Published online Apr 26, 2022. doi: 10.4252/wjsc.v14.i4.287
Peer-review started: March 28, 2021
First decision: May 12, 2021
Revised: May 19, 2021
Accepted: April 21, 2022
Article in press: April 21, 2022
Published online: April 26, 2022
Processing time: 393 Days and 16.6 Hours
Mesenchymal stem cells (MSC) effects on tissue regeneration are mainly mediated by their secreted substances (secretome), inducing their paracrine activity. This Conditioned medium (CM), including soluble factors (proteins, nucleic acids, lipids) and extracellular vesicles is emerging as a potential alternative to cell therapy. However, the manufacturing of CM suffers from variable procedures and protocols leading to varying results between studies. Besides, there is no well-defined optimized procedure targeting specific applications in regenerative medicine.
To focus on conditioned medium produced from dental MSC (DMSC-CM), we reviewed the current parameters and manufacturing protocols, in order to propose a standardization and optimization of these manufacturing procedures.
We have selected all publications investigating the effects of dental MSC secretome in in vitro and in vivo models of tissue regeneration, in accordance with the PRISMA guidelines.
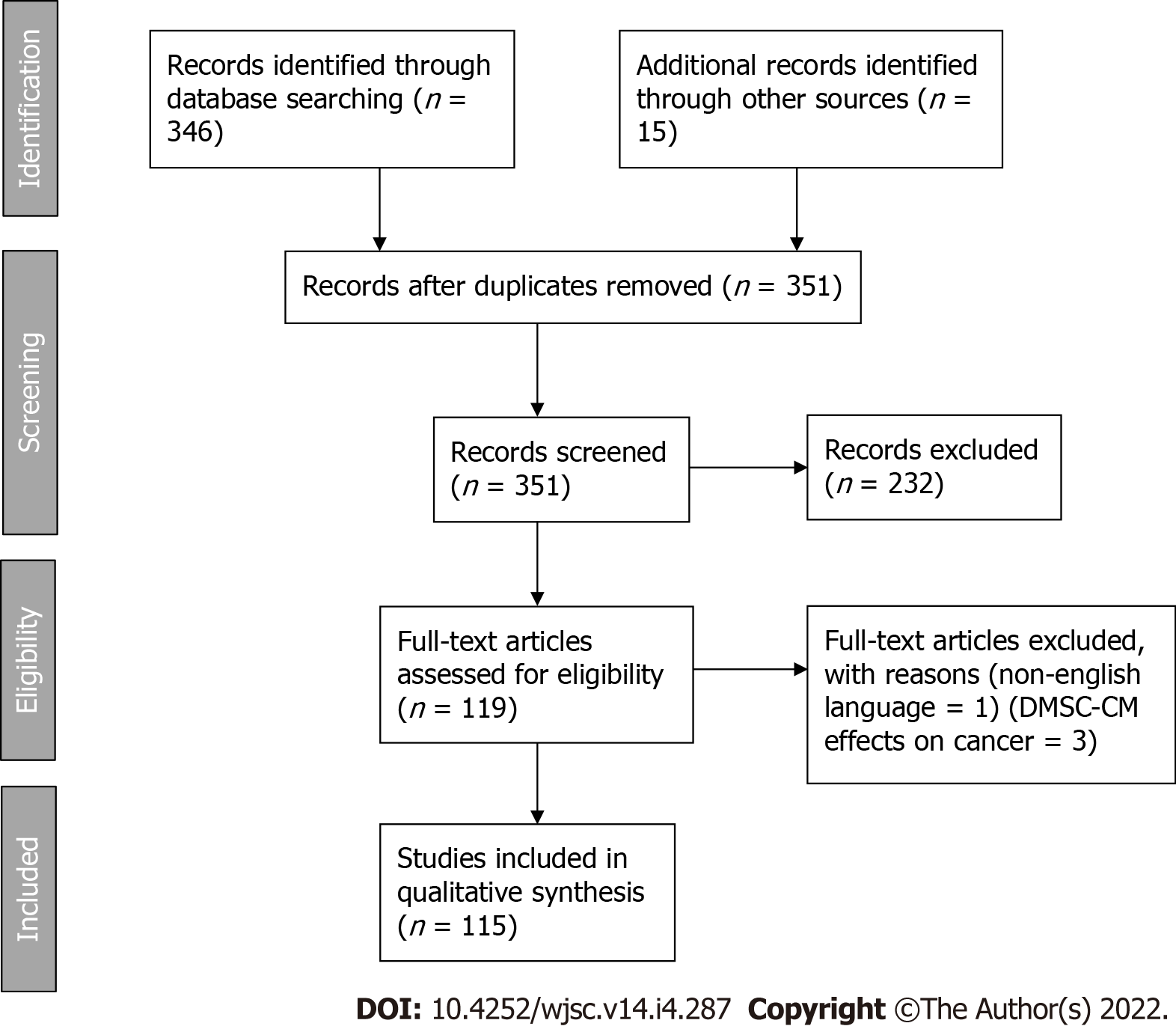
A total of 351 results were identified. And based on the inclusion criteria described above, 118 unique articles were included in the systematic review. DMSC-CM production was considered at three stages: before CM recovery (cell sources for CM), during CM production (culture conditions) and after production (CM treatment).
No clear consensus could be recovered as evidence-based methods, but we were able to describe the most commonly used protocols: donors under 30 years of age, dental pulp stem cells and exfoliated deciduous tooth stem cells with cell passage between 1 and 5, at a confluence of 70% to 80%. CM were often collected during 48 h, and stored at -80 °C. It is important to point out that the preconditioning environment had a significant impact on DMSC-CM content and efficiency.
Core Tip: Dental Mesenchymal stem cells (DMSC) effects on tissue regeneration are highly mediated by their secreted substances [conditioned medium (CM)] such as soluble factors and extracellular vesicles. The manufacturing of CM products suffers from variable procedures and protocols leading to different results between studies. Focusing on CM produced from DMSC (DMSC-CM), we reviewed the current parameters and manufacturing protocols, aiming to facilitate the standardization and optimization of manufacturing procedures, in accordance with PRISMA guideline. No clear consensus could be recovered as evidence-based methods, but it clearly appeared that the preconditioning environment had a significant impact on DMSC-CM content and efficiency.
- Citation: Chouaib B, Cuisinier F, Collart-Dutilleul PY. Dental stem cell-conditioned medium for tissue regeneration: Optimization of production and storage. World J Stem Cells 2022; 14(4): 287-302
- URL: https://www.wjgnet.com/1948-0210/full/v14/i4/287.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v14.i4.287
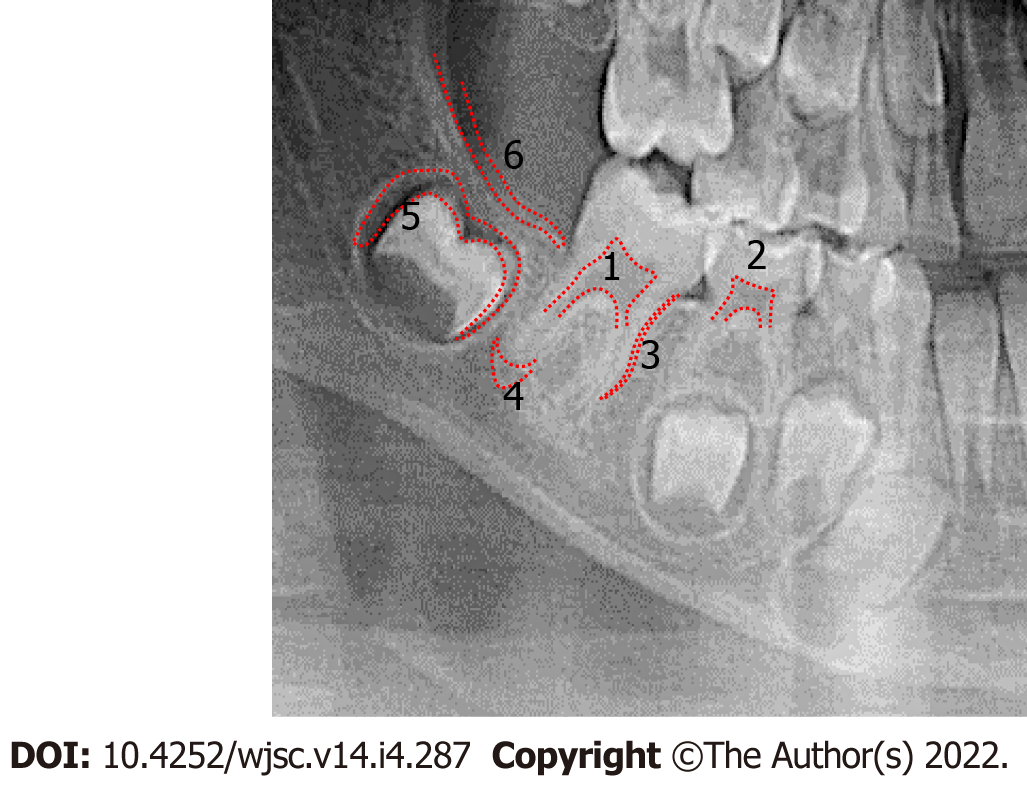
Although mesenchymal stem cells (MSC) were initially isolated from bone marrow, MSC from dental and periodontal tissue (DMSC) have attracted international attention for future therapies because of their practical and technical advantages[1]. Since 2000, when Gronthos et al[2] described a population of pluripotent progenitors in adult dental pulp, studies have shown that dental tissues can be an important resource of MSCs: dental pulp stem cells (DPSC), exfoliated deciduous tooth stem cells (SHED), apical papilla stem cells (SCAP) which are situated at the ends of growing dental roots[3], periodontal ligament stem cells (PDLSC), dental follicle stem cells (DFPC) located around the tooth germ, and responsible for cementum, periodontal ligament and alveolar bone formation during tooth development[4], gingiva-derived mesenchymal stem cells (GMSC) (Figure 1). For teeth at early stage of development (bell stage), multipotent progenitors from dental mesenchyme have been described, named as tooth germ progenitor cells (TGPC)[5].
An important advantage of these sources of MSC is the absence of morbidity and the fact that no additional surgical procedures is required[6]. DMSC are obtained from exfoliated teeth, teeth extracted for orthodontic or medical needs, and supernumerary teeth. While generally discarded as medical waste, teeth could be an abundant source of mesenchymal stem cells.
Numerous studies have indicated that MSC effects on tissue regeneration are mostly mediated by their secreted substances[7] defined as secretome, or MSC-conditioned medium (MSC-CM), which are endowed with paracrine activity. These MSC-CM include soluble factors (proteins, nucleic acids, lipids) and extracellular vesicles (EV)[8]. MSC-CM appears as a potential substitute for cell therapy, with considerable potential to be developed into pharmaceutical products for use in regenerative medicine[9].
Compared to non-dental MSC-CM, dental mesenchymal stem cell-conditioned medium (DMSC-CM) have greater amounts of transcriptional, metabolic and proliferation-associated proteins, neurotrophins and chemokines. They also present reduced levels of proteins required for extracellular matrix production and adhesion, and superior effects on cell differentiation, maturation and tissue regeneration[10].
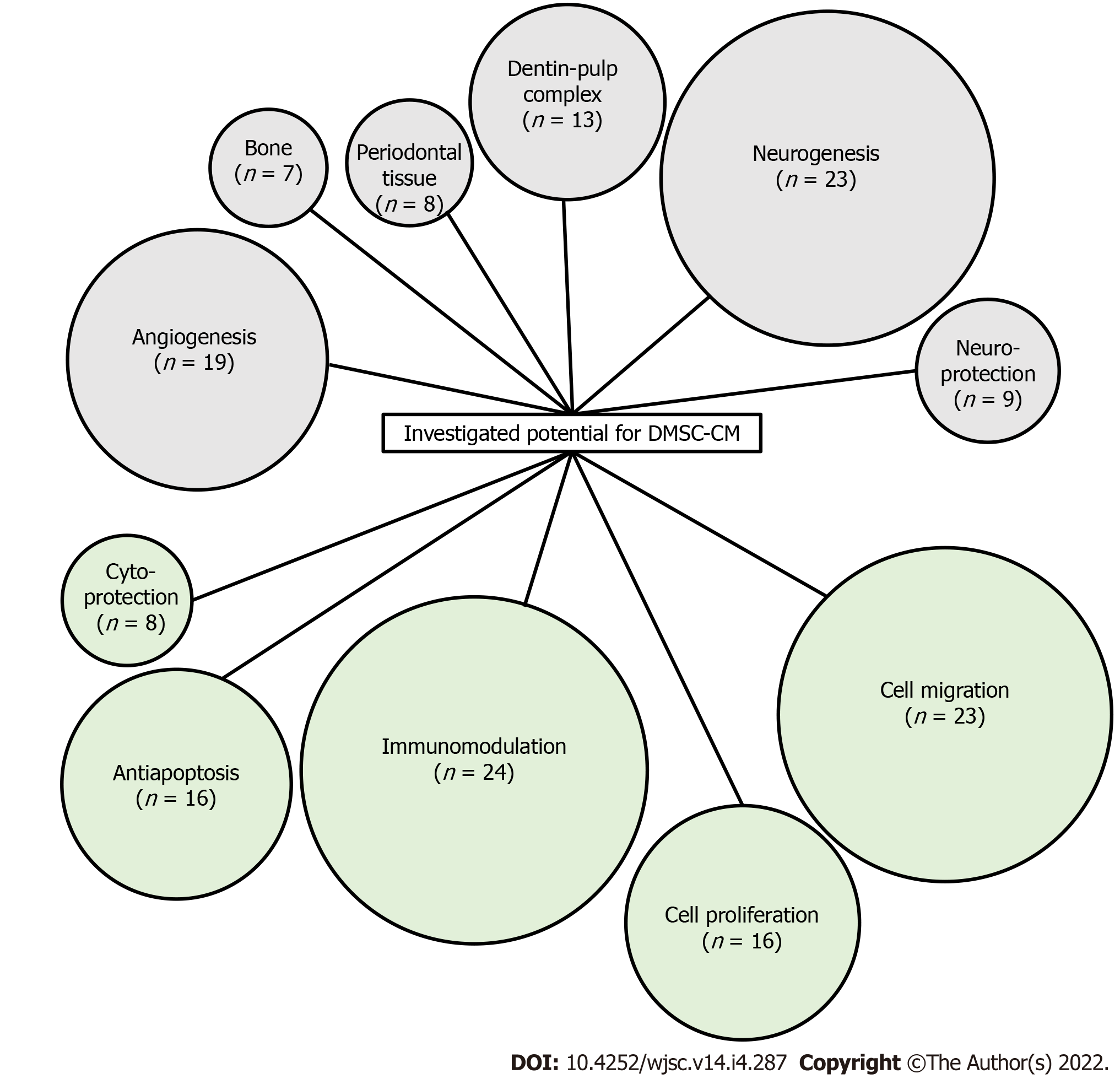
Many research works have described the application of DMSC secretome for various diseases treatment and for tissue regeneration[8,10-12]. Dental MSC-CM have been investigated for the repair of neurological disorders[13-18], cardiac lesions[19], diabetic disorders[20], liver diseases[21,22], pulmonary lesions[23], immunity problems[24-26], dental and bone defects[27-30], and growth of hair[31]. These potentials are assigned to positive effects on cell proliferation-migration-protection, to specific effects such as anti-apoptotic, pro-angiogenic, immunomodulation, or to cell differentiation and further tissue regeneration like osteodifferentiation, neuron-like regeneration, dentin-pulp complex formation, periodontal regeneration (Figure 2 and Table 1).
| Type of DMSC | Ref. |
| Dental pulp stem cells | Kumar[33], 2017; Kumar[34], 2018; Kumar[35], 2017; Gervois[36], 2017; Horibe[37], 2014; Zhou[38], 2020; Venugopal[39], 2018; Caseiro[40], 2019; Shen[41], 2015; Mead[42], 2014; Wada[43], 2009; Kolar[44], 2017; Bronckaers[45], 2013; Paschalidis[46], 2014; Piva[47], 2017; Gharaei[48], 2018; Murakami[49], 2013; Li[50], 2019; Yamamoto[51], 2014; Sakai[52], 2012; Hu[53], 2019; Wang[17], 2019; Nakayama[54], 2017; Merckx[55], 2020; Swanson[56], 2020; Gervois[57], 2019; Ivica[58], 2020; Zhang[59], 2020; Yamamoto[60], 2016; Ahmed[13], 2016; Xian[61], 2018; Song[62], 2015; Joo[63], 2018; Shen[64], 2020; Akazawa[65], 2015; De rosa[66], 2011; Ji[67], 2019; Aranha[68], 2010; Huang[69], 2016; Lambricht[70], 2017; Iohara[71], 2008; Ishizaka[72], 2013; Kawamura[73], 2016; Hayashi[74], 2015; Iohara[75], 2014; Iohara[76], 2013; Murakami[77], 2015; Omi[78], 2017; Makino[79], 2019; Omi[80], 2016; Chen[81], 2019 |
| Stem cells from human exfoliated deciduous teeth | Li[82], 2017; Pivoraite[25], 2015; Jarmalaviciute[16], 2015; Kano[83], 2017; Matsubara[84], 2015; Matsushita[21], 2017; Omori[27], 2015; Yamagata[85], 2013; Fujii[86], 2015; Tsuruta[87], 2018; Shimojima[24], 2016; Yamaguchi[19], 2015; Sugimura-Wakayama[88], 2015; Han[89], 2020; Sakai[52], 2012; Yamamoto[51], 2014; Chen[15], 2020; Mussano[90], 2018; Gunawardena[31], 2019; De cara[29], 2019; Wang[91], 2020; Hiraki[28], 2020; Miura-Yura[92], 2020; Ishikawa[26], 2016; Wakayama[23], 2015; Izumoto-Akita[20], 2015; Ogasawara[93], 2020; Sakai[94], 2020; Hirata[22], 2016; Asadi-Golshan[14], 2018; Mita[18], 2015; Inoue[95], 2013; Wei[96], 2020; Li[97], 2019 |
| Periodontal ligament stem cells | Kang[98], 2018; Diomede[99], 2018; Aghamohamadi[100], 2020; Kolar[44], 2017; Nagata[101], 2017; Cianci[102], 2016; Wada[43], 2009; Qiu[30], 2020; Zhang[103], 2020 |
| Stem cells from apical papilla | Kolar[44], 2017; Kumar[35], 2017; Kumar[33], 2017; Kumar[34], 2018; Bakopoulou[104], 2015; Zhuang[105], 2020; Yu[106], 2016; Yu[107], 2020; Yu[108], 2020 |
| Dental follicule stem cells | Kumar[35], 2017; Kumar[33], 2017; Kumar[34], 2018; Chen[109], 2018; Wen[110], 2015; Wen[111], 2011; Liu[112], 2014; Wu[113], 2013 |
| Gingiva-derived mesenchymal stem cells | Jin[114], 2020; Qiu[30], 2020; Mao[115], 2019; Wang[116], 2020; Zhang[117], 2019; Rajan[118], 2017; Rao[119], 2019, Diomede[120], 2018; Silvestro[121], 2020 |
| Tooth germ progenitor cells | Wang[122], 2011; Huo[123], 2010; Ye[124], 2015; Yu[125], 2006; Shan[126], 2015; Yang[127], 2009; Yang[128], 2009 |
However, MSC-CM manufacturing suffers from variable procedures and protocols, leading to different results between studies. Besides, there is no well-defined optimized procedure targeting specific applications in regenerative medicine.
In the present article, we focus on conditioned medium produced from DMSC and their derivative products. We review the current parameters and DMSC culture conditions used in the manufacturing protocols. The ultimate objective is to facilitate the standardization and optimization of manufacturing procedures needed for the clinical translation of DMSC-CM and their derivative products.
This systematic review was performed in accordance with the PRISMA guidelines[32].
We have selected all publications investigating the effects of dental MSC secretome (CM, EV, exosomes) in in vitro and in vivo models of tissue regeneration by using the PubMed electronic database and the following search terms: ((dental stem cells) AND ((conditioned medium) OR (secretome))) / ((dental stem cells) AND ((extravesicles) OR (exosomes))). The bibliographic search considered articles meeting the inclusion criteria, and published between 2006 and July 2020. Articles taken into account had studied the DMSC secretome as a therapeutic agent, had analyzed the profiles of DMSC-CM, or included experiments based on DMSC-CM or its derivatives (EV, exosomes) to assess the paracrine activity of DMSC. Only articles in English were considered. All results were screened based on titles and abstracts. Full texts of the potentially selected records were obtained for definitive inclusion.
A total of 351 results were identified. And based on the inclusion criteria described above, 118 unique articles were included in the systematic review. Flow diagram is available as Figure 1.
