Published online Apr 26, 2019. doi: 10.4252/wjsc.v11.i4.222
Peer-review started: January 8, 2019
First decision: January 21, 2019
Revised: January 30, 2019
Accepted: March 16, 2019
Article in press: March 16, 2019
Published online: April 26, 2019
Processing time: 108 Days and 5.8 Hours
Osteoarthritis (OA) refers to a chronic joint disease characterized by degenerative changes of articular cartilage and secondary bone hyperplasia. Since articular cartilage has a special structure, namely the absence of blood vessels as well as the low conversion rate of chondrocytes in the cartilage matrix, the treatment faces numerous clinical challenges. Traditional OA treatment (e.g., arthroscopic debridement, microfracture, autologous or allogeneic cartilage transplantation, chondrocyte transplantation) is primarily symptomatic treatment and pain management, which cannot contribute to regenerating degenerated cartilage or reducing joint inflammation. Also, the generated mixed fibrous cartilage tissue is not the same as natural hyaline cartilage. Mesenchymal stem cells (MSCs) have turned into the most extensively explored new therapeutic drugs in cell-based OA treatment as a result of their ability to differentiate into chondrocytes and their immunomodulatory properties. In this study, the preliminary results of preclinical (OA animal model)/clinical trials regarding the effects of MSCs on cartilage repair of knee joints are briefly summarized, which lay a solid application basis for more and deeper clinical studies on cell-based OA treatment.
Core tip: The key points include: (1) Animal studies have reported that the expanded culture of mesenchymal stem cells (MSCs) is conducive to repairing cartilage and subchondral bone, and regulating the progression of secondary osteoarthritis (OA); (2) Recent studies on the treatment of OA by MSCs have progressed to clinical trials, and most clinical trials have achieved significant positive results with minimal side effects; and (3) Intra-articular injection of MSCs can offer OA patients a safe and effective treatment, yet some problems still remain to be solved for the clinical application of MSC in the treatment of OA.
- Citation: Wang AT, Feng Y, Jia HH, Zhao M, Yu H. Application of mesenchymal stem cell therapy for the treatment of osteoarthritis of the knee: A concise review. World J Stem Cells 2019; 11(4): 222-235
- URL: https://www.wjgnet.com/1948-0210/full/v11/i4/222.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v11.i4.222
Osteoarthritis (OA) refers to a common chronic degenerative joint disease, namely the degenerative injury of articular cartilage caused by still multiple factors (e.g., aging, obesity, fatigue injury, trauma, joint congenital abnormalities, joint deformity, etc). Pathological changes largely include articular cartilage destruction, subchondral osteosclerosis and synovial hyperplasia[1]. OA occurs primarily after middle age, and it is more widespread in women than in men. Clinical manifestations include joint pain, joint stiffness and loss of function, which impairs patient mobility, and OA will turn out to be the fourth most disabling disease by 2020[2,3]. The cartilage has poor self-repair and regeneration abilities since the hyaline cartilage tissue on the joint surface has no nerves nor blood vessels, and it is hard to recover by itself once damaged. At present, the main clinical treatment methods for OA include non-drug therapy, drug therapy and surgical treatment, which is only capable of relieving pain, and can to a certain extent improve symptoms, delay illness and correct malformation. Nevertheless, the progressive degeneration of articular cartilage cannot be thoroughly delayed for patients with OA disease[4-8]. Autologous chondrocyte transplantation has been successfully employed to repair damaged cartilage, yet in vitro cultured chondrocytes show dedifferentiation and decreased chondrocyte-specific gene expression, thereby affecting its therapeutic effect. In recent years, new stem cell-based therapies for OA have aroused increasing attention. Mesenchymal stem cells (MSCs) have the potential of self-renewal and directional differentiation, which can repair cartilage tissue and suppress chondrocyte secretion of inflammatory factors and homing characteristics, which make MSCs the ideal seed cells for gradual OA treatment. This study reviews the potential applications of MSCs in preclinical models, as well as the clinical applications of OA.
MSCs are adult stem cells that are not hematopoietic stem cells, and exist in various tissues (e.g., bone marrow, umbilical cord, placenta, tendon, periodontal, adipose, and many other tissues)[9]. In the 1970s, Friedenstein isolated MSCs from whole bone marrow cultures, and the cells were subsequently extensively studied. In 1995, Lazarus et al[10] reported in the journal of bone marrow transplant the first clinical study of bone marrow derived from MSCs for the treatment of marrow transplant patients. The international society for cell therapy (ISCT) defines MSCs with three criteria: (1) Plastic-adherent; (2) Expression of CD105, CD73 and CD90, and lack of CD45, CD34, CD14 or CD11b, CD79α or CD19 and HLA-DR surface molecules; and (3) MSC must differentiate into osteoblasts, adipocytes and chondroblasts in vitro[11]. Besides their differentiation potential, MSCs also express enzymes and secrete numerous nutritional factors involved in paracrine activities, including growth factors, cytokines and chemokines[12], which nourish cartilage by activating cellular and angiogenesis pathways. Moreover, it is noteworthy that MSCs participate in the local immune regulation mechanism, which can suppress T cell proliferation, dendritic cell maturation, as well as the activation, proliferation and antibody secretion of B cells, thereby affecting the polarization of macrophages and the differentiation of antibody-secreting cells, thus essentially eliminating the risk of rejection and disease transmission[13]. However, the immunomodulatory function of MSCs may vary among individuals, species, tissue sources, culture conditions and activation states. ISCT proposed the standardization of MSC immunomodulatory characteristics[14]. Finally, MSCs also play a homing role, actively migrating to cartilage ischemia or damaged sites under the action of the microenvironment in vivo. Besides, repair and reconstruction can be performed by secreting growth factors, cytokines and extracellular matrix[15]. In brief, further understanding of MSC function will have therapeutic significance for slowing cartilage degeneration in OA patients.
In vivo experiments on various animal models have been performed in the literature. These studies include the following models: Sodium iodoacetate (MIA) model in guinea pigs/rabbits, oophorectomy in rats, and anterior cruciate ligament amputation in rats/rabbits (ACLT). In addition, some chemical agents (e.g., papain, quinolone and collagenase) can induce the OA model in animals[16,17]. ACLT on the anterior feet of rabbits is one of the classic ways to build an OA model in rabbits. This type of rabbit model has been successfully modeled in 3 to 8 wk, which also exhibits similar biochemical and pathological variations to those of humans[17]. It was reported in animal experiments that local intra-articular injection of MSCs, MSC-derived exosomes, implants with MSC-laden scaffolds, and MSC suspensions with carrier media can effectively alleviate OA disease.
MSCs can serve as cartilage progenitor cells or regenerative cells, which can be seeded onto three-dimensional scaffolds in order to repair damaged cartilage through the stimulation of endogenous cells[18]. MSCs can be differentiated into chondrocytes in vitro, which is similar to the structural characteristics of hyaline cartilage. However, there are differences in the chondrocyte differentiation capacity of MSCs derived from different sources, cells can tend to hypertrophy during differentiation, and the phenotypic stability of mature chondrocytes remains difficult to ensure[19]. Many previous experiments have verified that connective tissue growth is vital for cartilage repair, i.e., it can promote cartilage and extracellular matrix repair. Accordingly, studies show that tissue growth factors can be loaded onto scaffolds to assist cartilage repair and increase the degree of integration of new cartilage units with surrounding tissues[20,21]. However, this method is usually employed to repair the small area cartilage defect model, yet it does not address the large area of cartilage defects related to OA. At present, several scaffolds [polylactic-co-glycolic acid, polyethylene glycol, polylactic acid, polyglycolic acid, collagen, gelatin, hyaluronic acid (HA), and fibrin] are applied for the implantation of articular cartilage defects in experimental animal models[22]. They are still not used as routine treatments in clinical practice, although several studies have shown the safety and efficacy of MSC-based tissue engineering methods. This is largely because: (1) Since both allogeneic MSCs and scaffold materials may cause unnecessary graft-versus-host reactions, the acquisition and culture of autologous MSCs and the selection of scaffold materials are major limitations to clinical application; and (2) At present, the selection of cytokines is more diversified, and the function of promoting chondrogenic and osteogenic differentiation is also favored by researchers. However, studies have demonstrated that different levels of growth factors have bidirectional effects on promoting chondrogenic and osteogenic differentiation. How to minimize osteogenic differentiation in the new cartilage area while maximizing chondrogenic differentiation ability remains one of the problems to be solved. Thus, more studies are required to prove their effectiveness in larger groups of OA patients before they can be implemented at a large scale.
In recent years, a growing number of researchers think that exosomes secreted by MSCs also play a role in the treatment of OA[23]. Exosomes are generally hypothesized to be intercellular communication vehicles and function to transfer lipids, nucleic acids (mRNAs and microRNAs) and proteins between cells to elicit biological responses in recipient cells that are reflective of the cargo contents[24]. MSC exosomes are abundant in a considerable amount of microRNA, which can specifically bind to transcribed mRNA from their target genes, thereby silencing the expressed target genes or forming an interaction network of multiple signals[24-26]. Accordingly, microRNA may be vital to mediate the efficacies of MSC exosomes in the treatment of OA[27-30]. For example, Tao et al[30] reported that exosomes derived from human synovial MSCs overexpressed with microRNA-140-5p can promote cartilage regeneration and suppress OA in rat models, suggesting that miroRNA-140 may be a protective factor in the pathogenesis of OA. It can also prevent and alleviate OA by upregulating the expression of SOX9 and aggrecan (ACAN) to maintain cartilage homeostasis[27-30]. Toh et al[23] reported that microRNA-23b, 92a, 125b, 320, 145, 22 and 221 were involved in the regulation of chondrogenesis and homeostasis. Besides, MSC exosomes are rich in ECM proteins and enzymes, thereby regulating and restoring ECM balance. The increase in enzyme activity is proportional to the loss of normal equilibrium, i.e., exosome-based enzymes promote tissue repair and regeneration by restoring homeostasis during injury and disease. In contrast, homeostasis was restored, and exosome enzyme activity was terminated after subsided injury[31]. According to the study on both the pathogenesis of OA and the drug treatment of OA, MSC exosomes exhibit infinite potential, with a good tolerance and minimal risk of immunogenicity and toxicity. However, how to obtain large-scale purified exosomes, as well as how to improve the utilization efficiency, biosafety and therapeutic efficacy of exosomes, should be further explored and studied. The study on the effect and mechanism of MSC exosomes on OA will remain one of the important hotspots for future research. In brief, MSC exosomes will soon become the main treatment modality for clinical OA with the continuous innovation of technology and in-depth research.
In recent years, local intra-articular injection of MSCs has been reported to promote the regeneration and repair of cartilage tissue and alleviate the degeneration caused by OA. MSCs are capable of significantly improving local microenvironmental, immune-regulation and anti-inflammatory biological activities through the secretion of exosomes, growth factors, cytokines, anti-inflammatory factors and other bioactive molecules, thereby gradually becoming the simplest and easiest method to treat OA. For example, Zhou et al[32] found that local intra-articular injection of adipose-derived MSCs (AD-MSCs) can effectively alleviate the condition in rat OA models through autophagy induction to reduce the secretion of pro-inflammatory cytokines. Toghraie et al[33] reported the establishment of an OA model by resection of anterior cruciate ligaments in rabbits. Radiology revealed OA symptoms after 12 wk, and then a single dose of 1 × 106/mL AD-MSCs was injected into the joint cavity of the OA model. It was found that cartilage tissue was significantly repaired and improved as the result of imaging, morphology and histology at 20 wk. In the meantime, platelet-rich plasma (PRP) with the active substance can promote cell proliferation, collagen synthesis and inflammatory chemotaxis. Thus, it is conducive to tissue repair and can assist tissue reconstruction. Pre-clinical studies have verified that PRP/MSCs can also improve knee joint function, and the repaired tissue exhibits good compatibility with the original articular facial cartilage tissue by MRI analysis. Additionally, HA combined with MSCs can effectively repair damaged cartilage, and its mechanism may be to promote the repair of damaged cartilage by suppressing the inflammatory response and apoptosis of chondrocyte. It has been reported that PRP/MSCs or HA/MSCs has a significantly better effect on the repair of damaged cartilage than the individual treatment group in the OA animal model (HA, PRP or MSCs were used alone, respectively). Table 1 shows the summary of pre-clinical trials of MSCs in the treatment of the OA animal model from 2015 to 2018.
| Animal models (osteoarthritis) | MSC type | Interventions | Results | Ref. |
| Sheep | AD-MSCs | AD-MSCs/HA vs HA | µCT, MRI and immunohistochemistry: AD-MSCs/HA > HA | Lv et al[34], 2018 |
| Sheep | Allogeneic AD-MSCs | AD-MSCs/HA vs HA | MRI and macroscopy examinations: AD-MSCs/HA > HA | Feng et al[35], 2017 |
| Rabbits | BMSCs | BMSCs/HA vs PRP vs PRP/HA | Histological scores and immunohistochemistry: BMSCs/HA > PRP/HA > PRP | Desando et al[36], 2017 |
| Rabbits | Allogeneic BMSCs | BMSCs/HA vs HA | Histological scores and cartilage content: BMSCs/HA > HA | Chiang et al[37], 2016 |
| Dogs | AD-MSCs | AD-MSCs/PRP vs PRP | Focal compressive strength: AD-MSCs/PRP > PRP function and pain: AD-MSCs/PRP > PRP | Yun et al[38], 2016 |
| Rabbits | AD-MSCs | AD-MSCs/PRP vs PRP | Macroscopic and histological examinations: AD-MSCs/PRP > PRP | Hermeto et al[39], 2016 |
Immunomodulatory effects of MSCs is one of the vital mechanisms of its treatment of OA. MSCs can be activated by inflammatory factors, then the secretion of PGE2, IDO, NO and other factors by MSCs can directly or indirectly suppress immune cells[40]. For instance, PGE2 secreted by MSCs can promote the production of immunosuppressive IL-10 by binding EP2 and EP4 receptors on macrophages, and participate in the regulation of CD4+ effector T cells[41]. Moreover, MSCs have been shown to suppress T cell proliferation and induce T cell apoptosis, resulting in fragments that stimulate phagocytes to produce tumor growth factor beta and increase the number of regulatory T cells[42]. MSCs also regulate innate immunity by inhibiting dendritic cell maturation and reducing natural killer (NK) cytotoxicity[43]. MSCs can also reverse the polarization of macrophages from pro-inflammatory (M1) to anti-inflammatory (M2) phenotypes[44]. Jo et al[45] found that MSCs can interact with macrophages to suppress the activation of macrophages and the secretion of IL-1β, TGF-α and another inflammatory factors.
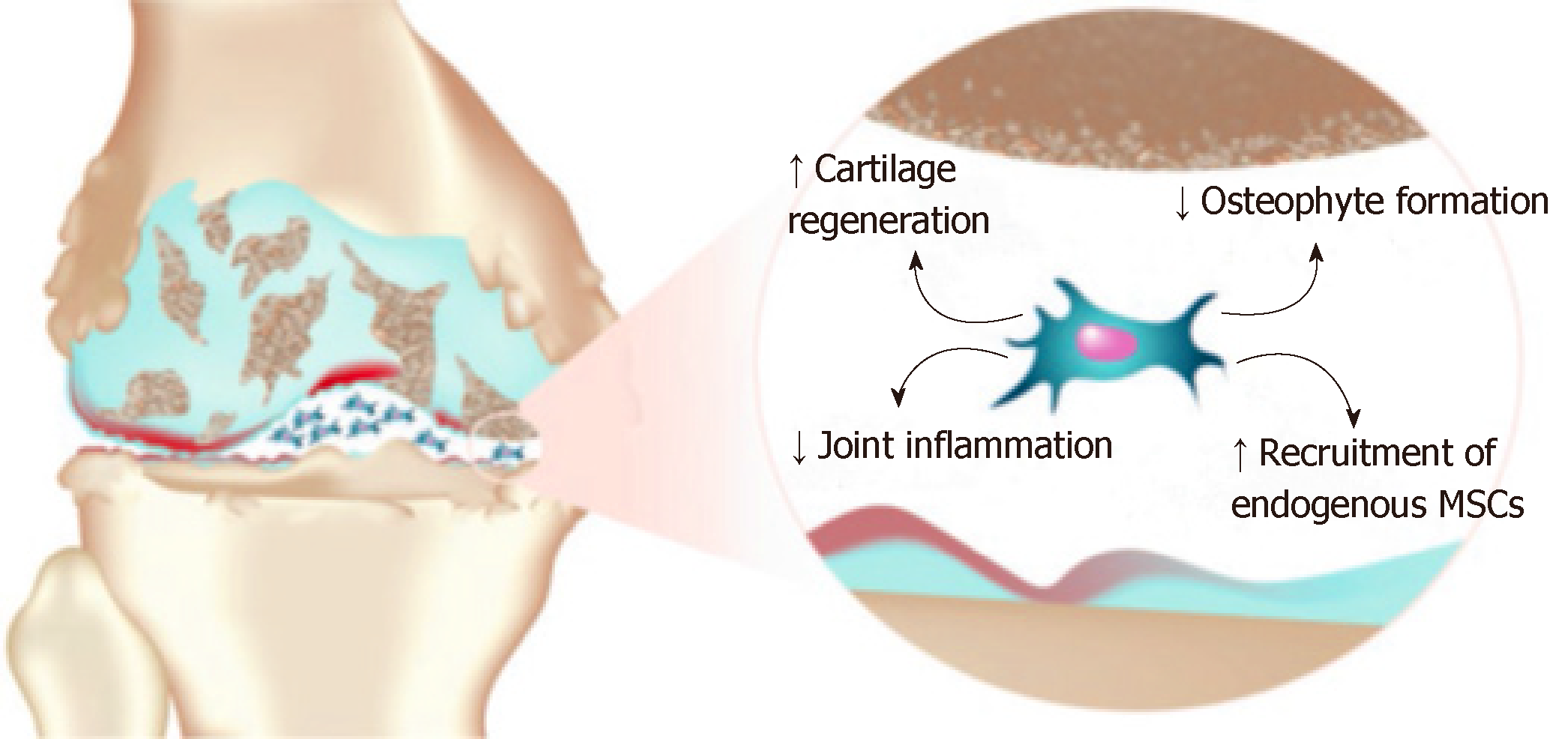
The supernatant from MSCs stimulated by INF-γ and IL-1β can increase the expression of arginine, IDO and nitric oxide synthase (iNOS) in macrophages, which lead to the transformation of macrophages from M1 to M2 types. MSCs also secrete an abundant of chemokines (SDF-1α, MCP-1 and MCP-2), which can attract monocytes, macrophages, lymphocytes and dendritic cells, etc, and then these cells are recruited to sites of injury and inflammation by chemotaxis, which participate in the repair of tissue injury[46]. A study reported that mature chondrocytes and the secretion of cytokines can promote the differentiation of MSCs into chondrocytes. In the meantime, cytokines secreted by MSCs can also promote the proliferation of chondrocytes and the synthesis of an ECM matrix, which can repair damaged bone and cartilage[47,48]. It has been reported that cytokines secreted by MSCs can target synovial membranes and chondrocytes, which can regulate anabolic and catabolic factors, as well as induce the expression of anti-inflammatory and chondrogenic molecules[49]. However, in recent years, most studies have suggested that MSCs primarily regulate local inflammation, apoptosis and proliferation of cells through paracrine mechanisms, rather than directly differentiating into chondrocytes to participate in tissue repair (Figure 1). Barry and Murphy thought that endogenous MSCs contribute to the maintenance of healthy tissues by acting as reservoirs for cell repair or as immunomodulatory sentinels to reduce inflammation, but also, paracrine signaling by MSCs might be more important than differentiation in stimulating repair responses[50]. In other words, MSCs are not specifically designed to replace damaged and lost cartilage, but rather coordinate and enhance this repair response.
Mixed injections means that MSCs are combined with growth factors, cytokines and scaffolds in order to improve efficacy. The commonly used support scaffolds are polymer scaffolds such as HA, fibrin gel, and nutrient-rich liquid such as serum platelet rich plasma (PRP). Among them, there are many studies on the treatment of OA by injecting MSCs/PRP suspensions into the articular cavity. Details of the case report of MSCs combined with PRP in the treatment of OA are shown in Table 2. It is generally known that PRP is an autologous tissue, rich in chondrogenic growth factors (e.g., TGF-β and platelet-derived growth factor). It can serve as a source of tissue for the treatment of damaged cartilage[51]. PRP composite scaffolds have high osteogenic induction activity, and are capable of promoting bone healing. The combination of PRP with MSCs (adipose MSCs: AD-MSCs/vascular stroma of adipose tissue: SVF) is used for treating knee OA, which can create a suitable microenvironment for MSC growth (promote the supplying of blood, reduce the responding of local inflammatory), promote the synthesis of cartilage matrix, and also improve the therapeutic effect of MSCs in knee arthritis[52-54]. The problem of PRP still lies in its preparation and the variability of the synthesis number of bioactive factors it expresses. Some growth factors secreted in PRP (e.g., vascular endothelial growth factor) may have adverse effects on both joints and MSCs[51,52].
| Defect type | MSC type | Delivery system | Type of study | Results | Ref. |
| OA | AD-MSC | MSCs/PRP | Case series (n = 21); Final follow-up: 6 mo | Significant positive changes at MRI | Bui et al[55], 2014 |
| OA | AD-MSC | MSCs/PRP | Case series (n = 18); Final follow-up: 24.3 mo | Clinical improvement; Function and pain improvement at 24.3 mo | Koh et al[52], 2013 |
| OA | AD-MSC | MSCs/PRP | Case series (n = 30); Final follow-up: 24 mo | Reducing pain and improving function in patients with knee OA | Koh et al[56], 2012 |
| OA | AD-MSC | MSCs/PRP | Case series (n = 21); Final follow-up: 24 mo | Function and pain improvement as compared with PRP only | Koh et al[57], 2014 |
| OA | Autologous SVF | SVF/PRP | Case series (n = 21); Final follow-up: 24 mo | All patients’ scores of pain improved to > 96; and quality of life scores to > 93 | Gibbs et al[58], 2015 |
| OA | Autologous SVF | SVF/PRP | Case series (n = 10); Final follow-up: 24 mo | Cartilage thickness improvement | Bansal et al[59], 2017 |
MSCs were first proposed to reside in bone marrow and have since been demonstrated to exist in other tissues (e.g., fat, placenta, umbilical cord, dental pulp, peripheral blood, and synovium)[60,61]. With the increase in evidence for the application of stem cell technology in animal and in vitro experiments, the application of MSC-based transplantation technology in the treatment of OA to achieve cartilage regeneration has shown promise. Thus far, clinical studies on mesenchymal stem cell therapy for OA have been conducted globally, and 74 of them have been registered on clinicaltrial.gov, some of which have completed clinical trials as well as preliminary evaluations of safety and efficacy. In China, research on the treatment of OA with MSCs is also in full swing. Currently, there are six studies registered on clinicaltrial.gov, taking up 8.1%, four of which (one UC-MSCs and three AD-MSCs) focus on the treatment of OA have been completed, and one study (UC-MSCs) is in the recruitment state. According to the results of the completed studies, mesenchymal stem cell (bone marrow, adipose and umbilical cord) therapy shows highly efficacy in the research of OA diseases, and has great potential to replace traditional therapies in the future. PubMed, Wiley, Elsevier ScienceDirect, Springer, Taylor and Francis were searched for the relevant studies published from 2015 to 2018. The search strategy included the keywords “mesenchymal stem cells”, “bone marrow-derived mesenchymal stem cells (BM-MSCs)”, “umbilical cord-derived mesenchymal stem cells (UC-MSCs)”, “adipose-derived mesenchymal stem cells (AD-MSCs)”, “stem cell therapy”, “osteoarthritis” and “clinical trial”. Inclusion criteria: (1) Clinical research journal articles or reviews were included; (2) The content of this study closely links to the application of MSC therapy in OA treatment; and (3) Select articles that have been recently published or published in an authoritative journal in the same field. Exclusion criteria: (1) Non-English literature in foreign languages; (2) Literature with repetitive content; and (3) Cannot get the full text of the document. In the end, 14 studies were included here, including eight on the clinical study of BM-MSCs in OA treatment (Table 3), three on the clinical study of UC-MSCs in OA treatment (Table 4), and three on the clinical study of AD-MSCs in OA treatment (Table 5).
| Cell type | Type of study | Experimental design | Cell dosage | Measurement | Results | Ref. |
| Autologous | Case series (n = 61); Final follow up: 6 mo | Phase I/II study | Not mentioned | VAS, WOMAC and X-ray | Significantly reductions in knee pain and increased quality of life at 6 mo follow-up | Garay-Mendoza et al[68], 2018 |
| Autologous | Case series (n = 13); Final follow up: 24 mo | Phase I/II study | Intra-articular injection of 30.5 × 106 MSCs | MRI and KOOS | After intra-articular injection with BM-MSCs had significantly improved the KOOS and knee cartilage thickness | Al-Najar et al[69], 2017 |
| Allogeneic | Case series (n = 60); Final follow up: 24 mo | Double-blind, multicentric, placebo-controlled, phase II study | Four dose levels were studied in this trial: 25 × 106, 50 × 106, 75 × 106, and 150 × 106 | VAS, ICOAP and WOMAC | A 25 × 106 cell dose may be the most effective among the doses; WOMAC, ICOAP, and VAS scores decreased by the time of the final follow-up period | Gupta et al[70], 2016 |
| Autologous | Case series (n = 30); Final follow up: 12 mo | Double-blind, multicentric, phase I/II study | Two dose levels were studied in this trial: 10 × 106 and 100 × 106 | VAS, WOMAC, X-ray and MRI | A clinical and functional improvement of knee OA by the injection of 100 × 106 cell dose; Improvement of pain and knee function of OA patients at 12 mo follow-up | Lamo-Espinosa et al[71], 2016 |
| Autologous | Case series (n = 4); Final follow up: 60 mo | Phase I study | Intra-articular injection of 8-9 × 106 MSCs | Walking time, X-ray and VAS | Earlier transplantation may give better results in long-term follow-up | Soler et al[72], 2016 |
| Allogeneic | Case series (n = 30); Final follow up: 12 mo | Multicentric, phase I/II study | Intra-articular injection of 40 × 106 MSCs | VAS, WOMAC, and LEQUESNE; MRI | Significantly improves cartilage quality and provides pain relief | Vega et al[73], 2015 |
| Autologous | Case series (n = 30); Final follow up: 30 mo | Not mentioned | Intra-articular injection of 0.5 × 106 MSCs | Walking distance, VAS, WOMAC and MRI | Significantly improves cartilage quality and knee function, and reduces pain level | Emadedin et al[74], 2015 |
| Autologous | Case series (n = 4); Final follow up: 60 mo | Phase I study,open label | Intra-articular injection of 8 × 106 MSCs | VAS, Knee motion, Range, X-ray | Earlier transplantation may give better results in long-term follow-up | Davatch et al[75], 2016 |
| Cell type | Type of study | Experimental design | Cell dosage | Measurement | Results | Ref. |
| Allogeneic | Case series (n = 7); Final follow-up: 60 mo | Open-label, single-arm, single-center, phase I/II study | A dose of 500 µL/cm2 of the defect area with a cell concentration of 0.5 × 107 MSCs per milliliter | ICRS, VAS, IKDC and MRI | Improvements in pain and knee function at 6 mo follow-up; Without significant deterioration over 7 yr of follow-up; Efficacy and safety | Park et al[66], 2017 |
| Allogeneic | Case series (n = 36); Final follow up: 12 mo | Not mentioned | Intra-articular injection of (2-3) × 107 MSCs | Lysholm, WOMAC and SF-36 scale score | Improvement of the joint function and quality of life | Wang et al[76], 2016 |
| Allogeneic | Case series (n = 40); Final follow up: 12 mo | randomized, triple-blind trial, phase I/II trial | Intra-articular injection of 20 × 106 (single-dose and repeated doses) MSCs | OARSI, WOMAC, VAS and SF-36 score | Efficacy and safety; Repeated injections of UC-MSCs had lower scores than others at 12 mo; Improvement of pain and knee function of OA patients at 12 mo follow-up | Matas et al[77], 2018 |
| Cell type | Type of study | Experimental design | Cell dosage | Measurement | Results | Ref. |
| Autologous | Case series (n = 18); Final follow-up: 24 mo | Randomized and Double-blinded, A phase I/II study | Three dose groups: The low-dose (1 × 107), mid-dose (2 × 107) and high-dose group (5 × 107) cells | WOMAC, SF-36 and NRS-11 | The dosage of 5 × 107 MSCs exhibited the highest improvement in pain, function and cartilage volume of the knee joint | Song et al[78], 2018 |
| Autologous | Case series (n = 18); Final follow-up: 24 mo | A phase I/II study | Phase I: 10 × 106 (low-dose), 50 × 106 (mid-dose), 100 × 106 (high-dose); Phase II:100 × 106 (high-dose) | VAS, WOMAC and MRI | A 100 × 106 cell dose may be the most effective among the doses | Jo et al[79], 2017 |
| Autologous | Case series (n = 18); Final follow-up: 20 mo | A phase I, bicentric, single-arm, open-label | Three dose levels were studied in this trial: 2 × 106 (low-dose), 10 × 106 (mid-dose) and 50 × 106 (high-dose) cells | WOMAC, VAS, SF-36, KOOS and OARSI | The group of patients injected with 2 × 106 cells exhibited the best response to MSC treatment, which can improve pain and induce structural benefit | Pers et al[80], 2016 |
Bone marrow is the most common and earliest effective source of MSCs for the treatment of OA diseases. BM-MSCs have achieved a promising effect in the clinical repair of knee articular cartilage using stem cell transplantation technology. In 2008, Centeno et al[62] reported a case of severe OA of the knee joint. Bone marrow MSCs in suspension culture with phosphate buffered saline were injected for treatment, and 10% platelet lysate (PL) and 10 ng dexamethasone injection were supplemented for cartilage stimulation. Six months after injection, MRIs showed the significant growth of articular cartilage and meniscus, ROM score increased and the pain score of modified VAS decreased. A single injection of BM-MSCs into the articular cavity without using adjuvant analgesics, anti-inflammatory drugs or immunosuppressants has also achieved positive results[62,63]. Studies have shown that BM-MSC tran-splantation is more effective than either autologous chondrocyte transplantation or no transplantation, with relatively fewer complications. Finally, though BM-MSCs have been extensively studied and its effectiveness and safety have been confirmed, further clinical application of BM-MSCs is limited by the fact that it is difficult to obtain sufficient numbers of primary generations due to factors such as trauma and differentiation ability affected by donor age. Intra-articular injection of AD-MSCs was also used in the treatment of OA. It is usually obtained by liposuction or is subpatellar fat pad-derived, and then the liposome is centrifuged and digested by collagenase I to prepare concentrated AD-MSCs[52,57,64]. It has been reported that intra-articular injection of 1.0 × 108 AD-MSCs can significantly improve knee joint pain (P < 0.001) and function (P < 0.001) without adverse events. Patients in the medium dose group (5.0 × 107) showed some improvement in clinical results, while those in the low dose group (1.0 × 107) showed no improvement in most outcome indicators[45]. These results suggest that intra-articular injection of MSCs has a significant dose-response effect, and that further large-scale trials are needed to confirm the long-term safety and clinical advantages of high-dose injection. However, comparative studies have shown that AD-MSCs have lower chondrogenic potential, lower cartilage specificity of matrix protein production, and low expression rate of the collagen type I gene as compared with BM-MSCs. Thus, scholars should work to further optimize the chondrogenic potential of AD-MSCs[65]. Umbilical cord-derived MSCs (UC-MSCs) are a type of pluripotent stem cell existing in neonatal umbilical cord tissues, which can be obtained from discarded umbilical cord or umbilical cord blood banks. At present, clinical trials have shown that injecting human umbilical cord-derived MSCs into the joint cavity for the treatment of degenerative knee OA can significantly improve the joint function and quality of life of patients[66]. In January 2012, the Korean Food and Drug Administration approved the manufacture and sale of Cartisem in South Korea as a safe and effective stem cell drug (containing UC-MSCs and sodium hyaluronate) for treating degenerative OA and cartilage injury. Since it was listed in South Korea in 2012, more than 5,000 patients have been treated at an effective rate of 97.67%, and the treatment effect is not limited by the age of the patients. More importantly, Cartistem uses allogeneic stem cells rather than autologous stem cells, and has become the world’s first user of allogeneic stem cells to produce therapeutic drugs. Cartistem utilizes umbilical cords to isolate and cultivate UC-MSCs that meet the needs of clinical treatment, and they are implanted into damaged cartilage. In the microenvironment of the implanted location, UC-MSCs coordinate and enhance the repair response of damaged cartilage tissue by a paracrine mechanism, thereby creating a new avenue for the treatment of OA. UC-MSCs are a little backwards compared with other MSCs because of their unique properties, whereas they are expected to be widely used in clinical practice and will make an important contribution to the repair of damaged cartilage, which will be the focus of future research.
Although the initial efficacy of intra-articular MSC injections in patients with severe knee OA deserves to be confirmed, prospective and placebo-controlled studies are still needed to verify the effectiveness of this method. New clinical trials should focus on the efficacy of MSC injections in patients with moderate OA and early radiology. Koh et al[67] showed that the effects of MSC implantation in level 3 OA patients were better than those in level 4 OA patients. Accordingly, MSC-based therapies should be more effective in preventing or limiting the progression of early stages of OA disease. Another important question is the optimal dose of the experimental cells. Cell dosages range from 2 × 106 to 3 × 108, with significant differences between clinical trials. However, the dose described by different researchers for the improvement of pain function and histological scores is also different, so there is still no clinical criteria for guiding treatment.
As early as 2005, Rubio et al[81] transplanted AD-MSCs into immunodeficient mice, and the results suggested that spontaneous stem cell transformations and malignant tumors occurred in mice. Later, several studies revealed that this malignant transformation is due to cell line contamination, and is therefore not correlated with MSCs themselves. Thus, this study was withdrawn[81,82]. In recent years, numerous animal studies have reported that intra-articular injection of MSCs can promote cartilage regeneration and reduce joint inflammation to improve the OA function of joints, and no malignant transformation of MSCs has been found. A total of 14 studies reported intra-articular injection of MSCs for the treatment of OA in clinical trials from 2015 to 2018. In general, whether intra-articular injection of autogenous and allogeneic MSCs (bone marrow, adipose and umbilical cord) were used, the clinical manifestations, radiological and histological scores of OA patients were improved, no graft-related death, tumorigenesis and infection occurred, and no serious adverse reactions were observed. However, there are still some problems with the intra-articular injection of MSCs for the treatment of OA in clinical trials: (1) It has been reported that MSCs could promote cartilage repair via the secretion/stimulation of biomolecules, and if these results are true, the duration of stimulation and whether the biomolecules secreted by MSCs can be characterized as drugs and used accumulatively should be considered; (2) How to improve the effectiveness of MSCs in the OA microenvironment. Also, the transfer of cells from in vitro atmospheric culture conditions to the in vivo niche may affect the survival rate of MSCs after transplantation; (3) How to accurately assess the progress of OA repair. There are many different clinical scoring systems that have been widely used until now, but the popularity of scoring systems and the debate over their relative merits suggest that they do not accurately assess the progression of OA disease; (4) How to eliminate the blindness of clinical research. While MSCs are usually packaged into syringes, there is a tendency for cells to aggregate and become fuzzy at the bottom of the syringe, which may affect the results of blind clinical trials compared with transparent placebos; and (5) Transport problem: how can cells be effectively transported from the laboratory to OA patients without losing their efficacy and quantity.
Since analgesics and anti-inflammatory drugs often cause gastrointestinal, liver, kidney and heart problems, many common side effects arise from current arthritis treatments, which may cause significant injury to the patient. Also, ACI surgery may cause morbidity in the donor site, and requires two operations under general anesthesia. With the advancement of research on the characteristics, pre-clinical and clinical applications of MSCs, regenerative medicine based on stem cell therapy has gradually presented its advantages in the treatment of OA disease. Previous studies have injected bone marrow-, umbilical cord- and adipose-derived MSCs into the joint cavity using the ultrasound detection technique. This study summarizes the contents of preclinical and clinical trials in the recent three years as follows: intra-articular injection of MSCs can lead to the reduction of index-pain, improve the function and significantly increase the volume of cartilage.
Despite many researchers’ initial worries about mesenchymal stem cell therapy, a systematic review of clinical trials has suggested that MSCs are relatively safe for both intravascular and intra-articular injection. It is noteworthy that umbilical cord MSCs can serve as allogeneic stem cell drugs, which can replace damaged tissue in the microenvironment of the implanted site, which creates a new approach for OA treatment. Finally, although these initial studies show promising therapeutic effects, their long-term therapeutic effects need further investigation. Furthermore, more reliable studies with larger sample sizes and randomized controls are also required for higher levels of evidence, and to comprehensively standardize and optimize MSC therapy in the treatment of OA diseases.
This work was supported by Cell products of National Engineering Research Center and National Stem Cell Engineering Research Center.
Manuscript source: Invited manuscript
Specialty type: Cell and tissue engineering
Country of origin: China
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Chivu-Economescu M, Liu SH, Maraldi T, Pixley JS, Scarfì S S-Editor: Ji FF L-Editor: Filipodia E-Editor: Wu YXJ
| 1. | Redler LH, Caldwell JM, Schulz BM, Levine WN. Management of articular cartilage defects of the knee. Phys Sportsmed. 2012;40:20-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 2. | Murray CJ, Vos T, Lozano R, Naghavi M, Flaxman AD, Michaud C, Ezzati M, Shibuya K, Salomon JA, Abdalla S, Aboyans V, Abraham J, Ackerman I, Aggarwal R, Ahn SY, Ali MK, Alvarado M, Anderson HR, Anderson LM, Andrews KG, Atkinson C, Baddour LM, Bahalim AN, Barker-Collo S, Barrero LH, Bartels DH, Basáñez MG, Baxter A, Bell ML, Benjamin EJ, Bennett D, Bernabé E, Bhalla K, Bhandari B, Bikbov B, Bin Abdulhak A, Birbeck G, Black JA, Blencowe H, Blore JD, Blyth F, Bolliger I, Bonaventure A, Boufous S, Bourne R, Boussinesq M, Braithwaite T, Brayne C, Bridgett L, Brooker S, Brooks P, Brugha TS, Bryan-Hancock C, Bucello C, Buchbinder R, Buckle G, Budke CM, Burch M, Burney P, Burstein R, Calabria B, Campbell B, Canter CE, Carabin H, Carapetis J, Carmona L, Cella C, Charlson F, Chen H, Cheng AT, Chou D, Chugh SS, Coffeng LE, Colan SD, Colquhoun S, Colson KE, Condon J, Connor MD, Cooper LT, Corriere M, Cortinovis M, de Vaccaro KC, Couser W, Cowie BC, Criqui MH, Cross M, Dabhadkar KC, Dahiya M, Dahodwala N, Damsere-Derry J, Danaei G, Davis A, De Leo D, Degenhardt L, Dellavalle R, Delossantos A, Denenberg J, Derrett S, Des Jarlais DC, Dharmaratne SD, Dherani M, Diaz-Torne C, Dolk H, Dorsey ER, Driscoll T, Duber H, Ebel B, Edmond K, Elbaz A, Ali SE, Erskine H, Erwin PJ, Espindola P, Ewoigbokhan SE, Farzadfar F, Feigin V, Felson DT, Ferrari A, Ferri CP, Fèvre EM, Finucane MM, Flaxman S, Flood L, Foreman K, Forouzanfar MH, Fowkes FG, Fransen M, Freeman MK, Gabbe BJ, Gabriel SE, Gakidou E, Ganatra HA, Garcia B, Gaspari F, Gillum RF, Gmel G, Gonzalez-Medina D, Gosselin R, Grainger R, Grant B, Groeger J, Guillemin F, Gunnell D, Gupta R, Haagsma J, Hagan H, Halasa YA, Hall W, Haring D, Haro JM, Harrison JE, Havmoeller R, Hay RJ, Higashi H, Hill C, Hoen B, Hoffman H, Hotez PJ, Hoy D, Huang JJ, Ibeanusi SE, Jacobsen KH, James SL, Jarvis D, Jasrasaria R, Jayaraman S, Johns N, Jonas JB, Karthikeyan G, Kassebaum N, Kawakami N, Keren A, Khoo JP, King CH, Knowlton LM, Kobusingye O, Koranteng A, Krishnamurthi R, Laden F, Lalloo R, Laslett LL, Lathlean T, Leasher JL, Lee YY, Leigh J, Levinson D, Lim SS, Limb E, Lin JK, Lipnick M, Lipshultz SE, Liu W, Loane M, Ohno SL, Lyons R, Mabweijano J, MacIntyre MF, Malekzadeh R, Mallinger L, Manivannan S, Marcenes W, March L, Margolis DJ, Marks GB, Marks R, Matsumori A, Matzopoulos R, Mayosi BM, McAnulty JH, McDermott MM, McGill N, McGrath J, Medina-Mora ME, Meltzer M, Mensah GA, Merriman TR, Meyer AC, Miglioli V, Miller M, Miller TR, Mitchell PB, Mock C, Mocumbi AO, Moffitt TE, Mokdad AA, Monasta L, Montico M, Moradi-Lakeh M, Moran A, Morawska L, Mori R, Murdoch ME, Mwaniki MK, Naidoo K, Nair MN, Naldi L, Narayan KM, Nelson PK, Nelson RG, Nevitt MC, Newton CR, Nolte S, Norman P, Norman R, O'Donnell M, O'Hanlon S, Olives C, Omer SB, Ortblad K, Osborne R, Ozgediz D, Page A, Pahari B, Pandian JD, Rivero AP, Patten SB, Pearce N, Padilla RP, Perez-Ruiz F, Perico N, Pesudovs K, Phillips D, Phillips MR, Pierce K, Pion S, Polanczyk GV, Polinder S, Pope CA, Popova S, Porrini E, Pourmalek F, Prince M, Pullan RL, Ramaiah KD, Ranganathan D, Razavi H, Regan M, Rehm JT, Rein DB, Remuzzi G, Richardson K, Rivara FP, Roberts T, Robinson C, De Leòn FR, Ronfani L, Room R, Rosenfeld LC, Rushton L, Sacco RL, Saha S, Sampson U, Sanchez-Riera L, Sanman E, Schwebel DC, Scott JG, Segui-Gomez M, Shahraz S, Shepard DS, Shin H, Shivakoti R, Singh D, Singh GM, Singh JA, Singleton J, Sleet DA, Sliwa K, Smith E, Smith JL, Stapelberg NJ, Steer A, Steiner T, Stolk WA, Stovner LJ, Sudfeld C, Syed S, Tamburlini G, Tavakkoli M, Taylor HR, Taylor JA, Taylor WJ, Thomas B, Thomson WM, Thurston GD, Tleyjeh IM, Tonelli M, Towbin JA, Truelsen T, Tsilimbaris MK, Ubeda C, Undurraga EA, van der Werf MJ, van Os J, Vavilala MS, Venketasubramanian N, Wang M, Wang W, Watt K, Weatherall DJ, Weinstock MA, Weintraub R, Weisskopf MG, Weissman MM, White RA, Whiteford H, Wiebe N, Wiersma ST, Wilkinson JD, Williams HC, Williams SR, Witt E, Wolfe F, Woolf AD, Wulf S, Yeh PH, Zaidi AK, Zheng ZJ, Zonies D, Lopez AD, AlMazroa MA, Memish ZA. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2197-2223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5870] [Cited by in RCA: 6064] [Article Influence: 466.5] [Reference Citation Analysis (0)] |
| 3. | Glyn-Jones S, Palmer AJ, Agricola R, Price AJ, Vincent TL, Weinans H, Carr AJ. Osteoarthritis. Lancet. 2015;386:376-387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1429] [Cited by in RCA: 1990] [Article Influence: 199.0] [Reference Citation Analysis (0)] |
| 4. | Makris EA, Gomoll AH, Malizos KN, Hu JC, Athanasiou KA. Repair and tissue engineering techniques for articular cartilage. Nat Rev Rheumatol. 2015;11:21-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 893] [Cited by in RCA: 879] [Article Influence: 87.9] [Reference Citation Analysis (0)] |
| 5. | Anandacoomarasamy A, March L. Current evidence for osteoarthritis treatments. Ther Adv Musculoskelet Dis. 2010;2:17-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 52] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 6. | Lubis AM, Lubis VK. Adult bone marrow stem cells in cartilage therapy. Acta Med Indones. 2012;44:62-68. [PubMed] |
| 7. | Steinert AF, Ghivizzani SC, Rethwilm A, Tuan RS, Evans CH, Nöth U. Major biological obstacles for persistent cell-based regeneration of articular cartilage. Arthritis Res Ther. 2007;9:213. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 243] [Cited by in RCA: 238] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 8. | Mithoefer K, McAdams T, Williams RJ, Kreuz PC, Mandelbaum BR. Clinical efficacy of the microfracture technique for articular cartilage repair in the knee: an evidence-based systematic analysis. Am J Sports Med. 2009;37:2053-2063. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 708] [Cited by in RCA: 698] [Article Influence: 43.6] [Reference Citation Analysis (0)] |
| 9. | Chen FH, Tuan RS. Mesenchymal stem cells in arthritic diseases. Arthritis Res Ther. 2008;10:223. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 223] [Cited by in RCA: 213] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 10. | Lazarus HM, Haynesworth SE, Gerson SL, Rosenthal NS, Caplan AI. Ex vivo expansion and subsequent infusion of human bone marrow-derived stromal progenitor cells (mesenchymal progenitor cells): implications for therapeutic use. Bone Marrow Transplant. 1995;16:557-564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 11. | Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop Dj, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11055] [Cited by in RCA: 12689] [Article Influence: 704.9] [Reference Citation Analysis (2)] |
| 12. | Maumus M, Jorgensen C, Noël D. Mesenchymal stem cells in regenerative medicine applied to rheumatic diseases: role of secretome and exosomes. Biochimie. 2013;95:2229-2234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 191] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 13. | Glenn JD, Whartenby KA. Mesenchymal stem cells: Emerging mechanisms of immunomodulation and therapy. World J Stem Cells. 2014;6:526-539. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 293] [Cited by in RCA: 302] [Article Influence: 27.5] [Reference Citation Analysis (1)] |
| 14. | Krampera M, Galipeau J, Shi Y, Tarte K, Sensebe L; MSC Committee of the International Society for Cellular Therapy (ISCT). Immunological characterization of multipotent mesenchymal stromal cells--The International Society for Cellular Therapy (ISCT) working proposal. Cytotherapy. 2013;15:1054-1061. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 297] [Cited by in RCA: 342] [Article Influence: 28.5] [Reference Citation Analysis (0)] |
| 15. | Fong EL, Chan CK, Goodman SB. Stem cell homing in musculoskeletal injury. Biomaterials. 2011;32:395-409. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 165] [Cited by in RCA: 148] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 16. | Kuyinu EL, Narayanan G, Nair LS, Laurencin CT. Animal models of osteoarthritis: classification, update, and measurement of outcomes. J Orthop Surg Res. 2016;11:19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 251] [Cited by in RCA: 381] [Article Influence: 42.3] [Reference Citation Analysis (0)] |
| 17. | Kim JE, Song DH, Kim SH, Jung Y, Kim SJ. Development and characterization of various osteoarthritis models for tissue engineering. PLoS One. 2018;13:e0194288. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 61] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 18. | Demoor M, Ollitrault D, Gomez-Leduc T, Bouyoucef M, Hervieu M, Fabre H, Lafont J, Denoix JM, Audigié F, Mallein-Gerin F, Legendre F, Galera P. Cartilage tissue engineering: molecular control of chondrocyte differentiation for proper cartilage matrix reconstruction. Biochim Biophys Acta. 2014;1840:2414-2440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 185] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 19. | Li Q, Tang J, Wang R, Bei C, Xin L, Zeng Y, Tang X. Comparing the chondrogenic potential in vivo of autogeneic mesenchymal stem cells derived from different tissues. Artif Cells Blood Substit Immobil Biotechnol. 2011;39:31-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 37] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 20. | Barron V, Merghani K, Shaw G, Coleman CM, Hayes JS, Ansboro S, Manian A, O'Malley G, Connolly E, Nandakumar A, van Blitterswijk CA, Habibovic P, Moroni L, Shannon F, Murphy JM, Barry F. Evaluation of Cartilage Repair by Mesenchymal Stem Cells Seeded on a PEOT/PBT Scaffold in an Osteochondral Defect. Ann Biomed Eng. 2015;43:2069-2082. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 21. | Qi BW, Yu AX, Zhu SB, Zhou M, Wu G. Chitosan/poly(vinyl alcohol) hydrogel combined with Ad-hTGF-β1 transfected mesenchymal stem cells to repair rabbit articular cartilage defects. Exp Biol Med (Maywood). 2013;238:23-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 22. | Tribe HC, McEwan J, Taylor H, Oreffo ROC, Tare RS. Mesenchymal Stem Cells: Potential Role in the Treatment of Osteochondral Lesions of the Ankle. Biotechnol J. 2017;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 23. | Toh WS, Lai RC, Hui JHP, Lim SK. MSC exosome as a cell-free MSC therapy for cartilage regeneration: Implications for osteoarthritis treatment. Semin Cell Dev Biol. 2017;67:56-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 364] [Article Influence: 40.4] [Reference Citation Analysis (0)] |
| 24. | Lai RC, Yeo RW, Lim SK. Mesenchymal stem cell exosomes. Semin Cell Dev Biol. 2015;40:82-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 301] [Cited by in RCA: 420] [Article Influence: 42.0] [Reference Citation Analysis (0)] |
| 25. | Chen TS, Lai RC, Lee MM, Choo AB, Lee CN, Lim SK. Mesenchymal stem cell secretes microparticles enriched in pre-microRNAs. Nucleic Acids Res. 2010;38:215-224. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 450] [Cited by in RCA: 509] [Article Influence: 31.8] [Reference Citation Analysis (0)] |
| 26. | Yu XM, Meng HY, Yuan XL, Wang Y, Guo QY, Peng J, Wang AY, Lu SB. MicroRNAs' Involvement in Osteoarthritis and the Prospects for Treatments. Evid Based Complement Alternat Med. 2015;2015:236179. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 46] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 27. | Miyaki S, Sato T, Inoue A, Otsuki S, Ito Y, Yokoyama S, Kato Y, Takemoto F, Nakasa T, Yamashita S, Takada S, Lotz MK, Ueno-Kudo H, Asahara H. MicroRNA-140 plays dual roles in both cartilage development and homeostasis. Genes Dev. 2010;24:1173-1185. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 492] [Cited by in RCA: 474] [Article Influence: 31.6] [Reference Citation Analysis (0)] |
| 28. | Karlsen TA, Jakobsen RB, Mikkelsen TS, Brinchmann JE. microRNA-140 targets RALA and regulates chondrogenic differentiation of human mesenchymal stem cells by translational enhancement of SOX9 and ACAN. Stem Cells Dev. 2014;23:290-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 104] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 29. | Liang Y, Duan L, Xiong J, Zhu W, Liu Q, Wang D, Liu W, Li Z, Wang D. E2 regulates MMP-13 via targeting miR-140 in IL-1β-induced extracellular matrix degradation in human chondrocytes. Arthritis Res Ther. 2016;18:105. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 75] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 30. | Tao SC, Yuan T, Zhang YL, Yin WJ, Guo SC, Zhang CQ. Exosomes derived from miR-140-5p-overexpressing human synovial mesenchymal stem cells enhance cartilage tissue regeneration and prevent osteoarthritis of the knee in a rat model. Theranostics. 2017;7:180-195. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 380] [Cited by in RCA: 550] [Article Influence: 68.8] [Reference Citation Analysis (0)] |
| 31. | Lai RC, Chen TS, Lim SK. Mesenchymal stem cell exosome: a novel stem cell-based therapy for cardiovascular disease. Regen Med. 2011;6:481-492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 393] [Cited by in RCA: 423] [Article Influence: 30.2] [Reference Citation Analysis (0)] |
| 32. | Zhou J, Wang Y, Liu Y, Zeng H, Xu H, Lian F. Adipose derived mesenchymal stem cells alleviated osteoarthritis and chondrocyte apoptosis through autophagy inducing. J Cell Biochem. 2018;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 39] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 33. | Toghraie F, Razmkhah M, Gholipour MA, Faghih Z, Chenari N, Torabi Nezhad S, Nazhvani Dehghani S, Ghaderi A. Scaffold-free adipose-derived stem cells (ASCs) improve experimentally induced osteoarthritis in rabbits. Arch Iran Med. 2012;15:495-499. [PubMed] |
| 34. | Lv X, He J, Zhang X, Luo X, He N, Sun Z, Xia H, Liu V, Zhang L, Lin X, Lin L, Yin H, Jiang D, Cao W, Wang R, Zhou G, Wang W. Comparative Efficacy of Autologous Stromal Vascular Fraction and Autologous Adipose-Derived Mesenchymal Stem Cells Combined With Hyaluronic Acid for the Treatment of Sheep Osteoarthritis. Cell Transplant. 2018;27:1111-1125. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 45] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 35. | Feng C, Luo X, He N, Xia H, Lv X, Zhang X, Li D, Wang F, He J, Zhang L, Lin X, Lin L, Yin H, He J, Wang J, Cao W, Wang R, Zhou G, Wang W. Efficacy and Persistence of Allogeneic Adipose-Derived Mesenchymal Stem Cells Combined with Hyaluronic Acid in Osteoarthritis After Intra-articular Injection in a Sheep Model. Tissue Eng Part A. 2018;24:219-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 57] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 36. | Desando G, Bartolotti I, Cavallo C, Schiavinato A, Secchieri C, Kon E, Filardo G, Paro M, Grigolo B. Short-Term Homing of Hyaluronan-Primed Cells: Therapeutic Implications for Osteoarthritis Treatment. Tissue Eng Part C Methods. 2018;24:121-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 37. | Chiang ER, Ma HL, Wang JP, Liu CL, Chen TH, Hung SC. Allogeneic Mesenchymal Stem Cells in Combination with Hyaluronic Acid for the Treatment of Osteoarthritis in Rabbits. PLoS One. 2016;11:e0149835. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 88] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 38. | Yun S, Ku SK, Kwon YS. Adipose-derived mesenchymal stem cells and platelet-rich plasma synergistically ameliorate the surgical-induced osteoarthritis in Beagle dogs. J Orthop Surg Res. 2016;11:9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 98] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 39. | Hermeto LC, DeRossi R, Oliveira RJ, Pesarini JR, Antoniolli-Silva AC, Jardim PH, Santana AE, Deffune E, Rinaldi JC, Justulin LA. Effects of intra-articular injection of mesenchymal stem cells associated with platelet-rich plasma in a rabbit model of osteoarthritis. Genet Mol Res. 2016;15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 40. | Kuroda K, Kabata T, Hayashi K, Maeda T, Kajino Y, Iwai S, Fujita K, Hasegawa K, Inoue D, Sugimoto N, Tsuchiya H. The paracrine effect of adipose-derived stem cells inhibits osteoarthritis progression. BMC Musculoskelet Disord. 2015;16:236. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 72] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 41. | Duffy MM, Pindjakova J, Hanley SA, McCarthy C, Weidhofer GA, Sweeney EM, English K, Shaw G, Murphy JM, Barry FP, Mahon BP, Belton O, Ceredig R, Griffin MD. Mesenchymal stem cell inhibition of T-helper 17 cell- differentiation is triggered by cell-cell contact and mediated by prostaglandin E2 via the EP4 receptor. Eur J Immunol. 2011;41:2840-2851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 174] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 42. | Pers YM, Ruiz M, Noël D, Jorgensen C. Mesenchymal stem cells for the management of inflammation in osteoarthritis: state of the art and perspectives. Osteoarthritis Cartilage. 2015;23:2027-2035. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 144] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 43. | Wang S, Zhu R, Li H, Li J, Han Q, Zhao RC. Mesenchymal stem cells and immune disorders: from basic science to clinical transition. Front Med. 2018;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 37] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 44. | Kim J, Hematti P. Mesenchymal stem cell-educated macrophages: a novel type of alternatively activated macrophages. Exp Hematol. 2009;37:1445-1453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 628] [Cited by in RCA: 607] [Article Influence: 37.9] [Reference Citation Analysis (0)] |
| 45. | Jo CH, Lee YG, Shin WH, Kim H, Chai JW, Jeong EC, Kim JE, Shim H, Shin JS, Shin IS, Ra JC, Oh S, Yoon KS. Intra-articular injection of mesenchymal stem cells for the treatment of osteoarthritis of the knee: A proof-of-concept clinical trial. Stem Cells. 2014;32:1254-1266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 566] [Cited by in RCA: 645] [Article Influence: 58.6] [Reference Citation Analysis (0)] |
| 46. | Samsonraj RM, Rai B, Sathiyanathan P, Puan KJ, Rötzschke O, Hui JH, Raghunath M, Stanton LW, Nurcombe V, Cool SM. Establishing criteria for human mesenchymal stem cell potency. Stem Cells. 2015;33:1878-1891. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 137] [Cited by in RCA: 163] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 47. | Mamidi MK, Das AK, Zakaria Z, Bhonde R. Mesenchymal stromal cells for cartilage repair in osteoarthritis. Osteoarthritis Cartilage. 2016;24:1307-1316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 87] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 48. | Kolf CM, Cho E, Tuan RS. Mesenchymal stromal cells. Biology of adult mesenchymal stem cells: regulation of niche, self-renewal and differentiation. Arthritis Res Ther. 2007;9:204. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 728] [Cited by in RCA: 664] [Article Influence: 36.9] [Reference Citation Analysis (0)] |
| 49. | Im GI. Tissue Engineering in Osteoarthritis: Current Status and Prospect of Mesenchymal Stem Cell Therapy. BioDrugs. 2018;32:183-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 43] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 50. | Barry F, Murphy M. Mesenchymal stem cells in joint disease and repair. Nat Rev Rheumatol. 2013;9:584-594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 269] [Cited by in RCA: 299] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 51. | Freitag J, Bates D, Boyd R, Shah K, Barnard A, Huguenin L, Tenen A. Mesenchymal stem cell therapy in the treatment of osteoarthritis: reparative pathways, safety and efficacy - a review. BMC Musculoskelet Disord. 2016;17:230. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 187] [Cited by in RCA: 195] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 52. | Koh YG, Jo SB, Kwon OR, Suh DS, Lee SW, Park SH, Choi YJ. Mesenchymal stem cell injections improve symptoms of knee osteoarthritis. Arthroscopy. 2013;29:748-755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 257] [Cited by in RCA: 258] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 53. | Pintat J, Silvestre A, Magalon G, Gadeau AP, Pesquer L, Perozziello A, Peuchant A, Mounayer C, Dallaudière B. Intra-articular Injection of Mesenchymal Stem Cells and Platelet-Rich Plasma to Treat Patellofemoral Osteoarthritis: Preliminary Results of a Long-Term Pilot Study. J Vasc Interv Radiol. 2017;28:1708-1713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 39] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 54. | Xie X, Wang Y, Zhao C, Guo S, Liu S, Jia W, Tuan RS, Zhang C. Comparative evaluation of MSCs from bone marrow and adipose tissue seeded in PRP-derived scaffold for cartilage regeneration. Biomaterials. 2012;33:7008-7018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 226] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 55. | Bui HT, Duong TD, Nguyen NT, Le VT, Mai VT, Phan NL, Dũng LM, Phan NK, Pham PV. Symptomatic knee osteoarthritis treatment using autologous adipose derived stem cells and platelet-rich plasma: A clinical study. Biomed Res Ther. 2014;1:2-8. [RCA] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 35] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 56. | Koh YG, Choi YJ. Infrapatellar fat pad-derived mesenchymal stem cell therapy for knee osteoarthritis. Knee. 2012;19:902-907. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 285] [Cited by in RCA: 300] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 57. | Koh YG, Kwon OR, Kim YS, Choi YJ. Comparative outcomes of open-wedge high tibial osteotomy with platelet-rich plasma alone or in combination with mesenchymal stem cell treatment: a prospective study. Arthroscopy. 2014;30:1453-1460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 161] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 58. | Gibbs N, Diamond R, Sekyere EO, Thomas WD. Management of knee osteoarthritis by combined stromal vascular fraction cell therapy, platelet-rich plasma, and musculoskeletal exercises: a case series. J Pain Res. 2015;8:799-806. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 53] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 59. | Bansal H, Comella K, Leon J, Verma P, Agrawal D, Koka P, Ichim T. Intra-articular injection in the knee of adipose derived stromal cells (stromal vascular fraction) and platelet rich plasma for osteoarthritis. J Transl Med. 2017;15:141. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 90] [Cited by in RCA: 87] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 60. | Kristjánsson B, Honsawek S. Current perspectives in mesenchymal stem cell therapies for osteoarthritis. Stem Cells Int. 2014;2014:194318. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 62] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 61. | de Windt TS, Vonk LA, Slaper-Cortenbach IC, van den Broek MP, Nizak R, van Rijen MH, de Weger RA, Dhert WJ, Saris DB. Allogeneic Mesenchymal Stem Cells Stimulate Cartilage Regeneration and Are Safe for Single-Stage Cartilage Repair in Humans upon Mixture with Recycled Autologous Chondrons. Stem Cells. 2017;35:256-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 166] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 62. | Centeno CJ, Busse D, Kisiday J, Keohan C, Freeman M, Karli D. Increased knee cartilage volume in degenerative joint disease using percutaneously implanted, autologous mesenchymal stem cells. Pain Physician. 2008;11:343-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 63. | Davatchi F, Abdollahi BS, Mohyeddin M, Shahram F, Nikbin B. Mesenchymal stem cell therapy for knee osteoarthritis. Preliminary report of four patients. Int J Rheum Dis. 2011;14:211-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 269] [Cited by in RCA: 277] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 64. | Emadedin M, Aghdami N, Taghiyar L, Fazeli R, Moghadasali R, Jahangir S, Farjad R, Baghaban Eslaminejad M. Intra-articular injection of autologous mesenchymal stem cells in six patients with knee osteoarthritis. Arch Iran Med. 2012;15:422-428. [PubMed] |
| 65. | Jayaram P, Ikpeama U, Rothenberg JB, Malanga GA. Bone Marrow-Derived and Adipose-Derived Mesenchymal Stem Cell Therapy in Primary Knee Osteoarthritis: A Narrative Review. PM R. 2018;pii:S1934-1482(18)30377-0. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 43] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 66. | Park YB, Ha CW, Lee CH, Yoon YC, Park YG. Cartilage Regeneration in Osteoarthritic Patients by a Composite of Allogeneic Umbilical Cord Blood-Derived Mesenchymal Stem Cells and Hyaluronate Hydrogel: Results from a Clinical Trial for Safety and Proof-of-Concept with 7 Years of Extended Follow-Up. Stem Cells Transl Med. 2017;6:613-621. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 203] [Cited by in RCA: 292] [Article Influence: 32.4] [Reference Citation Analysis (0)] |
| 67. | Kim YS, Kwon OR, Choi YJ, Suh DS, Heo DB, Koh YG. Comparative Matched-Pair Analysis of the Injection Versus Implantation of Mesenchymal Stem Cells for Knee Osteoarthritis. Am J Sports Med. 2015;43:2738-2746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 84] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 68. | Garay-Mendoza D, Villarreal-Martínez L, Garza-Bedolla A, Pérez-Garza DM, Acosta-Olivo C, Vilchez-Cavazos F, Diaz-Hutchinson C, Gómez-Almaguer D, Jaime-Pérez JC, Mancías-Guerra C. The effect of intra-articular injection of autologous bone marrow stem cells on pain and knee function in patients with osteoarthritis. Int J Rheum Dis. 2018;21:140-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 69] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 69. | Al-Najar M, Khalil H, Al-Ajlouni J, Al-Antary E, Hamdan M, Rahmeh R, Alhattab D, Samara O, Yasin M, Abdullah AA, Al-Jabbari E, Hmaid D, Jafar H, Awidi A. Intra-articular injection of expanded autologous bone marrow mesenchymal cells in moderate and severe knee osteoarthritis is safe: a phase I/II study. J Orthop Surg Res. 2017;12:190. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 101] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 70. | Gupta PK, Chullikana A, Rengasamy M, Shetty N, Pandey V, Agarwal V, Wagh SY, Vellotare PK, Damodaran D, Viswanathan P, Thej C, Balasubramanian S, Majumdar AS. Efficacy and safety of adult human bone marrow-derived, cultured, pooled, allogeneic mesenchymal stromal cells (Stempeucel®): preclinical and clinical trial in osteoarthritis of the knee joint. Arthritis Res Ther. 2016;18:301. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 256] [Cited by in RCA: 242] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 71. | Lamo-Espinosa JM, Mora G, Blanco JF, Granero-Moltó F, Nuñez-Córdoba JM, Sánchez-Echenique C, Bondía JM, Aquerreta JD, Andreu EJ, Ornilla E, Villarón EM, Valentí-Azcárate A, Sánchez-Guijo F, Del Cañizo MC, Valentí-Nin JR, Prósper F. Intra-articular injection of two different doses of autologous bone marrow mesenchymal stem cells versus hyaluronic acid in the treatment of knee osteoarthritis: multicenter randomized controlled clinical trial (phase I/II). J Transl Med. 2016;14:246. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 181] [Cited by in RCA: 231] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 72. | Soler R, Orozco L, Munar A, Huguet M, López R, Vives J, Coll R, Codinach M, Garcia-Lopez J. Final results of a phase I-II trial using ex vivo expanded autologous Mesenchymal Stromal Cells for the treatment of osteoarthritis of the knee confirming safety and suggesting cartilage regeneration. Knee. 2016;23:647-654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 129] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 73. | Vega A, Martín-Ferrero MA, Del Canto F, Alberca M, García V, Munar A, Orozco L, Soler R, Fuertes JJ, Huguet M, Sánchez A, García-Sancho J. Treatment of Knee Osteoarthritis With Allogeneic Bone Marrow Mesenchymal Stem Cells: A Randomized Controlled Trial. Transplantation. 2015;99:1681-1690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 373] [Cited by in RCA: 450] [Article Influence: 45.0] [Reference Citation Analysis (0)] |
| 74. | Emadedin M, Ghorbani Liastani M, Fazeli R, Mohseni F, Moghadasali R, Mardpour S, Hosseini SE, Niknejadi M, Moeininia F, Aghahossein Fanni A, Baghban Eslaminejhad R, Vosough Dizaji A, Labibzadeh N, Mirazimi Bafghi A, Baharvand H, Aghdami N. Long-Term Follow-up of Intra-articular Injection of Autologous Mesenchymal Stem Cells in Patients with Knee, Ankle, or Hip Osteoarthritis. Arch Iran Med. 2015;18:336-344. [PubMed] |
| 75. | Davatchi F, Sadeghi Abdollahi B, Mohyeddin M, Nikbin B. Mesenchymal stem cell therapy for knee osteoarthritis: 5 years follow-up of three patients. Int J Rheum Dis. 2016;19:219-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 145] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 76. | Wang Y, Jin W, Liu H, Cui Y, Mao Q, Fei Z, Xiang C. [Curative effect of human umbilical cord mesenchymal stem cells by intra-articular injection for degenerative knee osteoarthritis]. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2016;30:1472-1477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 77. | Matas J, Orrego M, Amenabar D, Infante C, Tapia-Limonchi R, Cadiz MI, Alcayaga-Miranda F, González PL, Muse E, Khoury M, Figueroa FE, Espinoza F. Umbilical Cord-Derived Mesenchymal Stromal Cells (MSCs) for Knee Osteoarthritis: Repeated MSC Dosing Is Superior to a Single MSC Dose and to Hyaluronic Acid in a Controlled Randomized Phase I/II Trial. Stem Cells Transl Med. 2018;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 130] [Cited by in RCA: 259] [Article Influence: 37.0] [Reference Citation Analysis (0)] |
| 78. | Song Y, Du H, Dai C, Zhang L, Li S, Hunter DJ, Lu L, Bao C. Human adipose-derived mesenchymal stem cells for osteoarthritis: a pilot study with long-term follow-up and repeated injections. Regen Med. 2018;13:295-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 176] [Article Influence: 25.1] [Reference Citation Analysis (0)] |
| 79. | Jo CH, Chai JW, Jeong EC, Oh S, Shin JS, Shim H, Yoon KS. Intra-articular Injection of Mesenchymal Stem Cells for the Treatment of Osteoarthritis of the Knee: A 2-Year Follow-up Study. Am J Sports Med. 2017;45:2774-2783. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 170] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 80. | Pers YM, Rackwitz L, Ferreira R, Pullig O, Delfour C, Barry F, Sensebe L, Casteilla L, Fleury S, Bourin P, Noël D, Canovas F, Cyteval C, Lisignoli G, Schrauth J, Haddad D, Domergue S, Noeth U, Jorgensen C; ADIPOA Consortium. Adipose Mesenchymal Stromal Cell-Based Therapy for Severe Osteoarthritis of the Knee: A Phase I Dose-Escalation Trial. Stem Cells Transl Med. 2016;5:847-856. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 293] [Cited by in RCA: 354] [Article Influence: 39.3] [Reference Citation Analysis (0)] |
| 81. | Rubio D, Garcia-Castro J, Martín MC, de la Fuente R, Cigudosa JC, Lloyd AC, Bernad A. Spontaneous human adult stem cell transformation. Cancer Res. 2005;65:3035-3039. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 805] [Cited by in RCA: 737] [Article Influence: 36.9] [Reference Citation Analysis (0)] |
| 82. | de la Fuente R, Bernad A, Garcia-Castro J, Martin MC, Cigudosa JC. Retraction: Spontaneous human adult stem cell transformation. Cancer Res. 2010;70:6682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 87] [Article Influence: 5.8] [Reference Citation Analysis (0)] |