Published online Jan 26, 2023. doi: 10.4252/wjsc.v15.i1.1
Peer-review started: August 24, 2022
First decision: November 14, 2022
Revised: November 23, 2022
Accepted: December 23, 2022
Article in press: December 23, 2022
Published online: January 26, 2023
Processing time: 91 Days and 23.4 Hours
The therapeutic effects of various stem cells in acute liver failure (ALF) have been demonstrated in preclinical studies. However, the specific type of stem cells with the highest therapeutic potential has not been determined.
To validate the efficacy of stem cells in ALF model and to identify the most promising stem cells.
A search was conducted on the PubMed, Web of Science, Embase, Scopus, and Cochrane databases from inception to May 3, 2022, and updated on November 16, 2022 to identify relevant studies. Two independent reviewers performed the literature search, identification, screening, quality assessment, and data extraction.
A total of 89 animal studies were included in the analysis. The results of traditional meta-analysis showed that stem cell therapy could significantly reduce the serum levels of alanine aminotransferase [weighted mean difference (WMD) = -181.05 (-191.71, -170.39)], aspartate aminotransferase [WMD = -309.04 (-328.45, -289.63)], tumor necrosis factor-alpha [WMD = -8.75 (-9.93, -7.56)], and interleukin-6 [WMD = -10.43 (-12.11, -8.76)] in animal models of ALF. Further subgroup analysis and network meta-analysis showed that although mesenchymal stem cells are the current research hotspot, the effect of liver stem cells (LSCs) on improving liver function is significantly better than that of the other five types of stem cells. In addition, the ranking results showed that the possibility of LSCs improving liver function ranked first. This fully proves the great therapeutic potential of LSCs, which needs to be paid more attention in the future.
LSCs may have a higher therapeutic potential. Further high-quality animal experiments are needed to explore the most effective stem cells for ALF.
Core Tip: Determination of stem cells with the highest therapeutic potential in acute liver failure is crucial for future animal and clinical research. In this pioneer study, we conducted traditional meta-analysis and network meta-analysis to explore the efficacy of stem cells from four aspects: Alanine aminotransferase, aspartate aminotransferase, tumor necrosis factor-alpha and interleukin-6. We found that although mesenchymal stem cells are the current research hotspot, liver stem cells may have higher therapeutic potential and should be the focus of future research. In addition, we point out the problems existing and the direction of improvement in the future through the quality evaluation.
- Citation: Ma JF, Gao JP, Shao ZW. Acute liver failure: A systematic review and network meta-analysis of optimal type of stem cells in animal models. World J Stem Cells 2023; 15(1): 1-15
- URL: https://www.wjgnet.com/1948-0210/full/v15/i1/1.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v15.i1.1
Acute liver failure (ALF) is a clinical syndrome in which a large number of hepatocytes undergo necrosis resulting in severe liver function damage within a short period time. This condition occurs in the absence of chronic liver disease and is associated with severe complications, such as hepatic encephalopathy and even death, with a mortality rate of about 40%-62.2%[1-3]. The etiologies of ALF include heat pain poisoning, liver ischemia, viral hepatitis and autoimmune hepatitis, as well as prescription drugs, herbs and dietary supplements[1]. Currently, patients with ALF mainly receive medical supportive treatment due to the lack of specific therapy. Although liver transplantation is the only proven treatment, it is limited by the unavailability of donor organs, high medical costs, and the need for lifelong immunosuppressive drugs. Therefore, there is a need to develop newer and more effective therapeutic strategies.
Advances in clinical application of stem cells have provided a new perspective for ALF treatment[4]. Stem cells have many advantages, including self-renewal capacities, high proliferative activities, multi-directional differentiation, anti-inflammation, anti-apoptosis, and immunomodulatory activities. They can be obtained from various sources, such as the bone marrow, umbilical cord, adipose tissue, amniotic fluid and embryos among others[5,6]. After transplantation, naive stem cells or differentiated hepatocyte-like cells rapidly restore essential liver functions by replenishing functional hepatocytes and/or by stimulating endogenous liver regeneration processes through paracrine actions. Stem cell transplantation has been shown to increase the cumulative survival rate of ALF patients from 55.6% to 73.2%[7]. Stem cells improve damaged liver functions by increasing the activities of serum albumin, cholinesterase and prothrombin and reducing the levels of serum total bilirubin and alanine aminotransferase (ALT)[8].
Although the significance of stem cells in the treatment of ALF has been demonstrated in preclinical and clinical trials, there are still some controversies and obstacles that need to be resolved. For example, bone marrow mesenchymal stem cells (BMSCs) can be easily obtained without ethical problems and are considered the first-choice for autologous transplantation. However, their therapeutic effects are limited by easy aging and lack of vitality[9]. On the contrary, umbilical cord MSCs (UCMSCs) have become a new research hotspot because of their non-aging, low immunogenicity, and high proliferative activities[10]. Adipose MSCs (ADMSCs) can be prepared by minimally invasive methods, grow faster in vitro, and secrete abundant cytokines as well as growth factors related to immune regulation. Dysregulation of ADMSCs in clinical applications is significantly lower than that of embryonic stem cells (ESCs) or induced pluripotent stem cells (IPSCs)[11,12]. Therefore, ADMSCs are good candidates for ALF treatment. The non-invasive stem cell population, menstrual blood-derived stem cells (MenSCs), has been recently studied[13-15]. It has been postulated that MenSCs can express markers for both MSCs and ESCs, and can differentiate into the three germ layers. Fathi-Kazerooni et al[16] found that compared with BMSCs, MenSCs significantly reduced serum ALT and aspartate aminotransferase (AST) levels in ALF mice models. Therefore, the therapeutic potential of MenSCs cannot be ignored.
In conclusion, stem cells can improve liver failure. However, it is not clear which kind of stem cells has the best therapeutic effect. Solving this problem will improve the therapeutic effect and accelerate the clinical translation of stem cells. To date, few studies have directly compared the therapeutic effects of different types of stem cells. In the absence of direct comparisons, systematic reviews and network meta-analyses can be used to perform direct and indirect comparisons between different interventions and estimate the ranking probabilities[17]. Therefore, we intend to compare the therapeutic potential of different types of stem cells through subgroup analysis of traditional meta-analysis and network meta-analysis. Our findings inform on optimal types of stem cells and provide a reference for animal experiments and clinical research.
Patients and diseases (P): Animal models of ALF. Interventions (I): Stem cells. Control (C): (1) Positive control: Comparisons between different stem cells; and (2) Negative control: Blank, DMEM, PBS, saline. Outcomes (O): (1) Primary outcomes: ALT and AST levels; and (2) Secondary outcomes: Expression levels of genes or proteins of tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6). Type of studies (S): Controlled studies.
Candidate studies were identified via searching PubMed, Web of Science, Embase, Scopus, Reference Citation Analysis, and Cochrane databases from their inceptions until February 3, 2022, and updated on November 16, 2022. Search terms were: “stem cell” OR “stem cells” OR “stromal cells” OR “stromal cell” OR “mesenchymal cell” OR “mesenchymal cells” OR “cell therapy” OR “cellular therapy” OR “progenitor cell” OR “progenitor cells” OR “cytotherapy” AND “liver failure” OR “hepatic failure”. The retrieval processes for each database are shown in Supplementary Table 1.
Two independent reviewers performed the literature search, identification, screening, quality assessment and data extraction. Literature screening was based on the inclusion and exclusion criteria, and data extraction was based the pre-established information (including basic information, outcome indicators, and key elements of quality evaluation) shown in Supplementary Table 2. Based on SYRCLE’s risk of bias tool for animal studies[18], 10 items in 6 aspects including selection bias, performance bias, detection bias, attrition bias, reporting bias, and other biases were used to evaluate the quality of included studies.
Traditional meta-analysis was performed using the Stata16.0 software. Odds ratios were used for count data, while weighted mean differences (WMD) rather than standardized mean differences (SMD) were used as effect indicators for measured data. In fact, when performing meta-analysis, WMD eliminates the influence of the absolute value on the results, so that the original weight and measure can truly reflect the experimental effect, and it is easy to understand when applied. However, SMD not only eliminates the influence of the absolute value, but also eliminates the influence of weights and measures on the results, which makes the results difficult to interpret and requires caution when interpreting the results. Therefore, it is a better practice to choose WMD in our study. Confidence intervals were also reported. Heterogeneity was analyzed by the χ2 test while I2 was used to determine the degree of heterogeneity. If the findings were not statistically different, the fixed effects model was used for meta-analysis. Conversely, the source of heterogeneity was further analyzed, and the random-effects model used for meta-analysis. Reliability of the traditional meta-analysis results were determined via sensitivity analyses.
