Published online Jul 26, 2019. doi: 10.4252/wjsc.v11.i7.383
Peer-review started: February 20, 2019
First decision: April 16, 2019
Revised: April 23, 2019
Accepted: June 12, 2019
Article in press: June13, 2019
Published online: July 26, 2019
Processing time: 157 Days and 2.5 Hours
Ovarian cancer is the deadliest gynecological malignancy. It is typically diagnosed at advanced stages of the disease, with metastatic sites disseminated widely within the abdominal cavity. Ovarian cancer treatment is challenging due to high disease recurrence and further complicated pursuant to acquired chemoresistance. Cancer stem cell (CSC) theory proposes that both tumor development and progression are driven by undifferentiated stem cells capable of self-renewal and tumor-initiation. The most recent evidence revealed that CSCs in terms of ovarian cancer are not only responsible for primary tumor growth, metastasis and relapse of disease, but also for the development of chemoresistance. As the elimination of this cell population is critical for increasing treatment success, a deeper understanding of ovarian CSCs pathobiology, including epithelial-mesenchymal transition, signaling pathways and tumor microenvironment, is needed. Finally, before introducing new therapeutic agents for ovarian cancer, targeting CSCs, accurate identification of different ovarian stem cell subpopulations, including the very small embryonic-like stem cells suggested as progenitors, is necessary. To these ends, reliable markers of ovarian CSCs should be identified. In this review, we present the current knowledge and a critical discussion concerning ovarian CSCs and their clinical role.
Core tip: Ovarian carcinoma, based on its biological profile and clinical course, presents a prototype of cancer stem-cell-driven disease. Ovarian cancer stem cells (CSCs) might be responsible for tumor formation, invasion, and metastasis, disease relapse and chemoresistance acquisition. For the successful treatment of this deadly disease, target therapy directed toward ovarian CSCs should be implemented. But to this, identification and characterisation of CSC subpopulations, such as very small embryonic-like stem cells, should be derived. This review’s objective is to consolidate present knowledge and to discuss future perspectives in the field of ovarian CSCs.
- Citation: Kenda Suster N, Virant-Klun I. Presence and role of stem cells in ovarian cancer. World J Stem Cells 2019; 11(7): 383-397
- URL: https://www.wjgnet.com/1948-0210/full/v11/i7/383.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v11.i7.383
Ovarian cancer is the leading cause of death amongst all the gynecological tumors affecting a large population of women worldwide. According to National Cancer Institute data from the United States of America, incidence between 2008 and 2014 was 11.6 per 100000 women per year. As the five-year surviving rate in this period only reached 47.4%, the number of deaths increased to 7.2 per 100000 women per year.
Ovarian cancer presents a heterogeneous group of tumors: Epithelial, germ cell, and stromal cell tumors. Approximately 90% of ovarian cancer belong to the malignant epithelial tumor (carcinomas) group and, based on histopathology, immunohisto-chemistry, and molecular genetic analysis, five main types of carcinoma are currently known: high grade serous carcinoma, 70%; low grade serous carcinoma < 5%; endometrioid carcinoma, 10%; clear cell carcinoma, 10%; and mucinous carcinoma 3%[1,2]. Different epithelial malignancies have, in addition to their different origin and morphologies, different biological behaviour[3]. Low grade serous carcinoma arises from fallopian tube, endometrioid carcinoma, clear cell carcinoma, and seromucinous carcinoma arise from endometriosis, mucinous carcinoma arises from germ cells, and malignant Brenner tumor arises from transitional epithelium[3]. All are slow-growing tumors which develop progressively from benign and borderline precursor lesions to malignancy[4]. They are genetically stable, characterized by mutations in different genes: KRAS, BRAF, PTEN, ß-catenin, and others[4]. High grade ovarian serous carcinoma, on the other hand, has a high level of genetic instability and is characterized by TP53 mutation, and loss of BRCA1 and BRCA2 function[3]. It is fast-growing and highly aggressive neoplasm, with massive disease in the omentum and the mesentery, usually accompanied by ascites[3]. There are two models considered, high grade ovarian serous carcinoma arising from the ovarian surface epithelium or from the fallopian tube[5]. As both tissues are derived from the same embryologic origin, high grade ovarian serous carcinoma may arise from two different sites that undergo similar changes[5]. Progenitor cells from different sites may respond similarly[5]. However, BRCA deficiency and simultaneously presence of the intraepithelial carcinoma in the fallopian tube (serous tubal intraepithelial carcinoma) make fallopian tube model of high grade ovarian serous carcinoma origin more relevant[3,5].
Pursuant to insufficient screening and nonspecific symptoms, such as abdominal discomfort and bloating, early diagnosis of the disease is challenging[6]. Consequently, 70% of ovarian cancer patients are usually diagnosed at advanced stages (III and IV), with metastatic sites disseminated widely within the peritoneal cavity, retroperi-toneum, and even in distant organs[7]. Treating disease in its advanced course is demanding and often unsuccessful, so defining the origin of ovarian cancer and performing suitable prophylactic surgery like oophorectomy or salpingectomy may save many lives[8].
To achieve complete removal of macroscopic tumors, patients with advanced disease receive radical debulking surgery in combination with neoadjuvant and/or adjuvant platinum and taxane combined chemotherapy[9,10]. The majority of patients initially respond well to treatment; however, tumors eventually relapse in over 70% of cases, resulting in chemoresistance and fatal disease[11]. The general opinion is that the microscopic tumor residue that remains after surgical debulking and standard chemotherapeutics’ limitations contribute to the likelihood of tumor relapse. Therefore, the five-year survival rate for advanced tumors is less than 30%, with only modest improvement in survival evidenced in recent decades[12,13].
Recent findings in the field of cancer stem cells (CSCs) in ovarian cancer are important, in terms of its explanation of tumor initiation pathogenesis, dissemination and recurrence after treatment, and also in terms of using CSC components as targets for ovarian cancer target therapy[11,14]. In this review article, we will discuss the current research on CSCs in ovarian cancer, focusing on CSCs development and their role in tumor formation, progression and recurrence after, allegedly, successful treatment.
The CSC model proposes that tumor initiation, growth and progression are fueled and sustained by undifferentiated cancer cells endowed with self-renewal on the one hand and differentiation on the other[15]. Ovarian carcinoma, based on its biological behavior and clinical course, represents a typical example of CSC-driven disease[15]. It is a highly aggressive cancer which spreads widely within the abdominal cavity and distant organs, even when primary ovarian tumors are still small and barely detectable. Despite aggressive treatment with debulking surgery and cytostatic chemotherapeutics, which at first reduce the size of tumors and temporarily improve patient signs and symptoms, ovarian cancer relapses in over 70% of all cases. It is believed that a highly-potent subpopulation of ovarian CSCs that “survive” treatment cause disease relapse[16]. Moreover, dormant ovarian CSCs able to repopulate again, lead to even more aggressive, drug-resistant disease[16]. The phenotype and molecular status of ovarian CSC population have still not been defined. It is known that CSC phenotype is not uniform amongst the various cancer types and even of those tumors of the same histological type, and it can change vis à vis in vitro culture condition[17]. Ovarian cancer manifestation seems to involve different types of stem cells interplaying in this complex process. Cells heterogeneity within tumors may influence disease course and its response to treatment in terms of drug resistance[18]. We know little about ovarian CSC location and CSC progenitors, but recent studies have aided in understanding CSC evolution and location within tumors[19,20]. An important task for the future is to determine the population of progenitor stem cells involved in ovarian cancer formation and progression.
The exact mechanisms by which normal ovarian cells transform into aggressive ovarian cancer cells still remain elusive. As with other adult tissue and organs, different research groups have detected very small embryonic-like (VSEL) stem cells with diameters of up to 5 μm in human adult ovaries[21-23]. These cells have been further characterised and differentiated in vitro[21-23]. It has been proposed that these small stem cells originate in the embryonic epiblast and remain dormant in adult human tissue and organs from the embryonic period of life[24]. VSEL stem cells in ovaries are mostly located in the ovarian surface epithelium of healthy women[22]. They express several genes related to pluripotency and germinal lineage, especially primordial germ cells[25].
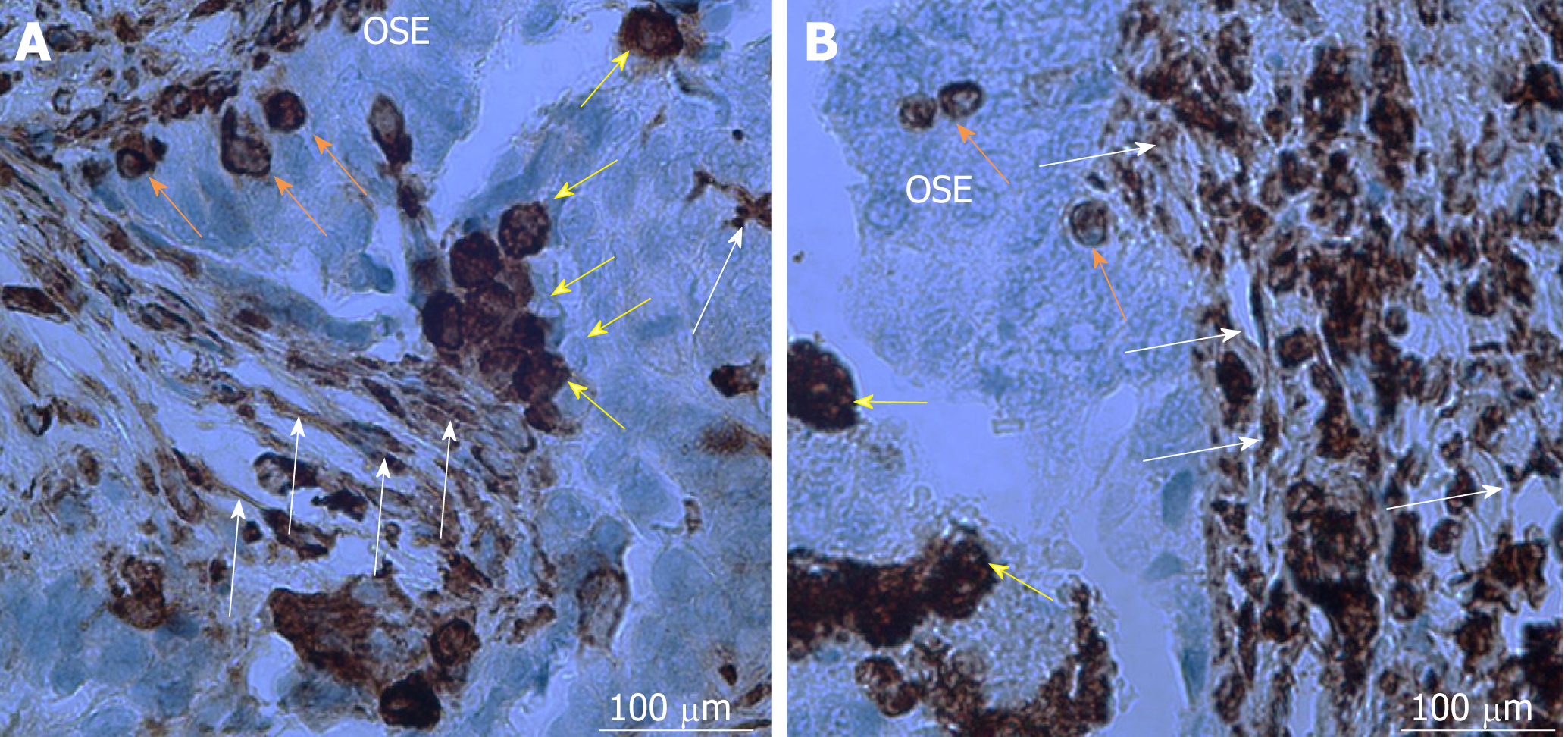
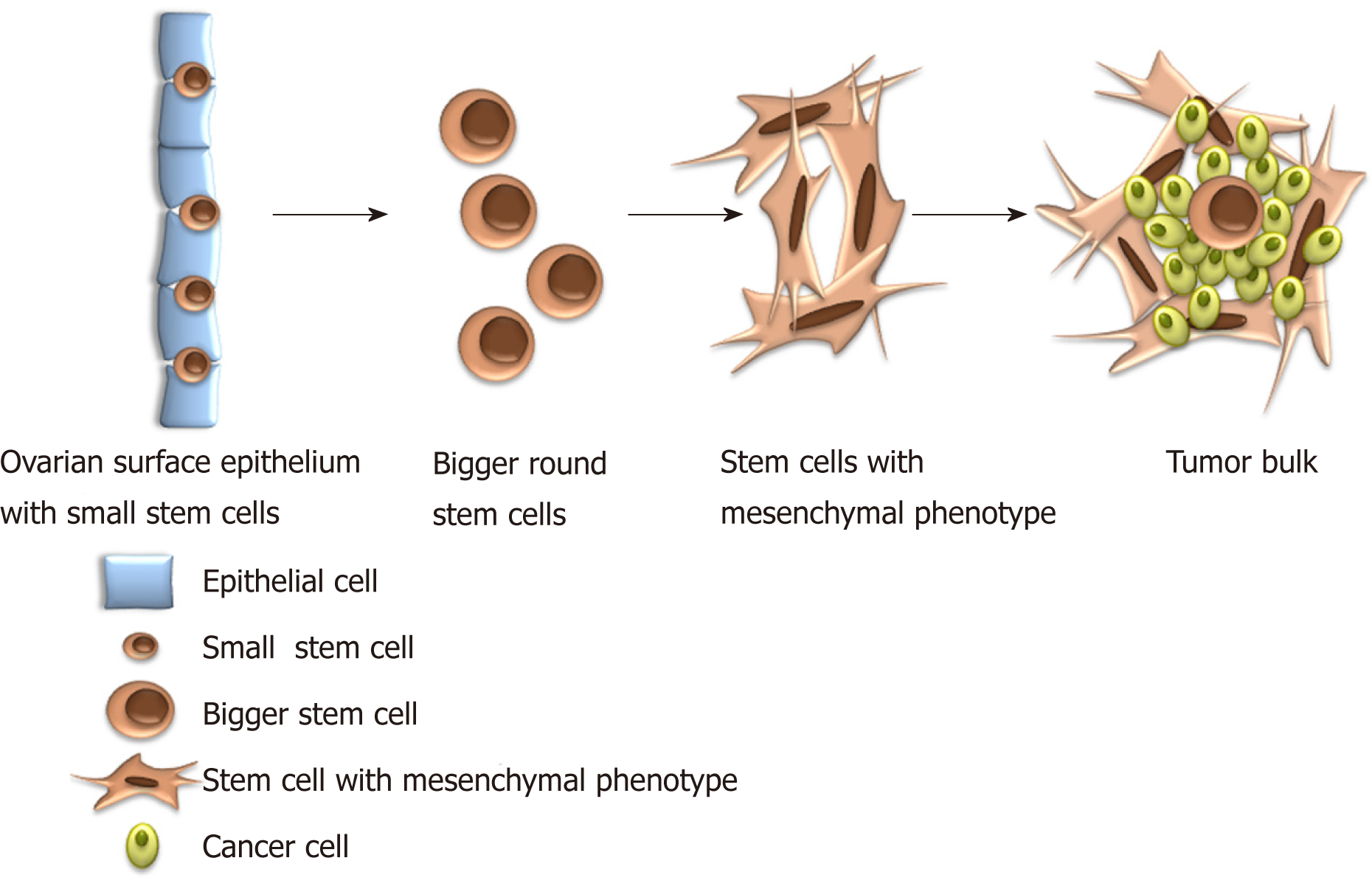
Small tumor-initiating stem cells were also discovered in the ovaries and ovarian cell cultures of patients with borderline ovarian tumors [26]. These cells spontaneously formed tumor-like structures in vitro and, after fluorescence-activated cell sorting, evidenced different gene expression profile in comparison to their counterparts from healthy, non-malignant ovarian tissue[26]. Results revealed that SOX17, forkhead box (FOXQ1, FOXL2), and homeobox (HOXD9) genes, known to regulate the cell growth, proliferation, differentiation, embryo development, and the establishment of body axes during embryogenesis, may play an important role in the transformation of “healthy” small stem cells into “cancerous” cells[26]. Amongst the multitude of interesting genes, MUC16/CA125, the most widely-used ovarian cancer biomarker, was highly upregulated in small putative stem cells from borderline ovarian tumors in comparison to cells from healthy ovaries, hESCs, and fibroblasts[26]. Interestingly, the small stem cells from healthy ovarian tissue did not form tumor-like structures in vitro[26]. A similar population of small stem cells was later on observed in situ in ovarian serous carcinoma slides[20,27]. A population of small stem cells with diameters of up to 5 μm contain NANOG-positive cells with nuclei, which made up almost all cell volume, and were clearly seen amongst the epithelial cells of the ovarian surface epithelium[27]. In this vicinity, there was also present a population of bigger round cells with diameters of 10-15 μm with similarly large nuclei, positively stained for mesenchymally-derived cell (vimentin) and pluripotency (NANOG, SSEA-4, and SOX2) markers[27] (Figure 1). Different stem cells observed in ovarian tumor tissue indicate the presence of different types of stem cells in ovarian cancer. Smaller stem cells resembling VSEL stem cells are suggested as progenitor stem cells which remain dormant from the embryonic period of life to regenerate damaged tissue, but can, in cases of inappropriate body condition, transform into bigger-CSCs involved in cancer manifestation.
Other researchers, likewise focusing on ovarian CSCs, observed similar cells, smaller spherical with diameters of up to 5 μm and larger elliptical or fibroblast like with diameters of up to ≥ 10 μm, both with stem cells characteristics[19,28]. Cells with co-expression of stem cell markers (OCT4, SSEA4), CSC markers (ALDH1/2, CD44 and LGR5) and proliferation marker (KI67)[19] were evidenced in the ovarian cortex. Interestingly, in a few cases, only non-proliferating stems or CSCs (SSEA4+/KI67- or ALDH1/2+/KI67-), or only proliferating (KI67+) cells were observed, which indicates a dynamic process in which different populations of cells are involved forming fascinating cellular hierarchy[19].
Many studies report that ovarian carcinogenesis is associated with epithelial-mesenchymal transition (EMT)[19,27]. Based on our own experimental experience and data from the literature, we suggest that small, spherical VSEL stem cells present amongst epithelial cells in the ovarian surface epithelium induce EMT by growing into larger (diameter approximately 10-15 μm), NANOG and vimentin-positive spindle/elliptical shaped cells, and transform into mesenchymal-like stem cells (Figures 1 and 2); surrounding epithelial cells are not excluded from the EMT process and support it in unknown ways[27]. Different signals from tumor microenvironment might be involved in a network of interactions that activate so-called EMT programs[29-31]. Cancer cells are, beside epithelial cells, surrounded by a large variety of stromal cells, such as fibroblasts, myoblasts, lymphocytes and macrophages, and endothelial cells and pericytes recruited to tumor vasculature[32]. Paracrine and juxtacrine signals in such microenvironment include different members of the transforming growth factor superfamily, epidermal growth factor, fibroblast growth factor, hypoxia-inducible factor, Wnt, Notch, and many others[33].
EMT is a basic physiological cell reprogramming event active in tissue remodeling during embryogenesis and, later in life, during the regeneration of adult tissue in cases of injury. During EMT, cells acquire unique mesenchymal cells characteristics, like epithelial cell polarity, intracellular adhesion and loss of specific cell surface markers. Pursuant to cytoskeletal change, cells subsequently gain a mesenchymal-like phenotype. The acquisition of mesenchymal characteristics during EMT occurs progressively, where fully epithelial and mesenchymal phenotype represent just the extreme edges[34]. This plastic and dynamic process involves several intermediate states, including so-called hybrid phenotypes in which cells express epithelial and mesenchymal features[35,36]. Cells expressing such hybrid epithelial/mesenchymal phenotype do not only play a fundamental role in embryogenesis, but also in cancer formation and progression[37,38].
Epithelial trait loss and mesenchymal characteristic acquisition, such as vimentin and myosin presence, which occurs during EMT, enable invasive cellular motility which enhances tumor progression[39]. Elliptical fibroblast-like cells with stem cell characteristics and positive pluripotency and mesenchymally-derived cell markers seen in high grade ovarian serous carcinoma, invaded ovarian tissue by changing their round shape into mesenchymal–like phenotype involving elongations and protrusions[27]. By promoting high degrees of cell invasion, EMT increases tumor malignancy[36,40].
EMT is also considered a key step in CSC metastases[41]. Mesenchymal properties, acquired by carcinoma cells during EMT, promote invasion into the extracellular matrix and further dissemination. Transformed tumor cells spread throughout the organism, where they present a reservoir that expands and refills cancer cell populations[42]. Circulating tumor cells with a fully-mesenchymal state display lower metastatic potential compared to hybrid epithelial/mesenchymal cells undergoing partial EMT[43]. Interestingly, not all cancer cells are able to undergo EMT at the same time and even those which have activated EMT may not be sufficient for metastasis[44]. Furthermore, in cases of colorectal cancer, it has been shown that EMT occurs only in a subset of cells at the invasive front of primary carcinoma, usually associated with stromal components[45].
The presence of a major cytoskeletal component of mesenchymal cell vimentin was, further to metastatic progression, also related to primary (intrinsic) resistance or poor response to chemotherapy[46,47]. Tumor cells undergoing EMT gain the ability to disarm body antitumor defence, resist apoptosis and anticancer drugs[47]. Morphological and functional changes involved in EMT related processes (generation and maintenance of CSCs, tumor invasion, metastasis formation and chemoresistance) require robust reprogramming of gene expression, which is partially accomplished at the transcriptional level of gene expression and partially in gene expression’s post-transcriptional regulation[44]. A well established factor regulating EMT is the activation of the signal transducer and activator of the transcription 3 (STAT3) pathway[48]. As EMT represents the main event in terms of cancer manifestation and progression, it is becoming a promising target for therapeutic intervention[42,49].
In conclusion, EMT as well as its reversal, mesenchymal-epithelial transition, represents a highly dynamic process in tumor cells which may be triggered by VSEL stem cells present amongst epithelial cells in the ovarian surface epithelium layer. Cancer stemness seems to be more associated with partial-EMT phenotype than fully-driven EMT[34,44,50]. In line with this concept is the concept of CSC plasticity, which postulates that CSCs are able to switch between different states, including a non-stem state[51].
Defining the niche that supports ovarian CSCs must consider the clinical course of the disease. The evolution of ovarian cancer, its origin in the ovarian surface epithelium or in the distal part of fallopian tube, its progression, and especially its peritoneal dissemination, indicates the existence of multiple types of niches. Within primary tumors, multiple stromal cell types are involved in the formation of a pro-tumorigenic microenvironment[52]. Tumor cells release several soluble factors and proteins that mobilize the population of tumor cells to settle in distant organs and tissues[53]. 3D cultures that displayed the early dissemination of ovarian cancer into peritoneal mesothelium reveled, that cancer cells induce mesothelial cells to synthesize fibronectin via secretion of transforming growth factor beta-1[54]. Ascites, frequently accompanying advanced disease, represents a unique type of ovarian CSCs microenvironment. Interleukin 6, being elevated in ascites, triggers the JAK /STAT3 signaling pathway, which plays an important role in ovarian CSC function[55-57]. The Wnt/beta-catenin pathway is another pathway involved in communication between ovarian cancer cells and ascites, thus its inhibition presents potential therapeutic target for ovarian cancer[58]. The adipose tissue, especially omentum, provides another microenvironment, optimal for ovarian cancer lesions. Omental adipocytes enable nesting, invasion, and migration of ovarian cancer cells, and provide energy for rapid tumor growth[59].
Beside soluble factors and proteins secreted by ovarian cancer cells, the role of extracellular vesicles in the formation of pre-metastatic niche and metastatic colonization has been investigated[60]. Exosomes play an important role in intercellular signaling and in transportation of genetic information [60]. They are coordinators between tumor cells, stromal cells and the extracellular matrix through the shuttling of different lipids, proteins, double-stranded DNAs, RNAs, non-transcribed RNAs, and microRNAs[60]. Ovarian cancer derived exosomes were reported to transfer CD44 into mesothelial cells, upregulating matrix metalloproteinase 9 that facilitates cancer cell nesting and invasion[61]. Exosomes in epithelial ovarian cancer have also promising therapeutic potential as they are related to immune system, tumor microenvironment and tumor angiogenesis[62]. Extracellular vesicles have created new perspective on diagnosis, prognosis, treatment, and drug resistance in ovarian cancer, however, knowledgebase has so far been limited, so further research is needed.
Identification of CSCs relies on the presence of markers. In ovarian cancer many markers are used to confirm the presence of CSCs (Table 1). Isolated CSCs can then be tested for stemness in vitro through spheroid forming assay, and in vivo with limiting dilution assays to examine the tumorigenicity of the sample on the animal model[26,63,64].
| Marker | Type of protein | Suspected role in cancer stem cells |
| CD24 | Cell surface transmembrane glycoprotein | Stem gene expression[118,119], tumor formation[73,74], metastasis[75,76], chemoresistance[75], poor prognosis[75,77], recurrence of disease[77] |
| CD44 | Cell surface transmembrane glycoprotein | Tumor formation[78], progression[79], chemoresistance[78], poor prognoses[80], recurrence of disease[81] |
| CD117 | Tyrosine kinase receptor | Tumor formation[65,66,120,121], chemoresistance[66,122], poor prognosis of disease[65,67] |
| CD133 | Cell surface transmembrane glycoprotein | Tumor formation[68], progression[69], chemoresistance[70,71], poor prognosis[71], successful treatment[72] |
| EpCAM | Cell surface transmembrane glycoprotein | Tumor growth inhibition[123], poor survival and chemoresistance[124] |
| ROR1 | Tyrosine kinase receptor | Spheroid and tumor formation[125], poor prognosis[126] |
| ALDH | Cytosolic aldehyde dehydrogenase enzyme | Cell proliferation and migration promotion[82], poor survival[83,85], chemoresistance[83-85] |
| SOX2 | Transcription factor | (Cancer) stem cell maintenance and self-renewal[86,87], poor prognosis and chemoresistance[88] |
| OCT4 | Transcription factor | (Cancer) stem cell maintenance and self-renewal[86,87], tumor formation and drug resistance[89] |
| NANOG | Transcription factor | (Cancer) stem cell maintenance and self-renewal[86,87], poor prognosis[90-92], chemoresistance[91,93] |
| MYC | Transcription factor | Tumor formation[94], chemoresistance[95] |
| ABCB1, ABCG2 | ATP binding cassette transporter | Chemoresistance[96-99] |
There are multiple surface biomarkers used to identify CSCs in ovarian cancer. CD117 was demonstrated to be the first cell surface marker for the ovarian CSCs. Its expression correlates with tumor formation, chemoresistance, and poor prognosis of disease[65-67]. CD 133 is one of the most commonly reported ovarian CSC surface markers. It is associated with a number of stem characteristics, like tumor formation, disease progression, chemoresistance, and poor prognosis[68-71]. It was also studied as a target for a cancer target therapy[72]. Other common CSC surface markers are CD24, CD44, EpCAM and ROR1. CD24 is associated with tumor formation, metastasis, poor prognosis, chemoresistance, and recurrence of disease[73-77]. Similar characteristics are correlated with CD 44[78-81]. Surface markers can be used alone or in combination with other ovarian CSC markers.
In addition to cell surface markers, the enzyme aldehyde dehydrogenase 1 (ALDH1) is used to identify CSCs in ovarian cancer. Several studies correlate ALDH1 expression with cell proliferation, migration promotion, poor survival, and chemoresistance[82-84]. Conversely, inhibition of ALDH1A1 in a mouse model sensitized the tumors to treatment[85]. The expression of ALDH1 alone or in combination with cell surface stem cell markers is an accepted method for CSC identification in ovarian cancer.
NANOG is a transcription factor that, along with transcription factors OCT4 and SOX2, plays a key role in pluripotency and self-renewal maintenance in undifferentiated embryonic stem cells[86]. NANOG, OCT4 and SOX2 are commonly expressed also in ovarian CSCs[87,88]. Their expression is associated with poor prognosis and chemoresistance[88-92]. NANOG also regulates epithelial-mesenchymal transition[93]. c-Myc is another key oncogenic transcription factor that participates in tumor pathogenesis[94]. Its knockdown by let-7d increases ovarian cancer cell sensitivity to a genistein analog[95]. Future studies on pluripotency factors expressed in ovarian CSCs will provide additional data on how cancer stemness is maintained.
Another way in which ovarian CSCs can be identified is by the ability to efflux DNA-binding dyes resulting in a side population using flow cytometry. For dye effluxion CSCs should express ATP binding cassette transporters such as MDR1/ABCB1 and ABCG2 that can efflux chemotherapeutic agents and so contribute to chemoresistance[96-99].
Although CSCs can cause different processes such as tumor initiation, malignant proliferation, relapse and multi-drug resistance, the way to eliminate CSCs remains unknown. The modern molecular genetic methods enable to study the ovarian CSCs in more detail, in terms of their gene expression profile (e.g., whole genome sequencing). An increasing number of studies try to elucidate whether the different stem cells compartments that are apparently present in ovarian cancer differ at the DNA level. These studies showed a niche-dependent gene expression profile of heterogeneous intratumoral populations of stem cells, which make a task to target ovarian CSCs even more difficult[100].
It was found that TP53 is the most frequently mutated gene in high grade ovarian cancer[101]. About 50% of these tumors showed defective homologous recombination due to germline and somatic BRCA mutations, epigenetic inactivation of BRCA and abnormalities of DNA repair genes. Along this, somatic copy number alterations are frequent in these tumors, especially defective NOTCH, RAS/MEK, PI3K and FOXM1 pathway signaling[101]. Some of them are associated with patients’ prognosis[101]. Other subtypes of ovarian cancer were characterised by a different mutational spectrum: Low grade ovarian serous carcinoma has increased frequency of BRAF and RAS mutations, mucinous cancers have a mutation in ARID1A, PIK3CA, PTEN, CTNNB1 and in RAS genes[101]. Some data also suggest that TERT C228T promoter mutations may have an important role in progression of adult granulosa cell tumors[102]. Intensive research was focused on relation between the gene expression profile and ovarian CSCs. For identification of CSCs positivity for some markers, including CD133, CD44, CD117, CD24, EpCAM, LY6A, and ALDH1, was used.
An important task remains to elucidate the originating CSCs (e.g., VSELs) and common characteristics of different populations of stem cells involved at different stages of ovarian cancer. Some studies compared the gene expression profile of ovarian CSCs in ovarian cancer of different grades, including advanced disease compared to normal ovarian surface epithelium, to identify the key pathways, and specific molecular signatures involved in the manifestation of ovarian cancer at different stages[103]. Comparison of genome-wide expression profiles in ovarian CSCs revealed a mass of differentially expressed genes. Among these genes, NAB1 and NPIPL1 were commonly upregulated, whereas the genes PROS1, GREB1, KLF9 and MTUS1 were commonly downregulated, regardless of the stage of a disease[103]. These genes regulate the cellular components such as centrosome, plasma membrane receptors, and basal lamina, and may participate in biological processes such as cell cycle regulation, chemoresistance and stemness induction. Moreover, the gene co-expression extrapolation screening by the Connectivity Map revealed several small-molecule compounds (such as SC-560, disulfiram, thapsigargin, esculetin and cinchonine) with potential anti-ovarian CSCs properties targeting ovarian CSC signature genes[103].
All these and several other data indicate that the gene expression profile and the presence of specific mutation/genomic aberration may help to identify the culprit of the CSC compartment. The improvements in our understanding of the molecular and stem cell basis of ovarian cancer should lead to more efficacious treatment.
As previously mentioned, ovarian cancer, according to its biological features and clinical characteristics, represents a typical CSC-driven malignancy[15]. Ovarian CSCs, including VSEL stem cells, play an important role in tumor formation and dissemination, thus advancing disease progression[19,104]. Tumor cell spheroids from ascites, which frequently accompany ovarian cancer, can survive and proliferate even in the absence of abdominal wall and/or abdominal organ adhesion. This characteristic represents one of the fundamental properties of CSCs, which is also used in vitro when assessing cell stemness as sphere formation presence in suspension culture[105]. Ascites, being rich in tumor cells with stem-like properties, is an important CSC source[106-109].
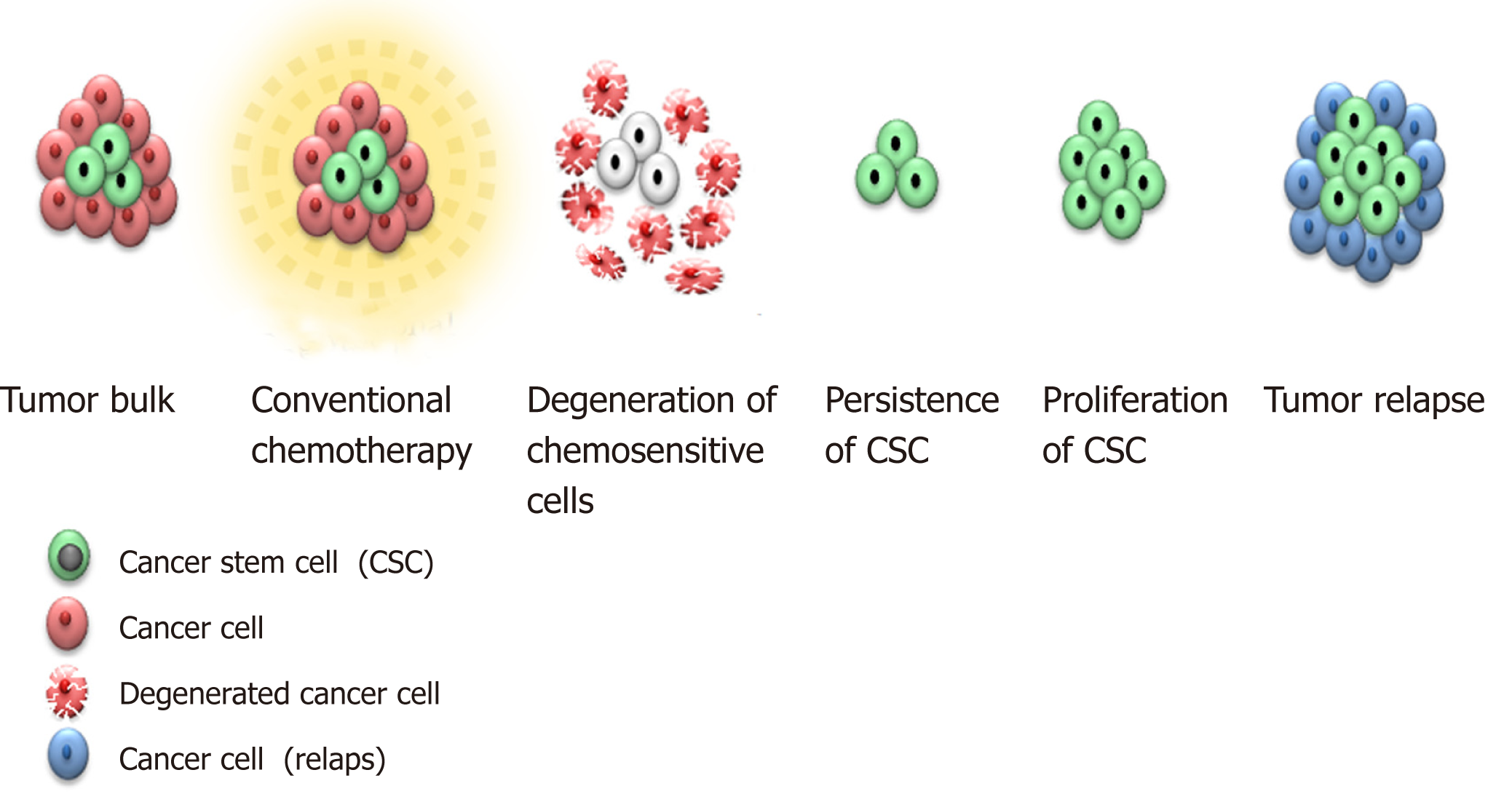
CSCs often display a slow-cycling rate, making them resistant to standard chemotherapy and radiotherapy, which target actively proliferating cells[110,111]. By remaining in the G0 phase of the cell cycle, CSCs maintain inactivity. As most treatment regimens target actively-dividing cells in the S or M phase of the cell cycle, CSC quiescence presents a significant issue in terms of successful treatment. Subpopulations of inactive ovarian CSCs, which survive, allegedly, successful treatment with cytostatic chemotherapy, might be the cause of high-frequency ovarian cancer relapse[15]. Resistant dormant CSCs “wake up” later and cause tumor recurrence[15]. As relapsed tumor is richer in CSCs, it becomes more chemoresistant than primary ovarian tumor (Figure 3).
Many studies support this thesis. In metastatic and relapsed ovarian cancer, a remarkable increase in CD44 level, a putative CSC marker, was observed in comparison to primary tumors[112]. CD44 was found overexpressed in drug-resistant ovarian cancer cell lines and up-regulated in tumor recurrence following chemotherapy[112]. Increased nuclear NANOG expression, another putative CSC marker, was significantly associated with reduced chemosensitivity and poor overall and disease-free survival[90]. NANOG has been suggested as a potential predictor of survival and is involved in the ovarian carcinoma chemoresistance mechanism[91]. Several other studies have investigated various putative ovarian CSC marker expression in correlation with clinico-pathological signs of aggression, poor disease prognosis and chemoresistance, and they have all provided similar results[65,71,81,85,113].
Ovarian CSCs’ strong implication in terms of disease clinical course is, similarly, supported by functional genomics studies which, based on gene expression profiles, identified different tumor subtypes rich in stemness-associated genes which, when expressed, relate to poor prognosis[114,115].
There are multiple pathways involved in promoting stem cell phenotype and chemoresistance in terms of ovarian cancer. Each pathway could potentially be therapeutically targeted with pathway inhibitors. If a therapeutic goal is to eliminate CSCs, more studies are needed to define which subpopulation of CSCs should to be targeted. Small, VSEL stem cells, proposed to be progenitor stem cells involved in ovarian cancer triggering and progression via EMT and interaction with other types of stem cells, might represent potential targets.
Before introducing CSC target therapy in clinical practice, we should think about the many challenges concerning stem cell inhibition. CSCs share epitopes with normal stem cells, so treatment regimens targeting CSCs may also harm normal stem cells, which would increase drug toxicity and the risk of adverse side effects. An ideal therapeutic agent should selectively target CSCs above normal stem cells, so further biological CSC characterization, particularly the underlying mechanisms regulating their function, is required.
The development of novel CSC treatments also requires a thorough understanding of the complex genomic profile of ovarian cancer, since its heterogeneity might influence treatment response[104,116].
Finally, only a small fraction of cells within the tumor would be eliminated by CSC target therapy, so treatment response, shrinking of the tumor bulk, may require some time to become visible. Therefore, a combined therapeutic approach with cytotoxic chemotherapy and/or other treatment regime should be introduced to reduce tumor bulk[117]. Treatment success should then be estimated in terms of tumor bulk shrinkage and the eradication of CSC populations for which identification of reliable ovarian CSC biomarkers is critical, so it can be used in clinical practice.
Successful ovarian cancer treatment depends on ovarian CSC eradication, as CSCs present the driving force of disease manifestation, progression and recurrence pursuant to conventional treatment. There are three potential targets for ovarian CSC eradication: CSC markers, CSC signaling pathways implicated in renewal and CSC niche.
Due to the heterogeneous nature of ovarian cancer, there are probably more markers identifying different subpopulations of ovarian CSCs, and diverse signaling pathways involved in CSC renewal. In terms of identifying CSC specific markers and signaling pathways, and additionally exploring ovarian CSC microenvironment, cancer cell lines are helpful, but in vitro tumor formation analysis should be upgraded by analysing ovarian cancer patient tumor tissue in vivo. Selected ovarian CSC markers, signaling pathways and factors from CSC microenvironment should then be tested in clinical practice, where their expression, influence and inhibition should be correlated, not only with disease outcome, but also in terms of their influence on chemoresistance. In vitro and in vivo investigation of CSC properties and their microenvironment properties may lead to novel therapeutic regimens for ovarian cancer elimination and relapse prevention.
Despite the tremendous progress made in recent years in the field of ovarian CSCs, a number of issues should be considered. Firstly, ovarian cancer presents a heterogeneous group of tumors and stem cells. A high level of heterogeneity makes identifying a target for a wide population of ovarian cancer patients difficult; to mitigate this, more phenotypic, genetic and epigenetic studies of ovarian cancer patient CSCs need to be performed. Secondly, we still don’t know if different CSC populations arise from common progenitor cells and which CSC populations are the most critical to target. We suggest a population of small, VSEL stem cells as progenitor stem cells needs be better elucidated. CSC subpopulations probably change during cancer progress, and specific CSC subpopulations might play a role at certain disease phases. Finally, do varying marker profiles signify different subpopulation of CSCs and how does CSCs microenvironment influence markers expression? These and many other questions in the field of CSCs should be answered in order to introducing personalised medicine in the treatment of ovarian cancer.
The authors would like to thank Shawn Nicholas Thomson for proofreading the article and Eva Skuk for technical preparation of figures.
Manuscript source: Invited manuscript
Specialty type: Cell and tissue engineering
Country of origin: Slovenia
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Huang YC, Liu L, Wiemer EAC S-Editor: Ji FF L-Editor: E-Editor: Ma YJ
| 1. | Prat J. Ovarian carcinomas: five distinct diseases with different origins, genetic alterations, and clinicopathological features. Virchows Arch. 2012;460:237-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 433] [Cited by in RCA: 403] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 2. | Kurman RJ, Carcangiu ML, Herrington CS, Young RH. Who classification of tumours of female reproductive organs. 4th ed. Lyon: International Agency for Research on Cancer 2014; 2. |
| 3. | Kurman RJ. Shih IeM. The Dualistic Model of Ovarian Carcinogenesis: Revisited, Revised, and Expanded. Am J Pathol. 2016;186:733-747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 518] [Cited by in RCA: 712] [Article Influence: 79.1] [Reference Citation Analysis (33)] |
| 4. | Kurman RJ. Shih IeM. Pathogenesis of ovarian cancer: lessons from morphology and molecular biology and their clinical implications. Int J Gynecol Pathol. 2008;27:151-160. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 209] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 5. | Klotz DM, Wimberger P. Cells of origin of ovarian cancer: ovarian surface epithelium or fallopian tube? Arch Gynecol Obstet. 2017;296:1055-1062. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 41] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 6. | Dong X, Men X, Zhang W, Lei P. Advances in tumor markers of ovarian cancer for early diagnosis. Indian J Cancer. 2014;51 Suppl 3:e72-e76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 59] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 7. | Ottevanger PB. Ovarian cancer stem cells more questions than answers. Semin Cancer Biol. 2017;44:67-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 114] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 8. | Chao X, Wang X, Xiao Y, Ji M, Wang S, Shi H, Fan Q, Zhu L, Leng J, Sun D, Lang J. Effects of hysterectomy with simultaneous bilateral salpingectomy on the subsequent pelvic mass. J Ovarian Res. 2019;12:27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 9. | Schorge JO, McCann C, Del Carmen MG. Surgical debulking of ovarian cancer: what difference does it make? Rev Obstet Gynecol. 2010;3:111-117. [PubMed] |
| 10. | Ozols RF. Treatment goals in ovarian cancer. Int J Gynecol Cancer. 2005;15 Suppl 1:3-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 158] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 11. | Pieterse Z, Amaya-Padilla MA, Singomat T, Binju M, Madjid BD, Yu Y, Kaur P. Ovarian cancer stem cells and their role in drug resistance. Int J Biochem Cell Biol. 2019;106:117-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 47] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 12. | Kroeger PT, Drapkin R. Pathogenesis and heterogeneity of ovarian cancer. Curr Opin Obstet Gynecol. 2017;29:26-34. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 137] [Cited by in RCA: 225] [Article Influence: 28.1] [Reference Citation Analysis (0)] |
| 13. | Kehoe S, Hook J, Nankivell M, Jayson GC, Kitchener H, Lopes T, Luesley D, Perren T, Bannoo S, Mascarenhas M, Dobbs S, Essapen S, Twigg J, Herod J, McCluggage G, Parmar M, Swart AM. Primary chemotherapy versus primary surgery for newly diagnosed advanced ovarian cancer (CHORUS): an open-label, randomised, controlled, non-inferiority trial. Lancet. 2015;386:249-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 799] [Cited by in RCA: 949] [Article Influence: 94.9] [Reference Citation Analysis (0)] |
| 14. | Roy L, Cowden Dahl KD. Can Stemness and Chemoresistance Be Therapeutically Targeted via Signaling Pathways in Ovarian Cancer? Cancers (Basel). 2018;10:pii: E241. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 45] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 15. | Lupia M, Cavallaro U. Ovarian cancer stem cells: still an elusive entity? Mol Cancer. 2017;16:64. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 100] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 16. | Clevers H. The cancer stem cell: premises, promises and challenges. Nat Med. 2011;17:313-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1349] [Cited by in RCA: 1449] [Article Influence: 103.5] [Reference Citation Analysis (0)] |
| 17. | Jiang H, Lin X, Liu Y, Gong W, Ma X, Yu Y, Xie Y, Sun X, Feng Y, Janzen V, Chen T. Transformation of epithelial ovarian cancer stemlike cells into mesenchymal lineage via EMT results in cellular heterogeneity and supports tumor engraftment. Mol Med. 2012;18:1197-1208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 33] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 18. | Zhou BB, Zhang H, Damelin M, Geles KG, Grindley JC, Dirks PB. Tumour-initiating cells: challenges and opportunities for anticancer drug discovery. Nat Rev Drug Discov. 2009;8:806-823. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 615] [Cited by in RCA: 651] [Article Influence: 40.7] [Reference Citation Analysis (0)] |
| 19. | Parte SC, Batra SK, Kakar SS. Characterization of stem cell and cancer stem cell populations in ovary and ovarian tumors. J Ovarian Res. 2018;11:69. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 63] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 20. | Virant-Klun I, Kenda-Suster N, Smrkolj S. Small putative NANOG, SOX2, and SSEA-4-positive stem cells resembling very small embryonic-like stem cells in sections of ovarian tissue in patients with ovarian cancer. J Ovarian Res. 2016;9:12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 21. | Virant-Klun I, Zech N, Rozman P, Vogler A, Cvjeticanin B, Klemenc P, Malicev E, Meden-Vrtovec H. Putative stem cells with an embryonic character isolated from the ovarian surface epithelium of women with no naturally present follicles and oocytes. Differentiation. 2008;76:843-856. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 185] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 22. | Virant-Klun I, Skutella T, Kubista M, Vogler A, Sinkovec J, Meden-Vrtovec H. Expression of pluripotency and oocyte-related genes in single putative stem cells from human adult ovarian surface epithelium cultured in vitro in the presence of follicular fluid. Biomed Res Int. 2013;2013:861460. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 23. | Parte S, Bhartiya D, Telang J, Daithankar V, Salvi V, Zaveri K, Hinduja I. Detection, characterization, and spontaneous differentiation in vitro of very small embryonic-like putative stem cells in adult mammalian ovary. Stem Cells Dev. 2011;20:1451-1464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 187] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 24. | Ratajczak MZ, Zuba-Surma EK, Wysoczynski M, Ratajczak J, Kucia M. Very small embryonic-like stem cells: characterization, developmental origin, and biological significance. Exp Hematol. 2008;36:742-751. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 95] [Cited by in RCA: 83] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 25. | Virant-Klun I, Stimpfel M, Cvjeticanin B, Vrtacnik-Bokal E, Skutella T. Small SSEA-4-positive cells from human ovarian cell cultures: related to embryonic stem cells and germinal lineage? J Ovarian Res. 2013;6:24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 26. | Virant-Klun I, Stimpfel M. Novel population of small tumour-initiating stem cells in the ovaries of women with borderline ovarian cancer. Sci Rep. 2016;6:34730. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 42] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 27. | Kenda Suster N, Smrkolj S, Virant-Klun I. Putative stem cells and epithelial-mesenchymal transition revealed in sections of ovarian tumor in patients with serous ovarian carcinoma using immunohistochemistry for vimentin and pluripotency-related markers. J Ovarian Res. 2017;10:11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 28. | Parte SC, Smolenkov A, Batra SK, Ratajczak MZ, Kakar SS. Ovarian Cancer Stem Cells: Unraveling a Germline Connection. Stem Cells Dev. 2017;26:1781-1803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 29. | Massagué J. TGFbeta in Cancer. Cell. 2008;134:215-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2725] [Cited by in RCA: 3126] [Article Influence: 183.9] [Reference Citation Analysis (0)] |
| 30. | Yang J, Weinberg RA. Epithelial-mesenchymal transition: at the crossroads of development and tumor metastasis. Dev Cell. 2008;14:818-829. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2157] [Cited by in RCA: 2333] [Article Influence: 137.2] [Reference Citation Analysis (0)] |
| 31. | Francí C, Takkunen M, Dave N, Alameda F, Gómez S, Rodríguez R, Escrivà M, Montserrat-Sentís B, Baró T, Garrido M, Bonilla F, Virtanen I, García de Herreros A. Expression of Snail protein in tumor-stroma interface. Oncogene. 2006;25:5134-5144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 178] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 32. | Balkwill FR, Capasso M, Hagemann T. The tumor microenvironment at a glance. J Cell Sci. 2012;125:5591-5596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1050] [Cited by in RCA: 1328] [Article Influence: 110.7] [Reference Citation Analysis (0)] |
| 33. | Lindsey S, Langhans SA. Crosstalk of Oncogenic Signaling Pathways during Epithelial-Mesenchymal Transition. Front Oncol. 2014;4:358. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 98] [Cited by in RCA: 126] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 34. | Nieto MA, Huang RY, Jackson RA, Thiery JP. EMT: 2016. Cell. 2016;166:21-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2533] [Cited by in RCA: 3379] [Article Influence: 422.4] [Reference Citation Analysis (0)] |
| 35. | Ye X, Weinberg RA. Epithelial-Mesenchymal Plasticity: A Central Regulator of Cancer Progression. Trends Cell Biol. 2015;25:675-686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 653] [Cited by in RCA: 793] [Article Influence: 79.3] [Reference Citation Analysis (0)] |
| 36. | Jordan NV, Johnson GL, Abell AN. Tracking the intermediate stages of epithelial-mesenchymal transition in epithelial stem cells and cancer. Cell Cycle. 2011;10:2865-2873. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 183] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 37. | Lee JM, Dedhar S, Kalluri R, Thompson EW. The epithelial-mesenchymal transition: new insights in signaling, development, and disease. J Cell Biol. 2006;172:973-981. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1482] [Cited by in RCA: 1596] [Article Influence: 84.0] [Reference Citation Analysis (0)] |
| 38. | Jolly MK, Boareto M, Huang B, Jia D, Lu M, Ben-Jacob E, Onuchic JN, Levine H. Implications of the Hybrid Epithelial/Mesenchymal Phenotype in Metastasis. Front Oncol. 2015;5:155. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 419] [Cited by in RCA: 505] [Article Influence: 50.5] [Reference Citation Analysis (0)] |
| 39. | Hay ED. The mesenchymal cell, its role in the embryo, and the remarkable signaling mechanisms that create it. Dev Dyn. 2005;233:706-720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 454] [Cited by in RCA: 464] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 40. | Yan H, Sun Y. Evaluation of the mechanism of epithelial-mesenchymal transition in human ovarian cancer stem cells transfected with a WW domain-containing oxidoreductase gene. Oncol Lett. 2014;8:426-430. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 41. | Cioce M, Ciliberto G. On the connections between cancer stem cells and EMT. Cell Cycle. 2012;11:4301-4302. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 42. | Lam SS, Mak AS, Yam JW, Cheung AN, Ngan HY, Wong AS. Targeting estrogen-related receptor alpha inhibits epithelial-to-mesenchymal transition and stem cell properties of ovarian cancer cells. Mol Ther. 2014;22:743-751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 55] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 43. | Aceto N, Bardia A, Miyamoto DT, Donaldson MC, Wittner BS, Spencer JA, Yu M, Pely A, Engstrom A, Zhu H, Brannigan BW, Kapur R, Stott SL, Shioda T, Ramaswamy S, Ting DT, Lin CP, Toner M, Haber DA, Maheswaran S. Circulating tumor cell clusters are oligoclonal precursors of breast cancer metastasis. Cell. 2014;158:1110-1122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1463] [Cited by in RCA: 1793] [Article Influence: 179.3] [Reference Citation Analysis (0)] |
| 44. | Pradella D, Naro C, Sette C, Ghigna C. EMT and stemness: flexible processes tuned by alternative splicing in development and cancer progression. Mol Cancer. 2017;16:8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 159] [Cited by in RCA: 230] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 45. | Brabletz T, Jung A, Reu S, Porzner M, Hlubek F, Kunz-Schughart LA, Knuechel R, Kirchner T. Variable beta-catenin expression in colorectal cancers indicates tumor progression driven by the tumor environment. Proc Natl Acad Sci U S A. 2001;98:10356-10361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 807] [Cited by in RCA: 857] [Article Influence: 35.7] [Reference Citation Analysis (0)] |
| 46. | Davidson B, Holth A, Hellesylt E, Tan TZ, Huang RY, Tropé C, Nesland JM, Thiery JP. The clinical role of epithelial-mesenchymal transition and stem cell markers in advanced-stage ovarian serous carcinoma effusions. Hum Pathol. 2015;46:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 49] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 47. | Li X, Yang J, Wang X, Li X, Liang J, Xing H. Role of TWIST2, E-cadherin and Vimentin in epithelial ovarian carcinogenesis and prognosis and their interaction in cancer progression. Eur J Gynaecol Oncol. 2016;37:100-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 48. | Liu S, Sun J, Cai B, Xi X, Yang L, Zhang Z, Feng Y, Sun Y. NANOG regulates epithelial-mesenchymal transition and chemoresistance through activation of the STAT3 pathway in epithelial ovarian cancer. Tumour Biol. 2016;37:9671-9680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 59] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 49. | Marcucci F, Stassi G, De Maria R. Epithelial-mesenchymal transition: a new target in anticancer drug discovery. Nat Rev Drug Discov. 2016;15:311-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 267] [Article Influence: 29.7] [Reference Citation Analysis (0)] |
| 50. | Chaffer CL, San Juan BP, Lim E, Weinberg RA. EMT, cell plasticity and metastasis. Cancer Metastasis Rev. 2016;35:645-654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 465] [Cited by in RCA: 632] [Article Influence: 79.0] [Reference Citation Analysis (0)] |
| 51. | Boesch M, Sopper S, Zeimet AG, Reimer D, Gastl G, Ludewig B, Wolf D. Heterogeneity of Cancer Stem Cells: Rationale for Targeting the Stem Cell Niche. Biochim Biophys Acta. 2016;1866:276-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 52. | Peinado H, Zhang H, Matei IR, Costa-Silva B, Hoshino A, Rodrigues G, Psaila B, Kaplan RN, Bromberg JF, Kang Y, Bissell MJ, Cox TR, Giaccia AJ, Erler JT, Hiratsuka S, Ghajar CM, Lyden D. Pre-metastatic niches: organ-specific homes for metastases. Nat Rev Cancer. 2017;17:302-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1003] [Cited by in RCA: 1319] [Article Influence: 164.9] [Reference Citation Analysis (0)] |
| 53. | Robado de Lope L, Alcíbar OL, Amor López A, Hergueta-Redondo M, Peinado H. Tumour-adipose tissue crosstalk: fuelling tumour metastasis by extracellular vesicles. Philos Trans R Soc Lond B Biol Sci. 2018;373:pii: 20160485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 56] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 54. | Kenny HA, Chiang CY, White EA, Schryver EM, Habis M, Romero IL, Ladanyi A, Penicka CV, George J, Matlin K, Montag A, Wroblewski K, Yamada SD, Mazar AP, Bowtell D, Lengyel E. Mesothelial cells promote early ovarian cancer metastasis through fibronectin secretion. J Clin Invest. 2014;124:4614-4628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 253] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 55. | Abubaker K, Luwor RB, Zhu H, McNally O, Quinn MA, Burns CJ, Thompson EW, Findlay JK, Ahmed N. Inhibition of the JAK2/STAT3 pathway in ovarian cancer results in the loss of cancer stem cell-like characteristics and a reduced tumor burden. BMC Cancer. 2014;14:317. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 80] [Cited by in RCA: 100] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 56. | Bharti R, Dey G, Mandal M. Cancer development, chemoresistance, epithelial to mesenchymal transition and stem cells: A snapshot of IL-6 mediated involvement. Cancer Lett. 2016;375:51-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 188] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 57. | Kim S, Gwak H, Kim HS, Kim B, Dhanasekaran DN, Song YS. Malignant ascites enhances migratory and invasive properties of ovarian cancer cells with membrane bound IL-6R in vitro. Oncotarget. 2016;7:83148-83159. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 37] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 58. | Arend RC, Londoño-Joshi AI, Samant RS, Li Y, Conner M, Hidalgo B, Alvarez RD, Landen CN, Straughn JM, Buchsbaum DJ. Inhibition of Wnt/β-catenin pathway by niclosamide: a therapeutic target for ovarian cancer. Gynecol Oncol. 2014;134:112-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 137] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 59. | Nieman KM, Kenny HA, Penicka CV, Ladanyi A, Buell-Gutbrod R, Zillhardt MR, Romero IL, Carey MS, Mills GB, Hotamisligil GS, Yamada SD, Peter ME, Gwin K, Lengyel E. Adipocytes promote ovarian cancer metastasis and provide energy for rapid tumor growth. Nat Med. 2011;17:1498-1503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1310] [Cited by in RCA: 1728] [Article Influence: 123.4] [Reference Citation Analysis (0)] |
| 60. | Cheng L, Wu S, Zhang K, Qing Y, Xu T. A comprehensive overview of exosomes in ovarian cancer: emerging biomarkers and therapeutic strategies. J Ovarian Res. 2017;10:73. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 65] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 61. | Nakamura K, Sawada K, Kinose Y, Yoshimura A, Toda A, Nakatsuka E, Hashimoto K, Mabuchi S, Morishige KI, Kurachi H, Lengyel E, Kimura T. Exosomes Promote Ovarian Cancer Cell Invasion through Transfer of CD44 to Peritoneal Mesothelial Cells. Mol Cancer Res. 2017;15:78-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 172] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 62. | Li X, Wang X. The emerging roles and therapeutic potential of exosomes in epithelial ovarian cancer. Mol Cancer. 2017;16:92. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 86] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 63. | Visvader JE, Lindeman GJ. Cancer stem cells in solid tumours: accumulating evidence and unresolved questions. Nat Rev Cancer. 2008;8:755-768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2552] [Cited by in RCA: 2622] [Article Influence: 154.2] [Reference Citation Analysis (0)] |
| 64. | Agro L, O'Brien C. In vitro and in vivo Limiting Dilution Assay for Colorectal Cancer. Bio Protoc. 2015;5:1-11. [PubMed] |
| 65. | Stemberger-Papić S, Vrdoljak-Mozetic D, Ostojić DV, Rubesa-Mihaljević R, Krigtofić I, Brncić-Fisher A, Kragević M, Eminović S. Expression of CD133 and CD117 in 64 Serous Ovarian Cancer Cases. Coll Antropol. 2015;39:745-753. [PubMed] |
| 66. | Luo L, Zeng J, Liang B, Zhao Z, Sun L, Cao D, Yang J, Shen K. Ovarian cancer cells with the CD117 phenotype are highly tumorigenic and are related to chemotherapy outcome. Exp Mol Pathol. 2011;91:596-602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 97] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 67. | Conic I, Stanojevic Z, Jankovic Velickovic L, Stojnev S, Ristic Petrovic A, Krstic M, Stanojevic M, Bogdanović D, Stefanovic V. Epithelial ovarian cancer with CD117 phenotype is highly aggressive and resistant to chemotherapy. J Obstet Gynaecol Res. 2015;41:1630-1637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 68. | Curley MD, Therrien VA, Cummings CL, Sergent PA, Koulouris CR, Friel AM, Roberts DJ, Seiden MV, Scadden DT, Rueda BR, Foster R. CD133 expression defines a tumor initiating cell population in primary human ovarian cancer. Stem Cells. 2009;27:2875-2883. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 276] [Cited by in RCA: 311] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 69. | Skubitz AP, Taras EP, Boylan KL, Waldron NN, Oh S, Panoskaltsis-Mortari A, Vallera DA. Targeting CD133 in an in vivo ovarian cancer model reduces ovarian cancer progression. Gynecol Oncol. 2013;130:579-587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 78] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 70. | Baba T, Convery PA, Matsumura N, Whitaker RS, Kondoh E, Perry T, Huang Z, Bentley RC, Mori S, Fujii S, Marks JR, Berchuck A, Murphy SK. Epigenetic regulation of CD133 and tumorigenicity of CD133+ ovarian cancer cells. Oncogene. 2009;28:209-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 301] [Cited by in RCA: 320] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 71. | Zhang J, Guo X, Chang DY, Rosen DG, Mercado-Uribe I, Liu J. CD133 expression associated with poor prognosis in ovarian cancer. Mod Pathol. 2012;25:456-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 105] [Article Influence: 8.1] [Reference Citation Analysis (1)] |
| 72. | Klapdor R, Wang S, Hacker U, Büning H, Morgan M, Dörk T, Hillemanns P, Schambach A. Improved Killing of Ovarian Cancer Stem Cells by Combining a Novel Chimeric Antigen Receptor-Based Immunotherapy and Chemotherapy. Hum Gene Ther. 2017;28:886-896. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 72] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 73. | Meirelles K, Benedict LA, Dombkowski D, Pepin D, Preffer FI, Teixeira J, Tanwar PS, Young RH, MacLaughlin DT, Donahoe PK, Wei X. Human ovarian cancer stem/progenitor cells are stimulated by doxorubicin but inhibited by Mullerian inhibiting substance. Proc Natl Acad Sci U S A. 2012;109:2358-2363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 101] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 74. | Burgos-Ojeda D, Rueda BR, Buckanovich RJ. Ovarian cancer stem cell markers: prognostic and therapeutic implications. Cancer Lett. 2012;322:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 129] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 75. | Nakamura K, Terai Y, Tanabe A, Ono YJ, Hayashi M, Maeda K, Fujiwara S, Ashihara K, Nakamura M, Tanaka Y, Tanaka T, Tsunetoh S, Sasaki H, Ohmichi M. CD24 expression is a marker for predicting clinical outcome and regulates the epithelial-mesenchymal transition in ovarian cancer via both the Akt and ERK pathways. Oncol Rep. 2017;37:3189-3200. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 83] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 76. | Jaggupilli A, Elkord E. Significance of CD44 and CD24 as cancer stem cell markers: an enduring ambiguity. Clin Dev Immunol. 2012;2012:708036. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 282] [Cited by in RCA: 358] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 77. | Choi YL, Kim SH, Shin YK, Hong YC, Lee SJ, Kang SY, Ahn G. Cytoplasmic CD24 expression in advanced ovarian serous borderline tumors. Gynecol Oncol. 2005;97:379-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 46] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 78. | Zhang S, Balch C, Chan MW, Lai HC, Matei D, Schilder JM, Yan PS, Huang TH, Nephew KP. Identification and characterization of ovarian cancer-initiating cells from primary human tumors. Cancer Res. 2008;68:4311-4320. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1023] [Cited by in RCA: 1007] [Article Influence: 59.2] [Reference Citation Analysis (0)] |
| 79. | Casagrande F, Cocco E, Bellone S, Richter CE, Bellone M, Todeschini P, Siegel E, Varughese J, Arin-Silasi D, Azodi M, Rutherford TJ, Pecorelli S, Schwartz PE, Santin AD. Eradication of chemotherapy-resistant CD44+ human ovarian cancer stem cells in mice by intraperitoneal administration of Clostridium perfringens enterotoxin. Cancer. 2011;117:5519-5528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 56] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 80. | Zhang J, Chang B, Liu J. CD44 standard form expression is correlated with high-grade and advanced-stage ovarian carcinoma but not prognosis. Hum Pathol. 2013;44:1882-1889. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 81. | Meng E, Long B, Sullivan P, McClellan S, Finan MA, Reed E, Shevde L, Rocconi RP. CD44+/CD24- ovarian cancer cells demonstrate cancer stem cell properties and correlate to survival. Clin Exp Metastasis. 2012;29:939-948. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 138] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 82. | Wang Y, Shao F, Chen L. ALDH1A2 suppresses epithelial ovarian cancer cell proliferation and migration by downregulating STAT3. Onco Targets Ther. 2018;11:599-608. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 83. | Wang YC, Yo YT, Lee HY, Liao YP, Chao TK, Su PH, Lai HC. ALDH1-bright epithelial ovarian cancer cells are associated with CD44 expression, drug resistance, and poor clinical outcome. Am J Pathol. 2012;180:1159-1169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 134] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 84. | Januchowski R, Wojtowicz K, Sterzyſska K, Sosiſska P, Andrzejewska M, Zawierucha P, Nowicki M, Zabel M. Inhibition of ALDH1A1 activity decreases expression of drug transporters and reduces chemotherapy resistance in ovarian cancer cell lines. Int J Biochem Cell Biol. 2016;78:248-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 80] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 85. | Landen CN, Goodman B, Katre AA, Steg AD, Nick AM, Stone RL, Miller LD, Mejia PV, Jennings NB, Gershenson DM, Bast RC, Coleman RL, Lopez-Berestein G, Sood AK. Targeting aldehyde dehydrogenase cancer stem cells in ovarian cancer. Mol Cancer Ther. 2010;9:3186-3199. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 308] [Cited by in RCA: 312] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 86. | Ng PM, Lufkin T. Embryonic stem cells: protein interaction networks. Biomol Concepts. 2011;2:13-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 87. | Yu Z, Pestell TG, Lisanti MP, Pestell RG. Cancer stem cells. Int J Biochem Cell Biol. 2012;44:2144-2151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 396] [Cited by in RCA: 498] [Article Influence: 38.3] [Reference Citation Analysis (0)] |
| 88. | Wen Y, Hou Y, Huang Z, Cai J, Wang Z. SOX2 is required to maintain cancer stem cells in ovarian cancer. Cancer Sci. 2017;108:719-731. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 71] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 89. | Ruan Z, Yang X, Cheng W. OCT4 accelerates tumorigenesis through activating JAK/STAT signaling in ovarian cancer side population cells. Cancer Manag Res. 2018;11:389-399. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 45] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 90. | Siu MK, Wong ES, Kong DS, Chan HY, Jiang L, Wong OG, Lam EW, Chan KK, Ngan HY, Le XF, Cheung AN. Stem cell transcription factor NANOG controls cell migration and invasion via dysregulation of E-cadherin and FoxJ1 and contributes to adverse clinical outcome in ovarian cancers. Oncogene. 2013;32:3500-3509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 125] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 91. | Lee M, Nam EJ, Kim SW, Kim S, Kim JH, Kim YT. Prognostic impact of the cancer stem cell-related marker NANOG in ovarian serous carcinoma. Int J Gynecol Cancer. 2012;22:1489-1496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 49] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 92. | Pan Y, Jiao J, Zhou C, Cheng Q, Hu Y, Chen H. Nanog is highly expressed in ovarian serous cystadenocarcinoma and correlated with clinical stage and pathological grade. Pathobiology. 2010;77:283-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 93. | Qin S, Li Y, Cao X, Du J, Huang X. NANOG regulates epithelial-mesenchymal transition and chemoresistance in ovarian cancer. Biosci Rep. 2017;37:pii: BSR20160247. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 94. | Di J, Duiveman-de Boer T, Zusterzeel PL, Figdor CG, Massuger LF, Torensma R. The stem cell markers Oct4A, Nanog and c-Myc are expressed in ascites cells and tumor tissue of ovarian cancer patients. Cell Oncol (Dordr). 2013;36:363-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 51] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 95. | Ning YX, Luo X, Xu M, Feng X, Wang J. Let-7d increases ovarian cancer cell sensitivity to a genistein analog by targeting c-Myc. Oncotarget. 2017;8:74836-74845. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 96. | Garson K, Vanderhyden BC. Epithelial ovarian cancer stem cells: underlying complexity of a simple paradigm. Reproduction. 2015;149:R59-R70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 52] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 97. | Hu L, McArthur C, Jaffe RB. Ovarian cancer stem-like side-population cells are tumourigenic and chemoresistant. Br J Cancer. 2010;102:1276-1283. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 212] [Cited by in RCA: 236] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 98. | Dou J, Jiang C, Wang J, Zhang X, Zhao F, Hu W, He X, Li X, Zou D, Gu N. Using ABCG2-molecule-expressing side population cells to identify cancer stem-like cells in a human ovarian cell line. Cell Biol Int. 2011;35:227-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 40] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 99. | Kobayashi Y, Seino K, Hosonuma S, Ohara T, Itamochi H, Isonishi S, Kita T, Wada H, Kojo S, Kiguchi K. Side population is increased in paclitaxel-resistant ovarian cancer cell lines regardless of resistance to cisplatin. Gynecol Oncol. 2011;121:390-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 36] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 100. | Abelson S, Shamai Y, Berger L, Skorecki K, Tzukerman M. Niche-dependent gene expression profile of intratumoral heterogeneous ovarian cancer stem cell populations. PLoS One. 2013;8:e83651. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 101. | Testa U, Petrucci E, Pasquini L, Castelli G, Pelosi E. Ovarian Cancers: Genetic Abnormalities, Tumor Heterogeneity and Progression, Clonal Evolution and Cancer Stem Cells. Medicines (Basel). 2018;5:pii: E16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 123] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 102. | Pilsworth JA, Cochrane DR, Xia Z, Aubert G, Färkkilä AEM, Horlings HM, Yanagida S, Yang W, Lim JLP, Wang YK, Bashashati A, Keul J, Wong A, Norris K, Brucker SY, Taran FA, Krämer B, Staebler A, van Meurs H, Oliva E, Shah SP, Kommoss S, Kommoss F, Gilks CB, Baird DM, Huntsman DG. TERT promoter mutation in adult granulosa cell tumor of the ovary. Mod Pathol. 2018;31:1107-1115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 42] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 103. | Huang Y, Ju B, Tian J, Liu F, Yu H, Xiao H, Liu X, Liu W, Yao Z, Hao Q. Ovarian cancer stem cell-specific gene expression profiling and targeted drug prescreening. Oncol Rep. 2014;31:1235-1248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 104. | Zhang T, Xu J, Deng S, Zhou F, Li J, Zhang L, Li L, Wang QE, Li F. Core signaling pathways in ovarian cancer stem cell revealed by integrative analysis of multi-marker genomics data. PLoS One. 2018;13:e0196351. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 105. | Dontu G, Abdallah WM, Foley JM, Jackson KW, Clarke MF, Kawamura MJ, Wicha MS. In vitro propagation and transcriptional profiling of human mammary stem/progenitor cells. Genes Dev. 2003;17:1253-1270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1841] [Cited by in RCA: 1912] [Article Influence: 86.9] [Reference Citation Analysis (0)] |
| 106. | Ho CM, Chang SF, Hsiao CC, Chien TY, Shih DT. Isolation and characterization of stromal progenitor cells from ascites of patients with epithelial ovarian adenocarcinoma. J Biomed Sci. 2012;19:23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 42] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 107. | Latifi A, Luwor RB, Bilandzic M, Nazaretian S, Stenvers K, Pyman J, Zhu H, Thompson EW, Quinn MA, Findlay JK, Ahmed N. Isolation and characterization of tumor cells from the ascites of ovarian cancer patients: molecular phenotype of chemoresistant ovarian tumors. PLoS One. 2012;7:e46858. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 150] [Cited by in RCA: 181] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 108. | Liao J, Qian F, Tchabo N, Mhawech-Fauceglia P, Beck A, Qian Z, Wang X, Huss WJ, Lele SB, Morrison CD, Odunsi K. Ovarian cancer spheroid cells with stem cell-like properties contribute to tumor generation, metastasis and chemotherapy resistance through hypoxia-resistant metabolism. PLoS One. 2014;9:e84941. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 219] [Cited by in RCA: 271] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 109. | Mo L, Bachelder RE, Kennedy M, Chen PH, Chi JT, Berchuck A, Cianciolo G, Pizzo SV. Syngeneic Murine Ovarian Cancer Model Reveals That Ascites Enriches for Ovarian Cancer Stem-Like Cells Expressing Membrane GRP78. Mol Cancer Ther. 2015;14:747-756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 110. | Chen W, Dong J, Haiech J, Kilhoffer MC, Zeniou M. Cancer Stem Cell Quiescence and Plasticity as Major Challenges in Cancer Therapy. Stem Cells Int. 2016;2016:1740936. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 174] [Cited by in RCA: 274] [Article Influence: 30.4] [Reference Citation Analysis (0)] |
| 111. | Takeishi S, Nakayama KI. To wake up cancer stem cells, or to let them sleep, that is the question. Cancer Sci. 2016;107:875-881. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 75] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 112. | Gao Y, Foster R, Yang X, Feng Y, Shen JK, Mankin HJ, Hornicek FJ, Amiji MM, Duan Z. Up-regulation of CD44 in the development of metastasis, recurrence and drug resistance of ovarian cancer. Oncotarget. 2015;6:9313-9326. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 102] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 113. | KendaŠuster N. Frković Grazio S, Virant-Klun I, Verdenik I, Smrkolj Š. Cancer Stem Cell-Related Marker NANOG Expression in Ovarian Serous Tumors: A Clinicopathological Study of 159 Cases. Int J Gynecol Cancer. 2017;27:2006-2013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 114. | Zhang W, Liu Y, Sun N, Wang D, Boyd-Kirkup J, Dou X, Han JD. Integrating genomic, epigenomic, and transcriptomic features reveals modular signatures underlying poor prognosis in ovarian cancer. Cell Rep. 2013;4:542-553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 72] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 115. | Tan TZ, Miow QH, Huang RY, Wong MK, Ye J, Lau JA, Wu MC, Bin Abdul Hadi LH, Soong R, Choolani M, Davidson B, Nesland JM, Wang LZ, Matsumura N, Mandai M, Konishi I, Goh BC, Chang JT, Thiery JP, Mori S. Functional genomics identifies five distinct molecular subtypes with clinical relevance and pathways for growth control in epithelial ovarian cancer. EMBO Mol Med. 2013;5:1051-1066. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 164] [Cited by in RCA: 176] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 116. | Zhan Q, Wang C, Ngai S. Ovarian cancer stem cells: a new target for cancer therapy. Biomed Res Int. 2013;2013:916819. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 52] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 117. | Kenda Suster N, Virant-Klun I, Frkovic Grazio S, Smrkolj S. The significance of the pluripotency and cancer stem cell-related marker NANOG in diagnosis and treatment of ovarian carcinoma. Eur J Gynaecol Oncol. 2016;37:604-612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 118. | Su D, Deng H, Zhao X, Zhang X, Chen L, Chen X, Li Z, Bai Y, Wang Y, Zhong Q, Yi T, Qian Z, Wei Y. Targeting CD24 for treatment of ovarian cancer by short hairpin RNA. Cytotherapy. 2009;11:642-652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 30] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 119. | Gao MQ, Choi YP, Kang S, Youn JH, Cho NH. CD24+ cells from hierarchically organized ovarian cancer are enriched in cancer stem cells. Oncogene. 2010;29:2672-2680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 277] [Cited by in RCA: 306] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 120. | Helland Å. Anglesio MS, George J, Cowin PA, Johnstone CN, House CM, Sheppard KE, Etemadmoghadam D, Melnyk N, Rustgi AK, Phillips WA, Johnsen H, Holm R, Kristensen GB, Birrer MJ; Australian Ovarian Cancer Study Group, Pearson RB, Børresen-Dale AL, Huntsman DG, deFazio A, Creighton CJ, Smyth GK, Bowtell DD. Deregulation of MYCN, LIN28B and LET7 in a molecular subtype of aggressive high-grade serous ovarian cancers. PLoS One. 2011;6:e18064. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 144] [Cited by in RCA: 158] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 121. | Schilder RJ, Sill MW, Lee RB, Shaw TJ, Senterman MK, Klein-Szanto AJ, Miner Z, Vanderhyden BC. Phase II evaluation of imatinib mesylate in the treatment of recurrent or persistent epithelial ovarian or primary peritoneal carcinoma: a Gynecologic Oncology Group Study. J Clin Oncol. 2008;26:3418-3425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 81] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 122. | Raspollini MR, Amunni G, Villanucci A, Baroni G, Taddei A, Taddei GL. c-KIT expression and correlation with chemotherapy resistance in ovarian carcinoma: an immunocytochemical study. Ann Oncol. 2004;15:594-597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 41] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 123. | Zheng J, Zhao S, Yu X, Huang S, Liu HY. Simultaneous targeting of CD44 and EpCAM with a bispecific aptamer effectively inhibits intraperitoneal ovarian cancer growth. Theranostics. 2017;7:1373-1388. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 88] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 124. | Tayama S, Motohara T, Narantuya D, Li C, Fujimoto K, Sakaguchi I, Tashiro H, Saya H, Nagano O, Katabuchi H. The impact of EpCAM expression on response to chemotherapy and clinical outcomes in patients with epithelial ovarian cancer. Oncotarget. 2017;8:44312-44325. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 62] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 125. | Zhang S. Cui B, Lai H, Liu G, Ghia EM, Widhopf GF 2nd, Zhang Z, Wu CC, Chen L, Wu R, Schwab R, Carson DA, Kipps TJ. Ovarian cancer stem cells express ROR1, which can be targeted for anti-cancer-stem-cell therapy. Proc Natl Acad Sci U S A. 2014;111:17266-17271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 154] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 126. | Zhang H, Qiu J, Ye C, Yang D, Gao L, Su Y, Tang X, Xu N, Zhang D, Xiong L, Mao Y, Li F, Zhu J. ROR1 expression correlated with poor clinical outcome in human ovarian cancer. Sci Rep. 2014;4:5811. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 92] [Article Influence: 8.4] [Reference Citation Analysis (0)] |