Published online Dec 26, 2021. doi: 10.4252/wjsc.v13.i12.1905
Peer-review started: May 5, 2021
First decision: June 23, 2021
Revised: July 6, 2021
Accepted: December 11, 2021
Article in press: December 11, 2021
Published online: December 26, 2021
Processing time: 233 Days and 23.4 Hours
As a cellular mode of therapy, bone marrow mesenchymal stem cells (BMSCs) are used to treat stroke. However, their mechanisms in stroke treatment have not been established. Recent evidence suggests that regulation of dysregulated gut flora after stroke affects stroke outcomes.
To investigate the effects of BMSCs on gut microbiota after ischemic stroke.
A total of 30 Sprague-Dawley rats were randomly divided into three groups, including sham operation control group, transient middle cerebral artery occlusion (MCAO) group, and MCAO with BMSC treatment group. The modified Neurological Severity Score (mNSS), beam walking test, and Morris water maze test were used to evaluate neurological function recovery after BMSC transplantation. Nissl staining was performed to elucidate on the pathology of nerve cells in the hippocampus. Feces from each group of rats were collected and analyzed by 16s rDNA sequencing.
BMSC transplantation significantly reduced mNSS (P < 0.01). Rats performed better in the beam walking test in the BMSC group than in the MCAO group (P < 0.01). The Morris water maze test revealed that the BMSC treatment group exhibited a significant improvement in learning and memory. Nissl staining for neuronal damage assessment after stroke showed that in the BMSC group, cells were orderly arranged with significantly reduced necrosis. Moreover, BMSCs regulated microbial structure composition. In rats treated with BMSCs, the abundance of potential short-chain fatty acid producing bacteria and Lactobacillus was increased.
BMSC transplantation is a potential therapeutic option for ischemic stroke, and it promotes neurological functions by regulating gut microbiota dysbiosis.
Core Tip: Bone marrow mesenchymal stem cell (BMSC) transplantation provides a novel approach for ischemic stroke therapy. Studies on the “gut-brain axis” indicate that gut microbiota dysbiosis affects stroke prognosis. We investigated the interactions between BMSCs and gut microbiota. Our findings indicate that the therapeutic mechanism of BMSCs on ischemic stroke treatment may involve the regulation of microbiome structure and function.
- Citation: Zhao LN, Ma SW, Xiao J, Yang LJ, Xu SX, Zhao L. Bone marrow mesenchymal stem cell therapy regulates gut microbiota to improve post-stroke neurological function recovery in rats. World J Stem Cells 2021; 13(12): 1905-1917
- URL: https://www.wjgnet.com/1948-0210/full/v13/i12/1905.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v13.i12.1905
Globally, stroke is a lethal disability-causing disease that affects up to 13 million people annually[1]. The latest data from the American Heart Association shows that in the United States, one person suffers a stroke after every 40 s[2]. Stroke patients exhibit recurrent attacks, which exerts a huge socio-economic burden on the society and families. Ischemic stroke is the most prevalent stroke type, accounting for 70%-80% of all stroke types[3]. Intravenous thrombolysis and endovascular thrombectomy are the primary treatment options for stroke. However, they are associated with time and technical limitations[4,5]. Therefore, it is important to develop novel therapeutic approaches for ischemic stroke.
Stem cell transplantation is considered a potential therapeutic strategy for patients after ischemic stroke[6]. Bone marrow mesenchymal stem cells (BMSCs) are a group of stem cells with various characteristics, including autologous harvesting, rapid proliferation, easy in vitro culture, and low immunogenicity. Moreover, they are not limited by ethical restrictions. BMSCs have the effects of neuroprotection, modulation of inflammation, immune responses, endogenous neurogenesis, and astrogenesis[7]. Specifically, their inflammatory regulatory function has been investigated in various inflammatory diseases.
An estimated 100 trillion microorganisms reside in the human gut. They are closely associated with human health and diseases[8]. The understanding of gut microbiota is only at the rudimentary stage; however, studies have confirmed the existence of bidirectional communication in the microbiota-gut-brain axis, which influences stroke treatment and prognosis[9-11]. After a stroke, the central nervous system (CNS) is injured, then, as a stress response mechanism, the hypothalamic-pituitary-adrenal axis triggers the release of adrenocorticotropic hormone-releasing factor (CRF) and glucocorticoids[12]. Sympathetic and parasympathetic nerves directly affect gastrointestinal functions via communication with the enteric nervous system[10]. This induces suppressed gut motility, increased gut permeability, gut microbiota dysbiosis, and immune cell activation. Studies have documented significant microbial diversity changes in feces of stroke patients[13,14]. Severe stroke destroys the intestinal barrier, therefore, commensal gut microbiota migrates to other organs; this is the primary cause of systemic infections after stroke[15]. A few bacterial species in gut microbiota or their metabolites regulate intestinal immunity, which regulates post-stroke immunity[16]. Animal model experiments have established that changing the gut microbiota improves the prognosis of stroke[17,18]. Despite the documented efficacy of stem cell therapy in altering the populations of gut microbiota in several inflammatory diseases, it has not determined whether it has a similar effect on ischemic stroke.
Therefore, we used a rat model of transient middle cerebral artery occlusion (MCAO) to investigate whether BMSCs can improve abnormal intestinal flora after ischemic stroke.
Adult male Sprague-Dawley (SD) rats, 5-6 week old, weighing 220–250 g, were purchased from Beijing Huafukang Biotechnology Company (Beijing, China). The rats were housed in pathogen-free conditions under a 12 h-light/12 h-dark cycle at 25 °C. The Ethics Committee of Tianjin University of Traditional Chinese Medicine approved this study (approval number: TCM-LAEC2019038).
In this study, 4-wk-old SD rats were cervically dislocated. The femur and tibia were isolated and removed under sterile conditions. The Dulbecco's modified Eagle medium (DMEM) was used for flushing the bone marrow cavity, and the bone marrow flush was collected. The isolated cell suspension was sieved through a 200-mesh nylon sieve and then centrifuged (1000 r/min) for 10 min at 4 °C. The supernatant was discarded, and the cells were re-suspended with DMEM containing 10% fetal bovine serum (FBS; BI). The cell density was adjusted to 2 × 106 cells into 25 cm2 culture flasks and incubated in a cell incubator (37 °C, 5% CO2). The cells were passaged every 3-4 d, and the third-passage cells were used for further experiments. BMSCs were incubated with fluorescence antibodies, including CD90-PE, CD29-APC, CD45-PerCP, and CD31-FITC (1:100, Miltenyi, Germany), to identify the phenotype by flow cytometry (FACS Calibur, BD, San Jose, CA, United States).
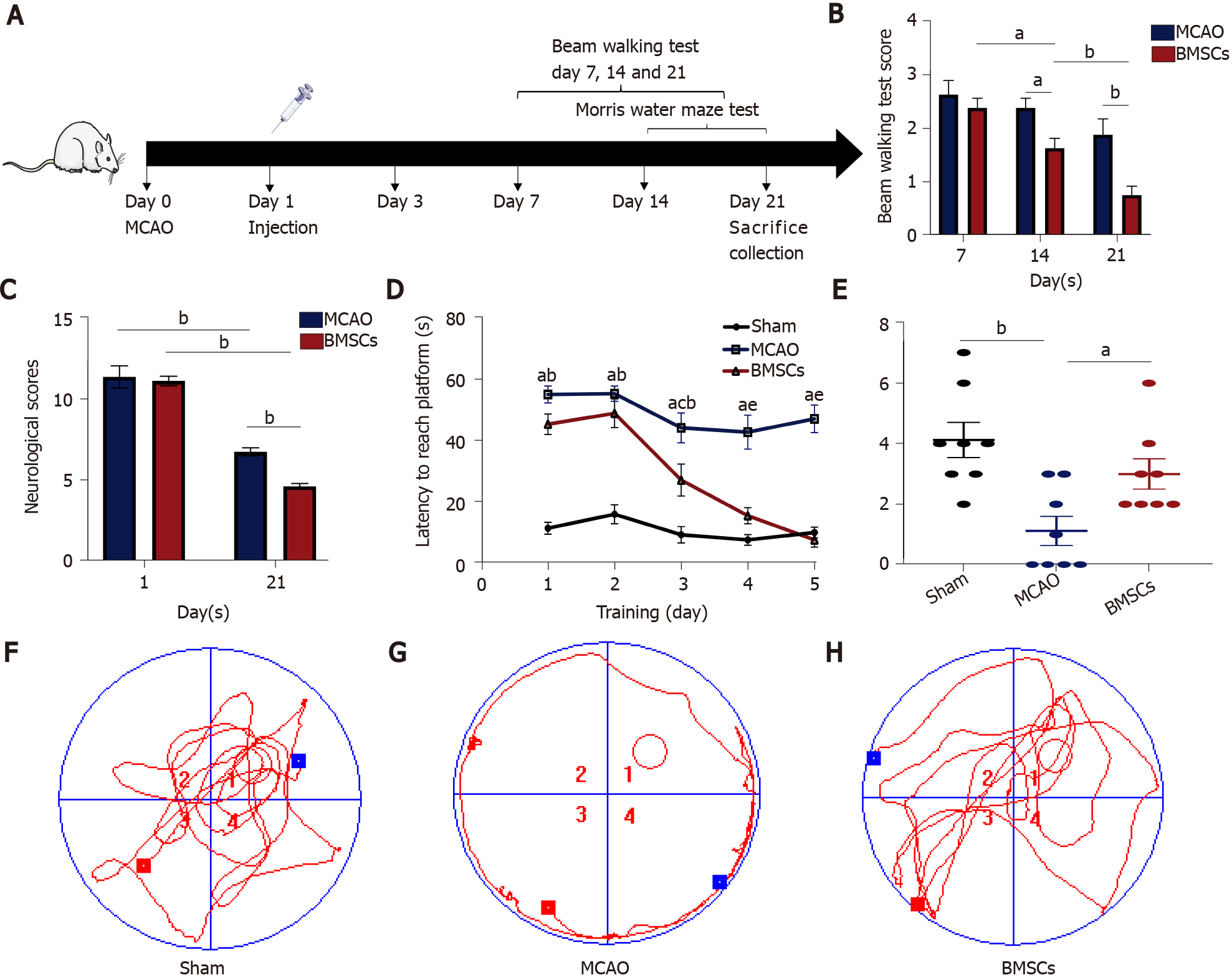
Rats were randomly divided into three groups (n = 10 each): Sham operation control group (Sham), transient MCAO group, and MCAO with BMSC treatment group. The Sham and MCAO groups were injected with normal saline (PBS), and the BMSCs group was injected with 1 × 106 BMSCs through the tail vein 24 h after reperfusion. Rats were killed after 21 d of reperfusion to collect feces and brain tissue for analysis (Figure 1A).
The intraluminal filament model was used to induce transient MCAO as described by Jackman et al[19]. Rats were anesthetized with 4% isoflurane and fixed in a supine position, and a longitudinal incision was made 0.3 cm to the right of the midline of their neck. Then, the muscles and tissues were separated to expose the right common carotid artery (CCA), external carotid artery (ECA), and internal carotid artery (ICA). Subsequently, a filament nylon suture was inserted into the right ECA and pushed until the middle cerebral artery (MCA) was obstructed. After 90 min of ischemia, the filament was removed carefully and reperfusion performed. During surgery, the rats were placed on a thermostat system to maintain body temperature.
The Longa 5-point scale was used to judge whether MCAO surgery is successful: 4, the animal died; 3, the animal could not walk in a straight line, and its body was tilted to one side; 2, the animal turned to one side during crawling; 1, the animal could not straighten its limbs and was stiff; 0, the animal was normal. If the score was 1-3, the model was considered successful, and the experiment can be carried out later; 0 and 4 were rejected. Animals with a score of 1 to 3 will be grouped for later experiments.
The modified Neurological Severity Score (mNSS) was used to score the neur
Two blinded investigators observed all behavioral tests at regular times of the day. The apparatus was washed with 70% ethanol after each animal was tested to eliminate olfactory cues.
Beam walking test: For detecting motor coordination and balance, the beam walking test was evaluated at 7, 14, and 21 d after reperfusion. The rats were placed on a balance beam that was 1 m long, 2.5 cm wide, and 20 cm high from the ground. A soft cushion was placed under the balance beam to prevent the mouse from falling. Every mouse was scored according to the following rules: (1) If the rat crossed the balance beam smoothly without the hind limbs slipping; (2) If the rat gripped the edge of the balance beam, but the hind limbs did not dangle; (3) If the rat clutched the balance beam, and one limb dropped from the balance beam; (4) If the rat clutched the balance beam, and two limbs dropped from the balance beam or rotated on the balance beam (> 60 s); (5) If the rat tried to balance on the balance beam but fell (> 40 s); (6) If the rat tried to balance on the balance beam but failed (> 20 s); and (7) If the rats fell and did not attempt to balance on the beam (< 20 s).
Morris water maze test: The Morris water maze test was performed 14 d after surgery for six consecutive days to test rats' spatial memory ability. The water maze was a circular black pool (Shanghai Xinsoft Information Technology Co., Ltd.), 150 cm in diameter, 50 cm high, and 25 cm deep, with the water temperature maintained at 20 ± 1 °C. The pool was divided into four quadrants (1, 2, 3, and 4), and the circular platform was located in quadrant 1, 2 cm below the water surface. The rats were tested twice daily for 60 s for the first 5 d and were allowed to remain on the platform for 10 s after each test. On day 6, a probe trial was performed by removing the platform and allowing the rat to swim freely in the pool for the 60 s. The time and route taken by the rats to complete the task were recorded. Finally, the data were exported and analyzed using Morris water maze analysis software.
The rats were fixed by perfusion in 4% paraformaldehyde (PFA). The brains were quickly removed and fixed in 4% PFA at 4 °C for 24 h. After dehydration, they were embedded in paraffin and serially sectioned into 4 μm tissue sections for histological analysis. Nissl staining was performed to evaluate neuron damage. The histo
The rat feces from each group were collected into 2 mL sterile freezing tubes on day 21 and stored at -80 °C until the bacterial DNA was extracted. Total bacterial DNA was extracted using DNA Extraction Kit (QIAGEN, Germany) following the manuf
α-diversity is a measure of the abundance and diversity of microbial communities in a sample. In this paper, the Shannon index and Chao index were used to represent α-diversity[21,22]. The Shannon index is an alpha diversity statistic for estimating the index of microbial diversity in a sample. A higher value indicates that the community is more diverse. The Chao index assesses the number of OTUs in a sample. The larger the Chao index, the higher the number of OTUs, indicating that the number of species in the sample is more numerous. The functional pathways of microbial communities for each sample were inferred using Phylogenetic Investigation of Communities by Reconstruction of Unobserved States (PICRUSt) software[23]. The PICRUSt software predicts the metabolic function of microorganisms by comparing the resulting 16S sequencing data with a genomic reference database of microorganisms with known metabolic functions.
The results are expressed as the mean ± SEM. The data were analyzed using one-way analysis of variance (ANOVA) and t-test. The difference was considered significant at P < 0.05.
The mNSS, beam walking test, and Morris water maze test were used to estimate the neurological function after ischemic stroke. The neurological deficit scores of each group of rats were evaluated at 1 and 21 d after ischemia-reperfusion (Figure 1B). Compared with the MCAO group, the BMSCs group had significantly improved neurological function. The mNSS scores of both the MCAO and BMSCs groups were substantially lower at 21 d than on the first day (P < 0.01). However, the BMSCs group had a more significant decrease in mNSS scores at day 21 than the MCAO group (P < 0.01). Beam walking test showed that rats subjected to BMSCs transplantation presented a larger motor functional improvement (14 d, P < 0.05; 21 d, P < 0.01; Figure 1C)
To assess the spatial learning and memory capacity of BMSC-treated rats after stroke, the Morris water maze test was used to detect the escape latency of a random search for the hidden platform during the first 5 d. Compared to the MCAO group, the BMSCs group showed a significantly shorter duration of escaping latency (P < 0.05; Figure 1D). After removing the hidden platform at 6 d, rats of the BMSCs group were easier to find the previous location of the platform site compared to those of the MCAO group, which passed over the platform site more times (P < 0.05; Figure 1E). The typical swimming tracks of each group (Figure 1F-H) also indicated that rats treated with BMSCs had significantly improved spatial memory.
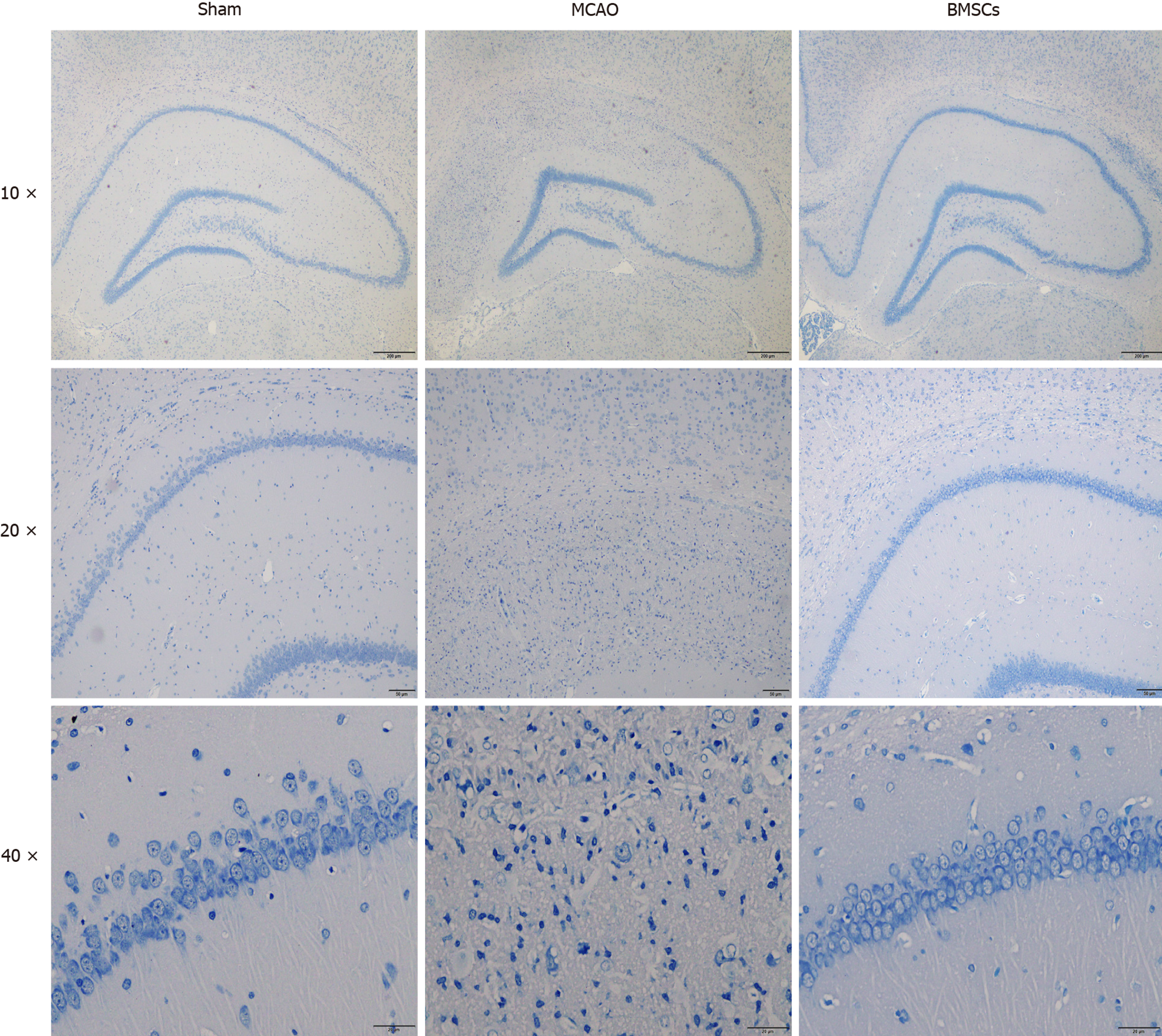
Nissl staining demonstrated no significant changes in neurons in the hippocampal CA1 area of the brain in the Sham group on day 21. In the MCAO group, the boundaries of the hippocampal CA1 area were irregular, the number of Nissl bodies was reduced, and a large number of neurons underwent necrosis. Compared with the MCAO group, the rat hippocampal neurons in the group treated with BMSCs were arranged in an orderly manner, and necrotic cells were significantly reduced (Figure 2). These results suggest that stroke causes severe neuronal damage in rats and that BMSC treatment can effectively protect neurons and prevent neuronal loss.
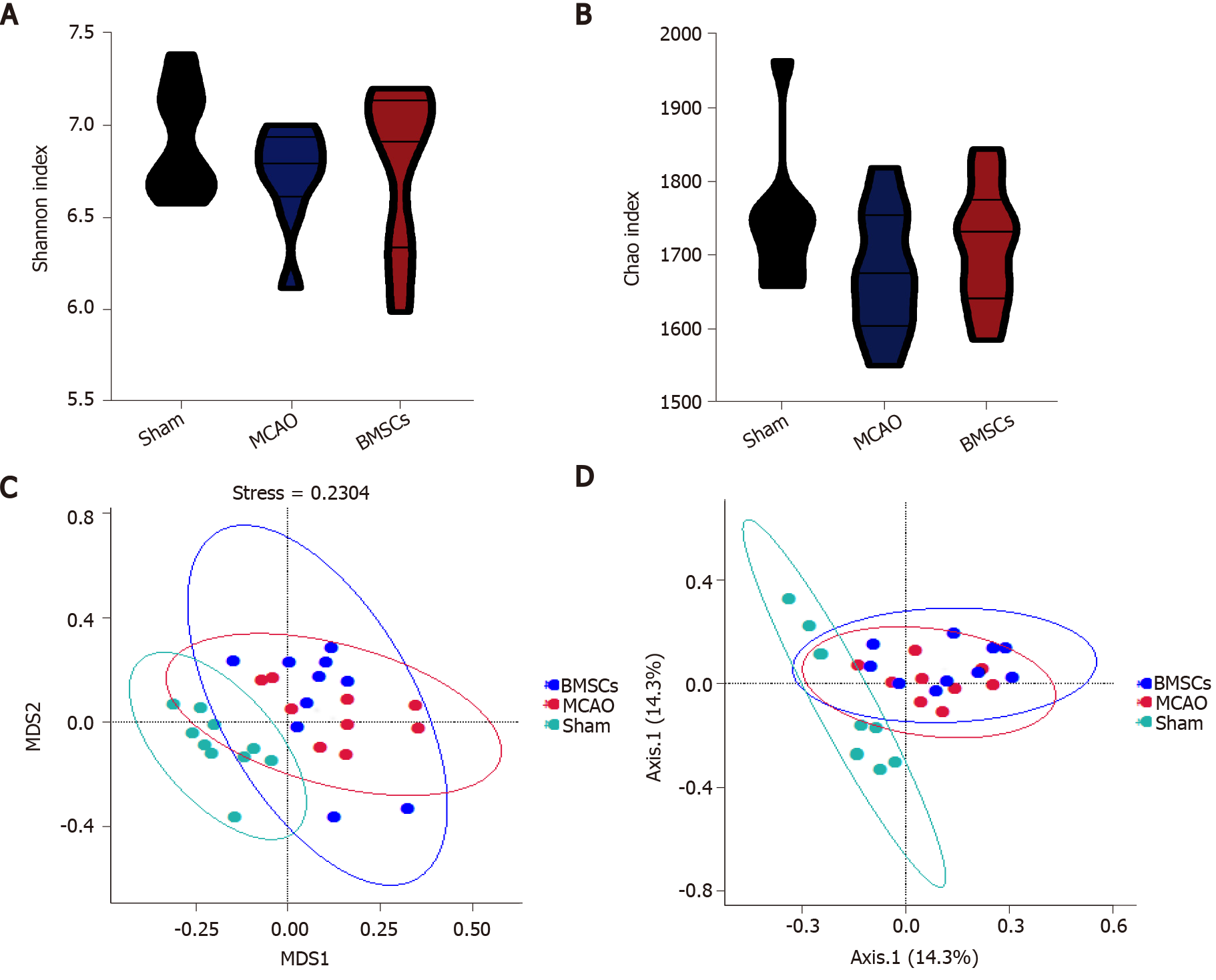
To identify whether treatment with BMSCs influences the gut microbiota after ischemic stroke, we analyzed differences in species complexity and bacterial communities between populations based on OTUS and species annotation results. We obtained a total of 1494295 quality filtered 16s rRNA gene sequences from three groups of 30 samples, with an average of 49810 ± 1281 reads per sample. We compared microbial α-diversity between the Sham, MCAO, and BMSCs groups, and both Shannon and Chao index results showed no statistical difference between the three groups (Figure 3A and B).
We calculated inter-sample distances between the three groups to analyze the differences in community species composition among individual samples within each group. We demonstrate the nonmetric multi-dimensional scaling (nMDS) plot, and the principle co-ordinates analysis (PCoA) plots in Figure 3C and D. Different groups are presented in different colors in the figure, and samples from the same group are clustered together. The nMDS analysis and PCoA showed that MCAO and BMSCs could alter the microbiota composition significantly compared to the Sham group. However, there was no significant difference in microbiota structure between the two groups of MCAO and BMSCs. To further investigate the variability of microbial communities between the two groups, the ANOSIM test was used to test both Bray-Curtis and Unweighted Unifrac algorithms (Bray-Curtis, r = 0.0769, P = 0.042; Unweighted Unifrac, r = 0.0679, P = 0.0415, respectively). The results showed significant differences in the microbial communities between the two groups.
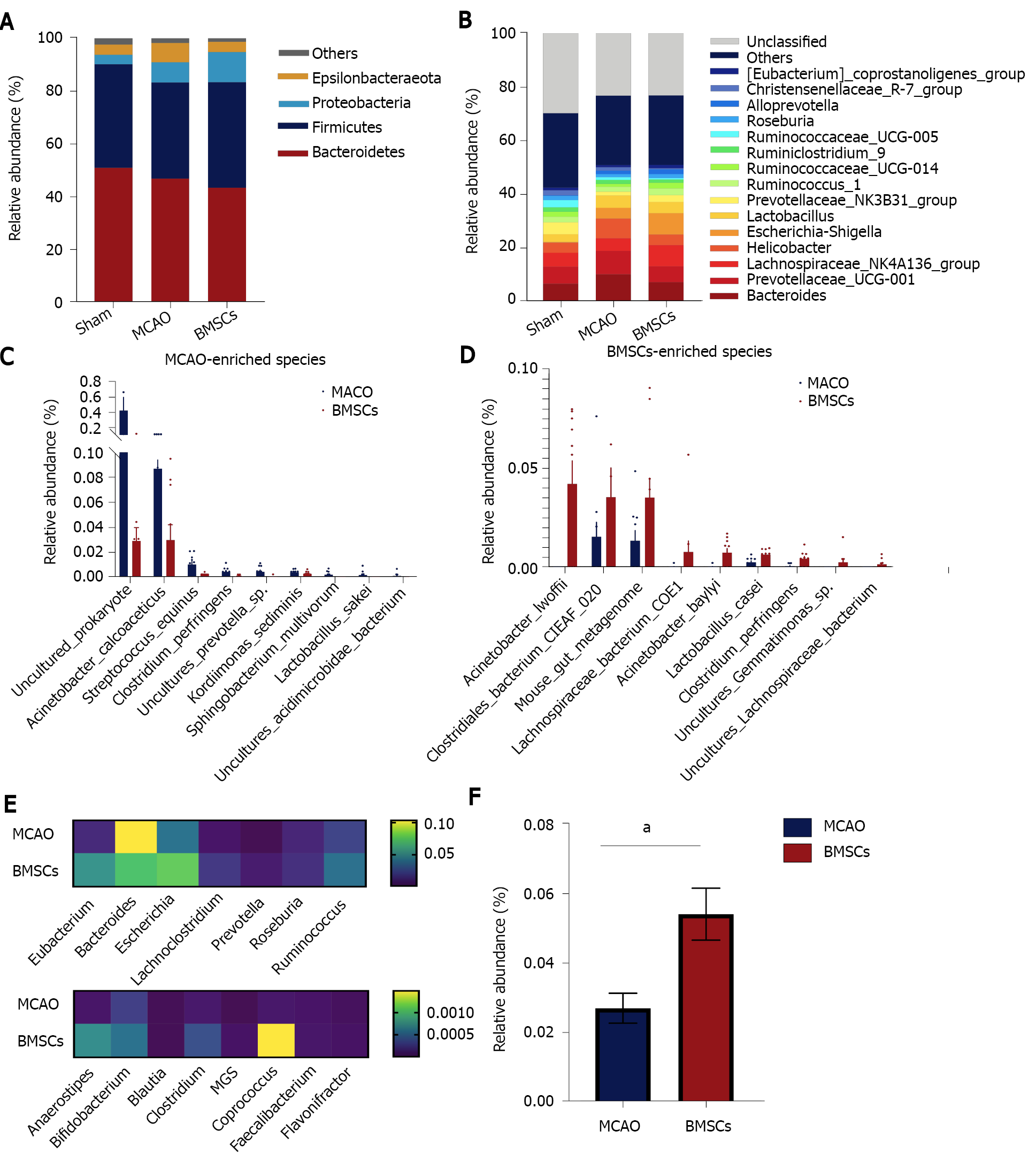
We next sought to explore the effect of treatment with BMSCs on the composition of the microbial structure. Figure 4A shows the abundance of microorganisms in the three groups, in which Bacteroidetes, Firmicutes, Proteobacteria, and Epsilonbacteraeota were the most significant contributors at the microbial phylum level. Compared with the Sham group, MCAO and BMSC increased the relative abundance of Proteobacteria, suggesting significant differences in the gut microbiota structure after stroke. Furthermore, we analyzed the differences in the relative abundance of microorganisms between the three groups at the level of genus (Figure 4B). The data showed that the relative abundance of Ruminococcaceae_UCG−005, Mycoplasma, Ruminiclostridium_5, Oceanimonas, and Marvinbryantia was significantly decreased, and the relative abundance of Escherichia−Shigella, Alloprevotella, Butyricimonas, ASF356, and Enterococcus was increased in the MCAO group compared with the Sham group. BMSC treatment increased the relative abundance of Ruminiclostridium_5 and decreased Butyricimonas and ASF356 at the species level. The dominant bacteria of MCAO and BMSCs are shown separately at the species level in Figure 4C and D. We concluded that species enriched in the BH group included Clostridium spp and Lachnospiraceae spp, which are the potential species to produce short-chain fatty acid (SCFA). A comparison of potential SCFA producing bacteria in the feces revealed that depletion occurred in the MCAO group (Figure 4E). Additionally, it was observed that the relative abundance of Lactobacillus was significantly increased at the genus level after BMSC treatment (Figure 4F).
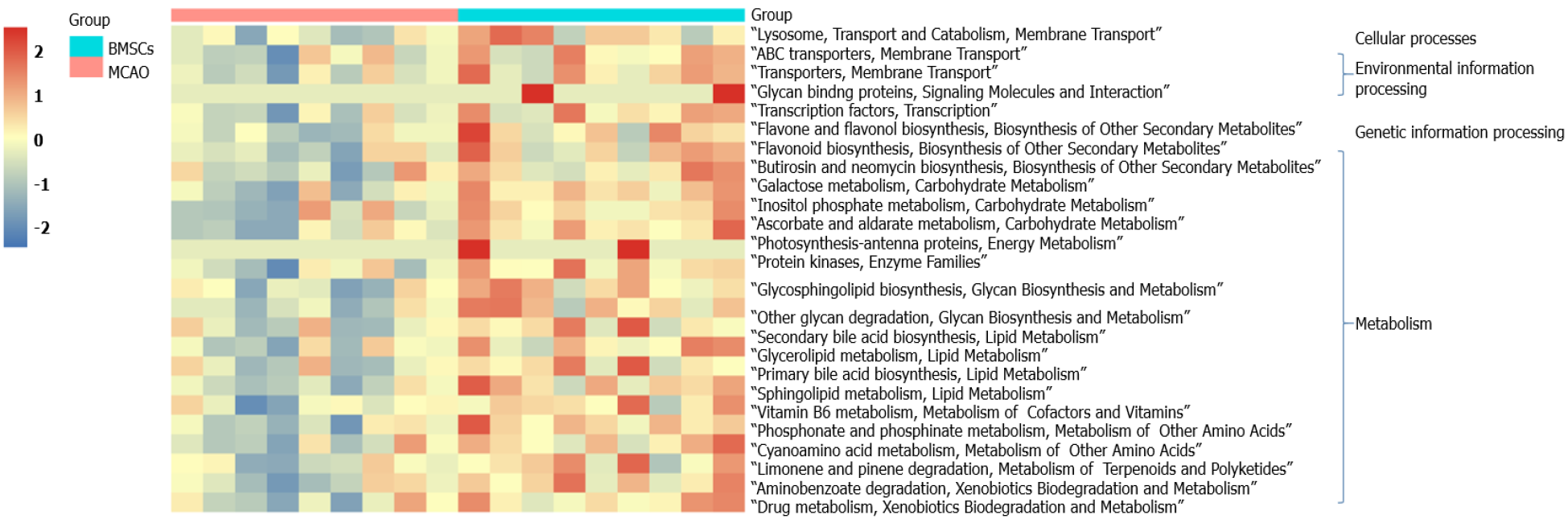
PICRUSt functional prediction analysis was based on 16S sequencing data annotated in the Greengenes database. Using PICRUSt software can predict the composition of known microbial gene functions and thus statistically different functions between groups. In this study, the Kyoto Encyclopedia of Genes and Genomes (KEGG) was used to assess microbial function, and 25 differentially KEGG functional pathways were identified between MCAO and BMSCs (Figure 5). The gut microbiota of BMSCs influenced the pathways of metabolism, including “Carbohydrate Metabolism”, “Biosynthesis of Other Secondary Metabolites”, “Glycan Biosynthesis and Metabolism”, “Lipid Metabolism”, “Metabolism of Cofactors and Vitamins”, “Metabolism of Other Amino Acids”, and “Xenobiotics Biodegradation and Metabolism”. We also found that BMSCs-enriched function pathways were associated with “Membrane Transport”, “Signaling Molecules and Interaction”, “Transport and Catabolism”, and “Transcription”.
For the first time, this study showed changes in gut microbiota after ischemic stroke treatment using BMSCs. BMSCs disrupted the composition and structure of gut microbiota, thereby affecting metabolic pathways in ischemic stroke.
Evidence from basic and clinical studies show that BMSCs can effectively treat patients with ischemic stroke[24]. Transplantation of BMSCs significantly enhances neurological functions after stroke[25], consistent with our results. We established that treatment with BMSCs significantly reduced mNSS scores and enhanced balance, coordination abilities, and learning memory in rats. Notably, cerebral ischemia caused neuronal damage in the hippocampus, striatum, thalamus, and cerebral and cerebellar cortices, with the CA1 region of the hippocampus being one of the most sensitive brain regions. Nissl staining revealed serious neuronal damage in rats after ischemic stroke, which explains memory impairment in the Morris water maze test. In contrast, BMSCs effectively protected the nerve cells.
Studies have confirmed complex interactions between gut microbiota and stroke. Xia et al[26] reported that Parabacteroides, Oscillospira, and Enterobacteriaceae among others were enriched in stroke patients, whereas Prevotella, Roseburia, and Fecalibacterium were enriched in healthy individuals[26]. In stroke patients, dysbiosis is closely associated with metabolism and inflammation. Besides, a specific genus of gut microbiota and associated metabolites are used as potential indicators for stroke prediction and prognosis[13,27]. In stroke animal models, similar alterations in gut microbiota have been detected. Singh et al[9] found that the most abundant phyla of Firmicutes, Bacteroidetes, and Actinobacteria overgrew in MCAO mice[9]. Chen et al[28] reported that after stroke, rats exhibited an increase in the abundance of opportunistic pathogens, including Alistipes, Bacteroides, Klebsiella, Shuttleworthia, Haemophilus, Fusobacterium, Faecalibacterium, Proteus, and Papillibacter[28]. After transplantation of BMSCs, we analyzed the changes in gut microbiota to investigate the role of gut microbiota in post-stroke rats. We found that BMSCs did not alter the α-diversity and structure of gut microbiota after stroke. Further assessments of the composition of microbiota structure suggested that BMSCs significantly increased the abundance of potential SCFA-producing bacteria.
Lachnospiraceae and Clostridium are the main groups of SCFA-producing bacteria[29]. For mammals, SCFA is a critical gut microbial metabolite. It can be used as a substrate for the metabolism of cholesterol, glucose, and lipids, which provide nearly 10% of daily caloric requirements[30]. Besides, it achieves its anti-inflammatory effects by activating G protein-coupled receptors (GPCR) to regulate T cells[31]. Additionally, SCFA protects and repairs the intestinal mucosal barrier by secreting mucus and stimulating tight junction protein expression[32].
The abundance of Lactobacillus has been shown to be significantly increased in cerebral infarction patients[33]. Interestingly, we found a significantly high abundance of Lactobacillus in the fecal matter of the BMSCs group. Bourriaud et al[34] realized that butyrate-producing bacteria ferment lactic acid to produce butyrate, which reduces inflammatory responses, thereby protecting the injured brain[34]. Given that BMSCs increase the abundance of potential SCFA-producing bacteria, an increase in Lactobacillus leads to the production of more lactic acid to be fermented to butyrate, thereby improving neuroinflammation during stroke.
This is the first study to elucidate on alterations in gut microbiota after BMSC treatment in an ischemic stroke condition. We found that BMSCs potentially improve neurological damage after stroke by regulating gut microbiota. This provides a basis for future research into the role of BMSCs from the perspective of the "gut-brain axis".
Ischemic stroke is a highly lethal and disabling disease that has a severe impact on the quality of life of patients. Gut microbiota is closely related to the treatment and prognosis of stroke. The improvement of neurological function by bone marrow mesenchymal stem cells (BMSCs) may be related to the regulation of gut microbiota.
Many studies have shown that gut microbiota plays an important role in immunity after stroke through the gut-brain axis.
To observe the regulation of gut microbiota after BMSC treatment.
Rats were divided into three groups [Sham, middle cerebral artery occlusion (MCAO), and BMSCs]. Recovery of neurological function in rats after BMSC transplantation was observed by the modified Neurological Severity Scores (mNSS), beam walking test, and Morris water maze test. Pathological observation of hippocampal neuronal cells was conducted by Nissl staining. 16S rDNA sequencing was used to analyze the composition of gut microbiota.
Transplantation of BMSCs significantly reduced mNSS scores (P < 0.01), and improved balance and coordination (P < 0.01), learning, and memory in rats. The structure of the C1 region of the hippocampus was clear and necrotic cells were significantly reduced after the intervention of BMSCs. Compared with the MCAO group, BMSCs effectively increased the relative abundance of short-chain fatty acid-producing bacteria and Lactobacillus in feces.
Transplantation of BMSCs can regulate gut microbiota, which provides a potential therapeutic mechanism for stroke treatment.
We demonstrated the modulatory effect of BMSCs on the gut microbiota after stroke, which provided an experimental basis for elucidating the gut-brain axis.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Cell and tissue engineering
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Jin W S-Editor: Fan JR L-Editor: Wang TQ P-Editor: Fan JR
| 1. | GBD 2016 Neurology Collaborators. Global, regional, and national burden of neurological disorders, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18:459-480. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3259] [Cited by in RCA: 2793] [Article Influence: 465.5] [Reference Citation Analysis (1)] |
| 2. | Virani SS, Alonso A, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Delling FN, Djousse L, Elkind MSV, Ferguson JF, Fornage M, Khan SS, Kissela BM, Knutson KL, Kwan TW, Lackland DT, Lewis TT, Lichtman JH, Longenecker CT, Loop MS, Lutsey PL, Martin SS, Matsushita K, Moran AE, Mussolino ME, Perak AM, Rosamond WD, Roth GA, Sampson UKA, Satou GM, Schroeder EB, Shah SH, Shay CM, Spartano NL, Stokes A, Tirschwell DL, VanWagner LB, Tsao CW; American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. Heart Disease and Stroke Statistics-2020 Update: A Report From the American Heart Association. Circulation. 2020;141:e139-e596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3254] [Cited by in RCA: 5451] [Article Influence: 1090.2] [Reference Citation Analysis (1)] |
| 3. | Campbell BCV, De Silva DA, Macleod MR, Coutts SB, Schwamm LH, Davis SM, Donnan GA. Ischaemic stroke. Nat Rev Dis Primers. 2019;5:70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 510] [Cited by in RCA: 1050] [Article Influence: 175.0] [Reference Citation Analysis (0)] |
| 4. | Emberson J, Lees KR, Lyden P, Blackwell L, Albers G, Bluhmki E, Brott T, Cohen G, Davis S, Donnan G, Grotta J, Howard G, Kaste M, Koga M, von Kummer R, Lansberg M, Lindley RI, Murray G, Olivot JM, Parsons M, Tilley B, Toni D, Toyoda K, Wahlgren N, Wardlaw J, Whiteley W, del Zoppo GJ, Baigent C, Sandercock P, Hacke W; Stroke Thrombolysis Trialists' Collaborative Group. Effect of treatment delay, age, and stroke severity on the effects of intravenous thrombolysis with alteplase for acute ischaemic stroke: a meta-analysis of individual patient data from randomised trials. Lancet. 2014;384:1929-1935. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1556] [Cited by in RCA: 1851] [Article Influence: 168.3] [Reference Citation Analysis (0)] |
| 5. | Saver JL, Goyal M, van der Lugt A, Menon BK, Majoie CB, Dippel DW, Campbell BC, Nogueira RG, Demchuk AM, Tomasello A, Cardona P, Devlin TG, Frei DF, du Mesnil de Rochemont R, Berkhemer OA, Jovin TG, Siddiqui AH, van Zwam WH, Davis SM, Castaño C, Sapkota BL, Fransen PS, Molina C, van Oostenbrugge RJ, Chamorro Á, Lingsma H, Silver FL, Donnan GA, Shuaib A, Brown S, Stouch B, Mitchell PJ, Davalos A, Roos YB, Hill MD; HERMES Collaborators. Time to Treatment With Endovascular Thrombectomy and Outcomes From Ischemic Stroke: A Meta-analysis. JAMA. 2016;316:1279-1288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1307] [Cited by in RCA: 1575] [Article Influence: 175.0] [Reference Citation Analysis (0)] |
| 6. | Boltze J, Modo MM, Mays RW, Taguchi A, Jolkkonen J, Savitz SI; STEPS 4 Consortium. Stem Cells as an Emerging Paradigm in Stroke 4: Advancing and Accelerating Preclinical Research. Stroke. 2019;50:3299-3306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 74] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 7. | Dabrowska S, Andrzejewska A, Lukomska B, Janowski M. Neuroinflammation as a target for treatment of stroke using mesenchymal stem cells and extracellular vesicles. J Neuroinflammation. 2019;16:178. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 123] [Cited by in RCA: 219] [Article Influence: 36.5] [Reference Citation Analysis (0)] |
| 8. | Lee JY, Tuazon JP, Ehrhart J, Sanberg PR, Borlongan CV. Gutting the brain of inflammation: A key role of gut microbiome in human umbilical cord blood plasma therapy in Parkinson's disease model. J Cell Mol Med. 2019;23:5466-5474. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 9. | Singh V, Roth S, Llovera G, Sadler R, Garzetti D, Stecher B, Dichgans M, Liesz A. Microbiota Dysbiosis Controls the Neuroinflammatory Response after Stroke. J Neurosci. 2016;36:7428-7440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 307] [Cited by in RCA: 535] [Article Influence: 66.9] [Reference Citation Analysis (0)] |
| 10. | Houlden A, Goldrick M, Brough D, Vizi ES, Lénárt N, Martinecz B, Roberts IS, Denes A. Brain injury induces specific changes in the caecal microbiota of mice via altered autonomic activity and mucoprotein production. Brain Behav Immun. 2016;57:10-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 266] [Article Influence: 29.6] [Reference Citation Analysis (0)] |
| 11. | Spychala MS, Venna VR, Jandzinski M, Doran SJ, Durgan DJ, Ganesh BP, Ajami NJ, Putluri N, Graf J, Bryan RM, McCullough LD. Age-related changes in the gut microbiota influence systemic inflammation and stroke outcome. Ann Neurol. 2018;84:23-36. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 165] [Cited by in RCA: 319] [Article Influence: 45.6] [Reference Citation Analysis (0)] |
| 12. | Li XJ, You XY, Wang CY, Li XL, Sheng YY, Zhuang PW, Zhang YJ. Bidirectional Brain-gut-microbiota Axis in increased intestinal permeability induced by central nervous system injury. CNS Neurosci Ther. 2020;26:783-790. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 64] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 13. | Yamashiro K, Tanaka R, Urabe T, Ueno Y, Yamashiro Y, Nomoto K, Takahashi T, Tsuji H, Asahara T, Hattori N. Gut dysbiosis is associated with metabolism and systemic inflammation in patients with ischemic stroke. PLoS One. 2017;12:e0171521. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 127] [Cited by in RCA: 189] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 14. | Yin J, Liao SX, He Y, Wang S, Xia GH, Liu FT, Zhu JJ, You C, Chen Q, Zhou L, Pan SY, Zhou HW. Dysbiosis of Gut Microbiota With Reduced Trimethylamine-N-Oxide Level in Patients With Large-Artery Atherosclerotic Stroke or Transient Ischemic Attack. J Am Heart Assoc. 2015;4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 405] [Cited by in RCA: 486] [Article Influence: 48.6] [Reference Citation Analysis (0)] |
| 15. | Stanley D, Mason LJ, Mackin KE, Srikhanta YN, Lyras D, Prakash MD, Nurgali K, Venegas A, Hill MD, Moore RJ, Wong CH. Translocation and dissemination of commensal bacteria in post-stroke infection. Nat Med. 2016;22:1277-1284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 327] [Article Influence: 36.3] [Reference Citation Analysis (0)] |
| 16. | Benakis C, Brea D, Caballero S, Faraco G, Moore J, Murphy M, Sita G, Racchumi G, Ling L, Pamer EG, Iadecola C, Anrather J. Commensal microbiota affects ischemic stroke outcome by regulating intestinal γδ T cells. Nat Med. 2016;22:516-523. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 515] [Cited by in RCA: 806] [Article Influence: 89.6] [Reference Citation Analysis (0)] |
| 17. | Chen R, Xu Y, Wu P, Zhou H, Lasanajak Y, Fang Y, Tang L, Ye L, Li X, Cai Z, Zhao J. Transplantation of fecal microbiota rich in short chain fatty acids and butyric acid treat cerebral ischemic stroke by regulating gut microbiota. Pharmacol Res. 2019;148:104403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 263] [Article Influence: 43.8] [Reference Citation Analysis (0)] |
| 18. | Sadler R, Cramer JV, Heindl S, Kostidis S, Betz D, Zuurbier KR, Northoff BH, Heijink M, Goldberg MP, Plautz EJ, Roth S, Malik R, Dichgans M, Holdt LM, Benakis C, Giera M, Stowe AM, Liesz A. Short-Chain Fatty Acids Improve Poststroke Recovery via Immunological Mechanisms. J Neurosci. 2020;40:1162-1173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 232] [Article Influence: 46.4] [Reference Citation Analysis (0)] |
| 19. | Jackman K, Kunz A, Iadecola C. Modeling focal cerebral ischemia in vivo. Methods Mol Biol. 2011;793:195-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 48] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 20. | Chen J, Sanberg PR, Li Y, Wang L, Lu M, Willing AE, Sanchez-Ramos J, Chopp M. Intravenous administration of human umbilical cord blood reduces behavioral deficits after stroke in rats. Stroke. 2001;32:2682-2688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 940] [Cited by in RCA: 1014] [Article Influence: 42.3] [Reference Citation Analysis (0)] |
| 22. | Chao A, Chazdon RL, Colwell RK, Shen TJ. A new statistical approach for assessing similarity of species composition with incidence and abundance data. Ecology Letters. 2005;8:148-159. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1256] [Cited by in RCA: 797] [Article Influence: 38.0] [Reference Citation Analysis (0)] |
| 23. | Langille MG, Zaneveld J, Caporaso JG, McDonald D, Knights D, Reyes JA, Clemente JC, Burkepile DE, Vega Thurber RL, Knight R, Beiko RG, Huttenhower C. Predictive functional profiling of microbial communities using 16s rRNA marker gene sequences. Nat Biotechnol. 2013;31:814-821. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5694] [Cited by in RCA: 6283] [Article Influence: 523.6] [Reference Citation Analysis (0)] |
| 24. | Bang OY, Kim EH, Cha JM, Moon GJ. Adult Stem Cell Therapy for Stroke: Challenges and Progress. J Stroke. 2016;18:256-266. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 75] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 25. | Cai Y, Liu W, Lian L, Xu Y, Bai X, Xu S, Zhang J. Stroke treatment: Is exosome therapy superior to stem cell therapy? Biochimie. 2020;179:190-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 45] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 26. | Xia GH, You C, Gao XX, Zeng XL, Zhu JJ, Xu KY, Tan CH, Xu RT, Wu QH, Zhou HW, He Y, Yin J. Stroke Dysbiosis Index (SDI) in Gut Microbiome Are Associated With Brain Injury and Prognosis of Stroke. Front Neurol. 2019;10:397. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 146] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 27. | Li N, Wang X, Sun C, Wu X, Lu M, Si Y, Ye X, Wang T, Yu X, Zhao X, Wei N. Change of intestinal microbiota in cerebral ischemic stroke patients. BMC Microbiol. 2019;19:191. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 200] [Article Influence: 33.3] [Reference Citation Analysis (0)] |
| 28. | Chen H, Nwe PK, Yang Y, Rosen CE, Bielecka AA, Kuchroo M, Cline GW, Kruse AC, Ring AM, Crawford JM, Palm NW. A Forward Chemical Genetic Screen Reveals Gut Microbiota Metabolites That Modulate Host Physiology. Cell. 2019;177:1217-1231.e18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 245] [Article Influence: 40.8] [Reference Citation Analysis (0)] |
| 29. | Chen R, Wu P, Cai Z, Fang Y, Zhou H, Lasanajak Y, Tang L, Ye L, Hou C, Zhao J. Puerariae Lobatae Radix with chuanxiong Rhizoma for treatment of cerebral ischemic stroke by remodeling gut microbiota to regulate the brain-gut barriers. J Nutr Biochem. 2019;65:101-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 127] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 30. | LeBlanc JG, Chain F, Martín R, Bermúdez-Humarán LG, Courau S, Langella P. Beneficial effects on host energy metabolism of short-chain fatty acids and vitamins produced by commensal and probiotic bacteria. Microb Cell Fact. 2017;16:79. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 368] [Cited by in RCA: 566] [Article Influence: 70.8] [Reference Citation Analysis (0)] |
| 31. | Al Nabhani Z, Dulauroy S, Marques R, Cousu C, Al Bounny S, Déjardin F, Sparwasser T, Bérard M, Cerf-Bensussan N, Eberl G. A Weaning Reaction to Microbiota Is Required for Resistance to Immunopathologies in the Adult. Immunity. 2019;50:1276-1288.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 241] [Cited by in RCA: 412] [Article Influence: 68.7] [Reference Citation Analysis (0)] |
| 32. | Zhao L, Yang L, Guo Y, Xiao J, Zhang J, Xu S. New Insights into Stroke Prevention and Treatment: Gut Microbiome. Cell Mol Neurobiol. 2021;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 33. | Li H, Zhang X, Pan D, Liu Y, Yan X, Tang Y, Tao M, Gong L, Zhang T, Woods CR, Du Y, Gao R, Qin H. Dysbiosis characteristics of gut microbiota in cerebral infarction patients. Transl Neurosci. 2020;11:124-133. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 34. | Bourriaud C, Robins RJ, Martin L, Kozlowski F, Tenailleau E, Cherbut C, Michel C. Lactate is mainly fermented to butyrate by human intestinal microfloras but inter-individual variation is evident. J Appl Microbiol. 2005;99:201-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 245] [Article Influence: 12.3] [Reference Citation Analysis (0)] |