 56. | Selleri S, Bifsha P, Civini S, Pacelli C, Dieng MM, Lemieux W, Jin P, Bazin R, Patey N, Marincola FM, Moldovan F, Zaouter C, Trudeau LE, Benabdhalla B, Louis I, Beauséjour C, Stroncek D, Le Deist F, Haddad E. Human mesenchymal stromal cell-secreted lactate induces M2-macrophage differentiation by metabolic reprogramming. Oncotarget. 2016;7:30193-30210. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 90] [Cited by in RCA: 115] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 57. | Islam MN, Das SR, Emin MT, Wei M, Sun L, Westphalen K, Rowlands DJ, Quadri SK, Bhattacharya S, Bhattacharya J. Mitochondrial transfer from bone-marrow-derived stromal cells to pulmonary alveoli protects against acute lung injury. Nat Med. 2012;18:759-765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 916] [Cited by in RCA: 1121] [Article Influence: 86.2] [Reference Citation Analysis (0)] |
| 58. | Pang SHM, D'Rozario J, Mendonca S, Bhuvan T, Payne NL, Zheng D, Hisana A, Wallis G, Barugahare A, Powell D, Rautela J, Huntington ND, Dewson G, Huang DCS, Gray DHD, Heng TSP. Mesenchymal stromal cell apoptosis is required for their therapeutic function. Nat Commun. 2021;12:6495. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 131] [Article Influence: 32.8] [Reference Citation Analysis (0)] |
| 59. | Lv H, Liu Q, Sun Y, Yi X, Wei X, Liu W, Zhang Q, Yi H, Chen G. Mesenchymal stromal cells ameliorate acute lung injury induced by LPS mainly through stanniocalcin-2 mediating macrophage polarization. Ann Transl Med. 2020;8:334. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 60. | Xu H, Nie G, Yin T, Shao C, Ding D, Zou M. Umbilical Cord-derived Mesenchymal Stem Cells with Surfactant Protein B Alleviates Inflammatory Response in Acute Respiratory Distress Syndrome by Regulating Macrophage Polarization. Balkan Med J. 2022;39:130-139. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 61. | Gao S, Mao F, Zhang B, Zhang L, Zhang X, Wang M, Yan Y, Yang T, Zhang J, Zhu W, Qian H, Xu W. Mouse bone marrow-derived mesenchymal stem cells induce macrophage M2 polarization through the nuclear factor-κB and signal transducer and activator of transcription 3 pathways. Exp Biol Med (Maywood). 2014;239:366-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 102] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 62. | Yu ZX, Ji MS, Yan J, Cai Y, Liu J, Yang HF, Li Y, Jin ZC, Zheng JX. The ratio of Th17/Treg cells as a risk indicator in early acute respiratory distress syndrome. Crit Care. 2015;19:82. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 103] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 63. | Risso K, Kumar G, Ticchioni M, Sanfiorenzo C, Dellamonica J, Guillouet-de Salvador F, Bernardin G, Marquette CH, Roger PM. Early infectious acute respiratory distress syndrome is characterized by activation and proliferation of alveolar T-cells. Eur J Clin Microbiol Infect Dis. 2015;34:1111-1118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 64. | Noack M, Miossec P. Th17 and regulatory T cell balance in autoimmune and inflammatory diseases. Autoimmun Rev. 2014;13:668-677. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 566] [Cited by in RCA: 692] [Article Influence: 62.9] [Reference Citation Analysis (0)] |
| 65. | Kleinewietfeld M, Hafler DA. Regulatory T cells in autoimmune neuroinflammation. Immunol Rev. 2014;259:231-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 177] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 66. | LaMarca B, Cornelius DC, Harmon AC, Amaral LM, Cunningham MW, Faulkner JL, Wallace K. Identifying immune mechanisms mediating the hypertension during preeclampsia. Am J Physiol Regul Integr Comp Physiol. 2016;311:R1-R9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 69] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 67. | D'Alessio FR, Tsushima K, Aggarwal NR, West EE, Willett MH, Britos MF, Pipeling MR, Brower RG, Tuder RM, McDyer JF, King LS. CD4+CD25+Foxp3+ Tregs resolve experimental lung injury in mice and are present in humans with acute lung injury. J Clin Invest. 2009;119:2898-2913. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 359] [Cited by in RCA: 454] [Article Influence: 28.4] [Reference Citation Analysis (0)] |