Network meta-analysis was performed using the Winbugs1.4.3 software. The evidence network diagram of the comparisons for each treatment was drawn to show the current research status in the field. Then, a comparison-correction funnel chart was established to evaluate the possibility of publication bias and small sample effects in the included studies. The Bayesian network meta-analysis was performed using the Markov Chain Monte Carlo method, and ranking probability for each intervention calculated[19]. In addition, potential false positive factor (PSRF) was used to evaluate the convergence of the results. A PSRF value is between 1-1.05 indicated complete convergence, good model stability, and reliable analysis[20].
Traditional meta-analysis was performed by subgroups to explore the therapeutic effects of different types of stem cells. Only ADMSCs, amniotic fluid MSCs (AFMSCs), BMSCs, liver stem cells (LSCs), MenSCs, and UCMSCs were subjected to the network meta-analysis because studies using these six stem cells reported all the primary and secondary outcomes. In this case, the results of network meta-analysis based on different outcome indicators are comparable.
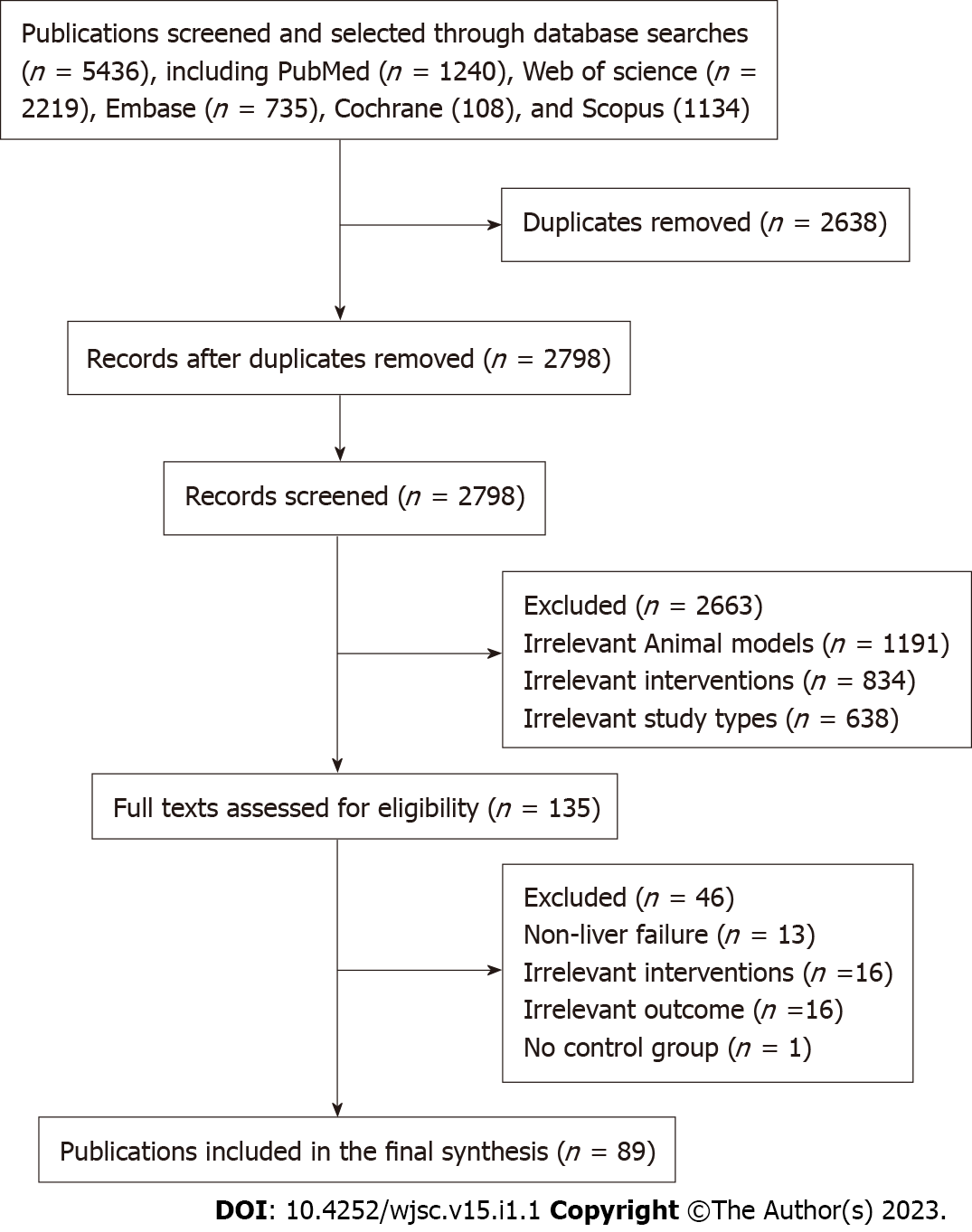
A total of 5436 articles were obtained from the preliminary search. After excluding repetitive articles and those that did not meet the inclusion criteria, 89 articles were finally included (Figure 1).
Among the 89 studies, there were 74 randomized controlled trials and 15 controlled studies. The animal species involved in the studies included Sprague-Dawley rats (22 studies), Wistar rats (6 studies), Albino rats (2 study), BALB/c mice (16 studies), C57BL/6 mice (29 studies), ICR mice (3 studies), NOD-SCID mice (2 studies), Swiss albino mice (1 study), dogs (1 study), pigs (6 studies), and F344-Fischer rats (1 study). In terms of gender, 66 studies included male animals, 10 studies included female animals while 13 studies did not report the gender of the animals. The animals weighed between 18 g-18 kg, were aged between 4 wk and 1.5 years, and the sample sizes were between 6-94. Modeling methods for ALF included partial hepatectomy (7 studies), carbon tetrachloride (25 studies), Concanavalin A (6 studies), D-galactosamine (D-GalN, 10 studies), D-GalN/lipopolysaccharide (27 studies), acetaminophen (9 studies), thioacetamide (4 studies), and ischemia-reperfusion injury (1 study). The types of stem cells that were assessed included ADMSCs (21 studies), UCMSCs (21 studies), AFMSCs (2 studies), BMSCs (39 studies), ESCs (1 study), IPSCs (2 studies), LSCs (2 studies), MenSCs (4 studies), and placental stem cells (PMSCs, 1 study). The transplantation routes for the stem cells included intraperitoneal injection (4 studies), liver parenchyma (3 studies), portal vein (6 studies), splenic vein (2 studies), tail vein (71 studies) and stent loading (3 studies). The transplantation timing for the stem cells was within 4 d after modeling, while the transplantation dose was between (1 × 105)-(1 × 108). The negative control groups were treated with normal saline (32 studies), PBS (35 studies), Blank (16 studies) and DMEM (6 studies). The basic information of the included studies is shown in Supplementary Table 2.
Although there were 74 randomized controlled trials among the included studies, there were no reports on specific randomized grouping methods or whether covert grouping was implemented. Eighty-one studies reported that the baseline characteristics of experimental animals, such as age, sex and weight were balanced. Stem cell transplantation before modeling was performed in 4 studies. Seventy-eight studies reported randomized placement of animals during the experiments. Due to limited information provided by the included studies, it was difficult to determine whether animal breeders and/or researchers had been blinded. Only eight studies reported randomly selecting animals in measurement of the results. Thirty-one studies had blinded outcome evaluators. There was no study that reported on the loss or follow-up of animals. Although protocols were not available for all studies, all expected results were clearly reported. Findings from the risk of bias assessment are shown in Figure 2.
