Published online May 26, 2021. doi: 10.4252/wjsc.v13.i5.439
Peer-review started: January 16, 2021
First decision: February 14, 2021
Revised: February 27, 2021
Accepted: April 22, 2021
Article in press: April 22, 2021
Published online: May 26, 2021
Processing time: 130 Days and 8.1 Hours
On February 11, 2020, the World Health Organization officially announced the coronavirus disease 2019 (COVID-19) caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), as an emerging recent pandemic illness, which currently has approximately taken the life of two million persons in more than 200 countries. Medical, clinical, and scientific efforts have focused on searching for new prevention and treatment strategies. Regenerative medicine and tissue engineering focused on using stem cells (SCs) have become a promising tool, and the regenerative and immunoregulatory capabilities of mesenchymal SCs (MSCs) and their exosomes have been demonstrated. Moreover, it has been essential to establishing models to reproduce the viral life cycle and mimic the pathology of COVID-19 to understand the virus's behavior. The fields of pluripotent SCs (PSCs), induced PSCs (iPSCs), and artificial iPSCs have been used for this purpose in the development of infection models or organoids. Nevertheless, some inconveniences have been declared in SC use; for example, it has been reported that SARS-CoV-2 enters human cells through the angiotensin-converting enzyme 2 receptor, which is highly expressed in MSCs, so it is important to continue investigating the employment of SCs in COVID-19, taking into consideration their advantages and disadvantages. In this review, we expose the use of different kinds of SCs and their derivatives for studying the SARS-CoV-2 behavior and develop treatments to counter COVID-19.
Core Tip: The use of stem cells (SCs) to address the coronavirus disease 2019 (COVID-19) pandemic has been widely studied in various fields; for example, human embryonic SCs and human induced pluripotent SCs have been used to generate functional human cells, tissues, and organoids that are used for modeling COVID-19 and discovering drugs. Mesenchymal SCs and their exosomes have been used in clinical trials to control the severe acute respiratory syndrome coronavirus 2 immune response, showing absorption of pulmonary lesions and clinical improvement.
- Citation: Mata-Miranda MM, Sanchez-Brito M, Vazquez-Zapien GJ. Different kinds of stem cells in the development of SARS-CoV-2 treatments. World J Stem Cells 2021; 13(5): 439-451
- URL: https://www.wjgnet.com/1948-0210/full/v13/i5/439.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v13.i5.439
In the last two decades, humanity has experienced outbreaks of Ebola, Severe acute respiratory syndrome (SARS), H1N1, and now severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). On February 11, 2020, the World Health Organization (WHO) and the International Committee on Taxonomy of Viruses officially announced coronavirus disease 2019 (COVID-19) and designated the virus SARS-CoV-2[1,2]. Although the majority of patients evolve clinically well and recover quickly, showing mild symptoms, a significant portion of the affected patients develop acute lung injury (ALI), devastating pulmonary edema and atelectasis caused by capillary membrane injury, which can subsequently trigger a cascade of serious complications, such as severe pneumonia with acute respiratory distress syndrome (ARDS), resulting in multiorgan failure and death[3-6].
Cases of this pandemic illness have been reported in more than 200 countries, taking the lives of more than two million persons, which is the reason that medical, clinical, and scientific efforts are needed[4,6]. Currently, basic research and clinical investigation are urgently required. For basic research, it is essential to establish models to reproduce the viral life cycle and mimic the pathology of COVID-19[7] and to develop new prevention or treatment strategies. In this sense, regenerative medicine and tissue engineering focusing on the use of stem cells (SCs) have become promising tools. In these approaches, cells are utilized to replace or rebuild damaged organs and tissues. There are currently 1135 clinical trials related to COVID-19 registered in the International Clinical Trials Registry Platform (ICTRP), which is an initiative of the WHO, and 16 of these 1135 trials involve the use of SCs[2]. On the other hand, in a recent review in ClinicalTrials.gov (February 2021), a resource provided by the United States. National Library of Medicine, 4793 COVID-19 studies were reported; 88 of them used different types of SCs or their derivatives. However, it is essential to mention that the use of SCs in this pandemic illness is focused not only on treatment options but also on organoids’ development to study the virus’s behavior.
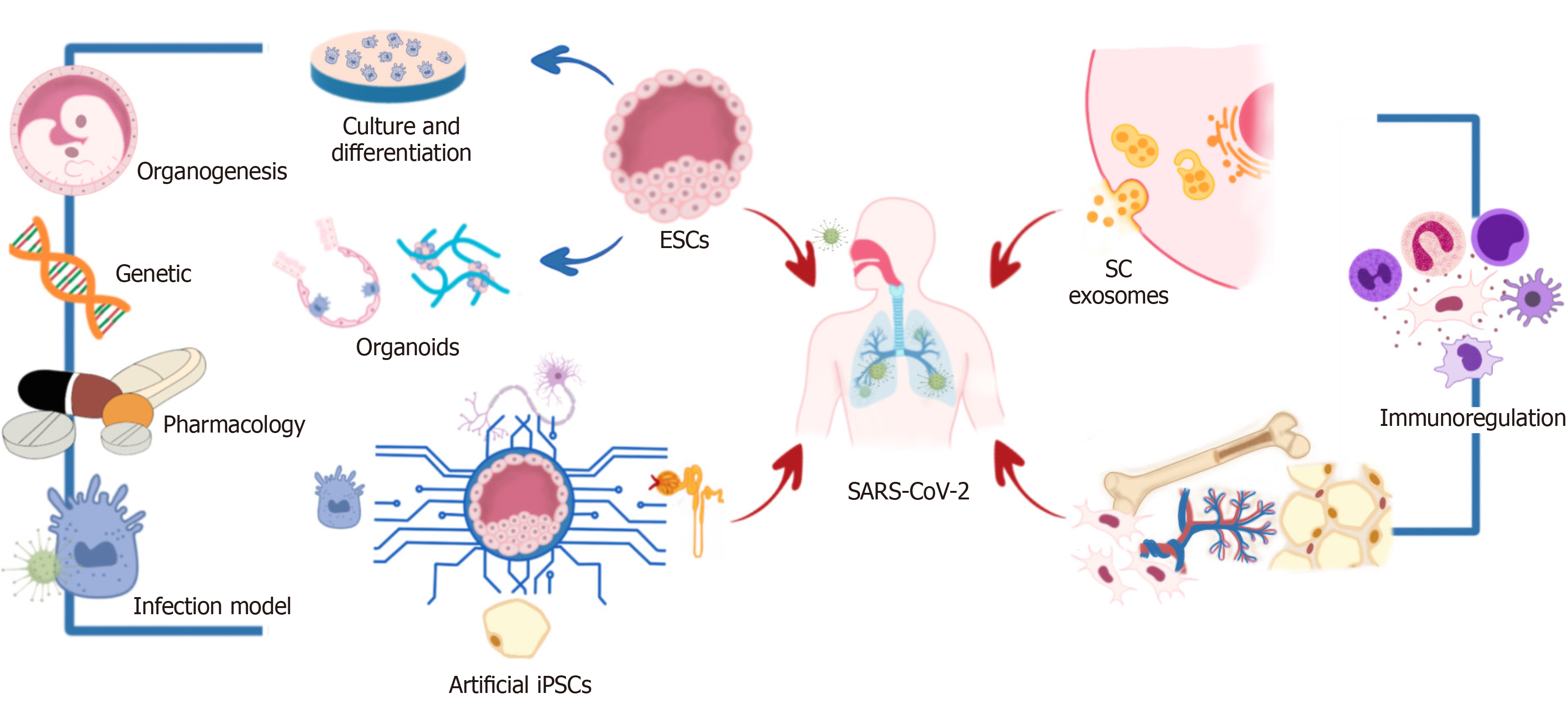
In this review, we expose the use of different kinds of SCs and their derivatives for studying the SARS-CoV-2 behavior and develop treatments to counter COVID-19 (Figure 1).
