Published online Jun 26, 2022. doi: 10.4252/wjsc.v14.i6.365
Peer-review started: January 3, 2022
First decision: March 13, 2022
Revised: March 15, 2022
Accepted: May 27, 2022
Article in press: May 27, 2022
Published online: June 26, 2022
Processing time: 171 Days and 14.1 Hours
In a recent article, the authors provide a detailed summary of the characteristics and biological functions of mesenchymal stem cells (MSCs), as well as a discussion on the potential mechanisms of action of MSC-based therapies. They describe the morphology, biogenesis, and current isolation techniques of exosomes, one of the most important fractions of the MSC-derived secretome. They also summarize the characteristics of MSC-derived exosomes and highlight their functions and therapeutic potential for tissue/organ regeneration and for kidney, liver, cardiovascular, neurological, and musculoskeletal diseases, as well as cutaneous wound healing. Despite the fact that MSCs are regarded as an important pillar of regenerative medicine, their regenerative potential has been demonstrated to be limited in a number of pathological conditions. The negative effects of MSC-based cell therapy have heightened interest in the therapeutic use of MSC-derived secretome. On the other hand, MSC-derived exosomes and microvesicles possess the potential to have a significant impact on disease development, including cancer. MSCs can interact with tumor cells and promote mutual exchange and induction of cellular markers by exchanging secretome. Furthermore, enzymes secreted into and activated within exosomes can result in tumor cells acquiring new properties. As a result, therapeutic applications of MSC-derived secretomes must be approached with extreme caution.
Core Tip: The authors of a recent article provide a detailed summary of the properties and biological functions of mesenchymal stem cell (MSC)-derived exosomes, one of the most important fractions of the MSC-derived secretome. However, in addition to their undeniable benefits, there are a number of risks associated with their use. Exosomes have the potential to have a significant impact on the development of diseases such as cancer. The use of MSC-derived secretomes for therapeutic purposes must be approached with extreme caution.
- Citation: Sipos F, Műzes G. Disagreements in the therapeutic use of mesenchymal stem cell-derived secretome. World J Stem Cells 2022; 14(6): 365-371
- URL: https://www.wjgnet.com/1948-0210/full/v14/i6/365.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v14.i6.365
Stem cell and tissue engineering studies appear to be critical components of regenerative medicine. Stem cells are characterized as totipotent, pluripotent, multipotent, or unipotent depending on their ability to differentiate into new cell lines. While allogeneic cells can create complications such as immunological rejection, when autologous cells are utilized, rejection can be avoided, making this a less risky mode of treatment.
Adult stem cells, such as mesenchymal stem cells (MSCs) and hematopoietic stem cells, are the most commonly used types in clinical practice, owing to their availability from individuals with various medical conditions (e.g., aplastic anemia, Duchenne muscular dystrophy, ankylosing spondylitis, etc.)[1].
MSCs have the ability to self-renew while also possessing a limited potential to distinguish from one another. Bone marrow, adipose tissue, liver, skin, lungs, cord blood, and fallopian tubes are their primary sources[2].
MSC-based treatments are widely used around the world, with their effects mediated via induced differentiation, immunological modulation, cell fusion, paracrine actions, mRNA or micro-RNA (miRNA) carriage, and mitochondrial metastasis. MSCs for therapeutic purposes face challenges such as maintaining a homogeneous culture and, further, characterization of the cells[3]. In addition to cell replacement, MSCs possess a diverse array of functional characteristics (i.e., angiogenesis, fibrosis inhibitory as well as anti-apoptotic capacity, directed migration, immunomodulation, growth and differentiation supporting activity on other stem cells)[4-7]. The release of bioactive components, referred to as the secretome, into the conditioned media of cell culture is one of their most intriguing qualities[8]. The secretome is composed of two fractions: Soluble and vesicular. Immunomodulatory molecules, chemokines, cytokines, and growth factors are abundant in the soluble fraction. The vesicular fraction consists of extracellular vesicles that can be categorized as apoptotic bodies, microvesicles, and exosomes based on their diameter and synthesis route. Exosomes and microvesicles containing lipids, proteins, or nucleic acids comprise the secretome derived from MSCs[8]. As indicated above, the secretome has the potential to directly stimulate target cells through endocytosis and to exert a wide range of actions[9]. However, it is critical to keep in mind that, depending on where the MSCs come from, the secretome's therapeutic potential may differ[10].
