Published online Dec 26, 2019. doi: 10.4252/wjsc.v11.i12.1104
Peer-review started: March 4, 2019
First decision: August 1, 2019
Revised: August 25, 2019
Accepted: September 13, 2019
Article in press: September 13, 2019
Published online: December 26, 2019
Processing time: 273 Days and 5.3 Hours
Stem cells have shown great potential in vascular repair. Numerous evidence indicates that mechanical forces such as shear stress and cyclic strain can regulate the adhesion, proliferation, migration, and differentiation of stem cells via serious signaling pathways. The enrichment and differentiation of stem cells play an important role in the angiogenesis and maintenance of vascular homeostasis. In normal tissues, blood flow directly affects the microenvironment of vascular endothelial cells (ECs); in pathological status, the abnormal interactions between blood flow and vessels contribute to the injury of vessels. Next, the altered mechanical forces are transduced into cells by mechanosensors to trigger the reformation of vessels. This process occurs when signaling pathways related to EC differentiation are initiated. Hence, a deep understanding of the responses of stem cells to mechanical stresses and the underlying mechanisms involved in this process is essential for clinical translation. In this the review, we provide an overview of the role of stem cells in vascular repair, outline the performance of stem cells under the mechanical stress stimulation, and describe the related signaling pathways.
Core tip: Stem cells and biomechanical stresses are very important for the success of stem cell-based therapy. In this review paper, we first summarize the application of stem cells for vascular repair, then discuss the response of stem cells to the biomechanical stresses in blood vessels, and finally describe the underlying mechanisms. This paper should be very beneficial to researchers in this field, as it provides a deeper understanding of the interactions between stem cells and biomechanical stresses for vascular repair.
- Citation: Tian GE, Zhou JT, Liu XJ, Huang YC. Mechanoresponse of stem cells for vascular repair. World J Stem Cells 2019; 11(12): 1104-1114
- URL: https://www.wjgnet.com/1948-0210/full/v11/i12/1104.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v11.i12.1104
Adult stem cells derived from the same precursors have potential functions for tissue development and regeneration including bone regeneration, wound healing, and vessel repair[1,2]. Traditionally, the damaged endothelium in vessel wall was thought to be replaced by nearby endothelial cell (EC) replication. However, recent findings challenge this notion and point out that stem cells also participate in the process of vascular repair. In fact, the promising role of stem cells in vascular repair has been well determined by numerous studies in vitro and in vivo experimental settings[3,4].
The repair processes includes relative signaling pathway activation, gene expression, oxidative balance and alignment of cytoskeletal filaments[5]. Based on these scientific outcomes, it is feasible for scientists to use stem/progenitor cells with or without scaffolds to create bioengineered vessels in vitro that are suitable for grafting clinically[6]. However, the key factors influencing the successful utilization of bioengineered vessel is the replication of different physical forces generated by blood flow during the cardiac cycle and the understanding of how these physical forces affect the biological behaviors of the grafted and resident stem cells[7].
The biomechanical patterns of blood flow in vessels are very complex[8]. Among these types of biomechanical stress, shear stress and strain stress are the major components[9]. Many studies have determined that these two stresses contribute to the repair of vessel lesion, as well as vessel injury and remodeling[10,11]. Importantly, they are involved in the process of rearrangement of vascular ECs and smooth muscle cells (SMCs), and they also regulate the differentiation of several types of stem cells, including resident or circulating progenitor cells[12] and stem cells derived from other sources[13]. To more deeply understand the response of stem cells to biomechanical stress and the underlying mechanisms, in this review paper, we summarize the use of stem cells for vascular repair, outline the role of biomechanical stress for vascular injury and repair, and emphasize how shear stress and strain stress regulate the behavior of stem cells for vascular repair. Furthermore, the transduction of biomechanical signals into stem cells are discussed.
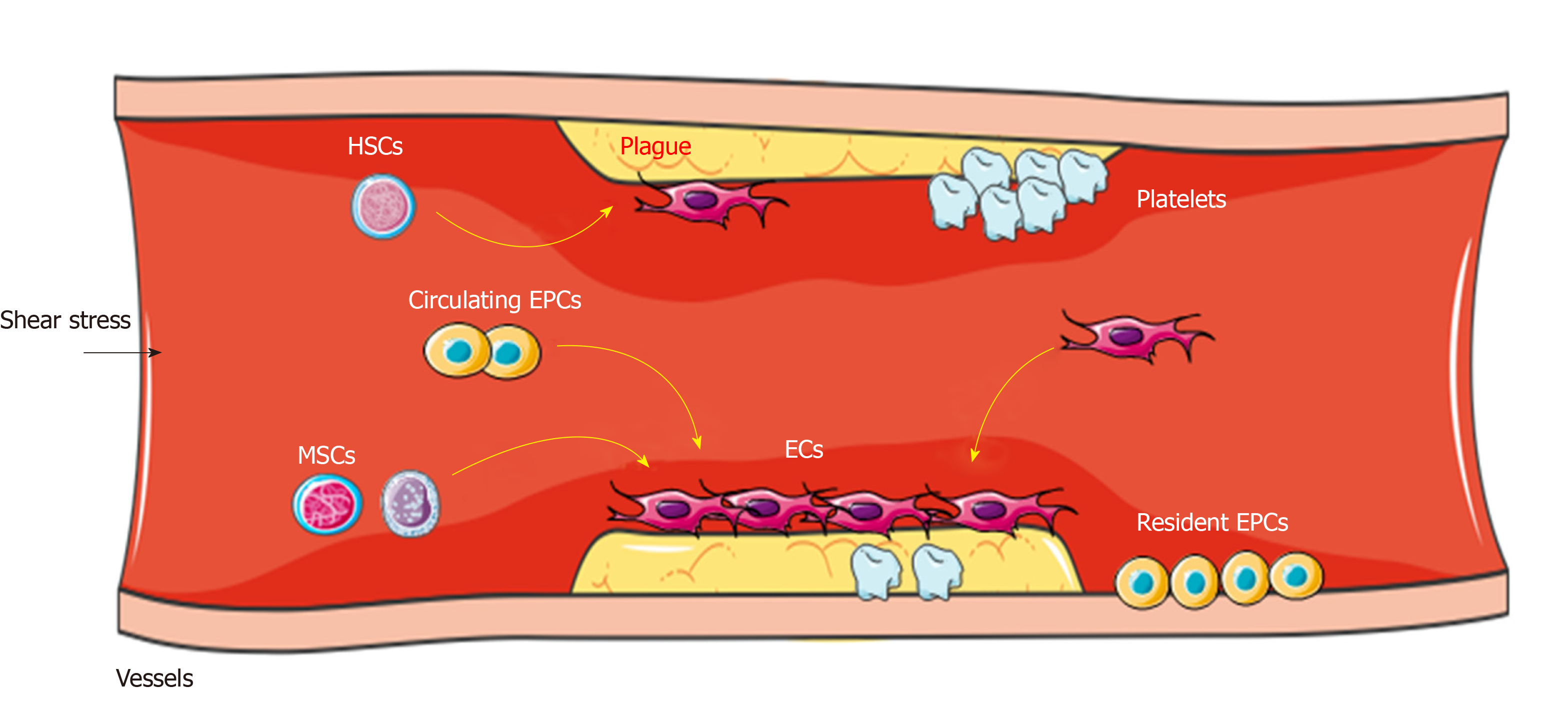
In the formation of blood vessels of angioblasts during embryogenesis, the peripheral endothelial progenitor cells (EPCs) and inner hematopoietic stem cells (HSCs) form blood islands and they participate in vascular repair[14]. In the process of vascular repair, quiescent stem cells such as HSCs and mesenchymal stem cells (MSCs) mobilize from bone marrow into the circulation, differentiate into EPCs (as circulating EPCs), and are home to the area of lesion (where they can contact and sense the blood flow) to participate in neovessel formation[15] (Figure 1). This phenomenon indicates that neovascular formation in adults may be the result of proliferation, migration, and remodeling of stem cells.
Several studies have shown that CD34+CD133+ EPCs can differentiate into ECs and enhance angiogenesis in injury vessels, as well as grafted vessels[16,17]. Endothelial colony-forming cells as a rare population of ECs, can be isolated from peripheral blood mononuclear cells and share characteristics with EPCs, including expression of the endothelial marker and vessel regeneration ability[18]. In addition, MSCs derived from bone marrow can differentiate into a variety of cell types and contribute to vascular reconstruction[3]. Beyond that, MSCs derived from umbilical cord, adipose tissue, dental pulp, and hair follicle also have the potential to differentiate into EPCs in injury conditions[18-20].
At the beginning of vessel repair, a part of EPCs directly incorporate into vessel intima and differentiate into ECs with active angiogenesis, while the other part of EPCs display a proliferative potential[21]. The mechanism of EPCs promoting the angiogenesis varies greatly, including the direct formation of neovessels and the production of paracrine signals such as vascular endothelial growth factor (VEGF), stromal cell-derived factor, and platelet-derived growth factor (PDGF), which further activate the proliferation and vascular repair of ECs[22]; this process depends on the recognition of markers on the cell surface. These findings suggest that vascular repair is probably induced by the interaction between stem cells and the certain microenvironment of injured vessels such as biomechanical stresses. Recently, stem cells combined with mechanical forces have been used in research and the clinic. For instance, the functional vessels constructed with scaffold and stem cells have the potential to promote stem cell differentiation into ECs during vessel grafting or damage[23]. In vitro experiments have found that the decellularized vessel scaffold surrounded by stem cells on both inner surface and the adventitial side can sense biomechanical forces under the pressure-driven perfusion with medium. Then these cells differentiate into both ECs and SMCs, which are induced by shear stress and strain stress respectively, in the bioengineered vessels[23-25]. A study reported that vascular grafts via EPCs seeding and maturation can rely on a controllable flow formed by bioreactor[26]; this strategy may be beneficial for utilizing EPCs in vascular repair.
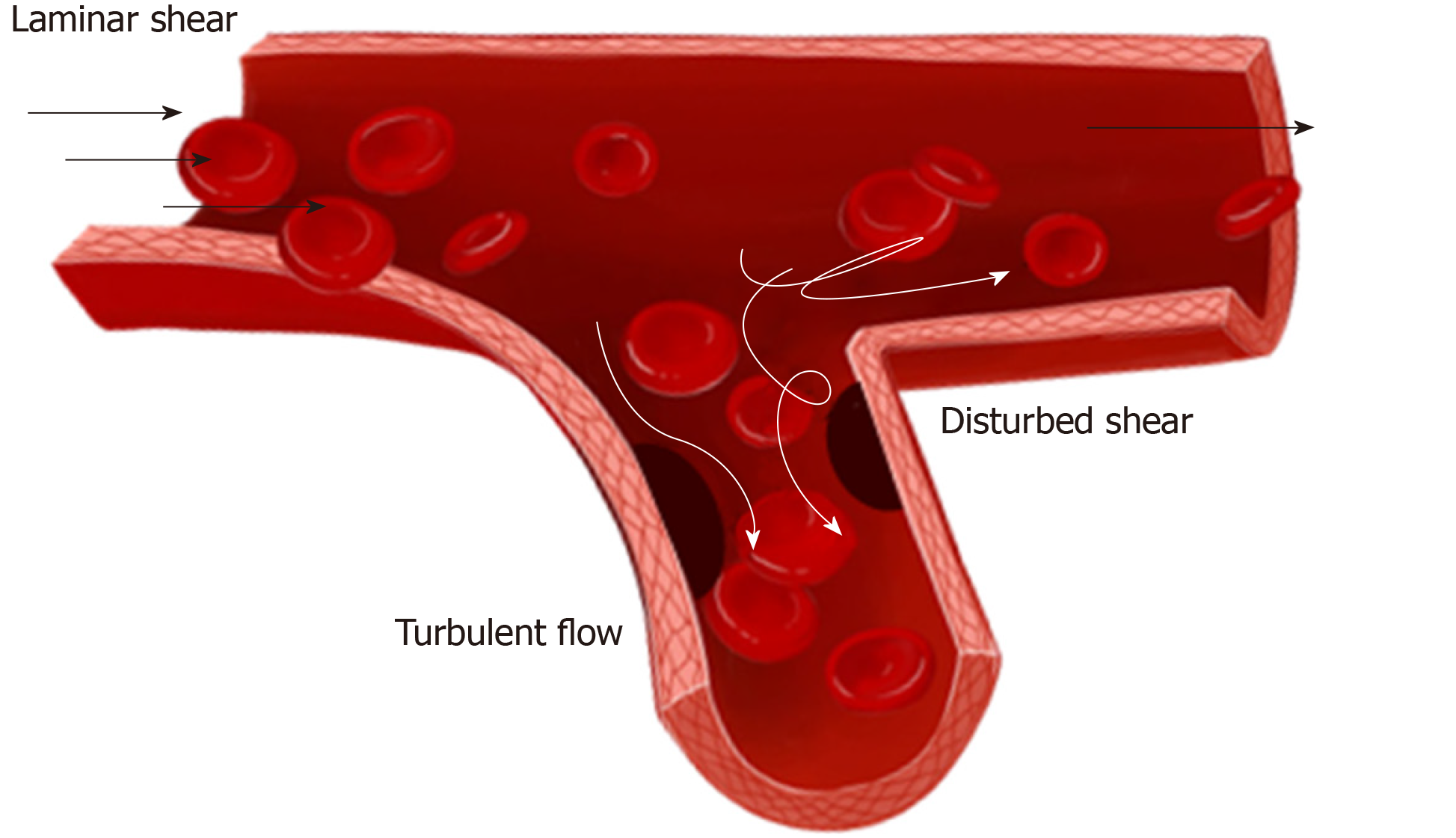
Studies on biomechanical forces have focused on their role in balancing the microenvironment of vessel, which is closely related to the vascular injury and repair[27]. Blood flow consists of two types: laminar and turbulent flows. There are three types of laminar flow, namely steady flow, pulsatile flow, and oscillatory flow. Among them, steady flow does not occur in arteries, while pulsatile and oscillatory flows are unsteady. In straight arterial areas, ECs are exposed to pulsatile shear stress generally between 10-20 and 40 dynes/cm2 as maximum[28]. In branch points, bifurcations, and curvatures, ECs are exposed to oscillatory shear stress of ± 4 dynes/cm2, where they easily develop atherosclerosis[28,29] (Figure 2). In physiological conditions, vessel intima is subjected to a fluid shear stress (average 10-20 dynes/cm2) caused by blood flowing[28].
The ECs display a fast turnover rate in certain regions such as bifurcations, branch points, and curved regions, while overall rates of cell turnover in the artery are very low because ECs experience various flow patterns[30]. Indeed, areas of low shear stress in human arteries have a relatively high rate of endothelial death, supporting the statement that high turnover rate of ECs is crucial to maintain vessel homeostasis[31]. Low and oscillatory shear stress is thought to play a causative role in endothelial dysfunction[32]. It is generally believed that endothelial dysfunction/loss is a common characteristic of vascular injury, which may cause severe cardiovascular disease such as atherosclerosis, hypertension, thrombosis, and ischemia/reperfusion injury[32,33].
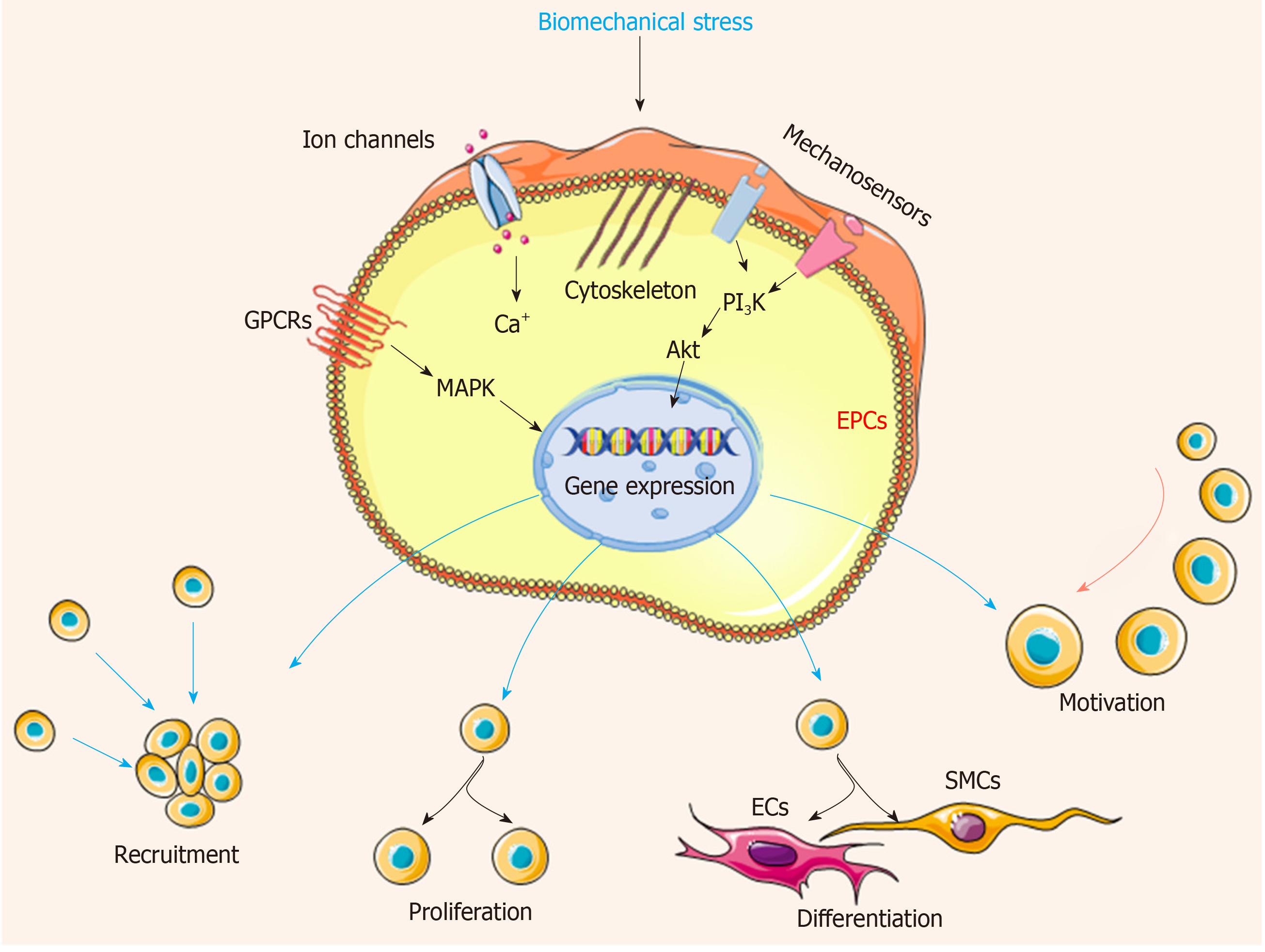
ECs with a variety of receptors can sense the altered flow and transmit mechanical signals through mechanosensitive signaling pathways, then activating a series of signaling cascades and cell events. Several potential mechanosensors including ion channels, cell surface or cytoplasmic receptors, integrins, kinases, and extracellular matrix components have been well determined[34,35]. When the blood flow changes, the mechanosensors quickly sense the signal, transduce to the downstream, and activate a series of cascades, finally triggering the physiological response including atherosclerosis, proliferation, angiogenesis, and inflammation[5,36]. Recently, the influence of biomechanical stresses in EPCs, MSCs, and other types of stem cells has been investigated. Mechanical stresses have been shown to increase the proliferation, differentiation, motivation, and recruitment of EPCs in the process of vascular repair[37] (Figure 3). In addition, cell functions are influenced by biomechanical stress including the activation of flow-sensitive ion channels, increased cell membrane permeability, release of several types of agonists (adenosine triphosphate, acetylcholine, and nitrous oxide), and mobilization of intracellular calcium (Ca2+), which keep the homeostasis of vascular system[38,39]. Subsequently, the following responses are triggered: increased cyclic guanosine monophosphate levels, cytoskeletal deformation, activation of mitogen-activated protein (MAP) kinase signaling cascades, transcription factors nuclear factor-kappa B (NF-κB) and nuclear factor activator protein-1[40]. Meanwhile, mechanical stresses regulate the expression of critical vasoactive and growth factors such as endothelin-1, nitric oxide synthase, PDGF A and B chains (PDGF-A and PDGF-B), and transforming growth factor β1, which have protective roles against atherosclerosis[41].
Over the past several years, it has been suggested that EPCs and other types of stem cells are home to the area of vascular damage to re-establish an intact endothelial layer after endothelium injury or damage[42]. Among various mechanical stimuli, shear stress is a critical factor to stimulate stem cells and activate downstream signaling. As a physical stimulus, shear stress plays a crucial role in signal transduction at focal adhesions, where the cell-extracellular matrix contacts[43]. In recent decades, researchers have explored the potential signaling pathways, but the precise mechanism is still unclear.
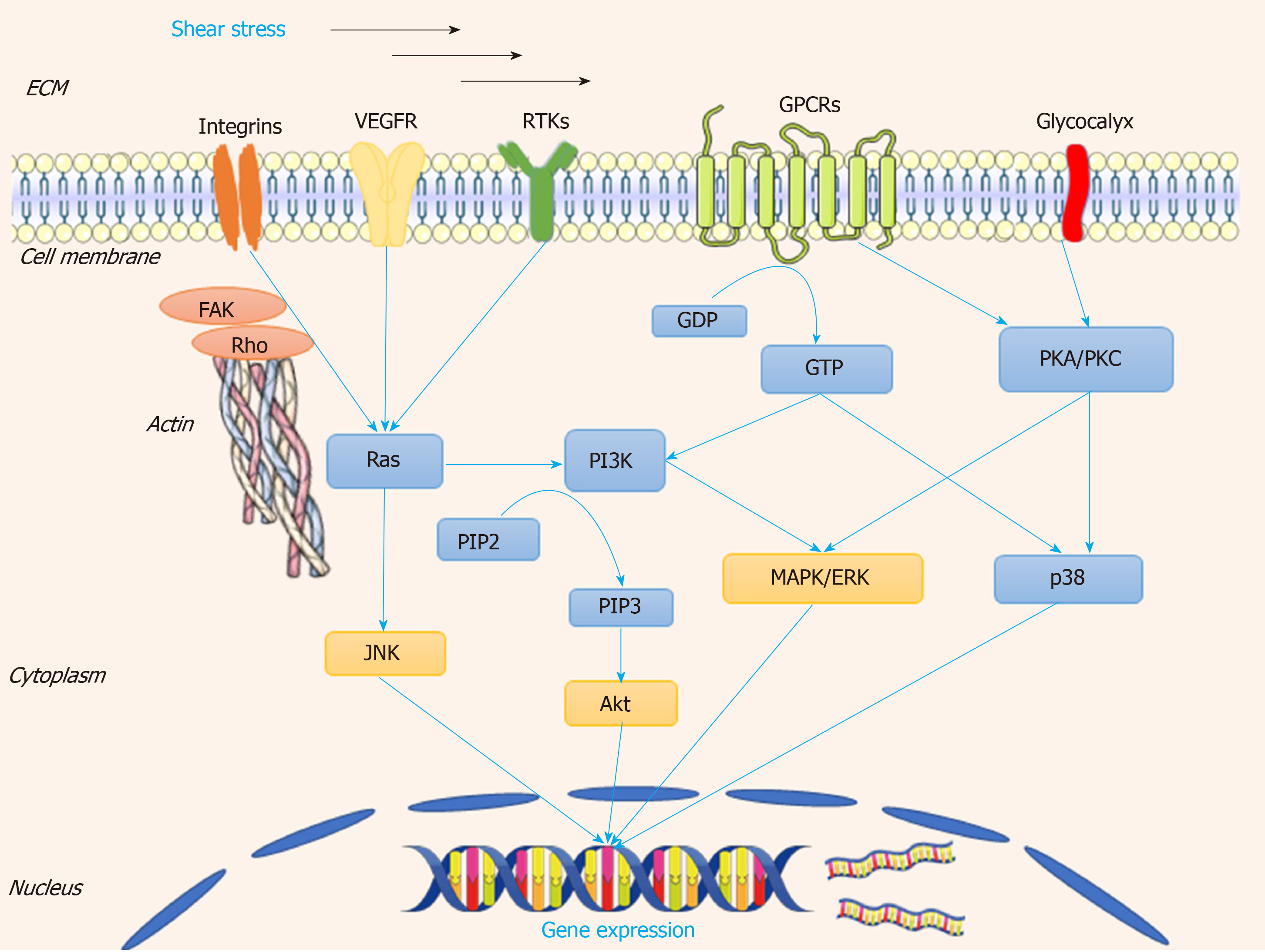
Once cells are stimulated by shear stress, at the early stage of response to the shear stress, several kinds of cells transform it to biochemical signals, and transmit into the nucleus to tune their physiological response. Several mechanosensitive molecules and/or compounds serve as “gatekeepers” in the whole process. The overall mechanosensors and the downstream are descripted in Figure 4.
Integrin has been verified to work as a mechanosensor[44]. It can be activated by shear stress and it accumulates at the vascular peripheral areas and is located along the stress fibers. Integrin and the associated RhoA small GTPase have been confirmed to participate in the process of sensing the shear stress and converting it to cascades of molecular signaling, which modulate gene expression[45]. These changes are involved in the process of anti-apoptosis, arrest of cell cycle, and morphological remodeling[46]. Inhibition of integrin β1 suppresses the formation of focal adhesions, which reversely verifies its role in vascular repair[20,47]. As one of the small G proteins, Ras is the earliest link between mechanical perception and transduction; Ras affects the downstream signal transduction cascades, which can be activated by integrin β1-related signals[45]. Then the above process is mediated by G protein-dependent activation of extracellular signal-regulated kinase (ERK) and JNK[48]. It has been demonstrated that Rho, Cdc42, and Rac (belonging to the Ras super-family of proteins) can modulate cytoskeletal rearrangement, EPC differentiation, and permeability of the endothelium after shear stress is applied[45].
Junctional adhesion receptors also play important roles in mechanoresponses[49,50]. VEGF receptor-2 (VEGFR-2) is required for the activation of most biomechanical stress-dependent signaling pathways[51,52]. VEGFR-2 can be activated by shear stress in a ligand-independent manner without the involvement of VEGF via two distinct signaling pathways in ECs: the phosphoinositide 3-kinase (PI3K)-Akt pathway and the protein kinase C-mitogen-activated protein kinase-ERK (PKC-MAPK-ERK). The PI3K-Akt pathway is a key regulator in shear stress-induced endothelial differentiation of EPCs[53,54]. In addition, suppression of the shear stress-induced phosphorylation of VEGFR2 by the VEGFR kinase inhibitor SU1498 abolishes the induction of EPC differentiation into vascular ECs; the inhibitor SU1498 blocks the shear stress-induced Notch cleavage in EPCs and suppresses expression of ephrinB2, which exerts a functional role in vascular repair[54]. It has also been reported that VE-cadherin works as an adapter, which is polarized activation of Rac1 in response to shear stress[55]. These results indicate the ability to repair damage vessels of EPCs by differentiation, and this ability has been verified using animal models[56].
PKC-MAPK-ERK is regulated by glycocalyx, which works as soon as the blood flow is initiated, and this signal activation is required for normal vascular development[57]. Previous studies have shown that in arteries, glycocalyx components are synthesized much more quickly under high shear stress than under low shear stress[58,59]. In a model of three-dimensional collagen-1 gel culture of SMCs, the flow-induced mechanotransduction could be sensed by glycocalyx biosynthesis, and then activated FAK-ERK1/2-C-Jun signaling pathway, leading to the up-regulation of MMP expression, cell migration and motility[60].
G protein-coupled receptors (GPCRs) show the ability to sense fluid shear stress, and the precise molecular mechanisms of mechanotransduction has been extensively studied[61,62]. Several GPCRs such as angiotensin II receptor type1 and bradykinin receptor B2 work as mechanosensors in vascular physiology[63]. It has been demonstrated that acute shear stresses induce various downstream signaling pathways such as phospholipase C, which further increases the intracellular Ca2+ concentration[64].
Endothelial injury is associated with activation of the coagulation system and recruitment of platelets[65]. The areas abundant with platelets promote recruitment and homing of EPCs which further lead to vessel formation[66]. Platelet EC adhesion molecule contains an immunoreceptor tyrosine-based inhibitory motif, which becomes the phosphorylated form when responding to shear stress, and directly induces the activation of ERK[67]. It was found that shear stress regulates the expression of endothelial markers von Willebrand factor and platelet EC adhesion molecule 1 in late EPCs, resulting in cytoskeletal arrangement, cell differentiation, and the activation of various mechanosensitive molecules including integrin β1, Ras, ERK1/2, paxillin, and FAK[68,69]. Mechanosensitive PPAP2B, an integral membrane protein involved in maintaining vascular integrity and EC rearrangement, is reduced as the result of low shear stress caused by vessel plaques[70].
Except EPCs, there are other types of stem cells involved in the process of vascular repair, for instance MSCs and EPCs derived from adipose[18], liver[71] and muscle[72]. It was found that when exposed to laminar shear stress at 0.5 dynes/cm2 with 30 min, MSCs contribute to the lack of polarity and upregulation of β-catenin downstream proteins, which are associated with cardiovascular development, EC protection, and angiogenesis[73]. When vascular injury occurs, MSCs resident in the medial intima of a healthy vessel can migrate to damaged areas and differentiate into SMCs[12]. Additionally, ECFCs isolated from the white adipose possess large expansion potential, stable differentiation, and robust in vivo vessel-forming capacity[18].
The vascular wall is subject to cyclic stretch of about 100-150 kPa, which is generated by the pulsatile blood pressure[74]. The excessive and pathological mechanical stretch occurring during hypertension is harmful as these high magnitude strain stress perturbs the vascular tone and causes improper cellular response of vascular wall, leading to cardiovascular diseases[32].
Venous bypass grafting is one of the most commonly used surgery for atherosclerosis patients; the insertion of a grafted vein into the arterial system probably exposes the vascular wall to the new hemodynamic environment, which has been considered to be a critical stimulus for vascular remodeling[74]. Cyclic strain stress generated after venous bypass grafting have been reported to regulate and change the functions of vascular smooth muscle cells (VSMCs) such as excessive proliferation, differentiation, and apoptosis[75].
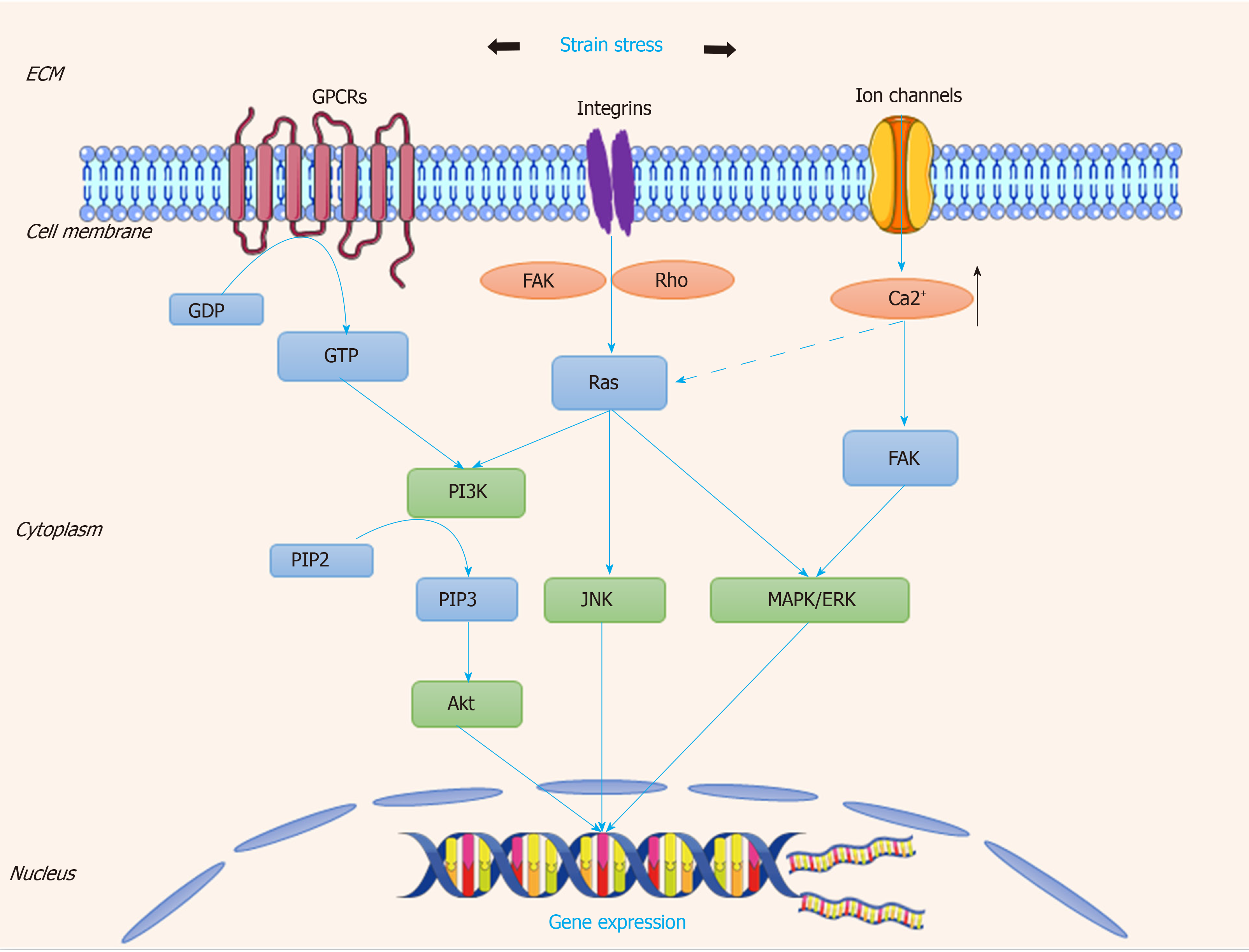
The cyclic strain is generated by the pulsatile of flow blood throughout one cardiac cycle to ensure SMCs within the wall maintain an active and contractile status[76]. Several membrane proteins or compounds have been found to be mechanosensitive to stretch, consisting of integrins, G-proteins, receptor tyrosine kinases (RTKs), and ion channels[77]. The overall signaling pathway is described in Figure 5.
Similarly, under the condition of shear stress, integrin molecules are involved in the pathway of extracellular matrix-integrin-cytoskeleton[78]. Normally, the strain stress applied to focal adhesion activates integrins and its downstream cascades including the focal adhesion kinase, G-proteins, Rho, and various signaling pathways related to stem cell differentiation[79]. A previous study showed that strain stress could induce stem cell-derived Sca-1+ progenitors to differentiate into SMCs via collagen IV- integrin-FAK-PI3-kinase-MAP kinase and PDGF receptor-beta signaling pathways[80].
G-proteins are another type of important mechanosensors in response to biomechanical stresses. Strain stress on cells allows structure changes in the G-proteins receptors, transducing mechanosignal into chemical signal and activating further signaling cascades[62]. These alternations may be related to iron channels and RTKs, which are considered to be the regulators for the development of stem cells. Thompson et al[81] found a novel mechanical target-mTORC2, which is critical for the proliferation, adipogenic, and cytoskeletal architecture of MSCs. Activation of mTORC2 requires focal adhesions and the binding of Fyn and FAK in vascular repair through the similar signaling pathway[81].
Ion channels also regulate the transduction of cyclically mechanical strain. As a ubiquitous secondary messenger, Ca2+ connects the inside and outside of cell, to maintain the homeostasis of cellular microenvironment. A study reported that VEGF-induced Ca2+ oscillations promote EPC growth and tubulogenesis by activating NF-κB[82,83]. It has been revealed that the ion channels stimulated by stretch induce Ca2+ influx in VSMCs and active the PKC signaling pathway, enhancing VSMC migration to lesion areas and accelerating the wound closure[84]. Interactions are present between these mechanosensors to strengthen the impacts of strain stress on activating the stem cells. TPRV4 ion channels can be activated by cyclic stretch, leading to cytoskeletal remodeling and cell reorientation via integrin-PI3K signaling[85]. Thus, strain stress can stimulate several types of mechanosensors, transmit biomechanical signaling into the nucleus, and regulate the related gene expression.
In this review, we first summarized the role of stem cells in vascular repair and then discussed the responses of stem cells to biomechanical stress and the underlying mechanisms. As the direct stimuli of vessel walls, mechanical forces play a crucial role in vascular injury and repair, which can directly activate the mechanosensing molecules. Mechanosensors of stem cells such as integrins, ion channels, GPCRs, RTKs, and VEGFR are able to sense the mechanical stresses and then are involved in the cytoskeleton rearrangement and finally regeneration of the endothelium. Manipulation of stem cells’ mechanosensors should be beneficial for vascular repair in clinics and the development of new therapeutic strategies. Therefore, identification of the mechanosensors and a full understanding of the molecular mechanism are essential to design effective treatments.
Many authors have proposed that increasing the number of stem cells is necessary to achieve sufficient vascular recovery and regeneration; hence, the safe and effective strategies to obtain enough number of stem cells which maintain the mechanical sensing potential are still a major challenge for the basic scientists and surgeons. Stem cells represent a promising tool for mechanical stresses sensing in the vasculature, but the methods to activate the resident and circulating stem cells and the underlying mechanisms for vascular repair remain unclear. The deeper understanding of how stem cells respond to mechanical forces should open a new dimension for the treatment of vascular disease, and enhance the clinical translation of stem cell-based strategy.
Manuscript source: Invited manuscript
Specialty type: Cell and tissue engineering
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Khan I, Sariboyaci AEE S-Editor: Dou Y L-Editor: Filipodia E-Editor: Ma YJ
| 1. | Ransom RC, Carter AC, Salhotra A, Leavitt T, Marecic O, Murphy MP, Lopez ML, Wei Y, Marshall CD, Shen EZ, Jones RE, Sharir A, Klein OD, Chan CKF, Wan DC, Chang HY, Longaker MT. Mechanoresponsive stem cells acquire neural crest fate in jaw regeneration. Nature. 2018;563:514-521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 129] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 2. | Ennis WJ, Sui A, Bartholomew A. Stem Cells and Healing: Impact on Inflammation. Adv Wound Care (New Rochelle). 2013;2:369-378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 86] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 3. | Kramann R, Goettsch C, Wongboonsin J, Iwata H, Schneider RK, Kuppe C, Kaesler N, Chang-Panesso M, Machado FG, Gratwohl S, Madhurima K, Hutcheson JD, Jain S, Aikawa E, Humphreys BD. Adventitial MSC-like Cells Are Progenitors of Vascular Smooth Muscle Cells and Drive Vascular Calcification in Chronic Kidney Disease. Cell Stem Cell. 2016;19:628-642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 246] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 4. | Eming SA, Martin P, Tomic-Canic M. Wound repair and regeneration: mechanisms, signaling, and translation. Sci Transl Med. 2014;6:265sr6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2114] [Cited by in RCA: 2136] [Article Influence: 194.2] [Reference Citation Analysis (0)] |
| 5. | Browning E, Wang H, Hong N, Yu K, Buerk DG, DeBolt K, Gonder D, Sorokina EM, Patel P, De Leon DD, Feinstein SI, Fisher AB, Chatterjee S. Mechanotransduction drives post ischemic revascularization through K(ATP) channel closure and production of reactive oxygen species. Antioxid Redox Signal. 2014;20:872-886. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 6. | Marrella A, Lee TY, Lee DH, Karuthedom S, Syla D, Chawla A, Khademhosseini A, Jang HL. Engineering vascularized and innervated bone biomaterials for improved skeletal tissue regeneration. Mater Today (Kidlington). 2018;21:362-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 203] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 7. | Potter CM, Lao KH, Zeng L, Xu Q. Role of biomechanical forces in stem cell vascular lineage differentiation. Arterioscler Thromb Vasc Biol. 2014;34:2184-2190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 48] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 8. | Welsh DG, Tran CHT, Hald BO, Sancho M. The Conducted Vasomotor Response: Function, Biophysical Basis, and Pharmacological Control. Annu Rev Pharmacol Toxicol. 2018;58:391-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 39] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 9. | Qi YX, Han Y, Jiang ZL. Mechanobiology and Vascular Remodeling: From Membrane to Nucleus. Adv Exp Med Biol. 2018;1097:69-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 10. | Xu Q. The role of stem cells in vein graft remodelling. Biochem Soc Trans. 2007;35:895-899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 11. | Zhou J, Li YS, Chien S. Shear stress-initiated signaling and its regulation of endothelial function. Arterioscler Thromb Vasc Biol. 2014;34:2191-2198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 285] [Cited by in RCA: 422] [Article Influence: 38.4] [Reference Citation Analysis (0)] |
| 12. | Swaminathan G, Stoilov I, Broekelmann T, Mecham R, Ramamurthi A. Phenotype-based selection of bone marrow mesenchymal stem cell-derived smooth muscle cells for elastic matrix regenerative repair in abdominal aortic aneurysms. J Tissue Eng Regen Med. 2018;12:e60-e70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 13. | Bobryshev YV, Orekhov AN, Chistiakov DA. Vascular stem/progenitor cells: current status of the problem. Cell Tissue Res. 2015;362:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 14. | Rafii S, Butler JM, Ding BS. Angiocrine functions of organ-specific endothelial cells. Nature. 2016;529:316-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 536] [Cited by in RCA: 701] [Article Influence: 77.9] [Reference Citation Analysis (0)] |
| 15. | Xu Q, Zhang Z, Davison F, Hu Y. Circulating progenitor cells regenerate endothelium of vein graft atherosclerosis, which is diminished in ApoE-deficient mice. Circ Res. 2003;93:e76-e86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 146] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 16. | Asahara T, Masuda H, Takahashi T, Kalka C, Pastore C, Silver M, Kearne M, Magner M, Isner JM. Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ Res. 1999;85:221-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2328] [Cited by in RCA: 2221] [Article Influence: 85.4] [Reference Citation Analysis (0)] |
| 17. | Del Papa N, Pignataro F. The Role of Endothelial Progenitors in the Repair of Vascular Damage in Systemic Sclerosis. Front Immunol. 2018;9:1383. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 41] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 18. | Lin RZ, Moreno-Luna R, Muñoz-Hernandez R, Li D, Jaminet SC, Greene AK, Melero-Martin JM. Human white adipose tissue vasculature contains endothelial colony-forming cells with robust in vivo vasculogenic potential. Angiogenesis. 2013;16:735-744. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 54] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 19. | Wang Y, Liu J, Tan X, Li G, Gao Y, Liu X, Zhang L, Li Y. Induced pluripotent stem cells from human hair follicle mesenchymal stem cells. Stem Cell Rev Rep. 2013;9:451-460. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 48] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 20. | Brown MA, Wallace CS, Angelos M, Truskey GA. Characterization of umbilical cord blood-derived late outgrowth endothelial progenitor cells exposed to laminar shear stress. Tissue Eng Part A. 2009;15:3575-3587. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 62] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 21. | Haybar H, Shahrabi S, Rezaeeyan H, Shirzad R, Saki N. Endothelial Cells: From Dysfunction Mechanism to Pharmacological Effect in Cardiovascular Disease. Cardiovasc Toxicol. 2019;19:13-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 92] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 22. | Lu W, Li X. Vascular stem/progenitor cells: functions and signaling pathways. Cell Mol Life Sci. 2018;75:859-869. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 23. | Wang K, Lin RZ, Melero-Martin JM. Bioengineering human vascular networks: trends and directions in endothelial and perivascular cell sources. Cell Mol Life Sci. 2019;76:421-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 42] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 24. | Margariti A, Winkler B, Karamariti E, Zampetaki A, Tsai TN, Baban D, Ragoussis J, Huang Y, Han JD, Zeng L, Hu Y, Xu Q. Direct reprogramming of fibroblasts into endothelial cells capable of angiogenesis and reendothelialization in tissue-engineered vessels. Proc Natl Acad Sci USA. 2012;109:13793-13798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 199] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 25. | Cooke JP. Therapeutic transdifferentiation: a novel approach for vascular disease. Circ Res. 2013;112:748-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (1)] |
| 26. | Melchiorri AJ, Bracaglia LG, Kimerer LK, Hibino N, Fisher JP. In Vitro Endothelialization of Biodegradable Vascular Grafts Via Endothelial Progenitor Cell Seeding and Maturation in a Tubular Perfusion System Bioreactor. Tissue Eng Part C Methods. 2016;22:663-670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 58] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 27. | Van der Heiden K, Gijsen FJ, Narracott A, Hsiao S, Halliday I, Gunn J, Wentzel JJ, Evans PC. The effects of stenting on shear stress: relevance to endothelial injury and repair. Cardiovasc Res. 2013;99:269-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 90] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 28. | Chiu JJ, Usami S, Chien S. Vascular endothelial responses to altered shear stress: pathologic implications for atherosclerosis. Ann Med. 2009;41:19-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 162] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 29. | Chatzizisis YS, Coskun AU, Jonas M, Edelman ER, Feldman CL, Stone PH. Role of endothelial shear stress in the natural history of coronary atherosclerosis and vascular remodeling: molecular, cellular, and vascular behavior. J Am Coll Cardiol. 2007;49:2379-2393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 969] [Cited by in RCA: 1021] [Article Influence: 56.7] [Reference Citation Analysis (0)] |
| 30. | Min E, Schwartz MA. Translocating transcription factors in fluid shear stress-mediated vascular remodeling and disease. Exp Cell Res. 2019;376:92-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 31. | Kwak BR, Bäck M, Bochaton-Piallat ML, Caligiuri G, Daemen MJ, Davies PF, Hoefer IE, Holvoet P, Jo H, Krams R, Lehoux S, Monaco C, Steffens S, Virmani R, Weber C, Wentzel JJ, Evans PC. Biomechanical factors in atherosclerosis: mechanisms and clinical implications. Eur Heart J. 2014;35:3013-3020, 3020a-3020d. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 261] [Cited by in RCA: 348] [Article Influence: 31.6] [Reference Citation Analysis (0)] |
| 32. | Cecchi E, Giglioli C, Valente S, Lazzeri C, Gensini GF, Abbate R, Mannini L. Role of hemodynamic shear stress in cardiovascular disease. Atherosclerosis. 2011;214:249-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 260] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 33. | Gisterå A, Hansson GK. The immunology of atherosclerosis. Nat Rev Nephrol. 2017;13:368-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 466] [Cited by in RCA: 665] [Article Influence: 83.1] [Reference Citation Analysis (0)] |
| 34. | Wang KC, Yeh YT, Nguyen P, Limqueco E, Lopez J, Thorossian S, Guan KL, Li YJ, Chien S. Flow-dependent YAP/TAZ activities regulate endothelial phenotypes and atherosclerosis. Proc Natl Acad Sci USA. 2016;113:11525-11530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 345] [Article Influence: 38.3] [Reference Citation Analysis (0)] |
| 35. | Rizzo V, Morton C, DePaola N, Schnitzer JE, Davies PF. Recruitment of endothelial caveolae into mechanotransduction pathways by flow conditioning in vitro. Am J Physiol Heart Circ Physiol. 2003;285:H1720-H1729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 156] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 36. | Dorland YL, Huveneers S. Cell-cell junctional mechanotransduction in endothelial remodeling. Cell Mol Life Sci. 2017;74:279-292. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 105] [Cited by in RCA: 127] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 37. | Xu Q. Progenitor cells in vascular repair. Curr Opin Lipidol. 2007;18:534-539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 44] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 38. | Lim CG, Jang J, Kim C. Cellular machinery for sensing mechanical force. BMB Rep. 2018;51:623-629. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 41] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 39. | Chistiakov DA, Orekhov AN, Bobryshev YV. Effects of shear stress on endothelial cells: go with the flow. Acta Physiol (Oxf). 2017;219:382-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 292] [Article Influence: 36.5] [Reference Citation Analysis (0)] |
| 40. | Zemskov EA, Lu Q, Ornatowski W, Klinger CN, Desai AA, Maltepe E, Yuan JX, Wang T, Fineman JR, Black SM. Biomechanical Forces and Oxidative Stress: Implications for Pulmonary Vascular Disease. Antioxid Redox Signal. 2019;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 41. | Hu X, Margadant FM, Yao M, Sheetz MP. Molecular stretching modulates mechanosensing pathways. Protein Sci. 2017;26:1337-1351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 55] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 42. | Campioni D, Zauli G, Gambetti S, Campo G, Cuneo A, Ferrari R, Secchiero P. In vitro characterization of circulating endothelial progenitor cells isolated from patients with acute coronary syndrome. PLoS One. 2013;8:e56377. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 43. | Yamamoto K, Ando J. Emerging Role of Plasma Membranes in Vascular Endothelial Mechanosensing. Circ J. 2018;82:2691-2698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 44. | Shyy JY, Chien S. Role of integrins in endothelial mechanosensing of shear stress. Circ Res. 2002;91:769-775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 421] [Cited by in RCA: 407] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 45. | Tzima E. Role of small GTPases in endothelial cytoskeletal dynamics and the shear stress response. Circ Res. 2006;98:176-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 216] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 46. | Kroon J, Heemskerk N, Kalsbeek MJT, de Waard V, van Rijssel J, van Buul JD. Flow-induced endothelial cell alignment requires the RhoGEF Trio as a scaffold protein to polarize active Rac1 distribution. Mol Biol Cell. 2017;28:1745-1753. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 34] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 47. | Egorova AD, DeRuiter MC, de Boer HC, van de Pas S, Gittenberger-de Groot AC, van Zonneveld AJ, Poelmann RE, Hierck BP. Endothelial colony-forming cells show a mature transcriptional response to shear stress. In Vitro Cell Dev Biol Anim. 2012;48:21-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 40] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 48. | Hall A, Nobes CD. Rho GTPases: molecular switches that control the organization and dynamics of the actin cytoskeleton. Philos Trans R Soc Lond B Biol Sci. 2000;355:965-970. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 373] [Cited by in RCA: 386] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 49. | Kim DH, Khatau SB, Feng Y, Walcott S, Sun SX, Longmore GD, Wirtz D. Actin cap associated focal adhesions and their distinct role in cellular mechanosensing. Sci Rep. 2012;2:555. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 135] [Cited by in RCA: 144] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 50. | Krishna L, Dhamodaran K, Jayadev C, Chatterjee K, Shetty R, Khora SS, Das D. Nanostructured scaffold as a determinant of stem cell fate. Stem Cell Res Ther. 2016;7:188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 86] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 51. | Tzima E, Irani-Tehrani M, Kiosses WB, Dejana E, Schultz DA, Engelhardt B, Cao G, DeLisser H, Schwartz MA. A mechanosensory complex that mediates the endothelial cell response to fluid shear stress. Nature. 2005;437:426-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1220] [Cited by in RCA: 1294] [Article Influence: 64.7] [Reference Citation Analysis (0)] |
| 52. | Prasai PK, Shrestha B, Orr AW, Pattillo CB. Decreases in GSH:GSSG activate vascular endothelial growth factor receptor 2 (VEGFR2) in human aortic endothelial cells. Redox Biol. 2018;19:22-27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 53. | Ye C, Bai L, Yan ZQ, Wang YH, Jiang ZL. Shear stress and vascular smooth muscle cells promote endothelial differentiation of endothelial progenitor cells via activation of Akt. Clin Biomech (Bristol, Avon). 2008;23 Suppl 1:S118-S124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 43] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 54. | Masumura T, Yamamoto K, Shimizu N, Obi S, Ando J. Shear stress increases expression of the arterial endothelial marker ephrinB2 in murine ES cells via the VEGF-Notch signaling pathways. Arterioscler Thromb Vasc Biol. 2009;29:2125-2131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 145] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 55. | Liu Y, Collins C, Kiosses WB, Murray AM, Joshi M, Shepherd TR, Fuentes EJ, Tzima E. A novel pathway spatiotemporally activates Rac1 and redox signaling in response to fluid shear stress. J Cell Biol. 2013;201:863-873. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 59] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 56. | Xu Q. Mouse models of arteriosclerosis: from arterial injuries to vascular grafts. Am J Pathol. 2004;165:1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 80] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 57. | Henderson-Toth CE, Jahnsen ED, Jamarani R, Al-Roubaie S, Jones EA. The glycocalyx is present as soon as blood flow is initiated and is required for normal vascular development. Dev Biol. 2012;369:330-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 58. | Koo A, Dewey CF, García-Cardeña G. Hemodynamic shear stress characteristic of atherosclerosis-resistant regions promotes glycocalyx formation in cultured endothelial cells. Am J Physiol Cell Physiol. 2013;304:C137-C146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 114] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 59. | Fu BM, Tarbell JM. Mechano-sensing and transduction by endothelial surface glycocalyx: composition, structure, and function. Wiley Interdiscip Rev Syst Biol Med. 2013;5:381-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 128] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 60. | Shi ZD, Wang H, Tarbell JM. Heparan sulfate proteoglycans mediate interstitial flow mechanotransduction regulating MMP-13 expression and cell motility via FAK-ERK in 3D collagen. PLoS One. 2011;6:e15956. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 72] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 61. | Chachisvilis M, Zhang YL, Frangos JA. G protein-coupled receptors sense fluid shear stress in endothelial cells. Proc Natl Acad Sci USA. 2006;103:15463-15468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 350] [Cited by in RCA: 363] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 62. | Xu J, Mathur J, Vessières E, Hammack S, Nonomura K, Favre J, Grimaud L, Petrus M, Francisco A, Li J, Lee V, Xiang FL, Mainquist JK, Cahalan SM, Orth AP, Walker JR, Ma S, Lukacs V, Bordone L, Bandell M, Laffitte B, Xu Y, Chien S, Henrion D, Patapoutian A. GPR68 Senses Flow and Is Essential for Vascular Physiology. Cell. 2018;173:762-775.e16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 218] [Article Influence: 36.3] [Reference Citation Analysis (0)] |
| 63. | Mederos y Schnitzler M, Storch U, Meibers S, Nurwakagari P, Breit A, Essin K, Gollasch M, Gudermann T. Gq-coupled receptors as mechanosensors mediating myogenic vasoconstriction. EMBO J. 2008;27:3092-3103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 273] [Cited by in RCA: 273] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 64. | Melchior B, Frangos JA. Gαq/11-mediated intracellular calcium responses to retrograde flow in endothelial cells. Am J Physiol Cell Physiol. 2012;303:C467-C473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 65. | Baeyens N. Fluid shear stress sensing in vascular homeostasis and remodeling: Towards the development of innovative pharmacological approaches to treat vascular dysfunction. Biochem Pharmacol. 2018;158:185-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 66. | Chatterjee S. Endothelial Mechanotransduction, Redox Signaling and the Regulation of Vascular Inflammatory Pathways. Front Physiol. 2018;9:524. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 126] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 67. | Osawa M, Masuda M, Kusano K, Fujiwara K. Evidence for a role of platelet endothelial cell adhesion molecule-1 in endothelial cell mechanosignal transduction: is it a mechanoresponsive molecule? J Cell Biol. 2002;158:773-785. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 233] [Cited by in RCA: 234] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 68. | Shivashankar GV. Mechanosignaling to the cell nucleus and gene regulation. Annu Rev Biophys. 2011;40:361-378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 125] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 69. | Cheng M, Guan X, Li H, Cui X, Zhang X, Li X, Jing X, Wu H, Avsar E. Shear stress regulates late EPC differentiation via mechanosensitive molecule-mediated cytoskeletal rearrangement. PLoS One. 2013;8:e67675. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 70. | Wu C, Huang RT, Kuo CH, Kumar S, Kim CW, Lin YC, Chen YJ, Birukova A, Birukov KG, Dulin NO, Civelek M, Lusis AJ, Loyer X, Tedgui A, Dai G, Jo H, Fang Y. Mechanosensitive PPAP2B Regulates Endothelial Responses to Atherorelevant Hemodynamic Forces. Circ Res. 2015;117:e41-e53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 76] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 71. | Aicher A, Rentsch M, Sasaki K, Ellwart JW, Fändrich F, Siebert R, Cooke JP, Dimmeler S, Heeschen C. Nonbone marrow-derived circulating progenitor cells contribute to postnatal neovascularization following tissue ischemia. Circ Res. 2007;100:581-589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 175] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 72. | Beckman SA, Chen WC, Tang Y, Proto JD, Mlakar L, Wang B, Huard J. Beneficial effect of mechanical stimulation on the regenerative potential of muscle-derived stem cells is lost by inhibiting vascular endothelial growth factor. Arterioscler Thromb Vasc Biol. 2013;33:2004-2012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 73. | Chen WT, Hsu WT, Yen MH, Changou CA, Han CL, Chen YJ, Cheng JY, Chang TH, Lee OK, Ho JH. Alteration of mesenchymal stem cells polarity by laminar shear stimulation promoting β-catenin nuclear localization. Biomaterials. 2019;190-191:1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 74. | Hoefer IE, den Adel B, Daemen MJ. Biomechanical factors as triggers of vascular growth. Cardiovasc Res. 2013;99:276-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 90] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 75. | Walters B, Uynuk-Ool T, Rothdiener M, Palm J, Hart ML, Stegemann JP, Rolauffs B. Engineering the geometrical shape of mesenchymal stromal cells through defined cyclic stretch regimens. Sci Rep. 2017;7:6640. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 33] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 76. | Frismantiene A, Philippova M, Erne P, Resink TJ. Smooth muscle cell-driven vascular diseases and molecular mechanisms of VSMC plasticity. Cell Signal. 2018;52:48-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 288] [Article Influence: 41.1] [Reference Citation Analysis (0)] |
| 77. | Liu YS, Lee OK. In search of the pivot point of mechanotransduction: mechanosensing of stem cells. Cell Transplant. 2014;23:1-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 78. | Andersen JI, Pennisi CP, Fink T, Zachar V. Focal Adhesion Kinase Activation Is Necessary for Stretch-Induced Alignment and Enhanced Differentiation of Myogenic Precursor Cells. Tissue Eng Part A. 2018;24:631-640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 79. | Shattil SJ, Kim C, Ginsberg MH. The final steps of integrin activation: the end game. Nat Rev Mol Cell Biol. 2010;11:288-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 812] [Cited by in RCA: 797] [Article Influence: 53.1] [Reference Citation Analysis (0)] |
| 80. | Zhang L, Issa Bhaloo S, Chen T, Zhou B, Xu Q. Role of Resident Stem Cells in Vessel Formation and Arteriosclerosis. Circ Res. 2018;122:1608-1624. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 96] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 81. | Thompson WR, Guilluy C, Xie Z, Sen B, Brobst KE, Yen SS, Uzer G, Styner M, Case N, Burridge K, Rubin J. Mechanically activated Fyn utilizes mTORC2 to regulate RhoA and adipogenesis in mesenchymal stem cells. Stem Cells. 2013;31:2528-2537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 63] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 82. | Dragoni S, Laforenza U, Bonetti E, Lodola F, Bottino C, Berra-Romani R, Carlo Bongio G, Cinelli MP, Guerra G, Pedrazzoli P, Rosti V, Tanzi F, Moccia F. Vascular endothelial growth factor stimulates endothelial colony forming cells proliferation and tubulogenesis by inducing oscillations in intracellular Ca2+ concentration. Stem Cells. 2011;29:1898-1907. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 137] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 83. | Carlson BE, Beard DA. Mechanical control of cation channels in the myogenic response. Am J Physiol Heart Circ Physiol. 2011;301:H331-H343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 84. | van Helvert S, Storm C, Friedl P. Mechanoreciprocity in cell migration. Nat Cell Biol. 2018;20:8-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 309] [Cited by in RCA: 389] [Article Influence: 48.6] [Reference Citation Analysis (0)] |
| 85. | Thodeti CK, Matthews B, Ravi A, Mammoto A, Ghosh K, Bracha AL, Ingber DE. TRPV4 channels mediate cyclic strain-induced endothelial cell reorientation through integrin-to-integrin signaling. Circ Res. 2009;104:1123-1130. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 312] [Cited by in RCA: 282] [Article Influence: 17.6] [Reference Citation Analysis (0)] |