Published online Jun 26, 2021. doi: 10.4252/wjsc.v13.i6.568
Peer-review started: March 8, 2021
First decision: March 29, 2021
Revised: April 7, 2021
Accepted: June 3, 2021
Article in press: June 3, 2021
Published online: June 26, 2021
Processing time: 110 Days and 0.1 Hours
The therapeutic value of mesenchymal stem cells (MSCs) for the treatment of infectious diseases and the repair of disease-induced tissue damage has been explored extensively. MSCs inhibit inflammation, reduce pathogen load and tissue damage encountered during infectious diseases through the secretion of antimicrobial factors for pathogen clearance and they phagocytose certain bacteria themselves. MSCs dampen tissue damage during infection by downregulating the levels of pro-inflammatory cytokines, and inhibiting the excessive recruitment of neutrophils and proliferation of T cells at the site of injury. MSCs aid in the regeneration of damaged tissue by differentiating into the damaged cell types or by releasing paracrine factors that direct tissue regeneration, differentiation, and wound healing. In this review, we discuss in detail the various mechanisms by which MSCs help combat pathogens, tissue damage associated with infectious diseases, and challenges in utilizing MSCs for therapy.
Core Tip: This review discusses the therapeutic benefits of utilizing mesenchymal stem cells (MSCs) to treat infectious diseases and repair tissue damage induced by the disease-causing infectious agents. The immunomodulatory and regenerative properties of MSCs are modulated by the inflammatory milieu generated by the disease and should be considered while utilizing MSCs for treatment.
- Citation: Sharma A, Chakraborty A, Jaganathan BG. Review of the potential of mesenchymal stem cells for the treatment of infectious diseases. World J Stem Cells 2021; 13(6): 568-593
- URL: https://www.wjgnet.com/1948-0210/full/v13/i6/568.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v13.i6.568
Infectious diseases are a leading cause of morbidity and mortality worldwide; respiratory infections and pneumonia are among the major causes of death globally. Failure of commonly used therapies, drugs and the rising number of new infectious disease outbreaks have increased the necessity to identify novel therapeutic strategies to combat infections, the resulting organ and tissue damage associated with the diseases. Mesenchymal stem cells (MSCs) are non-hematopoietic cells found in the bone marrow and other tissues such as adipose tissue, placenta, dental pulp, synovial membrane, endometrium, umbilical cord blood, Wharton's jelly, and ocular tissues[1-4]. Tissues are mechanically or enzymatically dissociated to isolate MSCs, giving rise to plastic adherent cell populations[5]. MSCs can also be separated by flow cytometry sorting based on their cell surface marker expression[6,7]. MSCs possess extensive self-renewal, proliferative, and multilineage differentiation potential. They are identified based on the expression of cell surface markers cluster of differentiation 105 (CD105), CD90, CD73, CD 44, CD29 and are negative for markers such as CD45, CD34, CD14, CD11b, CD79α, CD19, and human leukocyte antigen (HLA)-DR[1]. However, when stimulated with interferon-gamma (IFN-γ), MSCs express HLA-DR[8-10].
MSCs have multilineage differentiation ability and give rise to adipocytes, osteoblasts, and chondrocytes under standard differentiation conditions. Additionally, MSCs play an important role in tissue repair and homeostasis; thus, they have become an attractive therapeutic option for the treatment of several infectious and degenerative diseases[11-17]. In addition, MSCs possess immunomodulatory and immunosuppressive properties, reduce inflammation, and display immune protective functions[1,18,19]. Due to the rising number of infectious diseases and associated organ damage, MSCs have been explored as a possible treatment option in recent years. Several pre-clinical and clinical trials with MSCs have yielded encouraging results, improved therapeutic outcomes, and provided the opportunity to utilize MSCs for the treatment of infectious diseases in addition to existing therapeutic options. Further, intravenous administration of MSCs is effective in treating pathogen-induced organ damage in several disease models[20-22].
This review summarizes various studies that tested the therapeutic advantages of MSCs in treating infectious diseases and repairing disease-induced tissue damage. We also discuss the various modes in which MSCs function to clear pathogens and rebuild the damaged tissue, the signaling pathways modulated by MSCs in the host cells during infections, and finally, some of the challenges associated with utilizing MSCs for therapy.
The objective of this review was to analyze various pre-clinical and clinical studies that utilized MSCs for the treatment of infectious diseases and associated tissue damage. PubMed, Scopus, and Web of Science databases were searched without any language restrictions. Studies that utilized MSCs with or without modification in disease models of infection or pathogen-induced tissue damage were selected for inclusion in the review. The research articles were grouped as follows based on their major findings when MSCs were injected: direct anti-pathogen effects, immunomodulatory effects, differentiation into cells of target tissues, and clinical trials.
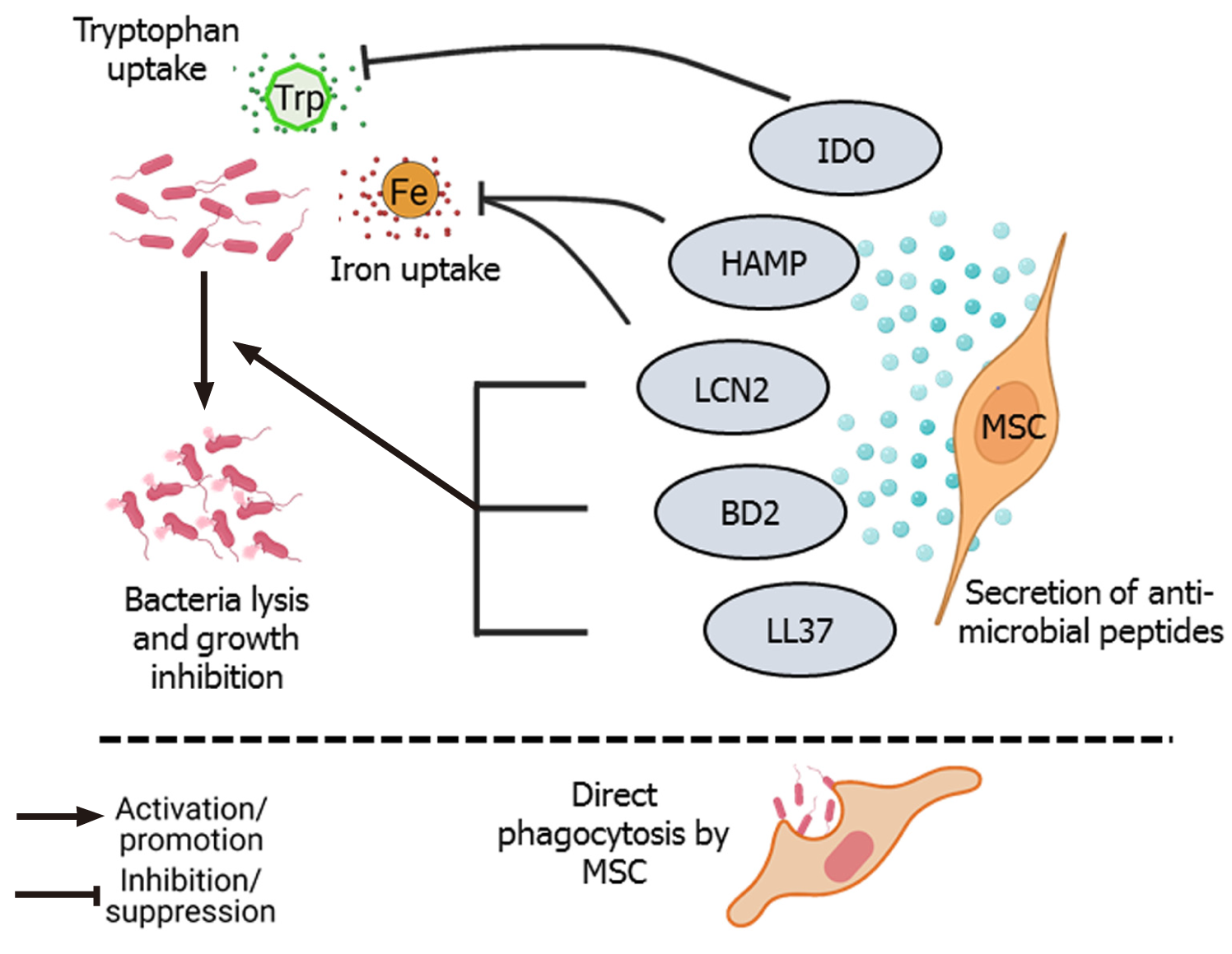
Several studies have reported that administration of MSCs during lung injury and sepsis significantly reduce the bacterial load. MSCs secrete four types of antimicrobial peptides (AMPs): LL-37, hepcidin AMP (HAMP), lipocalin 2 (LCN2), and beta-defensin-2 (BD2) (Figure 1). Besides AMPs, several other paracrine factors secreted by MSCs also contribute to the antimicrobial defense. LL-37 is an amphipathic AMP that belongs to the cathelicidin family of AMPs that induces bacterial lysis and enhances antibiotic sensitivity. LL-37 directly binds to and inactivates lipopolysaccharides (LPS), thereby disrupting the bacterial outer membrane. LL-37 can also neutralize the LPS (endotoxin) released by bacteria. LL-37 has chemotactic activity, recruits immune cells to enhance pathogen clearance at the site of infection. However, this recruitment of immune cells such as macrophages does not increase pro-inflammatory cytokines such as tumor necrosis factor α (TNF-α)[23,24]. LL-37 also promotes regeneration and angiogenesis by binding to formyl peptide receptor-like 1 expressed on endothelial cells[25]. LL-37 secreted by either bone marrow-derived MSCs (BM-MSCs) or adipose tissue-derived MSCs (AD-MSCs) increased the effectiveness of antibiotics, enhanced pathogen killing, and slowed bacterial growth in a pulmonary infection model of cystic fibrosis induced by Pseudomonas aeruginosa, Staphylococcus aureus, and Streptococcus pneumonia[26]. HAMP, another AMP secreted by MSCs, promotes bacterial clearance by preventing iron uptake by the pathogens. HAMP promotes transport of the cellular iron storage protein, ferritin, into the macrophages and subsequent destruction in lysosomes. This causes iron to be stored inside the macrophages, making it unavailable for bacterial survival. So, by depleting iron, HAMP hampers the growth and survival of bacteria[27]. LCN secreted by MSCs also promotes bacterial clearance by blocking iron uptake by the bacterial cells[28,29]. BD2 secreted by MSCs reportedly play an important role in pathogen clearance. Sung et al[30] reported that in an Escherichia coli-induced pneumonia model, intratracheal administration of human umbilical cord-derived MSCs (UC-MSCs) resulted in the attenuation of lung injury and led to a significant reduction in inflammation and increase in bacterial clearance from the infected site. Microarray analysis found that toll-like receptor 2 (TLR-2), TLR-4, and BD2 expression levels were significantly upregulated in lung tissue. The TLR-4 signaling pathway is important for BD2 secretion and silencing of TLR-4 but not TLR-2 abolished the anti-bacterial effect of MSCs against E. coli[30]. Depletion of the essential amino acid, tryptophan, by indoleamine 2,3-dioxygenase (IDO) secreted by MSCs also has antimicrobial effects on various pathogens such as toxoplasma, plasmodium, chlamydia, rickettsia, streptococci, staphylococci, and herpes virus[31]. In addition, MSCs directly phagocytose bacteria through scavenger receptors (Figure 1). Khan et al[32] found that human MSCs internalized M. tuberculosis through two types of scavenger receptors, namely the macrophage receptor with collagenous structure and scavenger receptor class B member 1. These endocytosed mycobacteria were killed by activation of intrinsic autophagy and nitric oxide secreted by MSCs[32].
In addition to the anti-bacterial properties, MSCs also exert anti-viral effects. Rodrigues et al[33] found that MSCs had suppressive effects on human T-lymphotropic virus (HTLV)-infected T cells, similar to that seen with healthy T cells. IDO and prostaglandin E2 (PGE2) secreted by MSCs suppressed the proliferation of infected T cells, and the co-culture of infected T cells with MSCs reduced the expression of HTLV1 pol gene[33]. In a mouse model of lethal herpes simplex virus (HSV-1) infection, MSC administration significantly increased the survival percentage and exerted anti-viral effects by upregulating IFN-γ levels, while decreasing IL-6 and TNF-α serum levels[34].
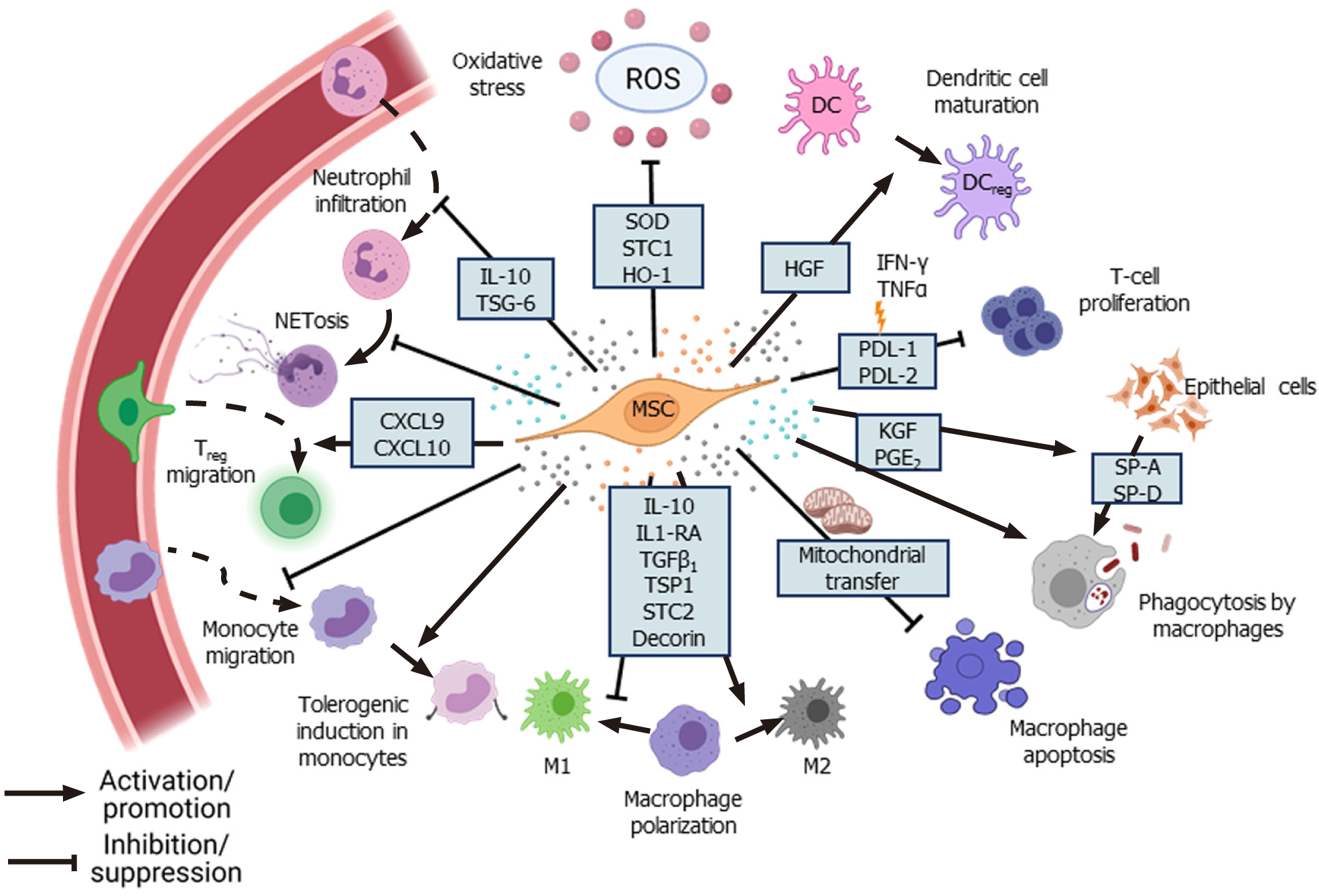
MSCs reduce the infiltration and accumulation of neutrophils and other immune cells at the site of tissue damage and infection. Neutrophils constitute the first line of defense against infections, but their excessive accumulation at the site of infection results in increased secretion of various proteolytic enzymes, matrix metalloproteinases, reactive oxygen species (ROS), and pro-inflammatory cytokines leading to neutrophil extracellular traps (NETosis). Although NETosis helps in pathogen clearance, it also results in tissue damage due to the exaggerated inflammatory response[35]. Excessive NET formation and its poor degradation results in tissue damage and has been implicated in sepsis[36,37] and coronavirus disease 2019 (COVID-19)[38]. MSCs alleviate the excessive influx of neutrophils through TNF-α-stimulated gene-6 (TSG-6) secretion, which inhibits the recruitment of neutrophils by IL-8[39]. In addition, MSCs diminish NET formation by delaying the apoptosis of neutrophils and inducing intercellular adhesion molecule 1 expression in neutrophils to facilitate their phagocytosis[40]. MSCs also control the tissue damage caused by toxic reactive oxygen and nitrogen species produced by neutrophils through the secretion of antioxidant enzymes such as superoxide dismutase (SOD)[40].
MSCs regulate the function of macrophages during infection. Macrophages play an important role in mediating the inflammatory response and can exist as pro-inflammatory M1-type, which mounts the immune response against pathogens, and anti-inflammatory M2-type, which helps in resolving inflammation through secretion of anti-inflammatory cytokines[41]. However, during acute respiratory distress syndrome (ARDS), the M1 phenotype is upregulated, disrupting the balance between M1 and M2 macrophages[42]. Several studies have reported that MSCs moderate the inflammatory response by promoting the polarization of macrophages towards M2 phenotype through secretion of various factors such as IL-1 receptor antagonist[43], decorin[44], stanniocalcin-2[45], and TSG-6[12]. In an LPS-induced acute lung injury (ALI) model, transforming growth factor-β3 (TGF-β3) and thrombospondin 1 (TSP-1) secreted by dental follicle-derived MSCs upregulated M2 phenotype in alveolar macrophages, marked by the increased expression of enzyme arginase 1 and downregulation of M1 macrophage markers such as inducible nitric oxide synthase and CD86[46]. Conversely, co-culture of rat BM-MSCs with LPS-treated alveolar macrophages promoted the survival of macrophages through the upregulation of anti-apoptotic B-cell lymphoma 2 (Bcl-2) and inhibition of caspase-3 and Bcl-2-associated X protein expression by modulating the Wnt/β-catenin pathway[47]. Furthermore, PGE2 secreted by MSCs upregulated the bactericidal activity of M1 macrophages through phosphoinositide 3-kinase and mediated the increase in NADPH oxidase 2 activity and ROS production[41]. Interestingly, in a pre-clinical ARDS model, Jackson et al[48] found that MSCs enhanced pathogen clearance and survival of alveolar macrophages by donating mitochondria via tunneling microtubules. In addition, intravenous injection of murine BM-MSCs overexpressing hepatocyte growth factor (HGF) attenuated the damage in an LPS-induced ALI model by modulating the function of dendritic cells (DCs). HGF secreted by the MSCs induced mature DCs to differentiate into “tolerogenic” regulatory DCs by activation of the HGF/Akt pathway[49].
Injection of MSCs inhibited the proliferation of septic natural killer (sNK) cells and significantly improved the survival of the experimental animals in a cecal ligation puncture mouse model. Injection of MSCs altered the cytokine profile in the serum and altered the sNK cell function, possibly through modulation of the Janus kinase/signal transducer and activator of transcription (STAT) pathway[50]. In pre-clinical models of acute liver injury and liver necrosis, injection of murine MSCs significantly downregulated the IL-17 level and decreased IL-17-producing NKT cells but enhanced FOXP3+ IL10+ NKT cells[51]. MSCs also suppressed the differentiation of CD4+ T cells into IFN-γ-producing T helper type 1 (Th1) cells or IL-17-producing Th17 cells but increased the number of regulatory T cells (Tregs)[52]. In a mouse model of Aspergillus hyphal extract-induced inflammation, administration of human BM-MSCs decreased IL-4, IL-5, and IL-17 levels and ameliorated inflammation[53]. In the presence of IFN-γ and TNF-α, MSCs enhanced the secretion of programmed death-ligand 1 (PD-L1) and PD-L2, respectively, which in turn inhibited T-cell proliferation and upregulated FOXO3 expression in these cells[54]. CD200, a cell surface protein highly expressed in Wharton’s jelly-derived MSCs (WJ-MSCs), has been implicated in inducing immune tolerance by interacting with CD200R present on CD4+ and CD8+ T cells[55].
Although MSCs are considered immune privileged, some studies have reported that they are susceptible to NK-mediated killing in an IL-2-dependent manner. Stimulation of TLRs on MSCs leads to shedding of NK cell-interacting ligands such as major histocompatibility complex I (MHC I) and NK group 2 member D, making them less susceptible to killing by activated NK cells[56]. TLR4 stimulation increases the survival of MSCs under stress conditions through the upregulation of extracellular signal-related kinase 1/2 (ERK1/2)[57]. Studies that have tracked MSCs in vivo found that MSCs died 24 h post-intravenous injection and accumulated in the lungs and liver[58,59]. de Witte et al[58] reported that the in vivo-injected UC-MSCs were rapidly phagocytosed by the monocytes, which then expressed PD-L1 and IL-10 and downregulated TNF-α expression, resulting in acquisition of the regulatory phenotype by these monocytes. Furthermore, phagocytosis of UC-MSCs by lung phagocytes induced the production of (C-X-C motif) ligand (CXCL) 9 and CXCL10 by these cells, which helped to recruit CXCR3+ Tregs[60]. Keratinocyte growth factor (KGF) secreted by MSCs promotes the survival of monocytes by enhancing Akt phosphorylation, thereby facilitating bacterial clearance[61]. In a mouse model of Coxsackie virus infection, secretion of CX3CL1 by the injected human BM-MSCs inhibited the migration of pro-inflammatory Ly6Chigh cells but promoted anti-inflammatory LyC6low monocyte migration. By modulating monocyte trafficking to the heart, MSCs reduced inflammation and damage in heart tissue[62]. Treatment with BM-MSCs improved lung function and reduced inflammatory cytokines in H9N2-[63] and H5N1-infected mice[64]. However, treatment with UC-MSCs was more effective than BM-MSCs in restoring alveolar fluid clearance (AFC) and reducing inflammation in H5N1-infected mice[64]. Thus, modulation of immune cells forms the basis for long-term therapeutic effects of MSCs in facilitating pathogen clearance and reducing inflammation-mediated tissue damage (Figure 2).
Several studies have shown that when subjected to an inflammatory environment, MSCs secrete higher levels of anti-inflammatory factors such as TSG-6, IL-10, and PGE2 and inhibit nuclear factor kappa B (NF-κB) signaling, which leads to the decreased expression of pro-inflammatory cytokines such as IL-6, TNF-α, and IL-1β[43,51,65-68]. In the absence of pro-inflammatory stimulus, MSCs secrete low levels of cyclooxygenase 2 (COX2), PGE2, TGF-β1, HGF, IL-10, PD-1, PD-L1, and PD-L2[69]. In contrast, when subjected to an inflammatory environment consisting of TNF-α and IFN-γ, MSCs significantly upregulate the expression of PGE2, COX2, PD1, IDO, HGF, and TGF-β1, which contribute to their immunomodulatory properties[67,69]. Furthermore, in the presence of pro-inflammatory cytokines, MSCs also supplement the production of anti-inflammatory lipid mediator lipoxinA4 (LXA4) by alveolar type II epithelial (AT-II) cells[70]. Secretion of IDO by MSCs, a rate-limiting enzyme involved in the catabolism of tryptophan via the kynurenine pathway, has been implicated in MSCs-mediated reduction of inflammation[33,71]. Inhibition of IDO with 1-methyltryptophan abolished the anti-inflammatory effects of MSCs on a murine hepatitis model[51], and inhibition of kynurenine, a downstream metabolite of IDO, downregulated TSG-6 secretion by MSCs[71]. Similarly, in an ALI mouse model, the anti-inflammatory effects of MSCs were abolished when TSG-6 or HGF was silenced[20,72], indicating the role of MSCs-secreted factors in controlling the inflammation. Additionally, netrin-1 expressed by MSCs inhibited neutrophil migration[73]. LXA4 and PGE2 secreted by MSCs induce heme oxygenase-1 (HO-1) expression in macrophages, resulting in cytoprotection during oxidative stress-mediated inflammation[74,75]. HO-1, along with angiopoietin-1 (Ang1), inhibits the TNF-α stimulated migration of leukocytes[76,77]. Secretion of antioxidants such as SOD, catalase, glutathione peroxidase, and glutathione reductase by MSCs also reduces oxidative stress[78].
Some studies have also identified a pro-inflammatory role of MSCs, in which MSCs promote the migration of neutrophils, macrophages, and monocytes to the infection site and expedite pathogen clearance[79,80]. Petri et al[81] found that secretion of IFN-γ by MSCs in the early stages of bacterial infection augmented the function of NK cells but induced the regulatory phenotype in NK cells at the later stages. In a P. aeruginosa-induced chronic lung injury model, injection of a high dose of AD-MSCs inhibited bacterial load and downregulated bacteria-induced secretion of PGE2 by alveolar cells[82]. Downregulation of PGE2 levels indirectly enhanced the immune response, leading to higher bacterial clearance[26,83]. Further, injection of BM-MSCs in Paracoccidioides brasiliensis-infected mice led to increased fungal levels and exaggerated immune responses, with increased accumulation of neutrophils, eosinophils, and M2 macrophages, leading to congestion and edema in lungs[84]. Similarly, treatment with BM-MSCs in mice with latent M. bovis infection resulted in significantly higher mycobacterial number and granuloma formation[85]. However, if the MSCs were conditioned with TLR-3 ligand, poly (A:U) prior to the injection, it significantly reduced the pathogen load, suggesting that priming of MSCs was necessary for their anti-mycobacterial effect[85].
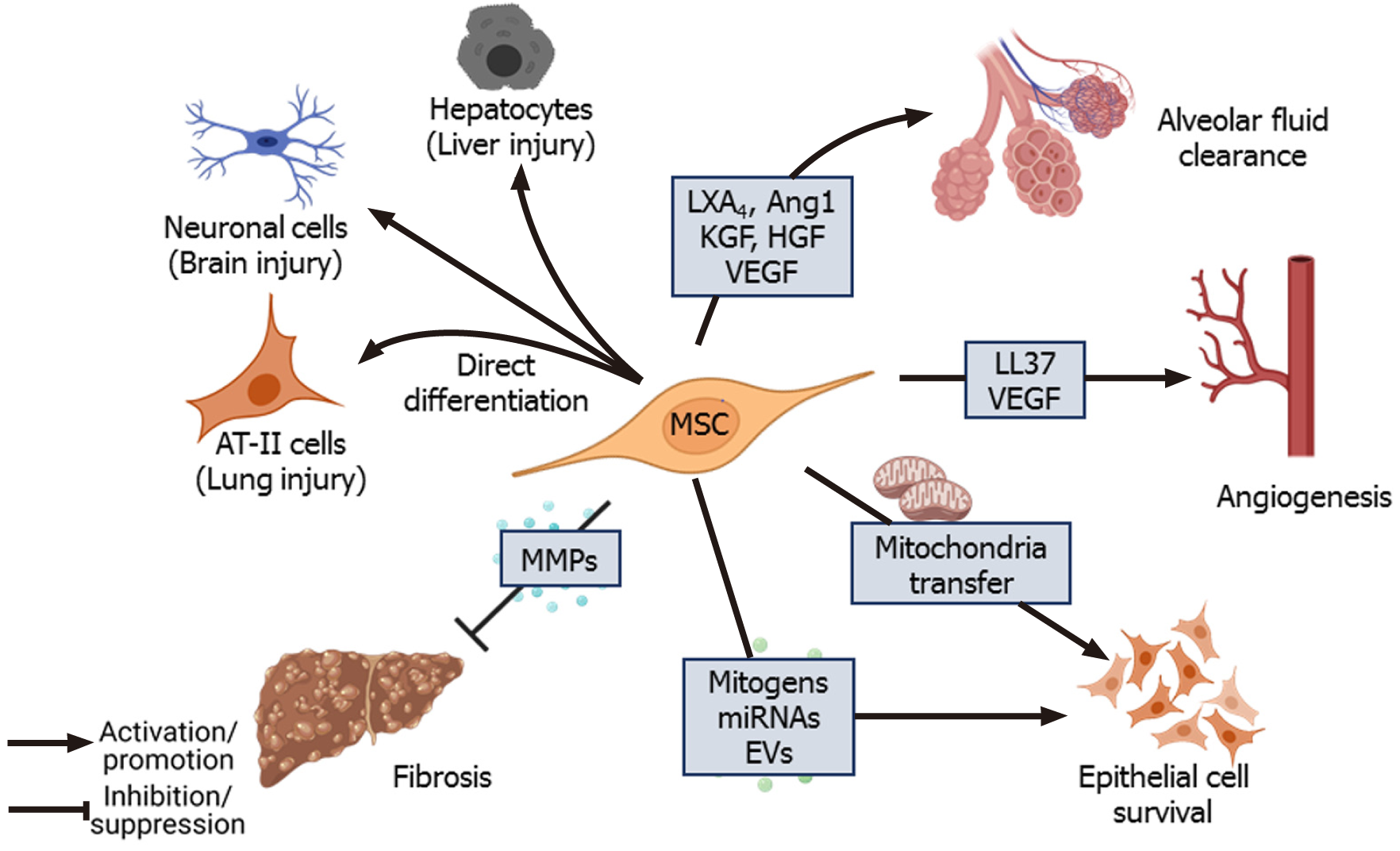
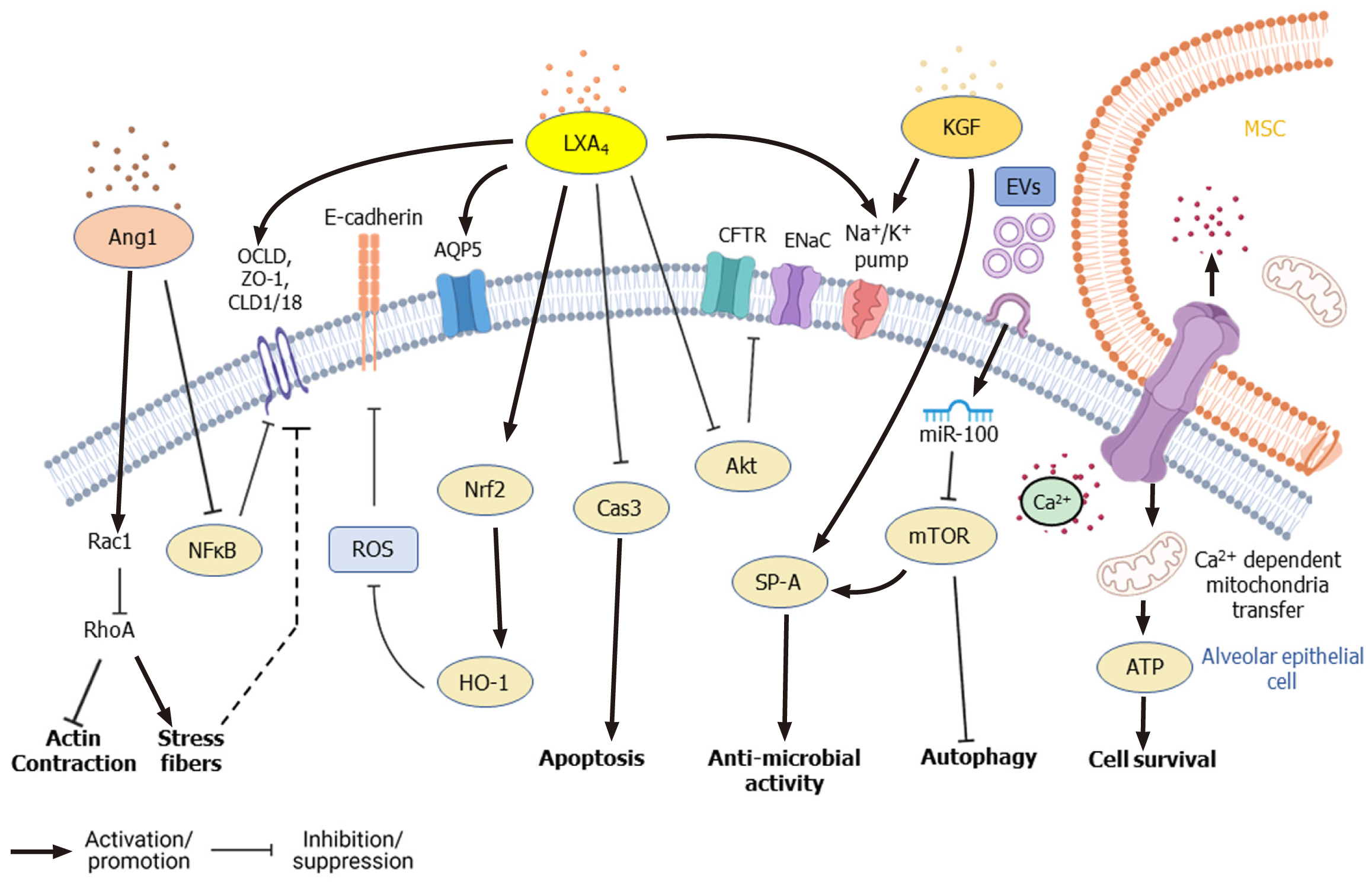
The regenerative and multipotent differentiation ability of MSCs also aids in the repair of tissue damage caused by infection. Despite employing different therapeutic strategies, the clinical outcome of ALI and acute respiratory distress syndrome is still poor and remains a significant healthcare burden necessitating novel therapeutic interventions[21,86]. Apart from controlling infection and inflammation, the paracrine factors secreted by MSCs repair and regenerate the damaged epithelial and endothelial barriers of the alveoli[64]. In the alveolar region, AT-I and AT-II epithelial cells (pneumocytes) constitute the continuous alveolar epithelium, separated from the endothelium by a layer of connective tissue. KGF and TSP-1 secreted by MSCs induce the proliferation of epithelial cells and induce the differentiation of AT-II cells into AT-I cells, which further promote the regeneration of alveolar epithelium[87,88]. Under normal conditions, tight junctions and other cellular junctions maintain the integrity of cellular barriers, allowing the selective flow of fluid. During ALI/ARDS, however, the barrier becomes compromised, and disruption of the ion channel proteins and aquaporins (AQPs) causes fluid leakage into the interstitium and alveolar spaces resulting in edema and compromised gas exchange in the lungs[89,90]. Transepithelial ion exchange through Na+ ion channels (ENaC), Na+/K+ ATPase, and cystic fibrosis transmembrane conductance regulator (CFTR) present on alveolar epithelial cells creates an osmotic gradient that drives the movement of water required for normal AFC. Inflammatory cytokines such as TNF-α, TGF-β1, IFN-γ, IL-4, IL-13, and IL-1β downregulate the expression of ion channel and junction proteins in the alveolar epithelial and endothelial layer leading to the dysregulation of AFC[91]. Lee et al[92] found that KGF secreted by MSCs promotes AFC and ameliorates edema during lung injury. KGF secreted by MSCs upregulates the expression of catalytic α1 subunit of Na+/K+ ATPase and surfactant protein (SP A) in AT-II cells[87]. Also, KGF-silenced MSCs failed to dampen pulmonary edema in an LPS-induced ALI mice model[93].
Furthermore, paracrine factors such as LXA4, KGF, Ang1, vascular endothelial growth factor (VEGF), and HGF secreted by MSCs induce the expression of ion channel, cellular junction, and tight junction proteins in epithelial cells, which facilitate repair of alveolar epithelium and restore normal AFC[68,70,94,95]. In an ALI mouse model, Fang et al[70] found that intratracheal administration of MSCs significantly increased LXA4 level in bronchoalveolar lavage fluid (BALF) and improved the survival of the experimental animals. LXA4 was found to enhance CFTR expression in AT-II cells damaged by LPS treatment through downregulation of Akt pho
MSCs were found to enhance the survival of pulmonary epithelial cells, he
The ability of MSCs to differentiate into different cell types contributes to the repair of damaged tissue during diseases. In LPS-induced ALI models, activation of canonical Wnt signaling promoted differentiation of mouse BM-MSCs into AT II cells and inhibited lung fibrosis[121]. Liu et al[122,123] found significantly high levels of Wnt3a in the lung tissue of ALI mice and in vitro co-culture of mouse BM-MSCs with AT-II cells of ALI or normal mice in the presence of Wnt ligands induced the differentiation of MSCs into AT II cells expressing AQP5, SPB and SPC. Further, intratracheal transplantation of murine MSCs overexpressing receptor tyrosine kinase-like orphan receptor 2 (ROR2), a Wnt5a receptor, into ARDS mice led to differentiation of MSCs into AT II cells, suppressed LPS-induced inflammation, and significantly improved the alveolar epithelial permeability[114]. However, under non-inflammatory conditions, inhibition of Wnt signaling promoted epithelial differentiation of murine lung resident MSCs (LR-MSCs)[124]. Fang et al[125] found that resident Dermo1+ LR-MSCs con
Few studies have also explored the therapeutic role of MSCs during prion infection and found that transplantation of human BM-MSCs intravenously or intrahippocampally improved the survival of prion-infected mice. The transplanted MSCs differentiated into neuronal and glial cells[13]. Migration of human MSCs to the site of prion infection was found to be mediated by CCR3, CCR5, CXCR3, and CXCR4, and blocking these receptors in the MSCs inhibited their migration to the infected site[129]. Furthermore, treatment with MSCs has been found to promote tissue regeneration and reduction of pathogen load in various parasitic infections such as malaria[14], Chagas disease[130,131], schistosomiasis[116,132,133], and leishmaniasis[134]. Thus, MSCs repair pathogen-induced tissue damage by direct differentiation or through the secretion of various mitogens and regulatory factors.
MSCs secrete bilayered lipid microvesicles (100-1000 nm) and exosomes (30-100 nm) that contain cytokines, microRNAs (miRNAs), chemokines, and AMPs[135-138]. MiRNAs present in MSCs-derived exosomes play an important role in mediating therapeutic effects. Exosome-derived miR-27a-3p was found to inhibit NF-κB expression and induce M2 polarization in macrophages[139]. miRNA-146a found in the exosomes of IL1-β primed MSCs-induced M2 polarization in macrophages by modulating IRAK1, TRAF6, and IRF5 signaling[140]. Furthermore, microvesicles from IFN-γ-primed MSCs were more efficient than those of naïve MSCs in inducing M2 phenotype and phagocytosis in macrophages[141]. EVs secreted by MSCs contain mRNA of KGF[142] and Ang1[68], which mediate anti-inflammatory effects on LPS-induced ALI mice models. In an E. coli-induced pneumonia mouse model, MSCs-derived EVs upregulated the BALF levels of leukotriene B4 (LTB4), a lipid mediator that acts as a chemoattractant for T cells, neutrophils, macrophages, and other immune cells, thereby facilitating pathogen elimination[143,144]. miRNA-145 present in EVs of MSCs was found to suppress the expression of multidrug resistant protein 1, leading to increased LTB4 production, which enhanced microbial clearance[143]. miR-100 found in WJ-MSCs-derived EVs enhanced autophagy through mTOR downregulation and improved the survival of alveolar epithelial cells[110] (Figure 4). Treatment with MSCs-derived EVs upregulated KGF, PGE2, IL-10 levels and reduced lung inflammation and endothelial permeability in a pre-clinical model of ischemia reperfusion-induced lung injury[145]. Wang et al[146] reported that HGF present in EVs and conditioned media (CM) of BM-MSCs reduced the permeability of endothelial barrier by modulating VE-cadherin and occludin expression. Khatri et al[147] reported that swine BM-MSCs and the EVs derived from them have similar surface marker expression, and treatment with EVs had similar anti-inflammatory effects as that of MSCs themselves in pig ALI.
UC-MSCs-derived EVs inhibited viral replication of hepatitis C virus (HCV) in a pre-clinical disease model, and the anti-viral effect was found to be mediated by miRNAs let-7f, miR-145, miR-199a, and miR-221[148]. Human BM-MSCs-derived exosomes were found to induce autophagy but inhibit D-GalN/LP-induced apoptosis of hepatocytes[149] as well as coxsackievirus B3-induced myocarditis[150]. Treatment with MSCs-derived exosomes modulated AMPK/mTOR signaling in human cardiomyocytes in vitro and promoted their survival[150].
CM derived from MSCs cultured in xenofree conditions has been hypothesized as a reasonable approach to cell-free therapy. The CM was found to be rich in exosomes, EVs, and several paracrine factors[151]. In an LPS-induced ALI mouse model, Su et al[152] reported that mice injected intravenously with the CM of MSCs showed reduced neutrophil infiltration and accumulation. MSCs-CM was also shown to induce apoptosis in neutrophils both in vitro and in vivo by inhibiting NF-κB signaling. BALF of MSCs-CM-treated mice had reduced levels of anti-apoptotic proteins such as Bcl-xL and Mcl-1[152]. Treatment with MSCs-derived CM reduced TNF-α, IL-6 levels and increased IL-10 secretion by macrophages stimulated with TLR ligands or live S. pneumoniae[153]. However, Hayes et al[154] showed that administration of MSCs was significantly more effective in improving ventilation-induced lung injury than treatment with MSCs-CM alone. In an ex-vivo perfusion lung injury model of pneumonia, Park et al[155] found that EVs derived from human BM-MSCs treated with TLR3 ligand, poly (I:C) significantly reduced the bacterial load, inflammation, and protein permeability compared to EVs derived from naïve MSCs. CM of murine BM-MSCs was found to exhibit pathogen-related differences in their therapeutic effect, where treatment with MSCs-CM inhibited herpes virus replication but not dengue or enterovirus[148].
Several studies have reported that genetic modifications of MSCs improved their efficacy and therapeutic potential (Table 1). Martínez-González et al[156] found that intravenous injection of MSCs overexpressing sST2, a soluble decoy receptor for IL-33, was highly effective at reducing inflammation and preserving the lung architecture compared to naïve MSCs in a murine ALI model. IL-33/IL-1 receptor-like (ST2) signaling 'alarms' and activates the immune cells upon damage of epithelial or endothelial cells[157]. Similarly, administration of MSCs overexpressing Ang1 or HO-1 reduced vascular endothelial permeability and inflammatory cells in the lungs of LPS-induced ALI animal models[78,158,159]. Intratracheal administration of TGF-β1 overexpressing MSCs increased Treg cells but decreased Th17 cells in the lungs of LPS-induced ARDS mice. MSCs expressing TGF-β1 induced occludin protein expression and improved vascular permeability[160]. In an E. coli-induced pneumosepsis experimental model, injection of human UC-MSCs that overexpressed IL-10 were highly effective in reducing the percentage of alveolar neutrophils and macrophages and also increased the phagocytic function of macrophages compared to naïve MSCs, leading to significantly reduced bacterial counts[75]. Murine BM-MSCs overexpressing either developmental endothelial locus-1 or FGF2 were found to attenuate lung injury and infiltration of immune cells and reduced TNF-α levels compared to control MSCs in an LPS-induced ALI mouse model[161,162]. HGF overexpressing MSCs reduced apoptosis and cell permeability in LPS-treated pulmonary endothelial cells by upregulating occludin via the mTOR/STAT3 signaling pathway[163]. BM-MSCs overexpressing β-catenin, Wnt5a receptor, ROR2, or p130/E2F4 showed higher retention in the lungs and differentiation into AT II cells compared to control MSCs, leading to significant improvement in lung tissue structure in LPS-induced ARDS mouse models[114,121,164]. Angiotensin-converting enzyme (ACE) and its homolog ACE2 are cell membrane-linked enzymes that have important catalytic functions in the rennin-angiotensin system. Although ACE, angiotensin II type 1a receptor, and angiotensin II are involved in the progression of ARDS by increasing edema and disturbing lung function, its homolog ACE2 and angiotensin II type 2 receptor play a protective role during sepsis-induced lung injury[165]. He et al[166] reported that treatment with MSCs expressing ACE2 led to a significant reduction in neutrophil counts and inflammatory cytokines, IL-6, and TNF-α levels in the lungs of LPS-induced ARDS mice compared to the control BM-MSCs-treated group. However, ACE2 was found to be the functional receptor for coronaviruses including severe acute respiratory syndrome (SARS)-associated coronavirus (SARS-CoV-1), and SARS-CoV-2 and has been implicated in the progression of SARS-induced ARDS[167,168]. Thus, ACE2 overexpressing MSCs might not be a suitable option for the treatment of SARS-induced ARDS.
| Overexpressed genes | Source of MSCs | Disease model | Experimental Outcome | Ref. |
| sST2, Ang1, HO1 | Human AD-MSCs, Mice BM-MSCs | LPS-induced ALI mouse model | Improved preservation of lung architecture and reduced inflammation | Xu et al[77], Martínez-González et al[156], Mei et al[159], Chen et al[160], and Chen et al[220] |
| TGF-β1 | Mice BM-MSCs | LPS-induced ARDS mice | Increased Treg, induced occludin expression and reduced vascular permeability | Chen et al[160] |
| IL-10 | Human UC-MSCs | E. coli-induced pneumosepsis mouse model | Reduced bacterial load by increasing phagocytosis in macrophages | Jerkic et al[75] |
| Del-1, FGF2 | Mice BM-MSCs | LPS-induced ALI mouse model | Reduction in inflammation and lung injury | Zhao et al[161], Zhao et al[161,162] |
| HGF | Mice BM-MSCs | In vitro LPS treatment of pulmonary endothelial cells | Upregulated occludin and reduced permeability | Meng et al[163] |
| β-catenin, ROR2, p130/E2F4 | Mice BM-MSCs | LPS-induced ARDS mouse model | Higher retention in lungs and increased differentiation into AT II cells | Cai et al [114], Cai et al[121], Zhang et al[114,121,164] |
| ACE2 | Mice BM-MSCs | LPS-induced ALI mouse model | Reduced permeability and lung Ang-II levels | He et al[166] |
| CXCR4, EP2 CXCR2 | Mice BM-MSCs | ALI/ARDS mouse model | Increased homing to the site of injury | Yang et al [169], Han et al[170], Shen et al[169-171] |
Migration of MSCs to the site of infection and injury is required for MSCs to exhibit their therapeutic effects. Overexpression of CXCR4, a receptor for stromal cell-derived factor-1α (SDF-1) in MSCs, improved their migration potential to the injured lungs, which in turn contributed significantly to controlling the tissue damage compared to control MSCs[169]. Because PGE2 levels increase significantly during lung injury, Han et al[170] overexpressed the E-prostanoid receptor (EP-2), a receptor for PGE2, in MSCs to improve their homing to the injured lung. EP-2 expressing MSCs showed significantly high migration to the injured lung and repaired the damaged tissue in the ARDS model[171]. In an oral mucositis rat model, Shen et al[172] found that overexpression of CXCR2 facilitated the migration of MSCs to the infected site. CXCR2 is a receptor for NAP2, secreted by NK cells at the injury site, and can act as a chemoattractant for MSCs expressing CXCR2[171].
MSCs modify their paracrine secretome depending on the environmental cues. Pre-conditioning MSCs with different environmental cues was found to alter their immunomodulation and differentiation abilities. MSCs pre-conditioned with the serum of ARDS mice or inflammatory cytokines had high expression of IL-10, IL-6, and IL1RA but significantly lower expression of inflammatory cytokines[140,173,174]. Similarly, stimulation of MSCs with pro-inflammatory cytokines TNF-α and IL1-β induced the expression of HGF, FGF2, heparin-binding EGF-like growth factor that contributed to the healing of airway epithelial cells in vitro by modulating ERK1/2 phosphorylation via EGFR activation[66]. Similarly, MSCs primed with IL1-β were more effective at reducing TNF-α and IL-6 levels and increasing IL-10 levels in serum of ARDS mice[140]. During co-culture of human BM-MSCs and macrophages, TNF-α secreted by activated M1 macrophages induced MSCs into the immunosuppressive phenotype. This effect was amplified by IL-10 produced by M2 macrophages, which further increased PGE2 secretion by MSCs[175]. Treatment of MSCs with either IFN-γ or TNF-α increased PGE2 expression, but IDO and PD-L1 levels increased only in IFN-γ-treated MSCs[69], which suggests that the composition of the inflammatory milieu alters the function of MSCs and their anti-inflammatory potential. Pre-treatment of UC-MSCs with TGF-β1 prior to transplantation improved their long-term survival in the lungs[176]. Long-chain fatty acids such as eicosapentaenoic acid treatment improved the therapeutic effects of MSCs, which led to a reduction in lung injury and increased secretion of inflammation resolving factors such as resolvin D1, IL-10, TGF-β, and PGE2 in a CLP-induced sepsis model[177]. Exposure to IFN-γ or TLR3 ligand poly (I:C) increased HLA-I expression in MSCs, which protected the cells from killing by NK cells[56,178]. Furthermore, murine BM-MSCs pre-conditioned with TLR3 ligand poly (A:U) were more efficient than naïve MSCs in eliminating M. bovis in an experimental model[85]. TLR4 activation in LPS-stimulated MSCs expedited wound healing by promoting neutrophil migration and NETosis at the site of infection[79]. Extracellular vesicles from IFN-γ-primed MSCs significantly increased the phagocytic ability of THP1 monocytic cells in vitro and improved the lung histopathology and survival of E. coli-induced ARDS mice[141].
In a pre-clinical model, Liu et al[117] reported that transplantation of MSCs expressing short hairpin RNA against hepatitis viral proteins (HBV S and HBV X) significantly reduced HBV antigens in the liver and serum. Masalova et al[179] tested the efficacy of utilizing MSCs as an immunization agent against HCV and found that murine BM-MSCs expressing five non-structural HCV proteins induced significantly higher proliferation of lymphocytes, IFN-γ secretion, and IgG2a levels compared to naked DNA immunizations suggesting the feasibility of utilizing modified MSCs as vaccine agents. Hypoxic pre-conditioning also improves the migration, survival, and anti-inflammatory properties of MSCs[174,180,181]. MSCs cultured under hypoxic conditions (1% O2) had high expression of SDF-1α receptors CXCR4 and CXCR7, which promoted their migration to the site of infection[180]. Although both short- and long-term hypoxia increased metabolic activity of BM-MSCs compared to normoxic conditions, short-term hypoxia was superior to long-term hypoxia in augmenting the therapeutic characteristic of MSCs. Hypoxic treatment altered the secretome of porcine BM-MSCs and human BM-MSCs differently, indicating species-specific variations in MSCs characteristics[181]. Pre-conditioning of MSCs from human bone marrow and adipose tissue with hypoxia (2% O2) significantly inhibited the differentiation potential but increased the metabolic activity of MSCs. Treatment with cytokine mix consisting of IL-1β, TNF-α, and IFN-γ increased the secretion of anti-inflammatory cytokines such as IL1RA and IL-10 as well as thrombogenic tissue factor in both AD-MSCs and BM-MSCs[174]. Similarly, subpopulations of MSCs selected based on the expression of specific cell surface markers showed a higher therapeutic effect compared to the bulk population. Masterson et al[7] found that intravenous administration of a homogenous population of syndecan2 (CD362)-positive BM-MSCs significantly improved the lung function and reduced inflammatory response during lung injury induced by E. coli compared to CD362-negative BM-MSCs. Similarly, PDGFR+Sca1+TER119- (PαS) BM-MSCs with high CFU-F ability were reported to reduce bacterial load, ameliorate inflammation, and increase survival in mice model of ALI induced by Klebsiella pneumonia[6]; albeit, the results were not compared with the effects seen with bulk MSCs population.
The route of administration of MSCs can also modify the therapeutic outcome. Danchuk et al[20] found that, whereas administration of human BM-MSCs through intravenous, oropharyngeal, or intraperitoneal routes reduced the pulmonary inflammation to a similar extent in an ALI mouse model, MSCs were not detected in the lung after intraperitoneal injection during their analysis period. Interestingly, intravenous administration of murine BM-MSCs was found to be beneficial in ameliorating ALI caused by intratracheal rather than intravenous injection of LPS. Extrapulmonary organ damage induced by intravenous LPS injection reduced the migration and retention of MSCs in the lungs and accounted for the difference in therapeutic effects observed between these two modes of injury[182]. In a mouse model of prion infection, intravenous or intrahippocampal administration of human BM-MSCs enhanced the survival of infected mice; however, the survival rate was higher in the experimental group where MSCs were transplanted intrahippocampally[13].
In a clinical trial involving 56 patients with hepatitis B infection, intra-hepatic administration of autologous BM-MSCs along with anti-viral drug Entecavir resulted in a significant reduction of inflammation and improvement in liver function[183]. Similar therapeutic benefits were observed in HBV-induced decompensated liver cirrhosis patients when UC-MSCs were intravenously administrated along with standard therapy[184]; however, UC-MSCs administration did not alter the prognosis in HBV infection-related acute-on-chronic liver failure patients[185]. Conversely, prolonged treatment with UC-MSCs for more than 4 wk was found to be effective at improving some but not all liver injury markers in HBV-related liver failure and liver cirrhosis patients[186]. In a clinical trial involving hepatitis C patients with end-stage liver disease, intravenous administration of autologous BM-MSCs was found to be well tolerated and effective at reducing liver injury markers and fibrosis[187].
Respiratory tract infections claim more than 1.5 million lives annually, and with epidemic and pandemic outbreaks, the number of deaths and disabilities can be devastatingly high (SARS outbreak in 2002, H1N1 flu in 2009, Middle East respiratory syndrome coronavirus outbreak in 2012, and COVID-19 outbreak in 2020)[188]. Based on the successful outcomes observed in pre-clinical models of bacterial pneumonia and respiratory infection, the potential benefits of MSCs administration were explored for treating infection-associated lung injury. Avian influenza viruses associated with high mortalities in poultry pose a risk to cross the interspecies barrier to give rise to influenza strains that can cause pandemics[189,190]. In a clinical trial involving 17 patients infected with H7N9, treatment with allogeneic menstrual blood-derived MSCs significantly reduced the death rate (54.5% in control vs 17.6% in MSCs treated) and improved lung function without any adverse side effects over a follow-up period of 5 years[191]. In a case study, intra-atrial injection of allogeneic BM-MSCs facilitated resolution of ARDS in a deteriorating critically ill 58-year-old patient with H1N1 infection[192].
MSCs are immune to infection by SARS-CoV-2 as they lack the expression of ACE2 and serine protease TMRSS2, which are essential for SARS-CoV-2 infection[193]. In a clinical trial of a critically ill 54-year-old man with COVID-19 pneumonia, administration of allogeneic WJ-MSCs showed no side effects, improved lung function, and diminished the infection by the 6th d of transplantation[194]. In another clinical trial involving seven COVID-19 patients with severe pneumonia, MSCs treatment was found to be safe and effective at reducing inflammation[193]. MSCs transplantation was reported to act synergistically with convalescent plasma therapy and improve lung injury in another critically ill 66-year-old COVID-19 patient[195]. Severely ill COVID-19 patients are at a risk of thromboembolism that can lead to multiorgan failure. The rationale for using MSCs in treating COVID-19 was discussed in several reports[196-198]; however, treatment of COVID-19 patients with MSCs requires further analysis considering certain aspects of COVID-19-related pathology. Since MSCs often have a high expression of procoagulant tissue factor CD142, intravenous administration of MSCs can be detrimental in patients at risk of systemic coagulation[199], and intratracheal or intramuscular administration can obviate this risk. In another clinical trial of 25 COVID-19 patients receiving MSCs transplantation once, twice, or thrice at intervals of 5 d, three patients developed complications such as liver failure, heart failure, and allergic reactions[200].
Although MSCs have potent anti-inflammatory and multipotent differentiation properties, some studies have reported that MSCs can act as “safe harbors” for some bacterial and viral pathogens and help them evade the immune response and therapeutic drugs. An in vitro study by Naik et al[201] reported that BM-MCs could be infected by both virulent (M. tuberculosis) and avirulent (M. bovis, M. smegmatis) mycobacteria. However, MSCs effectively eliminated the intracellular avirulent species but not the virulent mycobacteria. M. bovis elimination was mediated by activation of TLR2/4 pathway. In contrast, intracellular survival of M. tuberculosis was facilitated by bacteria-induced downregulation of CRAMP, an AMP expressed in BM-MSCs[201]. Intracellular M. tuberculosis in MSCs were drug-resistant, attributed to the expression of drug-efflux pumps ABCC1 and ABCG2, and immune protected, due to PGE2 secretion by MSCs[202]. Lopes et al[203] found that CD271+Sca1+ BM-MSCs served as a niche for Leishmania infantum in vivo, which protected the parasite from anti-parasite drugs, possibly through active drug pump ABCG2 expressed by MSCs.
Qiao et al[204]and Soland et al[205] reported that human MSCs were fully permissive to human cytomegalovirus infection, and the highest infection rate was observed in lung perivascular MSCs, suggesting that MSCs in different organs might act as a viral reservoir in humans. Further, Meisel et al[206] found that MSCs infected with CMV lose their immunosuppressive and antimicrobial properties, and Sundin et al[207] found that parvovirus B19 persisted in BM-MSCs even after several years of infection. Human placenta-derived MSCs were found to be permissible to infection with HSV such as HSV1 and HSV2, and fetal membrane-derived MSCs are susceptible to infection with Varicella Zoster Virus[208]. Human BM-MSCs were found to be susceptible to HBV infection[209], and Wang et al[210] reported that while BM-MSCs from patients with chronic HBV infection had defective differentiation potential, AD-MSCs were not permissible to HBV infection and differentiated effectively into functional hepatocyte-like cells. Therefore, AD-MSCs might be a better therapeutic option than BM-MSCs in patients with HBV infection. Similarly, avian influenza virus H5N1 was also reported to infect and induce cell death in human BM-MSCs and cord blood-derived MSCs[211]. MSCs are also susceptible to HIV infection since they express receptors and co-receptors for HIV-1. Cotter et al[212] reported that HIV-1 infection alters the differentiation potential of MSCs, and MSCs exposed to sera from patients with high viral load showed proadipogenic phenotype. BM-MSCs from HIV transgenic mice showed a reduction in proliferation and therapeutic effects on an acute kidney injury model compared to normal BM-MSCs[213]. Cervenakova et al[214] reported that BM-MSCs from mice infected with prions were able to propagate TSE agents or prions when transplanted into healthy animals. Thus, due considerations on the susceptibility of MSCs to various infectious agents should be given while utilizing MSCs for therapy.
Although several studies have reported that MSCs are non-immunogenic due to lack of MHC II and the co-stimulatory molecules CD40, CD80, or CD86 and that the allogenic MSCs are well tolerated[191,192], some studies have found that allogenic MSCs elicit an immune response in the recipients leading to transplantation failure[215-217]. Furthermore, MSCs from different tissue sources have varied differentiation ability and secrete a unique set of immunomodulatory factors which might influence the clinical outcome, and these source-specific differences are reviewed in detail elsewhere[198,218]. Further studies are required to understand the immune response elicited by allogeneic MSCs transplantation as well as the diverse effects of utilizing MSCs isolated from different tissue sources. An important point to consider is that several pre-clinical studies were performed in animal models with non-human MSCs or human MSCs from various tissue sources. The non-human inflammatory milieu might not exactly resemble the disease conditions seen in humans, and thus additional precautions should be taken while interpreting the potential benefits of utilizing MSCs for the treatment of infectious diseases.
Exaggerated immune response and inflammation during infections cause tissue damage, which is one of the major reasons for infectious disease-induced mortality. However, treatment with MSCs was reported to provide therapeutic benefits by reducing inflammation, pathogen load, and tissue damage in several disease models. By expediting pathogen clearance through secretion of AMPs and direct phagocytosis and by reducing inflammation through secretion of several anti-inflammatory cytokines, MSCs combat tissue damage at the site of infection. MSCs play an important role in tissue regeneration by secreting various mitogens as well as differentiating into cells of the target tissue. During ARDS, secretion of LXA4, Ang1, HGF, and VEGF by MSCs upregulate the expression of ion channel and tight junction proteins and thus restore AFC and reduce endothelial permeability. MSCs-derived EVs contain several therapeutically beneficial cytokines, miRNAs, and treatment with MSCs-EVs showed promising results in clinical trials involving patients with liver injuries and severe COVID-19 pneumonia. However, caution should be exercised in utilizing MSCs for treatment as they can harbor harmful pathogens and might cause unfavorable outcomes in patients with pre-existing conditions.
Manuscript source: Invited manuscript
Specialty type: Infectious diseases
Country/Territory of origin: India
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Liu L, Mousa HAL S-Editor: Liu M L-Editor: Filipodia P-Editor: Zhang YL
| 1. | Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop Dj, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11055] [Cited by in RCA: 12689] [Article Influence: 704.9] [Reference Citation Analysis (2)] |
| 2. | Mawrie D, Bhattacharjee K, Sharma A, Sharma R, Bhattacharyya J, Bhattacharjee H, Deori N, Kumar A, Jaganathan BG. Human orbital adipose tissue-derived mesenchymal stem cells possess neuroectodermal differentiation and repair ability. Cell Tissue Res. 2019;378:531-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 3. | Mawrie D, Kumar A, Magdalene D, Bhattacharyya J, Jaganathan BG. Mesenchymal Stem Cells from Human Extra Ocular Muscle Harbor Neuroectodermal Differentiation Potential. PLoS One. 2016;11:e0156697. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 4. | Tetro JA. From hidden outbreaks to epidemic emergencies: the threat associated with neglecting emerging pathogens. Microbes Infect. 2019;21:4-9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 5. | Mushahary D, Spittler A, Kasper C, Weber V, Charwat V. Isolation, cultivation, and characterization of human mesenchymal stem cells. Cytometry A. 2018;93:19-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 397] [Article Influence: 49.6] [Reference Citation Analysis (0)] |
| 6. | Hackstein H, Lippitsch A, Krug P, Schevtschenko I, Kranz S, Hecker M, Dietert K, Gruber AD, Bein G, Brendel C, Baal N. Prospectively defined murine mesenchymal stem cells inhibit Klebsiella pneumoniae-induced acute lung injury and improve pneumonia survival. Respir Res. 2015;16:123. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 42] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 7. | Masterson C, Devaney J, Horie S, O'Flynn L, Deedigan L, Elliman S, Barry F, O'Brien T, O'Toole D, Laffey JG. Syndecan-2-positive, Bone Marrow-derived Human Mesenchymal Stromal Cells Attenuate Bacterial-induced Acute Lung Injury and Enhance Resolution of Ventilator-induced Lung Injury in Rats. Anesthesiology. 2018;129:502-516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 45] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 8. | Grau-Vorster M, Laitinen A, Nystedt J, Vives J. HLA-DR expression in clinical-grade bone marrow-derived multipotent mesenchymal stromal cells: a two-site study. Stem Cell Res Ther. 2019;10:164. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 42] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 9. | Grau-Vorster M, Rodríguez L, Torrents-Zapata S, Vivas D, Codinach M, Blanco M, Oliver-Vila I, García-López J, Vives J. Levels of IL-17F and IL-33 correlate with HLA-DR activation in clinical-grade human bone marrow-derived multipotent mesenchymal stromal cell expansion cultures. Cytotherapy. 2019;21:32-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 10. | Polchert D, Sobinsky J, Douglas G, Kidd M, Moadsiri A, Reina E, Genrich K, Mehrotra S, Setty S, Smith B, Bartholomew A. IFN-gamma activation of mesenchymal stem cells for treatment and prevention of graft vs host disease. Eur J Immunol. 2008;38:1745-1755. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 485] [Cited by in RCA: 457] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 11. | Adak S, Magdalene D, Deshmukh S, Das D, Jaganathan BG. A Review on Mesenchymal Stem Cells for Treatment of Retinal Diseases. Stem Cell Rev Rep. 2021;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 39] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 12. | Liu HM, Liu YT, Zhang J, Ma LJ. Bone marrow mesenchymal stem cells ameliorate lung injury through anti-inflammatory and antibacterial effect in COPD mice. J Huazhong Univ Sci Technolog Med Sci. 2017;37:496-504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 13. | Song CH, Honmou O, Ohsawa N, Nakamura K, Hamada H, Furuoka H, Hasebe R, Horiuchi M. Effect of transplantation of bone marrow-derived mesenchymal stem cells on mice infected with prions. J Virol. 2009;83:5918-5927. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 31] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 14. | Thakur RS, Tousif S, Awasthi V, Sanyal A, Atul PK, Punia P, Das J. Mesenchymal stem cells play an important role in host protective immune responses against malaria by modulating regulatory T cells. Eur J Immunol. 2013;43:2070-2077. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 15. | Tsuchiya A, Takeuchi S, Iwasawa T, Kumagai M, Sato T, Motegi S, Ishii Y, Koseki Y, Tomiyoshi K, Natsui K, Takeda N, Yoshida Y, Yamazaki F, Kojima Y, Watanabe Y, Kimura N, Tominaga K, Kamimura H, Takamura M, Terai S. Therapeutic potential of mesenchymal stem cells and their exosomes in severe novel coronavirus disease 2019 (COVID-19) cases. Inflamm Regen. 2020;40:14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 59] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 16. | Van Linthout S, Savvatis K, Miteva K, Peng J, Ringe J, Warstat K, Schmidt-Lucke C, Sittinger M, Schultheiss HP, Tschöpe C. Mesenchymal stem cells improve murine acute coxsackievirus B3-induced myocarditis. Eur Heart J. 2011;32:2168-2178. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 85] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 17. | Yudintceva NM, Bogolyubova IO, Muraviov AN, Sheykhov MG, Vinogradova TI, Sokolovich EG, Samusenko IA, Shevtsov MA. Application of the allogenic mesenchymal stem cells in the therapy of the bladder tuberculosis. J Tissue Eng Regen Med. 2018;12:e1580-e1593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 18. | Fu X, Liu G, Halim A, Ju Y, Luo Q, Song AG. Mesenchymal Stem Cell Migration and Tissue Repair. Cells. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 562] [Cited by in RCA: 662] [Article Influence: 110.3] [Reference Citation Analysis (36)] |
| 19. | Tse WT, Pendleton JD, Beyer WM, Egalka MC, Guinan EC. Suppression of allogeneic T-cell proliferation by human marrow stromal cells: implications in transplantation. Transplantation. 2003;75:389-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1141] [Cited by in RCA: 1088] [Article Influence: 49.5] [Reference Citation Analysis (0)] |
| 20. | Danchuk S, Ylostalo JH, Hossain F, Sorge R, Ramsey A, Bonvillain RW, Lasky JA, Bunnell BA, Welsh DA, Prockop DJ, Sullivan DE. Human multipotent stromal cells attenuate lipopolysaccharide-induced acute lung injury in mice via secretion of tumor necrosis factor-α-induced protein 6. Stem Cell Res Ther. 2011;2:27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 170] [Cited by in RCA: 182] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 21. | Dushianthan A, Grocott MP, Postle AD, Cusack R. Acute respiratory distress syndrome and acute lung injury. Postgrad Med J. 2011;87:612-622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 215] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 22. | Zhao W, Li JJ, Cao DY, Li X, Zhang LY, He Y, Yue SQ, Wang DS, Dou KF. Intravenous injection of mesenchymal stem cells is effective in treating liver fibrosis. World J Gastroenterol. 2012;18:1048-1058. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 95] [Cited by in RCA: 101] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 23. | Dürr UH, Sudheendra US, Ramamoorthy A. LL-37, the only human member of the cathelicidin family of antimicrobial peptides. Biochim Biophys Acta. 2006;1758:1408-1425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 668] [Cited by in RCA: 771] [Article Influence: 40.6] [Reference Citation Analysis (0)] |
| 24. | Krasnodembskaya A, Song Y, Fang X, Gupta N, Serikov V, Lee JW, Matthay MA. Antibacterial effect of human mesenchymal stem cells is mediated in part from secretion of the antimicrobial peptide LL-37. Stem Cells. 2010;28:2229-2238. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 660] [Cited by in RCA: 587] [Article Influence: 39.1] [Reference Citation Analysis (0)] |
| 25. | Duplantier AJ, van Hoek ML. The Human Cathelicidin Antimicrobial Peptide LL-37 as a Potential Treatment for Polymicrobial Infected Wounds. Front Immunol. 2013;4:143. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 125] [Cited by in RCA: 174] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 26. | Sutton MT, Fletcher D, Ghosh SK, Weinberg A, van Heeckeren R, Kaur S, Sadeghi Z, Hijaz A, Reese J, Lazarus HM, Lennon DP, Caplan AI, Bonfield TL. Antimicrobial Properties of Mesenchymal Stem Cells: Therapeutic Potential for Cystic Fibrosis Infection, and Treatment. Stem Cells Int. 2016;2016:5303048. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 115] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 27. | Johnson EE, Wessling-Resnick M. Iron metabolism and the innate immune response to infection. Microbes Infect. 2012;14:207-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 185] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 28. | Gupta N, Krasnodembskaya A, Kapetanaki M, Mouded M, Tan X, Serikov V, Matthay MA. Mesenchymal stem cells enhance survival and bacterial clearance in murine Escherichia coli pneumonia. Thorax. 2012;67:533-539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 262] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 29. | Xiao X, Yeoh BS, Vijay-Kumar M. Lipocalin 2: An Emerging Player in Iron Homeostasis and Inflammation. Annu Rev Nutr. 2017;37:103-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 274] [Article Influence: 34.3] [Reference Citation Analysis (0)] |
| 30. | Sung DK, Chang YS, Sung SI, Yoo HS, Ahn SY, Park WS. Antibacterial effect of mesenchymal stem cells against Escherichia coli is mediated by secretion of beta- defensin- 2 via toll- like receptor 4 signalling. Cell Microbiol. 2016;18:424-436. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 101] [Cited by in RCA: 136] [Article Influence: 13.6] [Reference Citation Analysis (1)] |
| 31. | MacKenzie CR, Heseler K, Müller A, Däubener W. Role of indoleamine 2,3-dioxygenase in antimicrobial defence and immuno-regulation: tryptophan depletion vs production of toxic kynurenines. Curr Drug Metab. 2007;8:237-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 110] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 32. | Khan A, Mann L, Papanna R, Lyu MA, Singh CR, Olson S, Eissa NT, Cirillo J, Das G, Hunter RL, Jagannath C. Mesenchymal stem cells internalize Mycobacterium tuberculosis through scavenger receptors and restrict bacterial growth through autophagy. Sci Rep. 2017;7:15010. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 48] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 33. | Rodrigues ES, de Macedo MD, Orellana MD, Takayanagui OM, Palma PVB, Pinto MT, de Oliveira GLV, Malmegrim KCR, Slavov SN, Covas DT, Kashima S. Short Communication: Human Bone Marrow Stromal Cells Exhibit Immunosuppressive Effects on Human T Lymphotropic Virus Type 1 T Lymphocyte from Infected Individuals. AIDS Res Hum Retroviruses. 2019;35:164-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 34. | Klimova RR, Momotyuk ЕD, Demidova NA, Yarigina EI, Kushch AA. [Mesenchymal stem cells enhance immune response and protect mice against lethal herpes viral infection. Vopr Virusol. 2018;63:261-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 35. | Abraham E, Carmody A, Shenkar R, Arcaroli J. Neutrophils as early immunologic effectors in hemorrhage- or endotoxemia-induced acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2000;279:L1137-L1145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 298] [Cited by in RCA: 309] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 36. | Li T, Zhang Z, Li X, Dong G, Zhang M, Xu Z, Yang J. Neutrophil Extracellular Traps: Signaling Properties and Disease Relevance. Mediators Inflamm. 2020;2020:9254087. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 54] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 37. | Liu S, Su X, Pan P, Zhang L, Hu Y, Tan H, Wu D, Liu B, Li H, Li Y, Dai M, Hu C, Tsung A. Neutrophil extracellular traps are indirectly triggered by lipopolysaccharide and contribute to acute lung injury. Sci Rep. 2016;6:37252. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 137] [Cited by in RCA: 185] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 38. | Leppkes M, Knopf J, Naschberger E, Lindemann A, Singh J, Herrmann I, Stürzl M, Staats L, Mahajan A, Schauer C, Kremer AN, Völkl S, Amann K, Evert K, Falkeis C, Wehrfritz A, Rieker RJ, Hartmann A, Kremer AE, Neurath MF, Muñoz LE, Schett G, Herrmann M. Vascular occlusion by neutrophil extracellular traps in COVID-19. EBioMedicine. 2020;58:102925. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 357] [Cited by in RCA: 345] [Article Influence: 69.0] [Reference Citation Analysis (0)] |
| 39. | Dyer DP, Thomson JM, Hermant A, Jowitt TA, Handel TM, Proudfoot AE, Day AJ, Milner CM. TSG-6 inhibits neutrophil migration via direct interaction with the chemokine CXCL8. J Immunol. 2014;192:2177-2185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 146] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 40. | Jiang D, Muschhammer J, Qi Y, Kügler A, de Vries JC, Saffarzadeh M, Sindrilaru A, Beken SV, Wlaschek M, Kluth MA, Ganss C, Frank NY, Frank MH, Preissner KT, Scharffetter-Kochanek K. Suppression of Neutrophil-Mediated Tissue Damage-A Novel Skill of Mesenchymal Stem Cells. Stem Cells. 2016;34:2393-2406. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 125] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 41. | Rabani R, Volchuk A, Jerkic M, Ormesher L, Garces-Ramirez L, Canton J, Masterson C, Gagnon S, Tatham KC, Marshall J, Grinstein S, Laffey JG, Szaszi K, Curley GF. Mesenchymal stem cells enhance NOX2-dependent reactive oxygen species production and bacterial killing in macrophages during sepsis. Eur Respir J. 2018;51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 61] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 42. | Orecchioni M, Ghosheh Y, Pramod AB, Ley K. Macrophage Polarization: Different Gene Signatures in M1(LPS+) vs. Classically and M2(LPS-) vs. Alternatively Activated Macrophages. Front Immunol. 2019;10:1084. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 765] [Cited by in RCA: 1375] [Article Influence: 229.2] [Reference Citation Analysis (0)] |
| 43. | Luz-Crawford P, Djouad F, Toupet K, Bony C, Franquesa M, Hoogduijn MJ, Jorgensen C, Noël D. Mesenchymal Stem Cell-Derived Interleukin 1 Receptor Antagonist Promotes Macrophage Polarization and Inhibits B Cell Differentiation. Stem Cells. 2016;34:483-492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 202] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 44. | Kwon JH, Kim M, Bae YK, Kim GH, Choi SJ, Oh W, Um S, Jin HJ. Decorin Secreted by Human Umbilical Cord Blood-Derived Mesenchymal Stem Cells Induces Macrophage Polarization via CD44 to Repair Hyperoxic Lung Injury. Int J Mol Sci. 2019;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 45. | Lv H, Liu Q, Sun Y, Yi X, Wei X, Liu W, Zhang Q, Yi H, Chen G. Mesenchymal stromal cells ameliorate acute lung injury induced by LPS mainly through stanniocalcin-2 mediating macrophage polarization. Ann Transl Med. 2020;8:334. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 46. | Chen X, Yang B, Tian J, Hong H, Du Y, Li K, Li X, Wang N, Yu X, Wei X. Dental Follicle Stem Cells Ameliorate Lipopolysaccharide-Induced Inflammation by Secreting TGF-β3 and TSP-1 to Elicit Macrophage M2 Polarization. Cell Physiol Biochem. 2018;51:2290-2308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 57] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 47. | Li B, Zhang H, Zeng M, He W, Li M, Huang X, Deng DY, Wu J. Bone marrow mesenchymal stem cells protect alveolar macrophages from lipopolysaccharide-induced apoptosis partially by inhibiting the Wnt/β-catenin pathway. Cell Biol Int. 2015;39:192-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 52] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 48. | Jackson MV, Morrison TJ, Doherty DF, McAuley DF, Matthay MA, Kissenpfennig A, O'Kane CM, Krasnodembskaya AD. Mitochondrial Transfer via Tunneling Nanotubes is an Important Mechanism by Which Mesenchymal Stem Cells Enhance Macrophage Phagocytosis in the In Vitro and In Vivo Models of ARDS. Stem Cells. 2016;34:2210-2223. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 285] [Cited by in RCA: 398] [Article Influence: 44.2] [Reference Citation Analysis (0)] |
| 49. | Lu Z, Chang W, Meng S, Xu X, Xie J, Guo F, Yang Y, Qiu H, Liu L. Mesenchymal stem cells induce dendritic cell immune tolerance via paracrine hepatocyte growth factor to alleviate acute lung injury. Stem Cell Res Ther. 2019;10:372. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 109] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 50. | Liu W, Gao Y, Li H, Wang H, Ye M, Jiang G, Chen Y, Liu Y, Kong J, Liu W, Sun M, Hou M, Yu K. Intravenous transplantation of mesenchymal stromal cells has therapeutic effects in a sepsis mouse model through inhibition of septic natural killer cells. Int J Biochem Cell Biol. 2016;79:93-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 51. | Milosavljevic N, Gazdic M, Simovic Markovic B, Arsenijevic A, Nurkovic J, Dolicanin Z, Djonov V, Lukic ML, Volarevic V. Mesenchymal stem cells attenuate acute liver injury by altering ratio between interleukin 17 producing and regulatory natural killer T cells. Liver Transpl. 2017;23:1040-1050. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 66] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 52. | Luz-Crawford P, Kurte M, Bravo-Alegría J, Contreras R, Nova-Lamperti E, Tejedor G, Noël D, Jorgensen C, Figueroa F, Djouad F, Carrión F. Mesenchymal stem cells generate a CD4+CD25+Foxp3+ regulatory T cell population during the differentiation process of Th1 and Th17 cells. Stem Cell Res Ther. 2013;4:65. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 286] [Cited by in RCA: 367] [Article Influence: 30.6] [Reference Citation Analysis (0)] |
| 53. | Cruz FF, Borg ZD, Goodwin M, Sokocevic D, Wagner D, McKenna DH, Rocco PR, Weiss DJ. Freshly thawed and continuously cultured human bone marrow-derived mesenchymal stromal cells comparably ameliorate allergic airways inflammation in immunocompetent mice. Stem Cells Transl Med. 2015;4:615-624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 67] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 54. | Davies LC, Heldring N, Kadri N, Le Blanc K. Mesenchymal Stromal Cell Secretion of Programmed Death-1 Ligands Regulates T Cell Mediated Immunosuppression. Stem Cells. 2017;35:766-776. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 180] [Cited by in RCA: 267] [Article Influence: 29.7] [Reference Citation Analysis (0)] |
| 55. | Najar M, Raicevic G, Jebbawi F, De Bruyn C, Meuleman N, Bron D, Toungouz M, Lagneaux L. Characterization and functionality of the CD200-CD200R system during mesenchymal stromal cell interactions with T-lymphocytes. Immunol Lett. 2012;146:50-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 58] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 56. | Giuliani M, Bennaceur-Griscelli A, Nanbakhsh A, Oudrhiri N, Chouaib S, Azzarone B, Durrbach A, Lataillade JJ. TLR ligands stimulation protects MSC from NK killing. Stem Cells. 2014;32:290-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 66] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 57. | Gupta N, Sinha R, Krasnodembskaya A, Xu X, Nizet V, Matthay MA, Griffin JH. The TLR4-PAR1 Axis Regulates Bone Marrow Mesenchymal Stromal Cell Survival and Therapeutic Capacity in Experimental Bacterial Pneumonia. Stem Cells. 2018;36:796-806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 58. | de Witte SFH, Luk F, Sierra Parraga JM, Gargesha M, Merino A, Korevaar SS, Shankar AS, O'Flynn L, Elliman SJ, Roy D, Betjes MGH, Newsome PN, Baan CC, Hoogduijn MJ. Immunomodulation By Therapeutic Mesenchymal Stromal Cells (MSC) Is Triggered Through Phagocytosis of MSC By Monocytic Cells. Stem Cells. 2018;36:602-615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 266] [Cited by in RCA: 373] [Article Influence: 53.3] [Reference Citation Analysis (0)] |
| 59. | Gonçalves FDC, Luk F, Korevaar SS, Bouzid R, Paz AH, López-Iglesias C, Baan CC, Merino A, Hoogduijn MJ. Membrane particles generated from mesenchymal stromal cells modulate immune responses by selective targeting of pro-inflammatory monocytes. Sci Rep. 2017;7:12100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 80] [Cited by in RCA: 70] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 60. | Li W, Chen W, Huang S, Yao G, Tang X, Sun L. Mesenchymal stem cells prevent overwhelming inflammation and reduce infection severity via recruiting CXCR3+ regulatory T cells. Clin Transl Immunology. 2020;9:e1181. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 61. | Lee JW, Krasnodembskaya A, McKenna DH, Song Y, Abbott J, Matthay MA. Therapeutic effects of human mesenchymal stem cells in ex vivo human lungs injured with live bacteria. Am J Respir Crit Care Med. 2013;187:751-760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 246] [Cited by in RCA: 287] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 62. | Miteva K, Pappritz K, El-Shafeey M, Dong F, Ringe J, Tschöpe C, Van Linthout S. Mesenchymal Stromal Cells Modulate Monocytes Trafficking in Coxsackievirus B3-Induced Myocarditis. Stem Cells Transl Med. 2017;6:1249-1261. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 59] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 63. | Li Y, Xu J, Shi W, Chen C, Shao Y, Zhu L, Lu W, Han X. Mesenchymal stromal cell treatment prevents H9N2 avian influenza virus-induced acute lung injury in mice. Stem Cell Res Ther. 2016;7:159. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 99] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 64. | Loy H, Kuok DIT, Hui KPY, Choi MHL, Yuen W, Nicholls JM, Peiris JSM, Chan MCW. Therapeutic Implications of Human Umbilical Cord Mesenchymal Stromal Cells in Attenuating Influenza A(H5N1) Virus-Associated Acute Lung Injury. J Infect Dis. 2019;219:186-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 99] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 65. | Ahmed AR, Owens RJ, Stubbs CD, Parker AW, Hitchman R, Yadav RB, Dumoux M, Hawes C, Botchway SW. Direct imaging of the recruitment and phosphorylation of S6K1 in the mTORC1 pathway in living cells. Sci Rep. 2019;9:3408. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 66. | Broekman W, Amatngalim GD, de Mooij-Eijk Y, Oostendorp J, Roelofs H, Taube C, Stolk J, Hiemstra PS. TNF-α and IL-1β-activated human mesenchymal stromal cells increase airway epithelial wound healing in vitro via activation of the epidermal growth factor receptor. Respir Res. 2016;17:3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 74] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 67. | Liang C, Jiang E, Yao J, Wang M, Chen S, Zhou Z, Zhai W, Ma Q, Feng S, Han M. Interferon-γ mediates the immunosuppression of bone marrow mesenchymal stem cells on T-lymphocytes in vitro. Hematology. 2018;23:44-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 53] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 68. | Tang XD, Shi L, Monsel A, Li XY, Zhu HL, Zhu YG, Qu JM. Mesenchymal Stem Cell Microvesicles Attenuate Acute Lung Injury in Mice Partly Mediated by Ang-1 mRNA. Stem Cells. 2017;35:1849-1859. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 136] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 69. | English K, Barry FP, Field-Corbett CP, Mahon BP. IFN-gamma and TNF-alpha differentially regulate immunomodulation by murine mesenchymal stem cells. Immunol Lett. 2007;110:91-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 294] [Cited by in RCA: 338] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 70. | Fang X, Abbott J, Cheng L, Colby JK, Lee JW, Levy BD, Matthay MA. Human Mesenchymal Stem (Stromal) Cells Promote the Resolution of Acute Lung Injury in Part through Lipoxin A4. J Immunol. 2015;195:875-881. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 124] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 71. | Wang G, Cao K, Liu K, Xue Y, Roberts AI, Li F, Han Y, Rabson AB, Wang Y, Shi Y. Kynurenic acid, an IDO metabolite, controls TSG-6-mediated immunosuppression of human mesenchymal stem cells. Cell Death Differ. 2018;25:1209-1223. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 168] [Cited by in RCA: 157] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 72. | Hu S, Li J, Xu X, Liu A, He H, Xu J, Chen Q, Liu S, Liu L, Qiu H, Yang Y. The hepatocyte growth factor-expressing character is required for mesenchymal stem cells to protect the lung injured by lipopolysaccharide in vivo. Stem Cell Res Ther. 2016;7:66. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 73] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 73. | Prieto CP, Ortiz MC, Villanueva A, Villarroel C, Edwards SS, Elliott M, Lattus J, Aedo S, Meza D, Lois P, Palma V. Netrin-1 acts as a non-canonical angiogenic factor produced by human Wharton's jelly mesenchymal stem cells (WJ-MSC). Stem Cell Res Ther. 2017;8:43. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 74. | Jerkic M, Gagnon S, Rabani R, Ward-Able T, Masterson C, Otulakowski G, Curley GF, Marshall J, Kavanagh BP, Laffey JG. Human Umbilical Cord Mesenchymal Stromal Cells Attenuate Systemic Sepsis in Part by Enhancing Peritoneal Macrophage Bacterial Killing via Heme Oxygenase-1 Induction in Rats. Anesthesiology. 2020;132:140-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 75. | Jerkic M, Masterson C, Ormesher L, Gagnon S, Goyal S, Rabani R, Otulakowski G, Zhang H, Kavanagh BP, Laffey JG. Overexpression of IL-10 Enhances the Efficacy of Human Umbilical-Cord-Derived Mesenchymal Stromal Cells in E. coli Pneumosepsis. J Clin Med. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 76. | Gamble JR, Drew J, Trezise L, Underwood A, Parsons M, Kasminkas L, Rudge J, Yancopoulos G, Vadas MA. Angiopoietin-1 is an antipermeability and anti-inflammatory agent in vitro and targets cell junctions. Circ Res. 2000;87:603-607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 355] [Cited by in RCA: 371] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 77. | Xu J, Qu J, Cao L, Sai Y, Chen C, He L, Yu L. Mesenchymal stem cell-based angiopoietin-1 gene therapy for acute lung injury induced by lipopolysaccharide in mice. J Pathol. 2008;214:472-481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 177] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 78. | Shalaby SM, El-Shal AS, Abd-Allah SH, Selim AO, Selim SA, Gouda ZA, Abd El Motteleb DM, Zanfaly HE, El-Assar HM, Abdelazim S. Mesenchymal stromal cell injection protects against oxidative stress in Escherichia coli-induced acute lung injury in mice. Cytotherapy. 2014;16:764-775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 51] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 79. | Munir S, Basu A, Maity P, Krug L, Haas P, Jiang D, Strauss G, Wlaschek M, Geiger H, Singh K, Scharffetter-Kochanek K. TLR4-dependent shaping of the wound site by MSCs accelerates wound healing. EMBO Rep. 2020;21:e48777. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 44] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 80. | Shi C, Jia T, Mendez-Ferrer S, Hohl TM, Serbina NV, Lipuma L, Leiner I, Li MO, Frenette PS, Pamer EG. Bone marrow mesenchymal stem and progenitor cells induce monocyte emigration in response to circulating toll-like receptor ligands. Immunity. 2011;34:590-601. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 416] [Cited by in RCA: 405] [Article Influence: 28.9] [Reference Citation Analysis (0)] |
| 81. | Petri RM, Hackel A, Hahnel K, Dumitru CA, Bruderek K, Flohe SB, Paschen A, Lang S, Brandau S. Activated Tissue-Resident Mesenchymal Stromal Cells Regulate Natural Killer Cell Immune and Tissue-Regenerative Function. Stem Cell Reports. 2017;9:985-998. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 72] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 82. | Mao YX, Xu JF, Seeley EJ, Tang XD, Xu LL, Zhu YG, Song YL, Qu JM. Adipose Tissue-Derived Mesenchymal Stem Cells Attenuate Pulmonary Infection Caused by Pseudomonas aeruginosa via Inhibiting Overproduction of Prostaglandin E2. Stem Cells. 2015;33:2331-2342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 51] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 83. | Agard M, Asakrah S, Morici LA. PGE(2) suppression of innate immunity during mucosal bacterial infection. Front Cell Infect Microbiol. 2013;3:45. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 110] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 84. | Arango JC, Puerta-Arias JD, Pino-Tamayo PA, Arboleda-Toro D, González Á. Bone marrow-derived mesenchymal stem cells transplantation alters the course of experimental paracoccidioidomycosis by exacerbating the chronic pulmonary inflammatory response. Med Mycol. 2018;56:884-895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 85. | Schwartz YS, Belogorodtsev SN, Filimonov PN, Cherednichenko AG, Pustylnikov SV, Krasnov VA. BCG infection in mice is promoted by naïve mesenchymal stromal cells (MSC) and suppressed by poly(A:U)-conditioned MSC. Tuberculosis (Edinb). 2016;101:130-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 86. | Johnson ER, Matthay MA. Acute lung injury: epidemiology, pathogenesis, and treatment. J Aerosol Med Pulm Drug Deliv. 2010;23:243-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 519] [Cited by in RCA: 606] [Article Influence: 40.4] [Reference Citation Analysis (0)] |
| 87. | Atabai K, Ishigaki M, Geiser T, Ueki I, Matthay MA, Ware LB. Keratinocyte growth factor can enhance alveolar epithelial repair by nonmitogenic mechanisms. Am J Physiol Lung Cell Mol Physiol. 2002;283:L163-L169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 75] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 88. | Esquivel D, Mishra R, Soni P, Seetharaman R, Mahmood A, Srivastava A. Stem Cells Therapy as a Possible Therapeutic Option in Treating COVID-19 Patients. Stem Cell Rev Rep. 2021;17:144-152. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 89. | Knudsen L, Ochs M. The micromechanics of lung alveoli: structure and function of surfactant and tissue components. Histochem Cell Biol. 2018;150:661-676. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 155] [Cited by in RCA: 234] [Article Influence: 33.4] [Reference Citation Analysis (0)] |
| 90. | Mowery NT, Terzian WTH, Nelson AC. Acute lung injury. Curr Probl Surg. 2020;57:100777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 196] [Article Influence: 39.2] [Reference Citation Analysis (0)] |
| 91. | Wynne BM, Zou L, Linck V, Hoover RS, Ma HP, Eaton DC. Regulation of Lung Epithelial Sodium Channels by Cytokines and Chemokines. Front Immunol. 2017;8:766. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 40] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 92. | Lee JW, Fang X, Gupta N, Serikov V, Matthay MA. Allogeneic human mesenchymal stem cells for treatment of E. coli endotoxin-induced acute lung injury in the ex vivo perfused human lung. Proc Natl Acad Sci USA. 2009;106:16357-16362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 534] [Cited by in RCA: 558] [Article Influence: 34.9] [Reference Citation Analysis (0)] |
| 93. | Li J, Huang S, Zhang J, Feng C, Gao D, Yao B, Wu X, Fu X. Mesenchymal stem cells ameliorate inflammatory cytokine-induced impairment of AT-II cells through a keratinocyte growth factor-dependent PI3K/Akt/mTOR signaling pathway. Mol Med Rep. 2016;13:3755-3762. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 94. | Fang X, Neyrinck AP, Matthay MA, Lee JW. Allogeneic human mesenchymal stem cells restore epithelial protein permeability in cultured human alveolar type II cells by secretion of angiopoietin-1. J Biol Chem. 2010;285:26211-26222. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 201] [Cited by in RCA: 208] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 95. | Yang Y, Chen QH, Liu AR, Xu XP, Han JB, Qiu HB. Synergism of MSC-secreted HGF and VEGF in stabilising endothelial barrier function upon lipopolysaccharide stimulation via the Rac1 pathway. Stem Cell Res Ther. 2015;6:250. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 75] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 96. | Yang Y, Cheng Y, Lian QQ, Yang L, Qi W, Wu DR, Zheng X, Liu YJ, Li WJ, Jin SW, Smith FG. Contribution of CFTR to alveolar fluid clearance by lipoxin A4 via PI3K/Akt pathway in LPS-induced acute lung injury. Mediators Inflamm. 2013;2013:862628. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 97. | Wang Q, Lian QQ, Li R, Ying BY, He Q, Chen F, Zheng X, Yang Y, Wu DR, Zheng SX, Huang CJ, Smith FG, Jin SW. Lipoxin A(4) activates alveolar epithelial sodium channel, Na,K-ATPase, and increases alveolar fluid clearance. Am J Respir Cell Mol Biol. 2013;48:610-618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 49] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 98. | Ba F, Zhou X, Zhang Y, Wu C, Xu S, Wu L, Li J, Yin Y, Gu X. Lipoxin A4 ameliorates alveolar fluid clearance disturbance in lipopolysaccharide-induced lung injury via aquaporin 5 and MAPK signaling pathway. J Thorac Dis. 2019;11:3599-3608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 99. | Shi Z, Ye W, Zhang J, Zhang F, Yu D, Yu H, Chen B, Zhou M, Sun H. LipoxinA4 attenuates acute pancreatitis-associated acute lung injury by regulating AQP-5 and MMP-9 expression, anti-apoptosis and PKC/SSeCKS-mediated F-actin activation. Mol Immunol. 2018;103:78-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 100. | Grumbach Y, Quynh NV, Chiron R, Urbach V. LXA4 stimulates ZO-1 expression and transepithelial electrical resistance in human airway epithelial (16HBE14o-) cells. Am J Physiol Lung Cell Mol Physiol. 2009;296:L101-L108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 56] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 101. | Kellner M, Noonepalle S, Lu Q, Srivastava A, Zemskov E, Black SM. ROS Signaling in the Pathogenesis of Acute Lung Injury (ALI) and Acute Respiratory Distress Syndrome (ARDS). Adv Exp Med Biol. 2017;967:105-137. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 246] [Cited by in RCA: 283] [Article Influence: 35.4] [Reference Citation Analysis (0)] |
| 102. | Zorov DB, Juhaszova M, Sollott SJ. Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol Rev. 2014;94:909-950. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2139] [Cited by in RCA: 3820] [Article Influence: 347.3] [Reference Citation Analysis (0)] |
| 103. | Cheng X, He S, Yuan J, Miao S, Gao H, Zhang J, Li Y, Peng W, Wu P. Lipoxin A4 attenuates LPS-induced mouse acute lung injury via Nrf2-mediated E-cadherin expression in airway epithelial cells. Free Radic Biol Med. 2016;93:52-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 57] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 104. | Yang JX, Li M, Chen XO, Lian QQ, Wang Q, Gao F, Jin SW, Zheng SX. Lipoxin A4 ameliorates lipopolysaccharide-induced lung injury through stimulating epithelial proliferation, reducing epithelial cell apoptosis and inhibits epithelial-mesenchymal transition. Respir Res. 2019;20:192. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 36] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 105. | Cascone I, Audero E, Giraudo E, Napione L, Maniero F, Philips MR, Collard JG, Serini G, Bussolino F. Tie-2-dependent activation of RhoA and Rac1 participates in endothelial cell motility triggered by angiopoietin-1. Blood. 2003;102:2482-2490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 53] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 106. | Mammoto T, Parikh SM, Mammoto A, Gallagher D, Chan B, Mostoslavsky G, Ingber DE, Sukhatme VP. Angiopoietin-1 requires p190 RhoGAP to protect against vascular leakage in vivo. J Biol Chem. 2007;282:23910-23918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 150] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 107. | Chen QH, Liu AR, Qiu HB, Yang Y. Interaction between mesenchymal stem cells and endothelial cells restores endothelial permeability via paracrine hepatocyte growth factor in vitro. Stem Cell Res Ther. 2015;6:44. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 71] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 108. | Yang Y, Hu S, Xu X, Li J, Liu A, Han J, Liu S, Liu L, Qiu H. The Vascular Endothelial Growth Factors-Expressing Character of Mesenchymal Stem Cells Plays a Positive Role in Treatment of Acute Lung Injury In Vivo. Mediators Inflamm. 2016;2016:2347938. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 49] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 109. | Hu Y, Lou J, Mao YY, Lai TW, Liu LY, Zhu C, Zhang C, Liu J, Li YY, Zhang F, Li W, Ying SM, Chen ZH, Shen HH. Activation of MTOR in pulmonary epithelium promotes LPS-induced acute lung injury. Autophagy. 2016;12:2286-2299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 166] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 110. | Chen WX, Zhou J, Zhou SS, Zhang YD, Ji TY, Zhang XL, Wang SM, Du T, Ding DG. Microvesicles derived from human Wharton's jelly mesenchymal stem cells enhance autophagy and ameliorate acute lung injury via delivery of miR-100. Stem Cell Res Ther. 2020;11:113. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 41] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 111. | Takeda K, Ning F, Domenico J, Okamoto M, Ashino S, Kim SH, Jeong YY, Shiraishi Y, Terada N, Sutherland ER, Gelfand EW. Activation of p70S6 Kinase-1 in Mesenchymal Stem Cells Is Essential to Lung Tissue Repair. Stem Cells Transl Med. 2018;7:551-558. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 112. | Islam MN, Das SR, Emin MT, Wei M, Sun L, Westphalen K, Rowlands DJ, Quadri SK, Bhattacharya S, Bhattacharya J. Mitochondrial transfer from bone-marrow-derived stromal cells to pulmonary alveoli protects against acute lung injury. Nat Med. 2012;18:759-765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 916] [Cited by in RCA: 1123] [Article Influence: 86.4] [Reference Citation Analysis (0)] |
| 113. | Ahmad T, Mukherjee S, Pattnaik B, Kumar M, Singh S, Rehman R, Tiwari BK, Jha KA, Barhanpurkar AP, Wani MR, Roy SS, Mabalirajan U, Ghosh B, Agrawal A. Miro1 regulates intercellular mitochondrial transport & enhances mesenchymal stem cell rescue efficacy. EMBO J. 2014;33:994-1010. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 305] [Article Influence: 27.7] [Reference Citation Analysis (0)] |
| 114. | Cai SX, Liu AR, Chen S, He HL, Chen QH, Xu JY, Pan C, Yang Y, Guo FM, Huang YZ, Liu L, Qiu HB. The Orphan Receptor Tyrosine Kinase ROR2 Facilitates MSCs to Repair Lung Injury in ARDS Animal Model. Cell Transplant. 2016;25:1561-1574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 115. | Li L, Dong L, Zhang J, Gao F, Hui J, Yan J. Mesenchymal stem cells with downregulated Hippo signaling attenuate lung injury in mice with lipopolysaccharideinduced acute respiratory distress syndrome. Int J Mol Med. 2019;43:1241-1252. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 116. | El-Shennawy SF, Abdel Aaty HE, Radwan NA, Abdel-Hameed DM, Alam-Eldin YH, El-Ashkar AM, Abu-Zahra FA. Therapeutic Potential of Mesenchymal Stem Cells on Early and Late Experimental Hepatic Schistosomiasis Model. J Parasitol. 2015;101:587-597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 117. | Liu D, Liu L, Wang L, Duan S, Song Y, Qu M, Gao N, Wu J, Zhang H, Wu H, Kong W, Yu B, Yu X. Therapeutic effects of mesenchymal stem cells combined with short hairpin RNA on liver injury induced by hepatitis B virus infection. Mol Med Rep. 2018;17:1731-1741. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 118. | Zhang GZ, Sun HC, Zheng LB, Guo JB, Zhang XL. In vivo hepatic differentiation potential of human umbilical cord-derived mesenchymal stem cells: Therapeutic effect on liver fibrosis/cirrhosis. World J Gastroenterol. 2017;23:8152-8168. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 49] [Cited by in RCA: 47] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 119. | Zhou Y, Chen Y, Wang S, Qin F, Wang L. MSCs helped reduce scarring in the cornea after fungal infection when combined with anti-fungal treatment. BMC Ophthalmol. 2019;19:226. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 120. | Itaba N, Kono Y, Watanabe K, Yokobata T, Oka H, Osaki M, Kakuta H, Morimoto M, Shiota G. Reversal of established liver fibrosis by IC-2-engineered mesenchymal stem cell sheets. Sci Rep. 2019;9:6841. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 121. | Cai SX, Liu AR, Chen S, He HL, Chen QH, Xu JY, Pan C, Yang Y, Guo FM, Huang YZ, Liu L, Qiu HB. Activation of Wnt/β-catenin signalling promotes mesenchymal stem cells to repair injured alveolar epithelium induced by lipopolysaccharide in mice. Stem Cell Res Ther. 2015;6:65. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 62] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 122. | Liu A, Chen S, Cai S, Dong L, Liu L, Yang Y, Guo F, Lu X, He H, Chen Q, Hu S, Qiu H. Wnt5a through noncanonical Wnt/JNK or Wnt/PKC signaling contributes to the differentiation of mesenchymal stem cells into type II alveolar epithelial cells in vitro. PLoS One. 2014;9:e90229. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 46] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 123. | Liu AR, Liu L, Chen S, Yang Y, Zhao HJ, Guo FM, Lu XM, Qiu HB. Activation of canonical wnt pathway promotes differentiation of mouse bone marrow-derived MSCs into type II alveolar epithelial cells, confers resistance to oxidative stress, and promotes their migration to injured lung tissue in vitro. J Cell Physiol. 2013;228:1270-1283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 63] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 124. | Shi C, Lv T, Xiang Z, Sun Z, Qian W, Han X. Role of Wnt/β-Catenin Signaling in Epithelial Differentiation of Lung Resident Mesenchymal Stem Cells. J Cell Biochem. 2015;116:1532-1539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 125. | Fang S, Zhang S, Dai H, Hu X, Li C, Xing Y. The role of pulmonary mesenchymal cells in airway epithelium regeneration during injury repair. Stem Cell Res Ther. 2019;10:366. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 126. | Tong L, Zhou J, Rong L, Seeley EJ, Pan J, Zhu X, Liu J, Wang Q, Tang X, Qu J, Bai C, Song Y. Fibroblast Growth Factor-10 (FGF-10) Mobilizes Lung-resident Mesenchymal Stem Cells and Protects Against Acute Lung Injury. Sci Rep. 2016;6:21642. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 54] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 127. | Chen X, Zhao C, Zhang C, Li Q, Chen J, Cheng L, Zhou J, Su X, Song Y. Vagal-α7nAChR signaling promotes lung stem cells regeneration via fibroblast growth factor 10 during lung injury repair. Stem Cell Res Ther. 2020;11:230. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 128. | Silva JD, Lopes-Pacheco M, Paz AHR, Cruz FF, Melo EB, de Oliveira MV, Xisto DG, Capelozzi VL, Morales MM, Pelosi P, Cirne-Lima E, Rocco PRM. Mesenchymal Stem Cells From Bone Marrow, Adipose Tissue, and Lung Tissue Differentially Mitigate Lung and Distal Organ Damage in Experimental Acute Respiratory Distress Syndrome. Crit Care Med. 2018;46:e132-e140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 54] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 129. | Song CH, Honmou O, Furuoka H, Horiuchi M. Identification of chemoattractive factors involved in the migration of bone marrow-derived mesenchymal stem cells to brain lesions caused by prions. J Virol. 2011;85:11069-11078. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 34] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 130. | Larocca TF, Souza BS, Silva CA, Kaneto CM, Alcantara AC, Azevedo CM, Castro MF, Macambira SG, Soares MB, Ribeiro-dos-Santos R. Transplantation of adipose tissue mesenchymal stem cells in experimental chronic chagasic cardiopathy. Arq Bras Cardiol. 2013;100:460-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 131. | Silva DN, de Freitas Souza BS, Azevedo CM, Vasconcelos JF, Carvalho RH, Soares MB, Dos Santos RR. Intramyocardial transplantation of cardiac mesenchymal stem cells reduces myocarditis in a model of chronic Chagas disease cardiomyopathy. Stem Cell Res Ther. 2014;5:81. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 132. | Hammam OA, Elkhafif N, Attia YM, Mansour MT, Elmazar MM, Abdelsalam RM, Kenawy SA, El-Khatib AS. Wharton's jelly-derived mesenchymal stem cells combined with praziquantel as a potential therapy for Schistosoma mansoni-induced liver fibrosis. Sci Rep. 2016;6:21005. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 133. | Xu H, Qian H, Zhu W, Zhang X, Yan Y, Mao F, Wang M, Xu H, Xu W. Mesenchymal stem cells relieve fibrosis of Schistosoma japonicum-induced mouse liver injury. Exp Biol Med (Maywood). 2012;237:585-592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 53] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 134. | Zanganeh E, Soudi S, Zavaran Hosseini A, Khosrojerdi A. Repeated intravenous injection of adipose tissue derived mesenchymal stem cells enhances Th1 immune responses in Leishmania major-infected BALB/c mice. Immunol Lett. 2019;216:97-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 135. | Bulut Ö, GÜrsel İ. Mesenchymal stem cell derived extracellular vesicles: promising immunomodulators against autoimmune, autoinflammatory disorders and SARS-CoV-2 infection. Turk J Biol. 2020;44:273-282. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 136. | Harrell CR, Jovicic N, Djonov V, Arsenijevic N, Volarevic V. Mesenchymal Stem Cell-Derived Exosomes and Other Extracellular Vesicles as New Remedies in the Therapy of Inflammatory Diseases. Cells. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 225] [Cited by in RCA: 535] [Article Influence: 89.2] [Reference Citation Analysis (0)] |
| 137. | Larabi A, Barnich N, Nguyen HTT. Emerging Role of Exosomes in Diagnosis and Treatment of Infectious and Inflammatory Bowel Diseases. Cells. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 33] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 138. | Mardpour S, Hamidieh AA, Taleahmad S, Sharifzad F, Taghikhani A, Baharvand H. Interaction between mesenchymal stromal cell-derived extracellular vesicles and immune cells by distinct protein content. J Cell Physiol. 2019;234:8249-8258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 111] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 139. | Wang J, Huang R, Xu Q, Zheng G, Qiu G, Ge M, Shu Q, Xu J. Mesenchymal Stem Cell-Derived Extracellular Vesicles Alleviate Acute Lung Injury Via Transfer of miR-27a-3p. Crit Care Med. 2020;48:e599-e610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 85] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 140. | Song Y, Dou H, Li X, Zhao X, Li Y, Liu D, Ji J, Liu F, Ding L, Ni Y, Hou Y. Exosomal miR-146a Contributes to the Enhanced Therapeutic Efficacy of Interleukin-1β-Primed Mesenchymal Stem Cells Against Sepsis. Stem Cells. 2017;35:1208-1221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 367] [Article Influence: 45.9] [Reference Citation Analysis (0)] |
| 141. | Varkouhi AK, Jerkic M, Ormesher L, Gagnon S, Goyal S, Rabani R, Masterson C, Spring C, Chen PZ, Gu FX, Dos Santos CC, Curley GF, Laffey JG. Extracellular Vesicles from Interferon-γ-primed Human Umbilical Cord Mesenchymal Stromal Cells Reduce Escherichia coli-induced Acute Lung Injury in Rats. Anesthesiology. 2019;130:778-790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 73] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 142. | Zhu YG, Feng XM, Abbott J, Fang XH, Hao Q, Monsel A, Qu JM, Matthay MA, Lee JW. Human mesenchymal stem cell microvesicles for treatment of Escherichia coli endotoxin-induced acute lung injury in mice. Stem Cells. 2014;32:116-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 451] [Cited by in RCA: 514] [Article Influence: 46.7] [Reference Citation Analysis (0)] |
| 143. | Hao Q, Gudapati V, Monsel A, Park JH, Hu S, Kato H, Lee JH, Zhou L, He H, Lee JW. Mesenchymal Stem Cell-Derived Extracellular Vesicles Decrease Lung Injury in Mice. J Immunol. 2019;203:1961-1972. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 93] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 144. | Saeki K, Yokomizo T. Identification, signaling, and functions of LTB4 receptors. Semin Immunol. 2017;33:30-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 76] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 145. | Stone ML, Zhao Y, Robert Smith J, Weiss ML, Kron IL, Laubach VE, Sharma AK. Mesenchymal stromal cell-derived extracellular vesicles attenuate lung ischemia-reperfusion injury and enhance reconditioning of donor lungs after circulatory death. Respir Res. 2017;18:212. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 103] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 146. | Wang H, Zheng R, Chen Q, Shao J, Yu J, Hu S. Mesenchymal stem cells microvesicles stabilize endothelial barrier function partly mediated by hepatocyte growth factor (HGF). Stem Cell Res Ther. 2017;8:211. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 70] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 147. | Khatri M, Richardson LA, Meulia T. Mesenchymal stem cell-derived extracellular vesicles attenuate influenza virus-induced acute lung injury in a pig model. Stem Cell Res Ther. 2018;9:17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 237] [Cited by in RCA: 258] [Article Influence: 36.9] [Reference Citation Analysis (0)] |
| 148. | Qian X, Xu C, Fang S, Zhao P, Wang Y, Liu H, Yuan W, Qi Z. Exosomal MicroRNAs Derived From Umbilical Mesenchymal Stem Cells Inhibit Hepatitis C Virus Infection. Stem Cells Transl Med. 2016;5:1190-1203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 124] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 149. | Zhao S, Liu Y, Pu Z. Bone marrow mesenchymal stem cell-derived exosomes attenuate D-GaIN/LPS-induced hepatocyte apoptosis by activating autophagy in vitro. Drug Des Devel Ther. 2019;13:2887-2897. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 79] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 150. | Gu X, Li Y, Chen K, Wang X, Wang Z, Lian H, Lin Y, Rong X, Chu M, Lin J, Guo X. Exosomes derived from umbilical cord mesenchymal stem cells alleviate viral myocarditis through activating AMPK/mTOR-mediated autophagy flux pathway. J Cell Mol Med. 2020;24:7515-7530. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 67] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 151. | Deffune E, Prudenciatti A, Moroz A. Mesenchymal stem cell (MSc) secretome: A possible therapeutic strategy for intensive-care COVID-19 patients. Med Hypotheses. 2020;142:109769. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 152. | Su VY, Lin CS, Hung SC, Yang KY. Mesenchymal Stem Cell-Conditioned Medium Induces Neutrophil Apoptosis Associated with Inhibition of the NF-κB Pathway in Endotoxin-Induced Acute Lung Injury. Int J Mol Sci. 2019;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 87] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 153. | Asami T, Ishii M, Namkoong H, Yagi K, Tasaka S, Asakura T, Suzuki S, Kamo T, Okamori S, Kamata H, Zhang H, Hegab AE, Hasegawa N, Betsuyaku T. Anti-inflammatory roles of mesenchymal stromal cells during acute Streptococcus pneumoniae pulmonary infection in mice. Cytotherapy. 2018;20:302-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 154. | Hayes M, Curley GF, Masterson C, Devaney J, O'Toole D, Laffey JG. Mesenchymal stromal cells are more effective than the MSC secretome in diminishing injury and enhancing recovery following ventilator-induced lung injury. Intensive Care Med Exp. 2015;3:29. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 64] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 155. | Park J, Kim S, Lim H, Liu A, Hu S, Lee J, Zhuo H, Hao Q, Matthay MA, Lee JW. Therapeutic effects of human mesenchymal stem cell microvesicles in an ex vivo perfused human lung injured with severe E. coli pneumonia. Thorax. 2019;74:43-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 160] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 156. | Martínez-González I, Roca O, Masclans JR, Moreno R, Salcedo MT, Baekelandt V, Cruz MJ, Rello J, Aran JM. Human mesenchymal stem cells overexpressing the IL-33 antagonist soluble IL-1 receptor-like-1 attenuate endotoxin-induced acute lung injury. Am J Respir Cell Mol Biol. 2013;49:552-562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 77] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 157. | Chan BCL, Lam CWK, Tam LS, Wong CK. IL33: Roles in Allergic Inflammation and Therapeutic Perspectives. Front Immunol. 2019;10:364. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 115] [Cited by in RCA: 205] [Article Influence: 34.2] [Reference Citation Analysis (0)] |
| 158. | Chen X, Wu S, Tang L, Ma L, Wang F, Feng H, Meng J, Han Z. Mesenchymal stem cells overexpressing heme oxygenase-1 ameliorate lipopolysaccharide-induced acute lung injury in rats. J Cell Physiol. 2019;234:7301-7319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 66] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 159. | Mei SH, McCarter SD, Deng Y, Parker CH, Liles WC, Stewart DJ. Prevention of LPS-induced acute lung injury in mice by mesenchymal stem cells overexpressing angiopoietin 1. PLoS Med. 2007;4:e269. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 467] [Cited by in RCA: 494] [Article Influence: 27.4] [Reference Citation Analysis (0)] |
| 160. | Chen J, Zhang X, Xie J, Xue M, Liu L, Yang Y, Qiu H. Overexpression of TGFβ1 in murine mesenchymal stem cells improves lung inflammation by impacting the Th17/Treg balance in LPS-induced ARDS mice. Stem Cell Res Ther. 2020;11:311. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 35] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 161. | Zhao YF, Luo YM, Xiong W, Ding W, Li YR, Zhao W, Zeng HZ, Gao HC, Wu XL. Mesenchymal stem cell-based FGF2 gene therapy for acute lung injury induced by lipopolysaccharide in mice. Eur Rev Med Pharmacol Sci. 2015;19:857-865. [PubMed] |
| 162. | Zhao YF, Xiong W, Wu XL. Mesenchymal stem cell-based developmental endothelial locus-1 gene therapy for acute lung injury induced by lipopolysaccharide in mice. Mol Med Rep. 2014;9:1583-1589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 163. | Meng SS, Guo FM, Zhang XW, Chang W, Peng F, Qiu HB, Yang Y. mTOR/STAT-3 pathway mediates mesenchymal stem cell-secreted hepatocyte growth factor protective effects against lipopolysaccharide-induced vascular endothelial barrier dysfunction and apoptosis. J Cell Biochem. 2019;120:3637-3650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 35] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 164. | Zhang X, Chen J, Xue M, Tang Y, Xu J, Liu L, Huang Y, Yang Y, Qiu H, Guo F. Overexpressing p130/E2F4 in mesenchymal stem cells facilitates the repair of injured alveolar epithelial cells in LPS-induced ARDS mice. Stem Cell Res Ther. 2019;10:74. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 37] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 165. | Imai Y, Kuba K, Rao S, Huan Y, Guo F, Guan B, Yang P, Sarao R, Wada T, Leong-Poi H, Crackower MA, Fukamizu A, Hui CC, Hein L, Uhlig S, Slutsky AS, Jiang C, Penninger JM. Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature. 2005;436:112-116. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1791] [Cited by in RCA: 2001] [Article Influence: 100.1] [Reference Citation Analysis (0)] |
| 166. | He H, Liu L, Chen Q, Liu A, Cai S, Yang Y, Lu X, Qiu H. Mesenchymal Stem Cells Overexpressing Angiotensin-Converting Enzyme 2 Rescue Lipopolysaccharide-Induced Lung Injury. Cell Transplant. 2015;24:1699-1715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 79] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 167. | Li W, Moore MJ, Vasilieva N, Sui J, Wong SK, Berne MA, Somasundaran M, Sullivan JL, Luzuriaga K, Greenough TC, Choe H, Farzan M. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426:450-454. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4113] [Cited by in RCA: 4603] [Article Influence: 209.2] [Reference Citation Analysis (0)] |
| 168. | Ni W, Yang X, Yang D, Bao J, Li R, Xiao Y, Hou C, Wang H, Liu J, Xu Y, Cao Z, Gao Z. Role of angiotensin-converting enzyme 2 (ACE2) in COVID-19. Crit Care. 2020;24:422. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 843] [Cited by in RCA: 703] [Article Influence: 140.6] [Reference Citation Analysis (1)] |
| 169. | Yang JX, Zhang N, Wang HW, Gao P, Yang QP, Wen QP. CXCR4 receptor overexpression in mesenchymal stem cells facilitates treatment of acute lung injury in rats. J Biol Chem. 2015;290:1994-2006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 118] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 170. | Han J, Lu X, Zou L, Xu X, Qiu H. E-Prostanoid 2 Receptor Overexpression Promotes Mesenchymal Stem Cell Attenuated Lung Injury. Hum Gene Ther. 2016;27:621-630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 171. | Almeida CR, Caires HR, Vasconcelos DP, Barbosa MA. NAP-2 Secreted by Human NK Cells Can Stimulate Mesenchymal Stem/Stromal Cell Recruitment. Stem Cell Reports. 2016;6:466-473. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 52] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 172. | Shen Z, Wang J, Huang Q, Shi Y, Wei Z, Zhang X, Qiu Y, Zhang M, Wang Y, Qin W, Huang S, Huang Y, Liu X, Xia K, Lin Z. Genetic modification to induce CXCR2 overexpression in mesenchymal stem cells enhances treatment benefits in radiation-induced oral mucositis. Cell Death Dis. 2018;9:229. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 35] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 173. | Bustos ML, Huleihel L, Meyer EM, Donnenberg AD, Donnenberg VS, Sciurba JD, Mroz L, McVerry BJ, Ellis BM, Kaminski N, Rojas M. Activation of human mesenchymal stem cells impacts their therapeutic abilities in lung injury by increasing interleukin (IL)-10 and IL-1RN levels. Stem Cells Transl Med. 2013;2:884-895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 67] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 174. | Rodriguez LA 2nd, Mohammadipoor A, Alvarado L, Kamucheka RM, Asher AM, Cancio LC, Antebi B. Preconditioning in an Inflammatory Milieu Augments the Immunotherapeutic Function of Mesenchymal Stromal Cells. Cells. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 175. | Saldaña L, Bensiamar F, Vallés G, Mancebo FJ, García-Rey E, Vilaboa N. Immunoregulatory potential of mesenchymal stem cells following activation by macrophage-derived soluble factors. Stem Cell Res Ther. 2019;10:58. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 121] [Cited by in RCA: 120] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 176. | Li D, Liu Q, Qi L, Dai X, Liu H, Wang Y. Low levels of TGF-β1 enhance human umbilical cord-derived mesenchymal stem cell fibronectin production and extend survival time in a rat model of lipopolysaccharide-induced acute lung injury. Mol Med Rep. 2016;14:1681-1692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 47] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 177. | Silva JD, Lopes-Pacheco M, de Castro LL, Kitoko JZ, Trivelin SA, Amorim NR, Capelozzi VL, Morales MM, Gutfilen B, de Souza SAL, Weiss DJ, Diaz BL, Rocco PRM. Eicosapentaenoic acid potentiates the therapeutic effects of adipose tissue-derived mesenchymal stromal cells on lung and distal organ injury in experimental sepsis. Stem Cell Res Ther. 2019;10:264. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 37] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 178. | Spaggiari GM, Capobianco A, Becchetti S, Mingari MC, Moretta L. Mesenchymal stem cell-natural killer cell interactions: evidence that activated NK cells are capable of killing MSCs, whereas MSCs can inhibit IL-2-induced NK-cell proliferation. Blood. 2006;107:1484-1490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 773] [Cited by in RCA: 780] [Article Influence: 39.0] [Reference Citation Analysis (0)] |
| 179. | Masalova OV, Lesnova EI, Klimova RR, Momotyuk ED, Kozlov VV, Ivanova AM, Payushina OV, Butorina NN, Zakirova NF, Narovlyansky AN, Pronin AV, Ivanov AV, Kushch AA. Genetically Modified Mouse Mesenchymal Stem Cells Expressing Non-Structural Proteins of Hepatitis C Virus Induce Effective Immune Response. Vaccines (Basel). 2020;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 180. | Meng SS, Xu XP, Chang W, Lu ZH, Huang LL, Xu JY, Liu L, Qiu HB, Yang Y, Guo FM. LincRNA-p21 promotes mesenchymal stem cell migration capacity and survival through hypoxic preconditioning. Stem Cell Res Ther. 2018;9:280. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 79] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 181. | Antebi B, Rodriguez LA 2nd, Walker KP 3rd, Asher AM, Kamucheka RM, Alvarado L, Mohammadipoor A, Cancio LC. Short-term physiological hypoxia potentiates the therapeutic function of mesenchymal stem cells. Stem Cell Res Ther. 2018;9:265. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 99] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 182. | Liu L, He H, Liu A, Xu J, Han J, Chen Q, Hu S, Xu X, Huang Y, Guo F, Yang Y, Qiu H. Therapeutic Effects of Bone Marrow-Derived Mesenchymal Stem Cells in Models of Pulmonary and Extrapulmonary Acute Lung Injury. Cell Transplant. 2015;24:2629-2642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 183. | Xu L, Gong Y, Wang B, Shi K, Hou Y, Wang L, Lin Z, Han Y, Lu L, Chen D, Lin X, Zeng Q, Feng W, Chen Y. Randomized trial of autologous bone marrow mesenchymal stem cells transplantation for hepatitis B virus cirrhosis: regulation of Treg/Th17 cells. J Gastroenterol Hepatol. 2014;29:1620-1628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 84] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 184. | Fang X, Liu L, Dong J, Zhang J, Song H, Song Y, Huang Y, Cui X, Lin J, Chen C, Liu B, Chen Z, Pan J, Chen X. A study about immunomodulatory effect and efficacy and prognosis of human umbilical cord mesenchymal stem cells in patients with chronic hepatitis B-induced decompensated liver cirrhosis. J Gastroenterol Hepatol. 2018;33:774-780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 41] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 185. | Xu WX, He HL, Pan SW, Chen YL, Zhang ML, Zhu S, Gao ZL, Peng L, Li JG. Combination Treatments of Plasma Exchange and Umbilical Cord-Derived Mesenchymal Stem Cell Transplantation for Patients with Hepatitis B Virus-Related Acute-on-Chronic Liver Failure: A Clinical Trial in China. Stem Cells Int. 2019;2019:4130757. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 46] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 186. | Jia Y, Shu X, Yang X, Sun H, Cao H, Zhang K, Xu Q, Li G, Yang Y. Enhanced therapeutic effects of umbilical cord mesenchymal stem cells after prolonged treatment for HBV-related liver failure and liver cirrhosis. Stem Cell Res Ther. 2020;11:277. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 187. | Salama H, Zekri AR, Medhat E, Al Alim SA, Ahmed OS, Bahnassy AA, Lotfy MM, Ahmed R, Musa S. Peripheral vein infusion of autologous mesenchymal stem cells in Egyptian HCV-positive patients with end-stage liver disease. Stem Cell Res Ther. 2014;5:70. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 95] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 188. | CDC. Middle East Respiratory Syndrome (MERS). 2019 [cited 5 March 2021]. In: Centers for Disease Control and Prevention [Internet]. Available from: https://www.cdc.gov/coronavirus/mers/about/index.html. |
| 189. | CDC. Influenza Type A Viruses, Avian Influenza (Flu); 2017. [cited 5 March 2021]. In: Centers for Disease Control and Prevention [Internet]. Available from: https://www.cdc.gov/flu/avianflu/influenza-a-virus-subtypes.htm. |
| 190. | WHO. Avian influenza: food safety issues; 2006. [cited 5 March 2021]. In: World Health Organization [Internet]. Available from: https://www.who.int/foodsafety/areas_work/zoonose/avian/en/index1.html. |
| 191. | Chen J, Hu C, Chen L, Tang L, Zhu Y, Xu X, Gao H, Lu X, Yu L, Dai X, Xiang C, Li L. Clinical Study of Mesenchymal Stem Cell Treatment for Acute Respiratory Distress Syndrome Induced by Epidemic Influenza A (H7N9) Infection: A Hint for COVID-19 Treatment. Engineering (Beijing). 2020;6:1153-1161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 169] [Article Influence: 33.8] [Reference Citation Analysis (0)] |
| 192. | Simonson OE, Mougiakakos D, Heldring N, Bassi G, Johansson HJ, Dalén M, Jitschin R, Rodin S, Corbascio M, El Andaloussi S, Wiklander OP, Nordin JZ, Skog J, Romain C, Koestler T, Hellgren-Johansson L, Schiller P, Joachimsson PO, Hägglund H, Mattsson M, Lehtiö J, Faridani OR, Sandberg R, Korsgren O, Krampera M, Weiss DJ, Grinnemo KH, Le Blanc K. In Vivo Effects of Mesenchymal Stromal Cells in Two Patients With Severe Acute Respiratory Distress Syndrome. Stem Cells Transl Med. 2015;4:1199-1213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 113] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 193. | Leng Z, Zhu R, Hou W, Feng Y, Yang Y, Han Q, Shan G, Meng F, Du D, Wang S, Fan J, Wang W, Deng L, Shi H, Li H, Hu Z, Zhang F, Gao J, Liu H, Li X, Zhao Y, Yin K, He X, Gao Z, Wang Y, Yang B, Jin R, Stambler I, Lim LW, Su H, Moskalev A, Cano A, Chakrabarti S, Min KJ, Ellison-Hughes G, Caruso C, Jin K, Zhao RC. Transplantation of ACE2- Mesenchymal Stem Cells Improves the Outcome of Patients with COVID-19 Pneumonia. Aging Dis. 2020;11:216-228. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 730] [Cited by in RCA: 852] [Article Influence: 170.4] [Reference Citation Analysis (0)] |
| 194. | Zhang Y, Ding J, Ren S, Wang W, Yang Y, Li S, Meng M, Wu T, Liu D, Tian S, Tian H, Chen S, Zhou C. Intravenous infusion of human umbilical cord Wharton's jelly-derived mesenchymal stem cells as a potential treatment for patients with COVID-19 pneumonia. Stem Cell Res Ther. 2020;11:207. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 93] [Cited by in RCA: 86] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 195. | Peng H, Gong T, Huang X, Sun X, Luo H, Wang W, Luo J, Luo B, Chen Y, Wang X, Long H, Mei H, Li C, Dai Y, Li H. A synergistic role of convalescent plasma and mesenchymal stem cells in the treatment of severely ill COVID-19 patients: a clinical case report. Stem Cell Res Ther. 2020;11:291. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 47] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 196. | Du J, Li H, Lian J, Zhu X, Qiao L, Lin J. Stem cell therapy: a potential approach for treatment of influenza virus and coronavirus-induced acute lung injury. Stem Cell Res Ther. 2020;11:192. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 36] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 197. | Golchin A, Seyedjafari E, Ardeshirylajimi A. Mesenchymal Stem Cell Therapy for COVID-19: Present or Future. Stem Cell Rev Rep. 2020;16:427-433. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 248] [Cited by in RCA: 225] [Article Influence: 45.0] [Reference Citation Analysis (1)] |
| 198. | Rogers CJ, Harman RJ, Bunnell BA, Schreiber MA, Xiang C, Wang FS, Santidrian AF, Minev BR. Rationale for the clinical use of adipose-derived mesenchymal stem cells for COVID-19 patients. J Transl Med. 2020;18:203. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 75] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 199. | Moll G, Drzeniek N, Kamhieh-Milz J, Geissler S, Volk HD, Reinke P. MSC Therapies for COVID-19: Importance of Patient Coagulopathy, Thromboprophylaxis, Cell Product Quality and Mode of Delivery for Treatment Safety and Efficacy. Front Immunol. 2020;11:1091. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 113] [Cited by in RCA: 129] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 200. | Chen X, Shan Y, Wen Y, Sun J, Du H. Mesenchymal stem cell therapy in severe COVID-19: A retrospective study of short-term treatment efficacy and side effects. J Infect. 2020;81:647-679. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 43] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 201. | Naik SK, Padhi A, Ganguli G, Sengupta S, Pati S, Das D, Sonawane A. Mouse Bone Marrow Sca-1+ CD44+ Mesenchymal Stem Cells Kill Avirulent Mycobacteria but Not Mycobacterium tuberculosis through Modulation of Cathelicidin Expression via the p38 Mitogen-Activated Protein Kinase-Dependent Pathway. Infect Immun. 2017;85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 202. | Jain N, Kalam H, Singh L, Sharma V, Kedia S, Das P, Ahuja V, Kumar D. Mesenchymal stem cells offer a drug-tolerant and immune-privileged niche to Mycobacterium tuberculosis. Nat Commun. 2020;11:3062. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 203. | Lopes CS, Daifalla N, Das B, Dias da Silva V, Campos-Neto A. CD271+ Mesenchymal Stem Cells as a Possible Infectious Niche for Leishmania infantum. PLoS One. 2016;11:e0162927. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 204. | Qiao GH, Zhao F, Cheng S, Luo MH. Multipotent mesenchymal stromal cells are fully permissive for human cytomegalovirus infection. Virol Sin. 2016;31:219-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 205. | Soland MA, Keyes LR, Bayne R, Moon J, Porada CD, St Jeor S, Almeida-Porada G. Perivascular stromal cells as a potential reservoir of human cytomegalovirus. Am J Transplant. 2014;14:820-830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 206. | Meisel R, Heseler K, Nau J, Schmidt SK, Leineweber M, Pudelko S, Wenning J, Zimmermann A, Hengel H, Sinzger C, Degistirici Ö, Sorg RV, Däubener W. Cytomegalovirus infection impairs immunosuppressive and antimicrobial effector functions of human multipotent mesenchymal stromal cells. Mediators Inflamm. 2014;2014:898630. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 207. | Sundin M, Lindblom A, Örvell C, Barrett AJ, Sundberg B, Watz E, Wikman A, Broliden K, Le Blanc K. Persistence of human parvovirus B19 in multipotent mesenchymal stromal cells expressing the erythrocyte P antigen: implications for transplantation. Biol Blood Marrow Transplant. 2008;14:1172-1179. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 208. | Avanzi S, Leoni V, Rotola A, Alviano F, Solimando L, Lanzoni G, Bonsi L, Di Luca D, Marchionni C, Alvisi G, Ripalti A. Susceptibility of human placenta derived mesenchymal stromal/stem cells to human herpesviruses infection. PLoS One. 2013;8:e71412. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 209. | Ma R, Xing Q, Shao L, Wang D, Hao Q, Li X, Sai L, Ma L. Hepatitis B virus infection and replication in human bone marrow mesenchymal stem cells. Virol J. 2011;8:486. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 34] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 210. | Wang Y, Wang F, Zhao H, Zhang X, Chen H, Zhang K. Human adipose-derived mesenchymal stem cells are resistant to HBV infection during differentiation into hepatocytes in vitro. Int J Mol Sci. 2014;15:6096-6110. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 211. | Thanunchai M, Kanrai P, Wiboon-Ut S, Puthavathana P, Hongeng S, Thitithanyanont A. Tropism of avian influenza A (H5N1) virus to mesenchymal stem cells and CD34+ hematopoietic stem cells. PLoS One. 2013;8:e81805. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 212. | Cotter EJ, Chew N, Powderly WG, Doran PP. HIV type 1 alters mesenchymal stem cell differentiation potential and cell phenotype ex vivo. AIDS Res Hum Retroviruses. 2011;27:187-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 47] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 213. | Cheng K, Rai P, Lan X, Plagov A, Malhotra A, Gupta S, Singhal PC. Bone-derived mesenchymal stromal cells from HIV transgenic mice exhibit altered proliferation, differentiation capacity and paracrine functions along with impaired therapeutic potential in kidney injury. Exp Cell Res. 2013;319:2266-2274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 214. | Cervenakova L, Akimov S, Vasilyeva I, Yakovleva O, McKenzie C, Cervenak J, Piccardo P, Asher DM. Fukuoka-1 strain of transmissible spongiform encephalopathy agent infects murine bone marrow-derived cells with features of mesenchymal stem cells. Transfusion. 2011;51:1755-1768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 215. | Berglund AK, Fortier LA, Antczak DF, Schnabel LV. Immunoprivileged no more: measuring the immunogenicity of allogeneic adult mesenchymal stem cells. Stem Cell Res Ther. 2017;8:288. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 130] [Cited by in RCA: 166] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 216. | Consentius C, Reinke P, Volk HD. Immunogenicity of allogeneic mesenchymal stromal cells: what has been seen in vitro and in vivo? Regen Med. 2015;10:305-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 50] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 217. | Griffin MD, Ryan AE, Alagesan S, Lohan P, Treacy O, Ritter T. Anti-donor immune responses elicited by allogeneic mesenchymal stem cells: what have we learned so far? Immunol Cell Biol. 2013;91:40-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 178] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 218. | Berebichez-Fridman R, Montero-Olvera PR. Sources and Clinical Applications of Mesenchymal Stem Cells: State-of-the-art review. Sultan Qaboos Univ Med J. 2018;18:e264-e277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 278] [Article Influence: 39.7] [Reference Citation Analysis (0)] |
| 219. | Miteva K, Pappritz K, Sosnowski M, El-Shafeey M, Müller I, Dong F, Savvatis K, Ringe J, Tschöpe C, Van Linthout S. Mesenchymal stromal cells inhibit NLRP3 inflammasome activation in a model of Coxsackievirus B3-induced inflammatory cardiomyopathy. Sci Rep. 2018;8:2820. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 59] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 220. | Chen X, Zhang Y, Wang W, Liu Z, Meng J, Han Z. Mesenchymal Stem Cells Modified with Heme Oxygenase-1 Have Enhanced Paracrine Function and Attenuate Lipopolysaccharide-Induced Inflammatory and Oxidative Damage in Pulmonary Microvascular Endothelial Cells. Cell Physiol Biochem. 2018;49:101-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 41] [Article Influence: 5.9] [Reference Citation Analysis (0)] |