| 68. | Garibaldi BT, D'Alessio FR, Mock JR, Files DC, Chau E, Eto Y, Drummond MB, Aggarwal NR, Sidhaye V, King LS. Regulatory T cells reduce acute lung injury fibroproliferation by decreasing fibrocyte recruitment. Am J Respir Cell Mol Biol. 2013;48:35-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 143] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 69. | Ghannam S, Pène J, Moquet-Torcy G, Jorgensen C, Yssel H. Mesenchymal stem cells inhibit human Th17 cell differentiation and function and induce a T regulatory cell phenotype. J Immunol. 2010;185:302-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 388] [Cited by in RCA: 412] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 70. | Luz-Crawford P, Kurte M, Bravo-Alegría J, Contreras R, Nova-Lamperti E, Tejedor G, Noël D, Jorgensen C, Figueroa F, Djouad F, Carrión F. Mesenchymal stem cells generate a CD4+CD25+Foxp3+ regulatory T cell population during the differentiation process of Th1 and Th17 cells. Stem Cell Res Ther. 2013;4:65. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 286] [Cited by in RCA: 367] [Article Influence: 30.6] [Reference Citation Analysis (0)] |
| 71. | Luz-Crawford P, Noël D, Fernandez X, Khoury M, Figueroa F, Carrión F, Jorgensen C, Djouad F. Mesenchymal stem cells repress Th17 molecular program through the PD-1 pathway. PLoS One. 2012;7:e45272. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 130] [Cited by in RCA: 158] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 72. | Chen J, Zhang X, Xie J, Xue M, Liu L, Yang Y, Qiu H. Overexpression of TGFβ1 in murine mesenchymal stem cells improves lung inflammation by impacting the Th17/Treg balance in LPS-induced ARDS mice. Stem Cell Res Ther. 2020;11:311. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 35] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 73. | Zhang S, Chen X, Devshilt I, Yun Q, Huang C, An L, Dorjbat S, He X. Fennel main constituent, transanethole treatment against LPSinduced acute lung injury by regulation of Th17/Treg function. Mol Med Rep. 2018;18:1369-1376. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 74. | Zhang X, Chen J, Xue M, Tang Y, Xu J, Liu L, Huang Y, Yang Y, Qiu H, Guo F. Overexpressing p130/E2F4 in mesenchymal stem cells facilitates the repair of injured alveolar epithelial cells in LPS-induced ARDS mice. Stem Cell Res Ther. 2019;10:74. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 37] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 75. | Xu G, Zhang L, Ren G, Yuan Z, Zhang Y, Zhao RC, Shi Y. Immunosuppressive properties of cloned bone marrow mesenchymal stem cells. Cell Res. 2007;17:240-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 110] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 76. | Liu L, He H, Liu A, Xu J, Han J, Chen Q, Hu S, Xu X, Huang Y, Guo F, Yang Y, Qiu H. Therapeutic Effects of Bone Marrow-Derived Mesenchymal Stem Cells in Models of Pulmonary and Extrapulmonary Acute Lung Injury. Cell Transplant. 2015;24:2629-2642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 77. | Li YY, Xu QW, Xu PY, Li WM. MSC-derived exosomal miR-34a/c-5p and miR-29b-3p improve intestinal barrier function by targeting the Snail/Claudins signaling pathway. Life Sci. 2020;257:118017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 78. | Wang H, Zheng R, Chen Q, Shao J, Yu J, Hu S. Mesenchymal stem cells microvesicles stabilize endothelial barrier function partly mediated by hepatocyte growth factor (HGF). Stem Cell Res Ther. 2017;8:211. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 70] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 79. | Meng SS, Guo FM, Zhang XW, Chang W, Peng F, Qiu HB, Yang Y. mTOR/STAT-3 pathway mediates mesenchymal stem cell-secreted hepatocyte growth factor protective effects against lipopolysaccharide-induced vascular endothelial barrier dysfunction and apoptosis. J Cell Biochem. 2019;120:3637-3650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 35] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 80. | Yang Y, Chen QH, Liu AR, Xu XP, Han JB, Qiu HB. Synergism of MSC-secreted HGF and VEGF in stabilising endothelial barrier function upon lipopolysaccharide stimulation via the Rac1 pathway. Stem Cell Res Ther. 2015;6:250. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 74] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 81. | Hu S, Park J, Liu A, Lee J, Zhang X, Hao Q, Lee JW. Mesenchymal Stem Cell Microvesicles Restore Protein Permeability Across Primary Cultures of Injured Human Lung Microvascular Endothelial Cells. Stem Cells Transl Med. 2018;7:615-624. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 96] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 82. | Matthay MA, Thompson BT, Read EJ, McKenna DH Jr, Liu KD, Calfee CS, Lee JW. Therapeutic potential of mesenchymal stem cells for severe acute lung injury. Chest. 2010;138:965-972. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 135] [Cited by in RCA: 131] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 83. | Doi R, Tsuchiya T, Mitsutake N, Nishimura S, Matsuu-Matsuyama M, Nakazawa Y, Ogi T, Akita S, Yukawa H, Baba Y, Yamasaki N, Matsumoto K, Miyazaki T, Kamohara R, Hatachi G, Sengyoku H, Watanabe H, Obata T, Niklason LE, Nagayasu T. Transplantation of bioengineered rat lungs recellularized with endothelial and adipose-derived stromal cells. Sci Rep. 2017;7:8447. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 54] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 84. | Murray JF. The structure and function of the lung. Int J Tuberc Lung Dis. 2010;14:391-396. [PubMed] |
| 85. | Yamada M. The Roles of MicroRNAs and Extracellular Vesicles in the Pathogeneses of Idiopathic Pulmonary Fibrosis and Acute Respiratory Distress Syndrome. Tohoku J Exp Med. 2020;251:313-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 86. | Zemans RL, Colgan SP, Downey GP. Transepithelial migration of neutrophils: mechanisms and implications for acute lung injury. Am J Respir Cell Mol Biol. 2009;40:519-535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 275] [Cited by in RCA: 265] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 87. | Matthay MA, Ware LB, Zimmerman GA. The acute respiratory distress syndrome. J Clin Invest. 2012;122:2731-2740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1156] [Cited by in RCA: 1389] [Article Influence: 106.8] [Reference Citation Analysis (0)] |
| 88. | Lee WL, Downey GP. Leukocyte elastase: physiological functions and role in acute lung injury. Am J Respir Crit Care Med. 2001;164:896-904. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 235] [Cited by in RCA: 229] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 89. | Madtes DK, Rubenfeld G, Klima LD, Milberg JA, Steinberg KP, Martin TR, Raghu G, Hudson LD, Clark JG. Elevated transforming growth factor-alpha levels in bronchoalveolar lavage fluid of patients with acute respiratory distress syndrome. Am J Respir Crit Care Med. 1998;158:424-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 86] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 90. | Snyder LS, Hertz MI, Peterson MS, Harmon KR, Marinelli WA, Henke CA, Greenheck JR, Chen B, Bitterman PB. Acute lung injury. Pathogenesis of intraalveolar fibrosis. J Clin Invest. 1991;88:663-673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 71] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 91. | Chandel NS, Budinger GR, Mutlu GM, Varga J, Synenki L, Donnelly HK, Zirk A, Eisenbart J, Jovanovic B, Jain M. Keratinocyte growth factor expression is suppressed in early acute lung injury/acute respiratory distress syndrome by smad and c-Abl pathways. Crit Care Med. 2009;37:1678-1684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 22] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 92. | Chen J, Si L, Zhou L, Deng Y. Role of bone marrow mesenchymal stem cells in the development of PQinduced pulmonary fibrosis. Mol Med Rep. 2019;19:3283-3290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 93. | He F, Wang Y, Li Y, Yu L. Human amniotic mesenchymal stem cells alleviate paraquat-induced pulmonary fibrosis in rats by inhibiting the inflammatory response. Life Sci. 2020;243:117290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 94. | Periera-Simon S, Xia X, Catanuto P, Coronado R, Kurtzberg J, Bellio M, Lee YS, Khan A, Smith R, Elliot SJ, Glassberg MK. Anti-fibrotic effects of different sources of MSC in bleomycin-induced lung fibrosis in C57BL6 male mice. Respirology. 2021;26:161-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 35] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 95. | Zhang E, Yang Y, Chen S, Peng C, Lavin MF, Yeo AJ, Li C, Liu X, Guan Y, Du X, Du Z, Shao H. Bone marrow mesenchymal stromal cells attenuate silica-induced pulmonary fibrosis potentially by attenuating Wnt/β-catenin signaling in rats. Stem Cell Res Ther. 2018;9:311. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 38] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 96. | Tzouvelekis A, Toonkel R, Karampitsakos T, Medapalli K, Ninou I, Aidinis V, Bouros D, Glassberg MK. Mesenchymal Stem Cells for the Treatment of Idiopathic Pulmonary Fibrosis. Front Med (Lausanne). 2018;5:142. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 52] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 97. | Harrell CR, Sadikot R, Pascual J, Fellabaum C, Jankovic MG, Jovicic N, Djonov V, Arsenijevic N, Volarevic V. Mesenchymal Stem Cell-Based Therapy of Inflammatory Lung Diseases: Current Understanding and Future Perspectives. Stem Cells Int. 2019;2019:4236973. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 136] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 98. | Wilson JG, Liu KD, Zhuo H, Caballero L, McMillan M, Fang X, Cosgrove K, Vojnik R, Calfee CS, Lee JW, Rogers AJ, Levitt J, Wiener-Kronish J, Bajwa EK, Leavitt A, McKenna D, Thompson BT, Matthay MA. Mesenchymal stem (stromal) cells for treatment of ARDS: a phase 1 clinical trial. Lancet Respir Med. 2015;3:24-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 575] [Cited by in RCA: 581] [Article Influence: 58.1] [Reference Citation Analysis (0)] |
| 99. | Matthay MA, Calfee CS, Zhuo H, Thompson BT, Wilson JG, Levitt JE, Rogers AJ, Gotts JE, Wiener-Kronish JP, Bajwa EK, Donahoe MP, McVerry BJ, Ortiz LA, Exline M, Christman JW, Abbott J, Delucchi KL, Caballero L, McMillan M, McKenna DH, Liu KD. Treatment with allogeneic mesenchymal stromal cells for moderate to severe acute respiratory distress syndrome (START study): a randomised phase 2a safety trial. Lancet Respir Med. 2019;7:154-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 323] [Cited by in RCA: 444] [Article Influence: 63.4] [Reference Citation Analysis (0)] |
| 100. | Yip HK, Fang WF, Li YC, Lee FY, Lee CH, Pei SN, Ma MC, Chen KH, Sung PH, Lee MS. Human Umbilical Cord-Derived Mesenchymal Stem Cells for Acute Respiratory Distress Syndrome. Crit Care Med. 2020;48:e391-e399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 64] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 101. | Simonson OE, Mougiakakos D, Heldring N, Bassi G, Johansson HJ, Dalén M, Jitschin R, Rodin S, Corbascio M, El Andaloussi S, Wiklander OP, Nordin JZ, Skog J, Romain C, Koestler T, Hellgren-Johansson L, Schiller P, Joachimsson PO, Hägglund H, Mattsson M, Lehtiö J, Faridani OR, Sandberg R, Korsgren O, Krampera M, Weiss DJ, Grinnemo KH, Le Blanc K. In Vivo Effects of Mesenchymal Stromal Cells in Two Patients With Severe Acute Respiratory Distress Syndrome. Stem Cells Transl Med. 2016;5:845. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 102. | Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, Wang B, Xiang H, Cheng Z, Xiong Y, Zhao Y, Li Y, Wang X, Peng Z. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA. 2020;323:1061-1069. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14113] [Cited by in RCA: 14767] [Article Influence: 2953.4] [Reference Citation Analysis (0)] |
| 103. | Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, Zhao X, Huang B, Shi W, Lu R, Niu P, Zhan F, Ma X, Wang D, Xu W, Wu G, Gao GF, Tan W; China Novel Coronavirus Investigating and Research Team. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N Engl J Med. 2020;382:727-733. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18987] [Cited by in RCA: 17645] [Article Influence: 3529.0] [Reference Citation Analysis (0)] |
| 104. | Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497-506. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35178] [Cited by in RCA: 30114] [Article Influence: 6022.8] [Reference Citation Analysis (3)] |
| 105. | Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, Guan L, Wei Y, Li H, Wu X, Xu J, Tu S, Zhang Y, Chen H, Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054-1062. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17476] [Cited by in RCA: 18199] [Article Influence: 3639.8] [Reference Citation Analysis (0)] |
| 106. | Hashemian SR, Aliannejad R, Zarrabi M, Soleimani M, Vosough M, Hosseini SE, Hossieni H, Keshel SH, Naderpour Z, Hajizadeh-Saffar E, Shajareh E, Jamaati H, Soufi-Zomorrod M, Khavandgar N, Alemi H, Karimi A, Pak N, Rouzbahani NH, Nouri M, Sorouri M, Kashani L, Madani H, Aghdami N, Vasei M, Baharvand H. Mesenchymal stem cells derived from perinatal tissues for treatment of critically ill COVID-19-induced ARDS patients: a case series. Stem Cell Res Ther. 2021;12:91. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 150] [Cited by in RCA: 144] [Article Influence: 36.0] [Reference Citation Analysis (0)] |
| 107. | Lanzoni G, Linetsky E, Correa D, Messinger Cayetano S, Alvarez RA, Kouroupis D, Alvarez Gil A, Poggioli R, Ruiz P, Marttos AC, Hirani K, Bell CA, Kusack H, Rafkin L, Baidal D, Pastewski A, Gawri K, Leñero C, Mantero AMA, Metalonis SW, Wang X, Roque L, Masters B, Kenyon NS, Ginzburg E, Xu X, Tan J, Caplan AI, Glassberg MK, Alejandro R, Ricordi C. Umbilical cord mesenchymal stem cells for COVID-19 acute respiratory distress syndrome: A double-blind, phase 1/2a, randomized controlled trial. Stem Cells Transl Med. 2021;10:660-673. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 266] [Cited by in RCA: 276] [Article Influence: 69.0] [Reference Citation Analysis (0)] |
| 108. | Dilogo IH, Aditianingsih D, Sugiarto A, Burhan E, Damayanti T, Sitompul PA, Mariana N, Antarianto RD, Liem IK, Kispa T, Mujadid F, Novialdi N, Luviah E, Kurniawati T, Lubis AMT, Rahmatika D. Umbilical cord mesenchymal stromal cells as critical COVID-19 adjuvant therapy: A randomized controlled trial. Stem Cells Transl Med. 2021;10:1279-1287. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 115] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 109. | Monsel A, Hauw-Berlemont C, Mebarki M, Heming N, Mayaux J, Nguekap Tchoumba O, Diehl JL, Demoule A, Annane D, Marois C, Demeret S, Weiss E, Voiriot G, Fartoukh M, Constantin JM, Mégarbane B, Plantefève G, Malard-Castagnet S, Burrel S, Rosenzwajg M, Tchitchek N, Boucher-Pillet H, Churlaud G, Cras A, Maheux C, Pezzana C, Diallo MH, Ropers J, Menasché P, Larghero J; APHP STROMA–CoV-2 Collaborative Research Group. Treatment of COVID-19-associated ARDS with mesenchymal stromal cells: a multicenter randomized double-blind trial. Crit Care. 2022;26:48. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 73] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 110. | Grégoire C, Layios N, Lambermont B, Lechanteur C, Briquet A, Bettonville V, Baudoux E, Thys M, Dardenne N, Misset B, Beguin Y. Bone Marrow-Derived Mesenchymal Stromal Cell Therapy in Severe COVID-19: Preliminary Results of a Phase I/II Clinical Trial. Front Immunol. 2022;13:932360. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 27] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 111. | Kaffash Farkhad N, Sedaghat A, Reihani H, Adhami Moghadam A, Bagheri Moghadam A, Khadem Ghaebi N, Khodadoust MA, Ganjali R, Tafreshian AR, Tavakol-Afshari J. Mesenchymal stromal cell therapy for COVID-19-induced ARDS patients: a successful phase 1, control-placebo group, clinical trial. Stem Cell Res Ther. 2022;13:283. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 40] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 112. | Zhang X, Wei X, Deng Y, Yuan X, Shi J, Huang W, Huang J, Chen X, Zheng S, Chen J, Chen K, Xu R, Wang H, Li W, Li S, Yi H, Xiang AP. Mesenchymal stromal cells alleviate acute respiratory distress syndrome through the cholinergic anti-inflammatory pathway. Signal Transduct Target Ther. 2022;7:307. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 25] [Reference Citation Analysis (0)] |
| 113. | Kakabadze Z, Kipshidze N, Paresishvili T, Vadachkoria Z, Chakhunashvili D. Human Placental Mesenchymal Stem Cells for the Treatment of ARDS in Rat. Stem Cells Int. 2022;2022:8418509. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 114. | Wang LT, Yen BL, Wang HH, Chao YY, Lee W, Huang LY, Chiu SK, Siu LK, Liu KJ, Sytwu HK, Yen ML. Placental mesenchymal stem cells boost M2 alveolar over M1 bone marrow macrophages via IL-1β in Klebsiella-mediated acute respiratory distress syndrome. Thorax. 2022;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 115. | Wang L, Feng Y, Dou M, Wang J, Bi J, Zhang D, Hou D, Chen C, Bai C, Zhou J, Tong L, Song Y. Study of mesenchymal stem cells derived from lung-resident, bone marrow and chorion for treatment of LPS-induced acute lung injury. Respir Physiol Neurobiol. 2022;302:103914. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 116. | Zhang X, Ye L, Liang G, Tang W, Yao L, Huang C. Different microRNAs contribute to the protective effect of mesenchymal stem cell-derived microvesicles in LPS induced acute respiratory distress syndrome. Iran J Basic Med Sci. 2021;24:1702-1708. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 117. | Xue M, Zhang X, Chen J, Liu F, Xu J, Xie J, Yang Y, Yu W, Qiu H. Mesenchymal Stem Cell-Secreted TGF-β1 Restores Treg/Th17 Skewing Induced by Lipopolysaccharide and Hypoxia Challenge via miR-155 Suppression. Stem Cells Int. 2022;2022:5522828. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 118. | He X, Li C, Yin H, Tan X, Yi J, Tian S, Wang Y, Liu J. Mesenchymal stem cells inhibited the apoptosis of alveolar epithelial cells caused by ARDS through CXCL12/CXCR4 axis. Bioengineered. 2022;13:9060-9070. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 17] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 119. | Zhang X, Ye L, Tang W, Ji Y, Zheng L, Chen Y, Ge Q, Huang C. Wnt/β-Catenin Participates in the Repair of Acute Respiratory Distress Syndrome-Associated Early Pulmonary Fibrosis via Mesenchymal Stem Cell Microvesicles. Drug Des Devel Ther. 2022;16:237-247. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 120. | Meng SS, Guo FM, Huang LL, Huang YZ, Xie JF, Yang CS, Qiu HB, Yang Y. mTORC2 Activation Mediated by Mesenchymal Stem Cell-Secreted Hepatocyte Growth Factors for the Recovery of Lipopolysaccharide-Induced Vascular Endothelial Barrier. Stem Cells Int. 2021;2021:9981589. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 121. | Ishii M, Tsuchiya T, Doi R, Morofuji Y, Fujimoto T, Muto H, Suematsu T, Mori R, Matsumoto K, Miyazaki T, Tomoshige K, Watanabe H, Iwatake M, Nagayasu T. Increased In Vitro Intercellular Barrier Function of Lung Epithelial Cells Using Adipose-Derived Mesenchymal Stem/Stromal Cells. Pharmaceutics. 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (1)] |
| 122. | Wang K, Du B, Zhang Y, Wu C, Wang X, Zhang X, Wang L. Vimentin-Rab7a Pathway Mediates the Migration of MSCs and Lead to Therapeutic Effects on ARDS. Stem Cells Int. 2021;2021:9992381. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 123. | Liu X, Gao C, Wang Y, Niu L, Jiang S, Pan S. BMSC-Derived Exosomes Ameliorate LPS-Induced Acute Lung Injury by miR-384-5p-Controlled Alveolar Macrophage Autophagy. Oxid Med Cell Longev. 2021;2021:9973457. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 51] [Article Influence: 12.8] [Reference Citation Analysis (0)] |