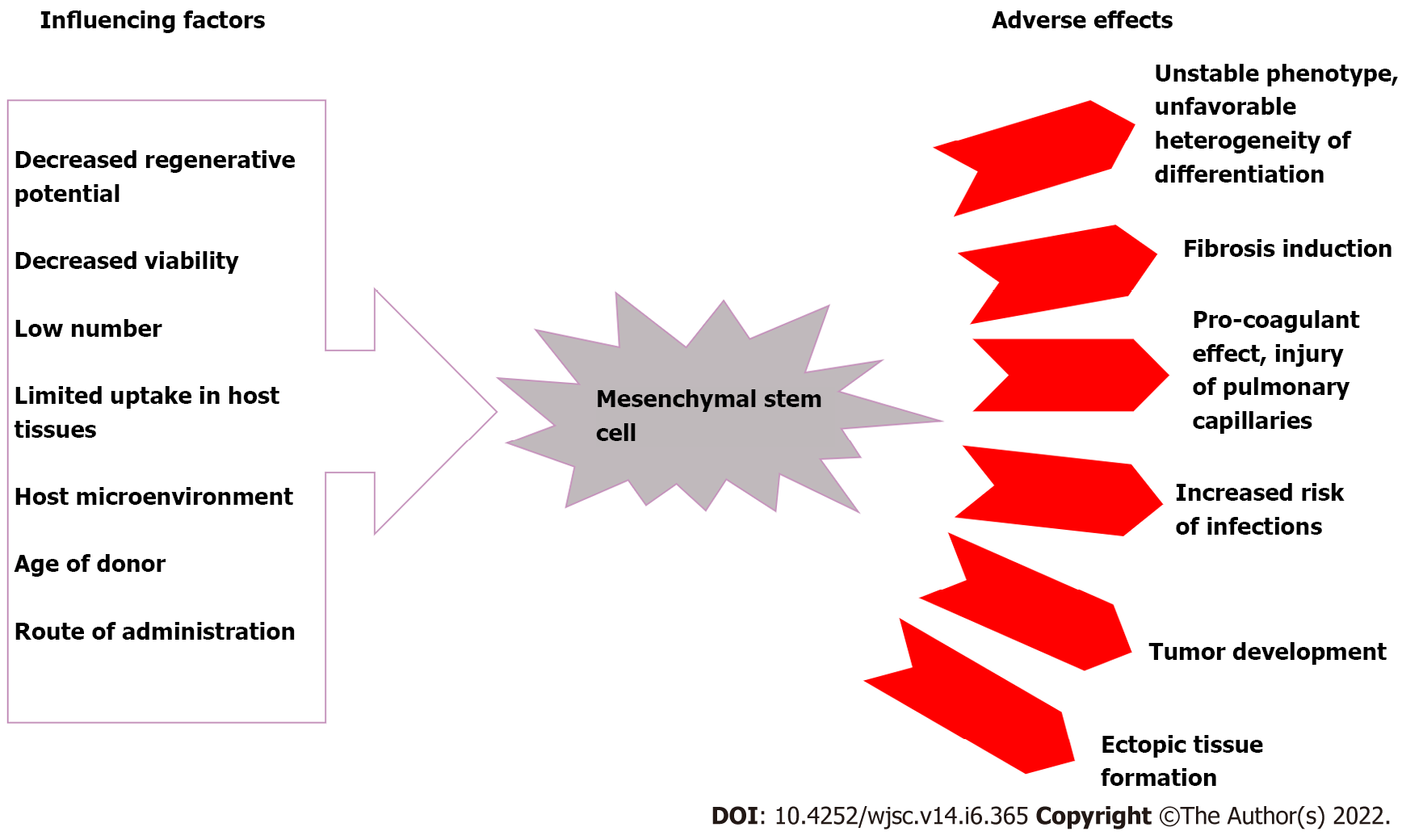
MSCs are an important pillar in regenerative medicine due to their wide range of functional capabilities. As a result, to ensure that no functional or genetic alterations occur during clinical use, their biosafety characteristics should be examined. MSCs have a number of disadvantages, including their detrimental effect on the pulmonary microvasculature, host cell rejection, and ectopic tissue formation[11-13]. Additionally, it has been demonstrated that MSCs have a very limited capacity for regeneration, particularly in pathological conditions. While MSCs are found in a variety of tissues, their numbers are relatively small. Furthermore, transplanted cells’ viability and uptake into host tissues are frequently compromised[14]. Also, a variety of factors, such as the donor’s age, the number of passages and culture conditions used during in vitro growth, administration procedure, and the deleterious host microenvironment encountered by the relocated MSCs, may have a negative effect on the cells’ proclivity for survival and engraftment in host tissues[15]. Recent studies have also indicated possible pro-tumorigenic activities of MSCs[16,17], along with pro-fibrogenic and pro-coagulant potentials[18,19], a higher risk of infections (e.g., zoonotic illnesses) during the in vitro growth process[20], and the unfavorable heterogeneity of their differentiation potential (Figure 1)[21]. Due to these drawbacks, their clinical application has been limited. As a result, it is necessary to develop alternative, complication-free MSC-based therapeutic strategies.
In a recent review by Ma et al[22], the authors provide a detailed summary of the characteristics and biological functions of MSCs and discuss the potential mechanisms of action of MSC-based therapies. They describe the morphology, biogenesis, and current isolating techniques of exosomes, one of the most important fractions of the MSC-derived secretome.
The consequences of the treatments with MSC-derived cells have heightened interest in the MSCs’ secretome for therapeutic purposes. The application of MSCs’ secretome has a number of significant benefits, including the complete absence of the necessity for an invasive solution to obtain cells, the capability of conducting pharmacological dosage and safety tests, the convenience of application, and the possibility of manipulating the composition[23]. Soluble and vesicular factors derived from MSCs exhibit a variety of unique properties that may make them a precious tool for therapeutic reasons[8]. Ma et al[22] compiled a list of the numerous regenerative medicine benefits of MSC-derived exosomes[22]. Simple collection, long-term stability, safety, optimal drug transport capacity, and tissue or microenvironment-specific targeting are the most critical of these. Additionally, they summarized recent research on the actions of MSC-derived exosomes in different diseases affecting the skin, bone, muscle, kidney, cardiovascular system, liver, and nervous system.
However, practical difficulties appear in cases of those entities, as their physical and biochemical properties frequently cause complications to obtain them as perfect and correctly characterized preparations. As a result, the International Society for Extracellular Vesicles developed guidelines for the field in 2014 (i.e., Minimal Information for Studies of Extracellular Vesicles), which were recently revised in 2018[24].
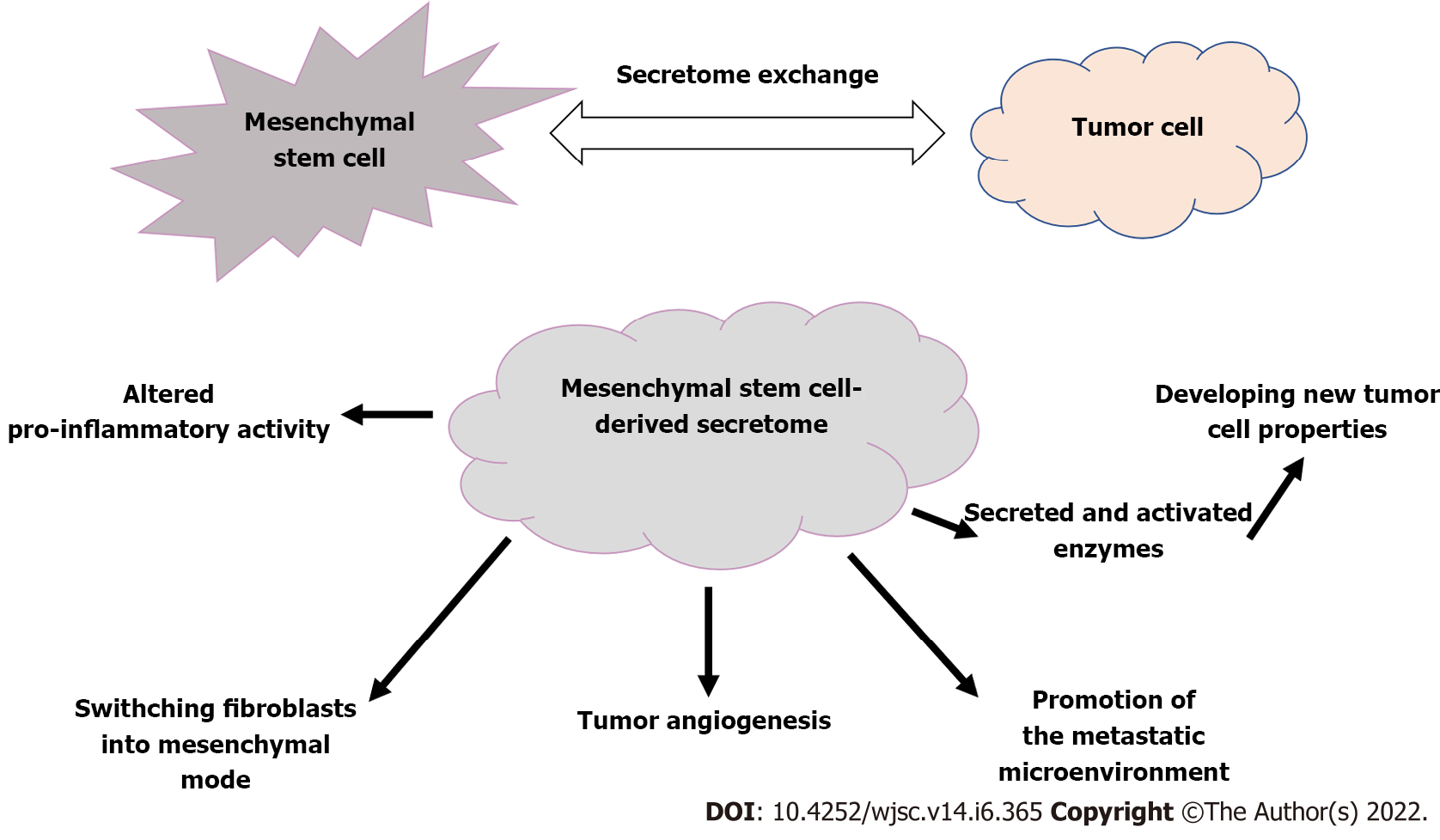
We must not forget that exosomes can also play a significant role in the development of diseases such as cancer. When tissue is damaged, MSCs are recruited to aid in the repair and regeneration of wounds. Also, aggressive tumor development results in inflammation-related tissue injury as a result of intense cell recruitment and cross-modulation. By exchanging secretome, MSCs have the potential to interact with tumor cells[25-28], promoting reciprocal interchange and induction of biological markers[29,30].
Not only the direct effect of the MSC-secreted soluble fraction, but enzymes excreted into and activated inside exosomes (primarily matrix metalloproteinases and their regulators) could make malignant cells have novel properties[25]. The secretome's vesicular fraction is involved in the formation of the pre-metastatic niche and tumor neovascularization. In addition, abnormalities in the extracellular matrix may influence cancer progression by promoting fibroblastic switching and acquisition of mesenchymal mode[26].
The incorporation of MSC-derived exosomes has been linked to the development of ecto-5′-nucleotidase activity in a subset of tumor cells[25]. Tumor cells equipped with this unique ability are capable of suppressing and modulating inflammation-inducing activity by way of the stimulation of adenosine receptor signaling located in the external membrane of the majority of immunocompetent cells, (e.g., tumor-infiltrating T-cell function)[31,32].
In the opposite direction, tumor cells can also affect and modify MSCs through the use of their secretome[22,26]. Extracellular vesicles produced by cancer stem cells are capable of establishing a metastasis supportive compartment and inducing an epithelial to mesenchymal transition, allowing tumors to spread more easily (Figure 2)[26].
Along with undesirable biological properties, current methods for isolating the vesicular secretome (e.g., membrane filtration, ultracentrifugation, precipitation, immunoaffinity capture technology, and size exclusion chromatography) are inefficient, yielding small quantities of low-purity, occasionally distorted extracellular vesicles. As a result, their further application presents difficulties[22,33-35].
In accordance with ClinicalTrials.gov, the number of studies utilizing the MSC-derived secretome is fairly small (i.e., ten), notwithstanding the fact that just three have been completed so far. While the restorative potential of MSC-originated secretome appears auspicious, care is advised. Not only is the content and function of the secretome formed from MSCs largely dependent on the environment from which they were derived (i.e., healthy, inflammatory or tumorous environment), but the therapeutic targeting of the secretome is also difficult at the moment[36]. Whichever method of application is employed, it is not yet feasible to be assured that the biologically active chemical will work on a particular cell type, nor is it totally likely to identify how the intended physiological action of the secretome is altered by the surrounding milieu.
Currently, we also lack knowledge on how drug combinations used in disease conditions affect MSCs and their secretome. By altering MSCs to carry anticancer miRNAs, oncolytic viruses, and anticancer drugs into tumor areas, scientists are able to overcome a number of barriers[37]. However, additional research is required to determine the influence of probable epigenetic or genetic alterations in MSCs on the content and biological functions of the secretome. This is critical to prevent the possibility of tumorigenicity[38].
Along with the technical challenges associated with locating and separating MSCs, laboratory approaches that are novel and efficient are required to extract the MSC-derived secretome in sufficient quality and quantity for application in daily routines. In addition, it would be advantageous to minimize the time and expense involved in these novel procedures, thereby effectively promoting their spread. In conclusion, there is no doubt that, in relation to cell-based techniques, cell-free bioactive components such as the secretome could serve as a significant option in translational medicine.
We would like to express our gratitude to Anika Scott for her assistance.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Cell and tissue engineering
Country/Territory of origin: Hungary
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Buchaim RL, Brazil; Cao HC, China; Liu L, China A-Editor: Flores AI, Spain S-Editor: Fan JR L-Editor: Webster JR P-Editor: Fan JR
| 1. | Vasanthan J, Gurusamy N, Rajasingh S, Sigamani V, Kirankumar S, Thomas EL, Rajasingh J. Role of Human Mesenchymal Stem Cells in Regenerative Therapy. Cells. 2020;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 91] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 2. | Mohammadian M, Shamsasenjan K, Lotfi Nezhad P, Talebi M, Jahedi M, Nickkhah H, Minayi N, Movassagh Pour A. Mesenchymal stem cells: new aspect in cell-based regenerative therapy. Adv Pharm Bull. 2013;3:433-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 33] [Reference Citation Analysis (0)] |
| 3. | Meirelles Lda S, Fontes AM, Covas DT, Caplan AI. Mechanisms involved in the therapeutic properties of mesenchymal stem cells. Cytokine Growth Factor Rev. 2009;20:419-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 951] [Cited by in RCA: 1011] [Article Influence: 63.2] [Reference Citation Analysis (0)] |
| 4. | Chang CJ, Yen ML, Chen YC, Chien CC, Huang HI, Bai CH, Yen BL. Placenta-derived multipotent cells exhibit immunosuppressive properties that are enhanced in the presence of interferon-gamma. Stem Cells. 2006;24:2466-2477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 198] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 5. | Jones BJ, Brooke G, Atkinson K, McTaggart SJ. Immunosuppression by placental indoleamine 2,3-dioxygenase: a role for mesenchymal stem cells. Placenta. 2007;28:1174-1181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 89] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 6. | Saeedi P, Halabian R, Imani Fooladi AA. A revealing review of mesenchymal stem cells therapy, clinical perspectives and Modification strategies. Stem Cell Investig. 2019;6:34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 180] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 7. | Patel DM, Shah J, Srivastava AS. Therapeutic potential of mesenchymal stem cells in regenerative medicine. Stem Cells Int. 2013;2013:496218. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 132] [Cited by in RCA: 152] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 8. | González-González A, García-Sánchez D, Dotta M, Rodríguez-Rey JC, Pérez-Campo FM. Mesenchymal stem cells secretome: The cornerstone of cell-free regenerative medicine. World J Stem Cells. 2020;12:1529-1552. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 103] [Cited by in RCA: 95] [Article Influence: 19.0] [Reference Citation Analysis (6)] |
| 9. | Hassanpour M, Rezabakhsh A, Rezaie J, Nouri M, Rahbarghazi R. Exosomal cargos modulate autophagy in recipient cells via different signaling pathways. Cell Biosci. 2020;10:92. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 70] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 10. | Zhao T, Sun F, Liu J, Ding T, She J, Mao F, Xu W, Qian H, Yan Y. Emerging Role of Mesenchymal Stem Cell-derived Exosomes in Regenerative Medicine. Curr Stem Cell Res Ther. 2019;14:482-494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 114] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 11. | Wang S, Guo L, Ge J, Yu L, Cai T, Tian R, Jiang Y, Zhao RCh, Wu Y. Excess Integrins Cause Lung Entrapment of Mesenchymal Stem Cells. Stem Cells. 2015;33:3315-3326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 91] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 12. | Fennema EM, Tchang LAH, Yuan H, van Blitterswijk CA, Martin I, Scherberich A, de Boer J. Ectopic bone formation by aggregated mesenchymal stem cells from bone marrow and adipose tissue: A comparative study. J Tissue Eng Regen Med. 2018;12:e150-e158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 63] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 13. | Kusuma GD, Menicanin D, Gronthos S, Manuelpillai U, Abumaree MH, Pertile MD, Brennecke SP, Kalionis B. Ectopic Bone Formation by Mesenchymal Stem Cells Derived from Human Term Placenta and the Decidua. PLoS One. 2015;10:e0141246. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 39] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 14. | Haque N, Kasim NH, Rahman MT. Optimization of pre-transplantation conditions to enhance the efficacy of mesenchymal stem cells. Int J Biol Sci. 2015;11:324-334. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 62] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 15. | Rezaie J, Mehranjani MS, Rahbarghazi R, Shariatzadeh MA. Angiogenic and Restorative Abilities of Human Mesenchymal Stem Cells Were Reduced Following Treatment With Serum From Diabetes Mellitus Type 2 Patients. J Cell Biochem. 2018;119:524-535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 44] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 16. | Barkholt L, Flory E, Jekerle V, Lucas-Samuel S, Ahnert P, Bisset L, Büscher D, Fibbe W, Foussat A, Kwa M, Lantz O, Mačiulaitis R, Palomäki T, Schneider CK, Sensebé L, Tachdjian G, Tarte K, Tosca L, Salmikangas P. Risk of tumorigenicity in mesenchymal stromal cell-based therapies--bridging scientific observations and regulatory viewpoints. Cytotherapy. 2013;15:753-759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 289] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 17. | Jeong JO, Han JW, Kim JM, Cho HJ, Park C, Lee N, Kim DW, Yoon YS. Malignant tumor formation after transplantation of short-term cultured bone marrow mesenchymal stem cells in experimental myocardial infarction and diabetic neuropathy. Circ Res. 2011;108:1340-1347. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 305] [Cited by in RCA: 304] [Article Influence: 21.7] [Reference Citation Analysis (1)] |
| 18. | Russo FP, Alison MR, Bigger BW, Amofah E, Florou A, Amin F, Bou-Gharios G, Jeffery R, Iredale JP, Forbes SJ. The bone marrow functionally contributes to liver fibrosis. Gastroenterology. 2006;130:1807-1821. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 388] [Cited by in RCA: 357] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 19. | Fischer UM, Harting MT, Jimenez F, Monzon-Posadas WO, Xue H, Savitz SI, Laine GA, Cox CS Jr. Pulmonary passage is a major obstacle for intravenous stem cell delivery: the pulmonary first-pass effect. Stem Cells Dev. 2009;18:683-692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 794] [Cited by in RCA: 925] [Article Influence: 57.8] [Reference Citation Analysis (0)] |
| 20. | Lepperdinger G, Brunauer R, Jamnig A, Laschober G, Kassem M. Controversial issue: is it safe to employ mesenchymal stem cells in cell-based therapies? Exp Gerontol. 2008;43:1018-1023. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 88] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 21. | McLeod CM, Mauck RL. On the origin and impact of mesenchymal stem cell heterogeneity: new insights and emerging tools for single cell analysis. Eur Cell Mater. 2017;34:217-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 143] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 22. | Ma ZJ, Yang JJ, Lu YB, Liu ZY, Wang XX. Mesenchymal stem cell-derived exosomes: Toward cell-free therapeutic strategies in regenerative medicine. World J Stem Cells. 2020;12:814-840. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 80] [Cited by in RCA: 71] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 23. | Baglio SR, Pegtel DM, Baldini N. Mesenchymal stem cell secreted vesicles provide novel opportunities in (stem) cell-free therapy. Front Physiol. 2012;3:359. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 346] [Cited by in RCA: 402] [Article Influence: 30.9] [Reference Citation Analysis (1)] |
| 24. | Théry C, Witwer KW, Aikawa E, Alcaraz MJ, Anderson JD, Andriantsitohaina R, Antoniou A, Arab T, Archer F, Atkin-Smith GK, Ayre DC, Bach JM, Bachurski D, Baharvand H, Balaj L, Baldacchino S, Bauer NN, Baxter AA, Bebawy M, Beckham C, Bedina Zavec A, Benmoussa A, Berardi AC, Bergese P, Bielska E, Blenkiron C, Bobis-Wozowicz S, Boilard E, Boireau W, Bongiovanni A, Borràs FE, Bosch S, Boulanger CM, Breakefield X, Breglio AM, Brennan MÁ, Brigstock DR, Brisson A, Broekman ML, Bromberg JF, Bryl-Górecka P, Buch S, Buck AH, Burger D, Busatto S, Buschmann D, Bussolati B, Buzás EI, Byrd JB, Camussi G, Carter DR, Caruso S, Chamley LW, Chang YT, Chen C, Chen S, Cheng L, Chin AR, Clayton A, Clerici SP, Cocks A, Cocucci E, Coffey RJ, Cordeiro-da-Silva A, Couch Y, Coumans FA, Coyle B, Crescitelli R, Criado MF, D'Souza-Schorey C, Das S, Datta Chaudhuri A, de Candia P, De Santana EF, De Wever O, Del Portillo HA, Demaret T, Deville S, Devitt A, Dhondt B, Di Vizio D, Dieterich LC, Dolo V, Dominguez Rubio AP, Dominici M, Dourado MR, Driedonks TA, Duarte FV, Duncan HM, Eichenberger RM, Ekström K, El Andaloussi S, Elie-Caille C, Erdbrügger U, Falcón-Pérez JM, Fatima F, Fish JE, Flores-Bellver M, Försönits A, Frelet-Barrand A, Fricke F, Fuhrmann G, Gabrielsson S, Gámez-Valero A, Gardiner C, Gärtner K, Gaudin R, Gho YS, Giebel B, Gilbert C, Gimona M, Giusti I, Goberdhan DC, Görgens A, Gorski SM, Greening DW, Gross JC, Gualerzi A, Gupta GN, Gustafson D, Handberg A, Haraszti RA, Harrison P, Hegyesi H, Hendrix A, Hill AF, Hochberg FH, Hoffmann KF, Holder B, Holthofer H, Hosseinkhani B, Hu G, Huang Y, Huber V, Hunt S, Ibrahim AG, Ikezu T, Inal JM, Isin M, Ivanova A, Jackson HK, Jacobsen S, Jay SM, Jayachandran M, Jenster G, Jiang L, Johnson SM, Jones JC, Jong A, Jovanovic-Talisman T, Jung S, Kalluri R, Kano SI, Kaur S, Kawamura Y, Keller ET, Khamari D, Khomyakova E, Khvorova A, Kierulf P, Kim KP, Kislinger T, Klingeborn M, Klinke DJ 2nd, Kornek M, Kosanović MM, Kovács ÁF, Krämer-Albers EM, Krasemann S, Krause M, Kurochkin IV, Kusuma GD, Kuypers S, Laitinen S, Langevin SM, Languino LR, Lannigan J, Lässer C, Laurent LC, Lavieu G, Lázaro-Ibáñez E, Le Lay S, Lee MS, Lee YXF, Lemos DS, Lenassi M, Leszczynska A, Li IT, Liao K, Libregts SF, Ligeti E, Lim R, Lim SK, Linē A, Linnemannstöns K, Llorente A, Lombard CA, Lorenowicz MJ, Lörincz ÁM, Lötvall J, Lovett J, Lowry MC, Loyer X, Lu Q, Lukomska B, Lunavat TR, Maas SL, Malhi H, Marcilla A, Mariani J, Mariscal J, Martens-Uzunova ES, Martin-Jaular L, Martinez MC, Martins VR, Mathieu M, Mathivanan S, Maugeri M, McGinnis LK, McVey MJ, Meckes DG Jr, Meehan KL, Mertens I, Minciacchi VR, Möller A, Møller Jørgensen M, Morales-Kastresana A, Morhayim J, Mullier F, Muraca M, Musante L, Mussack V, Muth DC, Myburgh KH, Najrana T, Nawaz M, Nazarenko I, Nejsum P, Neri C, Neri T, Nieuwland R, Nimrichter L, Nolan JP, Nolte-'t Hoen EN, Noren Hooten N, O'Driscoll L, O'Grady T, O'Loghlen A, Ochiya T, Olivier M, Ortiz A, Ortiz LA, Osteikoetxea X, Østergaard O, Ostrowski M, Park J, Pegtel DM, Peinado H, Perut F, Pfaffl MW, Phinney DG, Pieters BC, Pink RC, Pisetsky DS, Pogge von Strandmann E, Polakovicova I, Poon IK, Powell BH, Prada I, Pulliam L, Quesenberry P, Radeghieri A, Raffai RL, Raimondo S, Rak J, Ramirez MI, Raposo G, Rayyan MS, Regev-Rudzki N, Ricklefs FL, Robbins PD, Roberts DD, Rodrigues SC, Rohde E, Rome S, Rouschop KM, Rughetti A, Russell AE, Saá P, Sahoo S, Salas-Huenuleo E, Sánchez C, Saugstad JA, Saul MJ, Schiffelers RM, Schneider R, Schøyen TH, Scott A, Shahaj E, Sharma S, Shatnyeva O, Shekari F, Shelke GV, Shetty AK, Shiba K, Siljander PR, Silva AM, Skowronek A, Snyder OL 2nd, Soares RP, Sódar BW, Soekmadji C, Sotillo J, Stahl PD, Stoorvogel W, Stott SL, Strasser EF, Swift S, Tahara H, Tewari M, Timms K, Tiwari S, Tixeira R, Tkach M, Toh WS, Tomasini R, Torrecilhas AC, Tosar JP, Toxavidis V, Urbanelli L, Vader P, van Balkom BW, van der Grein SG, Van Deun J, van Herwijnen MJ, Van Keuren-Jensen K, van Niel G, van Royen ME, van Wijnen AJ, Vasconcelos MH, Vechetti IJ Jr, Veit TD, Vella LJ, Velot É, Verweij FJ, Vestad B, Viñas JL, Visnovitz T, Vukman KV, Wahlgren J, Watson DC, Wauben MH, Weaver A, Webber JP, Weber V, Wehman AM, Weiss DJ, Welsh JA, Wendt S, Wheelock AM, Wiener Z, Witte L, Wolfram J, Xagorari A, Xander P, Xu J, Yan X, Yáñez-Mó M, Yin H, Yuana Y, Zappulli V, Zarubova J, Žėkas V, Zhang JY, Zhao Z, Zheng L, Zheutlin AR, Zickler AM, Zimmermann P, Zivkovic AM, Zocco D, Zuba-Surma EK. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles. 2018;7:1535750. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6453] [Cited by in RCA: 7665] [Article Influence: 1095.0] [Reference Citation Analysis (1)] |
| 25. | Yang Y, Bucan V, Baehre H, von der Ohe J, Otte A, Hass R. Acquisition of new tumor cell properties by MSC-derived exosomes. Int J Oncol. 2015;47:244-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 109] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 26. | Nawaz M, Shah N, Zanetti BR, Maugeri M, Silvestre RN, Fatima F, Neder L, Valadi H. Extracellular Vesicles and Matrix Remodeling Enzymes: The Emerging Roles in Extracellular Matrix Remodeling, Progression of Diseases and Tissue Repair. Cells. 2018;7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 99] [Cited by in RCA: 134] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 27. | Mandel K, Yang Y, Schambach A, Glage S, Otte A, Hass R. Mesenchymal stem cells directly interact with breast cancer cells and promote tumor cell growth in vitro and in vivo. Stem Cells Dev. 2013;22:3114-3127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 111] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 28. | Hass R, Otte A. Mesenchymal stem cells as all-round supporters in a normal and neoplastic microenvironment. Cell Commun Signal. 2012;10:26. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 110] [Cited by in RCA: 104] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 29. | Yang Y, Otte A, Hass R. Human mesenchymal stroma/stem cells exchange membrane proteins and alter functionality during interaction with different tumor cell lines. Stem Cells Dev. 2015;24:1205-1222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 73] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 30. | Salimi L, Akbari A, Jabbari N, Mojarad B, Vahhabi A, Szafert S, Kalashani SA, Soraya H, Nawaz M, Rezaie J. Synergies in exosomes and autophagy pathways for cellular homeostasis and metastasis of tumor cells. Cell Biosci. 2020;10:64. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 103] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 31. | Ohta A, Sitkovsky M. Extracellular adenosine-mediated modulation of regulatory T cells. Front Immunol. 2014;5:304. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 187] [Cited by in RCA: 242] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 32. | Clayton A, Al-Taei S, Webber J, Mason MD, Tabi Z. Cancer exosomes express CD39 and CD73, which suppress T cells through adenosine production. J Immunol. 2011;187:676-683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 363] [Cited by in RCA: 436] [Article Influence: 31.1] [Reference Citation Analysis (0)] |
| 33. | Ahmadi M, Jafari R, Mahmoodi M, Rezaie J. The tumorigenic and therapeutic functions of exosomes in colorectal cancer: Opportunity and challenges. Cell Biochem Funct. 2021;39:468-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 34. | Ahmadi M, Rezaie J. Ageing and mesenchymal stem cells derived exosomes: Molecular insight and challenges. Cell Biochem Funct. 2021;39:60-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 70] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 35. | Babaei M, Rezaie J. Application of stem cell-derived exosomes in ischemic diseases: opportunity and limitations. J Transl Med. 2021;19:196. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 67] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 36. | Phelps J, Sanati-Nezhad A, Ungrin M, Duncan NA, Sen A. Bioprocessing of Mesenchymal Stem Cells and Their Derivatives: Toward Cell-Free Therapeutics. Stem Cells Int. 2018;2018:9415367. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 115] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 37. | Yassine S, Alaaeddine N. Mesenchymal Stem Cell Exosomes and Cancer: Controversies and Prospects. Adv Biol (Weinh). 2022;6:e2101050. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 38. | Hassanzadeh A, Rahman HS, Markov A, Endjun JJ, Zekiy AO, Chartrand MS, Beheshtkhoo N, Kouhbanani MAJ, Marofi F, Nikoo M, Jarahian M. Mesenchymal stem/stromal cell-derived exosomes in regenerative medicine and cancer; overview of development, challenges, and opportunities. Stem Cell Res Ther. 2021;12:297. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 122] [Article Influence: 30.5] [Reference Citation Analysis (0)] |