Published online Dec 26, 2022. doi: 10.4252/wjsc.v14.i12.839
Peer-review started: September 27, 2022
First decision: October 10, 2022
Revised: October 30, 2022
Accepted: December 7, 2022
Article in press: December 7, 2022
Published online: December 26, 2022
Processing time: 84 Days and 18.3 Hours
There is still no consensus on which concentration of mesenchymal stem cells (MSCs) to use for promoting fracture healing in a rat model of long bone fracture.
To assess the optimal concentration of MSCs for promoting fracture healing in a rat model.
Wistar rats were divided into four groups according to MSC concentrations: Normal saline (C), 2.5 × 106 (L), 5.0 × 106 (M), and 10.0 × 106 (H) groups. The MSCs were injected directly into the fracture site. The rats were sacrificed at 2 and 6 wk post-fracture. New bone formation [bone volume (BV) and percentage BV (PBV)] was evaluated using micro-computed tomography (CT). Histological analysis was performed to evaluate fracture healing score. The protein expression of factors related to MSC migration [stromal cell-derived factor 1 (SDF-1), transforming growth factor-beta 1 (TGF-β1)] and angiogenesis [vascular endothelial growth factor (VEGF)] was evaluated using western blot analysis. The expression of cytokines associated with osteogenesis [bone morphogenetic protein-2 (BMP-2), TGF-β1 and VEGF] was evaluated using real-time polymerase chain reaction.
Micro-CT showed that BV and PBV was significantly increased in groups M and H compared to that in group C at 6 wk post-fracture (P = 0.040, P = 0.009; P = 0.004, P = 0.001, respectively). Significantly more cartilaginous tissue and immature bone were formed in groups M and H than in group C at 2 and 6 wk post-fracture (P = 0.018, P = 0.010; P = 0.032, P = 0.050, respectively). At 2 wk post-fracture, SDF-1, TGF-β1 and VEGF expression were significantly higher in groups M and H than in group L (P = 0.031, P = 0.014; P < 0.001, P < 0.001; P = 0.025, P < 0.001, respectively). BMP-2 and VEGF expression were significantly higher in groups M and H than in group C at 6 wk post-fracture (P = 0.037, P = 0.038; P = 0.021, P = 0.010). Compared to group L, TGF-β1 expression was significantly higher in groups H (P = 0.016). There were no significant differences in expression levels of chemokines related to MSC migration, angiogenesis and cytokines associated with osteogenesis between M and H groups at 2 and 6 wk post-fracture.
The administration of at least 5.0 × 106 MSCs was optimal to promote fracture healing in a rat model of long bone fractures.
Core Tip: This study focused on the optimal concentration of mesenchymal stem cells (MSCs) that affect fracture healing in a rat model of long bone shaft fracture. Factors related to the homing effect of MSCs, osteogenesis and angiogenesis were analyzed by in vivo (radiographic and histologic evaluation) as well as in vitro (reverse transcriptase-polymerase chain reaction and western blot analysis). Among the various concentrations used, the administration of at least 5.0 × 106 MSCs was optimal to promote the therapeutic effect on fracture healing.
- Citation: Kim MS, Chung HJ, Kim KI. Optimal concentration of mesenchymal stem cells for fracture healing in a rat model with long bone fracture. World J Stem Cells 2022; 14(12): 839-850
- URL: https://www.wjgnet.com/1948-0210/full/v14/i12/839.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v14.i12.839
Long bone fractures, such as those of the femur, tibia, and humerus, occur mainly in working-age adults[1] and are caused by high-energy trauma[2]. Long bone fractures have a high incidence of nonunion owing to the complex and specific anatomical area of the fracture[3]. Intramedullary nailing is the treatment of choice for femoral shaft fractures[4]; however, the risk of nonunion in this procedure has been reported to be up to 13%, even after nailing[5]. As femur has a significant weight-bearing role, the fracture nonunion can cause an increase in morbidity[2]. Moreover, it may be impossible for patients to return to normal daily activities after an injury[6]. Ekegren et al[3] reported that among fracture healing complications, the post-operative readmission rate was highest for femoral shaft fractures, followed by tibial shaft fractures. Among these, nonunion has been reported to be the most common cause. The nonunion rate has been reported to be up to 33% after intramedullary nailing in humerus and femur shaft fractures, and a relatively high nonunion rate of approximately 5%-7% has also been reported in tibial shaft fractures[7]. Thus, when nonunion occurs in long bone fractures, significant disability occurs and quality of life deteriorates, resulting in a high socioeconomic burden during treatment[3,7]. Therefore, preventing nonunion during the initial surgery of shaft fractures in long bones is important for improving patient prognosis.
Autologous cells with regeneration potential have emerged as a novel method to replace the standard method of bone repair[8-10]. Mesenchymal stem cells (MSCs) have the potential to promote both osteoinduction and osteogenesis[11]. Stem cell therapy using this type of cell has an important effect in promoting the bone-healing process[8,10]; some animal studies have reported that MSCs improve fracture healing[12]. Wilson et al[12] evaluated the extent of bone defect regeneration in the ramus of swine with or without MSC injection. They reported that bone healing was accelerated in a group injected with MSCs[12,13]. Obermeyer et al[13] reported that the administration of MSCs increased the volume and biomechanical strength of the callus in an alcohol-induced impaired fracture healing mouse model, resulting in accelerated fracture healing. Some previous studies have reported that injection of MSCs improves fracture healing; however, no studies have specifically reported the most effective concentration of MSC. Although a concentration of 5.0 × 106 MSCs was mainly used in several previous studies[10-12], there was no rationale for this selection. Therefore, this study aimed to confirm the ability of MSCs and assess their optimal concentration to promote fracture healing in a rat model of long bone fracture. The authors hypothesized that administration of at least 5.0 × 106 MSCs would signi
Forty-eight adult male Wistar rats (8 wk old with 200-250 g weight) were obtained from the Orient Bio Institute, Seongnam City, Gyeonggi-do, Republic of Korea. All procedures and treatments involving animals in this study followed the requirements of the Institutional Animal Care and Use Committee of the Clinical Research Institute, and the final approval was obtained from the ethics committee of Kyung Hee University Hospital at Gangdong (KHNMC AP 2020-018). The rats had free access to food and water and were bred in a controlled environment at 21 ± 2 °C with a 12-h/12-h light/dark cycle.
The femoral shaft, which is a representative long bone, was used as the fracture model in this study. Under general anesthesia, the right lower extremities of the rats were shaved and disinfected. First, the approach was performed using an anterior midline incision. After exposing the right knee joint by dislocating the patella medially, the intercondylar groove of the femur was exposed by flexion of the knee joint. An 18-gauge needle was retrogradely inserted into the center of the intercondylar groove to prevent significant displacement during the fracture. Since the proximal end of the needle protruding into the knee joint can affect the knee joint range of motion, we cut it and inserted the proximal end of the needle into the distal femur. Next, the femoral shaft was approached through a lateral approach, taking care to avoid damage to the periosteum. After applying an oscillating thin saw at a depth of 1 mm, a fracture was generated in the femoral shaft using the 3-point bending technique[14]. Sterile saline was injected into the fracture site to minimize the periosteal damage owing to heat when applying the saw. After inserting the needle tip into the fracture site, the muscular fascia was closed, the adipose-derived (AD)-MSCs were mixed with 0.3 mL sterile normal saline, and the cells were injected once directly into the fracture site. The muscular fascia was repaired before direct injection of the cell suspension to prevent AD-MSCs from flowing out. Other weight-bearing activities were unrestricted post operatively.
Human AD-MSCs (Jointstem; R-Bio, Seoul, Korea) were used in this study[15,16]. Three weeks before injection, human adipose tissue was collected by lipoaspiration using the tumescent technique. The aspirated tissue was digested with collagenase I to obtain AD-MSCs, and the digested tissue was centrifuged after removing cellular debris. The obtained pellet was resuspended in Dulbecco’s modified Eagle’s medium (DMEM; Invitrogen)-based medium containing 0.2 mmol/L ascorbic acid and 10% fetal bovine serum, and the cell suspension was recentrifuged. When the cells reached 90% confluence by resuspension and recentrifugation, they were passaged[16]. AD-MSCs at passage 3 were used in this study. AD-MSCs were prepared under Good Manufacturing Practice conditions at the Stem Cell Research Center of RNL BIO. The expanded cells were then tested for number, viability, purity, identity, and fungal, bacterial, endotoxin, and mycoplasma contamination, as suggested by the Code of Federal Regulations Title 21, before further use. Cultured AD-MSCs showed a survival rate of over 80% between 2 and 8 °C for 72 h[15]. Immediately before injection, 2.5 × 106, 5.0 × 106, and 10.0 × 106 AD-MSCs were counted using a hemocytometer[17]. The prepared AD-MSCs were injected into rats within one day of arrival at the animal laboratory.
After breeding for one week, the rats were randomly divided into four groups (n = 6 in each group): Rats injected with normal saline (C), 2.5 × 106 (L), 5.0 × 106 (M), and 10.0 × 106 (H) groups. Several studies have reported the injection of 5 × 106 MSCs into animal models[10-12]. In particular, Wilson et al[12] injected 5.0 × 106 MSCs based on the study by Hou et al[18] that concluded that > 3.0 × 106 MSCs should be injected for bone healing. Therefore, a concentration of 5.0 × 106 MSCs were used as a reference in this study. The highest concentration was set as 10.0 × 106, according to a previous study that reported that the effective dose for fracture healing was between 2.0 × 106 and 10.0 × 106 MSCs[19]. Moreover, 2.5 × 106 cells, an intermediate concentration between normal saline and 5.0 × 106 cells, was set as the lowest concentration.
At 2 and 6 wk post-fracture, the rats were sacrificed to harvest femur specimens, and the intramedullary needle was removed. Six weeks post-fracture has been reported as an important time point for fracture healing in previous studies[20-22]; hence, 6 wk after fracture was chosen in this study to evaluate the late phase of fracture healing. Wang et al[20] reported that the expression of transforming growth factor-beta 1 (TGF-β1), a chemokine that has an important effect on MSC migration, peaked at 2 wk post-fracture. Moreover, it is known that the renewal phase, in which MSCs proliferate and differentiate, usually occurs 7-10 d post-fracture[23]. In this study, the expression levels of factors related to MSC migration were also analyzed. Referring to the above studies, the early phase was set at 2 wk post-fracture.
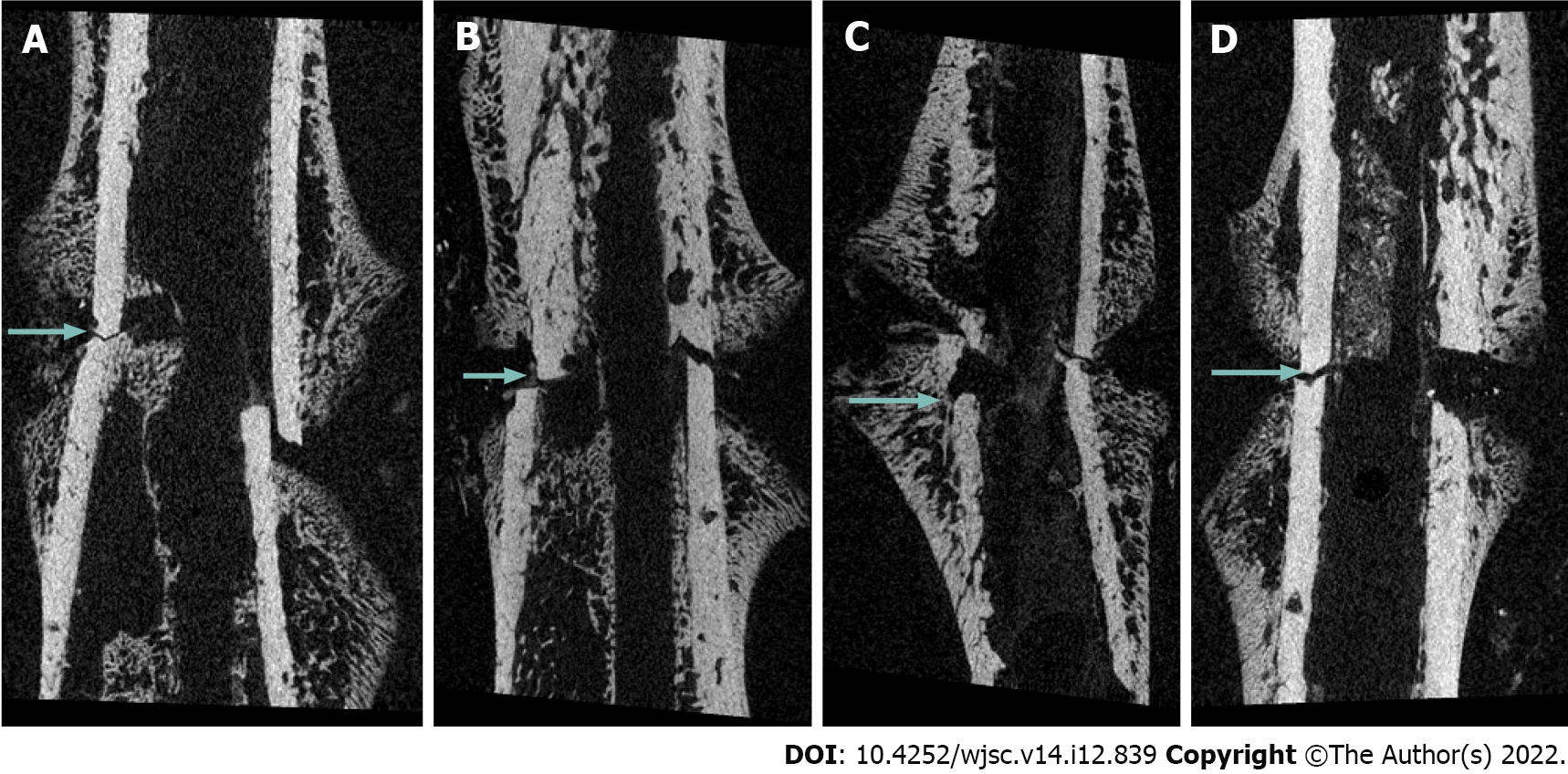
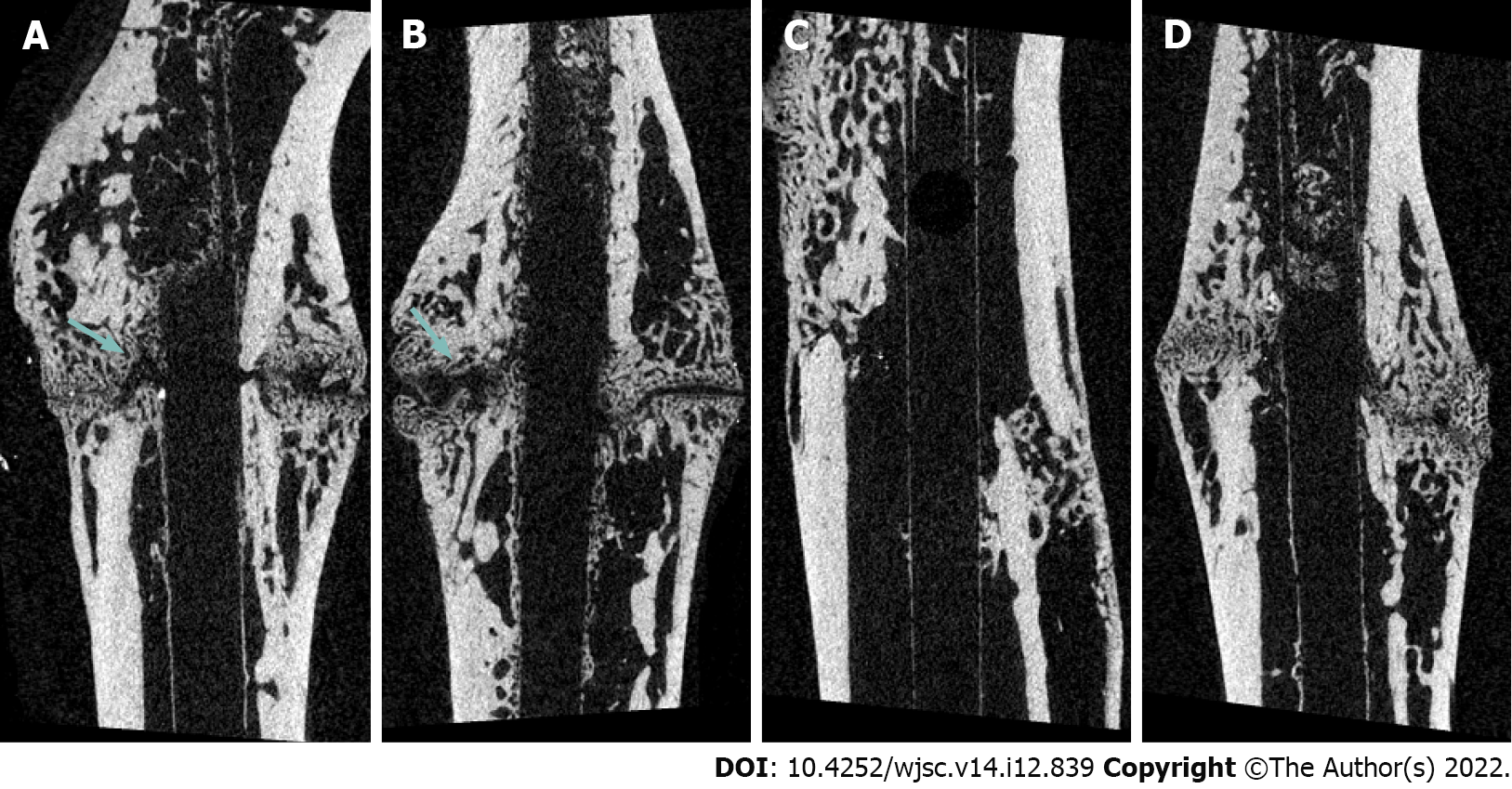
Radiologic evaluation through micro-computed tomography: Micro-computed tomography (micro-CT) was used to evaluate the volume of the newly formed callus [bone volume (BV)] and the percentage of BV [(PBV), calculated as BV/tissue volume]. A 6-mm long section centered on the fracture site was analyzed. Preexisting cortical bone and medullary canal volumes were excluded according to the method described by Wang et al[20]. The femur specimens were scanned using three-dimensional micro-focus micro-CT (Sky-Scan 1172TM, Skyscan, Kontich, Belgium) at 10 μm resolution, 440 ms exposure, 0.4° rotation step, 80 kV, and 167 μm with a 0.5 mm aluminum filter.
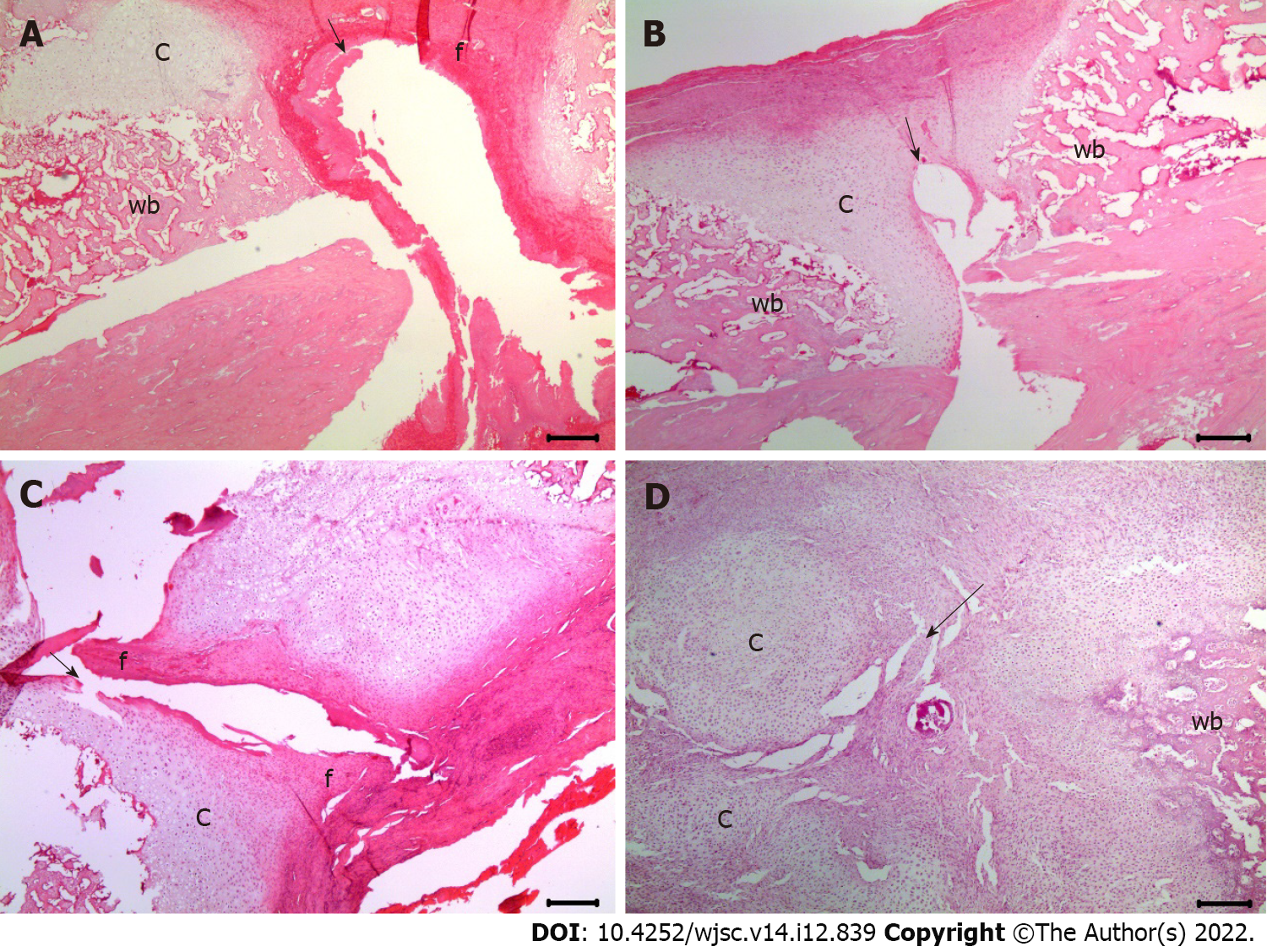
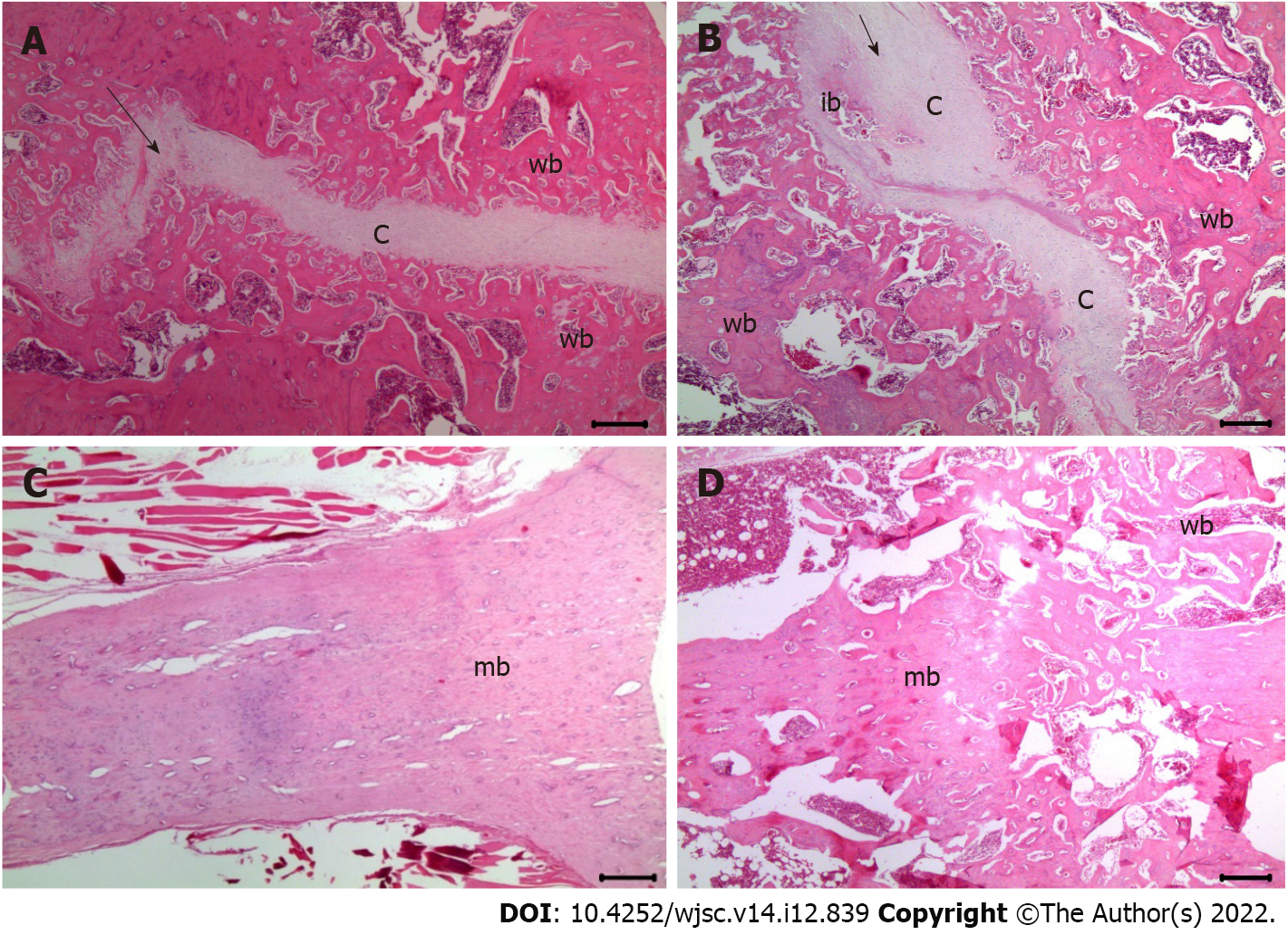
Histological evaluation: Decalcification was performed using a rapid decalcifier solution (RDO, Apex Engineering Products Corporation) at room temperature for three days. The RDO solution was replaced daily. The decalcification process and endpoint were assessed using a surgical blade and radiographic analysis, wherein in the opacity of the tissue suggested incomplete decalcification. The femur specimen was sagittally sectioned to a thickness of 3 μm and stained with hematoxylin and eosin (H&E) (Sigma-Aldrich) for histological analysis. The slides were visualized using an Olympus CX41 microscope (Olympus Co., Tokyo, Japan). Fracture healing was evaluated using a histological scoring tool for fracture healing[24] (Table 1).
| Score | Histological findings |
| 1 | Fibrous tissue |
| 2 | Predominantly fibrous tissue with small amount of cartilage |
| 3 | Equal mixture of fibrous and cartilaginous tissue |
| 4 | Predominantly cartilage with small amount of fibrous tissue |
| 5 | Cartilage |
| 6 | Predominantly cartilage with small amount of immature bone |
| 7 | Equal mixture of cartilage and immature bone |
| 8 | Predominantly immature bone with small amount of cartilage |
| 9 | Union of fracture fragments by immature bone |
| 10 | Union of fracture fragments by mature bone |
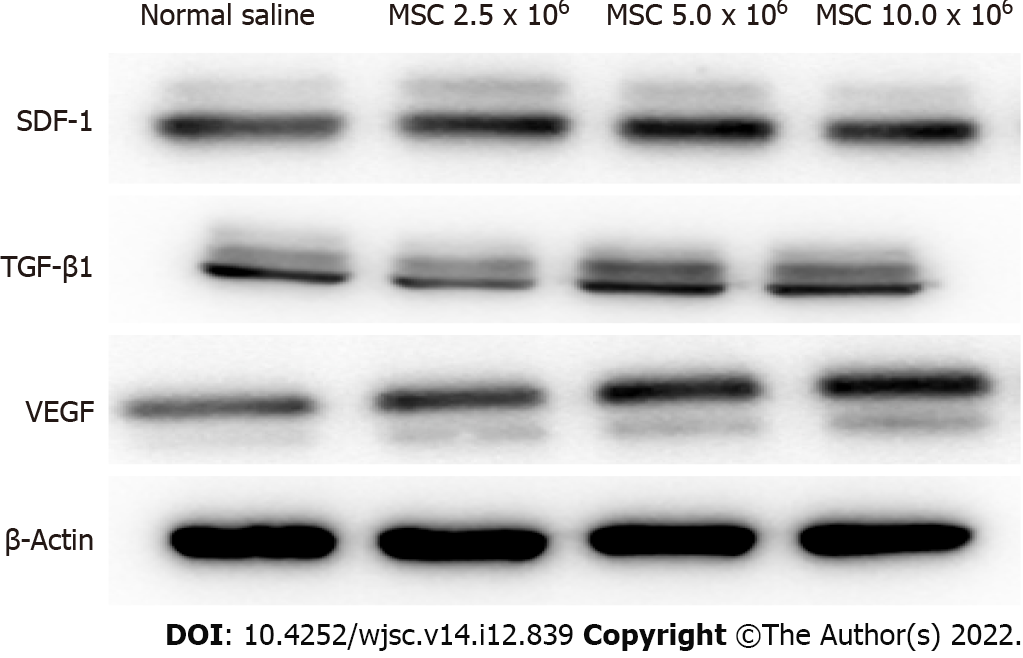
Western blot analysis: Rat femur specimens were ground in liquid nitrogen and incubated with lysis buffer containing 140 mmol/L NaCl, 50 mmol/L NaF, 1 mmol/L EDTA, 1 mmol/L Na3VO4, 1 mmol/L phenylmethylsulfonyl fluoride, 10 μg/mL aprotinin, and 1% (w/v) Nonidet P-40 in 20 mmol/L Tris-HCl (pH 7.4). Protein fractions were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis on 12% polyacrylamide gels and electrotransferred onto PVDF membranes (Bio-Rad, Hercules, CA, United States). The membranes were then blocked with Tris-buffered saline buffer consisting of 1% nonfat dry milk and 1% bovine serum albumin for 1 h. Next, membranes were then incubated overnight at 4 °C with primary antibodies against stromal cell-derived factor 1 (SDF-1) (Abcam, Cat # ab18919, 1:3000), TGF-β1 (Abcam, Cat # ab215715, 1:3000), vascular endothelial growth factor (VEGF) (Santa Cruz, SC-7269, 1:2000) and β-actin (Santa Cruz Biotechnology; 1:1000). The membranes were developed for 1 h peroxidase-conjugated anti-rabbit immunoglobulin G (Santa Cruz Biotechnology). The blots were visualized using a ChemicDoc XRS system (Bio-Rad), and protein concentrations were quantified using the Quantity One imaging software (Bio-Rad). All experiments were performed in triplicates.
Real-time quantitative polymerase chain reaction: Total RNA from the rat femur specimens was extracted using an RNeasy Mini Kit (Qiagen, Hilden, Germany), according to the manufacturer’s protocol. RNA was reverse transcribed using Superscript II reverse transcriptase (Life Technologies) at 42 °C via random hexamer priming. The quantitative polymerase chain reaction (qPCR) conditions were as follows: Pre-denaturation at 95 °C for 5 min, denaturation at 95 °C for 10 s, and annealing at 60 °C for 30 s for a total of 40 cycles, followed by fluorescence signal detection during annealing. Glyceraldehyde 3-phosphate dehydrogenase was used as an internal reference for normalization. The reactions were performed using the StepOnePlus Real-Time PCR System (Applied Biosystems, Foster City, CA, United States). The sequences of the primers used for reverse transcriptase-qPCR (RT-qPCR) are listed in Supplementary Table 1.
Statistical analysis: Based on the concentration of MSCs, one-way analysis of variance and post hoc analyses were performed to evaluate the differences in micro-CT, histological scores of fracture healing, and mRNA and protein expression (as evidenced by RT-qPCR and western blot results, respectively). Statistical significance was set P = 0.05, with 95% confidence interval. SPSS version 21.0 software (SPSS, Inc., Chicago, Illinois, United States) was used for all statistical analyses.
Fracture healing as evaluated via micro-CT analysis: At 2 and 6 wk post-fracture, BV was significantly higher in group M and H than in group C (P = 0.048, P = 0.040 and P = 0.023, P = 0.009, respectively). There was no significant difference in BV between M and H groups (P = 0.999 and P = 0.887). There was no significant difference in PBV between four groups at 2 wk post-fracture. However, PBV was significantly increased in groups M and H compared to that in group C (P = 0.004 and P = 0.001, respectively) and group L (P = 0.026 and P = 0.003, respectively) at 6 wk post-fracture. There was no significant difference in the PBV between groups M and H (P = 0.425) (Table 2, Figures 1 and 2).
| Normal saline | 2.5 × 106 | 5.0 × 106 | 10.0 × 106 | ||
| Two weeks post-fracture | BV (μm3) | 34.9 ± 3.1 | 37.3 ± 4.5 | 45.3 ± 2.9 | 45.7 ± 4.9 |
| Normal saline | - | 0.878 | 0.048 | 0.040 | |
| 2.5 × 106 | 0.878 | - | 0.137 | 0.112 | |
| 5.0 × 106 | 0.048 | 0.137 | - | 0.999 | |
| 10.0 × 106 | 0.040 | 0.112 | 0.999 | - | |
| PBV (TV/BV, %) | 7.3 ± 0.8 | 9.0 ± 0.5 | 10.8 ± 1.2 | 11.0 ± 2.6 | |
| Normal saline | - | 0.517 | 0.079 | 0.067 | |
| 2.5 × 106 | 0.517 | - | 0.509 | 0.445 | |
| 5.0 × 106 | 0.079 | 0.509 | - | 0.999 | |
| 10.0 × 106 | 0.067 | 0.445 | 0.999 | - | |
| Six weeks post-fracture | BV (μm3) | 71.3 ± 8.0 | 78.7 ± 8.8 | 101.4 ± 14.0 | 107.2 ± 6.9 |
| Normal saline | - | 0.798 | 0.023 | 0.009 | |
| 2.5 × 106 | 0.798 | - | 0.083 | 0.030 | |
| 5.0 × 106 | 0.023 | 0.083 | - | 0.887 | |
| 10.0 × 106 | 0.009 | 0.030 | 0.887 | - | |
| PBV (TV/BV, %) | 13.4 ± 2.2 | 15.0 ± 3.1 | 20.5 ± 2.2 | 23.6 ± 3.3 | |
| Normal saline | - | 0.769 | 0.004 | 0.001 | |
| 2.5 × 106 | 0.769 | - | 0.026 | 0.003 | |
| 5.0 × 106 | 0.004 | 0.026 | - | 0.425 | |
| 10.0 × 106 | 0.001 | 0.003 | 0.425 | - |
Histological scores of fracture healing evaluated using H&E staining: The formation of fibrous, cartilaginous, and immature bones was evaluated using histological scores. At 2 and 6 wk post-fracture, there was no significant difference in the fracture healing scores between groups C and L (2.8 ± 0.5 vs 3.8 ± 0.5, P = 1.000 and 5.3 ± 0.5 vs 6.5 ± 1.7, P = 1.000, respectively). Significantly more cartilaginous tissue was formed in groups M (5.5 ± 1.3) and H than in group C (P = 0.018 and P = 0.010, respectively) at 2 wk post-fracture (Figure 3). Moreover, significantly more immature bone was formed in groups M (8.8 ± 1.9) and H (8.5 ± 1.3) than in the group injected with normal saline (P = 0.032 and P = 0.050, respectively) at 6 wk post-fracture (Figure 4).
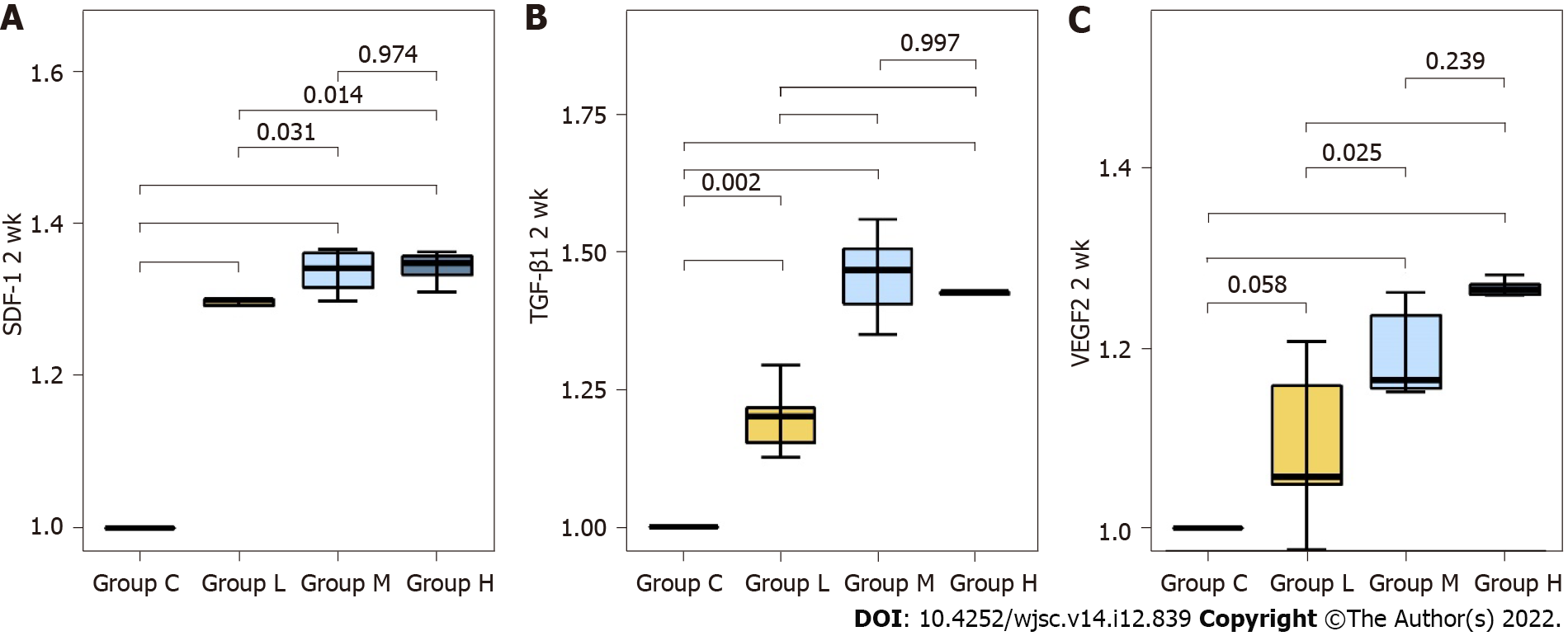
Comparison of protein expression levels of chemokines related to MSC migration and angiogenesis at 2 wk post-fracture: The fold change in mRNA expression (all reported values are fold-changes relative to the comparator) of SDF-1 was significantly higher in groups L, M, and H than in group C (P < 0.001 all). Compared to group L, groups M and H showed significantly higher SDF-1 expression (P = 0.031, P = 0.014, respectively). There was no significant difference in SDF-1 expression between groups M and H (P = 0.974). TGF-β1 expression was significantly higher in groups L, M and H than in group C (P = 0.003, P < 0.001, P < 0.001, respectively). Compared to group L, groups M and H also showed significantly higher TGF-β1 expression at 2 wk post-fracture (P < 0.001, all). There was no significant difference in TGF-β1 expression between groups M and H (P = 0.997). VEGF expression was significantly higher in groups M and H than in group C (P < 0.001, all). In addition, VEGF expression was significantly higher in groups M and H than in group L (P = 0.025, P < 0.001, respectively). There was no significant difference in VEGF expression between groups M and H (P = 0.239) (Figures 5 and 6) (Supplementary Table 2).
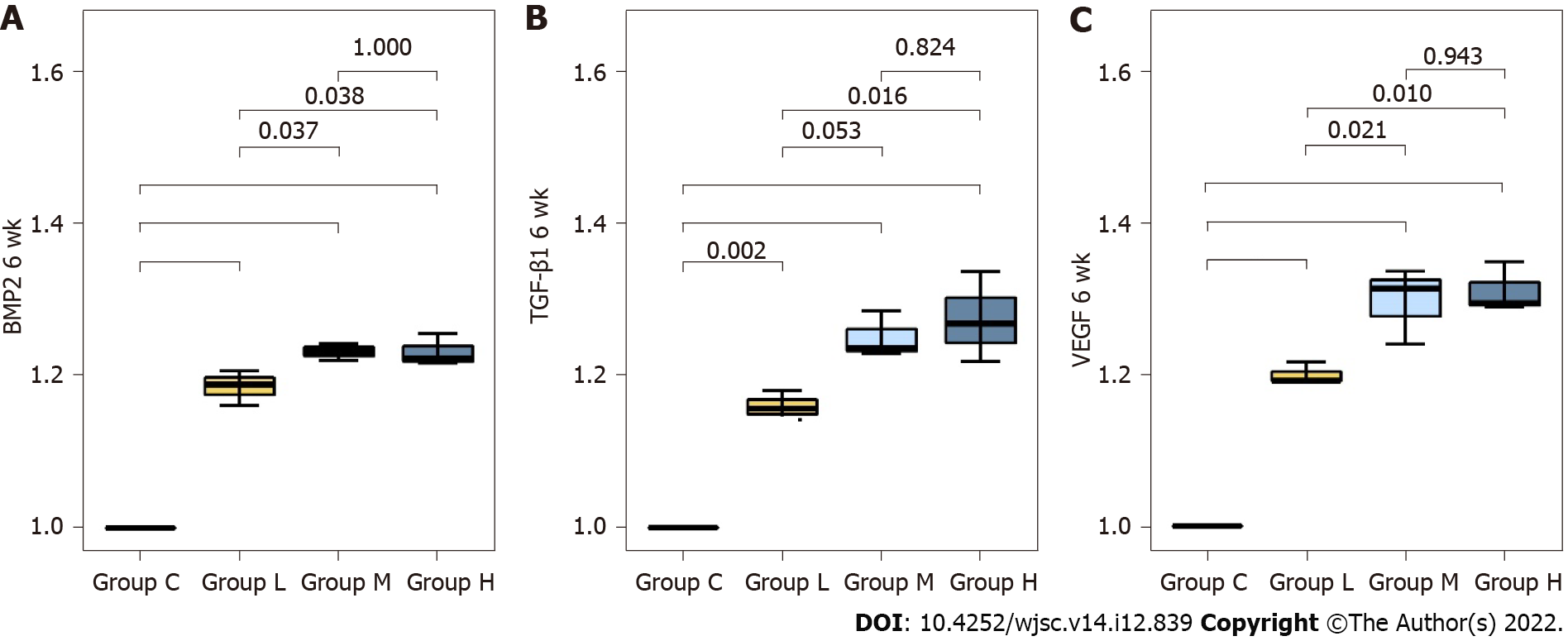
Comparison of the mRNA expression levels of osteogenesis-related factors and chemokine related to angiogenesis at 6 wk post-fracture: Bone morphogenetic protein-2 (BMP-2) expression was significantly higher in groups L, M, and H than in group C (P < 0.001, all). Compared to group L, BMP-2 expression was significantly higher in groups M and H (P = 0.037, P = 0.038, respectively). There was no significant difference in BMP-2 expression between groups M and H (P = 1.000). TGF-β1 expression was significantly higher in groups L, M and H than in group C (P = 0.002, P < 0.001, P < 0.001, respectively). Compared to group L, TGF-β1 expression was significantly higher in groups H (P = 0.016). There was no significant difference in TGF-β1 expression between groups M and H (P = 0.824).
VEGF expression was significantly higher in groups L, M and H than in group C (P < 0.001, all). In addition, VEGF expression was significantly higher in groups M and H than in group L (P = 0.021, P = 0.010, respectively). There was no significant difference in VEGF expression between groups M and H (P = 0.943) (Figure 7) (Supplementary Table 3). The protein expression levels of BMP-2, TGF- β1 and VEGF are listed in Supplementary Table 4.
In this study, fracture healing was significantly improved in the groups injected with MSCs compared with that in the control group. In addition, after injection of MSCs at different concentrations, the mRNA and protein expression of genes related to MSC migration, angiogenesis, and osteogenesis were higher in groups injected with 5.0 × 106 and 10 × 106 MSCs than in the group injected with 2.5 × 106 MSCs. This study is meaningful as it is the first animal study to confirm that an MSC concentration of 5.0 × 106 cells, which has been used in several previous studies, maximizes fracture healing.
The nonunion of long bone fractures results in a high socioeconomic burden and long treatment duration[7]. Studies aimed at enhancing fracture healing through bone regeneration[25,26] and those that used MSCs as a cell therapy have been conducted[12,13,27]. Obermeyer et al[13] compared fracture healing after the injection of MSCs and saline in an animal model of impaired fracture healing. They reported that significantly more callus formation was observed in the group injected with MSCs and that MSCs migrated and homed to the fracture site, contributing to fracture healing. However, they evaluated only the migration of labeled MSCs to the fracture site via immunofluorescence staining; they did not quantitatively evaluate the levels of factors related to MSC homing.
The homing of MSCs to the fracture site is a key mechanism during the early stages of fracture healing. After MSCs are recruited to a fracture site, they differentiate into osteogenic cells to enhance healing[28]. In addition, Caplan[29] reported that directly injected MSCs did not differentiate in the injured tissue, but homed to the injury site to secrete bioactive factors, thereby resulting in therapeutic effects. Therefore, in this study, we evaluated the homing effect by quantitatively analyzing the expression of SDF-1, which is known to be an important chemokine for the recruitment of MSCs to fracture sites[20], and TGF-β1, which is known to enhance MSC proliferation[30], using western blotting at 2 wk post-fracture. The expression of factors related to MSC homing to the fracture site was higher in the groups injected with 5.0 × 106 and 10.0 × 106 MSCs than in the control group injected with normal saline.
Known methods of injecting MSCs for fracture healing include systemic intravenous and direct injections[20,31]. Systemic MSC injection is convenient and minimally invasive[15], and repeated administration is possible[20]. Therefore, it has been used in many animal studies. Ra et al[15] evaluated the safety of systemic MSC injection in animals and humans and reported no serious side effects in any animal or patient. However, other than this study, no studies have analyzed the stability, toxicity, and possible adverse effects of systemic MSC injection. On the other hand, Galindo et al[32] suggested that systemic injection of MSCs may be associated with a high risk of side effects, and the number of cells reaching the target area may be small. The treatment of fractures often requires opening of the fracture site to fix the injured area. Therefore, in this study, the above-mentioned disadvantage of systemic injection was reflected, and direct injection of MSCs into the fracture site was performed instead.
Hou et al[18] reported that MSCs at a concentration higher than 3.0 × 106 should be administered in mice. Dreger et al[27] evaluated bone regeneration in a mouse femur shaft fracture model after the injection of 2.0 × 106 MSCs, which was less than that reported by Hou et al[27]. Dreger et al[27] reported that injected MSCs significantly accumulate at the fracture site and enhance bone regeneration. However, there is still no consensus on the optimal number of MSCs to be injected to enhance fracture healing. Janko et al[19] injected MSCs using scaffolds in a large-sized bone defect rat model and reported that a range of (2.0-10.0) × 106 MSCs was an effective dose window for fracture healing. However, they evaluated only healing at 8 wk post-fracture and did not analyze the effect of MSC concentration on fracture healing in the early phase. In addition, they analyzed only callus formation via histological analysis, not via radiologic evaluation, such as micro-CT. Furthermore, the expression of factors related to osteogenesis and angiogenesis, which are important in evaluating fracture healing, was not analyzed. In this study, fracture healing was analyzed at 2 and 6 wk post-fracture, that is, in both the early and late phases. Histological analysis, micro-CT, and the expression of factors related to MSC migration, osteogenesis, and angiogenesis were analyzed. Our results showed that fracture healing was enhanced in the groups injected with 5.0 × 106 and 10 × 106 MSCs compared to the groups injected with normal saline and 2.5 × 106 MSCs. There was no significant difference between the groups injected with 5.0 × 106 and 10 × 106 MSCs.
This study has several limitations. First, although previous reports have shown that human MSCs are safe when injected into animals[15], MSCs cultured in rats were not used in this study. However, because conventionally prepared MSCs are used, purity can be guaranteed. Second, in this study, fracture healing was compared by administering MSCs at different concentrations of 2.5 × 106, 5.0 × 106, and 10 × 106 cells; however, the criterion for determining the concentrations was ambiguous. Third, in cases of direct injection of MSCs mixed with normal saline, it may be difficult to retain MSCs at the fracture site for the long time. Additionally, we did not evaluate retention of the implanted MSC at the fracture site using fluorescence imaging analysis. Despite these limitations, this study is meaningful because it is the first animal study to analyze the optimal concentration of MSCs that maximizes the effect on fracture healing. In addition, this study could help to set the standard concentration of MSCs for evaluating fracture healing in an rat model of long bone fracture.
Direct injection of various concentrations of MSCs enhances fracture healing in a rat model of long bone fractures. Among the various concentrations used, 5.0 × 106 MSCs was optimal to promote fracture healing. Therefore, in order to evaluate the therapeutic effect on fracture healing of MSCs in a rat model of fractures, administration of at least 5.0 × 106 MSCs is suggested.
Previous studies have reported that injection of mesenchymal stem cells (MSCs) improves fracture healing. However, no studies have specifically reported the most effective concentration of MSC.
There is no consensus on which concentration of MSCs to use for promoting fracture healing in a rat model of long bone fracture.
The present study aimed to assess the optimal concentration of MSCs for promoting fracture healing in a rat model.
Wistar rats were divided into four groups according to MSC concentrations: Normal saline (C), 2.5 × 106 (L), 5.0 × 106 (M), and 10.0 × 106 (H) groups. New bone formation was evaluated using micro-computed tomography (micro-CT). Histological analysis was performed to evaluate fracture healing score. The protein expression of factors related to MSC migration and angiogenesis was evaluated using western blot analysis. The expression of cytokines associated with osteogenesis was evaluated using real-time polymerase chain reaction.
Micro-CT showed that new bone formation was significantly increased in groups M and H compared to that in group C at 6 wk post-fracture. Significantly more cartilaginous tissue and immature bone were formed in groups M and H than in group C at 2 and 6 wk post-fracture. At 2 post-fracture, the protein expression levels of factors related to MSC migration and angiogenesis were significantly higher in groups M and H than in group L. The mRNA levels of cytokines associated with osteogenesis and angiogenesis were significantly higher in groups M and H than in group C at 6 wk post-fracture. There were no significant differences between M and H groups.
Among the various concentrations used, 5.0 × 106 MSCs was the optimal concentration that promoted healing of long bone shaft fractures.
This study could help to set the standard concentration of MSCs for evaluating fracture healing in an animal model of fracture.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Cell and tissue engineering
Country/Territory of origin: South Korea
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Prasetyo EP, Indonesia; Ventura C, Italy S-Editor: Wang JJ L-Editor: A P-Editor: Wang JJ
| 1. | Bucholz RW, Jones A. Fractures of the shaft of the femur. J Bone Joint Surg Am. 1991;73:1561-1566. [PubMed] |
| 2. | Wu KJ, Li SH, Yeh KT, Chen IH, Lee RP, Yu TC, Peng CH, Liu KL, Yao TK, Wang JH, Wu WT. The risk factors of nonunion after intramedullary nailing fixation of femur shaft fracture in middle age patients. Medicine (Baltimore). 2019;98:e16559. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 3. | Ekegren CL, Edwards ER, de Steiger R, Gabbe BJ. Incidence, Costs and Predictors of Non-Union, Delayed Union and Mal-Union Following Long Bone Fracture. Int J Environ Res Public Health. 2018;15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 192] [Article Influence: 27.4] [Reference Citation Analysis (0)] |
| 4. | Ricci WM, Gallagher B, Haidukewych GJ. Intramedullary nailing of femoral shaft fractures: current concepts. J Am Acad Orthop Surg. 2009;17:296-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 169] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 5. | Song SH. Radiologic Outcomes of Intramedullary Nailing in Infraisthmal Femur-Shaft Fracture with or without Poller Screws. Biomed Res Int. 2019;2019:9412379. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 6. | Bell A, Templeman D, Weinlein JC. Nonunion of the Femur and Tibia: An Update. Orthop Clin North Am. 2016;47:365-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 98] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 7. | Rupp M, Biehl C, Budak M, Thormann U, Heiss C, Alt V. Diaphyseal long bone nonunions - types, aetiology, economics, and treatment recommendations. Int Orthop. 2018;42:247-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 144] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 8. | Henrich D. Focus on bone healing: new strategies for improvement of bone healing. Eur J Trauma Emerg Surg. 2020;46:229-230. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (1)] |
| 9. | Giuliani N, Lisignoli G, Magnani M, Racano C, Bolzoni M, Dalla Palma B, Spolzino A, Manferdini C, Abati C, Toscani D, Facchini A, Aversa F. New insights into osteogenic and chondrogenic differentiation of human bone marrow mesenchymal stem cells and their potential clinical applications for bone regeneration in pediatric orthopaedics. Stem Cells Int. 2013;2013:312501. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 67] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 10. | Oryan A, Kamali A, Moshiri A, Baghaban Eslaminejad M. Role of Mesenchymal Stem Cells in Bone Regenerative Medicine: What Is the Evidence? Cells Tissues Organs. 2017;204:59-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 251] [Article Influence: 31.4] [Reference Citation Analysis (1)] |
| 11. | Bajada S, Harrison PE, Ashton BA, Cassar-Pullicino VN, Ashammakhi N, Richardson JB. Successful treatment of refractory tibial nonunion using calcium sulphate and bone marrow stromal cell implantation. J Bone Joint Surg Br. 2007;89:1382-1386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 81] [Article Influence: 4.8] [Reference Citation Analysis (1)] |
| 12. | Wilson SM, Goldwasser MS, Clark SG, Monaco E, Bionaz M, Hurley WL, Rodriguez-Zas S, Feng L, Dymon Z, Wheeler MB. Adipose-derived mesenchymal stem cells enhance healing of mandibular defects in the ramus of swine. J Oral Maxillofac Surg. 2012;70:e193-e203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 40] [Article Influence: 3.1] [Reference Citation Analysis (1)] |
| 13. | Obermeyer TS, Yonick D, Lauing K, Stock SR, Nauer R, Strotman P, Shankar R, Gamelli R, Stover M, Callaci JJ. Mesenchymal stem cells facilitate fracture repair in an alcohol-induced impaired healing model. J Orthop Trauma. 2012;26:712-718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 2.4] [Reference Citation Analysis (1)] |
| 14. | Furuta T, Miyaki S, Ishitobi H, Ogura T, Kato Y, Kamei N, Miyado K, Higashi Y, Ochi M. Mesenchymal Stem Cell-Derived Exosomes Promote Fracture Healing in a Mouse Model. Stem Cells Transl Med. 2016;5:1620-1630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 341] [Article Influence: 37.9] [Reference Citation Analysis (0)] |
| 15. | Ra JC, Shin IS, Kim SH, Kang SK, Kang BC, Lee HY, Kim YJ, Jo JY, Yoon EJ, Choi HJ, Kwon E. Safety of intravenous infusion of human adipose tissue-derived mesenchymal stem cells in animals and humans. Stem Cells Dev. 2011;20:1297-1308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 392] [Cited by in RCA: 437] [Article Influence: 31.2] [Reference Citation Analysis (0)] |
| 16. | Lee WS, Kim HJ, Kim KI, Kim GB, Jin W. Intra-Articular Injection of Autologous Adipose Tissue-Derived Mesenchymal Stem Cells for the Treatment of Knee Osteoarthritis: A Phase IIb, Randomized, Placebo-Controlled Clinical Trial. Stem Cells Transl Med. 2019;8:504-511. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 147] [Cited by in RCA: 327] [Article Influence: 54.5] [Reference Citation Analysis (0)] |
| 17. | Green MR, Sambrook J. Estimation of Cell Number by Hemocytometry Counting. Cold Spring Harb Protoc. 2019;2019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 18. | Hou Z, Nguyen Q, Frenkel B, Nilsson SK, Milne M, van Wijnen AJ, Stein JL, Quesenberry P, Lian JB, Stein GS. Osteoblast-specific gene expression after transplantation of marrow cells: implications for skeletal gene therapy. Proc Natl Acad Sci U S A. 1999;96:7294-7299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 83] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 19. | Janko M, Pöllinger S, Schaible A, Bellen M, Schröder K, Heilani M, Fremdling C, Marzi I, Nau C, Henrich D, Verboket RD. Determination of the effective dose of bone marrow mononuclear cell therapy for bone healing in vivo. Eur J Trauma Emerg Surg. 2020;46:265-276. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 20. | Wang X, Wang C, Gou W, Xu X, Wang Y, Wang A, Xu W, Guo Q, Liu S, Lu Q, Meng H, Yuan M, Peng J, Lu S. The optimal time to inject bone mesenchymal stem cells for fracture healing in a murine model. Stem Cell Res Ther. 2018;9:272. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 42] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 21. | Gorter EA, Hamdy NA, Appelman-Dijkstra NM, Schipper IB. The role of vitamin D in human fracture healing: a systematic review of the literature. Bone. 2014;64:288-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 87] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 22. | Einhorn TA, Gerstenfeld LC. Fracture healing: mechanisms and interventions. Nat Rev Rheumatol. 2015;11:45-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1101] [Cited by in RCA: 1157] [Article Influence: 115.7] [Reference Citation Analysis (0)] |
| 23. | Majidinia M, Sadeghpour A, Yousefi B. The roles of signaling pathways in bone repair and regeneration. J Cell Physiol. 2018;233:2937-2948. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 294] [Article Influence: 36.8] [Reference Citation Analysis (0)] |
| 24. | Oetgen ME, Merrell GA, Troiano NW, Horowitz MC, Kacena MA. Development of a femoral non-union model in the mouse. Injury. 2008;39:1119-1126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 42] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 25. | Gómez-Barrena E, Rosset P, Müller I, Giordano R, Bunu C, Layrolle P, Konttinen YT, Luyten FP. Bone regeneration: stem cell therapies and clinical studies in orthopaedics and traumatology. J Cell Mol Med. 2011;15:1266-1286. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 101] [Cited by in RCA: 85] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 26. | Gómez-Barrena E, Padilla-Eguiluz N, Rosset P, Gebhard F, Hernigou P, Baldini N, Rouard H, Sensebé L, Gonzalo-Daganzo RM, Giordano R, García-Rey E, Cordero-Ampuero J, Rubio-Suárez JC, García-Simón MD, Stanovici J, Ehrnthaller C, Huber-Lang M, Flouzat-Lachaniette CH, Chevallier N, Donati DM, Spazzoli B, Ciapetti G, Fleury S, Fernandez MN, Cabrera JR, Avendaño-Solá C, Montemurro T, Panaitescu C, Veronesi E, Rojewski MT, Lotfi R, Dominici M, Schrezenmeier H, Layrolle P. Early efficacy evaluation of mesenchymal stromal cells (MSC) combined to biomaterials to treat long bone non-unions. Injury. 2020;51 Suppl 1:S63-S73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 44] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 27. | Dreger T, Watson JT, Akers W, Molligan J, Achilefu S, Schon LC, Zhang Z. Intravenous application of CD271-selected mesenchymal stem cells during fracture healing. J Orthop Trauma. 2014;28 Suppl 1:S15-S19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 28. | Li WY, Xiong H. [Study on MSCs homing and its research on osteodiseases]. Zhongguo Gu Shang. 2020;33:689-692. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 29. | Caplan AI. Mesenchymal Stem Cells: Time to Change the Name! Stem Cells Transl Med. 2017;6:1445-1451. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 558] [Cited by in RCA: 730] [Article Influence: 91.3] [Reference Citation Analysis (0)] |
| 30. | Sarahrudi K, Thomas A, Mousavi M, Kaiser G, Köttstorfer J, Kecht M, Hajdu S, Aharinejad S. Elevated transforming growth factor-beta 1 (TGF-β1) levels in human fracture healing. Injury. 2011;42:833-837. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 84] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 31. | Zhang Y, Hao Z, Wang P, Xia Y, Wu J, Xia D, Fang S, Xu S. Exosomes from human umbilical cord mesenchymal stem cells enhance fracture healing through HIF-1α-mediated promotion of angiogenesis in a rat model of stabilized fracture. Cell Prolif. 2019;52:e12570. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 137] [Cited by in RCA: 207] [Article Influence: 34.5] [Reference Citation Analysis (0)] |
| 32. | Galindo S, de la Mata A, López-Paniagua M, Herreras JM, Pérez I, Calonge M, Nieto-Miguel T. Subconjunctival injection of mesenchymal stem cells for corneal failure due to limbal stem cell deficiency: state of the art. Stem Cell Res Ther. 2021;12:60. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 42] [Article Influence: 10.5] [Reference Citation Analysis (0)] |