Published online Jun 26, 2020. doi: 10.4252/wjsc.v12.i6.500
Peer-review started: December 18, 2019
First decision: February 20, 2020
Revised: March 17, 2020
Accepted: April 8, 2020
Article in press: April 8, 2020
Published online: June 26, 2020
Processing time: 190 Days and 3.4 Hours
With continuous advancement of industrial society, environmental pollution has become more and more serious. There has been an increase in infertility caused by environmental factors. Nonylphenol (NP) is a stable degradation product widely used in daily life and production and has been proven to affect male fertility. However, the underlying mechanisms therein are unclear. Thus, it is necessary to study the effect and mechanism of NP on spermatogonial stem cells (SSCs).
To investigate the cytotoxic effect of NP on SSCs via the phosphatidylinositol-3-kinase/protein kinase B/mammalian target of rapamycin (PI3K/AKT/mTOR) pathway.
SSCs were treated with NP at 0, 10, 20 or 30 µmol. MTT assay was performed to evaluate the effect of NP on the proliferation of SSCs. Flow cytometry was conducted to measure SSC apoptosis. The expression of Bad, Bcl-2, cytochrome-c, pro-Caspase 9, SOX-2, OCT-4, Nanog, Nanos3, Stra8, Scp3, GFRα1, CD90, VASA, Nanos2, KIT, PLZF and PI3K/AKT/mTOR-related proteins was observed by western blot, and the mRNA expression of SOX-2, OCT-4 and Nanog was detected by quantitative reverse transcription polymerase chain reaction.
Compared with untreated cells (0 μmol NP), SSCs treated with NP at all concentrations showed a decrease in cell proliferation and expression of Bcl-2, Nanog, OCT-4, SOX-2, Nanos3, Stra8, Scp3, GFRα1, CD90, VASA, Nanos2, KIT, and PLZF (P < 0.05), whereas the expression of Bad, cytochrome-c, and pro-Caspase 9 increased significantly (P < 0.05). We further examined the PI3K/AKT/mTOR pathway and found that the phosphorylation of PI3K, AKT, mTORC1, and S6K was significantly decreased by NP at all concentrations compared to that in untreated SSCs (P < 0.05). NP exerted the greatest effect at 30 μmol among all NP concentrations.
NP attenuated the proliferation, differentiation and stemness maintenance of SSCs while promoting apoptosis and oxidative stress. The associated mechanism may be related to the PI3K/AKT/mTOR pathway.
Core tip: With continuous advancement of industrial society, environmental pollution has become more and more serious. There has been an increase in infertility caused by environmental factors. Nonylphenol is a stable degradation product widely used in daily life and production and has been proven to affect male fertility. Our study demonstrated that nonylphenol reduced the proliferation, differentiation and stemness maintenance of spermatogonial stem cells while promoting apoptosis and oxidative stress. The mechanism may be related to the phosphatidylinositol-3-kinase/protein kinase B/mammalian target of rapamycin pathway, providing a potential method for the treatment of male infertility.
- Citation: Lei JH, Yan W, Luo CH, Guo YM, Zhang YY, Wang XH, Su XJ. Cytotoxicity of nonylphenol on spermatogonial stem cells via phosphatidylinositol-3-kinase/protein kinase B/mammalian target of rapamycin pathway. World J Stem Cells 2020; 12(6): 500-513
- URL: https://www.wjgnet.com/1948-0210/full/v12/i6/500.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v12.i6.500
Spermatogonial stem cells (SSCs) are a class of primitive spermatogonia located at the basement membrane of convoluted seminiferous tubules that not only self-renew and maintain their population but also differentiate into spermatocytes. SSCs are transformed into primary and secondary spermatocytes, which then produce round and long spermatozoa cells, eventually forming sperm. SSCs are considered to be immortal because they can replicate themselves in adults and pass on from generation to generation.
With continuous advancement of industrial society, environmental pollution has become more and more serious. There has been an increase in infertility caused by environmental factors[1,2]. Environmental endocrine disruptors are exogenous substances that can interfere with the endocrine functions of the body, including those that mediate the synthesis, secretion, transport, binding, biological effects and clearance of hormones. This in turn leads to endocrine disorders and causes abnormal damage to body behavior, reproduction and development. Nonylphenol (NP) is a stable degradation product of alkylphenol that is widely used in plasticizers, pesticide emulsifiers and other industrial applications. Its molecular structure is similar to that of estradiol in the human body and can interfere with endocrine metabolism and damage human health[3-5]. Studies have shown that NP affects the body’s hormonal balance and damages the body’s reproductive functions[6,7]. NP can reduce the testicular weight and sperm production in rats without inducing pathological changes in the testis[8], but the underlying mechanisms therein are unclear. In this study, we observed the effect of different concentrations of NP on the proliferation, differentiation and apoptosis of isolated SSCs. Further molecular experiments were performed to verify whether these effects were achieved via the phosphatidylinositol-3-kinase/protein kinase B/mammalian target of rapamycin (PI3K/AKT/mTOR) signaling pathway.
Ten-day-old (specific pathogen-free, disinfected with 75% ethanol) neonatal mice were provided by the Hubei Province Disease Control Center (No. 211002300042744). After anesthesia, bilateral testicles were removed from the mice and placed in sterile Petri dishes. Testicular tissue was rinsed three times with phosphate-buffered saline containing 5% penicillin-streptomycin (PAB180086, Bioswamp). Primary SSCs were isolated according to the method of Yu et al[9]. The rinsed testicular tissue was peeled off under a microscope, placed in a Petri dish with a small amount of high-glucose Dulbecco's modified Eagle medium (DMEM, SH30022.01B, Hyclone) and cut into 1-mm3 pieces with surgical ophthalmic scissors. The tissue block was resuspended in a solution of 0.125% trypsin and 0.1% collagenase I (1:1) (17100-017, Gibco) and incubated at 37 °C in an incubator containing 5% CO2 for 30 min. Tissue digestion was terminated by adding an equal volume of DMEM, and the cells were centrifuged at 2500 × g for 5 min. Thereafter, the cells were resuspended in culture solution and cultured in a Petri dish at 37 °C for 1 h. The cells were then seeded into a feeder-free Petri dish and passaged when they reached 85% confluence.
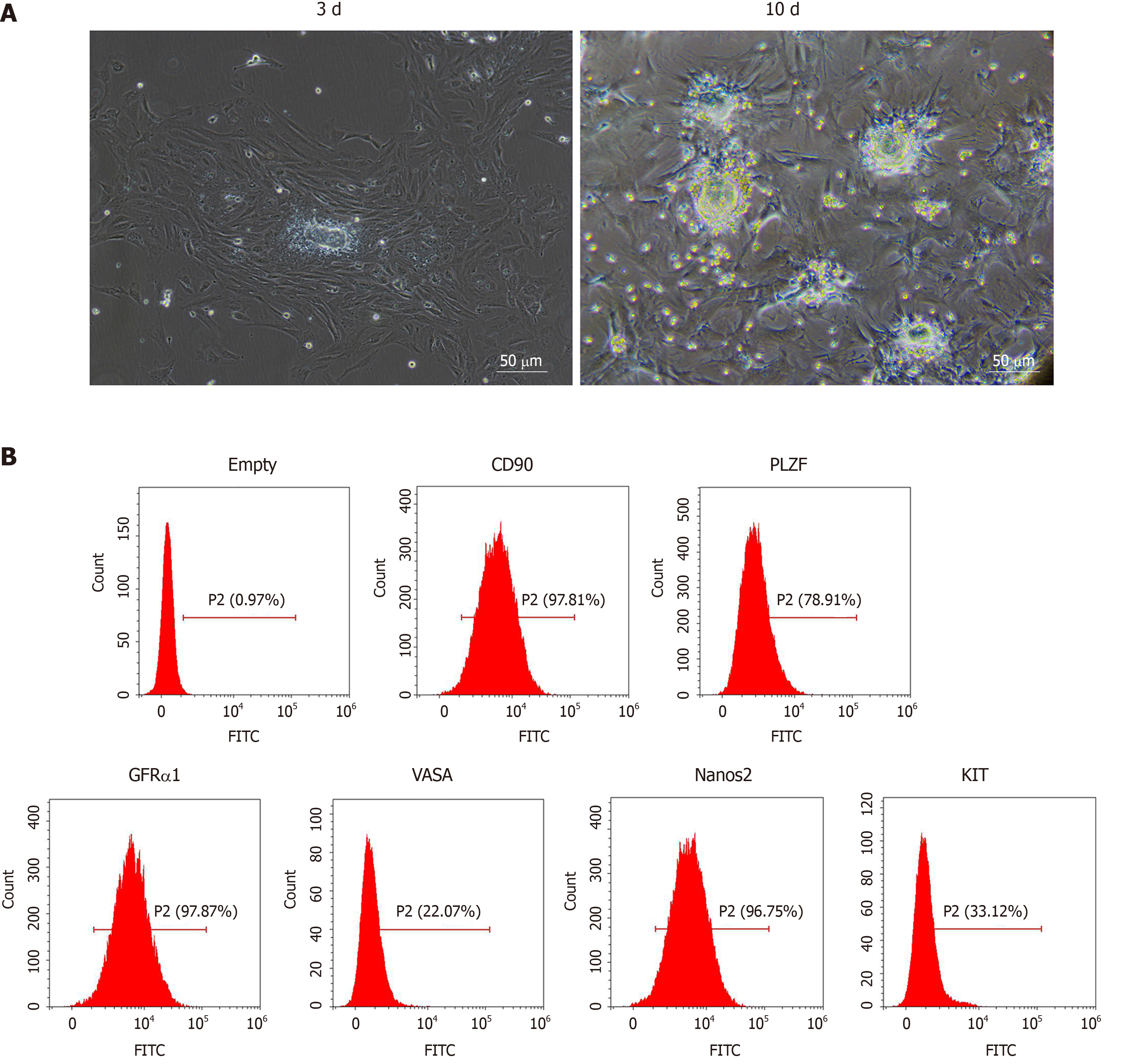
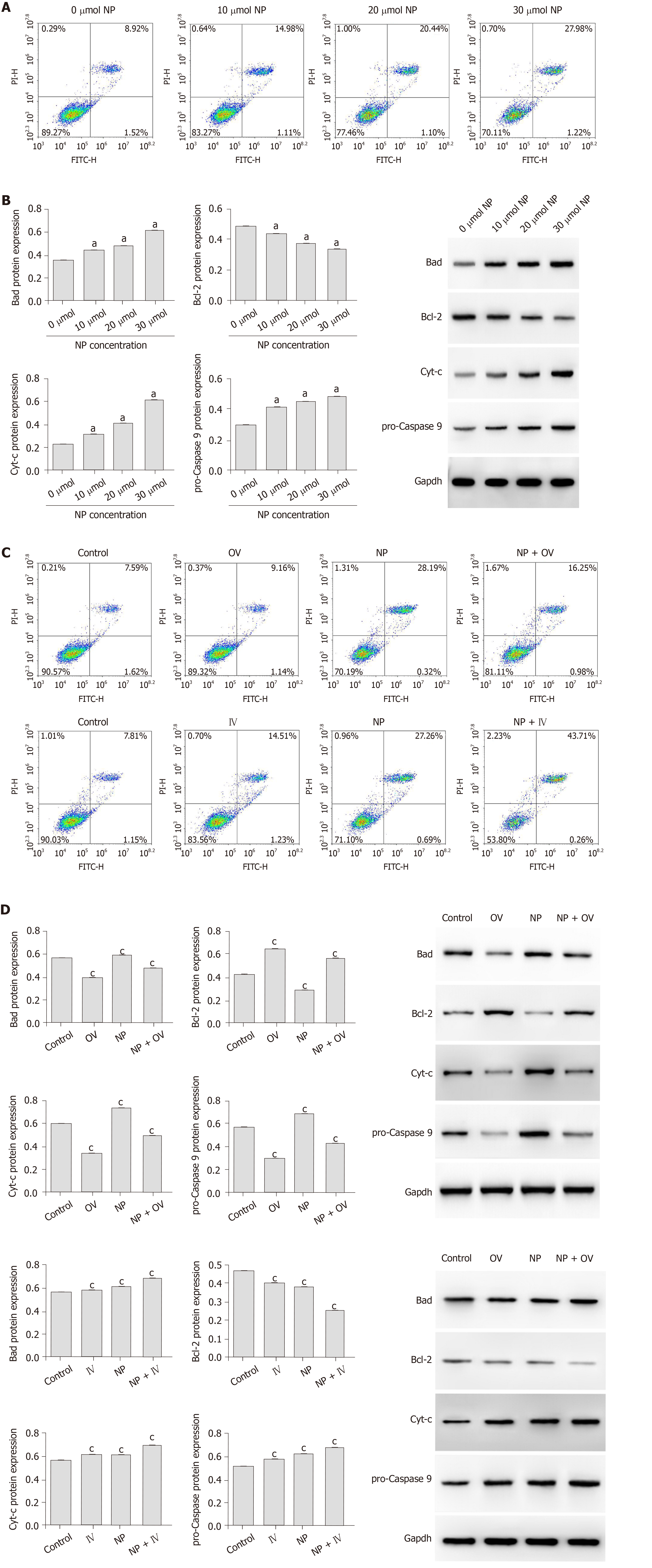
For flow cytometric identification of SSCs, seven Eppendorf tubes were prepared and 100 μL of single cell suspension was added to each tube. Antibodies against glial cell line-derived neurotrophic factor family receptor alpha-1 (GFRα1, 1:200, ab186855, Abcam), CD90 (11-0903-82, 0.125 μg/test, Invitrogen), VASA (PA5-23378r, 1:200, Invitrogen), Nanos2 (PA5-20553r, 1:50, Invitrogen), KIT (17-1171-82, 0.125 μg/test, Invitrogen) and promyelocytic leukemia zinc finger (PLZF, 1 μg/test, 53-9320-82, Invitrogen) were individually added to each tube (with one blank tube). The tubes were incubated at 4 °C for 45 min in the dark. After the addition of 2 μL of fluorescein (FITC)-conjugated Affinipure Goat Anti-Rabbit IgG (H+L) (PAB160016, Bioswamp) to each tube, they were further incubated at 4 °C for 45 min in the dark. After adding 400 μL of flow cytometry dyeing buffer to each tube, the cells were subjected to flow cytometry (CytoFLEX S, BECKMAN), and the results were analyzed using CYEXPERT software. After SSCs have been successfully isolated and identified, they were treated with NP (N109556, Aladdin) at 0, 10, 20 and 30 μmol for 7 d based on the method of Huang et al[10]. SSCs were treated with mTOR agonists (MHY1485, 10 μmol) and mTOR inhibitors (AZD8055, 10 μmol) according to the references[11,12] for 24 h. SSCs were respectively divided into four groups: Control (Control group, not subjected to any activator or inhibitor), NP (NP group), OV or IV (mTOR activator group; mTOR inhibitor group), OV/IV + NP (OV/IV + NP group) groups.
The proliferation of SSCs was detected using an MTT assay kit (PAB180013, Bioswamp) according to the manufacturer’s instructions. Cells in the logarithmic growth phase were collected and seeded at 5 × 103 cells/well in a 96-well plate, and 20 μL of MTT solution was added to each well. The cells were incubated overnight at 37 °C in an incubator containing 5% CO2, and 150 μL of dimethyl sulfoxide (D2650, Sigma) was added to each well. The optical density was measured at 490 nm with a microplate reader (Multiskan FC, Thermo).
SSCs (1.0 × 105 cells/mL) were treated once with different concentrations of NP (0, 10, 20 and 30 μmol) in sterile tubes and cultured for 7 d, after which 1 mL of pre-cooled phosphate-buffered saline was added. The cells were centrifuged at 1000 × g. Then, 10 μL of Annexin V-FITC and 10 μL of propidium iodide were added. The cell samples were analyzed by flow cytometry (CytoFLEX S, Beckman Coulter), and one-step fluorescence compensation strategy was used to eliminate interference in the FITC channel. After mTOR overexpression and mTOR inhibitor AZD8055 intervention, the effect of NP on apoptosis of SSCs was also examined.
The concentration of proteins extracted from cells was measured using a bicinchoninic acid protein assay kit (PAB180007, Bioswamp). Total protein was separated by 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to polyvinylidene fluoride membranes. The membranes were blocked with 5% milk in Tris-buffered saline (pH 7.6) containing 0.1% Tween-20, incubated with specific primary antibodies overnight at 4 °C and incubated with horseradish peroxidase-conjugated secondary goat anti-rabbit IgG (1:20000, PAB160011, Bioswamp) for 2 h at 4 °C. The following primary antibodies were used: Bad (1:1000, PAB0589, Bioswamp), Bcl-2 (1:100, PAB30041, Bioswamp), cytochrome-c (Cyt-c, 1:1000, ab90529, Abcam), pro-caspase 9 (1:500, ab135544, Abcam), Nanos3 (1:1000, ab216694, Abcam), Stra8 (1:1000, ab49602, Abcam), synaptonemal complex protein 3 (Scp3, 1:1000, ab150292, Abcam), GFRα1 (1:500, PA1-32476, Thermo), CD90 (1:1000, ab92574, Abcam), VASA (1:1000, ab209710, Abcam), Nanos2 (1:1000, ab70000, Abcam), KIT (1:1000, ab32363, Abcam), PLZF (1:1000, ab39354, Abcam), PI3K (1:1000, ab191606, Abcam), p-PI3K (1:1000, ab182651, Abcam), AKT (1:1000, ab227100, Abcam), p-AKT (1:1000, ab133458, Thermo), mTOR complex 1 (mTORC1, 1:1000, ab137341, Abcam), p-mTORC1 (1:2000, ab226957, Abcam), ribosomal protein S6 kinase (S6K, 1:1000, ab186753, Abcam), p-S6K (1:1000, ab59208, Abcam), sex determining region Y-box 2 (SOX-2, 1:1000, PAB30154, Bioswamp), octamer-binding transcription factor 4 (OCT-4, 1:1000, ab18976, Abcam), Nanog (1:1000, PAB33609, Bioswamp) and GAPDH (1:2000, PAB36264, Bioswamp). After three washes with phosphate-buffered saline/Tween 20, the membranes were incubated with horseradish peroxidase-conjugated secondary goat anti-rabbit IgG (1:20000, PAB160011, Bioswamp). Protein bands were visualized by enhanced chemiluminescence color detection (Tanon-5200, TANON) and analyzed using AlphaEase FC gel image analysis software.
Whole RNA was extracted from cell samples using Trizol reagent according to the manufacturer’s procedures, and cDNA was synthesized using a reverse transcriptase kit (639505, TAKARA). Quantitative reverse transcription polymerase chain reaction (qRT-PCR) was performed using a real-time system (CFX-96, Bio-RAD) with the SYBR Green PCR Kit (KM4101, KAPA Biosystems). Each qPCR reaction was performed in duplicate and the results were analyzed using the 2-ΔΔCt method. The primers were designed and configured by Nanjing Kingsy Biotechnology Co., Ltd. and are listed in Table 1.
| Primer | Sequence, 5’-3’ |
| OCT-4-F | CACTCACATCGCCAATC |
| OCT-4-R | AAGGTGTCCCTGTAGCC |
| SOX-2-F | AGCGGCGTAAGATGGC |
| SOX-2-R | CGGTCTCGGACAAAAGT |
| Nanog-F | AGCCCTGATTCTTCTACC |
| Nanog-R | TGAAACCTGTCCTTGAGT |
| GAPDH-F | CCACTCCTCCACCTTTG |
| GAPDH-R | CACCACCCTGTTGCTGT |
All data are presented as the mean ± standard deviation. The SPSS 19.0 software was used for statistical analyses and GraphPad Prism 5.0 was used to prepare the figures. The data were evaluated for statistical significance using one-way analysis of variance. Statistical significance was established at P < 0.05.
We observed SSCs on the 3rd and 10th d of culture with optical microscopy. As shown in Figure 1A, the isolated SSCs were round with large and round nuclei and less cytoplasm. Compared with the 3rd d, the number of SSCs increased significantly on the 10th d. We further identified SSCs by flow cytometry. As shown in Figure 1B, the SSCs showed high expression of GFRα1, CD90, VASA, Nanos2, KIT and PLZF, suggesting that mouse SSCs were successfully isolated and cultured.
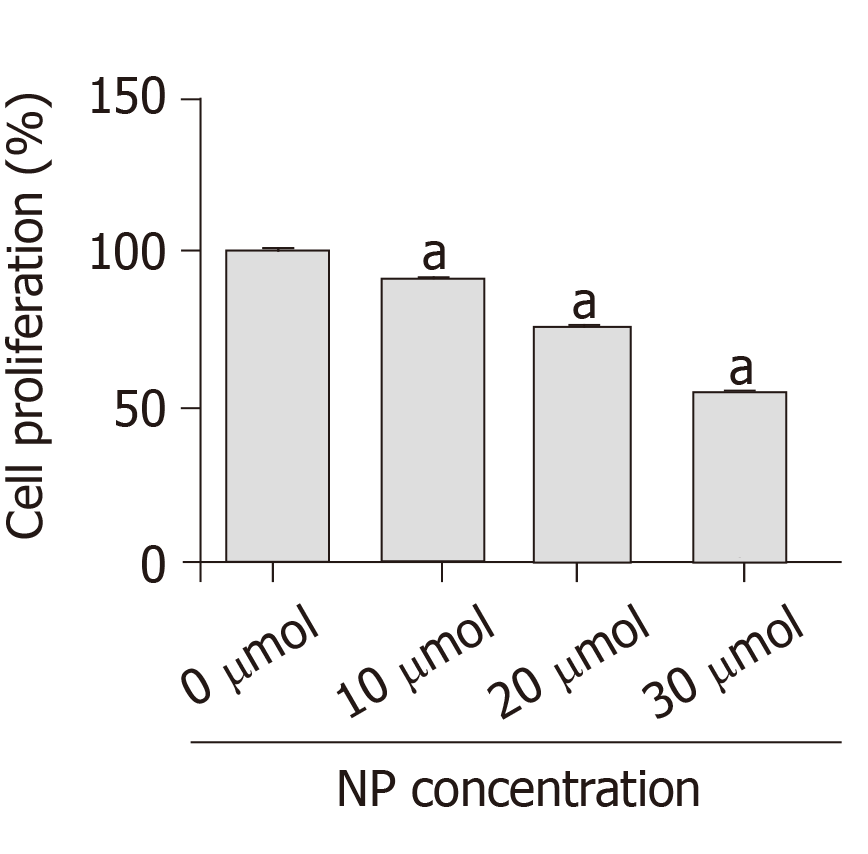
After SSCs were treated with different concentrations of NP (0, 10, 20 and 30 μmol) for 7 d, we examined the changes in cell proliferation (Figure 2). Compared with untreated cells (0 μmol), the proliferation of cells treated with NP at 10, 20 and 30 μmol NP decreased significantly (P < 0.05) in a concentration-dependent manner. This suggests that NP had an inhibitory effect on SSC proliferation.
To study the effect of NP on SSCs apoptosis, flow cytometry and western blot were performed. As the NP concentration increased, the percentage of SSC apoptosis increased gradually (Figure 3A). Examination of the expression of apoptosis-related proteins revealed that compared to untreated SSCs (0 μmol NP), those treated with 10, 20 and 30 μmol NP showed a significant increase in the expression of Bad, Cyt-c and pro-Caspase 9 (P < 0.05), while the expression of Bcl-2 decreased significantly (P < 0.05) (Figure 3B). We next observed the cell apoptosis after mTOR activator or inhibitor intervention, the results showed that the apoptosis rate of NP group decreased significantly after increased mTOR activity, while the apoptosis rate of SSCs in the NP group increased significantly after inhibiting mTOR activity (Figure 3C). The expression levels of apoptosis-related proteins are similar to the above trend (P < 0.05) (Figure 3D).
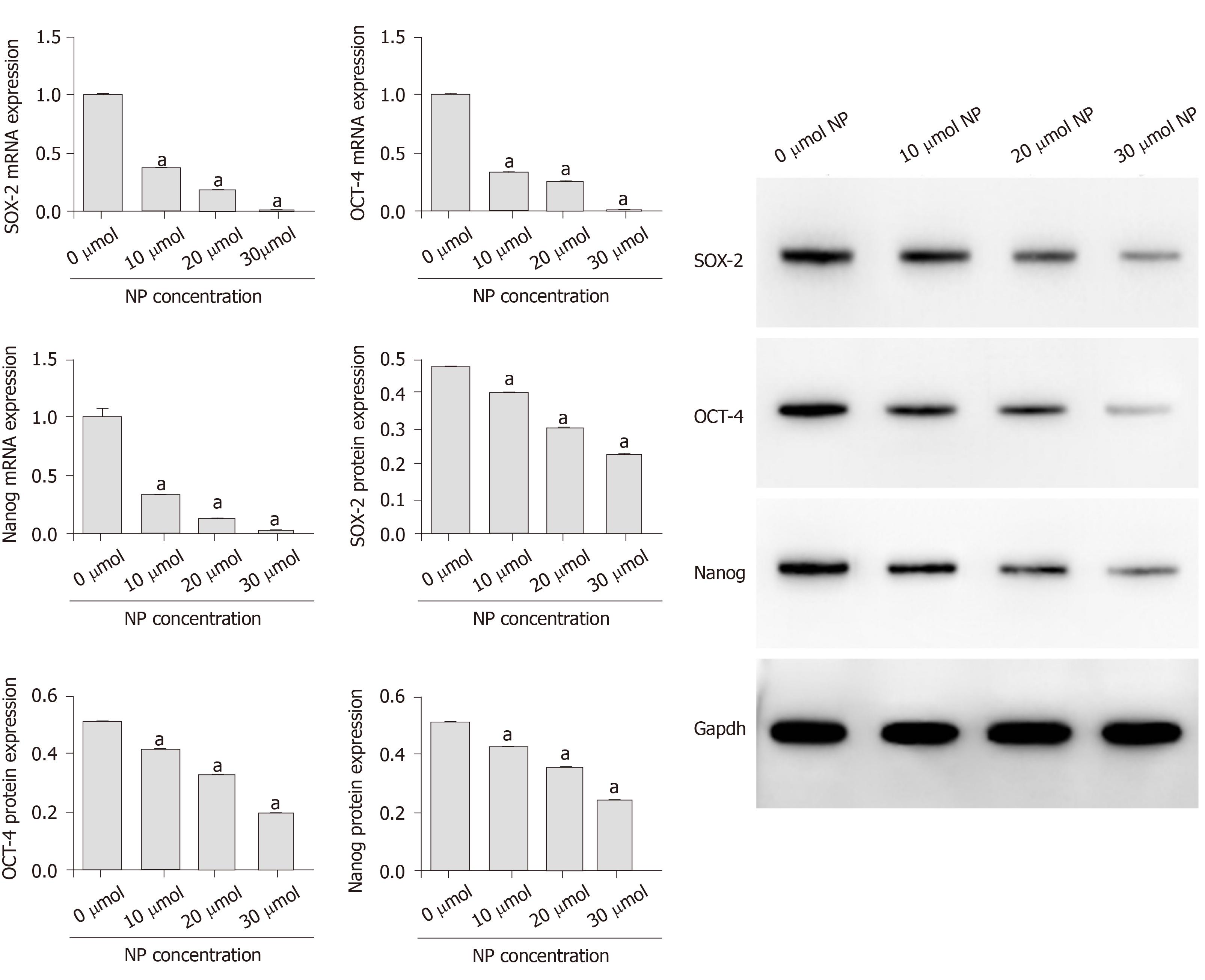
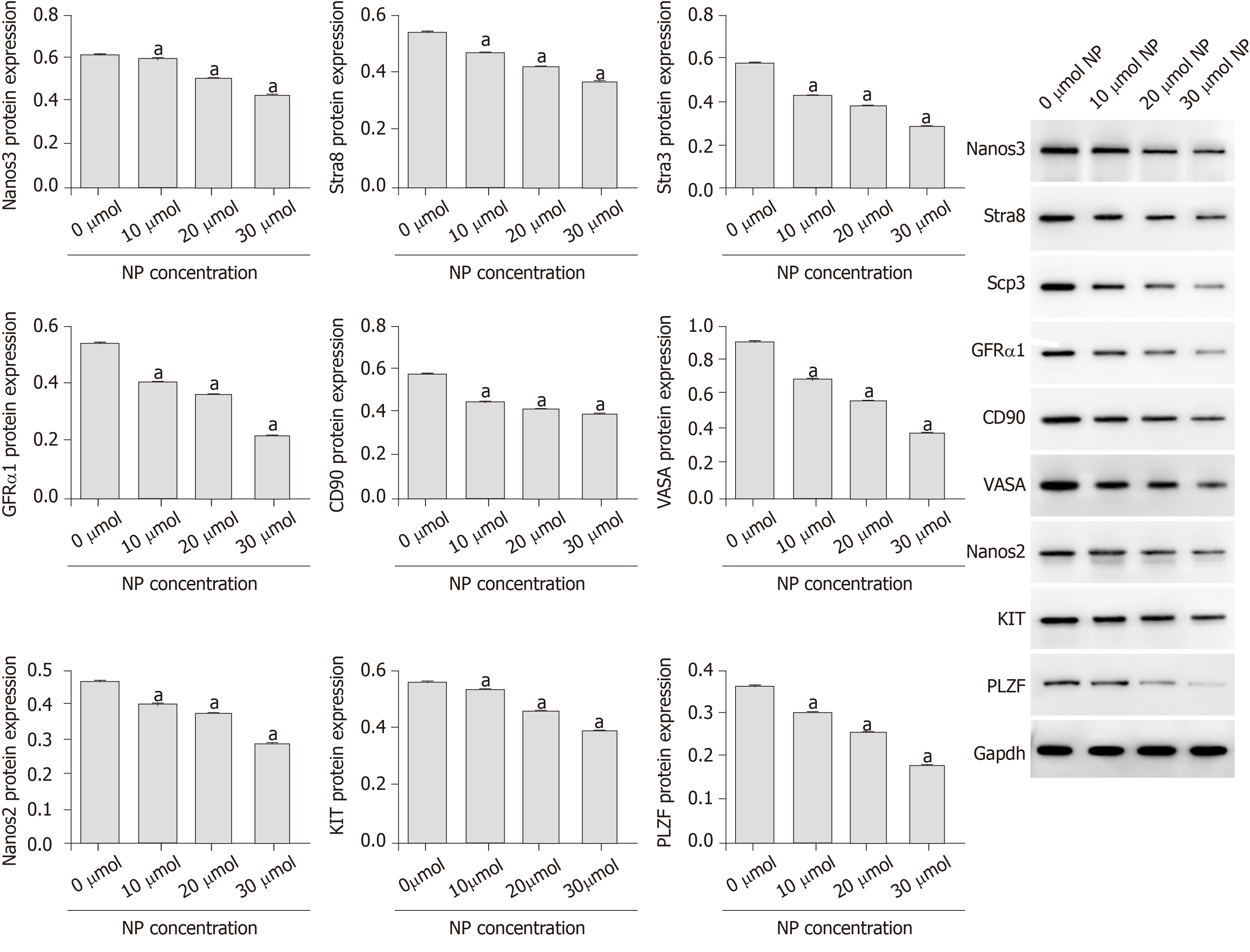
As shown in Figure 4, the mRNA and protein expression of stemness maintenance markers Nanog, OCT-4 and SOX-2 in SSCs treated with 10, 20 and 30 μmol NP was decreased compared with those in untreated cells (P < 0.05), and the decrease was directly proportional to the NP concentration. Further detection of SSC differentiation markers showed that the protein expression of Nanos3, Stra8, Scp3, GFRα1, CD90, VASA, Nanos2, KIT and PLZF in SSCs treated with NP was decreased significantly (P < 0.05) compared with that in untreated cells (Figure 5). These results indicated that NP inhibited the stemness maintenance and differentiation of SSCs.
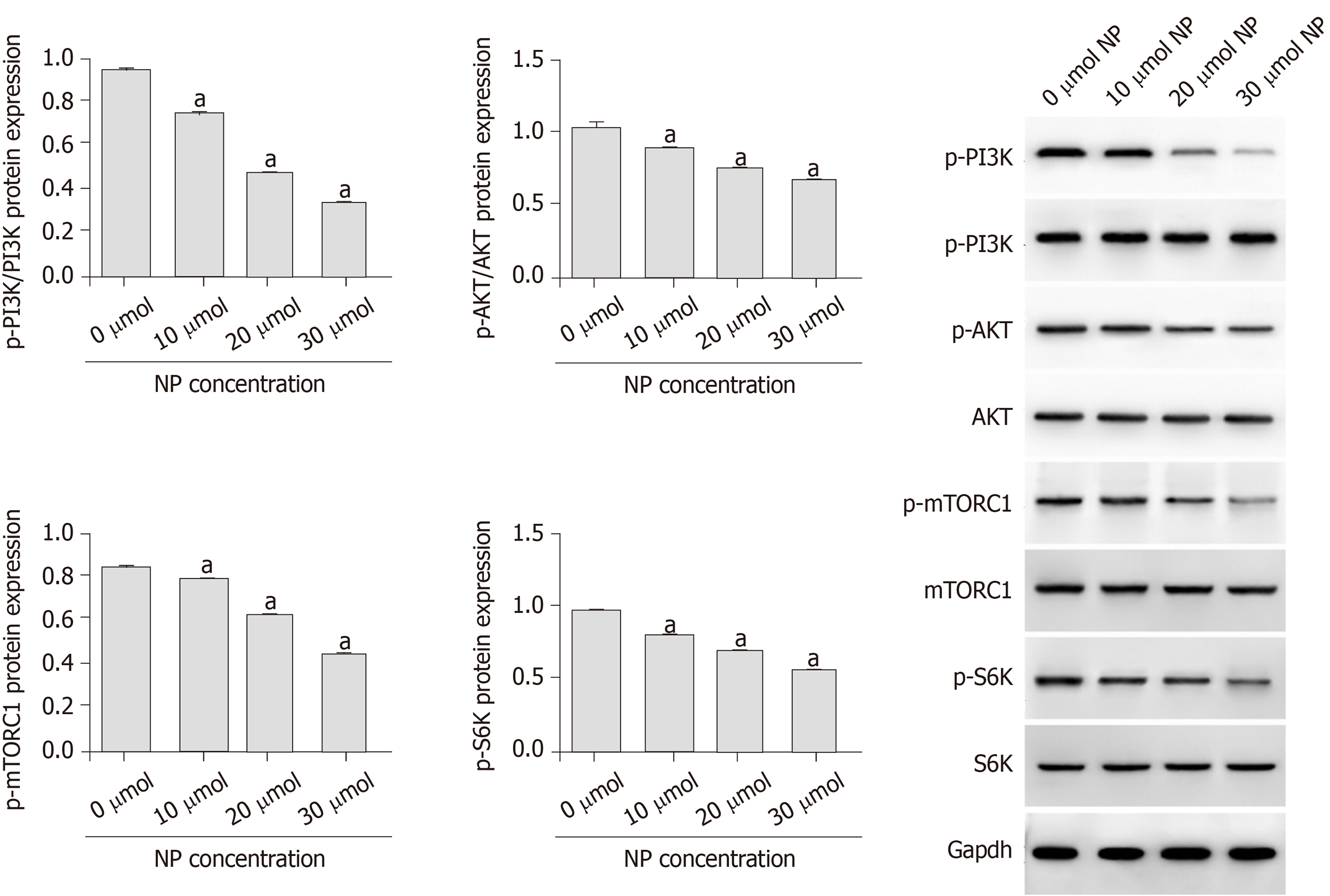
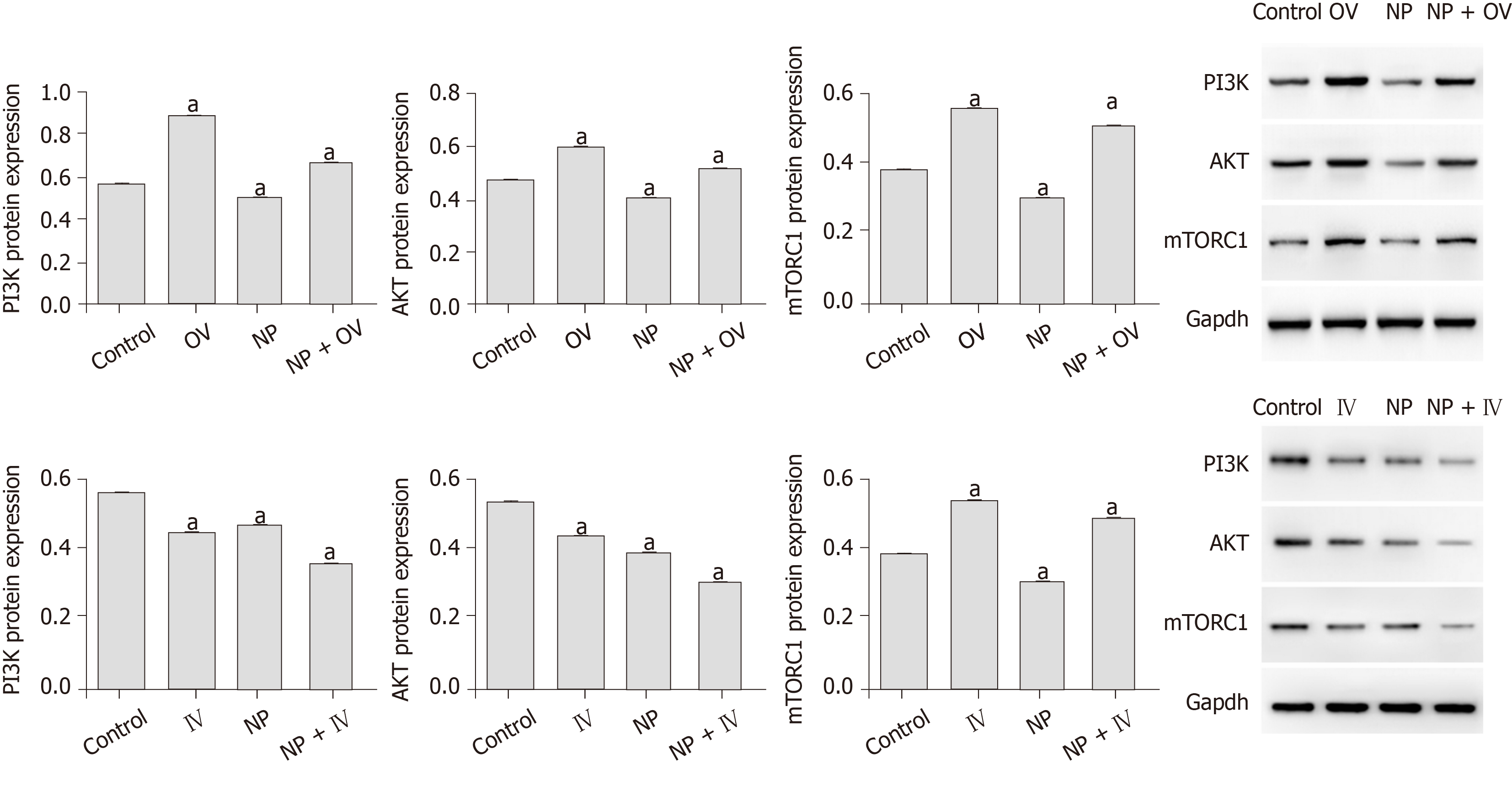
To investigate the influence of NP on PI3K/AKT/mTOR signaling in SSCs, we measured the protein expression of p-PI3K, p-Akt, p-mTORC1 and p-S6K using western blot (Figure 6). We observed that compared with untreated cells (0 μmol), the expression of p-PI3K, p-Akt, p-mTORC1 and p-S6K was significantly decreased by NP at all concentrations (P < 0.05), with 30 μmol NP exerting the greatest effect among all concentrations. We further observed the effect of NP on the PI3K/AKT/mTOR pathway in SSCs after activation or inhibition of mTOR. The results showed that after activation of mTOR, the expression of PI3K, AKT and mTORC1 protein in the NP group decreased significantly compared with the control group (P < 0.05), while that in the NP + OV group increased significantly (P < 0.05). When mTOR was inhibited, the trend reversed (P < 0.05) (Figure 7).
Numerous self-renewal systems exist in male mammals, but in terms of species continuity, the most important process is spermatogenesis, which is crucial in gene transmission for the generation and evolution of male gametes. A large number of sperm is produced via spermatogenesis through orderly and strict cell proliferation and differentiation, and SSCs form the basis of this system. NP is an important fine chemical raw material and intermediate that is mainly used in the production of surfactants, antioxidants, lubricant additives, pesticide emulsifiers, resins and rubber stabilizers and is known as a “sperm killer”[13]. NP has certain toxic effects on sperm, which are differentiated from SSCs. Shaliutina et al[14] found that NP induced oxidative stress in fish sperm, reduced sperm activity and destroyed the integrity of the sperm cell membrane. Meng et al[15] found that NP decreased the number and activity of sperm and increased the rate of sperm deformity with significant NP concentration-dependent response. Though the impact of NP on sperm health has been demonstrated, whether NP has similar toxic effects on SSCs is unclear.
While SSCs can maintain their relative number by self-proliferation, they also undergo mitosis and meiosis to form spermatocytes, which eventually form sperm. In addition, they have the potential to maintain self-renewal and proliferation to cope with possible damage[16]. The lifetime amplification and immortality of SSCs guarantee sperm production. In this experiment, we treated SSCs with different concentrations of NP for 7 consecutive days. We found that with the increase in NP concentration, the proliferation of SSCs decreased while apoptosis increased gradually. We detected apoptosis-related proteins and found that the pro-apoptotic proteins Bad, Cyt-c and pro-Caspase 9 were upregulated significantly, while the anti-apoptotic protein Bcl-2 was downregulated, suggesting that NP promoted apoptosis and inhibited SSC proliferation by inducing oxidative stress.
Stemness maintenance and differentiation potential are important characteristics of SSCs, and the continuous differentiation of SSCs is necessary for spermatogenesis. We showed that NP decreased the expression of markers of SSC stemness maintenance and differentiation. Stra8, which can be specifically activated by retinoic acid, is expressed in mouse embryonic ovary and adult testis, as well as before meiosis in mammalian germ cells[17,18]. Male Stra8-knockout mice were sterile and had severe defects in spermatogenesis and decreased testicular volume, suggesting that Stra8 plays a vital role in spermatogenesis[19]. Anderson et al[20] confirmed that Stra8 is not necessary for DNA replication in pre-filament spermatocytes but is essential for chromosome condensation and DNA homologous recombination in pre-filament spermatocytes during meiosis. Scp3 is an essential component of synaptonemal complex formation that plays an important role in male germ cell meiosis[21]. Nanos3 is a homologous gene of Drosophila Nanos that is specifically expressed in mouse germ cells. It is also an endogenous factor that initiates the process of male germ cell differentiation and plays a critical role in germ cell meiosis[22,23]. In this study, NP downregulated the expression of Stra8, Nanos3 and Scp3, suggesting that NP may inhibit the differentiation and stemness maintenance of SSCs by regulating pre-meiotic factors.
The PI3K/AKT/mTOR signaling pathway is essential for the proliferation of SSCs. The AKT/mTOR signaling pathway plays an important role in spermatogenesis by regulating the growth and transport of sperm cells in the testis and maintaining the normal function of SSCs[24,25]. The mTOR signal is directly involved in the late differentiation of SSCs. Inhibition of mTOR expression can lead to the accumulation of a large number of SSCs[26,27], and the promotion of mTOR expression can promote the proliferation of SSCs[28,29]. Duan et al[30] observed that NP inhibits the expression of mTOR and mediates autophagy, apoptosis and necrosis of sperm cells. Duan et al[31] also revealed that NP inhibits the activity of mTOR-p70S6K/4EBP1 signaling pathway and inhibits sperm differentiation and proliferation. Ma et al[32] found that Lin28a, a conserved RNA-binding protein, is involved in SSC development, pluripotency, stemness maintenance, proliferation and self-renewal through the PI3K/AKT/mTOR pathway. Huang et al[33] observed that exposure to NP during pregnancy led to endocrine dysfunction in male offspring, inhibition of AKT/mTOR signaling and induction of apoptosis and autophagy in testicular tissue. We showed that NP significantly reduced the expression of p-PI3K, p-Akt, p-mTORC1 and p-S6K, suggesting that the effect of NP may be exerted through the PI3K/AKT/mTOR signaling pathway.
NP is produced by biodegradation of NP polyoxyethylene ether and is soluble in water. NP polyoxyethylene ether is widely used in industry and household. NP is widely found in rivers and silts in Japan, the United States, Germany and other countries. In heavily polluted rivers in the United Kingdom, the concentration of NP is as high as 534 μg/L[34-36]. Similar findings have been found in some rivers in China, and the pollution of NP in China’s current water system has significantly exceeded that in foreign countries. Compared with the significant increase in the concentration of NP in water, the reproduction rate of wild animals and humans is greatly reduced[37,38]. More and more families are childless, and women have been criticized for their inability to reproduce. Gender discrimination has become increasingly serious worldwide. Male oligospermia or azoospermia is also one of the important reasons for the lack of offspring, which is often overlooked. In this experiment, we confirmed that NP can not only reduce the number and activity of sperm but also affect the proliferation and differentiation of SSCs. The mechanism may be related to the PI3K/AKT/mTOR pathway. Therefore, we speculate that promoting the activity of the PI3K/AKT/mTOR pathway may help relieve male oligozoospermia caused by NP.
In conclusion, our study demonstrated that NP reduced the proliferation, differentiation and stemness maintenance of SSCs while promoting apoptosis and oxidative stress, and the mechanism may be related to the PI3K/AKT/mTOR pathway. In future studies, we will investigate whether promoting PI3K/AKT/mTOR activity has therapeutic effects on NP-induced oligospermia.
Infertility has become a social problem that needs to be solved urgently in the world. Apart from the infertility caused by female causes, infertility caused by male oligozoospermia has gradually been valued. Previous studies have confirmed that nonylphenol (NP) widely used in daily life can reduce male sperm counts, but the underlying mechanism is still unclear. Studying the specific mechanism of NP-induced oligospermia could provide some ideas for the treatment of NP-induced oligospermia.
NP has been shown to affect sperm activity, but the mechanism is currently unknown. Spermatogonial stem cells (SCCs) can eventually differentiate into sperm. We aim to study whether NP can affect the proliferation, differentiation and potential mechanism of SSCs in order to provide ideas for clinical treatment of male oligospermia caused by NP.
To study the effect and potential mechanism of NP on SSCs.
SSCs were treated with NP at 0, 10, 20 or 30 μmol. MTT was used to detect the effect of NP on the proliferation of SSCs. Flow cytometry, reverse transcription polymerase chain reaction and western blot were used to detect the effect of NP on the proliferation, apoptosis, oxidative stress and stemness maintenance of SSCs. The effects of NP on phosphatidylinositol-3-kinase/protein kinase B/mammalian target of rapamycin (PI3K/AKT/mTOR) pathway was also measured by western blot.
Different concentrations of NP (10, 20 or 30 μmol) could inhibit the proliferation of SSCs, reduce the expression of cell differentiation and stem maintenance related factors and promote apoptosis and the release of oxidative stress factors. We further examined the effect of NP on the PI3K/AKT/mTOR pathway, and the results showed that NP can significantly inhibit the activity of the PI3K/AKT/mTOR pathway. Among all NP concentrations, 30 μmol had the greatest effect.
NP reduced the proliferation, differentiation and stemness maintenance of SSCs while promoting apoptosis and oxidative stress, and the mechanism may be related to the PI3K/AKT/mTOR pathway, providing a potential method for the treatment of male infertility.
In this study, we demonstrated in vitro that NP could promote apoptosis and oxidative stress of SSCs and reduce the proliferation, differentiation and stem maintenance of SSCs, and the mechanism may be related to the PI3K/AKT/mTOR pathway. Therefore, we speculate that promoting the activity of the PI3K/AKT/mTOR pathway may help relieve male oligozoospermia caused by NP, and we will use PI3K/AKT/mTOR pathway agonist to verify our conjecture in the following studies in vivo.
Manuscript source: Unsolicited manuscript
Specialty type: Cell and tissue engineering
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Cheon YP, Giovannetti E S-Editor: Dou Y L-Editor: Filipodia E-Editor: Xing YX
| 1. | Sharpe RM, Franks S. Environment, lifestyle and infertility--an inter-generational issue. Nat Cell Biol. 2002;4 Suppl:s33-s40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 74] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 2. | Huo X, Chen D, He Y, Zhu W, Zhou W, Zhang J. Bisphenol-A and Female Infertility: A Possible Role of Gene-Environment Interactions. Int J Environ Res Public Health. 2015;12:11101-11116. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 75] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 3. | Lee HJ, Chattopadhyay S, Gong EY, Ahn RS, Lee K. Antiandrogenic effects of bisphenol A and nonylphenol on the function of androgen receptor. Toxicol Sci. 2003;75:40-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 338] [Cited by in RCA: 325] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 4. | Laws SC, Carey SA, Ferrell JM, Bodman GJ, Cooper RL. Estrogenic activity of octylphenol, nonylphenol, bisphenol A and methoxychlor in rats. Toxicol Sci. 2000;54:154-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 433] [Cited by in RCA: 382] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 5. | Masuyama H, Hiramatsu Y, Kunitomi M, Kudo T, MacDonald PN. Endocrine disrupting chemicals, phthalic acid and nonylphenol, activate Pregnane X receptor-mediated transcription. Mol Endocrinol. 2000;14:421-428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 102] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 6. | Capela D, Dombret C, Poissenot K, Poignant M, Malbert-Colas A, Franceschini I, Keller M, Mhaouty-Kodja S. Adult male mice exposure to nonylphenol alters courtship vocalizations and mating. Sci Rep. 2018;8:2988. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 7. | Fang H, Cui Y, Wang Z, Wang S. Toxicological assessment of multi-walled carbon nanotubes combined with nonylphenol in male mice. PLoS One. 2018;13:e0200238. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 8. | Qiu YL. Effect of environmental endocrine disruptors on p-np on the function of supporting cells in rat testis and its mechanism [MSc. Thesis]. [Sichuan]: Sichuan University; 2005. Available from: http://cdmd.cnki.com.cn/article/cdmd-10610-2005147188.htm. |
| 9. | Yu M, Mu H, Niu Z, Chu Z, Zhu H, Hua J. miR-34c enhances mouse spermatogonial stem cells differentiation by targeting Nanos2. J Cell Biochem. 2014;115:232-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 82] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 10. | Huang W, Quan C, Duan P, Tang S, Chen W, Yang K. Nonylphenol induced apoptosis and autophagy involving the Akt/mTOR pathway in prepubertal Sprague-Dawley male rats in vivo and in vitro. Toxicology. 2016;373:41-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 54] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 11. | Rakhmanova V, Jin M, Shin J. Inhibition of Mast Cell Function and Proliferation by mTOR Activator MHY1485. Immune Netw. 2018;18:e18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 12. | Grasso CS, Tang Y, Truffaux N, Berlow NE, Liu L, Debily MA, Quist MJ, Davis LE, Huang EC, Woo PJ, Ponnuswami A, Chen S, Johung TB, Sun W, Kogiso M, Du Y, Qi L, Huang Y, Hütt-Cabezas M, Warren KE, Le Dret L, Meltzer PS, Mao H, Quezado M, van Vuurden DG, Abraham J, Fouladi M, Svalina MN, Wang N, Hawkins C, Nazarian J, Alonso MM, Raabe EH, Hulleman E, Spellman PT, Li XN, Keller C, Pal R, Grill J, Monje M. Functionally defined therapeutic targets in diffuse intrinsic pontine glioma. Nat Med. 2015;21:827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 99] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 13. | Uguz C, Varisli O, Agca C, Agca Y. Effects of nonylphenol on motility and subcellular elements of epididymal rat sperm. Reprod Toxicol. 2009;28:542-549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 30] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 14. | Shaliutina O, Shaliutina-Kolešová A, Lebeda I, Rodina M, Gazo I. The in vitro effect of nonylphenol, propranolol, and diethylstilbestrol on quality parameters and oxidative stress in sterlet (Acipenser ruthenus) spermatozoa. Toxicol In Vitro. 2017;43:9-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 15. | Meng Y, Xu WB, Li QZ, Cao YH, Qian QZ, Wang Q. Effects of nonylphenol on spermatogenic ability and testosterone in male mice. Huanjing Yu Zhiye Yixue. 2015;32:1067-1070. |
| 16. | Mahla RS, Reddy N, Goel S. Spermatogonial stem cells (SSCs) in buffalo (Bubalus bubalis) testis. PLoS One. 2012;7:e36020. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 17. | Snyder EM, Davis JC, Zhou Q, Evanoff R, Griswold MD. Exposure to retinoic acid in the neonatal but not adult mouse results in synchronous spermatogenesis. Biol Reprod. 2011;84:886-893. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 49] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 18. | Imudia AN, Wang N, Tanaka Y, White YA, Woods DC, Tilly JL. Comparative gene expression profiling of adult mouse ovary-derived oogonial stem cells supports a distinct cellular identity. Fertil Steril. 2013;100:1451-1458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 19. | Mark M, Jacobs H, Oulad-Abdelghani M, Dennefeld C, Féret B, Vernet N, Codreanu CA, Chambon P, Ghyselinck NB. STRA8-deficient spermatocytes initiate, but fail to complete, meiosis and undergo premature chromosome condensation. J Cell Sci. 2008;121:3233-3242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 171] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 20. | Anderson EL, Baltus AE, Roepers-Gajadien HL, Hassold TJ, de Rooij DG, van Pelt AM, Page DC. Stra8 and its inducer, retinoic acid, regulate meiotic initiation in both spermatogenesis and oogenesis in mice. Proc Natl Acad Sci USA. 2008;105:14976-14980. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 499] [Cited by in RCA: 465] [Article Influence: 27.4] [Reference Citation Analysis (0)] |
| 21. | Hou Y, Wu YJ, Luo FH, Su HN, Zhang XM, Zhang Y, Bai YBT, Liu TD. Cloning of Scp3 gene in cashmere goats and the occurrence of the first round of meiosis in testicles. Zool Res. 2009;30. [DOI] [Full Text] |
| 22. | Mochizuki K, Tachibana M, Saitou M, Tokitake Y, Matsui Y. Implication of DNA demethylation and bivalent histone modification for selective gene regulation in mouse primordial germ cells. PLoS One. 2012;7:e46036. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 23. | Yu M, Lian ZM, Hua JL. Nanos 3 and germ cell development and differentiation. Zhongguo Shengwuhuaxue Yu Fenzishengwu Xuebao. 2012;4:326. |
| 24. | Riera MF, Regueira M, Galardo MN, Pellizzari EH, Meroni SB, Cigorraga SB. Signal transduction pathways in FSH regulation of rat Sertoli cell proliferation. Am J Physiol Endocrinol Metab. 2012;302:E914-E923. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 82] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 25. | Quan C, Wang C, Duan P, Huang W, Yang K. Prenatal bisphenol a exposure leads to reproductive hazards on male offspring via the Akt/mTOR and mitochondrial apoptosis pathways. Environ Toxicol. 2017;32:1007-1023. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 36] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 26. | Busada JT, Niedenberger BA, Velte EK, Keiper BD, Geyer CB. Mammalian target of rapamycin complex 1 (mTORC1) Is required for mouse spermatogonial differentiation in vivo. Dev Biol. 2015;407:90-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 80] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 27. | Boyer A, Girard M, Thimmanahalli DS, Levasseur A, Céleste C, Paquet M, Duggavathi R, Boerboom D. mTOR Regulates Gap Junction Alpha-1 Protein Trafficking in Sertoli Cells and Is Required for the Maintenance of Spermatogenesis in Mice. Biol Reprod. 2016;95:13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 58] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 28. | Feng LX, Ravindranath N, Dym M. Stem cell factor/c-kit up-regulates cyclin D3 and promotes cell cycle progression via the phosphoinositide 3-kinase/p70 S6 kinase pathway in spermatogonia. J Biol Chem. 2000;275:25572-25576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 192] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 29. | Busada JT, Chappell VA, Niedenberger BA, Kaye EP, Keiper BD, Hogarth CA, Geyer CB. Retinoic acid regulates Kit translation during spermatogonial differentiation in the mouse. Dev Biol. 2015;397:140-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 109] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 30. | Duan P, Hu C, Quan C, Yu T, Zhou W, Yuan M, Shi Y, Yang K. 4-Nonylphenol induces apoptosis, autophagy and necrosis in Sertoli cells: Involvement of ROS-mediated AMPK/AKT-mTOR and JNK pathways. Toxicology. 2016;341-343:28-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 116] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 31. | Duan P, Hu C, Quan C, Yu T, Huang W, Chen W, Tang S, Shi Y, Martin FL, Yang K. 4-Nonylphenol induces autophagy and attenuates mTOR-p70S6K/4EBP1 signaling by modulating AMPK activation in Sertoli cells. Toxicol Lett. 2017;267:21-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 59] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 32. | Ma F, Zhou Z, Li N, Zheng L, Wu C, Niu B, Tang F, He X, Li G, Hua J. Lin28a promotes self-renewal and proliferation of dairy goat spermatogonial stem cells (SSCs) through regulation of mTOR and PI3K/AKT. Sci Rep. 2016;6:38805. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 33. | Huang WT. Role of Akt/mTOR signaling pathway in male reproductive toxicity induced by nonylphenol. [Ph.D. Thesis]. [Wuhan]: Huazhong University of Science and Technology; 2017. Available from: http://cdmd.cnki.com.cn/Article/CDMD-10487-1017166921.htm. |
| 34. | Verslycke TA, Vethaak AD, Arijs K, Janssen CR. Flame retardants, surfactants and organotins in sediment and mysid shrimp of the Scheldt estuary (The Netherlands). Environ Pollut. 2005;136:19-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 104] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 35. | Jin X, Huang G, Jiang G, Zhou Q, Liu J. Distribution of 4-nonylphenol isomers in surface water of the Haihe River, People's Republic of China. Bull Environ Contam Toxicol. 2004;73:1109-1116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 36. | Johnson AC, Aerni HR, Gerritsen A, Gibert M, Giger W, Hylland K, Jürgens M, Nakari T, Pickering A, Suter MJ, Svenson A, Wettstein FE. Comparing steroid estrogen, and nonylphenol content across a range of European sewage plants with different treatment and management practices. Water Res. 2005;39:47-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 151] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 37. | Colborn T, vom Saal FS, Soto AM. Developmental effects of endocrine-disrupting chemicals in wildlife and humans. Environ Health Perspect. 1993;101:378-384. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2242] [Cited by in RCA: 1997] [Article Influence: 62.4] [Reference Citation Analysis (0)] |
| 38. | McLachlan JA, Korach KS. Symposium on estrogens in the environment, III. Environ Health Perspect. 1995;103 Suppl 7:3-4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 71] [Article Influence: 2.4] [Reference Citation Analysis (0)] |