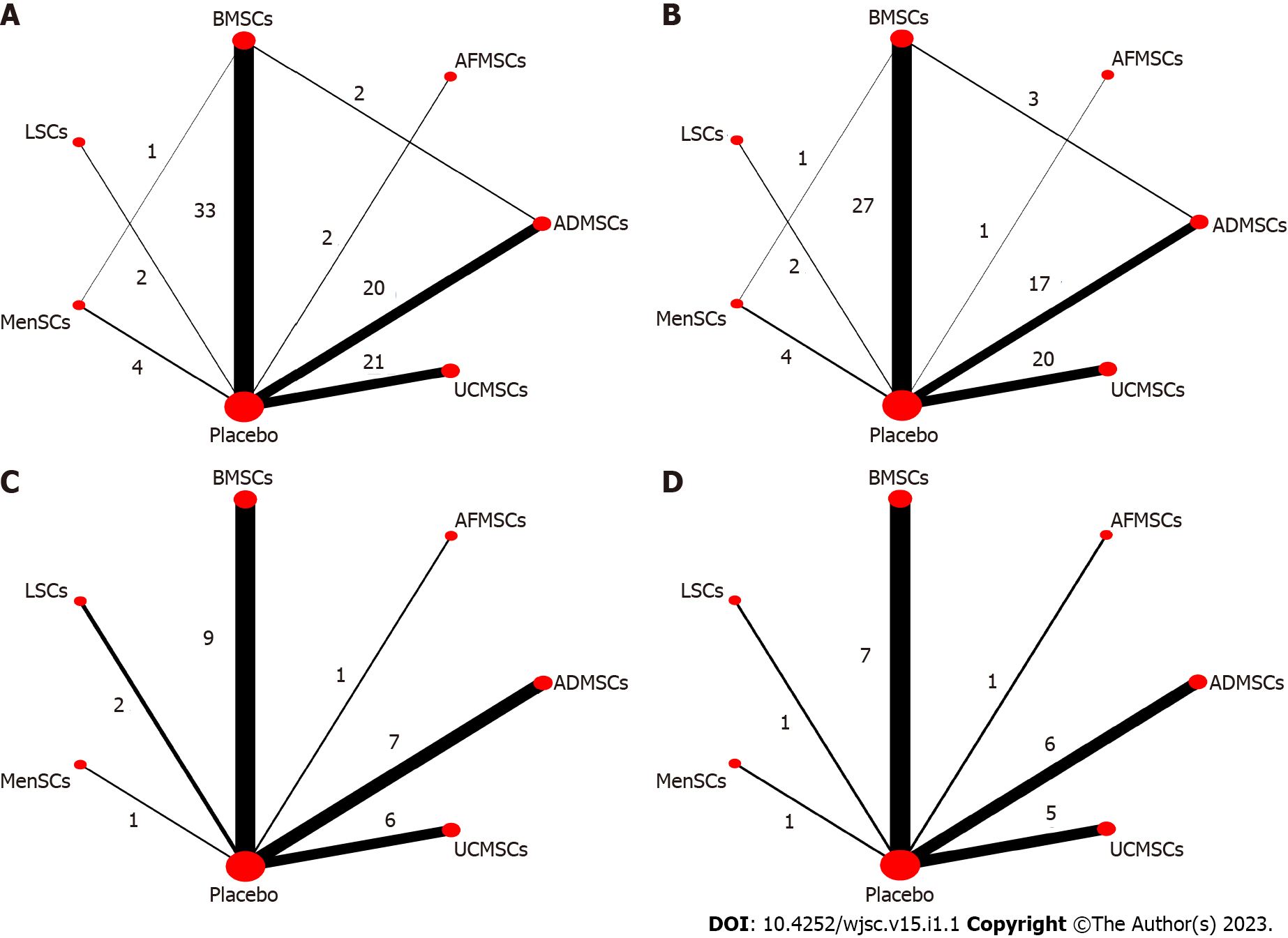
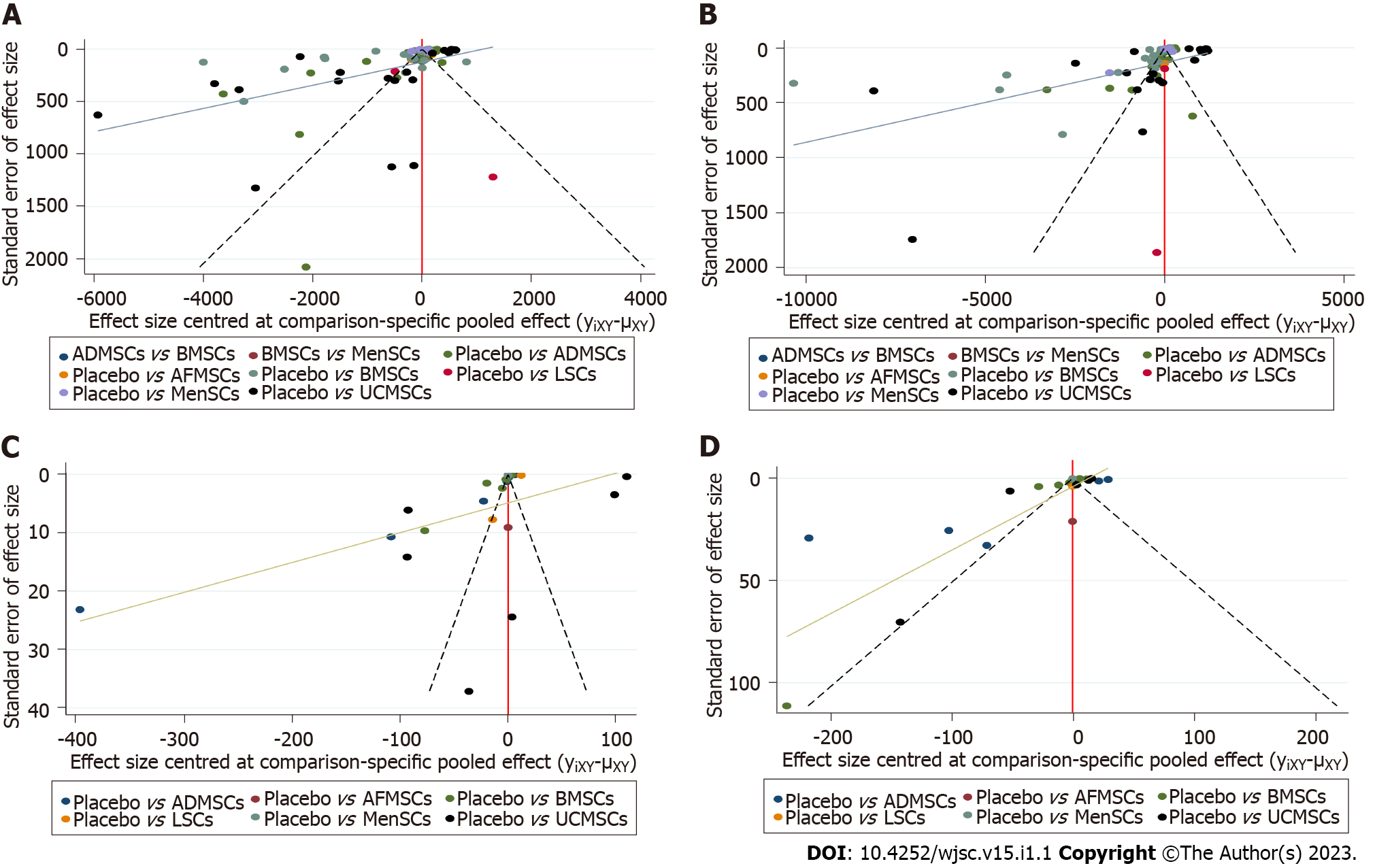
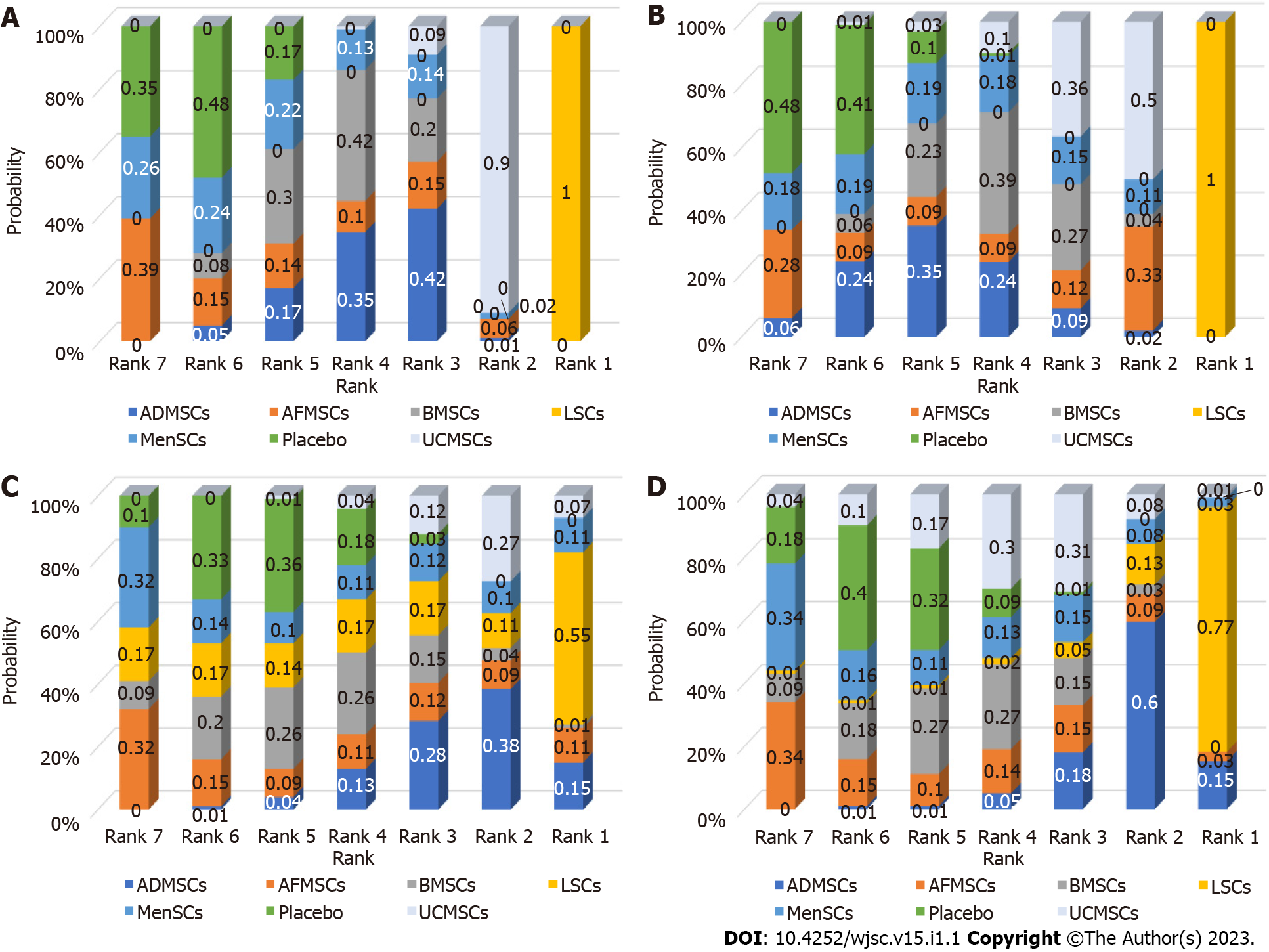
Serum ALT levels: A total of 78 studies reported on serum ALT levels. Meta-analysis of the random effects model showed that ALT levels in the stem cell group were significantly suppressed, relative to those of the negative control group [WMD = -181.05 (-191.71, -170.39)]. Subgroup analysis revealed that the trend of improving liver functions was: IPSCs, LSCs, UCMSCs, ADMSCs, PMSCs, MenSCs, BMSCs. Notably, the treatment effects of AFMSCs and ESCs were not markedly different from those of the control group (Supplementary Figure 1). The evidence network diagram showed that BMSCs and ADMSCs are the current research hotspots (Figure 3A). Network meta-analysis revealed that the effects of LSCs in reducing ALT levels were significantly better than those of the other five stem cells (Table 1). The asymmetrical comparative correction funnel chart suggested that there was publication bias and small sample effects (Figure 4A). The ranking results showed that LSCs had the best effects in reducing ALT levels (Figure 5A).
| Interventions | Outcomes | |||
| ALT | AST | TNF-α | IL-6 | |
| ADMSCs vs AFMSCs | -564.66 (-2299.61, 1244.94) | 333.03 (-3589.84, 4179.61) | -75.75 (-288.40, 135.39) | -83.75 (-240.12, 67.73) |
| ADMSCs vs BMSCs | -128.07 (-785.76, 520.77) | 360.87 (-715.94, 1438.35) | -63.71 (-161.64, 37.04) | -72.42 (-155.32, 11.73) |
| ADMSCs vs LSCs | 5933.08 (3909.44, 7997.17)1 | 9937.57 (6697.32, 13196.61)1 | -55.42 (-215.17, 101.23) | 64.61 (-91.38, 208.00) |
| ADMSCs vs MenSCs | -469.19 (-1740.82, 814.19) | 45.27 (-2014.75, 2083.32) | -74.89 (-284.96, 141.77) | -86.05 (-240.23, 59.90) |
| ADMSCs vs Placebo | -667.52 (-1203.62, -120.99)1 | -606.72 (-1506.56, 287.46) | -77.20 (-152.74, -1.88)1 | -89.51 (-156.08, -28.79)1 |
| ADMSCs vs UCMSCs | 795.81 (46.64, 1560.45)1 | 1050.35 (-188.25, 2288.34) | 37.30 (-72.01, 147.61) | -55.68 (-148.03, 34.58) |
| AFMSCs vs BMSCs | 436.30 (-1349.95, 2166.93) | 39.47 (-3802.42, 3952.36) | 12.93 (-194.78, 220.48) | 11.84 (-137.80, 164.77) |
| AFMSCs vs LSCs | 6488.58 (3913.57, 9087.80)1 | 9604.11 (4643.34, 14441.26)1 | 18.65 (-224.35, 261.70) | 148.58 (-49.07, 342.37) |
| AFMSCs vs MenSCs | 82.07 (-1917.03, 2135.48) | -286.68 (-4498.58, 3913.27) | 2.59 (-277.20, 281.02) | -2.79 (-200.21, 193.85) |
| AFMSCs vs Placebo | -114.65 (-1822.82, 1576.48) | -928.62 (-4714.44, 2892.00) | -1.11 (-201.89, 197.43) | -6.71 (-143.71, 133.84) |
| AFMSCs vs UCMSCs | 1352.71 (-432.10, 3142.15) | 724.02 (-3142.84, 4626.69) | 114.19 (-99.91, 324.03) | 28.52 (-122.68, 187.29) |
| BMSCs vs LSCs | 6070.03 (4071.59, 8102.74)1 | 9576.33 (6336.42, 12727.97)1 | 7.85 (-144.90, 158.13) | 136.42 (-15.99, 284.09) |
| BMSCs vs MenSCs | -341.05 (-1545.56, 858.89) | -322.93 (-2219.61, 1608.47) | -10.36 (-218.54, 200.58) | -13.77 (-163.34, 128.40) |
| BMSCs vs Placebo | -533.83 (-957.97, -124.80)1 | -968.24 (-1654.55, -302.11)1 | -13.45 (-79.67, 51.73) | -16.82 (-77.28, 33.66) |
| BMSCs vs UCMSCs | 924.27 (248.03, 1604.46)1 | 693.88 (-410.09, 1801.40) | 101.07 (-3.29, 205.02) | 16.77 (-68.35, 99.68) |
| LSCs vs MenSCs | -6409.29 (-8678.76, -4138.55)1 | -9871.16 (-13492.79, -6276.98)1 | -19.14 (-258.98, 224.58) | -151.36 (-340.66, 43.49) |
| LSCs vs Placebo | -6600.26 (-8583.58, -4625.55)1 | -10542.45 (-13603.53, -7407.19)1 | -20.86 (-158.68, 119.97) | -154.33 (-290.19, -15.83)1 |
| LSCs vs UCMSCs | -5138.64 (-7210.04, -3092.11)1 | -8886.10 (-12104.64, -5633.36)1 | 93.80 (-66.86, 257.57) | -119.18 (-268.00, 37.35) |
| MenSCs vs Placebo | -204.31 (-1344.63, 944.68) | -639.98 (-2497.51, 1193.49) | -3.78 (-207.01, 193.79) | -3.14 (-138.85, 129.45) |
| MenSCs vs UCMSCs | 1270.03 (-1.67, 2541.86) | 1019.16 (-1023.95, 3022.83) | 111.17 (-106.96, 327.48) | 30.02 (-116.58, 179.83) |
| Placebo vs UCMSCs | 1461.02 (918.17, 2019.69)1 | 1657.56 (793.12, 2525.40)1 | 114.68 (33.79, 197.01)1 | 33.87 (-27.82, 100.16) |
Serum AST levels: Seventy-one studies reported serum AST levels. The meta-analysis of the random effects model showed that serum AST levels in the stem cell group were significantly lower than those of the negative control group [WMD = -309.04 (-328.45, -289.63)]. Subgroup analysis showed that the trend for effects of improving liver functions was: LSCs, IPSCs, UCMSCs, AFMSCs, ADMSCs, MenSCs, BMSCs (Supplementary Figure 2). The evidence network diagram showed that BMSCs and ADMSCs are the current research hotspots (Figure 3B). Network meta-analysis showed that the effects of LSCs in reducing AST levels were significantly better than those of the other five stem cells (Table 1). The asymmetrical comparative correction funnel chart revealed the possibility of publication bias and small sample effects (Figure 4B). The rank ranking results showed that LSCs have the best effects in reducing AST levels (Figure 5B).
Serum TNF-α levels: Twenty-six studies reported the expression of TNF-α at gene or protein levels. The meta-analysis of random effects model showed that TNF-α levels in the stem cell group were significantly suppressed, relative to those of the negative control group [WMD = -8.75 (-9.93, -7.56)]. Subgroup analysis showed that the reduction trend in TNF-α levels was UCMSCs, LSCs, ADMSCs, MenSCs, BMSCs. The treatment effects of AFMSCs were not significantly different from those of the negative control group (Supplementary Figure 3). The evidence network diagram showed that BMSCs and ADMSCs are the current research hotspots (Figure 3C). Network meta-analysis showed that differences in the effects of all stem cells on reducing TNF-α levels were insignificant (Table 1). The asymmetrical comparative correction funnel chart suggested the existence of publication bias and small sample effects (Figure 4C). The rank ranking results showed that LSCs was most effective in reducing TNF-α levels (Figure 5C).
Serum IL-6 levels: A total of 20 studies reported the expression of IL-6 at gene or proteins levels. A meta-analysis of the random effects model showed that that IL-6 levels in the stem cell group were significantly lower than those of the negative control group [WMD = -10.43 (-12.11, -8.76)]. Subgroup analysis showed that the trend in reduction of IL-6 was LSCs, ADMSCs, UCMSCs, BMSCs, MenSCs. The treatment effects of AFMSCs were not markedly different from those of the negative control group (Supplementary Figure 4). The evidence network diagram showed that BMSCs and ADMSCs are the current research hotspots (Figure 3D). Network meta-analysis did not reveal significant differences in the effects of all stem cells in reducing IL-6 levels (Table 1). The asymmetrical comparative correction funnel chart showed the possibility of publication bias and small sample effects (Figure 4D). The rank ranking results revealed that LSCs have the best effects in reducing IL-6 levels (Figure 5D).
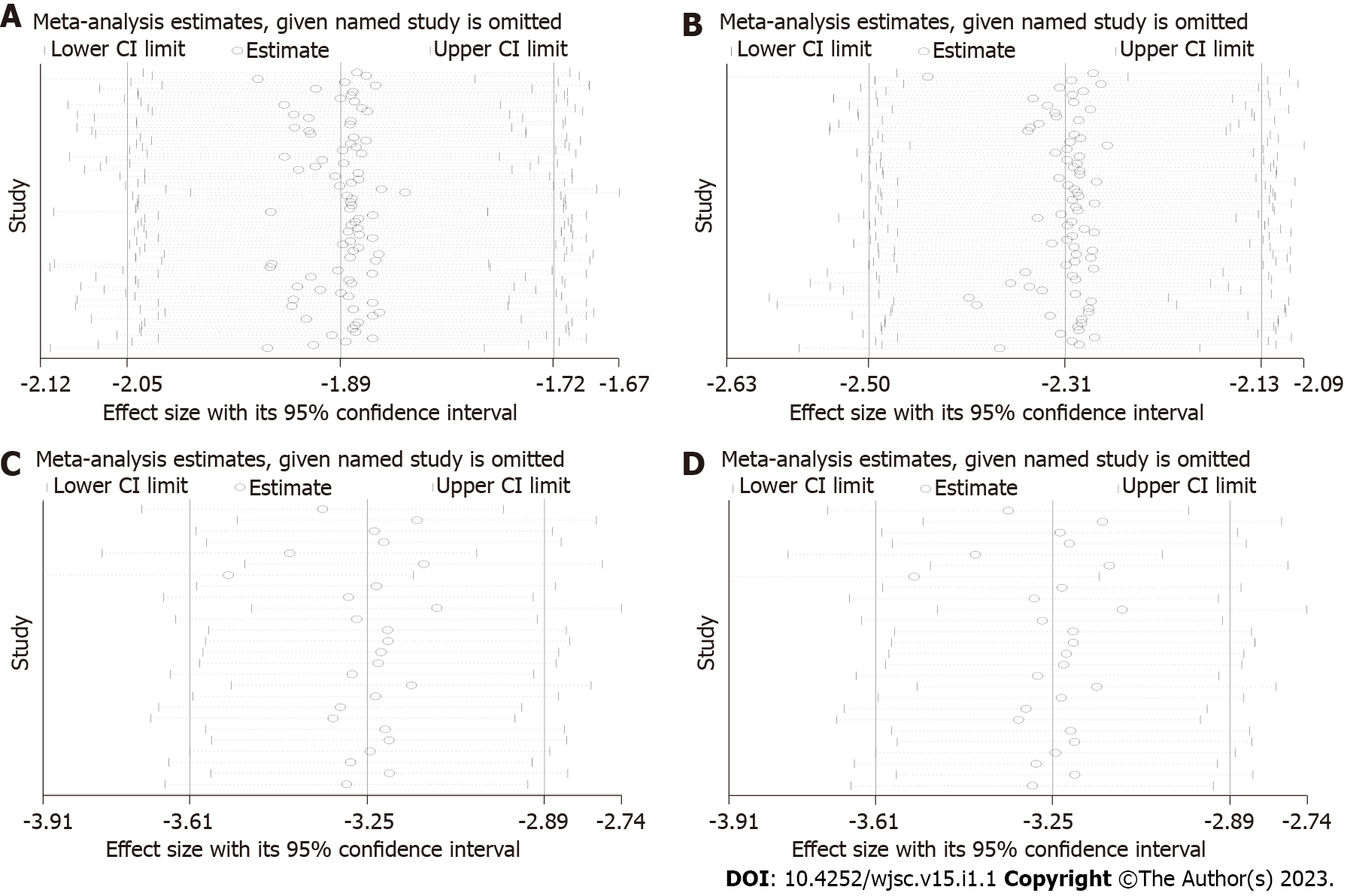
Reliability test of meta-analysis results: Sensitivity analysis was performed to test the reliability of the results from the traditional meta-analysis. The four outcome indicators showed that after one by one exclusion of certain studies, directions of the confidence intervals of the combined results of the remaining studies did not change, indicating that findings from the meta-analysis were robust and reliable (Figure 6).
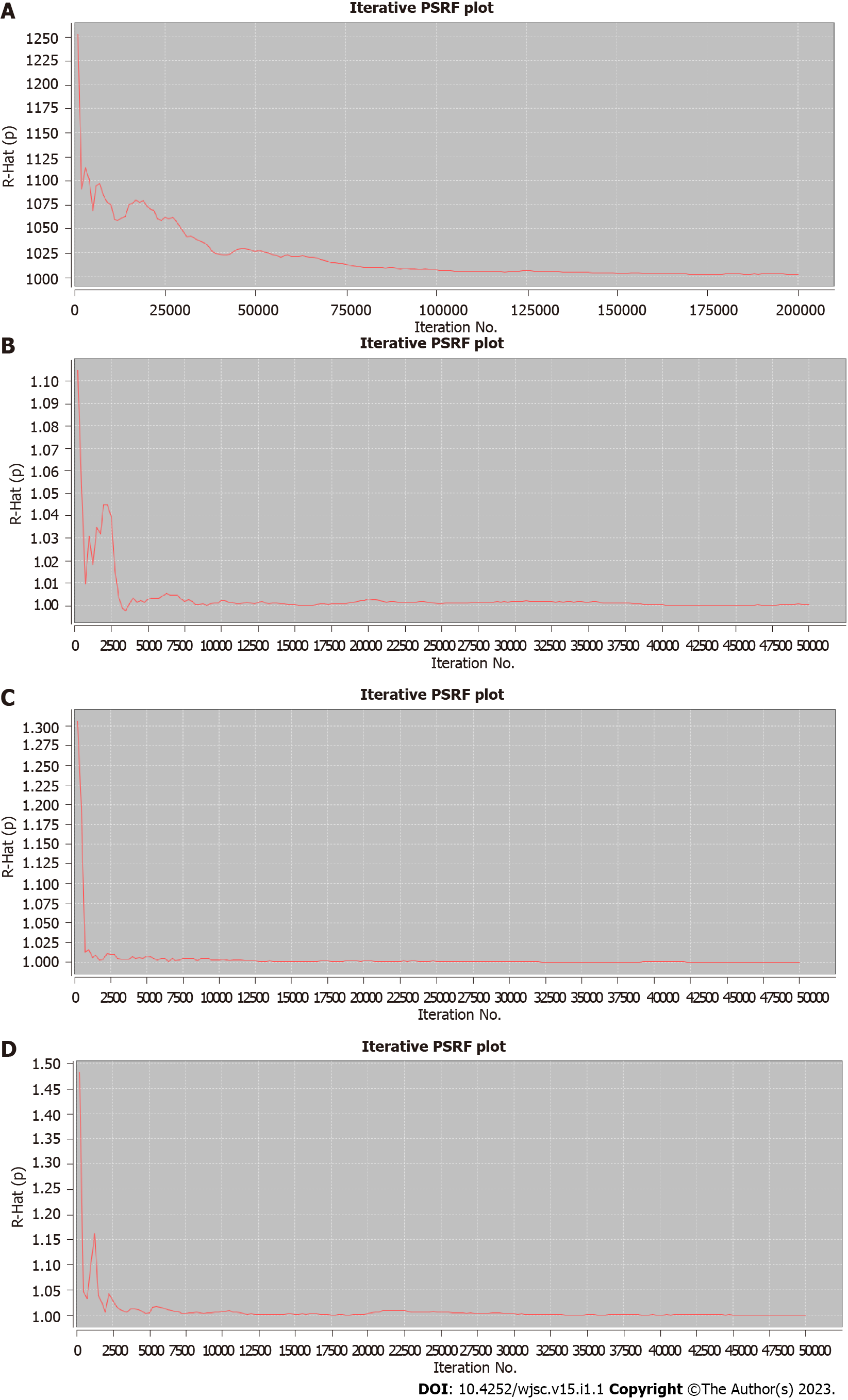
Then, PSRF was used to evaluate the fitting effects of the model, after which the reliability of the network meta-analysis results was determined. The four outcome indicators showed that PSRF converged to 1 after a certain number of iterations, indicating that robustness and reliability of the network meta-analysis results were good (Figure 7).
The mechanism for occurrence of ALF begins with hepatocyte necrosis[21], followed by damage to the membrane structure of hepatocytes and the release of AST and glutamic pyruvic transaminase (ALT) from hepatocytes into the bloodstream. Therefore, the serum levels of AST and ALT are the most reliable markers of liver injury[22]. Stem cells can protect the damaged liver by promoting hepatocyte regeneration and inhibiting hepatocyte apoptosis[23]. Consistent with findings from current animal studies, our meta-analysis showed that different types of stem cell transplantation can significantly suppress serum ALT and AST levels. This indicates the great therapeutic potential of stem cells in ALF. By confirming that stem cells can improve liver functions, there is a need to elucidate on their therapeutic potential to accelerate their clinical translation. About 10 different types of stem cells have been studied in ALF, however, a limited number of studies have compared the therapeutic effects of different types of stem cells. We found that only four studies compared the therapeutic potentials of different types of stem cells in ALF. Among them, three studies compared ADMSCs with BMSCs. They showed that the effects of the two kinds of stem cells on improving liver functions were comparable[24-26]. In another study, MenSCs were found to be significantly better than BMSCs in reducing ALT and AST levels[16]. Studies have reported that MSCs have the advantages of easy access, multipotential, anti-apoptosis, immunosuppression, and paracrine characteristics[27]. Therefore, MSCs are the most studied stem cells in the field of ALF. This is consistent with results shown in the evidence map of our network meta-analysis, that is, MSCs derived from the bone marrow, fat and umbilical cord are the current research focus. However, further subgroup analysis and network meta-analysis showed that they are not the best in improving liver functions. Besides, LSCs are more effective in reducing serum ALT and AST levels. LSCs are MSC-like cells in the liver. In addition to having the same surface markers as MSCs, such as CD29, CD73, CD44, CD105 and CD146, they also express specific hepatocyte markers (albumin, cytokeratin8, and cytokeratin-18) and embryonic markers (Nanog, Oct3/4, Sox2, Musashi, SSEA4, and Pax2)[28,29]. Studies have shown that LSCs improve liver functions by directly differentiating into hepatocytes and secreting cytokines that inhibit injury progression[29]. A possible reason why LSCs showed a better ability to repair liver functions is their greater ability to migrate to the damaged liver. For example, after intravenous transplantation, LSCs preferentially aggregate and play a role in the damaged liver, while MSCs are mainly trapped in the lungs and do not migrate to the damaged liver[29,30]. In addition, human LSCs do not express human leukocyte antigen II molecules and costimulatory molecules (CD40, CD80 and CD86), which reduces the severity of immune responses[31].
The most common causes of death in ALF patients is systemic complications with release of proinflammatory cytokines and impairment of associated molecular patterns of the necrotic hepat
Considerable heterogeneity among the included studies: The included studies involved 11 different animal species, 8 modeling methods, and there were wide variations in baseline characteristics of animals, such as body weight and age. In addition, there were differences in stem cell types, transplantation doses and routes, as well as in measurement and reporting of outcome indicators (such as protein levels, gene levels, etc.), resulting in greater heterogeneity among the included studies, which reduced the reliability of meta-analysis results. There is a need to standardize ALF animal models, experimental implementation and outcome measurements to improve the authenticity of experimental results and the reliability of systematic review results.
Insufficient internal authenticity of animal experiments: (1) Selection bias: Randomization and covert grouping of animals while ensuring the balance of their baseline characteristics is an important measure to avoid selection bias[18]. Although about 90% of the studies were randomized controlled trials and the baseline characteristics of the animals were balanced, no study reported the specific randomization method and whether they implemented concealed grouping, resulting in a certain selection bias; (2) Performance bias: Placement of animals, such as different light and temperature conditions, has an important impact on experimental results[18]. Although 87.64% of the studies randomized the placement of animals, no study had blinded animal breeders and researchers, resulting in a certain implementation bias; (3) Detection bias: Failure to blind the outcome assessors in animal experiments may lead to exaggeration of effects and produce false positive results[44,45]. Only 34.83% of the studies reported blinding the outcome assessors, and no study reported a specific blinding process. Therefore, studies should pay more attention to applications of blinding in experimental designs, and should provide more experimental details to improve the reporting quality; (4) Reporting bias: Selective reporting of results can lead to publication bias, which affects the reliability of experimental results[46]. Moreover, protocols were not available for all studies. Therefore, we suggest that animal experimental researchers prospectively register protocols to improve transparency; and (5) Publication bias: Negative results from animal experiments are difficult to publish[47]. If negative results are not included in systematic reviews, the effects of intervention will be overestimated. The corrected funnel chart revealed a certain publication bias in this field.
Limited external authenticity of included studies: External authenticity in animal experiments refers to reproducibility of results and feasibility of their translation to clinical research[48]. The lack of external authenticity in this study is reflected in the following aspects: (1) Although this study has preliminarily determined the best type of stem cells, the routes, dose, and timing of stem cell transplantation are also key factors for achieving desirable outcomes[49,50]. Until these experimental conditions are clearly explored, clinical translation of stem cells will remain immature; (2) In animal experiments, stem cells are often transplanted in early stages. However, clinical practice must be to recruit patients a few days or weeks after ALF; (3) Clinically, factors such as medical histories and physical conditions of patients may affect the efficacies of stem cells. For example, aging and diabetes can lead to impaired stem cell proliferation, decreased angiogenesis, and reduced wound healing. Animal experiments are difficult to simulate[51]; (4) The clinically used stem cells are all human stem cells, but in animal experiments, stem cells are obtained from various sources, which may lead to strong immune responses, affecting the authenticity of animal experimental results[50]; and (5) Stem cells have a potential risk of developing tumors because of their multi-directional differentiation ability, but no research has focused on the potential side effects of stem cells[49]. Therefore, studies should pay attention to effectiveness and safety of stem cell therapy.
Strengths of this study: (1) As the first study in the ALF field, we systematically evaluated and analyzed the effects of different types of stem cells by subgroups, and pointed out the challenges and directions for improvement; (2) After testing the robustness and reliability of the results of traditional meta-analysis and network meta-analysis, we found that our results are very reliable; (3) In the absence of evidence for direct comparisons, the therapeutic potential of different stem cells was comprehensively compared through subgroup analyses of traditional meta-analyses and network meta-analyses; and (4) Based on the SYRCLE bias risk assessment tool, the internal authenticity of animal experiments was evaluated. At the same time, external authenticity was also evaluated.
