Published online Dec 26, 2019. doi: 10.4252/wjsc.v11.i12.1084
Peer-review started: March 28, 2019
First decision: April 15, 2019
Revised: September 13, 2019
Accepted: October 14, 2019
Article in press: October 14, 2019
Published online: December 26, 2019
Processing time: 247 Days and 16 Hours
Mesenchymal stem cells (MSCs) are adult stem cells harboring self-renewal and multilineage differentiation potential that are capable of differentiating into osteoblasts, adipocytes, or chondrocytes in vitro, and regulating the bone marrow microenvironment and adipose tissue remodeling in vivo. The process of fate determination is initiated by signaling molecules that drive MSCs into a specific lineage. Impairment of MSC fate determination leads to different bone and adipose tissue-related diseases, including aging, osteoporosis, and insulin resistance. Much progress has been made in recent years in discovering small molecules and their underlying mechanisms control the cell fate of MSCs both in vitro and in vivo. In this review, we summarize recent findings in applying small molecules to the trilineage commitment of MSCs, for instance, genistein, medicarpin, and icariin for the osteogenic cell fate commitment; isorhamnetin, risedronate, and arctigenin for pro-adipogenesis; and atractylenolides and dihydroartemisinin for chondrogenic fate determination. We highlight the underlying mechanisms, including direct regulation, epigenetic modification, and post-translational modification of signaling molecules in the AMPK, MAPK, Notch, PI3K/AKT, Hedgehog signaling pathways etc. and discuss the small molecules that are currently being studied in clinical trials. The target-based manipulation of lineage-specific commitment by small molecules offers substantial insights into bone marrow microenvironment regulation, adipose tissue homeostasis, and therapeutic strategies for MSC-related diseases.
Core tip: Mesenchymal stem cells (MSCs), also called MSCs, are adult stem cells with multilineage differentiation potential. They serve crucial physiological roles, regulating the bone marrow microenvironment and adipose tissue remodeling in vivo. A complex regulatory network and signaling pathways are involved in governing MSC fate commitment. Much progress has been made in recent years in discovering small molecules and their underlying mechanisms that control the cell fate of MSCs. In this review, we summarize recent findings in applying small molecules to the trilineage cell fate commitment of MSCs, highlighting the underlying mechanisms and the current clinical trials. The small molecules for MSC fate determination offer substantial insights into bone marrow and adipose tissue homeostasis and therapeutic strategies for MSC-related diseases.
- Citation: Cheng YH, Dong JC, Bian Q. Small molecules for mesenchymal stem cell fate determination. World J Stem Cells 2019; 11(12): 1084-1103
- URL: https://www.wjgnet.com/1948-0210/full/v11/i12/1084.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v11.i12.1084
Mesenchymal stem cells (MSCs) are a rare cell population originally identified in the bone marrow stroma[1]. In addition to bone marrow, MSCs have been isolated from a multitude of adult tissues, such as adipose tissue[2], synovial membrane[3], and umbilical cord blood[4]. MSCs exhibit distinctive stem cell properties of self-renewal and multipotency defined by the International Society for Cellular Therapy as the competence of differentiation into three mesodermal lineage cells, which are the osteocytes, the adipocytes and the chondrocytes in vitro[5-7]. Beyond well-known trilineage differentiation potential, MSCs have also been reported to be capable of differentiating into other cell types[8], including endothelial cells[9,10], hepatocytes[11-13] and neurons [14,15].
The physiological role of MSCs has been widely investigated in both bone marrow and adipose tissue. The bone marrow MSCs (BM-MSCs) lie in the perivascular region[16,17], and replenish osteoblasts and adipocytes that govern early hematopoiesis with opposing effects[18,19]. An imbalanced ratio of adipocytes and osteoblasts in the bone marrow is found in several pathological conditions, such as aging and osteoporosis, which is the most common bone disorder and presents an increased ratio of marrow fat content[20]. The physiological role of adipose tissue-derived MSCs (AD-MSCs), which are isolated from the stromal vascular fraction of adipose tissue, has also been widely explored[21]. AD-MSCs are the cardinal regulators that govern adipogenesis in adipose tissue and play a critical role in metabolic homeostasis[22]. Impairment of AD-MSCs affects adipose tissue remodeling and expansion, which leads to obesity-associated insulin resistance and increases the risk of cardiovascular diseases[23,24]. Understanding the mechanisms and conceiving a better regimen to control MSC fate in vitro and in vivo will not only advance the translation of stem-cell-based treatment approaches into clinical treatment but also facilitate the development of novel therapeutic strategies for shaping the bone marrow microenvironment, adipose tissue remodeling and managing MSC-related bone and metabolic diseases.
The fate determination of MSCs is controlled by several intrinsic factors, such as regulation of signaling pathways[20], activation of lineage-specific transcription factors[25-27] and epigenetic modification[28-30], which can be governed by diverse extrinsic factors. The extrinsic factors, including mechanical induction[31,32], growth factors, and small molecules, deliver signal cues and activate downstream signaling pathways to guide the fate commitment of MSCs. Small molecules are one of the earliest approaches that researchers used to modulate cell fate and function of MSCs[33]. Small molecules not only have distinct manipulative features - fast and reversible, providing precise control in compared with genetic or epigenetic strategies[34] but also have discrete functional groups that are modifiable for future large-scale screening and biopharmaceutical application.
Investigations into the effect of small molecules on the fate determination of MSCs will undoubtedly offer insights into bone marrow microenvironment regulation and therapeutic strategies for pathological conditions such as obesity, osteoporosis, and aging. Recent studies based on traditional treatment strategies or large-scale screening allow the identification of many candidates that regulate the cell fate of MSCs. In this article, we review the small molecules that modulate the fate determination of MSCs through the PubMed literature searches (last search conducted on March 23, 2019), and summarize their underlying mechanisms (Table 1).
| Small molecules | Origin/Natural source | Effect | Pathway/Targets | Experimental model and dose | Comments | Ref. |
| 5-azacytidine | Synthetic | Anti-adipogenic | Inhibit DNA methyltransferase | ST2 cells (0.5 μmol/L) | Reduce expression level of PPARγ, aP2, FAS and C/EBPα | [13,8] |
| 8-HUDE | Synthetic | Anti-adipogenic | Activate HO-1 pathway | Human BM-MSCs (1 μmol/L) | Reduce expression level of Fas, Pparγ, and Cebpα | [132] |
| Albiflorin | Paeonia lactiflora | Beige-Adipoinductive | Activate AMPK, PI3K/AKT/mTOR pathways | Human AD-MSCs (5, 10, 20 μmol/L)/ HFD mice (5 mg/kg per day, in an unknown solvent, orally) | Cell viability decrease when AF exceeds 20 μmol/L. Induce expression of thermogenic marker Ucp1. | [126] |
| Arctigenin | Arctii fructus, Forsythia fruit | Anti-adipogenic | Activate AMPK pathway | Human AD-MSCs (10, 50, 100 μmol/L)/HFD mice (50 mg/kg per day, in 10% DMSO, orally) | Cell viability was not affected by ARC. | [125] |
| Atractylenolides | Atractylodis macrocephalae | Chondroinductive | Activate Shh pathway | Rat BM-MSCs (10, 100, 500 μg/mL) | Induce Sox9 collagen type II and aggrecan expression | [149] |
| AUDA | Synthetic | Anti-adipogenic | Activate HO-1 pathway | Human BM-MSCs (1 μmol/L) | Reduce expression level of Fas, Pparγ, and Cebpα | [132] |
| Baicalin | Scutellaria baicalensis | Osteoinductive | Activate Wnt pathway | Rat BM-MSCs (0.1, 0.5, 1, 5, 10, 50 μmol/L)/ Radix Scutellariae extract (2 and 50 mg/kg per day, orally) | Enhance ALP activity | [54,55] |
| Cordycepin | Cordyceps militaris | Osteoinductive | Activate Wnt pathway | Human BM-MSCs with H2O2 treatment (1, 5, 10, 20, 40, 80 μg/mL)/ Human BM-MSCs with ethanol treatment/ rat alcohol-induced osteonecrosis of the femoral head model (10 mg/kg per day in saline intraperitoneal) | Induce osteogenic markers osteopontin and collagen type I expression | [59,61] |
| Cyanidin-3-O-glucoside | Black rice | Anti-adipogenic | Activate Wnt pathway | C3H10T1/2 cells (black rice extract 10, 20, 40, and 80 μg/ mL)/ HFD mice (black rice extract 10% corn oil and 90% water, 100 mg/kg per day, orally) | Induce Wnt-specific target genes such as Axin2, Wisp2, and Cyclin d1 | [114] |
| Dihydroartemisinin | Artemisia apiacea | Anti-chondrogenic | Activate Notch pathway | C3H10T1/2 cells (1, 10, 50, 200, 300 μmol/L) | Suppress Sox9, COMP and Col2a1 expression | [151] |
| Er-Xian Decoction extracts | Er-Xian Decoction | Osteoinductive | Activate MAPK/ERK pathway | OVX mice (30g/kg per day, orally)/ Mice BM-MSCs (isolated from OVX and treated mice) | Elevate ALP activity. | [94] |
| Ethanol | Alcohol | Anti-osteogenic | Inhibit TGFβ pathway | MSCs (25 mmol/L)/ Tibial fracture mice (20% in saline, intraperitoneal) | Inhibit Col I and Sox9 expression | [62] |
| Genistein | Soybean | Osteoinductive | Activate BMP pathway | Human BM-MSCs (10 -0.01 μmol/L, MAX at 1 μmol/L) | Induce Runx2 and osteocalcin expression, inhibit BMP pathway inhibitor SMAD6, 7 | [44] |
| Ginkgo biloba extract | Ginkgo biloba | Adipoinductive Osteoinductive | Activate BMP and Wnt pathways | Mouse BM-MSCs (50, 100, 150, 200, 400 μg/mL)/ human BM-MSCs (25, 50, 70, 100) | Induce Runx2, Col 1a1 and BMP-2 experssion | [64,65] |
| Ginkgolic acid | Ginkgo biloba | Adipoinductive | Inhibit sumoylation | Mouse BM-MSCs (50 μmol/L) | Promote adipogenic commitment and inhibit adipocyte terminal maturation. | [136] |
| Icariin | Herba epimedii | Osteoinductive | Activate MAPK, BMP, WNT and PI3K/AKT pathway | MC3T3-E1 cells, rat BM-MSCs (0.0001, 0.001, 0.01, 0.1, 1, 10 μmol/L; 5, 10, 20, 40 μmol/L)/ OVX rats (150 mg/kg in saline, orally) | Induce Runx2, BMP4, and Col I expression | [88,89,90,92] |
| Isorhamnetin | Sea buckthorn | Anti-adipogenic | Activate Wnt pathway | Human AD-MSCs (1, 25 μmol/L) | Downregulate Wnt antagonist Sfrp1 and Dkk1 | [113] |
| Medicarpin | Medicago truncatula | Beige-Adipoinductive | Activate AMPK pathway | C3H10T1/2 cells (10μmol/L) | Induce expression of thermogenic marker Ucp1. | [121] |
| Osteoinductive | Activate Wnt and Notch pathways | OVX mice (1 mg or 10 mg/kg per day, orally)/ OVX + Drill hole mice (0.5, 1 mg/kg per day, orally) | Induce Runx2, Osteocalcin and TGF-β expression | [57,58] | ||
| Naringin | Rhizoma Drynariae | Osteoinductive | Activate AMPK and AKT pathway | Human BM-MSCs (1, 10, 100 μg/mL)/ OVX mice (0, 0.5, 1, 5 and 10 mg/kg per day, orally) | Induce osteocalcin, collagen type I, osteopontin and ALP expression. | [81,82] |
| N-methyl pyrrolidone | Synthetic | Anti-adipogenic | Inhibit Brd4 | Human BM-MSCs (5, 10 mM)/ OVX mice (10.5 mM/100 g/wk, intraperitoneal injection) | Reduce PPARγ expression level | [140] |
| Oleanolic acid | Glossy privet | Osteoinductive | Activate Notch pathway | Rat BM-MSCs (1, 10, 100 μmol/L) / OVX mice (2 wks, 3 mo, dissolve in normal saline, 20 mg/kg per day, orally) | KEGG analysis on differential gene patterns | [69] |
| Peonidin-3-O-glucoside | Black rice | Anti-adipogenic | Activate Wnt pathway | C3H10T1/2 cells (black rice extract 10, 20, 40, and 80 μg/ mL)/ HFD mice (black rice extract in 10% corn oil and 90% water, 100 mg/kg per day, orally) | Induce Wnt-specific target genes such as Axin2, Wisp2, and Cyclin d1 | [114] |
| Plastrum testudinis extracts | Plastrum testudinis | Osteoinductive | Activate PI3K/AKT pathway | Rat BM-MSCs (0.03, 0.3, 3, 30, 300 μg/mL)/ Dexamethasone induced osteoporosis rat (30 mg/kg per day, subcutaneous injection) | Induce β-catenin, Runx2 and osteocalcin expression | [76,77] |
| Platycodin D | Platycodi radix | Anti-adipogenic Beige-Adipoinductive | Activate AMPK pathway | Human AD-MSCs (0.5-5 μmol/L)/ db/db mice (2, 5 mg/kg per day) | Suppress adipogenic genes, such as Pparγ, Cebpα, Fabp4, Adipoq, and Resistin | [127] |
| Psoralen | Psoralea corylifolia | Anti-adipogenic Osteoinductive | Activate Notch pathway | Rat BM-MSCs (? μmol/L) / OVX mice (2 wks, 3 mo, dissolve in normal saline, 20 mg/kg per day, orally) | KEGG analysis on differential gene patterns | [68] |
| Psoralidin | Psoralea corylifolia | Anti-adipogenic Osteoinductive | Activate PI3K/AKT pathway | OVX rat (in sesame oil, 10 mg/kg per day, orally) | Promote osteogenic differentiation of BM-MSCs isolated from the treated OVX mice | [78] |
| Resveratrol | Wine, grape | Osteoinductive | Activate MAPK/ERK pathway | Human BM-MSCs (0.01, 0.1, 1, 10, 100 μmol/L) | Induce Runx2, osterix and osteocalcin expression | [96] |
| Risedronic acid | Synthetic | Anti-adipogenic | Inhibit PI3K/AKT/mTOR pathway | Human BM-MSCs (1, 5, 10, 25 μmol/L) | Inhibit mTOR1 downstream effector S6 phosphorylation | [118] |
| Salvianolic acid B | Salvia miltiorrhiza | Osteoinductive | Activate MAPK/ERK pathway | Rat BM-MSCs (50, 100, 500, 1000 nmol/L)/ Steroid induced osteoporotic rat (40, 80 mg/kg per day) | Enhance ALP activity and osteocalcin expression | [101,102] |
| SKL2001 | Synthetic | Osteoinductive | Activate Wnt pathway | ST2 cells (5, 10, 30 μmol/L) | Stabilize β-catenin without affecting expression level | [63] |
| T63 | Synthetic | Anti-adipogenic Osteoinductive | Activate BMP and Wnt pathway | C3H10T1/2 (1-40 μmol/L)/ OVX mice (5 mg/kg and 20 mg/kg, orally) | Induce Runx2, Bglap and, Spp1 expression. | [47] |
| Tithonia diversifolia extracts | Tithonia diversifolia | Anti-adipogenic | Activate HO-1 pathway | Human AD-MSCs (175 μg/mL) | Oil red staining quantitatively decrease in a dosage-dependent manner | [130] |
| Tricin | Rice bran | Osteoinductive | Activate Wnt pathway | Human AD-MSCs (5, 10, 15, 20, 25 μmol/L) | Induce bone sialoprotein, osteocalcin, ALP, Runx2, Col I, osterix, osteopontin | [53] |
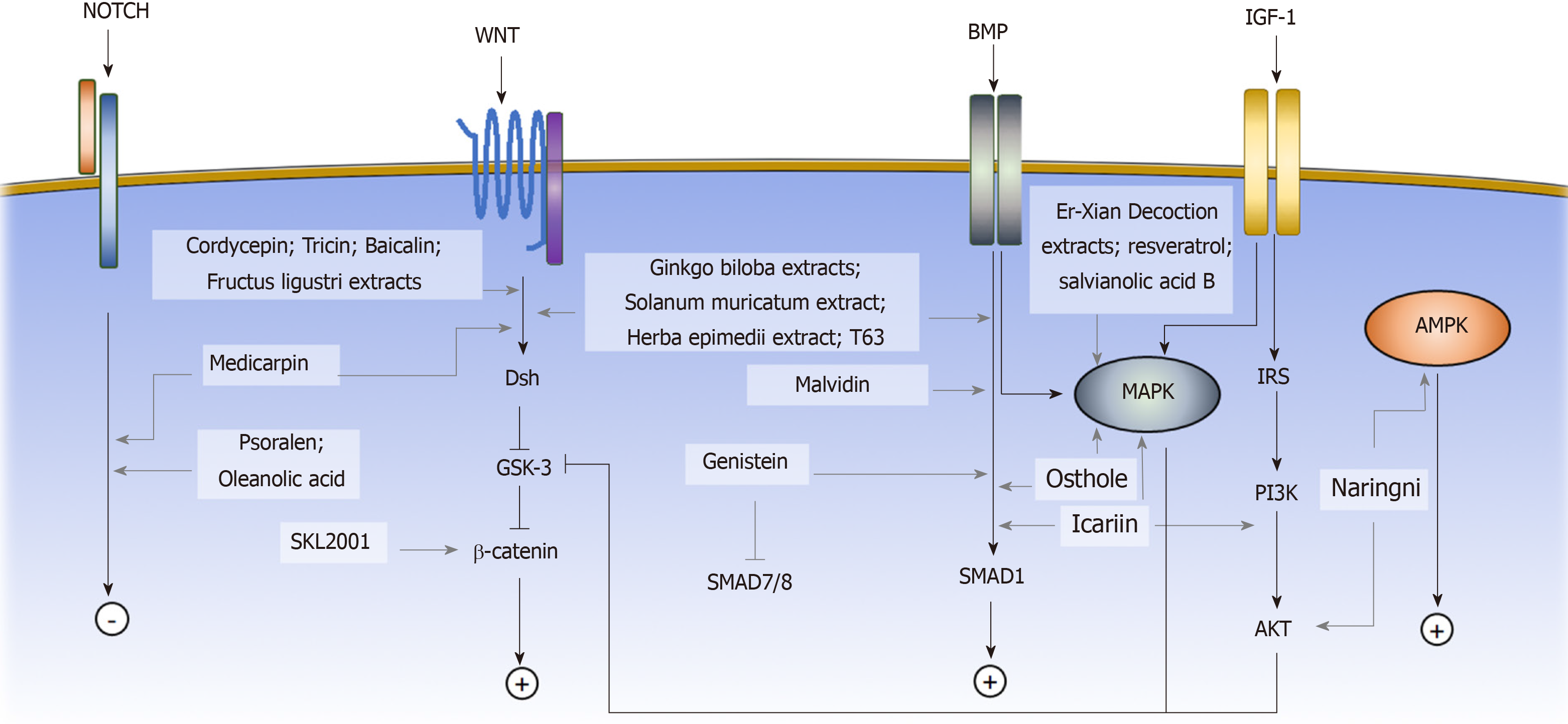
Osteogenic differentiation of MSCs is commonly induced by the small molecule supplements of ascorbic acid, β-glycerophosphate, and dexamethasone, resulting in an increase in alkaline phosphatase (ALP) activity and calcium deposition[7,35]. Ascorbic acid and β-glycerophosphate increase type I collagen secretion and stimulate the formation of hydroxyapatite-rich mineralized matrix[7]. Dexamethasone induces the expression of osteogenic-associated genes, including runt-related transcription factor 2 (Runx2), Osterix, and bone matrix proteins. Runx2 is the master regulator of osteoblastic commitment. The Runx2 expression is controlled by many signaling pathways; among them, the BMP, Wnt, and Notch signaling pathways are the major cascades that promote both Runx2 expression and osteogenesis[36]. In this section, we will review the small molecules that affect the osteogenic commitment of MSCs based on their mechanisms (Figure 1).
BMPs are growth factors belonging to the transforming growth factor beta (TGF-β) superfamily. Upon ligand binding, the BMP receptors form an activated quaternary complex, which subsequently phosphorylates and activates intracellular Smad proteins and downstream cascades. The detailed mechanisms of BMP-mediated osteogenesis are not well-characterized; however, BMP triggers MSCs to express downstream osteogenic genes, such as ALP and type I collagen (Col I)[37]. Many small molecules have been identified to exert their osteogenic effect on MSCs by interfering with the BMP signaling pathway. Some examples include genistein, Solanum muricatum extract[38], Herba epimedii extract[39], malvidin[40], T63 and osthole[41-43].
Genistein is a phytoestrogen enriched in soybean products. Dai et al[44] showed that genistein promoted osteogenic differentiation of human BM-MSCs through BMP-dependent SMAD signaling. A concentration ranging from 0.1 to 10 μmol/L Genistein was tested, and the osteogenic stimulations were statistically significant at 0.1 and 1 μmol/L with the highest ALP activity at 1 μmol/L. The gene expression profile showed that osteogenic genes, such as Runx2 and osteocalcin, were highly expressed in genistein-treated cells compared with untreated cells. In addition, the BMP signaling pathway related mediators were upregulated, while BMP signaling pathway inhibitors such as SMAD6 and 7 were downregulated[44]. Soybean products have been reported to prevent bone loss in ovariectomy-induced (OVX) osteoporotic mice in the 1990s, the Dai et al[45] study provided a possible explanation for the underlying mechanism. A clinical trial was carried out to assess the effect of genistein on osteopenic postmenopausal women. The results demonstrated an increase in bone mineral density at both the anteroposterior lumbar spine and the femoral neck[46]. However, the study didn’t calculate the fracture rate, so more concrete evidence is needed to evaluate the osteoprotective effect of genistein.
Zhao et al[47] identified a small molecule, named as T63, by high-throughput screening with the Runx2-responsive 3T3 luciferase cell line. Through ALP activity validation, treatment of T63 showed the most significant increase compared with other screening molecules. The addition of T63 to the osteogenic induction media during C3H10T1/2 cell differentiation showed an upregulation of osteogenic genes, including Runx2, Bglap, and Spp1. When T63 was added to the adipogenic induction medium, the adipogenic markers, including Pparγ2, Srebf1, and Fabp4, were significantly suppressed. Treatment with T63 upregulated the expression levels of the Bmp2, Bmp4 and Bmp7 genes in the BMP signaling pathway and increased the phosphorylation of the BMP downstream mediators Smad1/5/8 in a dose-dependent manner. Meanwhile, the phosphorylation of GSK-3β, an upstream regulator of β-catenin in the Wnt signaling pathway, also increased, indicating that T63 was also involved in the regulation of the Wnt signaling pathway. The addition of the BMP signaling pathway inhibitor Noggin or the Wnt signaling pathway inhibitor DKK-1 reduced the osteogenic effect of T63, confirming that T63 exerted an osteoinductive effect via the BMP and Wnt pathways. In the OVX mouse model, after three mo of a dose of 5 mg/kg or 20 mg/kg T63 increased both the bone mineral density and the bone mineral content in femurs and lumbar vertebrae, suggesting that T63 promoted bone formation in vivo.
The Wnt signaling pathway has been shown to play a critical role in bone formation and osteogenic differentiation of MSCs. Upon Wnt proteins binding to the frizzled (Fzd) receptors, the canonical Wnt signaling pathway is activated, and cytoplasmic β-catenin is stabilized via glycogen synthase kinase-3 (GSK3) inhibition[48,49]. β-catenin accumulates in the cytosol and subsequently translocates to the nucleus, where it promotes the transcription of target genes[50]. Activation of the canonical Wnt signaling pathway upregulates the gene expression of the osteogenic regulators Runx2, Dlx5, and Osterix[51] and suppresses the expression of the adipogenic inducers Pparγ and Cebpα[52]. The small molecules that affect the osteogenic cell fate of MSCs via the Wnt signaling pathway include medicarpin, cordycepin, SKL2001, tricin[53], baicalin[54,55], Ginkgo biloba extracts (GBE), and Fructus ligustri extracts[56].
Medicarpin (Med), a pterocarpan compound, is present in many leguminous species, such as chickpea and Butea monosperma. Tyagi et al[57] demonstrated the osteoprotective effect of Med on OVX osteoporotic rats. Med was given at a dosage of 1mg or 10 mg/kg per day. MicroCT revealed that the osteoporotic phenotype was significantly improved under the Med treatment, giving a higher total trabecular volume and number. With Med administration, BM-MSCs isolated from the treated OVX rats presented a superior mineralization level under osteogenic medium induction compared with the BM-MSCs from the untreated rat[57]. Another study used the OVX drill-hole injury animal model to assess the regenerative effect of Med in vivo. The OVX rats underwent the dill-hole procedure at the sites of femur mid-diaphysis and received treatment of 0.5, 1 or 5 mg/kg of Med. The results showed that Med administration not only increased the bone mineral density but also upregulated several osteogenic markers, including Runx2 and osteocalcin. The gene expression profile comparison demonstrated the effect to be mediated by both the canonical Wnt and the Notch signaling pathways, evidenced by the increased expression level of Wnt signaling pathway mediators β-catenin, Dishevelled and Fzd, and the Notch signaling pathway mediators Notch-1 and Jagged-1 at the defect region[58].
Cordycepin is one of the major compounds of Cordyceps militaris. Wang et al[59] showed that cordycepin prevented oxidative stress-induced inhibition of osteogenesis through activation of the Wnt signaling pathway. The H2O2-induced inhibition of human BM-MSC osteogenesis was used as the baseline, and treatment with 10 μg/mL cordycepin reversed the osteogenic dysfunction with the increase in ALP staining and mineralization. The osteogenic genes osteopontin and Col I were upregulated under cordycepin treatment, while the osteoclast promoting agent RANKL was downregulated. The H2O2 negatively regulated the Wnt signaling pathway, but cordycepin treatment recovered the downregulation[60]. The addition of the Wnt signaling pathway inhibitor DKK1 reduced the osteoinductive effects of cordycepin on ALP activity, calcium quantification, and Runx2 expression, confirming that Wnt signaling was involved in exerting the osteoprotective effect of cordycepin[59].
The osteoprotective effect of cordycepin has also been examined in alcohol-induced osteonecrosis by using both in vitro and in vivo models. Chen et al[61]demonstrated the osteoinductive effect of cordycepin on human BM-MSCs under ethanol treatment. Previous studies have shown that ethanol treatment impaired the osteogenic differentiation of BM-MSC[62]. The cordycepin treatment at a dose of 1 or 10 μg/mL attenuated the osteogenic inhibitory effect of ethanol. The rat model of alcohol-induced osteonecrosis of the femoral head (ONFH) was established to assess the effect of cordycepin in vivo. Intraperitoneal injection of Cordycepin at a dose of 10 mg/kg per day decreased the development rate of ONFH from 70% to 20%, and both trabecular volume and thickness were significantly increased by cordycepin treatment[61].
Gwak et al[63] performed a cell-based chemical screening assay with a library of 270000 small molecules on HEK293 reporter cells and identified a compound named as SKL2001 that strongly stimulated the Wnt signaling pathway. When SKL2001 was added, the ST2 BM-MSC cell line expressed higher levels of type I collagen and Runx2 under osteogenic induction. The ALP activity also increased in a dosage-dependent manner. Assessment of Wnt signaling pathway mediators revealed that SKL2001 enhanced the protein expression level of both cytoplasmic and nuclear β-catenin without affecting the RNA expression level, suggesting SKL2001 was involved in the protein degradation of β-catenin. The subsequent findings that SKL2001 inhibited the phosphorylation of β-catenin and hindered the interaction of β-catenin with its degradation mediator β-TrCP confirmed that SKL2001 affected the osteogenic commitment of MSCs by stabilizing β-catenin.
GBE was shown by Wu et al[64] to enhance osteogenic differentiation and inhibit adipogenic differentiation in murine BM-MSCs. The addition of 150 μg/mL GBE into osteogenic differentiation medium prominently enhanced both calcium deposits and ALP activity. The expression levels of the osteogenic markers Runx2, Col 1, and BMP-2 were upregulated, whereas GBE treatment decreased lipid accumulation in the differentiated adipocytes and suppressed the expression levels of the adipogenic genes Pparγ and Fabp4. The osteoprotective effect was also examined in human BM-MSCs, whose results were similar to murine BM-MSCs, showing an increase in ALP activity and upregulation in the expression of the osteogenic genes osteopontin and Col I. A loss-of-function assay was performed to identify the signaling pathway involved in the GBE treatment. When the Wnt or the BMP signaling pathway was inhibited, the ALP activity under GBE treatment decreased significantly, confirming that GBE exerted its effect through regulation of both Wnt and BMP signaling pathways[65].
Notch signaling is a highly conserved signaling pathway related to cell-fate determination, self-renewal potential, and apoptosis[66]. Induction of Notch signaling enhances the osteogenic differentiation of human BM-MSCs and inhibits adipogenic commitment[67]. The small molecules that are involved in osteogenic differentiation of MSCs by regulating the Notch signaling pathway are psoralen (PSO) and oleanolic acid (OA).
PSO is the active ingredient of Psoralea corylifolia, which is commonly prescribed for treating fractures, bone diseases and joint diseases in traditional Chinese medicine. To elucidate the osteoprotective mechanism of PSO, we investigated the effects of PSO on adipogenic and osteogenic differentiation of rat BM-MSCs. In the OVX osteoporotic rats, PSO significantly increased trabecular number and thickness. The in vitro assays demonstrated that PSO inhibited adipogenic differentiation and promoted osteogenic differentiation. Using the Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analysis on the microarray data, the differentially expressed genes were highly enriched in the Notch signaling pathway, suggesting that PSO exerts its osteogenic effect via the Notch signaling pathway[68].
OA isolated from glossy privet was reported to prevent bone loss by inhibiting osteoclast formation. We discovered that OA not only affected osteoclastogenesis but also stimulated the osteoblastic differentiation of BM-MSCs in vitro. In the OVX osteoporotic rats, administration of OA at a dosage of 20 mg/kg per day significantly increased the trabecular number and thickness. The expression levels of both osteocalcin and Runx2, which are markers for bone formation and osteogenic differentiation, also increased compared with untreated mice. KEGG pathway analysis of the differentially expressed genes revealed that the Notch signaling pathway is involved in the osteogenic effect of OA[69].
Insulin-like growth factor 1 (IGF-1) is an important osteogenic regulator during skeletal development. IGF-1 receptor autophosphorylation occurs under IGF-1 stimulation and subsequently activates downstream PI3K/AKT and MAPK signaling pathways[70,71]. Previous studies have shown that loss of IGF-1 receptors could lead to retardation of skeletal development and defects in trabecular bone[72,73]. Evidence has shown that the PI3K/AKT pathway is among the most critical signaling pathways for osteogenic differentiation and bone growth[74,75]. The small molecules that regulate MSC osteogenesis through that PI3K/AKT signaling pathway include Plastrum testudinis extracts (PTE) and psoralidin.
Plastrum testudinis is an herbal medication commonly used in traditional Chinese medicine for treating bone diseases. Liang et al[76]demonstrated that injecting PTE into the steroid-induced osteoporosis rat at a dosage of 30 mg/kg per day improved not only the histological features, promoting a more orderly trabecular structure, but also the biomechanical properties, promoting bone strength and energy absorption capacity compared with untreated rats. The underlying osteoprotective mechanism of PTE was further investigated by Shen et al[77], who demonstrated that PTE promoted BM-MSC proliferation and osteogenic differentiation. Five different concentrations, 0.03, 0.3, 3, 30 and 300 μg/mL, were tested in the study. The ALP activity and mineralization of differentiated cells increased in a dosage-dependent manner. The osteogenic genes, including β-catenin, Runx2, and osteocalcin, were all upregulated. The study also revealed that PTE promoted p-PI3K, p-AKT, and p-GSK3β protein expression during osteogenesis, indicating that the effect of PTE on osteogenic differentiation was dependent on the PI3K/AKT signaling pathway.
Psoralidin is a compound enriched in the seeds of Psoralea corylifolia. Zhai et al[78] found that administration of 10 mg/kg per day of psoralidin could prevent bone loss in the OVX-induced osteoporosis model, improving both bone density and biomechanical properties. The BM-MSCs were isolated from both treated and untreated rats. Under osteogenic induction, BM-MSCs from the psoralidin-treated rats were prone to undergo osteogenic differentiation, while adipogenic differentiation was suppressed. Psoralidin treatment increased the levels of p-PI3K and p-AKT and p-GSK3β, which led to an accumulation of β-catenin, confirming that psoralidin promoted MSC osteogenesis through the PI3K/AKT pathway.
The AMP-activated protein kinase (AMPK) signaling pathway has recently been shown to regulate MSC osteogenesis. During osteogenic differentiation, both AMPK expression and phosphorylation increases[79]. Inhibition of the AMPK signaling pathway leads to a reduction in mineral deposition and suppresses the expression of osteogenic genes, including Runx2, ALP, and osteocalcin, indicating that AMPK activation favors MSC osteogenic differentiation[79].
Naringin is a major compound of Rhizoma Drynariae that enhances BMP expression level in osteoblast[80]. Zhang et al[81] showed that the treatment with naringin promoted proliferation and osteogenic differentiation of human BM-MSCs. Naringin dose-dependently increased the expression of osteoblast-related markers osteocalcin, Col I, osteopontin, and ALP. The mechanism of naringin was further studied by the Wang group. In the OVX mice, feeding 5 mg/kg per day naringin showed the most significant enhancement in the expression of the osteogenic genes, and improved the total bone density at the distal femur, proximal tibia, and lumbar spine. The addition of AMPK and AKT inhibitor reversed the osteoprotective effect given by naringin, suggesting that the AMPK and AKT signaling pathways could be a possible mechanism for the osteogenic induction of naringin[82].
MAPKs are a family of kinases that transmitted extracellular stimuli into intracellular signaling cascade and regulate crucial cell behaviors, including proliferation and differentiation[83]. Conventional MAPK members are the extracellular signal-regulated kinases 1/2 and ERK5, c-Jun amino (N)-terminal kinases 1/2/3, and the p38 isoforms. Activation of the MAPK signaling pathway promotes human MSC osteogenic commitment[84]. The small molecules that regulate MSC osteogenic differentiation through the MAPK signaling pathway include icariin, Er-Xian decoction (EXD) extracts, resveratrol, and salvianolic acid B.
Icariin (ICA) is the main active component of Herba epimedii, which is a well-known traditional Chinese medicine for treating osteoporosis[85]. Previous studies have shown that ICA promoted osteogenic differentiation in vitro[86,87]. Wu et al[88] recently found that the effect of ICA was mainly mediated by MAPK pathway activation, as it increased the phosphorylation of MAPK signaling molecules, including ERK and JNK, upon ICA treatment. Subsequently, the gene expression of osteogenic markers, including Col I, osteocalcin and osteopontin and the ALP activity increased in a dosage-dependent manner. The osteogenic effect of ICA was suppressed by either ERK or JNK inhibitors, suggesting that the MAPK pathway is necessary for the induction of osteogenesis of BM-MSCs by ICA. In addition to the MAPK pathway, ICA is involved in regulating osteogenesis through other osteogenic-associated signaling pathways, including BMP[89], WNT[90], and PI3K/AKT signaling pathways[91]. Cao et al[92] showed that the daily intragastric administration of ICA to the fractured OVX rat at a dosage of 150 mg/kg significantly increased bone mineral density and accelerated fracture healing within 5 mo. These findings demonstrated that, following bone fracture in OVX rats, the administration of ICA accelerated bone mineralization and improved fracture healing. A double-blind randomized controlled trial showed that the administration of a daily dose of 60 mg ICA, 15 mg daidzein, and 3 mg genistein for 12 mo or 24 mo significantly reduced bone loss in late-postmenopausal women in comparison with the placebo group, demonstrating a positive effect of epimedium-extract small molecules on preventing bone loss[93].
EXD, which is a common Chinese medicine mixing of six different herbs clinicians prescribed to treat menopausal symptoms. We studied the extracts from EXD and demonstrated their stimulatory effect on the osteoblastic differentiation of murine BM-MSCs[94]. The BM-MSCs isolated from the EXD extract-treated mice showed an increased ALP activity under osteogenic induction compared with those from OVX mice, suggesting the osteoprotective role of EXD extracts. The gene expression profiles showed that the common genes that were upregulated during EXD extract treatment were related to the MAPK signaling pathway, indicating that EXD exerted its effect by regulating the MAPK signaling pathway. A clinical trial showed that EXD improved bone mineral density at both the lumbar spine and the femoral head in postmenopausal women, demonstrating an osteoprotective effect[95]. However, no study has demonstrated its effect on the incidence rate of fracture. Future studies of longer duration with calculation of fracture rates are needed to confirm the clinical benefit of EXD.
Resveratrol (RSVL) is a phenolic compound enriched in wine and grape and famous for its antioxidant effect. Dai et al[96] tested the osteogenic regulatory effect of RSVL by treating BM-MSCs with RSVL at different doses from 0.01 to 100 μmol/L and measured the ALP activity. The results showed that under 1 μmol/L RSVL treatment, the differentiated BM-MSCs presented the maximal increase in ALP activity. The osteogenic genes Runx2, osterix, and osteocalcin were all upregulated during the treatment with RSVL. The addition of the ERK inhibitor PD98059 reversed the expression level of osteogenic markers and ALP activity, confirming that RSVL affects the MSC osteogenesis through the MAPK signaling pathway. In addition to the MAPK signaling pathway, other studies have also shown that RSVL activated SIRT1, a nicotinamide adenine dinucleotide (NAD+)-dependent deacetylase, and subsequently upregulated FOXO3A protein expression, which promoted SIRT1-FOXFO3A complex formation and upregulated Runx2 expression[97,98]. A clinical trial was performed to assess the osteoprotective effect of RSVL on osteoporotic obese patients. The results showed that RSVL increased lumbar spine bone mineral density in a dose-dependent manner, with a maximal increase of 2.6% in the high-dose RSVL group[99].
Salvianolic acid B (SalB) is the active compound of Salvia miltiorrhiza, which is commonly used in treating cardiovascular diseases in Chinese medicine[100]. Cui et al[101] demonstrated the association between SalB and osteogenesis by showing that administration of SalB at a dosage of 40 mg/kg per day to the steroid induced osteoporotic rats reversed the osteoporotic phenotype. The rats presented elevated bone mineral density, increased cancellous bone mass, and thicker trabeculae after the treatment. This effect was consistent with the finding that SalB promoted osteogenic differentiation of rat BM-MSCs in vitro at the dosages ranging between 100 and 500 nmol/L. The differentiated cells showed a significantly higher ALP activity along with an increase in osteocalcin expression[101]. The underlying mechanism of SalB was studied by the same group in human BM-MSCs. The addition of the ERK inhibitor U0126 diminished the effect of Sal B on osteogenesis, suggesting that the Sal B regulated the osteogenesis of BM-MSCs through the MAPK/ERK signaling pathway[102].
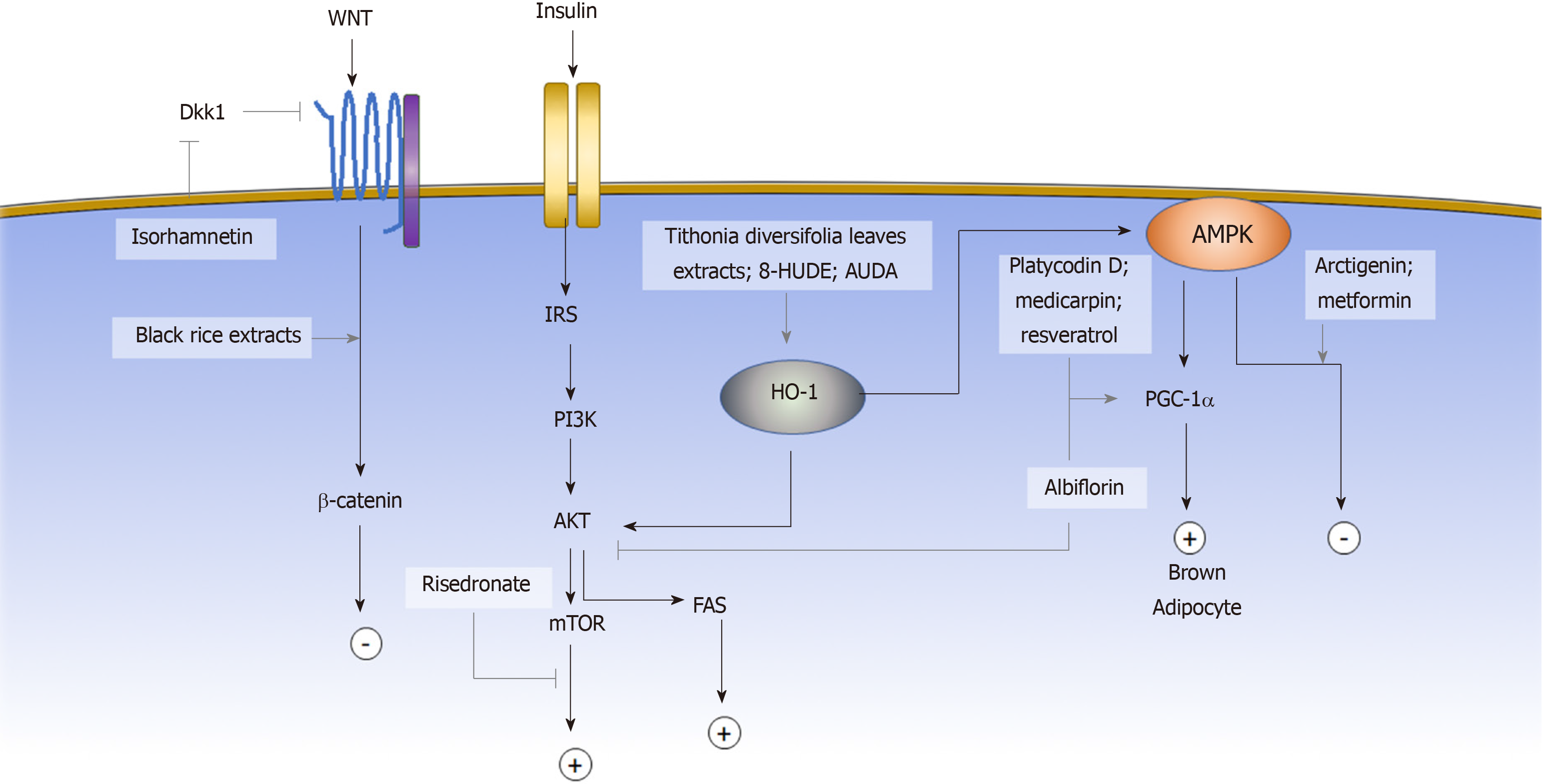
The induction of adipogenic differentiation in MSCs in vitro is traditionally achieved by the activation of the adipogenic master regulator Cebpα and Pparγ through a small molecule cocktail of 3-isobutyl-1-methylxanthine (IBMX), indomethacin, dexamethasone, and insulin[7]. IBMX is a phosphodiesterase inhibitor that increases the intracellular cyclic AMP (cAMP) and activates the downstream PKA signaling pathway to induce Cebpα and Pparγ expression[103-105]. Dexamethasone, on the other hand, binds to the intracellular glucocorticoid receptor and subsequently enhances the expression of the adipogenic transcription factor C/EBPβ[106]. Indomethacin is a well-known COX1/2 inhibitor; however, its adipogenic activity is not due to the inhibition of COX but through activation of PPARγ. Insulin promotes glucose uptake and stimulates triglyceride synthesis in adipocytes. In the past decade, more signaling pathways have been identified to be involved in regulating the adipogenesis of MSCs. Some of the pathways are the Wnt, AKT and AMPK signaling pathways[103]. Activating the AKT signaling pathway promotes the differentiation of adipose stem cells, whereas activating the Wnt[107,108] or AMPK[79] signaling pathways inhibits adipogenesis. Aside from regulating gene expression through a signaling pathway cascade, the post-translational modification also affects adipogenic fate determination through post-translational modification, such as sumoylation and epigenetic modification. In this section, we will review the small molecules that have been reported to affect the adipogenic commitment of MSCs based on their mechanisms (Figure 2).
The canonical and noncanonical signaling pathways present different effects on MSC adipogenesis. The canonical signaling pathway mediates signaling through the stabilization of β-catenin, and activation of the Wnt canonical signaling pathway was shown to block the induction of PPARγ and C/EBPα and to inhibit adipogenesis[109]. Wnt also activates noncanonical signaling pathways. Genetic evidence indicates that noncanonical signaling through Wnt5a antagonizes the canonical signaling pathway[110]. Wnt antagonists exert a crucial role during the adipogenic commitment of MSCs[111,112]. Some of the small molecules that have been shown to exert adipogenic regulatory effects via the Wnt signaling pathway include isorhamnetin, cyanidin-3-O-glucoside, and peonidin-3-O-glucoside.
Isorhamnetin (IsR) is a flavonoid extracted from sea buckthorn. IsR was first identified by Lee et al to inhibit adipogenic differentiation of MSCs. The triglyceride level was significantly lower under treatment of 25 μmol/L IsR. The Wnt signaling pathway antagonists Sfrp1 and Dkk1 were downregulated under IsR treatment, thereby stabilizing and increasing the protein level of β-catenin without affecting the mRNA expression level. The finding indicated that IsR affected adipogenesis through activation of the Wnt signaling pathway[113].
Cyanidin-3-O-glucoside (C3G) and peonidin-3-O-glucoside (P3G) are two anthocyanin components of black rice extract (BRE). Both compounds were shown by Jang et al[114] to inhibit adipogenic differentiation of the murine MSC line C3H10T1/2. Under treatment with C3G or P3G, lipid accumulation in the differentiated cells decreased in a dose-dependent manner, and the adipogenic gene Pparγ was significantly suppressed. Although the mechanism of C3G and P3G were not investigated, the original BRE has been shown to activate Wnt signaling and downstream targets, exerting both anti-adipogenic and osteoinductive effects.
The PI3K/AKT signaling pathway can be activated by a range of extracellular factors through the receptor tyrosine kinases (RTKs). Upon RTK activation, the IRS1/2 phosphorylates and activates PI3K. PI3K subsequently activates AKT, which regulates many functional mediators, including GSK3, FoxO, mTOR, which in turn form complex regulatory circuits that govern the manifold response. Among them, activation of mTOR leads to upregulation in Pparγ and promotes adipogenesis[115-117].
Risedronate is a bisphosphonate medication that is used to treat osteoporosis clinically by inhibiting osteoclastic differentiation. Jin et al[118] demonstrated that risedronate, in addition to affecting osteoclast development, also inhibited human BM-MSC adipogenesis through the PI3K/AKT signaling pathway. The ratio of adipocyte formation under the adipogenic induction decreased in a dose-dependent manner while increasing the concentration of risedronate from 1 μmol/L to 25 μmol/L. Further exploration of the mechanism showed that phosphorylation of mTOR downstream effectors was inhibited under risedronate, suggesting that its effect on adipogenesis of BM-MSCs was mediated by mTOR signaling pathway regulation[118].
AMPK is highly involved in cellular energy homeostasis, and the AMPK signaling pathway has been shown to regulate the adipogenic differentiation of MSC, as inhibition of AMPK signaling pathway promotes lipid droplet formation and adipogenesis[79]. Aside from typical white adipogenesis, the other important cell fate modulation associated with the AMPK signaling pathway is brown adipogenesis. Unlike white adipocytes, brown adipocytes exert a significant thermogenic effect, which has a promising effect for obesity control. Activation of the AMPK signaling pathway enhances the gene expression of PGC-1α[119] and subsequently mediates MSCs to differentiate into brown adipocytes[120]. Compounds that have been shown to affect adipogenic fate commitment via the AMPK signaling pathway include arctigenin (ARC), albiflorin (AF), medicarpin[121], platycodin D (PD), metformin[122] and resveratrol[123,124].
ARC is a major lignan component of Arctii fructus and Forsythia fruit. Han et al[125] discovered that ARC inhibited adipogenesis in human AD-MSCs. The viability of MSCs did not change between the concentration of 10 and 100 μmol/L. Under ARC treatment, AMPK phosphorylation was significantly increased, suggesting that the adipogenic regulatory effect of ARC was mediated via activation of the AMPK signaling pathway. The in vivo experiments showed that the administration of ARC at a dose of 50 mg/kg per day reversed the body weight gain in high-fat diet (HFD) induced obesity mice, which is consistent with the in vitro findings.
AF is a major monoterpene glycoside compound of Paeonia lactiflora. Jeong et al[126] demonstrated that AF enhanced brown adipocyte differentiation via the AMPK and the PI3K/AKT/mTOR signaling pathways. In the presence of AF, lipid accumulation increased and beige-specific markers, including UCP1, PGC-1α, and CIDEA, were upregulated. After a 6-week administration of AF, body weight gain in HFD mice was significantly reduced, while the worsening of liver function, as measured by total cholesterol, LDL, ALT, and AST, reversed[126].
PD is an active compound of Platycodi radix. Kim et al[127] PD showed that administration of PD at a dosage of 5 mg/kg per day reduced the weight gain of db/db mice. However, the anti-obeisty effect of PD was not observed at the lower dosage of 2 mg/kg per day. In vitro, PD treatment suppressed the expression of adipogenic genes, such as Pparγ, Cebpα, Fabp4, Adipoq, and resistin, during the adipogenic differentiation of human AD-MSCs, suggesting the anti-adipogenic role of PD. Meanwhile, PD increased the expression of thermogenic factors UCP1 and PGC1 in both db/db mice and in vitro, which indicated the beige-adipoinductive role of PD. The phosphorylation of AMPK was significantly elevated under PD treatment, suggesting that PD is involved in regulating the AMPK signaling pathway.
Heme oxygenase (HO) is the enzyme that digests heme proteins and generates carbon monoxide, biliverdin, and iron. HO-1 induction results in increased levels of phosphorylated AMPK and AKT. The upregulation of HO-1 expression inhibits MSC adipogenic differentiation and favors osteogenic differentiation[128,129]. The small molecules that regulate MSC adipogenic cell fate via HO-1 activation include Tithonia diversifolia extracts and epoxyeicosatrienoic acid agonists.
Tithonia diversifolia extracts (TDE) are frequently used in traditional medicine for treating diabetes and wound healing. Giacomo et al[130] showed that TDE inhibited adipogenesis by inducing the AMPK signaling pathway via HO-1 activation. Upon TDE treatment, phosphorylated AMPK and HO-1 protein expression levels were significantly increased. A functional assay showed a dose-dependent effect of TDE on decreasing lipid accumulation in differentiated adipocytes.
Epoxyeicosatrienoic acids (EETs) are the derivative of arachidonic acid and act as an inducer of HO-1 activity[131]. The formed EETs can be metabolized by soluble epoxide hydrolase (sEH) into dihydroxyeicosatrienoic acids (DHETs). Kim et al[132] showed that adding the sEH inhibitor, 12-(3-hexylureido) dodec-8(Z)-enoic acid (8-HUDE) and 12-(3-adamantan-1-yl-ureido)-dodecanoic acid (AUDA) activated HO-1 and inhibited human BM-MSC adipogenesis. The inhibitory effects of 8-HUDE and AUDA were reduced by inhibition of HO activity, which demonstrated the role of AUDA and 8-HUDE in regulating adipogenesis of MSCs via the HO-1 signaling pathway.
Sumoylation is a post-translational modification process that is important in regulating the functional features of many proteins. Some of the transcription factors closely related to adipogenesis, such as PPARγ, C/EBPα, and C/EBPβ, are targets of sumoylation. The transcriptional activity of these master regulators can be negatively regulated by sumoylation and affect the commitment of adipogenic cell fate of MSCs[133,134].
Ginkgolic acid (GA), a compound that is enriched in the leaves of Ginkgo biloba, impairs SUMOylation by blocking the formation of the E1-SUMO thioester complex and functioning as a sumoylation inhibitor[135]. Liu et al[136] investigated the effect of GA on adipogenesis and demonstrated that the addition of GA in the early stage of adipogenesis promoted the commitment of mouse BM-MSCs into adipocytes, whereas addition at a later stage inhibited adipocyte differentiation.
DNA and histone methylation are the key components in the epigenetic machinery, regulating gene expression profiles. Some small molecules inhibit acetlyltransferase or methyltransferase and change the epigenetic landscape[137], which is recognized by other proteins that subsequently affect the expression of adipogenic genes. For MSC adipogenesis, these small molecules include 5-azacytidine and N-methyl pyrrolidone.
5-Azacytidine (5-aza) is a DNA methyltransferase inhibitor. Chen et al[138] showed that the methylation levels of Wnt10a chromatin regions were significantly reduced under the treatment with 5-aza and subsequently activated the expression of Wnt10a. Wnt10a then downregulated the expression level of adipogenic markers PPARγ, aP2, FAS, and C/EBPα and inhibited MSC commitment to adipogenic lineage.
Brd4 is a member of the bromodomain and extraterminal domain (BET) family that binds to active enhancers through recognition of acetyl-lysine residues of histones and controls PPARγ downstream adipogenic genes[139]. Gjoksi et al[140] showed that N-methyl pyrrolidone, a Brd4 inhibitor, reduced transcriptional activation of PPARγ and inhibited adipogenesis of human BM-MSCs. Furthermore, in the OVX rat model, administration of N-methyl pyrrolidone reduced fat accumulation and adipogenesis in the bone marrow tissue further validating the anti-adipogenic effect of Brd4.
The chondrogenic differentiation of MSCs is induced with a high cell-density pellet supplemented with transforming growth factor (TGF)-β in a serum-free medium, leading to an elevated production of cartilage-specific proteins, such as proteoglycans and type II collagen (Col 2). In addition to the TGF-β signaling pathway, there are also other signaling pathways involved in regulating the chondrogenesis of MSCs, including includes BMP[141], Wnt[142,143], fibroblast growth factor[144], hedgehog (HH), and Notch signaling pathways.
The HH signaling pathway is known to be important for cartilage development in vivo[145]. Implantation of fibroblasts expressing sonic hedgehog protein (Shh) protein or indian hedgehog protein in the nude mice forms cartilage at the donor site[146]. The other important role of HH signaling is the proliferative impact on the chondrocytes. Activation of the HH signaling pathway induces GLI, a transcriptional factor that promotes cell proliferation. Therefore, continuous activation of the HH signaling pathway causes uncontrolled cell proliferation and leads to the development of enchondromatosis[147,148].
Atractylenolides are enriched in Atractylodis macrocephalae, a kind of herbal medicine that is commonly prescribed to treat arthritis. Li et al[149] demonstrated that the addition of atractylenolides induced the Gli promoter and promoted chondrogenic differentiation in rat BM-MSCs. The chondrogenic markers Sox9, Col 2 and aggrecan were upregulated compared with the untreated group. When Shh signaling was inhibited by the signaling pathway inhibitor cyclopamine, the effect of atractylenolides on promoting chondrogenic differentiation was reduced, confirming that the chondrogenic effect of Atractylenolides was dependent on the Shh signaling pathway.
The Notch intracellular domain is the main mediator for regulating the chondrogenesis of the MSCs in the Notch signaling pathway. Upon ligand stimulation, the NOTCH protein undergoes proteolytic cleavage and releases the intracellular domain. The intracellular domain subsequently translocates into the nucleus and induces the expression of the HES gene family. The HES gene family, including HES-1 and HEY-1, acts on the Sox9 binding site at the Col2a1 enhancer and consequently prevents Sox9-mediated transcriptional activation of Col2a1, suppressing the chondrogenesis of MSC[150].
Dihydroartemisinin (DHA) is a major compound derived from Artemisia apiacea. Cao et al[151] showed that DHA inhibited chondrogenic differentiation of the C3H10T1/2 cell line in vitro. After treatment with DHA containing chondrogenic medium for 14 d, the chondrogenic-specific markers Sox9, COMP and Col2a1 were significantly suppressed compared with the untreated cells. The key factors in different signaling pathways were subsequently assessed, revealing upregulation of Hey1 expression, indicating that the Notch signaling pathway is involved in the DHA inhibition of chondrogenesis of the MSCs.
The regulation of MSC differentiation is multifaceted, governed by multiple signaling pathways, epigenetic regulation, and post-translational modification. Beyond the above small molecules illustrated, there were many others that have recently been identified to affect the lineage commitment of MSCs, but the underlying mechanisms are still elusive. For instance, the Cornus walteri extracts, the Oryza sativa extracts, and piceatannol (enriched in Aiphanes horrida) were shown to inhibit adipogenesis of MSC[152-154]. Ajuga decumbens extracts were shown to stimulate osteogenesis of MSCs[155]. Honokiol improved the chondrogenesis of MSCs[156]. However, the underlying mechanisms of all the above compounds have not yet been explored. Understanding the mechanism by which different small molecules affect MSC cell determination will benefit the application utility of small molecules as precisive modulators, offering researchers a useful probe in guiding MSC differentiation.
Some of the small molecules identified have been investigated in clinical trials for the treatment of MSC-related diseases, while many others identified recently remained unexplored. Even though the in vitro cell culture experiments and the animal studies offered promising results, there are several aspects that can hinder investigators from translating these small molecules into clinical use. One of the major obstacles to success is that the cells and animal models cannot fully reflect the effect of small molecules in humans for many reasons, such as differences in physiological background, length of administration and subjective endpoints. The other obstacle is that, for those small molecules identified from a complex compound, one kind of small molecule may not be sufficient to have the maximal effect, but require other small molecules from the original compound to synergize with it.
Overall, the ongoing discovery of new small molecules facilitating MSC cell fate commitment will continue to play critical roles in basic science research and potentially become novel therapeutic agents in treating various MSC-related diseases.
Manuscript source: Invited Manuscript
Specialty type: Cell and tissue engineering
Country of origin: China
Peer-review report classification
Grade A (Excellent): A, A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Bonartsev AP, Khan I, Ruiz MA S-Editor: Tang JZ L-Editor: A E-Editor: Ma YJ
| 1. | Friedenstein AJ, Gorskaja JF, Kulagina NN. Fibroblast precursors in normal and irradiated mouse hematopoietic organs. Exp Hematol. 1976;4:267-274. [PubMed] |
| 2. | Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, Alfonso ZC, Fraser JK, Benhaim P, Hedrick MH. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13:4279-4295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4817] [Cited by in RCA: 5015] [Article Influence: 218.0] [Reference Citation Analysis (0)] |
| 3. | De Bari C, Dell'Accio F, Tylzanowski P, Luyten FP. Multipotent mesenchymal stem cells from adult human synovial membrane. Arthritis Rheum. 2001;44:1928-1942. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 4. | Erices A, Conget P, Minguell JJ. Mesenchymal progenitor cells in human umbilical cord blood. Br J Haematol. 2000;109:235-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1137] [Cited by in RCA: 1077] [Article Influence: 43.1] [Reference Citation Analysis (0)] |
| 5. | Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop Dj, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11055] [Cited by in RCA: 12689] [Article Influence: 704.9] [Reference Citation Analysis (2)] |
| 6. | Muraglia A, Cancedda R, Quarto R. Clonal mesenchymal progenitors from human bone marrow differentiate in vitro according to a hierarchical model. J Cell Sci. 2000;113:1161-1166. [PubMed] |
| 7. | Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15372] [Cited by in RCA: 15203] [Article Influence: 584.7] [Reference Citation Analysis (0)] |
| 8. | Jiang Y, Jahagirdar BN, Reinhardt RL, Schwartz RE, Keene CD, Ortiz-Gonzalez XR, Reyes M, Lenvik T, Lund T, Blackstad M, Du J, Aldrich S, Lisberg A, Low WC, Largaespada DA, Verfaillie CM. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002;418:41-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4340] [Cited by in RCA: 3918] [Article Influence: 170.3] [Reference Citation Analysis (0)] |
| 9. | Liu C, Tsai AL, Li PC, Huang CW, Wu CC. Endothelial differentiation of bone marrow mesenchyme stem cells applicable to hypoxia and increased migration through Akt and NFκB signals. Stem Cell Res Ther. 2017;8:29. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 10. | Janeczek Portalska K, Leferink A, Groen N, Fernandes H, Moroni L, van Blitterswijk C, de Boer J. Endothelial differentiation of mesenchymal stromal cells. PLoS One. 2012;7:e46842. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 142] [Cited by in RCA: 145] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 11. | Seo MJ, Suh SY, Bae YC, Jung JS. Differentiation of human adipose stromal cells into hepatic lineage in vitro and in vivo. Biochem Biophys Res Commun. 2005;328:258-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 401] [Cited by in RCA: 394] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 12. | Sgodda M, Aurich H, Kleist S, Aurich I, König S, Dollinger MM, Fleig WE, Christ B. Hepatocyte differentiation of mesenchymal stem cells from rat peritoneal adipose tissue in vitro and in vivo. Exp Cell Res. 2007;313:2875-2886. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 140] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 13. | Lee KD, Kuo TK, Whang-Peng J, Chung YF, Lin CT, Chou SH, Chen JR, Chen YP, Lee OK. In vitro hepatic differentiation of human mesenchymal stem cells. Hepatology. 2004;40:1275-1284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 668] [Cited by in RCA: 661] [Article Influence: 31.5] [Reference Citation Analysis (0)] |
| 14. | Tao YX, Xu HW, Zheng Q Y, FitzGibbon T. Noggin induces human bone marrow-derived mesenchymal stem cells to differentiate into neural and photoreceptor cells. Indian J Exp Biol. 2010;48:444-452. [PubMed] |
| 15. | Fan CG, Tang FW, Zhang QJ, Lu SH, Liu HY, Zhao ZM, Liu B, Han ZB, Han ZC. Characterization and neural differentiation of fetal lung mesenchymal stem cells. Cell Transplant. 2005;14:311-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 68] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 16. | Corselli M, Chen CW, Crisan M, Lazzari L, Péault B. Perivascular ancestors of adult multipotent stem cells. Arterioscler Thromb Vasc Biol. 2010;30:1104-1109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 235] [Cited by in RCA: 228] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 17. | Méndez-Ferrer S, Michurina TV, Ferraro F, Mazloom AR, Macarthur BD, Lira SA, Scadden DT, Ma'ayan A, Enikolopov GN, Frenette PS. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature. 2010;466:829-834. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2810] [Cited by in RCA: 2581] [Article Influence: 172.1] [Reference Citation Analysis (0)] |
| 18. | Naveiras O, Nardi V, Wenzel PL, Hauschka PV, Fahey F, Daley GQ. Bone-marrow adipocytes as negative regulators of the haematopoietic microenvironment. Nature. 2009;460:259-263. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 921] [Cited by in RCA: 854] [Article Influence: 53.4] [Reference Citation Analysis (0)] |
| 19. | Garrett RW, Emerson SG. Bone and blood vessels: the hard and the soft of hematopoietic stem cell niches. Cell Stem Cell. 2009;4:503-506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 77] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 20. | Chen Q, Shou P, Zheng C, Jiang M, Cao G, Yang Q, Cao J, Xie N, Velletri T, Zhang X, Xu C, Zhang L, Yang H, Hou J, Wang Y, Shi Y. Fate decision of mesenchymal stem cells: adipocytes or osteoblasts? Cell Death Differ. 2016;23:1128-1139. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 606] [Cited by in RCA: 914] [Article Influence: 101.6] [Reference Citation Analysis (0)] |
| 21. | Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, Benhaim P, Lorenz HP, Hedrick MH. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7:211-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5703] [Cited by in RCA: 5763] [Article Influence: 240.1] [Reference Citation Analysis (0)] |
| 22. | Cawthorn WP, Scheller EL, MacDougald OA. Adipose tissue stem cells: the great WAT hope. Trends Endocrinol Metab. 2012;23:270-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 71] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 23. | Moreno-Indias I, Tinahones FJ. Impaired adipose tissue expandability and lipogenic capacities as ones of the main causes of metabolic disorders. J Diabetes Res. 2015;2015:970375. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 72] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 24. | Klein S, Fontana L, Young VL, Coggan AR, Kilo C, Patterson BW, Mohammed BS. Absence of an effect of liposuction on insulin action and risk factors for coronary heart disease. N Engl J Med. 2004;350:2549-2557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 551] [Cited by in RCA: 490] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 25. | Hong JH, Hwang ES, McManus MT, Amsterdam A, Tian Y, Kalmukova R, Mueller E, Benjamin T, Spiegelman BM, Sharp PA, Hopkins N, Yaffe MB. TAZ, a transcriptional modulator of mesenchymal stem cell differentiation. Science. 2005;309:1074-1078. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 744] [Cited by in RCA: 814] [Article Influence: 40.7] [Reference Citation Analysis (0)] |
| 26. | Almalki SG, Agrawal DK. Key transcription factors in the differentiation of mesenchymal stem cells. Differentiation. 2016;92:41-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 307] [Article Influence: 34.1] [Reference Citation Analysis (0)] |
| 27. | Barzilay R, Melamed E, Offen D. Introducing transcription factors to multipotent mesenchymal stem cells: making transdifferentiation possible. Stem Cells. 2009;27:2509-2515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 82] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 28. | Yin B, Yu F, Wang C, Li B, Liu M, Ye L. Epigenetic Control of Mesenchymal Stem Cell Fate Decision via Histone Methyltransferase Ash1l. Stem Cells. 2019;37:115-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 52] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 29. | Hemming S, Cakouros D, Isenmann S, Cooper L, Menicanin D, Zannettino A, Gronthos S. EZH2 and KDM6A act as an epigenetic switch to regulate mesenchymal stem cell lineage specification. Stem Cells. 2014;32:802-815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 213] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 30. | Ye L, Fan Z, Yu B, Chang J, Al Hezaimi K, Zhou X, Park NH, Wang CY. Histone demethylases KDM4B and KDM6B promotes osteogenic differentiation of human MSCs. Cell Stem Cell. 2012;11:50-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 275] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 31. | Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677-689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9969] [Cited by in RCA: 9694] [Article Influence: 510.2] [Reference Citation Analysis (0)] |
| 32. | Yang C, Tibbitt MW, Basta L, Anseth KS. Mechanical memory and dosing influence stem cell fate. Nat Mater. 2014;13:645-652. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 789] [Cited by in RCA: 827] [Article Influence: 75.2] [Reference Citation Analysis (0)] |
| 33. | Xu Y, Shi Y, Ding S. A chemical approach to stem-cell biology and regenerative medicine. Nature. 2008;453:338-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 267] [Cited by in RCA: 255] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 34. | Riesenberg S, Maricic T. Targeting repair pathways with small molecules increases precise genome editing in pluripotent stem cells. Nat Commun. 2018;9:2164. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 95] [Cited by in RCA: 129] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 35. | Jaiswal N, Haynesworth SE, Caplan AI, Bruder SP. Osteogenic differentiation of purified, culture-expanded human mesenchymal stem cells in vitro. J Cell Biochem. 1997;64:295-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 36. | Fakhry M, Hamade E, Badran B, Buchet R, Magne D. Molecular mechanisms of mesenchymal stem cell differentiation towards osteoblasts. World J Stem Cells. 2013;5:136-148. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 177] [Cited by in RCA: 191] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 37. | Beederman M, Lamplot JD, Nan G, Wang J, Liu X, Yin L, Li R, Shui W, Zhang H, Kim SH, Zhang W, Zhang J, Kong Y, Denduluri S, Rogers MR, Pratt A, Haydon RC, Luu HH, Angeles J, Shi LL, He TC. BMP signaling in mesenchymal stem cell differentiation and bone formation. J Biomed Sci Eng. 2013;6:32-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 228] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 38. | Wang N, Wang F, Gao Y, Zhou Z, Liu W, Pan C, Yin P, Tang M, Yu X. Solanum Muricatum Promotes Osteogenic Differentiation of Rat Bone Marrow Stromal Cells. J Food Sci. 2017;82:1775-1780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 39. | Zhang JF, Li G, Chan CY, Meng CL, Lin MC, Chen YC, He ML, Leung PC, Kung HF. Flavonoids of Herba Epimedii regulate osteogenesis of human mesenchymal stem cells through BMP and Wnt/beta-catenin signaling pathway. Mol Cell Endocrinol. 2010;314:70-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 111] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 40. | Saulite L, Jekabsons K, Klavins M, Muceniece R, Riekstina U. Effects of malvidin, cyanidin and delphinidin on human adipose mesenchymal stem cell differentiation into adipocytes, chondrocytes and osteocytes. Phytomedicine. 2019;53:86-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 47] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 41. | Kuo PL, Hsu YL, Chang CH, Chang JK. Osthole-mediated cell differentiation through bone morphogenetic protein-2/p38 and extracellular signal-regulated kinase 1/2 pathway in human osteoblast cells. J Pharmacol Exp Ther. 2005;314:1290-1299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 92] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 42. | Hu H, Chen M, Dai G, Du G, Wang X, He J, Zhao Y, Han D, Cao Y, Zheng Y, Ding D. An Inhibitory Role of Osthole in Rat MSCs Osteogenic Differentiation and Proliferation via Wnt/β-Catenin and Erk1/2-MAPK Pathways. Cell Physiol Biochem. 2016;38:2375-2388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 43. | Tang DZ, Hou W, Zhou Q, Zhang M, Holz J, Sheu TJ, