Published online Jul 26, 2021. doi: 10.4252/wjsc.v13.i7.776
Peer-review started: February 9, 2021
First decision: March 30, 2021
Revised: April 29, 2021
Accepted: June 22, 2021
Article in press: June 22, 2021
Published online: July 26, 2021
Processing time: 164 Days and 7.6 Hours
Mesenchymal stem/stromal cells (MSCs) are extensively studied as cell-therapy agents for neurological diseases. Recent studies consider exosomes secreted by MSCs as important mediators for MSCs’ neuroprotective functions. Exosomes transfer functional molecules including proteins, lipids, metabolites, DNAs, and coding and non-coding RNAs from MSCs to their target cells. Emerging evidence shows that exosomal microRNAs (miRNAs) play a key role in the neuroprotective properties of these exosomes by targeting several genes and regulating various biological processes. Multiple exosomal miRNAs have been identified to have neuroprotective effects by promoting neurogenesis, neurite remodeling and survival, and neuroplasticity. Thus, exosomal miRNAs have significant therapeu
Core Tip: Mesenchymal stem/stromal cells (MSCs) are multipotent stem cells, which, due to their high availability and their reparative abilities, have been developed as therapeutic agents for various neurological diseases. MSC-derived exosomes have been receiving increased attention for their therapeutic capacity and low adverse effects. This review summarizes recent research on the neuroprotective effects of selected MSC-derived exosomal microRNAs (miRNAs) and provides an overview of their application potential in different neurological disorder disease models. It also discusses practical bioengineering approaches for isolating MSC-derived exosomes, manipulating their miRNA cargos, and improving their therapeutic abilities.
- Citation: Nasirishargh A, Kumar P, Ramasubramanian L, Clark K, Hao D, Lazar SV, Wang A. Exosomal microRNAs from mesenchymal stem/stromal cells: Biology and applications in neuroprotection. World J Stem Cells 2021; 13(7): 776-794
- URL: https://www.wjgnet.com/1948-0210/full/v13/i7/776.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v13.i7.776
Mesenchymal stem/stromal cells (MSCs) are multipotent stem cells with self-renewing capacities that can be isolated from a variety of human tissues including adipose, peripheral blood, muscle, amniotic fluid, placenta, skin and dental pulp[1-3]. MSCs are used as therapeutic agents for a broad range of diseases due to their immunomodulatory, angiogenic, and neuroprotective functions[1]. It has been suggested that the in vivo therapeutic effects of MSCs are achieved predominantly through a paracrine signaling mechanism that includes free proteins (growth factors and cytokines) and extracellular vesicles (EVs)[1,2,4-7]. EVs are membrane-bound vesicles secreted by cells and can be categorized into three subtypes, exosomes (50-150 nm), microvesicles (100-1000 nm), and apoptotic bodies (500-5000 nm) apoptotic bodies are formed during the late stages of apoptosis and contain intact organelles and chromatins along with small amounts of glycosylated proteins[8]. Exosomes and microvesicles are involved in the transfer of biological information and have shown to have potentials in the treatment of neurological disorders[9,10]. MSC-derived exosomes include bioactive components on their surface such as glycocalyx, membrane-bound signaling receptors and proteins. The internal exosomal compartment includes proteins, lipids, metabolites, DNA and coding and non-coding RNAs[11-13]. This multi-functional quality of MSC-derived exosomes allows them to be used for cell free therapies and reduces the risk for potential challenges that are associated with MSC-based therapy, such as microvascular occlusion and immune rejection[12]. It has been shown that the RNA content of exosomes consists primarily of small RNAs (less than 500 nucleotides) rather than mRNAs (around 1000 nucleotides)[14,15]. High-throughput sequencing of bone marrow-derived MSCs (BM-MSCs) demonstrated that over half of total non-coding RNAs in EVs are composed of microRNA (miRNAs)[16]. MSC-derived exosomes have been shown to have antiapoptotic, anti-necrotic, and antioxidant effects to protect neurons from degeneration[17-19]. Several studies have established that exosomal miRNAs played an essential role in the neuroprotective function of MSCs[17]. There is still ongoing research to improve stem cell therapeutic efficacy for clinical applications using bioengineering approaches. This review summarizes recent research on the neuroprotective effect of selected MSC-derived exosomal miRNAs and discusses potential approaches to manipulate exosome cargos and methodologies to improve EV yield for therapeutic treatment.
English language peer reviewed studies on neuroprotective MSC-derived exosomal miRNAs were located through PubMed online search using keywords: Mesenchymal stem cells, extracellular vesicles, and miRNA. This review includes papers in the past decade that studied neuroprotective potential of MSC-derived exosomal miRNAs and did not exclude studies on basis of methodologies. Letters and conference abstracts were excluded.
MiRNAs are a family of small single-stranded non-coding RNAs, ranging between 20 and 25 bases in length, that regulate gene expression in target cells to mediate protein translation and overall cellular functions[20]. MiRNAs function as a part of the RNA-induced silencing complex to downregulate translation of mRNAs or result in their decay by acting at the mRNA 3′UTR[21,22]. A correlation matrix analysis study showed a weak relationship between the RNA content of MSC-derived exosomes and the original MSCs, indicating that miRNAs were selectively loaded into the exosomes[23]. It has been suggested that RNA-binding proteins such as hnRNPA2B1 and hnRNPA1 directly bind to miRNAs and regulate their selective loading into exosomes[22,24]. Endosomal sorting complex required for transport (ESCRT) plays an important role in loading protein-miRNA complexes into exosomes. In addition, ESCRT-independent pathways, such as ceramide–mediated mechanisms, have been shown to be involved in cargo sorting into exosomes[25,26]. Further research is needed to uncover the specifics of the selective miRNA loading process.
It has been reported that environmental conditions affect miRNA levels in cells. For instance, hypoxic conditions have been reported to increase the expression of exosomal miR-21 in BM-MSCs[27]. The heterogeneity of miRNA content in exosomes have led to the discovery of diverse exosomal miRNAs with different impacts on regulating the phenotype and physiological state of their target cells, such as downregulating neuronal apoptosis and upregulating neurogenesis[28]. In this study the clinically relevant miRNAs were selected based on their involvement in neurological pathologies and their potential translational applications in neurodegenerative pathways.
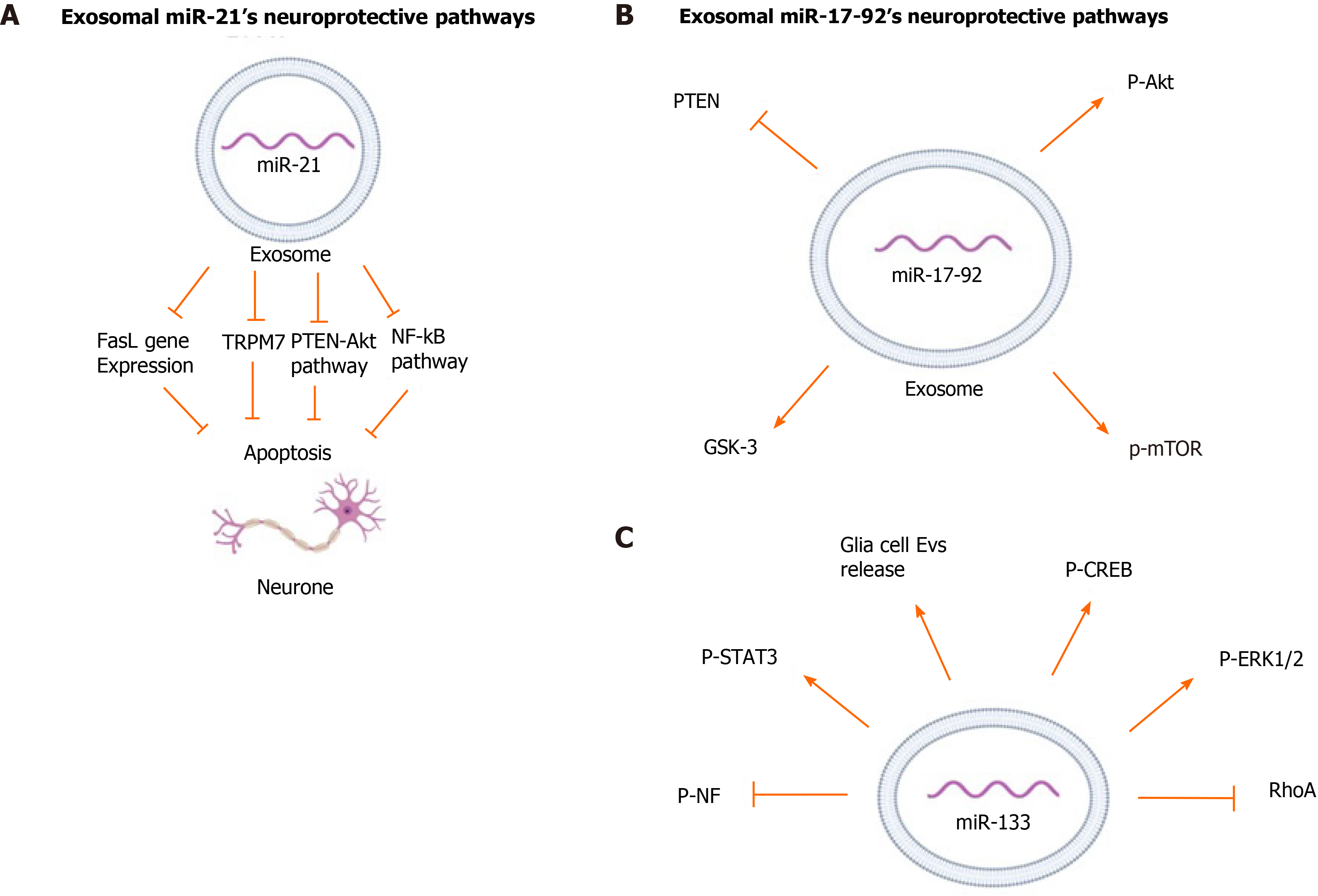
miR-21 is one of the most widely studied exosomal miRNAs due to its role in regulating several physiological conditions, such as immune cell function, cardiovascular function, and neuroprotection[29]. High-throughput sequencing of BM-MSC-derived exosomes has shown that 60% of miRNA content of these vesicles consists of 7 unique miRNAs, with miR-21-5p accounting for 22.5% of the content[16]. miR-21 is well known for its role in cell survival by stimulating cell proliferation, inhibiting apoptosis, and regulating differentiation[30].
Studies have shown that miR-21 acts through multiple pathways for different treatments. The neuroprotective function of miR-21 has been related to decreased apoptotic rates and downregulates expression of apoptosis-related proteins by MSCs[31]. To investigate the mechanism of action of miR-21, online databases were screened for direct mRNA targets of miR-21 and led to the identification of a conserved binding site in the 3′-UTR region of transient receptor potential cation channel subfamily m member 7 (TRPM7)[31]. miR-21 in BM-MSC-derived exosomes was implicated in significantly down-regulating expression of TRPM7 in rats with intracerebral hemorrhage (ICH)[31]. TRPM7 is a member of the transient receptor potential cation channel superfamily involved in a neurotoxic mechanism through regenerative calcium-dependent cellular reactive oxygen species production[32,33]. Suppression of these channels resulted in reduced anoxic neuronal death[33]. MiRNA-21 has also been shown to be involved in the Nuclear Factor-κB (NF-κB) pathway which regulates inflammatory responses, cellular growth and apoptosis[31,34]. It has been shown that the NF-κB pathway is related to neuronal death in brain tissue of patients following ICH[35]. Analysis of two downstream proteins of the NF-κB pathway, p65 and p-IκB-α, in PC12 cells line, cells commonly used as a neuron cell model, rats with ICH showed that miR-21 was involved in decreasing phosphorylation of IκB-α and decreasing p65 transport to the nucleus[31,36]. Thus, miR-21 overexpression can affect neuronal apoptosis by reducing activation of the NF-κB pathway.
The effect of miR-21 on decreasing Fas ligand (FasL) protein level has been widely studied[17,37,38]. FasL is a member of the tumor necrosis factor family (TNF) and functions by binding to Fas, a transmembrane protein of the TNF/neuron growth factor receptor family[39,40]. Analysis of binding sites for miR-21-5p in exosomes from BM-MSCs using software programs to predict miRNA targets, TargetScan and miRBase, shows that this miRNA has complementary binding sites to the 3′ UTR of the FasL gene[17]. Studies involving rat models of spinal cord injury (SCI) and middle cerebral artery occlusion (MCAO) used a luciferase reporter containing a portion of the FasL 3′-UTR and further confirmed that FasL is the direct target of miR-21-5p. These studies showed that exosomal miR-21 decreased FasL gene expression in neurons, thus protecting them from apoptosis[17,37].
Phosphatase and tensin homolog (PTEN) is a tumor suppressor phosphatase and inhibits the protein kinase B (Akt) pathway, which is a major pathway for cell survival[41]. Several studies have analyzed the regulatory effect of miR-21-5p as on the PTEN/Akt pathway in many disorders[42]. A study on rats with subarachnoid hemorrhage suggested that exosomal miR-21-5p from BM-MSCs regulated PTEN/Akt pathway by significantly inhibiting PTEN expression[16]. Another study using an in vitro model of traumatic brain injury showed that transfection of cortical neurons with miR-21 mimics (agomir) could enhance the PTEN-Akt signaling pathway[43]. Overexpression of miR-21 increased p-Akt levels while suppression of miR-21 decreased p-Akt levels. Western blot analysis of injured neurons after transfection with miR-21 agomir showed a significant increase in Bcl-2 and a slight decrease in Bcl-2-associated X protein (Bax), both downstream proteins of the Akt pathway, indicating the neuroprotective function of miR-21 in regulating the Akt pathway[43]. The neuroprotective pathways regulated by exosomal miR-21 are depicted in Figure 1A.
miR-17-92 is a 800-base pair long polycistronic miRNA which can be processed into 6 individual miRNAs (miR-17, miR-18a, miR-19a, miR-19b, miR-20a, and miR-92a)[44,45]. Administration of miR-17–92 enriched exosomes derived from BM-MSCs to rats with transient MCAO resulted in a significant improvement of neurological function and oligodendrogenesis. These miR-17-92 enriched exosomes also improved neurogenesis and neuronal dendrite plasticity of the rats, confirming the neuroprotective effect of this cluster of miRNAs[45].
Chondroitin sulfate proteoglycans (CSPGs), a type of extracellular matrix protein, have an inhibitory effect on axonal growth after brain injury[46]. In comparison to native MSC-derived exosomes, the BM-MSC derived exosomes enriched with miR-17-92 reversed the inhibitory effect of CSPG and significantly enhanced axonal growth as well as the speed of axonal elongation under CSPGs condition[47].
Similar to miR-21, miR-17-92 has been shown to target the PTEN/Akt pathway. Protein expression from rat brain tissues has shown that treatment with BM-MSC-derived exosomes enriched in miR-17-92 significantly decreased PTEN expression. This led to increased phosphorylation of Akt and its downstream proteins, mechanistic target of rapamycin and glycogen synthase kinase 3β, which play an essential role in axon regeneration[45,47]. miR-19a, another important miRNA in the miR-17-92 cluster, has shown to have anti-apoptotic effect by suppressing the PTEN/Akt pathway[48]. Attenuation of endogenous miR-19a has been linked to significant reduction of axonal outgrowth due to an increase in axonal PTEN levels[49]. Although the neuroprotective effect of MSC-derived exosomal miR-19a has not yet been studied, it may play a key role in miR-17–92 cluster’s anti-apoptotic function by targeting PTEN/Akt pathways. The neurprotective pathways regulated by exosomal miR-17–92 are summarized in Figure 1B.
miR-133 has been shown to induce functional recovery in several neurological disorders such as Parkinson's disease, spinal cord injury, and cerebral ischemia[7,50-52]. It is enriched in the midbrain and promotes neuronal density and attenuates neuronal apoptosis. In a murine model of Parkinson’s disease, it was demonstrated that miR-133 regulates maturation and function of dopaminergic neurons in the midbrain[51]. It targets Pitx3, a transcription factor restrictively expressed in the midbrain after birth and is involved in development of dopaminergic neurons and regulation of dopamine transporters production[53-55].
Similar to miR-17-92, miR-133 has been shown to reverse the inhibitory effect of CSPGs on axonal growth[56]. miR-133 also downregulates connective tissue growth factor (CTGF), a protein that inhibits axonal growth at injury sites in the CNS[52,57,58]. Immunohistochemical analysis of ischemic boundary zone in rats with MCAO showed that miR-133b present in MSCs regulates CTGF expression in astrocytes. Treatment with miR-133b enriched MSCs significantly decreased CTGF expression in MCAO rats, whereas administration of MSCs with down regulated miR-133 exhibited significantly elevated CTGF in MCAO rat brain tissues[57]. Treatment of the ischemic tissue of rats with MSC-derived exosomes showed a significant increase in the miR-133b level in the brain tissue, especially in astrocytes and neuronal cells[55]. Astrocytes and other glial cells in the CNS release exosomes that promote neuroplasticity, have neuroprotective function, and increase neuronal density[59]. Exosomes enriched in miR-133 significantly increased the secretion of these neuroprotective CNS-derived exosomes in MCAO rats compared to native MSCs and PBS treated controls. In contrast, incubation of astrocytes with miR-133-knockout exosomes significantly decreased exosome release from astrocytes[60].
Investigation of miR-133’s effect on neurite outgrowth showed Ras homolog family member A (RhoA), a small GTP-binding protein, is a target and mediator of miR-133b and negatively regulates the initiation of neuronal polarization and axonal outgrowth[52,56,61]. Inhibition of RhoA has been associated with increase in phosphorylation of extracellular signal-regulated kinase 1/2 (ERK1/2) and Akt and activation of ERK1/2 and the phosphoinositide-3 kinase/Akt pathways, which are known to improve cell survival and neurite outgrowth[56,62]. It has been shown that MSC-derived exosomes containing miR-133 significantly increased the phosphorylation of ERK1/2 in the injured neurons[63]. In addition, administration of miR-133 enriched MSC-derived exosomes increased phosphorylation and enhanced activity of signal transducer and activator of transcription 3 and cAMP response element-binding protein (CREB), both transcription factors essential for neuronal growth and regeneration of axons after injury[50,63]. CREB is a part of the ERK1/2-CREB pathway, which is regulated by RhoA[50]. Transfection of neuronal cells with miR-133b inhibitors prior to exosome treatment reversed this effect and led to a significant decrease in neurite branch number and total neurite length, and it significantly increased expression of RhoA[52]. Treatment of rats with CNS injuries with MSC-derived miR-133b enriched exosomes significantly promoted neurite branch number and total neurite length while decreasing RhoA[57,60,63].
BM-MSC-derived exosomes enriched in miR-133 enhanced neurite remodeling and neuroplasticity, hence leading to functional recovery after stroke and SCI[57,60,63]. Hematoxylin and eosin staining of injured neurons of rats with a SCI treated with miR-133b exosomes showed a significant decrease in the lesion area cavity at day 4 following injury, and showed an increase in mature neuron numbers when compared to the control group[63]. Neurofilament (NF) is a major structural element of the neuronal cytoskeleton, and its phosphorylation is essential for its function[64]. Administration of BM-MSC-derived exosomes enriched in miR-133b increased the p-NF level in the injured rat CNS tissue and promoted neuronal growth[57,63]. The neuroprotective pathways regulated by exosomal miR-133 are summarized in Figure 1C.
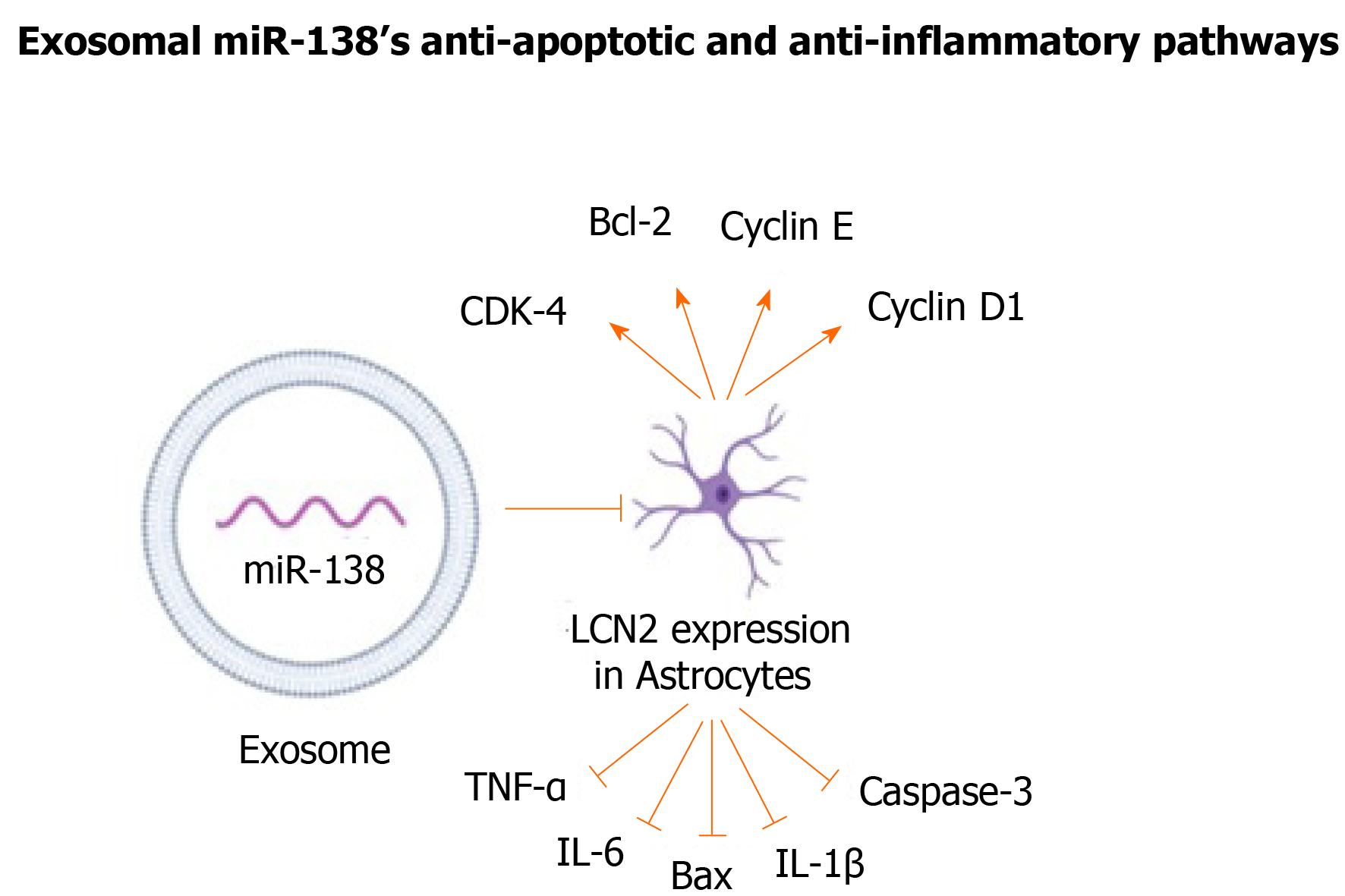
Endogenous miR-138 is essential in neuronal development and primarily expressed in synaptic sites in the neocortex[65]. miR-138 was found to robustly regulate the growth of dendritic spines and mediate their morphology by downregulating Acyl protein thioesterase 1, an enzyme responsible for depalmitoylation of several signaling proteins[66,67]. This results in actomyosin contraction and spine shrinkage through Rho-dependent pathways[67]. The structural and functional plasticity of dendritic spines correlates with long-lasting changes in synaptic function related to higher cognitive functions, and any abnormalities may lead to neurological diseases, such as memory dysfunctions and learning disabilities[65].
miR-138 has also been reported to be responsible for oligodendrocyte differentiation and maturation[68]. By inhibiting SRY-box transcription factor 4, a transcription factor that represses oligodendrocyte maturation, miR-138 can prolong the ability of terminally differentiating oligodendrocytes to myelinated axons[68,69]. In addition, ectopic overexpression of miR-138 was found to enhance migration of hypothalamic neuronal and glial cells in vitro by targeting expression of the extracellular matrix glycoprotein Reelin[65]. It also regulated axonal regeneration, neuronal development and axonal survival by regulating NF-κB activity via targeting sirtuins1 in human immunodeficiency virus transgenic rats[70].
Despite miR-138’s wide range of neuroprotective functions, limited studies have been conducted on the therapeutic use of MSC-derived exosomal miR-138s. A study on neurological changes in a mouse model of MCAO has addressed this gap in knowledge by using BM-MSC derived exosomes[71]. Lipocalin 2 (LCN2), a protein involved in neuronal death and inflammation and a known target for miR-138, was markedly upregulated in injured CNS tissues and was highly expressed in mice with ischemic stroke[71,72]. The exosomal miR-138-5p negatively regulated the expression of LCN2 in oxygen-glucose deprived (OGD) astrocytes in context of ischemic stroke. Through this mechanism, MSC-derived exosomal miR-138 alleviated neuronal injury in mice with ischemic stroke and promoted proliferation of astrocytes by repressing inflammatory and pro-apoptotic factors, such as TNF-α, IL-6, IL-1B, Bax, and caspase-3. It also functions by increasing anti-apoptotic and cell cycle markers, including CDK-4, Bcl-2, cyclin E, and cyclin D1, in astrocytes (Figure 2)[71].
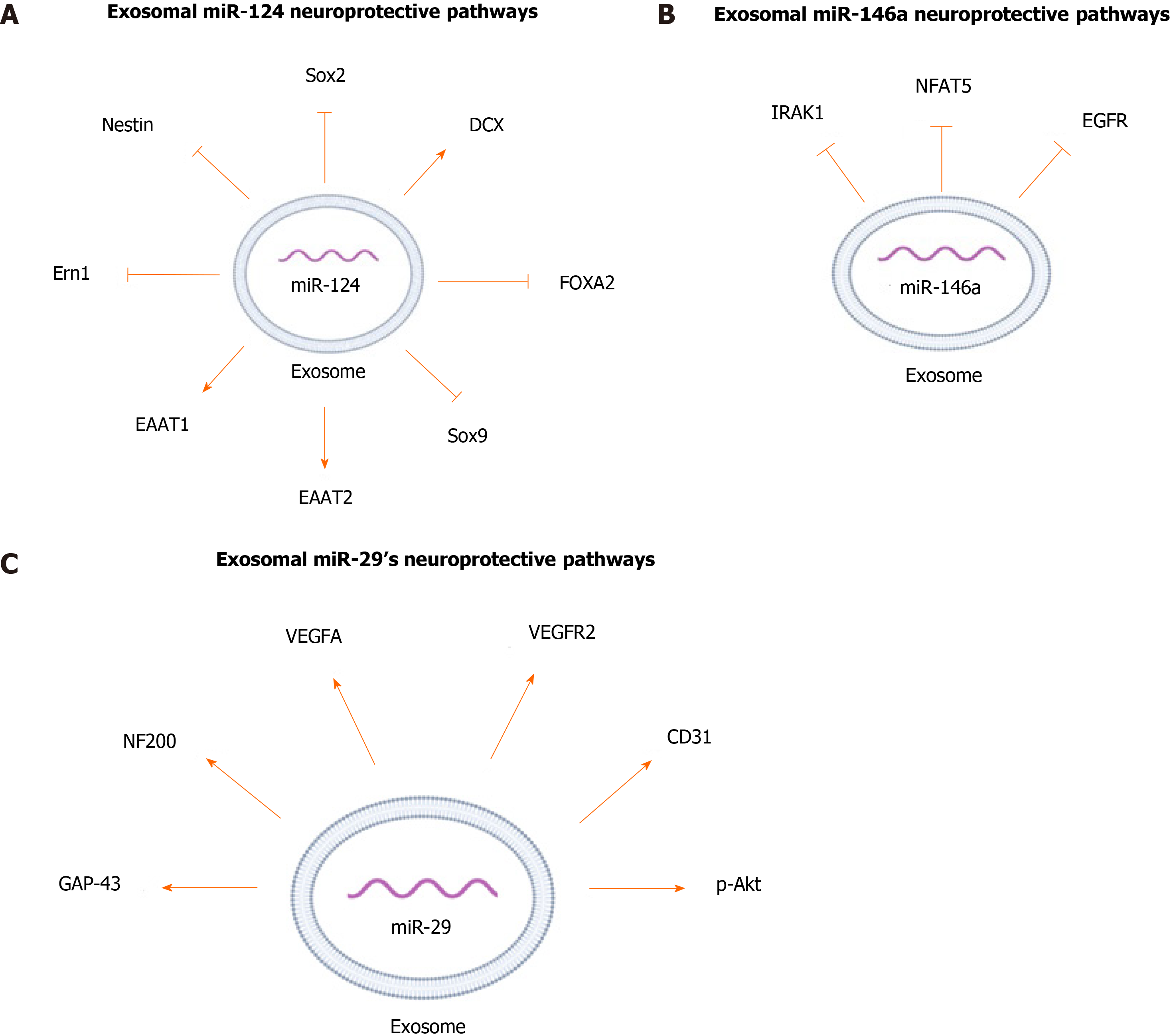
miR-124 is one of the highly expressed miRNAs in the CNS and is involved in a wide range of neurological functions[73-76]. In conjunction with other miRNAs, miR-124 promotes neural induction and growth by regulating the differentiation of MSCs into mature neurons[73,75-78]. To study the neuroprotective effect of exosomal miR-124, exosomes from BM-MSCs were incubated with human neural progenitor cells transfected with Sox9 3’-UTR-luciferase plasmid. Sox9 is a transcription factor important for differentiation and maintenance of multipotent neural stem cells[79]. miR-124 decreased the expression of Sox9, which suggests that it has a crucial role in the maintenance and differentiation of stem cells[79,80]. To further support the potential role of MSC-derived miR-124 in regulating differentiation of neuronal progenitor cells, mice with focal cortical ischemia were treated with BM-MSC-derived exosomes enriched inmiR-124, and analysis of brain tissues demonstrated a notable reduction of Nestin and Sox2, markers for neuronal progenitors[81]. Also, administration of the exosomes enriched in miR-124 to the infarct site doubled the expression of doublecortin, an immature neuronal marker. These changes in the expression of the markers indicate that exosomal miR-124 functions in cortical neurogenesis by promoting neural progenitors to differentiate into neuronal lineages[81].
Culturing human neuronal progenitor cells and astrocytes with MSCs that were transfected with miR-124 mimics increased the expression of the glutamate transporters excitatory amino-acid transporter 1 (EAAT1) and EAAT2[79]. Glutamate is the main excitatory neurotransmitter in the CNS and the upregulation of its transporters has been associated with neuroprotection[82,83]. Exosomal miR-124 had a neuroprotective effect in a rat model of spinal cord ischemia-reperfusion injury (SCIRI). Treating the rats with exosomal miR-124 derived from BM-MSCs led to higher motor function and fewer low-degree injuries. This treatment also resulted in a significant increase in the blood-spinal cord barrier integrity compared to that of the control rats[84]. In addition, administration of BM-MSC-derived exosomal miR-124 to rats with SCIRI has been shown to have anti-inflammatory effects by mediating polarization of neuroprotective and anti-inflammatory macrophages (M2) by downregulating the expression of endoplasmic reticulum to nucleus signaling 1, a signaling protein that negatively impacts polarization of M2[84].
To find a treatment for glioblastoma, BM-MSC exosomes were engineered to express an upregulated level of miR-124[85]. Glioma stem cells (GSCs) are subpopulations of cells in the glioblastoma that are involved in the radioresistance and chemore
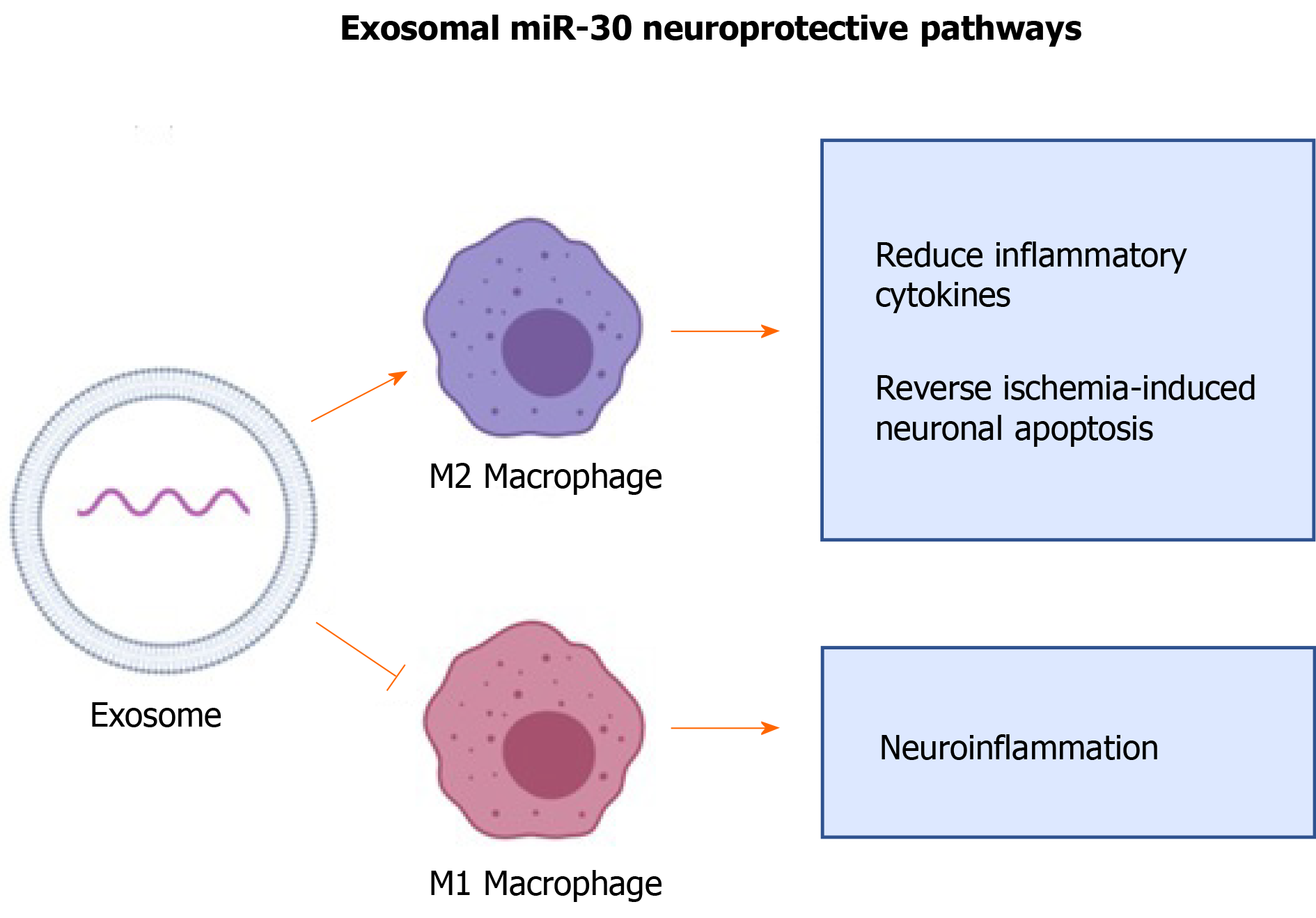
miR-30 is known to regulate neuronal development and recovery from injuries and participates in fine-tuning brain-derived neurotrophic factor expression levels[87]. miR-30 has a neuroprotective effect in the CNS of rats with ischemic injury by inhibiting autophagy via suppression of Beclin-1, Atg-5, and LC3B, all of which are regulators of autophagy[88-90]. Suppression of autophagy has been shown to decrease inflammatory response to CNS injuries[88,90].
A study showed that miR-30 enriched exosomes from adipose-derived MSCs suppress microglia polarization to M1 macrophages in ischemia-induced nerve injury[88]. M1 macrophages are pro-inflammatory and are involved in propagating inflammatory signals[91]. The same study used an in vivo approach in murine models of MCAO and showed that miR-30 enriched exosomes promote polarization of microglia into M2, reduce inflammatory cytokines, and reverse ischemia-induced neuronal apoptosis (Figure 4)[88]. Thus, MSC-derived exosomal miR-30 has significant potential for promoting tissue healing and treating neurological disorders such as stroke.
miR-146a is commonly found in astrocytes, and its expression increases in response to neuronal cell loss and astrocyte-mediated inflammatory response[92,93]. Treating brain tissue of rats with ICH with miR-146a-enriched BM-MSC derived exosomes showed more significant decrease in neuronal degeneration and neuronal apoptosis than in the tissues treated with the BM-MSC derived exosomes[94]. BM-MSC derived exosomal miR-146a suppressed oxidative stress imbalance, pro-inflammatory factors, and M1 microglia polarization in brain tissue of rats with ICH[94]. The anti-inflammatory effect of BM-MSC derived miR-146a is likely due to targeting and reducing expression of interleukin-1 receptor associated kinase 1 (IRAK1), a mediator of inflammation, and nuclear factors of activated T-cells 5, a pro-inflammatory transcription factor which induces polymerization of M1 macrophages[94-96]. Also, an increase in endogenous expression of miR-146a in BM-MSCs exerted anti-inflammatory effects on astrocytes in diabetic rats by suppressing IRAK1 expression which leads to subsequent reduction in NF-κB and TNF-α expression[95]. Similar to miR-124, BM-MSC -derived exosomal miR-146 has therapeutic potential for treating gliomas[97]. It has been shown that glioma cells express lower levels of miR-146 compared to normal astrocytes and in vitro transfection of human glioblastoma cells with miR-146 mimics decreased glioma invasiveness and migration[98]. MSC-derived exosomal miR-146 also suppressed epidermal growth factor receptor (EGFR) activity[97]. EGFR belongs to a family of tyrosine kinase superfamily receptors, and its expression has been shown to positively correlate with invasiveness of glioma[98]. In vitro administration of exosomal miR-146 significantly reduced growth, motility, and invasiveness of the glioma cells[97]. The same study showed that in vivo intra-tumor injection of miR-146 enriched MSC-derived exosomes significantly reduced tumor volume compared to tumors treated with miR-67, which had no known mRNA binding site in rats, or with PBS vehicle[97]. These findings suggested that MSC exosomal miR-146 is a promising treatment for oncological and inflammatory neurological diseases, especially astrocyte-mediated disorders. The neuroprotective pathways regulated by exosomal miR-146 are summarized in Figure 3B.
miR-29b has been shown to induce neuronal maturation and inhibit apoptosis in neuronal cells by targeting multiple members of the Bcl-2 family proteins[99]. miR-29b was reported to contribute to MCAO-induced brain injury in neuralcells and primary cortical neurons, and it was depleted at the infarct site after stroke[100,101]. Administration of miR-29b mimics resulted in significant protection against arachidonic acid-induced neuronal apoptosis and prevented loss of the cells’ mitochondrial membrane potential in a mouse model of stroke[101]. In addition, an in vivo murine model of MCAO also found that BM-MSC-derived exosomal miR-29b mimic significantly improved stroke outcomes[100]. These studies collectively suggest that the exosomal miR-29b-3p might have a potential for the treatment of stroke-related injuries.
BM-MSC-derived exosomal miR-29 has been studied in the context of SCI[102]. In rats that were injured at the T10 vertebrae in the spinal cord, exosomal miR-29b increased levels of neurofilament protein 200 and growth-associated protein-43[102]. These proteins are significantly involved in neuronal regeneration and their upregulation accelerates the repair of SCI[102]. In addition, injection of miRNA-29b exosomes alleviated histopathological damage in spinal cord tissues of SCI rats, and improved their hind-limb motor dysfunctions[102]. Thus, miR-29b possesses an important clinical potential for treating SCIs and other CNS injuries.
Exosomes from BM-MSCs were also used in an attempt to investigate the anti-apoptotic effect of miR-29b[100]. Decreased levels of miR-29b-3p in the brain of MCAO rats, and in rats with OGD cortical neurons and injured brain microvascular endothelial cells, was found to be accompanied by decreased angiogenesis[100]. Exosomes released from MSCs overexpressing miR-29b-3p were able to suppress neuronal apoptosis and induce expression of angiogenesis-stimulating factors such as vascular endothelial growth factor (VEGF) A, VEGF receptor 2, and CD31[100]. Also, exosomal miR-29b-3p inhibited neuronal apoptosis by regulating the PTEN/Akt pathway and elevating Akt phosphorylation in a rat model of stroke, thus highlighting the functional ability of MSC-derived exosomal miR-29b to modulate hypoxia-ischemia CNS injuries[100]. The neuroprotective pathways regulated by exosomal miR-29 summarized in Figure 3C.
MSC-derived exosomes are receiving increased attention for their therapeutic potential and lower adverse effects[28,103,104]. The nano size and the surface lipid and protein composition of exosomes allow them to cross the blood-brain barrier, thus overcoming the limitations of MSC cell therapy such as obstruction of microvasculature and rapid clearance by the mononuclear phagocyte system[7,88,105]. Autologous exosomes are non-immunogenic and are personalized sources of MSC-derived neuroprotective miRNA delivery[106]. These exosomes provide advantages over synthetic delivery vehicles, such as liposomes, in avoiding host immune response, endosomal-lysosomal degradation and inflammation[81,107]. Manipulating exosomes to transfer desired cargos to CNS could be therapeutically significant in improving their potentials for treating neurological disorders.
To accelerate the efficacy of miRNA therapeutic applications and to optimize their therapeutic properties, it is essential to purify and isolate the exosomes and manipulate their cargo[108]. Size exclusion chromatography, ultrafiltration, and immunoaffinity are examples of purification and isolation techniques that have been commonly used[108,109]. It has been shown that the composition of exosomes is closely associated with the health, the growth environment, and the origin of their “mother” MSCs[108,110]. For instance, exposing MSC culture to brain tissue extract of rats subjected to MCAO elevated the expression of miR-133b in the MSCs and their exosomes[52]. Also, exosomes from TNFα-primed MSCs showed significantly increased neuroprotective effect on retinal ganglion cells compared with exosomes from unprimed MSCs[111].
Multiple studies have implemented techniques to manipulate environmental factors, such as 2D and 3D scaffolds/scaffold-free culture, to induce proliferation of MSCs and to promote their differentiation in a more physiologically relevant environment[108,109]. 3D culture can mimic native extracellular matrix structure and functions and provides a three-dimensional environment for enhanced cell growth and exosome production[109]. In comparison with the exosomes secreted by cells grown in common 2D cultures, the exosomes derived from MSCs grown in 3D culture conditions contained a greater amount of protein and had better outcomes in promoting functional recovery in rats with traumatic brain injury[108]. In addition, the composition of the 3D scaffolds can be engineered to regulate the content of exosomes. For instance, the exosomal concentration of vascular endothelial growth factor and exosomal miR-126 Levels were higher when the placenta-derived mesenchymal stem cells (PMSCs) were exposed to a nitric oxide-releasing chitosan scaffold[112].
Different expansion techniques have been shown to increase the exosome yield without significantly sacrificing therapeutic properties[109]. For example, the hollow-fiber bioreactor, which is based on culturing the cells in a hollow and semi-permeable cartridge scaffold, is a time efficient method and can increase yield up to 19.4-fold and more concentrated exosomes (15.5-fold) than exosomes from MSCs cultured in 2D scaffold[113]. Endolysosomal pathways could also be targeted to increase yield and efficiency of the exosomes. Activating P2X7 receptors and SNARES, which are receptors involved in exosomes' secretion and neuronal internalization respectively, have been suggested as potentials for this approach[47,109].
The content of MSC-derived exosomes can be directly engineered to upregulate levels of neuroprotective miRNAs by preconditioning MSCs (Table 1). Transducing MSCs with lentivirus is an approach used to upregulate miRNAs in exosomes[57,85,100,102]. For example, transducing MSCs with lentivirus containing the precursor of miR-124a upregulated the loading of this miRNA into exosomes[85]. Another study showed that transfecting BM-MSCs with a miR-17-92 cluster plasmid was effective in increasing the level of miR-17-92 in the exosomes derived from the cells, as compared to BM-MSCs treated with empty vectors[47].
| Technique | Description | Direct/indirect exosomal loading |
| Transfection | A lentiviral vector or a plasmid is encoded with the desired miRNA and introduced to the MSCs[57,85,100,102] | Indirect |
| Electroporation | Electrical pulses in microseconds to milliseconds durations are applied to cause a temporary loss of the stability of the membranes of both MSCs and exosomes, which allows cargo to pass into the cell or exosomes[45,47,81,97] | Direct and indirect |
| Sonication | Low-frequency ultrasound is applied to disrupt the membrane integrity of the exosomes and form small pores in their membrane to allow small RNAs into the exosomes[114,116] | Direct |
| Modified calcium chloride transfection | Phosphate-buffered saline is slowly mixed with a CaCl2 solution containing the desired small RNA which leads to formation of RNA-calcium phosphate precipitates on the cell/exosomes . A heat shock is added to the solution to change the fluidity of the exosomes' plasma membranes for introducing[118] | Direct |
| Co-incubation of exosome with hydrophobically modified RNA | Conjugating the small RNA with a cholesterol moiety enhances hydrophobicity of the RNA and allows for diffusing the exosomal membrane during simple incubation[119] | Direct |
Electroporation has been shown to be effective in gene transfection and introduction of exogenous RNAs into exosomes and MSCs[45,47,81,97]. In electroporation, electrical pulses in microsecond to millisecond durations are applied to cells to cause a temporary loss of membrane stability and increase membrane permeability. This technique is commonly used to increase cell uptake of drugs, molecular probes, and genetic materials[26]. For instance, electroporation was used to load miR-17-92 in MSCs to improve neuroprotective functions[45]. One of the major limitations of direct loading of exosomes using electroporation is the aggregation and fusion of exosomes[114]. Adding trehalose to the exosome solution has shown to improve the exosome colloidal stability and reduce aggregation[114].
Sonication is another method that can be used to load small RNAs in exosomes. In sonication, low-frequency ultrasound is applied to disrupt exosome membrane integrity and form small pores in their membrane to allow RNAs to transfer into the exosomes[115]. It has been shown that sonication increases miRNA loading in exosomes by 267% compared to passive loading of miRNA into exosomes without sonication[116]. Sonication and electroporation both have high loading efficiency and prevent exosomes from breaking down[117]. To load exosomes with siRNAs, sonication has been observed to have less siRNA aggregates compared to electroporation[116]. Also, siRNA delivery to cells was higher when the cells were treated with exosomes loaded by sonication compared to when cells were exposed to exosomes loaded by electroporation[116]. However, the overall amount of siRNA delivered to the cells via exosomes was still low when using sonication[26,116]. Sonication is also considered the most damaging technique for exosomal membranes and may cause exosome rupture[114]. Proper experimental design and effective duration of sonication have to be used to prevent disruption of membrane integrity using this technique[114,116]. In addition, sonication has mostly been studied in the context of siRNA exosomal loading[26,116]. More research must be done on the effectiveness of this technique for sorting miRNAs in MSC-derived exosomes.
Direct transfection of exosomes with a modified calcium chloride transfection method has been suggested to be more convenient and easier to use than electroporation[118]. It includes slow mixing of phosphate-buffered saline with a CaCl2 solution containing the desired small RNA. This leads to formation of RNA-calcium phosphate precipitates on the cell/exosomes surface. By adding a heat shock step to the conventional calcium chloride transfection method, it is possible to change the fluidity of the exosomes' plasma membrane and directly load miRNA mimics or inhibitors in isolated exosomes for delivery to target cells[118].
Hydrophobic compounds can passively enter exosomes through direct incubation[117]. Simple co-incubation of exosomes with their cargo allows for loading of the exosomes without any damage to the exosome's membrane integrity[117]. RNAs are hydrophilic compounds and are not able to passively diffuse across the hydrophobic exosome lipid bilayer membrane during incubation[117]. Modifying siRNAs to be more hydrophobic by conjugating them with cholesterol moiety and stabilizing them with a fully phosphorothioate tail has shown to be effective in silencing Huntington mRNA in a mouse model[119]. Co-incubation of the hydrophobically modified siRNAs with exosomes allows efficient and stable loading of exosomes and successful delivery to neuronal cells[119]. Given the therapeutic potential of this method for treating neurological disorders, more research needs to be done to evaluate effectiveness of this technique for modifying the miRNA content of MSC-derived exosomes.
MSCs are multipotent stem cells with neuroprotective functions[45,120]. Due to their high availability and their reparative potential, MSCs derived from various tissue sources have been widely studied over the past three decades as therapeutic agents[121-126]. Exosomes derived from these cells have shown to be ideal candidates for transferring proteins and RNA cargos to specific target cells. The advantages of exosomes over cell-therapies have led to an increasing interest in the therapeutic potential of this class of EVs[104,127]. Recent studies suggest that miRNAs play a major role in mediating the neuroprotective effect of exosomes by regulating signaling pathways and gene expression in the target cells[17,28,81].
The content and yield of exosomes vary depending on the cell that they are derived forms. For instance, exosomes from amniotic fluid stem cells had 1.3 times more particles/mL compared to BM-MSCs[128]. For example, a study compared the effect of MSC-derived exosomes harvested from bone marrow, umbilical cord, chorionic, and menstrual fluid on neurite outgrowth. It was shown that exosomes derived from menstrual-MSCs and BM-MSCs increased the rate of neurite growth while umbilical cord and chorionic stem cell-derived exosomes did not promote neurite growth[129]. Although it is clear that the exosomal content vary depending on the origin of the MSCs, the relationship between the sources of MSCs and their exosomal miRNA content needs to be investigated in the future studies. Also, exosomes derived from BM-MSCs are most widely studied for MSC treatments, and the majority of studies about neuroprotective miRNAs have used bone marrow as the source of MSCs[130]. However, limited research has been done on the therapeutic potential of neuroprotective miRNAs that are abundant in MSCs derived from other types of tissues, such as the placenta and peripheral blood[28,131,132]. The age of the MSCs has also been shown to influence the content of EVs and their neuroprotective potentials[133,134]. Fetal MSCs have been reported to have higher growth kinetics and differentiation potential than adult MSCs[132]. Embryonic stem cell derived MSCs have been shown to have higher neuroprotective potential than fetal MSCs[132]. Such studies suggest that to optimally take advantage of neuroprotective exosomal miRNAs, different tissues must be investigated in the future studies.
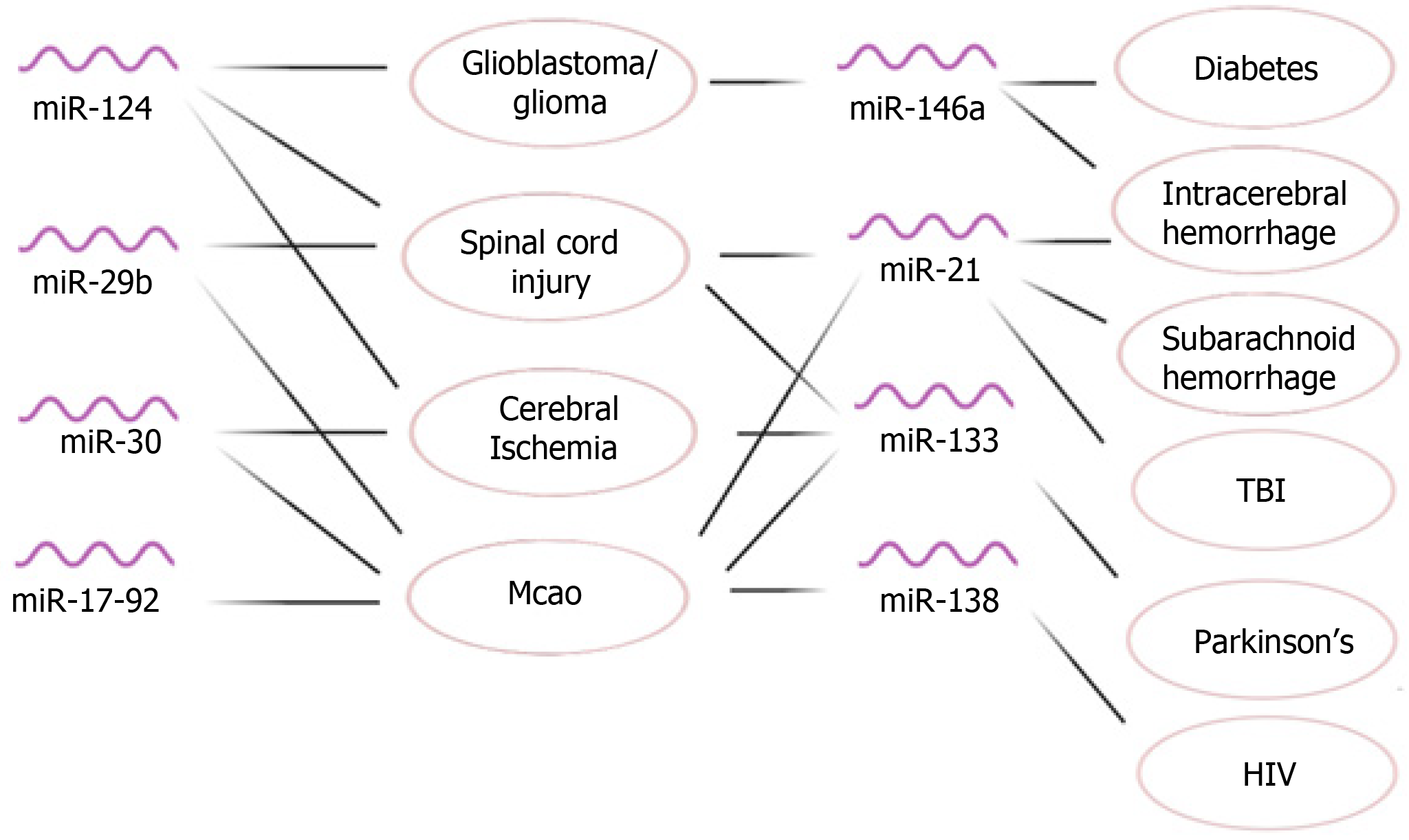
In this review article, we discussed the neuroprotective effect of selected miRNAs that are abundant in MSCs and provided an overview of their application in modulating different neurological disorders (Figure 5). Further research is warranted to explore the therapeutic effect of MSCs-derived exosomal miRNAs in treating these disorders. In addition, some neuroprotective miRNAs that are abundant in MSCs have received limited attention for their functions as exosomal miRNAs. For instance, miR-128 has a significant neuroprotective function in CNS, and is present in human adipose-derived MSCs, PMSCs, and BM-MSCs[28,135-137]. However, there is a gap in knowledge about its neuroprotective effect as an exosomal miRNA. More research can be done about the neuroprotective function of miR-128 and many other MSC-derived miRNAs when they are delivered to the target cells via exosomes.
For MSC-derived exosomal treatments to be effective, it is important to properly purify and isolate the exosomes. Different methods, including ultracentrifugation, ultrafiltration, and immunoaffinity, have shown to be effective to serve these purposes[109]. To enhance the function of exosomes, the expression of exosomal miRNAs can be manipulated by transfecting MSCs with miRNA mimics or inhibitors[16,52,72,84]. Studies have used this approach to investigate the mechanisms of action of different miRNAs. However, such procedures may also have the potential to help customize the exosomes for therapeutic use. For instance, injecting miR-206-knockdown exosomes that were obtained from human umbilical cord-derived MSCs into rats with subarachnoid hemorrhage has been shown to promote neuronal survival and improve neurological deficits[138].
Despite their significant neuroprotective properties, limited research has been done on the potential of MSC-derived miRNAs in developing exosome-based therapies. There is a gap in knowledge for the clinical implications of exosomal miRNA treatments. A clinical trial that used BM-MSC-derived exosomes enriched in miR-124 in five patients with acute ischemic stroke has been reported by Clinicaltrials.gov[139]. While no result for this trial has been published so far, the primary outcome of this study is safety at 12 mo following therapy and the secondary outcome is measurement of disability at 12 mo after treatment[139,140]. With the increasing focus on the neuroprotective effect of MSC-derived exosomal miRNAs and advancements in bioengineering and technology, the clinical implications of exosomal miRNAs in treating neurological disorders warrants further investigation.
Manuscript source: Invited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Alshammary S S-Editor: Zhang H L-Editor: A P-Editor: Li JH
| 1. | Zhang Y, Chopp M, Meng Y, Katakowski M, Xin H, Mahmood A, Xiong Y. Effect of exosomes derived from multipluripotent mesenchymal stromal cells on functional recovery and neurovascular plasticity in rats after traumatic brain injury. J Neurosurg. 2015;122:856-867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 419] [Cited by in RCA: 533] [Article Influence: 53.3] [Reference Citation Analysis (0)] |
| 2. | Chen TS, Lim SK. Measurement of precursor miRNA in exosomes from human ESC-derived mesenchymal stem cells. Methods Mol Biol. 2013;1024:69-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 3. | Mishra VK, Shih HH, Parveen F, Lenzen D, Ito E, Chan TF, Ke LY. Identifying the Therapeutic Significance of Mesenchymal Stem Cells. Cells. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 103] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 4. | Caplan AI, Dennis JE. Mesenchymal stem cells as trophic mediators. J Cell Biochem. 2006;98:1076-1084. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2126] [Cited by in RCA: 2224] [Article Influence: 117.1] [Reference Citation Analysis (0)] |
| 5. | Phinney DG, Di Giuseppe M, Njah J, Sala E, Shiva S, St Croix CM, Stolz DB, Watkins SC, Di YP, Leikauf GD, Kolls J, Riches DW, Deiuliis G, Kaminski N, Boregowda SV, McKenna DH, Ortiz LA. Mesenchymal stem cells use extracellular vesicles to outsource mitophagy and shuttle microRNAs. Nat Commun. 2015;6:8472. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 519] [Cited by in RCA: 736] [Article Influence: 73.6] [Reference Citation Analysis (0)] |
| 6. | Ionescu L, Byrne RN, van Haaften T, Vadivel A, Alphonse RS, Rey-Parra GJ, Weissmann G, Hall A, Eaton F, Thébaud B. Stem cell conditioned medium improves acute lung injury in mice: in vivo evidence for stem cell paracrine action. Am J Physiol Lung Cell Mol Physiol. 2012;303:L967-L977. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 261] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 7. | Eleuteri S, Fierabracci A. Insights into the Secretome of Mesenchymal Stem Cells and Its Potential Applications. Int J Mol Sci. 2019;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 220] [Cited by in RCA: 208] [Article Influence: 34.7] [Reference Citation Analysis (0)] |
| 8. | Doyle LM, Wang MZ. Overview of Extracellular Vesicles, Their Origin, Composition, Purpose, and Methods for Exosome Isolation and Analysis. Cells. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1394] [Cited by in RCA: 2137] [Article Influence: 356.2] [Reference Citation Analysis (35)] |
| 9. | Kourembanas S. Exosomes: vehicles of intercellular signaling, biomarkers, and vectors of cell therapy. Annu Rev Physiol. 2015;77:13-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 425] [Cited by in RCA: 571] [Article Influence: 51.9] [Reference Citation Analysis (0)] |
| 10. | Lee JY, Kim E, Choi SM, Kim DW, Kim KP, Lee I, Kim HS. Microvesicles from brain-extract-treated mesenchymal stem cells improve neurological functions in a rat model of ischemic stroke. Sci Rep. 2016;6:33038. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 77] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 11. | Li P, Kaslan M, Lee SH, Yao J, Gao Z. Progress in Exosome Isolation Techniques. Theranostics. 2017;7:789-804. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 870] [Cited by in RCA: 1387] [Article Influence: 173.4] [Reference Citation Analysis (1)] |
| 12. | Toh WS, Lai RC, Zhang B, Lim SK. MSC exosome works through a protein-based mechanism of action. Biochem Soc Trans. 2018;46:843-853. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 152] [Cited by in RCA: 279] [Article Influence: 39.9] [Reference Citation Analysis (0)] |
| 13. | Dong R, Liu Y, Yang Y, Wang H, Xu Y, Zhang Z. MSC-Derived Exosomes-Based Therapy for Peripheral Nerve Injury: A Novel Therapeutic Strategy. Biomed Res Int. 2019;2019:6458237. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 102] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 14. | Chen TS, Lai RC, Lee MM, Choo AB, Lee CN, Lim SK. Mesenchymal stem cell secretes microparticles enriched in pre-microRNAs. Nucleic Acids Res. 2010;38:215-224. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 450] [Cited by in RCA: 509] [Article Influence: 31.8] [Reference Citation Analysis (0)] |
| 15. | Milo R, Jorgensen P, Springer M. Charactaristic average size of mRNA - Bacteria Escherichia coli - BNID 100022. 2010. Available from: https://bionumbers.hms.harvard.edu/bionumber.aspx?&id=100022. |
| 16. | Gao X, Xiong Y, Li Q, Han M, Shan D, Yang G, Zhang S, Xin D, Zhao R, Wang Z, Xue H, Li G. Extracellular vesicle-mediated transfer of miR-21-5p from mesenchymal stromal cells to neurons alleviates early brain injury to improve cognitive function via the PTEN/Akt pathway after subarachnoid hemorrhage. Cell Death Dis. 2020;11:363. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 79] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 17. | Zhou X, Chu X, Yuan H, Qiu J, Zhao C, Xin D, Li T, Ma W, Wang H, Wang Z, Wang D. Mesenchymal stem cell derived EVs mediate neuroprotection after spinal cord injury in rats via the microRNA-21-5p/FasL gene axis. Biomed Pharmacother. 2019;115:108818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 82] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 18. | Venugopal C, Shamir C, Senthilkumar S, Babu JV, Sonu PK, Nishtha KJ, Rai KS, K S, Dhanushkodi A. Dosage and Passage Dependent Neuroprotective Effects of Exosomes Derived from Rat Bone Marrow Mesenchymal Stem Cells: An In Vitro Analysis. Curr Gene Ther. 2017;17:379-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 19. | Yang Y, Ye Y, Su X, He J, Bai W, He X. MSCs-Derived Exosomes and Neuroinflammation, Neurogenesis and Therapy of Traumatic Brain Injury. Front Cell Neurosci. 2017;11:55. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 100] [Cited by in RCA: 170] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 20. | Leavitt RJ, Limoli CL, Baulch JE. miRNA-based therapeutic potential of stem cell-derived extracellular vesicles: a safe cell-free treatment to ameliorate radiation-induced brain injury. Int J Radiat Biol. 2019;95:427-435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 35] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 21. | Gu S, Jin L, Zhang F, Sarnow P, Kay MA. Biological basis for restriction of microRNA targets to the 3' untranslated region in mammalian mRNAs. Nat Struct Mol Biol. 2009;16:144-150. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 368] [Cited by in RCA: 365] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 22. | Yu X, Odenthal M, Fries JW. Exosomes as miRNA Carriers: Formation-Function-Future. Int J Mol Sci. 2016;17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 166] [Cited by in RCA: 299] [Article Influence: 33.2] [Reference Citation Analysis (0)] |
| 23. | Baglio SR, Rooijers K, Koppers-Lalic D, Verweij FJ, Pérez Lanzón M, Zini N, Naaijkens B, Perut F, Niessen HW, Baldini N, Pegtel DM. Human bone marrow- and adipose-mesenchymal stem cells secrete exosomes enriched in distinctive miRNA and tRNA species. Stem Cell Res Ther. 2015;6:127. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 443] [Cited by in RCA: 599] [Article Influence: 59.9] [Reference Citation Analysis (0)] |
| 24. | Villarroya-Beltri C, Gutiérrez-Vázquez C, Sánchez-Cabo F, Pérez-Hernández D, Vázquez J, Martin-Cofreces N, Martinez-Herrera DJ, Pascual-Montano A, Mittelbrunn M, Sánchez-Madrid F. Sumoylated hnRNPA2B1 controls the sorting of miRNAs into exosomes through binding to specific motifs. Nat Commun. 2013;4:2980. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1165] [Cited by in RCA: 1515] [Article Influence: 137.7] [Reference Citation Analysis (0)] |
| 25. | Thind A, Wilson C. Exosomal miRNAs as cancer biomarkers and therapeutic targets. J Extracell Vesicles. 2016;5:31292. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 294] [Cited by in RCA: 306] [Article Influence: 34.0] [Reference Citation Analysis (0)] |
| 26. | Li SP, Lin ZX, Jiang XY, Yu XY. Exosomal cargo-loading and synthetic exosome-mimics as potential therapeutic tools. Acta Pharmacol Sin. 2018;39:542-551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 284] [Cited by in RCA: 283] [Article Influence: 40.4] [Reference Citation Analysis (0)] |
| 27. | Cui GH, Wu J, Mou FF, Xie WH, Wang FB, Wang QL, Fang J, Xu YW, Dong YR, Liu JR, Guo HD. Exosomes derived from hypoxia-preconditioned mesenchymal stromal cells ameliorate cognitive decline by rescuing synaptic dysfunction and regulating inflammatory responses in APP/PS1 mice. FASEB J. 2018;32:654-668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 291] [Article Influence: 41.6] [Reference Citation Analysis (0)] |
| 28. | Kumar P, Becker JC, Gao K, Carney RP, Lankford L, Keller BA, Herout K, Lam KS, Farmer DL, Wang A. Neuroprotective effect of placenta-derived mesenchymal stromal cells: role of exosomes. FASEB J. 2019;33:5836-5849. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 49] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 29. | Ti D, Hao H, Fu X, Han W. Mesenchymal stem cells-derived exosomal microRNAs contribute to wound inflammation. Sci China Life Sci. 2016;59:1305-1312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 120] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 30. | Feng YH, Tsao CJ. Emerging role of microRNA-21 in cancer. Biomed Rep. 2016;5:395-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 352] [Article Influence: 39.1] [Reference Citation Analysis (0)] |
| 31. | Zhang H, Wang Y, Lv Q, Gao J, Hu L, He Z. MicroRNA-21 Overexpression Promotes the Neuroprotective Efficacy of Mesenchymal Stem Cells for Treatment of Intracerebral Hemorrhage. Front Neurol. 2018;9:931. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 52] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 32. | Nuñez-Villena F, Becerra A, Echeverría C, Briceño N, Porras O, Armisén R, Varela D, Montorfano I, Sarmiento D, Simon F. Increased expression of the transient receptor potential melastatin 7 channel is critically involved in lipopolysaccharide-induced reactive oxygen species-mediated neuronal death. Antioxid Redox Signal. 2011;15:2425-2438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 39] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 33. | Aarts M, Iihara K, Wei WL, Xiong ZG, Arundine M, Cerwinski W, MacDonald JF, Tymianski M. A key role for TRPM7 channels in anoxic neuronal death. Cell. 2003;115:863-877. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 590] [Cited by in RCA: 606] [Article Influence: 28.9] [Reference Citation Analysis (0)] |
| 34. | Park MH, Hong JT. Roles of NF-κB in Cancer and Inflammatory Diseases and Their Therapeutic Approaches. Cells. 2016;5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 305] [Cited by in RCA: 461] [Article Influence: 51.2] [Reference Citation Analysis (0)] |
| 35. | Zhang Z, Liu Y, Huang Q, Su Y, Zhang Y, Wang G, Li F. NF-κB activation and cell death after intracerebral hemorrhage in patients. Neurol Sci. 2014;35:1097-1102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 51] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 36. | Hu R, Cao Q, Sun Z, Chen J, Zheng Q, Xiao F. A novel method of neural differentiation of PC12 cells by using Opti-MEM as a basic induction medium. Int J Mol Med. 2018;41:195-201. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 37. | Buller B, Liu X, Wang X, Zhang RL, Zhang L, Hozeska-Solgot A, Chopp M, Zhang ZG. MicroRNA-21 protects neurons from ischemic death. FEBS J. 2010;277:4299-4307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 197] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 38. | Wu MF, Yang J, Xiang T, Shi YY, Liu LJ. miR-21 targets Fas ligand-mediated apoptosis in breast cancer cell line MCF-7. J Huazhong Univ Sci Technolog Med Sci. 2014;34:190-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 39. | Takahashi T, Tanaka M, Inazawa J, Abe T, Suda T, Nagata S. Human Fas ligand: gene structure, chromosomal location and species specificity. Int Immunol. 1994;6:1567-1574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 311] [Cited by in RCA: 326] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 40. | Wang R, Zhang L, Yin D, Mufson RA, Shi Y. Protein kinase C regulates Fas (CD95/APO-1) expression. J Immunol. 1998;161:2201-2207. [PubMed] |
| 41. | Georgescu MM. PTEN Tumor Suppressor Network in PI3K-Akt Pathway Control. Genes Cancer. 2010;1:1170-1177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 261] [Cited by in RCA: 357] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 42. | Reza-Zaldivar EE, Hernández-Sapiéns MA, Minjarez B, Gutiérrez-Mercado YK, Márquez-Aguirre AL, Canales-Aguirre AA. Potential Effects of MSC-Derived Exosomes in Neuroplasticity in Alzheimer's Disease. Front Cell Neurosci. 2018;12:317. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 124] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 43. | Han Z, Chen F, Ge X, Tan J, Lei P, Zhang J. miR-21 alleviated apoptosis of cortical neurons through promoting PTEN-Akt signaling pathway in vitro after experimental traumatic brain injury. Brain Res. 2014;1582:12-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 106] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 44. | Concepcion CP, Bonetti C, Ventura A. The microRNA-17-92 family of microRNA clusters in development and disease. Cancer J. 2012;18:262-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 222] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 45. | Xin H, Katakowski M, Wang F, Qian JY, Liu XS, Ali MM, Buller B, Zhang ZG, Chopp M. MicroRNA cluster miR-17-92 Cluster in Exosomes Enhance Neuroplasticity and Functional Recovery After Stroke in Rats. Stroke. 2017;48:747-753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 401] [Cited by in RCA: 436] [Article Influence: 54.5] [Reference Citation Analysis (0)] |
| 46. | Haas CA, Rauch U, Thon N, Merten T, Deller T. Entorhinal cortex lesion in adult rats induces the expression of the neuronal chondroitin sulfate proteoglycan neurocan in reactive astrocytes. J Neurosci. 1999;19:9953-9963. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 106] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 47. | Zhang Y, Chopp M, Liu XS, Katakowski M, Wang X, Tian X, Wu D, Zhang ZG. Exosomes Derived from Mesenchymal Stromal Cells Promote Axonal Growth of Cortical Neurons. Mol Neurobiol. 2017;54:2659-2673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 242] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 48. | Olive V, Bennett MJ, Walker JC, Ma C, Jiang I, Cordon-Cardo C, Li QJ, Lowe SW, Hannon GJ, He L. miR-19 is a key oncogenic component of mir-17-92. Genes Dev. 2009;23:2839-2849. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 464] [Cited by in RCA: 511] [Article Influence: 31.9] [Reference Citation Analysis (0)] |
| 49. | Zhang Y, Ueno Y, Liu XS, Buller B, Wang X, Chopp M, Zhang ZG. The MicroRNA-17-92 cluster enhances axonal outgrowth in embryonic cortical neurons. J Neurosci. 2013;33:6885-6894. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 129] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 50. | Shen H, Yao X, Li H, Li X, Zhang T, Sun Q, Ji C, Chen G. Role of Exosomes Derived from miR-133b Modified MSCs in an Experimental Rat Model of Intracerebral Hemorrhage. J Mol Neurosci. 2018;64:421-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 101] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 51. | Kim J, Inoue K, Ishii J, Vanti WB, Voronov SV, Murchison E, Hannon G, Abeliovich A. A MicroRNA feedback circuit in midbrain dopamine neurons. Science. 2007;317:1220-1224. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 976] [Cited by in RCA: 929] [Article Influence: 51.6] [Reference Citation Analysis (0)] |
| 52. | Xin H, Li Y, Buller B, Katakowski M, Zhang Y, Wang X, Shang X, Zhang ZG, Chopp M. Exosome-mediated transfer of miR-133b from multipotent mesenchymal stromal cells to neural cells contributes to neurite outgrowth. Stem Cells. 2012;30:1556-1564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 613] [Cited by in RCA: 702] [Article Influence: 54.0] [Reference Citation Analysis (0)] |
| 53. | Li J, Dani JA, Le W. The role of transcription factor Pitx3 in dopamine neuron development and Parkinson's disease. Curr Top Med Chem. 2009;9:855-859. [PubMed] |
| 54. | Smidt MP, Smits SM, Burbach JP. Homeobox gene Pitx3 and its role in the development of dopamine neurons of the substantia nigra. Cell Tissue Res. 2004;318:35-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 94] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 55. | Sanchez-Simon FM, Zhang XX, Loh HH, Law PY, Rodriguez RE. Morphine regulates dopaminergic neuron differentiation via miR-133b. Mol Pharmacol. 2010;78:935-942. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 94] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 56. | Lu XC, Zheng JY, Tang LJ, Huang BS, Li K, Tao Y, Yu W, Zhu RL, Li S, Li LX. MiR-133b Promotes neurite outgrowth by targeting RhoA expression. Cell Physiol Biochem. 2015;35:246-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 61] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 57. | Xin H, Li Y, Liu Z, Wang X, Shang X, Cui Y, Zhang ZG, Chopp M. MiR-133b promotes neural plasticity and functional recovery after treatment of stroke with multipotent mesenchymal stromal cells in rats via transfer of exosome-enriched extracellular particles. Stem Cells. 2013;31:2737-2746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 588] [Cited by in RCA: 585] [Article Influence: 48.8] [Reference Citation Analysis (0)] |
| 58. | Duisters RF, Tijsen AJ, Schroen B, Leenders JJ, Lentink V, van der Made I, Herias V, van Leeuwen RE, Schellings MW, Barenbrug P, Maessen JG, Heymans S, Pinto YM, Creemers EE. miR-133 and miR-30 regulate connective tissue growth factor: implications for a role of microRNAs in myocardial matrix remodeling. Circ Res. 2009;104:170-178, 6p following 178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 644] [Cited by in RCA: 685] [Article Influence: 40.3] [Reference Citation Analysis (0)] |
| 59. | Frühbeis C, Fröhlich D, Kuo WP, Krämer-Albers EM. Extracellular vesicles as mediators of neuron-glia communication. Front Cell Neurosci. 2013;7:182. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 244] [Cited by in RCA: 282] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 60. | Xin H, Wang F, Li Y, Lu QE, Cheung WL, Zhang Y, Zhang ZG, Chopp M. Secondary Release of Exosomes From Astrocytes Contributes to the Increase in Neural Plasticity and Improvement of Functional Recovery After Stroke in Rats Treated With Exosomes Harvested From MicroRNA 133b-Overexpressing Multipotent Mesenchymal Stromal Cells. Cell Transplant. 2017;26:243-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 216] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 61. | Dupraz S, Hilton BJ, Husch A, Santos TE, Coles CH, Stern S, Brakebusch C, Bradke F. RhoA Controls Axon Extension Independent of Specification in the Developing Brain. Curr Biol. 2019;29:3874-3886.e9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 70] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 62. | Li F, Jiang Q, Shi KJ, Luo H, Yang Y, Xu CM. RhoA modulates functional and physical interaction between ROCK1 and Erk1/2 in selenite-induced apoptosis of leukaemia cells. Cell Death Dis. 2013;4:e708. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 63. | Li D, Zhang P, Yao X, Li H, Shen H, Li X, Wu J, Lu X. Exosomes Derived From miR-133b-Modified Mesenchymal Stem Cells Promote Recovery After Spinal Cord Injury. Front Neurosci. 2018;12:845. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 134] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 64. | Pant HC, Veeranna. Neurofilament phosphorylation. Biochem Cell Biol. 1995;73:575-592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 143] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 65. | Bicker S, Lackinger M, Weiß K, Schratt G. MicroRNA-132, -134, and -138: a microRNA troika rules in neuronal dendrites. Cell Mol Life Sci. 2014;71:3987-4005. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 75] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 66. | Bielefeld P, Mooney C, Henshall DC, Fitzsimons CP. miRNA-Mediated Regulation of Adult Hippocampal Neurogenesis; Implications for Epilepsy. Brain Plast. 2017;3:43-59. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 67. | Castañeda P, Muñoz M, García-Rojo G, Ulloa JL, Bravo JA, Márquez R, García-Pérez MA, Arancibia D, Araneda K, Rojas PS, Mondaca-Ruff D, Díaz-Véliz G, Mora S, Aliaga E, Fiedler JL. Association of N-cadherin levels and downstream effectors of Rho GTPases with dendritic spine loss induced by chronic stress in rat hippocampal neurons. J Neurosci Res. 2015;93:1476-1491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 42] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 68. | Cho KHT, Xu B, Blenkiron C, Fraser M. Emerging Roles of miRNAs in Brain Development and Perinatal Brain Injury. Front Physiol. 2019;10:227. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 85] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 69. | Potzner MR, Griffel C, Lütjen-Drecoll E, Bösl MR, Wegner M, Sock E. Prolonged Sox4 expression in oligodendrocytes interferes with normal myelination in the central nervous system. Mol Cell Biol. 2007;27:5316-5326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 62] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 70. | Hu G, Liao K, Yang L, Pendyala G, Kook Y, Fox HS, Buch S. Tat-Mediated Induction of miRs-34a & -138 Promotes Astrocytic Activation via Downregulation of SIRT1: Implications for Aging in HAND. J Neuroimmune Pharmacol. 2017;12:420-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 33] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 71. | Deng Y, Chen D, Gao F, Lv H, Zhang G, Sun X, Liu L, Mo D, Ma N, Song L, Huo X, Yan T, Zhang J, Miao Z. Exosomes derived from microRNA-138-5p-overexpressing bone marrow-derived mesenchymal stem cells confer neuroprotection to astrocytes following ischemic stroke via inhibition of LCN2. J Biol Eng. 2019;13:71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 143] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 72. | Tang XJ, Yang MH, Cao G, Lu JT, Luo J, Dai LJ, Huang KM, Zhang LI. Protective effect of microRNA-138 against cerebral ischemia/reperfusion injury in rats. Exp Ther Med. 2016;11:1045-1050. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 73. | Zammit V, Brincat MR, Cassar V, Muscat-Baron Y, Ayers D, Baron B. MiRNA influences in mesenchymal stem cell commitment to neuroblast lineage development. Noncoding RNA Res. 2018;3:232-242. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 74. | Lee ST, Im W, Ban JJ, Lee M, Jung KH, Lee SK, Chu K, Kim M. Exosome-Based Delivery of miR-124 in a Huntington's Disease Model. J Mov Disord. 2017;10:45-52. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 122] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 75. | Xu W, Li P, Qin K, Wang X, Jiang X. miR-124 regulates neural stem cells in the treatment of spinal cord injury. Neurosci Lett. 2012;529:12-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 76. | Wang Y, Wang D, Guo D. MiR-124 Promote Neurogenic Transdifferentiation of Adipose Derived Mesenchymal Stromal Cells Partly through RhoA/ROCK1, but Not ROCK2 Signaling Pathway. PLoS One. 2016;11:e0146646. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 77. | Zou D, Chen Y, Han Y, Lv C, Tu G. Overexpression of microRNA-124 promotes the neuronal differentiation of bone marrow-derived mesenchymal stem cells. Neural Regen Res. 2014;9:1241-1248. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 43] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 78. | Guo L, Zhao RC, Wu Y. The role of microRNAs in self-renewal and differentiation of mesenchymal stem cells. Exp Hematol. 2011;39:608-616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 106] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 79. | Lee HK, Finniss S, Cazacu S, Xiang C, Brodie C. Mesenchymal stem cells deliver exogenous miRNAs to neural cells and induce their differentiation and glutamate transporter expression. Stem Cells Dev. 2014;23:2851-2861. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 99] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 80. | Sabelström H, Petri R, Shchors K, Jandial R, Schmidt C, Sacheva R, Masic S, Yuan E, Fenster T, Martinez M, Saxena S, Nicolaides TP, Ilkhanizadeh S, Berger MS, Snyder EY, Weiss WA, Jakobsson J, Persson AI. Driving Neuronal Differentiation through Reversal of an ERK1/2-miR-124-SOX9 Axis Abrogates Glioblastoma Aggressiveness. Cell Rep. 2019;28:2064-2079.e11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 36] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 81. | Yang J, Zhang X, Chen X, Wang L, Yang G. Exosome Mediated Delivery of miR-124 Promotes Neurogenesis after Ischemia. Mol Ther Nucleic Acids. 2017;7:278-287. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 286] [Cited by in RCA: 454] [Article Influence: 56.8] [Reference Citation Analysis (0)] |
| 82. | Verma R, Mishra V, Sasmal D, Raghubir R. Pharmacological evaluation of glutamate transporter 1 (GLT-1) mediated neuroprotection following cerebral ischemia/reperfusion injury. Eur J Pharmacol. 2010;638:65-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 95] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 83. | Rothstein JD, Patel S, Regan MR, Haenggeli C, Huang YH, Bergles DE, Jin L, Dykes Hoberg M, Vidensky S, Chung DS, Toan SV, Bruijn LI, Su ZZ, Gupta P, Fisher PB. Beta-lactam antibiotics offer neuroprotection by increasing glutamate transporter expression. Nature. 2005;433:73-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1079] [Cited by in RCA: 1165] [Article Influence: 58.3] [Reference Citation Analysis (0)] |
| 84. | Li R, Zhao K, Ruan Q, Meng C, Yin F. Bone marrow mesenchymal stem cell-derived exosomal microRNA-124-3p attenuates neurological damage in spinal cord ischemia-reperfusion injury by downregulating Ern1 and promoting M2 macrophage polarization. Arthritis Res Ther. 2020;22:75. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 121] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 85. | Lang FM, Hossain A, Gumin J, Momin EN, Shimizu Y, Ledbetter D, Shahar T, Yamashita S, Parker Kerrigan B, Fueyo J, Sawaya R, Lang FF. Mesenchymal stem cells as natural biofactories for exosomes carrying miR-124a in the treatment of gliomas. Neuro Oncol. 2018;20:380-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 195] [Article Influence: 32.5] [Reference Citation Analysis (0)] |
| 86. | Liebelt BD, Shingu T, Zhou X, Ren J, Shin SA, Hu J. Glioma Stem Cells: Signaling, Microenvironment, and Therapy. Stem Cells Int. 2016;2016:7849890. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 105] [Cited by in RCA: 133] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 87. | Mellios N, Huang HS, Grigorenko A, Rogaev E, Akbarian S. A set of differentially expressed miRNAs, including miR-30a-5p, act as post-transcriptional inhibitors of BDNF in prefrontal cortex. Hum Mol Genet. 2008;17:3030-3042. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 229] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 88. | Jiang M, Wang H, Jin M, Yang X, Ji H, Jiang Y, Zhang H, Wu F, Wu G, Lai X, Cai L, Hu R, Xu L, Li L. Exosomes from MiR-30d-5p-ADSCs Reverse Acute Ischemic Stroke-Induced, Autophagy-Mediated Brain Injury by Promoting M2 Microglial/Macrophage Polarization. Cell Physiol Biochem. 2018;47:864-878. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 245] [Article Influence: 35.0] [Reference Citation Analysis (0)] |
| 89. | Kang R, Zeh HJ, Lotze MT, Tang D. The Beclin 1 network regulates autophagy and apoptosis. Cell Death Differ. 2011;18:571-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1794] [Cited by in RCA: 1946] [Article Influence: 139.0] [Reference Citation Analysis (0)] |
| 90. | He HY, Ren L, Guo T, Deng YH. Neuronal autophagy aggravates microglial inflammatory injury by downregulating CX3CL1/fractalkine after ischemic stroke. Neural Regen Res. 2019;14:280-288. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 51] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 91. | Saqib U, Sarkar S, Suk K, Mohammad O, Baig MS, Savai R. Phytochemicals as modulators of M1-M2 macrophages in inflammation. Oncotarget. 2018;9:17937-17950. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 149] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 92. | Iyer A, Zurolo E, Prabowo A, Fluiter K, Spliet WG, van Rijen PC, Gorter JA, Aronica E. MicroRNA-146a: a key regulator of astrocyte-mediated inflammatory response. PLoS One. 2012;7:e44789. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 244] [Cited by in RCA: 264] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 93. | Aronica E, Fluiter K, Iyer A, Zurolo E, Vreijling J, van Vliet EA, Baayen JC, Gorter JA. Expression pattern of miR-146a, an inflammation-associated microRNA, in experimental and human temporal lobe epilepsy. Eur J Neurosci. 2010;31:1100-1107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 259] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 94. | Duan S, Wang F, Cao J, Wang C. Exosomes Derived from MicroRNA-146a-5p-Enriched Bone Marrow Mesenchymal Stem Cells Alleviate Intracerebral Hemorrhage by Inhibiting Neuronal Apoptosis and Microglial M1 Polarization. Drug Des Devel Ther. 2020;14:3143-3158. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 105] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 95. | Kubota K, Nakano M, Kobayashi E, Mizue Y, Chikenji T, Otani M, Nagaishi K, Fujimiya M. An enriched environment prevents diabetes-induced cognitive impairment in rats by enhancing exosomal miR-146a secretion from endogenous bone marrow-derived mesenchymal stem cells. PLoS One. 2018;13:e0204252. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 64] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 96. | Tellechea M, Buxadé M, Tejedor S, Aramburu J, López-Rodríguez C. NFAT5-Regulated Macrophage Polarization Supports the Proinflammatory Function of Macrophages and T Lymphocytes. J Immunol. 2018;200:305-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 45] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 97. | Katakowski M, Buller B, Zheng X, Lu Y, Rogers T, Osobamiro O, Shu W, Jiang F, Chopp M. Exosomes from marrow stromal cells expressing miR-146b inhibit glioma growth. Cancer Lett. 2013;335:201-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 466] [Cited by in RCA: 589] [Article Influence: 49.1] [Reference Citation Analysis (0)] |
| 98. | Katakowski M, Zheng X, Jiang F, Rogers T, Szalad A, Chopp M. MiR-146b-5p suppresses EGFR expression and reduces in vitro migration and invasion of glioma. Cancer Invest. 2010;28:1024-1030. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 143] [Cited by in RCA: 138] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 99. | Kole AJ, Swahari V, Hammond SM, Deshmukh M. miR-29b is activated during neuronal maturation and targets BH3-only genes to restrict apoptosis. Genes Dev. 2011;25:125-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 179] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 100. | Hou K, Li G, Zhao J, Xu B, Zhang Y, Yu J, Xu K. Bone mesenchymal stem cell-derived exosomal microRNA-29b-3p prevents hypoxic-ischemic injury in rat brain by activating the PTEN-mediated Akt signaling pathway. J Neuroinflammation. 2020;17:46. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 70] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 101. | Khanna S, Rink C, Ghoorkhanian R, Gnyawali S, Heigel M, Wijesinghe DS, Chalfant CE, Chan YC, Banerjee J, Huang Y, Roy S, Sen CK. Loss of miR-29b following acute ischemic stroke contributes to neural cell death and infarct size. J Cereb Blood Flow Metab. 2013;33:1197-1206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 93] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 102. | Yu T, Zhao C, Hou S, Zhou W, Wang B, Chen Y. Exosomes secreted from miRNA-29b-modified mesenchymal stem cells repaired spinal cord injury in rats. Braz J Med Biol Res. 2019;52:e8735. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 99] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 103. | Xin H, Li Y, Chopp M. Exosomes/miRNAs as mediating cell-based therapy of stroke. Front Cell Neurosci. 2014;8:377. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 180] [Cited by in RCA: 227] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 104. | Yeo RW, Lai RC, Zhang B, Tan SS, Yin Y, Teh BJ, Lim SK. Mesenchymal stem cell: an efficient mass producer of exosomes for drug delivery. Adv Drug Deliv Rev. 2013;65:336-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 464] [Cited by in RCA: 639] [Article Influence: 53.3] [Reference Citation Analysis (0)] |
| 105. | Fujita Y, Kadota T, Araya J, Ochiya T, Kuwano K. Clinical Application of Mesenchymal Stem Cell-Derived Extracellular Vesicle-Based Therapeutics for Inflammatory Lung Diseases. J Clin Med. 2018;7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 95] [Cited by in RCA: 127] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 106. | Akuma P, Okagu OD, Udenigwe CC. Naturally Occurring Exosome Vesicles as Potential Delivery Vehicle for Bioactive Compounds. Front Sustain Food Syst. 2019;3. [RCA] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 112] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 107. | Momen-Heravi F, Bala S, Bukong T, Szabo G. Exosome-mediated delivery of functionally active miRNA-155 inhibitor to macrophages. Nanomedicine. 2014;10:1517-1527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 239] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 108. | Park KS, Bandeira E, Shelke GV, Lässer C, Lötvall J. Enhancement of therapeutic potential of mesenchymal stem cell-derived extracellular vesicles. Stem Cell Res Ther. 2019;10:288. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 116] [Cited by in RCA: 182] [Article Influence: 30.3] [Reference Citation Analysis (0)] |
| 109. | Phan J, Kumar P, Hao D, Gao K, Farmer D, Wang A. Engineering mesenchymal stem cells to improve their exosome efficacy and yield for cell-free therapy. J Extracell Vesicles. 2018;7:1522236. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 239] [Cited by in RCA: 231] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 110. | Phinney DG, Pittenger MF. Concise Review: MSC-Derived Exosomes for Cell-Free Therapy. Stem Cells. 2017;35:851-858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 831] [Cited by in RCA: 1230] [Article Influence: 153.8] [Reference Citation Analysis (0)] |
| 111. | Mead B, Chamling X, Zack DJ, Ahmed Z, Tomarev S. TNFα-Mediated Priming of Mesenchymal Stem Cells Enhances Their Neuroprotective Effect on Retinal Ganglion Cells. Invest Ophthalmol Vis Sci. 2020;61:6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 55] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 112. | Du W, Zhang K, Zhang S, Wang R, Nie Y, Tao H, Han Z, Liang L, Wang D, Liu J, Liu N, Kong D, Zhao Q, Li Z. Enhanced proangiogenic potential of mesenchymal stem cell-derived exosomes stimulated by a nitric oxide releasing polymer. Biomaterials. 2017;133:70-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 183] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 113. | Cao J, Wang B, Tang T, Lv L, Ding Z, Li Z, Hu R, Wei Q, Shen A, Fu Y, Liu B. Three-dimensional culture of MSCs produces exosomes with improved yield and enhanced therapeutic efficacy for cisplatin-induced acute kidney injury. Stem Cell Res Ther. 2020;11:206. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 178] [Article Influence: 35.6] [Reference Citation Analysis (0)] |
| 114. | Donoso-Quezada J, Ayala-Mar S, González-Valdez J. State-of-the-art exosome loading and functionalization techniques for enhanced therapeutics: a review. Crit Rev Biotechnol. 2020;40:804-820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 87] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 115. | Li J, Ma L, Liao X, Liu D, Lu X, Chen S, Ye X, Ding T. Ultrasound-Induced Escherichia coli O157:H7 Cell Death Exhibits Physical Disruption and Biochemical Apoptosis. Front Microbiol. 2018;9:2486. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 116. | Lamichhane TN, Jeyaram A, Patel DB, Parajuli B, Livingston NK, Arumugasaamy N, Schardt JS, Jay SM. Oncogene Knockdown via Active Loading of Small RNAs into Extracellular Vesicles by Sonication. Cell Mol Bioeng. 2016;9:315-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 262] [Article Influence: 29.1] [Reference Citation Analysis (0)] |
| 117. | Zhu Q, Heon M, Zhao Z, He M. Microfluidic engineering of exosomes: editing cellular messages for precision therapeutics. Lab Chip. 2018;18:1690-1703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 89] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 118. | Zhang D, Lee H, Zhu Z, Minhas JK, Jin Y. Enrichment of selective miRNAs in exosomes and delivery of exosomal miRNAs in vitro and in vivo. Am J Physiol Lung Cell Mol Physiol. 2017;312:L110-L121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 241] [Article Influence: 30.1] [Reference Citation Analysis (1)] |
| 119. | Didiot MC, Hall LM, Coles AH, Haraszti RA, Godinho BM, Chase K, Sapp E, Ly S, Alterman JF, Hassler MR, Echeverria D, Raj L, Morrissey DV, DiFiglia M, Aronin N, Khvorova A. Exosome-mediated Delivery of Hydrophobically Modified siRNA for Huntingtin mRNA Silencing. Mol Ther. 2016;24:1836-1847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 366] [Cited by in RCA: 378] [Article Influence: 42.0] [Reference Citation Analysis (0)] |
| 120. | Kassis I, Grigoriadis N, Gowda-Kurkalli B, Mizrachi-Kol R, Ben-Hur T, Slavin S, Abramsky O, Karussis D. Neuroprotection and immunomodulation with mesenchymal stem cells in chronic experimental autoimmune encephalomyelitis. Arch Neurol. 2008;65:753-761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 235] [Cited by in RCA: 230] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 121. | Pittenger MF, Discher DE, Péault BM, Phinney DG, Hare JM, Caplan AI. Mesenchymal stem cell perspective: cell biology to clinical progress. NPJ Regen Med. 2019;4:22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 773] [Cited by in RCA: 1254] [Article Influence: 209.0] [Reference Citation Analysis (0)] |
| 122. | Williams AR, Hare JM. Mesenchymal stem cells: biology, pathophysiology, translational findings, and therapeutic implications for cardiac disease. Circ Res. 2011;109:923-940. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 628] [Cited by in RCA: 652] [Article Influence: 46.6] [Reference Citation Analysis (0)] |
| 123. | Wang A, Brown EG, Lankford L, Keller BA, Pivetti CD, Sitkin NA, Beattie MS, Bresnahan JC, Farmer DL. Placental mesenchymal stromal cells rescue ambulation in ovine myelomeningocele. Stem Cells Transl Med. 2015;4:659-669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 99] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 124. | Lankford L, Selby T, Becker J, Ryzhuk V, Long C, Farmer D, Wang A. Early gestation chorionic villi-derived stromal cells for fetal tissue engineering. World J Stem Cells. 2015;7:195-207. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 125. | Brown EG, Keller BA, Lankford L, Pivetti CD, Hirose S, Farmer DL, Wang A. Age Does Matter: A Pilot Comparison of Placenta-Derived Stromal Cells for in utero Repair of Myelomeningocele Using a Lamb Model. Fetal Diagn Ther. 2016;39:179-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 126. | Vanover M, Wang A, Farmer D. Potential clinical applications of placental stem cells for use in fetal therapy of birth defects. Placenta. 2017;59:107-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 127. | Hao D, Swindell HS, Ramasubramanian L, Liu R, Lam KS, Farmer DL, Wang A. Extracellular Matrix Mimicking Nanofibrous Scaffolds Modified With Mesenchymal Stem Cell-Derived Extracellular Vesicles for Improved Vascularization. Front Bioeng Biotechnol. 2020;8:633. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 44] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 128. | Tracy SA, Ahmed A, Tigges JC, Ericsson M, Pal AK, Zurakowski D, Fauza DO. A comparison of clinically relevant sources of mesenchymal stem cell-derived exosomes: Bone marrow and amniotic fluid. J Pediatr Surg. 2019;54:86-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 44] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 129. | Lopez-Verrilli MA, Caviedes A, Cabrera A, Sandoval S, Wyneken U, Khoury M. Mesenchymal stem cell-derived exosomes from different sources selectively promote neuritic outgrowth. Neuroscience. 2016;320:129-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 154] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 130. | Klingemann H, Matzilevich D, Marchand J. Mesenchymal Stem Cells - Sources and Clinical Applications. Transfus Med Hemother. 2008;35:272-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 107] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 131. | Nichols JE, Niles JA, DeWitt D, Prough D, Parsley M, Vega S, Cantu A, Lee E, Cortiella J. Neurogenic and neuro-protective potential of a novel subpopulation of peripheral blood-derived CD133+ ABCG2+CXCR4+ mesenchymal stem cells: development of autologous cell-based therapeutics for traumatic brain injury. Stem Cell Res Ther. 2013;4:3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 67] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 132. | Hawkins KE, Corcelli M, Dowding K, Ranzoni AM, Vlahova F, Hau KL, Hunjan A, Peebles D, Gressens P, Hagberg H, de Coppi P, Hristova M, Guillot PV. Embryonic Stem Cell-Derived Mesenchymal Stem Cells (MSCs) Have a Superior Neuroprotective Capacity Over Fetal MSCs in the Hypoxic-Ischemic Mouse Brain. Stem Cells Transl Med. 2018;7:439-449. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 72] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 133. | Fafián-Labora J, Lesende-Rodriguez I, Fernández-Pernas P, Sangiao-Alvarellos S, Monserrat L, Arntz OJ, van de Loo FAJ, Mateos J, Arufe MC. Corrigendum: Effect of age on pro-inflammatory miRNAs contained in mesenchymal stem cell-derived extracellular vesicles. Sci Rep. 2017;7:46850. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 134. | Bang OY, Kim EH. Mesenchymal Stem Cell-Derived Extracellular Vesicle Therapy for Stroke: Challenges and Progress. Front Neurol. 2019;10:211. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 98] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 135. | Guidi M, Muiños-Gimeno M, Kagerbauer B, Martí E, Estivill X, Espinosa-Parrilla Y. Overexpression of miR-128 specifically inhibits the truncated isoform of NTRK3 and upregulates BCL2 in SH-SY5Y neuroblastoma cells. BMC Mol Biol. 2010;11:95. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 73] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 136. | Fang H, Li HF, Yang M, Wang RR, Wang QY, Zheng PC, Zhang FX, Zhang JP. microRNA-128 enhances neuroprotective effects of dexmedetomidine on neonatal mice with hypoxic-ischemic brain damage by targeting WNT1. Biomed Pharmacother. 2019;113:108671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 137. | Xu T, Luo Y, Wang J, Zhang N, Gu C, Li L, Qian D, Cai W, Fan J, Yin G. Exosomal miRNA-128-3p from mesenchymal stem cells of aged rats regulates osteogenesis and bone fracture healing by targeting Smad5. J Nanobiotechnology. 2020;18:47. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 100] [Cited by in RCA: 98] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 138. | Zhao H, Li Y, Chen L, Shen C, Xiao Z, Xu R, Wang J, Luo Y. HucMSCs-Derived miR-206-Knockdown Exosomes Contribute to Neuroprotection in Subarachnoid Hemorrhage Induced Early Brain Injury by Targeting BDNF. Neuroscience. 2019;417:11-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 56] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 139. | Allogenic Mesenchymal Stem Cell Derived Exosome in Patients With Acute Ischemic Stroke. [accessed 2020 April 25]. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https://clinicaltrials.gov/ct2/show/NCT03384433 ClinicalTrials.gov Identifier: NCT03384433. |
| 140. | Mendt M, Rezvani K, Shpall E. Mesenchymal stem cell-derived exosomes for clinical use. Bone Marrow Transplant. 2019;54:789-792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 347] [Article Influence: 69.4] [Reference Citation Analysis (0)] |