Published online Sep 26, 2021. doi: 10.4252/wjsc.v13.i9.1215
Peer-review started: February 27, 2021
First decision: May 5, 2021
Revised: May 20, 2021
Accepted: August 30, 2021
Article in press: August 30, 2021
Published online: September 26, 2021
Processing time: 202 Days and 10.3 Hours
Neurodegenerative disease is a brain disorder caused by the loss of structure and function of neurons that lowers the quality of human life. Apart from the limited potential for endogenous regeneration, stem cell-based therapies hold considerable promise for maintaining homeostatic tissue regeneration and enhancing plasticity. Despite many studies, there remains insufficient evidence for stem cell tracing and its correlation with endogenous neural cells in brain tissue with three-dimensional structures. Recent advancements in tissue optical clearing techniques have been developed to overcome the existing shortcomings of cross-sectional tissue analysis in thick and complex tissues. This review focuses on recent progress of stem cell treatments to improve neurodegenerative disease, and introduces tissue optical clearing techniques that can implement a three-dimensional image as a proof of concept. This review provides a more comprehensive understanding of stem cell tracing that will play an important role in evaluating therapeutic efficacy and cellular interrelationship for regeneration in neurodegenerative diseases.
Core Tip: Although the use of stem cells in neurodegenerative disease has become widespread, a proof of concept (PoC) for three-dimensional analysis of the interrelationships in brain structure has not been performed in vivo. This review will introduce recent stem cell research for therapies and PoC for a three-dimensional analysis based on tissue optical clearing.
- Citation: Kim IK, Park JH, Kim B, Hwang KC, Song BW. Recent advances in stem cell therapy for neurodegenerative disease: Three dimensional tracing and its emerging use. World J Stem Cells 2021; 13(9): 1215-1230
- URL: https://www.wjgnet.com/1948-0210/full/v13/i9/1215.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v13.i9.1215
Most brain disorders lead to irreversible consequences in intra- and inter-cellular responses depending on their severity, which commonly causes deterioration of physical or intellectual function. In general, it is known that the adult central nervous system is not capable of neurogenesis, but recent research on stem cells has negated this precept[1,2]. To improve neural regeneration to replace damaged neural cells and/or re-establish dendritic connections, two basic strategies have been established over the past two decades[3-5]. First, endogenous neural stem cells (NSCs) participate in the self-repair process in the subventricular zone (SVZ) lining the lateral ventricles and the subgranular zone within the dentate gyrus of the hippocampus, despite limitations in cell number and regenerative ability[6]. Even if symptomatic treatment is performed when the boundaries of endogenous regeneration in the brain are crossed, they have limited implications, including sustainability and efficiency in the repair of neurodegenerative diseases. Second, exogenous transplantation of stem cells is expected to become a source of neurogenesis. Stem cells derived from pluripotent ‘embryonic’ stem cells (ESCs), which are more lineage-committed reprogrammed ‘embryonic-like’ pluripotent stem cells (PSCs) have been used as a therapeutic source in neurodegenerative diseases[7,8]. The fundamental mechanism underlying all therapies is a positive regulation of progressive loss of brain structure, function, or neuronal survival. Although stem cell-derived NSCs or neural progenitors can affect cell replacement therapy, direct transplantation of stem cells or stem cell-free therapy is mostly known to be exerted through paracrine effects, including cytokines, chemokines, and neurotrophic factors at the molecular level[9-11]. Unfortunately, the therapy currently available does not fully account for the mechanism of stem cell function in vivo, and it does not prove the relationship between exogenous stem cells and existing brain cells from the site of neurodegenerative disease[12]. To analyze an interconnected network with a molecular biological approach, an integrative descri
This review will focus on recent improvements of stem cell therapy for neurodegenerative disease, the methodological approach of cell tracking for the definition of stem cell proof-of-concept (PoC), and on the advanced technique of cell tracking for a three-dimensional structure description after stem cell treatment. This clarification will influence future studies by providing insights into the three-dimensional structure approach of stem cell tracing for many therapies of neurodegenerative diseases.
Neurodegenerative diseases are mainly classified by clinical characteristics which are based on major symptoms and the site of involvement, including Alzheimer’s disease (AD), Parkinson’s disease (PD), and Huntington’s disease (HD). AD and HD are caused by neuronal loss in the brain, and PD is known to involve a specific local loss of dopaminergic neurons in the substantia nigra of the brain[13]. In fact, AD is the most common neurodegenerative disease, usually chronic and progressive, showing a decline in intellectual function, such as memory, judgment, and language skills, and impairments in daily life ability, personality, and behavior. HD is also accompanied by abnormal behavioral movements and cognitive impairment. PD is a degenerative neurological disease with the second highest incidence after Alzheimer’s disease, which is chronically manifested, as well as movement disorders such as tremors or paralysis of the arms, legs, and face, stiffness, stiffness, and postural instability, as well as pain, depression, and dementia. Although there are limited treatment options, the viability of cell therapy treatment has been the focus of recent research.
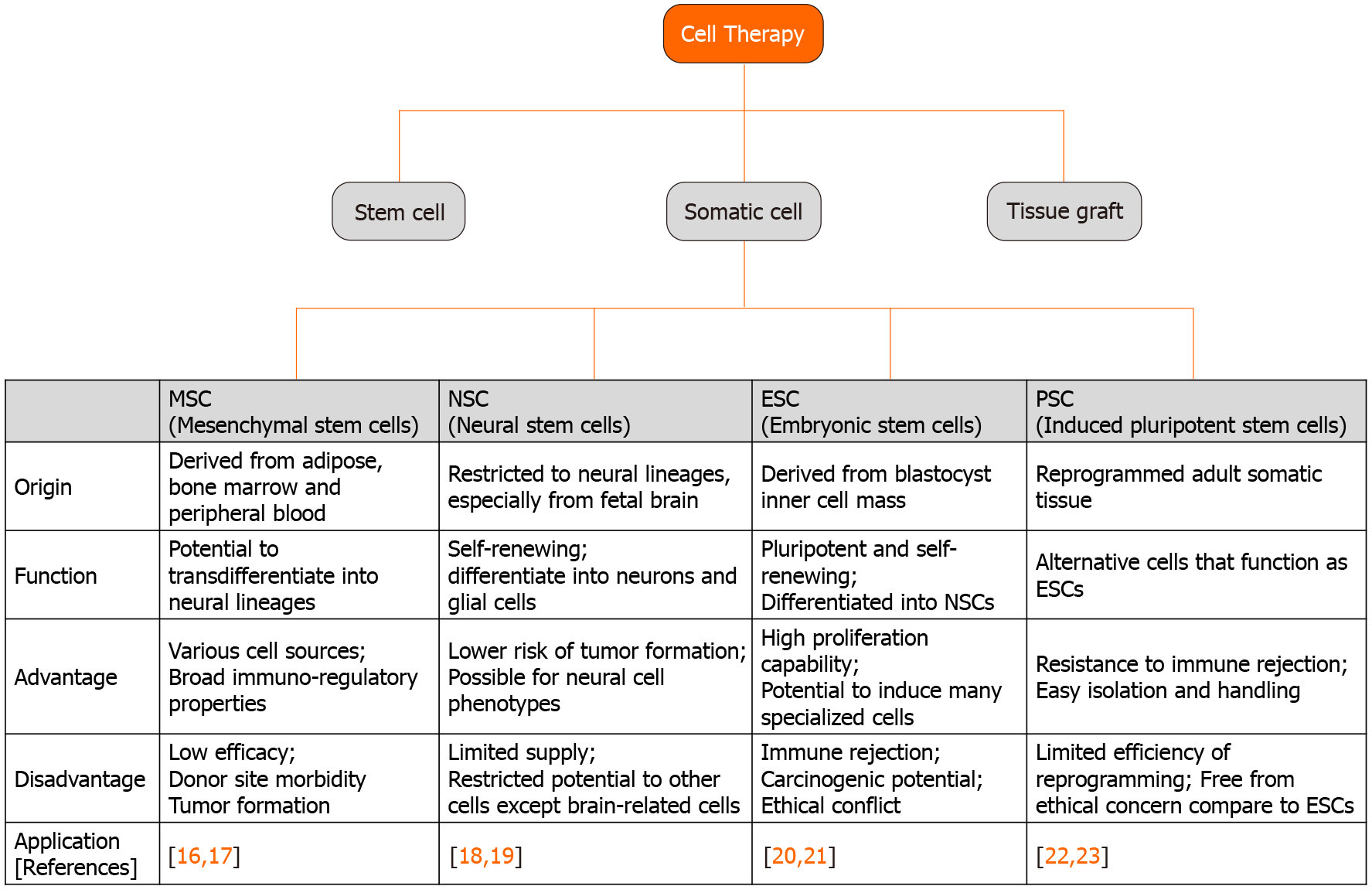
Stem cells were discovered in the early 1960s and are generally capable of continuous self-renewal and have the ability to differentiate into several types of cell lineages[14,15]. Stem cells include ESCs, progenitor cells, mesenchymal stem cells (MSCs), and PSCs, and are classified as totipotent, pluripotent, or multipotent according to their differentiation ability. Totipotent stem cells such as ESCs and PSCs can be isolated from the four cell stages of the embryo and can differentiate into all types of cells in the body, including tissues outside the embryo. Multipotent stem cells such as MSCs and progenitor cells can be isolated from various tissues in the adult human body and can differentiate into various cells, but only those of a closely related family type of cells. In recent years, the development of stem cell technology has expanded to many human body tissues, including treatment for degenerative neurological diseases using stem cells. The application fields of stem cells used in the treatment of neurodegenerative diseases are shown in Figure 1[16-23]. Because organizations with ineffective recovery systems cannot easily return after injury or extensive degenerative events, it is important to understand the characteristics of the available stem cell type and the specific mechanisms of neurodegenerative diseases, including AD, PD, and HD[12].
The 2018 Global Alzheimer's Disease Report stated that 50 million people worldwide have the disease, and it is the most common cause of dementia, accounting for 50%-70% of cases of dementia cases[24,25]. AD has been shown to cause intracellular formation of nerve fiber tangles caused by the deposition of β-amyloid (Aβ) peptides on the extracellular matrix between neurons and the accumulation of hyperphosphorylated tau proteins in neurons[26].
MSCs play a major role in the treatment of AD, such as immune regulation, reduction of Aβ plaque burden through internalization and Aβ degradation of endosomal–lysosomal pathway oligomers and neurotrophic/regenerative potential[25,27]. Injection of green fluorescent protein (GFP)-labeled bone marrow (BM) MSCs in the hippocampus of an AD animal model has been shown to reduce the size of Aβ plaques and regulate functional immunity[28]. Transplantation of MSCs was shown to increase neurogenesis as demonstrated by immunostaining brain sections with an anti-polysialylated form of the neural cell adhesion molecule and doublecortin antibodies[29]. It was also confirmed that MSCs labeled with PKH26-111 were injected into AD mice through the tail vein to reach the brain, and the radioactivity of BMSCs was significantly higher in the AD model than in the control group in the gamma counter and gamma camera imaging[30]. The effect of intravenous injection of BM MSCs in a mouse model of Alzheimer's disease was confirmed through β-amyloid positron emission tomography imaging, memory function studies, and histopathological evaluation[31]. Another technique for tissue repair involves paracrine effects using the secretion of extracellular vesicles from MSCs. The secretion of MSC extracellular vesicles can target Aβ deposition and is being studied as an important method for AD treatment, including siRNA and enzymes[29,32,33]. MSC-derived cytokines and vas
The mammalian brain has the capacity to repair itself through neurogenesis and gliogenesis to a limited degree; however, endogenous neurogenesis and gliogenesis decrease significantly with age and are unable to regenerate enough brain cells alone. Research using NSCs that express a phenotype similar to that of brain cells has great potential in the treatment of AD. Several recent studies have shown that NSCs can increase the survival and regeneration of endogenous neurons by producing neuro
Significant experimental and clinical progress has been made with PSCs since they were discovered 10 years ago. They are now widely used in the treatment of AD to regulate endogenous neurogenesis, neuronal loss, and pathological changes. Administration of PSCs derived from mouse skin fibroblasts by treating protein extracts of ESCs has been shown to mitigate plaque deposition and cognitive dysfunction in a 5XFAD transgenic mouse model[39]. From human sources, PSC-or PSC-derived cells have been used to ameliorate degenerative disorders. Human iPSC-derived macrophage-like cells genetically modified to express neprilysin-2 or to mutate Tau Ex10+16, Aβ-degrading activity, differentiated into functional neurons, and reduced Aβ levels after xenograft administration to the 5XFAD or APP PS1 tg/wt NOD-SCID transgenic AD mouse model[40,41].
PD is a common neurodegenerative disease characterized by impaired motor function, which is known to be caused by the selective loss of dopamine (DA) neurons in the human midbrain. Various studies have been conducted extensively on both motor and non-motor deficits. Cognitive impairment begins to develop motor impairment at an early stage and continues to progress. Non-motor symptoms are also a cause of deterioration in the quality of life of patients and treatments that can resolve cognitive impairment and dysfunction may be possible. Stem cells are generally used to consider neuroprotection, neuroplasticity, and immunomodulatory properties in PD pathogenesis.
Transplantation of human MSCs into 6-hydroxydopamine (6-OHDA)-induced lesions protected dopaminergic neurons and induced neurogenesis, resulting in therapeutic effects due to the release of soluble factors such as brain-derived neuro
NSC transplantation allowed parkinsonian rats to be recovered through the regulation of SDF-1/chemokine receptor 4 (CXCR4) expression. Intraperitoneal injection of the CXCR4 antagonist, AMD3100, increased mRNA and protein expression of SDF-1 and CXCR4 in the NSC-transplanted site of the right substantia nigra. Furthermore, apomorphine-induced rotational behavior was reduced significantly in a rat model of PD[48]. In the xenograft model, the characterization of PD sites was examined using a high-throughput quantitative proteomic approach at the SN, striatum, olfactory bulb, and SVZ after human NSC treatment. These effects demon
In a study conducted 20 years ago, transplantation of low-dose undifferentiated mouse ESCs into mice increased the proliferation of differentiated DA neurons and restored cerebral function in a PD animal model[51]. Another study demonstrated that a highly enriched population of midbrain neural stem cells derived from ESCs improved the electrophysiological and behavioral properties in a rodent model of PD[52,53]. Recently, studies on ESC-derived DA neurons capable of translational use have been actively conducted. Using a two-step WNT signaling activation strategy, human ESCs were induced to midbrain DA neurons at the clinical-grade level, and engraftment of these cells upregulated their behavioral recovery of amphetamine-induced rotation in a 6-OHDA model[54]. Furthermore, clinical-grade midbrain DA neurons, named MSK-DA01, safely demonstrated survival of the transplanted cells and behavioral amelioration in parkinsonian rats under GLP conditions without adverse effects[55]. PSCs were also examined for the improvement of PD at the pre-clinical level. In a primate PD model, hPSC-derived DA neurons showed an improvement in long-term survival of cells and spontaneous movement, dopaminergic progenitors derived from a clinical-grade human PSC line were produced, and their therapeutic effects were confirmed in 6-OHDA-lesioned rats[56,57].
HD is a common degenerative brain disease with autosomal dominant inheritance. It is the least researched of the three major neurodegenerative diseases[58]. HD, characterized by progressive neuronal death, has various symptoms such as cognitive decline, behavioral changes, motor dysfunction, weight loss, sleep disturbance, and mental disorders[59]. This situation begins in the striatal part of the basal ganglia by increasing the number of CAG repeats in exon 1 of the huntingtin (HTT) gene, which encodes the huntingtin protein, leading to an atypically long polyglutamine region at the protein N-terminus[60]. Numerous therapies are aimed at slowing disease sym
MSCs are a promising HD treatment because they are not only simple to acquire and cultivate, but also have unique nutritive activity and immunomodulatory functions. Simple treatment of human MSCs has been demonstrated to enhance neural differentiation capacity, neurotrophic factor stimulation, and anti-apoptotic effects using the R6/2-J2 animal model. Transplanted MSCs can integrate with host cells to increase the level of secretory factors such as von Willebrand factor, SDF-1, and CXCR4[62]. Both intranasal deliveries showed the possibility of improving the thera
Pluripotent cell lines targeting HD have been developed by several research groups. The QA-lesioned HD rat model was monitored using an apomorphine-induced behavioral test and immunohistochemical staining after implantation of BDNF-overexpressing human NSCs (HB1.F3.BDNF) on the opposite side of the striatum. NSC PoC confirmed that the transplanted cells were moved to the QA lesion site with striatal GABAergic medium spiny neurons, containing DARPP-32 in the host brain[69]. A clonal conditionally immortalized NSC line (CTX0E03), which already showed safety and efficacy signals in patients with chronic ischemic stroke, was examined using the QA-lesioned HD model. Thirteen weeks post-transplantation, CTX0E03 survived in the striatum and cortex of the brain with QA lesions, differentiated into striatal neurons, and showed progenitor-palatal connections with the host tissue. Survived CTX0E03 reduced gliosis and host immune responses, but increased endogenous neurogenesis and angiogenesis[70]. The same research group also demonstrated the therapeutic potential of PSCs in a rodent model of HD. NSCs derived from a human PSC line (1231A3-NPCs) also showed reconstruction of the damaged neuronal connections and behavioral improvement for 12 wk post- transplantation[71]. Human embryonic stem cell-derived NSC lines were also reported in a therapeutic study in the striatum of R6/2 mouse HD fragment model (first confirmation) to confirm the efficacy of improving motor deficits and rescue synaptic alterations. The second confirmation for improving motor and late cognitive impairment was done using the Q140 knock-in mouse HD model[72]. Combination therapy related to transduction of HTT gene-regulated PSCs was also conducted. PSCs derived from fibroblast/dental pulp of wild or HD rhesus monkeys were transfected with shRNA targeting the HTT transcript and transplanted into the N171-82Q mouse model. The mutant HTT-PSC-transplanted group was encouraged in their lifespan counterpart, with motor function and pathological changes, including integration and differentiation[73].
To track transplanted stem cells, many preclinical studies on brain injury use bromodeoxyuridine, PKH26, and 4,6-diamidino-2-phenylindole as fluorophores[74-76]. Stem cells pre-labeled with fluorophores can be identified via immunohistochemistry in fixed tissue using anti-fluorescent-tagged antibodies or staining methods that use color-changing substrates. At present, histology is the gold standard to test whether transplanted stem cells survive or differentiate into tissue cells in an animal model study[77]. However, this requires the sacrifice of numerous animals and provides no longitudinal or whole-body monitoring. With a lack of information on stem cell behavior, in vivo longitudinal, non-invasive, and repeatable methods have been developed to monitor transplanted cells. In addition, it is crucial to track the capabilities of transplanted stem cells to reconstruct brain functions and biological roles.
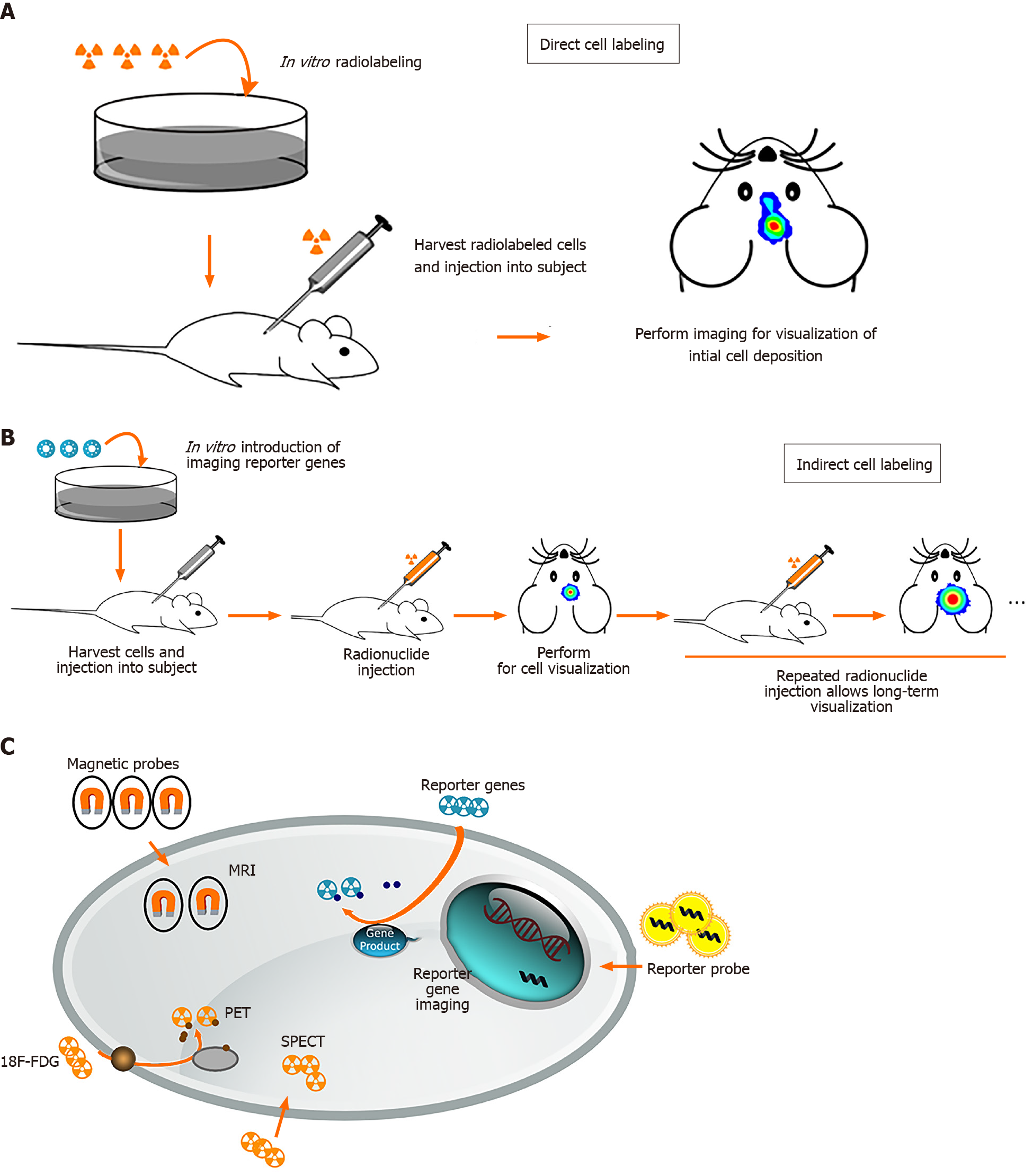
Stem cell imaging methods can be divided into direct and indirect cell labeling depending on the possibility of re-imaging over a long period of time. Direct cell labeling is the most frequently used method and consists of incubation prior to implantation and labeling cells in vitro using reporter probes containing fluorophores, radiotracers, or paramagnetic nanoparticles (Figure 2)[78,79-81]. These reporter probes can bind to specific epitopes on the cell membrane, such as copper-64-labeled antibody or zirconium-89-desferrioxamine-NCS (89Zr-DBN)[82,83], or can be absorbed by passive diffusion or transporters such as indium-111- and 89Zr-oxine or 2-[18F]-fluoro-2-deoxyglucose (18F-FDG)[30,79,84]. After incubation, cells are injected in vivo for monitoring by magnetic resonance imaging (MRI), positron emission tomography (PET), single photon emission computed tomography (SPECT), and optical imaging.
The first study of MR tracking of progenitor cells transplanted into the central nervous system was reported in 1992, and a superparamagnetic contrast agent was used for imaging rat brain cells[85]. Direct tracking through MRI offers benefits such as morphological characterization, high spatial resolution, lack of radiation, and long-term stem cell monitoring[86,87]. MRI requires the use of a contrast agent to visualize cells. For example, superparamagnetic iron oxide nanoparticles (SPIONs) have been shown to allow in vivo maintenance of neural progenitor cell viability, phenotype, proliferation, and differentiation[88,89]. Successful labeling of MSCs and progenitor cells with SPION has also been demonstrated in long-term, multimodal imaging and found no consequences on viability, differentiation capacity, or biological characteristics[90-92].
However, there are two limitations in labeling stem cells with magnetic contrast agents. After transplantation, the label was diluted because the stem cells continued to proliferate rapidly. Therefore, the MR signal decreased and lost rapidly over time because of cellular proliferation. In addition, SPIONs can be deposited in extracellular tissues when dead transplanted cells are entrapped by immune cells such as microglia in the central nervous system, leading to false signals in MRI[93].
PET and SPECT are nuclear medicine imaging techniques that represent promising imaging modality for tracking stem cells widely used in experimental trials. Before stem cells were transplanted into the host, radiotracers such as 18F-FDG, lipophilic 99mTc-D,L-hexamethylene-propyleneamine oxime, and 111In-oxine are required to label the stem cells to detect the transplanted cells via PET or SPECT scanner[79,94-96]. There was no difference in viability or differentiation ability after labeling with radiotracers. No microstructural changes were observed. The positron emitted from the radioactive isotope rapidly loses kinetic energy while traveling through the surrounding tissue, and then interacts with the electron to emit two high-energy photons of 511keVat (high-frequency photons) moving in almost the opposite direction. PET camera scanners can detect and image these photons. SPECT is very similar to PET in the use of radioactive tracers and the detection of gamma rays. These methods provide sensitivity in the picomolar range and the ability to use the same tracer across multiple species. SPECT imaging has the added advantage of having a lower false-positive signal compared to MRI. However, they do not provide anatomical information and must be used in conjunction with MRI, computerized tomography, or X-rays[97].
Optical imaging, compared to MRI, PET, and SPET, has the benefits of lower cost, rapid acquisition, no radiation toxicity, and relatively high sensitivity[98]. Semiconductor nanocrystals, also known as quantum dots (QDs), are a new class of biocompatible fluorescent materials that are relatively photostable and have a narrow luminescence band used for cell tracking. Near-infrared-emitting QDs may be particularly useful for tracking transplanted cells in the human brain, because longer wavelengths allow easier penetration of tissues such as bone and skin[99]. Bioluminescence imaging (BLI) has been widely applied in preclinical studies of stem cell imaging in the brain for several years. BLI has also been used to quantify gene expression and stem cell localization in mice and rats[100,101]. BLI is only limited to small animals, but not to large animals, because BLI can only penetrate a few centimeters of tissue.
Indirect cell labeling was modified by inserting an exogenous reporter gene into the cells. These reporter genes can produce specific proteins that function as radioactive probes, so the probe signal is not limited to the half-life of the tracer used and can be detected by PET, SPECT, and MRI for a long time. It not only allows long-term nonin
Tissue imaging techniques for the depiction of three-dimensional structures and their molecular information are a growing trend that researchers need to facilitate volu
| Hydrogel-based method | Solvent-based method | Aqueous-based method | |
| Types | CLARITY, MAP, SHIELD, PACT, PARS | 3DISCO, iDISCO, BABB | FocusClear, CUBIC, Scale12, SeeDB2 |
| Component | FocusClear/80% glycerol or histodenz | Benzyl/alcohol series | Urea, glycerol or sucrose |
| Process | Hydrogel monomer infusion → hydrogel-tissue hybridization → Electrophoretic tissue clearing | Dehydration with lipid solvation → Clearing by RI matching | Decolorization by pigment removal → Delipidation using mild detergents → Expansion and RI matching |
| RI match | 1.38–1.48 | 1.44–1.56 | 1.38–1.48 |
| Features | Minimizing structural damage and loss of biomolecules | Fast and easy clearing. Permanent preservation of the endogenous fluorescent signal | Biocompatibility, biosafety and preservation of protein function. Penetrating more rapidly and deeply into tissues |
| Limitations | Expansion of tissue size. Longer incubation | Toxic nature of many solvents, substantial shrinkage of tissue (up to 50%) | Expansion of tissue size. Longer incubation |
| References | [107,109] | [110,111] | [112,113] |
Intact tissue clearing methods continue to grow for three-dimensional imaging of the brain, centered on labeling options and imaging analysis tools. It is expected that this process may prove the discovery of novel physiological and pathological mecha
There are no PoC studies of three-dimensional stem cell tracing for treatment of neurodegenerative diseases. The only research on the existence of stem cells represented the spatial relationship with endogenous Gli1 positive MSCs in adult calvarial bones during postnatal craniofacial development, and indicated the osteogenesis mechanism for craniofacial research using the bone specific poly (ethylene glycol)- associated solvent system tissue clearing method[117].
The identification of the transplanted stem cells that can participate in the specific circuit and the host neurons that provide inputs to them may be critical for successful cell tracing for stem cell-based therapies for neurological disorders. To trace full or limited area projections in the brain, researchers need to be complemented by labeling or genetic manipulation in vivo before stem cell transplantation and by using high-resolution image system including in vivo multi-photon or light-sheet microscopy[118]. Neuro-specific proteins, DNA/RNA-conjugated fluorescent dyes, and viral/non-viral constructs have been used to explore the connectivity between reciprocal hosts and stem cell grafts[119]. For reliable three-dimensional analysis, the membrane-bound protein-specific phenotype of stem cells and the target circuits with strong can be set and verified in a genetic animal model of neurodegenerative disease[120,121]. Furthermore, stem cell labeling based on gene delivery can be important to define the correlation analysis in three dimensions between PoC phenotypes of endogenous or exogenous stem cells, state-modified/unmodified proteins, and state-altering genes to understand the physiology and pathology of degenerative brains[108,122].
For many decades, appropriate cell tracing strategies for PoC and the connectivity between host neurons and grafted stem cells have been observed using traditional two-dimensional tracing techniques. Through the development of tissue optical clearing techniques and their convergence technologies, however, it is possible to demonstrate tracing in three dimensions and to analyze the molecular pathological changes associated with endogenous cells functioning in neurodegenerative diseases. Studies on the ability of three-dimensional host-graft integration in diseases will help to serve from the basic application to the clinical monitoring of the potential strategies of stem cell therapy. An understanding of the three-dimensional imaging of stem cells may also help to approach fundamental questions regarding the cell conditions, that is, dose, time, phase, and disease mechanism, when regenerating naturally or therapeutically in neurodegenerative disease.
Manuscript source: Invited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: South Korea
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Fang FC, Prodromidou K, Wang YF S-Editor: Wang JL L-Editor: A P-Editor: Wu RR
| 1. | Ma DK, Bonaguidi MA, Ming GL, Song H. Adult neural stem cells in the mammalian central nervous system. Cell Res. 2009;19:672-682. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 264] [Cited by in RCA: 239] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 2. | Dantuma E, Merchant S, Sugaya K. Stem cells for the treatment of neurodegenerative diseases. Stem Cell Res Ther. 2010;1:37. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 69] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 3. | Horner PJ, Gage FH. Regenerating the damaged central nervous system. Nature. 2000;407:963-970. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 590] [Cited by in RCA: 562] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 4. | Thompson-Peer KL, DeVault L, Li T, Jan LY, Jan YN. In vivo dendrite regeneration after injury is different from dendrite development. Genes Dev. 2016;30:1776-1789. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 5. | Xiao L, Saiki C, Ide R. Stem cell therapy for central nerve system injuries: glial cells hold the key. Neural Regen Res. 2014;9:1253-1260. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 6. | Basak O, Taylor V. Stem cells of the adult mammalian brain and their niche. Cell Mol Life Sci. 2009;66:1057-1072. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 40] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 7. | Malgrange B, Borgs L, Grobarczyk B, Purnelle A, Ernst P, Moonen G, Nguyen L. Using human pluripotent stem cells to untangle neurodegenerative disease mechanisms. Cell Mol Life Sci. 2011;68:635-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 8. | Neirinckx V, Coste C, Rogister B, Wislet-Gendebien S. Concise review: adult mesenchymal stem cells, adult neural crest stem cells, and therapy of neurological pathologies: a state of play. Stem Cells Transl Med. 2013;2:284-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 75] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 9. | Drago D, Cossetti C, Iraci N, Gaude E, Musco G, Bachi A, Pluchino S. The stem cell secretome and its role in brain repair. Biochimie. 2013;95:2271-2285. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 238] [Cited by in RCA: 257] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 10. | Lo Furno D, Mannino G, Giuffrida R. Functional role of mesenchymal stem cells in the treatment of chronic neurodegenerative diseases. J Cell Physiol. 2018;233:3982-3999. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 119] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 11. | Bonafede R, Scambi I, Peroni D, Potrich V, Boschi F, Benati D, Bonetti B, Mariotti R. Exosome derived from murine adipose-derived stromal cells: Neuroprotective effect on in vitro model of amyotrophic lateral sclerosis. Exp Cell Res. 2016;340:150-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 142] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 12. | Zakrzewski W, Dobrzyński M, Szymonowicz M, Rybak Z. Stem cells: past, present, and future. Stem Cell Res Ther. 2019;10:68. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 937] [Cited by in RCA: 958] [Article Influence: 159.7] [Reference Citation Analysis (35)] |
| 13. | Lunn JS, Sakowski SA, Hur J, Feldman EL. Stem cell technology for neurodegenerative diseases. Ann Neurol. 2011;70:353-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 175] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 14. | Becker AJ, McCulloch EA, TILL JE. Cytological demonstration of the clonal nature of spleen colonies derived from transplanted mouse marrow cells. Nature. 1963;197:452-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 930] [Cited by in RCA: 799] [Article Influence: 29.6] [Reference Citation Analysis (0)] |
| 15. | Siminovitch L, McCulloch EA, Till JE. The distribution of colony-forming cells among spleen colonies. J Cell Comp Physiol. 1963;62:327-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 582] [Cited by in RCA: 527] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 16. | Kim J, Lee Y, Lee S, Kim K, Song M, Lee J. Mesenchymal Stem Cell Therapy and Alzheimer's Disease: Current Status and Future Perspectives. J Alzheimers Dis. 2020;77:1-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 54] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 17. | Fričová D, Korchak JA, Zubair AC. Challenges and translational considerations of mesenchymal stem/stromal cell therapy for Parkinson's disease. NPJ Regen Med. 2020;5:20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 55] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 18. | Zhang W, Gu GJ, Shen X, Zhang Q, Wang GM, Wang PJ. Neural stem cell transplantation enhances mitochondrial biogenesis in a transgenic mouse model of Alzheimer's disease-like pathology. Neurobiol Aging. 2015;36:1282-1292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 60] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 19. | Madrazo I, Kopyov O, Ávila-Rodríguez MA, Ostrosky F, Carrasco H, Kopyov A, Avendaño-Estrada A, Jiménez F, Magallón E, Zamorano C, González G, Valenzuela T, Carrillo R, Palma F, Rivera R, Franco-Bourland RE, Guízar-Sahagún G. Transplantation of Human Neural Progenitor Cells (NPC) into Putamina of Parkinsonian Patients: A Case Series Study, Safety and Efficacy Four Years after Surgery. Cell Transplant. 2019;28:269-285. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 38] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 20. | Zhao J, Su M, Lin Y, Liu H, He Z, Lai L. Administration of Amyloid Precursor Protein Gene Deleted Mouse ESC-Derived Thymic Epithelial Progenitors Attenuates Alzheimer's Pathology. Front Immunol. 2020;11:1781. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 21. | Adler AF, Cardoso T, Nolbrant S, Mattsson B, Hoban DB, Jarl U, Wahlestedt JN, Grealish S, Björklund A, Parmar M. hESC-Derived Dopaminergic Transplants Integrate into Basal Ganglia Circuitry in a Preclinical Model of Parkinson's Disease. Cell Rep. 2019;28:3462-3473.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 71] [Article Influence: 14.2] [Reference Citation Analysis (37)] |
| 22. | Seo J, Kritskiy O, Watson LA, Barker SJ, Dey D, Raja WK, Lin YT, Ko T, Cho S, Penney J, Silva MC, Sheridan SD, Lucente D, Gusella JF, Dickerson BC, Haggarty SJ, Tsai LH. Inhibition of p25/Cdk5 Attenuates Tauopathy in Mouse and iPSC Models of Frontotemporal Dementia. J Neurosci. 2017;37:9917-9924. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 126] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 23. | Ford E, Pearlman J, Ruan T, Manion J, Waller M, Neely GG, Caron L. Human Pluripotent Stem Cells-Based Therapies for Neurodegenerative Diseases: Current Status and Challenges. Cells. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 48] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 24. | Caprnda M, Kubatka P, Gazdikova K, Gasparova I, Valentova V, Stollarova N, La Rocca G, Kobyliak N, Dragasek J, Mozos I, Prosecky R, Siniscalco D, Büsselberg D, Rodrigo L, Kruzliak P. Immunomodulatory effects of stem cells: Therapeutic option for neurodegenerative disorders. Biomed Pharmacother. 2017;91:60-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 25. | Liu XY, Yang LP, Zhao L. Stem cell therapy for Alzheimer's disease. World J Stem Cells. 2020;12:787-802. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 107] [Cited by in RCA: 96] [Article Influence: 19.2] [Reference Citation Analysis (15)] |
| 26. | Angelopoulou E, Paudel YN, Shaikh MF, Piperi C. Flotillin: A Promising Biomarker for Alzheimer's Disease. J Pers Med. 2020;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 27. | Elia CA, Losurdo M, Malosio ML, Coco S. Extracellular Vesicles from Mesenchymal Stem Cells Exert Pleiotropic Effects on Amyloid-β, Inflammation, and Regeneration: A Spark of Hope for Alzheimer's Disease from Tiny Structures? Bioessays. 2019;41:e1800199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 28. | Naaldijk Y, Jäger C, Fabian C, Leovsky C, Blüher A, Rudolph L, Hinze A, Stolzing A. Effect of systemic transplantation of bone marrow-derived mesenchymal stem cells on neuropathology markers in APP/PS1 Alzheimer mice. Neuropathol Appl Neurobiol. 2017;43:299-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 91] [Article Influence: 10.1] [Reference Citation Analysis (2)] |
| 29. | Reza-Zaldivar EE, Hernández-Sapiéns MA, Gutiérrez-Mercado YK, Sandoval-Ávila S, Gomez-Pinedo U, Márquez-Aguirre AL, Vázquez-Méndez E, Padilla-Camberos E, Canales-Aguirre AA. Mesenchymal stem cell-derived exosomes promote neurogenesis and cognitive function recovery in a mouse model of Alzheimer's disease. Neural Regen Res. 2019;14:1626-1634. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 161] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 30. | Park BN, Lim TS, Yoon JK, An YS. In vivo tracking of intravenously injected mesenchymal stem cells in an Alzheimer's animal model. Cell Transplant. 2018;27:1203-1209. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 31. | Park BN, Kim JH, Lim TS, Park SH, Kim TG, Yoon BS, Son KS, Yoon JK, An YS. Therapeutic effect of mesenchymal stem cells in an animal model of Alzheimer's disease evaluated by β-amyloid positron emission tomography imaging. Aust N Z J Psychiatry. 2020;54:883-891. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 32. | Alvarez-Erviti L, Seow Y, Yin H, Betts C, Lakhal S, Wood MJ. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat Biotechnol. 2011;29:341-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3883] [Cited by in RCA: 3583] [Article Influence: 255.9] [Reference Citation Analysis (0)] |
| 33. | Katsuda T, Tsuchiya R, Kosaka N, Yoshioka Y, Takagaki K, Oki K, Takeshita F, Sakai Y, Kuroda M, Ochiya T. Human adipose tissue-derived mesenchymal stem cells secrete functional neprilysin-bound exosomes. Sci Rep. 2013;3:1197. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 327] [Cited by in RCA: 427] [Article Influence: 35.6] [Reference Citation Analysis (0)] |
| 34. | Garcia KO, Ornellas FL, Martin PK, Patti CL, Mello LE, Frussa-Filho R, Han SW, Longo BM. Therapeutic effects of the transplantation of VEGF overexpressing bone marrow mesenchymal stem cells in the hippocampus of murine model of Alzheimer's disease. Front Aging Neurosci. 2014;6:30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 92] [Cited by in RCA: 134] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 35. | Li B, Gao Y, Zhang W, Xu JR. Regulation and effects of neurotrophic factors after neural stem cell transplantation in a transgenic mouse model of Alzheimer disease. J Neurosci Res. 2018;96:828-840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 36. | Gu G, Zhang W, Li M, Ni J, Wang P. Transplantation of NSC-derived cholinergic neuron-like cells improves cognitive function in APP/PS1 transgenic mice. Neuroscience. 2015;291:81-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 38] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 37. | Lee IS, Jung K, Kim IS, Lee H, Kim M, Yun S, Hwang K, Shin JE, Park KI. Human neural stem cells alleviate Alzheimer-like pathology in a mouse model. Mol Neurodegener. 2015;10:38. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 122] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 38. | Park D, Choi EK, Cho TH, Joo SS, Kim YB. Human Neural Stem Cells Encoding ChAT Gene Restore Cognitive Function via Acetylcholine Synthesis, Aβ Elimination, and Neuroregeneration in APPswe/PS1dE9 Mice. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 39. | Cha MY, Kwon YW, Ahn HS, Jeong H, Lee YY, Moon M, Baik SH, Kim DK, Song H, Yi EC, Hwang D, Kim HS, Mook-Jung I. Protein-Induced Pluripotent Stem Cells Ameliorate Cognitive Dysfunction and Reduce Aβ Deposition in a Mouse Model of Alzheimer's Disease. Stem Cells Transl Med. 2017;6:293-305. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 63] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 40. | Takamatsu K, Ikeda T, Haruta M, Matsumura K, Ogi Y, Nakagata N, Uchino M, Ando Y, Nishimura Y, Senju S. Degradation of amyloid beta by human induced pluripotent stem cell-derived macrophages expressing Neprilysin-2. Stem Cell Res. 2014;13:442-453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 43] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 41. | Espuny-Camacho I, Arranz AM, Fiers M, Snellinx A, Ando K, Munck S, Bonnefont J, Lambot L, Corthout N, Omodho L, Vanden Eynden E, Radaelli E, Tesseur I, Wray S, Ebneth A, Hardy J, Leroy K, Brion JP, Vanderhaeghen P, De Strooper B. Hallmarks of Alzheimer's Disease in Stem-Cell-Derived Human Neurons Transplanted into Mouse Brain. Neuron. 2017;93:1066-1081.e8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 189] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 42. | Cova L, Armentero MT, Zennaro E, Calzarossa C, Bossolasco P, Busca G, Lambertenghi Deliliers G, Polli E, Nappi G, Silani V, Blandini F. Multiple neurogenic and neurorescue effects of human mesenchymal stem cell after transplantation in an experimental model of Parkinson's disease. Brain Res. 2010;1311:12-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 118] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 43. | Chi H, Guan Y, Li F, Chen Z. The Effect of Human Umbilical Cord Mesenchymal Stromal Cells in Protection of Dopaminergic Neurons from Apoptosis by Reducing Oxidative Stress in the Early Stage of a 6-OHDA-Induced Parkinson's Disease Model. Cell Transplant. 2019;28:87S-99S. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 44. | Wang F, Yasuhara T, Shingo T, Kameda M, Tajiri N, Yuan WJ, Kondo A, Kadota T, Baba T, Tayra JT, Kikuchi Y, Miyoshi Y, Date I. Intravenous administration of mesenchymal stem cells exerts therapeutic effects on parkinsonian model of rats: focusing on neuroprotective effects of stromal cell-derived factor-1alpha. BMC Neurosci. 2010;11:52. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 110] [Cited by in RCA: 117] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 45. | Cerri S, Greco R, Levandis G, Ghezzi C, Mangione AS, Fuzzati-Armentero MT, Bonizzi A, Avanzini MA, Maccario R, Blandini F. Intracarotid Infusion of Mesenchymal Stem Cells in an Animal Model of Parkinson's Disease, Focusing on Cell Distribution and Neuroprotective and Behavioral Effects. Stem Cells Transl Med. 2015;4:1073-1085. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 54] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 46. | Mendes-Pinheiro B, Anjo SI, Manadas B, Da Silva JD, Marote A, Behie LA, Teixeira FG, Salgado AJ. Bone Marrow Mesenchymal Stem Cells' Secretome Exerts Neuroprotective Effects in a Parkinson's Disease Rat Model. Front Bioeng Biotechnol. 2019;7:294. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 72] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 47. | Oh SH, Kim HN, Park HJ, Shin JY, Bae EJ, Sunwoo MK, Lee SJ, Lee PH. Mesenchymal Stem Cells Inhibit Transmission of α-Synuclein by Modulating Clathrin-Mediated Endocytosis in a Parkinsonian Model. Cell Rep. 2016;14:835-849. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 65] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 48. | Xu JT, Qian Y, Wang W, Chen XX, Li Y, Yang ZY, Song XB, Lu D, Deng XL. Effect of stromal cell-derived factor-1/CXCR4 axis in neural stem cell transplantation for Parkinson's disease. Neural Regen Res. 2020;15:112-119. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 49. | Zuo F, Xiong F, Wang X, Li X, Wang R, Ge W, Bao X. Intrastriatal Transplantation of Human Neural Stem Cells Restores the Impaired Subventricular Zone in Parkinsonian Mice. Stem Cells. 2017;35:1519-1531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 50. | Carelli S, Giallongo T, Rey F, Barzaghini B, Zandrini T, Pulcinelli A, Nardomarino R, Cerullo G, Osellame R, Cereda C, Zuccotti GV, Raimondi MT. Neural precursors cells expanded in a 3D micro-engineered niche present enhanced therapeutic efficacy in vivo. Nanotheranostics. 2021;5:8-26. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 51. | Bjorklund LM, Sánchez-Pernaute R, Chung S, Andersson T, Chen IY, McNaught KS, Brownell AL, Jenkins BG, Wahlestedt C, Kim KS, Isacson O. Embryonic stem cells develop into functional dopaminergic neurons after transplantation in a Parkinson rat model. Proc Natl Acad Sci U S A. 2002;99:2344-2349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 880] [Cited by in RCA: 811] [Article Influence: 35.3] [Reference Citation Analysis (0)] |
| 52. | Kim JH, Auerbach JM, Rodríguez-Gómez JA, Velasco I, Gavin D, Lumelsky N, Lee SH, Nguyen J, Sánchez-Pernaute R, Bankiewicz K, McKay R. Dopamine neurons derived from embryonic stem cells function in an animal model of Parkinson's disease. Nature. 2002;418:50-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1216] [Cited by in RCA: 1096] [Article Influence: 47.7] [Reference Citation Analysis (0)] |
| 53. | Nishimura F, Yoshikawa M, Kanda S, Nonaka M, Yokota H, Shiroi A, Nakase H, Hirabayashi H, Ouji Y, Birumachi J, Ishizaka S, Sakaki T. Potential use of embryonic stem cells for the treatment of mouse parkinsonian models: improved behavior by transplantation of in vitro differentiated dopaminergic neurons from embryonic stem cells. Stem Cells. 2003;21:171-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 78] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 54. | Kim TW, Piao J, Koo SY, Kriks S, Chung SY, Betel D, Socci ND, Choi SJ, Zabierowski S, Dubose BN, Hill EJ, Mosharov EV, Irion S, Tomishima MJ, Tabar V, Studer L. Biphasic Activation of WNT Signaling Facilitates the Derivation of Midbrain Dopamine Neurons from hESCs for Translational Use. Cell Stem Cell. 2021;28:343-355.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 142] [Article Influence: 35.5] [Reference Citation Analysis (0)] |
| 55. | Piao J, Zabierowski S, Dubose BN, Hill EJ, Navare M, Claros N, Rosen S, Ramnarine K, Horn C, Fredrickson C, Wong K, Safford B, Kriks S, El Maarouf A, Rutishauser U, Henchcliffe C, Wang Y, Riviere I, Mann S, Bermudez V, Irion S, Studer L, Tomishima M, Tabar V. Preclinical Efficacy and Safety of a Human Embryonic Stem Cell-Derived Midbrain Dopamine Progenitor Product, MSK-DA01. Cell Stem Cell. 2021;28:217-229.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 167] [Article Influence: 41.8] [Reference Citation Analysis (0)] |
| 56. | Kikuchi T, Morizane A, Doi D, Magotani H, Onoe H, Hayashi T, Mizuma H, Takara S, Takahashi R, Inoue H, Morita S, Yamamoto M, Okita K, Nakagawa M, Parmar M, Takahashi J. Human iPS cell-derived dopaminergic neurons function in a primate Parkinson's disease model. Nature. 2017;548:592-596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 396] [Cited by in RCA: 492] [Article Influence: 70.3] [Reference Citation Analysis (0)] |
| 57. | Doi D, Magotani H, Kikuchi T, Ikeda M, Hiramatsu S, Yoshida K, Amano N, Nomura M, Umekage M, Morizane A, Takahashi J. Pre-clinical study of induced pluripotent stem cell-derived dopaminergic progenitor cells for Parkinson's disease. Nat Commun. 2020;11:3369. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 105] [Cited by in RCA: 228] [Article Influence: 45.6] [Reference Citation Analysis (0)] |
| 58. | Bora E, Velakoulis D, Walterfang M. Social cognition in Huntington's disease: A meta-analysis. Behav Brain Res. 2016;297:131-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 82] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 59. | Paoli RA, Botturi A, Ciammola A, Silani V, Prunas C, Lucchiari C, Zugno E, Caletti E. Neuropsychiatric Burden in Huntington's Disease. Brain Sci. 2017;7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 88] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 60. | Reiner A, Deng YP. Disrupted striatal neuron inputs and outputs in Huntington's disease. CNS Neurosci Ther. 2018;24:250-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 65] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 61. | Bachoud-Lévi AC, Massart R, Rosser A. Cell therapy in Huntington's disease: Taking stock of past studies to move the field forward. Stem Cells. 2021;39:144-155. [PubMed] |
| 62. | Lin YT, Chern Y, Shen CK, Wen HL, Chang YC, Li H, Cheng TH, Hsieh-Li HM. Human mesenchymal stem cells prolong survival and ameliorate motor deficit through trophic support in Huntington's disease mouse models. PLoS One. 2011;6:e22924. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 83] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 63. | Linares GR, Chiu CT, Scheuing L, Leng Y, Liao HM, Maric D, Chuang DM. Preconditioning mesenchymal stem cells with the mood stabilizers lithium and valproic acid enhances therapeutic efficacy in a mouse model of Huntington's disease. Exp Neurol. 2016;281:81-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 54] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 64. | Yu-Taeger L, Stricker-Shaver J, Arnold K, Bambynek-Dziuk P, Novati A, Singer E, Lourhmati A, Fabian C, Magg J, Riess O, Schwab M, Stolzing A, Danielyan L, Nguyen HHP. Intranasal Administration of Mesenchymal Stem Cells Ameliorates the Abnormal Dopamine Transmission System and Inflammatory Reaction in the R6/2 Mouse Model of Huntington Disease. Cells. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 52] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 65. | Sadan O, Shemesh N, Barzilay R, Dadon-Nahum M, Blumenfeld-Katzir T, Assaf Y, Yeshurun M, Djaldetti R, Cohen Y, Melamed E, Offen D. Mesenchymal stem cells induced to secrete neurotrophic factors attenuate quinolinic acid toxicity: a potential therapy for Huntington's disease. Exp Neurol. 2012;234:417-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 64] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 66. | Olson SD, Kambal A, Pollock K, Mitchell GM, Stewart H, Kalomoiris S, Cary W, Nacey C, Pepper K, Nolta JA. Examination of mesenchymal stem cell-mediated RNAi transfer to Huntington's disease affected neuronal cells for reduction of huntingtin. Mol Cell Neurosci. 2012;49:271-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 68] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 67. | Wu TT, Su FJ, Feng YQ, Liu B, Li MY, Liang FY, Li G, Li XJ, Zhang Y, Cai ZQ, Pei Z. Mesenchymal stem cells alleviate AQP-4-dependent glymphatic dysfunction and improve brain distribution of antisense oligonucleotides in BACHD mice. Stem Cells. 2020;38:218-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (1)] |
| 68. | Giampà C, Alvino A, Magatti M, Silini AR, Cardinale A, Paldino E, Fusco FR, Parolini O. Conditioned medium from amniotic cells protects striatal degeneration and ameliorates motor deficits in the R6/2 mouse model of Huntington's disease. J Cell Mol Med. 2019;23:1581-1592. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 38] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 69. | Kim HS, Jeon I, Noh JE, Lee H, Hong KS, Lee N, Pei Z, Song J. Intracerebral Transplantation of BDNF-overexpressing Human Neural Stem Cells (HB1.F3.BDNF) Promotes Migration, Differentiation and Functional Recovery in a Rodent Model of Huntington's Disease. Exp Neurobiol. 2020;29:130-137. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 70. | Yoon Y, Kim HS, Jeon I, Noh JE, Park HJ, Lee S, Park IH, Stevanato L, Hicks C, Corteling R, Barker RA, Sinden JD, Song J. Implantation of the clinical-grade human neural stem cell line, CTX0E03, rescues the behavioral and pathological deficits in the quinolinic acid-lesioned rodent model of Huntington's disease. Stem Cells. 2020;38:936-947. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 71. | Yoon Y, Kim HS, Hong CP, Li E, Jeon I, Park HJ, Lee N, Pei Z, Song J. Neural Transplants From Human Induced Pluripotent Stem Cells Rescue the Pathology and Behavioral Defects in a Rodent Model of Huntington's Disease. Front Neurosci. 2020;14:558204. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 72. | Reidling JC, Relaño-Ginés A, Holley SM, Ochaba J, Moore C, Fury B, Lau A, Tran AH, Yeung S, Salamati D, Zhu C, Hatami A, Cepeda C, Barry JA, Kamdjou T, King A, Coleal-Bergum D, Franich NR, LaFerla FM, Steffan JS, Blurton-Jones M, Meshul CK, Bauer G, Levine MS, Chesselet MF, Thompson LM. Human Neural Stem Cell Transplantation Rescues Functional Deficits in R6/2 and Q140 Huntington's Disease Mice. Stem Cell Reports. 2018;10:58-72. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 76] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 73. | Cho IK, Hunter CE, Ye S, Pongos AL, Chan AWS. Combination of stem cell and gene therapy ameliorates symptoms in Huntington's disease mice. NPJ Regen Med. 2019;4:7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 74. | Wang JW, Qiu YR, Fu Y, Liu J, He ZJ, Huang ZT. Transplantation with hypoxia-preconditioned mesenchymal stem cells suppresses brain injury caused by cardiac arrest-induced global cerebral ischemia in rats. J Neurosci Res. 2017;95:2059-2070. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 45] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 75. | Lin QM, Zhao S, Zhou LL, Fang XS, Fu Y, Huang ZT. Mesenchymal stem cells transplantation suppresses inflammatory responses in global cerebral ischemia: contribution of TNF-α-induced protein 6. Acta Pharmacol Sin. 2013;34:784-792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 40] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 76. | Wang T, Tang W, Sun S, Xu T, Wang H, Guan J, Huang Z, Weil MH. Intravenous infusion of bone marrow mesenchymal stem cells improves brain function after resuscitation from cardiac arrest. Crit Care Med. 2008;36:S486-S491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 77. | Kubis N, Tomita Y, Tran-Dinh A, Planat-Benard V, André M, Karaszewski B, Waeckel L, Pénicaud L, Silvestre JS, Casteilla L, Seylaz J, Pinard E. Vascular fate of adipose tissue-derived adult stromal cells in the ischemic murine brain: A combined imaging-histological study. Neuroimage. 2007;34:1-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 25] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 78. | Wolfs E, Verfaillie CM, Van Laere K, Deroose CM. Radiolabeling strategies for radionuclide imaging of stem cells. Stem Cell Rev Rep. 2015;11:254-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 79. | Arbab AS, Thiffault C, Navia B, Victor SJ, Hong K, Zhang L, Jiang Q, Varma NR, Iskander A, Chopp M. Tracking of In-111-labeled human umbilical tissue-derived cells (hUTC) in a rat model of cerebral ischemia using SPECT imaging. BMC Med Imaging. 2012;12:33. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 80. | Yahyapour R, Farhood B, Graily G, Rezaeyan A, Rezapoor S, Abdollahi H, Cheki M, Amini P, Fallah H, Najafi M, Motevaseli E. Stem Cell Tracing Through MR Molecular Imaging. Tissue Eng Regen Med. 2018;15:249-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 81. | Holvoet B, De Waele L, Quattrocelli M, Gheysens O, Sampaolesi M, Verfaillie CM, Deroose CM. Increased Understanding of Stem Cell Behavior in Neurodegenerative and Neuromuscular Disorders by Use of Noninvasive Cell Imaging. Stem Cells Int. 2016;2016:6235687. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 82. | Tarantal AF, Lee CC, Kukis DL, Cherry SR. Radiolabeling human peripheral blood stem cells for positron emission tomography (PET) imaging in young rhesus monkeys. PLoS One. 2013;8:e77148. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 83. | Bansal A, Pandey MK, Demirhan YE, Nesbitt JJ, Crespo-Diaz RJ, Terzic A, Behfar A, DeGrado TR. Novel (89)Zr cell labeling approach for PET-based cell trafficking studies. EJNMMI Res. 2015;5:19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 102] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 84. | Sato N, Wu H, Asiedu KO, Szajek LP, Griffiths GL, Choyke PL. (89)Zr-Oxine Complex PET Cell Imaging in Monitoring Cell-based Therapies. Radiology. 2015;275:490-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 123] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 85. | Sykova E, Jendelova P. In vivo tracking of stem cells in brain and spinal cord injury. Prog Brain Res. 2007;161:367-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 94] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 86. | Mishra SK, Khushu S, Singh AK, Gangenahalli G. Homing and Tracking of Iron Oxide Labelled Mesenchymal Stem Cells After Infusion in Traumatic Brain Injury Mice: a Longitudinal In Vivo MRI Study. Stem Cell Rev Rep. 2018;14:888-900. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 87. | Ngen EJ, Wang L, Kato Y, Krishnamachary B, Zhu W, Gandhi N, Smith B, Armour M, Wong J, Gabrielson K, Artemov D. Imaging transplanted stem cells in real time using an MRI dual-contrast method. Sci Rep. 2015;5:13628. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 42] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 88. | Shen WB, Plachez C, Chan A, Yarnell D, Puche AC, Fishman PS, Yarowsky P. Human neural progenitor cells retain viability, phenotype, proliferation, and lineage differentiation when labeled with a novel iron oxide nanoparticle, Molday ION Rhodamine B. Int J Nanomedicine. 2013;8:4593-4600. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 89. | Shen WB, Plachez C, Tsymbalyuk O, Tsymbalyuk N, Xu S, Smith AM, Michel SL, Yarnell D, Mullins R, Gullapalli RP, Puche A, Simard JM, Fishman PS, Yarowsky P. Cell-Based Therapy in TBI: Magnetic Retention of Neural Stem Cells In Vivo. Cell Transplant. 2016;25:1085-1099. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 90. | Taylor A, Herrmann A, Moss D, Sée V, Davies K, Williams SR, Murray P. Assessing the efficacy of nano- and micro-sized magnetic particles as contrast agents for MRI cell tracking. PLoS One. 2014;9:e100259. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 91. | Huang X, Zhang F, Wang Y, Sun X, Choi KY, Liu D, Choi JS, Shin TH, Cheon J, Niu G, Chen X. Design considerations of iron-based nanoclusters for noninvasive tracking of mesenchymal stem cell homing. ACS Nano. 2014;8:4403-4414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 82] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 92. | Pacak CA, Hammer PE, MacKay AA, Dowd RP, Wang KR, Masuzawa A, Sill B, McCully JD, Cowan DB. Superparamagnetic iron oxide nanoparticles function as a long-term, multi-modal imaging label for non-invasive tracking of implanted progenitor cells. PLoS One. 2014;9:e108695. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 93. | Li Z, Wang J, Zhao C, Ren K, Xia Z, Yu H, Jiang K. Acute Blockage of Notch Signaling by DAPT Induces Neuroprotection and Neurogenesis in the Neonatal Rat Brain After Stroke. Transl Stroke Res. 2016;7:132-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 94. | Peng Z, Gao W, Yue B, Jiang J, Gu Y, Dai J, Chen L, Shi Q. Promotion of neurological recovery in rat spinal cord injury by mesenchymal stem cells loaded on nerve-guided collagen scaffold through increasing alternatively activated macrophage polarization. J Tissue Eng Regen Med. 2018;12:e1725-e1736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 36] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 95. | Meseguer-Olmo L, Montellano AJ, Martínez T, Martínez CM, Revilla-Nuin B, Roldán M, Mora CF, López-Lucas MD, Fuente T. Intraarticular and intravenous administration of 99MTc-HMPAO-labeled human mesenchymal stem cells (99MTC-AH-MSCS): In vivo imaging and biodistribution. Nucl Med Biol. 2017;46:36-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 96. | Mitkari B, Kerkelä E, Nystedt J, Korhonen M, Mikkonen V, Huhtala T, Jolkkonen J. Intra-arterial infusion of human bone marrow-derived mesenchymal stem cells results in transient localization in the brain after cerebral ischemia in rats. Exp Neurol. 2013;239:158-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 66] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 97. | Li G, Bonamici N, Dey M, Lesniak MS, Balyasnikova IV. Intranasal delivery of stem cell-based therapies for the treatment of brain malignancies. Expert Opin Drug Deliv. 2018;15:163-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 36] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 98. | Sabapathy V, Mentam J, Jacob PM, Kumar S. Noninvasive Optical Imaging and In Vivo Cell Tracking of Indocyanine Green Labeled Human Stem Cells Transplanted at Superficial or In-Depth Tissue of SCID Mice. Stem Cells Int. 2015;2015:606415. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 99. | Chen G, Lin S, Huang D, Zhang Y, Li C, Wang M, Wang Q. Revealing the Fate of Transplanted Stem Cells In Vivo with a Novel Optical Imaging Strategy. Small. 2018;14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 53] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 100. | De Vocht N, Lin D, Praet J, Hoornaert C, Reekmans K, Le Blon D, Daans J, Pauwels P, Goossens H, Hens N, Berneman Z, Van der Linden A, Ponsaerts P. Quantitative and phenotypic analysis of mesenchymal stromal cell graft survival and recognition by microglia and astrocytes in mouse brain. Immunobiology. 2013;218:696-705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 33] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 101. | Bernau K, Lewis CM, Petelinsek AM, Benink HA, Zimprich CA, Meyerand ME, Suzuki M, Svendsen CN. In vivo tracking of human neural progenitor cells in the rat brain using bioluminescence imaging. J Neurosci Methods. 2014;228:67-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 102. | Peeters M, van Rijn S, Vergroesen PP, Paul CP, Noske DP, Vandertop WP, Wurdinger T, Helder MN. Bioluminescence-mediated longitudinal monitoring of adipose-derived stem cells in a large mammal ex vivo organ culture. Sci Rep. 2015;5:13960. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 103. | Qiao H, Zhang R, Gao L, Guo Y, Wang J, Li X, Li C, Chen Y, Cao F. Molecular Imaging for Comparison of Different Growth Factors on Bone Marrow-Derived Mesenchymal Stromal Cells' Survival and Proliferation In Vivo. Biomed Res Int. 2016;2016:1363902. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 104. | Aswendt M, Vogel S, Schäfer C, Jathoul A, Pule M, Hoehn M. Quantitative in vivo dual-color bioluminescence imaging in the mouse brain. Neurophotonics. 2019;6:025006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 105. | Guglielmetti C, Praet J, Rangarajan JR, Vreys R, De Vocht N, Maes F, Verhoye M, Ponsaerts P, Van der Linden A. Multimodal imaging of subventricular zone neural stem/progenitor cells in the cuprizone mouse model reveals increased neurogenic potential for the olfactory bulb pathway, but no contribution to remyelination of the corpus callosum. Neuroimage. 2014;86:99-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 106. | Zhang F, Duan X, Lu L, Zhang X, Chen M, Mao J, Cao M, Shen J. In Vivo Long-Term Tracking of Neural Stem Cells Transplanted into an Acute Ischemic Stroke model with Reporter Gene-Based Bimodal MR and Optical Imaging. Cell Transplant. 2017;26:1648-1662. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 107. | Chung K, Wallace J, Kim SY, Kalyanasundaram S, Andalman AS, Davidson TJ, Mirzabekov JJ, Zalocusky KA, Mattis J, Denisin AK, Pak S, Bernstein H, Ramakrishnan C, Grosenick L, Gradinaru V, Deisseroth K. Structural and molecular interrogation of intact biological systems. Nature. 2013;497:332-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1381] [Cited by in RCA: 1416] [Article Influence: 118.0] [Reference Citation Analysis (0)] |
| 108. | Ueda HR, Ertürk A, Chung K, Gradinaru V, Chédotal A, Tomancak P, Keller PJ. Tissue clearing and its applications in neuroscience. Nat Rev Neurosci. 2020;21:61-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 389] [Cited by in RCA: 365] [Article Influence: 73.0] [Reference Citation Analysis (0)] |
| 109. | Yang B, Treweek JB, Kulkarni RP, Deverman BE, Chen CK, Lubeck E, Shah S, Cai L, Gradinaru V. Single-cell phenotyping within transparent intact tissue through whole-body clearing. Cell. 2014;158:945-958. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 755] [Cited by in RCA: 713] [Article Influence: 64.8] [Reference Citation Analysis (0)] |
| 110. | Ertürk A, Becker K, Jährling N, Mauch CP, Hojer CD, Egen JG, Hellal F, Bradke F, Sheng M, Dodt HU. Three-dimensional imaging of solvent-cleared organs using 3DISCO. Nat Protoc. 2012;7:1983-1995. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 650] [Cited by in RCA: 706] [Article Influence: 54.3] [Reference Citation Analysis (0)] |
| 111. | d'Esposito A, Nikitichev D, Desjardins A, Walker-Samuel S, Lythgoe MF. Quantification of light attenuation in optically cleared mouse brains. J Biomed Opt. 2015;20:80503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 112. | Ke MT, Fujimoto S, Imai T. SeeDB: a simple and morphology-preserving optical clearing agent for neuronal circuit reconstruction. Nat Neurosci. 2013;16:1154-1161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 636] [Cited by in RCA: 654] [Article Influence: 54.5] [Reference Citation Analysis (0)] |
| 113. | Susaki EA, Tainaka K, Perrin D, Kishino F, Tawara T, Watanabe TM, Yokoyama C, Onoe H, Eguchi M, Yamaguchi S, Abe T, Kiyonari H, Shimizu Y, Miyawaki A, Yokota H, Ueda HR. Whole-brain imaging with single-cell resolution using chemical cocktails and computational analysis. Cell. 2014;157:726-739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 843] [Cited by in RCA: 931] [Article Influence: 84.6] [Reference Citation Analysis (0)] |
| 114. | Weber MT, Arena JD, Xiao R, Wolf JA, Johnson VE. CLARITY reveals a more protracted temporal course of axon swelling and disconnection than previously described following traumatic brain injury. Brain Pathol. 2019;29:437-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 115. | Gail Canter R, Huang WC, Choi H, Wang J, Ashley Watson L, Yao CG, Abdurrob F, Bousleiman SM, Young JZ, Bennett DA, Delalle I, Chung K, Tsai LH. 3D mapping reveals network-specific amyloid progression and subcortical susceptibility in mice. Commun Biol. 2019;2:360. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 59] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 116. | Phillips J, Laude A, Lightowlers R, Morris CM, Turnbull DM, Lax NZ. Development of passive CLARITY and immunofluorescent labelling of multiple proteins in human cerebellum: understanding mechanisms of neurodegeneration in mitochondrial disease. Sci Rep. 2016;6:26013. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 39] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 117. | Luo W, Yi Y, Jing D, Zhang S, Men Y, Ge WP, Zhao H. Investigation of Postnatal Craniofacial Bone Development with Tissue Clearing-Based Three-Dimensional Imaging. Stem Cells Dev. 2019;28:1310-1321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 118. | Falkner S, Grade S, Dimou L, Conzelmann KK, Bonhoeffer T, Götz M, Hübener M. Transplanted embryonic neurons integrate into adult neocortical circuits. Nature. 2016;539:248-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 124] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 119. | Song BW. In Vivo Assessment of Stem Cells for Treating Neurodegenerative Disease: Current Approaches and Future Prospects. Stem Cells Int. 2017;2017:9751583. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 120. | Treweek JB, Gradinaru V. Extracting structural and functional features of widely distributed biological circuits with single cell resolution via tissue clearing and delivery vectors. Curr Opin Biotechnol. 2016;40:193-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 121. | Menegas W, Bergan JF, Ogawa SK, Isogai Y, Umadevi Venkataraju K, Osten P, Uchida N, Watabe-Uchida M. Dopamine neurons projecting to the posterior striatum form an anatomically distinct subclass. Elife. 2015;4:e10032. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 175] [Cited by in RCA: 227] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 122. | Robinson JE, Gradinaru V. Dopaminergic dysfunction in neurodevelopmental disorders: recent advances and synergistic technologies to aid basic research. Curr Opin Neurobiol. 2018;48:17-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |