Published online Nov 26, 2020. doi: 10.4252/wjsc.v12.i11.1366
Peer-review started: March 12, 2020
First decision: May 26, 2020
Revised: July 2, 2020
Accepted: September 8, 2020
Article in press: September 8, 2020
Published online: November 26, 2020
Processing time: 259 Days and 6.4 Hours
High humidity and temperature in Taiwan have significant effects on the reproductivity of Holstein cattle, resulting in the occurrence of bovine ovarian follicular cyst (OFC). Because of economic loss from OFC, manual rupture and hormone injection have been advocated for the management of OFC. However, these incomplete treatments increase hormone resistance in cattle. Mesenchymal stem cells (MSCs) derived from placental stem cells (PSCs) demonstrate potential properties for the treatment of several diseases via promoting angiogenesis and immune modulation.
To establish the possibility of cattle placental stem cells (CPSCs) as a treatment modality for OFC of cows in Taiwan.
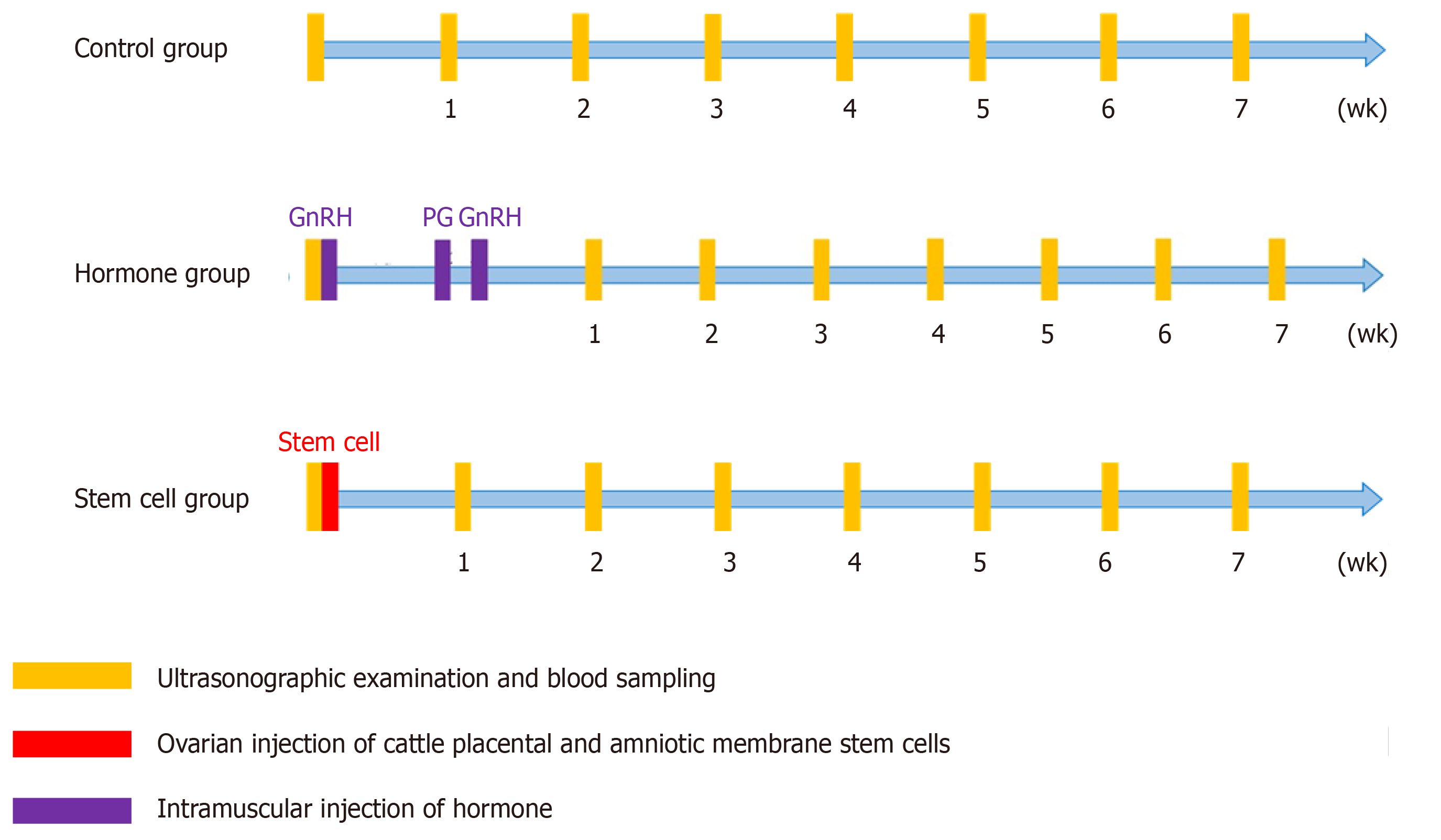
The cows with OFC were divided into three groups: control (BC1 and BC2), hormone (H1 and H2), and CPSC (PS1 and PS2) treatment groups. In the hormone treatment group, the cows were given gonadotrophin-releasing hormone (GnRH)-prostaglandin-GnRH intramuscular injection with or without drainage of follicular fluid. In the CPSC treatment group, CPSCs were isolated from the placenta after labor. With the identification of surface antigen on stem cells, the cows were administered ovarian injection of 1 × 106 or 6 × 106 CPSCs with drainage. In all groups, OFC was scanned by ultrasound once a week for a total of seven times. The concentrations of estradiol and progesterone in serum were tested in the same period. The estrus cycle was analyzed by food intake and activity. If estrus was detected, artificial insemination was conducted. Then the cow was monitored by ultrasound for confirmation of pregnancy.
After 7 d of culture, CPSCs were successfully isolated from placental pieces. CPSCs significantly proliferated every 24 h and had high expression of MSC markers such as cluster of differentiation 44, as determined by flow cytometry. Ultrasound showed lower numbers of OFCs with drainage of follicular fluid. We achieved recovery rates of 0%, 50%, 50%, 75%, 75% and 75% in BC1, BC2, H1, H2, PS1, and PS2, respectively. Higher concentrations of progesterone were detected in the CPSC treatment groups. However, both hormone and CPSC treatment groups had no significant difference in the concentration of estradiol. The estrus rate was 0%, 100%, 25%, 75%, 75% and 75% in BC1, BC2, H1, H2, PS1, and PS2, respectively. The two fetuses were born in H2 and PS1. In brief, cows with CPSC injection achieved higher recovery, estrus, and inseminated conception rates.
CPSCs have efficacy in treating cows with OFC, and thus, may serve as an alternative treatment for reproductive disorders.
Core Tip: Ovarian follicular cysts (OFCs) harm the reproductivity and milk production of cows. To deal with economic loss, this study established the possibility of using cattle placental stem cells (CPSCs) for treating OFC. We drained the follicular fluid and injected CPSCs into the ovaries. Then the CPSCs significantly proliferated and expressed high levels of cluster of differentiation 44. Elevated concentrations of progesterone in serum were observed. Decreased numbers of OFCs were shown by ultrasound. Cows with CPSC injection had higher recovery, estrus, and inseminated conception rates. These outcomes indicate the therapeutic potential of CPSCs for OFCs in cattle.
- Citation: Peng SY, Wu TH, Lin TY, Hii LY, Chan KS, Fu TY, Chang SC, Shen PC, Liu KY, Shaw SW.. Application of cattle placental stem cells for treating ovarian follicular cyst. World J Stem Cells 2020; 12(11): 1366-1376
- URL: https://www.wjgnet.com/1948-0210/full/v12/i11/1366.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v12.i11.1366
Taiwan is located in subtropical and tropical areas with high humidity and temperature. Such heat stress seriously affects the physiological and reproductive function of Holstein, including milk yield and conception[1]. To produce more milk, cows should take in more nutrition. However, decreased food intake due to heat stress leads to poor reproductive function[2,3]. This reproductive disorder represents the main economic loss in Taiwan.
Ovarian follicular cyst (OFC) is a common reproductive disorder caused by an imbalance in hormones from the hypothalamus and pituitary gland. This abnormal condition causes ovulation failure and decreases the profits for farmers[4-6]. To solve this problem, manual rupture of ovarian follicles is used for treating OFC. However, this method decreases the conception rate if hemorrhage in ovaries occurs[7]. Consequently, an alternative approach is using hormones, such as human chorionic gonadotrophin[8] and gonadotrophin-releasing hormone (GnRH)[7,9]. Unfortunately, this treatment increases hormone resistance or has no effect on cattle[7-9].
Recently, the use of stem cells to repair or replace damaged tissue has been extensively studied[10]. Stem cells secrete growth factors and anti-inflammatory factors[11] and reduce fibrosis[12]. Several studies have reported the success of stem cells for treating retinal[13], liver[14], and cardiac diseases[15]. On the other hand, the placenta can be non-invasively collected as great sources of stem cells without much ethical concern[16]. Many placental stem cells (PSCs) can be identified by the expression of mesenchymal stem cells (MSCs) and pluripotency markers[17]. These cells can be differentiated into mesodermal-relative cells[18]. In addition, PSCs have therapeutic properties for the management of several diseases such as wound healing[19], diabetes[20], lung fibrosis[21], among others[22,23]. To date, the effect of cattle placental stem cells (CPSCs) on treating OFC is still under research.
The objective of this study was to establish the possibility of using CPSCs for the treatment of cattle with OFCs in Taiwan.
The placental tissues were collected from 3- to 4-year-old laboring cows of Holstein species from the National Pingtung University of Science and Technology. Holstein cows from three different pastures were all fed a total mixed ration composed of alfalfa, bermuda, sweet oats, and silage corn in free-stall barns. The OFCs were diagnosed by follicle size (> 25 mm), existence (> 10 d), and thickness of the follicular wall (< 3 mm).
Isolation of stem cells: After labor, the placenta was collected and disinfected by 75% alcohol. Then the cleaned placenta was cut into small pieces and cultured in medium (αMEM containing 10% fetal bovine serum, M0894; Sigma, St. Louis, MO, United States and 1% penicillin/streptococcus, 10437 and 15140; Gibco, Mexico and the United States, respectively) in a 38.5 °C, 5% CO2 incubator (Thermo, Electron Corporation, Waltham, MA, United States) for 7 d to isolate the cells.
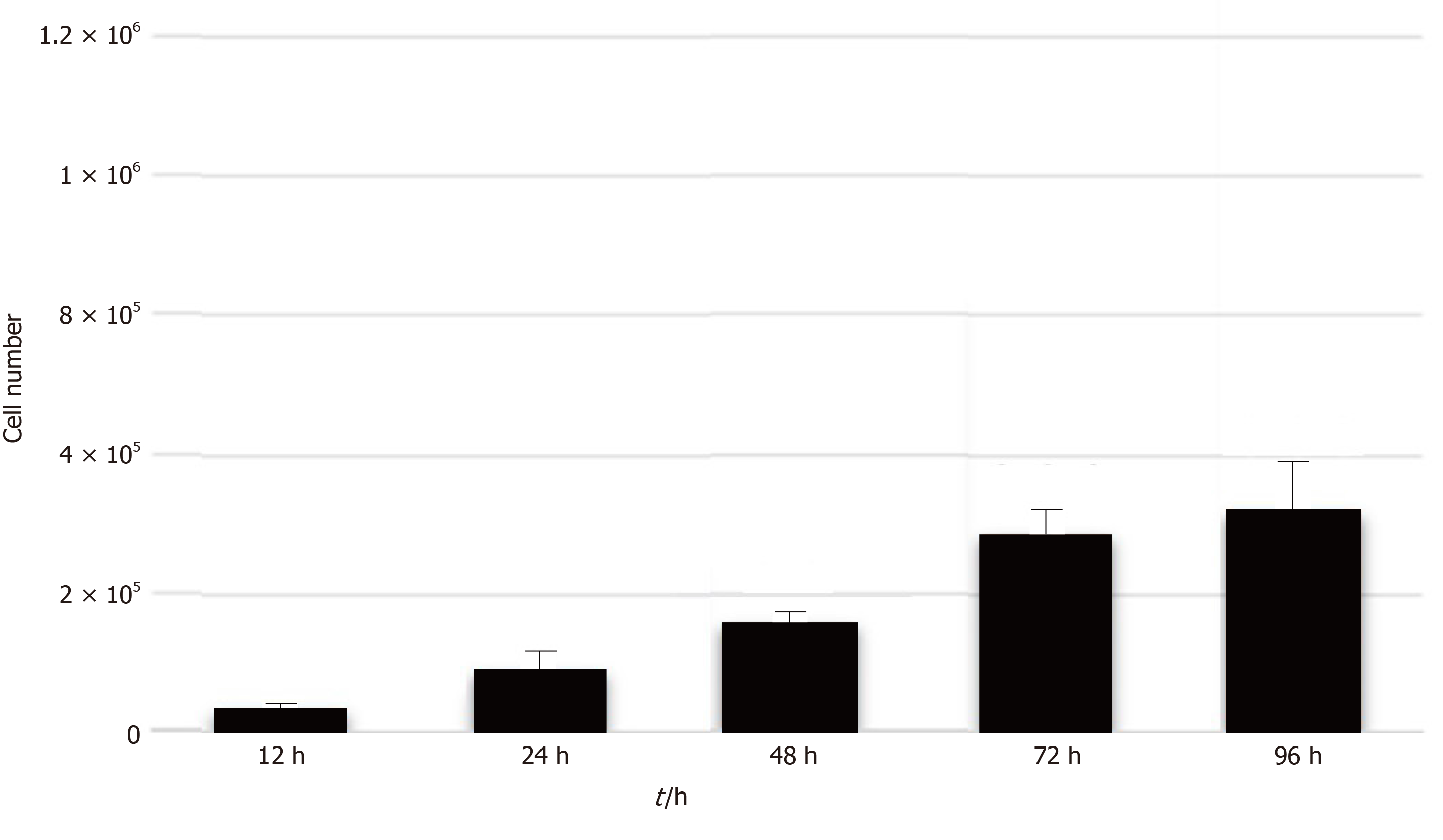
Analysis of stem cell proliferation: After the sixth passage, CPSCs were suspended in 0.25% trypsin-EDTA (25200072; Gibco, Vancouver, Canada). Then 2 × 104 cells were plated into 6-well dishes. The cell number was measured with trypan blue staining at 12, 24, 48, 72, and 96 h.
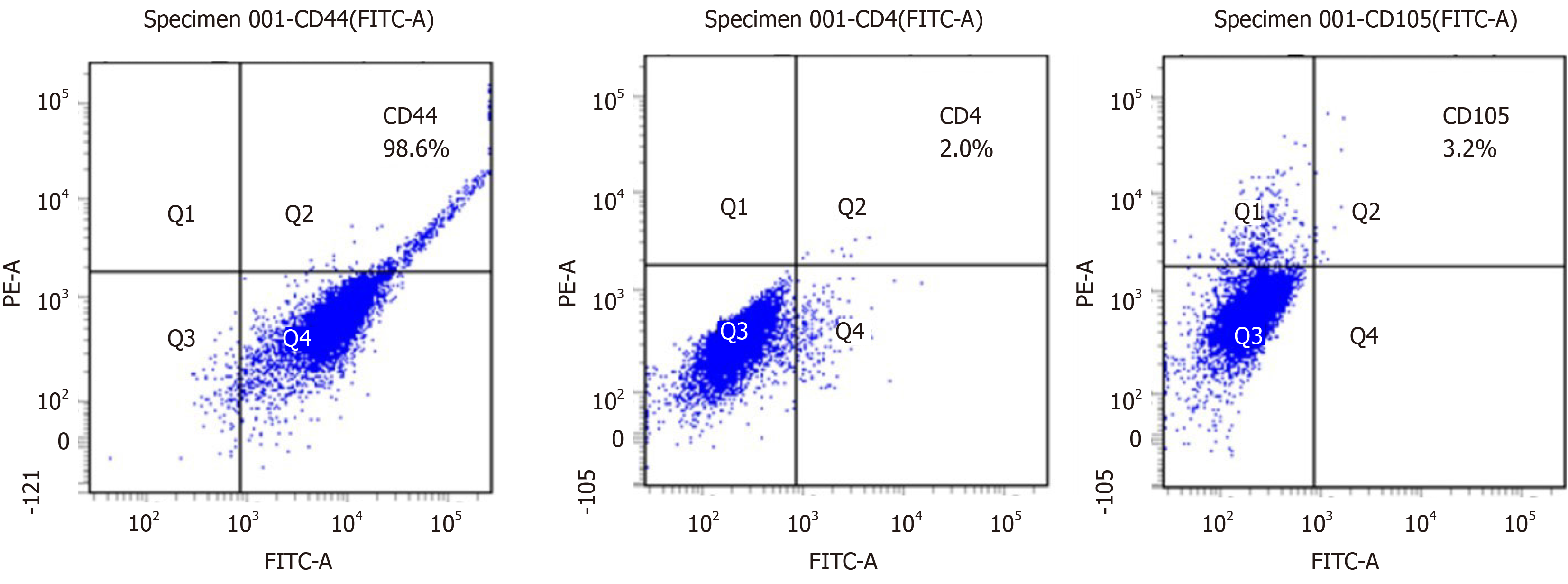
Analysis of stem cell surface antigen: After calculating the number of CPSCs, 2.5 × 105 cells were centrifuged, followed by staining with the cell surface antigens cluster of differentiation 4 (CD4, MCA 1653F; Bio-Rad, Hercules, CA, United States), CD44 (MCA 2433F; Bio-Rad), and CD105 (MA5-11854; Thermo Fisher Scientific) for 30 min. The antibodies were removed by centrifugation at 270 × g for 5 min. Finally, the stained cells were analyzed by flow cytometry (BD FACS Aria II; BD Biosciences, Franklin Lakes, NJ, United States).
CPSC therapeutic potential: The study design is shown in Figure 1. A total of 19 cows with OFC were divided into three groups: control, hormone, and CPSC treatment groups. The BC1 control group was not treated. The BC2 control group was treated with follicular fluid drainage and saline injection. The H1 hormone treatment group was given intramuscular injection of GnRH (250 μg)-prostaglandin (PG) (500 μg)–GnRH (250 μg) without drainage of follicular fluid. The H2 hormone treatment group was given intramuscular injection of GnRH-PG–GnRH with drainage of follicular fluid. The PS1 CPSC treatment group was given ovarian injection of 1 × 106 CPSCs with follicular fluid drainage. The PS2 CPSC treatment group was given ovarian injection of 6 × 106 CPSCs (Figure 1) with follicular fluid drainage. All of the suspended CPSCs were stored in Dulbecco’s phosphate-buffered saline (21300; Gibco, United States) at 4 °C before use.
Observation of ovarian follicle, estrus, and pregnancy status: The status of ovarian follicles was observed by the B-mode ultrasound scanner (iScan Draminski B-Mode) after treatment. The estrus detection system was used to determine the estrus cycle by analyzing feed intake and activity. When estrus was detected, artificial insemination was done after 8 to 12 h. Then ultrasound assessment was performed to confirm pregnancy.
Analysis of the hormone in serum: A total of 10 mL blood was collected in an anticoagulation-EDTA tube (367525; BD Vacutainer, Becton Dickinson, London, United Kingdom) from the tail veins of each experimental group, and stored at 4 °C for 30 min. The blood was centrifuged at 3000 rpm for 10 min to collect the serum. Then the serum was stored at -80 °C. Chemiluminescence was utilized to measure the concentrations of estradiol and progesterone in the serum (Centaur Immunoassay System; SIEMENS, Munich, Germany).
The data were analyzed with a statistical analysis system (SAS 9.4). A general linear model and Duncan’s New Multiple Range Test were generated with statistical significance if P < 0.05.
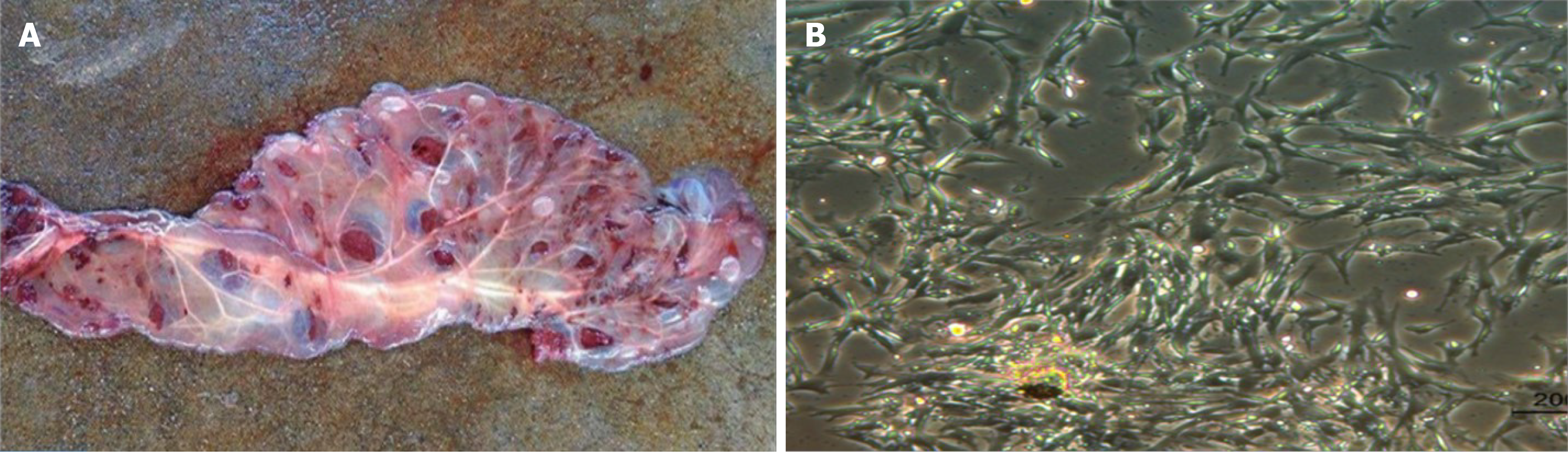
After 3 to 7 d of culture, the spindle-shaped cells were isolated from placental pieces (Figure 2). There was a higher possibility of isolating the cells from the smaller placental pieces. The CPSCs calculated every 24 h in the sixth passage showed strong proliferation potential (Figure 3). The CPSCs expressed CD44 and slightly expressed CD4 and CD105 (Figure 4).
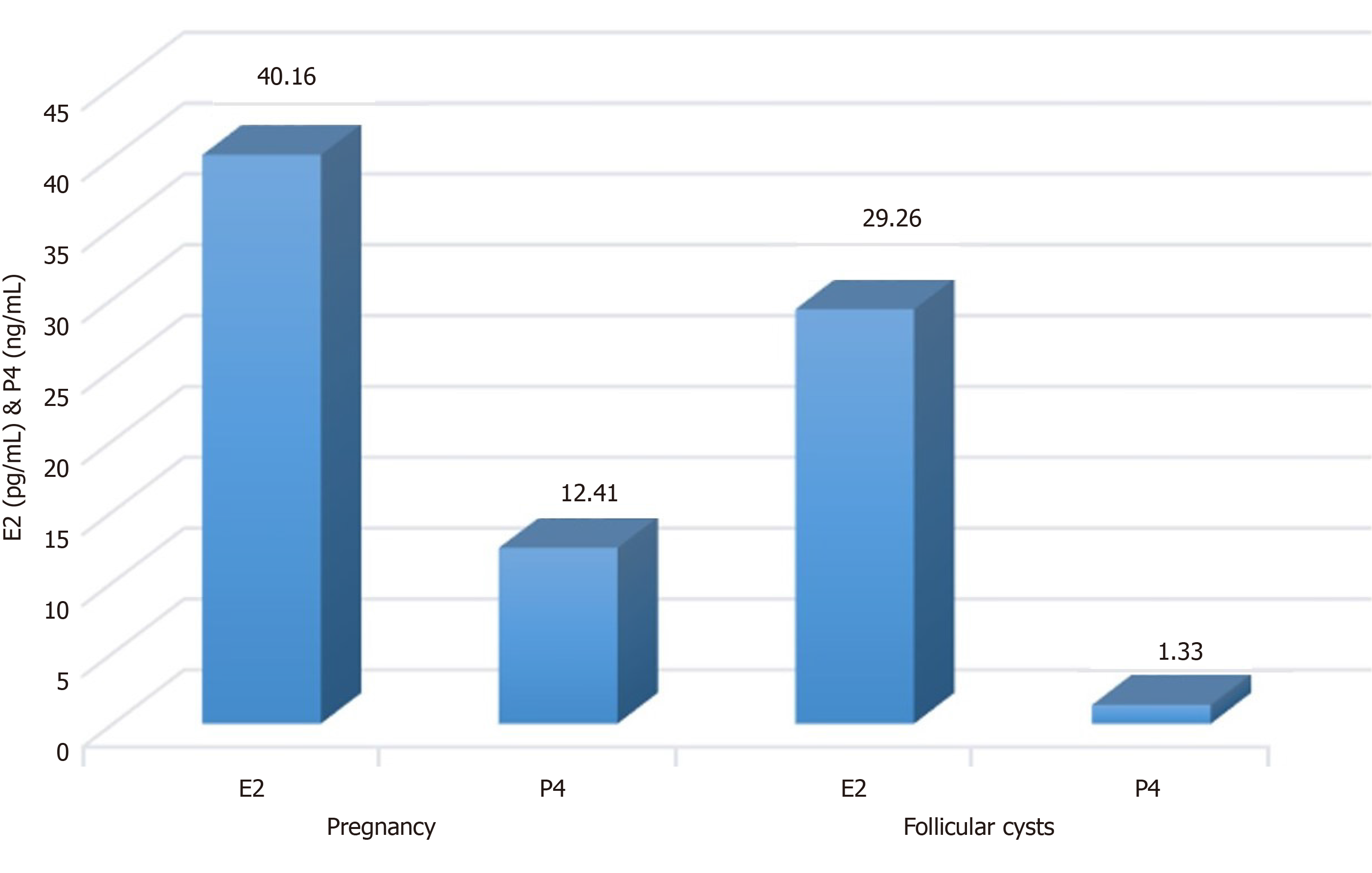
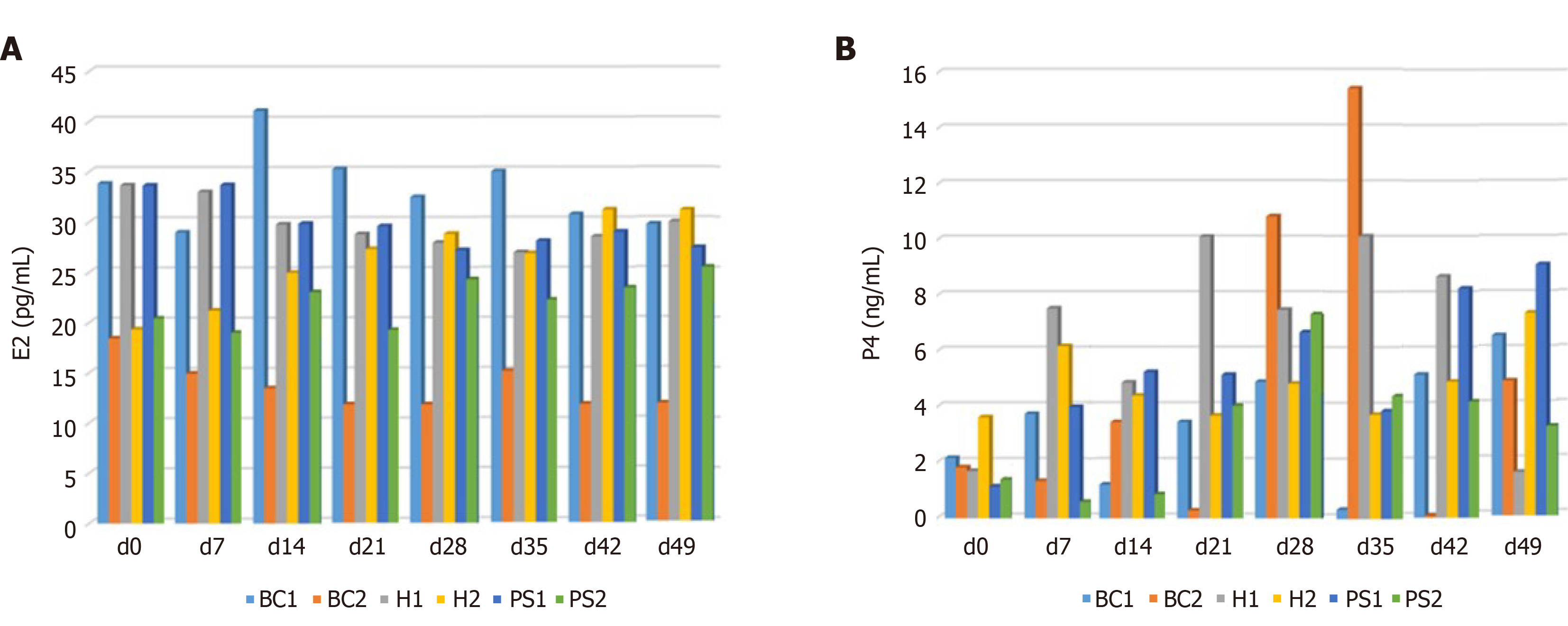
To the best of our knowledge, the status of the ovary varies in the estrus cycle with a change in hormones, such as progesterone (P4) and estradiol (E2). A high concentration of E2 and P4 confirmed the pregnancy, but cows with OFC had a low concentration of P4 (Figure 5). Both the hormone and CPSC treatment groups had no significant difference in E2 concentration, which may originate from variation in individual bovines (Figure 6A). Interestingly, the concentration of P4 increased in the hormone and CPSC treatment groups, showing the therapeutic potential for both treatments (Figure 6B).
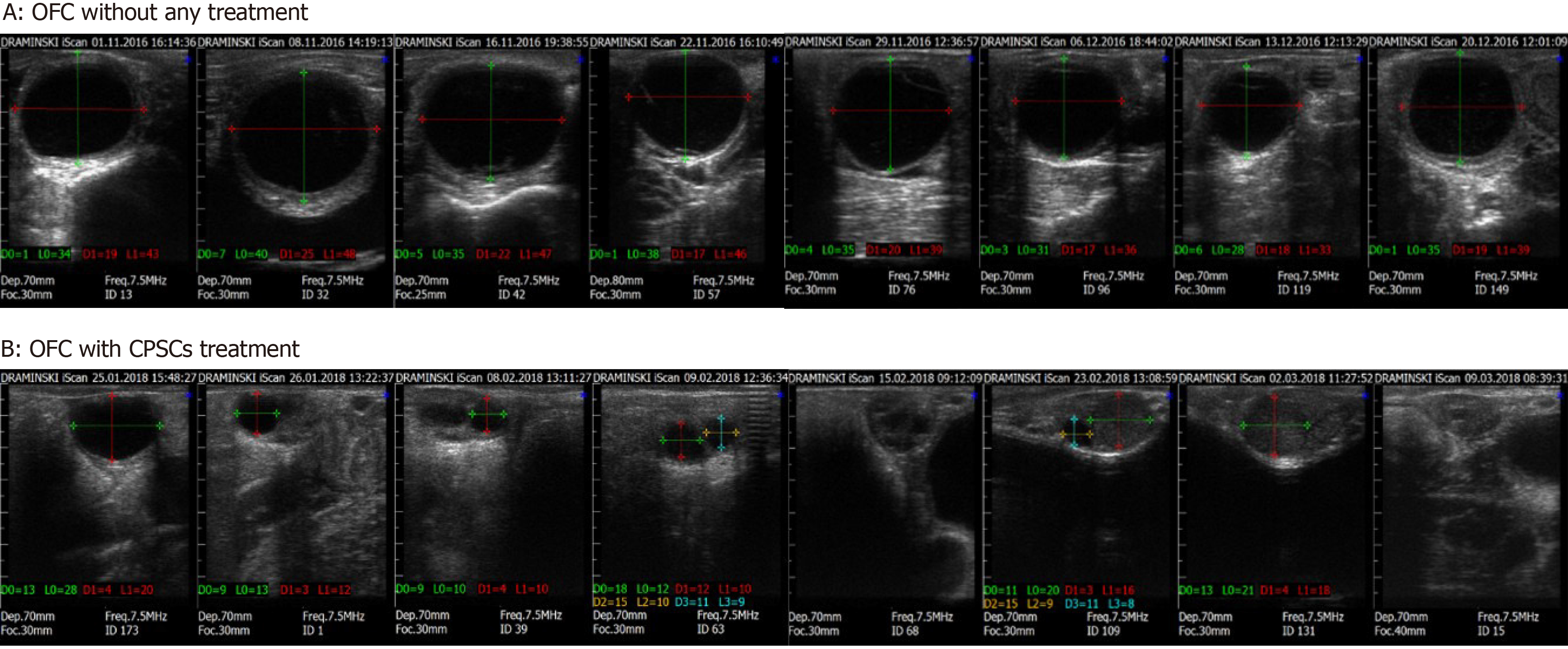
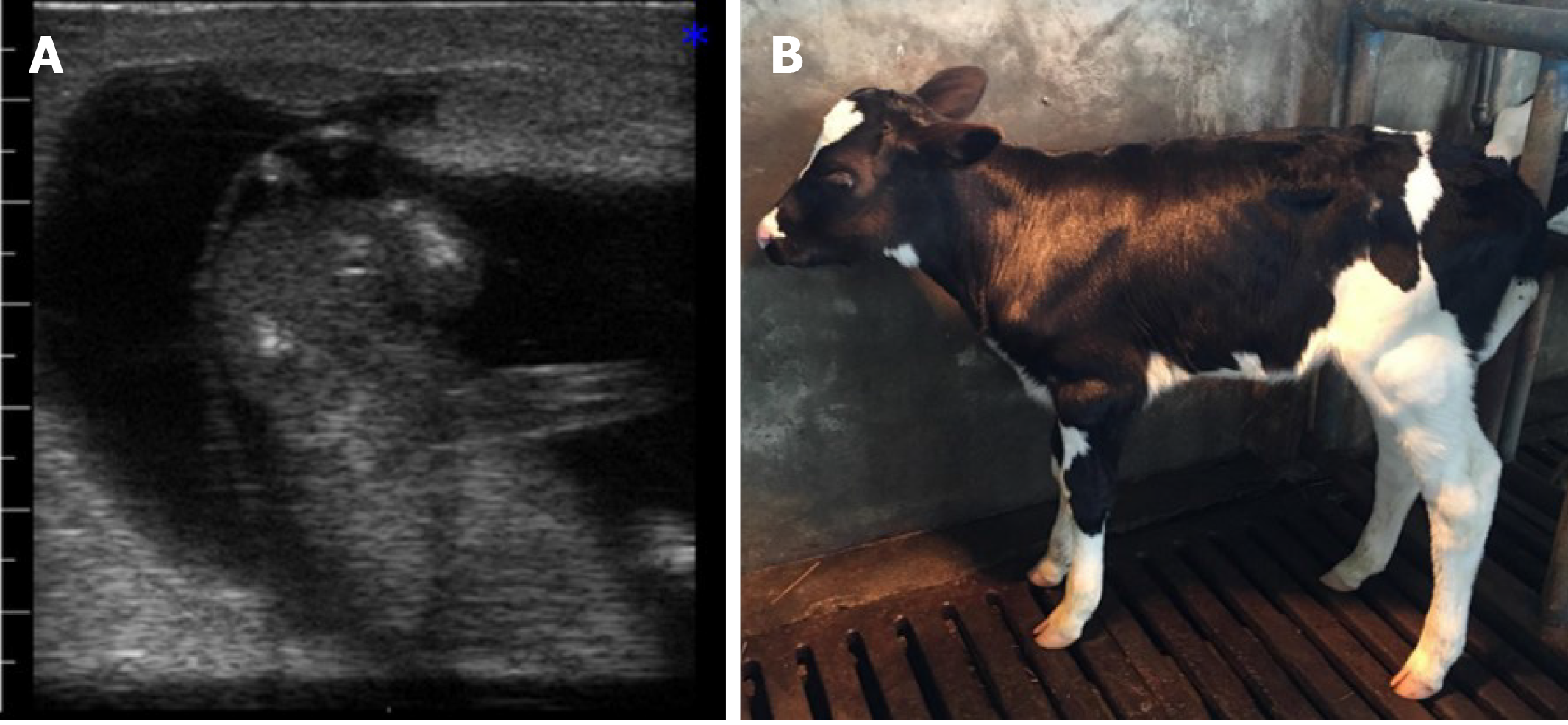
Without treatment, the reproductive function of cows with OFC does not recover. The follicular cyst causes excessively sexual excitement. Then, artificial insemination might fail. These non-lactating cows cause significant economic loss for farmers. In our study, OFC subsided with artificial drainage of ovarian follicular fluid, but recurrence is probable (Figure 7). Therefore, treatment for OFC is needed. Hormone and CPSC injections were effective in managing OFC, as we achieved recovery rates of 50%, 75%, 75%, and 75% in H1, H2, PS1, and PS2, respectively (Table 1). Furthermore, the estrus rate was 100%, 25%, 75%, 75% and 75% in BC2, H1, H2, PS1, and PS2, respectively. Both H2 and PS1 demonstrated a 25% inseminated conception rate and gave birth to a heifer (Figure 8).
| Group | Number of cows | Recovery rate, % | Estrus rate, % | Inseminated conception rate, % |
| BC1 | 1 | 0 (0/1) | 0 (0/1) | 0 (0/1) |
| BC2 | 2 | 50 (1/2) | 100 (2/2) | 0 (0/2) |
| H1 | 4 | 50 (2/4) | 25 (1/4) | 0 (0/4) |
| H2 | 4 | 75 (3/4) | 75 (3/4) | 25 (1/4) |
| PS1 | 4 | 75 (3/4) | 75 (3/4) | 25 (1/4) |
| PS2 | 4 | 75 (3/4) | 75 (3/4) | 0 (0/4) |
Placenta originating from the trophoblast forms chorionic villi, which allow healthy development of the embryo and fetus[24]. Generally, the placenta is useless after delivery. Some researchers have begun using placenta as source of stem cells to improve the treatment of intractable disease[17,18]. Our research showed that CPSCs had strong properties of proliferation and expression of MSC markers, similar to previous studies[25,26]. CD44 secreted by MSCs is associated with cell homing and immuno-regulation. Therefore, CD44 is able to reduce the inflammatory process in damaged tissues[27,28]. Based on this characteristic, CPSCs seem to have the potential to treat OFC.
Interestingly, PSCs can modulate the maternal immune system during conception[29] after transplantation[19,30,31]. Besides, drainage of ovarian follicular fluid plays an essential role, because the groups with drainage had higher recovery and estrus rates in this study, consistent with previous research[32]. Both the hormone and CPSC treatments can treat cows with OFC. The recovery rate in the H2 treatment group was consistent with previous research[9]. However, the overall conception rates were lower in all treatment groups than shown in previous studies, although two fetuses were produced in the H2 and PS1 treatment groups[9,32]. This outcome might be derived from the use of cows eliminated by local farmers.
From the aspect of food safety, the hormone secreted in milk might harm consumers[33]. Hence, cell therapy seems to be an alternative method to deal with hormone problem of OFC. Growth differentiation factor-9 and bone morphogenetic protein-15 are important factors in the development of ovarian follicles[34] and regulation of the structure of follicular or granulosa cells[35]. Thus, it is important to acquire cystic ovarian with less expression of these factors[36]. CPSCs can treat OFC in cows with paracrine therapeutic effect by improving tissue repair[37] and reducing inflammation[38]. In addition, PSCs enhance angiogenesis and cutaneous reconstruction through paracrine effects[39-41] in animal models[42,43]. In summary, CPSCs have the therapeutic potential of MSCs to reduce the use of hormone in cows with OFC.
To avoid economic loss from OFC, several treatments have been proposed to promote reproductive function and milk production. Aside from manual rupture and hormone injection, a novel therapy, CPSC transplantation, demonstrated therapeutic potential for treating OFC. CPSCs can be easily obtained from the placenta and proliferate significantly to repair tissue. Compared with hormone injection, higher recovery rate, estrus rate, and inseminated conception rate were noted in CPSC transplantation. As a consequence, this therapy can serve as an alternative approach to cure cows with OFC.
High humidity and temperature in Taiwan have significant effects on the reproductivity of Holstein cattle, which results in the occurrence of bovine ovarian follicular cyst (OFC). Because of economic loss from OFC, manual rupture and hormone injection have been advocated. However, these incomplete treatments decrease the conception rate and increase hormone resistance in cattle. In recent years, stem cells have been extensively utilized to repair or replace damaged tissues. Injection of stem cells might become an effective modality to treat OFC.
Through angiogenesis promotion and immune modulation, mesenchymal stem cells (MSCs) showed the potential in the treatment of several diseases. Besides, MSCs can be non-invasively collected from the placenta without much ethical concern. With a great source of stem cells, transplantation treatment could be conducted practically.
This study established the possibility of using cattle placental stem cells (CPSCs) as a treatment modality for OFC in cows.
The cattle with OFC were divided into three groups: control (BC1 and BC2), hormone (H1 and H2), and CPSC (PS1 and PS2) treatment groups. In the hormone treatment group, the cows were given gonadotrophin-releasing hormone (GnRH)-prostaglandin-GnRH injection with or without drainage of follicular fluid. In the CPSC treatment groups, CPSCs were isolated from the placenta. The cows were given ovarian injection of 1 × 106 or 6 × 106 CPSCs with drainage. Then OFC was scanned by ultrasound once a week for a total of seven times. The concentrations of estradiol and progesterone in serum were tested in the same period. The estrus cycle was analyzed by food intake and activity. If estrus was detected, artificial insemination was conducted. The cow was monitored by ultrasound for confirmation of pregnancy.
After 7 d of culture, CPSCs were successfully isolated from placental pieces. CPSCs proliferated significantly every 24 h and highly expressed MSC markers, such as CD44. In an ultrasound study, more subsided OFCs were observed with drainage of follicular fluid. The recovery rates were 0%, 50%, 50%, 75%, 75%, and 75% in BC1, BC2, H1, H2, PS1, and PS2, respectively. The estrus rate was 0%, 100%, 25%, 75%, 75%, and 75% in BC1, BC2, H1, H2, PS1, and PS2, respectively. Two fetuses were born in H2 and PS1.
Cows with CPSC injection achieved higher recovery, estrus, and inseminated conception rates. This approach shows efficacy in treating cows with OFC.
CPSC injection could serve as an alternative treatment for OFC. In the future, other reproductive disorders might also be investigated with stem cell therapy.
Manuscript source: Invited manuscript
Specialty type: Obstetrics and gynecology
Country/Territory of origin: Taiwan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ventura C S-Editor: Wang DM L-Editor: Filipodia P-Editor: Xing YX
| 1. | De Rensis F, Lopez-Gatius F, García-Ispierto I, Morini G, Scaramuzzi RJ. Causes of declining fertility in dairy cows during the warm season. Theriogenology. 2017;91:145-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 65] [Article Influence: 7.2] [Reference Citation Analysis (1)] |
| 2. | Butler WR. Energy balance relationships with follicular development, ovulation and fertility in postpartum dairy cows. Livest Prod Sci. 2003;83:211-218. [RCA] [DOI] [Full Text] [Cited by in Crossref: 324] [Cited by in RCA: 342] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 3. | Sakaguchi M, Sasamoto Y, Suzuki T, Takahashi Y, Yamada Y. Postpartum ovarian follicular dynamics and estrous activity in lactating dairy cows. J Dairy Sci. 2004;87:2114-2121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 46] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 4. | Garverick HA. Ovarian follicular cysts in dairy cows. J Dairy Sci. 1997;80:995-1004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 183] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 5. | Silvia WJ, Hatler TB, Nugent AM, Laranja da Fonseca LF. Ovarian follicular cysts in dairy cows: an abnormality in folliculogenesis. Domest Anim Endocrinol. 2002;23:167-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 124] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 6. | Braw-Tal R, Pen S, Roth Z. Ovarian cysts in high-yielding dairy cows. Theriogenology. 2009;72:690-698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 7. | Jeengar K, Chaudhary V, Kumar A, Raiya S, Gaur M, Purohit G N. Ovarian cysts in dairy cows: old and new concepts for definition, diagnosis and therapy. Anim Reprod. 2014;11:63-73. |
| 8. | Drost M, Thatcher WW. Application of gonadotrophin releasing hormone as therapeutic agent in animal reproduction. Anim Reprod Sci 1992; 28: 11-19. . [RCA] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 9. | Probo M, Comin A, Mollo A, Cairoli F, Stradaioli G, Veronesi MC. Reproductive performance of dairy cows with luteal or follicular ovarian cysts after treatment with buserelin. Anim Reprod Sci. 2011;127:135-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 10. | Ting AE, Mays RW, Frey MR, Hof WV, Medicetty S, Deans R. Therapeutic pathways of adult stem cell repair. Crit Rev Oncol Hematol. 2008;65:81-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 11. | Liang X, Ding Y, Zhang Y, Tse HF, Lian Q. Paracrine mechanisms of mesenchymal stem cell-based therapy: current status and perspectives. Cell Transplant. 2014;23:1045-1059. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 513] [Cited by in RCA: 683] [Article Influence: 68.3] [Reference Citation Analysis (0)] |
| 12. | Luo J, Zhao S, Wang J, Luo L, Li E, Zhu Z, Liu Y, Kang R, Zhao Z. Bone marrow mesenchymal stem cells reduce ureteral stricture formation in a rat model via the paracrine effect of extracellular vesicles. J Cell Mol Med. 2018;22:4449-4459. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 13. | Park SS, Moisseiev E, Bauer G, Anderson JD, Grant MB, Zam A, Zawadzki RJ, Werner JS, Nolta JA. Advances in bone marrow stem cell therapy for retinal dysfunction. Prog Retin Eye Res. 2017;56:148-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 89] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 14. | Forbes SJ, Newsome PN. New horizons for stem cell therapy in liver disease. J Hepatol. 2012;56:496-499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 68] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 15. | Duelen R, Sampaolesi M. Stem Cell Technology in Cardiac Regeneration: A Pluripotent Stem Cell Promise. EBioMedicine. 2017;16:30-40. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 64] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 16. | Peng SY, Chou CW, Kuo YH, Shen PC, Shaw SWS. Potential differentiation of islet-like cells from pregnant cow-derived placental stem cells. Taiwan J Obstet Gynecol. 2017;56:306-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 17. | Antoniadou E, David AL. Placental stem cells. Best Pract Res Clin Obstet Gynaecol. 2016;31:13-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 71] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 18. | Vanover M, Wang A, Farmer D. Potential clinical applications of placental stem cells for use in fetal therapy of birth defects. Placenta. 2017;59:107-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 19. | Wang H, Chen L, Liu Y, Luo B, Xie N, Tan T, Song L, Erli P, Luo M. Implantation of placenta-derived mesenchymal stem cells accelerates murine dermal wound closure through immunomodulation. Am J Transl Res. 2016;8:4912-4921. [PubMed] |
| 20. | Kadam S, Muthyala S, Nair P, Bhonde R. Human placenta-derived mesenchymal stem cells and islet-like cell clusters generated from these cells as a novel source for stem cell therapy in diabetes. Rev Diabet Stud. 2010;7:168-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 85] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 21. | Cargnoni A, Gibelli L, Tosini A, Signoroni PB, Nassuato C, Arienti D, Lombardi G, Albertini A, Wengler GS, Parolini O. Transplantation of allogeneic and xenogeneic placenta-derived cells reduces bleomycin-induced lung fibrosis. Cell Transplant. 2009;18:405-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 186] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 22. | Lee MJ, Jung J, Na KH, Moon JS, Lee HJ, Kim JH, Kim GI, Kwon SW, Hwang SG, Kim GJ. Anti-fibrotic effect of chorionic plate-derived mesenchymal stem cells isolated from human placenta in a rat model of CCl(4)-injured liver: potential application to the treatment of hepatic diseases. J Cell Biochem. 2010;111:1453-1463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 104] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 23. | Farmer D. Placental stem cells: The promise of curing diseases before birth. Placenta. 2017;59:113-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 24. | Gude NM, Roberts CT, Kalionis B, King RG. Growth and function of the normal human placenta. Thromb Res. 2004;114:397-407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 478] [Cited by in RCA: 609] [Article Influence: 30.5] [Reference Citation Analysis (0)] |
| 25. | Abumaree MH, Al Jumah MA, Kalionis B, Jawdat D, Al Khaldi A, AlTalabani AA, Knawy BA. Phenotypic and functional characterization of mesenchymal stem cells from chorionic villi of human term placenta. Stem Cell Rev Rep. 2013;9:16-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 115] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 26. | Mohammadi Z, Afshari JT, Keramati MR, Alamdari DH, Ganjibakhsh M, Zarmehri AM, Jangjoo A, Sadeghian MH, Ameri MA, Moinzadeh L. Differentiation of adipocytes and osteocytes from human adipose and placental mesenchymal stem cells. Iran J Basic Med Sci. 2015;18:259-266. [PubMed] |
| 27. | Gee K, Kryworuchko M, Kumar A. Recent advances in the regulation of CD44 expression and its role in inflammation and autoimmune diseases. Arch Immunol Ther Exp (Warsz). 2004;52:13-26. [PubMed] |
| 28. | Basakran NS. CD44 as a potential diagnostic tumor marker. Saudi Med J. 2015;36:273-279. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 95] [Cited by in RCA: 119] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 29. | Bansal AS, Bora SA, Saso S, Smith JR, Johnson MR, Thum MY. Mechanism of human chorionic gonadotrophin-mediated immunomodulation in pregnancy. Expert Rev Clin Immunol. 2012;8:747-753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 50] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 30. | Fisher-Shoval Y, Barhum Y, Sadan O, Yust-Katz S, Ben-Zur T, Lev N, Benkler C, Hod M, Melamed E, Offen D. Transplantation of placenta-derived mesenchymal stem cells in the EAE mouse model of MS. J Mol Neurosci. 2012;48:176-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 71] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 31. | Wang A, Brown EG, Lankford L, Keller BA, Pivetti CD, Sitkin NA, Beattie MS, Bresnahan JC, Farmer DL. Placental mesenchymal stromal cells rescue ambulation in ovine myelomeningocele. Stem Cells Transl Med. 2015;4:659-669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 99] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 32. | Nessan GK, King GJ, McKay GW, Thomson JD, Bertrand W. Treatment of cystic ovarian degeneration in dairy cows with gonadotrophic releasing hormone or human chorionic gonadotrophic hormone. Can Vet J. 1977;18:33-37. [PubMed] |
| 33. | Nachman KE, Smith TJ. Hormone Use in Food Animal Production: Assessing Potential Dietary Exposures and Breast Cancer Risk. Curr Environ Health Rep. 2015;2:1-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 34. | Sanfins A, Rodrigues P, Albertini DF. GDF-9 and BMP-15 direct the follicle symphony. J Assist Reprod Genet. 2018;35:1741-1750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 130] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 35. | Alam MH, Lee J, Miyano T. GDF9 and BMP15 induce development of antrum-like structures by bovine granulosa cells without oocytes. J Reprod Dev. 2018;64:423-431. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 36. | Zhao SY, Qiao J, Chen YJ, Liu P, Li J, Yan J. Expression of growth differentiation factor-9 and bone morphogenetic protein-15 in oocytes and cumulus granulosa cells of patients with polycystic ovary syndrome. Fertil Steril. 2010;94:261-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 56] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 37. | Ho CH, Lan CW, Liao CY, Hung SC, Li HY, Sung YJ. Mesenchymal stem cells and their conditioned medium can enhance the repair of uterine defects in a rat model. J Chin Med Assoc. 2018;81:268-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 38. | Pouya S, Heidari M, Baghaei K, Asadzadeh Aghdaei H, Moradi A, Namaki S, Zali MR, Hashemi SM. Study the effects of mesenchymal stem cell conditioned medium injection in mouse model of acute colitis. Int Immunopharmacol. 2018;54:86-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 46] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 39. | König J, Weiss G, Rossi D, Wankhammer K, Reinisch A, Kinzer M, Huppertz B, Pfeiffer D, Parolini O, Lang I. Placental mesenchymal stromal cells derived from blood vessels or avascular tissues: what is the better choice to support endothelial cell function? Stem Cells Dev. 2015;24:115-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 40. | Komaki M, Numata Y, Morioka C, Honda I, Tooi M, Yokoyama N, Ayame H, Iwasaki K, Taki A, Oshima N, Morita I. Exosomes of human placenta-derived mesenchymal stem cells stimulate angiogenesis. Stem Cell Res Ther. 2017;8:219. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 102] [Cited by in RCA: 148] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 41. | Rameshbabu AP, Bankoti K, Datta S, Subramani E, Apoorva A, Ghosh P, Maity PP, Manchikanti P, Chaudhury K, Dhara S. Silk Sponges Ornamented with a Placenta-Derived Extracellular Matrix Augment Full-Thickness Cutaneous Wound Healing by Stimulating Neovascularization and Cellular Migration. ACS Appl Mater Interfaces. 2018;10:16977-16991. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 53] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 42. | Zahavi-Goldstein E, Blumenfeld M, Fuchs-Telem D, Pinzur L, Rubin S, Aberman Z, Sher N, Ofir R. Placenta-derived PLX-PAD mesenchymal-like stromal cells are efficacious in rescuing blood flow in hind limb ischemia mouse model by a dose- and site-dependent mechanism of action. Cytotherapy. 2017;19:1438-1446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 43. | Ertl J, Pichlsberger M, Tuca AC, Wurzer P, Fuchs J, Geyer SH, Maurer-Gesek B, Weninger WJ, Pfeiffer D, Bubalo V, Parvizi D, Kamolz LP, Lang I. Comparative study of regenerative effects of mesenchymal stem cells derived from placental amnion, chorion and umbilical cord on dermal wounds. Placenta. 2018;65:37-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 41] [Article Influence: 5.9] [Reference Citation Analysis (0)] |