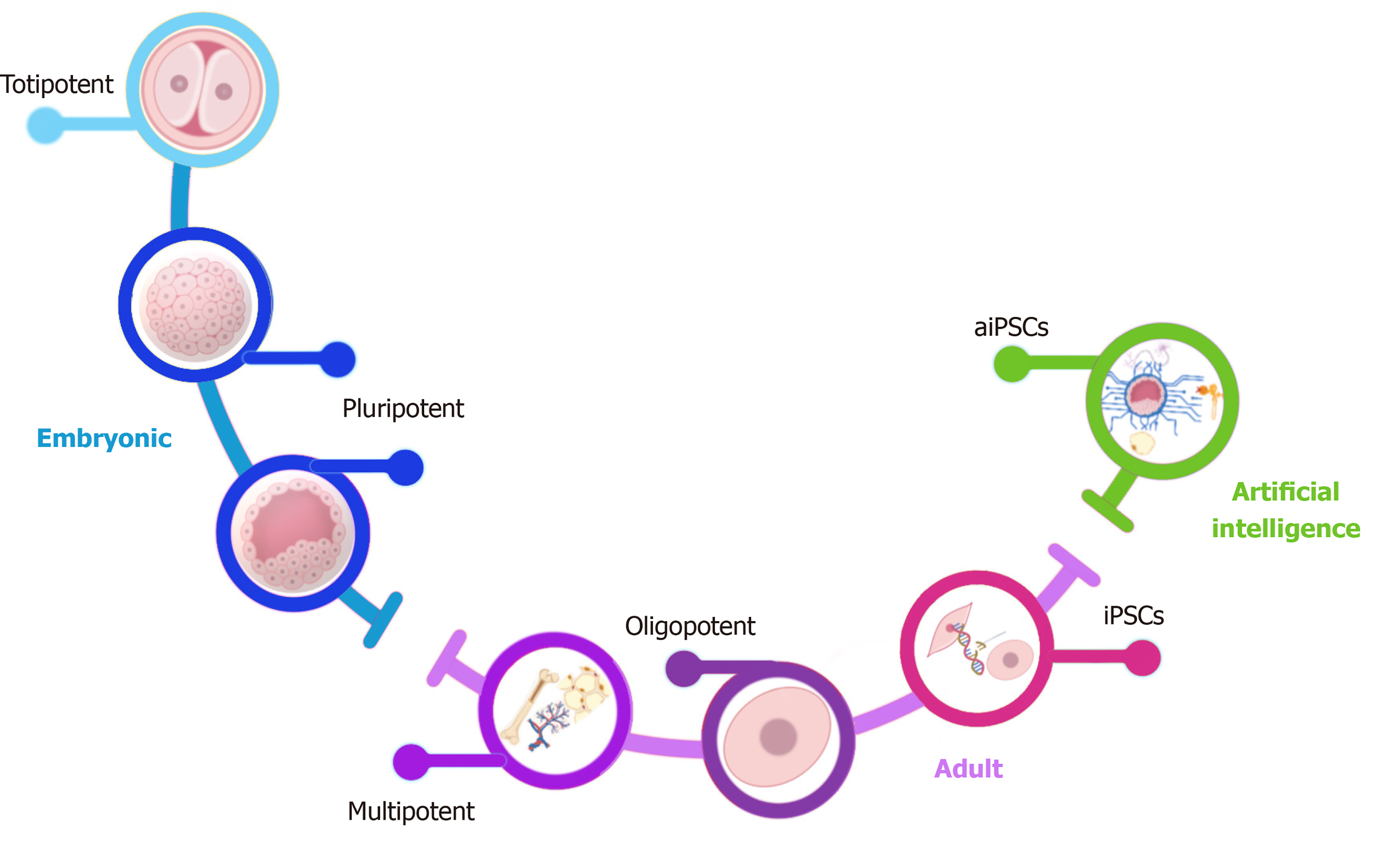
SCs are unspecialized cells with the potential to differentiate into any organism’s cell and have the capability of self-renewal. According to their origin, SCs can divide into embryonic and adult cells. Embryonic SCs (ESCs) can be obtained from the zygote, morula, or the blastocyst's inner cell mass and possess high potentiality (range of differentiation potential). Contrary, adult SCs can be isolated from neonatal and adult tissues such as the umbilical cord, placenta, bone marrow, adipose tissue, dental pulp, and peripheral blood; adult SCs show restricted potentiality. Regarding their potentiality, they are divided into totipotent, pluripotent, multipotent, and oligopotent SCs (Figure 2)[4,8].
SCs have the highest differentiation potential; there are two definitions for totipotency. One declares that a totipotent cell is a single cell that can give rise to a new organism given appropriate maternal support, and the other states that a totipotent cell can give rise to all the extraembryonic tissues plus all the tissues of the body, differentiating into any of the three germ layers. One example of a totipotent cell is the zygote[8,9].
Pluripotent SCs (PSCs) are defined by their capability of differentiating into cell types derived from the three embryonic germ layers but not extraembryonic structures. PSCs were initially established in culture as ESCs and obtained from the morula or the blastocyst's inner cell mass (4-14 d after oocyte fertilization). Induced pluripotent SCs (iPSCs) are also a type of PSC derived from adult somatic cells that have been genetically reprogrammed into PSCs. The advantage of reprogramming iPSCs has created new opportunities for understanding human diseases and physiopathology, including a growing number of viral infections[6].
Multipotent SCs (MSCs) have a narrower spectrum of differentiation than PSCs. However, they can differentiate into all cell types of one particular lineage; one example is a hematopoietic stem cell, which can differentiate into several blood cells.
Oligopotent SCs are characterized by the narrowest differentiation capabilities and have the competency to differentiate into only one lineage[8,10,11]. Considering the characteristics mentioned above, we will discuss some clinical trials and basic science assays focused on using SCs and their derivatives in COVID-19.
Human pluripotent SCs (hPSCs), human embryonic SCs (hESCs), and human induced pluripotent SCs (hiPSCs) are being used to generate functional human cells, tissues, and organoids that are used for modeling human disease and drug discovery, including modeling infectious disease. hPSC derived neuronal progenitor cells (hNPCs), and brain organoids were used to study the Zika virus's impact on human brain development[12]. Likewise, another research group demonstrated the infection capacity of the protozoan Trypanosoma cruzi in hiPSC-derived cardiomyocytes, demonstrating the potential of these cells as a human model for studying cardiomyopathy in Chagas disease and for the development of new therapies against the parasite. In the same way, these cells have been used to study hepatitis B[11].
Considering those mentioned above, currently, the use of these cells has been proposed in differentiation protocols for generating lung airway, lung alveolar, and intestinal epithelial cells. Abo et al[13] declared that iPSC-derived lung and intestinal epithelial cells derived in their protocols could be banked and used to generate multiple organ lineages from a single individual cell. Likewise, these cells can recapitulate appropriate cell-intrinsic phenotypes of a genetic disease and respond to immune stimuli. Moreover, it is essential to mention that they established a novel iPSC-derived alveolar epithelial type 2 cell air-liquid interface culture system to enable modeling of environmental exposures of the human alveolar epithelium, including viral infection, observing the expression of angiotensin-converting enzyme 2 (ACE2) and TMPRSS2, two genes encoding host cell proteins essential for SARS-CoV-2 cell entry in the iPSC-derived airway, alveolar, and intestinal epithelial cells.
In biological research history, one of the significant challenges to understanding human biology and disease and the clinical development of novel drugs includes a limited number of suitable animal models, which try to recapitulate human physiology in vivo. However, there are significant differences in metabolism between humans and laboratory models, and common drugs for humans, such as ibuprofen and warfarin, are toxic to rats, highlighting that humans are not inbred in contrast to all animal models[5,14].
The most used models for SARS-CoV-2 studies have been African green monkey-derived Vero cells or human cancer cell lines, which show limitations for modeling complex human organ systems. Therefore, relevant human models to study SARS-CoV-2 infection are critically important[15,16]. Recently, with the discovery of PSCs, knowledge about human development and morphogenesis in healthy and disease contexts has significantly improved. With the recapitulation of human organogenesis in vitro, the concept of an “organoid” emerged. Currently, the term organoid refers to three-dimensional systems formed by self-assembly of SCs in vitro that allow the recreation of the architecture and physiology of human organs in detail; as the functions are similar to natural organs in the body, organoids have provided opportunities for studying human diseases, infectious diseases, genetic disorders, and cancers[14,17,18].
The use of these organoids in SARS-CoV-2 research is not an exception. Pei et al[18] developed an optimized method to differentiate human airway organoids and alveolar organoids from hESCs, carrying out differentiation through six stages (ESCs, definitive endoderm, anterior foregut endoderm, ventralized anterior foregut endoderm, lung progenitors, and human airway or alveolar organoids), demonstrating that SARS-CoV-2 infects and extensively replicates in these organoids.
In the same way, Han et al[16] developed a lung organoid model and colonic organoids using hPSCs, reporting a robust induction of chemokines following SARS-CoV-2 infection in the lung organoid, similar to what is seen in patients with COVID-19, and that multiple colonic cell types, especially enterocytes, express ACE2 and are permissive to SARS-CoV-2 infection.
Therefore, organoids recapitulate many biological parameters, including the spatial organization of heterogeneous tissue-specific cells, cell-cell interactions, cell-matrix interactions, and certain physiological functions generated by tissue-specific cells within the organoid. Moreover, organoids provide a stable system amenable to extended cultivation and manipulation while being more representative of in vivo physiology[19,20] and thereby have significant advantages in studying this pandemic illness. Table 1 resumes the organoid infection models developed for the SARS-CoV-2 study.
| Ref. | Organoid infection model | Reported advantages |
| Yang et al[15] | hPSC-derived cells/organoids, including pancreatic endocrine cells, liver organoids, endothelial cells, cardiomyocytes, macrophages, microglia, cortical neurons, and dopaminergic neurons | Permissiveness to SARS-CoV-2 infection; ACE2 expression was detected; Chemokine induction |
| Han et al[16] | hPSC-LOs | hPSC-LOs (particularly alveolar type-II-like cells) are permissive to SARS-CoV-2 infection; Robust chemokine induction; Discovery and test therapeutic drugs |
| hPSC-COs | Permissiveness to SARS-CoV-2 infection hPSC-Cos especially enterocytes, express ACE2; Discovery and test therapeutic drugs | |
| Pei et al[18] | hAWOs and hALOs from hESCs | Permissiveness to SARS-CoV-2 infection and replication; Infected cells express ACE2 but not all ACE2 expressing cells were infected; Chemokine induction; Discovery and test therapeutic drugs |
| Yiangou et al[20] | hPSC-derived cardiac models | Permissiveness to SARS-CoV-2 infection; Activation of the innate inflammatory response; Show contractility, electrophysiology, and sarcomeric fragmentation |
| Monteil et al[22] | Human capillary organoids from iPSCs; Kidney organoids from hESCs | Permissiveness to SARS-CoV-2 infection and significantly inhibited by human recombinant soluble ACE2. ACE2 expression |
Although significant advances have been reported in the use of organoids in SARS-CoV-2, one possible concern is whether hPSC-derived cells can recapitulate the biology of SARS-CoV-2 infection in adults since the vertical infection of the fetus is not entirely clear[15]. Moreover, Tindle et al[21] have declared that existing lung organoid models available for modeling COVID-19 do not recapitulate all the heterogeneous epithelial cellularity of both proximal and distal airways, lack propagability, and/or cannot be reproducibly generated for biobanking. Similarly, Monteil et al[22] have stated that their studies' design focuses on the early stages of infection, limiting predictions concerning the effect in later phases. So, organoids remain rudimentary compared to the adult human organ[21].
Despite significant advances in iPSCs, cellular reprogramming remains a challenge due to the high costs, time-consuming, and the tendency of iPSCs to revert to their original somatic genotypes over time, adding ethical limitations; reason by which new technologies have been used to avoid these limitations. Artificial intelligence (AI) has demonstrated that it can shorten the process and increase efficiency.
AI is the way to model human intelligence to accomplish specific tasks without much intervention of human beings. It is defined as “the science and engineering of making intelligent machines.” The term was first used in 1956 with The Logic Theorist program, designed to simulate human beings' problem-solving ability. Since then, a significant subset of AI called machine learning (ML) has emerged at the forefront of AI research. An ML is conceptualized as "a field of study that gives the computer the ability to self-learn without being explicitly programmed.” The impact of AI has been transferred to the field of healthcare with its use in pharmaceutical and biomedical studies[23,24], is useful in a wide range of applications across public health, disease prediction, and drug development, including the analysis of real-time data for disease detection, and ML-based disease risk models. Moreover, AI has also helped model human behavior[25], and currently, SCs have also been evolved in this field.
Esmail and Danter[6] created the DeepNEU platform, a validated hybrid deep-machine learning system[6]. This platform enables the generation of artificial iPSCs (aiPSCs) (Figure 2). In the same way, they also reported the generation of artificially induced neural SCs and artificially induced cardiomyocytes from aiPSCs[23]. The authors have also used DeepNEU v5.0 for creating computer simulations of artificially induced type 1 (AT1) and type 2 (AT2) alveolar lung cells (aiLUNGs). Moreover, they exposed aiLUNGs to the simulated SARS-CoV-2, reproducing the genotypic and phenotypic profiles associated with the infection. Furthermore, these infected cells were treated with drug repurposing of a small group of approved drugs with well-known action mechanisms. This study also demonstrated that aiLUNG-COVID-19 simulations could be used to rapidly repurpose novel and known drug combinations with anti-SARS-CoV-2 therapeutic potential for animal and human trial validation[6].
Nonetheless, this technology's employment must consider ethical and societal implications, in addition to the requirement of systematic examination (e.g., issues around security, privacy, and confidentiality). Even though significant advances have been developed, it is mandatory to recognize that AI is still at the early stages of its development for its application across the healthcare industry[25].
As previously mentioned, MSCs have high proliferative potential and limited differentiation capacity; nevertheless, one of their most promising characteristics is their immunomodulatory properties because they secrete many types of cytokines by paracrine secretion or make direct interactions with immune cells, leading to immunomodulation. In this sense, these cells help modulate the proliferation, activation, and function of various immune cells, altering innate and adaptive immune responses. These immunomodulatory effects are triggered by the activation of TLRs in MSCs stimulated by pathogen-associated molecules such as lipopolysaccharides[4,26].
It has been reported that MSCs, as well as human bone marrow SCs (BMSCs), possess anti-inflammatory properties, which have also been demonstrated in virus-induced lung injury models. Intravenous injection of mouse BMSCs into H9N2 virus-infected mice reduced inflammatory cell recruitment into the lungs and provoked a reduction in chemokine and proinflammatory cytokine levels. Similarly, human umbilical cord-derived MSCs showed a similar effect on the inflammatory response, secreting growth factors in an in vitro lung injury model induced by the H5N1 virus[5].
MSC therapeutic effectiveness is probably limited to a niche of immunological disorders and immune-mediated illnesses such as graft-vs-host-disease, for which MSCs have demonstrated varying levels of efficacy in phase 1/2 clinical studies[27].
In SARS-CoV-2 research, it has been reported that inflammation is the driving force behind coronavirus infections. The majority of deaths caused by COVID-19 are the result of ALI and ARDS due to rapid virus replication, massive inflammatory cell infiltration, and elevated proinflammatory cytokine/chemokine responses (cytokine storm), which are events that are associated with a dysregulation of the immune response and are crucial to controlling inflammation as early as possible[4,28]. ALI and ARDS are severe clinical manifestations of COVID-19, and while administration of MSCs to subjects with ARDS was well tolerated, efficacy data at the clinical level is not as compelling[27].
Due to no pharmacological therapies halt the disease and progress very fast, immunomodulation using SCs seems to be an effective therapy. In this sense, MSCs represent one of the most promising candidates since their safety and efficacy have been shown in pre-clinical models of ARDS[1].
For the reasons mentioned above, MSCs have been widely used in cell-based therapy, from basic research to clinical trials, highlighting that most of the trials that use MSCs are focused on the immune response; for example, the clinical trial reported by Leng et al[26] showed that the inflammatory and immune functions were corrected, based on measurement of cytokine levels [i.e., tumor necrosis factor (TNF)-α, IL-10] and a subset of immune cells (D4, CD8, NK, DC cells) without adverse effects.
All cells in the organism release exosomes, described as extracellular vesicles enclosed by a membrane, transporting biologically active molecules, such as lipids, chemokines, growth factors, nucleic acids, metabolites, and proteins. The molecular contents of exosomes differ depending on their cellular origin, environment, developmental phase, and epigenetic modification, among other factors. Active molecules are taken up by surrounding cells or circulate in the blood and eventually are taken up by distant cells, mediating autocrine, paracrine, and endocrine effects that can be exploited therapeutically[29-31].
The terms “exosomes,” “microvesicles,” “microparticles” are used interchangeably, including all extracellular vesicles produced by SCs. These were first described in the 1970s. Exosomes' therapeutic potential was first described in MSC exosomes when it was observed that they were cardioprotective in a murine model of acute myocardial ischemia/reperfusion injury[32,33].
In this sense, many research types have focused on SC exosomes, emerging the exciting prospect of “cell therapy without the cells”[29]. In addition to the great regenerative potential, the content of SC exosomes has anti-inflammatory and immunomodulatory properties. Moreover, unlike SCs, exosomes do not raise immunological reactions, survive in an inflamed medium, do not develop teratomas, and protect their content against degrading enzymes[34].
It is also important to mention that exosomes play a significant role in intercellular communication and trigger physiological responses, and also have the ability to transfer horizontal micro ribonucleic acid. All these processes are facilitated through the exosome׳s cargo function[33]; they deliver their cargo to the cell of interest, enter the cell, interact with cellular organelles, and contribute to chemical reactions at the cellular level with their enzymes, respectively. For this reason, the employment of exosomes to living tissues is more feasible and less threatening in comparison to SCs[34].
As of June 13, 2020, 174 clinical trials have been registered to utilize exosomes (Clinicaltrials.gov). However, there are no Food and Drug Administration-approved exosome products available on the market[28].
Moreover, preclinical studies have shown encouraging effects of exosomes in animal models of acute respiratory distress syndrome and other respiratory diseases, showing reduced alveolar inflammation and restoration of leaky epithelial membranes[4]. Besides, it has been reported that the MSC secretome can be administered through inhalation, which is a beneficial characteristic of respiratory diseases. Preliminary studies suggest that MSC exosomes might also be efficient for the treatment of COVID-19[35].
Nevertheless, it is essential to mention that some other authors have declared the role of exosomes in COVID-19 reinfection or reactivation[36], supporting this declaration of basic virus biology and physiopathology. Viruses enter the cells using the endocytic pathway and exit the host cell by direct budding through the membrane. During viral infections, exosomes incorporate pathogen-derived nucleic acids, proteins, and lipids, becoming a vector of viral materials for the “Trojan exosome hypothesis,” promoting the viral spread and evading the immune response, which discourages the use of exosomes[35].
As a perspective, these exosomes could also be considered for the design of SARS-CoV-2 vaccine trials, since a research group in Italy at the Istituto Superiore di Sanità in Rome declared that they might have a platform for vaccines for the emerging diseases based on exosomes, using deoxyribonucleic acid vectors where the antigen is fused to an exosome-anchoring protein, which was demonstrated for the Ebola and influenza virus, as well as Crimean-Congo hemorrhagic fever, West Nile virus, and hepatitis C virus[37].
Many countries, including the United States, Italy, the United Kingdom, France, Germany, Brazil, and Jordon, have proposed SCs as a COVID-19 treatment; as expected, China hosts almost 50% of these trials. As of June 12, 2020, over 2100 clinical trials were officially registered for COVID-19 treatment (ClinicalTrials.gov). These clinical trials range from the application of antiviral drugs to novel therapies such as cell therapies. At the same time, 169 “cell therapy” trials were registered on ClinicalTrials.gov. As of August 2020, 38 registered clinical trials were using MSCs, of which seven used exosomes for the treatment of COVID-19 (https://clinicaltrials.gov/)[11,35]; only 16 trials were registered in the ICTRP[2,4,29].
In a recent review in ClinicalTrials.gov (February 2021), we found 4793 COVID-19 studies, 88 of them used different types of SCs or their derivatives (one employing ESCs, two PSCs, three SC exosomes, and 82 MSCs), highlighting that nine of these studies are reported as completed, which are summarized in Table 2.
| No. | Title | Conditions | Interventions | Locations |
| 1 | Study Evaluating the Safety and Efficacy of Autologous Non-Hematopoietic Peripheral Blood SCs in COVID-19 | COVID-19 | Biological: Autologous NHPBSC; Drug: COVID-19 standard care | Abu Dhabi SCs Center, Abu Dhabi, United Arab Emirates |
| 2 | Mesenchymal SCs Therapy in Patients With COVID-19 Pneumonia | COVID-19, Pneumonia | Other: Mesenchymal SCs | University of Health Sciences, Istanbul, Turkey |
| 3 | Treatment with Human Umbilical Cord-derived Mesenchymal SCs for Severe Corona Virus Disease 2019 (COVID-19) | COVID-19 | Biological: UC-MSCs; Biological: Saline containing 1% Human serum albumin solution without UC-MSCs | General Hospital of Central Theater Command, Wuhan, Hubei, China |
| 4 | Mesenchymal SCs for the Treatment of COVID-19 | COVID-19, Prophylaxis | Biological: PrimePro (UC-MSCs); Other: Placebo | Southern California Hospital at Culver City/Southern California Hospital at Hollywood, Culver City, California, United States |
| 5 | Use of UC-MSCs for COVID-19 Patients | COVID-19, ARDS | Biological: Umbilical Cord Mesenchymal; SCs + Heparin along with best supportive care. Other: Vehicle + Heparin along with best supportive care | Diabetes Research Institute, University of Miami Miller School of Medicine, Miami, Florida, United States |
| 6 | Therapeutic Study to Evaluate the Safety and Efficacy of DWMSC in COVID-19 Patients | COVID-19, SAR | Drug: allogeneic mesenchymal stem cell; Other: Placebo | Site 550: University of Hassanudin/Dr. Wahidin Sudirohusodo Hospital, Makassar, Indonesia |
| 7 | Investigational Treatments for COVID-19 in Tertiary Care Hospital of Pakistan | COVID-19, Cytokine Release Syndrome, Critical Illness, ARDS | Procedure: Therapeutic Plasma exchange; Biological: Convalescent Plasma; Drug: Tocilizumab; Drug: Remdesivir; Biological: Mesenchymal stem cell therapy | Pak Emirates Military Hospital, Rawalpindi, Punjab, Pakistan |
| 8 | Evaluation of Safety and Efficiency of Method of Exosome Inhalation in SARS-CoV-2 Associated Pneumonia. | COVID-19, Pneumonia | Drug: EXO 1 inhalation; Drug: EXO 2 inhalation; Drug: Placebo inhalation | Medical Centre Dinasty, Samara, Russian Federation |
| 9 | A Pilot Clinical Study on Inhalation of Mesenchymal SCs Exosomes Treating Severe Novel Coronavirus Pneumonia | COVID-19 | Biological: MSCs-derived exosomes | Ruijin Hospital Shanghai Jiao Tong University School of Medicine, Shanghai, Shanghai, China |
One of these trials was conducted in Beijing's YouAn Hospital in China, from January 23, 2020, to February 16, 2020, and seven confirmed COVID-19 patients were included in the study and received an intravenous transplant of 1 × 106 MSCs per kilogram body weight. The patients had a clinical follow-up of 14 d in which no adverse effects were observed, reporting a decreased ratio of serum proinflammatory cytokine TNF-α and a subset of immune cells; in the same way, the symptoms of these seven patients improved two days after MSC transplantation[1,27].
In another case report, Liang et al[38] administered three doses of UCMSCs to a 65-year-old woman who had tested positive for SARS-CoV-2 and had clinical signs of ALI and severe organ injury caused by an inflammatory response; no side effects were reported, and most of the laboratory indexes and computed tomography (CT) images showed remission of the inflammation symptoms. The patient was subsequently transferred out of the intensive care unit (ICU), and the throat swab test was negative four days later. Sánchez-Guijo et al[39] described an additional case report in which 13 adult COVID-19 patients under invasive mechanical ventilation were included. All the patients previously received antiviral and/or anti-inflammatory treatments, including steroids. Ten out of thirteen patients received two doses of allogenic adipose tissue-derived MSCs (AT-MSCs), two patients received one dose, and one patient received three doses. Each dose contained 0.98 × 106 AT-MSCs/kg of body weight. As a result, no adverse effects were reported, and 70% of the patients exhibited clinical improvement and were discharged from the ICU; however, four patients remained intubated, and two patients died.
As previously mentioned, MSC exosomes have also been used in clinical trials. Chu et al[40], from February 26, 2020, to April 30, 2020, recruited six patients diagnosed with COVID-19 pneumonia (two patients with severe symptoms and four patients with minor symptoms) who received nebulization of MSC-derived exosomes in different modalities at the end of the beginning of antiviral treatment for a while. No acute allergic reactions or secondary allergic reactions were observed. Their results indicated that MSC-derived exosome nebulization is a safe and feasible therapeutic approach for treating patients with COVID-19 pneumonia, showing clinical benefits. Moreover, chest CT revealed absorption of pulmonary lesions. Although the research has shortcomings, it is considered an essential antecedent for further research.
Another clinical trial that evaluates the use of exosomes is being developed in Ruijin Hospital Shanghai Jiao Tong University School of Medicine in Shanghai, China, by Qu et al[41], who evaluate aerosol's safety and efficiency inhalation of allogenic AT-MSC-derived exosomes in the treatment of severe COVID-19 pneumonia patients. In this research, participants will receive conventional treatment plus one dose of aerosol inhalation of MSC-derived exosomes a day at 2 × 108 nanovesicles/3 mL for five consecutive days.
One published study used BMSC-derived exosomes in COVID-19-infected patients and evaluated the treatment's safety and effectiveness, reporting an overall improvement in clinical symptoms and laboratory tests within 3-4 d after treatment without adverse effects[36].
However, it is crucial to recognize that all these clinical trials have shown advantages and disadvantages as any other treatment in development, which are resumed in Table 3.
| Advantages | Disadvantages | |
| ESCs | ||
| High differentiation capability which can recapitulate appropriate cell-intrinsic phenotypes | Ethical dilemma | |
| Grow up in the laboratory from a single cell for later transplantation | Tumorigenesis risk | |
| MSCs | ||
| Immunomodulatory properties | A narrower spectrum of differentiation | |
| Widespread availability and accessibility | High expression of ACE2 receptor | |
| Limited ethical concerns | Expression of MHC I and II |
As previously mentioned, the use of MSCs as an immunomodulatory and regenerative treatment has been suggested to treat COVID-19. Nevertheless, there are many challenges associated with MSC therapy, including low in vivo survival rates, dosing, cell isolation and growth strategies, and donor variability issues[27]. Moreover, it is essential to mention the intervention of the ACE2 receptor in COVID-19, as Desterke et al[42] reported that ACE2 is highly expressed in MSCs from adult bone marrow, adipose tissue, or umbilical cord[42].
ACE2 is a type I transmembrane metallocarboxypeptidase that is homologous to ACE, an enzyme that plays a vital role in the renin-angiotensin system. It is mainly expressed in alveolar type I and II cells, fibroblasts, endothelial cells, and macrophages. Currently, ACE2 protein expression has also been reported in the kidney and gastrointestinal tract, tissues that have been shown to harbor SARS-CoV-2, highlighting treatments with ACE inhibitors or the receptor antagonist angiotensin II notably increase the expression of ACE2. The reason is that patients with these pathologies who are treated with these medicines have an increased risk of developing COVID-19[42].
Some studies have demonstrated that SARS-CoV-2 enters the human cell through the receptor ACE2, acting as a receptor-binding domain for the virus spike complex, allowing viral attachment, fusion, intracellular entry, and COVID-19 infection[43,44]. Once the virus reaches the circulatory system, after replicating in type II pneumocytes, it infects other organs that express ACE2, which can generate multiple organ failure[45].
Considering the abovementioned findings, the expression level of ACE2 in MSCs could make MSCs not beneficial in COVID-19 patients if its expression is high. Currently, there are no data concerning ACE2 expression levels in MSCs of different origins used for therapy. Moreover, Desterke et al[42] reported that ACE2 expression was significantly higher in MSCs derived from adipose tissue and adult bone marrow, and lower expression levels were found in placenta-derived MSCs but only in early passages of cultures. However, it is imperative to highlight that Desterke et al[42] have also declared that hESCs and hiPSCs express deficient levels of ACE2. Therefore, it is crucial to determine if the SC population that would be transfused to patients could also be a target for SARS-CoV-2 entry into the human body.
As previously mentioned, many clinical trials employ SCs in the COVID-19 illness. Nevertheless, there has always been insecurity in the use of this type of cells. The ESCs show the disadvantage of tumorigenesis risk, widely discussed in the literature[46]. However, a fact of great weight that limits its use is the ethical dilemma. Since the beginning of the ESCs use in the research field, the thought of human embryo destruction has existed. Being the fundamental question: whether it is morally acceptable to pursue novel therapies for curing illnesses at the expense of destroying an early human embryo? Reason by which many countries such as Italy and United States have forbidden the research using hESCs. Since then, new strategies to avoid this problem have been proposed; in this sense, iPSCs technology has provided new opportunities[47].
Moreover, MSCs have arisen as a leading contender for cell sources due to their limited ethical concerns and low risk of tumor formation. Additionally, MSCs show widespread availability and accessibility[48]. However, no matter what type of SCs are used as novel treatments, there is a concern in the unproven commercial practices marketing that involve SC treatments, reason by which the International Society for Stem Cell Research in 2007 established a task force of scientific, medical, and bioethical experts to develop guidelines for the clinical translation of SC research. These guidelines address any attempt to develop novel clinical applications of SCs and their direct derivatives[49]. It is essential to mention that there are two central aspects to the definition of innovative therapies: the departure from standard medical therapy and that the employed therapy has not been validated or there is not enough available evidence to support the safety and efficiency of the therapy. Moreover, the only adult stem cell therapy currently accepted for therapeutic use as standard best practice are hematopoietic SCs[50].
With all the above, pre-clinical and clinical trials in early-stage have demonstrated the efficiency and safety of SC treatment in COVID-19 patients; however, it is important to continue investigating different types of SCs and their derivatives in large-scale researches to confirm and validate the safety and efficacy profile of these therapies with reliable evidence. Likewise, considering their advantages and disadvantages, it is essential to change the paradigm using some types of SCs that could help obtain better results and are not used by the persistence of some taboos.
Finally, it is imperative to recognize that we are not prepared to face outbreaks of this magnitude. Worldwide medicine must be prepared for future pandemics since it needs to face globalization and the factors it carries, such as international travel, global economic exchange, and social behavior. We must advance in regenerative medicine and SCs therapies to improve the immune response, regenerate damaged tissues or systems, and understand virus behaviors in cultured cells and organoids.
Manuscript source: Invited manuscript
Specialty type: Research and experimental medicine
Country/Territory of origin: Mexico
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Chen J, Nabil A, Wang DW S-Editor: Zhang L L-Editor: A P-Editor: Xing YX
| 1. | Yu F, Jia R, Tang Y, Liu J, Wei B. SARS-CoV-2 infection and stem cells: Interaction and intervention. Stem Cell Res. 2020;46:101859. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 2. | Irmak DK, Darıcı H, Karaöz E. Stem Cell Based Therapy Option in COVID-19: Is It Really Promising? Aging Dis. 2020;11:1174-1191. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 3. | COVID-19 Treatment Guidelines Panel. Coronavirus Disease 2019 (COVID-19). Treatment Guidelines. National Institutes of Health [cited 3 March 2021]. Available from: https://www.covid19treatmentguidelines.nih.gov/. |
| 4. | Choudhery MS, Harris DT. Stem cell therapy for COVID-19: Possibilities and challenges. Cell Biol Int. 2020;44:2182-2191. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 36] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 5. | Du J, Li H, Lian J, Zhu X, Qiao L, Lin J. Stem cell therapy: a potential approach for treatment of influenza virus and coronavirus-induced acute lung injury. Stem Cell Res Ther. 2020;11:192. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 36] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 6. | Esmail S, Danter WR. Viral pandemic preparedness: A pluripotent stem cell‐based machine‐learning platform for simulating SARS‐CoV‐2 infection to enable drug discovery and repurposing. STEM CELLS Transl Med. 2020;10:239-250. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 7. | Pei R, Feng J, Zhang Y, Sun H, Li L, Yang X, He J, Xiao S, Xiong J, Lin Y, Wen K, Zhou H, Chen J, Rong Z, Chen X. Human Embryonic Stem Cell-derived Lung Organoids: a Model for SARS-CoV-2 Infection and Drug Test. BioRxiv. 2020;12:1-17. [DOI] [Full Text] |
| 8. | Zakrzewski W, Dobrzyński M, Szymonowicz M, Rybak Z. Stem cells: past, present, and future. Stem Cell Res Ther. 2019;10:68. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 937] [Cited by in RCA: 953] [Article Influence: 158.8] [Reference Citation Analysis (35)] |
| 9. | Baker CL, Pera MF. Capturing Totipotent Stem Cells. Cell Stem Cell. 2018;22:25-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 78] [Article Influence: 13.0] [Reference Citation Analysis (1)] |
| 10. | Sobhani A, Khanlarkhani N, Baazm M, Mohammadzadeh F, Najafi A, Mehdinejadiani S, Sargolzaei Aval F. Multipotent Stem Cell and Current Application. Acta Med Iran. 2017;55:6-23. [PubMed] |
| 11. | Saldanha-Araujo F, Melgaço Garcez E, Silva-Carvalho AE, Carvalho JL. Mesenchymal Stem Cells: A New Piece in the Puzzle of COVID-19 Treatment. Front Immunol. 2020;11:1563. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 12. | Zhou T, Tan L, Cederquist GY, Fan Y, Hartley BJ, Mukherjee S, Tomishima M, Brennand KJ, Zhang Q, Schwartz RE, Evans T, Studer L, Chen S. High-Content Screening in hPSC-Neural Progenitors Identifies Drug Candidates that Inhibit Zika Virus Infection in Fetal-like Organoids and Adult Brain. Cell Stem Cell 2017; 21: 274-283. e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 204] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 13. | Abo KM, Ma L, Matte T, Huang J, Alysandratos KD, Werder RB, Mithal A, Beermann ML, Lindstrom-Vautrin J, Mostoslavsky G, Ikonomou L, Kotton DN, Hawkins F, Wilson A, Villacorta-Martin C. Human iPSC-derived alveolar and airway epithelial cells can be cultured at air-liquid interface and express SARS-CoV-2 host factors. bioRxiv. 2020;2020:; 132639. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 38] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 14. | Kim J, Koo BK, Knoblich JA. Human organoids: model systems for human biology and medicine. Nat Rev Mol Cell Biol. 2020;21:571-584. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 535] [Cited by in RCA: 1234] [Article Influence: 246.8] [Reference Citation Analysis (0)] |
| 15. | Yang L, Han Y, Nilsson-Payant BE, Gupta V, Wang P, Duan X, Tang X, Zhu J, Zhao Z, Jaffré F, Zhang T, Kim TW, Harschnitz O, Redmond D, Houghton S, Liu C, Naji A, Ciceri G, Guttikonda S, Bram Y, Nguyen DT, Cioffi M, Chandar V, Hoagland DA, Huang Y, Xiang J, Wang H, Lyden D, Borczuk A, Chen HJ, Studer L, Pan FC, Ho DD, tenOever BR, Evans T, Schwartz RE, Chen S. A Human Pluripotent Stem Cell-based Platform to Study SARS-CoV-2 Tropism and Model Virus Infection in Human Cells and Organoids. Cell Stem Cell 2020; 27: 125-136. e7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 529] [Cited by in RCA: 512] [Article Influence: 102.4] [Reference Citation Analysis (0)] |
| 16. | Han Y, Duan X, Yang L, Nilsson-Payant BE, Wang P, Duan F, Tang X, Yaron TM, Zhang T, Uhl S, Bram Y, Richardson C, Zhu J, Zhao Z, Redmond D, Houghton S, Nguyen DT, Xu D, Wang X, Jessurun J, Borczuk A, Huang Y, Johnson JL, Liu Y, Xiang J, Wang H, Cantley LC, tenOever BR, Ho DD, Pan FC, Evans T, Chen HJ, Schwartz RE, Chen S. Identification of SARS-CoV-2 inhibitors using lung and colonic organoids. Nature. 2021;589:270-275. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 393] [Cited by in RCA: 397] [Article Influence: 99.3] [Reference Citation Analysis (0)] |
| 17. | Silva TP, Cotovio JP, Bekman E, Carmo-Fonseca M, Cabral JMS, Fernandes TG. Design Principles for Pluripotent Stem Cell-Derived Organoid Engineering. Stem Cells Int. 2019;2019:4508470. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 18. | Pei R, Feng J, Zhang Y, Sun H, Li L, Yang X, He J, Xiao S, Xiong J, Lin Y, Wen K, Zhou H, Chen J, Rong Z, Chen X. Host metabolism dysregulation and cell tropism identification in human airway and alveolar organoids upon SARS-CoV-2 infection. Protein Cell. 2020;12:; 1-17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 67] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 19. | Yin X, Mead BE, Safaee H, Langer R, Karp JM, Levy O. Engineering Stem Cell Organoids. Cell Stem Cell. 2016;18:25-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 520] [Cited by in RCA: 596] [Article Influence: 66.2] [Reference Citation Analysis (0)] |
| 20. | Yiangou L, Davis RP, Mummery CL. Using Cardiovascular Cells from Human Pluripotent Stem Cells for COVID-19 Research: Why the Heart Fails. Stem Cell Reports. 2021;16:385-397. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 21. | Tindle C, Fuller M, Fonseca A, Taheri S, Ibeawuchi SR, Beutler N, Claire A, Castillo V, Hernandez M, Russo H, Duran J, Crotty Alexander LE, Tipps A, Lin G, Thistlethwaite PA, Chattopadhyay R, Rogers TF, Sahoo D, Ghosh P, Das S. Adult Stem Cell-derived Complete Lung Organoid Models Emulate Lung Disease in COVID-19. bioRxiv. 2020;2020:; 344002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 22. | Monteil V, Kwon H, Prado P, Hagelkrüys A, Wimmer RA, Stahl M, Leopoldi A, Garreta E, Hurtado Del Pozo C, Prosper F, Romero JP, Wirnsberger G, Zhang H, Slutsky AS, Conder R, Montserrat N, Mirazimi A, Penninger JM. Inhibition of SARS-CoV-2 Infections in Engineered Human Tissues Using Clinical-Grade Soluble Human ACE2. Cell 2020; 181: 905-913. e7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1779] [Cited by in RCA: 1660] [Article Influence: 332.0] [Reference Citation Analysis (0)] |
| 23. | Esmail S, Danter WR. DeepNEU: Artificially Induced Stem Cell (aiPSC) and Differentiated Skeletal Muscle Cell (aiSkMC) Simulations of Infantile Onset POMPE Disease (IOPD) for Potential Biomarker Identification and Drug Discovery. Front Cell Dev Biol. 2019;7:325. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 24. | Thakur A, Mishra AP, Panda B, Rodríguez DCS, Gaurav I, Majhi B. Application of Artificial Intelligence in Pharmaceutical and Biomedical Studies. Curr Pharm Des. 2020;26:3569-3578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 25. | Park Y, Casey D, Joshi I, Zhu J, Cheng F. Emergence of New Disease: How Can Artificial Intelligence Help? Trends Mol Med. 2020;26:627-629. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 26. | Leng Z, Zhu R, Hou W, Feng Y, Yang Y, Han Q, Shan G, Meng F, Du D, Wang S, Fan J, Wang W, Deng L, Shi H, Li H, Hu Z, Zhang F, Gao J, Liu H, Li X, Zhao Y, Yin K, He X, Gao Z, Wang Y, Yang B, Jin R, Stambler I, Lim LW, Su H, Moskalev A, Cano A, Chakrabarti S, Min KJ, Ellison-Hughes G, Caruso C, Jin K, Zhao RC. Transplantation of ACE2- Mesenchymal Stem Cells Improves the Outcome of Patients with COVID-19 Pneumonia. Aging Dis. 2020;11:216-228. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 730] [Cited by in RCA: 852] [Article Influence: 170.4] [Reference Citation Analysis (0)] |
| 27. | Durand N, Mallea J, Zubair AC. Insights into the use of mesenchymal stem cells in COVID-19 mediated acute respiratory failure. NPJ Regen Med. 2020;5:17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 35] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 28. | Shafiee A, Moradi L, Lim M, Brown J. Coronavirus disease 2019: A tissue engineering and regenerative medicine perspective. Stem Cells Transl Med. 2021;10:27-38. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 29. | Barile L, Vassalli G. Exosomes: Therapy delivery tools and biomarkers of diseases. Pharmacol Ther. 2017;174:63-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 527] [Cited by in RCA: 795] [Article Influence: 99.4] [Reference Citation Analysis (0)] |
| 30. | Zhang ZG, Buller B, Chopp M. Exosomes - beyond stem cells for restorative therapy in stroke and neurological injury. Nat Rev Neurol. 2019;15:193-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 260] [Cited by in RCA: 388] [Article Influence: 64.7] [Reference Citation Analysis (0)] |
| 31. | Mianehsaz E, Mirzaei HR, Mahjoubin-Tehran M, Rezaee A, Sahebnasagh R, Pourhanifeh MH, Mirzaei H, Hamblin MR. Mesenchymal stem cell-derived exosomes: a new therapeutic approach to osteoarthritis? Stem Cell Res Ther. 2019;10:340. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 121] [Cited by in RCA: 197] [Article Influence: 32.8] [Reference Citation Analysis (0)] |
| 32. | Yeo RWY, Lai RC, Tan KH, Lim SK. Exosome: A Novel and Safer Therapeutic Refinement of Mesenchymal Stem Cell. EXMV. 2013;1:1-12. [RCA] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 33. | Ha D, Yang N, Nadithe V. Exosomes as therapeutic drug carriers and delivery vehicles across biological membranes: current perspectives and future challenges. Acta Pharm Sin B. 2016;6:287-296. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 903] [Cited by in RCA: 973] [Article Influence: 108.1] [Reference Citation Analysis (0)] |
| 34. | Kharazi U, Badalzadeh R. A review on the stem cell therapy and an introduction to exosomes as a new tool in reproductive medicine. Reprod Biol. 2020;20:447-459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 38] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 35. | Pocsfalvi G, Mammadova R, Ramos Juarez AP, Bokka R, Trepiccione F, Capasso G. COVID-19 and Extracellular Vesicles: An Intriguing Interplay. Kidney Blood Press Res. 2020;45:661-670. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 48] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 36. | Elrashdy F, Aljaddawi AA, Redwan EM, Uversky VN. On the potential role of exosomes in the COVID-19 reinfection/reactivation opportunity. J Biomol Struct Dyn. 2020;1-12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 53] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 37. | Jungbauer A. Exosomes Enter Vaccine Development: Strategies Meeting Global Challenges of Emerging Infections. Biotechnol J. 2018;13:e1700749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 38. | Liang B, Chen J, Li T, Wu H, Yang W, Li Y, Li J, Yu C, Nie F, Ma Z, Yang M, Xiao M, Nie P, Gao Y, Qian C, Hu M. Clinical remission of a critically ill COVID-19 patient treated by human umbilical cord mesenchymal stem cells: A case report. Medicine (Baltimore). 2020;99:e21429. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 194] [Cited by in RCA: 226] [Article Influence: 45.2] [Reference Citation Analysis (0)] |
| 39. | Sánchez-Guijo F, García-Arranz M, López-Parra M, Monedero P, Mata-Martínez C, Santos A, Sagredo V, Álvarez-Avello JM, Guerrero JE, Pérez-Calvo C, Sánchez-Hernández MV, Del-Pozo JL, Andreu EJ, Fernández-Santos ME, Soria-Juan B, Hernández-Blasco LM, Andreu E, Sempere JM, Zapata AG, Moraleda JM, Soria B, Fernández-Avilés F, García-Olmo D, Prósper F. Adipose-derived mesenchymal stromal cells for the treatment of patients with severe SARS-CoV-2 pneumonia requiring mechanical ventilation. A proof of concept study. EClinicalMedicine. 2020;25:100454. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 101] [Cited by in RCA: 122] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 40. | Chu M, Wang H, Bian L, Huang J, Wu D, Fei F, Zhang R, Chen Y, Xia J. Nebulization Therapy for COVID-19 Pneumonia with Embryonic Mesenchymal Stem Cells-derived Exosomes. 2020; Preprint. [DOI] [Full Text] |
| 41. | Qu JM, Wang C, Cao B; Chinese Thoracic Society and Chinese Association of Chest Physicians. Guidance for the management of adult patients with coronavirus disease 2019. Chin Med J (Engl). 2020;133:1575-1594. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 42. | Desterke C, Griscelli F, Imeri J, Marcoux P, Lemonnier T, Latsis T, Turhan AG, Bennaceur-Griscelli A. Molecular investigation of adequate sources of mesenchymal stem cells for cell therapy of COVID-19-associated organ failure. Stem Cells Transl Med. 2021;10:568-571. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 43. | Kumar A, Ghosh SB. Emerging Treatment Options of Regenerative Medicine in Severe Corona Virus/COVID 19 Infections. Int J Stem Cells. 2020;13:305-311. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 44. | McLachlan CS. The angiotensin-converting enzyme 2 (ACE2) receptor in the prevention and treatment of COVID-19 are distinctly different paradigms. Clin Hypertens. 2020;26:14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 35] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 45. | Montaño LM, Flores-Soto E. COVID-19 y su asociación con los inhibidores de la enzima convertidora de angiotensina y los antagonistas de los receptores para angiontensina II. Rev Fac Med UNAM. 2020;63:30-34. [RCA] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 46. | Musiał-Wysocka A, Kot M, Majka M. The Pros and Cons of Mesenchymal Stem Cell-Based Therapies. Cell Transplant. 2019;28:801-812. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 160] [Cited by in RCA: 355] [Article Influence: 59.2] [Reference Citation Analysis (0)] |
| 47. | Volarevic V, Markovic BS, Gazdic M, Volarevic A, Jovicic N, Arsenijevic N, Armstrong L, Djonov V, Lako M, Stojkovic M. Ethical and Safety Issues of Stem Cell-Based Therapy. Int J Med Sci. 2018;15:36-45. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 359] [Cited by in RCA: 526] [Article Influence: 75.1] [Reference Citation Analysis (0)] |
| 48. | Fričová D, Korchak JA, Zubair AC. Challenges and translational considerations of mesenchymal stem/stromal cell therapy for Parkinson's disease. NPJ Regen Med. 2020;5:20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 55] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 49. | Munsie M, Hyun I. A question of ethics: selling autologous stem cell therapies flaunts professional standards. Stem Cell Res. 2014;13:647-653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 50. | McLean AK, Stewart C, Kerridge I. Untested, unproven, and unethical: the promotion and provision of autologous stem cell therapies in Australia. Stem Cell Res Ther. 2015;6:33. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 39] [Article Influence: 3.9] [Reference Citation Analysis (0)] |