Published online Feb 26, 2019. doi: 10.4252/wjsc.v11.i2.55
Peer-review started: October 10, 2018
First decision: November 27, 2018
Revised: December 30, 2018
Accepted: January 22, 2019
Article in press: January 23, 2019
Published online: February 26, 2019
Processing time: 140 Days and 3 Hours
Autism and autism spectrum disorders (ASD) refer to a range of conditions characterized by impaired social and communication skills and repetitive behaviors caused by different combinations of genetic and environmental influences. Although the pathophysiology underlying ASD is still unclear, recent evidence suggests that immune dysregulation and neuroinflammation play a role in the etiology of ASD. In particular, there is direct evidence supporting a role for maternal immune activation during prenatal life in neurodevelopmental conditions. Currently, the available options of behavioral therapies and pharmacological and supportive nutritional treatments in ASD are only symptomatic. Given the disturbing rise in the incidence of ASD, and the fact that there is no effective pharmacological therapy for ASD, there is an urgent need for new therapeutic options. Mesenchymal stem cells (MSCs) possess immunomodulatory properties that make them relevant to several diseases associated with inflammation and tissue damage. The paracrine regenerative mechanisms of MSCs are also suggested to be therapeutically beneficial for ASD. Thus the underlying pathology in ASD, including immune system dysregulation and inflammation, represent potential targets for MSC therapy. This review will focus on immune dysfunction in the pathogenesis of ASD and will further discuss the therapeutic potential for MSCs in mediating ASD-related immunological disorders.
Core tip: Autism spectrum disorder (ASD) is a complex, behaviorally defined disorder characterized by severe impairments in social communication and repetitive behavior. Because of an incomplete understanding of the pathology of ASD, available treatment options in ASD are only symptomatic. We discuss the role of immune dysfunction in the etiology of ASD and function of mesenchymal stem cells. We summarize the pre-clinical and clinical evidence for mesenchymal stem cell therapy in ASD and suggest that more basic experiments are needed to better understand the therapeutic mechanisms of mesenchymal stem cells in ASD.
- Citation: Liu Q, Chen MX, Sun L, Wallis CU, Zhou JS, Ao LJ, Li Q, Sham PC. Rational use of mesenchymal stem cells in the treatment of autism spectrum disorders. World J Stem Cells 2019; 11(2): 55-72
- URL: https://www.wjgnet.com/1948-0210/full/v11/i2/55.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v11.i2.55
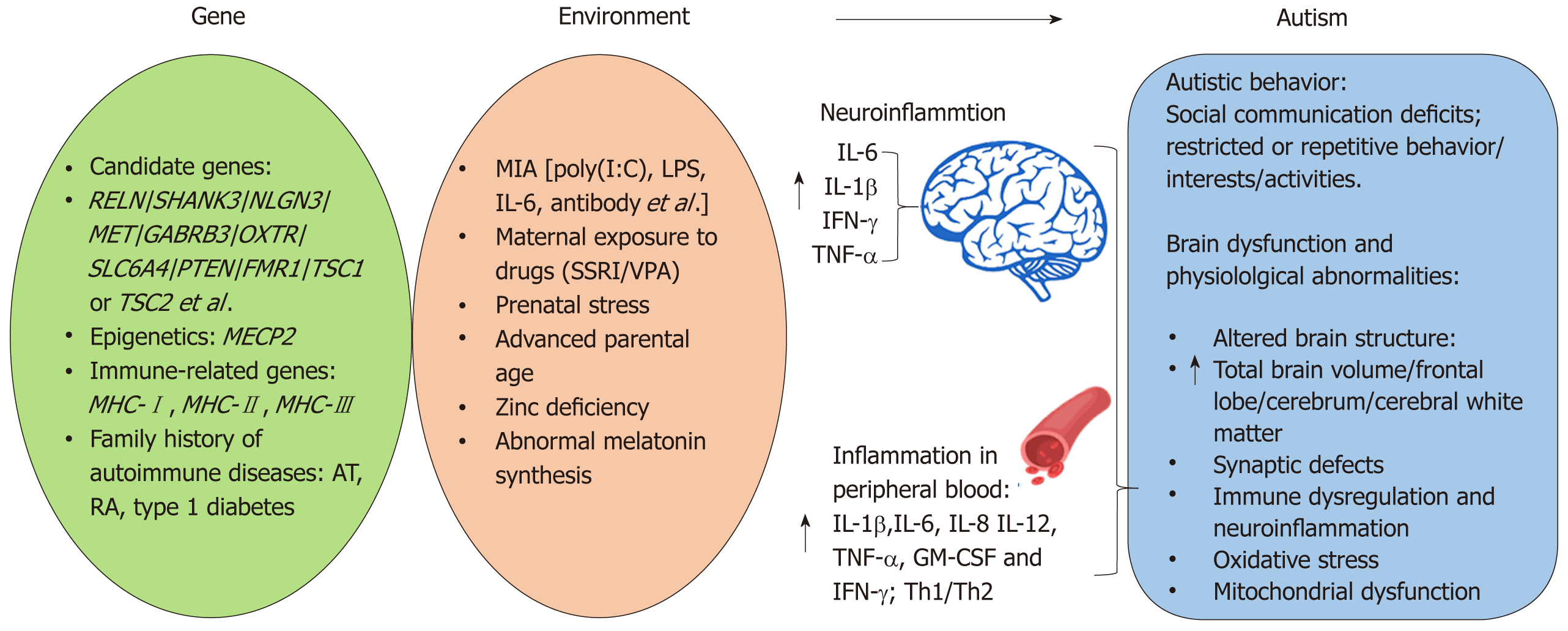
Autism spectrum disorder (ASD) is a complex, behaviorally defined disorder characterized by severe and pervasive impairments in social communication and repetitive behavior. According to the 5th edition of the diagnostic and statistical manual of mental disorders, ASD is diagnosed in individuals exhibiting three social communication and interaction deficits, at least two symptoms of restricted or repetitive behavior/interests/activities, and a variety of specific symptoms classified within each diagnostic category. ASD is one of the most common psychiatric disorders affecting 1 in 59 children aged 8 years based on the most recent estimates calculated by the United States Center of Disease Control[1]. There has recently been a steady and highly significant rise in the estimated prevalence of ASD, due both to a greater awareness of the disorder and broader diagnostic criteria[1,2]. ASD is a complex and heterogeneous psychiatric disorder, and early studies suggest a strong genetic component to autism. For example, identical twin studies estimate concordance for ASD to be between 70% and 90%[3-5]. However, growing evidence suggests that these previous studies may have overestimated the genetic component of autism because the heritability of autism and shared twin environment were similar[6]. Meanwhile, large numbers of ASD candidate genes have been uncovered by whole-genome linkage studies, gene association studies, copy number variation screening, and SNP analysis[7]. Many of the candidate genes, such as reelin (RELN)[8], SH3 and multiple ankyrin repeat domains 3 (SHANK3)[9], neuroligin 3 (NLGN3), NLGN4X[10], MET[11], gamma-aminobutyric acid type-A receptor beta3 subunit (GABRB3)[12], oxytocin receptor (OXTR)[13], serotonin transporter (SLC6A4)[14], and phosphatase and tensin homolog (PTEN)[15] have been demonstrated to be associated with ASD (Figure 1). Furthermore, single gene mutations cause several ASD-related syndromes, including Rett’s syndrome (methyl CpG binding protein 2, MECP2)[16], Fragile X (fragile X mental retardation 1, FMR1)[17], and tuberous sclerosis (TSC1 or TSC2)[18]. Proteins within the phosphoinositide-3-kinase pathway, including MET, PTEN, TSC1, and TSC2 have a major role in regulating interleukin (IL)-12 production and are involved in both innate and adaptive immunity[19]. Additionally, some of the ASD candidate genes, including the major histocompatibility complex class genes are traditionally thought to play a role exclusively in the immune system[20] (Figure 1). Even with the recent advances in identifying candidate genes involved in ASD, all identified genes account for < 20% of ASD cases[21]. Moreover, a number of these genetic risk factors are present in individuals without ASD suggesting additional risk factors are also necessary. For example, recent studies provided evidence for altered DNA methylation in ASD[22,23]. Thus through epigenetic mechanisms, exposure to specific environmental factors may be responsible for triggering the development of ASD in some individuals (Figure 1). A variety of environmental risk factors have been identified to increase ASD risk including: maternal immune activation (MIA)[24-27]; prenatal or perinatal exposure to valproic acid (VPA)[28,29] and selective serotonin reuptake inhibitors (SSRI)[30-32]; early life exposure to stress[33,34]; advanced parental age, zinc deficiency, abnormal melatonin synthesis[35]; and environmental toxins[36] (Figure 1). MIA and maternal exposure to drugs such as SSRI and VPA are of particular interest given evidence from clinical and animal studies supporting the role for immune dysfunction and inflammation in the etiology of ASD.
The first part of this review discusses immune-related genetic and environmental risk factors for ASD, from both human and animal studies, and the role of immune activation in the etiology of ASD-related behavioral and neuropathological abnormalities. Understanding how immune abnormalities are involved in the etiology of ASD will provide a valuable starting point for further work towards potential stem cell therapies for ASD. There is great potential for the use of stem cells in the future of molecular and regenerative medicine. Amongst the various stem cell subtypes, mesenchymal stem cells (MSCs) are the most promising clinical candidate for the treatment of several diseases related with inflammation, tissue damage, and subsequent regeneration and repair[37]. Therefore, the second part of this review will focus on underlying treatment mechanism of MSCs in ASD.
Major histocompatibility complex molecules: Major histocompatibility complex (MHC) occurs on the short arm of chromosome 6 and is divided into three regions; MHC class I, II, and III (MHC-I, MHC-II, and MHC-III). The human leukocyte antigen refers to the MHC locus in humans, which contains a large number of genes involved in integrating the innate and adaptive immune system. MHC-I molecules are found on all nucleated cells, and present epitopes to T-cell receptor proteins on cytotoxic CD8+ T lymphocytes[38]. As a result of MHC-I presentation, cytotoxic CD8+ T lymphocytes become activated and play an important role in the clearance of bacterial and viral infections. While MHC-I molecules have long been known for their primary role in adaptive immunity, they also bind to inhibitory receptors on natural killer (NK) cells, which are part of the innate immune system[39,40]. Interestingly, some recent studies have demonstrated novel roles of MHC-I molecules in regulating synaptic function, plasticity of the cerebral cortex, and cortical glutamatergic connectivity[41-43]. Several lines of evidence have indicated that abnormalities in the balance between excitatory (glutamate-mediated) and inhibitory (gamma-Aminobutyric acid-mediated) neurotransmission may be a key pathological mechanism in autism[44-46]. MHC may also function in social communication and the formation of social memories[47,48]. Moreover, the class one allele of human leukocyte antigen-A2, an important MHC-I antigen presenting molecule, is linked to higher incidence of autism[49]. Thus, it is interesting to speculate that human leukocyte antigen polymorphisms might contribute to the abnormal social communication in ASD by altering excitatory/inhibitory balance in the brain.
Unlike MHC-I, MHC-II molecules are expressed exclusively by the antigen presenting cells, including B cells, dendritic cells, and macrophages, in response to inflammation signals[50]. In general, helper CD4+ T lymphocytes can recognize exogenous antigen presented on MHC-II through T cell receptors. Once activated, helper CD4+ T lymphocytes promote B cell differentiation and antibody production and secrete many cytokines and chemokines. MHC-II alleles are associated with autoimmune disease[51], and interestingly, many studies report that a family history of autoimmune disease is a significant risk factor for ASD[52]. Moreover, in the developing and adult brain, MHC-II molecules are expressed mainly on microglia, astrocytes, and perivascular monocytes[53-55]. In vitro experiments suggest that the expression of MHC-II differs in astrocytes and microglia. For example, glutamate, an excitatory neurotransmitter abundantly present in the central nervous system (CNS), inhibits expression of MHC-II induced by interferon-gamma (IFN-γ) on astrocytes, but not on microglia cells[54]. Hellendall and Ting[56] reported that cytokine (IFN-γ) induced expression of MHC-II on astrocytes is mediated through a cAMP and protein kinase C-dependent pathway. Whilst a mitogen-activated protein kinase (MAPK) signal pathway including extracellular signal-regulated kinases 1/2, c-Jun N-terminal kinase, and p38 MAPK and cyclic AMP responding element binding protein, may be involved in lipopolysaccharide (LPS)-activated microglia[57]. Altered microglial activation in the brain is accompanied by the behavioral phenotype of autism (e.g., anxiety, abnormal social interaction, and learning impairment) in MIA animal models[58,59]. Meanwhile, an increased average microglia somal volume in white matter and microglial density in grey matter has been reported in post-mortem studies of ASD[60,61]. Furthermore, several studies have reported that the DRβ1*04 allele of the MHC-II region is associated with ASD[62-64].
The MHC-III region encodes a cluster of proteins with immune functions including complement proteins (C2 and C4), tumor necrosis factor (TNF)-α, and heat shock proteins. The CB4 null allele of MHC-III has been implicated in ASD[65]. In addition, strong evidence has demonstrated that MHC-III molecules play an important role in brain development and function. For example, TNF-α enhances dendrite growth and synaptic connectivity, balances neuronal excitation and inhibition, and alters synaptic plasticity[66-68].
Clearly, the MHC molecules play a vital role in the formation, refinement, maintenance, and plasticity of the brain. Thus, disruptions in the expression of MHC molecules in the developing brain induced by mutations and/or immune dysregulation might contribute to the altered brain function and endophenotypes of ASD.
MIA and ASD: Epidemiological studies indicate that generalized activation of the maternal immune system caused by maternal infection during prenatal life is a strong risk factor for ASD[69-72]. Consistent with these reports, our research group and others have demonstrated non-specific induction of MIA using viral analogues such as the double stranded RNA poly(I:C), and this is sufficient to bring about neuropathologic, neuroimaging, and behavioral phenotypic changes in the offspring, which are analogous to those observed in human ASD[22,24-26,73,74]. In addition, MIA can be induced in both rodent and non-human primate models with influenza[75], IL-6[76], maternal anti-fetal brain antibody[77], and LPS[78]. Altogether, these large epidemiological findings and animal experiments point to a primary role for MIA in the etiology of ASD.
It is now well understood that shortly after maternal injection with poly(I:C), pro-inflammatory cytokines, including IL-1β, IL-6, and TNF-α are elevated in the maternal bloodstream, placenta, and fetal brain[59,79]. IL-6 in particular may be a crucial immunological mediator of the link between maternal immune activation and altered adult brain functions. This is because, unlike IL-1β and TNF-α, IL-6 may cross the placenta and enter the fetal brain after MIA[80,81]. Indeed, maternal IL-6 injection is sufficient to precipitate offspring prepulse inhibition and latent inhibition deficits usually consequent on poly(I:C) exposure[76]. Simultaneous injection of an anti-IL-6 antibody can prevent behavioral maldevelopment and gene expression changes caused by MIA[76]. More convincingly, IL-6 knock-out mice are resistant to the effects of prenatal poly(I:C) exposure[76]. There is also evidence that maternal IL-6 dependent activation of the Janus kinase/signal transducer and activator of transcription 3 pathway in the placenta demonstrates a direct transfer of the MIA response from maternal to fetal cells[79]. Interestingly, pathways downstream of the Janus kinase/signal transducer and activator of transcription 3 signaling including the MAPK cascade that contains Ras/Raf, mitogen-activated protein kinase kinase 1, and phosphorylated extracellular signal-regulated kinases, have been demonstrated to contribute to the fetal brain dysfunction observed in the MIA mice model[82]. Moreover, several recent studies from our group and others report that MIA induces epigenetic alterations in the brain, suggesting that stable DNA methylation is a plausible mechanism underlying the disruption of gene transcription, brain development, and behavioral functions in response to immune challenge in utero[22,83-85].
Maternal exposure to SSRI and ASD: Depression during pregnancy is not uncommon; the prevalence is reported to be around 7%-12%[86,87]. SSRIs are the most frequently prescribed antidepressants during pregnancy because they are thought to be relatively safe for the fetus compared to other antidepressants. However, recent meta-analyses have suggested that SSRI exposure during pregnancy increases the risk for preterm birth and low birth weight[88], congenital malformation[89], and unfavorable effects on language or behavioral development in children[90]. SSRIs can cross the placenta and are able to reach the fetal brain, which might have long-term neurobehavioral and neurodevelopmental consequences in the offspring[91]. An imbalance of serotonin (5-HT) in prenatal life may be a risk factor for ASD. Experimental investigations have demonstrated that SSRIs have the potential to cause changes in brain circuitry and maladaptive behaviors, due to elevated levels of 5-HT[92]. In utero, exposure to an SSRI during a key developmental window lead to dysfunctional 5-HT signaling, loss of 5-HT terminals, and behavioral abnormalities in animals[92]. 5-HT levels are reported to be decreased in ASD patients[93]. Additionally, several clinical studies have reported that the use of SSRIs during pregnancy increases the risk of ASD in children[30,94-96]. Although several reviews and meta-analyses have recently been published addressing this issue, there are conflicting conclusions when controlling for maternal psychiatric disease and other confounding factors, such as genetic syndromes and congenital anomalies that are associated with ASD-like behavior[97-99]. To answer this question more accurately further investigation is warranted, in particular focusing on maternal psychiatric conditions and/or SSRI treated and untreated siblings.
Given that 5-HT plays a role as an immunomodulator, it is possible that prenatal SSRI exposure may contribute to the pathophysiology of ASD through interactions between an altered serotonergic system and the immune system. 5-HT modulates the function of a wide range of immune cells, including macrophages, NK cells, dendritic cells, T-cells, and B-cells through binding to 5-HT receptors during the immune response[100]. In addition, there is an association between serum 5-HT levels and the presence of certain MHC genes in ASD children[101]. It is possible that abnormal synaptic or extracellular levels of 5-HT may affect the immune system, triggering abnormalities as seen in ASD. However, a direct experimental investigation is needed to verify the 5-HT-mediated neuro-immune crosstalk in ASD.
Valproic acid and ASD: VPA has been used for the treatment of seizures and mood swings for more than 30 years. Several lines of clinical evidence have suggested that maternal exposure to VPA is associated with increased risk of ASD[102-104]. Our research group and others have shown that rodents exposed to VPA prenatally develop behavioral traits and neurochemical alterations that may be relevant to ASD[28,105]. Interestingly, prenatal exposure to VPA on gestation day 9 before neural tube closure disrupts the maturation of serotonergic neurons thereby interrupting early development of the serotonergic system[106]. In addition, prenatal exposure to VPA on gestation day 9 results in an elevated level of 5-HT in the hippocampus and hyperserotonemia in blood[107]. Furthermore, Dufour-Rainfray et al[108] reported that decreased 5-HT levels in the hippocampus of rats exposed to VPA at gestation day 9 may be associated with behavioral impairments. Therefore, these results suggest prenatal VPA exposure may play a role in the development of ASD through disruption of the normal development of the serotonin system. However, further research is required to elucidate the mechanisms by which this occurs. Clinical use of VPA is often associated with hepatotoxicity and the pathology of VPA-induced hepatotoxicity has been studied extensively. Oxidative stress and hepatic inflammation are apparent; elevated levels of nuclear NF-κB in the liver is accompanied by the induction of IL-1β, IL-6, and TNF-α, and these play important roles in the pathology of VPA-induced hepatotoxicity[109]. Moreover, moderate or high doses of prenatal exposure to VPA can also induce toxicity and even death in the offspring in animals[28]. However, the underlying mechanism of VPA-induced toxicity in the CNS is not clear yet. We suspect that oxidative stress and/or neuroinflammation may also play an important role in the altered brain function observed in prenatal exposure to VPA. Further study is required to improve understanding of the mechanisms by which prenatal VPA exposure may induce ASD, through investigation of the serotoninergic system and immune responses in the fetal brain.
A consistent body of data has suggested that there is active inflammation in the CNS in ASD patients. Increased activation of astroglia and microglia has been found in the postmortem brain and cerebrospinal fluid samples in ASD patients[61]. In addition, elevated macrophage chemoattractant protein-1 and tumor growth factor-β1 derived from neuroglia are the most prominent cytokines in the brain samples of ASD patients; marked expression of a prominent inflammatory cytokine profile, macrophage chemoattractant protein-1, IL-6, IL-8, and IFN-γ is shown in the cerebrospinal fluid of ASD patients[61]. Another study further demonstrates that pro-inflammatory cytokines including TNF-α, IL-6, IL-8, granulocyte macrophage-colony stimulating factor, and IFN-γ (Th1 cytokines) are significantly increased in the brains of ASD patients[110]. However, there is no increase in IL-4 or IL-5 (Th2 cytokines), thus Th1/Th2 ratio is significantly raised in ASD patients, suggesting that the Th1 pathway is activated in ASD[110].
A number of studies have shown that IL-1β, IL-12, TNF-α, and IFN-γ are increased in the peripheral blood of autistic patients[111]. Two recent large case-control studies comparing ASD and typically developing children have further confirmed increased levels of plasma cytokines including the Th1-like IL-12p40 and pro-inflammatory cytokines IL-1β, IL-6, IL-8, and granulocyte macrophage-colony stimulating factor[112], and chemokines, including MCP-1, regulated on activation normal T cell expressed and secreted, and eotaxin[113]. These elevated levels of cytokines and chemokines are associated with behavioral and cognitive impairments[112,113].
MSCs are a population of progenitor cells of mesodermal origin found principally in the bone marrow, which possess the capacity of self-renewal and also exhibit multilineage differentiation[114,115]. In addition to bone marrow, MSC populations can also be obtained readily from adipose tissue[116], placenta[117], skin[118], umbilical cord blood[119], umbilical cord perivascular cells[120], umbilical cord Wharton’s jelly[121], amniotic fluid[122], synovial membrane[123], breast milk[124], alveolar epithelium[125], myocardium[126], menstrual blood[127], and endometrium[128] (Table 1). MSCs are relatively easy to isolate and expand in culture and capable of self-renewal and differentiation, making them a promising treatment option for a variety of clinical conditions. Although the multipotency of MSCs is demonstrated in vitro[129], this is still not definite in vivo. Until now, it is also still unclear whether MSCs isolated from different tissue sources have similar therapeutic potentials[130]. Furthermore, it is uncertain whether systematic delivery (i.e., intravenous) of MSCs is sufficient to reach the brain as compared to direct implantation of MSCs[131,132]. Though intranasal application of cells provides an alternative, non-invasive method to deliver MSCs directly into the CNS[133]. At present, neither intravenous nor direct injection of MSCs have been able to yield consistent clinical results because infused cells exhibit limited survival and transient functionality in host tissues[134-136].
| Tissue sources | MSCs | Ref. |
| Bone marrow | BM-MSCs | [115] |
| Adipose | Ad-MSCs | [116] |
| Placenta | Pl-MSCs | [117] |
| Skin | S-MSCs | [118] |
| Umbilical cord blood | UCB-MSCs | [119] |
| Umbilical cord perivascular cells | UCPVC-MSCs | [120] |
| Umbilical cord Wharton’s jelly | WJ-MSCs | [121] |
| Amniotic fluid | AF-MSCs | [122] |
| Synovial membrane | SM-MSCs | [123] |
| Breast milk | M-MSCs | [124] |
| Alveolar epithelium (lung) | AE-MSCs | [125] |
| Myocardium (heart) | Myo-MSCs | [126] |
| Menstrual blood | Men-MSCs | [127] |
| Endometrium | En-MSCs | [128] |
As well as the ability to self-renew and differentiate, MSCs can also secrete immunomodulatory, anti-apoptotic, anti-inflammatory, pro-angiogenic, pro-mitogenic, and antibacterial molecules that contribute to immunomodulatory and trophic effects[137]. Thus recent recognition of the immunomodulatory functions of MSCs may result in the exploration and development of new therapies for ASD.
Although the mechanism of action of MSCs on the nervous system remains largely unknown, recent research suggests that neuroprotection, neurogenesis, and synaptogenesis may be involved[138]. Genetic findings linking ASD to synapse-associated genes, such as SH3 and multiple ankyrin repeat domains 3 (SHANK3) and mutations of other synaptic cell adhesion molecules, suggest that ASD may result, at least partially, from disruption of synapse function and plasticity[139]. MSCs act through several possible mechanisms to regulate synaptic function and plasticity, that is, secreting survival-promoting growth factors (e.g., brain-derived neurotrophic factor; nerve growth factor), sustaining synaptic plasticity, restoring synaptic transmitter release by providing local re-innervations, integrating into existing synaptic networks, and re-establishing functional afferent and efferent connec-tions[138,140,141].
There is a considerable body of literature documenting the effects of MSCs on the immune system. MSCs act on both the adaptive and innate immune system by suppressing pro-inflammatory activities, inhibiting dendritic cell maturation, polarizing macrophages towards anti-inflammatory M2-like state, promoting the generation of regulatory T cells via IL-10, suppressing proliferation and cytotoxicity of NK cells, and reducing B cell activation and proliferation. These functions of MSCs on the immune system have been covered extensively in several reviews[142-146]. As discussed above in this review, ASD patients show an imbalance between Th1 and Th2, as well as NK cells, overproduction of pro-inflammation, and reduction of anti-inflammation. MSCs immunoregulatory effects have the potential to restore this immune imbalance, inhibit TNF-α, IL-1β and IFN-γ production, and increase IL-10 and IL-4 levels[147].
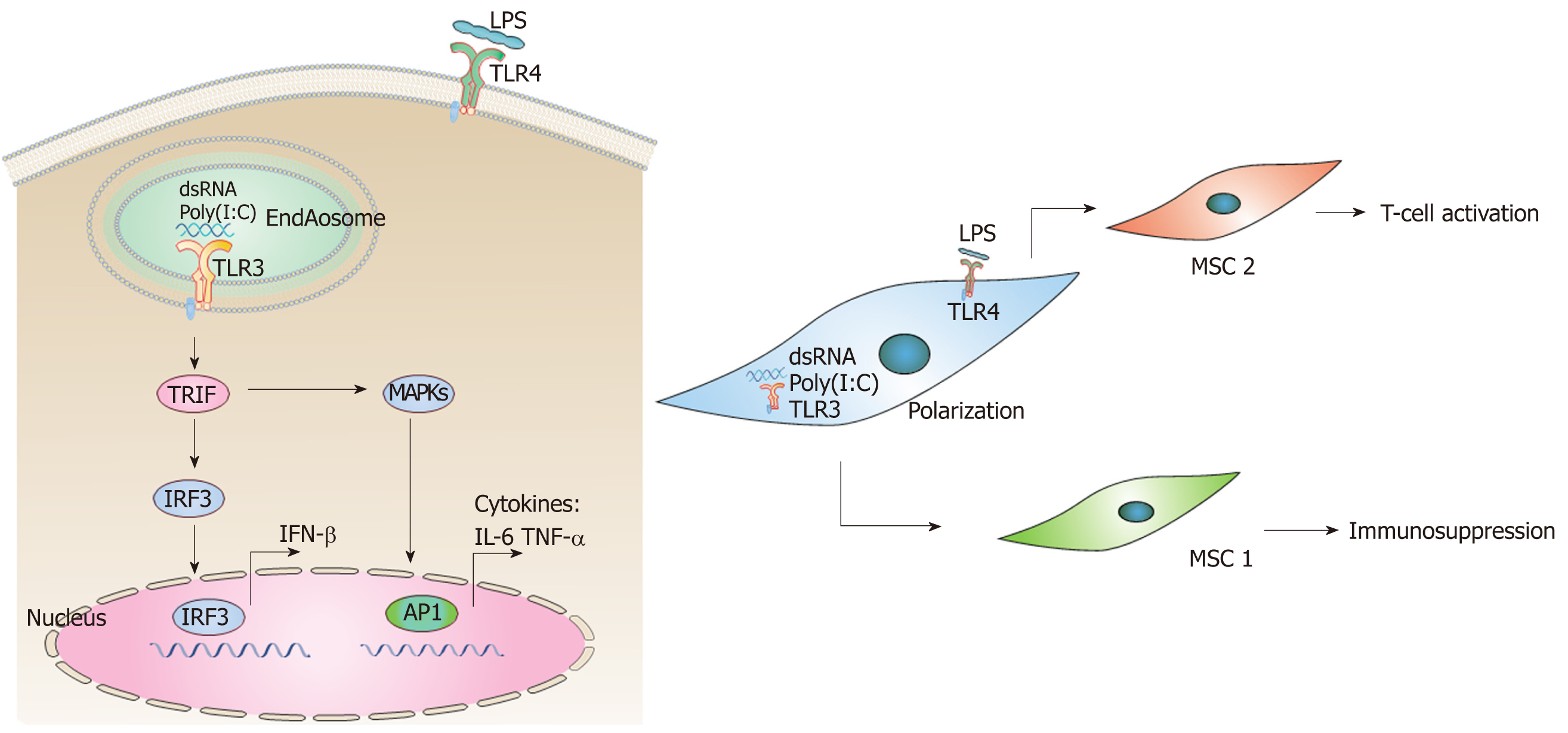
In addition, MSCs are capable of crossing the blood-brain-barrier and migrating to sites of tissue injury and inflammation[148,149]. MSCs act through Toll-like receptor (TLR) signaling to initiate the clearance of pathogens and promote the repair of injured tissue. These TLRs respond to so-called “danger signals” from microbial invasion, such as double-stranded RNA (dsRNA), LPS, and heat shock proteins, triggering intracellular signaling pathways. This results in the induction of inflammatory cytokines, type I IFNs, and upregulation of co-stimulatory molecules leading to the activation of the adaptive immune response[150]. As mentioned above, prenatal exposure to poly(I:C), a synthetic analog of dsRNA, elicits a plethora of intracellular signaling pathways through binding to TLR3 in a MIA model of ASD[151], whilst LPS elicits distinct molecular profiles through binding to TLR4[152]. In TLR3- and TLR4-mediated signaling pathways, toll–IL-1 receptor domain-containing adaptor inducing IFN-β (TRIF) leads to activation of the transcription factors interferon regulatory factor 3 (IRF3), which are responsible for induction of IFN-β[153] (Figure 2A). TRIF-dependent signaling pathway, both downstream of TLR-3 and TLR-4, also leads to activation of MAPKs and production of cytokines, such as IL-6 and TNF-α[153,154]. Interestingly, TLRs may polarize MSCs toward pro-inflammatory (MSC1) or anti-inflammatory (MSC2) phenotypes. For example, TLR4 (LPS) priming results in production of pro-inflammatory cytokines such as IL-6 or IL-8 (MSC1), while TLR3 (dsRNA, ployI:C) priming induces secretion of anti-inflammatory molecules such as IL-10, IL-4, indoleamine 2,3-dioxygenase, or prostaglandin (MSC2)[155,156] (Figure 2B). These polarizing effects of TLR priming depend on the ligand concentration, timing, and kinetics of activation. This may also explain the contradictory results obtained so far regarding the effects of TLRs on immunomodulation by MSCs[155,156]. However, in contradiction to the reported LPS polarizing process (MSC1 phenotype) observed in vitro, several studies have reported beneficial effects of MSC treatment in animal models of LPS-induced tissue injury[157-159]. Therefore, the in vivo modulation of MSCs by TLR ligands deserves further investigation and clarification. In particular, the MIA model of prenatal exposure to poly(I:C) represents a good animal model in which to explore the underlying mechanism of MSC treatment in ASD.
Numerous autoimmune conditions have been associated with ASD, including autoimmune thyroiditis, rheumatoid arthritis, ulcerative colitis, celiac disease, and type 1 diabetes[160] (Table 2). The potential use of MSC therapy has been investigated in many of these conditions. Preclinical experiments and clinical trials have demonstrated the safety and efficacy of MSC therapy in rheumatoid arthritis animal models and patients[161]. In addition, MSC therapy has been reported to increase regulatory T cells, restore Th1/Th2 balance in blood and induce apoptosis of infiltrated leukocytes in pancreatic islet cells in mice with type 1 diabetes[162]. However, in order to translate this finding from bench to bedside, further in-depth mechanistic studies of the therapeutic effects of MSCs on type 1 diabetes are warranted. Recent pre-clinical research has shown the restorative effect of MSCs in mice with autoimmune thyroiditis through the MAPK signaling pathway[163]. Furthermore, graft-versus-host-disease and multiple sclerosis have been targeted for MSC treatment in both animal experiments and clinical trials[164-167] (Table 2). However, the use of MSCs in the treatment of graft-versus-host-disease has failed to give consistent results in animal experiments[167].
| Autoimmune diseases | ASD | MSCs |
| Autoimmune thyroiditis | + | Pre-clinical experiment |
| Rheumatoid arthritis | + | Pre-clinical experiment; Clinical trials on-going |
| GVHD | - | Pre-clinical experiment; Clinical trials |
| MS | - | Pre-clinical experiment; Clinical trials on-going |
| Type 1 diabetes | + | Pre-clinical experiment; Clinical trials on-going |
To date, only a few pre-clinical studies have demonstrated the therapeutic potential of MSC treatment in animal models of ASD. Ha et al[168] reported that adipose MSCs are transplanted intraventricularly into the brains of neonatal fetal pups at a very early stage. This early intervention reduces repetitive behavior and anxiety, and improves social deficits in mice prenatally exposed to VPA through the rescue of decreased IL-10 and vascular endothelial growth factor levels together with upregulation of reduced PTEN proteins in the brain. In addition, it has been demonstrated that by promoting the maturation of newly formed neurons in the granular cell layer of the dentate gyrus, MSC transplantation restores post-developmental hippocampal neurogenesis in VPA-exposed mice[169]. This is associated with improvements in cognitive and social behavior 2 wk after transplantation of the MSCs and thus may be related to the modulation of hippocampal neurogenesis[169].
A widely accepted mouse model of ASD is the BTBR T+, tf/J (Black and Tan Brachyury, BTBR) inbred mouse strain, which display autistic-like behavior and neuroanatomical abnormalities, including absence of corpus callosum and reduced hippocampal commissure, analogous to the core endophenotype of autism[170-172]. It has been shown that intracerebroventricular transplant of human MSCs into BTBR mice results in a reduction of stereotypical behaviors and cognitive rigidity and an improvement in social behavior[173]. Furthermore, elevated brain-derived neurotrophic factor levels and hippocampal neurogenesis were detected in the MSCs-transplanted BTBR mice[173]. This finding then promoted an investigation of the behavioral effects of transplanted MSCs, which were induced to secrete a higher amount of neurotrophic factors (NurOwn®) in BTBR mice[174]. This study demonstrated NurOwn®[175] are superior to MSCs without induced neurotrophic factors in several aspects. In particular, NurOwn® contains 2 and 5 fold levels of brain-derived neurotrophic factor and glial cell-derived neurotrophic factor, respectively, compared to MSCs from the same donor[176]. Moreover, NurOwn® transplantation increases male-female social interaction, decreases repetitive behavior (changes which can be sustained for 6 mo after treatment), and improves cognitive flexibility in BTBR mice[174]. Exosomes derived from MSCs serve as the main mediators of the therapeutic effect of MSC, with an involvement in repairing damaged tissues, suppressing inflammatory responses, and modulating the immune system[177,178]. Their potential as a surrogate of therapeutic MSCs has been widely explored. Recently, it has been shown that BTBR mice treated with exosomes derived from MSCs via intranasal administration present with significant behavioral improvements in social interaction and ultrasonic communication and reduced repetitive behavior. Interestingly, BTBR mothers that were treated with exosomes derived from MSCs showed improvements in maternal behaviors such as pup retrieval behavior[179].
Although there have been few pre-clinical studies of MSC therapy for ASD, several clinical trials on human have been conducted. Lv et al[180] performed a non-randomized, open-label, controlled, proof-of-concept clinical trial to exam the treatment, safety and efficacy of umbilical cord blood MSCs and/or cord blood mononuclear cells in children with autism. At 24 wk post-treatment, significant reductions in symptom severity are observed with the greatest improvement in the combined group (umbilical cord blood-MSCs + cord blood mononuclear cells), suggesting a synergic effect of dual therapy[180]. There is no significant safety issue related to the treatment and no observed severe adverse effects.
Meanwhile, Sharma et al[181] conducted another open-label proof of concept study and reported on the use of intrathecal transplantation of autologous bone marrow mononuclear cells that contain MSCs in 32 patients with ASD. This study included children as well as adults with ASD (age 3-33). Most of the patients showed improved scores in various behavioral scales after a 26 mo follow up, including improvements in social relationships and reciprocity, emotional responsiveness, speech, language, communication, behavior patterns, sensory aspects, and cognition. Only a few adverse events (including seizures and hyperactivity) were observed, and these were controlled with medications[181]. It has been reported that cerebral hypoperfusion or insufficient blood flow in the brain occurs in many brain regions in ASD[182], and interestingly, their study suggested that the cell transplantation may have had a balancing effect on the brain metabolism[181]. Comparative Positron Emission Tomography-Computed Tomography scans before and 6 mo after cell transplantation showed increased 18F-fluorodeoxyglucose uptake in the areas of frontal lobe, cerebellum, amygdala, hippocampus, parahippocampus, and mesial temporal lobe[181].
Another small pilot open label study recently investigated the clinical benefits of bone marrow aspirate concentrate stem cell with intrathecal transplantation in 10 ASD children (4-12 years of age)[183]. The maximal effect of cell therapy was observed within the first 12 mo following the treatment. Interestingly they also found that improvement decreased as the age of ASD child increased[183]. However, there was no control group and the number of subjects in this study was quite small. Dawson et al[184] conducted an open-label phase I clinical trial of a single intravenous infusion of autologous UCB (AUCB) on 25 ASD children aged between 2 and 5 years. They found that most of the significant improvements in behavior occurred during the first 6 mo and were sustained between 6 and 12 mo post-infusion. Thus whilst a single therapy did not improve all autistic symptoms, this work has demonstrated that it is safe and feasible to perform AUCB infusions for the effective treatment of ASD in young children[184]. Dawson’s research team[185] performed a secondary follow up study and reported changes in electroencephalography spectral power by 12-mo post-treatment of AUCB on ASD children. Baseline posterior electroencephalography beta power was positively associated with an improvement in social communication symptoms in ASD children, suggesting the electroencephalography may be a useful biomarker to predict the outcome of clinical trials for ASD.
Recently, the first randomized, double-blinded, placebo-controlled clinical trial provided further evidence that AUCB was safe, but there was minimal clinical efficacy compared to the findings of the previous open-label trial[186]. Twenty-nine ASD children 2-6 years of age were infused with either AUCB or placebo, and evaluated at baseline, 12 wk, and 24 wk[186]. This study suggested that infusion of AUCB has no serious adverse events for the treatment of ASD and potentially had an impact on socialization for children with ASD.
While the clinical trials discussed above have generally reported a good safety profile for MSC transplantation in ASD children, the follow-up checks are currently only up to 12 mo after treatment. Thus caution should still prevail as no data of long-term effects such as 5 to 20 years posttreatment are currently available.
Despite the increasing incidence of ASD, autism currently remains untreatable. The available options of behavioral, pharmacological, and nutritional therapies are only supportive treatments[84,187,188]. The underlying pathology of ASD involves immune system dysregulation, autoimmunity, and inflammation[189], and these processes are targetable with MSC therapy. MSCs can be transplanted directly without genetic modification or pretreatment, differentiated according to the cues from the surrounding tissues, and do not cause uncontrollable growth or tumors[190]. Several proof-of-concept clinical studies mentioned above and meta-analyses have shown the safety and/or efficacy of MSCs treatment in autistic patients or other clinical conditions of immune dysregulation[180,181,184,190]. Although MSCs have the potential for clinical use in ASD, a number of methodological, technical, and safety challenges still need to be considered[191]. Additionally, their response to other pharmacological interventions, tissue distribution upon administration, and their long-term safety profile are key areas in need of further investigation. Currently, it is unclear how long a single dose of MSC can sustain anti-inflammatory effects or when would be the ideal age for intervention (the early the better?). Furthermore, the most recent randomized, double-blinded, placebo-controlled clinical trial, which had a much more rigorous design than other clinical trials mentioned in this review reported lack of efficacy of AUCB for the treatment of ASD. Given that the long-term safety and efficacy of MSC treatment cannot be fully ascertained, standardized trial design needs to be considered when designing future clinical trials.
More importantly, our understanding of basic MSC biology and underlying etiology of ASD is still limited. Further basic research into endogenous functions of MSC is warranted to elucidate the mechanism by which therapeutic MSCs for the treatment of ASD mediate their action. Animal models such as the MIA and BTBR mouse models may be vital for this as they allow the simultaneous measurement of peripheral and central immune function, quantitative neuronal modification, and behavioral changes in response to MSC treatment, thus enabling a better understanding of the therapeutic mechanisms of MSCs in ASD.
Manuscript source: Unsolicited manuscript
Specialty type: Cell and tissue engineering
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Fatkhudinov T, Miloso M, Oltra E S- Editor: Ma YJ L- Editor: Filipodia E- Editor: Bian YN
| 1. | Baio J, Wiggins L, Christensen DL, Maenner MJ, Daniels J, Warren Z, Kurzius-Spencer M, Zahorodny W, Robinson Rosenberg C, White T, Durkin MS, Imm P, Nikolaou L, Yeargin-Allsopp M, Lee LC, Harrington R, Lopez M, Fitzgerald RT, Hewitt A, Pettygrove S, Constantino JN, Vehorn A, Shenouda J, Hall-Lande J, Van Naarden Braun K, Dowling NF. Prevalence of Autism Spectrum Disorder Among Children Aged 8 Years - Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2014. MMWR Surveill Summ. 2018;67:1-23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2119] [Cited by in RCA: 2099] [Article Influence: 299.9] [Reference Citation Analysis (0)] |
| 2. | Croen LA, Grether JK, Hoogstrate J, Selvin S. The changing prevalence of autism in California. J Autism Dev Disord. 2002;32:207-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 316] [Cited by in RCA: 265] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 3. | Bailey A, Le Couteur A, Gottesman I, Bolton P, Simonoff E, Yuzda E, Rutter M. Autism as a strongly genetic disorder: evidence from a British twin study. Psychol Med. 1995;25:63-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1644] [Cited by in RCA: 1481] [Article Influence: 49.4] [Reference Citation Analysis (0)] |
| 4. | Rosenberg RE, Law JK, Yenokyan G, McGready J, Kaufmann WE, Law PA. Characteristics and concordance of autism spectrum disorders among 277 twin pairs. Arch Pediatr Adolesc Med. 2009;163:907-914. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 289] [Cited by in RCA: 263] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 5. | Steffenburg S, Gillberg C, Hellgren L, Andersson L, Gillberg IC, Jakobsson G, Bohman M. A twin study of autism in Denmark, Finland, Iceland, Norway and Sweden. J Child Psychol Psychiatry. 1989;30:405-416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 581] [Cited by in RCA: 529] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 6. | Hallmayer J, Cleveland S, Torres A, Phillips J, Cohen B, Torigoe T, Miller J, Fedele A, Collins J, Smith K, Lotspeich L, Croen LA, Ozonoff S, Lajonchere C, Grether JK, Risch N. Genetic heritability and shared environmental factors among twin pairs with autism. Arch Gen Psychiatry. 2011;68:1095-1102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1226] [Cited by in RCA: 1247] [Article Influence: 89.1] [Reference Citation Analysis (0)] |
| 7. | Abrahams BS, Geschwind DH. Advances in autism genetics: on the threshold of a new neurobiology. Nat Rev Genet. 2008;9:341-355. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1404] [Cited by in RCA: 1193] [Article Influence: 70.2] [Reference Citation Analysis (0)] |
| 8. | Skaar DA, Shao Y, Haines JL, Stenger JE, Jaworski J, Martin ER, DeLong GR, Moore JH, McCauley JL, Sutcliffe JS, Ashley-Koch AE, Cuccaro ML, Folstein SE, Gilbert JR, Pericak-Vance MA. Analysis of the RELN gene as a genetic risk factor for autism. Mol Psychiatry. 2005;10:563-571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 133] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 9. | Moessner R, Marshall CR, Sutcliffe JS, Skaug J, Pinto D, Vincent J, Zwaigenbaum L, Fernandez B, Roberts W, Szatmari P, Scherer SW. Contribution of SHANK3 mutations to autism spectrum disorder. Am J Hum Genet. 2007;81:1289-1297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 479] [Cited by in RCA: 507] [Article Influence: 28.2] [Reference Citation Analysis (0)] |
| 10. | Jamain S, Quach H, Betancur C, Råstam M, Colineaux C, Gillberg IC, Soderstrom H, Giros B, Leboyer M, Gillberg C, Bourgeron T; Paris Autism Research International Sibpair Study. Mutations of the X-linked genes encoding neuroligins NLGN3 and NLGN4 are associated with autism. Nat Genet. 2003;34:27-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1323] [Cited by in RCA: 1300] [Article Influence: 59.1] [Reference Citation Analysis (0)] |
| 11. | Campbell DB, Sutcliffe JS, Ebert PJ, Militerni R, Bravaccio C, Trillo S, Elia M, Schneider C, Melmed R, Sacco R, Persico AM, Levitt P. A genetic variant that disrupts MET transcription is associated with autism. Proc Natl Acad Sci USA. 2006;103:16834-16839. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 324] [Cited by in RCA: 312] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 12. | Buxbaum JD, Silverman JM, Smith CJ, Greenberg DA, Kilifarski M, Reichert J, Cook EH, Fang Y, Song CY, Vitale R. Association between a GABRB3 polymorphism and autism. Mol Psychiatry. 2002;7:311-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 229] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 13. | Wu S, Jia M, Ruan Y, Liu J, Guo Y, Shuang M, Gong X, Zhang Y, Yang X, Zhang D. Positive association of the oxytocin receptor gene (OXTR) with autism in the Chinese Han population. Biol Psychiatry. 2005;58:74-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 404] [Cited by in RCA: 375] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 14. | Brune CW, Kim SJ, Salt J, Leventhal BL, Lord C, Cook EH. 5-HTTLPR Genotype-Specific Phenotype in Children and Adolescents With Autism. Am J Psychiatry. 2006;163:2148-2156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 96] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 15. | Frazier TW, Embacher R, Tilot AK, Koenig K, Mester J, Eng C. Molecular and phenotypic abnormalities in individuals with germline heterozygous PTEN mutations and autism. Mol Psychiatry. 2015;20:1132-1138. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 107] [Cited by in RCA: 129] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 16. | Lam CW, Yeung WL, Ko CH, Poon PM, Tong SF, Chan KY, Lo IF, Chan LY, Hui J, Wong V, Pang CP, Lo YM, Fok TF. Spectrum of mutations in the MECP2 gene in patients with infantile autism and Rett syndrome. J Med Genet. 2000;37:E41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 111] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 17. | Belmonte MK, Bourgeron T. Fragile X syndrome and autism at the intersection of genetic and neural networks. Nat Neurosci. 2006;9:1221-1225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 221] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 18. | Wiznitzer M. Autism and tuberous sclerosis. J Child Neurol. 2004;19:675-679. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 134] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 19. | Fukao T, Tanabe M, Terauchi Y, Ota T, Matsuda S, Asano T, Kadowaki T, Takeuchi T, Koyasu S. PI3K-mediated negative feedback regulation of IL-12 production in DCs. Nat Immunol. 2002;3:875-881. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 427] [Cited by in RCA: 426] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 20. | Needleman LA, McAllister AK. The major histocompatibility complex and autism spectrum disorder. Dev Neurobiol. 2012;72:1288-1301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 49] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 21. | Abrahams BS, Geschwind DH. Connecting genes to brain in the autism spectrum disorders. Arch Neurol. 2010;67:395-399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 132] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 22. | Basil P, Li Q, Dempster EL, Mill J, Sham PC, Wong CC, McAlonan GM. Prenatal maternal immune activation causes epigenetic differences in adolescent mouse brain. Transl Psychiatry. 2014;4:e434. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 91] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 23. | Wong CC, Meaburn EL, Ronald A, Price TS, Jeffries AR, Schalkwyk LC, Plomin R, Mill J. Methylomic analysis of monozygotic twins discordant for autism spectrum disorder and related behavioural traits. Mol Psychiatry. 2014;19:495-503. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 218] [Cited by in RCA: 211] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 24. | Li Q, Cheung C, Wei R, Cheung V, Hui ES, You Y, Wong P, Chua SE, McAlonan GM, Wu EX. Voxel-based analysis of postnatal white matter microstructure in mice exposed to immune challenge in early or late pregnancy. Neuroimage. 2010;52:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 49] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 25. | Li Q, Cheung C, Wei R, Hui ES, Feldon J, Meyer U, Chung S, Chua SE, Sham PC, Wu EX, McAlonan GM. Prenatal immune challenge is an environmental risk factor for brain and behavior change relevant to schizophrenia: evidence from MRI in a mouse model. PLoS One. 2009;4:e6354. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 102] [Cited by in RCA: 108] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 26. | Meyer U. Prenatal poly(i:C) exposure and other developmental immune activation models in rodent systems. Biol Psychiatry. 2014;75:307-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 427] [Cited by in RCA: 474] [Article Influence: 43.1] [Reference Citation Analysis (0)] |
| 27. | Brown AS. Epidemiologic studies of exposure to prenatal infection and risk of schizophrenia and autism. Dev Neurobiol. 2012;72:1272-1276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 296] [Cited by in RCA: 327] [Article Influence: 25.2] [Reference Citation Analysis (0)] |
| 28. | Wei R, Li Q, Lam S, Leung J, Cheung C, Zhang X, Sham PC, Chua SE, McAlonan GM. A single low dose of valproic acid in late prenatal life alters postnatal behavior and glutamic acid decarboxylase levels in the mouse. Behav Brain Res. 2016;314:190-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 29. | Christensen J, Grønborg TK, Sørensen MJ, Schendel D, Parner ET, Pedersen LH, Vestergaard M. Prenatal valproate exposure and risk of autism spectrum disorders and childhood autism. JAMA. 2013;309:1696-1703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 802] [Cited by in RCA: 867] [Article Influence: 72.3] [Reference Citation Analysis (0)] |
| 30. | Croen LA, Grether JK, Yoshida CK, Odouli R, Hendrick V. Antidepressant use during pregnancy and childhood autism spectrum disorders. Arch Gen Psychiatry. 2011;68:1104-1112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 325] [Cited by in RCA: 322] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 31. | Olivier JD, Vallès A, van Heesch F, Afrasiab-Middelman A, Roelofs JJ, Jonkers M, Peeters EJ, Korte-Bouws GA, Dederen JP, Kiliaan AJ, Martens GJ, Schubert D, Homberg JR. Fluoxetine administration to pregnant rats increases anxiety-related behavior in the offspring. Psychopharmacology (Berl). 2011;217:419-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 111] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 32. | Simpson KL, Weaver KJ, de Villers-Sidani E, Lu JY, Cai Z, Pang Y, Rodriguez-Porcel F, Paul IA, Merzenich M, Lin RC. Perinatal antidepressant exposure alters cortical network function in rodents. Proc Natl Acad Sci USA. 2011;108:18465-18470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 145] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 33. | Ronald A, Pennell CE, Whitehouse AJ. Prenatal Maternal Stress Associated with ADHD and Autistic Traits in early Childhood. Front Psychol. 2011;1:223. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 142] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 34. | Fine R, Zhang J, Stevens HE. Prenatal stress and inhibitory neuron systems: implications for neuropsychiatric disorders. Mol Psychiatry. 2014;19:641-651. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 73] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 35. | Grabrucker AM. Environmental factors in autism. Front Psychiatry. 2013;3:118. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 80] [Cited by in RCA: 117] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 36. | Landrigan PJ. What causes autism? Exploring the environmental contribution. Curr Opin Pediatr. 2010;22:219-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 265] [Cited by in RCA: 246] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 37. | Keating A. Mesenchymal stromal cells: new directions. Cell Stem Cell. 2012;10:709-716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 560] [Cited by in RCA: 607] [Article Influence: 46.7] [Reference Citation Analysis (0)] |
| 38. | Machold RP, Ploegh HL. Intermediates in the assembly and degradation of class I major histocompatibility complex (MHC) molecules probed with free heavy chain-specific monoclonal antibodies. J Exp Med. 1996;184:2251-2259. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 38] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 39. | Krzewski K, Strominger JL. The killer's kiss: the many functions of NK cell immunological synapses. Curr Opin Cell Biol. 2008;20:597-605. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 73] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 40. | Long EO. Negative signaling by inhibitory receptors: the NK cell paradigm. Immunol Rev. 2008;224:70-84. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 286] [Cited by in RCA: 279] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 41. | Garay PA, McAllister AK. Novel roles for immune molecules in neural development: implications for neurodevelopmental disorders. Front Synaptic Neurosci. 2010;2:136. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 139] [Cited by in RCA: 163] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 42. | Needleman LA, Liu XB, El-Sabeawy F, Jones EG, McAllister AK. MHC class I molecules are present both pre- and postsynaptically in the visual cortex during postnatal development and in adulthood. Proc Natl Acad Sci USA. 2010;107:16999-17004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 85] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 43. | Glynn MW, Elmer BM, Garay PA, Liu XB, Needleman LA, El-Sabeawy F, McAllister AK. MHCI negatively regulates synapse density during the establishment of cortical connections. Nat Neurosci. 2011;14:442-451. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 168] [Cited by in RCA: 153] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 44. | Yizhar O, Fenno LE, Prigge M, Schneider F, Davidson TJ, O'Shea DJ, Sohal VS, Goshen I, Finkelstein J, Paz JT, Stehfest K, Fudim R, Ramakrishnan C, Huguenard JR, Hegemann P, Deisseroth K. Neocortical excitation/inhibition balance in information processing and social dysfunction. Nature. 2011;477:171-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1540] [Cited by in RCA: 1770] [Article Influence: 126.4] [Reference Citation Analysis (0)] |
| 45. | Rubenstein JL, Merzenich MM. Model of autism: increased ratio of excitation/inhibition in key neural systems. Genes Brain Behav. 2003;2:255-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1988] [Cited by in RCA: 1921] [Article Influence: 87.3] [Reference Citation Analysis (0)] |
| 46. | Chao HT, Chen H, Samaco RC, Xue M, Chahrour M, Yoo J, Neul JL, Gong S, Lu HC, Heintz N, Ekker M, Rubenstein JL, Noebels JL, Rosenmund C, Zoghbi HY. Dysfunction in GABA signalling mediates autism-like stereotypies and Rett syndrome phenotypes. Nature. 2010;468:263-269. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 970] [Cited by in RCA: 911] [Article Influence: 60.7] [Reference Citation Analysis (0)] |
| 47. | Ruff JS, Nelson AC, Kubinak JL, Potts WK. MHC signaling during social communication. Adv Exp Med Biol. 2012;738:290-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 45] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 48. | St Pourcain B, Whitehouse AJ, Ang WQ, Warrington NM, Glessner JT, Wang K, Timpson NJ, Evans DM, Kemp JP, Ring SM, McArdle WL, Golding J, Hakonarson H, Pennell CE, Smith GD. Common variation contributes to the genetic architecture of social communication traits. Mol Autism. 2013;4:34. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 49. | Torres AR, Sweeten TL, Cutler A, Bedke BJ, Fillmore M, Stubbs EG, Odell D. The association and linkage of the HLA-A2 class I allele with autism. Hum Immunol. 2006;67:346-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 75] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 50. | Kambayashi T, Laufer TM. Atypical MHC class II-expressing antigen-presenting cells: can anything replace a dendritic cell? Nat Rev Immunol. 2014;14:719-730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 298] [Cited by in RCA: 365] [Article Influence: 33.2] [Reference Citation Analysis (0)] |
| 51. | Unanue ER, Turk V, Neefjes J. Variations in MHC Class II Antigen Processing and Presentation in Health and Disease. Annu Rev Immunol. 2016;34:265-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 190] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 52. | Wu S, Ding Y, Wu F, Li R, Xie G, Hou J, Mao P. Family history of autoimmune diseases is associated with an increased risk of autism in children: A systematic review and meta-analysis. Neurosci Biobehav Rev. 2015;55:322-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 89] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 53. | Wierzba-Bobrowicz T, Kosno-Kruszewska E, Lewandowska E, Lechowicz W, Schmidt-Sidor B. Major histocompatibility complex class II (MHC II) expression during development of human fetal brain and haemopoietic organs. Adv Exp Med Biol. 2001;495:93-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 54. | Lee SC, Collins M, Vanguri P, Shin ML. Glutamate differentially inhibits the expression of class II MHC antigens on astrocytes and microglia. J Immunol. 1992;148:3391-3397. [PubMed] |
| 55. | Perlmutter LS, Scott SA, Barrón E, Chui HC. MHC class II-positive microglia in human brain: association with Alzheimer lesions. J Neurosci Res. 1992;33:549-558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 179] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 56. | Hellendall RP, Ting JP. Differential regulation of cytokine-induced major histocompatibility complex class II expression and nitric oxide release in rat microglia and astrocytes by effectors of tyrosine kinase, protein kinase C, and cAMP. J Neuroimmunol. 1997;74:19-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 39] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 57. | Ajmone-Cat MA, De Simone R, Nicolini A, Minghetti L. Effects of phosphatidylserine on p38 mitogen activated protein kinase, cyclic AMP responding element binding protein and nuclear factor-kappaB activation in resting and activated microglial cells. J Neurochem. 2003;84:413-416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 55] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 58. | Singer HS, Morris C, Gause C, Pollard M, Zimmerman AW, Pletnikov M. Prenatal exposure to antibodies from mothers of children with autism produces neurobehavioral alterations: A pregnant dam mouse model. J Neuroimmunol. 2009;211:39-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 121] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 59. | Meyer U, Nyffeler M, Engler A, Urwyler A, Schedlowski M, Knuesel I, Yee BK, Feldon J. The time of prenatal immune challenge determines the specificity of inflammation-mediated brain and behavioral pathology. J Neurosci. 2006;26:4752-4762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 567] [Cited by in RCA: 609] [Article Influence: 32.1] [Reference Citation Analysis (0)] |
| 60. | Morgan JT, Chana G, Pardo CA, Achim C, Semendeferi K, Buckwalter J, Courchesne E, Everall IP. Microglial activation and increased microglial density observed in the dorsolateral prefrontal cortex in autism. Biol Psychiatry. 2010;68:368-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 470] [Cited by in RCA: 526] [Article Influence: 35.1] [Reference Citation Analysis (0)] |
| 61. | Vargas DL, Nascimbene C, Krishnan C, Zimmerman AW, Pardo CA. Neuroglial activation and neuroinflammation in the brain of patients with autism. Ann Neurol. 2005;57:67-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1337] [Cited by in RCA: 1472] [Article Influence: 73.6] [Reference Citation Analysis (0)] |
| 62. | Johnson WG, Buyske S, Mars AE, Sreenath M, Stenroos ES, Williams TA, Stein R, Lambert GH. HLA-DR4 as a risk allele for autism acting in mothers of probands possibly during pregnancy. Arch Pediatr Adolesc Med. 2009;163:542-546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 54] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 63. | Lee LC, Zachary AA, Leffell MS, Newschaffer CJ, Matteson KJ, Tyler JD, Zimmerman AW. HLA-DR4 in families with autism. Pediatr Neurol. 2006;35:303-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 73] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 64. | Torres AR, Maciulis A, Stubbs EG, Cutler A, Odell D. The transmission disequilibrium test suggests that HLA-DR4 and DR13 are linked to autism spectrum disorder. Hum Immunol. 2002;63:311-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 87] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 65. | Odell D, Maciulis A, Cutler A, Warren L, McMahon WM, Coon H, Stubbs G, Henley K, Torres A. Confirmation of the association of the C4B null allelle in autism. Hum Immunol. 2005;66:140-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 79] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 66. | Beattie EC, Stellwagen D, Morishita W, Bresnahan JC, Ha BK, Von Zastrow M, Beattie MS, Malenka RC. Control of synaptic strength by glial TNFalpha. Science. 2002;295:2282-2285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 991] [Cited by in RCA: 1043] [Article Influence: 45.3] [Reference Citation Analysis (0)] |
| 67. | Stellwagen D. The contribution of TNFα to synaptic plasticity and nervous system function. Adv Exp Med Biol. 2011;691:541-557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 68. | Albensi BC, Mattson MP. Evidence for the involvement of TNF and NF-kappaB in hippocampal synaptic plasticity. Synapse. 2000;35:151-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 69. | Chess S. Autism in children with congenital rubella. J Autism Child Schizophr. 1971;1:33-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 298] [Cited by in RCA: 250] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 70. | Atladóttir HÓ, Henriksen TB, Schendel DE, Parner ET. Autism after infection, febrile episodes, and antibiotic use during pregnancy: an exploratory study. Pediatrics. 2012;130:e1447-e1454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 254] [Cited by in RCA: 272] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 71. | Zerbo O, Iosif AM, Walker C, Ozonoff S, Hansen RL, Hertz-Picciotto I. Is maternal influenza or fever during pregnancy associated with autism or developmental delays? Results from the CHARGE (CHildhood Autism Risks from Genetics and Environment) study. J Autism Dev Disord. 2013;43:25-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 189] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 72. | Lee BK, Magnusson C, Gardner RM, Blomström Å, Newschaffer CJ, Burstyn I, Karlsson H, Dalman C. Maternal hospitalization with infection during pregnancy and risk of autism spectrum disorders. Brain Behav Immun. 2015;44:100-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 246] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 73. | Meyer U, Feldon J, Schedlowski M, Yee BK. Immunological stress at the maternal-foetal interface: a link between neurodevelopment and adult psychopathology. Brain Behav Immun. 2006;20:378-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 200] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 74. | McAlonan GM, Li Q, Cheung C. The timing and specificity of prenatal immune risk factors for autism modeled in the mouse and relevance to schizophrenia. Neurosignals. 2010;18:129-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 75. | Fatemi SH, Earle J, Kanodia R, Kist D, Emamian ES, Patterson PH, Shi L, Sidwell R. Prenatal viral infection leads to pyramidal cell atrophy and macrocephaly in adulthood: implications for genesis of autism and schizophrenia. Cell Mol Neurobiol. 2002;22:25-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 189] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 76. | Smith SE, Li J, Garbett K, Mirnics K, Patterson PH. Maternal immune activation alters fetal brain development through interleukin-6. J Neurosci. 2007;27:10695-10702. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1327] [Cited by in RCA: 1223] [Article Influence: 67.9] [Reference Citation Analysis (0)] |
| 77. | Martin LA, Ashwood P, Braunschweig D, Cabanlit M, Van de Water J, Amaral DG. Stereotypies and hyperactivity in rhesus monkeys exposed to IgG from mothers of children with autism. Brain Behav Immun. 2008;22:806-816. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 188] [Cited by in RCA: 171] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 78. | Romero E, Guaza C, Castellano B, Borrell J. Ontogeny of sensorimotor gating and immune impairment induced by prenatal immune challenge in rats: implications for the etiopathology of schizophrenia. Mol Psychiatry. 2010;15:372-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 128] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 79. | Hsiao EY, Patterson PH. Activation of the maternal immune system induces endocrine changes in the placenta via IL-6. Brain Behav Immun. 2011;25:604-615. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 327] [Cited by in RCA: 311] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 80. | Xing Z, Gauldie J, Cox G, Baumann H, Jordana M, Lei XF, Achong MK. IL-6 is an antiinflammatory cytokine required for controlling local or systemic acute inflammatory responses. J Clin Invest. 1998;101:311-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1053] [Cited by in RCA: 1123] [Article Influence: 41.6] [Reference Citation Analysis (0)] |
| 81. | Zaretsky MV, Alexander JM, Byrd W, Bawdon RE. Transfer of inflammatory cytokines across the placenta. Obstet Gynecol. 2004;103:546-550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 288] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 82. | Deng MY, Lam S, Meyer U, Feldon J, Li Q, Wei R, Luk L, Chua SE, Sham P, Wang Y, McAlonan GM. Frontal-subcortical protein expression following prenatal exposure to maternal inflammation. PLoS One. 2011;6:e16638. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 83. | Richetto J, Massart R, Weber-Stadlbauer U, Szyf M, Riva MA, Meyer U. Genome-wide DNA Methylation Changes in a Mouse Model of Infection-Mediated Neurodevelopmental Disorders. Biol Psychiatry. 2017;81:265-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 118] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 84. | Basil P, Li Q, Gui H, Hui TCK, Ling VHM, Wong CCY, Mill J, McAlonan GM, Sham PC. Prenatal immune activation alters the adult neural epigenome but can be partly stabilised by a n-3 polyunsaturated fatty acid diet. Transl Psychiatry. 2018;8:125. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 85. | Labouesse MA, Dong E, Grayson DR, Guidotti A, Meyer U. Maternal immune activation induces GAD1 and GAD2 promoter remodeling in the offspring prefrontal cortex. Epigenetics. 2015;10:1143-1155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 101] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 86. | Le Strat Y, Dubertret C, Le Foll B. Prevalence and correlates of major depressive episode in pregnant and postpartum women in the United States. J Affect Disord. 2011;135:128-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 184] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 87. | Gavin NI, Gaynes BN, Lohr KN, Meltzer-Brody S, Gartlehner G, Swinson T. Perinatal depression: a systematic review of prevalence and incidence. Obstet Gynecol. 2005;106:1071-1083. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2030] [Cited by in RCA: 2230] [Article Influence: 117.4] [Reference Citation Analysis (0)] |
| 88. | Huang H, Coleman S, Bridge JA, Yonkers K, Katon W. A meta-analysis of the relationship between antidepressant use in pregnancy and the risk of preterm birth and low birth weight. Gen Hosp Psychiatry. 2014;36:13-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 123] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 89. | Myles N, Newall H, Ward H, Large M. Systematic meta-analysis of individual selective serotonin reuptake inhibitor medications and congenital malformations. Aust N Z J Psychiatry. 2013;47:1002-1012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 74] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 90. | Malm H. Prenatal exposure to selective serotonin reuptake inhibitors and infant outcome. Ther Drug Monit. 2012;34:607-614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 91. | Lattimore KA, Donn SM, Kaciroti N, Kemper AR, Neal CR, Vazquez DM. Selective serotonin reuptake inhibitor (SSRI) use during pregnancy and effects on the fetus and newborn: a meta-analysis. J Perinatol. 2005;25:595-604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 111] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 92. | Borue X, Chen J, Condron BG. Developmental effects of SSRIs: lessons learned from animal studies. Int J Dev Neurosci. 2007;25:341-347. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 48] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 93. | McDougle CJ, Naylor ST, Cohen DJ, Aghajanian GK, Heninger GR, Price LH. Effects of tryptophan depletion in drug-free adults with autistic disorder. Arch Gen Psychiatry. 1996;53:993-1000. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 192] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 94. | Rai D, Lee BK, Dalman C, Golding J, Lewis G, Magnusson C. Parental depression, maternal antidepressant use during pregnancy, and risk of autism spectrum disorders: population based case-control study. BMJ. 2013;346:f2059. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 223] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 95. | Gidaya NB, Lee BK, Burstyn I, Yudell M, Mortensen EL, Newschaffer CJ. In utero exposure to selective serotonin reuptake inhibitors and risk for autism spectrum disorder. J Autism Dev Disord. 2014;44:2558-2567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 75] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 96. | Man KK, Tong HH, Wong LY, Chan EW, Simonoff E, Wong IC. Exposure to selective serotonin reuptake inhibitors during pregnancy and risk of autism spectrum disorder in children: a systematic review and meta-analysis of observational studies. Neurosci Biobehav Rev. 2015;49:82-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 91] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 97. | Gentile S. Prenatal antidepressant exposure and the risk of autism spectrum disorders in children. Are we looking at the fall of Gods? J Affect Disord. 2015;182:132-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 49] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 98. | Brown HK, Hussain-Shamsy N, Lunsky Y, Dennis CE, Vigod SN. The Association Between Antenatal Exposure to Selective Serotonin Reuptake Inhibitors and Autism: A Systematic Review and Meta-Analysis. J Clin Psychiatry. 2017;78:e48-e58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 39] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 99. | Kaplan YC, Keskin-Arslan E, Acar S, Sozmen K. Prenatal selective serotonin reuptake inhibitor use and the risk of autism spectrum disorder in children: A systematic review and meta-analysis. Reprod Toxicol. 2016;66:31-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 44] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 100. | Jaiswal P, Mohanakumar KP, Rajamma U. Serotonin mediated immunoregulation and neural functions: Complicity in the aetiology of autism spectrum disorders. Neurosci Biobehav Rev. 2015;55:413-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 101. | Warren RP, Singh VK. Elevated serotonin levels in autism: association with the major histocompatibility complex. Neuropsychobiology. 1996;34:72-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 31] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 102. | Williams G, King J, Cunningham M, Stephan M, Kerr B, Hersh JH. Fetal valproate syndrome and autism: additional evidence of an association. Dev Med Child Neurol. 2001;43:202-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 202] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 103. | Evatt ML, DeLong MR, Grant WB, Cannell JJ, Tangpricha V. Autism spectrum disorders following in utero exposure to antiepileptic drugs. Neurology. 2009;73:997. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 104. | Dean JC, Hailey H, Moore SJ, Lloyd DJ, Turnpenny PD, Little J. Long term health and neurodevelopment in children exposed to antiepileptic drugs before birth. J Med Genet. 2002;39:251-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 152] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 105. | Wagner GC, Reuhl KR, Cheh M, McRae P, Halladay AK. A new neurobehavioral model of autism in mice: pre- and postnatal exposure to sodium valproate. J Autism Dev Disord. 2006;36:779-793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 192] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 106. | Miyazaki K, Narita N, Narita M. Maternal administration of thalidomide or valproic acid causes abnormal serotonergic neurons in the offspring: implication for pathogenesis of autism. Int J Dev Neurosci. 2005;23:287-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 144] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 107. | Narita N, Kato M, Tazoe M, Miyazaki K, Narita M, Okado N. Increased monoamine concentration in the brain and blood of fetal thalidomide- and valproic acid-exposed rat: putative animal models for autism. Pediatr Res. 2002;52:576-579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 67] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 108. | Dufour-Rainfray D, Vourc'h P, Le Guisquet AM, Garreau L, Ternant D, Bodard S, Jaumain E, Gulhan Z, Belzung C, Andres CR, Chalon S, Guilloteau D. Behavior and serotonergic disorders in rats exposed prenatally to valproate: a model for autism. Neurosci Lett. 2010;470:55-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 123] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 109. | Jin J, Xiong T, Hou X, Sun X, Liao J, Huang Z, Huang M, Zhao Z. Role of Nrf2 activation and NF-κB inhibition in valproic acid induced hepatotoxicity and in diammonium glycyrrhizinate induced protection in mice. Food Chem Toxicol. 2014;73:95-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 51] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 110. | Li X, Chauhan A, Sheikh AM, Patil S, Chauhan V, Li XM, Ji L, Brown T, Malik M. Elevated immune response in the brain of autistic patients. J Neuroimmunol. 2009;207:111-116. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 621] [Cited by in RCA: 569] [Article Influence: 35.6] [Reference Citation Analysis (0)] |
| 111. | Onore C, Careaga M, Ashwood P. The role of immune dysfunction in the pathophysiology of autism. Brain Behav Immun. 2012;26:383-392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 415] [Cited by in RCA: 458] [Article Influence: 35.2] [Reference Citation Analysis (0)] |
| 112. | Ashwood P, Krakowiak P, Hertz-Picciotto I, Hansen R, Pessah I, Van de Water J. Elevated plasma cytokines in autism spectrum disorders provide evidence of immune dysfunction and are associated with impaired behavioral outcome. Brain Behav Immun. 2011;25:40-45. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 712] [Cited by in RCA: 644] [Article Influence: 46.0] [Reference Citation Analysis (0)] |
| 113. | Ashwood P, Krakowiak P, Hertz-Picciotto I, Hansen R, Pessah IN, Van de Water J. Associations of impaired behaviors with elevated plasma chemokines in autism spectrum disorders. J Neuroimmunol. 2011;232:196-199. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 230] [Cited by in RCA: 216] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 114. | Collins E, Gu F, Qi M, Molano I, Ruiz P, Sun L, Gilkeson GS. Differential efficacy of human mesenchymal stem cells based on source of origin. J Immunol. 2014;193:4381-4390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 115. | Friedenstein AJ, Gorskaja JF, Kulagina NN. Fibroblast precursors in normal and irradiated mouse hematopoietic organs. Exp Hematol. 1976;4:267-274. [PubMed] |
| 116. | Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, Alfonso ZC, Fraser JK, Benhaim P, Hedrick MH. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13:4279-4295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4817] [Cited by in RCA: 5013] [Article Influence: 218.0] [Reference Citation Analysis (0)] |
| 117. | In't Anker PS, Scherjon SA, Kleijburg-van der Keur C, de Groot-Swings GM, Claas FH, Fibbe WE, Kanhai HH. Isolation of mesenchymal stem cells of fetal or maternal origin from human placenta. Stem Cells. 2004;22:1338-1345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 824] [Cited by in RCA: 818] [Article Influence: 40.9] [Reference Citation Analysis (0)] |
| 118. | Shih DT, Lee DC, Chen SC, Tsai RY, Huang CT, Tsai CC, Shen EY, Chiu WT. Isolation and characterization of neurogenic mesenchymal stem cells in human scalp tissue. Stem Cells. 2005;23:1012-1020. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 170] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 119. | Erices A, Conget P, Minguell JJ. Mesenchymal progenitor cells in human umbilical cord blood. Br J Haematol. 2000;109:235-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1137] [Cited by in RCA: 1077] [Article Influence: 43.1] [Reference Citation Analysis (0)] |
| 120. | Sarugaser R, Lickorish D, Baksh D, Hosseini MM, Davies JE. Human umbilical cord perivascular (HUCPV) cells: a source of mesenchymal progenitors. Stem Cells. 2005;23:220-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 600] [Cited by in RCA: 559] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 121. | Wang HS, Hung SC, Peng ST, Huang CC, Wei HM, Guo YJ, Fu YS, Lai MC, Chen CC. Mesenchymal stem cells in the Wharton's jelly of the human umbilical cord. Stem Cells. 2004;22:1330-1337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 946] [Cited by in RCA: 917] [Article Influence: 45.9] [Reference Citation Analysis (0)] |
| 122. | Nadri S, Soleimani M. Comparative analysis of mesenchymal stromal cells from murine bone marrow and amniotic fluid. Cytotherapy. 2007;9:729-737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 39] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 123. | De Bari C, Dell'Accio F, Tylzanowski P, Luyten FP. Multipotent mesenchymal stem cells from adult human synovial membrane. Arthritis Rheum. 2001;44:1928-1942. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 124. | Patki S, Kadam S, Chandra V, Bhonde R. Human breast milk is a rich source of multipotent mesenchymal stem cells. Hum Cell. 2010;23:35-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 111] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 125. | Griffiths MJ, Bonnet D, Janes SM. Stem cells of the alveolar epithelium. Lancet. 2005;366:249-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 95] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 126. | Beltrami AP, Barlucchi L, Torella D, Baker M, Limana F, Chimenti S, Kasahara H, Rota M, Musso E, Urbanek K, Leri A, Kajstura J, Nadal-Ginard B, Anversa P. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell. 2003;114:763-776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2692] [Cited by in RCA: 2439] [Article Influence: 110.9] [Reference Citation Analysis (0)] |
| 127. | Hida N, Nishiyama N, Miyoshi S, Kira S, Segawa K, Uyama T, Mori T, Miyado K, Ikegami Y, Cui C, Kiyono T, Kyo S, Shimizu T, Okano T, Sakamoto M, Ogawa S, Umezawa A. Novel cardiac precursor-like cells from human menstrual blood-derived mesenchymal cells. Stem Cells. 2008;26:1695-1704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 239] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 128. | Gargett CE, Schwab KE, Zillwood RM, Nguyen HP, Wu D. Isolation and culture of epithelial progenitors and mesenchymal stem cells from human endometrium. Biol Reprod. 2009;80:1136-1145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 363] [Cited by in RCA: 361] [Article Influence: 22.6] [Reference Citation Analysis (1)] |
| 129. | Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15372] [Cited by in RCA: 15199] [Article Influence: 584.6] [Reference Citation Analysis (0)] |
| 130. | Prasanna SJ, Gopalakrishnan D, Shankar SR, Vasandan AB. Pro-inflammatory cytokines, IFNgamma and TNFalpha, influence immune properties of human bone marrow and Wharton jelly mesenchymal stem cells differentially. PLoS One. 2010;5:e9016. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 324] [Cited by in RCA: 355] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 131. | Kim SM, Jeong CH, Woo JS, Ryu CH, Lee JH, Jeun SS. In vivo near-infrared imaging for the tracking of systemically delivered mesenchymal stem cells: tropism for brain tumors and biodistribution. Int J Nanomedicine. 2015;11:13-23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 132. | Karp JM, Leng Teo GS. Mesenchymal stem cell homing: the devil is in the details. Cell Stem Cell. 2009;4:206-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1012] [Cited by in RCA: 1086] [Article Influence: 67.9] [Reference Citation Analysis (0)] |
| 133. | Danielyan L, Schäfer R, von Ameln-Mayerhofer A, Buadze M, Geisler J, Klopfer T, Burkhardt U, Proksch B, Verleysdonk S, Ayturan M, Buniatian GH, Gleiter CH, Frey WH 2nd. Intranasal delivery of cells to the brain. Eur J Cell Biol. 2009;88:315-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 226] [Cited by in RCA: 256] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 134. | Eggenhofer E, Benseler V, Kroemer A, Popp FC, Geissler EK, Schlitt HJ, Baan CC, Dahlke MH, Hoogduijn MJ. Mesenchymal stem cells are short-lived and do not migrate beyond the lungs after intravenous infusion. Front Immunol. 2012;3:297. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 439] [Cited by in RCA: 586] [Article Influence: 45.1] [Reference Citation Analysis (0)] |
| 135. | Liu XB, Chen H, Chen HQ, Zhu MF, Hu XY, Wang YP, Jiang Z, Xu YC, Xiang MX, Wang JA. Angiopoietin-1 preconditioning enhances survival and functional recovery of mesenchymal stem cell transplantation. J Zhejiang Univ Sci B. 2012;13:616-623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 136. | Eggenhofer E, Luk F, Dahlke MH, Hoogduijn MJ. The life and fate of mesenchymal stem cells. Front Immunol. 2014;5:148. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 270] [Cited by in RCA: 342] [Article Influence: 31.1] [Reference Citation Analysis (0)] |
| 137. | Caplan AI, Correa D. The MSC: an injury drugstore. Cell Stem Cell. 2011;9:11-15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1372] [Cited by in RCA: 1224] [Article Influence: 87.4] [Reference Citation Analysis (0)] |
| 138. | Gesundheit B, Ashwood P, Keating A, Naor D, Melamed M, Rosenzweig JP. Therapeutic properties of mesenchymal stem cells for autism spectrum disorders. Med Hypotheses. 2015;84:169-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 139. | Ebert DH, Greenberg ME. Activity-dependent neuronal signalling and autism spectrum disorder. Nature. 2013;493:327-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 410] [Cited by in RCA: 483] [Article Influence: 40.3] [Reference Citation Analysis (0)] |
| 140. | Chopp M, Li Y. Treatment of neural injury with marrow stromal cells. Lancet Neurol. 2002;1:92-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 478] [Cited by in RCA: 475] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 141. | Spejo AB, Carvalho JL, Goes AM, Oliveira AL. Neuroprotective effects of mesenchymal stem cells on spinal motoneurons following ventral root axotomy: synapse stability and axonal regeneration. Neuroscience. 2013;250:715-732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 53] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 142. | Nauta AJ, Fibbe WE. Immunomodulatory properties of mesenchymal stromal cells. Blood. 2007;110:3499-3506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1289] [Cited by in RCA: 1335] [Article Influence: 74.2] [Reference Citation Analysis (1)] |
| 143. | Chen PM, Yen ML, Liu KJ, Sytwu HK, Yen BL. Immunomodulatory properties of human adult and fetal multipotent mesenchymal stem cells. J Biomed Sci. 2011;18:49. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 107] [Cited by in RCA: 126] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 144. | Uccelli A, Moretta L, Pistoia V. Mesenchymal stem cells in health and disease. Nat Rev Immunol. 2008;8:726-736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2360] [Cited by in RCA: 2699] [Article Influence: 168.7] [Reference Citation Analysis (0)] |
| 145. | Li N, Hua J. Interactions between mesenchymal stem cells and the immune system. Cell Mol Life Sci. 2017;74:2345-2360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 233] [Article Influence: 29.1] [Reference Citation Analysis (0)] |
| 146. | Gao F, Chiu SM, Motan DA, Zhang Z, Chen L, Ji HL, Tse HF, Fu QL, Lian Q. Mesenchymal stem cells and immunomodulation: current status and future prospects. Cell Death Dis. 2016;7:e2062. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 611] [Cited by in RCA: 826] [Article Influence: 91.8] [Reference Citation Analysis (0)] |
| 147. | Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105:1815-1822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3271] [Cited by in RCA: 3277] [Article Influence: 156.0] [Reference Citation Analysis (0)] |
| 148. | Girard SD, Virard I, Lacassagne E, Paumier JM, Lahlou H, Jabes F, Molino Y, Stephan D, Baranger K, Belghazi M, Deveze A, Khrestchatisky M, Nivet E, Roman FS, Féron F. From Blood to Lesioned Brain: An In Vitro Study on Migration Mechanisms of Human Nasal Olfactory Stem Cells. Stem Cells Int. 2017;2017:1478606. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 149. | Rüster B, Göttig S, Ludwig RJ, Bistrian R, Müller S, Seifried E, Gille J, Henschler R. Mesenchymal stem cells display coordinated rolling and adhesion behavior on endothelial cells. Blood. 2006;108:3938-3944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 415] [Cited by in RCA: 412] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 150. | Delarosa O, Dalemans W, Lombardo E. Toll-like receptors as modulators of mesenchymal stem cells. Front Immunol. 2012;3:182. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 115] [Cited by in RCA: 140] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 151. | Park C, Lee S, Cho IH, Lee HK, Kim D, Choi SY, Oh SB, Park K, Kim JS, Lee SJ. TLR3-mediated signal induces proinflammatory cytokine and chemokine gene expression in astrocytes: differential signaling mechanisms of TLR3-induced IP-10 and IL-8 gene expression. Glia. 2006;53:248-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 121] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 152. | Joh EH, Gu W, Kim DH. Echinocystic acid ameliorates lung inflammation in mice and alveolar macrophages by inhibiting the binding of LPS to TLR4 in NF-κB and MAPK pathways. Biochem Pharmacol. 2012;84:331-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 111] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 153. | Meylan E, Tschopp J, Karin M. Intracellular pattern recognition receptors in the host response. Nature. 2006;442:39-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 865] [Cited by in RCA: 880] [Article Influence: 46.3] [Reference Citation Analysis (0)] |
| 154. | Pisegna S, Pirozzi G, Piccoli M, Frati L, Santoni A, Palmieri G. p38 MAPK activation controls the TLR3-mediated up-regulation of cytotoxicity and cytokine production in human NK cells. Blood. 2004;104:4157-4164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 93] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 155. | Waterman RS, Tomchuck SL, Henkle SL, Betancourt AM. A new mesenchymal stem cell (MSC) paradigm: polarization into a pro-inflammatory MSC1 or an Immunosuppressive MSC2 phenotype. PLoS One. 2010;5:e10088. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 798] [Cited by in RCA: 949] [Article Influence: 63.3] [Reference Citation Analysis (0)] |
| 156. | Shirjang S, Mansoori B, Solali S, Hagh MF, Shamsasenjan K. Toll-like receptors as a key regulator of mesenchymal stem cell function: An up-to-date review. Cell Immunol. 2017;315:1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 79] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 157. | Mei SH, McCarter SD, Deng Y, Parker CH, Liles WC, Stewart DJ. Prevention of LPS-induced acute lung injury in mice by mesenchymal stem cells overexpressing angiopoietin 1. PLoS Med. 2007;4:e269. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 467] [Cited by in RCA: 494] [Article Influence: 27.4] [Reference Citation Analysis (0)] |
| 158. | Xu J, Woods CR, Mora AL, Joodi R, Brigham KL, Iyer S, Rojas M. Prevention of endotoxin-induced systemic response by bone marrow-derived mesenchymal stem cells in mice. Am J Physiol Lung Cell Mol Physiol. 2007;293:L131-L141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 270] [Cited by in RCA: 270] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 159. | Nemeth K, Mayer B, Mezey E. Modulation of bone marrow stromal cell functions in infectious diseases by toll-like receptor ligands. J Mol Med (Berl). 2010;88:5-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 58] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 160. | Gesundheit B, Rosenzweig JP, Naor D, Lerer B, Zachor DA, Procházka V, Melamed M, Kristt DA, Steinberg A, Shulman C, Hwang P, Koren G, Walfisch A, Passweg JR, Snowden JA, Tamouza R, Leboyer M, Farge-Bancel D, Ashwood P. Immunological and autoimmune considerations of Autism Spectrum Disorders. J Autoimmun. 2013;44:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 133] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 161. | Pistoia V, Raffaghello L. Mesenchymal stromal cells and autoimmunity. Int Immunol. 2017;29:49-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 55] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 162. | Zhao Y, Lin B, Darflinger R, Zhang Y, Holterman MJ, Skidgel RA. Human cord blood stem cell-modulated regulatory T lymphocytes reverse the autoimmune-caused type 1 diabetes in nonobese diabetic (NOD) mice. PLoS One. 2009;4:e4226. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 62] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 163. | Ma S, Chen X, Wang L, Wei Y, Ni Y, Chu Y, Liu Y, Zhu H, Zheng R, Zhang Y. Repairing effects of ICAM-1-expressing mesenchymal stem cells in mice with autoimmune thyroiditis. Exp Ther Med. 2017;13:1295-1302. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 164. | Uccelli A, Laroni A, Freedman MS. Mesenchymal stem cells for the treatment of multiple sclerosis and other neurological diseases. Lancet Neurol. 2011;10:649-656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 231] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 165. | Le Blanc K, Frassoni F, Ball L, Locatelli F, Roelofs H, Lewis I, Lanino E, Sundberg B, Bernardo ME, Remberger M, Dini G, Egeler RM, Bacigalupo A, Fibbe W, Ringdén O; Developmental Committee of the European Group for Blood and Marrow Transplantation. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet. 2008;371:1579-1586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2047] [Cited by in RCA: 2028] [Article Influence: 119.3] [Reference Citation Analysis (0)] |
| 166. | El-Akabawy G, Rashed LA. Beneficial effects of bone marrow-derived mesenchymal stem cell transplantation in a non-immune model of demyelination. Ann Anat. 2015;198:11-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 167. | Boieri M, Shah P, Dressel R, Inngjerdingen M. The Role of Animal Models in the Study of Hematopoietic Stem Cell Transplantation and GvHD: A Historical Overview. Front Immunol. 2016;7:333. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 35] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 168. | Ha S, Park H, Mahmood U, Ra JC, Suh YH, Chang KA. Human adipose-derived stem cells ameliorate repetitive behavior, social deficit and anxiety in a VPA-induced autism mouse model. Behav Brain Res. 2017;317:479-484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 169. | Gobshtis N, Tfilin M, Wolfson M, Fraifeld VE, Turgeman G. Transplantation of mesenchymal stem cells reverses behavioural deficits and impaired neurogenesis caused by prenatal exposure to valproic acid. Oncotarget. 2017;8:17443-17452. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 33] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 170. | Ellegood J, Crawley JN. Behavioral and Neuroanatomical Phenotypes in Mouse Models of Autism. Neurotherapeutics. 2015;12:521-533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 95] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 171. | Wahlsten D, Metten P, Crabbe JC. Survey of 21 inbred mouse strains in two laboratories reveals that BTBR T/+ tf/tf has severely reduced hippocampal commissure and absent corpus callosum. Brain Res. 2003;971:47-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 157] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 172. | Faraji J, Karimi M, Lawrence C, Mohajerani MH, Metz GAS. Non-diagnostic symptoms in a mouse model of autism in relation to neuroanatomy: the BTBR strain reinvestigated. Transl Psychiatry. 2018;8:234. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 173. | Segal-Gavish H, Karvat G, Barak N, Barzilay R, Ganz J, Edry L, Aharony I, Offen D, Kimchi T. Mesenchymal Stem Cell Transplantation Promotes Neurogenesis and Ameliorates Autism Related Behaviors in BTBR Mice. Autism Res. 2016;9:17-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 80] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 174. | Perets N, Segal-Gavish H, Gothelf Y, Barzilay R, Barhum Y, Abramov N, Hertz S, Morozov D, London M, Offen D. Long term beneficial effect of neurotrophic factors-secreting mesenchymal stem cells transplantation in the BTBR mouse model of autism. Behav Brain Res. 2017;331:254-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 39] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 175. | Bahat-Stroomza M, Barhum Y, Levy YS, Karpov O, Bulvik S, Melamed E, Offen D. Induction of adult human bone marrow mesenchymal stromal cells into functional astrocyte-like cells: potential for restorative treatment in Parkinson's disease. J Mol Neurosci. 2009;39:199-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 62] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 176. | Gothelf Y, Abramov N, Harel A, Offen D. Safety of repeated transplantations of neurotrophic factors-secreting human mesenchymal stromal stem cells. Clin Transl Med. 2014;3:21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 177. | Yu B, Zhang X, Li X. Exosomes derived from mesenchymal stem cells. Int J Mol Sci. 2014;15:4142-4157. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 624] [Cited by in RCA: 589] [Article Influence: 53.5] [Reference Citation Analysis (0)] |
| 178. | Xin H, Li Y, Buller B, Katakowski M, Zhang Y, Wang X, Shang X, Zhang ZG, Chopp M. Exosome-mediated transfer of miR-133b from multipotent mesenchymal stromal cells to neural cells contributes to neurite outgrowth. Stem Cells. 2012;30:1556-1564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 613] [Cited by in RCA: 702] [Article Influence: 54.0] [Reference Citation Analysis (0)] |
| 179. | Perets N, Hertz S, London M, Offen D. Intranasal administration of exosomes derived from mesenchymal stem cells ameliorates autistic-like behaviors of BTBR mice. Mol Autism. 2018;9:57. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 110] [Cited by in RCA: 119] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 180. | Lv YT, Zhang Y, Liu M, Qiuwaxi JN, Ashwood P, Cho SC, Huan Y, Ge RC, Chen XW, Wang ZJ, Kim BJ, Hu X. Transplantation of human cord blood mononuclear cells and umbilical cord-derived mesenchymal stem cells in autism. J Transl Med. 2013;11:196. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 118] [Cited by in RCA: 112] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 181. | Sharma A, Gokulchandran N, Sane H, Nagrajan A, Paranjape A, Kulkarni P, Shetty A, Mishra P, Kali M, Biju H, Badhe P. Autologous bone marrow mononuclear cell therapy for autism: an open label proof of concept study. Stem Cells Int. 2013;2013:623875. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 70] [Article Influence: 5.8] [Reference Citation Analysis (1)] |
| 182. | Bjørklund G, Kern JK, Urbina MA, Saad K, El-Houfey AA, Geier DA, Chirumbolo S, Geier MR, Mehta JA, Aaseth J. Cerebral hypoperfusion in autism spectrum disorder. Acta Neurobiol Exp (Wars). 2018;78:21-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 183. | Bansal H, Verma P, Agrawal A, Leon J, Sundell IB, Koka PS. A Short Study Report on Bone Marrow Aspirate Concentrate Cell Therapy in Ten South Asian Indian Patients with Autism. J Stem Cells. 2016;11:25-36. [PubMed] |
| 184. | Dawson G, Sun JM, Davlantis KS, Murias M, Franz L, Troy J, Simmons R, Sabatos-DeVito M, Durham R, Kurtzberg J. Autologous Cord Blood Infusions Are Safe and Feasible in Young Children with Autism Spectrum Disorder: Results of a Single-Center Phase I Open-Label Trial. Stem Cells Transl Med. 2017;6:1332-1339. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 82] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 185. | Murias M, Major S, Compton S, Buttinger J, Sun JM, Kurtzberg J, Dawson G. Electrophysiological Biomarkers Predict Clinical Improvement in an Open-Label Trial Assessing Efficacy of Autologous Umbilical Cord Blood for Treatment of Autism. Stem Cells Transl Med. 2018;7:783-791. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (1)] |
| 186. | Chez M, Lepage C, Parise C, Dang-Chu A, Hankins A, Carroll M. Safety and Observations from a Placebo-Controlled, Crossover Study to Assess Use of Autologous Umbilical Cord Blood Stem Cells to Improve Symptoms in Children with Autism. Stem Cells Transl Med. 2018;7:333-341. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 44] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 187. | Li Q, Leung YO, Zhou I, Ho LC, Kong W, Basil P, Wei R, Lam S, Zhang X, Law AC, Chua SE, Sham PC, Wu EX, McAlonan GM. Dietary supplementation with n-3 fatty acids from weaning limits brain biochemistry and behavioural changes elicited by prenatal exposure to maternal inflammation in the mouse model. Transl Psychiatry. 2015;5:e641. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 41] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 188. | Sharma SR, Gonda X, Tarazi FI. Autism Spectrum Disorder: Classification, diagnosis and therapy. Pharmacol Ther. 2018;190:91-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 303] [Article Influence: 43.3] [Reference Citation Analysis (0)] |
| 189. | Estes ML, McAllister AK. Immune mediators in the brain and peripheral tissues in autism spectrum disorder. Nat Rev Neurosci. 2015;16:469-486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 300] [Cited by in RCA: 351] [Article Influence: 35.1] [Reference Citation Analysis (0)] |
| 190. | Lalu MM, McIntyre L, Pugliese C, Fergusson D, Winston BW, Marshall JC, Granton J, Stewart DJ; Canadian Critical Care Trials Group. Safety of cell therapy with mesenchymal stromal cells (SafeCell): a systematic review and meta-analysis of clinical trials. PLoS One. 2012;7:e47559. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 901] [Cited by in RCA: 848] [Article Influence: 65.2] [Reference Citation Analysis (0)] |
| 191. | Simberlund J, Ferretti CJ, Hollander E. Mesenchymal stem cells in autism spectrum and neurodevelopmental disorders: pitfalls and potential promises. World J Biol Psychiatry. 2015;16:368-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |