Published online Nov 26, 2021. doi: 10.4252/wjsc.v13.i11.1783
Peer-review started: May 5, 2021
First decision: June 23, 2021
Revised: June 25, 2021
Accepted: October 15, 2021
Article in press: October 15, 2021
Published online: November 26, 2021
Processing time: 203 Days and 16.5 Hours
Adipose-derived stem cells (ASCs) have been increasingly explored for cell-based medicine because of their numerous advantages in terms of easy availability, high proliferation rate, multipotent differentiation ability and low immunogenicity. In this respect, they have been widely investigated in the last two decades to develop therapeutic strategies for a variety of human pathologies including eye disease. In ocular diseases involving the retina, various cell types may be affected, such as Müller cells, astrocytes, photoreceptors and retinal pigment epithelium (RPE), which plays a fundamental role in the homeostasis of retinal tissue, by secreting a variety of growth factors that support retinal cells.
To test ASC neural differentiation using conditioned medium (CM) from an RPE cell line (ARPE-19).
ASCs were isolated from adipose tissue, harvested from the subcutaneous region of healthy donors undergoing liposuction procedures. Four ASC culture condi
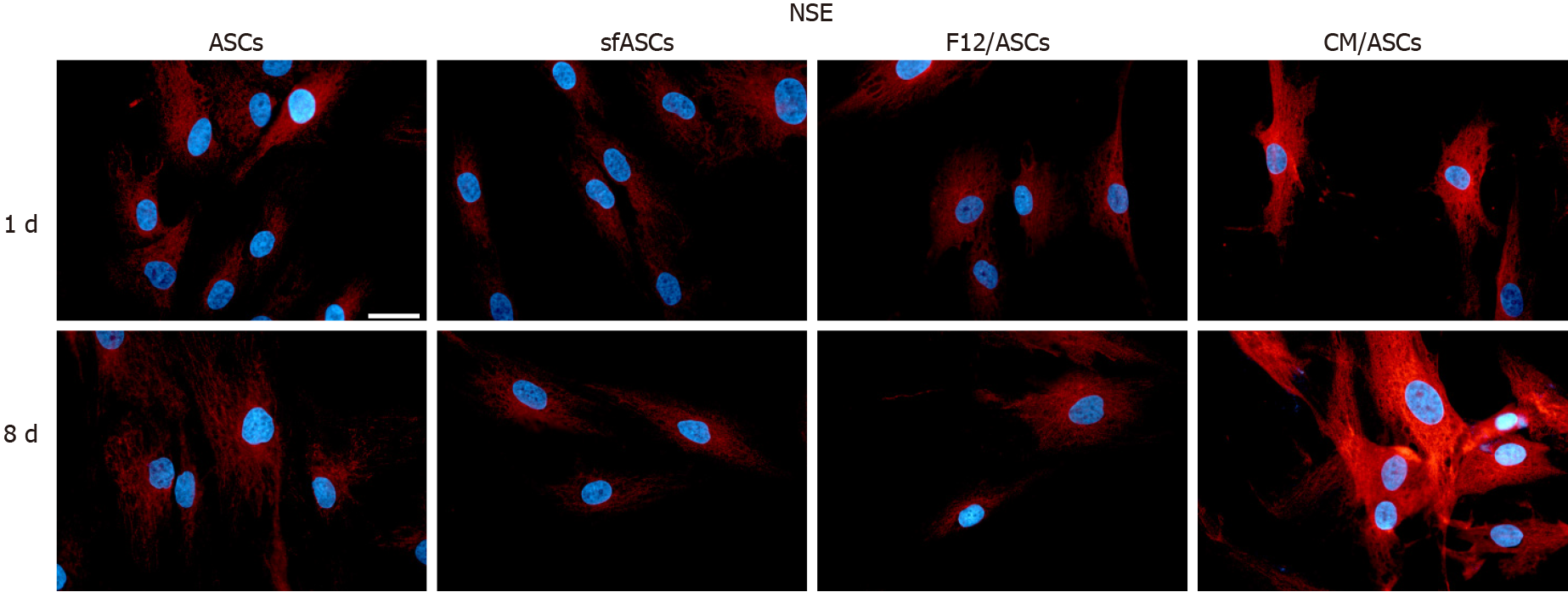
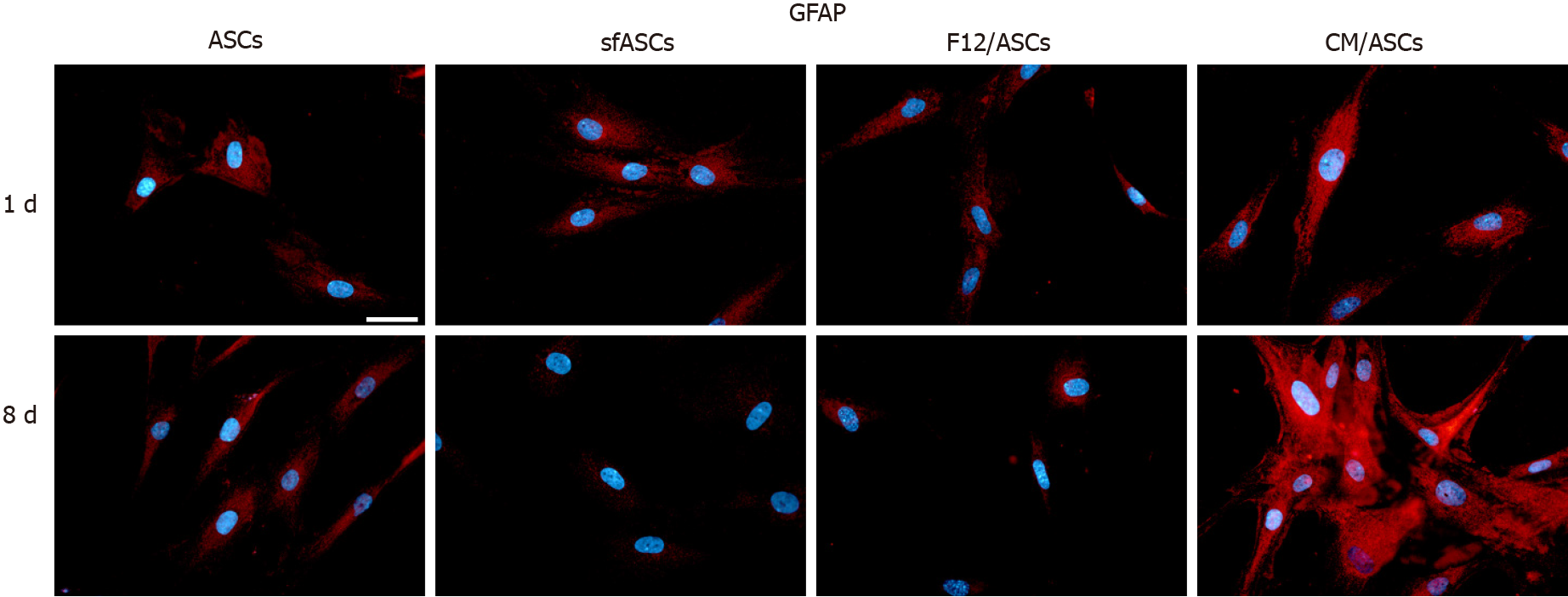
Depending on the culture medium, ASC proliferation rate and viability showed some significant differences. Overall, less dense populations were observed in serum-free cultures, except for ASCs cultured in ARPE-19 serum-free CM. Moreover, a different cell morphology was seen in these cultures after 8 d of treatment, with more elongated cells, often showing cytoplasmic ramifications. Immunofluorescence results and western blot analysis were indicative of ASC neural differentiation. In fact, basal levels of neural markers detected under control conditions significantly increased when cells were cultured in ARPE-19 CM. Specifically, neural marker overexpression was more marked at 8 d. The most evident increase was observed for NSE and GFAP, a modest increase was observed for nestin, and less relevant changes were observed for PGP9.5.
The presence of growth factors produced by ARPE-19 cells in tissue culture induces ASCs to express neural differentiation markers typical of the neuronal and glial cells of the retina.
Core Tip: Neural-like differentiation of adipose-derived stem cells (ASCs) was tested using a conditioned medium from ARPE-19 cells, a cell line derived from human retinal pigment epithelium. Following this treatment, the expression of typical glial and neuronal markers increased in a time-dependent manner. Neural-like differentiated ASCs may represent a valuable tool for cell-based therapeutic approaches in the field of regenerative medicine for the treatment of eye diseases.
- Citation: Mannino G, Cristaldi M, Giurdanella G, Perrotta RE, Lo Furno D, Giuffrida R, Rusciano D. ARPE-19 conditioned medium promotes neural differentiation of adipose-derived mesenchymal stem cells. World J Stem Cells 2021; 13(11): 1783-1796
- URL: https://www.wjgnet.com/1948-0210/full/v13/i11/1783.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v13.i11.1783
Mesenchymal stem cells (MSCs) have been widely investigated in the last two decades in order to develop cell-based therapeutic strategies for a variety of human pathologies including eye disease[1-4]. Based on their multipotent differentiation ability, MSCs of different sources (bone marrow, adipose tissue, umbilical cord) have been successfully differentiated not only into cells of mesodermal origin, but also into cells of different derivation, such as epithelial and neural cells[5,6]. In particular, adipose-derived stem cells (ASCs) have been increasingly explored because they offer numerous advantages: They can be obtained in a large amount from subcutaneous tissue with minimal discomfort for the donors; they feature a high proliferation rate; they can also be used for allogeneic transplantation because of their low immunogenicity.
In a recent study, we were able to induce pericyte-like differentiated human ASCs[7], suitable for the treatment of diabetic retinopathy, characterized by extensive pericyte loss. However, several other cell types may be affected in retinal diseases, such as Müller cells[8], astrocytes[9], and photoreceptors[4]. Moreover, the visual loss occurring in diabetic retinopathy or in glaucoma is related to retinal sensory dysfunction, mainly due to retinal ganglion cell (RGC) loss.
The purpose of this study was to test whether growth of ASCs in serum free tissue culture medium conditioned by retinal pigment epithelium (RPE) could trigger their neural differentiation.
RPE is a specialized epithelium lying between the neural retina and the capillary lamina of the choroid[10]. Early in development, RPE is required for the normal growth of the eye. However, it is also fundamental to maintain the correct retina homeostasis also in adults[11].
It has multiple functions, such as absorption of light and protection against photo-oxidation, transport of nutrients, water, and ions. Other than playing a crucial role in the constitution of the outer blood-retina barrier, RPE cells govern differentiation and regeneration of photoreceptors and retinal progenitor cells through a variety of growth factors within the retinal stem cell niche[12]. RPE-secreted factors are able to rescue degenerating photoreceptors by prolonging their survival. In addition, they can transdifferentiate and give rise to photoreceptors, bipolar or multipolar (ganglionic and amacrine) cells[13]. In addition, RPE conditioned medium (CM) drives differentiation of retinal progenitor cells towards photoreceptors, depending on the cell density.
Indeed, the effects of human or porcine RPE cell CM on ASCs were tested in a work by Vossmerbaeumer et al[14], reporting a possible ASC differentiation toward the RPE lineage, as suggested by the increased expression of typical RPE markers such as bestrophin, cytokeratins 8 and 18, and RPE 65. However, a systematic study on neural marker expression was not carried out.
In the present work, ASC neural differentiation was induced by culture in CM from ARPE-19, a spontaneously arising cell line with a normal karyotype derived from human retinal pigmented epithelium[15]. In this way, ASCs would grow in an in vitro environment resembling the environment existing in the normal eye, without addition of chemical agents that might be potentially toxic. ASC differentiation was verified by immunocytochemical techniques and western blot analysis for nestin, neuronal specific enolase (NSE), protein gene product (PGP) 9.5 and glial fibrillary acidic protein (GFAP).
The human retinal pigment epithelial cell line (ARPE-19) was purchased from the American Type Culture Collection (CRL-2302™) and cultured at 37 °C in Dulbecco's Modified Eagle Medium (DMEM)/F12 medium (ATCC 30-2006, Washington, DC, United States) containing 10% phosphate buffered saline (FBS) and 1% penicillin/ streptomycin. For CM preparation, ARPE-19 cells were seeded and cultured until 80% confluence was reached, usually after 24 h, when the medium was replaced with fresh, serum-free, DMEM/F12. The day after, the medium was collected, filtered to remove debris and floating cells, and stored at −20 °C before further use.
ASCs were isolated from adipose tissue, harvested from the subcutaneous region of four healthy female donors (32–38-years-old) undergoing liposuction procedures at the Cannizzaro Hospital, Catania (Italy). Lipoaspirate was obtained after donors signed a written informed consent to allow the use of the adipose tissue for experimental investigations, which were carried out in accordance with the Declaration of Helsinki. The protocol was approved by the local ethics committee (Comitato etico Catania1; Authorization n. 155/2018/PO).
Red blood cells and debris were removed by washing the raw lipoaspirate (50–100 mL) with sterile PBS (Invitrogen). It was then incubated for 3 h at 37 °C with DMEM containing 0.075% of type I collagenase (GIBCO 17100017, Thermo Fisher Scientific, Waltham, MA, United States). The collagenase was then inactivated by adding an equal volume of DMEM (Lonza 12-707F, Basel, CH) containing 10% FBS (DMEM/FBS) and the lipoaspirate was centrifuged for 10 min at 1200 rpm. After pellet resuspension in PBS, cells were filtered through a 100 μm nylon cell strainer (Falcon BD Biosciences, Milan, Italy). Following a final centrifugation/resuspension procedure, cells were plated in T75 culture flasks (Falcon BD Biosciences) with DMEM/FBS, 1% penici
The MSC nature of ASCs used in the present study had been verified in previous studies, where cells of the same stock had been investigated[7]. Virtually the entire population (above 98% of cells) was immunopositive for typical MSC markers (CD44, CD73, CD90, and CD105), whereas only a few cells (less than 1%) were immunostained for typical hematopoietic stem cell markers (CD14, CD34, and CD45).
For the present investigation, four groups of ASC cultures were prepared. In the first group ASCs were maintained in DMEM/FBS (ASCs); in the second group, ASCs were cultured in serum-free DMEM (sfASCs); in the third group ASCs were cultured in serum-free DMEM/F12 (F12/ASCs); and in the fourth group, ASCs were grown in ARPE-19 CM (CM/ASCs). From each group, some samples were processed at 1 d of culture; other samples were processed at day 4; further samples were processed at day 8. At each time point, cell proliferation and viability assays, fluorescence immunocytochemistry and western blot procedures were carried out for specific signal detection.
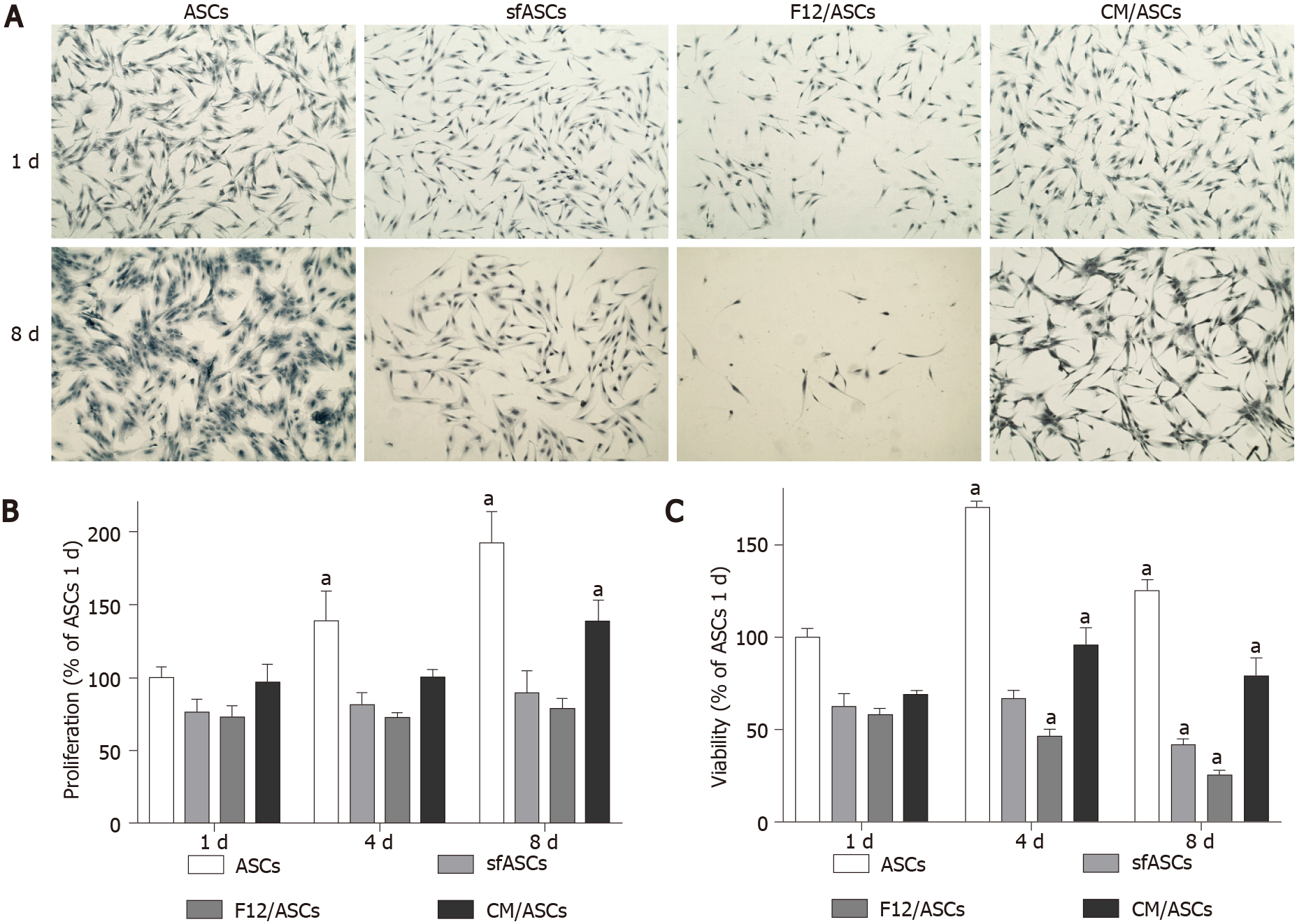
The crystal violet assay was used to evaluate the proliferation rate of ASCs of each group at 1, 4 and 8 d of culture. To this purpose, cells were stained with 0.5% crystal violet solution in 20% methanol for 10 min. After photomicrographs were taken (Leica Microscope), the crystal violet was solubilized in 1% sodium dodecyl sulphate (SDS) and absorbance values were measured at 570 nm with a microplate reader (Synergy 2-BioTek). Each assay was carried out in triplicate, from three independent experiments.
Cell viability was evaluated in ASCs of each group at 1, 4 and 8 d of culture. To this purpose, 3-[4,5-dimethylthiazol-2-y l]-2,5-diphenyl tetrasodium bromide (MTT assay, Chemicon, Temecula, CA, United States) was added to each sample and incubated for 3 h at 37 °C. The supernatant was then removed and 100 μL Dimethyl Sulfoxide (DMSO) were used to dissolve the precipitate. Absorbance values were determined at 570 nm in a plate reader (Synergy 2-BioTek). Each assay was carried out in triplicate, from three independent experiments.
Immunocytochemical staining was carried out following a protocol previously described[6]. Briefly, cells were washed with PBS, fixed with 4% paraformaldehyde and incubated for 30 min with a 5% solution of normal goat serum (Sigma–Aldrich) in PBS containing 0.1% Triton (Sigma–Aldrich). They were then exposed overnight at 4 °C to primary antibodies: Mouse anti-nestin (1:100, Abcam, ab22035 Cambridge, MA, United States); mouse anti-NSE (1:100, Abcam ab16808); rabbit anti-PGP9.5 (1:100, Abcam ab108986), and mouse anti-GFAP (1:100; Novus Biologicals NB120-10062, Centennial, CO, United States). The following day, cells were washed with PBS and incubated for 60 min at room temperature with secondary antibodies conjugated to different fluorochromes: FITC conjugated goat anti-rabbit (Abcam ab96899) and/or Cy3-conjugated goat anti-mouse (Abcam ab96880). Finally, DAPI was applied for 10 min to stain cell nuclei. In each experiment, specificity of immunostaining was verified in some samples by omitting the primary antibody. Immunofluorescence was detected using a Leica DMRB Fluorescence Microscope. Digital images were acquired through a 40 × oil objective and a computer-assisted digital camera (Leica DFC 320).
Immunostaining quantification was carried out using the FIJI-ImageJ measure tool (NIH, Bethesda, MD, United States). At each time point, at least three samples of each group were examined. Three digital photomicrographs were randomly selected from each sample. Up to five immunofluorescent cells were analyzed from each photomicrograph. Values were calculated from the average grayscale intensity. For each cell, the integrated density, the cell area and the mean fluorescence value were assessed. Three replicate measurements were performed for each capture region. The same procedure was applied to three different background areas, close to the selected cell. The corrected total cell fluorescence (CTCF) was then calculated, using the following equation:
CTCF = Integrated density - (cell area × background mean fluorescence).
Percentages of immunopositive cells were estimated counting immunostained cells and DAPI-stained nuclei in randomly selected microscopic fields.
Immunoblots were carried out on samples of each treatment group (ASCs, sfASCs, F12/ASCs and CM/ASCs) at day 1, 4 and 8 of growth. Cells were trypsinized, centrifuged and resuspended in RIPA buffer (Life Technologies), in the presence of a protease inhibitor cocktail (Sigma), serine/threonine phosphatase inhibitors (Sigma) and tyrosine protein phosphatase inhibitors (Sigma). Protein concentrations were determined by the BCA protein assay using BSA as the standard. Cell lysates (50 μg protein) were loaded onto 4%-20% SDS-PAGE, blotted and probed for different target proteins.
Membranes were incubated overnight at 4 °C with the same primary antibodies used for immunofluorescence: Mouse anti-nestin (1:1000); rabbit anti-PGP9.5 (1:2000); mouse anti-NSE (1:1500); and mouse anti-GFAP (1:1500). The following day, the membranes were incubated with the respective secondary antibodies (1:2000) for 1 h at room temperature, and the immunocomplexes were detected by the ChemiDocTM Touch Imaging System (BIO-RAD). All blots were checked for equal loading by probing with GAPDH (rabbit, 1:1000; Cell Signaling). Densitometry analysis was performed using free software Image J (NIH, Bethesda, MD, United States).
Statistical analysis was performed by using GraphPad Prism 7.0 (GraphPad Software, La Jolla, CA, United States). For each experimental condition, values are reported as mean ± SD. Differences between samples were assessed using two-way analysis of variance (two-way ANOVA) followed by post hoc Tukey’s multiple comparisons test. P values of 0.05 or less were considered statistically significant. The statistical methods of this study were reviewed by Dr Vincenzo Guardabasso, Specialist in Public Health Statistics, University of Catania, Italy.
Depending on the culture medium, ASCs showed some significant differences. At day 1 (Figure 1A), all samples exhibited the typical fibroblast-like morphology. However, a decrease in population density was observed in serum-free cultures, especially in F12/ASCs. A lower decrease was seen in CM/ASCs. More marked differences were observed at day 8. At this time, a denser cell population was observed in control ASCs, still conserving the same shape as day 1. Moreover, a decreased population density was observed for ASCs cultured under serum-free conditions; however, this was less evident in CM/ASCs. It is worth noting that under the CM/ASC condition, a different cell morphology was apparent, with more elongated cells, often showing cytoplasmic ramifications. Data illustrated in representative pictures are supported by quantitative measurements, for cell proliferation (Figure 1B) and viability (Figure 1C).
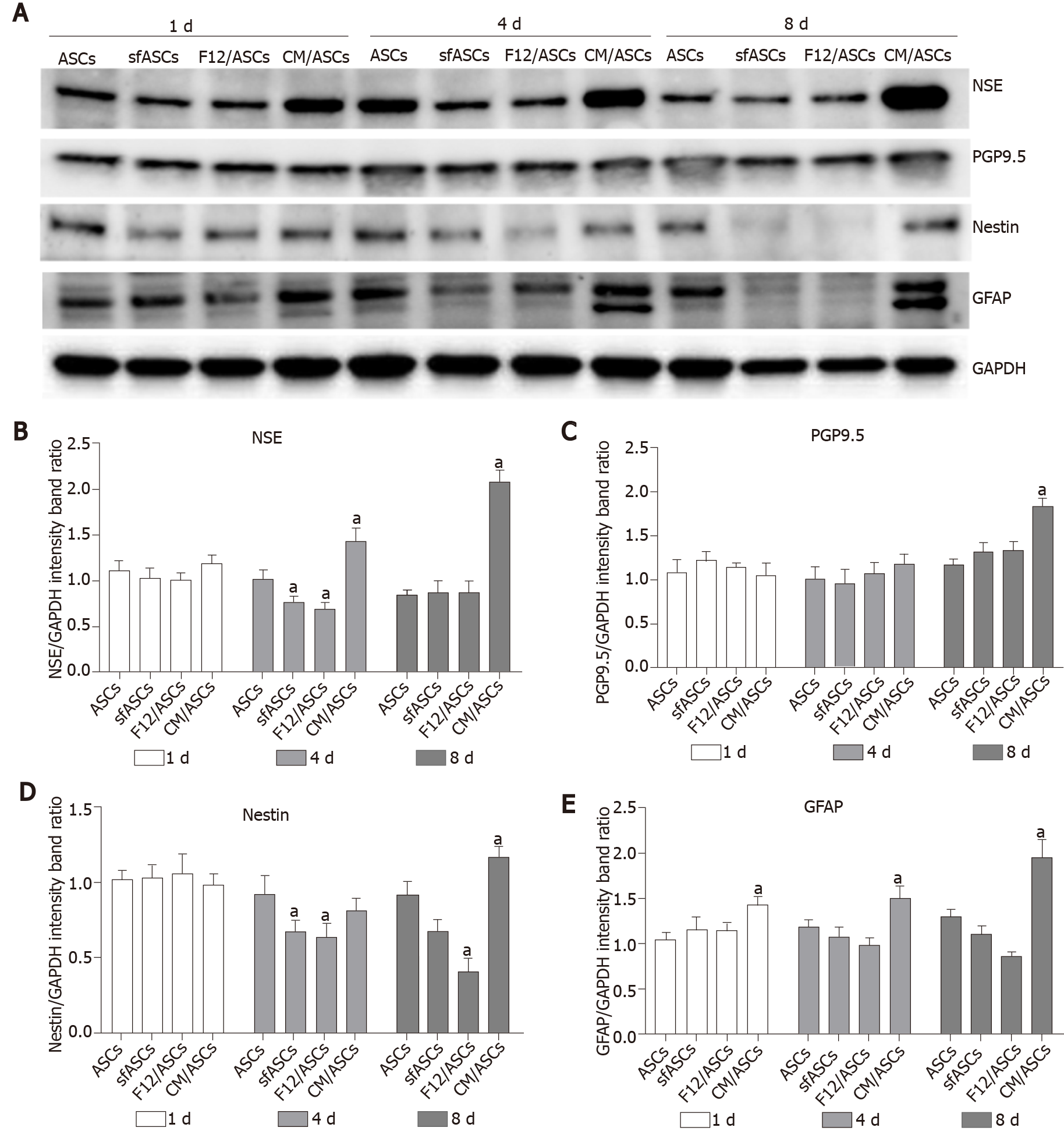
Immunofluorescence results and western blot analyses indicated that a neural differentiation likely occurs when ASCs were cultured in ARPE-19 CM. Overall, although to a different extent, all neural markers increased their expression in a time-dependent fashion.
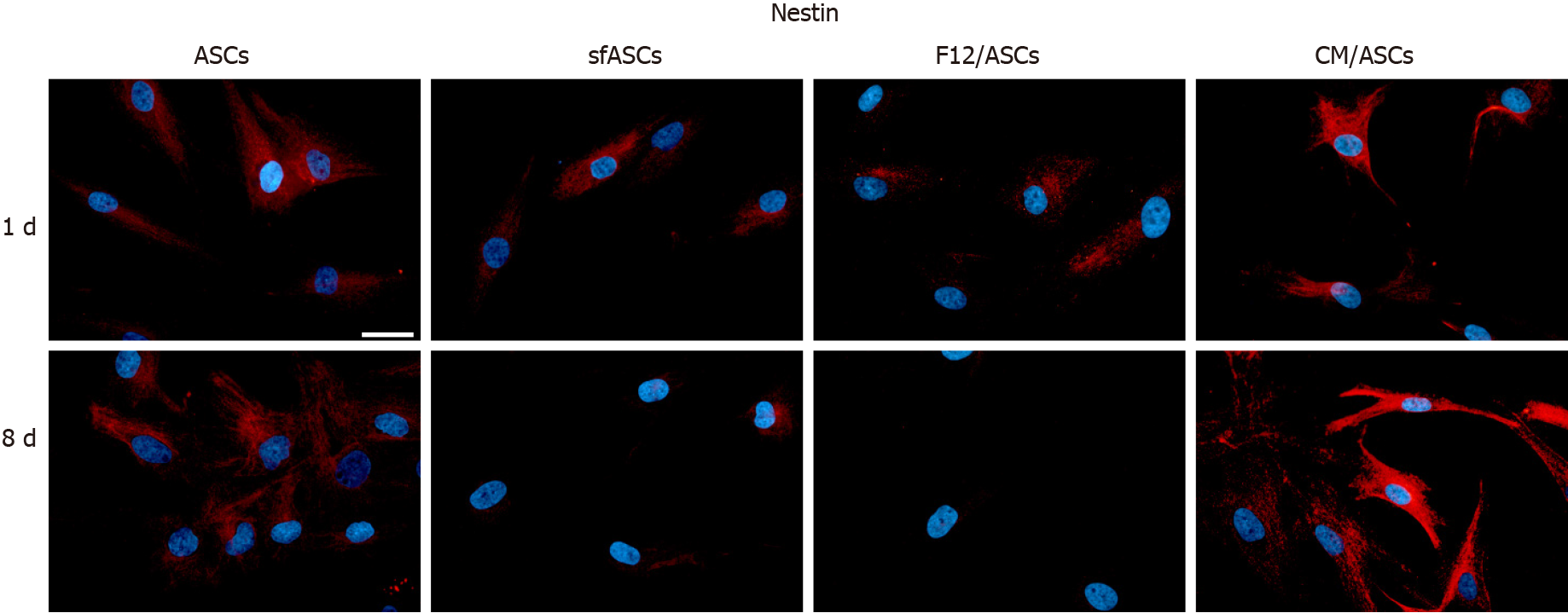
Immunofluorescence photomicrographs (Figure 2) and western blot (Figure 3) analyses revealed that a basal level of nestin could be detected in a considerable portion (62%) of cells in all ASC samples at 1 d of culture (Table 1). At day 4, these basal levels were reduced in serum-free cultures (sfASCs and F12/ASCs), whereas comparable values were maintained in CM/ASCs. At day 8, basal nestin levels were still present in control ASCs, while they were strongly decreased in serum-free-cultures, especially in F12/ASCs. On the contrary, increased nestin levels were observed in CM/ASCs.
| Marker | Day 1 | Day 8 | ||||||
| ASCs | sfASCs | F12/ASCs | CM/ASCs | ASCs | sfASCs | F12/ASCs | CM/ASCs | |
| Nestin | 62 ± 5 | 53 ± 4 | 51 ± 3 | 78 ± 4 | 74 ± 6 | 39 ± 2 | 31 ± 3 | 88 ± 4 |
| NSE | 63 ± 3 | 52 ± 6 | 54 ± 7 | 82 ± 3 | 61 ± 4 | 49 ± 3 | 50 ± 4 | 93 ± 5 |
| PGP9.5 | 68 ± 4 | 65 ± 3 | 66 ± 5 | 75 ± 5 | 78 ± 5 | 62 ± 3 | 67 ± 6 | 89 ± 3 |
| GFAP | 55 ± 7 | 56 ± 5 | 53 ± 4 | 77 ± 3 | 53 ± 6 | 41 ± 5 | 45 ± 7 | 87 ± 5 |
These observations were in agreement with quantitative immunofluorescence estimates (Figure 4). No evident changes were observed at day 1, except for a modest increase in CM/ASCs. At day 8, fluorescence intensity and percentages of immunopositive cells (Table 1) were lower in serum-free cultures, whereas both parameters were increased in CM/ASCs.
A similar trend was observed for NSE expression modifications (Figures 3 and 5). Comparable basal values were detected at day 1 in all ASC samples. At day 4 a decreased NSE expression was observed in sfASCs and F12/ASCs, whereas increased values were measured in CM/ASCs. A further increase was found at day 8 for CM/ASCs. Quantitative immunofluorescence measurements (Figure 4) and percentages of immunopositive cells (Table 1) confirmed that the most evident effects were detected for CM/ASCs, showing a marked increase at day 8.
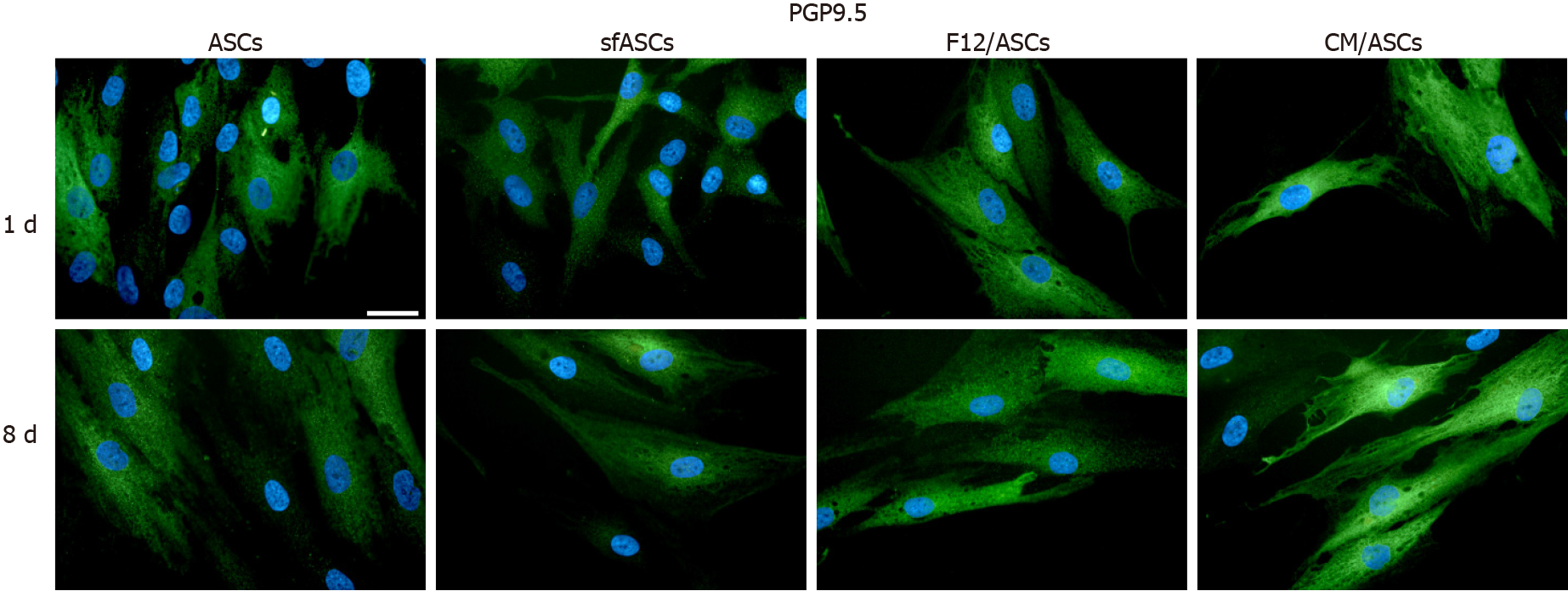
When compared to control ASCs, all detection methods showed that no evident differences were noted between the different samples, except for CM/ASCs at day 8 of treatment (Figures 3, 4 and 6 and Table 1)
Basal GFAP values at day 1 and day 4 were similar in control and serum-free ASCs (Figures 3 and 7), whereas a significant increase was observed in CM/ASCs at both times. This increase was even more evident at day 8, whereas lower values were observed in serum-free cultures (sfASCs and F12/ASCs). These observations largely match the quantitative estimates reported in Figure 4 and Table 1.
As is well known, native ASCs exhibit a variety of cellular markers, some of them belonging to cell lineages quite different from each other. This characteristic is probably related to their multipotent differentiation ability, which is evident by the different cell types that can be obtained following different induction strategies. In fact, a wide range of differentiated cells can be obtained starting from native ASCs; from insulin-producing pancreatic cells[16] to neurons or glial cells[6].
The results presented here show that CM obtained from ARPE-19 can trigger differentiation of ASCs towards a neural-like phenotype. This is not unexpected, since RPE has tight interactions with the neural retina, secreting factors necessary for its homeostasis and function.
RPE derived growth factors include pigment epithelium-derived factor (PEDF), ciliary neurotrophic factor (CNTF)[17], basic fibroblast growth factor (FGF-2), epidermal growth factor (EGF), and nerve growth factor (NGF)[8,12,18]. Both FGF-2 and EGF have been shown to generate retinal neurons from human retinal precursor cells[19]. Secreted into the interphotoreceptor matrix, the neurotrophic factor PEDF induces antiapoptotic, antioxidative, and anti-inflammatory effects. The intraocular injection of PEDF delayed photoreceptor cell degeneration and apoptosis. Moreover, it also acted in neuronal differentiation and survival. PEDF-related effects may explain our observation about the different proliferation rates observed in the various samples examined in the present study. In fact, as already reported for human umbilical cord MSCs, the addition of PEDF significantly reduced apoptosis when cells were cultured in a serum-free medium[20]. In particular, the authors showed that this PEDF-induced apoptosis reduction was due to a decreased p53 expression. This is particularly important since this method allows for a significant cell expansion even in serum-free cultures, thus reducing safety related problems for possible clinical applications. ARPE-19 effects, weakly visible at day 1, was more pronounced at day 4 and, particularly, at day 8.
A panel of neural markers was chosen to verify the differentiating phenotype of ASCs under the described culture conditions. Nestin is an intermediate filament protein that is expressed in the early development stages of the central/peripheral nervous system, muscle and other tissues. During differentiation, it is downregulated and replaced by tissue-specific intermediate filament proteins[21]. PGP9.5 was originally detected as a “brain-specific protein”, accounting for about 5% of total neuronal proteins[22,23]. NSE is currently considered a useful marker of neural maturation, being highly specific for neurons and peripheral neuroendocrine cells[24]. NSE may also induce neurotrophic functions as it controls neuronal survival, differentiation, and neurite regeneration[25,26]. GFAP expression is commonly considered specific of astrocytes[27], also present in activated Müller cells of the retina[28] and multipotent neural stem cells of the adult mammalian brain[29].
Several studies report the presence of these markers also within the mammalian retina, some of them at different stages of development and under different conditions. According to Mayer et al[30], nestin-positive cells in the normal retina represent a population of progenitor cells that differentiate to protect the structural integrity of the retina and RGCs. In the adult retina, they show morphological similarities to neural cells, such as RGCs, and Müller cells. Subpopulations of nestin -positive cells were also positive for GFAP. Nestin-positive cells are probably involved in regenerative processes, since their number increases following optic nerve transection[31]. PGP9.5 immunoreactivity has been detected in the retina of several mammalian species, especially in ganglion cells, suggesting that PGP9.5 can be used as a specific neuronal marker for these neurons[32]. In fact, PGP9.5 immunoreactivity was found in about 80% of ganglion cells retrogradely labeled after injection of peroxidase into the optic nerve[33]. Widely distributed in small to medium size ganglion cells, it is suggested that PGP9.5 modulates the early stages of retina development[34]. Experiments in rats show that NSE immunopositive neurons can be clearly detected in the retina only during embryonic development and early neonatal stages[35]. The first appearance of NSE immunoreactivity was identified in pigment epithelium, then in ganglion cells, photoreceptors and amacrine cells. Further retinal neurons became NSE immunopositive by postnatal day 14. It is suggested that NSE expression occurs in retinal neurons just after their migration to their final location and before establishing synaptic contacts. High GFAP levels in the mammalian retina during the first neonatal week rapidly decline during animal growth. In fact, in the adult organism, only astrocytes are GFAP-positive, while Müller cells only weakly express GFAP. However, high levels of GFAP can be detected in Müller cells following photoreceptor degeneration or in cases of retinal degeneration/detachment. It is possible that GFAP upregulation occurs in activated "dedifferentiating" Müller cells because of a disruption of normal neuron-glia interactions[28].
Overall, it can be speculated that, even though some of these markers may be found in tissues different from the nervous system, their increased expression in morphologically changed cells induced by ARPE-19 CM is suggestive of ASC neural differentiation. Vossmerbaeumer et al[14] reported less induction of GFAP and nestin levels in ASCs exposed to pig-derived primary RPE-CM, in a study mainly designed to monitor RPE markers, while neural markers were only marginally explored, to exclude neural stem cell contamination in their ASC samples. Possible differences could be related to a different antibody sensitivity and/or different experimental procedures. In fact, the lack of nestin immunostaining in their samples was contradicted by their quantitative real-time polymerase chain reaction results that, also in primary ASC cultures, revealed a basal nestin level. Moreover, this basal expression was found “unexpectedly” increased after porcine RPE CM treatment. In the present study, a systematic investigation by immunostaining and western blot analyses showed that an increased expression of both GFAP and nestin was consistently observed in a time dependent manner. In fact, although some differences could already be noted at day 1, they were more clearly appreciable at day 8. It is important to point out that striking differences were observed between basal F12/ASCs and CM/ASCS. In fact, since the same culture medium was used in both cases, the differences observed must be attributed to the release of soluble factors or extracellular vesicles by ARPE-19 during their growth. In this respect, it should be pointed out that serum-free cultures were also preferred to avoid interferences on ASC differentiation properties between factors present in ARPE-19 CM and FBS[36].
Since both neuronal and glial markers were found overexpressed in the same cell population, a likely possibility is that neural-like differentiating ASCs might still be at early stages of differentiation, similarly to neural progenitor cells, where both types of markers normally coexist[37-39]. An alternative explanation is that this might be a combined effect of the factors present in the CM and the particular in vitro situation, in the absence of a dynamic physiological environment, which would more specifically address the cell differentiation fate. It is reasonable to hypothesize that under in vivo conditions, on the basis of real microenvironment cues, their fate would be more precisely traced. For the same reason, some neural markers such as GFAP and NSE might be more expressed in neural-like differentiating ASCs. In fact, high levels of these markers, other than in response to retina damage, can be physiologically found at early stages of development.
Since different neural elements are present within a functional retina, further investigation will be carried out by using more specific markers to better clarify the type of neural cells into which ASCs preferentially differentiate. Moreover, it will be interesting to identify which component (growth factors and soluble molecules) might be responsible for the effects described in the present work. Finally, the presence of extracellular vesicles in ARPE-19 CM cannot be excluded and will be investigated in future studies.
ASC neural-like differentiation obtained by the protocol used in the present study offers some advantages. ASCs can be easily isolated for both autologous and heterologous use. A CM from an RPE cell line may closely mimic the physiologic enviro
Based on their multipotent differentiation ability, mesenchymal stem cells (MSCs) have been widely investigated in the last two decades in order to develop cell-based therapeutic strategies for a variety of human pathologies including eye disease.
In many cases, available therapeutic approaches are not satisfactory to counteract the loss of retinal cells. Thus, administration of pre-differentiated MSCs may produce beneficial outcomes and improve the quality of life of patients suffering ocular diseases.
The aim of the investigation was to obtain a neural-like differentiation of adipose-derived stem cells (ASCs) using a serum-free culture medium, resembling the physiologic eye microenvironment.
A serum-free conditioned medium (CM) from ARPE-19, a cell line derived from human retinal pigment epithelium, has been used to promote ASC neural differentiation. Immunofluorescence and western blot analysis were used to evaluate modifications of typical neural marker expression: Nestin, neuronal specific enolase, protein gene product 9.5, and glial fibrillary acidic protein.
Neural marker expression was increased in a time-dependent manner. In fact, CM effects were particularly evident after 8 d of treatment. Moreover, cell proliferation and viability were favored by the presence of ARPE-19 CM.
The method adopted in the present study provided encouraging results to develop cell-based strategies for ocular diseases characterized by neural cell loss or degeneration.
At the next stage of the study, neural-like pre-differentiated ASCs would be implanted in rodent models of ocular diseases to verify their survival rate and possible beneficial effects.
Provenance and peer review: Invited article; Externally peer reviewed.
Specialty type: Cell and tissue engineering
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Jiang L, Limnios IJ, Long X S-Editor: Fan JR L-Editor: Filipodia P-Editor: Fan JR
| 1. | Elshaer SL, Evans W, Pentecost M, Lenin R, Periasamy R, Jha KA, Alli S, Gentry J, Thomas SM, Sohl N, Gangaraju R. Adipose stem cells and their paracrine factors are therapeutic for early retinal complications of diabetes in the Ins2Akita mouse. Stem Cell Res Ther. 2018;9:322. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 41] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 2. | Fiori A, Hammes HP, Bieback K. Adipose-derived mesenchymal stromal cells reverse high glucose-induced reduction of angiogenesis in human retinal microvascular endothelial cells. Cytotherapy. 2020;22:261-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 3. | Ji S, Xiao J, Liu J, Tang S. Human Umbilical Cord Mesenchymal Stem Cells Attenuate Ocular Hypertension-Induced Retinal Neuroinflammation via Toll-Like Receptor 4 Pathway. Stem Cells Int. 2019;2019:9274585. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 4. | Limoli PG, Limoli CSS, Morales MU, Vingolo EM. Mesenchymal stem cell surgery, rescue and regeneration in retinitis pigmentosa: clinical and rehabilitative prognostic aspects. Restor Neurol Neurosci. 2020;38:223-237. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 5. | Lo Furno D, Graziano AC, Avola R, Giuffrida R, Perciavalle V, Bonina F, Mannino G, Cardile V. A Citrus bergamia Extract Decreases Adipogenesis and Increases Lipolysis by Modulating PPAR Levels in Mesenchymal Stem Cells from Human Adipose Tissue. PPAR Res. 2016;2016:4563815. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 6. | Lo Furno D, Mannino G, Giuffrida R, Gili E, Vancheri C, Tarico MS, Perrotta RE, Pellitteri R. Neural differentiation of human adipose-derived mesenchymal stem cells induced by glial cell conditioned media. J Cell Physiol. 2018;233:7091-7100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 7. | Mannino G, Gennuso F, Giurdanella G, Conti F, Drago F, Salomone S, Lo Furno DL, Bucolo C, Giuffrida R. Pericyte-like differentiation of human adipose-derived mesenchymal stem cells: An in vitro study. World J Stem Cells. 2020;12:1152-1170. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 8. | Devoldere J, Peynshaert K, De Smedt SC, Remaut K. Müller cells as a target for retinal therapy. Drug Discov Today. 2019;24:1483-1498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 50] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 9. | Puñal VM, Paisley CE, Brecha FS, Lee MA, Perelli RM, Wang J, O'Koren EG, Ackley CR, Saban DR, Reese BE, Kay JN. Large-scale death of retinal astrocytes during normal development is non-apoptotic and implemented by microglia. PLoS Biol. 2019;17:e3000492. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 57] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 10. | Simó R, Villarroel M, Corraliza L, Hernández C, Garcia-Ramírez M. The retinal pigment epithelium: something more than a constituent of the blood-retinal barrier--implications for the pathogenesis of diabetic retinopathy. J Biomed Biotechnol. 2010;2010:190724. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 239] [Cited by in RCA: 329] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 11. | Raymond SM, Jackson IJ. The retinal pigmented epithelium is required for development and maintenance of the mouse neural retina. Curr Biol. 1995;5:1286-1295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 154] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 12. | Sheedlo HJ, Bartosh TJ, Wang Z, Srinivasan B, Brun-Zinkernagel AM, Roque RS. RPE-derived factors modulate photoreceptor differentiation: a possible role in the retinal stem cell niche. In Vitro Cell Dev Biol Anim. 2007;43:361-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 13. | Dutt K, Douglas P, Cao Y. RPE-secreted factors: influence differentiation in human retinal cell line in dose- and density-dependent manner. J Ocul Biol Dis Infor. 2010;3:144-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 14. | Vossmerbaeumer U, Ohnesorge S, Kuehl S, Haapalahti M, Kluter H, Jonas JB, Thierse HJ, Bieback K. Retinal pigment epithelial phenotype induced in human adipose tissue-derived mesenchymal stromal cells. Cytotherapy. 2009;11:177-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 56] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 15. | Dunn KC, Aotaki-Keen AE, Putkey FR, Hjelmeland LM. ARPE-19, a human retinal pigment epithelial cell line with differentiated properties. Exp Eye Res. 1996;62:155-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 836] [Cited by in RCA: 982] [Article Influence: 33.9] [Reference Citation Analysis (0)] |
| 16. | Wada Y, Ikemoto T, Morine Y, Imura S, Saito Y, Yamada S, Shimada M. The Differences in the Characteristics of Insulin-producing Cells Using Human Adipose-tissue Derived Mesenchymal Stem Cells from Subcutaneous and Visceral Tissues. Sci Rep. 2019;9:13204. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 17. | Li R, Wen R, Banzon T, Maminishkis A, Miller SS. CNTF mediates neurotrophic factor secretion and fluid absorption in human retinal pigment epithelium. PLoS One. 2011;6:e23148. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 41] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 18. | Nadal-Nicolas FM, Becerra SP. Pigment Epithelium-derived Factor Protects Retinal Pigment Epithelial Cells Against Cytotoxicity "In Vitro". Adv Exp Med Biol. 2018;1074:457-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 19. | Ezeonu I, Derrickson B, Dutt K. Cell fate decisions in a human retinal precursor cell line: basic fibroblast growth factor- and transforming growth factor-alpha-mediated differentiation. DNA Cell Biol. 2000;19:527-537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 20. | Ding DC, Wen YT, Tsai RK. Pigment epithelium-derived factor from ARPE19 promotes proliferation and inhibits apoptosis of human umbilical mesenchymal stem cells in serum-free medium. Exp Mol Med. 2017;49:e411. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 21. | Michalczyk K, Ziman M. Nestin structure and predicted function in cellular cytoskeletal organisation. Histol Histopathol. 2005;20:665-671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 128] [Reference Citation Analysis (0)] |
| 22. | Day IN, Thompson RJ. UCHL1 (PGP 9.5): neuronal biomarker and ubiquitin system protein. Prog Neurobiol. 2010;90:327-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 200] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 23. | Bishop P, Rocca D, Henley JM. Ubiquitin C-terminal hydrolase L1 (UCH-L1): structure, distribution and roles in brain function and dysfunction. Biochem J. 2016;473:2453-2462. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 123] [Cited by in RCA: 197] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 24. | Isgrò MA, Bottoni P, Scatena R. Neuron-Specific Enolase as a Biomarker: Biochemical and Clinical Aspects. Adv Exp Med Biol. 2015;867:125-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 355] [Article Influence: 35.5] [Reference Citation Analysis (0)] |
| 25. | Polcyn R, Capone M, Matzelle D, Hossain A, Chandran R, Banik NL, Haque A. Enolase inhibition alters metabolic hormones and inflammatory factors to promote neuroprotection in spinal cord injury. Neurochem Int. 2020;139:104788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 26. | Haque A, Polcyn R, Matzelle D, Banik NL. New Insights into the Role of Neuron-Specific Enolase in Neuro-Inflammation, Neurodegeneration, and Neuroprotection. Brain Sci. 2018;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 139] [Cited by in RCA: 165] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 27. | Zhang Z, Ma Z, Zou W, Guo H, Liu M, Ma Y, Zhang L. The Appropriate Marker for Astrocytes: Comparing the Distribution and Expression of Three Astrocytic Markers in Different Mouse Cerebral Regions. Biomed Res Int. 2019;2019:9605265. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 73] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 28. | Sarthy PV, Fu M, Huang J. Developmental expression of the glial fibrillary acidic protein (GFAP) gene in the mouse retina. Cell Mol Neurobiol. 1991;11:623-637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 46] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 29. | Middeldorp J, Boer K, Sluijs JA, De Filippis L, Encha-Razavi F, Vescovi AL, Swaab DF, Aronica E, Hol EM. GFAPdelta in radial glia and subventricular zone progenitors in the developing human cortex. Development. 2010;137:313-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 69] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 30. | Mayer EJ, Hughes EH, Carter DA, Dick AD. Nestin positive cells in adult human retina and in epiretinal membranes. Br J Ophthalmol. 2003;87:1154-1158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 48] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 31. | Wang L, Li P. Expressions of nestin and glial fibrillary acidic protein in rat retina after optic nerve transection. Int J Ophthalmol. 2017;10:1510-1515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 32. | Chen ST, von Bussmann KA, Garey LJ, Jen LS. Protein gene product 9.5-immunoreactive retinal neurons in normal developing rats and rats with optic nerve or tract lesion. Brain Res Dev Brain Res. 1994;78:265-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 33. | Bonfanti L, Candeo P, Piccinini M, Carmignoto G, Comelli MC, Ghidella S, Bruno R, Gobetto A, Merighi A. Distribution of protein gene product 9.5 (pgp 9.5) in the vertebrate retina: Evidence that immunoreactivity is restricted to mammalian horizontal and ganglion cells. J Comp Neurol. 1992;322:35-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 34. | Wang JP, Chen ST, Chien CH, Yao CJ, Chou LS. Protein gene product 9.5-immunoreactive neurons in the retina of striped dolphin (Stenella coeruleoalba) and Fraser dolphin (Lagenodelphis hosei). Kaibogaku Zasshi. 1999;74:441-446. [PubMed] |
| 35. | Fujieda H, Sato T, Wake K. Expression of neuron-specific enolase in the developing rat retina as revealed by immunocytochemistry. Brain Res Dev Brain Res. 1994;82:69-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 36. | Liu Q, Lü L, Sun H, Zhang J, Ma W, Zhang T. [Effect of serum on the differentiation of neural stem cells]. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2018;32:223-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 37. | Wei LC, Shi M, Chen LW, Cao R, Zhang P, Chan YS. Nestin-containing cells express glial fibrillary acidic protein in the proliferative regions of central nervous system of postnatal developing and adult mice. Brain Res Dev Brain Res. 2002;139:9-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 106] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 38. | Kempermann G, Jessberger S, Steiner B, Kronenberg G. Milestones of neuronal development in the adult hippocampus. Trends Neurosci. 2004;27:447-452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1011] [Cited by in RCA: 1106] [Article Influence: 52.7] [Reference Citation Analysis (0)] |
| 39. | Vinci L, Ravarino A, Fanos V, Naccarato AG, Senes G, Gerosa C, Bevilacqua G, Faa G, Ambu R. Immunohistochemical markers of neural progenitor cells in the early embryonic human cerebral cortex. Eur J Histochem. 2016;60:2563. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 35] [Article Influence: 3.9] [Reference Citation Analysis (0)] |