Limitations of this study: (1) Due to the small number of studies and the large differences in treatment strategies of stem cells in different studies, subgroup analysis of transplantation doses and route of stem cell administration were not performed; (2) Searches were only performed in English databases, which may have led to a certain linguistic bias; and (3) Failure to search grey literature and conference abstracts may have led to a publication bias.
This analysis showed that stem cell therapy can significantly reduce ALT, AST, TNF-α and IL-6 levels in ALF animal models through a comprehensive analysis of 89 studies. Importantly, although MSCs are the current research hotspot, we found that LSCs have superior therapeutic effects in ALF. However, because of the low quality of evidence from internal and external authenticity of animal studies, future high-quality animal studies should aim at identifying the best stem cells for therapeutic purposes.
The therapeutic effects of various stem cells in acute liver failure (ALF) have been demonstrated in preclinical studies, however, it has not been determined which stem cells have the best therapeutic implications.
The efficacy of stem cells in ALF has been demonstrated in preclinical and clinical trials. However, it remains unclear which stem cells have the most therapeutic potential. Addressing this issue is critical to improve the efficacy of stem cells and to accelerate the progress of basic and clinical research of stem cells.
To explore the best type of stem cells for ALF treatment and to promote the clinical translation of stem cell therapy.
A systematic review and meta-analysis of stem cell therapy for ALF. A search was conducted on the PubMed, Web of Science, Embase, Scopus, and Cochrane databases. Two independent reviewers performed the literature search, identification, screening, quality assessment, and data extraction. The data was analyzed by STATA 16 and Winbugs1.4.3 software.
Stem cell therapy can significantly reduce serum levels of alanine aminotransferase, aspartate aminotransferase, tumor necrosis factor-alpha, and interleukin-6 in animals with ALF. Although mesenchymal stem cells are the current research focus, among the six types of stem cells included in the analysis, liver stem cells (LSCs) have the greatest therapeutic potential.
LSCs have the best effects in treating ALF.
In ALF treatment, stem cell therapy, especially LSCs should be paid more attention to. In addition, to improve on the quality of research, future animal studies should be carefully designed and reported.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Cell and tissue engineering
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Jukic I, Croatia; Muthu S, India S-Editor: Wang JJ L-Editor: A P-Editor: Wang JJ
| 1. | Stravitz RT, Lee WM. Acute liver failure. Lancet. 2019;394:869-881. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 513] [Cited by in RCA: 572] [Article Influence: 95.3] [Reference Citation Analysis (0)] |
| 2. | Punzalan CS, Barry CT. Acute Liver Failure: Diagnosis and Management. J Intensive Care Med. 2016;31:642-653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 3. | Nie H, An F, Mei J, Yang C, Zhan Q, Zhang Q. IL-1β Pretreatment Improves the Efficacy of Mesenchymal Stem Cells on Acute Liver Failure by Enhancing CXCR4 Expression. Stem Cells Int. 2020;2020:1498315. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 4. | Sun K, Xie X, Xie J, Jiao S, Chen X, Zhao X, Wang X, Wei L. Cell-based therapy for acute and chronic liver failures: distinct diseases, different choices. Sci Rep. 2014;4:6494. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 5. | Volarevic V, Nurkovic J, Arsenijevic N, Stojkovic M. Concise review: Therapeutic potential of mesenchymal stem cells for the treatment of acute liver failure and cirrhosis. Stem Cells. 2014;32:2818-2823. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 144] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 6. | Lee CW, Chen YF, Wu HH, Lee OK. Historical Perspectives and Advances in Mesenchymal Stem Cell Research for the Treatment of Liver Diseases. Gastroenterology. 2018;154:46-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 82] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 7. | Lin BL, Chen JF, Qiu WH, Wang KW, Xie DY, Chen XY, Liu QL, Peng L, Li JG, Mei YY, Weng WZ, Peng YW, Cao HJ, Xie JQ, Xie SB, Xiang AP, Gao ZL. Allogeneic bone marrow-derived mesenchymal stromal cells for hepatitis B virus-related acute-on-chronic liver failure: A randomized controlled trial. Hepatology. 2017;66:209-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 202] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 8. | Shi M, Zhang Z, Xu R, Lin H, Fu J, Zou Z, Zhang A, Shi J, Chen L, Lv S, He W, Geng H, Jin L, Liu Z, Wang FS. Human mesenchymal stem cell transfusion is safe and improves liver function in acute-on-chronic liver failure patients. Stem Cells Transl Med. 2012;1:725-731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 274] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 9. | Schubert T, Xhema D, Vériter S, Schubert M, Behets C, Delloye C, Gianello P, Dufrane D. The enhanced performance of bone allografts using osteogenic-differentiated adipose-derived mesenchymal stem cells. Biomaterials. 2011;32:8880-8891. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 77] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 10. | Baksh D, Yao R, Tuan RS. Comparison of proliferative and multilineage differentiation potential of human mesenchymal stem cells derived from umbilical cord and bone marrow. Stem Cells. 2007;25:1384-1392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 719] [Cited by in RCA: 740] [Article Influence: 41.1] [Reference Citation Analysis (0)] |
| 11. | Traktuev DO, Merfeld-Clauss S, Li J, Kolonin M, Arap W, Pasqualini R, Johnstone BH, March KL. A population of multipotent CD34-positive adipose stromal cells share pericyte and mesenchymal surface markers, reside in a periendothelial location, and stabilize endothelial networks. Circ Res. 2008;102:77-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 638] [Cited by in RCA: 633] [Article Influence: 35.2] [Reference Citation Analysis (0)] |
| 12. | Yukawa H, Watanabe M, Kaji N, Okamoto Y, Tokeshi M, Miyamoto Y, Noguchi H, Baba Y, Hayashi S. Monitoring transplanted adipose tissue-derived stem cells combined with heparin in the liver by fluorescence imaging using quantum dots. Biomaterials. 2012;33:2177-2186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 106] [Article Influence: 7.6] [Reference Citation Analysis (1)] |
| 13. | Darzi S, Werkmeister JA, Deane JA, Gargett CE. Identification and Characterization of Human Endometrial Mesenchymal Stem/Stromal Cells and Their Potential for Cellular Therapy. Stem Cells Transl Med. 2016;5:1127-1132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 81] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 14. | Khanmohammadi M, Khanjani S, Edalatkhah H, Zarnani AH, Heidari-Vala H, Soleimani M, Alimoghaddam K, Kazemnejad S. Modified protocol for improvement of differentiation potential of menstrual blood-derived stem cells into adipogenic lineage. Cell Prolif. 2014;47:615-623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 15. | Chen D, Zeng R, Teng G, Cai C, Pan T, Tu H, Lin H, Du Q, Wang H, Chen Y. Menstrual blood-derived mesenchymal stem cells attenuate inflammation and improve the mortality of acute liver failure combining with A2AR agonist in mice. J Gastroenterol Hepatol. 2021;36:2619-2627. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 16. | Fathi-Kazerooni M, Kazemnejad S, Khanjani S, Saltanatpour Z, Tavoosidana G. Down-regulation of miR-122 after transplantation of mesenchymal stem cells in acute liver failure in mice model. Biologicals. 2019;58:64-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 17. | Shang Z, Li D, Chen J, Wang R, Wang M, Zhang B, Wang X, Wanyan P. What Is the Optimal Timing of Transplantation of Neural Stem Cells in Spinal Cord Injury? Front Immunol. 2022;13:855309. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 18. | Hooijmans CR, Rovers MM, de Vries RB, Leenaars M, Ritskes-Hoitinga M, Langendam MW. SYRCLE's risk of bias tool for animal studies. BMC Med Res Methodol. 2014;14:43. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2511] [Cited by in RCA: 2463] [Article Influence: 223.9] [Reference Citation Analysis (2)] |
| 20. | Brooks SP, Gelman A. General methods for monitoring convergence of iterative simulations. J Comput Graph Stat. 1998;7:434-455. [RCA] [DOI] [Full Text] [Cited by in Crossref: 472] [Cited by in RCA: 514] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 21. | Donnelly MC, Hayes PC, Simpson KJ. Role of inflammation and infection in the pathogenesis of human acute liver failure: Clinical implications for monitoring and therapy. World J Gastroenterol. 2016;22:5958-5970. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 43] [Cited by in RCA: 45] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 22. | Dufour DR, Lott JA, Nolte FS, Gretch DR, Koff RS, Seeff LB. Diagnosis and monitoring of hepatic injury. I. Performance characteristics of laboratory tests. Clin Chem. 2000;46:2027-2049. [PubMed] |
| 23. | Zhang Y, Li Y, Li W, Cai J, Yue M, Jiang L, Xu R, Zhang L, Li J, Zhu C. Therapeutic Effect of Human Umbilical Cord Mesenchymal Stem Cells at Various Passages on Acute Liver Failure in Rats. Stem Cells Int. 2018;2018:7159465. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 24. | Xu L, Wang S, Sui X, Wang Y, Su Y, Huang L, Zhang Y, Chen Z, Chen Q, Du H, Yan L. Mesenchymal Stem Cell-Seeded Regenerated Silk Fibroin Complex Matrices for Liver Regeneration in an Animal Model of Acute Liver Failure. ACS Appl Mater Interfaces. 2017;9:14716-14723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 39] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 25. | Zare H, Jamshidi S, Dehghan MM, Saheli M, Piryaei A. Bone marrow or adipose tissue mesenchymal stem cells: Comparison of the therapeutic potentials in mice model of acute liver failure. J Cell Biochem. 2018;119:5834-5842. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 26. | Xu LJ, Wang SF, Wang DQ, Ma LJ, Chen Z, Chen QQ, Wang J, Yan L. Adipose-derived stromal cells resemble bone marrow stromal cells in hepatocyte differentiation potential in vitro and in vivo. World J Gastroenterol. 2017;23:6973-6982. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 16] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 27. | Le Blanc K, Ringdén O. Immunomodulation by mesenchymal stem cells and clinical experience. J Intern Med. 2007;262:509-525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 530] [Cited by in RCA: 512] [Article Influence: 28.4] [Reference Citation Analysis (0)] |
| 28. | Herrera MB, Bruno S, Buttiglieri S, Tetta C, Gatti S, Deregibus MC, Bussolati B, Camussi G. Isolation and characterization of a stem cell population from adult human liver. Stem Cells. 2006;24:2840-2850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 309] [Cited by in RCA: 316] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 29. | Herrera MB, Fonsato V, Bruno S, Grange C, Gilbo N, Romagnoli R, Tetta C, Camussi G. Human liver stem cells improve liver injury in a model of fulminant liver failure. Hepatology. 2013;57:311-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 79] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 30. | Saat TC, van den Engel S, Bijman-Lachger W, Korevaar SS, Hoogduijn MJ, IJzermans JN, de Bruin RW. Fate and Effect of Intravenously Infused Mesenchymal Stem Cells in a Mouse Model of Hepatic Ischemia Reperfusion Injury and Resection. Stem Cells Int. 2016;2016:5761487. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 31. | Bruno S, Grange C, Tapparo M, Pasquino C, Romagnoli R, Dametto E, Amoroso A, Tetta C, Camussi G. Human Liver Stem Cells Suppress T-Cell Proliferation, NK Activity, and Dendritic Cell Differentiation. Stem Cells Int. 2016;2016:8468549. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 32. | Jalan R, Pollok A, Shah SH, Madhavan K, Simpson KJ. Liver derived pro-inflammatory cytokines may be important in producing intracranial hypertension in acute liver failure. J Hepatol. 2002;37:536-538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 71] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 33. | Chung RT, Stravitz RT, Fontana RJ, Schiodt FV, Mehal WZ, Reddy KR, Lee WM. Pathogenesis of liver injury in acute liver failure. Gastroenterology. 2012;143:e1-e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 80] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 34. | Rolando N, Wade J, Davalos M, Wendon J, Philpott-Howard J, Williams R. The systemic inflammatory response syndrome in acute liver failure. Hepatology. 2000;32:734-739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 535] [Cited by in RCA: 507] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 35. | Li F, Miao L, Sun H, Zhang Y, Bao X, Zhang D. Establishment of a new acute-on-chronic liver failure model. Acta Pharm Sin B. 2017;7:326-333. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 36. |
Gao H, Liu L, Zhao Y, Hara H, Chen P, Xu J, Tang J, Wei L, Li Z, Cooper DKC, Cai Z, Mou L. Human IL-6, IL-17, |
| 37. | Gao H, Zhang Q, Chen J, Cooper DKC, Hara H, Chen P, Wei L, Zhao Y, Xu J, Li Z, Cai Z, Luan S, Mou L. Porcine IL-6, IL-1β, and TNF-α regulate the expression of pro-inflammatory-related genes and tissue factor in human umbilical vein endothelial cells. Xenotransplantation. 2018;25:e12408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 38. | Xu L, Zheng X, Wang Y, Fan Q, Zhang M, Li R, Ye J, Wu X, Zhao W, Zhang Y. Berberine protects acute liver failure in mice through inhibiting inflammation and mitochondria-dependent apoptosis. Eur J Pharmacol. 2018;819:161-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 44] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 39. | Guo G, Zhu Y, Wu Z, Ji H, Lu X, Zhou Y, Li Y, Cao X, Lu Y, Talbot P, Liao J, Shi Y, Bu H. Circulating monocytes accelerate acute liver failure by IL-6 secretion in monkey. J Cell Mol Med. 2018;22:4056-4067. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 40. | Wüstefeld T, Rakemann T, Kubicka S, Manns MP, Trautwein C. Hyperstimulation with interleukin 6 inhibits cell cycle progression after hepatectomy in mice. Hepatology. 2000;32:514-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 97] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 41. | Yu J, Cao H, Yang J, Pan Q, Ma J, Li J, Li Y, Wang Y, Li L. In vivo hepatic differentiation of mesenchymal stem cells from human umbilical cord blood after transplantation into mice with liver injury. Biochem Biophys Res Commun. 2012;422:539-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 32] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 42. | Mou XZ, Lin J, Chen JY, Li YF, Wu XX, Xiang BY, Li CY, Ma JM, Xiang C. Menstrual blood-derived mesenchymal stem cells differentiate into functional hepatocyte-like cells. J Zhejiang Univ Sci B. 2013;14:961-972. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 58] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 43. | Pascual-Miguelañez I, Salinas-Gomez J, Fernandez-Luengas D, Villar-Zarra K, Clemente LV, Garcia-Arranz M, Olmo DG. Systemic treatment of acute liver failure with adipose derived stem cells. J Invest Surg. 2015;28:120-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 44. | Vesterinen HM, Sena ES, ffrench-Constant C, Williams A, Chandran S, Macleod MR. Improving the translational hit of experimental treatments in multiple sclerosis. Mult Scler. 2010;16:1044-1055. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 131] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 45. | Sena E, van der Worp HB, Howells D, Macleod M. How can we improve the pre-clinical development of drugs for stroke? Trends Neurosci. 2007;30:433-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 279] [Cited by in RCA: 288] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 46. | Korevaar DA, Hooft L, ter Riet G. Systematic reviews and meta-analyses of preclinical studies: publication bias in laboratory animal experiments. Lab Anim. 2011;45:225-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 77] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 47. | Ioannidis JP. Extrapolating from animals to humans. Sci Transl Med. 2012;4:151ps15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 82] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 48. | Kennedy-Martin T, Curtis S, Faries D, Robinson S, Johnston J. A literature review on the representativeness of randomized controlled trial samples and implications for the external validity of trial results. Trials. 2015;16:495. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 405] [Cited by in RCA: 606] [Article Influence: 60.6] [Reference Citation Analysis (0)] |
| 49. | Wang J, Cen P, Chen J, Fan L, Li J, Cao H, Li L. Role of mesenchymal stem cells, their derived factors, and extracellular vesicles in liver failure. Stem Cell Res Ther. 2017;8:137. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 39] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 50. | Shang Z, Wang R, Li D, Chen J, Zhang B, Wang M, Wang X, Wanyan P. Spinal Cord Injury: A Systematic Review and Network Meta-Analysis of Therapeutic Strategies Based on 15 Types of Stem Cells in Animal Models. Front Pharmacol. 2022;13:819861. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 24] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 51. | El-Ftesi S, Chang EI, Longaker MT, Gurtner GC. Aging and diabetes impair the neovascular potential of adipose-derived stromal cells. Plast Reconstr Surg. 2009;123:475-485. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 83] [Article Influence: 5.2] [Reference Citation Analysis (0)] |