Published online Nov 26, 2020. doi: 10.4252/wjsc.v12.i11.1377
Peer-review started: May 28, 2020
First decision: July 30, 2020
Revised: July 31, 2020
Accepted: September 1, 2020
Article in press: September 1, 2020
Published online: November 26, 2020
Processing time: 178 Days and 1.9 Hours
Multipotent mesenchymal stromal cells (MSCs) are widely used in the clinic due to their unique properties, namely, their ability to differentiate in all mesenchymal directions and their immunomodulatory activity. Healthy donor MSCs were used to prevent the development of acute graft vs host disease (GVHD) after allogeneic bone marrow transplantation (allo-BMT). The administration of MSCs to patients was not always effective. The MSCs obtained from different donors have individual characteristics. The differences between MSC samples may affect their clinical efficacy.
To study the differences between effective and ineffective MSCs.
MSCs derived from the bone marrow of a hematopoietic stem cells donor were injected intravenously into allo-BMT recipients for GVHD prophylaxis at the moment of blood cell reconstitution. Aliquots of 52 MSC samples that were administered to patients were examined, and the same cells were cultured in the presence of peripheral blood mononuclear cells (PBMCs) from a third-party donor or treated with the pro-inflammatory cytokines IL-1β, IFN and TNF. Flow cytometry revealed the immunophenotype of the nontreated MSCs, the MSCs cocultured with PBMCs for 4 d and the MSCs exposed to cytokines. The proportions of CD25-, CD146-, CD69-, HLA-DR- and PD-1-positive CD4+ and CD8+ cells and the distribution of various effector and memory cell subpopulations in the PBMCs cocultured with the MSCs were also determined.
Differences in the immunophenotypes of effective and ineffective MSCs were observed. In the effective samples, the mean fluorescence intensity (MFI) of HLA-ABC, HLA-DR, CD105, and CD146 was significantly higher. After MSCs were treated with IFN or cocultured with PBMCs, the HLA-ABC, HLA-DR, CD90 and CD54 MFI showed a stronger increase in the effective MSCs, which indicated an increase in the immunomodulatory activity of these cells. When PBMCs were cocultured with effective MSCs, the proportions of CD4+ and CD8+central memory cells significantly decreased, and the proportion of CD8+CD146+ lymphocytes increased more than in the subpopulations of lymphocytes cocultured with MSC samples that were ineffective in the prevention of GVHD; in addition, the proportion of CD8+effector memory lymphocytes decreased in the PBMCs cocultured with the effective MSC samples but increased in the PBMCs cocultured with the ineffective MSC samples. The proportion of CD4+CD146+ lymphocytes increased only when cocultured with the inefficient samples.
For the first time, differences were observed between MSC samples that were effective for GVHD prophylaxis and those that were ineffective. Thus, it was shown that the immunomodulatory activity of MSCs depends on the individual characteristics of the MSC population.
Core Tip: An attempt was made to identify the main differences between multipotent mesenchymal stromal cells (MSCs) samples that are effective and those that are ineffective in preventing the development of acute graft vs host disease after allogeneic bone marrow transplantation. The mean fluorescence intensity of HLA-ABC, HLA-DR, CD105, and CD146 was shown to be significantly lower on the surface of samples that were ineffective for prophylaxis. Significant differences were revealed between effective and ineffective MSCs in terms of their responses to interaction with lymphocytes and stimulation by pro-inflammatory cytokines. The patterns observed here indicate a possible mechanism of the immunosuppressive action of these cells in clinical use.
- Citation: Petinati N, Kapranov N, Davydova Y, Bigildeev A, Pshenichnikova O, Karpenko D, Drize N, Kuzmina L, Parovichnikova E, Savchenko V. Immunophenotypic characteristics of multipotent mesenchymal stromal cells that affect the efficacy of their use in the prevention of acute graft vs host disease. World J Stem Cells 2020; 12(11): 1377-1395
- URL: https://www.wjgnet.com/1948-0210/full/v12/i11/1377.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v12.i11.1377
Graft vs host disease (GVHD) is the main complication after allogeneic haematopoietic stem cell transplantation (allo-HSCT). In GVHD, donor T cells attack recipient cells. The immune conflict that develops causes clinically significant damage to organs and tissues in 20%-70% of patients[1].
The most common first-line therapy for the treatment of GVHD is glucocorti-costeroids. Glucocorticosteroid refractoriness occurs in approximately 30% of patients. Aggressive immunosuppressive or anti-inflammatory drugs are not effective in all patients with steroid resistance. Second- and third-line treatments are often not effective and significantly increase the risk of infectious complications. In this regard, alternative approaches to the treatment of acute GVHD have been proposed, in particular, the introduction of multipotent mesenchymal stromal cells (MSCs)[2,3].
Human bone marrow MSCs are a heterogeneous population of fibroblast-like cells; this population includes multipotent stem cells, which are able to form bone, cartilage, and adipose tissue in vitro[4], and stromal cell components that regulate blood formation in stem cell niches due to specific intercellular interactions and soluble factors[5]. In 2006, the International Society for Cell and Gene Therapy established the minimal criteria for identifying MSCs: These cells adhere to plastic, express CD73, CD90, and CD105 on the membrane surface, do not express markers of haematopoietic cells - CD14, CD31, CD34, CD45, and weakly express HLA-ABC[6].
A common functional feature of the MSC population, including the early and differentiating precursors, is the ability to affect both innate and adaptive immune cells. This process depends on the presence of inflammatory cytokines, such as interferon gamma (IFN), tumour necrosis factor alpha (TNF) and interleukin-1 (IL)-1 α or β, in the microenvironment[7,8]. For successful immunomodulation, MSCs require licensing of the anti-inflammatory phenotype through exposure to an environment rich in cytokines. Cytokines are produced primarily by activated T cells. It is well known that IFN licenses MSCs, and its removal significantly reduces the antiproliferative effect of MSCs on T cells[9]. TNF is also important for enhancing the immunomodulatory activity of MSCs; in contrast to IFN, it regulates the expression of haemoxigenase 1 and insulin-like growth factor 1[10].
Based on their immunomodulatory properties, MSCs are now used for the prevention and treatment of GVHD after allo-HSCT[11,12]. Previous studies have reported conflicting results regarding the efficacy of MSCs in the prevention and treatment of GVHD. Morata-Tarif and coauthors conducted a meta-analysis to elucidate whether the introduction of MSCs can improve the overall survival of these patients[13]. The authors performed a dichotomous analysis of 11 studies providing data on the overall survival of the control (n = 298) and MSC (n = 213) groups, which showed that the introduction of MSCs leads to a 17% increase in survival (95%CI: 1.02-1.33, I2 = 0%). The data related to the incidence of acute GVHD in the control (n = 235) and MSC (n = 150) groups were collected from 10 studies. The frequency of GVHD was lower in the MSC group than in the control group. In the analysis of 4 studies (144 patients), the frequency of severe acute grade IV GVHD was significantly lower in the group of patients that received MSCs (RR = 0.22; 95%CI: 0.06-0.81). The vast majority of the studies used MSCs obtained from a third-party donor and provided by manufacturers[14]. In this regard, it was impossible to study the differences between the effective and ineffective MSC samples.
For 10 years, a randomized study on the prevention of GVHD with the use of MSCs from a haematopoietic cell donor was conducted at the National Research Center for Hematology. The study was registered on the website https://clinicaltrials.gov NCT01941394. Such a study made it possible to investigate the differences in the properties of the individual MSC samples that can affect the efficiency of the use of these cells in preventing the development of GVHD.
The aim of this work was to study the immunophenotypes of non-activated MSCs that were administered to patients and the same cell populations that were activated by the indicated pro-inflammatory cytokines (IL-1β, IFN and TNF) or by interaction with PBMCs.
The differences in the samples of MSCs that were effective and ineffective in the prevention of GVHD were revealed.
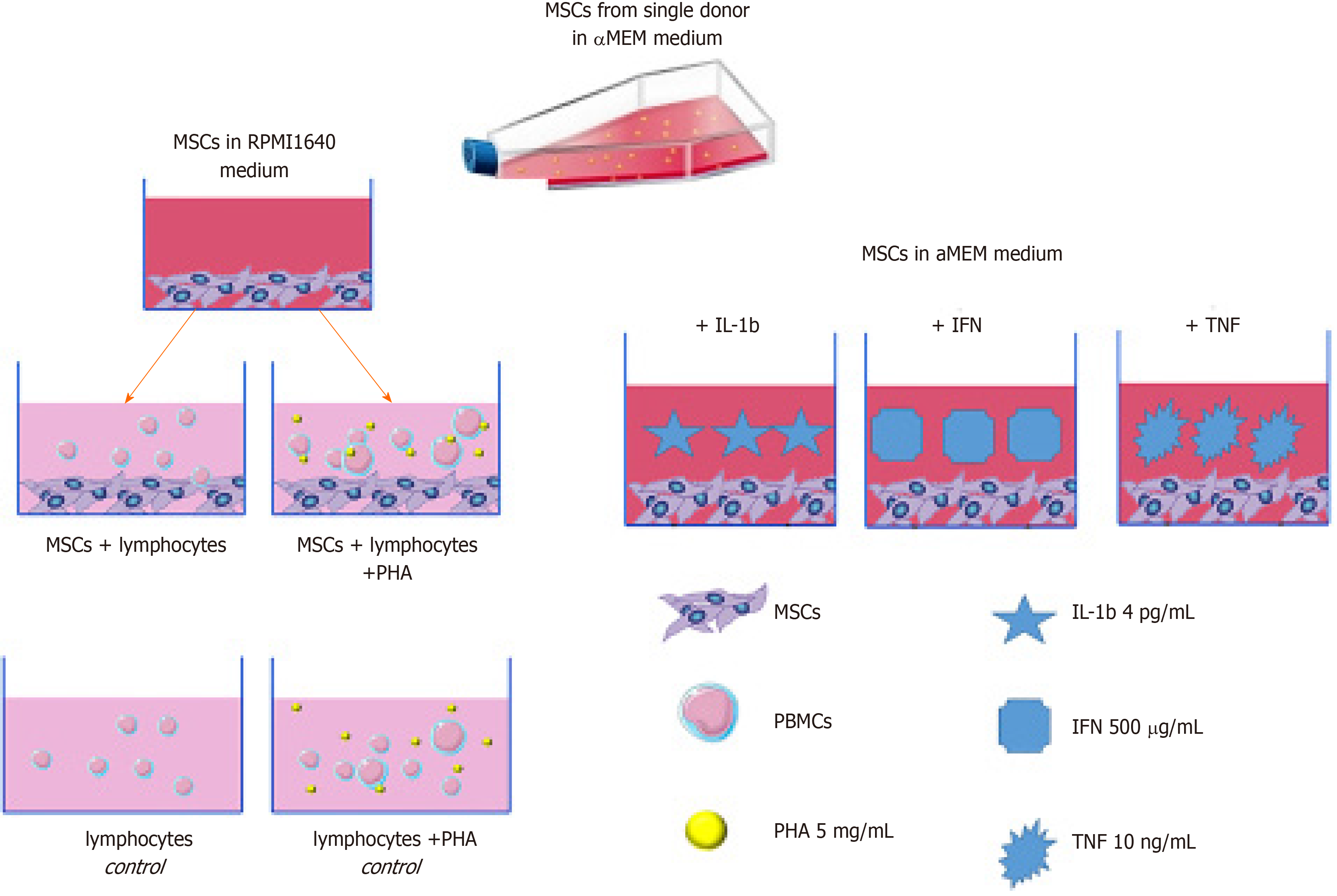
All the bone marrow samples were obtained from donors who provided signed informed consent and were collected during the harvesting of bone marrow for allogeneic transplantation at the Department of High-Dose Chemotherapy, Depressions of Hematopoiesis, and Bone Marrow Transplantation of the National Research Center for Hematology, Moscow, Russia. The bone marrow samples of 52 healthy donors, including 29 males and 23 females aged 13 to 66 years (median: 33 years), were studied. The MSCs were derived from 5-10 mL of donor bone marrow. To separate the mononuclear cells, the bone marrow was mixed with an equal volume of alpha-МЕМ (HyClone, United States) containing 0.2% methylcellulose (1500 cP, Sigma-Aldrich Corporation, United States) and incubated at room temperature. After 40 min, the erythrocytes and granulocytes had mostly precipitated, while the mononuclear cells remained in suspension. The upper fraction (suspension) was aspirated and centrifuged for 10 min at 450 g. The standard cultivation medium consisted of αMEM supplemented with 10% foetal bovine serum (FBS) (HyClone), 2 mM-glutamine (HyClone), 100 U/mL penicillin (Ferein, Russia) and 50 mg/mL streptomycin (Ferein). The cells were cultivated at a density of 3 × 106 cells in T25 culture flasks (Corning-Costar, United States). When a confluent monolayer of cells had formed, the cells were washed with 0.02% EDTA (ICN, United States) in a physiological solution (Sigma-Aldrich) and then detached from the surface by incubation in 0.25% trypsin solution (MP Biomedicals, France) for 10-15 min at room temperature. The cells were then reseeded at a density of 4 × 103 cells per cm2 of flask area. The cultures were maintained at 37°C in 5% CO2. The number of harvested cells was directly counted; the cell viability was assessed using Trypan blue dye exclusion staining.
IL-1β (4 pg/mL; Sigma) or TNF (10 ng/mL; Sigma) was added to some cultures and incubated for 4 d. IFN (Sigma) dissolved in αMEM medium at a concentration of 500 IU/mL was added to some of the experimental flasks for 4 h. Then, the flasks were washed and cultured for 4 d.
For analysis of the interactions between MSCs and lymphocytes, PBMCs from two nonrelated healthy donors were separated using Lymphoprep solution at a density of 1.077 g/cm3 (MP Biomedicals). The obtained PBMC fraction was washed 3 times with RPMI-1640 medium without serum. PBMCs and MSCs were cocultured for 4 d at 37°C in 5% CO2. PBMCs cultured without MSCs were used as controls.
MSCs at 2-3 passages were seeded at 105 cells per T25 flask, and a day later, the flasks were washed, and 106 PBMCs suspended in RPMI-1640 medium supplemented with 10% FBS were added. In some experiments, the lymphocytes were activated with 5 mg/mL phytohemagglutinin (PHA-lymphocytes)[15].
After culture, the PBMCs were removed from the flasks and washed first in RPMI-1640 medium and then in CellWash buffer (BD Biosciences, United States). A schematic of the experimental conditions is shown in Figure 1. After coculture, the proportion of viable cells was analysed. In all the samples, the proportion of viable cells was 90%-95%.
Determination of the level of antigen expression on MSCs was performed by flow cytometry. After removing the MSCs from the bottom of the flask, they were washed twice with CellWash solution (BD Biosciences), and then, 2 × 104 cells were incubated for 20 min in the dark with anti-CD90 monoclonal antibodies labelled with PE (BD Pharmingen, United States), anti-HLA-ABC labelled with FITC (BioLegend, United States), anti-HLA-DR labelled with APC (BioLegend), anti-CD54 labelled with APC (BD Biosciences), anti-CD73 labelled with PE (BD Biosciences), anti-CD105 labelled with FITC (BioLegend), anti-CD146 labelled with PE (BD Biosciences), and anti-CD274 labelled with FITC (BD Biosciences, United States). The appropriate isotypic controls were used for each fluorochrome.
The MSC population was identified by the parameters of forward and side light scattering. In this cell population, the mean fluorescence intensity (MFI) of the fluorescently labelled antibodies bound to the HLA-ABC, CD105, CD274, CD90, CD73, CD146, HLA-DR, and CD54 antigens was estimated. Then, based on the obtained MFI values, the MFI of the isotypic controls was subtracted, which provided a description of the presence or absence of the studied marker on the cells. When calculating the relative change in antigen expression (MFI) on the surface of MSCs under various conditions, the MFI was equated to unity if expression of the antigen was not detected on the cells.The cytometric analysis of lymphocytes was performed using the following monoclonal antibodies labelled with fluorochrome dyes: CD4 APC-Cy7, CD95 PE-Cy7, CD69 FITC, CD45RO FITC, and CD127 PE (BioLegend, United States) and CD28 APC, CD197 PE, HLA-DR APC, CD8 PerCP-Cy 5.5, CD25 FITC, CD146 PE, and CD279 APC (BD Biosciences, United States). The cytometric analysis was performed using a BD FACSCanto II flow cytometer (BD Biosciences) and the BD FACS Diva program (v6.0, BD Biosciences).
The analysis of activated and non-activated lymphocytes was carried out in the same way. First, the lymphocyte population was determined by the parameters of forward and side light scattering, and then, the populations of CD4+ and CD8+ cells were isolated from these lymphocytes. The subpopulations of memory cells and naive and effector cells were determined according to Mahnke (2013) and others[16] based on the levels of expression of the CD28, CD197 (CCR7), CD45RO, and CD95 (FAS) antigens on the surface of T cells. The immunophenotype of various subpopulations of T cells and the gating strategy have been described previously[17]. Among the non-activated lymphocytes, the relative number of regulatory T cells (CD4 + CD25 + CD127-) and the number of activated T helper cells (CD4 + CD25 + CD127 +) were determined. The number of CD4+ and CD8+ T cells expressing HLA-DR, PD-1, and CD146 was determined in cultures of both activated and non-activated lymphocytes. In cultures of PHA-activated lymphocytes, the proportion of activated CD69+ and CD25+ CD4+ and CD8+ lymphocytes was also determined.
The data are presented as the mean ± SE. For each sample of experimental data, a normality test was performed using the Shapiro-Wilk test (at P < 0.05, the distribution was assumed to be different from normal). To analyse the changes in the average values of the parameters within one group on different days of cultivation, a paired t-test or Wilcoxon test was used, depending on whether the distribution was normal. The significance of the differences in the data between different cultivation conditions was determined using the Student's t-test when comparing the samples corresponding to the normal distribution and the Mann-Whitney test for abnormal distributions. The differences were considered statistically significant at P < 0.05. Statistical analysis was performed using GraphPad Prism 8.03. The significance of differences was assessed by the method of multiple comparisons in GraphPad. Forward stepwise discriminant function analysis was performed using Statistica 8.0 software.
The preliminary results of a clinical study on the prevention of acute GVHD using MSCs have been described previously[18-20]. In this study, MSCs obtained from a haematopoietic cell donor were intravenously administered to patients on the day of restoration of the white blood cell count to 1 × 109/L at a dose of 106 cells per kilogram of body weight. Of the 52 patients included in the study, 11 developed grade II-IV acute GVHD (Table 1).
| Donor MSCs | Gender male/female | Age (median), yr | Body mass index | CFU-F per 106 BM cells | Total MSC production for 4 passages, × 106 | Time to P0, d |
| No GVHD | 24/17 | 33.19 ± 1.70 (32) | 24.88 ± 0.82 (23) | 17.27 ± 3.39 | 13.93 ± 2.14 | 14.44 ± 0.42 |
| Acute GVHD | 5/6 | 41.27 ± 4.15 (45) | 26.36 ± 1.58 (27) | 11.46 ± 4.50 | 7.71 ± 2.40 | 14.73 ± 0.87 |
GVHD developed in patients who were administered MSCs from older donors with a higher body mass index and lower concentration of CFU-F in the bone marrow. The ineffective MSCs had lower proliferative potential.
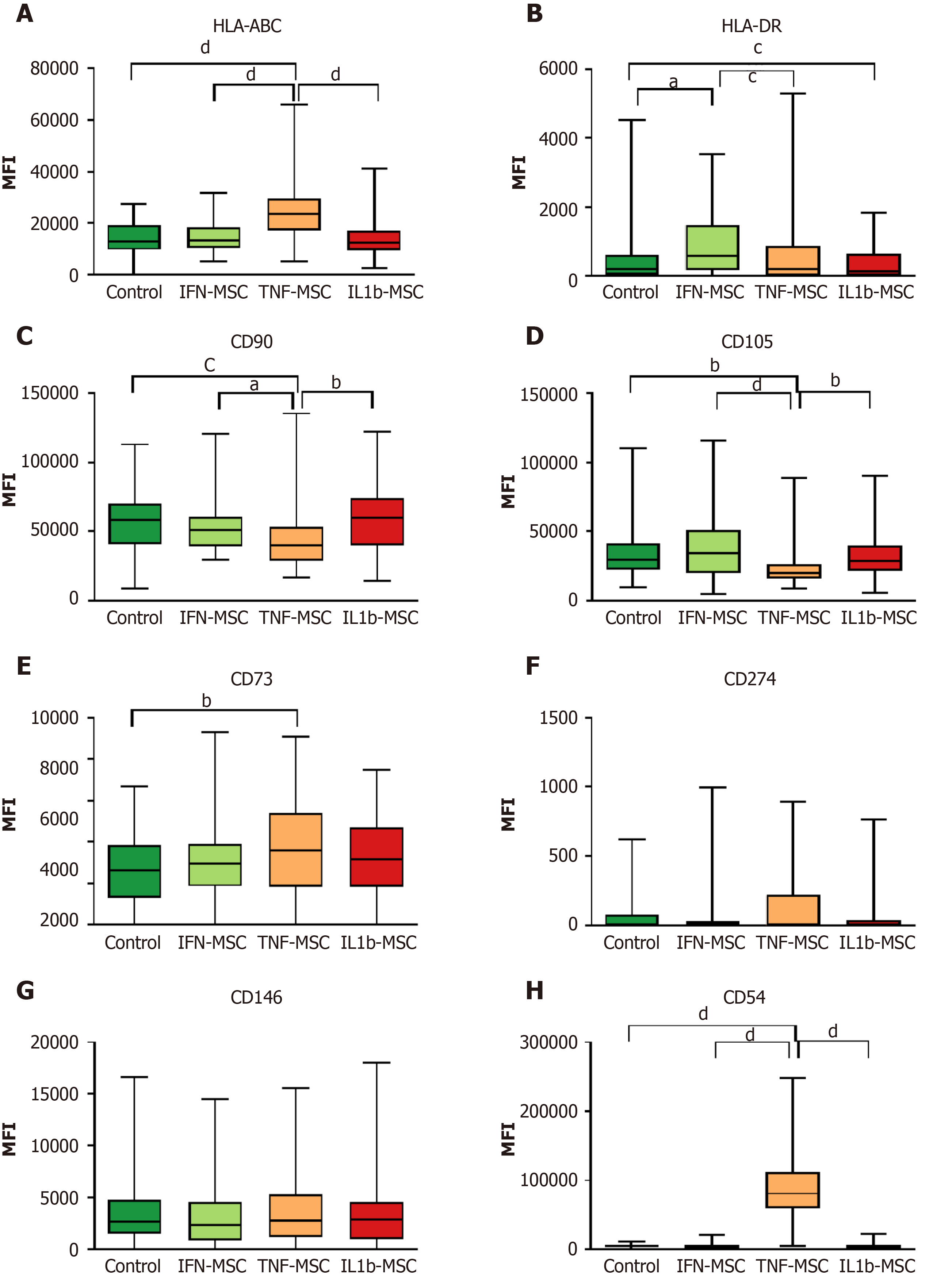
After intravenous administration, MSCs are exposed to cytokines, mainly IL-1β, IFN and TNF. These cytokines change the MSC properties. Modelling this interaction in vitro has shown that exposure to IL-1β, IFN, and TNF changed the immunophenotype of MSCs in various ways. When cultured MSCs were treated with IFN, the MFI of HLA-DR significantly increased compared with that of the control MSCs. The cultivation of MSCs in the presence of TNF led to a significant increase in the MFI of HLA-ABC, CD73, and CD54, while the MFI of CD90 and CD105 decreased. The treatment of MSCs with IL-1β led to a decrease in the MFI of HLA-DR (Figure 2).
Analysis of the expression of clusters of differentiation on the surface of MSCs showed that IFN and TNF induced the expression of HLA molecules and that IL-1β reduced this expression. TNF influenced the expression of adhesion molecules. Moreover, given that all pro-inflammatory cytokines act differently in culture and in the body, their effect certainly depends on their ratio in the patient's blood. In addition, differences in the sensitivity of MSC samples to cytokines may affect their clinical effectiveness.
Analysis of the immunophenotypic characteristics of the MSCs in the effective samples showed that the MFIs of HLA-ABC, HLA-DR, CD105 and CD146 were increased compared with those of the ineffective samples (Table 2). Upon MSC activation with IL-1β, the difference in the expression of HLA-ABC and HLA-DR between the effective and ineffective samples remained, and the CD54 MFI sharply increased in the effective MSC samples (4-fold) due to the effect of IL-1β but did not change in the ineffective MSC samples. IFN also did not alter the expression of HLA-ABC and HLA-DR, and the difference between the effective and ineffective samples remained; however, additional differences in the expression of CD90 were revealed. Similar to the MSCs treated with IL-1β, the MFI of CD54 increased in the effective MSC samples due to the influence of IFN but did not change in the ineffective MSC samples. After MSC treatment with TNF, only an increase in HLA-DR expression in the effective samples was maintained compared with the ineffective samples. Under the influence of this factor, the expression of HLA-ABC and CD146 in the ineffective MSC samples increased, which offset the differences between the effective and ineffective samples. The expression of CD54 increased by more than 12-fold in both the effective and ineffective samples.
| MFI | ||||||||
| MSCs | MSCs treated with IL1β | MSCs treated with IFN | MSCs treated with TNF | |||||
| Antigen | Effective | Ineffective | Effective | Ineffective | Effective | Ineffective | Effective | Ineffective |
| HLA-ABC | 14561 ± 7741 | 8767 ± 1434 | 14740 ± 10211 | 8850 ± 994 | 15200 ± 9601 | 10208 ± 1189 | 21174 ± 1615 | 15706 ± 2938 |
| HLA-DR | 752 ± 1501 | 188 ± 42 | 610 ± 1541 | 149 ± 25 | 1077 ± 1531 | 423 ± 163 | 659 ± 1371 | 169 ± 38 |
| CD54 | 4881 ± 371 | 4447 ± 1203 | 13773 ± 32181 | 4234 ± 1612 | 7889 ± 8161 | 4378 ± 1566 | 61708 ± 5998 | 65461 ± 11324 |
| CD73 | 3047 ± 253 | 2354 ± 343 | 3396 ± 305 | 2226 ± 543 | 3414 ± 383 | 2678 ± 655 | 3395 ± 305 | 3370 ± 605 |
| CD90 | 57679 ± 2626 | 49241 ± 7022 | 60117 ± 3990 | 45739 ± 9525 | 56500 ± 30721 | 40335 ± 4502 | 55799 ± 3471 | 45198 ± 7142 |
| CD105 | 35682 ± 26231 | 21471 ± 3247 | 34402 ± 2490 | 29665 ± 6032 | 41171 ± 3118 | 30525 ± 5609 | 30431 ± 2471 | 24260 ± 3466 |
| CD146 | 4345 ± 5341 | 1807 ± 482 | 3546 ± 497 | 3124 ± 688 | 3762 ± 479 | 2378 ± 707 | 3137 ± 387 | 3463 ± 539 |
| CD274 | 293 ± 37 | 261 ± 38 | 227 ± 204 | 400 ± 104 | 304 ± 219 | 341 ± 54 | 322 ± 81 | |
| %CD54 | 56 ± 3 | 50 ± 9 | 57 ± 4 | 42 ± 8 | 66 ± 3 | 55 ± 8 | 89 ± 2 | 93 ± 4 |
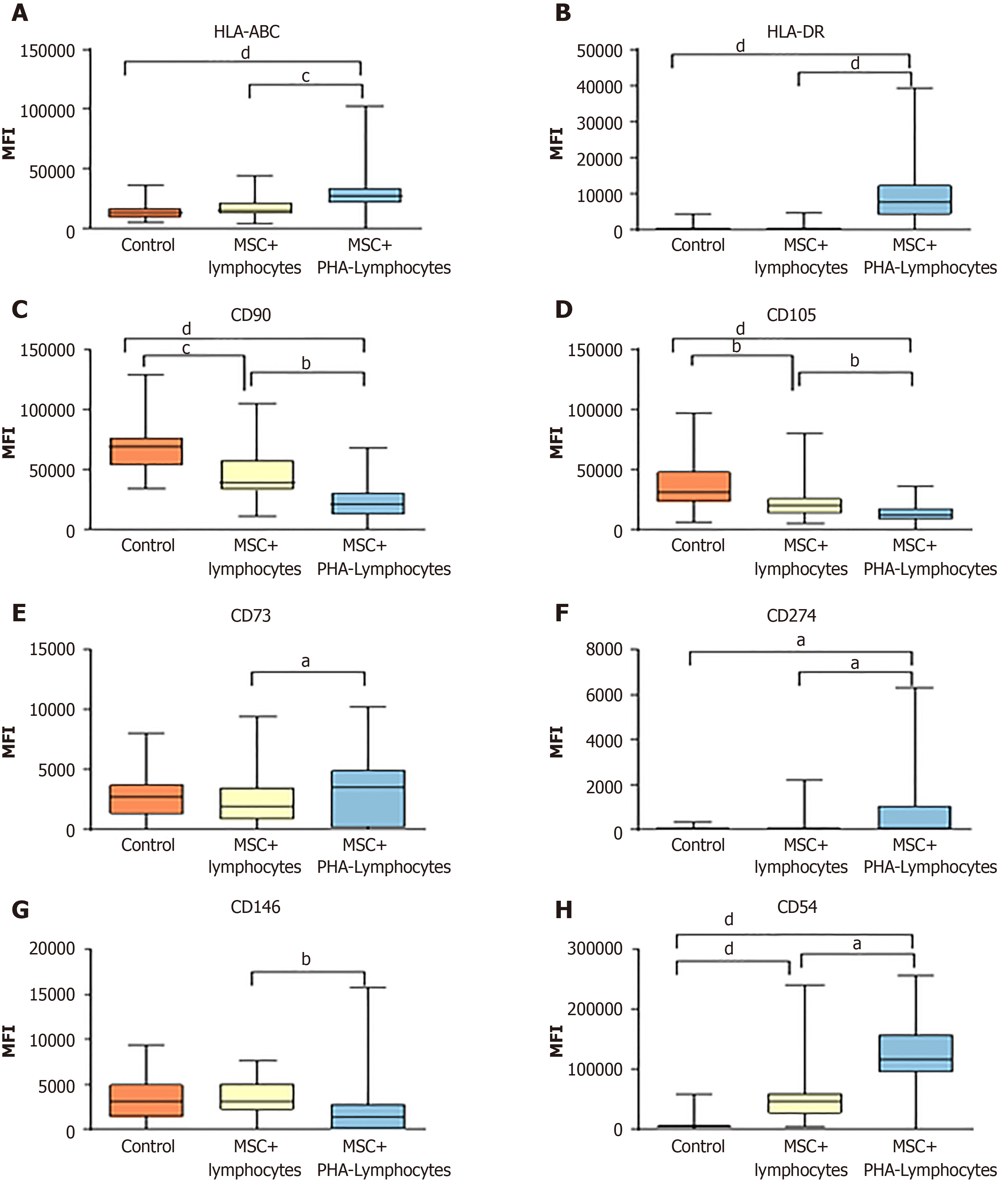
MSCs: In addition to cytokines, MSCs in the bloodstream interact with lymphocytes. The interactions of MSCs with lymphocytes were studied in vitro. Co-cultivation of MSCs with PBMCs for 4 d altered the expression of MSC surface markers (Figure 3). In the MSCs cocultured with non-activated lymphocytes, the MFI of CD54 was significantly increased, and the expression of CD90 and CD105 was reduced. Activated lymphocytes caused an increase in the MSC expression of HLA-ABC, HLA-DR, CD274 and CD54 and a decrease in the expression of CD90 and CD105.
When MSCs were cocultured with PBMCs, the level of HLA-ABC and HLA-DR expression on the MSCs increased regardless of the effectiveness of the samples in GVHD prevention (Table 3). The level of CD90 expression significantly decreased on the surface of the ineffective MSCs after one day of co-cultivation with PBMCs, and then, the MFI of this molecule continued to decrease on both the effective and ineffective cells. The MFI of CD105 also decreased during co-cultivation with PBMCs and was noticeably stronger, but this increase was not significant in the inefficient samples. The expression of CD54 and CD274 increased in the effective samples more than in the ineffective samples. The CD73 MFI decreased in the ineffective samples on the first day of co-cultivation with PBMCs and practically did not increase by day 4, which was different to the results observed in the effective samples.
| Time of co-cultivation | 1 d | 4 d | ||
| Group of MSC samples | Effective | Ineffective | Effective | Ineffective |
| HLA-ABC | 1.90 ± 0.11 | 1.53 ± 0.30 | 1.90 ± 0.15 | 1.67 ± 0.33 |
| CD90 | 0.95 ± 0.04 | 0.79 ± 0.061 | 0.55 ± 0.04 | 0.49 ± 0.10 |
| HLA-DR | 134.37 ± 63.90 | 654.74 ± 363.49 | 682.42 ± 326.38 | 151.88 ± 96.44 |
| CD105 | 1.08 ± 0.06 | 0.98 ± 0.14 | 0.64 ± 0.05 | 0.42 ± 0.07 |
| CD146 | 0.82 ± 0.07 | 0.86 ± 0.30 | 22.16 ± 16.28 | 0.81 ± 0.21 |
| CD54 | 60.59 ± 12.29 | 44.76 ± 8.84 | 33.08 ± 5.30 | 23.19 ± 7.64 |
| CD274 | 434.64 ± 93.56 | 1.27 ± 0.281 | 573.44 ± 172.78 | 319.25 ± 233.40 |
| CD73 | 1.05 ± 0.08 | 0.56 ± 0.131 | 150.36 ± 103.94 | 1.60 ± 0.54 |
Lymphocytes: Lymphocyte populations were analysed in PBMCs co-cultivated with MSCs. The composition of the lymphocyte populations changed during co-cultivation with MSCs.
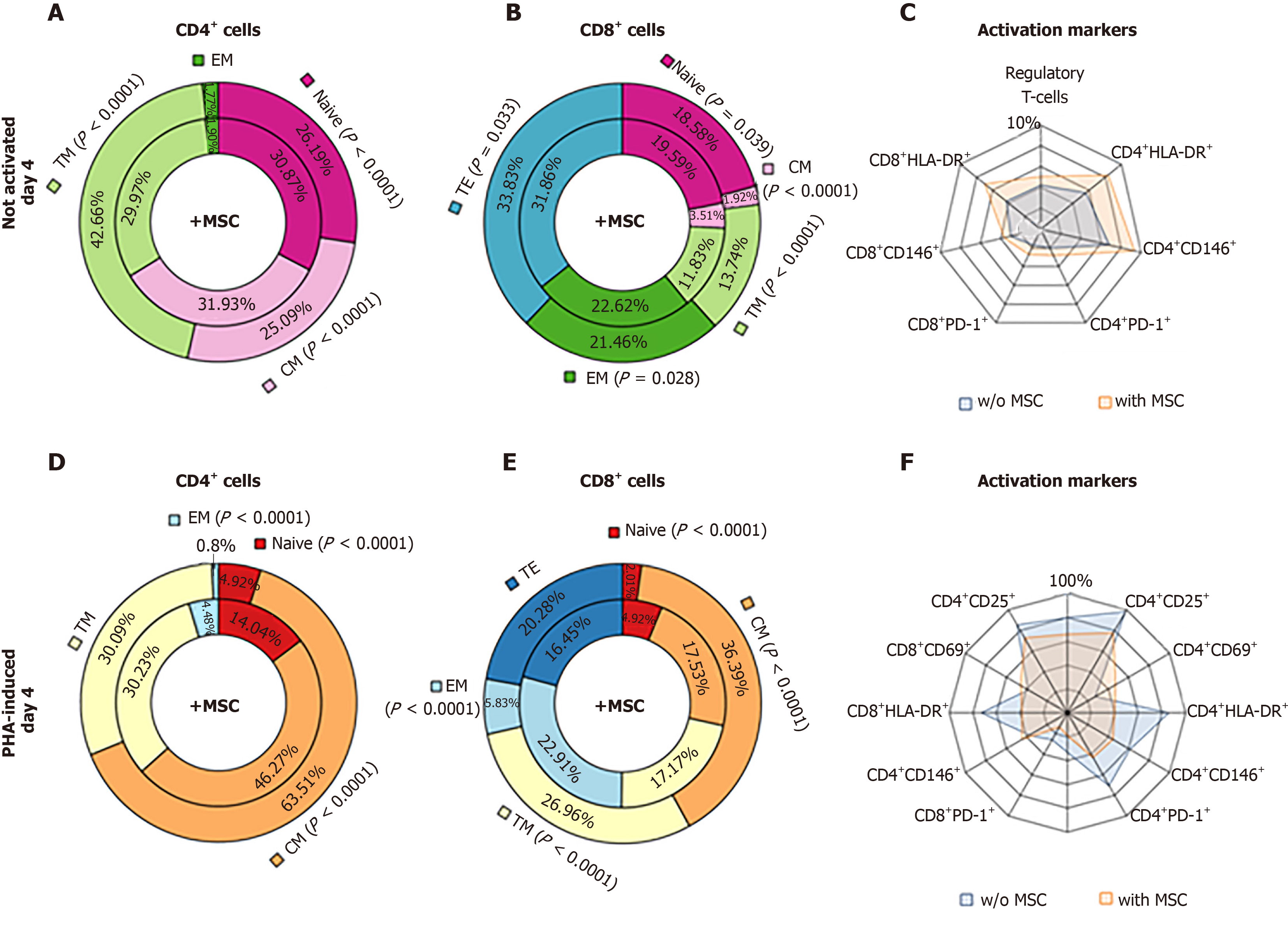
After 4 d of MSC co-cultivation with non-activated lymphocytes, the proportion of terminal memory (TM) and effector memory (TE) lymphocytes was lower than that in the control cultures, while the proportions of both CD4+ and CD8+ EM, T regulatory (Treg), and HLA-DR+CD146+PD-1+ lymphocytes was higher than those in the control cultures (Figure 4A-C).
Co-cultivation of MSCs with PHA-activated lymphocytes for 4 days resulted in higher proportions of naive and effector memory cells among the CD4+ cells, CD8+ cells, and CD8+ TM cells, but the proportion of central memory (CM) cells was lower than that in the control cultures (Figure 4D-F).
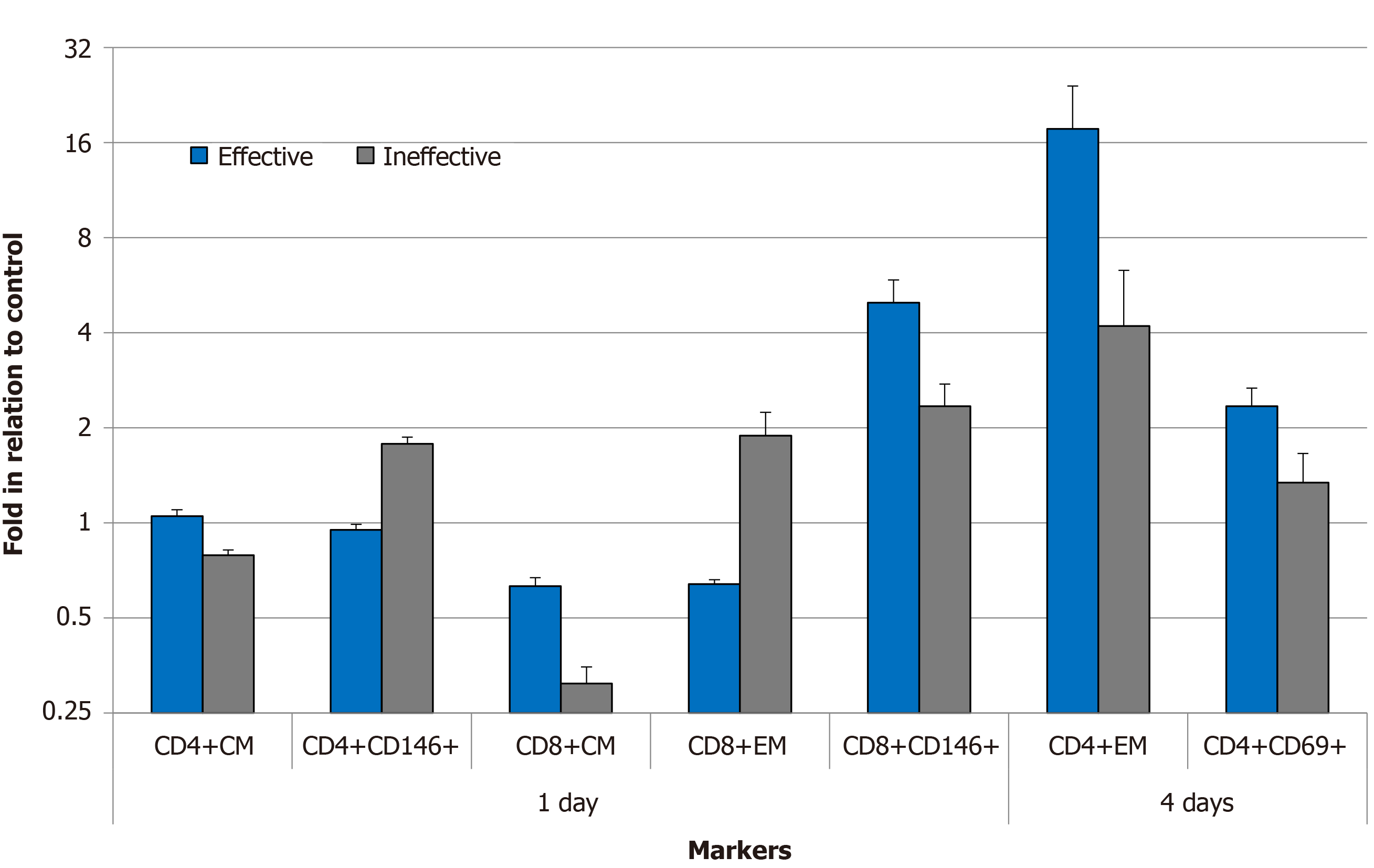
On the first day of co-cultivation, significant differences were observed in the subpopulations of both CD4+ and CD8+ CM; CD8+ EM; and activated CD146+ T cells; moreover, the CD8+CD146+ proportion was higher in the PBMCs cocultured with the effective samples, and the CD4+CD146+ proportion was significantly lower (Figure 5). A significantly stronger increase in the proportion of CD4+EM and activated CD4+CD69+ cells was detected after 4 days of PBMC coculture with the effective MSC samples. The data are presented as fold changes relative to the control lymphocytes cultured without MSCs.
The MSC population is heterogeneous, and these cells have different properties and act differently in organisms. MSCs obtained from various sources are known to secrete different factors[21]. The properties of MSCs obtained from different donors also differ[22]. Moreover, different MSC clones within the same population have different immunomodulatory properties[23]. Thus, there may be factors that affect the efficacy of MSCs in the clinic, but at the moment, these factors are not known. It was shown that the concentration of CFU-F and the total cellular production of MSCs are significantly higher in younger donors[22]. In our study, in patients who developed GVHD, that is, in patients in whom the MSCs were ineffective, the age of the donors was greater, and the total cellular production of MSCs and the concentration of CFU-F were lower. These differences were not significant between groups; however, they are interrelated and indicate the preference for choosing MSCs from younger donors.
The MFIs of HLA-ABC, HLA-DR, CD105 and CD146 were significantly higher in the effective MSC samples. The molecules of the main histocompatibility complex are weakly expressed on MSCs[24]; thus, MSCs were considered immunoprivileged cells. Subsequent work has shown that this immunoprivileged classification is not accurate[25]. Moreover, the presence of the molecules of the main histocompatibility complex during the administration of MSCs from a third-party donor does not worsen the clinical results[14]. According to our data, the MFIs of HLA-ABC and HLA-DR on the effective MSCs were 1.7 and 4 times higher than those on the ineffective MSCs, respectively. The study analysed the introduction of MSCs obtained from the bone marrow of a haematopoietic cell donor; thus, an increase in the expression of the molecules of the main histocompatibility complex on the MSCs does not induce an immune response but indicates the activation of these cells, as occurs after exposure to IFN[26,27]. We suggest that the increased expression of the HLA-ABC and HLA-DR molecules in the effective MSC samples suggests that they are initially more activated to perform their immunomodulatory functions. This conclusion is also supported by a higher response to all the investigated antigens in the group of effective MSCs. Furthermore, upon activation of these MSC samples by IL-1β, IFN and TNF, the expression of HLA-ABC and HLA-DR did not change as much as we previously observed[28].
MSCs exhibit their immunomodulatory properties not only due to the secretion of many factors but also due to direct intercellular interactions. Numerous adhesion molecules were expressed on the surface of MSCs, and the combination of the functions of these molecules leads to modulation of the T cell response[29]. Three types of molecules involved in the adhesion of T lymphocytes to stromal cells — CD54, CD105, and CD146 — were studied.
The expression of CD105 and CD146 on the surface of the effective MSC samples was increased, which indicates an increase in their adhesive properties.
CD105 (endoglin, SH2) is a transmembrane glycoprotein that is expressed on endothelial cells; CD105 functions as a coreceptor for several ligands of the transforming growth factor β family and plays a vital role in the development and remodelling of blood vessels[30,31]. White blood cells and haematopoietic stem cells also express endoglin[32,33], which regulates leukocyte migration and the quiescence state of haematopoietic stem cells. The extracellular domain of endoglin is involved in cell adhesion, which is mediated by integrins through the RGD motif. A role of endothelial endoglin in leukocyte migration has also been described[34]. The expression of CD105 varies significantly among MSCs obtained from different tissue sources[35,36] and varies among MSC samples from different donors. Thus, it can be assumed that the increased expression of CD105 on the surface of the effective MSC samples enhances their interaction with lymphocytes, thereby increasing their immunomodulatory function.
CD146 is a membrane glycoprotein that functions as a Ca2+-independent cell adhesion molecule and is involved in heterophilic intercellular interactions[37]. The level of CD146 expression on MSCs varies depending on the source of the cells and the culture conditions[38,39]. It was shown that the expression of CD146 decreases in culture, which is associated with a decrease in the frequency of CFU-F[40] and MSC ageing[41]. These data are consistent with a reduced concentration of CFU-F in the inefficient samples. CD146 is responsible for the adhesion of lymphocytes to endothelial cells and MSCs and is involved in cell migration, differentiation, and the immune response[42]. Thus, the reduced expression of CD146 in the ineffective samples is involved in the weakening of their immunomodulatory potential.
CD54 (ICAM-1) plays an important role in the immunosuppressive effect of MSCs[43]. CD54 enhances the interaction of MSCs with type 1 macrophages, which activates MSCs and increases their immunosuppressive function[44]. When MSCs are licensed by IFN or IL-1β, the expression of CD54 and, accordingly, the immunomodulatory effect increase[43]. The effective and ineffective MSC samples not treated with cytokines or activated by lymphocytes expressed equal levels of CD54. However, upon activation by IFN and IL-1β, the CD54 MFI in the effective samples increased 1.8 and 3.3 times, respectively. In the ineffective samples, the MFI of this molecule did not change. Upon MSCs activation by TNF, the CD54 MFI in both the effective and ineffective samples increased by more than 12 times; thus, the effect of TNF helps MSCs overcome a certain “threshold” in CD54 expression. Such an increase demonstrates the potential ability of ineffective MSCs to be activated. The CD54 MFI increased significantly more on the surface of MSCs cocultured with lymphocytes compared with that on the surface of MSCs after exposure to pro-inflammatory cytokines, enhancing the adhesive properties of these cells. In the effective MSC samples, this increase was more intense than in the ineffective samples, but this difference was not significant.
It was shown that the suppression of T lymphocyte proliferation by IFN–licensed MSCs correlated with that by resting MSCs[45]. Co-cultivation of MSCs with PBMCs led to more pronounced changes than licensing by one pro-inflammatory cytokine at the concentration used in this study. Lymphocytes secrete IFN[46], which is a factor that induces an increase in the expression level of HLA-ABC and HLA-DR on MSCs[47,48]. The expression levels of HLA-ABC and HLA-DR increased regardless of the effectiveness of the samples in the prevention of GVHD. The increase in the expression of the molecules of the main histocompatibility complex is significantly greater after co-cultivation with PBMCs than after treatment with IFN, which is most likely because lymphocytes, especially activated lymphocytes, secrete more IFN than was used in the first part of the experiments. In addition, lymphocytes secrete other cytokines and chemokines that affect MSCs.
The CD90 MFI decreased during the co-cultivation of MSCs with PBMCs, and this effect was much stronger in the inefficient samples. CD90 (Thy-1) is a glycoprotein that is bound to the glycosylphosphatidylinositol expressed on many types of cells in addition to MSCs, including T cells, thymocytes, neurons, endothelial cells and fibroblasts[49]. CD90 is involved in the activation of T cells[50]. The expression of CD90 is reduced upon the differentiation of MSCs[51]. The CD90 MFI declined more in the ineffective samples upon co-cultivation with PBMCs and after MSC activation. This result indicates a reduction in the immunomodulatory potential of these samples.
The CD274 gene (PD-L1) encodes an immune inhibitory receptor ligand that is expressed by haematopoietic and non-haematopoietic cells, such as T cells and B cells, and various types of tumour cells. The interaction of this ligand with its receptor inhibits T cell activation and cytokine production. The PD-1 protein is an important regulator of T cell activation. The binding of PD-L1 to its receptors inhibits T cell migration, proliferation and cytotoxic mediator secretion and limits tumour cell death. The interaction of PD-L1 with PD-1 protects the host from overactive effector T cells, not only in cancer processes but also in infectious diseases. It was shown that this pathway may be important in contact-dependent immunomodulation using MSCs. MSCs express and secrete PD-L1 and PD-L2, and this expression is regulated by exposure to IFN and TNF. MSCs secrete PD-1 ligands, inhibiting the activation of CD4+ T cells and the secretion of IL-2 and inducing irreversible hyporeactivity and cell death[52]. CD274 is weakly expressed on the surface of MSCs. In some cases, the differences from the isotypic control are very small. In the case of the licensing of MSCs by pro-inflammatory cytokines, no significant differences were found in the CD274 MFI; nevertheless, it was always higher on the effective MSC samples than on the ineffective samples. When cocultured with lymphocytes, the CD274 MFI of the effective samples significantly exceeded that of the ineffective samples.
CD73 (NT5E) is an ecto-5 primary nucleotidase that catalyses the conversion of purine mononucleotides to nucleosides at neutral pH, and adenosine 5'-monophosphate (AMP) is the preferred substrate. Adenosine is actively produced from AMP by CD73 on MSCs and extracellular vesicles derived from MSCs. Adenosinergic signalling plays a role in inhibiting T cell proliferation in vitro. Adenosinergic signal transmission has been shown to be an important immunoregulatory mechanism of MSCs, especially in situations where ATP is present in the extracellular environment, for example, in the case of tissue damage. The effective synthesis of immunosuppressive adenosine depends on the coordinated action of CD39+ immune cells with CD73+ cells, such as MSCs or their vesicles[53]. In some cases, the adenosinergic pathway acts as a key mechanism by which MSCs perform haemostatic and immunomodulatory functions[54]. The CD73 MFI was higher on the surface of effective MSCs than on the surface of ineffective MSCs in all the experimental conditions. During the co-cultivation of MSCs with lymphocytes, the CD73 MFI in the ineffective MSCs was significantly reduced. These data also reveal differences in the functional potencies of MSCs obtained from different donors. All surface markers that identify MSCs[6] are expressed at different intensities on these cells and participate in their immunosuppressive effect. It is not possible to identify key players in this process; however, the set of MFIs of all the investigated markers on MSCs clearly shows that a decrease in the MFIs leads to a decrease in the efficiency of these cells in modulating the immune response.
Significant differences in the subpopulations of lymphocytes were revealed only for the activated cells cocultured with the effective and ineffective MSCs. This result probably occurred because when lymphocytes are activated by PHA, significant subpopulation changes occur. It is known that the ratio of naive, effector, and memory cells changes, and the appearance of molecules indicating cell activation, CD25, CD38, CD69, PD-1, and HLA-DR, is observed[55]. The ability of MSCs to suppress T lymphocyte proliferation varies between donors[45]. The proportion of CD4 + CM lymphocytes did not change, while the proportion of CD8 + CM and CD8 + EM decreased upon co-cultivation with the effective samples, which is consistent with the immunomodulatory properties of MSCs. During co-cultivation with the inefficient samples, a paradoxical effect of an increased proportion of CD8 + EM was observed. Such a large difference in the effects of different MSC samples once again confirms the presence of individual MSC characteristics that affect their efficiency. The increase in the proportion of CD4 + CD146+ lymphocytes after coculture with the ineffective samples can be explained by the fact that the expression of the adhesion molecule CD146 is involved in the initial stages of the interaction between the endothelium and lymphocytes, which exhibit adhesion receptor activity, including possible homophilic interactions of CD146-CD146[56]; in addition, the expression of CD146 is increased in many diseases that are associated with inflammation[57,58].
An attempt was made to summarize all the data obtained regarding the differences between the effective and ineffective MSC samples. We analysed the differences between the effective and ineffective MSC samples in two ways. First, the analysis was based on the immunophenotypes of the MSCs one day after co-cultivation with activated and non-activated lymphocytes. As the result of linear discriminant analysis, 6 variables were revealed that were sufficient to separate the samples into groups of samples that were effective and ineffective in the prevention of GVHD (Table 4). Based on the obtained coefficients, posterior probabilities of assigning the MSC samples to a particular group were determined (Table 5). The prediction was incorrect for only 1 of the studied samples. This sample (# 19) fell fairly close to the centroids of both groups. When analysing the changes in the MSC immunophenotypes during co-cultivation with lymphocytes in relation to the control MSCs, the greatest differences were observed at the level of HLA-DR expression. In the ineffective samples, HLA-DR expression increased sharply after one day, which was different from HLA-DR expression in the effective samples. The ineffective samples became immunogenic and were possibly eliminated much faster than the effective samples. The mechanism of action of MSCs in this case requires further rigorous study. Nevertheless, using a rather simple functional test, the MSC immunophenotype after 24 h of co-cultivation with lymphocytes, one can try to predict the effectiveness of the MSC sample in preventing the development of acute GVHD.
| MSCs markers after 1 d co-cultivation with non-activated and activated lymphocytes (PHA) | Wilks'Lambda | Partial | F-remove | P-level | Coefficients of discriminant function | |
| No GVHD1 | GVHD2 | |||||
| HLA-DR | 0.572005 | 0.625895 | 11.95423 | 0.002488 | -0.00358 | 0.0213 |
| CD146 | 0.439602 | 0.814407 | 4.55774 | 0.045330 | -0.01545 | 0.0095 |
| CD73 PHA | 0.517153 | 0.692280 | 8.89005 | 0.007375 | 4.30313 | -0.6335 |
| CD105 | 0.499794 | 0.716325 | 7.92028 | 0.010713 | 9.05802 | -1.9000 |
| CD105 PHA | 0.419957 | 0.852503 | 3.46032 | 0.077629 | 2.65834 | 11.3187 |
| CD54 | 0.404184 | 0.885773 | 2.57915 | 0.123954 | -0.01228 | 0.0118 |
| Sample number | GVHD development after prophylaxis | Posterior probabilities of sample classification | Squared mahalanobis distances from group centroids | ||
| No GVHD | GVHD | No GVHD | GVHD | ||
| 1 | No GVHD | 0.999996 | 0.000004 | 4.81908 | 26.37491 |
| 2 | No GVHD | 0.999831 | 0.000169 | 4.69845 | 18.56620 |
| 3 | No GVHD | 0.999937 | 0.000063 | 8.69194 | 24.53850 |
| 4 | No GVHD | 0.999973 | 0.000027 | 5.37220 | 22.94517 |
| 5 | No GVHD | 0.999999 | 0.000001 | 4.25232 | 27.68426 |
| 6 | No GVHD | 0.999513 | 0.000487 | 1.87460 | 13.62827 |
| 6 | No GVHD | 0.957820 | 0.042180 | 4.51571 | 7.26276 |
| 8 | No GVHD | 0.999812 | 0.000188 | 0.52715 | 14.18290 |
| 9 | No GVHD | 0.999849 | 0.000151 | 1.49937 | 15.60338 |
| 10 | No GVHD | 0.999975 | 0.000025 | 1.27143 | 18.96579 |
| 11 | No GVHD | 0.999962 | 0.000038 | 1.37772 | 18.21698 |
| 12 | No GVHD | 0.984365 | 0.015635 | 6.84328 | 11.62984 |
| 13 | No GVHD | 0.999843 | 0.000157 | 0.26067 | 14.27728 |
| 14 | No GVHD | 0.999493 | 0.000507 | 1.71004 | 13.38532 |
| 15 | No GVHD | 0.970256 | 0.029744 | 4.22516 | 7.69663 |
| 16 | No GVHD | 0.999991 | 0.000009 | 7.74281 | 27.47988 |
| 17 | No o GVHD | 0.999810 | 0.000190 | 6.84972 | 20.48673 |
| 18 | No GVHD | 0.999978 | 0.000022 | 2.57251 | 20.55185 |
| 191 | GVHD | 0.986823 | 0.013177 | 2.37048 | 7.50407 |
| 20 | No GVHD | 0.999810 | 0.000190 | 23.00573 | 36.64553 |
| 21 | No GVHD | 0.997370 | 0.002630 | 1.20348 | 9.58174 |
| 22 | GVHD | 0.000015 | 0.999985 | 44.40075 | 18.74916 |
| 23 | GVHD | 0.000033 | 0.999967 | 42.58649 | 18.44380 |
| 24 | No GVHD | 0.990716 | 0.009284 | 2.92989 | 8.77180 |
| 25 | No GVHD | 0.998626 | 0.001374 | 0.63394 | 10.31299 |
| 26 | No GVHD | 0.999170 | 0.000830 | 0.42912 | 11.11676 |
| 27 | GVHD | 0.096789 | 0.903211 | 15.96187 | 7.99662 |
The second model used the data on the immunophenotypes of MSCs co-cultured with PBMCs and activated lymphocytes, data on the immunophenotypes of MSCs after activation by pro-inflammatory cytokines, and data on the alterations in the subpopulation composition of non-activated and activated lymphocytes after co-cultivation with MSCs. Based on complex calculations, a graphic image is presented in Figure 6. A comprehensive analysis of the differences between the effective and ineffective MSC samples showed that the ineffective samples mainly differ from the general group of the effective samples. There is a small area of intersection. The presence of such an area is because the group of effective MSC samples includes those administered to patients who would not have developed GVHD without MSCs. Presenting data on a single scale is similar to developing a probabilistic scale for the effectiveness of MSCs in GVHD prevention. It is possible that not all of the investigated parameters have predictive value. In this study, the CD54 MFI after incubation with IFN, the CD105 MFI on MSCs after exposure to IL-1β, and the proportions of CD4+ and CD8+ EM and CD4+CD146+ lymphocytes after co-cultivation with MSCs were the most significant parameters. The list of the most significant parameters may change after increasing the number of samples and additional analysis of samples using OMIX technology.
Despite the relatively small number of ineffective MSC samples, all the data obtained are interrelated and fit into the general current understanding of the mechanisms of the immunomodulatory action of MSCs.
Multipotent mesenchymal stromal cells (MSCs) are widely used in the clinic due to their unique properties, namely, their immunomodulatory activity. Healthy donor MSCs were used to prevent the development of graft vs host disease (GVHD) after allogeneic bone marrow transplantation (allo-BMT). The administration of MSCs to patients was not always effective. The MSCs obtained from different donors have individual characteristics. The differences between MSC samples may affect their clinical efficacy.
It is necessary to increase the efficiency of MSCs use for GVHD prevention after allo-BMT.
The present study aimed to identify the differences between effective and ineffective MSCs.
Aliquots of 52 MSC samples that were used for GVHD prophylaxis were examined. These cells were cultured in the presence of peripheral blood mononuclear cells (PBMCs) from a third-party donor or treated with the pro-inflammatory cytokines IL-1β, IFN and TNF. The immunophenotype of untreated MSCs, the MSCs cocultured with PBMCs for 4 days or the MSCs exposed to cytokines was investigated by flow cytometry. The proportions of CD25-, CD146-, CD69-, HLA-DR- and PD-1-positive CD4+ and CD8+ cells and the distribution of various effector and memory cell subpopulations in the PBMCs cocultured with MSCs were also determined.
Differences in the immunophenotypes of effective and ineffective MSCs were observed. In the effective samples, the mean fluorescence intensity (MFI) of HLA-ABC, HLA-DR, CD105, and CD146 was significantly higher. After MSCs were treated with IFN or cocultured with PBMCs, the HLA-ABC, HLA-DR, CD90 and CD54 MFI showed a stronger increase in the effective MSCs, which indicated an increase in the immunomodulatory activity of these cells. When PBMCs were cocultured with effective MSCs, the proportions of CD4+ and CD8+central memory cells significantly decreased, and the proportion of CD8+CD146+ lymphocytes increased more than in the subpopulations of lymphocytes cocultured with MSC samples that were ineffective in GVHD prevention. In addition, the proportion of CD8+effector memory lymphocytes decreased in the PBMCs cocultured with the effective MSC samples but increased in the PBMCs cocultured with the ineffective MSC samples. The proportion of CD4+CD146+ lymphocytes increased only when cocultured with the inefficient samples.
For the first time, differences were observed between MSC samples that were effective for GVHD prophylaxis and those that were ineffective. Thus, it was shown that the immunomodulatory activity of MSCs depends on the individual characteristics of the MSC population.
Determination of the main differences between effective and ineffective samples will improve the clinical results of MSCs use. The list of the most significant parameters showing the differences between effective and ineffective MSC samples may change after increasing the number of samples and additional analysis of samples using OMIX technology.
Manuscript source: Invited manuscript
Corresponding Author's Membership in Professional Societies: American Society of Hematology, No. 423595; International Society for Experimental Hematology, No. 9295857; European Hematology Association, No. 003726.
Specialty type: Cell and tissue engineering
Country/Territory of origin: Russia
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): E
P-Reviewer: Valtieri M S-Editor: Ma YJ L-Editor: Webster JR P-Editor: Xing YX
| 1. | Ghimire S, Weber D, Mavin E, Wang XN, Dickinson AM, Holler E. Pathophysiology of GvHD and Other HSCT-Related Major Complications. Front Immunol. 2017;8:79. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 92] [Cited by in RCA: 170] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 2. | Elgaz S, Kuçi Z, Kuçi S, Bönig H, Bader P. Clinical Use of Mesenchymal Stromal Cells in the Treatment of Acute Graft-versus-Host Disease. Transfus Med Hemother. 2019;46:27-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 58] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 3. | Galipeau J, Sensébé L. Mesenchymal Stromal Cells: Clinical Challenges and Therapeutic Opportunities. Cell Stem Cell. 2018;22:824-833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1009] [Cited by in RCA: 1208] [Article Influence: 172.6] [Reference Citation Analysis (0)] |
| 4. | Bianco P, Robey PG, Simmons PJ. Mesenchymal stem cells: revisiting history, concepts, and assays. Cell Stem Cell. 2008;2:313-319. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1248] [Cited by in RCA: 1126] [Article Influence: 66.2] [Reference Citation Analysis (0)] |
| 5. | Greim H, Kaden DA, Larson RA, Palermo CM, Rice JM, Ross D, Snyder R. The bone marrow niche, stem cells, and leukemia: impact of drugs, chemicals, and the environment. Ann N Y Acad Sci. 2014;1310:7-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 48] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 6. | Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop Dj, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11055] [Cited by in RCA: 12689] [Article Influence: 704.9] [Reference Citation Analysis (2)] |
| 7. | Krampera M. Mesenchymal stromal cell 'licensing': a multistep process. Leukemia. 2011;25:1408-1414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 256] [Cited by in RCA: 283] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 8. | Miettinen JA, Pietilä M, Salonen RJ, Ohlmeier S, Ylitalo K, Huikuri HV, Lehenkari P. Tumor necrosis factor alpha promotes the expression of immunosuppressive proteins and enhances the cell growth in a human bone marrow-derived stem cell culture. Exp Cell Res. 2011;317:791-801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 9. | Liu S, Ginestier C, Ou SJ, Clouthier SG, Patel SH, Monville F, Korkaya H, Heath A, Dutcher J, Kleer CG, Jung Y, Dontu G, Taichman R, Wicha MS. Breast cancer stem cells are regulated by mesenchymal stem cells through cytokine networks. Cancer Res. 2011;71:614-624. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 485] [Cited by in RCA: 500] [Article Influence: 35.7] [Reference Citation Analysis (0)] |
| 10. | Chen H, Min XH, Wang QY, Leung FW, Shi L, Zhou Y, Yu T, Wang CM, An G, Sha WH, Chen QK. Pre-activation of mesenchymal stem cells with TNF-α, IL-1β and nitric oxide enhances its paracrine effects on radiation-induced intestinal injury. Sci Rep. 2015;5:8718. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 98] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 11. | Le Blanc K, Rasmusson I, Sundberg B, Götherström C, Hassan M, Uzunel M, Ringdén O. Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet. 2004;363:1439-1441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2044] [Cited by in RCA: 2029] [Article Influence: 96.6] [Reference Citation Analysis (0)] |
| 12. | Introna M, Rambaldi A. Mesenchymal stromal cells for prevention and treatment of graft-versus-host disease: successes and hurdles. Curr Opin Organ Transplant. 2015;20:72-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 43] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 13. | Morata-Tarifa C, Macías-Sánchez MDM, Gutiérrez-Pizarraya A, Sanchez-Pernaute R. Mesenchymal stromal cells for the prophylaxis and treatment of graft-versus-host disease-a meta-analysis. Stem Cell Res Ther. 2020;11:64. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 47] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 14. | Fisher SA, Cutler A, Doree C, Brunskill SJ, Stanworth SJ, Navarrete C, Girdlestone J. Mesenchymal stromal cells as treatment or prophylaxis for acute or chronic graft-versus-host disease in haematopoietic stem cell transplant (HSCT) recipients with a haematological condition. Cochrane Database Syst Rev. 2019;1:CD009768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 56] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 15. | Greaves M, Janossy G, Doenhoff M. Selective triggering of human T and B lymphocytes in vitro by polyclonal mitogens. J Exp Med. 1974;140:1-18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 187] [Cited by in RCA: 220] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 16. | Mahnke YD, Brodie TM, Sallusto F, Roederer M, Lugli E. The who's who of T-cell differentiation: human memory T-cell subsets. Eur J Immunol. 2013;43:2797-2809. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 556] [Cited by in RCA: 654] [Article Influence: 54.5] [Reference Citation Analysis (0)] |
| 17. | Kapranov NM, Davydova YO, Galtseva IV, Petinati NA, Drize NI, Kuzmina LA, Parovichnikova EN, Savchenko VG. Effect of Priming of Multipotent Mesenchymal Stromal Cells with Interferon γ on Their Immunomodulating Properties. Biochemistry (Mosc). 2017;82:1158-1168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 18. | Kuzmina LA, Petinati NA, Parovichnikova EN, Lubimova LS, Gribanova EO, Gaponova TV, Shipounova IN, Zhironkina OA, Bigildeev AE, Svinareva DA, Drize NJ, Savchenko VG. Multipotent Mesenchymal Stromal Cells for the Prophylaxis of Acute Graft-versus-Host Disease-A Phase II Study. Stem Cells Int. 2012;2012:968213. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 87] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 19. | Kuzmina LA, Petinati NA, Shipounova IN, Sats NV, Bigildeev AE, Zezina EA, Popova MD, Drize NJ, Parovichnikova EN, Savchenko VG. Analysis of multipotent mesenchymal stromal cells used for acute graft-versus-host disease prophylaxis. Eur J Haematol. 2016;96:425-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 20. | Shipounova IN, Petinati NA, Bigildeev AE, Zezina EA, Drize NI, Kuzmina LA, Parovichnikova EN, Savchenko VG. Analysis of results of acute graft-versus-host disease prophylaxis with donor multipotent mesenchymal stromal cells in patients with hemoblastoses after allogeneic bone marrow transplantation. Biochemistry (Mosc). 2014;79:1363-1370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 21. | Pires AO, Mendes-Pinheiro B, Teixeira FG, Anjo SI, Ribeiro-Samy S, Gomes ED, Serra SC, Silva NA, Manadas B, Sousa N, Salgado AJ. Unveiling the Differences of Secretome of Human Bone Marrow Mesenchymal Stem Cells, Adipose Tissue-Derived Stem Cells, and Human Umbilical Cord Perivascular Cells: A Proteomic Analysis. Stem Cells Dev. 2016;25:1073-1083. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 172] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 22. | Shipounova IN, Petinati NA, Bigildeev AE, Sats NV, Drize NJ, Kuzmina LA, Parovichnikova EN, Savchenko VG. Hierarchy of mesenchymal stem cells: Comparison of multipotent mesenchymal stromal cells with fibroblast colony forming units. J Biomed Sci Eng. 2013;6:66-73. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 23. | Sempere JM, Martinez-Peinado P, Arribas MI, Reig JA, De La Sen ML, Zubcoff JJ, Fraga MF, Fernández AF, Santana A, Roche E. Single cell-derived clones from human adipose stem cells present different immunomodulatory properties. Clin Exp Immunol. 2014;176:255-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 24. | Jones BJ, McTaggart SJ. Immunosuppression by mesenchymal stromal cells: from culture to clinic. Exp Hematol. 2008;36:733-741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 175] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 25. | Ankrum JA, Ong JF, Karp JM. Mesenchymal stem cells: immune evasive, not immune privileged. Nat Biotechnol. 2014;32:252-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 872] [Cited by in RCA: 1131] [Article Influence: 102.8] [Reference Citation Analysis (0)] |
| 26. | Petinati NA, Kapranov NM, Bigil'deev AE, Popova MD, Davydova YO, Gal'tseva IV, Drize NI, Kuz'mina LA, Parovichnikova EN, Savchenko VG. Changing the Properties of Multipotent Mesenchymal Stromal Cells by IFNγ Administration. Bull Exp Biol Med. 2017;163:230-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 27. | Schepers K, Fibbe WE. Unraveling mechanisms of mesenchymal stromal cell-mediated immunomodulation through patient monitoring and product characterization. Ann N Y Acad Sci. 2016;1370:15-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 28. | Kapranov NM, Davydova JO, Petinati NA, Bakshinskayte MV, Galtseva IV, Drize NI, Kuzmina LA, Parovichnikova EN, Savchenko VG. Alterations in multipotent mesenchymal stromal cells properties: In vitro model of their interactions with allogeneic lymphocytes. Cell Ther Transplant 2016. 5. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 29. | Benvenuto F, Voci A, Carminati E, Gualandi F, Mancardi G, Uccelli A, Vergani L. Human mesenchymal stem cells target adhesion molecules and receptors involved in T cell extravasation. Stem Cell Res Ther. 2015;6:245. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 30. | Duff SE, Li C, Garland JM, Kumar S. CD105 is important for angiogenesis: evidence and potential applications. FASEB J. 2003;17:984-992. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 361] [Cited by in RCA: 371] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 31. | Mo M, Wang S, Zhou Y, Li H, Wu Y. Mesenchymal stem cell subpopulations: phenotype, property and therapeutic potential. Cell Mol Life Sci. 2016;73:3311-3321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 94] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 32. | Pierelli L, Bonanno G, Rutella S, Marone M, Scambia G, Leone G. CD105 (endoglin) expression on hematopoietic stem/progenitor cells. Leuk Lymphoma. 2001;42:1195-1206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 53] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 33. | Borges L, Oliveira VKP, Baik J, Bendall SC, Perlingeiro RCR. Serial transplantation reveals a critical role for endoglin in hematopoietic stem cell quiescence. Blood. 2019;133:688-696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 34. | Rossi E, Bernabeu C, Smadja DM. Endoglin as an Adhesion Molecule in Mature and Progenitor Endothelial Cells: A Function Beyond TGF-β. Front Med (Lausanne). 2019;6:10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 83] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 35. | Arufe MC, De la Fuente A, Fuentes I, de Toro FJ, Blanco FJ. Chondrogenic potential of subpopulations of cells expressing mesenchymal stem cell markers derived from human synovial membranes. J Cell Biochem. 2010;111:834-845. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 84] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 36. | Gaebel R, Furlani D, Sorg H, Polchow B, Frank J, Bieback K, Wang W, Klopsch C, Ong LL, Li W, Ma N, Steinhoff G. Cell origin of human mesenchymal stem cells determines a different healing performance in cardiac regeneration. PLoS One. 2011;6:e15652. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 95] [Cited by in RCA: 97] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 37. | Shih IM. The role of CD146 (Mel-CAM) in biology and pathology. J Pathol. 1999;189:4-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 38. | Martin-Rendon E, Sweeney D, Lu F, Girdlestone J, Navarrete C, Watt SM. 5-Azacytidine-treated human mesenchymal stem/progenitor cells derived from umbilical cord, cord blood and bone marrow do not generate cardiomyocytes in vitro at high frequencies. Vox Sang. 2008;95:137-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 126] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 39. | Rider DA, Dombrowski C, Sawyer AA, Ng GH, Leong D, Hutmacher DW, Nurcombe V, Cool SM. Autocrine fibroblast growth factor 2 increases the multipotentiality of human adipose-derived mesenchymal stem cells. Stem Cells. 2008;26:1598-1608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 115] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 40. | Dmitrieva RI, Minullina IR, Bilibina AA, Tarasova OV, Anisimov SV, Zaritskey AY. Bone marrow- and subcutaneous adipose tissue-derived mesenchymal stem cells: differences and similarities. Cell Cycle. 2012;11:377-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 136] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 41. | Jin HJ, Kwon JH, Kim M, Bae YK, Choi SJ, Oh W, Yang YS, Jeon HB. Downregulation of Melanoma Cell Adhesion Molecule (MCAM/CD146) Accelerates Cellular Senescence in Human Umbilical Cord Blood-Derived Mesenchymal Stem Cells. Stem Cells Transl Med. 2016;5:427-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 58] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 42. | Wang Z, Yan X. CD146, a multi-functional molecule beyond adhesion. Cancer Lett. 2013;330:150-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 226] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 43. | Ren G, Zhao X, Zhang L, Zhang J, L'Huillier A, Ling W, Roberts AI, Le AD, Shi S, Shao C, Shi Y. Inflammatory cytokine-induced intercellular adhesion molecule-1 and vascular cell adhesion molecule-1 in mesenchymal stem cells are critical for immunosuppression. J Immunol. 2010;184:2321-2328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 483] [Cited by in RCA: 497] [Article Influence: 33.1] [Reference Citation Analysis (0)] |
| 44. | Espagnolle N, Balguerie A, Arnaud E, Sensebé L, Varin A. CD54-Mediated Interaction with Pro-inflammatory Macrophages Increases the Immunosuppressive Function of Human Mesenchymal Stromal Cells. Stem Cell Reports. 2017;8:961-976. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 69] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 45. | Guan Q, Li Y, Shpiruk T, Bhagwat S, Wall DA. Inducible indoleamine 2,3-dioxygenase 1 and programmed death ligand 1 expression as the potency marker for mesenchymal stromal cells. Cytotherapy. 2018;20:639-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 49] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 46. | Reddy M, Eirikis E, Davis C, Davis HM, Prabhakar U. Comparative analysis of lymphocyte activation marker expression and cytokine secretion profile in stimulated human peripheral blood mononuclear cell cultures: an in vitro model to monitor cellular immune function. J Immunol Methods. 2004;293:127-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 217] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 47. | Polchert D, Sobinsky J, Douglas G, Kidd M, Moadsiri A, Reina E, Genrich K, Mehrotra S, Setty S, Smith B, Bartholomew A. IFN-gamma activation of mesenchymal stem cells for treatment and prevention of graft versus host disease. Eur J Immunol. 2008;38:1745-1755. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 485] [Cited by in RCA: 457] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 48. | Chan WK, Lau AS, Li JC, Law HK, Lau YL, Chan GC. MHC expression kinetics and immunogenicity of mesenchymal stromal cells after short-term IFN-gamma challenge. Exp Hematol. 2008;36:1545-1555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 93] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 49. | Rege TA, Hagood JS. Thy-1 as a regulator of cell-cell and cell-matrix interactions in axon regeneration, apoptosis, adhesion, migration, cancer, and fibrosis. FASEB J. 2006;20:1045-1054. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 262] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 50. | Barboni E, Gormley AM, Pliego Rivero FB, Vidal M, Morris RJ. Activation of T lymphocytes by cross-linking of glycophospholipid-anchored Thy-1 mobilizes separate pools of intracellular second messengers to those induced by the antigen-receptor/CD3 complex. Immunology. 1991;72:457-463. [PubMed] |
| 51. | Moraes DA, Sibov TT, Pavon LF, Alvim PQ, Bonadio RS, Da Silva JR, Pic-Taylor A, Toledo OA, Marti LC, Azevedo RB, Oliveira DM. A reduction in CD90 (THY-1) expression results in increased differentiation of mesenchymal stromal cells. Stem Cell Res Ther. 2016;7:97. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 125] [Cited by in RCA: 144] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 52. | Davies LC, Heldring N, Kadri N, Le Blanc K. Mesenchymal Stromal Cell Secretion of Programmed Death-1 Ligands Regulates T Cell Mediated Immunosuppression. Stem Cells. 2017;35:766-776. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 180] [Cited by in RCA: 267] [Article Influence: 29.7] [Reference Citation Analysis (0)] |
| 53. | Kerkelä E, Laitinen A, Räbinä J, Valkonen S, Takatalo M, Larjo A, Veijola J, Lampinen M, Siljander P, Lehenkari P, Alfthan K, Laitinen S. Adenosinergic Immunosuppression by Human Mesenchymal Stromal Cells Requires Co-Operation with T cells. Stem Cells. 2016;34:781-790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 86] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 54. | Netsch P, Elvers-Hornung S, Uhlig S, Klüter H, Huck V, Kirschhöfer F, Brenner-Weiß G, Janetzko K, Solz H, Wuchter P, Bugert P, Bieback K. Human mesenchymal stromal cells inhibit platelet activation and aggregation involving CD73-converted adenosine. Stem Cell Res Ther. 2018;9:184. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 55. | Chadburn A, Inghirami G, Knowles DM. The kinetics and temporal expression of T-cell activation-associated antigens CD15 (LeuM1), CD30 (Ki-1), EMA, and CD11c (LeuM5) by benign activated T cells. Hematol Pathol. 1992;6:193-202. [PubMed] |
| 56. | Guezguez B, Vigneron P, Lamerant N, Kieda C, Jaffredo T, Dunon D. Dual role of melanoma cell adhesion molecule (MCAM)/CD146 in lymphocyte endothelium interaction: MCAM/CD146 promotes rolling via microvilli induction in lymphocyte and is an endothelial adhesion receptor. J Immunol. 2007;179:6673-6685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 95] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 57. | Wu C, Goodall JC, Busch R, Gaston JS. Relationship of CD146 expression to secretion of interleukin (IL)-17, IL-22 and interferon-γ by CD4(+) T cells in patients with inflammatory arthritis. Clin Exp Immunol. 2015;179:378-391. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 58. | Dagur PK, Tatlici G, Gourley M, Samsel L, Raghavachari N, Liu P, Liu D, McCoy JP Jr. CD146+ T lymphocytes are increased in both the peripheral circulation and in the synovial effusions of patients with various musculoskeletal diseases and display pro-inflammatory gene profiles. Cytometry B Clin Cytom. 2010;78:88-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |