Published online Jun 26, 2021. doi: 10.4252/wjsc.v13.i6.619
Peer-review started: February 27, 2021
First decision: March 29, 2021
Revised: April 3, 2021
Accepted: May 17, 2021
Article in press: May 17, 2021
Published online: June 26, 2021
Processing time: 118 Days and 19.1 Hours
Mesenchymal stem cells (MSCs) are a population of primary and non-specialized cells, which can be isolated from various tissues. Currently, MSCs are key players in cellular therapy and regenerative medicine. However, the possibility of using MSCs in the treatment of many diseases needs to be preceded, though, by in-depth analysis of their properties, especially by determining the mechanism of tissue homing as well as the mechanism, due to which cells contribute to tissue regeneration. This review is intended to present information on recent findings regarding the mechanism of recruitment and tissue homing by MSCs and discuss current hypotheses for how MSCs can reach target tissues.
Core Tip: Mesenchymal stem cells (MSCs) have been extensively studied for their therapeutic potential in clinical practice and regenerative medicine. MSCs can migrate towards damaged tissue and act as reservoirs for regenerative molecules and growth factors. Consequently, MSC-based therapies rely on the successful migration of these cells into the damaged tissue following administration. Here we look at the factors influencing the migration and colonization of damaged tissues by MSCs.
- Citation: Szydlak R. Biological, chemical and mechanical factors regulating migration and homing of mesenchymal stem cells . World J Stem Cells 2021; 13(6): 619-631
- URL: https://www.wjgnet.com/1948-0210/full/v13/i6/619.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v13.i6.619
Mesenchymal stem cells (MSCs) are a population of primary and non-specialized cells, which can be isolated from various tissues. Friedenstein et al[1] described a bone marrow-derived fibroblast-like cell for the first time, which later became the most extensively studied MSC and are sometimes regarded as the “gold standard.” Later, these cells were identified in nearly every tissue type (peripheral blood, adipose tissue, bone marrow, dental pulp)[1-4]. The human umbilical cord (UC), cord blood, placenta, and amniotic fluid have been shown to contain MSCs[5,6].
Independently of the tissue source, the harvested cells must have common characteristics to be defined as the MSCs. Therefore, to organize the nomenclature and define the characteristics of human MSCs, the International Society for Cellular Therapy proposed three minimum criteria characterizing human MSCs[7]. Accordingly, to classify cells as MSCs cumulatively three conditions must be met by cells: (1) Adhere to plastic during in vitro cultivation; (2) Express a set of surface markers, CD73, CD90, and CD105, simultaneously lacking CD34, CD45, CD14, CD11b, CD79a, CD19 and the major histocompatibility complex class II; and (3) Demonstrate multipotency and significant plasticity of trilinear differentiation to osteoblasts, adipocytes, and chondrocytes[7,8]. Even though a wide range of selection markers defining MSCs was identified, no single marker specific to them has been indicated.
Because of their unique properties, MSCs provide extraordinary therapeutic potential that is used to treat a wide range of disorders. MSCs show high proliferative potential and the ability to differentiate into derived cell lines from all germ leaves. Also, these cells have unique immunomodulatory properties and the ability of directional migration in response to inflammatory factors, and the ability to colonize damaged tissues, where they participate in their regeneration[9]. MSCs have a special ability to secrete many biological factors, including cytokines and growth factors, involved in various processes conducive to tissue remodeling, such as angiogenesis and immunomodulation, but these cells may stimulate endogenous repair mechanisms[10-12].
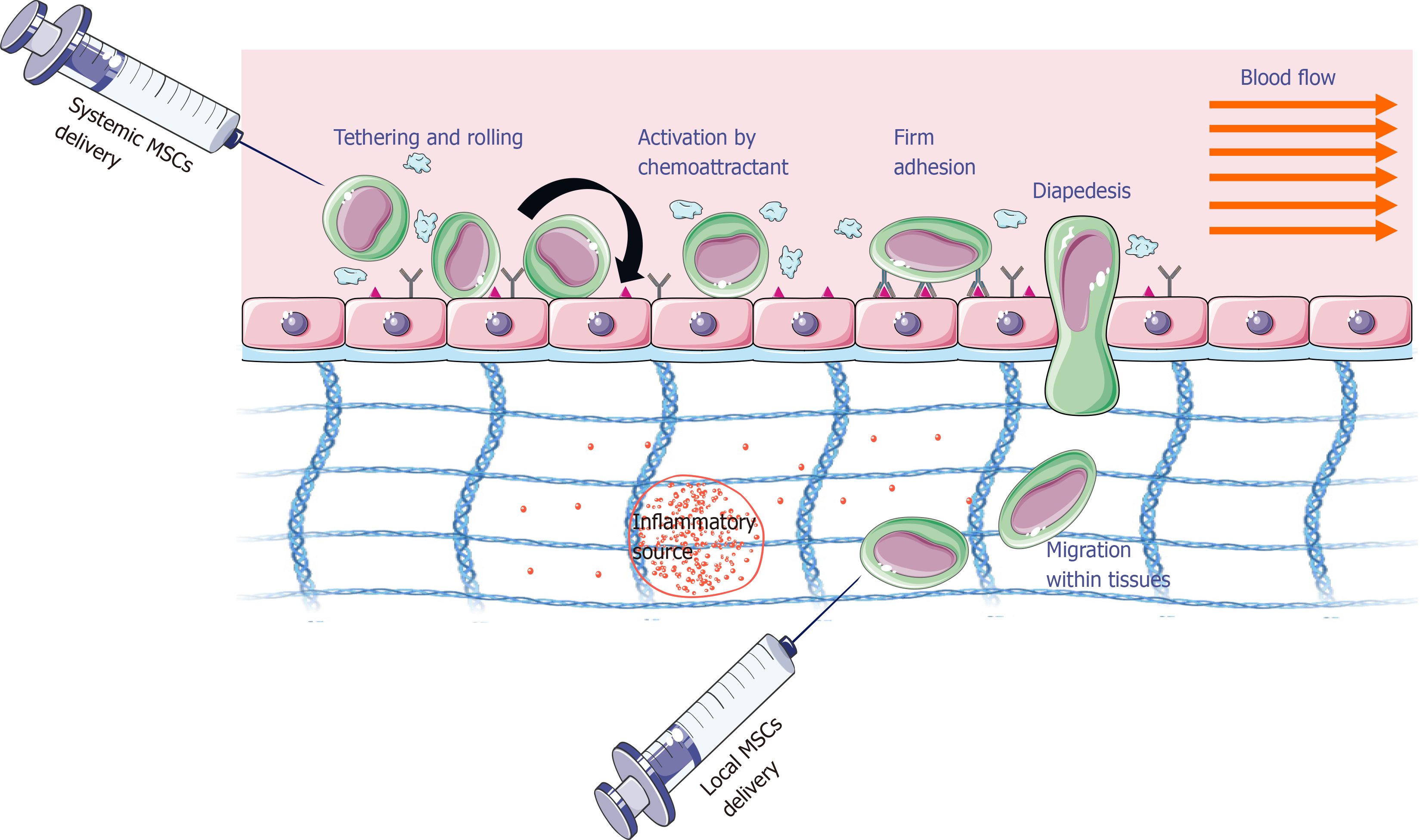
The success of MSC-based therapy depends on MSC homing efficiency, which here means the ability of these cells to reach the damaged tissue. This process is possible thanks to their ability to adhere, migrate, and implant in the target tissue. The homing process can be accomplished with both local and systemic injections (Figure 1)[13]. For local injection, MSCs are transplanted into the target tissue and then directed to the site of injury via a proinflammatory cytokine gradient. In systemic injection, MSCs are administered into the bloodstream and then have to go through a multistep process to leave the circulation and move to the site of the injury. In this case, it is assumed that MSCs exhibit migration mechanisms similar to leukocytes. However, it should be emphasized that they occur with the participation of other adhesion molecules, and MSCs are larger than leukocytes[13]. The therapeutic effectiveness and tissue colonization by MSCs are influenced by several factors that can be divided into biological (e.g., the presence of adhesive molecules, cell source, donor age, doubling rate), biochemical (e.g., cytokines, chemokines, growth factors), and biophysical (e.g., cell size, cell deformability, shear force). There are also other factors such as cultivation conditions, method of cell administration, number of injected and implanted cells, general health of the host, and compliance of the recipient[14-18]. This review summarizes information about the factors influencing tissue migration and colonization by MSCs.
The site used for the administration of MSCs for therapeutic purposes can influence the route taken by cells to reach the desired destination[19]. For therapy, MSCs can be administered through intracardiac, intra-arterial (IA), intraperitoneal, or intravenous (IV) injection. Although intravenous administration is least invasive, more excellent engraftment rates were demonstrated by IA and intracardiac administration as compared to IV administration in models of myocardial infarction[20-22]. They administered radiolabeled cells in models with brain injury and found that IA injection in the extracranial right internal carotid artery (near target) led to greater homing of cells in the brain as compared to IV injection in the femoral vein. Walczak et al[22] demonstrated that the IA injection near the desired organ gave better results than IV injection at a distant point[22]. In cases of IV administration, MSCs accumulated in filtering parts of the body such as the spleen, liver, or lung, but this accumulation was reduced in cases of IA injection[20,23,24]. However, there was a higher chance of microvascular occlusions with IA injection, a condition known as passive entrapment[22]. In cases of IA and intracardiac administration, a significantly more MSCs were able to reach and engraft at an ischemic site as the cells bypassing the lungs.
The intraperitoneal administration of MSCs is occasionally used. It was used to administer MSCs to fetuses in mice with muscular dystrophy as IV injection was considered to be inappropriate for this particular case[25]. The donor cells were detected in muscular as well as non-muscular tissues. Finally, one can also use the method of local delivery by injection of MSCs directly into the target site. Beggs et al[26] administered Dil-labeled MSCs into baboons through IV injection but could not detect cells in limb muscles[26].
On the other hand, when they injected the cells directly into the muscle, DiO-labeled MSCs could be caught there[26]. However, Muschler et al[27] reported that this method is not feasible in most clinical cases because it is too invasive, particularly in the brain or heart[27]. Moreover, locally injected cells may die before their role in healing because of a limited supply of oxygen and nutrients.
Because intravascular infusion is the most common form of therapy, it is crucial to understand the mechanisms by which MSCs might be delivered to the microcirculation, become adherent to the walls of blood vessels and subsequently migrate through them. It is also useful to consider whether endogenous MSCs can circulate ‘normally’ in the blood.
Most research to date has focused on the behavior of exogenous bone marrow MSCs (BM-MSCs), which may differ from MSCs obtained from other sources. Recent work suggests that MSCs obtained from perinatal tissues for therapeutic purposes may have more advantages, such as better cell availability and ethical aspects. Sheriff et al[28] conducted a study comparing the adhesive properties of UC-MSCs and BM-MSCs and their interactions with platelets, which may be of particular importance for systemic injections[28]. This study showed that UC-MSCs had a greater ability to adhere to extracellular matrix proteins compared to BM-MSCs and that UC-MSCs also caused platelet activation[28]. In another comparative study by Alanazi et al[29], BM-MSCs, UC-MSCs, and tubercular-MSCs isolated from trabecular bone were tested. They showed that there were some differences in adhesive properties as well as in migration through the porous filter[29].
The migration properties of MSCs can also be influenced by cultivation conditions. Rombouts et al[30] showed that freshly isolated MSCs have higher tissue colonization capacity compared to in vitro cultured cells[30]. Probably this is the result of aging and differentiation of MSCs under in vitro culture conditions[31,32]. Culture conditions also have a significant impact on the homing capacity as they can modify the expression of surface markers involved in this process. For example, hypoxia and the presence of cytokines [e.g., interleukin (IL)-6, hepatocyte growth factor] can regulate the expression of the chemokine receptor (CXCR)4 receptor, which is involved in the migration of MSCs[33,34].
Exogenous MSCs injected into the body can bind non-specifically in microvessels or with adhesion molecules such as integrins, bind to endothelial cells or extracellular matrix proteins (i.e. collagen, fibronectin, laminin), and then transmigrate through the endothelium and basal membrane to tissues[35-39]. MSCs are thought to use the same migration mechanism as leukocytes[13]. However, in contrast to the well-described mechanisms of leukocyte adhesion and migration, the mechanism of tissue homing by MSCs has not yet been fully understood, even though many studies are assessing MSC adhesive molecules and possible mechanisms of vascular wall adhesion and migration (Table 1) as well as the role of chemokines in guiding MSCs to target tissues[40].
| Molecules | Stage of homing | Ref. | |
| Adhesion molecules | CD24, CD29, CD44, CD49a-f, CD51/61VCAM-1, ICAM-1, ICAM-2, Pselectin | Rolling, adhesion, transendothelial migration | Langer et al[55], 2009 |
| Eseonu and De Bari[66], 2015 | |||
| Chemokines receptors and chemokines | CXCR1/2/3/4/5/6, CCR1/2/4/6/8/9, MIP-1α, MCP-1, SDF-1 | Chemotaxis | Kitaori et al[67], 2009 |
| Su et al[68], 2017 | |||
| Zhang et al[73], 2015 | |||
| Fu et al[74], 2019 | |||
| Proinflammatory cytokines and growth factors | TGF-β, IGF-1, TNF-α, IL-1β, IL-8, IL-6, IL-3, SCF, HGF, EGF, VEGF, FGF, PDGF, IGF | Chemotaxis | De Becker et al[70], 2007 |
| Yuan et al[75], 2012 | |||
| Gao et al[76], 2001 | |||
| Extracellular matrix metalloproteinases | MMP-1, MMP-2 | Invasion | Majumdar et al[43], 2003 |
| Schrepfer et al[82], 2007 |
Before MSCs migrate through the vessel wall, they “crawl” on its surface, looking for the best place for adhesion and then transmigration through the endothelium (Figure 1)[13]. Interactions of integrins that are expressed in the MSC cell membrane and adhesion molecules on the endothelial surface [(vascular cell adhesion molecule (VCAM)-1) and intercellular adhesion molecule (ICAM)] can lead to the formation of so-called docking structures and transmigration wells that are sites rich in ICAM-1, VCAM-1 molecules, proteins, and cytoskeleton components (e.g., α-actinin).
If the homing concept is correct, tissues would need to recruit circulating MSCs from the flow to ensure effective delivery to damaged sites. For this purpose, MSCs have on their surface many different adhesion molecules shared by leukocytes. These adhesion molecules include CD24, CD29 (β1-integrin), CD44, and CD49a-f (α1-α6-integrin), although other studies found no CD24[41,42]. Adhesion molecules that are found on endothelial cells are also expressed by MSCs. These molecules include VCAM-1, ICAM-1, and ICAM-2[43].
It seems that the number and type of adhesion molecules found to be present on MSCs may be influenced by the source of MSCs and the method used for their isolation and culture. For example, adhesion molecules expressed by MSCs at passage four and passage six were found to be different[44]. There was a linear relationship between passage number and the expression of CD49, but a decrease in the expression of CD44 was noted at passage six. However, other reports indicated no difference between the molecules expressed by MSCs at passages 3, 5, and 7 (e.g., CD73, CD90, and CD105)[45]. Concerning the origin of MSCs, it was found that adhesion molecules expressed by MSCs isolated from bone marrow and those isolated from adipose tissue differed. Differences in expression were noted for cell adhesion molecules CD49d (integrin α4), CD54 (ICAM-1), CD34, and CD106 (VCAM-1) with large variation in CD106 (VCAM1) and CD54 (ICAM-1)[45]. It is therefore likely that the source and methods of isolation and expansion must be taken into consideration when evaluating adhesive properties of MSC adhesion.
Several mechanisms involving different adhesion molecules have been proposed for recruiting flowing MSCs to the vasculature. During a study on MSC recruitment to the vasculature in mice, Rüster et al[42] found that P-selectin and α4β1-integrin/VCAM-1 played a significant role in recruitment in venules[42]. In comparison with the wild-type controls, the Pselectin-/- mice demonstrated a lesser degree of MSCs rolling in the ear venules. The function of other adhesion molecules was also investigated through in vitro studies that made use of endothelial cells as a substrate for the adhesion. During a flow-based assay, the number of MSCs demonstrating adherence decreased considerably when P-selectin was blocked on the tumor necrosis factor-α (TNF-α)-treated endothelial cells[42]. However, it was found that MSCs neither expressed Pselectin glycoprotein ligand-1 (CD162) nor the alternative P-selectin ligand, CD24, on their surface[42]. In the same study, adherence of MSCs to the TNF-α-treated endothelial cells was found to be reduced after blocking α4β1-integrin or VCAM-1 to a similar degree to each other, showing a role for this pathway[42]. It should be highlighted that in these studies the flow was reduced to very low shear stress to allow attachment followed by an increase in flow to “washout.” In another study, small numbers of MSCs adhered to cytokine-treated endothelial cells after prolonged perfusion at 0.1 Pa, also through VCAM-1[46].
In the studies conducted by Luu et al[47], MSCs were also perfused over endothelial cells treated with TNF-α[47]. It was found that MSC adhesion was negligible at a wall shear stress of 0.05 Pa, which resembles the low end of venular shear. If the flow was decreased to 0.01 Pa to allow attachment, then washed out at 0.05 Pa, adhesion could be detected on stimulated but not unstimulated endothelial cells[47]. MSCs adhered in large numbers if allowed to remain stationary and in contact with endothelial cells for 30 min before washout at 0.05 Pa. Chamberlain et al[48] also found little adhesion of perfused MSCs to endothelial cells unless flow was stopped, and the cells were allowed to settle before washing out[48]. These data suggested that attachment of flowing MSCs in intact vessels would be rare under normal circulatory conditions, but that MSCs could adhere to endothelium only if already arrested or trapped[48].
A wide range of different cells express the glycoprotein CD44 on their surface, which can act as a ligand to allow adhesion via several other molecules, including hyaluronan[49]. Its role as a ligand for P-selectin has also been reported, and it may be the ligand for Pselectin expressed by MSCs. Studies indicate that hematopoietic cells E-/L-selectin ligand is capable of binding with E-selectin[50,51]. While MSCs have a high expression of CD44 molecules on their surface, it was found that MSC adhesion was not decreased by blocking E-selectin on endothelial cells[42]. However, other researchers have found CD44 on MSCs to interact with E-selectin[52].
The molecular mechanisms involved in mouse MSC recruitment to the heart were investigated in animals suffering myocardial infarction[53]. Upregulation of several genes was recorded in the heart after infarct, and these included the genes for VCAM-1,ICAM-1-1, and Eselectin. Recruitment of murine MSCs in the infracted myocardium decreased when MSCs were treated with the antibody against β1-integrin. Blockade of α4β1-integrin (CD49d/CD29) did not affect recruitment, and the particular α-integrin subunit working in this process was not identified, although the presence of α9-, α6- and α8-integrins were demonstrated[53].
So far, several adhesion molecules have been identified to be involved in MSC transendothelial migration. These include very late antigen-4 (VLA-4), VCAM-1, ICAM-1, and P-selectin[42,53]. Adhesive particles, including integrins, selectins, and chemokine receptors, are involved in the rolling, adhesion, and transmigration of MSCs. MSCs have been shown to express a diversity of receptors associated with intercellular contacts and adherence to extracellular matrix proteins, such as integrins α1, α2, α3, α4, α5, αv, β1, β3 and β4, and other adhesive molecules, i.e. VCAM-1, ICAM-1, ICAM-3, and CD166. Some studies have shown that the interaction of MSCs with the endothelium is mediated by P-selectin. Other studies have shown that E-selectin and L-selectin are not expressed in the MSC cell membrane. and their involvement in the interaction with the wall of blood vessels is not significant[42]. It has also been reported that, if present, this mechanism only has an indirect function due to the strong interaction of VLA-4/VCAM-1 particles, which are crucial receptors for MSC transendothelial migration. Steingen et al[54] reported that MSCs can migrate through non-activated endothelium using VLA-4/VCAM-1 molecules and tend to integrate with the endothelial layer instead of undergoing full diapedesis. Among the integrin family, integrin α4β1, which is a cell surface heterodimer and mediates cell-cell and cell-environment contact, plays an essential role in migration and chemotaxis. However, because transendothelial migration of MSCs has not been entirely blocked by anti-VLA-4 and anti-VCAM-1 antibodies, it can be assumed that other integrins are also involved in this process[54].
Platelets have been reported to be involved in the recruitment of MSCs in both in vitro and in vivo models. In a flow-based adhesion assay, Langer et al[55] noticed an increase in the recruitment of MSCs to human arterial endothelial cells when the endothelial cells were preincubated with platelets[55]. In particular, preincubation with platelets caused more excellent MSC adhesion in comparison with the activation of endothelial cells with IL-1β. In vivo studies generated results that followed these findings. MSC adhesion was found to be decreased considerably in a murine model with carotid artery injury after treatment with anti-GPIb and platelet-depleting antibody. It was also demonstrated that αvβ3-integrin blockade reduced the adhesion of platelets to immobilized MSCs[55]. In a model of pulmonary arterial hypertension, infused rat MSCs protected a rise in right-sided blood pressure and cardiac hypertrophy[56]. MSCs were found in the lung, and their adhesion there was reduced by blockade of P-selectin and GpIIbIIIa. The same receptors were found to support the attachment of MSCs along with platelets to collagen in an in vitro flow assay. It was concluded that platelets mediated MSC homing to the lung. In a recent study, there was preferential trafficking of infused MSCs to an inflamed vs control ear, but this was decreased if platelets were depleted from the blood[57]. Direct observation of microvessels showed MSCs were adherent along with platelets and neutrophils. The above studies strongly suggest that MSCs will interact with platelets in the blood and that this interaction will modify their behavior in vivo.
In MSC trafficking, chemokines released from tissues and endothelial cells can promote the activation of ligands involved in adhesion, migration, chemotaxis, and homing of MSCs in target tissues. Many reports suggest that damaged tissue releases specific factors that act as chemoattractants to facilitate the adhesion, migration, and homing of MSCs in affected areas. Studies have shown that MSCs are capable of migrating to inflamed tissues in response to factors that are regulated under inflammation[4,58,59]. To date, many chemokines and growth factors have been identified that are involved in the migration process. These include inflammatory cytokines such as TNF-α, IL-1β, IL-6, and IL8 and many growth factors, e.g., epidermal growth factor, vascular endothelial-derived growth factor-A, fibroblast growth factor, platelet-derived growth factor (PDGF-AB), hepatocyte growth factor, transforming growth factor-β1, stromal cell-derived factor, and insulin-like growth factor (IGF1)[39,59-62].
Some studies have shown the expression of chemokine receptors by MSCs, including CXCR1, CXCR2, CXCR3, CXCR4, CXCR5, CXCR6, CCR1, CCR2, CCR4, CCR6, CCR7, CCR8, CCR9, and CCR10, and indicated the functional roles of some of them in the migration process of MSCs[60,63-65]. It has been proved that CXCR1, CXCR2, CXCR4, CCR1, CCR2, IL-8, macrophage inflammatory protein-1α, and monocyte chemoattractant protein-1 are involved in the migration of MSCs to damaged tissue[66]. Other studies have shown that the stromal cell-derived factor-1/CXCR4 axis plays an essential role in the movement of MSCs isolated from the bone marrow[67,68]. Thus, it is likely that the chemokines released from the tissues cause the intracellular CXCR4 receptor to move to the cell surface, which contributes to the migration of MSCs to the destination.
It has also been shown that an increase in IL-8 concentration in damaged tissues can activate MSC migration[63]. The active role of IL-6, PDGF, PDGFR-α, PDGFR-β, vascular endothelial growth factor receptor 1, and insulin-like growth factor-1 have been indicated in BM-MSC migration studies[66]. PDGFR is strongly expressed on the surface of BM-MSCs, and PDGF induces BM-MSCs migration. The migration test through a porous filter also showed that PDGF had a stronger effect on MSC chemotaxis than stromal cell-derived factor-1 and monocyte chemoattractant protein-1[69]. Inflammatory cytokines such as IL1β and TNF-α stimulate the production of matrix metalloproteinase (MMPs) by MSCs and trigger the activation of chemotactic migration through the extracellular matrix[35]. According to studies, many chemokines play a role in the induction of MSC migration, but characteristics including the settlement of MSCs require further in vitro and in vivo studies.
It has also been confirmed that an essential role of MSC migration is played by proteolytic enzymes-MMPs, which regulate the degradation of the extracellular matrix[36,70]. Different MMPs and their signaling pathways have been shown to affect MSC differentiation, migration, angiogenesis, and proliferation. The migration and invasion of MSCs into damaged tissues are facilitated by the expression of CXCR4, MMP-2, and MT1-MMP[71,72].
Exogenous MSCs injected into the body during migration through peripheral blood circulation towards the damaged tissue are exposed to various hemodynamic forces applied to the vessel walls, including shear stress and cyclic mechanical load. It has been observed that mechanical loads affect the migration of MSCs. As an example, studies have shown that cyclic mechanical stretching (10%, 8 h) promoted MSC migration but led to a decrease in the invasive potential of MSCs[73,74].
Shear stress is another type of force inside the blood vessels. However, few studies have focused on the effects of shear stress on MSC migration. It was observed that shear stress (approximately 0.2 Pa) promoted MSC migration in the wound healing test while higher shear stress (> 2 Pa) significantly inhibited MSC migration[75].
Although there is ample evidence that specific ligand-receptor pairs are involved in tissue homing MSCs, mechanical entrapment of MSCs at sites of injury or in a tumor occurs at least in part in limited environments. The key difference between MSCs and lymphocytes is their size, with cell diameters ranging from 15-30 μm to 4-12 μm, respectively[76,77]. This larger cell size, especially after ex vivo cultivation, can lead to the passive retention of MSCs in small diameter vessels such as terminal arterioles, capillaries, and extra-glomerular venules due to mechanical entrapment[78]. Indeed, the vast majority of MSCs administered intravenously are cleared rapidly from the blood and are found in the pulmonary capillaries within minutes of an injection[23,79,80]. In both, animal models and clinical trials, this rapid entrapment is followed by removal from the lungs and accumulation in the spleen and liver[23,79-81]. The mechanical entrapment in the lungs is because the pulmonary capillaries have a diameter of 10-15 μm[76,82-84]. Studies conducted by Dutly et al[84] with micros
It can be assumed that the ability of cells to deform may facilitate the movement of larger cells through smaller vessels[18,59,85]. Although cellular deformability may to some extent facilitate the traverse of larger cells through smaller vessels[85].
IA infusions can reduce the entrapment of MSCs in the lung by providing one pass through the systemic circulation and exposure to peripheral tissues before entering the lungs. However, mechanical confinement may still be the predominant driver of MSC biodistribution. To date, little research has been done to determine the importance of active and passive arrest in the lung or other tissues. However, it is likely that both mechanisms are important and can be manipulated to increase the efficiency of targeting MSCs.
The vast majority of exogenously injected MSCs have limited access to damaged target tissue due to mechanical entrapment. To partially overcome this barrier and improve targeting, preadministration of vasodilators such as sodium nitroprusside was used in mouse models[82,83] to reduce lung entrapment. In addition, many researchers have developed ex vivo expansion protocols by which MSC cultures with smaller mean cell diameters can be obtained[86,87].
In conclusion, although MSC-based therapies give hope for effective treatment of many incurable diseases, the low percentage of MSCs homing to damaged tissue remains a big challenge in regenerative medicine. Even though many factors have been identified to be involved in the MSC migration process, undoubtedly, one of the great needs of MSC-based therapy is the improvement of the effectiveness of MSC homing and obtaining high-grade target tissue uptake. To date, it has been observed that only a small percentage of the injected MSCs authentically reach and remain in the target tissue[88]. Why is the homing efficiency so low? Several factors are presumed to be involved. A lot of transplanted MSCs may be trapped in the lung capillaries[89,90]. Hence, to reduce lung entrapment, some research groups have taken an approach with vasodilators like heparin[76,91]. Moreover, some of the MSCs are distributed into the liver, spleen, and sites of inflammation or damage.
As discussed above, this migration of MSCs is regulated by a wide spectrum of factors. Essentially, the homing process is based on specific molecular interactions rather than passive distribution. Thus, effective migration of MSCs and implantation into the target tissue requires a high expression level of the appropriate adhesion molecules. For example, Wynn et al[92] observed that a small population of MSCs expressed the CXCR4 receptor and that only these cells can migrate specifically to the bone marrow[92]. Also, the expression of the surface markers involved in the homing process may be modified by culture conditions[31,60]. The methods of preparing the MSCs for injection as well as the methods of injecting these cells remain at the experimental level. Parallel studies on the biology of MSCs and clinical trials are still ongoing. While much remains to be done, addressing the basic biological mechanisms underlying tissue homing in MSCs in vivo will reveal new optimization pathways.
To overcome the problems with a low level of retention of regenerative cells, various strategies have been taken to improve the MSC homing as discussed elsewhere recently[93]. In general, these approaches include targeted administration, genetic modification, in vitro stimulation, cell surface engineering, magnetic guidance, radiotherapeutic techniques, and target tissue modification[93,94]. Many of these approaches are still debatable because many of them have not been validated in vivo yet. For example, targeted administration may not always be feasible or may be a highly invasive procedure depending on the target tissue. In addition, modifying MSCs does not prevent their distribution to organs other than the target. Also, modifying target tissue by chemical or genetic methods raises patient safety concerns. While it is still an active area of study, these limitations pose huge barriers to their application in the clinic. It is expected that future research will disclose which of these approaches provide the most effective treatment. Such research is essential for advancing the field of cell-based therapies and increasing the efficacy of therapies in applications ranging from immune modulation to regeneration.
I would like to express my sincere gratitude and deep appreciation to Prof. Piotr Laidler and Prof. Marcin Majka for all inspirational discussions about stem cell biology and all scientific advice.
Manuscript source: Invited manuscript
Specialty type: Cell and tissue engineering
Country/Territory of origin: Poland
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): E
P-Reviewer: Bian Q, Chen F, Tanabe S S-Editor: Fan JR L-Editor: Filipodia P-Editor: Xing YX
| 1. | Friedenstein AJ, Chailakhjan RK, Lalykina KS. The development of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells. Cell Tissue Kinet. 1970;3:393-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 408] [Cited by in RCA: 943] [Article Influence: 17.1] [Reference Citation Analysis (1)] |
| 2. | Murray IR, West CC, Hardy WR, James AW, Park TS, Nguyen A, Tawonsawatruk T, Lazzari L, Soo C, Péault B. Natural history of mesenchymal stem cells, from vessel walls to culture vessels. Cell Mol Life Sci. 2014;71:1353-1374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 200] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 3. | Berebichez-Fridman R, Montero-Olvera PR. Sources and Clinical Applications of Mesenchymal Stem Cells: State-of-the-art review. Sultan Qaboos Univ Med J. 2018;18:e264-e277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 276] [Article Influence: 39.4] [Reference Citation Analysis (0)] |
| 4. | Szydlak R. Mesenchymal stem cells' homing and cardiac tissue repair. Acta Biochim Pol. 2019;66:483-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 5. | In 't Anker PS, Scherjon SA, Kleijburg-van der Keur C, de Groot-Swings GM, Claas FH, Fibbe WE, Kanhai HH. Isolation of mesenchymal stem cells of fetal or maternal origin from human placenta. Stem Cells. 2004;22:1338-1345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 824] [Cited by in RCA: 818] [Article Influence: 40.9] [Reference Citation Analysis (0)] |
| 6. | Panepucci RA, Siufi JL, Silva WA Jr, Proto-Siquiera R, Neder L, Orellana M, Rocha V, Covas DT, Zago MA. Comparison of gene expression of umbilical cord vein and bone marrow-derived mesenchymal stem cells. Stem Cells. 2004;22:1263-1278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 206] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 7. | Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop Dj, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11055] [Cited by in RCA: 12657] [Article Influence: 703.2] [Reference Citation Analysis (2)] |
| 8. | Krampera M, Galipeau J, Shi Y, Tarte K, Sensebe L; MSC Committee of the International Society for Cellular Therapy (ISCT). Immunological characterization of multipotent mesenchymal stromal cells--The International Society for Cellular Therapy (ISCT) working proposal. Cytotherapy. 2013;15:1054-1061. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 297] [Cited by in RCA: 342] [Article Influence: 28.5] [Reference Citation Analysis (0)] |
| 9. | Rosenthal N. Prometheus's vulture and the stem-cell promise. N Engl J Med. 2003;349:267-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 108] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 10. | Gnecchi M, Danieli P, Cervio E. Mesenchymal stem cell therapy for heart disease. Vascul Pharmacol. 2012;57:48-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 111] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 11. | Hamid T, Prabhu SD. Immunomodulation Is the Key to Cardiac Repair. Circ Res. 2017;120:1530-1532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 12. | Vagnozzi RJ, Maillet M, Sargent MA, Khalil H, Johansen AKZ, Schwanekamp JA, York AJ, Huang V, Nahrendorf M, Sadayappan S, Molkentin JD. An acute immune response underlies the benefit of cardiac stem cell therapy. Nature. 2020;577:405-409. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 252] [Cited by in RCA: 414] [Article Influence: 69.0] [Reference Citation Analysis (0)] |
| 13. | Nitzsche F, Müller C, Lukomska B, Jolkkonen J, Deten A, Boltze J. Concise Review: MSC Adhesion Cascade-Insights into Homing and Transendothelial Migration. Stem Cells. 2017;35:1446-1460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 281] [Article Influence: 35.1] [Reference Citation Analysis (0)] |
| 14. | Beane OS, Fonseca VC, Cooper LL, Koren G, Darling EM. Impact of aging on the regenerative properties of bone marrow-, muscle-, and adipose-derived mesenchymal stem/stromal cells. PLoS One. 2014;9:e115963. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 210] [Cited by in RCA: 241] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 15. | Siegel G, Kluba T, Hermanutz-Klein U, Bieback K, Northoff H, Schäfer R. Phenotype, donor age and gender affect function of human bone marrow-derived mesenchymal stromal cells. BMC Med. 2013;11:146. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 308] [Cited by in RCA: 360] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 16. | Izadpanah R, Kaushal D, Kriedt C, Tsien F, Patel B, Dufour J, Bunnell BA. Long-term in vitro expansion alters the biology of adult mesenchymal stem cells. Cancer Res. 2008;68:4229-4238. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 286] [Cited by in RCA: 261] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 17. | Zhuang Y, Li D, Fu J, Shi Q, Lu Y, Ju X. Comparison of biological properties of umbilical cord-derived mesenchymal stem cells from early and late passages: immunomodulatory ability is enhanced in aged cells. Mol Med Rep. 2015;11:166-174. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 50] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 18. | McGrail DJ, McAndrews KM, Dawson MR. Biomechanical analysis predicts decreased human mesenchymal stem cell function before molecular differences. Exp Cell Res. 2013;319:684-696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 19. | Boltze J, Arnold A, Walczak P, Jolkkonen J, Cui L, Wagner DC. The Dark Side of the Force - Constraints and Complications of Cell Therapies for Stroke. Front Neurol. 2015;6:155. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 93] [Cited by in RCA: 118] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 20. | Barbash IM, Chouraqui P, Baron J, Feinberg MS, Etzion S, Tessone A, Miller L, Guetta E, Zipori D, Kedes LH, Kloner RA, Leor J. Systemic delivery of bone marrow-derived mesenchymal stem cells to the infarcted myocardium: feasibility, cell migration, and body distribution. Circulation. 2003;108:863-868. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 916] [Cited by in RCA: 898] [Article Influence: 40.8] [Reference Citation Analysis (0)] |
| 21. | Freyman T, Polin G, Osman H, Crary J, Lu M, Cheng L, Palasis M, Wilensky RL. A quantitative, randomized study evaluating three methods of mesenchymal stem cell delivery following myocardial infarction. Eur Heart J. 2006;27:1114-1122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 462] [Cited by in RCA: 455] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 22. | Walczak P, Zhang J, Gilad AA, Kedziorek DA, Ruiz-Cabello J, Young RG, Pittenger MF, van Zijl PC, Huang J, Bulte JW. Dual-modality monitoring of targeted intraarterial delivery of mesenchymal stem cells after transient ischemia. Stroke. 2008;39:1569-1574. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 335] [Cited by in RCA: 301] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 23. | Kraitchman DL, Tatsumi M, Gilson WD, Ishimori T, Kedziorek D, Walczak P, Segars WP, Chen HH, Fritzges D, Izbudak I, Young RG, Marcelino M, Pittenger MF, Solaiyappan M, Boston RC, Tsui BM, Wahl RL, Bulte JW. Dynamic imaging of allogeneic mesenchymal stem cells trafficking to myocardial infarction. Circulation. 2005;112:1451-1461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 450] [Cited by in RCA: 456] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 24. | Sackstein R, Merzaban JS, Cain DW, Dagia NM, Spencer JA, Lin CP, Wohlgemuth R. Ex vivo glycan engineering of CD44 programs human multipotent mesenchymal stromal cell trafficking to bone. Nat Med. 2008;14:181-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 464] [Cited by in RCA: 469] [Article Influence: 27.6] [Reference Citation Analysis (0)] |
| 25. | Chan J, Waddington SN, O'Donoghue K, Kurata H, Guillot PV, Gotherstrom C, Themis M, Morgan JE, Fisk NM. Widespread distribution and muscle differentiation of human fetal mesenchymal stem cells after intrauterine transplantation in dystrophic mdx mouse. Stem Cells. 2007;25:875-884. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 90] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 26. | Beggs KJ, Lyubimov A, Borneman JN, Bartholomew A, Moseley A, Dodds R, Archambault MP, Smith AK, McIntosh KR. Immunologic consequences of multiple, high-dose administration of allogeneic mesenchymal stem cells to baboons. Cell Transplant. 2006;15:711-721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 126] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 27. | Muschler GF, Nakamoto C, Griffith LG. Engineering principles of clinical cell-based tissue engineering. J Bone Jt Surg-Ser A. 2004;. [RCA] [DOI] [Full Text] [Cited by in Crossref: 646] [Cited by in RCA: 546] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 28. | Sheriff L, Alanazi A, Ward LSC, Ward C, Munir H, Rayes J, Alassiri M, Watson SP, Newsome PN, Rainger GE, Kalia N, Frampton J, McGettrick HM, Nash GB. Origin-Specific Adhesive Interactions of Mesenchymal Stem Cells with Platelets Influence Their Behavior After Infusion. Stem Cells. 2018;36:1062-1074. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 29. | Alanazi A, Munir H, Alassiri M, Ward LSC, McGettrick HM, Nash GB. Comparative adhesive and migratory properties of mesenchymal stem cells from different tissues. Biorheology. 2019;56:15-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 30. | Rombouts WJ, Ploemacher RE. Primary murine MSC show highly efficient homing to the bone marrow but lose homing ability following culture. Leukemia. 2003;17:160-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 430] [Cited by in RCA: 420] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 31. | Phinney DG, Prockop DJ. Concise review: mesenchymal stem/multipotent stromal cells: the state of transdifferentiation and modes of tissue repair--current views. Stem Cells. 2007;25:2896-2902. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1391] [Cited by in RCA: 1410] [Article Influence: 78.3] [Reference Citation Analysis (0)] |
| 32. | Le Blanc K, Tammik L, Sundberg B, Haynesworth SE, Ringdén O. Mesenchymal stem cells inhibit and stimulate mixed lymphocyte cultures and mitogenic responses independently of the major histocompatibility complex. Scand J Immunol. 2003;57:11-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1048] [Cited by in RCA: 996] [Article Influence: 45.3] [Reference Citation Analysis (0)] |
| 33. | Schioppa T, Uranchimeg B, Saccani A, Biswas SK, Doni A, Rapisarda A, Bernasconi S, Saccani S, Nebuloni M, Vago L, Mantovani A, Melillo G, Sica A. Regulation of the chemokine receptor CXCR4 by hypoxia. J Exp Med. 2003;198:1391-1402. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 596] [Cited by in RCA: 623] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 34. | Shi M, Li J, Liao L, Chen B, Li B, Chen L, Jia H, Zhao RC. Regulation of CXCR4 expression in human mesenchymal stem cells by cytokine treatment: role in homing efficiency in NOD/SCID mice. Haematologica. 2007;92:897-904. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 251] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 35. | Sohni A, Verfaillie CM. Mesenchymal stem cells migration homing and tracking. Stem Cells Int. 2013;2013:130763. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 248] [Cited by in RCA: 300] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 36. | Ries C, Egea V, Karow M, Kolb H, Jochum M, Neth P. MMP-2, MT1-MMP, and TIMP-2 are essential for the invasive capacity of human mesenchymal stem cells: differential regulation by inflammatory cytokines. Blood. 2007;109:4055-4063. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 378] [Cited by in RCA: 405] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 37. | Docheva D, Popov C, Mutschler W, Schieker M. Human mesenchymal stem cells in contact with their environment: surface characteristics and the integrin system. J Cell Mol Med. 2007;11:21-38. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 229] [Cited by in RCA: 217] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 38. | Berry MF, Engler AJ, Joseph Woo Y, Pirolli TJ, Bish LT, Jayasankar V, Morine KJ, Gardner TJ, Discher DE, Lee Sweeney H. Regulation and Function of Stem Cells in the Cardiovascular System Mesenchymal stem cell injection after myocardial infarction improves myocardial compliance. Am J Physiol Hear Circ Physiol. 2006;. |
| 39. | Fox JM, Chamberlain G, Ashton BA, Middleton J. Recent advances into the understanding of mesenchymal stem cell trafficking. Br J Haematol. 2007;137:491-502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 204] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 40. | Wang S, Wu Y. The role of chemokines in mesenchymal stromal cell homing to sites of inflammation, including infarcted myocardium. Biol Ther Appl Mesenchymal Cells. 2016;. [DOI] [Full Text] |
| 41. | Chamberlain G, Fox J, Ashton B, Middleton J. Concise review: mesenchymal stem cells: their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells. 2007;25:2739-2749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1843] [Cited by in RCA: 1670] [Article Influence: 92.8] [Reference Citation Analysis (0)] |
| 42. | Rüster B, Göttig S, Ludwig RJ, Bistrian R, Müller S, Seifried E, Gille J, Henschler R. Mesenchymal stem cells display coordinated rolling and adhesion behavior on endothelial cells. Blood. 2006;108:3938-3944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 415] [Cited by in RCA: 412] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 43. | Majumdar MK, Keane-Moore M, Buyaner D, Hardy WB, Moorman MA, McIntosh KR, Mosca JD. Characterization and functionality of cell surface molecules on human mesenchymal stem cells. J Biomed Sci. 2003;10:228-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 44. | Aldridge V, Garg A, Davies N, Bartlett DC, Youster J, Beard H, Kavanagh DP, Kalia N, Frampton J, Lalor PF, Newsome PN. Human mesenchymal stem cells are recruited to injured liver in a β1-integrin and CD44 dependent manner. Hepatology. 2012;56:1063-1073. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 53] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 45. | Lo Surdo J, Bauer SR. Quantitative approaches to detect donor and passage differences in adipogenic potential and clonogenicity in human bone marrow-derived mesenchymal stem cells. Tissue Eng Part C Methods. 2012;18:877-889. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 77] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 46. | Segers VF, Van Riet I, Andries LJ, Lemmens K, Demolder MJ, De Becker AJ, Kockx MM, De Keulenaer GW. Mesenchymal stem cell adhesion to cardiac microvascular endothelium: activators and mechanisms. Am J Physiol Heart Circ Physiol. 2006;290:H1370-H1377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 152] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 47. | Luu NT, McGettrick HM, Buckley CD, Newsome PN, Rainger GE, Frampton J, Nash GB. Crosstalk between mesenchymal stem cells and endothelial cells leads to downregulation of cytokine-induced leukocyte recruitment. Stem Cells. 2013;31:2690-2702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 61] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 48. | Chamberlain G, Smith H, Rainger GE, Middleton J. Mesenchymal stem cells exhibit firm adhesion, crawling, spreading and transmigration across aortic endothelial cells: effects of chemokines and shear. PLoS One. 2011;6:e25663. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 87] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 49. | Zhu H, Mitsuhashi N, Klein A, Barsky LW, Weinberg K, Barr ML, Demetriou A, Wu GD. The role of the hyaluronan receptor CD44 in mesenchymal stem cell migration in the extracellular matrix. Stem Cells. 2006;24:928-935. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 294] [Cited by in RCA: 303] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 50. | Alves CS, Burdick MM, Thomas SN, Pawar P, Konstantopoulos K. The dual role of CD44 as a functional P-selectin ligand and fibrin receptor in colon carcinoma cell adhesion. Am J Physiol Cell Physiol. 2008;294:C907-C916. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 79] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 51. | Dimitroff CJ, Lee JY, Fuhlbrigge RC, Sackstein R. A distinct glycoform of CD44 is an L-selectin ligand on human hematopoietic cells. Proc Natl Acad Sci USA. 2000;97:13841-13846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 108] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 52. | Thankamony SP, Sackstein R. Enforced hematopoietic cell E- and L-selectin ligand (HCELL) expression primes transendothelial migration of human mesenchymal stem cells. Proc Natl Acad Sci U S A. 2011;108:2258-2263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 82] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 53. | Ip JE, Wu Y, Huang J, Zhang L, Pratt RE, Dzau VJ. Mesenchymal stem cells use integrin beta1 not CXC chemokine receptor 4 for myocardial migration and engraftment. Mol Biol Cell. 2007;18:2873-2882. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 169] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 54. | Steingen C, Brenig F, Baumgartner L, Schmidt J, Schmidt A, Bloch W. Characterization of key mechanisms in transmigration and invasion of mesenchymal stem cells. J Mol Cell Cardiol. 2008;44:1072-1084. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 182] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 55. | Langer HF, Stellos K, Steingen C, Froihofer A, Schönberger T, Krämer B, Bigalke B, May AE, Seizer P, Müller I, Gieseke F, Siegel-Axel D, Meuth SG, Schmidt A, Wendel HP, Bloch W, Gawaz M. Platelet derived bFGF mediates vascular integrative mechanisms of mesenchymal stem cells in vitro. J Mol Cell Cardiol. 2009;47:315-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 63] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 56. | Jiang L, Song XH, Liu P, Zeng CL, Huang ZS, Zhu LJ, Jiang YZ, Ouyang HW, Hu H. Platelet-mediated mesenchymal stem cells homing to the lung reduces monocrotaline-induced rat pulmonary hypertension. Cell Transplant. 2012;21:1463-1475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 57. | Teo GS, Yang Z, Carman CV, Karp JM, Lin CP. Intravital imaging of mesenchymal stem cell trafficking and association with platelets and neutrophils. Stem Cells. 2015;33:265-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 60] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 58. | Ahmadian Kia N, Bahrami AR, Ebrahimi M, Matin MM, Neshati Z, Almohaddesin MR, Aghdami N, Bidkhori HR. Comparative analysis of chemokine receptor's expression in mesenchymal stem cells derived from human bone marrow and adipose tissue. J Mol Neurosci. 2011;44:178-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 79] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 59. | Szydlak R, Majka M, Lekka M, Kot M, Laidler P. AFM-based Analysis of Wharton's Jelly Mesenchymal Stem Cells. Int J Mol Sci. 2019;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 60. | Honczarenko M, Le Y, Swierkowski M, Ghiran I, Glodek AM, Silberstein LE. Human bone marrow stromal cells express a distinct set of biologically functional chemokine receptors. Stem Cells. 2006;24:1030-1041. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 498] [Cited by in RCA: 513] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 61. | Kortesidis A, Zannettino A, Isenmann S, Shi S, Lapidot T, Gronthos S. Stromal-derived factor-1 promotes the growth, survival, and development of human bone marrow stromal stem cells. Blood. 2005;105:3793-3801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 283] [Cited by in RCA: 289] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 62. | Leibacher J, Henschler R. Biodistribution, migration and homing of systemically applied mesenchymal stem/stromal cells. Stem Cell Res Ther. 2016;7:7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 211] [Cited by in RCA: 258] [Article Influence: 28.7] [Reference Citation Analysis (0)] |
| 63. | Ringe J, Strassburg S, Neumann K, Endres M, Notter M, Burmester GR, Kaps C, Sittinger M. Towards in situ tissue repair: human mesenchymal stem cells express chemokine receptors CXCR1, CXCR2 and CCR2, and migrate upon stimulation with CXCL8 but not CCL2. J Cell Biochem. 2007;101:135-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 257] [Cited by in RCA: 275] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 64. | Von Lüttichau I, Notohamiprodjo M, Wechselberger A, Peters C, Henger A, Seliger C, Djafarzadeh R, Huss R, Nelson PJ. Human adult CD34- progenitor cells functionally express the chemokine receptors CCR1, CCR4, CCR7, CXCR5, and CCR10 but not CXCR4. Stem Cells Dev. 2005;14:329-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 149] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 65. | Sordi V, Malosio ML, Marchesi F, Mercalli A, Melzi R, Giordano T, Belmonte N, Ferrari G, Leone BE, Bertuzzi F, Zerbini G, Allavena P, Bonifacio E, Piemonti L. Bone marrow mesenchymal stem cells express a restricted set of functionally active chemokine receptors capable of promoting migration to pancreatic islets. Blood. 2005;106:419-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 438] [Cited by in RCA: 441] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 66. | Eseonu OI, De Bari C. Homing of mesenchymal stem cells: mechanistic or stochastic? Rheumatology (Oxford). 2015;54:210-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 62] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 67. | Kitaori T, Ito H, Schwarz EM, Tsutsumi R, Yoshitomi H, Oishi S, Nakano M, Fujii N, Nagasawa T, Nakamura T. Stromal cell-derived factor 1/CXCR4 signaling is critical for the recruitment of mesenchymal stem cells to the fracture site during skeletal repair in a mouse model. Arthritis Rheum. 2009;60:813-823. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 423] [Cited by in RCA: 431] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 68. | Su G, Liu L, Yang L, Mu Y, Guan L. Homing of endogenous bone marrow mesenchymal stem cells to rat infarcted myocardium via ultrasound-mediated recombinant SDF-1α adenovirus in microbubbles. Oncotarget. 2018;9:477-487. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 69. | Lee JM, Kim BS, Lee H, Im GI. In vivo tracking of mesechymal stem cells using fluorescent nanoparticles in an osteochondral repair model. Mol Ther. 2012;20:1434-1442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 49] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 70. | De Becker A, Van Hummelen P, Bakkus M, Vande Broek I, De Wever J, De Waele M, Van Riet I. Migration of culture-expanded human mesenchymal stem cells through bone marrow endothelium is regulated by matrix metalloproteinase-2 and tissue inhibitor of metalloproteinase-3. Haematologica. 2007;92:440-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 171] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 71. | Almalki SG, Agrawal DK. Effects of matrix metalloproteinases on the fate of mesenchymal stem cells. Stem Cell Res Ther. 2016;7:129. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 93] [Cited by in RCA: 121] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 72. | Ponte AL, Marais E, Gallay N, Langonné A, Delorme B, Hérault O, Charbord P, Domenech J. The in vitro migration capacity of human bone marrow mesenchymal stem cells: comparison of chemokine and growth factor chemotactic activities. Stem Cells. 2007;25:1737-1745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 694] [Cited by in RCA: 723] [Article Influence: 40.2] [Reference Citation Analysis (0)] |
| 73. | Zhang B, Luo Q, Chen Z, Sun J, Xu B, Ju Y, Song G. Cyclic mechanical stretching promotes migration but inhibits invasion of rat bone marrow stromal cells. Stem Cell Res. 2015;14:155-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 38] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 74. | Fu X, Halim A, Tian B, Luo Q, Song G. MT1-MMP downregulation via the PI3K/Akt signaling pathway is required for the mechanical stretching-inhibited invasion of bone-marrow-derived mesenchymal stem cells. J Cell Physiol. 2019;234:14133-14144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 75. | Yuan L, Sakamoto N, Song G, Sato M. Migration of human mesenchymal stem cells under low shear stress mediated by mitogen-activated protein kinase signaling. Stem Cells Dev. 2012;21:2520-2530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 76. | Gao J, Dennis JE, Muzic RF, Lundberg M, Caplan AI. The dynamic in vivo distribution of bone marrow-derived mesenchymal stem cells after infusion. Cells Tissues Organs. 2001;169:12-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 700] [Cited by in RCA: 702] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 77. | Brennen WN, Kisteman LN, Isaacs JT. Rapid selection of mesenchymal stem and progenitor cells in primary prostate stromal cultures. Prostate. 2016;76:552-564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 78. | Toma C, Wagner WR, Bowry S, Schwartz A, Villanueva F. Fate of culture-expanded mesenchymal stem cells in the microvasculature: in vivo observations of cell kinetics. Circ Res. 2009;104:398-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 279] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 79. | Devine SM, Cobbs C, Jennings M, Bartholomew A, Hoffman R. Mesenchymal stem cells distribute to a wide range of tissues following systemic infusion into nonhuman primates. Blood. 2003;101:2999-3001. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 568] [Cited by in RCA: 554] [Article Influence: 25.2] [Reference Citation Analysis (0)] |
| 80. | Gholamrezanezhad A, Mirpour S, Bagheri M, Mohamadnejad M, Alimoghaddam K, Abdolahzadeh L, Saghari M, Malekzadeh R. In vivo tracking of 111In-oxine labeled mesenchymal stem cells following infusion in patients with advanced cirrhosis. Nucl Med Biol. 2011;38:961-967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 198] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 81. | Lee RH, Pulin AA, Seo MJ, Kota DJ, Ylostalo J, Larson BL, Semprun-Prieto L, Delafontaine P, Prockop DJ. Intravenous hMSCs improve myocardial infarction in mice because cells embolized in lung are activated to secrete the anti-inflammatory protein TSG-6. Cell Stem Cell. 2009;5:54-63. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1574] [Cited by in RCA: 1447] [Article Influence: 90.4] [Reference Citation Analysis (0)] |
| 82. | Schrepfer S, Deuse T, Reichenspurner H, Fischbein MP, Robbins RC, Pelletier MP. Stem cell transplantation: the lung barrier. Transplant Proc. 2007;39:573-576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 477] [Cited by in RCA: 493] [Article Influence: 27.4] [Reference Citation Analysis (0)] |
| 83. | Fischer UM, Harting MT, Jimenez F, Monzon-Posadas WO, Xue H, Savitz SI, Laine GA, Cox CS Jr. Pulmonary passage is a major obstacle for intravenous stem cell delivery: the pulmonary first-pass effect. Stem Cells Dev. 2009;18:683-692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 794] [Cited by in RCA: 925] [Article Influence: 57.8] [Reference Citation Analysis (0)] |
| 84. | Dutly AE, Kugathasan L, Trogadis JE, Keshavjee SH, Stewart DJ, Courtman DW. Fluorescent microangiography (FMA): an improved tool to visualize the pulmonary microvasculature. Lab Invest. 2006;86:409-416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 85. | Lipowsky HH, Bowers DT, Banik BL, Brown JL. Mesenchymal Stem Cell Deformability and Implications for Microvascular Sequestration. Ann Biomed Eng. 2018;46:640-654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 86. | Zanetti A, Grata M, Etling EB, Panday R, Villanueva FS, Toma C. Suspension-Expansion of Bone Marrow Results in Small Mesenchymal Stem Cells Exhibiting Increased Transpulmonary Passage Following Intravenous Administration. Tissue Eng Part C Methods. 2015;21:683-692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 87. | Sekiya I, Larson BL, Smith JR, Pochampally R, Cui JG, Prockop DJ. Expansion of human adult stem cells from bone marrow stroma: conditions that maximize the yields of early progenitors and evaluate their quality. Stem Cells. 2002;20:530-541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 723] [Cited by in RCA: 695] [Article Influence: 31.6] [Reference Citation Analysis (0)] |
| 88. | Lee WY, Wei HJ, Lin WW, Yeh YC, Hwang SM, Wang JJ, Tsai MS, Chang Y, Sung HW. Enhancement of cell retention and functional benefits in myocardial infarction using human amniotic-fluid stem-cell bodies enriched with endogenous ECM. Biomaterials. 2011;32:5558-5567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 67] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 89. | Rigol M, Solanes N, Roura S, Roqué M, Novensà L, Dantas AP, Martorell J, Sitges M, Ramírez J, Bayés-Genís A, Heras M. Allogeneic adipose stem cell therapy in acute myocardial infarction. Eur J Clin Invest. 2014;44:83-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 90. | von Bahr L, Batsis I, Moll G, Hägg M, Szakos A, Sundberg B, Uzunel M, Ringden O, Le Blanc K. Analysis of tissues following mesenchymal stromal cell therapy in humans indicates limited long-term engraftment and no ectopic tissue formation. Stem Cells. 2012;30:1575-1578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 380] [Cited by in RCA: 422] [Article Influence: 32.5] [Reference Citation Analysis (0)] |
| 91. | Yukawa H, Watanabe M, Kaji N, Okamoto Y, Tokeshi M, Miyamoto Y, Noguchi H, Baba Y, Hayashi S. Monitoring transplanted adipose tissue-derived stem cells combined with heparin in the liver by fluorescence imaging using quantum dots. Biomaterials. 2012;33:2177-2186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 106] [Article Influence: 7.6] [Reference Citation Analysis (1)] |
| 92. | Wynn RF, Hart CA, Corradi-Perini C, O'Neill L, Evans CA, Wraith JE, Fairbairn LJ, Bellantuono I. A small proportion of mesenchymal stem cells strongly expresses functionally active CXCR4 receptor capable of promoting migration to bone marrow. Blood. 2004;104:2643-2645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 565] [Cited by in RCA: 579] [Article Influence: 27.6] [Reference Citation Analysis (0)] |
| 93. | Ullah M, Liu DD, Thakor AS. Mesenchymal Stromal Cell Homing: Mechanisms and Strategies for Improvement. iScience. 2019;15:421-438. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 381] [Cited by in RCA: 367] [Article Influence: 61.2] [Reference Citation Analysis (0)] |
| 94. | De Becker A, Riet IV. Homing and migration of mesenchymal stromal cells: How to improve the efficacy of cell therapy? World J Stem Cells. 2016;8:73-87. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 320] [Cited by in RCA: 360] [Article Influence: 40.0] [Reference Citation Analysis (3)] |