Published online Nov 26, 2020. doi: 10.4252/wjsc.v12.i11.1396
Peer-review started: June 1, 2020
First decision: August 22, 2020
Revised: September 4, 2020
Accepted: September 25, 2020
Article in press: September 25, 202
Published online: November 26, 2020
Processing time: 178 Days and 3.5 Hours
Emerging evidence suggests that the spread of glioma to the subventricular zone (SVZ) is closely related to glioma recurrence and patient survival. Neural stem cells (NSCs) are the main cell type in the SVZ region and exhibit tumor-homing ability.
To evaluate the effects of conditioned medium (CM) derived from SVZ NSCs on the cancer-related behaviors of glioma cells.
The characteristics of SVZ hNSCs were identified by immunofluorescence. The normoxic-hNSC-CM and hypoxic-hNSC-CM (3% O2, oxygen-glucose deprived [OGD] culturing) were collected from 80%-90% confluent SVZ NSCs in sterile conditions. The CCK8 and Transwell assays were used to compare and evaluate the effects of normoxic-CM and hypoxic-CM on glioma proliferation and invasion. Then proteins secreted from SVZ NSCs into the CM were investigated by mass spectrometry, and the potential effects of candidate protein NCAN in the regulation of glioma progression were examined by CCK8 and Transwell assays.
The CM from SVZ NSCs significantly increased the proliferation and invasion of glioma cells, particularly the CM from OGD NSCs induced under hypoxic conditions. Furthermore, the secreted protein neurocan (NCAN) in CM from OGD NSCs was identified by proteomic analysis. NCAN was expressed in glioma cells and played regulatory roles in mediating the progression of glioma cells mainly via the Rho/Rho-associated protein kinase pathway.
Our study identified a potential interactive mechanism between SVZ NSCs and glioma cells, in which SVZ NSCs promote glioma progression via the secreted protein NCAN. These findings suggested that exploring the CM derived from cells could be a novel strategy for optimizing treatments and that NCAN derived from SVZ NSCs may be a potential new target in glioma progression.
Core Tip: The cell communications or crosstalk between glioma cells and non-glioma cells in the tumor microenvironment plays dominant roles in tumor therapy. The subventricular zone (SVZ) neural stem cells (NSCs) have been shown to be closely associated with glioma progression and recurrence. Interestingly, the conditioned medium (CM) from SVZ NSCs significantly promoted glioma cell proliferation and invasion, with oxygen-glucose deprived NSC-CM showing enhanced effects. Furthermore, the secreted protein NCAN presented in oxygen-glucose deprived NSC-CM played important roles in the progression of glioma cells mainly via the Rho/Rho-associated protein kinase pathway. Overall, this study provided important insights into the potential therapeutic application of NCAN derived from SVZ NSCs in the treatment of glioma.
- Citation: Zhang GL, Qian C, Zhang SZ, Tuo YH, Zeng BY, Ji YX, Wang YZ. Effect of conditioned medium from neural stem cells on glioma progression and its protein expression profile analysis. World J Stem Cells 2020; 12(11): 1396-1409
- URL: https://www.wjgnet.com/1948-0210/full/v12/i11/1396.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v12.i11.1396
Glioma is a common type of primary malignant tumor found in the brain. Glioblastoma multiforme (GBM) is the most aggressive type of primary brain tumor and is associated with a high mortality rate. Less than 6% of patients with GBM survive beyond 5 years, and the median overall survival is approximately 15-23 mo[1]. Currently, there are no effective treatment options for GBM. Despite extensive studies of novel therapeutic approaches, most strategies still lead to drug resistance or eventual tumor recurrence[2,3]. Recent studies have suggested that GBMs are heterogeneous, characterized by genetic, epigenetic, and molecular variations among cells[2-4]. These features are mainly affected by the tumor microenvironment (TME) surrounding the GBM. Thus, GBM growth occurs before the cells are able to communicate with or be manipulated by other cells (non-glioma cells) via secreted cytokines, growth factors, and chemokines in the TME, thereby promoting tumor progression and drug resistance[4-6].
GBM cells not only interact with immune cells, including microglia/macrophages, to alter their phenotypes and support tumor growth but also communicate with normal brain cells, including neurons, astrocytes, and endothelial cells, to create a favorable microenvironment for tumor proliferation[7-13]. Chen et al[12] found that PTEN deficiency in GBM increases macrophage infiltration via the YAP1-LOXb1 integrin-PYK2 axis. Additionally, the infiltrated macrophages secrete SPP1 to sustain glioma cell survival and stimulate angiogenesis. Venkatesh et al[11] showed that active neurons can promote glioma proliferation and growth. They used conditioned medium (CM) from optogenetically stimulated cortical slices in co-cultures with glioma cells and found that this CM significantly promoted cell proliferation. They identified synaptic protein neuroligin-3 present in active CM using a quantitative mass spectrometry approach. Moreover, Gao et al[14] showed that the communication mediated by extracellular vesicles (EVs) derived from glioma cells increased the synaptic activity of neurons. Blocking EV release caused a reduction in glioma growth in vivo. Henrik Heiland et al[10] also reported a distinct transcriptional phenotype of reactive astrocytes from glioblastoma in the tumor environment. They revealed a complex interaction of tumor-associated astrocytes and microglia cells, which induced an immunosup-pressive microenvironment. Griveau et al[9] showed that distinct glioma-vascular microenvironmental interactions may regulate glial-encoded pathways. They found that targeted Wnt7a/7b deletion or pharmacologic Wnt inhibition blocked Olig2+ glioma single-cell vessel co-option and enhanced responses to temozolomide. Thus, these findings indicated that glioblastoma involves multiple modes of cell communication and that the multidimensional cell communications or crosstalk between glioma cells and non-glioma brain cells in the TME may play dominant roles in glioma therapy[14,15].
Notably, the growth, invasion, and recurrence of GBM are known to be related to the subventricular zone (SVZ) of the lateral ventricles[16-18]. GBM contacting the SVZ is thought to have a higher risk of recurrence. Moreover, the involvement of the SVZ in glioma is associated with more rapid progression and further decrease in the overall survival of patients with GBM[16-18]. The SVZ is one of two dominating neurogenic zones located near neural stem cells (NSCs) in the adult brain. However, communication or crosstalk between neural stem cells in the SVZ (SVZ NSCs) and GBM via the TME remains poorly understood, especially the roles of SVZ NSCs in regulating glioma progression are still unclear.
Accordingly, in this study, we investigated the functions of SVZ NSCs in gliomas using CM derived from SVZ NSCs in cocultures with glioma cells. We also explored the potential functional molecules present in the CM by proteomics analysis. Our results provided important insights into the mechanisms of glioma invasion and suggested potential targets in the treatment of GBM.
SVZ NSCs obtained from the human fetal brain tissue were kept in our laboratory (approved by the Institutional Review Board of Zhongda Hospital Southeast University), as previously described[19-21]. NSCs were cultured in serum-free DMEM/F12 medium (Gibco) supplemented with 2% B27 (Gibco), 20 ng/mL basic FGF (bFGF, PeproTech), 20 ng/mL EGF (PeproTech), 2 nmol/L L-glutamine (Invitrogen), 2 μg/mL heparin sodium, and 1% penicillin-streptomycin (Gibco), and incubated at 37 °C in an incubator containing 5% CO2. Fresh medium was changed every 3 d, and cells were passaged at the 7th day. Neurospheres were detached to single cells with Accutase Dissociation Reagent (Sigma). In brief, cCM was collected from 80%-90% confluent SVZ NSCs in sterile conditions after culturing for 3 d, sequentially centrifuged to remove intact cells, and then filtered using a filter unit (Millipore) with a 0.22 μm membrane to remove cell debris. The concentration of CM was qualified by BCA method, and the same concentrations (from 1.5 μg/uL to 2.5 μg/uL) of normoxic-CM and hypoxic-CM were used for subsequent experiments.
Human glioma cell lines U87 MG and U251 MG were preserved in our laboratory, obtained from American Type Culture Collection. Glioma cells were maintained at 37 °C in 5% CO2 in DMEM (Gibco) supplemented with 10% fetal bovine serum (FBS, Hyclone) and 1% penicillin/streptomycin (Gibco). Fresh medium was changed every 2 d, and cells were passaged at the 4th day.
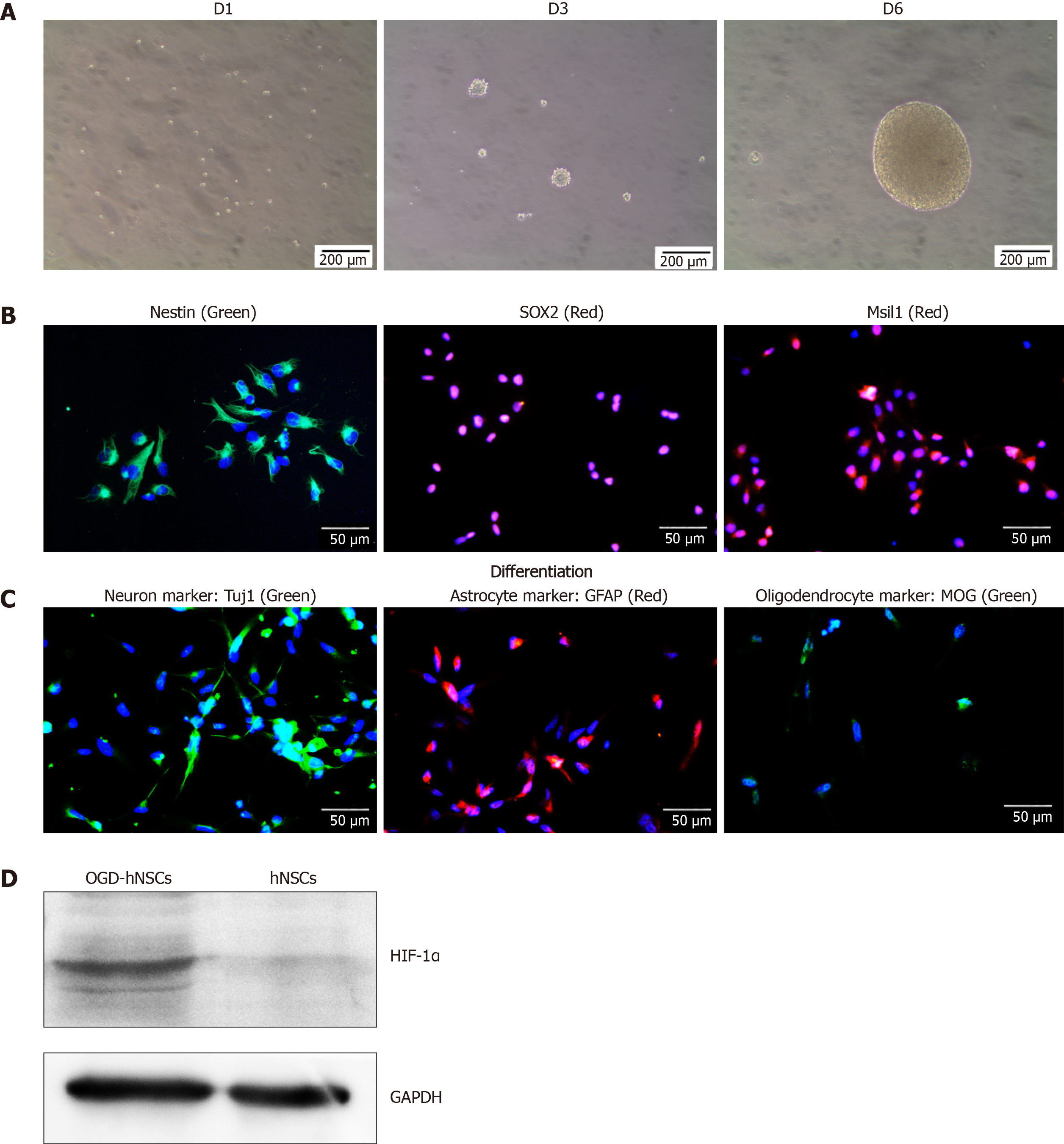
To evaluate the characteristic of NSCs, cells were incubated in a 24 well plate coated with laminin (Sigma) at 5 × 104 cells per well with complete medium. To induce cell differentiation, NSCs were dissociated with Accutase and cultured in differentiation medium (DMEM/F12 supplemented with 2% FBS and 1% Penicillin-Streptomycin, without growth factors) for 7 d. For immunofluorescence, cells were fixed in 4% paraformaldehyde, followed by Triton X-100 (Sigma), and then blocked in 10% donkey serum (Jackson). Then cells were incubated with different primary antibodies: Rabbit anti-Sox2 (1:500, Abcam), anti-Musashi1 (1:500, Abcam), mouse anti-Nestin (1:500, Abcam), anti-beta III-tubulin (Tuj1, 1:400, Abcam), goat anti-Myelin oligodendrocyte glycoprotein, (1:400, Abcam), and rabbit anti-GFAP (1:400, Bioss) overnight. Next day, the secondary antibodies donkey anti-rabbit-Alexa Fluor 647/488, anti-goat-Alexa Fluor 555/488(1:600, Abcam), or donkey anti-mouse-Alexa Fluor 488 (1:600, Jackson) were added. Finally, following staining with DAPI, the slides were cover slipped for capturing on a fluorescence microscope.
For OGD culturing, SVZ NSCs were incubated in the flask for 3 d. After new fresh medium was changed, cells were transferred into a CO2/O2 trigas incubator (Thermo) containing 3% O2 and 5% CO2 at 37 °C. Oxygen and CO2 contents were continuously maintained at a constant level by the tri-gas incubator for 3 d. Then cells and CM were collected. To confirm the OGD-NSCs model in vitro, the proteins of cells were extracted and collected for Western blot analysis.
The glioma cells were seeded in 96-well plates for cell viability analysis. The cells were cultured with fresh medium, different CM of SVZ NSCs, or recombinant protein NCAN of different concentrations for 24-48 h, and then 10 μL CCK-8 (KeyGen) was added into each well. After incubation for 1-4 h, absorbance value for each well was calorimetrically determined. This experiment was repeated at least three times.
U87 MG and U251 MG cells were added into the upper chamber of the 8 μm pore polycarbonate membrane (Transwell, Corning) at 5 × 104 cells and 2 × 104 cells per well with serum-free medium in the 24-well plates, and 800 μL of complete medium was added into the lower chamber to cultivate for 12-16 h. The 8 μm pore polycarbonate membrane was pre-coated with Matrigel (BD Biosciences). Then the liquid in the upper chamber was discarded, and the pores were fixed with 4% paraformaldehyde for 30 min, followed by staining with 0.1% crystal violet for 30 min. The cells that had migrated across the membrane were observed and photographed in six independent fields for each well under an inverted microscope.
CM was collected from normoxic and hypoxic SVZ NSCs cultured in serum-free complete medium after incubation for 3 d. The proteins were detected by Label Free Quantitative proteomics technology (Shanghai Applied Protein Technology). The process mainly includes protein extraction, peptide digestion, chromatographic fractionation, data collection by LC-MS/MS, protein identification and quantitative analysis, screening and cluster analysis of differentially expressed proteins, functional annotation, and pathway analysis. Proteins that were up- or down- regulated more than 1.5-fold (P < 0.05) in hypoxic and normoxic NSC-CM were further analyzed. Blast2Go (https://www.blast2go.com/com/) was used for functional annotation in the Gene Ontology (GO) database (http://www.geneontology.org) on all proteins identified in this project, and then GO function enrichment analysis was performed on differentially expressed proteins by Fisher's exact test method. The top 20 enriched biological pathways in the GO analysis were selected.
Total protein was extracted from glioma cells with lysis buffer (SD-001, Invent), and quantified with a BCA protein assay kit (Pierce). To perform Western blot analysis, equal amount of protein (20-40 μg) of each sample was subjected to sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS–PAGE, Solarbio) and transferred onto polyvinylidene difluoride membranes (PVDF, Millipore). PVDF was blocked with TBST containing 5% non-fat milk at room temperature for 1h, and then incubated with diluted primary antibodies at 4°C overnight, including anti-HIF1α (1:1000, Proteintech), anti-NCAN (1:1000, Abcam), anti-RhoA and anti-Rho-associated protein kinase (ROCK) (1:1000, Bioss), anti-β-actin(1:5000, Jackson), and anti-GAPDH (1:5000, Proteintech). The next day, the membranes were incubated with horseradish peroxidase conjugated goat anti rabbit or rabbit anti-goat secondary antibody (1:20000, Jackson) at room temperature for 1h, and then reacted with an enhanced ECL substrate (Pierce). Beta-actin was used as a control according to the protocol.
Data analyses and statistics were performed with GraphPad Prism version 8. The results are presented as the mean ± standard deviation (x ± s) of at least three independent experiments. Comparisons were performed by t-tests or ANOVA analysis. A P value < 0.05 was considered statistically significant, with P < 0.05 as significant and P < 0.01 as very significant.
SVZ NSCs were cultured in serum-free complete medium, and rapidly formed nonadherent neurospheres (Figure 1A). NSC special markers, including Nestin, SOX2, and Msil1, were identified by immunofluorescence (Figure 1B). After differentiation, NSCs were incubated in differentiation medium and showed significant expression of Tuj1 (a neuron marker), GFAP (an astrocyte marker), and myelin oligodendrocyte glycoprotein (an oligodendrocyte marker) by immunofluorescence (Figure 1C). These results identified the characteristics of NSCs. After culturing in hypoxia (3% O2, OGD conditions), the OGD NSCs showed significant overexpression of the hypoxic marker HIF-1α (Figure 1D). Subsequently, we collected the CM derived from normoxic and OGD preconditioned SVZ NSCs via centrifuging and filtration to enable us to discard cells and cell debris.
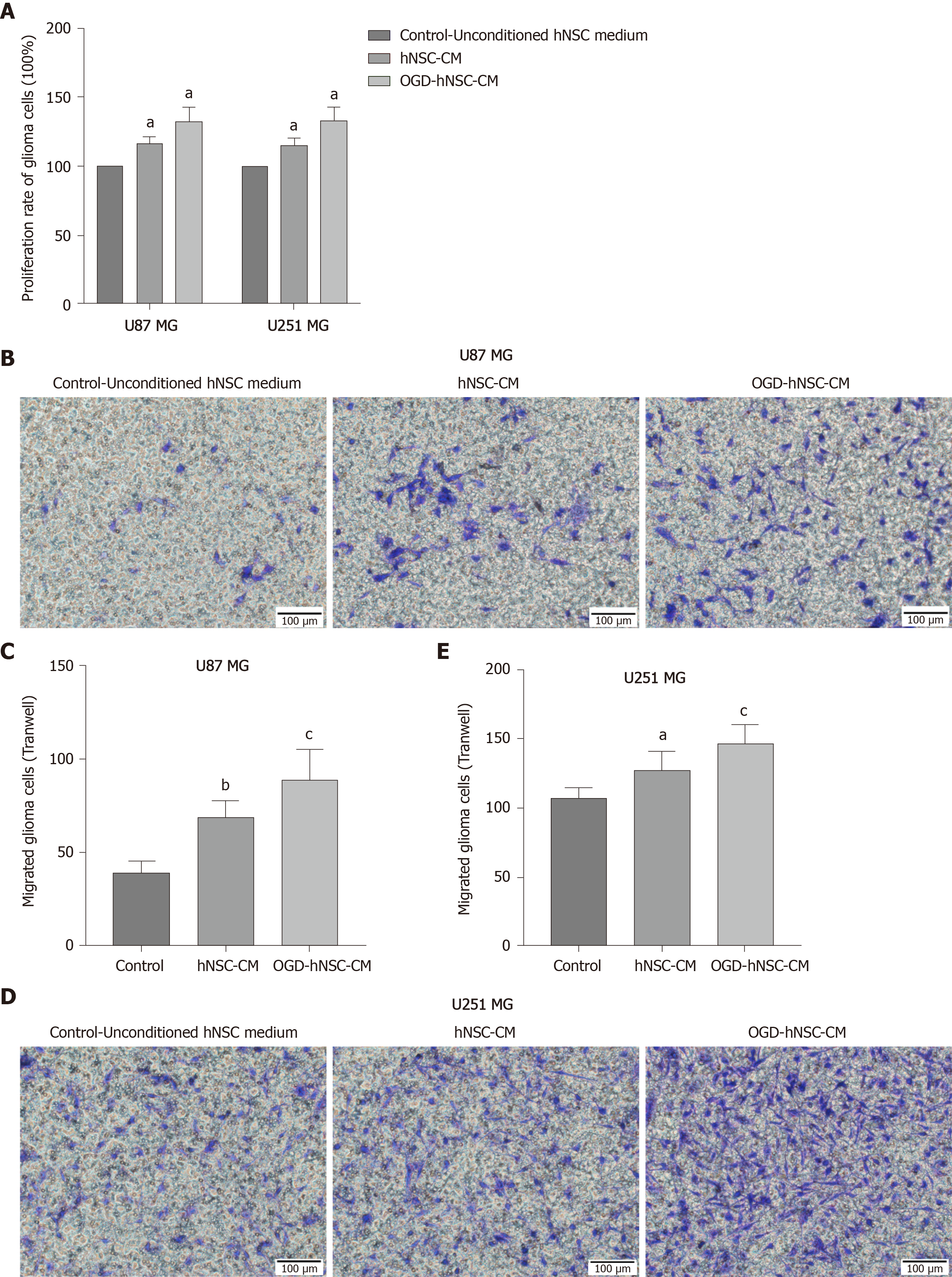
Next, we analyzed the effects of CM derived from SVZ NSCs on glioma proliferation and compared the results for normoxic-CM and hypoxic-CM using CCK8 assays. Our results showed that the rate of glioma cell proliferation was increased in the NSC-CM group compared with that in the control group, and the effect of OGD-CM was significantly superior to that of normoxic-CM (P < 0.05; Figure 2A). These findings indicated that OGD-NSC-CM further improved glioma cell activity. Moreover, Transwell invasion experiments revealed that NSC-CM promoted the invasion of tumor cells compared with that of control cells (Figure 2B-E), particularly in the OGD-CM group. These results showed that the proliferation and invasion of glioma cells were improved after co-incubation of NSC-CM with U87 and U251 glioma cells. Furthermore, hypoxia enhanced the effects of NSC-CM in glioma cells. Taken together, these findings confirmed the roles of NSC-CM in promoting glioma cell proliferation and invasion.
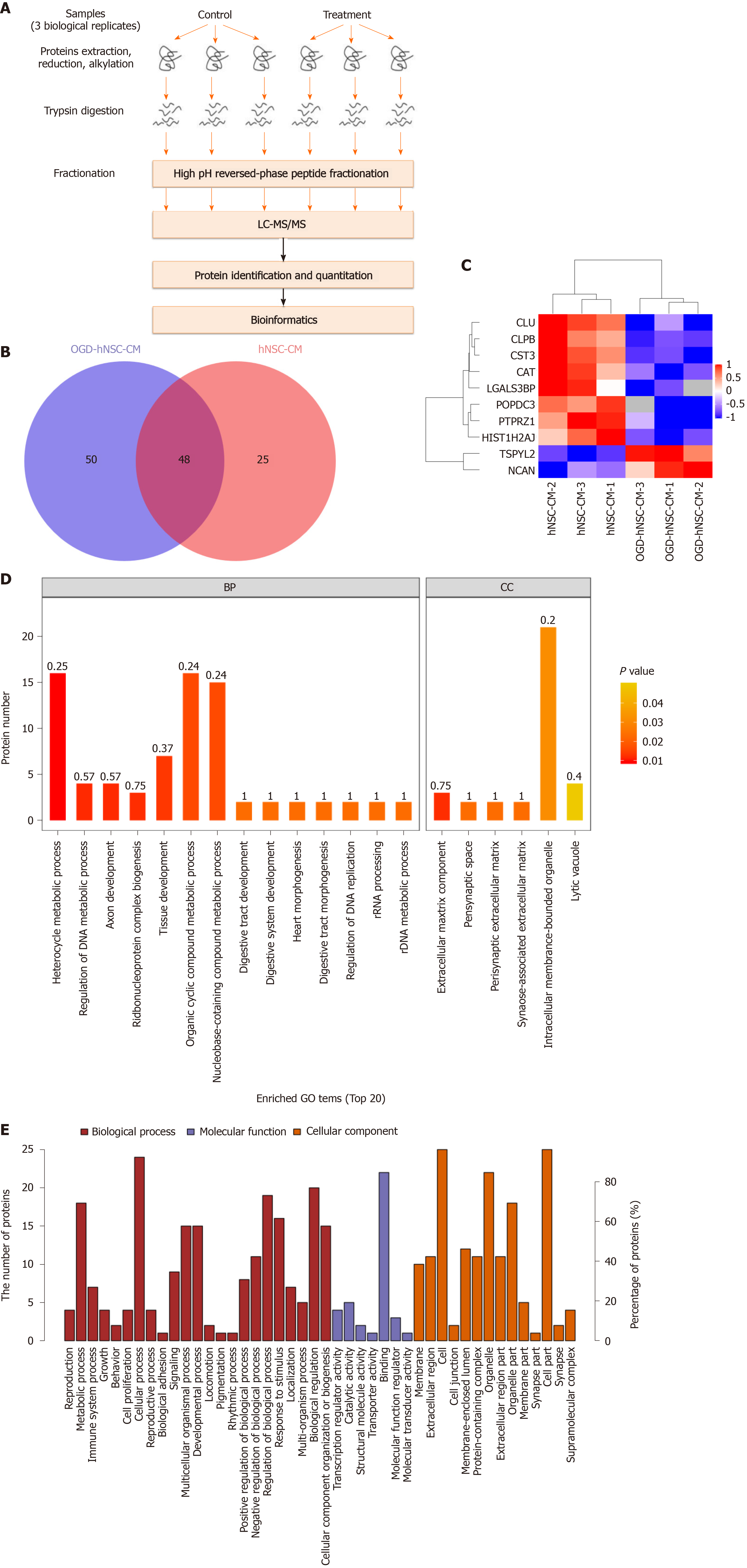
Next, proteins secreted from SVZ NSCs into the CM were investigated by protein mass spectrometry. The signal intensities of proteins from normoxic and hypoxic CM were detected and analyzed by SDS-PAGE. Proteomic analysis was performed with three pairs of biological replicates from SVZ NSC-CM, and OGD-NSC-CM was compared with normoxic NSC-CM. The experimental workflow was performed as shown in Figure 3A, and approximately 73–98 proteins were identified in each sample (Figure 3B). Proteins with a fold change of at least 1.5 and P value of less than 0.05 for the comparison between hypoxic and normoxic CM were chosen for further analysis. The results showed that two proteins were upregulated (TSPYL2 and NCAN) and eight were downregulated (PTPRZ1, HIST1H2AJ, CLPB, CST3, POPDC3, CAT, CLU, and LGALS3BP) in the CM of hypoxic NSCs compared with that in normoxic NSCs (Figure 3C).
Next, GO analysis was used to determine the top 20 enriched biological processes involving the differentially expressed proteins (Figure 3D). The biological process domain was enriched in the terms of cellular process, metabolic process, biological regulation, and regulation of biological process; the molecular function domain was enriched in the terms of binding and protein binding; moreover, the cellular component domain was enriched in the terms of cell, cell part, organelle, and organelle part (Figure 3E). Furthermore, the upregulated proteins in the hypoxic NSC-CM group were related to the progression of gliomas. The two upregulated proteins identified by MS included the nucleosome assembly protein TSPYL2, and the chondroitin sulfate proteoglycan protein NCAN. TSPYL2 is characterized by a predicted nucleosome assembly protein domain for nucleosome remodeling and gene expression regulation and functions as a tumor suppressor, cell growth regulator, and DNA damage response mediator. NCAN, which is primarily expressed in the central nervous system, accumulates in the matrix surrounding axonal bundles and neuronal cells and is involved in the modulation of cell adhesion, invasion, and migration through interactions with a variety of matrix or transmembrane molecules. Thus, we next evaluated the potential effects of the candidate protein NCAN in the regulation of glioma progression.
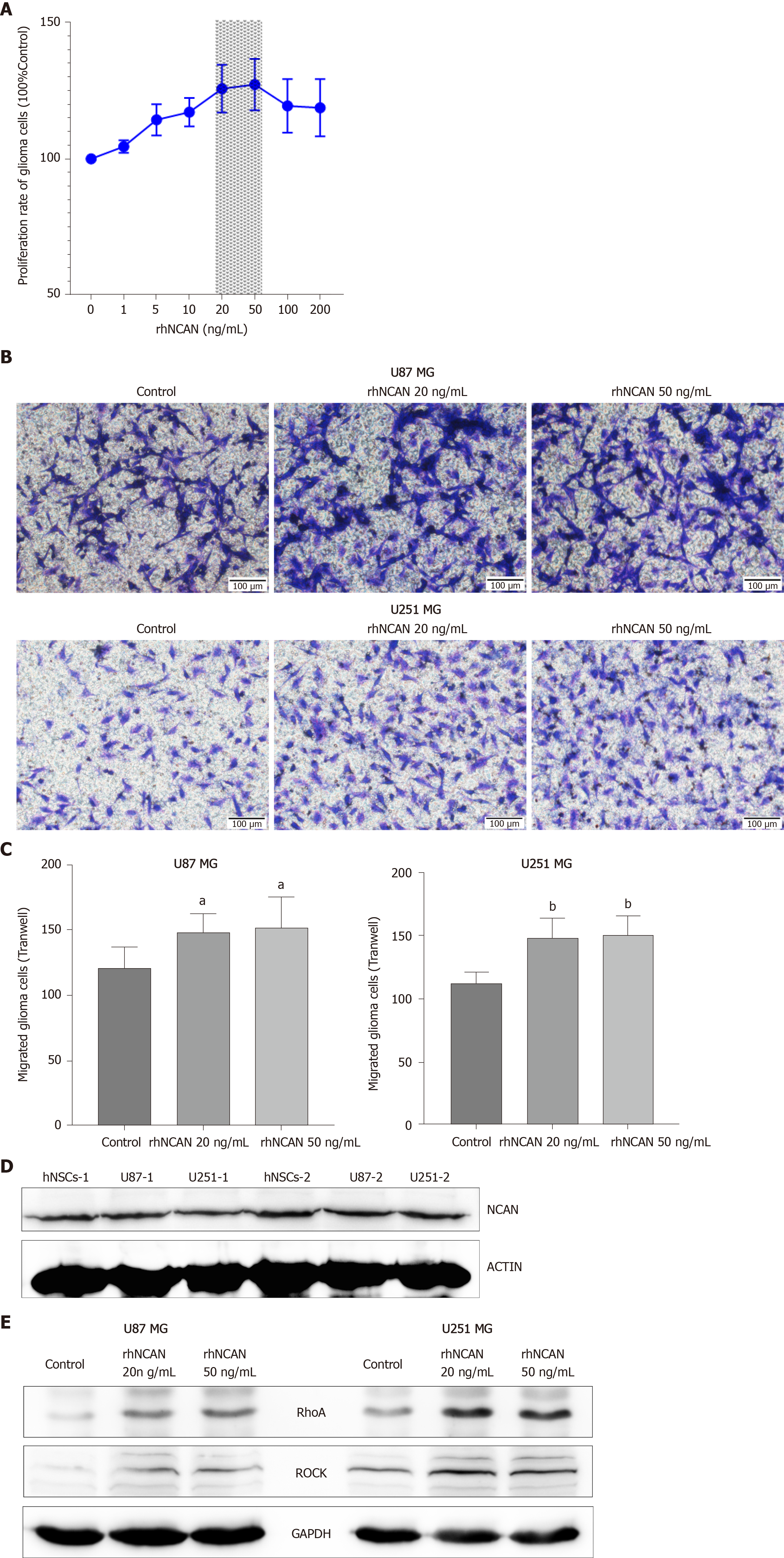
Based on the above findings, we next evaluated the potential roles of NCAN in glioma progression by incubating glioma cells with different concentrations (1–100 ng/mL) of recombinant human protein NCAN (rhNCAN). The results showed that rhNCAN increased the proliferation of glioma cells in a concentration-dependent manner, particularly at concentrations of 20 and 50 ng/mL (Figure 4A). We then examined the effects of rhNCAN on glioma cell invasion. Notably, cultivation of glioma cells with rhNCAN significantly augmented the invasion ability of glioma cells compared with that in the untreated control group (Figure 4B and C). Thus, these results suggested that NCAN had certain effects on glioma cells and mediated the proliferation and invasion of tumor cells. In addition, NCAN was also significantly expressed in hNSCs and glioma cells (Figure 4D), and treatment of glioma cells with rhNCAN resulted in dramatically increased expression levels of RhoA and ROCK compared with the control group, as demonstrated by Western blot analysis (Figure 4E). These findings suggested that the Rho–ROCK pathway may be mediated by NCAN during glioma progression and may be downstream of the NCAN signaling pathway.
In this study, we showed that culture of glioma cells with SVZ NSC-derived CM under hypoxic conditions further increased glioma cell proliferation and invasion compared with normoxic NSC-CM. The regulatory effects induced by OGD-NSC-CM were associated with NCAN, which was secreted from OGD NSCs. Moreover, NCAN was expressed in glioma cells and played an important role in mediating the progression of tumor cells mainly via the Rho/ROCK pathway.
GBM is an aggressive and incurable brain tumor, which harbors many gene mutations and genetic abnormalities, such as alterations in the neurofibromin 1, TP53, isocitrate dehydrogenase (IDH), and PTEN genes, which are related to the proliferation of glioma cells, and the forkhead box G1 and SOX2 genes, which affect the self-renewal of glioma cells[22-24]. Improving our understanding of the origin or disease progression of gliomas could provide a fundamental basis for developing new treatments. Recently, NSCs in the SVZ region have been considered as the origin of gliomagenesis. Moreover, Lee et al[25] found that normal SVZ tissue located at a distance from the tumor in 56.3% of patients with wild-type IDH GBM contained low levels of GBM driver mutations. Additionally, they suggested that astrocyte-like NSCs harboring driver mutations may have migrated from the SVZ and facilitated the development of high-grade malignant gliomas in distant brain regions. Bardella et al[22] showed that the expression of IDH1R132H in the SVZ of the adult mouse brain caused gliomagenesis and tumor infiltration.
Emerging data have confirmed that glioma spread to the SVZ is closely associated with decreased survival of patients and increased tumor recurrence[15,17,18,26], such as patients with GBMs contacting SVZ region have lower survival rates than those with GBMs contacting the subgranulazr zone, corpus callosum, or cortex. Clinical studies of radiation treatment of SVZ NSCs in patients with GBM have found that incidental radiation to the SVZ is robustly correlated with progression-free survival in patients[17,27]. However, direct genetic evidence, which may improve prognoses in patients with GBM, is still lacking. Although glioma often spreads to the SVZ, cell communication between NSCs and gliomas have not been thoroughly evaluated.
Recently, a novel strategy using CM derived from cells has been evaluated for the treatment of various diseases. Li et al[28] showed that the CM from human menstrual blood-derived endometrial stem cells had protective effects on neurotoxicant 1-methyl-4-phenylpyridinium (MPP+)-induced cytotoxicity and inflammation in patients with Parkinson’s disease and found at least 12 neurotrophic factors in the CM by protein assays, suggesting that these factors may contribute to the protective effects against Parkinson’s disease. Baez-Jurado et al[29] indicated that the CM of human adipose mesenchymal stem cells improved the viability of astrocytes, reduced nuclear fragmentation of cells, and improved the mitochondrial membrane potential of astrocytes. Additionally, Bordoni et al[30] cultured human CD34 cells for several weeks in hepatocyte CM and found that the expression of CD34 and CD133 markers was gradually decreased, whereas the expression of CD144 and CD14 cell markers was increased in vitro and in vivo. These findings indicated that the hematopoietic cells differentiated into endothelial cells following induction by hepatocyte CM in the cellular microenvironment. Gharaei et al[31] showed that CM from human dental pulp stem cells significantly accelerated the adhesion phase (from sedimentation to attachment and spreading), the proliferation rate, and migration of human umbilical vein endothelial cells. Doeppner et al[32] intravenously applied CM derived from SVZ neural progenitor cells in male C57BL6 mice with focal cerebral ischemia and found that CM dose-dependently reduced infarct volume and brain leukocyte infiltration and enhanced cell survival signaling, which was associated with increased brain glial cell line-derived neurotrophic factor and vascular endothelial growth factor. Thus, these findings suggested that neural progenitor cell-derived CM induced sustained neuroprotection and that the secreted factors derived from cells may have applications as an alternative to cell transplantation. Furthermore, Wang et al[33] confirmed that NSCs were involved in the recurrence of GBM mediated by GBM-derived EVs. They suggested that GBM-derived EVs may induce NSC differentiation into tumor-promoting cells.
The biological effects of SVZ NSCs associated with GBM development may be mediated by factors derived from the CM, including cytoskeletal proteins, tumor associated-proteins, transcription factors, and growth factors. Therefore, in this study, we co-cultured glioma cells with the CM of SVZ NSCs and found significant increases in the proliferation and invasion of glioma cells, particularly under hypoxic conditions, compared with those under normoxic conditions. Furthermore, protein assays demonstrated that NCAN, which was overexpressed in the CM of OGD-NSCs, induced important regulatory effects in the proliferation and invasion of glioma cells via activation of the Rho/ROCK pathway. Rho/ROCK signaling is a well-established pathway in cell invasion and migration. Active ROCK inhibits myosin light chain phosphatase, which promotes actin and myosin crosslinking and thus leads to cell motion and increased cell migration[34].
In conclusion, in this study, we have demonstrated a new role for the interaction between SVZ NSCs and glioma cells, in which SVZ NSCs promote glioma cell invasion via the secreted protein NCAN. Moreover, we have showed that SVZ NSC-derived CM under hypoxic conditions further increases glioma cell proliferation and invasion and that the secreted protein NCAN mediates the progression of glioma cells via activation of the Rho/ROCK pathway. Taken together, these findings highlight the prominent regulatory effects of SVZ NSCs in glioma progression and identify NCAN, which is secreted from NSCs, as a potential new therapeutic target for glioma treatment.
Glioma contacting with the subventricular zone (SVZ) is associated with a poor prognosis and decreased overall survival of patients. The most important cell type in the SVZ region is neural stem cells (NSCs), which can differentiate into neurons, astrocytes, and oligodendrocytes.
The roles of neural stem cells of the subventricular zone (SVZ NSCs) in glioma progression are still unclear.
To investigate the effects of SVZ NSCs in gliomas, especially the conditioned medium (CM) derived from SVZ NSCs, on the tumor-related behaviors of glioma cells.
We used the CM derived from SVZ NSCs under normoxic and hypoxic conditions to co-culture with glioma cells, and explored the potential functional molecules in the CM by mass spectrometry analysis.
After co-cultured with glioma cells, the CM derived from hypoxic SVZ NSCs further increased glioma cell proliferation and invasion compared with normoxic CM. The MS analysis identified the candidate protein NCAN in the hypoxic CM. Furthermore, NCAN played an important role in mediating the progression of tumor cells mainly via the Rho/ROCK pathway in gliomas.
SVZ NSC-derived CM under hypoxic condition further increases glioma cell proliferation and invasion, and the protein NCAN secreted from hypoxic SVZ NSCs plays prominent regulatory effects in the progression of glioma cells.
The study provided important insights into the mechanisms of tumor progression associated with glioma contacting SVZ region, and suggested a new molecular target of gene therapy for gliomas.
Manuscript source: Invited manuscript
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Bonartsev A, Durán Alonso MB, Kim BS, Kode JA S-Editor: Zhang L L-Editor: Wang TQ P-Editor: Li JH
| 1. | Lapointe S, Perry A, Butowski NA. Primary brain tumours in adults. Lancet. 2018;392:432-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 621] [Cited by in RCA: 905] [Article Influence: 129.3] [Reference Citation Analysis (0)] |
| 2. | Pine AR, Cirigliano SM, Nicholson JG, Hu Y, Linkous A, Miyaguchi K, Edwards L, Singhania R, Schwartz TH, Ramakrishna R, Pisapia DJ, Snuderl M, Elemento O, Fine HA. Tumor Microenvironment Is Critical for the Maintenance of Cellular States Found in Primary Glioblastomas. Cancer Discov. 2020;10:964-979. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 114] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 3. | Shergalis A, Bankhead A, Luesakul U, Muangsin N, Neamati N. Current Challenges and Opportunities in Treating Glioblastoma. Pharmacol Rev. 2018;70:412-445. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 631] [Cited by in RCA: 578] [Article Influence: 82.6] [Reference Citation Analysis (0)] |
| 4. | Schiffer D, Annovazzi L, Casalone C, Corona C, Mellai M. Glioblastoma: Microenvironment and Niche Concept. Cancers (Basel). 2018;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 98] [Cited by in RCA: 154] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 5. | Broekman ML, Maas SLN, Abels ER, Mempel TR, Krichevsky AM, Breakefield XO. Multidimensional communication in the microenvirons of glioblastoma. Nat Rev Neurol. 2018;14:482-495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 387] [Cited by in RCA: 416] [Article Influence: 59.4] [Reference Citation Analysis (0)] |
| 6. | Wang H, Xu T, Huang Q, Jin W, Chen J. Immunotherapy for Malignant Glioma: Current Status and Future Directions. Trends Pharmacol Sci. 2020;41:123-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 116] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 7. | Shevela E, Davydova M, Starostina N, Yankovskaya A, Ostanin A, Chernykh E. Intranasal delivery of m2 macrophage-derived soluble products reduces neuropsychological deficit in patients with cerebrovascular disease: A pilot study. Journal of Neurorestoratology. 2019;7:89-100. [RCA] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 8. | Yu T, Wang X, Zhi T, Zhang J, Wang Y, Nie E, Zhou F, You Y, Liu N. Delivery of MGMT mRNA to glioma cells by reactive astrocyte-derived exosomes confers a temozolomide resistance phenotype. Cancer Lett. 2018;433:210-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 74] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 9. | Griveau A, Seano G, Shelton SJ, Kupp R, Jahangiri A, Obernier K, Krishnan S, Lindberg OR, Yuen TJ, Tien AC, Sabo JK, Wang N, Chen I, Kloepper J, Larrouquere L, Ghosh M, Tirosh I, Huillard E, Alvarez-Buylla A, Oldham MC, Persson AI, Weiss WA, Batchelor TT, Stemmer-Rachamimov A, Suvà ML, Phillips JJ, Aghi MK, Mehta S, Jain RK, Rowitch DH. A Glial Signature and Wnt7 Signaling Regulate Glioma-Vascular Interactions and Tumor Microenvironment. Cancer Cell. 2018;33:874-889.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 181] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 10. | Henrik Heiland D, Ravi VM, Behringer SP, Frenking JH, Wurm J, Joseph K, Garrelfs NWC, Strähle J, Heynckes S, Grauvogel J, Franco P, Mader I, Schneider M, Potthoff AL, Delev D, Hofmann UG, Fung C, Beck J, Sankowski R, Prinz M, Schnell O. Tumor-associated reactive astrocytes aid the evolution of immunosuppressive environment in glioblastoma. Nat Commun. 2019;10:2541. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 118] [Cited by in RCA: 267] [Article Influence: 44.5] [Reference Citation Analysis (0)] |
| 11. | Venkatesh HS, Johung TB, Caretti V, Noll A, Tang Y, Nagaraja S, Gibson EM, Mount CW, Polepalli J, Mitra SS, Woo PJ, Malenka RC, Vogel H, Bredel M, Mallick P, Monje M. Neuronal Activity Promotes Glioma Growth through Neuroligin-3 Secretion. Cell. 2015;161:803-816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 362] [Cited by in RCA: 603] [Article Influence: 60.3] [Reference Citation Analysis (0)] |
| 12. | Chen P, Zhao D, Li J, Liang X, Li J, Chang A, Henry VK, Lan Z, Spring DJ, Rao G, Wang YA, DePinho RA. Symbiotic Macrophage-Glioma Cell Interactions Reveal Synthetic Lethality in PTEN-Null Glioma. Cancer Cell. 2019;35:868-884.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 253] [Article Influence: 42.2] [Reference Citation Analysis (0)] |
| 13. | Chen P, Hsu WH, Chang A, Tan Z, Lan Z, Zhou A, Spring DJ, Lang FF, Wang YA, DePinho RA. Circadian Regulator CLOCK Recruits Immune-Suppressive Microglia into the GBM Tumor Microenvironment. Cancer Discov. 2020;10:371-381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 159] [Article Influence: 31.8] [Reference Citation Analysis (0)] |
| 14. | Gao X, Zhang Z, Mashimo T, Shen B, Nyagilo J, Wang H, Wang Y, Liu Z, Mulgaonkar A, Hu XL, Piccirillo SGM, Eskiocak U, Davé DP, Qin S, Yang Y, Sun X, Fu YX, Zong H, Sun W, Bachoo RM, Ge WP. Gliomas Interact with Non-glioma Brain Cells via Extracellular Vesicles. Cell Rep. 2020;30:2489-2500.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 65] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 15. | Jung E, Alfonso J, Osswald M, Monyer H, Wick W, Winkler F. Emerging intersections between neuroscience and glioma biology. Nat Neurosci. 2019;22:1951-1960. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 105] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 16. | Yamaki T, Shibahra I, Matsuda KI, Kanemura Y, Konta T, Kanamori M, Yamakawa M, Tominaga T, Sonoda Y. Relationships between recurrence patterns and subventricular zone involvement or CD133 expression in glioblastoma. J Neurooncol. 2020;146:489-499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 17. | Chen L, Chaichana KL, Kleinberg L, Ye X, Quinones-Hinojosa A, Redmond K. Glioblastoma recurrence patterns near neural stem cell regions. Radiother Oncol. 2015;116:294-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 65] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 18. | Mistry AM, Dewan MC, White-Dzuro GA, Brinson PR, Weaver KD, Thompson RC, Ihrie RA, Chambless LB. Decreased survival in glioblastomas is specific to contact with the ventricular-subventricular zone, not subgranular zone or corpus callosum. J Neurooncol. 2017;132:341-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 67] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 19. | McGrath E, Gao J, Wu P. Proliferation and differentiation of human fetal brain neural stem cells in vitro. Journal of Neurorestoratology. 2018;19-27. [RCA] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 20. | Zhang G, Chen L, Guo X, Wang H, Chen W, Wu G, Gu B, Miao W, Kong J, Jin X, Yi G, You Y, Su X, Gu N. Comparative Analysis of microRNA Expression Profiles of Exosomes Derived from Normal and Hypoxic Preconditioning Human Neural Stem Cells by Next Generation Sequencing. J Biomed Nanotechnol. 2018;14:1075-1089. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 43] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 21. | Zhang G, Zhu Z, Wang H, Yu Y, Chen W, Waqas A, Wang Y, Chen L. Exosomes derived from human neural stem cells stimulated by interferon gamma improve therapeutic ability in ischemic stroke model. J Adv Res. 2020;24:435-445. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 88] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 22. | Bardella C, Al-Dalahmah O, Krell D, Brazauskas P, Al-Qahtani K, Tomkova M, Adam J, Serres S, Lockstone H, Freeman-Mills L, Pfeffer I, Sibson N, Goldin R, Schuster-Böeckler B, Pollard PJ, Soga T, McCullagh JS, Schofield CJ, Mulholland P, Ansorge O, Kriaucionis S, Ratcliffe PJ, Szele FG, Tomlinson I. Expression of Idh1R132H in the Murine Subventricular Zone Stem Cell Niche Recapitulates Features of Early Gliomagenesis. Cancer Cell. 2016;30:578-594. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 115] [Cited by in RCA: 119] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 23. | Bulstrode H, Johnstone E, Marques-Torrejon MA, Ferguson KM, Bressan RB, Blin C, Grant V, Gogolok S, Gangoso E, Gagrica S, Ender C, Fotaki V, Sproul D, Bertone P, Pollard SM. Elevated FOXG1 and SOX2 in glioblastoma enforces neural stem cell identity through transcriptional control of cell cycle and epigenetic regulators. Genes Dev. 2017;31:757-773. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 92] [Cited by in RCA: 90] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 24. | Alcantara Llaguno S, Sun D, Pedraza AM, Vera E, Wang Z, Burns DK, Parada LF. Cell-of-origin susceptibility to glioblastoma formation declines with neural lineage restriction. Nat Neurosci. 2019;22:545-555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 99] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 25. | Lee JH, Lee JE, Kahng JY, Kim SH, Park JS, Yoon SJ, Um JY, Kim WK, Lee JK, Park J, Kim EH, Lee JH, Lee JH, Chung WS, Ju YS, Park SH, Chang JH, Kang SG, Lee JH. Human glioblastoma arises from subventricular zone cells with low-level driver mutations. Nature. 2018;560:243-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 320] [Cited by in RCA: 442] [Article Influence: 63.1] [Reference Citation Analysis (0)] |
| 26. | Matarredona ER, Pastor AM. Neural Stem Cells of the Subventricular Zone as the Origin of Human Glioblastoma Stem Cells. Therapeutic Implications. Front Oncol. 2019;9:779. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 76] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 27. | Chen L, Guerrero-Cazares H, Ye X, Ford E, McNutt T, Kleinberg L, Lim M, Chaichana K, Quinones-Hinojosa A, Redmond K. Increased subventricular zone radiation dose correlates with survival in glioblastoma patients after gross total resection. Int J Radiat Oncol Biol Phys. 2013;86:616-622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 119] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 28. | Li H, Yahaya BH, Ng WH, Yusoff NM, Lin J. Conditioned Medium of Human Menstrual Blood-Derived Endometrial Stem Cells Protects Against MPP+-Induced Cytotoxicity in vitro. Front Mol Neurosci. 2019;12:80. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 29. | Baez-Jurado E, Hidalgo-Lanussa O, Guio-Vega G, Ashraf GM, Echeverria V, Aliev G, Barreto GE. Conditioned Medium of Human Adipose Mesenchymal Stem Cells Increases Wound Closure and Protects Human Astrocytes Following Scratch Assay In Vitro. Mol Neurobiol. 2018;55:5377-5392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 30. | Bordoni V, Alonzi T, Zanetta L, Khouri D, Conti A, Corazzari M, Bertolini F, Antoniotti P, Pisani G, Tognoli F, Dejana E, Tripodi M. Hepatocyte-conditioned medium sustains endothelial differentiation of human hematopoietic-endothelial progenitors. Hepatology. 2007;45:1218-1228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 31. | Gharaei MA, Xue Y, Mustafa K, Lie SA, Fristad I. Human dental pulp stromal cell conditioned medium alters endothelial cell behavior. Stem Cell Res Ther. 2018;9:69. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 32. | Doeppner TR, Traut V, Heidenreich A, Kaltwasser B, Bosche B, Bähr M, Hermann DM. Conditioned Medium Derived from Neural Progenitor Cells Induces Long-term Post-ischemic Neuroprotection, Sustained Neurological Recovery, Neurogenesis, and Angiogenesis. Mol Neurobiol. 2017;54:1531-1540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 33. | Wang J, Liu J, Sun G, Meng H, Wang J, Guan Y, Yin Y, Zhao Z, Dong X, Yin S, Li H, Cheng Y, Wu H, Wu A, Yu X, Chen L. Glioblastoma extracellular vesicles induce the tumour-promoting transformation of neural stem cells. Cancer Lett. 2019;466:1-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 37] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 34. | Campbell H, Fleming N, Roth I, Mehta S, Wiles A, Williams G, Vennin C, Arsic N, Parkin A, Pajic M, Munro F, McNoe L, Black M, McCall J, Slatter TL, Timpson P, Reddel R, Roux P, Print C, Baird MA, Braithwaite AW. ∆133p53 isoform promotes tumour invasion and metastasis via interleukin-6 activation of JAK-STAT and RhoA-ROCK signalling. Nat Commun. 2018;9:254. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 59] [Article Influence: 8.4] [Reference Citation Analysis (0)] |