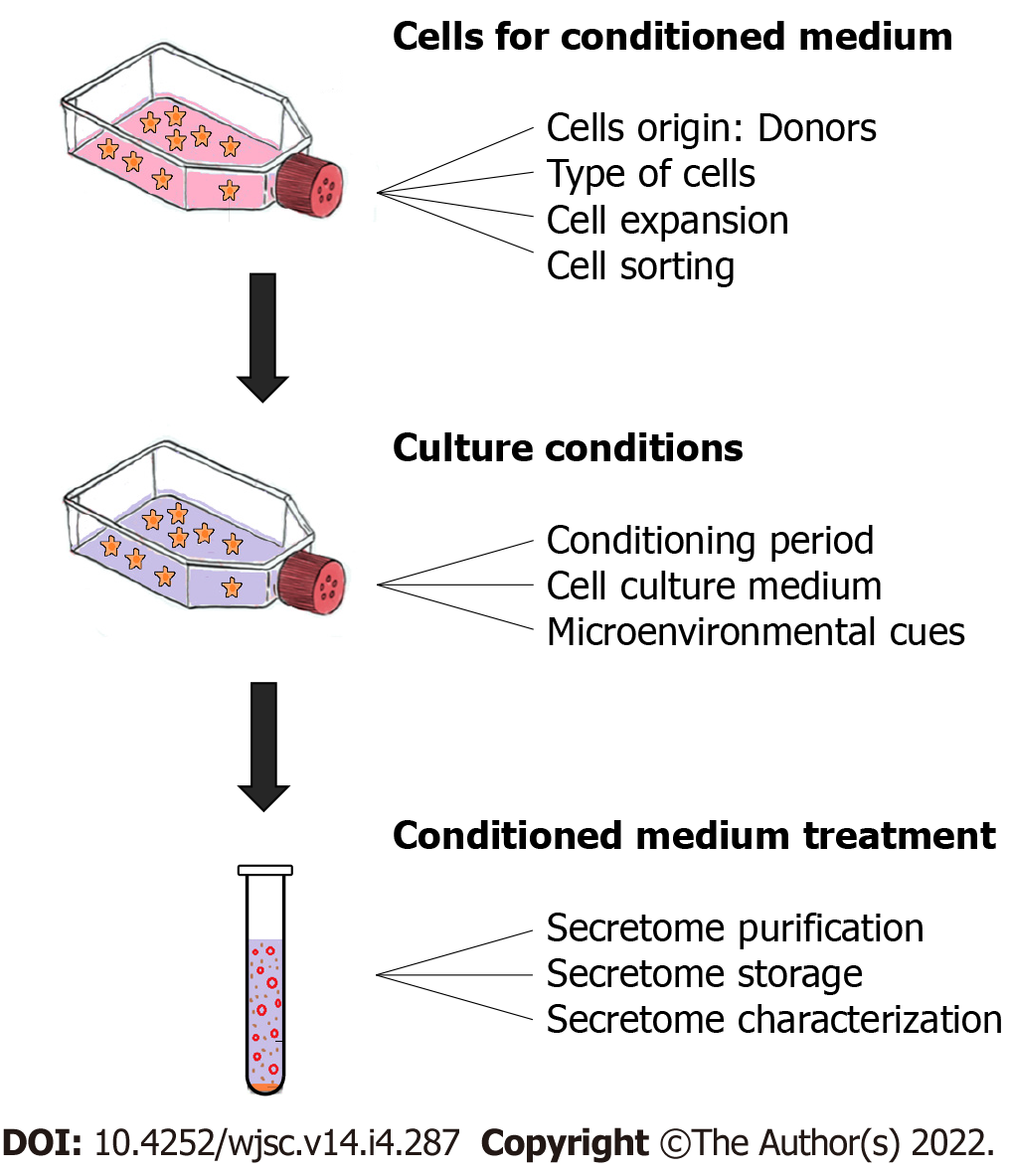
To organize the analysis and interpretation, DMSC-CM production was considered at three stages: Before CM recovery (cell sources for CM), during CM production (culture conditions) and after production (CM treatment). For these three steps, we identified key points that were often taken into account in the published experimental works. A schematic representation of these elements is shown in Figure 3.
Most of studies have investigated the secretome effects of DPSC and SHED. The other secretomes, from PDLSC, SCAP, DFSC, GMSC, or TGPC have been less investigated (Table 1).
Most of these studies analyzed DMSC to verify their stemness. Specific cell surface markers were verified before starting the experimentation: positive expression of CD90, CD73, CD29, CD44, and CD105 (MSC markers), and absence of CD34, CD45, CD11b/c, CD31, CD144, and HLA-DR (endothelial/hematopoietic markers). Cell differentiation capacities were controlled for the adipogenic, chondrogenic, and osteogenic lineage. However, there were several studies in which stemness character was not controlled. And few studies did not considered stemness character for dental cells[58,65,111-113,129] (Supplementary Table 1).
DPSC, SHED, PDLSC, SCAP, DFPC, GMSC and TGPC derive from oral and tooth related structures. However, the specific properties of these different dental stem cell populations are slightly different according to the location from which they are isolated, in term of marker expression and differentiation potencies[130]. Thus, their secretome may also vary. A previously published study compared the neural potential of DMSC-CM obtained from three sources (DPSC, DFSC, and SCAP) with BMSC-CM: the results showed that neurite extension in neural cells were significantly lowered when cells were incubated in BMSC-CM, compared to DMSC-CM. However, among these three dental CM, a significant difference was observed, in term of in vitro effects and secretome profiles. Thus, neurite length was increased with neural cells incubated with DPSC-CM, compared to DFSC-CM and SCAP-CM. Besides, significantly different levels of neurotrophins and cytokines were observed in the three secretomes[35]. Another study compared also the neural potential of DMSC-CM from three dental sources (DPSC, SCAP, and PDLSC) of the same donor; Although all CM significantly enhanced axon regeneration and showed neuroprotective effects on neurons, results were better with SCAP-CM. By protein quantification, it was revealed that the level of secreted proteins were very similar for DPSC-CM and SCAP-CM, but different from PDLSC-CM[44]. A third study, comparing SHED and DPSC, could not highlight any difference between SHED-CM and DPSC-CM, as both secretome promoted neurite extension[52].
DMSC-CM have been generally compared with ASC-CM and BMSC-CM. Small particles with molecular weights between 30 and 100 nm were more abundant in DPSC-CM, whereas the fraction of intermediate particles (100-300 nm) was larger in BM-MSC[55]. DPSC-CM seemed to be more potent in neurogenesis[35,72], neuroprotection[13,39,42], angiogenesis[72,74], and migration[74] than ADCS-CM and BMSC-CM. Furthermore, the anti-apoptotic potential of DPSC-CM was found to be greater than that of BMSC-CM and ADSC-CM[39, 74]. However, endothelial cell chemotaxis and in ovo neovascularization were less observed with DPSC-CM than BM-MSC[55]. Compared to umbilical cord mesen
Similarly, SHED and other MSC differed in their basal expression of several biomolecules. Some relevant factors were abundant in SHED-CM but hardly detectable in ASC-CM[90]. SHED-CM was more anti-inflammatory[19,21] and anti-apoptotic[19] compared to BMSC-CM and ASC-CM. It was more efficient than BMSC-CM for the treatment of arthritis[26] and diabetes[20], for neuroprotection[62] and repair[18].
Concerning SCAP-CM, studies have shown that compared to BMSC, SCAP secrete more proteins associated with metabolic processes and transcription, but less proteins involved in biological adhesion, immune function, and developmental processes. The amounts of chemokines and neurotrophins secreted by SCAP are significantly higher than those of BMSC, in contrast to extracellular matrix proteins and proangiogenic factors[106].
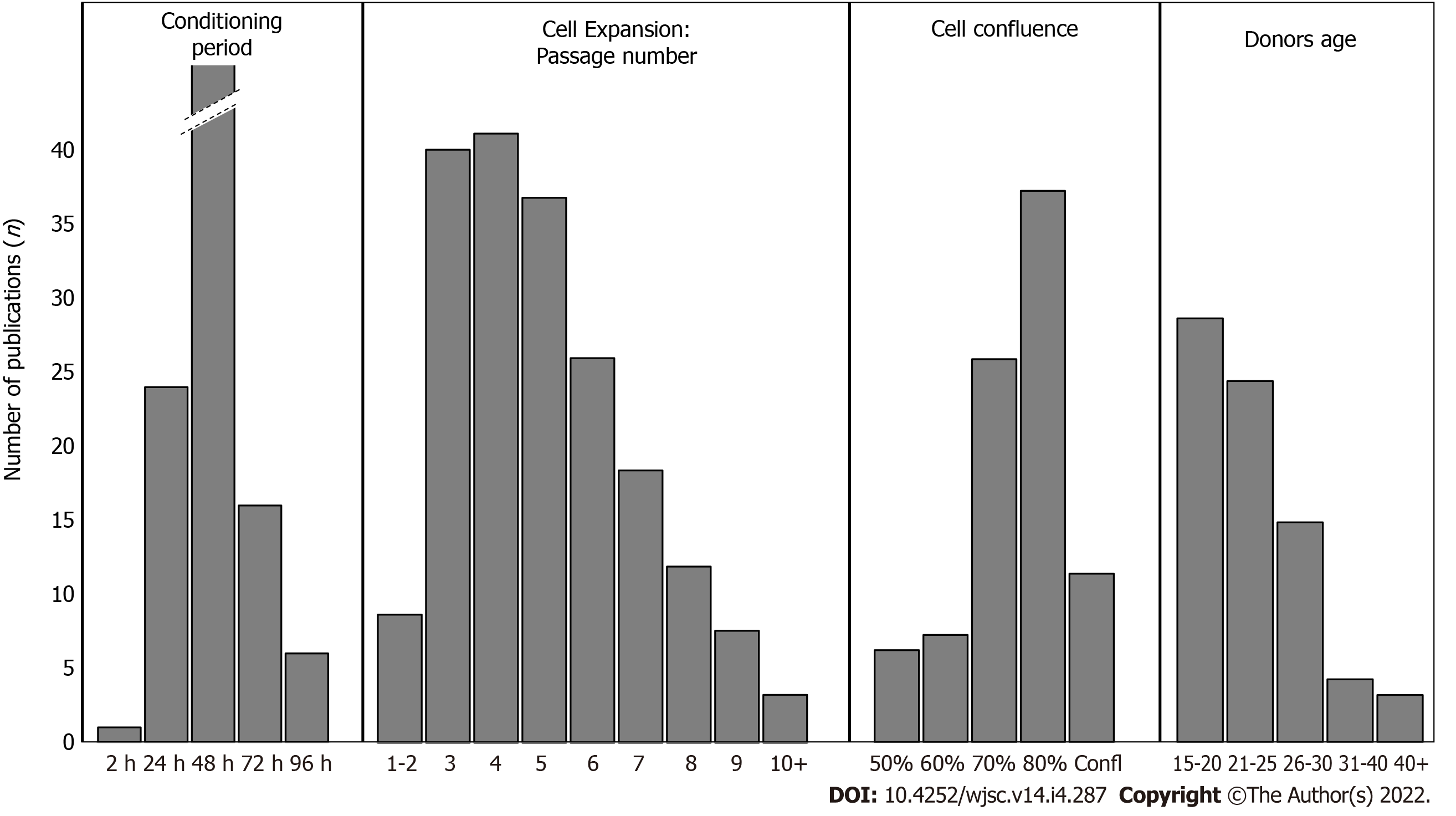
Aging has a clear influence on cell proliferation and capacity to differentiate. However, it is difficult to define a barrier discriminating optimal age for cell recovery. Decrease in dental MSC proliferation, migration, and differentiation has been correlated with age in the literature[131]. But among the publications considered in this systematic review, only one investigated the impact of donor age and could not highlight any significant difference between DPSC-CM from aged (44-70 years old) and young donors (19-30 years old), in term of trophic effects[37]. Taking the overall publications, donor age range in most of the studies is between 14 to 30 years old. Some studies considered donors over 30 years old[38,53,66,67,102], with good results overall. In general, the number of donors, their gender and medical status were not reported. Inter-donor variability has rarely been taken into account, although teeth have been extracted from several donors. In most cases, DPSC were isolated from erupted or impacted third molars, but they were also obtained from premolars, canines and incisors in some studies. SHED were always isolated from deciduous teeth, with donors under 12 years of age. CSDF were obtained mainly from molars and premolars. The developmental stages of the teeth were considered only in few studies, and their clinical status (decayed or not) were not systematically provided.
Some studies used dental cells from rats, dogs, and pigs, making comparison even more complicated, in term of donor. Thus, it is not possible to conclude about optimal donor age range, according to the available data. However, except for SHED, most of the investigations were conducted with donors under 30 years (Figure 4). Complete donors’ characteristics of studies investigating DMSC-CM are given in supplementary data (Supplementary Table 2).
To our knowledge, the impact of cell passage number on their secretions has never been examined, while its effect on the concentration of secreted growth factors has only been noted once by Miura-Yura et al[92]. The passage number of cells ranged from 1 to 12 in studies investigating the potential of DMSC secretomes (Supplementary Table 3). Some studies did not even precise cell passage numbers[6,16,20,29,30,60,66,68,72,82,85,87,88,92,98]. When considering cell expansion (through passage number), we can only observe that the majority of works were conducted with cells before passage 5, to prevent any risk of senescence during cell expansion (Figures 4 and 5).
Significant variability in proliferative and differentiation capabilities have been observed for individual DPSC populations expanded from human teeth from donors within a similar age range. Inherent differences were even identified between DPSC populations derived from the same patient[132]. In some studies, cell population was selected before investigating DMSC-CM. Side populations were used in some studies, targeting CD31 marker[72,74] or CD31/CD146 markers[71,133]. These sorted populations from dental pulps are enriched in stem/progenitor cells and can significantly stimulate angiogenesis/vasculogenesis[133]. Stem cell mobilization through G-CSF induction was also used in several studies to isolate stem cells[37,49,54,60,73,75-77]. This technique generated a mobilized subpopulation of dental pulp stem cells that are stable and age-independent, with high proliferative, migratory and regenerative potentials. Single cell clones derived from human deciduous tooth pulp cells were used by Akazawa et al[65]. Finally, De Rosa et al[66] selected DPSC based on their relative positivity profile for markers such as CD34, CD117, CD44, STRO-1, RUNX-2 and OC, before studying the secretome potential of osteodifferentiated DPSC.
No consensus could be described about cell sorting before CM recovery. This is not surprising as mesenchymal/stromal population are heterogeneous populations in which no specific/unique marker is described so far.
In most studies investigating DMSC-CM effects from secretome, cells were harvested for 48 h, starting with DMSC at 70%-80% of confluency (Histograms in Figure 4 and Supplementary Table 4 in supplementary data). Paschalidis et al[46] followed DPSC-CM collections from 4 d to 24 d, and described most pronounced effects within the first collection. The conditioning period was always considered with cells incubated in serum-free media. No clear difference could be enlightened from the various studies about protein concentration or DMSC-CM effects, but 48 h was the most used duration.
The principal culture medium used for DMSC-CM processing was DMEM. This medium was used for processing indifferently of the targeted application, or clinical objective: cardiac injury[19], diabetes[20], lung[23] or liver[22] injuries, bone regeneration[27], nerve regeneration[60], dental regeneration[73], and cerebral ischemia[95]. During the processing, in most of the studies, cells were kept in serum-free conditions, without Phosphate Buffer Saline (PBS) washing step before conditioning period (Supplementary Table 5).
It has been described that MSC could act as repair cells within the body when stimulated and recruited. This concept lead to MSC implication, not only by natural and constitutive secretion of regenerative factors, but also by producing specific factors in response to stimuli[134]. Thus, the secretion of different potential therapeutic factors by MSC can be modulated according to various physical, chemical, and biological cues.
During DMSC-CM production, the effects of hypoxia, 3D culture, LPS preconditioning, and osteodifferentiation were studied (Supplementary Table 6). Hypoxia is an environmental cue increasing VEGF levels[68,104] in the secretome. A study even described that hypoxia increased angiogenic potential of SCAP conditioned medium[104]. It was shown that 3D culture increased anti-apoptotic effects of SHED conditioned medium, when used with dopaminergic neurons[16]. LPS preconditioning increased DPSC conditioned medium potential for Schwann cells proliferation, migration, and odontogenic differentiation[50]. Secretome with LPS preconditioning also increased PDLSC anti-inflammatory effects[98]. The cytokine, chemokine and growth factor profiles of SHED-CM were significantly affected after osteoinduction of SHED cells[90], which also strengthened the effect of SHED-Exosomes on the osteogenic differentiation of PDLSC[91]. Osteogenesis of amniotic fluid stem cells was also stimulated by the secretome of osteodifferentiated DPSCs[66]. Kolar et al[44] stimulated DMSC using a previously published protocol[135], which increased significantly the secretion of VEGF-A and BDNF proteins and enhanced the effect of DMSC secretome on neurite outgrowth.
Each preconditioning regimen induced an individual expression profile with a wide variety of factors, including several growth factors and cytokines[136].
DMSC-CM was concentrated before use in some studies and diluted to 50% in others[35,46,109,110]. A 50% diluted DMSC-CM has sometimes been proposed as the most effective[46], probably because of an optimal balance achieved between paracrine stimulatory and metabolic inhibitory products[46,137]. Only protein fractions with a molecular weight between 10 kDa and 3 kDa were able to protect neurons from induced neurodegeneration, indicating a possible responsibility of these microproteins for the neuroprotective character of DPSC-CM[39]. Only the fraction smaller than 6 kDa of SHED-CM promoted neurite growth in dorsal root ganglion neurons[92]. Finally, in most studies, filtration with 0.2μm pore filters was performed, in order to sterilize the CM and/or remove debris. Supplementary Table 7 summarizes the CM purification procedures in studies using DMSC-CM for tissue regeneration.
Numerous studies have been performed to assess if DMSC functions are associated with EV-enriched fractions or not. Merckx et al[55] demonstrated that the main angiogenic effect of growth factors secreted by DMSC was not associated with EVs. Nevertheless, Zhou et al[38] showed a decrease in the proangiogenic effects of DPSC-CM when EV secretion was blocked. DPSC exosomes in comparison with DPSC-CM showed similar neuroprotective efficacy, with superior anti-necrotic properties and implication in aging processes[39,138]. SHED-exosomes, in contrast to SHED-derived microvesicles, have approved anti-apoptotic effects in dopaminergic neurons[16]. However, they did not show the neuritogenic potential observed in SHED-CM[92]. Supplementary Table 8 in supplementary data summarizes the methods used to purify the DMSC-EV in the literature.
DMSC-CM was stored at -80 °C in most studies, at -20 °C in three studies[78-80] and at +4 in two studies[87, 88]. These different kind of storage did not affect the potentials of CM. Protease inhibitors were added to secretomes just in few studies[13,16,73,74]. Supplementary Table 9 in supplementary data summarizes the storage conditions of DMSC-CM in literature.
Secretome characterization is necessary to identify DMSC-CM profiles and to confirm the reproducibility of manufacturing. Different technics were used in the literature (Supplementary Table 10). Protein concentration in the secretome was measured with the BCA and Bradford protein assays. Other techniques were used for qualitative and quantitative identification of CM. With the development of technology, proteomics has become a strong tool for identification, and mass spectrometry has emerged as the main technique applied for the detection of proteins in cell secretomes[139]. A high protein coverage was obtained by mass spectrometry in a study conducted by Tachida et al[140] to identify the secretion profile of DPSC-CM. In that study, CM was prepared with DPSC isolated from rat incisors, having a confluence of 80-90%. Three washes with PBS were done before starting the conditioning of serum-free alpha-MEM medium by DPSC for 72 h. A 0.2µm filtration and a 50-fold concentration with a 3 KDa MWCO centrifugal unit were then performed[140]. The CM profile described in this study cannot represent DPSC-CM in general, as the CM preparation protocol is different from those used in other DMSC-CM studies. Comparing protein profiles and concentrations directly between studies is hampered by the variability of all the factors detailed above, and a production standardization may be the key to obtaining clear DMSC-CM profiles and ensure appropriate use of each CM based on its profile.
Among all the considered key points, at the 3 Levels of CM production, no consensus could be highlighted as evidence-based methods. We could only describe the most commonly used protocols. However, the various microenviroenmental cues were shown many times to have a significant impact on CM protein content, and improved effect on specifically targeted applications. Hypoxia enhanced the VEGF secretion and proangiogenic; 3D culture increased anti-apoptotic effects on neurons; LPS preconditioning increased cell proliferation and migration, with enhanced anti-inflammatory activity; osteo-induction of DMSC enhanced the CM effect for further cell osteodifferentiation. Taken altogether, each preconditioning manipulation induced a specific protein expression profile.
More recently, we have demonstrated that specific preconditioning protocols could lead to DMSC-CM which significantly increase neurite growth in sensory neurons[141]. This neuroregenerative effect of CM due to preconditioning was linked to a change in secretome composition, with an increase of several factors involved in neurogenesis, neuroprotection, and angiogenesis.
We have reviewed here the different conditions and protocols used in the manufacturing of DMSC-CM and their derived products. This literature survey allowed us to describe that donors under 30 years of age are often used to produce CM. DPSC and SHED were the most commonly used cells, with cell passage between 1 and 5, and at a confluence of 70% to 80%. DMEM was the most commonly used culture medium for all applications. CM were often collected during the first 48 h, and frozen at -80 °C. The preconditioning environment (environmental cues) had a significant impact on DMSC-CM content and efficiency. Therefore, further studies should be conducted to confirm which environmental conditions specifically optimize the potentials of DMSC-CM for each application in tissue regeneration, and which parts of DMSC-CM are responsible for these potentials.
Dental Mesenchymal stem cells are progenitor populations recovered from dental and periodontal tissues easily accessible when a tooth is extracted (and usually discarded as medical waste). One of the main effects of Mesenchymal stem cells on tissue regeneration is due to their paracrine activity, mediated by their secreted substances defined as secretome or conditioned medium. Conditioned medium represents a cell-free product with wide potential applications for various diseases treatment and tissue regeneration.
Conditioned medium manufacturing suffers from variable procedures and protocols. Results presented by various studies are difficult to compare, due to the different methods of production. Moreover, there is no well-defined optimized procedure to specifically target specialized tissue in regenerative medicine.
To describe potential consensus for the standardization and optimization of manufacturing procedures, mandatory for further clinical applications. In this systematic review, we focused on conditioned medium produced from Dental Mesenchymal stem cells. We explored the current parameters and culture conditions used in the manufacturing protocols.
The bibliographic research was conducted in accordance with the PRISMA guidelines. All articles published between 2006 and 2020 investigating the effects of Dental Mesenchymal stem cells secretome on tissue regeneration were selected. We used the electronic PubMed database with these search terms: ((dental stem cells) AND ((conditioned medium) OR (secretome))) / ((dental stem cells) AND ((extravesicles) OR (exosomes))). Only publications in English were considered.
Based on the inclusion criteria, 118 articles were included in the systematic review. Conditioned medium production was considered at three levels: before recovery (cell sources), during production (culture conditions) and after production (secretome treatment). We identified key points that were often taken into account in the published experimental works.
Among all the considered key points, no consensus could be highlighted. However, some tendencies could be described: cells used were mainly from donors under 30 years of age, with cell passage between 1 and 5, at a confluence of 70% to 80%. Conditioned medium was usually collected during the first 48 h, and kept frozen at -80°C. The various microenviroenmental cues were shown many times to have a significant impact on protein content, and improved effects on specifically targeted applications: each preconditioning manipulation induced a specific protein expression profile.
Standardization of procedures is of prior importance to develop clinical-grade products. However, the protein contents of secretome is more linked to preconditioning than to specific technical methods. The challenge to overcome in the near future is to define specific preconditioning protocols to produce tissue specific conditioned medium (i.e, osteogenic environement, neuronal environment, cell incubation with inflammatory proteins…).
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Cell and tissue engineering
Country/Territory of origin: France
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ocak E, Turkey; Sukumaran A, Qatar S-Editor: Wang LL L-Editor: A P-Editor: Wang LL
| 1. | Karamzadeh R, Eslaminejad MB. Dental-related stem cells and their potential in regenerative medicine. In: Andrades JAs. Proceedings of the Regenerative Medicine and Tissue Engineering. IntechOpen. 2013;95-116. |
| 2. | Gronthos S, Mankani M, Brahim J, Robey PG, Shi S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci U S A. 2000;97:13625-13630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3174] [Cited by in RCA: 3364] [Article Influence: 134.6] [Reference Citation Analysis (0)] |
| 3. | Chen FM, Shi S. Periodontal Tissue Engineering. In: Lanza R, Langer R, Vacanti Js. Proceedings of the Principles of Tissue Engineering. Academic Press. 2014;1507-1540. |
| 4. | Zhou T, Pan J, Wu P, Huang R, Du W, Zhou Y, Wan M, Fan Y, Xu X, Zhou X, Zheng L. Dental Follicle Cells: Roles in Development and Beyond. Stem Cells Int. 2019;2019:9159605. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 70] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 5. | Ikeda E, Yagi K, Kojima M, Yagyuu T, Ohshima A, Sobajima S, Tadokoro M, Katsube Y, Isoda K, Kondoh M, Kawase M, Go MJ, Adachi H, Yokota Y, Kirita T, Ohgushi H. Multipotent cells from the human third molar: feasibility of cell-based therapy for liver disease. Differentiation. 2008;76:495-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 132] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 6. | Alkhalil M, Smajilagić A, Redžić A. Human dental pulp mesenchymal stem cells isolation and osteoblast differentiation. Med Glas (Zenica). 2015;12:27-32. [PubMed] |
| 7. | Fu Y, Karbaat L, Wu L, Leijten J, Both SK, Karperien M. Trophic Effects of Mesenchymal stem cells in Tissue Regeneration. Tissue Eng Part B Rev. 2017;23:515-528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 193] [Article Influence: 27.6] [Reference Citation Analysis (0)] |
| 8. | Kichenbrand C, Velot E, Menu P, Moby V. Dental Pulp Stem Cell-Derived Conditioned Medium: An Attractive Alternative for Regenerative Therapy. Tissue Eng Part B Rev. 2019;25:78-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 71] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 9. | Pawitan JA. Prospect of stem cell conditioned medium in regenerative medicine. Biomed Res Int. 2014;2014:965849. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 199] [Cited by in RCA: 290] [Article Influence: 26.4] [Reference Citation Analysis (0)] |
| 10. | El Moshy S, Radwan IA, Rady D, Abbass MMS, El-Rashidy AA, Sadek KM, Dörfer CE, Fawzy El-Sayed KM. Dental Stem Cell-Derived Secretome/Conditioned Medium: The Future for Regenerative Therapeutic Applications. Stem Cells Int. 2020;2020:7593402. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 82] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 11. | Muhammad SA, Nordin N, Fakurazi S. Regenerative potential of secretome from dental stem cells: a systematic review of preclinical studies. Rev Neurosci. 2018;29:321-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 12. | Stanko P, Altanerova U, Jakubechova J, Repiska V, Altaner C. Dental Mesenchymal Stem/Stromal Cells and Their Exosomes. Stem Cells Int. 2018;2018:8973613. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 76] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 13. | Ahmed Nel-M, Murakami M, Hirose Y, Nakashima M. Therapeutic Potential of Dental Pulp Stem Cell Secretome for Alzheimer's Disease Treatment: An In Vitro Study. Stem Cells Int. 2016;2016:8102478. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 65] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 14. | Asadi-Golshan R, Razban V, Mirzaei E, Rahmanian A, Khajeh S, Mostafavi-Pour Z, Dehghani F. Sensory and Motor Behavior Evidences Supporting the Usefulness of Conditioned Medium from Dental Pulp-Derived Stem Cells in Spinal Cord Injury in Rats. Asian Spine J. 2018;12:785-793. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 15. | Chen YR, Lai PL, Chien Y, Lee PH, Lai YH, Ma HI, Shiau CY, Wang KC. Improvement of Impaired Motor Functions by Human Dental Exfoliated Deciduous Teeth Stem Cell-Derived Factors in a Rat Model of Parkinson's Disease. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 42] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 16. | Jarmalavičiūtė A, Tunaitis V, Pivoraitė U, Venalis A, Pivoriūnas A. Exosomes from dental pulp stem cells rescue human dopaminergic neurons from 6-hydroxy-dopamine-induced apoptosis. Cytotherapy. 2015;17:932-939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 189] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 17. | Wang J, Zuzzio K, Walker CL. Systemic Dental Pulp Stem Cell Secretome Therapy in a Mouse Model of Amyotrophic Lateral Sclerosis. Brain Sci. 2019;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 18. | Mita T, Furukawa-Hibi Y, Takeuchi H, Hattori H, Yamada K, Hibi H, Ueda M, Yamamoto A. Conditioned medium from the stem cells of human dental pulp improves cognitive function in a mouse model of Alzheimer's disease. Behav Brain Res. 2015;293:189-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 118] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 19. | Yamaguchi S, Shibata R, Yamamoto N, Nishikawa M, Hibi H, Tanigawa T, Ueda M, Murohara T, Yamamoto A. Dental pulp-derived stem cell conditioned medium reduces cardiac injury following ischemia-reperfusion. Sci Rep. 2015;5:16295. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 93] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 20. | Izumoto-Akita T, Tsunekawa S, Yamamoto A, Uenishi E, Ishikawa K, Ogata H, Iida A, Ikeniwa M, Hosokawa K, Niwa Y, Maekawa R, Yamauchi Y, Seino Y, Hamada Y, Hibi H, Arima H, Ueda M, Oiso Y. Secreted factors from dental pulp stem cells improve glucose intolerance in streptozotocin-induced diabetic mice by increasing pancreatic β-cell function. BMJ Open Diabetes Res Care. 2015;3:e000128. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 21. | Matsushita Y, Ishigami M, Matsubara K, Kondo M, Wakayama H, Goto H, Ueda M, Yamamoto A. Multifaceted therapeutic benefits of factors derived from stem cells from human exfoliated deciduous teeth for acute liver failure in rats. J Tissue Eng Regen Med. 2017;11:1888-1896. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 44] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 22. | Hirata M, Ishigami M, Matsushita Y, Ito T, Hattori H, Hibi H, Goto H, Ueda M, Yamamoto A. Multifaceted Therapeutic Benefits of Factors Derived From Dental Pulp Stem Cells for Mouse Liver Fibrosis. Stem Cells Transl Med. 2016;5:1416-1424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 51] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 23. | Wakayama H, Hashimoto N, Matsushita Y, Matsubara K, Yamamoto N, Hasegawa Y, Ueda M, Yamamoto A. Factors secreted from dental pulp stem cells show multifaceted benefits for treating acute lung injury in mice. Cytotherapy. 2015;17:1119-1129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 73] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 24. | Shimojima C, Takeuchi H, Jin S, Parajuli B, Hattori H, Suzumura A, Hibi H, Ueda M, Yamamoto A. Conditioned Medium from the Stem Cells of Human Exfoliated Deciduous Teeth Ameliorates Experimental Autoimmune Encephalomyelitis. J Immunol. 2016;196:4164-4171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 65] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 25. | Pivoraitė U, Jarmalavičiūtė A, Tunaitis V, Ramanauskaitė G, Vaitkuvienė A, Kašėta V, Biziulevičienė G, Venalis A, Pivoriūnas A. Exosomes from Human Dental Pulp Stem Cells Suppress Carrageenan-Induced Acute Inflammation in Mice. Inflammation. 2015;38:1933-1941. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 67] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 26. | Ishikawa J, Takahashi N, Matsumoto T, Yoshioka Y, Yamamoto N, Nishikawa M, Hibi H, Ishigro N, Ueda M, Furukawa K, Yamamoto A. Factors secreted from dental pulp stem cells show multifaceted benefits for treating experimental rheumatoid arthritis. Bone. 2016;83:210-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 70] [Article Influence: 7.8] [Reference Citation Analysis (1)] |
| 27. | Omori M, Tsuchiya S, Hara K, Kuroda K, Hibi H, Okido M, Ueda M. A new application of cell-free bone regeneration: immobilizing stem cells from human exfoliated deciduous teeth-conditioned medium onto titanium implants using atmospheric pressure plasma treatment. Stem Cell Res Ther. 2015;6:124. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 28. | Hiraki T, Kunimatsu R, Nakajima K, Abe T, Yamada S, Rikitake K, Tanimoto K. Stem cell-derived conditioned media from human exfoliated deciduous teeth promote bone regeneration. Oral Dis. 2020;26:381-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 29. | de Cara SPHM, Origassa CST, de Sá Silva F, Moreira MSNA, de Almeida DC, Pedroni ACF, Carvalho GL, Cury DP, Câmara NOS, Marques MM. Angiogenic properties of dental pulp stem cells conditioned medium on endothelial cells in vitro and in rodent orthotopic dental pulp regeneration. Heliyon. 2019;5:e01560. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 40] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 30. | Qiu J, Wang X, Zhou H, Zhang C, Wang Y, Huang J, Liu M, Yang P, Song A. Enhancement of periodontal tissue regeneration by conditioned media from gingiva-derived or periodontal ligament-derived mesenchymal stem cells: a comparative study in rats. Stem Cell Res Ther. 2020;11:42. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 69] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 31. | Gunawardena TNA, Masoudian Z, Rahman MT, Ramasamy TS, Ramanathan A, Abu Kasim NH. Dental derived stem cell conditioned media for hair growth stimulation. PLoS One. 2019;14:e0216003. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 32. | Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18665] [Cited by in RCA: 17535] [Article Influence: 1095.9] [Reference Citation Analysis (1)] |
| 33. | Kumar A, Kumar V, Rattan V, Jha V, Pal A, Bhattacharyya S. Molecular spectrum of secretome regulates the relative hepatogenic potential of mesenchymal stem cells from bone marrow and dental tissue. Sci Rep. 2017;7:15015. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 43] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 34. | Kumar A, Kumar V, Rattan V, Jha V, Bhattacharyya S. Secretome proteins regulate comparative osteogenic and adipogenic potential in bone marrow and dental stem cells. Biochimie. 2018;155:129-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 43] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 35. | Kumar A, Kumar V, Rattan V, Jha V, Bhattacharyya S. Secretome Cues Modulate the Neurogenic Potential of Bone Marrow and Dental Stem Cells. Mol Neurobiol. 2017;54:4672-4682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 51] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 36. | Gervois P, Wolfs E, Dillen Y, Hilkens P, Ratajczak J, Driesen RB, Vangansewinkel T, Bronckaers A, Brône B, Struys T, Lambrichts I. Paracrine Maturation and Migration of SH-SY5Y Cells by Dental Pulp Stem Cells. J Dent Res. 2017;96:654-662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 37. | Horibe H, Murakami M, Iohara K, Hayashi Y, Takeuchi N, Takei Y, Kurita K, Nakashima M. Isolation of a sSupplementary Table ubpopulation of mobilized dental pulp stem cells (MDPSCs) with high proliferation, migration, and regeneration potential is independent of age. PLoS One. 2014;9:e98553. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 48] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 38. | Zhou H, Li X, Yin Y, He XT, An Y, Tian BM, Hong YL, Wu LA, Chen FM. The proangiogenic effects of extracellular vesicles secreted by dental pulp stem cells derived from periodontally compromised teeth. Stem Cell Res Ther. 2020;11:110. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 50] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 39. | Venugopal C, K S, Rai KS, Pinnelli VB, Kutty BM, Dhanushkodi A. Neuroprotection by Human Dental Pulp Mesenchymal stem cells: From Billions to Nano. Curr Gene Ther. 2018;18:307-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 40. | Caseiro AR, Santos Pedrosa S, Ivanova G, Vieira Branquinho M, Almeida A, Faria F, Amorim I, Pereira T, Maurício AC. Mesenchymal Stem/ Stromal Cells metabolomic and bioactive factors profiles: A comparative analysis on the umbilical cord and dental pulp derived Stem/ Stromal Cells secretome. PLoS One. 2019;14:e0221378. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 41. | Shen C, Li L, Feng T, Li J, Yu M, Lu Q, Li H. Dental pulp stem cells derived conditioned medium promotes angiogenesis in hindlimb ischemia. Tissue Engineering and Regenerative Medicine. 2015;12:59-68. [RCA] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 42. | Mead B, Logan A, Berry M, Leadbeater W, Scheven BA. Paracrine-mediated neuroprotection and neuritogenesis of axotomised retinal ganglion cells by human dental pulp stem cells: comparison with human bone marrow and adipose-derived mesenchymal stem cells. PLoS One. 2014;9:e109305. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 144] [Cited by in RCA: 183] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 43. | Wada N, Menicanin D, Shi S, Bartold PM, Gronthos S. Immunomodulatory properties of human periodontal ligament stem cells. J Cell Physiol. 2009;219:667-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 298] [Cited by in RCA: 306] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 44. | Kolar MK, Itte VN, Kingham PJ, Novikov LN, Wiberg M, Kelk P. The neurotrophic effects of different human dental mesenchymal stem cells. Sci Rep. 2017;7:12605. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 109] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 45. | Bronckaers A, Hilkens P, Fanton Y, Struys T, Gervois P, Politis C, Martens W, Lambrichts I. Angiogenic properties of human dental pulp stem cells. PLoS One. 2013;8:e71104. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 145] [Cited by in RCA: 161] [Article Influence: 13.4] [Reference Citation Analysis (1)] |
| 46. | Paschalidis T, Bakopoulou A, Papa P, Leyhausen G, Geurtsen W, Koidis P. Dental pulp stem cells' secretome enhances pulp repair processes and compensates TEGDMA-induced cytotoxicity. Dent Mater. 2014;30:e405-e418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 47. | Piva E, Tarlé SA, Nör JE, Zou D, Hatfield E, Guinn T, Eubanks EJ, Kaigler D. Dental Pulp Tissue Regeneration Using Dental Pulp Stem Cells Isolated and Expanded in Human Serum. J Endod. 2017;43:568-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 48] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 48. | Gharaei MA, Xue Y, Mustafa K, Lie SA, Fristad I. Human dental pulp stromal cell conditioned medium alters endothelial cell behavior. Stem Cell Res Ther. 2018;9:69. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 49. | Murakami M, Horibe H, Iohara K, Hayashi Y, Osako Y, Takei Y, Nakata K, Motoyama N, Kurita K, Nakashima M. The use of granulocyte-colony stimulating factor induced mobilization for isolation of dental pulp stem cells with high regenerative potential. Biomaterials. 2013;34:9036-9047. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 89] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 50. | Li J, Ju Y, Liu S, Fu Y, Zhao S. Exosomes derived from lipopolysaccharide-preconditioned human dental pulp stem cells regulate Schwann cell migration and differentiation. Connect Tissue Res. 2021;62:277-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 45] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 51. | Yamamoto A, Matsubara K, Kano F, Sakai K. Analysis of the neuroregenerative activities of mesenchymal stem cells in functional recovery after rat spinal cord injury. Methods Mol Biol. 2014;1213:321-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 52. | Sakai K, Yamamoto A, Matsubara K, Nakamura S, Naruse M, Yamagata M, Sakamoto K, Tauchi R, Wakao N, Imagama S, Hibi H, Kadomatsu K, Ishiguro N, Ueda M. Human dental pulp-derived stem cells promote locomotor recovery after complete transection of the rat spinal cord by multiple neuro-regenerative mechanisms. J Clin Invest. 2012;122:80-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 249] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 53. | Hu X, Zhong Y, Kong Y, Chen Y, Feng J, Zheng J. Lineage-specific exosomes promote the odontogenic differentiation of human dental pulp stem cells (DPSCs) through TGFβ1/smads signaling pathway via transfer of microRNAs. Stem Cell Res Ther. 2019;10:170. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 99] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 54. | Nakayama H, Iohara K, Hayashi Y, Okuwa Y, Kurita K, Nakashima M. Enhanced regeneration potential of mobilized dental pulp stem cells from immature teeth. Oral Dis. 2017;23:620-628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 55. | Merckx G, Hosseinkhani B, Kuypers S, Deville S, Irobi J, Nelissen I, Michiels L, Lambrichts I, Bronckaers A. Angiogenic Effects of Human Dental Pulp and Bone Marrow-Derived Mesenchymal Stromal Cells and their Extracellular Vesicles. Cells. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 58] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 56. | Swanson WB, Gong T, Zhang Z, Eberle M, Niemann D, Dong R, Rambhia KJ, Ma PX. Controlled release of odontogenic exosomes from a biodegradable vehicle mediates dentinogenesis as a novel biomimetic pulp capping therapy. J Control Release. 2020;324:679-694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 76] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 57. | Gervois P, Ratajczak J, Wolfs E, Vangansewinkel T, Dillen Y, Merckx G, Bronckaers A, Lambrichts I. Preconditioning of Human Dental Pulp Stem Cells with Leukocyte- and Platelet-Rich Fibrin-Derived Factors Does Not Enhance Their Neuroregenerative Effect. Stem Cells Int. 2019;2019:8589149. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 58. | Ivica A, Ghayor C, Zehnder M, Valdec S, Weber FE. Pulp-Derived Exosomes in a Fibrin-Based Regenerative Root Filling Material. J Clin Med. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 59. | Zhang W, Yu L, Han X, Pan J, Deng J, Zhu L, Lu Y, Huang W, Liu S, Li Q, Liu Y. The secretome of human dental pulp stem cells protects myoblasts from hypoxiainduced injury via the Wnt/βcatenin pathway. Int J Mol Med. 2020;45:1501-1513. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 60. | Yamamoto T, Osako Y, Ito M, Murakami M, Hayashi Y, Horibe H, Iohara K, Takeuchi N, Okui N, Hirata H, Nakayama H, Kurita K, Nakashima M. Trophic Effects of Dental Pulp Stem Cells on Schwann Cells in Peripheral Nerve Regeneration. Cell Transplant. 2016;25:183-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 57] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 61. | Xian X, Gong Q, Li C, Guo B, Jiang H. Exosomes with Highly Angiogenic Potential for Possible Use in Pulp Regeneration. J Endod. 2018;44:751-758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 68] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 62. | Song M, Jue SS, Cho YA, Kim EC. Comparison of the effects of human dental pulp stem cells and human bone marrow-derived mesenchymal stem cells on ischemic human astrocytes in vitro. J Neurosci Res. 2015;93:973-983. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 51] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 63. | Joo KH, Song JS, Kim S, Lee HS, Jeon M, Kim SO, Lee JH. Cytokine Expression of Stem Cells Originating from the Apical Complex and Coronal Pulp of Immature Teeth. J Endod. 2018;44:87-92.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 64. | Shen Z, Kuang S, Zhang Y, Yang M, Qin W, Shi X, Lin Z. Chitosan hydrogel incorporated with dental pulp stem cell-derived exosomes alleviates periodontitis in mice via a macrophage-dependent mechanism. Bioact Mater. 2020;5:1113-1126. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 180] [Article Influence: 36.0] [Reference Citation Analysis (0)] |
| 65. | Akazawa Y, Hasegawa T, Yoshimura Y, Chosa N, Asakawa T, Ueda K, Sugimoto A, Kitamura T, Nakagawa H, Ishisaki A, Iwamoto T. Recruitment of mesenchymal stem cells by stromal cell-derived factor 1α in pulp cells from deciduous teeth. Int J Mol Med. 2015;36:442-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 66. | De Rosa A, Tirino V, Paino F, Tartaglione A, Mitsiadis T, Feki A, d'Aquino R, Laino L, Colacurci N, Papaccio G. Amniotic fluid-derived mesenchymal stem cells lead to bone differentiation when cocultured with dental pulp stem cells. Tissue Eng Part A. 2011;17:645-653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 67. | Ji L, Bao L, Gu Z, Zhou Q, Liang Y, Zheng Y, Xu Y, Zhang X, Feng X. Comparison of immunomodulatory properties of exosomes derived from bone marrow mesenchymal stem cells and dental pulp stem cells. Immunol Res. 2019;67:432-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 78] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 68. | Aranha AM, Zhang Z, Neiva KG, Costa CA, Hebling J, Nör JE. Hypoxia enhances the angiogenic potential of human dental pulp cells. J Endod. 2010;36:1633-1637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 110] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 69. | Huang CC, Narayanan R, Alapati S, Ravindran S. Exosomes as biomimetic tools for stem cell differentiation: Applications in dental pulp tissue regeneration. Biomaterials. 2016;111:103-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 237] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 70. | Lambrichts I, Driesen RB, Dillen Y, Gervois P, Ratajczak J, Vangansewinkel T, Wolfs E, Bronckaers A, Hilkens P. Dental Pulp Stem Cells: Their Potential in Reinnervation and Angiogenesis by Using Scaffolds. J Endod. 2017;43:S12-S16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 62] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 71. | Iohara K, Zheng L, Wake H, Ito M, Nabekura J, Wakita H, Nakamura H, Into T, Matsushita K, Nakashima M. A novel stem cell source for vasculogenesis in ischemia: subfraction of side population cells from dental pulp. Stem Cells. 2008;26:2408-2418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 183] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 72. | Ishizaka R, Hayashi Y, Iohara K, Sugiyama M, Murakami M, Yamamoto T, Fukuta O, Nakashima M. Stimulation of angiogenesis, neurogenesis and regeneration by side population cells from dental pulp. Biomaterials. 2013;34:1888-1897. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 105] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 73. | Kawamura R, Hayashi Y, Murakami H, Nakashima M. EDTA soluble chemical components and the conditioned medium from mobilized dental pulp stem cells contain an inductive microenvironment, promoting cell proliferation, migration, and odontoblastic differentiation. Stem Cell Res Ther. 2016;7:77. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 74. | Hayashi Y, Murakami M, Kawamura R, Ishizaka R, Fukuta O, Nakashima M. CXCL14 and MCP1 are potent trophic factors associated with cell migration and angiogenesis leading to higher regenerative potential of dental pulp side population cells. Stem Cell Res Ther. 2015;6:111. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 62] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 75. | Iohara K, Murakami M, Nakata K, Nakashima M. Age-dependent decline in dental pulp regeneration after pulpectomy in dogs. Exp Gerontol. 2014;52:39-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 85] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 76. | Iohara K, Murakami M, Takeuchi N, Osako Y, Ito M, Ishizaka R, Utunomiya S, Nakamura H, Matsushita K, Nakashima M. A novel combinatorial therapy with pulp stem cells and granulocyte colony-stimulating factor for total pulp regeneration. Stem Cells Transl Med. 2013;2:521-533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 141] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 77. | Murakami M, Hayashi Y, Iohara K, Osako Y, Hirose Y, Nakashima M. Trophic Effects and Regenerative Potential of Mobilized Mesenchymal stem cells From Bone Marrow and Adipose Tissue as Alternative Cell Sources for Pulp/Dentin Regeneration. Cell Transplant. 2015;24:1753-1765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 84] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 78. | Omi M, Hata M, Nakamura N, Miyabe M, Ozawa S, Nukada H, Tsukamoto M, Sango K, Himeno T, Kamiya H, Nakamura J, Takebe J, Matsubara T, Naruse K. Transplantation of dental pulp stem cells improves long-term diabetic polyneuropathy together with improvement of nerve morphometrical evaluation. Stem Cell Res Ther. 2017;8:279. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 37] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 79. | Makino E, Nakamura N, Miyabe M, Ito M, Kanada S, Hata M, Saiki T, Sango K, Kamiya H, Nakamura J, Miyazawa K, Goto S, Matsubara T, Naruse K. Conditioned media from dental pulp stem cells improved diabetic polyneuropathy through anti-inflammatory, neuroprotective and angiogenic actions: Cell-free regenerative medicine for diabetic polyneuropathy. J Diabetes Investig. 2019;10:1199-1208. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 80. | Omi M, Hata M, Nakamura N, Miyabe M, Kobayashi Y, Kamiya H, Nakamura J, Ozawa S, Tanaka Y, Takebe J, Matsubara T, Naruse K. Transplantation of dental pulp stem cells suppressed inflammation in sciatic nerves by promoting macrophage polarization towards anti-inflammation phenotypes and ameliorated diabetic polyneuropathy. J Diabetes Investig. 2016;7:485-496. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 71] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 81. | Chen TF, Chen KW, Chien Y, Lai YH, Hsieh ST, Ma HY, Wang KC, Shiau CY. Dental Pulp Stem Cell-Derived Factors Alleviate Subarachnoid Hemorrhage-Induced Neuroinflammation and Ischemic Neurological Deficits. Int J Mol Sci. 2019;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 82. | Li Y, Yang YY, Ren JL, Xu F, Chen FM, Li A. Exosomes secreted by stem cells from human exfoliated deciduous teeth contribute to functional recovery after traumatic brain injury by shifting microglia M1/M2 polarization in rats. Stem Cell Res Ther. 2017;8:198. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 98] [Cited by in RCA: 146] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 83. | Kano F, Matsubara K, Ueda M, Hibi H, Yamamoto A. Secreted Ectodomain of Sialic Acid-Binding Ig-Like Lectin-9 and Monocyte Chemoattractant Protein-1 Synergistically Regenerate Transected Rat Peripheral Nerves by Altering Macrophage Polarity. Stem Cells. 2017;35:641-653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 84. | Matsubara K, Matsushita Y, Sakai K, Kano F, Kondo M, Noda M, Hashimoto N, Imagama S, Ishiguro N, Suzumura A, Ueda M, Furukawa K, Yamamoto A. Secreted ectodomain of sialic acid-binding Ig-like lectin-9 and monocyte chemoattractant protein-1 promote recovery after rat spinal cord injury by altering macrophage polarity. J Neurosci. 2015;35:2452-2464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 118] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 85. | Yamagata M, Yamamoto A, Kako E, Kaneko N, Matsubara K, Sakai K, Sawamoto K, Ueda M. Human dental pulp-derived stem cells protect against hypoxic-ischemic brain injury in neonatal mice. Stroke. 2013;44:551-554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 117] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 86. | Fujii H, Matsubara K, Sakai K, Ito M, Ohno K, Ueda M, Yamamoto A. Dopaminergic differentiation of stem cells from human deciduous teeth and their therapeutic benefits for Parkinsonian rats. Brain Res. 2015;1613:59-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 83] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 87. | Tsuruta T, Sakai K, Watanabe J, Katagiri W, Hibi H. Dental pulp-derived stem cell conditioned medium to regenerate peripheral nerves in a novel animal model of dysphagia. PLoS One. 2018;13:e0208938. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 88. | Sugimura-Wakayama Y, Katagiri W, Osugi M, Kawai T, Ogata K, Sakaguchi K, Hibi H. Peripheral Nerve Regeneration by Secretomes of Stem Cells from Human Exfoliated Deciduous Teeth. Stem Cells Dev. 2015;24:2687-2699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 71] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 89. | Han Y, Gong T, Zhang C, Dissanayaka WL. HIF-1α Stabilization Enhances Angio-/Vasculogenic Properties of SHED. J Dent Res. 2020;99:804-812. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 90. | Mussano F, Genova T, Petrillo S, Roato I, Ferracini R, Munaron L. Osteogenic Differentiation Modulates the Cytokine, Chemokine, and Growth Factor Profile of ASCs and SHED. Int J Mol Sci. 2018;19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 91. | Wang M, Li J, Ye Y, He S, Song J. SHED-derived conditioned exosomes enhance the osteogenic differentiation of PDLSCs via Wnt and BMP signaling in vitro. Differentiation. 2020;111:1-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 99] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 92. | Miura-Yura E, Tsunekawa S, Naruse K, Nakamura N, Motegi M, Nakai-Shimoda H, Asano S, Kato M, Yamada Y, Izumoto-Akita T, Yamamoto A, Himeno T, Kondo M, Kato Y, Nakamura J, Kamiya H. Secreted factors from cultured dental pulp stem cells promoted neurite outgrowth of dorsal root ganglion neurons and ameliorated neural functions in streptozotocin-induced diabetic mice. J Diabetes Investig. 2020;11:28-38. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 93. | Ogasawara N, Kano F, Hashimoto N, Mori H, Liu Y, Xia L, Sakamaki T, Hibi H, Iwamoto T, Tanaka E, Yamamoto A. Factors secreted from dental pulp stem cells show multifaceted benefits for treating experimental temporomandibular joint osteoarthritis. Osteoarthritis Cartilage. 2020;28:831-841. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 45] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 94. | Sakai K, Tsuruta T, Watanabe J, Sugimura Y, Sakaguchi K, Katagiri W, Hibi H. Peripheral Nerve Regeneration in a Novel Rat Model of Dysphagia. In: Kioussi Cs. Proceedings of the Stem Cells and Tissue Repair. New York: Springer, 2020: 107-113. |
| 95. | Inoue T, Sugiyama M, Hattori H, Wakita H, Wakabayashi T, Ueda M. Stem cells from human exfoliated deciduous tooth-derived conditioned medium enhance recovery of focal cerebral ischemia in rats. Tissue Eng Part A. 2013;19:24-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 117] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 96. | Wei J, Song Y, Du Z, Yu F, Zhang Y, Jiang N, Ge X. Exosomes derived from human exfoliated deciduous teeth ameliorate adult bone loss in mice through promoting osteogenesis. J Mol Histol. 2020;51:455-466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 58] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 97. | Li XX, Yuan XJ, Zhai Y, Yu S, Jia RX, Yang LP, Ma ZZ, Zhao YM, Wang YX, Ge LH. Treatment with Stem Cells from Human Exfoliated Deciduous Teeth and Their Derived Conditioned Medium Improves Retinal Visual Function and Delays the Degeneration of Photoreceptors. Stem Cells Dev. 2019;28:1514-1526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 98. | Kang H, Lee MJ, Park SJ, Lee MS. Lipopolysaccharide-Preconditioned Periodontal Ligament Stem Cells Induce M1 Polarization of Macrophages through Extracellular Vesicles. Int J Mol Sci. 2018;19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 48] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 99. | Diomede F, D'Aurora M, Gugliandolo A, Merciaro I, Ettorre V, Bramanti A, Piattelli A, Gatta V, Mazzon E, Fontana A, Trubiani O. A novel role in skeletal segment regeneration of extracellular vesicles released from periodontal-ligament stem cells. Int J Nanomedicine. 2018;13:3805-3825. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 77] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 100. | Aghamohamadi Z, Kadkhodazadeh M, Torshabi M, Tabatabaei F. A compound of concentrated growth factor and periodontal ligament stem cell-derived conditioned medium. Tissue Cell. 2020;65:101373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 101. | Nagata M, Iwasaki K, Akazawa K, Komaki M, Yokoyama N, Izumi Y, Morita I. Conditioned Medium from Periodontal Ligament Stem Cells Enhances Periodontal Regeneration. Tissue Eng Part A. 2017;23:367-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 121] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 102. | Cianci E, Recchiuti A, Trubiani O, Diomede F, Marchisio M, Miscia S, Colas RA, Dalli J, Serhan CN, Romano M. Human Periodontal Stem Cells Release Specialized Proresolving Mediators and Carry Immunomodulatory and Prohealing Properties Regulated by Lipoxins. Stem Cells Transl Med. 2016;5:20-32. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 82] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 103. | Zhang Z, Shuai Y, Zhou F, Yin J, Hu J, Guo S, Wang Y, Liu W. PDLSCs Regulate Angiogenesis of Periodontal Ligaments via VEGF Transferred by Exosomes in Periodontitis. Int J Med Sci. 2020;17:558-567. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 78] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 104. | Bakopoulou A, Kritis A, Andreadis D, Papachristou E, Leyhausen G, Koidis P, Geurtsen W, Tsiftsoglou A. Angiogenic Potential and Secretome of Human Apical Papilla Mesenchymal stem cells in Various Stress Microenvironments. Stem Cells Dev. 2015;24:2496-2512. [RCA] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 65] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 105. | Zhuang X, Ji L, Jiang H, Liu Y, Liu X, Bi J, Zhao W, Ding Z, Chen X. Exosomes Derived from Stem Cells from the Apical Papilla Promote Dentine-Pulp Complex Regeneration by Inducing Specific Dentinogenesis. Stem Cells Int. 2020;2020:5816723. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 64] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 106. | Yu S, Zhao Y, Ma Y, Ge L. Profiling the Secretome of Human Stem Cells from Dental Apical Papilla. Stem Cells Dev. 2016;25:499-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 49] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 107. | Yu S, Zhao Y, Fang TJ, Ge L. Effect of the Soluble Factors Released by Dental Apical Papilla-Derived Stem Cells on the Osteo/Odontogenic, Angiogenic, and Neurogenic Differentiation of Dental Pulp Cells. Stem Cells Dev. 2020;29:795-805. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 108. | Yu S, Li J, Zhao Y, Li X, Ge L. Comparative Secretome Analysis of Mesenchymal stem cells From Dental Apical Papilla and Bone Marrow During Early Odonto/Osteogenic Differentiation: Potential Role of Transforming Growth Factor-β2. Front Physiol. 2020;11:41. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 109. | Chen X, Yang B, Tian J, Hong H, Du Y, Li K, Li X, Wang N, Yu X, Wei X. Dental Follicle Stem Cells Ameliorate Lipopolysaccharide-Induced Inflammation by Secreting TGF-β3 and TSP-1 to Elicit Macrophage M2 Polarization. Cell Physiol Biochem. 2018;51:2290-2308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 57] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 110. | Wen X, Liu L, Deng M, Liu R, Zhang L, Nie X. In vitro cementoblast-like differentiation of postmigratory neural crest-derived p75(+) stem cells with dental follicle cell conditioned medium. Exp Cell Res. 2015;337:76-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 111. | Wen X, Nie X, Zhang L, Liu L, Deng M. Adipose tissue-deprived stem cells acquire cementoblast features treated with dental follicle cell conditioned medium containing dentin non-collagenous proteins in vitro. Biochem Biophys Res Commun. 2011;409:583-589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 112. | Liu N, Gu B, Liu N, Nie X, Zhang B, Zhou X, Deng M. Wnt/β-catenin pathway regulates cementogenic differentiation of adipose tissue-deprived stem cells in dental follicle cell-conditioned medium. PLoS One. 2014;9:e93364. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 113. | Wu J, Jia Q, He W, Liu J, Hou L, Zhang J, Niu Z, Ni L. Conditioned medium from periapical follicle cells induces the odontogenic differentiation of stem cells from the apical papilla in vitro. J Endod. 2013;39:1015-1022. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 114. | Jin S, Yang C, Huang J, Liu L, Zhang Y, Li S, Zhang L, Sun Q, Yang P. Conditioned medium derived from FGF-2-modified GMSCs enhances migration and angiogenesis of human umbilical vein endothelial cells. Stem Cell Res Ther. 2020;11:68. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 46] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 115. | Mao Q, Nguyen PD, Shanti RM, Shi S, Shakoori P, Zhang Q, Le AD. Gingiva-Derived Mesenchymal Stem Cell-Extracellular Vesicles Activate Schwann Cell Repair Phenotype and Promote Nerve Regeneration. Tissue Eng Part A. 2019;25:887-900. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 68] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 116. | Wang R, Ji Q, Meng C, Liu H, Fan C, Lipkind S, Wang Z, Xu Q. Role of gingival mesenchymal stem cell exosomes in macrophage polarization under inflammatory conditions. Int Immunopharmacol. 2020;81:106030. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 58] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 117. | Zhang Y, Shi S, Xu Q, Zhang Q, Shanti RM, Le AD. SIS-ECM Laden with GMSC-Derived Exosomes Promote Taste Bud Regeneration. J Dent Res. 2019;98:225-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 52] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 118. | Rajan TS, Diomede F, Bramanti P, Trubiani O, Mazzon E. Conditioned medium from human gingival mesenchymal stem cells protects motor-neuron-like NSC-34 cells against scratch-injury-induced cell death. Int J Immunopathol Pharmacol. 2017;30:383-394. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 41] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 119. | Rao F, Zhang D, Fang T, Lu C, Wang B, Ding X, Wei S, Zhang Y, Pi W, Xu H, Wang Y, Jiang B, Zhang P. Exosomes from Human Gingiva-Derived Mesenchymal stem cells Combined with Biodegradable Chitin Conduits Promote Rat Sciatic Nerve Regeneration. Stem Cells Int. 2019;2019:2546367. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 83] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 120. | Diomede F, Gugliandolo A, Scionti D, Merciaro I, Cavalcanti MF, Mazzon E, Trubiani O. Biotherapeutic Effect of Gingival Stem Cells Conditioned Medium in Bone Tissue Restoration. Int J Mol Sci. 2018;19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 63] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 121. | Silvestro S, Chiricosta L, Gugliandolo A, Pizzicannella J, Diomede F, Bramanti P, Trubiani O, Mazzon E. Extracellular Vesicles Derived from Human Gingival Mesenchymal stem cells: A Transcriptomic Analysis. Genes (Basel). 2020;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 52] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 122. | Wang YX, Ma ZF, Huo N, Tang L, Han C, Duan YZ, Jin Y. Porcine tooth germ cell conditioned medium can induce odontogenic differentiation of human dental pulp stem cells. J Tissue Eng Regen Med. 2011;5:354-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 123. | Huo N, Tang L, Yang Z, Qian H, Wang Y, Han C, Gu Z, Duan Y, Jin Y. Differentiation of dermal multipotent cells into odontogenic lineage induced by embryonic and neonatal tooth germ cell-conditioned medium. Stem Cells Dev. 2010;19:93-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 42] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 124. | Ye L, Chen L, Feng F, Cui J, Li K, Li Z, Liu L. Bone marrow-derived stromal cells are more beneficial cell sources for tooth regeneration compared with adipose-derived stromal cells. Cell Biol Int. 2015;39:1151-1161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 125. | Yu J, Deng Z, Shi J, Zhai H, Nie X, Zhuang H, Li Y, Jin Y. Differentiation of dental pulp stem cells into regular-shaped dentin-pulp complex induced by tooth germ cell conditioned medium. Tissue Eng. 2006;12:3097-3105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 113] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 126. | Shan T, Zhou C, Yang R, Yan F, Zhang P, Fu Y, Jiang H. Lithium chloride promotes the odontoblast differentiation of hair follicle neural crest cells by activating Wnt/β-catenin signaling. Cell Biol Int. 2015;39:35-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 127. | Yang ZH, Zhang XJ, Dang NN, Ma ZF, Xu L, Wu JJ, Sun YJ, Duan YZ, Lin Z, Jin Y. Apical tooth germ cell-conditioned medium enhances the differentiation of periodontal ligament stem cells into cementum/periodontal ligament-like tissues. J Periodontal Res. 2009;44:199-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 69] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 128. | Yang Z, Jin F, Zhang X, Ma D, Han C, Huo N, Wang Y, Zhang Y, Lin Z, Jin Y. Tissue engineering of cementum/periodontal-ligament complex using a novel three-dimensional pellet cultivation system for human periodontal ligament stem cells. Tissue Eng Part C Methods. 2009;15:571-581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 73] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 129. | Xia X, Chiu PWY, Lam PK, Chin WC, Ng EKW, Lau JYW. Secretome from hypoxia-conditioned adipose-derived mesenchymal stem cells promotes the healing of gastric mucosal injury in a rodent model. Biochim Biophys Acta Mol Basis Dis. 2018;1864:178-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 49] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 130. | Park YJ, Cha S, Park YS. Regenerative Applications Using Tooth Derived Stem Cells in Other Than Tooth Regeneration: A Literature Review. Stem Cells Int. 2016;2016:9305986. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 49] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 131. | Zhang J, An Y, Gao LN, Zhang YJ, Jin Y, Chen FM. The effect of aging on the pluripotential capacity and regenerative potential of human periodontal ligament stem cells. Biomaterials. 2012;33:6974-6986. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 128] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 132. | Alraies A, Alaidaroos NY, Waddington RJ, Moseley R, Sloan AJ. Variation in human dental pulp stem cell ageing profiles reflect contrasting proliferative and regenerative capabilities. BMC cell biology. 2017;18:12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 76] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 133. | Iohara K, Zheng L, Ito M, Ishizaka R, Nakamura H, Into T, Matsushita K, Nakashima M. Regeneration of dental pulp after pulpotomy by transplantation of CD31(-)/CD146(-) side population cells from a canine tooth. Regen Med. 2009;4:377-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 119] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 134. | Madrigal M, Rao KS, Riordan NH. A review of therapeutic effects of mesenchymal stem cell secretions and induction of secretory modification by different culture methods. J Transl Med. 2014;12:260. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 355] [Cited by in RCA: 432] [Article Influence: 39.3] [Reference Citation Analysis (0)] |
| 135. | Kingham PJ, Kolar MK, Novikova LN, Novikov LN, Wiberg M. Stimulating the neurotrophic and angiogenic properties of human adipose-derived stem cells enhances nerve repair. Stem Cells Dev. 2014;23:741-754. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 164] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 136. | Baer PC, Overath JM, Urbschat A, Schubert R, Koch B, Bohn AA, Geiger H. Effect of Different Preconditioning Regimens on the Expression Profile of Murine Adipose-Derived Stromal/Stem Cells. Int J Mol Sci. 2018;19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 137. | Hu L, Zhao J, Liu J, Gong N, Chen L. Effects of adipose stem cell-conditioned medium on the migration of vascular endothelial cells, fibroblasts and keratinocytes. Exp Ther Med. 2016;12:3137. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 138. | Iezzi I, Pagella P, Mattioli-Belmonte M, Mitsiadis TA. The effects of ageing on dental pulp stem cells, the tooth longevity elixir. Eur Cell Mater. 2019;37:175-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 38] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 139. | Stastna M, Van Eyk JE. Investigating the secretome: lessons about the cells that comprise the heart. Circ Cardiovasc Genet. 2012;5:o8-o18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 46] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 140. | Tachida Y, Sakurai H, Okutsu J, Suda K, Sugita R, Yaginuma Y, Ogura Y, Shimada K, Isono F, Kubota K, Kobayashi H. Proteomic comparison of the secreted factors of mesenchymal stem cells from bone marrow, adipose tissue and dental pulp. Journal of Proteomics & Bioinformatics. 2015;8:266-273. [RCA] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 141. | Chouaib B, Collart-Dutilleul PY, Blanc-Sylvestre N, Younes R, Gergely C, Raoul C, Scamps F, Cuisinier F, Romieu O. Identification of secreted factors in dental pulp cell-conditioned medium optimized for neuronal growth. Neurochem Int. 2021;144:104961. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |