Published online Jun 26, 2021. doi: 10.4252/wjsc.v13.i6.485
Peer-review started: January 27, 2021
First decision: February 28, 2021
Revised: April 2, 2021
Accepted: June 16, 2021
Article in press: June 16, 2021
Published online: June 26, 2021
Processing time: 149 Days and 22.8 Hours
Targeted genome editing is a continually evolving technology employing programmable nucleases to specifically change, insert, or remove a genomic sequence of interest. These advanced molecular tools include meganucleases, zinc finger nucleases, transcription activator-like effector nucleases and RNA-guided engineered nucleases (RGENs), which create double-strand breaks at specific target sites in the genome, and repair DNA either by homologous recombination in the presence of donor DNA or via the error-prone non-homologous end-joining mechanism. A recently discovered group of RGENs known as CRISPR/Cas9 gene-editing systems allowed precise genome manipulation revealing a causal association between disease genotype and phenotype, without the need for the reengineering of the specific enzyme when targeting different sequences. CRISPR/Cas9 has been successfully employed as an ex vivo gene-editing tool in embryonic stem cells and patient-derived stem cells to understand pancreatic beta-cell development and function. RNA-guided nucleases also open the way for the generation of novel animal models for diabetes and allow testing the efficiency of various therapeutic approaches in diabetes, as summarized and exemplified in this manuscript.
Core Tip: In this review, CRISPR/Cas9 nuclease is deployed in the generation of cellular and animal models of diabetes, which are suitable for the testing of the treatment efficacy of novel insulin gene therapy approaches.
- Citation: Eksi YE, Sanlioglu AD, Akkaya B, Ozturk BE, Sanlioglu S. Genome engineering and disease modeling via programmable nucleases for insulin gene therapy: Promises of CRISPR/Cas9 technology . World J Stem Cells 2021; 13(6): 485-502
- URL: https://www.wjgnet.com/1948-0210/full/v13/i6/485.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v13.i6.485
Approximately 25000 protein-coding and 25000 noncoding RNA genes totaling up to 50000 genes are estimated to exist over a meter of linear DNA in the human genome[1]. In recent years, decoding the function of individual genes became the major goal of human genome research. Comprehensive genetic analysis was required to disclose correlations between genetic variants and human diseases. The development of effective therapeutic drugs against human genetic diseases depends on understanding the function of genes. Although the connection between genetic changes and human diseases has been known for years, the mutations that cause the emergence of some disease phenotypes can only be treated by genetic modification[2,3]. Due to the complexity of the human genome, modification of genetic information represented a challenge requiring technologically advanced molecular tools[4,5]. Furthermore, the efficient and safe delivery of these genome engineering tools to targeted tissues of interest remains to be a concern for the clinical application of these technologies[6].
The idea of genome modification was first introduced by Rudin and Haber[7] in 1988, where they reported efficient repair of homothallic switching endonuclease enzyme-triggered chromosomal breaks in Saccharomyces cerevisiae (S. cerevisiae) by homologous recombination (HR). They demonstrated that the efficiency of gene targeting is increased via the creation of double-strand DNA breaks (DSBs) in yeasts[7]. Furthermore, the significance of HR in the repair of DSBs was first demonstrated in mammalian cells by Jasin’s research group[8]. Previously, it was thought that lethal chromosomal DSBs in mammalian cells were repaired by a mechanism that does not require homology to the break site. This was a rather contrast with S. cerevisiae where the major DSB repair pathway is HR. To determine if mammalian cells used recombinational repair at a notable level, the DNA cleavage sites for the rare-cutting endonuclease I-SceI from S. cerevisiae were integrated into the mammalian (mouse) genome. Thus, specific DSBs could be introduced into the mouse genome at the enzyme’s cleavage sites via an expression system for the I-SceI. The use of a homologous DNA resulted in targeted genetic modification of mammalian cells mainly by HR or to a lesser degree via error-prone non-homologous end joining (NHEJ) mechanism. These two studies not only demonstrated the importance of HR in the repair of DSBs in mammalian cells, but also laid the foundation for targeted genome modification. Yet it is really a challenge to reprogram the long DNA-binding recognition regions of the meganucleases for genomic modification. At this point, genome editing via programmable nucleases constitutes a very efficient alternative approach to modify any desired region in the genome.
Gene targeting via HR is not practical in higher eukaryotic cells due to low efficiency, hampering their routine use. On the other hand, programmable nucleases generating site-specific DSBs were found to increase HR efficiency by at least 100 fold and/or activated the error-prone NHEJ mechanism[9]. Following genome editing approaches via zinc finger nucleases (ZFNs) that remained as the primary modification option for researchers for a while, transcription activator-like effector nucleases (TALENs) were developed as novel tools for genome editing, followed by the development of a new class of genome-editing nucleases named RNA-guided engineered nucleases (RGENs). RGENs which displayed their specificity via small guide RNAs, brought excitement to the field as easier to modify targeted nuclease systems. While each programmable nuclease has its exclusive properties, they all cleave the nuclear DNA at specific target sites as a similar mechanism of action, activating endogenous DNA repair systems leading to targeted genome modification. In this review, we would like to first describe the general features of programmable nucleases then specifically focus on RGENs for genome modification.
In the early 1990s, a biochemist named Srinivasan Chandrasegaran from Johns Hopkins University speculated and have shown that the type IIS restriction enzyme Fok1 had two separate protein domains, namely the DNA-binding domain and the domain that exerted the endonuclease activity, which could be separated from each other with the help of a protease, without losing function[10]. This opened the way for the idea of combining the Fok1 nuclease site with DNA binding domains of other proteins to create sequence-specific nucleases, such as in the generation of ZFNs by binding of the Fok1 endonuclease to zinc finger proteins[11]. Inspired by Chandra
ZFNs are very useful to manipulate the genomes of plants, animals, and humans. They have successfully been used to correct disease-causing alleles in triplet repeat disorders, which are present in more than a dozen inherited neurological disorders including but not limited to Huntington's disease, myotonic dystrophy, and several spinocerebellar ataxias[16]. Targeted delivery of the therapeutic genes to a preselected chromosomal locus can be achieved using plasmids encoding ZFNs via the generation of DSBs in a specific gene in human cells. This would prevent all the complications associated with the viral delivery of therapeutic genes[17]. Furthermore, patients' stem cells can be modified using ZFNs ex vivo; after expanding them in culture, genetically modified stem cells can be put back into the patient to generate differentiated cells with corrected functions[18].
Unfortunately, many ZFNs have been shown to possess cytotoxic effects due to off-target DSBs[19]. Off-target cleavage activity of ZFNs is reported when the zinc finger domains are not specific enough for their target site or carry homology to other unintended sites. Generation of excessive amounts of DSBs may overwhelm the repair machinery leading to random integration of donor DNA and eventual cell death. To decrease off-target cleavage of 3-finger ZFNs that target two adjacent 9-basepair sites, the use of ZFNs with 4, 5, or 6 zinc fingers targeting longer and rare sites is suggested[20,21]. Intriguingly, the competition between HR and NHEJ repair pathways represents a handicap for ZFN-mediated gene modifications. Inactivation of the catalytic activity of one ZFN monomer in the ZFN dimer results in the generation of Zinc-finger nickases (ZFNickases) which has shown to provide a bias for HR-mediated gene modification[22]. ZFNickases possess a reduced spectrum of off-target alterations due to the reduction in NHEJ repairs. Unfortunately, the application of ZFNs to manipulate endogenous genes has been a challenging job due to difficulty in generating zinc finger domains that target the chosen sequence with sufficient sequence specificity.
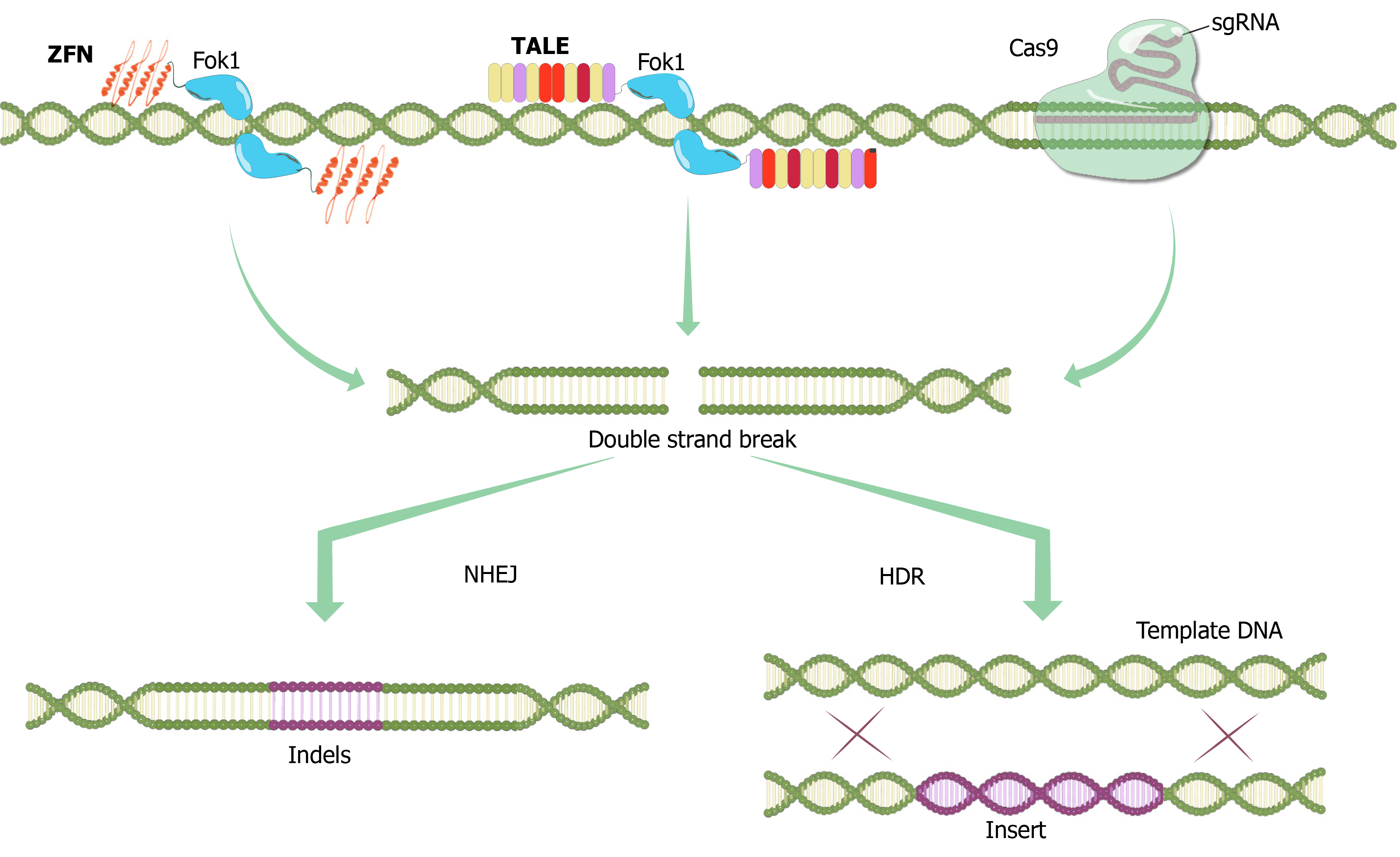
The solution to the cytotoxicity problem of ZFNs came in 2009 from naturally occurring transcription factors of a Gram-negative bacterial plant pathogen Xanthomonas. Two research groups, one led by Bogdanove et al[23] at Iowa State University and the other by Boch and Bonas[24] at Martin Luther University, revealed the interaction mechanisms between Xanthomonas-originated transcription activator-like effectors (TALEs) and DNA, and this led to the discovery that these proteins could also be used for genome editing with their simple DNA-binding code and relative ease of engineering. TALEs are proteins that are secreted by Xanthomonas bacteria by way of type III secretion system following infection of plants. The DNA-binding domain of a TALE carries a repetitive highly conserved 33–34 amino acid sequence with divergent 12th and 13th amino acids referred to as the repeat variable Di-residue[25]. These residues are highly flexible and used in specific nucleotide recognition. TALENs are generated by fusing a TAL effector DNA-binding domain to a DNA cleavage domain Fok1, which can be engineered to cleave specific sequences of DNA (Figure 1)[26]. Although ZFNs and TALENs contain the same Fok1 nuclease domain at their C-terminal ends, their DNA binding sites are different from each other (Figure 1). Unlike zinc finger proteins, each repeat of TALEs recognizes a single base.
TALENs have been used to efficiently modify human embryonic stem cell (hESC) and induced pluripotent stem cell (iPSC) clones and human erythroid cell lines[27,28], to generate knockout mice and rats[29,30]. TALENs have also been experimentally deployed to correct the genetic errors underlying diseases[31]. The genetic defects that cause disorders such as sickle cell disease[28,32], xeroderma pigmentosum, and epidermolysis bullosa[33] were all amenable to correction in vitro using TALENs. Moreover, T cells can be genetically modified by TALENs to become resistant to chemotherapeutic drugs and display antitumor activity[34,35].
Unfortunately, the lack of an efficient delivery mechanism, immunogenicity, and the nonspecific TALEN binding to unintended sites limited the in situ application of TALENs for the treatment of human diseases[31]. Furthermore, the generation of the DNA segment containing the TALE repeat sequences is very difficult and time-consuming. It is technically very hard to generate these sequences since they are likely to recombine with each other in cells. Although it is quite remarkable that TALENs showed minimal toxicity and off-targeting activity in human cells, the emergence of the CRISPR/Cas9 system has diverted much of the attention of the community to this new class of nucleases.
The studies that led to the discovery of clustered DNA repeats came independently from three different parts of the world. Ishino et al[36] and his colleagues from Osaka University accidentally cloned part of a clustered regularly interspaced short palindromic repeats (CRISPR) sequence together with their gene of interest, the isozyme conversion of alkaline phosphatase gene, in 1987 without knowing the function of the interrupted clustered repeats. In 1993, Groenen et al[37] have discovered a cluster of interrupted direct repeats (DRs) while working on DNA polymorphism in the DR cluster of Mycobacterium tuberculosis in the Netherlands. Later, Mojica et al[38] at the University of Alicante in Spain was studying the repeat sequences and their relevant function in the archaeal Haloferax and Haloarcula species when he observed the transcription of interrupted repeats for the first time in 2000. In 2001, while working on possible additional interrupted repeats, Mojica and Montoliu[39] suggested the use of the acronym CRISPR to prevent the misunderstanding curtailing from the numerous abbreviations utilized to define the sequences in the related literature. The evidence that showed some CRISPR spacers were derived from phage DNA and extrachromosomal DNA such as plasmids came from three separate research groups in 2005[40-42]. CRISPR, which was discovered in the genomes of prokaryotic organisms like bacteria and archaea, is a family of DNA sequences derived from DNA segments of certain bacteriophages that had previously infected the prokaryote[43]. CRISPR-associated protein 9 (Cas9) is an enzyme manifesting helicase and nuclease motifs that employs CRISPR sequences as a guide to identify and slice specific strands of DNA that are complementary to the CRISPR sequence. CRISPR sequences together with Cas9 enzymes established the foundation of a new technology known as CRISPR/Cas9 that is deployed to modify genes within organisms[44].
CRISPR/Cas systems with different properties identified from many bacteria and archaea species can be categorized into different groups. Class 1 systems use a complex of multiple Cas proteins to disrupt the target nucleic acids, while the Class 2 system exerts the same function via a single large Cas protein. Among these, the type II CRISPR/Cas9 system, which belongs to Streptococcus pyogenes (S. pyogenes) is the best known and the most commonly used technology as of today. This system functions as an immune system against viruses or foreign nucleic acids in bacteria[45]. If the bacteria are infected with a phage, the phage DNA entering the bacteria causes the bacterial defense mechanisms to be activated. Many Cas proteins are synthesized from the bacterial CRISPR locus and take a piece (protospacer) from the phage DNA in an unknown mechanism, and add it together with a repeat sequence to the region separated by palindromic repeat sequences. Subsequently, transcription of the CRISPR locus that contains the pre-CRISPR RNA (crRNA), transactivator crRNA (tracrRNA) and Cas9 enzymes takes place[46]. The pre-crRNA is processed to form the 20 nucleotide-long crRNA that is complementary to the viral DNA. Many Cas proteins are involved in this process. crRNA-tracrRNA and the Cas9 protein bind to the viral DNA by forming a complex. To bind to viral DNA, Cas enzymes specifically recognize the protospacer adjacent motif (PAM) sequences that must be present in the target DNA. These species-specific conserved sequences located near protospacers, matching the spacer sequences in CRISPR loci, were discovered via computational analyses. For the S. pyogenes Cas9 enzyme, this sequence is either "NAG" or "NGG", the latter being the most widely used PAM sequence in customized cleavage systems.
The Cas9 enzyme contains two nuclease activity domains. These are the HNH domain (creates a break in the complementary chain) and the RuvC domain (creates a break in the non-complementary chain). In the presence of the PAM sequence, the crRNA-tracrRNA-Cas9 complex binds to the viral DNA and creates double-strand breaks, thus prevents viral infection[47].
After this discovery, crRNA and tracrRNAs were combined without causing a decrease in Cas9 activity, thus the chimeric-hybrid guide RNA (sgRNA) was formed, also recognized by the Nobel Prize in Chemistry in 2020 as a very significant finding[48]. Genome editing in human cell cultures using CRISPR/Cas9 was reported for the first time simultaneously by Hsu et al[49], Cong et al[50] and Mali et al[51]. Later, various CRISPR/Cas9 and sgRNA expression plasmids were reported. In this new system, the desired target gene could be modified with the Cas9 expression vector by designing a target-specific oligonucleotide of 20 nucleotides in length and transferring it to the sgRNA expression plasmid.
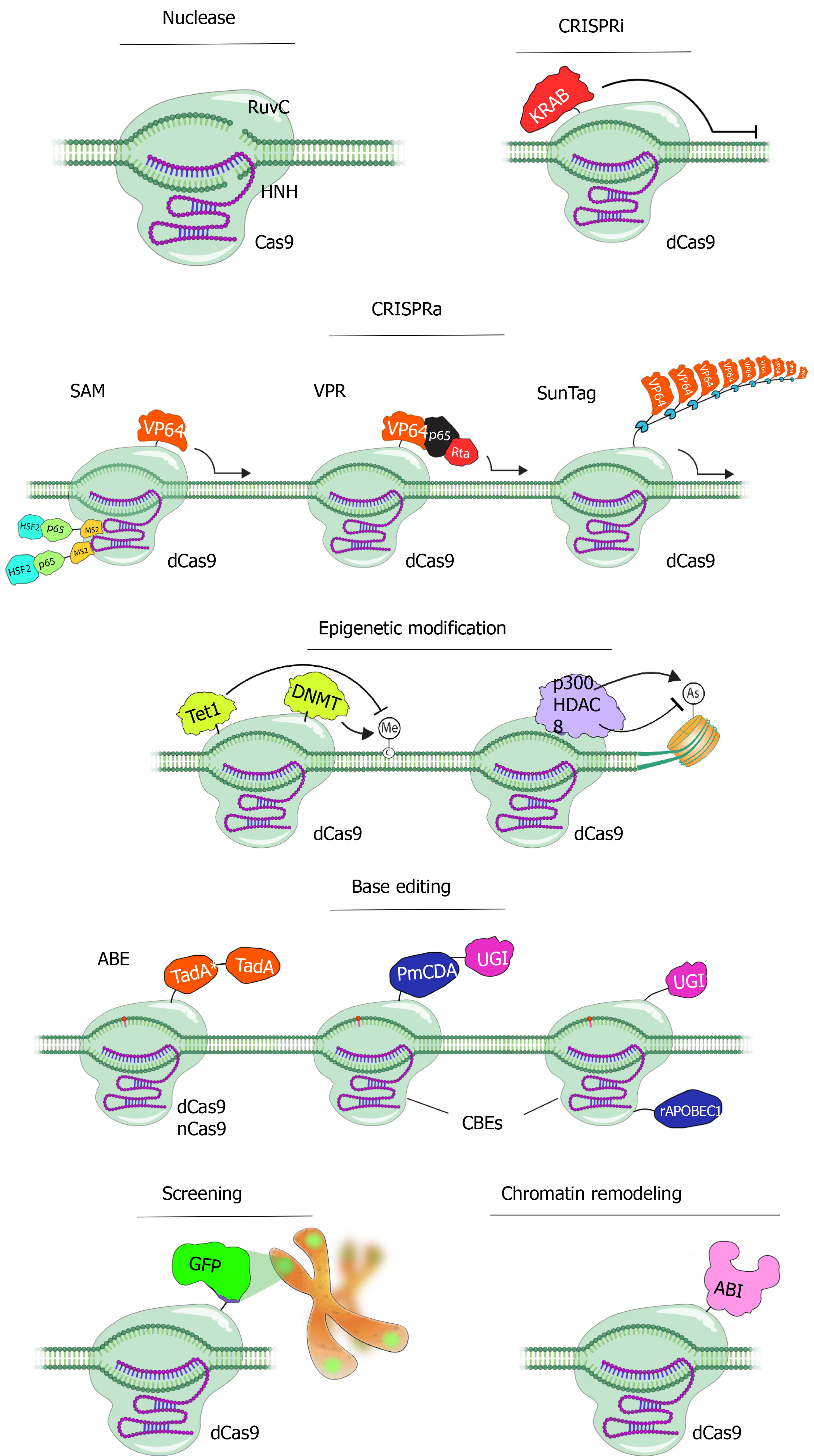
Potential off-target effects of the Cas enzymes, although the specificity of which is under strict control of the 20 nt guide sequence and the PAM region adjacent to the target sequence to be cleaved, are of major concern especially for therapeutic applications. Strategies to overcome this drawback include alteration of the sgRNA sequence for instance via truncation of the 3’ sequence, reducing the amount of the transfected DNA, or modifications in the Cas9 enzyme. In this context, a mutation was introduced to the 10th amino acid of the Cas9 enzyme, converting Aspartic acid to Alanine (D10A). This mutation caused the loss of RuvC nuclease activity of the Cas9 enzyme and enabled the creation of the nickase Cas9 (Cas9_D10A) mutant (Figure 2)[52]. Nickase activity introduces only single-strand DNA breaks, which are repaired by a "base excision" repair mechanism that does not induce mutations in the DNA sequence. Furthermore, two different gRNAs are needed to create double-strand DNA breaks with nickase Cas9[53]. Since it is very unlikely that two different sequences of 20 nucleotides exist in the same way in the genome, this makes Cas9_D10A-mediated DNA modifications safer[51].
After the nickase Cas9 protein, the ‘defective or dead Cas9’ (dCas9) protein was generated by silencing mutations in both the HNH and RuvC domains of the enzyme (D10A and H840A, respectively), with inactivated endonuclease activity thus retaining only the specific DNA-targeting function when guided by the sgRNA[52]. The dCas9 protein is fused with many different proteins and used for various purposes such as transcriptional control as well as DNA modifications (Figure 2). Fusion of the Fok1 nuclease domain to the dCas9 protein reduced off-target effects. Researchers fused dCas9 with a series of tandem repeats (such as VP48, VP64, VP160) of the Herpes simplex viral protein 16 (VP16) trans-activation domain, together with sgRNAs designed to target the upper part of the transcription start site, thus activating transcription in the target gene (Figure 2)[54,55]. Similarly, the fusion of a transcription-inhibiting protein blocks the target gene. The best defined and frequently used of these repressors is the Krüppel-associated box domain, a repressive chromatin-modifier domain, providing a 90%-99% knockdown of the target gene through heterochromatin spreading with minimal off-target effects[56,57]. Moreover, fusing the dCas9 to epigenetic enzymatic domains (such as p300, SID4x, PRDM9, DOT1L, Tet1) enables specific epigenetic alterations, while base editing in specific DNA sequences can be initiated via fusion of dCas9 with base editing enzymes (Figure 2)[56-59]. Another CRISPR/Cas9 technology called CRISPR-genome organization offers a programmable 3D platform for studying the relationship between the nuclear structure and gene regulation and function (Figure 2)[60].
Of all the programmable nucleases available with unique properties used for various purposes, today the CRISPR/Cas9 system is considered the best choice for genome editing as an easy to apply, cost-effective, and versatile system. Moreover, effective gene edits in human tripronuclear zygotes using the CRISPR/Cas9 system were first described by Chinese scientists Liang et al[61] in 2015. A successful cleavage of mutant Beta-Hemoglobin in 28 out of 54 embryos was achieved using the CRISPR/Cas9 system.
In 2015, another type of nuclease, Cas12a (formerly known as Cpf1), was discovered from the bacterium Francisella novicida[62]. Several key differences from Cas9 were observed in Cas12a such as requiring only a crRNA for successful targeting, relying on a T-rich PAM, and causing a staggered cut in double-stranded DNA. These features made Cas12a ideal for multiplexed genome editing. Also, Cas12a cleaves DNA 18–23 base pairs downstream from the PAM site without disrupting the recognition sequence after repair.
In 2016, a new RNA-guided RNA endonuclease, the nuclease Cas13a, was characterized from the bacterium Leptotrichia shahii[63]. Cas13a is directed by its crRNA to an ssRNA target and then cleaves other ssRNA molecules non-discriminately. This cleavage pattern of Cas13a called collateral cleavage has been exploited for the development of various diagnostic technologies[64-66].
Nonetheless, the CRISPR/Cas9 system, which functions as a bacterial defense system in nature, has been deployed in many areas of biotechnology as one of the most up-to-date gene modification methods today[67].
The mechanisms of beta cell function in health and disease conditions can experimentally be dissected using CRISPR/Cas9 technology via manipulation of the genome in pancreatic beta cells[68,69]. Moreover, the generation of novel genetically modified animal models for the testing of beta-cell function is now feasible using CRISPR/Cas9 technology. Pancreatic islet cell loss is one of the essential underlying causes of diabetes[70]. As a solution to this problem, three different strategies have been employed for the recovery of pancreatic beta cells over the years. These three approaches are stimulation of the proliferation of existing beta cells, reprogramming various types of cells into beta cells, and inducing differentiation of beta cells from PSCs. Pancreatic islet-cell proliferation can also be induced in vivo using gene therapy approaches as shown previously[71-73]. In this context of utilizing pluripotent stem cells, pancreatic beta cell-like insulin-producing cells (IPCs) differentiated from ESCs, which were first isolated from human embryos in 1989; and iPSCs, which were first obtained by reprogramming of differentiated cells, have created excitement as new potential sources of beta cells[74-76]. Ethical concerns, as well as the necessity of immune suppression, emerged in studies/potential treatment approaches related to ESCs led studies in this field focus mostly on iPSC-mediated approaches. Besides eliminating ethical concerns, this approach also facilitates disease modeling, drug development, and understanding of the pathogenesis of diabetes, paving the way for development of new-generation treatment strategies. Programmable nucleases (ZFNs, TALENs, CRISPR/Cas9) helped us understand the development, regeneration, insulin production, and secretion patterns of pancreatic beta cells in all aspects.
CRISPR/Cas9 system revealed species-specific differences in gene function. For an instance, McGrath et al[77] demonstrated that the lack of Neurogenin3 (NEUROG3), a basic helix-loop-helix transcription factor essential for endocrine pancreas formation in mice, did not interfere with the growth of the human endocrine pancreas. hESC lines carrying NEUROG3 knockout mutations efficiently formed endoderm and pancreatic progenitors but not the endocrine cells in vitro. Furthermore, a 75%–90% knockdown of NEUROG3 expression via lentivirus-mediated short hairpin RNA approach only reduced but did not prevent the pancreatic endocrine cell development, suggesting that although NEUROG3 is fundamental for human endocrine pancreas development, very little amounts are sufficient for pancreatic endocrine cell formation.
STAT3 has been shown to mediate the activation of Neurog3 in acinar cells reprogramming them into beta cells in diabetic mouse models[78]. Besides, activating germline mutations in STAT3 were recently reported as a cause of neonatal diabetes mellitus associated with beta-cell autoimmunity[79]. The investigation of the activating mutation, STAT3K392R, on pancreatic development using iPSCs derived from a patient with neonatal diabetes and pancreatic hypoplasia suggested that the STAT3K392R mutation caused premature endocrine differentiation through direct induction of NEUROG3 expression. Fortunately, the disease phenotype was completely reversed using CRISPR/Cas9 technology to correct STAT3 mutation.
An efficient genome-editing platform in human PSCs (hPSCs) (iCRISPR) has recently been established through the use of TALENs and the CRISPR/Cas system[80]. This approach combined the strength of genome editing and stem cell biology to methodically manipulate transcriptional control of pancreatic development and the developmental defects related to permanent neonatal diabetes mellitus[81]. In this study, Zhu et al[82] silenced eight of the transcription factors (PDX1, RFX6, PTF1A, GLIS3, MNX1, NGN3, HES1, and ARX) effective in beta cell development in hESCs using CRISPR/Cas9 and TALENs. In addition to defining the specific developmental steps affected by these mutations, this approach uncovered new insights into disease mechanisms, concerning the role of RFX6 in controlling the number of pancreatic progenitors, a prerequisite for PDX1 in pancreatic endocrine development, and a potentially differing role of NGN3 in humans and mice. The deployment of programmable nucleases enabled the identification of transcription factors regulating pancreatic development, advancing hPSC-based beta cell replacement therapies for the treatment of diabetes. Although experimental animal models are needed for reproducing human diseases, genetic and metabolic differences between the species can sometimes cause failure to properly mimic human disease, preventing the development of an effective treatment.
The CRISPR/Cas9 system also significantly contributed to the evaluation of the results gathered from genome-wide association studies (GWAS). Although a large number of genes associated with type 2 diabetes (T2D) have been identified containing single nucleotide polymorphisms, insertions, deletions, and copy number variations in GWAS studies, making the connection between the pathophysiology of the disease and any possible use of the information in drug discovery is challenging[83-86]. Utilizing CRISPR/Cas9, Zeng et al[87] silenced three genes (CDKAL1, KCN111, KCNQ1) in hESC-derived glucose-responsive cells, which were identified by GWAS studies as susceptibility genes for T2D, and observed that although the silencing of these genes did not affect the generation of insulin+ cells, no change was detected in the beta-cell differentiation potential. However, insulin+ beta-cells were hypersensitive to glucolipotoxicity in the absence of the CDKAL1 gene expression. These preliminary studies have shown that functional properties of gene loci identified by GWAS with possible roles in the pathogenesis of diseases can be better assessed by utilizing CRISPR/Cas9 technology.
As isolated human islets are rare and valuable materials for diabetes research, hPSCs represent a good alternative to pancreatic islet cell donors. iPSCs can be established from adult somatic cells via direct reprogramming and differentiated into beta cell-like insulin-producing cells[88]. Although full maturation of these cells into IPCs that secrete insulin in response to changes in blood glucose concentration cannot be achieved easily, this approach can be used to model developmental defects of genetic diseases like diabetes[68]. Genome editing mediated by CRISPR/Cas9 technology provides strong opportunities to examine the effects of specific genotypes and to develop disease models with genetic changes that cause diabetic syndromes, and likely to rapidly increase the possibilities for studying beta cell physiology[89].
Type 1 diabetes (T1D) results from the destruction of the pancreatic beta cells by the autoimmune system due to the impairment of multiple immune tolerance mechanisms[90]. T1D in humans is strongly associated with an allelic variant of protein tyrosine phosphatase nonreceptor type 22 (PTPN22), PTPN22R620W, the existence of which increases the risk of diabetes by two- to fourfold[91]. Since the NOD mouse is a spontaneous T1D model sharing many diabetes-related genetic pathways with humans, the introduction of the murine orthologous PTPNR619W mutation to the NOD genome was expected to enhance the spontaneous development of T1D. Microinjection of CRISPR/Cas9 and a homology-directed repair template into NOD single-cell zygotes resulted in mice with PTPNR619W mutation exhibiting increased insulin autoantibodies and earlier onset and higher penetrance of T1D[92].
Leptin receptor (Lepr) functions as a receptor for the fat cell-specific hormone leptin (ob), which regulates energy homeostasis, the balance between food intake and energy expenditure[93]. The Lepr-defective mice (db/db) is currently the most widely used mouse model of T2D, exhibiting severe obesity, hyperphagia, polydipsia, and polyuria[94]. The rat equivalent of the db/db mouse is the Zucker rat (fa/fa), which has a spontaneous autosomal mutation in the Lepr gene and exhibits a comparable phenotype of hyperphagia leading to glucose intolerance, insulin resistance, and morbid obesity[95]. Intriguingly, the Zucker rat does not exhibit hyperglycemia. To solve this, the Lepr knockout rats were generated using the CRISPR/Cas9 system[96]. These Lepr CRISPR/Cas9 knockout rats exhibited all the diabetes-related phenotypes expanding animal models for biomedical and pharmacological research on obesity and T2D. Besides, some problems observed in leptin receptor-deficient db/db mouse and Zucker rat animal models concerning persistent hyperglycemia and late appearance of glucose intolerance were overcome using CRISPR/Cas9-generated Lepr knockout rats.
Although rodent models are useful for studying diabetes, the use of animal models metabolically closer to humans is a more effective way to understand the development and pathogenesis of human diseases using the CRISPR/Cas9 system. Despite diabetes research can benefit from using pigs as a model owing to sharing similar physiology and metabolic pathways to humans, the scarcity of pig models exhibiting diabetes symptoms represents a clear handicap. For this purpose, Cho et al[97] first modified the insulin gene in somatic cells using the CRISPR/Cas9 system. Then, somatic cell nuclear transfer carrying the modified insulin gene resulted in the generation of pig embryos with insulin (INS) knockout phenotype. INS knockout piglets manifested hyperglycemia and glucosuria demonstrating the effectiveness of CRISPR/Cas9-mediated generation of novel pig models, which might be more suitable for drug development and islet transplantation studies compared to rodents.
Also, the CRISPR/Cas9 system can be an effective way to develop disease models of monogenic diabetes such as Maturity Onset Diabetes of the Young (MODY) referring to any of the several hereditary forms of diabetes mellitus caused by mutations interrupting insulin production[98]. Despite MODY is an autosomal dominant disease, which requires only one abnormal gene to manifest the disease symptoms, the severity of the disease is regulated by the presence of a second allele. While MODY 2 and MODY 3 are the most common forms, mutations in the insulin gene cause MODY10, which is often confused with T2D owing to the similarities in the clinical symptoms[99]. Although the course of the disease is mild, people with this genetic disorder require insulin use in the following years. Such single gene disorders, which cannot be treated with current treatment methods, can be effectively treated with gene therapy. Here, lentivirus-mediated insulin gene therapy may be a permanent solution for MODY10 treatment as shown previously for T1D[72,100].
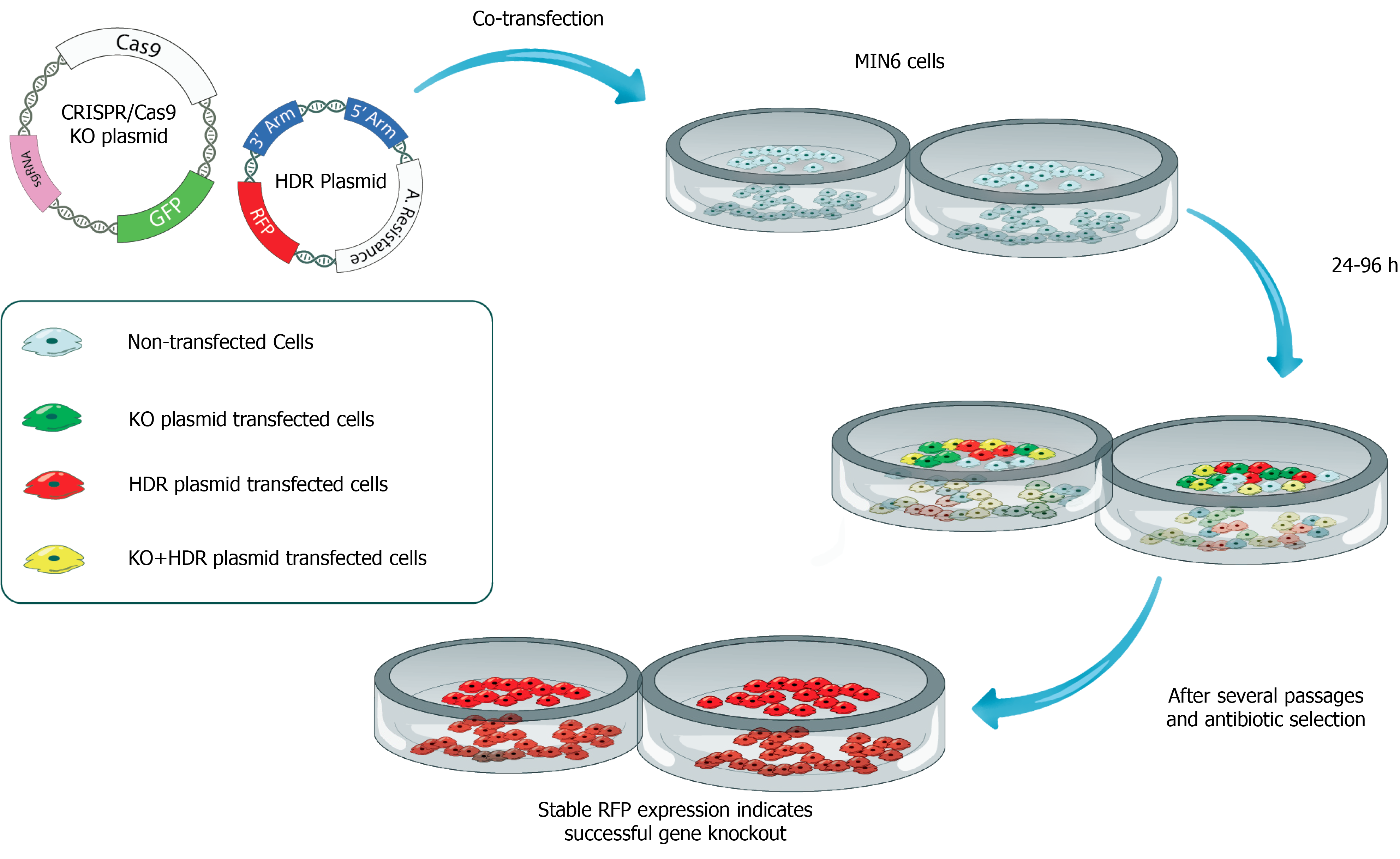
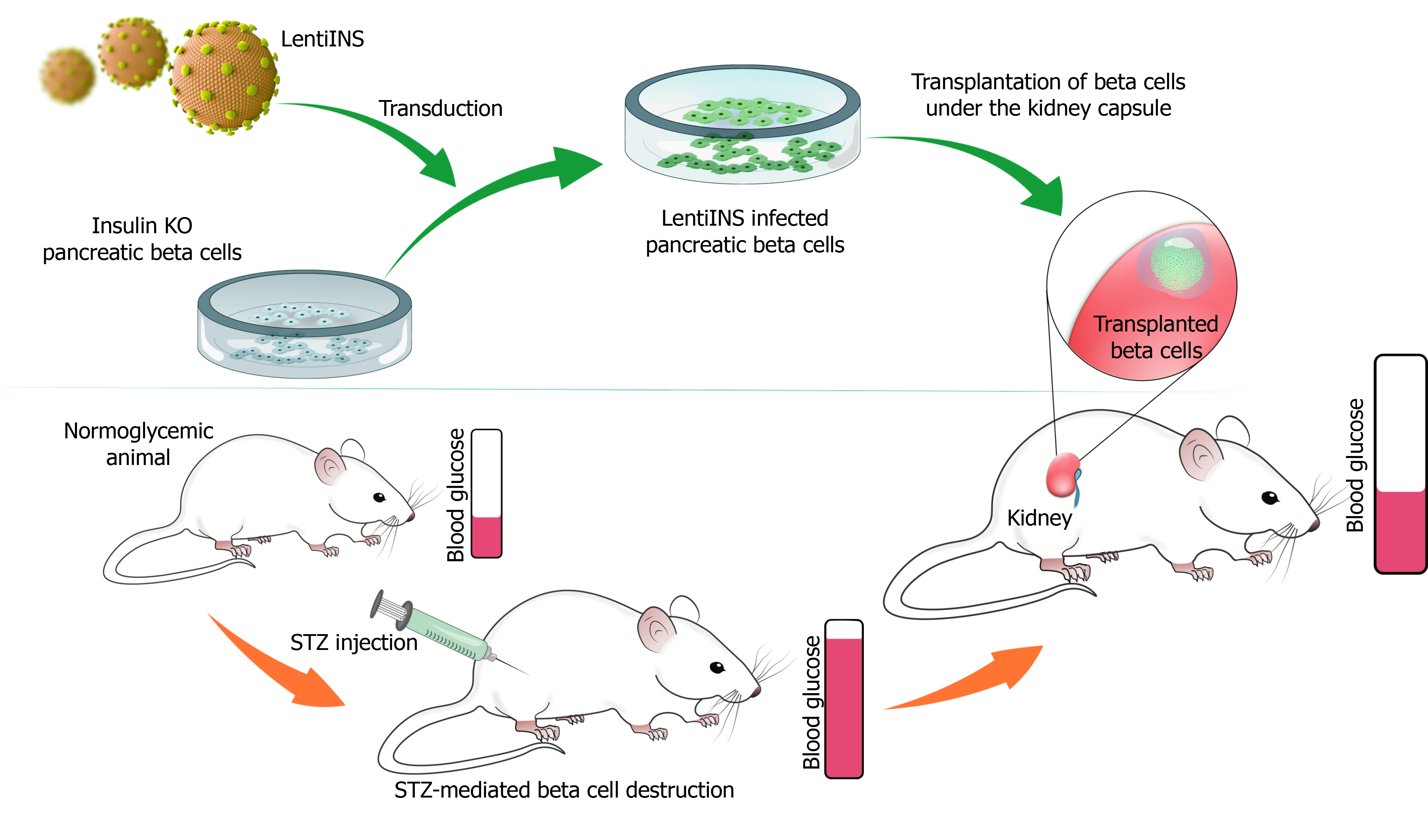
Although a certain reduction in the pancreatic beta cell mass is achieved in streptozotocin (STZ)-induced experimental diabetic animal models, beta cells that remain alive and capable of synthesizing and secreting insulin may interfere with the testing of insulin gene therapy strategies. To fully test the efficacy of insulin gene therapy vectors, pancreatic beta cells that do not synthesize insulin are needed. Since these cells are not commercially available, CRISPR/Cas9 system provides a suitable method to generate pancreatic beta cells without insulin synthesis. The insulin gene can be silenced at the DNA level with the help of the CRISPR/Cas9 using specific guide RNAs targeting the insulin gene. Since these cells will have all beta-cell characteristics except for insulin synthesis, it will be a suitable model for both ex vivo and in vivo testing of the efficacy of insulin gene transfer vectors. To create this model, pancreatic beta cells are transfected with a silencing plasmid encoding the CRISPR Cas9 protein, specific guide RNA, and a HR plasmid containing the homologous regions to the insulin gene (Figure 3). In this strategy, the silencing plasmid creates a double-strand break in the insulin gene, while the HR plasmid delivers an antibiotic resistance gene to selectively purify successful transformants, and a fluorescent protein-encoding gene (e.g. RFP) to confirm successful recombination in the region where the DSB occurs. Thus, a pancreatic beta-cell line is generated with stable fluorescent protein expression, that can be visually examined under a fluorescent microscope after transfection. These cells can be purified by flow cytometry due to the stable fluorescent protein expression they contain, or by antibiotic selection with the transferred antibiotic resistance gene (Figure 3). Then, the cells are cloned using the limited dilution method. Finally, the insulin secretion status of single-cell colonies can be confirmed by insulin ELISA, by western blot, or immunocytochemistry methods. After confirmation, the insulin gene can be delivered in situ by gene therapy vectors to determine if insulin secretion is restored following the transduction of INS knockout pancreatic beta cells. Then, INS knockout beta cells generated by CRISPR/Cas9 technology with or without insulin gene delivery can be transplanted under the kidney capsule of STZ-induced diabetic animals (Figure 4). By doing so, a pancreatic beta-cell line deficient in insulin gene synthesis can be generated and can be used for in vivo testing of the efficacy of insulin gene therapy vectors.
In summary, the CRISPR/Cas9 system is a unique technology that provides a very powerful and versatile platform for genome editing, with ease of design and implementation, high efficiency, and low cost[67]. Described as a game-changer in genetic engineering, one of the main purposes of using the CRISPR/Cas9 technology is to develop suitable models for drug testing and to understand the molecular and physiological mechanisms underlying the development of human diseases[18]. Among the diseases for which successful cell-based CRISPR/Cas9-mediated disease models were generated are cystic fibrosis, Barth syndrome, β-thalassemia, Duchenne muscular dystrophy, and hemophilia A[101-106]. Mouse models for tyrosinemia and lung cancer, and rat and primate models for muscular dystrophy have also been successfully created with the CRISPR/Cas9 technology, with many more that are worked on and to be developed[107-110]. As an additional approach, as described in this publication, a new in vivo diabetes disease model can be developed by allogenic transfer of an INS knockout pancreatic beta-cell line that is created by CRISPR/Cas9 technology under the kidney capsule of STZ-induced diabetic animals. To determine the therapeutic efficacy of insulin gene therapy, the same procedure needs to be repeated with insulin-deficient pancreatic beta cells only after the insulin gene delivery. With the current advances in the powerful CRISPR/Cas9 technology, there is a better chance for overcoming the challenge of generating and implementing the most accurate, specific, and predictive disease models to provide a better understanding and treatment of human diseases.
Manuscript source: Invited manuscript
Corresponding Author's Membership in Professional Societies: American Society of Gene and Cell Therapy; and European Society of Gene and Cell Therapy
Specialty type: Medicine, research and experimental
Country/Territory of origin: Turkey
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Duan W, Kurniawan A S-Editor: Liu M L-Editor: A P-Editor: Xing YX
| 1. | Salzberg SL. Open questions: How many genes do we have? BMC Biol. 2018;16:94. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 98] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 2. | Xiong X, Chen M, Lim WA, Zhao D, Qi LS. CRISPR/Cas9 for Human Genome Engineering and Disease Research. Annu Rev Genomics Hum Genet. 2016;17:131-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 69] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 3. | Porteus M. Genome Editing: A New Approach to Human Therapeutics. Annu Rev Pharmacol Toxicol. 2016;56:163-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 76] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 4. | Li H, Yang Y, Hong W, Huang M, Wu M, Zhao X. Applications of genome editing technology in the targeted therapy of human diseases: mechanisms, advances and prospects. Signal Transduct Target Ther. 2020;5:1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 949] [Cited by in RCA: 1091] [Article Influence: 218.2] [Reference Citation Analysis (0)] |
| 5. | Hanna RE, Doench JG. Design and analysis of CRISPR-Cas experiments. Nat Biotechnol. 2020;38:813-823. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 115] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 6. | Nelson CE, Gersbach CA. Engineering Delivery Vehicles for Genome Editing. Annu Rev Chem Biomol Eng. 2016;7:637-662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 88] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 7. | Rudin N, Haber JE. Efficient repair of HO-induced chromosomal breaks in Saccharomyces cerevisiae by recombination between flanking homologous sequences. Mol Cell Biol. 1988;8:3918-3928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 82] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 8. | Liang F, Han M, Romanienko PJ, Jasin M. Homology-directed repair is a major double-strand break repair pathway in mammalian cells. Proc Natl Acad Sci USA. 1998;95:5172-5177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 430] [Cited by in RCA: 489] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 9. | Kim H, Kim JS. A guide to genome engineering with programmable nucleases. Nat Rev Genet. 2014;15:321-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 839] [Cited by in RCA: 818] [Article Influence: 74.4] [Reference Citation Analysis (0)] |
| 10. | Li L, Wu LP, Chandrasegaran S. Functional domains in Fok I restriction endonuclease. Proc Natl Acad Sci USA. 1992;89:4275-4279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 155] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 11. | Kim YG, Shi Y, Berg JM, Chandrasegaran S. Site-specific cleavage of DNA-RNA hybrids by zinc finger/FokI cleavage domain fusions. Gene. 1997;203:43-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 56] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 12. | Bibikova M, Golic M, Golic KG, Carroll D. Targeted chromosomal cleavage and mutagenesis in Drosophila using zinc-finger nucleases. Genetics. 2002;161:1169-1175. [PubMed] |
| 13. | Bitinaite J, Wah DA, Aggarwal AK, Schildkraut I. FokI dimerization is required for DNA cleavage. Proc Natl Acad Sci USA. 1998;95:10570-10575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 362] [Cited by in RCA: 346] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 14. | Guo J, Gaj T, Barbas CF 3rd. Directed evolution of an enhanced and highly efficient FokI cleavage domain for zinc finger nucleases. J Mol Biol. 2010;400:96-107. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 176] [Cited by in RCA: 162] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 15. | Ramalingam S, Kandavelou K, Rajenderan R, Chandrasegaran S. Creating designed zinc-finger nucleases with minimal cytotoxicity. J Mol Biol. 2011;405:630-641. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 58] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 16. | Mittelman D, Moye C, Morton J, Sykoudis K, Lin Y, Carroll D, Wilson JH. Zinc-finger directed double-strand breaks within CAG repeat tracts promote repeat instability in human cells. Proc Natl Acad Sci USA. 2009;106:9607-9612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 75] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 17. | Sung YK, Kim SW. Recent advances in the development of gene delivery systems. Biomater Res. 2019;23:8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 163] [Cited by in RCA: 283] [Article Influence: 47.2] [Reference Citation Analysis (0)] |
| 18. | Ashmore-Harris C, Fruhwirth GO. The clinical potential of gene editing as a tool to engineer cell-based therapeutics. Clin Transl Med. 2020;9:15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 53] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 19. | Porteus MH. Mammalian gene targeting with designed zinc finger nucleases. Mol Ther. 2006;13:438-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 125] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 20. | Gupta A, Meng X, Zhu LJ, Lawson ND, Wolfe SA. Zinc finger protein-dependent and -independent contributions to the in vivo off-target activity of zinc finger nucleases. Nucleic Acids Res. 2011;39:381-392. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 95] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 21. | Pattanayak V, Ramirez CL, Joung JK, Liu DR. Revealing off-target cleavage specificities of zinc-finger nucleases by in vitro selection. Nat Methods. 2011;8:765-770. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 368] [Cited by in RCA: 392] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 22. | Ramirez CL, Certo MT, Mussolino C, Goodwin MJ, Cradick TJ, McCaffrey AP, Cathomen T, Scharenberg AM, Joung JK. Engineered zinc finger nickases induce homology-directed repair with reduced mutagenic effects. Nucleic Acids Res. 2012;40:5560-5568. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 140] [Cited by in RCA: 129] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 23. | Bogdanove AJ, Schornack S, Lahaye T. TAL effectors: finding plant genes for disease and defense. Curr Opin Plant Biol. 2010;13:394-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 321] [Cited by in RCA: 278] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 24. | Boch J, Bonas U. Xanthomonas AvrBs3 family-type III effectors: discovery and function. Annu Rev Phytopathol. 2010;48:419-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 722] [Cited by in RCA: 627] [Article Influence: 41.8] [Reference Citation Analysis (0)] |
| 25. | Boch J, Scholze H, Schornack S, Landgraf A, Hahn S, Kay S, Lahaye T, Nickstadt A, Bonas U. Breaking the code of DNA binding specificity of TAL-type III effectors. Science. 2009;326:1509-1512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2048] [Cited by in RCA: 1812] [Article Influence: 113.3] [Reference Citation Analysis (0)] |
| 26. | Boch J. TALEs of genome targeting. Nat Biotechnol. 2011;29:135-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 117] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 27. | Hockemeyer D, Wang H, Kiani S, Lai CS, Gao Q, Cassady JP, Cost GJ, Zhang L, Santiago Y, Miller JC, Zeitler B, Cherone JM, Meng X, Hinkley SJ, Rebar EJ, Gregory PD, Urnov FD, Jaenisch R. Genetic engineering of human pluripotent cells using TALE nucleases. Nat Biotechnol. 2011;29:731-734. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 996] [Cited by in RCA: 958] [Article Influence: 68.4] [Reference Citation Analysis (0)] |
| 28. | Wienert B, Funnell AP, Norton LJ, Pearson RC, Wilkinson-White LE, Lester K, Vadolas J, Porteus MH, Matthews JM, Quinlan KG, Crossley M. Editing the genome to introduce a beneficial naturally occurring mutation associated with increased fetal globin. Nat Commun. 2015;6:7085. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 103] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 29. | Tesson L, Usal C, Ménoret S, Leung E, Niles BJ, Remy S, Santiago Y, Vincent AI, Meng X, Zhang L, Gregory PD, Anegon I, Cost GJ. Knockout rats generated by embryo microinjection of TALENs. Nat Biotechnol. 2011;29:695-696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 473] [Cited by in RCA: 457] [Article Influence: 32.6] [Reference Citation Analysis (0)] |
| 30. | Davies B, Davies G, Preece C, Puliyadi R, Szumska D, Bhattacharya S. Site specific mutation of the Zic2 locus by microinjection of TALEN mRNA in mouse CD1, C3H and C57BL/6J oocytes. PLoS One. 2013;8:e60216. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 48] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 31. | Carlson DF, Fahrenkrug SC, Hackett PB. Targeting DNA With Fingers and TALENs. Mol Ther Nucleic Acids. 2012;1:e3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 75] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 32. | Ramalingam S, Annaluru N, Kandavelou K, Chandrasegaran S. TALEN-mediated generation and genetic correction of disease-specific human induced pluripotent stem cells. Curr Gene Ther. 2014;14:461-472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 53] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 33. | Osborn MJ, Starker CG, McElroy AN, Webber BR, Riddle MJ, Xia L, DeFeo AP, Gabriel R, Schmidt M, von Kalle C, Carlson DF, Maeder ML, Joung JK, Wagner JE, Voytas DF, Blazar BR, Tolar J. TALEN-based gene correction for epidermolysis bullosa. Mol Ther. 2013;21:1151-1159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 206] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 34. | Valton J, Guyot V, Marechal A, Filhol JM, Juillerat A, Duclert A, Duchateau P, Poirot L. A Multidrug-resistant Engineered CAR T Cell for Allogeneic Combination Immunotherapy. Mol Ther. 2015;23:1507-1518. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 85] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 35. | Poirot L, Philip B, Schiffer-Mannioui C, Le Clerre D, Chion-Sotinel I, Derniame S, Potrel P, Bas C, Lemaire L, Galetto R, Lebuhotel C, Eyquem J, Cheung GW, Duclert A, Gouble A, Arnould S, Peggs K, Pule M, Scharenberg AM, Smith J. Multiplex Genome-Edited T-cell Manufacturing Platform for "Off-the-Shelf" Adoptive T-cell Immunotherapies. Cancer Res. 2015;75:3853-3864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 372] [Cited by in RCA: 457] [Article Influence: 45.7] [Reference Citation Analysis (0)] |
| 36. | Ishino Y, Shinagawa H, Makino K, Amemura M, Nakata A. Nucleotide sequence of the iap gene, responsible for alkaline phosphatase isozyme conversion in Escherichia coli, and identification of the gene product. J Bacteriol. 1987;169:5429-5433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1229] [Cited by in RCA: 1290] [Article Influence: 33.9] [Reference Citation Analysis (0)] |
| 37. | Groenen PM, Bunschoten AE, van Soolingen D, van Embden JD. Nature of DNA polymorphism in the direct repeat cluster of Mycobacterium tuberculosis; application for strain differentiation by a novel typing method. Mol Microbiol. 1993;10:1057-1065. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 295] [Cited by in RCA: 284] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 38. | Mojica FJ, Díez-Villaseñor C, Soria E, Juez G. Biological significance of a family of regularly spaced repeats in the genomes of Archaea, Bacteria and mitochondria. Mol Microbiol. 2000;36:244-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 496] [Cited by in RCA: 467] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 39. | Mojica FJM, Montoliu L. On the Origin of CRISPR-Cas Technology: From Prokaryotes to Mammals. Trends Microbiol. 2016;24:811-820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 104] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 40. | Pourcel C, Salvignol G, Vergnaud G. CRISPR elements in Yersinia pestis acquire new repeats by preferential uptake of bacteriophage DNA, and provide additional tools for evolutionary studies. Microbiology (Reading). 2005;151:653-663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 887] [Cited by in RCA: 870] [Article Influence: 43.5] [Reference Citation Analysis (0)] |
| 41. | Mojica FJ, Díez-Villaseñor C, García-Martínez J, Soria E. Intervening sequences of regularly spaced prokaryotic repeats derive from foreign genetic elements. J Mol Evol. 2005;60:174-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1294] [Cited by in RCA: 1378] [Article Influence: 68.9] [Reference Citation Analysis (0)] |
| 42. | Bolotin A, Quinquis B, Sorokin A, Ehrlich SD. Clustered regularly interspaced short palindrome repeats (CRISPRs) have spacers of extrachromosomal origin. Microbiology (Reading). 2005;151:2551-2561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1103] [Cited by in RCA: 1100] [Article Influence: 55.0] [Reference Citation Analysis (0)] |
| 43. | Barrangou R. The roles of CRISPR-Cas systems in adaptive immunity and beyond. Curr Opin Immunol. 2015;32:36-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 139] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 44. | Zhang F, Wen Y, Guo X. CRISPR/Cas9 for genome editing: progress, implications and challenges. Hum Mol Genet. 2014;23:R40-R46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 331] [Cited by in RCA: 377] [Article Influence: 34.3] [Reference Citation Analysis (0)] |
| 45. | Sorek R, Lawrence CM, Wiedenheft B. CRISPR-mediated adaptive immune systems in bacteria and archaea. Annu Rev Biochem. 2013;82:237-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 448] [Cited by in RCA: 458] [Article Influence: 38.2] [Reference Citation Analysis (0)] |
| 46. | Jiang F, Doudna JA. CRISPR-Cas9 Structures and Mechanisms. Annu Rev Biophys. 2017;46:505-529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1144] [Cited by in RCA: 1183] [Article Influence: 147.9] [Reference Citation Analysis (0)] |
| 47. | Barrangou R, Fremaux C, Deveau H, Richards M, Boyaval P, Moineau S, Romero DA, Horvath P. CRISPR provides acquired resistance against viruses in prokaryotes. Science. 2007;315:1709-1712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4094] [Cited by in RCA: 4159] [Article Influence: 231.1] [Reference Citation Analysis (0)] |
| 48. | Jinek M, Chylinski K, Fonfara I, Hauer M, Doudna JA, Charpentier E. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science. 2012;337:816-821. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11979] [Cited by in RCA: 11001] [Article Influence: 846.2] [Reference Citation Analysis (0)] |
| 49. | Hsu PD, Lander ES, Zhang F. Development and applications of CRISPR-Cas9 for genome engineering. Cell. 2014;157:1262-1278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3671] [Cited by in RCA: 3901] [Article Influence: 354.6] [Reference Citation Analysis (0)] |
| 50. | Cong L, Ran FA, Cox D, Lin S, Barretto R, Habib N, Hsu PD, Wu X, Jiang W, Marraffini LA, Zhang F. Multiplex genome engineering using CRISPR/Cas systems. Science. 2013;339:819-823. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10336] [Cited by in RCA: 11164] [Article Influence: 930.3] [Reference Citation Analysis (0)] |
| 51. | Mali P, Yang L, Esvelt KM, Aach J, Guell M, DiCarlo JE, Norville JE, Church GM. RNA-guided human genome engineering via Cas9. Science. 2013;339:823-826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6555] [Cited by in RCA: 7005] [Article Influence: 583.8] [Reference Citation Analysis (0)] |
| 52. | Qi LS, Larson MH, Gilbert LA, Doudna JA, Weissman JS, Arkin AP, Lim WA. Repurposing CRISPR as an RNA-guided platform for sequence-specific control of gene expression. Cell. 2013;152:1173-1183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3647] [Cited by in RCA: 3561] [Article Influence: 296.8] [Reference Citation Analysis (0)] |
| 53. | Wang H, La Russa M, Qi LS. CRISPR/Cas9 in Genome Editing and Beyond. Annu Rev Biochem. 2016;85:227-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 819] [Cited by in RCA: 747] [Article Influence: 83.0] [Reference Citation Analysis (0)] |
| 54. | Maeder ML, Linder SJ, Cascio VM, Fu Y, Ho QH, Joung JK. CRISPR RNA-guided activation of endogenous human genes. Nat Methods. 2013;10:977-979. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 909] [Cited by in RCA: 927] [Article Influence: 77.3] [Reference Citation Analysis (0)] |
| 55. | Konermann S, Brigham MD, Trevino AE, Joung J, Abudayyeh OO, Barcena C, Hsu PD, Habib N, Gootenberg JS, Nishimasu H, Nureki O, Zhang F. Genome-scale transcriptional activation by an engineered CRISPR-Cas9 complex. Nature. 2015;517:583-588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1764] [Cited by in RCA: 2087] [Article Influence: 189.7] [Reference Citation Analysis (0)] |
| 56. | Gilbert LA, Larson MH, Morsut L, Liu Z, Brar GA, Torres SE, Stern-Ginossar N, Brandman O, Whitehead EH, Doudna JA, Lim WA, Weissman JS, Qi LS. CRISPR-mediated modular RNA-guided regulation of transcription in eukaryotes. Cell. 2013;154:442-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2731] [Cited by in RCA: 2716] [Article Influence: 226.3] [Reference Citation Analysis (0)] |
| 57. | Gilbert LA, Horlbeck MA, Adamson B, Villalta JE, Chen Y, Whitehead EH, Guimaraes C, Panning B, Ploegh HL, Bassik MC, Qi LS, Kampmann M, Weissman JS. Genome-Scale CRISPR-Mediated Control of Gene Repression and Activation. Cell. 2014;159:647-661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1983] [Cited by in RCA: 1995] [Article Influence: 181.4] [Reference Citation Analysis (0)] |
| 58. | Hess GT, Tycko J, Yao D, Bassik MC. Methods and Applications of CRISPR-Mediated Base Editing in Eukaryotic Genomes. Mol Cell. 2017;68:26-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 172] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 59. | Shimatani Z, Kashojiya S, Takayama M, Terada R, Arazoe T, Ishii H, Teramura H, Yamamoto T, Komatsu H, Miura K, Ezura H, Nishida K, Ariizumi T, Kondo A. Targeted base editing in rice and tomato using a CRISPR-Cas9 cytidine deaminase fusion. Nat Biotechnol. 2017;35:441-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 469] [Cited by in RCA: 459] [Article Influence: 57.4] [Reference Citation Analysis (0)] |
| 60. | Wang H, Xu X, Nguyen CM, Liu Y, Gao Y, Lin X, Daley T, Kipniss NH, La Russa M, Qi LS. CRISPR-Mediated Programmable 3D Genome Positioning and Nuclear Organization. Cell 2018; 175: 1405-1417. e14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 150] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 61. | Liang P, Xu Y, Zhang X, Ding C, Huang R, Zhang Z, Lv J, Xie X, Chen Y, Li Y, Sun Y, Bai Y, Songyang Z, Ma W, Zhou C, Huang J. CRISPR/Cas9-mediated gene editing in human tripronuclear zygotes. Protein Cell. 2015;6:363-372. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 715] [Cited by in RCA: 650] [Article Influence: 65.0] [Reference Citation Analysis (0)] |
| 62. | Zetsche B, Gootenberg JS, Abudayyeh OO, Slaymaker IM, Makarova KS, Essletzbichler P, Volz SE, Joung J, van der Oost J, Regev A, Koonin EV, Zhang F. Cpf1 is a single RNA-guided endonuclease of a class 2 CRISPR-Cas system. Cell. 2015;163:759-771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3250] [Cited by in RCA: 3131] [Article Influence: 313.1] [Reference Citation Analysis (0)] |
| 63. | Abudayyeh OO, Gootenberg JS, Konermann S, Joung J, Slaymaker IM, Cox DB, Shmakov S, Makarova KS, Semenova E, Minakhin L, Severinov K, Regev A, Lander ES, Koonin EV, Zhang F. C2c2 is a single-component programmable RNA-guided RNA-targeting CRISPR effector. Science. 2016;353:aaf5573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1521] [Cited by in RCA: 1525] [Article Influence: 169.4] [Reference Citation Analysis (0)] |
| 64. | Gootenberg JS, Abudayyeh OO, Lee JW, Essletzbichler P, Dy AJ, Joung J, Verdine V, Donghia N, Daringer NM, Freije CA, Myhrvold C, Bhattacharyya RP, Livny J, Regev A, Koonin EV, Hung DT, Sabeti PC, Collins JJ, Zhang F. Nucleic acid detection with CRISPR-Cas13a/C2c2. Science. 2017;356:438-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2307] [Cited by in RCA: 2271] [Article Influence: 283.9] [Reference Citation Analysis (0)] |
| 65. | Gootenberg JS, Abudayyeh OO, Kellner MJ, Joung J, Collins JJ, Zhang F. Multiplexed and portable nucleic acid detection platform with Cas13, Cas12a, and Csm6. Science. 2018;360:439-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1086] [Cited by in RCA: 1623] [Article Influence: 231.9] [Reference Citation Analysis (0)] |
| 66. | Iwasaki RS, Batey RT. SPRINT: a Cas13a-based platform for detection of small molecules. Nucleic Acids Res. 2020;48:e101. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 68] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 67. | Anzalone AV, Koblan LW, Liu DR. Genome editing with CRISPR-Cas nucleases, base editors, transposases and prime editors. Nat Biotechnol. 2020;38:824-844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 643] [Cited by in RCA: 1417] [Article Influence: 283.4] [Reference Citation Analysis (0)] |
| 68. | Balboa D, Prasad RB, Groop L, Otonkoski T. Genome editing of human pancreatic beta cell models: problems, possibilities and outlook. Diabetologia. 2019;62:1329-1336. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 69. | McCloskey AG, Miskelly MG, Moore CBT, Nesbit MA, Christie KA, Owolabi AI, Flatt PR, McKillop AM. CRISPR/Cas9 gene editing demonstrates metabolic importance of GPR55 in the modulation of GIP release and pancreatic beta cell function. Peptides. 2020;125:170251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 70. | Sanlioglu AD, Altunbas HA, Balci MK, Griffith TS, Sanlioglu S. Clinical utility of insulin and insulin analogs. Islets. 2013;5:67-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 71. | Tasyurek HM, Altunbas HA, Balci MK, Griffith TS, Sanlioglu S. Therapeutic Potential of Lentivirus-Mediated Glucagon-Like Peptide-1 Gene Therapy for Diabetes. Hum Gene Ther. 2018;29:802-815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 72. | Erendor F, Eksi YE, Sahin EO, Balci MK, Griffith TS, Sanlioglu S. Lentivirus Mediated Pancreatic Beta-Cell-Specific Insulin Gene Therapy for STZ-Induced Diabetes. Mol Ther. 2021;29:149-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 73. | Erendor F, Sahin EO, Sanlioglu AD, Balci MK, Griffith TS, Sanlioglu S. Lentiviral gene therapy vectors encoding VIP suppressed diabetes-related inflammation and augmented pancreatic beta-cell proliferation. Gene Ther. 2021;28:130-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 74. | Raikwar SP, Kim EM, Sivitz WI, Allamargot C, Thedens DR, Zavazava N. Human iPS cell-derived insulin producing cells form vascularized organoids under the kidney capsules of diabetic mice. PLoS One. 2015;10:e0116582. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 41] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 75. | Bar-Nur O, Russ HA, Efrat S, Benvenisty N. Epigenetic memory and preferential lineage-specific differentiation in induced pluripotent stem cells derived from human pancreatic islet beta cells. Cell Stem Cell. 2011;9:17-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 456] [Cited by in RCA: 457] [Article Influence: 35.2] [Reference Citation Analysis (0)] |
| 76. | Zhang D, Jiang W, Liu M, Sui X, Yin X, Chen S, Shi Y, Deng H. Highly efficient differentiation of human ES cells and iPS cells into mature pancreatic insulin-producing cells. Cell Res. 2009;19:429-438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 429] [Cited by in RCA: 415] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 77. | McGrath PS, Watson CL, Ingram C, Helmrath MA, Wells JM. The Basic Helix-Loop-Helix Transcription Factor NEUROG3 Is Required for Development of the Human Endocrine Pancreas. Diabetes. 2015;64:2497-2505. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 85] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 78. | Baeyens L, Bonné S, German MS, Ravassard P, Heimberg H, Bouwens L. Ngn3 expression during postnatal in vitro beta cell neogenesis induced by the JAK/STAT pathway. Cell Death Differ. 2006;13:1892-1899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 61] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 79. | Saarimäki-Vire J, Balboa D, Russell MA, Saarikettu J, Kinnunen M, Keskitalo S, Malhi A, Valensisi C, Andrus C, Eurola S, Grym H, Ustinov J, Wartiovaara K, Hawkins RD, Silvennoinen O, Varjosalo M, Morgan NG, Otonkoski T. An Activating STAT3 Mutation Causes Neonatal Diabetes through Premature Induction of Pancreatic Differentiation. Cell Rep. 2017;19:281-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 82] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 80. | González F, Zhu Z, Shi ZD, Lelli K, Verma N, Li QV, Huangfu D. An iCRISPR platform for rapid, multiplexable, and inducible genome editing in human pluripotent stem cells. Cell Stem Cell. 2014;15:215-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 338] [Cited by in RCA: 371] [Article Influence: 33.7] [Reference Citation Analysis (0)] |
| 81. | Aguilar-Bryan L, Bryan J. Neonatal diabetes mellitus. Endocr Rev. 2008;29:265-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 159] [Article Influence: 9.4] [Reference Citation Analysis (1)] |
| 82. | Zhu Z, Li QV, Lee K, Rosen BP, González F, Soh CL, Huangfu D. Genome Editing of Lineage Determinants in Human Pluripotent Stem Cells Reveals Mechanisms of Pancreatic Development and Diabetes. Cell Stem Cell. 2016;18:755-768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 138] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 83. | Voight BF, Scott LJ, Steinthorsdottir V, Morris AP, Dina C, Welch RP, Zeggini E, Huth C, Aulchenko YS, Thorleifsson G, McCulloch LJ, Ferreira T, Grallert H, Amin N, Wu G, Willer CJ, Raychaudhuri S, McCarroll SA, Langenberg C, Hofmann OM, Dupuis J, Qi L, Segrè AV, van Hoek M, Navarro P, Ardlie K, Balkau B, Benediktsson R, Bennett AJ, Blagieva R, Boerwinkle E, Bonnycastle LL, Bengtsson Boström K, Bravenboer B, Bumpstead S, Burtt NP, Charpentier G, Chines PS, Cornelis M, Couper DJ, Crawford G, Doney AS, Elliott KS, Elliott AL, Erdos MR, Fox CS, Franklin CS, Ganser M, Gieger C, Grarup N, Green T, Griffin S, Groves CJ, Guiducci C, Hadjadj S, Hassanali N, Herder C, Isomaa B, Jackson AU, Johnson PR, Jørgensen T, Kao WH, Klopp N, Kong A, Kraft P, Kuusisto J, Lauritzen T, Li M, Lieverse A, Lindgren CM, Lyssenko V, Marre M, Meitinger T, Midthjell K, Morken MA, Narisu N, Nilsson P, Owen KR, Payne F, Perry JR, Petersen AK, Platou C, Proença C, Prokopenko I, Rathmann W, Rayner NW, Robertson NR, Rocheleau G, Roden M, Sampson MJ, Saxena R, Shields BM, Shrader P, Sigurdsson G, Sparsø T, Strassburger K, Stringham HM, Sun Q, Swift AJ, Thorand B, Tichet J, Tuomi T, van Dam RM, van Haeften TW, van Herpt T, van Vliet-Ostaptchouk JV, Walters GB, Weedon MN, Wijmenga C, Witteman J, Bergman RN, Cauchi S, Collins FS, Gloyn AL, Gyllensten U, Hansen T, Hide WA, Hitman GA, Hofman A, Hunter DJ, Hveem K, Laakso M, Mohlke KL, Morris AD, Palmer CN, Pramstaller PP, Rudan I, Sijbrands E, Stein LD, Tuomilehto J, Uitterlinden A, Walker M, Wareham NJ, Watanabe RM, Abecasis GR, Boehm BO, Campbell H, Daly MJ, Hattersley AT, Hu FB, Meigs JB, Pankow JS, Pedersen O, Wichmann HE, Barroso I, Florez JC, Frayling TM, Groop L, Sladek R, Thorsteinsdottir U, Wilson JF, Illig T, Froguel P, van Duijn CM, Stefansson K, Altshuler D, Boehnke M, McCarthy MI; MAGIC investigators; GIANT Consortium. Twelve type 2 diabetes susceptibility loci identified through large-scale association analysis. Nat Genet. 2010;42:579-589. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1472] [Cited by in RCA: 1385] [Article Influence: 92.3] [Reference Citation Analysis (0)] |
| 84. | Gaulton KJ, Ferreira T, Lee Y, Raimondo A, Mägi R, Reschen ME, Mahajan A, Locke A, Rayner NW, Robertson N, Scott RA, Prokopenko I, Scott LJ, Green T, Sparso T, Thuillier D, Yengo L, Grallert H, Wahl S, Frånberg M, Strawbridge RJ, Kestler H, Chheda H, Eisele L, Gustafsson S, Steinthorsdottir V, Thorleifsson G, Qi L, Karssen LC, van Leeuwen EM, Willems SM, Li M, Chen H, Fuchsberger C, Kwan P, Ma C, Linderman M, Lu Y, Thomsen SK, Rundle JK, Beer NL, van de Bunt M, Chalisey A, Kang HM, Voight BF, Abecasis GR, Almgren P, Baldassarre D, Balkau B, Benediktsson R, Blüher M, Boeing H, Bonnycastle LL, Bottinger EP, Burtt NP, Carey J, Charpentier G, Chines PS, Cornelis MC, Couper DJ, Crenshaw AT, van Dam RM, Doney AS, Dorkhan M, Edkins S, Eriksson JG, Esko T, Eury E, Fadista J, Flannick J, Fontanillas P, Fox C, Franks PW, Gertow K, Gieger C, Gigante B, Gottesman O, Grant GB, Grarup N, Groves CJ, Hassinen M, Have CT, Herder C, Holmen OL, Hreidarsson AB, Humphries SE, Hunter DJ, Jackson AU, Jonsson A, Jørgensen ME, Jørgensen T, Kao WH, Kerrison ND, Kinnunen L, Klopp N, Kong A, Kovacs P, Kraft P, Kravic J, Langford C, Leander K, Liang L, Lichtner P, Lindgren CM, Lindholm E, Linneberg A, Liu CT, Lobbens S, Luan J, Lyssenko V, Männistö S, McLeod O, Meyer J, Mihailov E, Mirza G, Mühleisen TW, Müller-Nurasyid M, Navarro C, Nöthen MM, Oskolkov NN, Owen KR, Palli D, Pechlivanis S, Peltonen L, Perry JR, Platou CG, Roden M, Ruderfer D, Rybin D, van der Schouw YT, Sennblad B, Sigurðsson G, Stančáková A, Steinbach G, Storm P, Strauch K, Stringham HM, Sun Q, Thorand B, Tikkanen E, Tonjes A, Trakalo J, Tremoli E, Tuomi T, Wennauer R, Wiltshire S, Wood AR, Zeggini E, Dunham I, Birney E, Pasquali L, Ferrer J, Loos RJ, Dupuis J, Florez JC, Boerwinkle E, Pankow JS, van Duijn C, Sijbrands E, Meigs JB, Hu FB, Thorsteinsdottir U, Stefansson K, Lakka TA, Rauramaa R, Stumvoll M, Pedersen NL, Lind L, Keinanen-Kiukaanniemi SM, Korpi-Hyövälti E, Saaristo TE, Saltevo J, Kuusisto J, Laakso M, Metspalu A, Erbel R, Jöcke KH, Moebus S, Ripatti S, Salomaa V, Ingelsson E, Boehm BO, Bergman RN, Collins FS, Mohlke KL, Koistinen H, Tuomilehto J, Hveem K, Njølstad I, Deloukas P, Donnelly PJ, Frayling TM, Hattersley AT, de Faire U, Hamsten A, Illig T, Peters A, Cauchi S, Sladek R, Froguel P, Hansen T, Pedersen O, Morris AD, Palmer CN, Kathiresan S, Melander O, Nilsson PM, Groop LC, Barroso I, Langenberg C, Wareham NJ, O'Callaghan CA, Gloyn AL, Altshuler D, Boehnke M, Teslovich TM, McCarthy MI, Morris AP; DIAbetes Genetics Replication And Meta-analysis (DIAGRAM) Consortium. Genetic fine mapping and genomic annotation defines causal mechanisms at type 2 diabetes susceptibility loci. Nat Genet. 2015;47:1415-1425. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 370] [Cited by in RCA: 305] [Article Influence: 30.5] [Reference Citation Analysis (0)] |
| 85. | Ingelsson E, Langenberg C, Hivert MF, Prokopenko I, Lyssenko V, Dupuis J, Mägi R, Sharp S, Jackson AU, Assimes TL, Shrader P, Knowles JW, Zethelius B, Abbasi FA, Bergman RN, Bergmann A, Berne C, Boehnke M, Bonnycastle LL, Bornstein SR, Buchanan TA, Bumpstead SJ, Böttcher Y, Chines P, Collins FS, Cooper CC, Dennison EM, Erdos MR, Ferrannini E, Fox CS, Graessler J, Hao K, Isomaa B, Jameson KA, Kovacs P, Kuusisto J, Laakso M, Ladenvall C, Mohlke KL, Morken MA, Narisu N, Nathan DM, Pascoe L, Payne F, Petrie JR, Sayer AA, Schwarz PE, Scott LJ, Stringham HM, Stumvoll M, Swift AJ, Syvänen AC, Tuomi T, Tuomilehto J, Tönjes A, Valle TT, Williams GH, Lind L, Barroso I, Quertermous T, Walker M, Wareham NJ, Meigs JB, McCarthy MI, Groop L, Watanabe RM, Florez JC; MAGIC investigators. Detailed physiologic characterization reveals diverse mechanisms for novel genetic Loci regulating glucose and insulin metabolism in humans. Diabetes. 2010;59:1266-1275. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 224] [Cited by in RCA: 203] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 86. | Fuchsberger C, Flannick J, Teslovich TM, Mahajan A, Agarwala V, Gaulton KJ, Ma C, Fontanillas P, Moutsianas L, McCarthy DJ, Rivas MA, Perry JRB, Sim X, Blackwell TW, Robertson NR, Rayner NW, Cingolani P, Locke AE, Tajes JF, Highland HM, Dupuis J, Chines PS, Lindgren CM, Hartl C, Jackson AU, Chen H, Huyghe JR, van de Bunt M, Pearson RD, Kumar A, Müller-Nurasyid M, Grarup N, Stringham HM, Gamazon ER, Lee J, Chen Y, Scott RA, Below JE, Chen P, Huang J, Go MJ, Stitzel ML, Pasko D, Parker SCJ, Varga TV, Green T, Beer NL, Day-Williams AG, Ferreira T, Fingerlin T, Horikoshi M, Hu C, Huh I, Ikram MK, Kim BJ, Kim Y, Kim YJ, Kwon MS, Lee S, Lin KH, Maxwell TJ, Nagai Y, Wang X, Welch RP, Yoon J, Zhang W, Barzilai N, Voight BF, Han BG, Jenkinson CP, Kuulasmaa T, Kuusisto J, Manning A, Ng MCY, Palmer ND, Balkau B, Stančáková A, Abboud HE, Boeing H, Giedraitis V, Prabhakaran D, Gottesman O, Scott J, Carey J, Kwan P, Grant G, Smith JD, Neale BM, Purcell S, Butterworth AS, Howson JMM, Lee HM, Lu Y, Kwak SH, Zhao W, Danesh J, Lam VKL, Park KS, Saleheen D, So WY, Tam CHT, Afzal U, Aguilar D, Arya R, Aung T, Chan E, Navarro C, Cheng CY, Palli D, Correa A, Curran JE, Rybin D, Farook VS, Fowler SP, Freedman BI, Griswold M, Hale DE, Hicks PJ, Khor CC, Kumar S, Lehne B, Thuillier D, Lim WY, Liu J, van der Schouw YT, Loh M, Musani SK, Puppala S, Scott WR, Yengo L, Tan ST, Taylor HA Jr, Thameem F, Wilson G Sr, Wong TY, Njølstad PR, Levy JC, Mangino M, Bonnycastle LL, Schwarzmayr T, Fadista J, Surdulescu GL, Herder C, Groves CJ, Wieland T, Bork-Jensen J, Brandslund I, Christensen C, Koistinen HA, Doney ASF, Kinnunen L, Esko T, Farmer AJ, Hakaste L, Hodgkiss D, Kravic J, Lyssenko V, Hollensted M, Jørgensen ME, Jørgensen T, Ladenvall C, Justesen JM, Käräjämäki A, Kriebel J, Rathmann W, Lannfelt L, Lauritzen T, Narisu N, Linneberg A, Melander O, Milani L, Neville M, Orho-Melander M, Qi L, Qi Q, Roden M, Rolandsson O, Swift A, Rosengren AH, Stirrups K, Wood AR, Mihailov E, Blancher C, Carneiro MO, Maguire J, Poplin R, Shakir K, Fennell T, DePristo M, de Angelis MH, Deloukas P, Gjesing AP, Jun G, Nilsson P, Murphy J, Onofrio R, Thorand B, Hansen T, Meisinger C, Hu FB, Isomaa B, Karpe F, Liang L, Peters A, Huth C, O'Rahilly SP, Palmer CNA, Pedersen O, Rauramaa R, Tuomilehto J, Salomaa V, Watanabe RM, Syvänen AC, Bergman RN, Bharadwaj D, Bottinger EP, Cho YS, Chandak GR, Chan JCN, Chia KS, Daly MJ, Ebrahim SB, Langenberg C, Elliott P, Jablonski KA, Lehman DM, Jia W, Ma RCW, Pollin TI, Sandhu M, Tandon N, Froguel P, Barroso I, Teo YY, Zeggini E, Loos RJF, Small KS, Ried JS, DeFronzo RA, Grallert H, Glaser B, Metspalu A, Wareham NJ, Walker M, Banks E, Gieger C, Ingelsson E, Im HK, Illig T, Franks PW, Buck G, Trakalo J, Buck D, Prokopenko I, Mägi R, Lind L, Farjoun Y, Owen KR, Gloyn AL, Strauch K, Tuomi T, Kooner JS, Lee JY, Park T, Donnelly P, Morris AD, Hattersley AT, Bowden DW, Collins FS, Atzmon G, Chambers JC, Spector TD, Laakso M, Strom TM, Bell GI, Blangero J, Duggirala R, Tai ES, McVean G, Hanis CL, Wilson JG, Seielstad M, Frayling TM, Meigs JB, Cox NJ, Sladek R, Lander ES, Gabriel S, Burtt NP, Mohlke KL, Meitinger T, Groop L, Abecasis G, Florez JC, Scott LJ, Morris AP, Kang HM, Boehnke M, Altshuler D, McCarthy MI. The genetic architecture of type 2 diabetes. Nature. 2016;536:41-47. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 809] [Cited by in RCA: 780] [Article Influence: 86.7] [Reference Citation Analysis (0)] |
| 87. | Zeng H, Guo M, Zhou T, Tan L, Chong CN, Zhang T, Dong X, Xiang JZ, Yu AS, Yue L, Qi Q, Evans T, Graumann J, Chen S. An Isogenic Human ESC Platform for Functional Evaluation of Genome-wide-Association-Study-Identified Diabetes Genes and Drug Discovery. Cell Stem Cell. 2016;19:326-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 94] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 88. | Lim D, Sreekanth V, Cox KJ, Law BK, Wagner BK, Karp JM, Choudhary A. Engineering designer beta cells with a CRISPR-Cas9 conjugation platform. Nat Commun. 2020;11:4043. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 89. | Hu M, Cherkaoui I, Misra S, Rutter GA. Functional Genomics in Pancreatic β Cells: Recent Advances in Gene Deletion and Genome Editing Technologies for Diabetes Research. Front Endocrinol (Lausanne). 2020;11:576632. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 90. | Sanlioglu AD, Griffith TS, Omer A, Dirice E, Sari R, Altunbas HA, Balci MK, Sanlioglu S. Molecular mechanisms of death ligand-mediated immune modulation: a gene therapy model to prolong islet survival in type 1 diabetes. J Cell Biochem. 2008;104:710-720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 91. | Bottini N, Peterson EJ. Tyrosine phosphatase PTPN22: multifunctional regulator of immune signaling, development, and disease. Annu Rev Immunol. 2014;32:83-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 163] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 92. | Lin X, Pelletier S, Gingras S, Rigaud S, Maine CJ, Marquardt K, Dai YD, Sauer K, Rodriguez AR, Martin G, Kupriyanov S, Jiang L, Yu L, Green DR, Sherman LA. CRISPR-Cas9-Mediated Modification of the NOD Mouse Genome With Ptpn22R619W Mutation Increases Autoimmune Diabetes. Diabetes. 2016;65:2134-2138. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 93. | Israel D, Chua S Jr. Leptin receptor modulation of adiposity and fertility. Trends Endocrinol Metab. 2010;21:10-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 48] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 94. | Szabadfi K, Pinter E, Reglodi D, Gabriel R. Neuropeptides, trophic factors, and other substances providing morphofunctional and metabolic protection in experimental models of diabetic retinopathy. Int Rev Cell Mol Biol. 2014;311:1-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 96. | Bao D, Ma Y, Zhang X, Guan F, Chen W, Gao K, Qin C, Zhang L. Preliminary Characterization of a Leptin Receptor Knockout Rat Created by CRISPR/Cas9 System. Sci Rep. 2015;5:15942. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 36] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 97. | Cho B, Kim SJ, Lee EJ, Ahn SM, Lee JS, Ji DY, Lee K, Kang JT. Generation of insulin-deficient piglets by disrupting INS gene using CRISPR/Cas9 system. Transgenic Res. 2018;27:289-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 98. | Nkonge KM, Nkonge DK, Nkonge TN. The epidemiology, molecular pathogenesis, diagnosis, and treatment of maturity-onset diabetes of the young (MODY). Clin Diabetes Endocrinol. 2020;6:20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 100] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 99. | Peixoto-Barbosa R, Reis AF, Giuffrida FMA. Update on clinical screening of maturity-onset diabetes of the young (MODY). Diabetol Metab Syndr. 2020;12:50. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 47] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 100. | Sanlioglu AD, Altunbas HA, Balci MK, Griffith TS, Sanlioglu S. Insulin gene therapy from design to beta cell generation. Expert Rev Mol Med. 2012;14:e18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 101. | Schwank G, Koo BK, Sasselli V, Dekkers JF, Heo I, Demircan T, Sasaki N, Boymans S, Cuppen E, van der Ent CK, Nieuwenhuis EE, Beekman JM, Clevers H. Functional repair of CFTR by CRISPR/Cas9 in intestinal stem cell organoids of cystic fibrosis patients. Cell Stem Cell. 2013;13:653-658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 931] [Cited by in RCA: 990] [Article Influence: 90.0] [Reference Citation Analysis (0)] |
| 102. | Xie F, Ye L, Chang JC, Beyer AI, Wang J, Muench MO, Kan YW. Seamless gene correction of β-thalassemia mutations in patient-specific iPSCs using CRISPR/Cas9 and piggyBac. Genome Res. 2014;24:1526-1533. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 349] [Cited by in RCA: 308] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 103. | Wang G, McCain ML, Yang L, He A, Pasqualini FS, Agarwal A, Yuan H, Jiang D, Zhang D, Zangi L, Geva J, Roberts AE, Ma Q, Ding J, Chen J, Wang DZ, Li K, Wang J, Wanders RJ, Kulik W, Vaz FM, Laflamme MA, Murry CE, Chien KR, Kelley RI, Church GM, Parker KK, Pu WT. Modeling the mitochondrial cardiomyopathy of Barth syndrome with induced pluripotent stem cell and heart-on-chip technologies. Nat Med. 2014;20:616-623. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 612] [Cited by in RCA: 655] [Article Influence: 59.5] [Reference Citation Analysis (0)] |
| 104. | Xu P, Tong Y, Liu XZ, Wang TT, Cheng L, Wang BY, Lv X, Huang Y, Liu DP. Both TALENs and CRISPR/Cas9 directly target the HBB IVS2-654 (C > T) mutation in β-thalassemia-derived iPSCs. Sci Rep. 2015;5:12065. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 106] [Cited by in RCA: 122] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 105. | Li HL, Fujimoto N, Sasakawa N, Shirai S, Ohkame T, Sakuma T, Tanaka M, Amano N, Watanabe A, Sakurai H, Yamamoto T, Yamanaka S, Hotta A. Precise correction of the dystrophin gene in duchenne muscular dystrophy patient induced pluripotent stem cells by TALEN and CRISPR-Cas9. Stem Cell Reports. 2015;4:143-154. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 381] [Cited by in RCA: 391] [Article Influence: 35.5] [Reference Citation Analysis (0)] |
| 106. | Park CY, Kim DH, Son JS, Sung JJ, Lee J, Bae S, Kim JH, Kim DW, Kim JS. Functional Correction of Large Factor VIII Gene Chromosomal Inversions in Hemophilia A Patient-Derived iPSCs Using CRISPR-Cas9. Cell Stem Cell. 2015;17:213-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 226] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 107. | Yin H, Xue W, Chen S, Bogorad RL, Benedetti E, Grompe M, Koteliansky V, Sharp PA, Jacks T, Anderson DG. Genome editing with Cas9 in adult mice corrects a disease mutation and phenotype. Nat Biotechnol. 2014;32:551-553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 675] [Cited by in RCA: 710] [Article Influence: 64.5] [Reference Citation Analysis (0)] |
| 108. | Chen S, Sanjana NE, Zheng K, Shalem O, Lee K, Shi X, Scott DA, Song J, Pan JQ, Weissleder R, Lee H, Zhang F, Sharp PA. Genome-wide CRISPR screen in a mouse model of tumor growth and metastasis. Cell. 2015;160:1246-1260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 688] [Cited by in RCA: 681] [Article Influence: 68.1] [Reference Citation Analysis (0)] |
| 109. | Chen Y, Zheng Y, Kang Y, Yang W, Niu Y, Guo X, Tu Z, Si C, Wang H, Xing R, Pu X, Yang SH, Li S, Ji W, Li XJ. Functional disruption of the dystrophin gene in rhesus monkey using CRISPR/Cas9. Hum Mol Genet. 2015;24:3764-3774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 179] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 110. | Nakamura K, Fujii W, Tsuboi M, Tanihata J, Teramoto N, Takeuchi S, Naito K, Yamanouchi K, Nishihara M. Generation of muscular dystrophy model rats with a CRISPR/Cas system. Sci Rep. 2014;4:5635. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 121] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 111. | Yin H, Kanasty RL, Eltoukhy AA, Vegas AJ, Dorkin JR, Anderson DG. Non-viral vectors for gene-based therapy. Nat Rev Genet. 2014;15:541-555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2046] [Cited by in RCA: 2378] [Article Influence: 216.2] [Reference Citation Analysis (0)] |
| 112. | Sandoval A Jr, Elahi H, Ploski JE. Genetically Engineering the Nervous System with CRISPR-Cas. eNeuro. 2020;7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |