Published online May 26, 2022. doi: 10.4252/wjsc.v14.i5.347
Peer-review started: January 28, 2022
First decision: March 11, 2022
Revised: March 25, 2022
Accepted: May 8, 2022
Article in press: May 8, 2022
Published online: May 26, 2022
Processing time: 118 Days and 1.8 Hours
Human Wharton’s jelly-derived mesenchymal stromal/stem cells (hWJ-MSCs) have gained considerable attention in their applications in cell-based therapy due to several advantages offered by them. Recently, we reported that hWJ-MSCs and their conditioned medium have significant therapeutic radioprotective potential. This finding raised an obvious question to identify unique features of hWJ-MSCs over other sources of stem cells for a better understanding of its radioprotective mechanism.
To understand the radioprotective mechanism of soluble factors secreted by hWJ-MSCs and identification of their unique genes.
Propidium iodide staining, endogenous spleen colony-forming assay, and survival study were carried out for radioprotection studies. Homeostasis-driven proliferation assay was performed for in vivo lymphocyte proliferation. Analysis of RNAseq data was performed to find the unique genes of WJ-MSCs by comparing them with bone marrow mesenchymal stem cells, embryonic stem cells, and human fibroblasts. Gene enrichment analysis and protein-protein interaction network were used for pathway analysis.
Co-culture of irradiated murine splenic lymphocytes with WJ-MSCs offered significant radioprotection to lymphocytes. WJ-MSC transplantation increased the homeostasis-driven proliferation of the lymphocytes. Neutralization of WJ-MSC conditioned medium with granulocyte-colony stimulating factor antibody abolished therapeutic radioprotection. Transcriptome analysis showed that WJ-MSCs share several common genes with bone marrow MSCs and embryonic stem cells and express high levels of unique genes such as interleukin (IL)1-α, IL1-β, IL-6, CXCL3, CXCL5, CXCL8, CXCL2, CCL2, FLT-1, and IL-33. It was also observed that WJ-MSCs preferentially modulate several cellular pathways and processes that handle the repair and regeneration of damaged tissues compared to stem cells from other sources. Cytokine-based network analysis showed that most of the radiosensitive tissues have a more complex network for the elevated cytokines.
Systemic infusion of WJ-MSC conditioned media will have significant potential for treating accidental radiation exposed victims.
Core Tip: This study showed the potential role of cytokine granulocyte-colony stimulating factor in therapeutic radioprotection. Transcriptome analysis showed that Wharton’s jelly-derived mesenchymal stromal/stem cells (WJ-MSCs) have a unique set of genes compared to bone marrow MSCs and embryonic stem cells. WJ-MSCs secrete several cytokines that promote cellular pathways that handle tissue repair and regeneration.
- Citation: Maurya DK, Bandekar M, Sandur SK. Soluble factors secreted by human Wharton’s jelly mesenchymal stromal/stem cells exhibit therapeutic radioprotection: A mechanistic study with integrating network biology. World J Stem Cells 2022; 14(5): 347-361
- URL: https://www.wjgnet.com/1948-0210/full/v14/i5/347.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v14.i5.347
Exposure of mice to high doses of gamma radiation causes acute radiation syndrome (ARS), which includes damage to hematopoietic, gastrointestinal, and neurovascular systems depending on the dose. Currently, extensive progress has been made to understand the molecular players leading to ARS and therapeutic options. Several agents have been reported to protect mice from ARS, which include thiol-containing compounds, phytochemicals[1-3], and several cytoprotective cytokines and growth factors [interleukin (IL)-1, IL-6, tumor necrosis factor-α, granulocyte-colony stimulating factor (G-CSF), granulocyte-macrophage colony-stimulating factor], etc[4-6].
Stem cell therapy is one of the promising strategies for the amelioration of ARS because of its ability to regenerate by sensing the damage. Mesenchymal stromal/stem cells (MSCs) are one such type of cells that have the potential for clinical applications. They are easy to culture and possess low immunogenicity, high regenerative potential, multilineage differentiation abilities, and potent antiapoptotic and immunosuppressive effects[7]. Several reports have shown the ability of MSCs to repair various tissue injuries induced by radiation and other stressors[8-12]. The mechanism of action to prevent radiation-induced tissue injury by MSCs could be paracrine secretion of several soluble factors[13,14]. Various paracrine factors secreted by MSCs include cytokines, chemokines, pro-survival factors, and growth factors[15-19]. All these properties of the MSCs may help in recovery from hematopoietic and gas
The therapeutic potential of bone marrow MSCs (BM-MSCs) against radiation injury has been well documented in several experimental studies[20-24]. Lange et al[23] showed that a protective response was evoked in combating inflammatory events post-radiation exposure after systemic administration of MSCs[23]. Another study by Chang et al[11] showed protection against whole body irradiation (WBI)-induced damage to the intestinal mucosa by enhancing angiogenesis and chemotaxis after bone marrow transplantation[11]. Existing evidence shows that MSCs “home in” to bone marrow or other injured tissues and secrete different soluble factors that help in the recovery of radiation-induced hematopoietic damage via induction of hematopoietic stem/progenitor cell division and differentiation[23-25]. The trophic factors (granulocyte-macrophage colony-stimulating factor, thrombopoietin, and stem cell factor) secreted by MSCs can modulate the hematopoietic stem cell niche thereby rescuing endogenous hematopoiesis[23,25].
Stem cells isolated from Wharton’s jelly of the umbilical cord (WJ-MSCs) are a unique source of MSCs. These cells did not induce any adverse effects or teratoma formation in the recipients and are hence considered safe[26]. WJ-MSCs exhibit reduced immunogenicity[27] as compared to other MSC types. WJ-MSCs have low expression of costimulatory ligands, which otherwise can stimulate immune responses[28]. Interestingly, WJ-MSCs are known to express human leukocyte antigen G, which helps in the expansion of immunosuppressive regulatory T cells. Recently, our laboratory has shown the radioprotective potential of human WJ-MSCs and conditioned media collected from culturing WJ-MSCs against ionizing radiation-induced mortality in mice[8,29].
In the present study, we have established the involvement of cytokine G-CSF in the observed radioprotection offered by human WJ-MSC-conditioned medium (WJ-MSC-CM). We have also constructed the cytokine network using an experimentally determined list of the cytokines secreted in mouse blood serum 4 h after human WJ-MSC transplantation in the mice. Our study showed that the protein-protein interaction (PPI) network constructed using cytokines is more complex in the radiosensitive tissues as compared to other tissues such as muscle. Our transcriptome analysis showed that WJ-MSCs preferentially modulated several cellular pathways and processes that are responsible for the repair and regeneration of damaged tissues.
MSC expansion medium, RPMI-1640 medium, fetal bovine serum (FBS), and antibiotic–antimycotic mixture were purchased from Hi-Media Pvt (Mumbai, India). MSC qualified FBS was purchased from Thermo Fischer Scientific. Propidium iodide (PI), ribonuclease A, and Triton X-100 were purchased from Sigma Chemical Co. (St Louis, MO, United States). Hoechst 33342 and carboxyfluorescein diacetate succinimidyl ester (CFSE) was procured from Molecular Probes (Eugene, OR, United States). Anti-G-CSF antibody was procured from R&D Systems (Minneapolis, MN, United States). All other chemicals used were of analytical grade and were procured from local manufacturers.
Ethical approval for isolation of WJ-MSCs was obtained from the Institutional ethical review board at Bhabha Atomic Research Centre Hospital, Mumbai, India (project numbers IC-SCRBARC/2018/2 and BARCHMEC/14). Human umbilical cords were freshly collected soon after the delivery of a full-term infant by cesarean section from the donor after obtaining written consent[8]. WJ-MSCs were isolated from the umbilical cord and characterized as described earlier[8]. They were cultured in MSC expansion medium added with 20% MSC qualified FBS, and 1% antibiotic-antimycotic mixture.
Ten- to twelve-week-old male Swiss mice weighing approximately 20-25 g were selected from an inbred group maintained under standard condition of temperature (25 ± 2) ºC and reared in the animal house of the Bhabha Atomic Research Centre. The guidelines issued by the Institutional Animal Ethics Committee of Bhabha Atomic Research Centre, Government of India (project No. BAEC/15/18 dated April 2018) regarding maintenance and dissection of animals were strictly followed.
WJ-MSCs (5 × 104) were cultured in 1 mL WJ-MSC complete media for 24 h in a 24-well plate. After incubation, media was harvested, centrifuged at 1000 rpm for 2 min, and then filtered through a 0.22 μm sterile syringe filter. Conditioned media was kept in the ultra-deep freezer in aliquots and thawed as and when required.
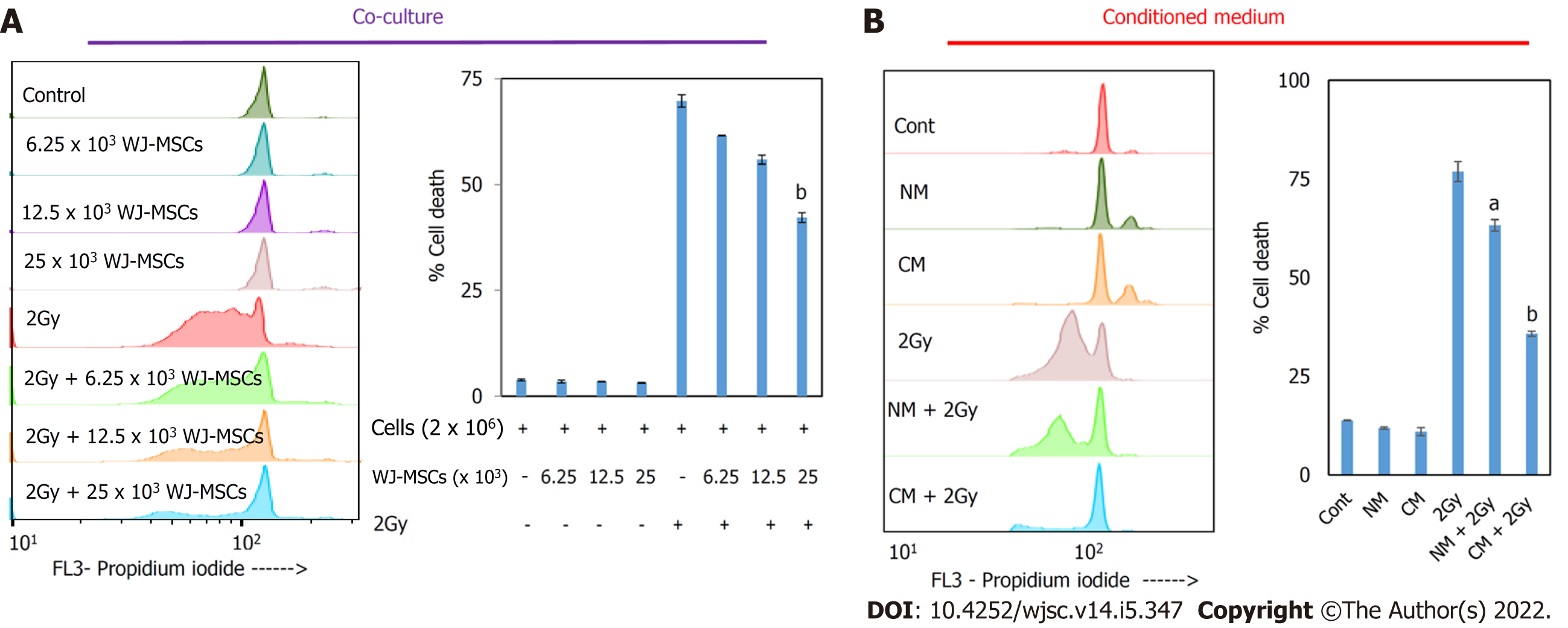
To study the radioprotective property of WJ-MSCs in vitro, different numbers of WJ-MSCs (6.25 × 103, 12.5 × 103, and 25 × 103) were seeded in a 24-well cell culture plate. The next day 2 Gy gamma-irradiated murine splenic lymphocytes were co-cultured with pre-seeded WJ-MSCs. Splenocytes were incubated for 24 h in complete RPMI-1640 medium (RPMI-1640 medium supplemented with 10% FBS and 100 units/mL penicillin/100 μg/mL streptomycin). To study the radioprotective property of WJ-MSC-CM, irradiated lymphocytes suspended in RPMI-1640 were mixed with normal medium or WJ-MSC-CM in a 1:1 ratio and cultured for 24 h with 10% FBS. Cells were then washed with PBS and stained with 1 mL of PI solution (0.5 μg/mL PI, 10 μg/mL ribonuclease A, 0.1% sodium citrate, and 0.1% Triton X-100) overnight. Cells were subjected to flow cytometry, and the data was analyzed using FlowJo v10 software. The pre-G1 population in the histogram represented apoptotic cells.
WJ-MSC-CM (24 h cultured) was treated with 5 μg/mL of human G-CSF neutralizing antibody. This conditioned media was incubated at 37 °C for 2 h. The G-CSF neutralized CM (200 μL) was infused to lethally irradiated mice to investigate the role of G-CSF in therapeutic radioprotection[8].
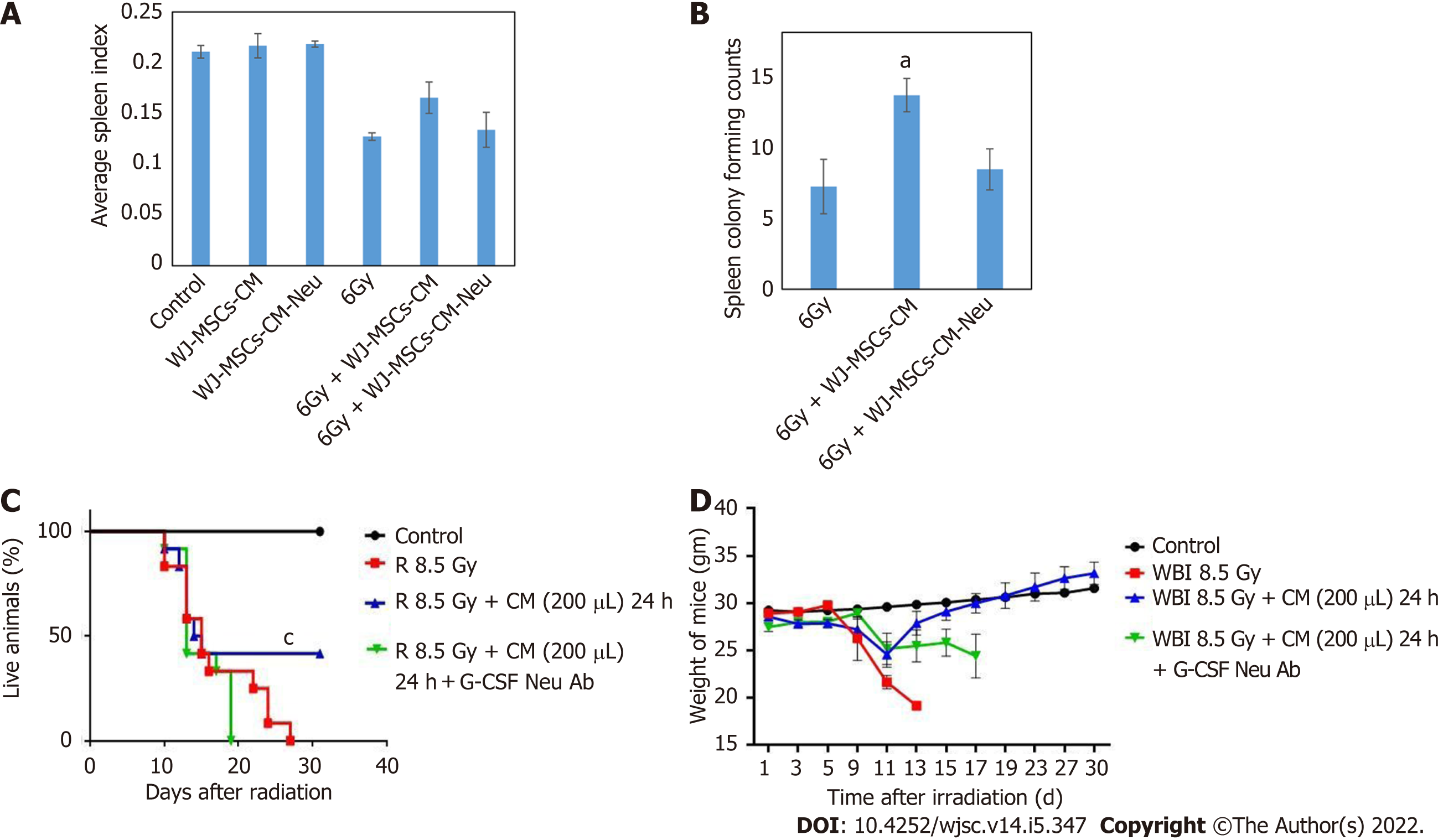
Endogenous spleen colony formation is considered a gold standard assay for the demonstration of radioprotection. To study the effect of WJ-MSC-CM or G-CSF neutralized WJ-MSC-CM on radiation-induced reduction in spleen index and endogenous spleen colony formation, mice were divided into four groups: Control, 6 Gy WBI, 6 Gy WBI + WJ-MSC-CM, and 6 Gy WBI + WJ-MSC-CM neutralized with G-CSF. Mice were exposed to 6 Gy WBI[8] and injected with 200 μL WJ-MSC-CM or WJ-MSC-CM neutralized with G-CSF 24 h after radiation exposure. Mice were sacrificed on day 15, and weights of mice and spleens were recorded. Spleens were then fixed in Bouin’s solution. Spleen index was calculated by taking the ratio of spleen weight to mouse weight. The spleen colony forming units were counted as macroscopic colonies formed on the surface of the spleen as a result of the proliferation of surviving BM stem/progenitor cells.
For studying the effect of WJ-MSC-CM infusion on the survival of mice, 8.5 Gy dose of radiation was selected based on our previous study[8]. For the survival study, mice were randomly divided into the following experimental groups: (1) Control: PBS alone; (2) 8.5 Gy WBI; (3) 8.5 Gy WBI + 24 h post-irradiation WJ-MSC-CM; and (4) 8.5 Gy WBI + 24 h post-irradiation WJ-MSC-CM neutralized with anti-G-CSF. The day of whole-body exposure was considered as day 0. WJ-MSC-CM or WJ-MSC-CM neutralized with anti-G-CSF (200 μL) were systemically infused through the lateral tail vein. Mice were monitored daily for 30 d.
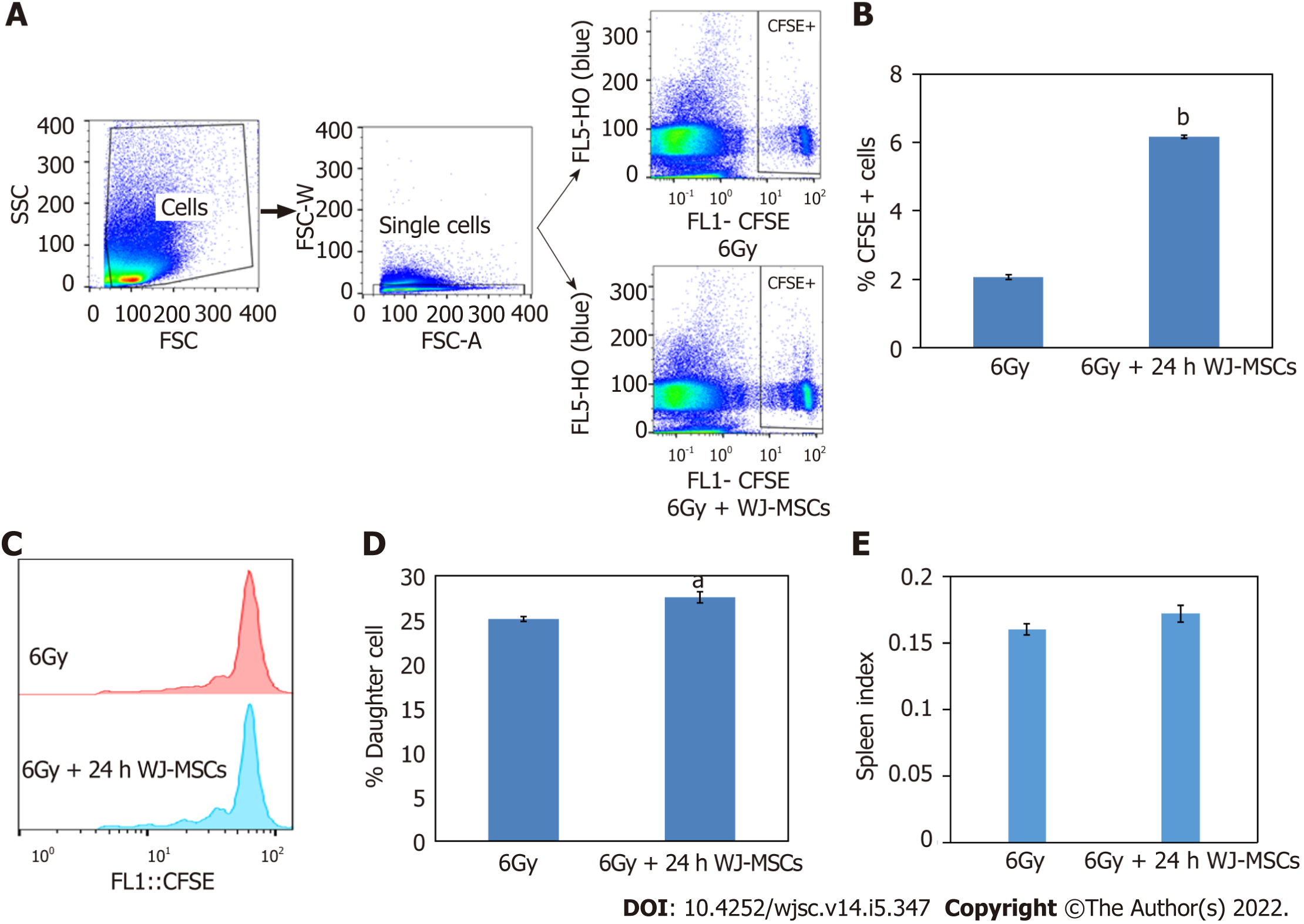
To perform homeostasis-driven proliferation, lymphopenia was induced in mice by exposing them to 6 Gy WBI and keeping them for 24 h. Further, CFSE (20 μM) labeled splenic lymphocytes (6 × 106 cells) were infused into lymphopenic mice with or without WJ-MSCs through the lateral tail vein. Each group consisted of four mice. Spleens were collected 96 h after injection. Spleen index was calculated by recording spleen weight and mice weight. Samples (250000 lymphocytes) from each group were acquired on a flow cytometer to enumerate the frequency of donor cells and monitor their cell proliferation.
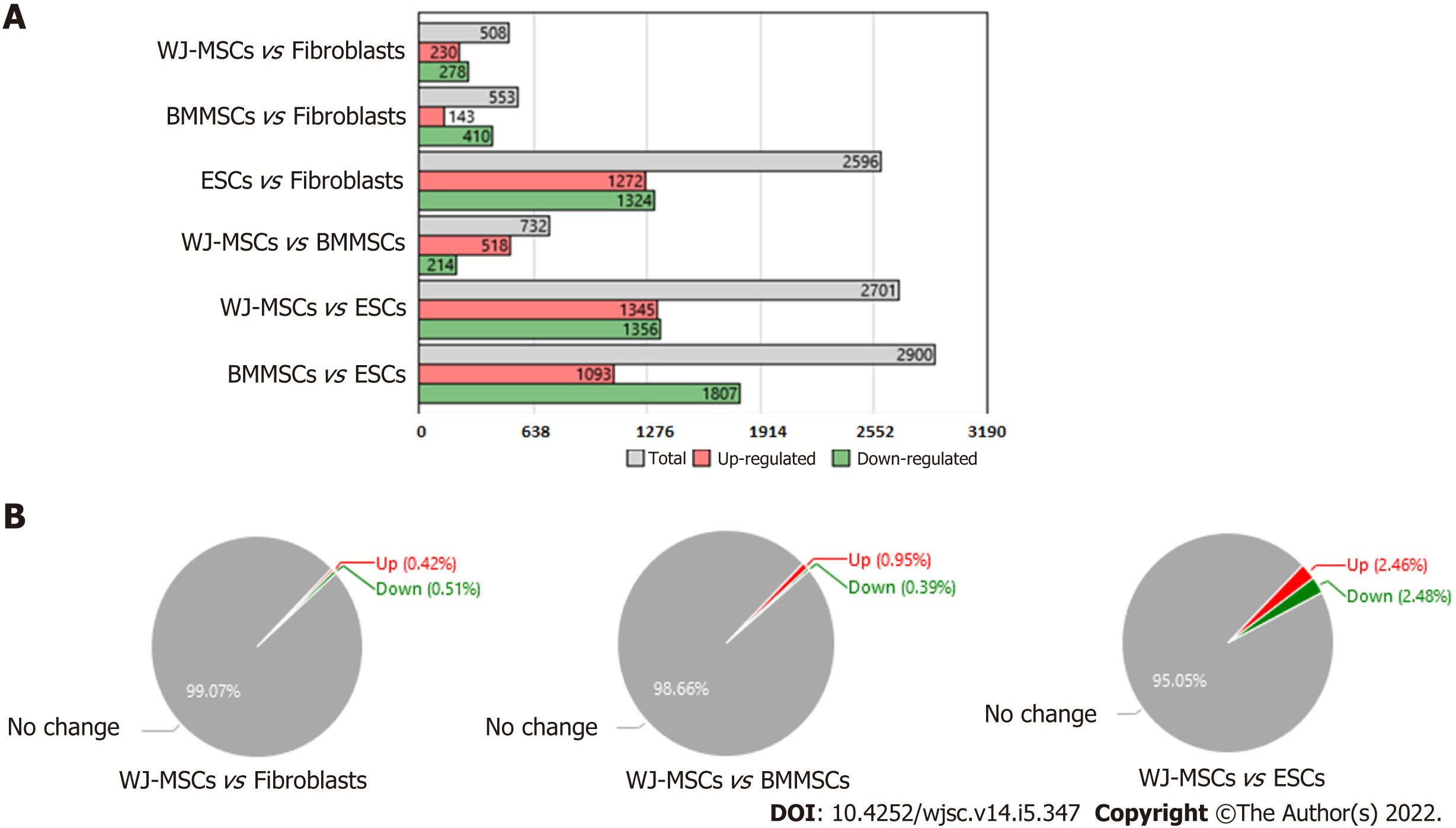
The raw data for different sources of stem cells were downloaded from the GEO database {GSE20124 and GSE48022 having GEO accession No. GSM1165510, GSM1165511 (WJ-MSCs); GSM503594, GSM503595 [embryonic stem cells (ESCs)]; GSM1165505, GSM1165506 (BM-MSCs); GSM503604, GSM503605 (Fibroblast), and platform Affymetrix Human Genome U133 plus 2.0 Array in CEL format} and analyzed using transcriptome analysis console (Applied Biosystems, Thermo Fisher Scientific, Waltham, MA, United States) for normalization of signal intensity, quality control, and statistical analysis. The differentially expressed genes in WJ-MSCs, BM-MSCs, and ESCs were compared with the fibroblasts using transcriptome analysis console. Further, t-test, Multiple Testing Corrections, and False Discovery Rate Prediction were employed to calculate the significance of differentially expressed genes. A threshold of 5-fold difference in gene expression between different stem cells compared to fibroblasts with a P < 0.05 was used for statistical significance.
The data obtained from the transcriptome analysis console was utilized to assess the biological significance. BiNGO plugin of Cytoscape was used for assigning specific biological function, molecular function, and functional pathways for genes that were enriched among the upregulated genes[30]. The list of genes was statistically assessed by gProfiler using the following settings: Organism-Homo sapiens, statistical domain scope-Only annotated genes, significant threshold-Benjamini Hochberg FDR, and threshold-0.05[31].
For creating PPI, a list of the cytokines elevated in mouse serum at 4 h post-WJ-MSC transplantation were obtained from our previous study[8]. A PPI network was constructed for these cytokines using Cytoscape (Ver 3.7.1). This constructed network was further extended using the STRING database. The Molecular Complex Detection was used to select modules of the PPI network with the following parameters: Degree cutoff = 2, node score cutoff = 0.2, core = 2, and max. depth = 100. Molecular Complex Detection encompasses an automated algorithm that detects densely connected regions in PPI networks that may represent molecular complexes. Further, BiNGO plugin of Cytoscape was used to obtain the biological significance of the predicted modules.
All the graphs were made using mean ± standard error using Microsoft Excel. Cellular data were analyzed for multiple comparison with Tukey-Kramer post-hoc test using KyPlot 5 statistical software (KyensLab Inc, Tokyo, Japan) with a statistical significance set at aP < 0.05 and bP < 0.001. For the survival study, Kaplan–Meier’s estimate of lifetime analysis was done using GraphPad Prism 5 software (GraphPad software, Inc., La Jolla, CA, United States). The difference between the estimated survival times of the two groups was evaluated by the log-rank test. The statistical significance of the log-rank test was considered if P < 0.05 according to χ2 distribution. cP < 0.0001.
In vitro therapeutic potential of WJ-MSCs was evaluated using a co-culture study. Our study showed that when irradiated lymphocytes were co-cultured with a different number of pre-cultured WJ-MSCs for 24 h that they offered protection to lymphocytes as monitored by PI staining (Figure 1A). The extent of protection increased with an increasing number of WJ-MSCs. Subsequently, WJ-MSC-CM was added to irradiated lymphocytes, and cell death was measured using the PI assay. It was found that WJ-MSC-CM offered therapeutic protection against radiation-induced apoptosis in lymphocytes (Figure 1B). The results showed that WJ-MSCs offered protection to irradiated lymphocytes by secreting certain soluble mediators that may help in repair.
Our previous findings showed that infusion of WJ-MSC-CM to lethally irradiated mice protected against mortality[8]. In the present study, infusion of conditioned media 24 h post-irradiation (6 Gy) led to better recovery in spleen index and endogenous spleen colony formation (Figure 2A and B). However, when WJ-MSC-CM was neutralized with anti-G-CSF and infused in irradiated mice, the protective effect of WJ-MSC-CM diminished as compared to mice that were injected with non-neutralized WJ-MSC-CM. Further, when non-neutralized WJ-MSC-CM was injected to lethally irradiated mice (8.5 Gy), it offered about 43% protection against mortality as compared to irradiated mice administered with WJ-MSC-CM neutralized with G-CSF (Figure 2C). It was also observed that surviving mice in the WJ-MSC-CM group recovered from WBI-induced morbidity (Figure 2D). These findings show that cytokine/growth factor G-CSF may be one factor responsible for the observed radioprotection offered by WJ-MSC-CM.
Transplantation of WJ-MSCs along with CFSE labeled lymphocytes into lymphopenic mice resulted in a higher frequency of CFSE positive lymphocytes in the host as compared to mice injected with CFSE labeled lymphocytes alone (Figure 3A and B). Further, these CFSE-labeled lymphocytes divided more times in the host when injected along with WJ-MSCs as compared to mice infused with CFSE labeled lymphocytes alone (Figure 3C and D). Figure 3E shows that the spleen index was higher in lymphopenic mice injected with lymphocytes along with WJ-MSCs as compared to mice that received only lymphocytes. These results indicated that WJ-MSCs or some of the soluble factors secreted by them may be helping in faster recovery from radiation-mediated injury.
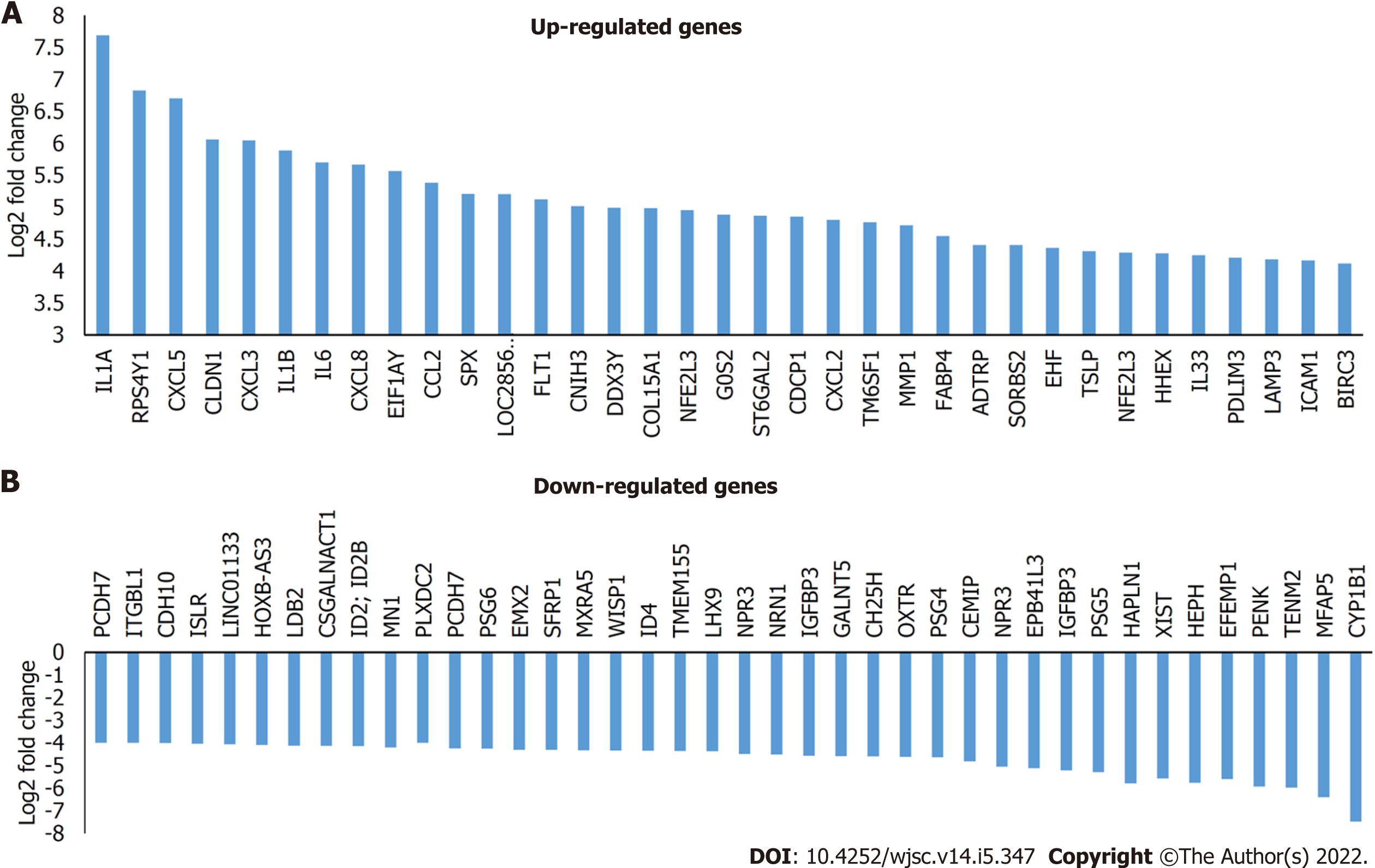
Our previous report[8] and the present findings have clearly shown that WJ-MSCs have distinct advantages over other stem cells like BM-MSCs and ESCs in ameliorating radiation injury. Hence, we wanted to understand the unique genes that are expressed by WJ-MSCs as compared to other stem cells and fibroblasts. For this purpose, gene expression data related to different stem cells were downloaded from the GEO database. Gene expression analysis was carried out by setting- gene-level fold change < -5 or > 5 and gene-level P < 0.05 (Anova method-Bayes). From Figure 4A, WJ-MSCs, BM-MSCs, and ESCs have 508, 553, and 2596 differentially expressed basal genes as compared to dermal fibroblasts, respectively. Among these, the number of downregulated genes was more. Interestingly, when WJ-MSCs were compared with BM-MSCs, 732 genes were differentially expressed, out of which 518 genes were upregulated, and 214 genes were downregulated in the WJ-MSCs. In contrast, when WJ-MSCs were compared with ESCs, 2701 genes were differentially expressed, out of which 1345 genes were upregulated, and 1356 genes were downregulated. On the other hand, when BM-MSCs were compared with the ESCs, 2900 genes were differentially expressed, out of which 1093 genes were upregulated in BM-MSCs, and 1807 genes were downregulated. Figure 4B shows the percentage of differentially expressed genes in WJ-MSCs when compared with fibroblasts, BM-MSCs, or ESCs, respectively. Supplementary Table 1 shows the list of the differentially expressed genes that are unique to WJ-MSCs. It is clear from Supplementary Table 1 that most of the differentially expressed genes of WJ-MSCs are different interleukins, growth factors (such as G-CSF), chemokines, various adhesion molecules, stemness markers, and many more that have a significant role in tissue regeneration and repair. Some of the highly upregulated and downregulated genes of the WJ-MSCs are plotted as shown in Figure 5.
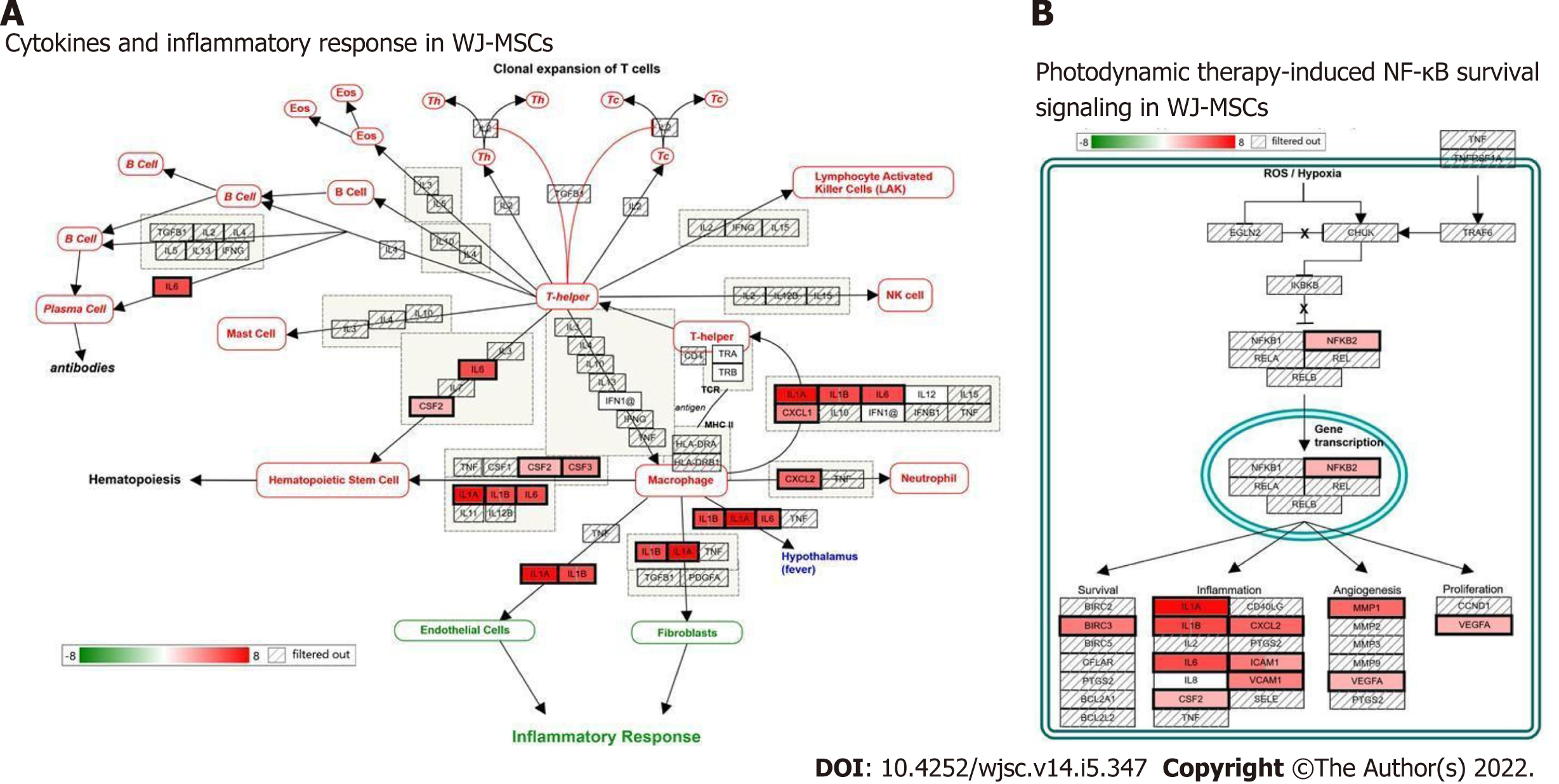
To explore the biological function of these identified unique genes of WJ-MSCs, gene enrichment analysis was performed using gProfiler for their gene ontology and functions. Supplementary Table 2 shows the gene ontology of unique genes from WJ-MSCs analyzed using gProfiler. A total of 32 biological processes were found to be influenced by these genes. Some of the functions of these genes are involved in the multicellular organismal process, positive regulation of macromolecule metabolic process, positive regulation of nitrogen compound metabolic process, positive regulation of the biological process, positive regulation of the metabolic process, and cytokine response. Some key molecular functions identified were cytokine activity, signaling receptor activator activity, receptor-ligand activity, cytokine receptor binding, molecular function regulator, and growth factor receptor binding. These genes belong to different cellular compartments like the extracellular matrix, extracellular space, and cell surface. To further understand how these differentially expressed genes influence the function of WJ-MSCs, we used prior knowledge of biological pathways reported in the literature. Figure 6 shows two of the major signaling pathways, cytokines, and inflammatory response pathway, and nuclear factor kappa B survival pathway, which regulates cell survival and death.
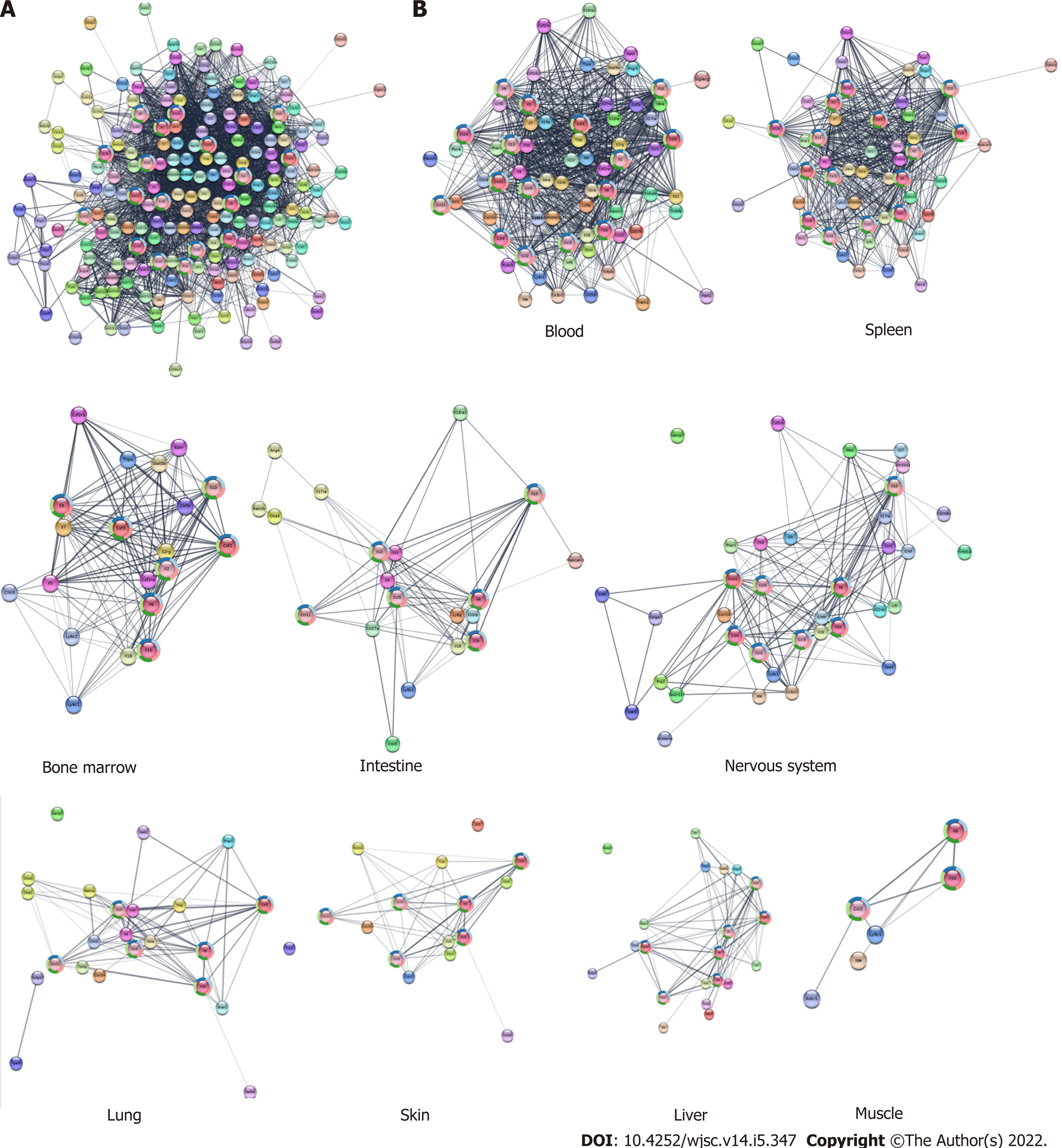
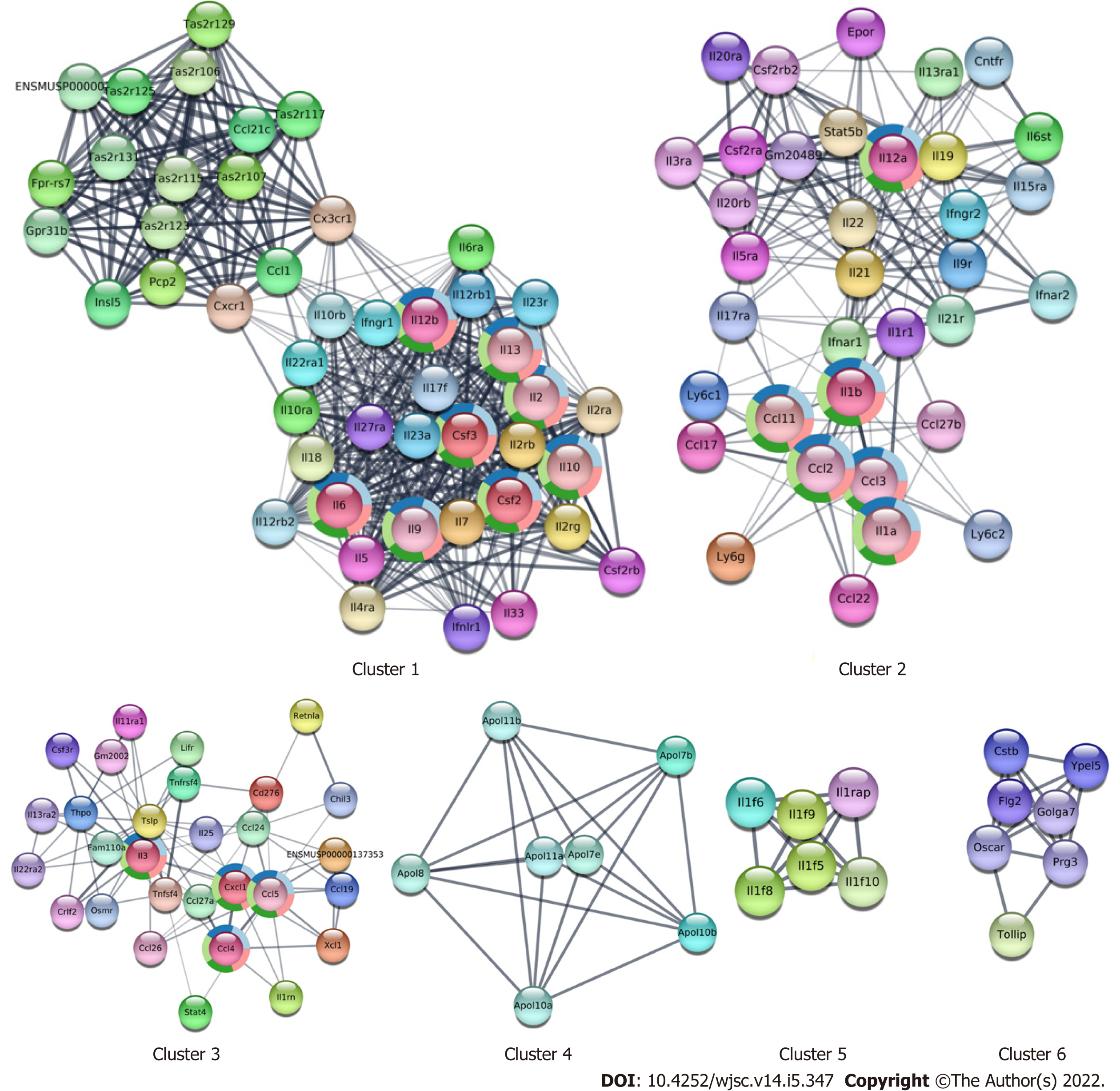
Our previous findings showed that CM from cultured WJ-MSCs or serum collected from WJ-MSC-infused mice contained several cytokines, chemokines, and growth factors[8]. The information obtained from serum samples was used to construct an expanded PPI network (Figure 7A). When this PPI was analyzed for their tissue specificity, most of the radiosensitive tissues such as blood, bone marrow, intestine, and spleen show a more complex network as compared to the radio-resistant tissues such as skin, liver, and muscles (Figure 7B) indicating that the WJ-MSC-secreted cytokines may play a major role in the radiosensitive tissues compared to radio-resistant tissues. When the extended cytokine PPI was subjected to cluster analysis using Molecular Complex Detection, six distinct clusters were obtained. These clusters were analyzed for their functions and found that they were responsible for the modulation of the immune system and related functions (Figure 8).
Stem cell-based therapy is receiving significant attention in the field of regenerative medicine for treating various disorders and injuries[32]. During unplanned radiation exposure (such as radiation accidents), significant tissue damage is observed, and if not repaired/regenerated it can develop into ARS. To avoid ARS, regenerative therapy will be a good therapeutic option. In the past, the therapeutic potential of BM-MSCs for radioprotection has been demonstrated in several experimental studies[20-24]. The first case of human cord blood transfusion [locus-mismatched, unrelated human umbilical cord blood (hUCB) transfusion] to a lethally irradiated 39-year-old male exposed with neutrons to 8-10 Gy in the uranium-processing plant was documented in a radiation accident in Tokai, Japan in 1999, and he lived for 210 d[33]. Azzam et al[34] showed that combinatorial administration of hUCB cells with an antibiotic (Levaquin) showed recovery from hematopoietic and gastrointestinal tract syndromes in lethally irradiated mice[34].
WJ is another alternate source of MSCs and are safe compared to other sources due to no adverse effects or teratoma formation in the recipients[26]. WJ-MSCs lack costimulatory ligands, which otherwise can activate robust immune responses in the host[28]. In addition, preclinical studies have shown anti-tumor activity of WJ-MSCs[35-38]. Recently, we have shown that infusion of WJ-MSCs either 4 h or 24 h post-irradiation significantly protected against lethal irradiation[8]. We also demonstrated that infusion of WJ-MSC-CM offered therapeutic radioprotection. The possible mechanism by which WJ-MSCs offered radioprotection could be via secreting cytoprotective cytokines such G-CSF, IL-6, and Nrf-2[8]. G-CSF has received the Food and Drug Administration approval for the treatment of myelosuppression induced by radiation during radiological incidents/accidents[39,40]. Shim et al[41] showed that hUCB-MSC treatment significantly elevated the number of peripheral leukocyte counts in irradiated mice. They also reported that treatment with hUCB-MSCs was more effective than G-CSF treatment in supporting the proliferation of various cells in the bone marrow[41].
The current study explored the possible mechanism by which WJ-MSCs were offering therapeutic radioprotection using network biology. Our findings showed that the co-culture of WJ-MSCs with irradiated lymphocytes led to protection against radiation-induced cell death in lymphocytes (Figure 1). The observed radioprotection could be due to the release of certain soluble mediators such as radioprotective cytokines, which other investigators had earlier reported[42]. A homeostasis-driven proliferation study showed that infusion of WJ-MSCs along with lymphocytes into lymphopenic mice led to increased lymphocyte division as compared to the group where lymphocytes alone were injected (Figure 3). We also observed an improved spleen index in these mice. This study indicated that WJ-MSCs alone or the soluble factors secreted by them may be helping the infused lymphocytes to survive and proliferate in a lymphopenic environment. These factors may be helping the host lymphocytes to survive radiation-induced death, which in turn can protect against opportunistic infections in irradiated mice.
Our earlier results have shown that WJ-MSCs homed to the radiosensitive tissues in irradiated mice[8]. Thus, the infused WJ-MSCs after reaching the target site favor the repair of damaged tissues and alter the microenvironment similar to other MSCs by secreting different cytokines and growth factors[42]. The experiments carried out using CM of WJ-MSCs also showed a survival benefit to lethally irradiated mice. Interestingly, when this CM was neutralized with an anti-G-CSF antibody, WJ-MSC-CM failed to protect mice against radiation-induced mortality. This finding corroborates well with the hypothesis that WJ-MSCs might be secreting radioprotective cytokines in the observed radioprotection. However, administration of WJ-MSC-CM to lethally irradiated mice offered only about 40% protection indicating that other mechanisms are also playing a role in WJ-MSC-mediated radioprotection.
Alternatively, the concentration of G-CSF present in the WJ-MSC-CM may not be equivalent to G-CSF secreted by infused WJ-MSCs in vivo. It is well known that tissues/organs like bone marrow, spleen, and gastrointestinal tract where there is active cell division taking place are highly sensitive to radiation-induced cell death. The cytokine/chemokine-based network analysis showed that they are more complex in the radiosensitive tissues as compared to radio-resistant tissues indicating their role in tissue radioprotection.
Inflammatory response during infection that offers a protective mechanism requires coordination among various types of immune cells. After exposure to lethal doses of radiation, proinflammatory mediators recruit inflammatory cells to the damaged sites and inflamed tissues. These events are regulated by a proinflammatory transcription factor nuclear factor kappa B, which translocates to the nucleus to initiate the transcriptional activation of pro-survival factors and inflammatory cytokines. Inflammatory cytokines, namely IL-1β and tumor necrosis factor-α, which are produced upon whole-body radiation exposure, evoke systemic responses leading to tissue injury/repair[43]. IL-6 is a pleiotropic cytokine that enhances hematopoietic cell recovery following WBI when combined with G-CSF[39]. The acute inflammation following exposure to ionizing radiation must be terminated soon after resolving the injury by elevating anti-inflammatory mechanisms at the damaged area and prevent the onset of chronic inflammation and subsequent damage to tissues. The pathway analysis carried out using the RNA-seq data taken from the GEO database revealed that many of the differentially expressed genes were: IL-1α, IL1-β, IL-6, CXCL3, CXCL5, CXCL8, CXCL2, CCL2, FLT-1, and IL-33 in WJ-MSCs as compared to fibroblasts (Figure 5).
When these sets of genes were further analyzed to get more insights into biological functions, cytokines and inflammatory response pathways and photodynamic-induced nuclear factor kappa B survival pathway seemed to be highly modulated (Figure 6), which was not seen in BM-MSCs and ESCs (Supplementary Figures 1 and 2). There are several reports on the beneficial role of cytokines in radioprotection. Infusion of recombinant IL-1 to mice protects against the lethal effects of ionizing radiation[44-46]. Interestingly, intravenous infusion of IL-1 and stem cell factor to mice before exposure to a lethal dose of WBI showed synergistic radioprotection[45,47,48]. CXCL5 has also been reported to play a role in the acute phase of radiation-induced salivary gland damage[49]. Bergonie and Tribondeau[50] have shown that IL-1 and tumor necrosis factor-α can induce IL-2 receptor, IL-6, colony-stimulating factor, and acute-phase proteins, which may be responsible for the observed radioprotective effects.
Our study showed that the co-culture of irradiated lymphocytes with WJ-MSCs protected the lymphocytes against radiation-induced cell death. Infusion of WJ-MSCs along with lymphocytes increased homeostasis-driven proliferation. Neutralization of WJ-MSC-CM with anti-G-CSF antibody reduced the radioprotective ability. Network biology and transcriptome profile analysis of WJ-MSCs indicated the radioprotective role of WJ-MSC-secreted cytokines. Thus, WJ-MSC-CM may be used as a therapeutic option during the recovery of radiation-exposed victims.
Exposure to high doses of ionizing radiation is known to cause acute radiation syndrome, such as damage to hematopoietic, gastrointestinal, and neurovascular systems depending on the dose. To avoid acute radiation syndrome, regenerative therapy will be a good therapeutic option. Therefore, stem cell therapy may be one of the promising candidates to ameliorate acute radiation syndrome because of its regenerative and damage sensing potential.
Stem cells isolated from Wharton’s jelly of the umbilical cord are a unique source of mesenchymal stromal/stem cells (MSCs), which have been reported to be safe when administered to recipients without inducing any adverse effects or teratoma formation. Recently, we reported that human Wharton’s jelly-MSCs (hWJ-MSCs) and their conditioned medium (CM) have significant therapeutic radioprotective potential in lethally irradiated mice. These findings motivated us to identify a unique feature of hWJ-MSCs over other sources of stem cells for the understanding of its radioprotective mechanism and deciphering the role of the granulocyte-colony stimulating factor (G-CSF) present in hWJ-MSC-CM.
The main objective was to understand the radioprotective mechanism of soluble factors secreted by hWJ-MSCs and identification of their unique genes.
Propidium iodide staining, endogenous spleen colony-forming assay, and survival study were carried out for radioprotection studies. Homeostasis-driven proliferation assay was performed for in vivo lymphocyte proliferation measurement. Neutralization of G-CSF with anti-G-CSF was done to investigate the role of G-CSF in therapeutic radioprotection. Analysis of RNAseq data was performed to find the unique genes of WJ-MSCs by comparing them with bone marrow mesenchymal stem cells, embryonic stem cells, and human fibroblasts. Gene enrichment analysis and protein-protein interaction network were used for pathway analysis.
Co-culture of irradiated murine splenic lymphocytes with WJ-MSCs offered significant radioprotection to lymphocytes. WJ-MSC transplantation increased the homeostasis-driven proliferation of the lymphocytes. Neutralization of WJ-MSC-CM with G-CSF antibody abolished therapeutic radioprotection. Transcriptome analysis showed that WJ-MSCs share several common genes with bone marrow MSCs and embryonic stem cells and express a high level of unique genes such as interleukin (IL)1-α, IL1-β, IL-6, CXCL3, CXCL5, CXCL8, CXCL2, CCL2, FLT-1, and IL-33. It was also observed that WJ-MSCs preferentially modulated several cellular pathways and processes that are responsible for the repair and regeneration of damaged tissues compared to other sources of stem cells. Cytokine-based network analysis showed that most of the radiosensitive tissues have a more complex network for the elevated cytokines.
This study showed the role of cytokine G-CSF present in WJ-MSC-CM in eliciting therapeutic radioprotection. Systemic infusion of WJ-MSC-CM may have significant potential for treating accidental radiation exposed victims.
WJ-MSC-CM holds significant therapeutic radioprotective ability and has translational potential for its use during radiation accidents.
The authors thank Dr. Amrita Misri and Dr. Nigamananda Mishra for their support in the project and helping in the collection of the umbilical cord. Authors also thank Ms. Binita Kumar for acquiring flow cytometry samples and Mr. Deepak Kathole and Mr. B. A. Naidu for maintaining animal and helping in animal experiments.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author’s Membership in Professional Societies: Society for Free radical Research-India.
Specialty type: Cell and tissue engineering
Country/Territory of origin: India
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Miceli V, Italy; Prasetyo EP, Indonesia; Shalaby MN, Egypt S-Editor: Fan JR L-Editor: Filipodia P-Editor: Zhang YL
| 1. | Bogdándi EN, Balogh A, Felgyinszki N, Szatmári T, Persa E, Hildebrandt G, Sáfrány G, Lumniczky K. Effects of low-dose radiation on the immune system of mice after total-body irradiation. Radiat Res. 2010;174:480-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 102] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 2. | Bharti D, Shivakumar SB, Park JK, Ullah I, Subbarao RB, Park JS, Lee SL, Park BW, Rho GJ. Comparative analysis of human Wharton's jelly mesenchymal stem cells derived from different parts of the same umbilical cord. Cell Tissue Res. 2018;372:51-65. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 72] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 3. | Checker R, Patwardhan RS, Jayakumar S, Maurya DK, Bandekar M, Sharma D, Sandur SK. Chemical and biological basis for development of novel radioprotective drugs for cancer therapy. Free Radic Res. 2021;55:595-625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 4. | Bentzen SM. Preventing or reducing late side effects of radiation therapy: radiobiology meets molecular pathology. Nat Rev Cancer. 2006;6:702-713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 710] [Cited by in RCA: 724] [Article Influence: 38.1] [Reference Citation Analysis (0)] |
| 5. | Bernard ME, Kim H, Berhane H, Epperly MW, Franicola D, Zhang X, Houghton F, Shields D, Wang H, Bakkenist CJ, Frantz MC, Forbeck EM, Goff JP, Wipf P, Greenberger JS. GS-nitroxide (JP4-039)-mediated radioprotection of human Fanconi anemia cell lines. Radiat Res. 2011;176:603-612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 6. | Bernier J, Hall EJ, Giaccia A. Radiation oncology: a century of achievements. Nat Rev Cancer. 2004;4:737-747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 393] [Cited by in RCA: 403] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 7. | Karp JM, Leng Teo GS. Mesenchymal stem cell homing: the devil is in the details. Cell Stem Cell. 2009;4:206-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1012] [Cited by in RCA: 1086] [Article Influence: 67.9] [Reference Citation Analysis (0)] |
| 8. | Bandekar M, Maurya DK, Sharma D, Checker R, Gota V, Mishra N, Sandur SK. Xenogeneic transplantation of human WJ-MSCs rescues mice from acute radiation syndrome via Nrf-2-dependent regeneration of damaged tissues. Am J Transplant. 2020;20:2044-2057. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 9. | Gao Z, Zhang Q, Han Y, Cheng X, Lu Y, Fan L, Wu Z. Mesenchymal stromal cell-conditioned medium prevents radiation-induced small intestine injury in mice. Cytotherapy. 2012;14:267-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 10. | Xue J, Li X, Lu Y, Gan L, Zhou L, Wang Y, Lan J, Liu S, Sun L, Jia L, Mo X, Li J. Gene-modified mesenchymal stem cells protect against radiation-induced lung injury. Mol Ther. 2013;21:456-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 60] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 11. | Chang YH, Lin LM, Lou CW, Chou CK, Ch'ang HJ. Bone marrow transplantation rescues intestinal mucosa after whole body radiation via paracrine mechanisms. Radiother Oncol. 2012;105:371-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 12. | Chang P, Qu Y, Liu Y, Cui S, Zhu D, Wang H, Jin X. Multi-therapeutic effects of human adipose-derived mesenchymal stem cells on radiation-induced intestinal injury. Cell Death Dis. 2013;4:e685. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 69] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 13. | Schmoldt A, Benthe HF, Haberland G. Digitoxin metabolism by rat liver microsomes. Biochem Pharmacol. 1975;24:1639-1641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1391] [Cited by in RCA: 1410] [Article Influence: 78.3] [Reference Citation Analysis (0)] |
| 14. | Bi B, Schmitt R, Israilova M, Nishio H, Cantley LG. Stromal cells protect against acute tubular injury via an endocrine effect. J Am Soc Nephrol. 2007;18:2486-2496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 393] [Cited by in RCA: 391] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 15. | Ionescu L, Byrne RN, van Haaften T, Vadivel A, Alphonse RS, Rey-Parra GJ, Weissmann G, Hall A, Eaton F, Thébaud B. Stem cell conditioned medium improves acute lung injury in mice: in vivo evidence for stem cell paracrine action. Am J Physiol Lung Cell Mol Physiol. 2012;303:L967-L977. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 261] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 16. | Hung SC, Pochampally RR, Chen SC, Hsu SC, Prockop DJ. Angiogenic effects of human multipotent stromal cell conditioned medium activate the PI3K-Akt pathway in hypoxic endothelial cells to inhibit apoptosis, increase survival, and stimulate angiogenesis. Stem Cells. 2007;25:2363-2370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 327] [Cited by in RCA: 350] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 17. | Boomsma RA, Geenen DL. Mesenchymal stem cells secrete multiple cytokines that promote angiogenesis and have contrasting effects on chemotaxis and apoptosis. PLoS One. 2012;7:e35685. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 227] [Cited by in RCA: 253] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 18. | Deans RJ, Moseley AB. Mesenchymal stem cells: biology and potential clinical uses. Exp Hematol. 2000;28:875-884. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1051] [Cited by in RCA: 1015] [Article Influence: 40.6] [Reference Citation Analysis (0)] |
| 19. | Majumdar MK, Thiede MA, Haynesworth SE, Bruder SP, Gerson SL. Human marrow-derived mesenchymal stem cells (MSCs) express hematopoietic cytokines and support long-term hematopoiesis when differentiated toward stromal and osteogenic lineages. J Hematother Stem Cell Res. 2000;9:841-848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 321] [Cited by in RCA: 330] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 20. | Mouiseddine M, François S, Semont A, Sache A, Allenet B, Mathieu N, Frick J, Thierry D, Chapel A. Human mesenchymal stem cells home specifically to radiation-injured tissues in a non-obese diabetes/severe combined immunodeficiency mouse model. Br J Radiol. 2007;80 Spec No 1:S49-S55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 124] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 21. | Hu KX, Sun QY, Guo M, Ai HS. The radiation protection and therapy effects of mesenchymal stem cells in mice with acute radiation injury. Br J Radiol. 2010;83:52-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 68] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 22. | Sémont A, Mouiseddine M, François A, Demarquay C, Mathieu N, Chapel A, Saché A, Thierry D, Laloi P, Gourmelon P. Mesenchymal stem cells improve small intestinal integrity through regulation of endogenous epithelial cell homeostasis. Cell Death Differ. 2010;17:952-961. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 117] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 23. | Lange C, Brunswig-Spickenheier B, Cappallo-Obermann H, Eggert K, Gehling UM, Rudolph C, Schlegelberger B, Cornils K, Zustin J, Spiess AN, Zander AR. Radiation rescue: mesenchymal stromal cells protect from lethal irradiation. PLoS One. 2011;6:e14486. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 84] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 24. | Yang X, Balakrishnan I, Torok-Storb B, Pillai MM. Marrow Stromal Cell Infusion Rescues Hematopoiesis in Lethally Irradiated Mice despite Rapid Clearance after Infusion. Adv Hematol. 2012;2012:142530. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 34] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 25. | Liu Y, Chen XH, Si YJ, Li ZJ, Gao L, Zhang C, Zhang X. Reconstruction of hematopoietic inductive microenvironment after transplantation of VCAM-1-modified human umbilical cord blood stromal cells. PLoS One. 2012;7:e31741. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 26. | Rachakatla RS, Marini F, Weiss ML, Tamura M, Troyer D. Development of human umbilical cord matrix stem cell-based gene therapy for experimental lung tumors. Cancer Gene Ther. 2007;14:828-835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 94] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 27. | Liu S, Yuan M, Hou K, Zhang L, Zheng X, Zhao B, Sui X, Xu W, Lu S, Guo Q. Immune characterization of mesenchymal stem cells in human umbilical cord Wharton's jelly and derived cartilage cells. Cell Immunol. 2012;278:35-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 84] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 28. | Racz GZ, Kadar K, Foldes A, Kallo K, Perczel-Kovach K, Keremi B, Nagy A, Varga G. Immunomodulatory and potential therapeutic role of mesenchymal stem cells in periodontitis. J Physiol Pharmacol. 2014;65:327-339. [PubMed] |
| 29. | Bandekar M, Maurya DK, Sharma D, Checker R, Gota V, Mishra N, Sandur SK. Therapeutic radioprotection by human umbilical cord derived Wharton's jelly mesenchymal stein cells: Role of cytokines and nrf‐2. Free Radic Biol Med. 2019;145:S18. [DOI] [Full Text] |
| 30. | Maere S, Heymans K, Kuiper M. BiNGO: a Cytoscape plugin to assess overrepresentation of gene ontology categories in biological networks. Bioinformatics. 2005;21:3448-3449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2998] [Cited by in RCA: 3108] [Article Influence: 155.4] [Reference Citation Analysis (0)] |
| 31. | Raudvere U, Kolberg L, Kuzmin I, Arak T, Adler P, Peterson H, Vilo J. g:Profiler: a web server for functional enrichment analysis and conversions of gene lists (2019 update). Nucleic Acids Res. 2019;47:W191-W198. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3393] [Cited by in RCA: 3445] [Article Influence: 574.2] [Reference Citation Analysis (0)] |
| 32. | Kwon SG, Kwon YW, Lee TW, Park GT, Kim JH. Recent advances in stem cell therapeutics and tissue engineering strategies. Biomater Res. 2018;22:36. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 80] [Cited by in RCA: 129] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 33. | Nagayama H, Misawa K, Tanaka H, Ooi J, Iseki T, Tojo A, Tani K, Yamada Y, Kodo H, Takahashi TA, Yamashita N, Shimazaki S, Asano S. Transient hematopoietic stem cell rescue using umbilical cord blood for a lethally irradiated nuclear accident victim. Bone Marrow Transplant. 2002;29:197-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 65] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 34. | Azzam EI, Yang Z, Li M, Kim S, Kovalenko OA, Khorshidi M, Ende N. The effect of human cord blood therapy on the intestinal tract of lethally irradiated mice: possible use for mass casualties. Int J Radiat Biol. 2010;86:467-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 35. | Ganta C, Chiyo D, Ayuzawa R, Rachakatla R, Pyle M, Andrews G, Weiss M, Tamura M, Troyer D. Rat umbilical cord stem cells completely abolish rat mammary carcinomas with no evidence of metastasis or recurrence 100 days post-tumor cell inoculation. Cancer Res. 2009;69:1815-1820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 87] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 36. | Ayuzawa R, Doi C, Rachakatla RS, Pyle MM, Maurya DK, Troyer D, Tamura M. Naïve human umbilical cord matrix derived stem cells significantly attenuate growth of human breast cancer cells in vitro and in vivo. Cancer Lett. 2009;280:31-37. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 103] [Cited by in RCA: 94] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 37. | Maurya DK, Doi C, Kawabata A, Pyle MM, King C, Wu Z, Troyer D, Tamura M. Therapy with un-engineered naïve rat umbilical cord matrix stem cells markedly inhibits growth of murine lung adenocarcinoma. BMC Cancer. 2010;10:590. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 38. | Matsuzuka T, Rachakatla RS, Doi C, Maurya DK, Ohta N, Kawabata A, Pyle MM, Pickel L, Reischman J, Marini F, Troyer D, Tamura M. Human umbilical cord matrix-derived stem cells expressing interferon-beta gene significantly attenuate bronchioloalveolar carcinoma xenografts in SCID mice. Lung Cancer. 2010;70:28-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 67] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 39. | Singh VK, Fatanmi OO, Singh PK, Whitnall MH. Role of radiation-induced granulocyte colony-stimulating factor in recovery from whole body gamma-irradiation. Cytokine. 2012;58:406-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 48] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 40. | Du R, Hu P, Liu Q, Zhang J, Deng G, Hu D. Granulocyte Colony-Stimulating Factor Treatment During Radiotherapy Is Associated With Survival Benefit in Patients With Lung Cancer. Technol Cancer Res Treat. 2018;17:1533033818816076. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 41. | Shim S, Lee SB, Lee JG, Jang WS, Lee SJ, Park S, Lee SS. Mitigating effects of hUCB-MSCs on the hematopoietic syndrome resulting from total body irradiation. Exp Hematol. 2013;41:346-53.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 42. | Yañez R, Lamana ML, García-Castro J, Colmenero I, Ramírez M, Bueren JA. Adipose tissue-derived mesenchymal stem cells have in vivo immunosuppressive properties applicable for the control of the graft-versus-host disease. Stem Cells. 2006;24:2582-2591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 518] [Cited by in RCA: 523] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 43. | Linard C, Marquette C, Mathieu J, Pennequin A, Clarençon D, Mathé D. Acute induction of inflammatory cytokine expression after gamma-irradiation in the rat: effect of an NF-kappaB inhibitor. Int J Radiat Oncol Biol Phys. 2004;58:427-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 143] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 44. | Neta R, Oppenheim JJ. Cytokines in therapy of radiation injury. Blood. 1988;72:1093-1095. [PubMed] |
| 45. | Bhang SH, Lee S, Shin JY, Lee TJ, Jang HK, Kim BS. Efficacious and clinically relevant conditioned medium of human adipose-derived stem cells for therapeutic angiogenesis. Mol Ther. 2014;22:862-872. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 132] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 46. | Neta R, Douches S, Oppenheim JJ. Interleukin 1 is a radioprotector. J Immunol. 1986;136:2483-2485. [PubMed] |
| 47. | Neta R, Oppenheim JJ, Wang JM, Snapper CM, Moorman MA, Dubois CM. Synergy of IL-1 and stem cell factor in radioprotection of mice is associated with IL-1 up-regulation of mRNA and protein expression for c-kit on bone marrow cells. J Immunol. 1994;153:1536-1543. [PubMed] |
| 48. | Bertho JM, Frick J, Prat M, Demarquay C, Dudoignon N, Trompier F, Gorin NC, Thierry D, Gourmelon P. Comparison of autologous cell therapy and granulocyte-colony stimulating factor (G-CSF) injection vs. G-CSF injection alone for the treatment of acute radiation syndrome in a non-human primate model. Int J Radiat Oncol Biol Phys. 2005;63:911-920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 40] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 49. | Berry SE. Concise review: mesoangioblast and mesenchymal stem cell therapy for muscular dystrophy: progress, challenges, and future directions. Stem Cells Transl Med. 2015;4:91-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 50. | Bergonie J, Tribondeau L. Interpretation of some results of radiotherapy and an attempt at determining a logical technique of treatment. Radiat Res. 1959;11:587-588. [PubMed] |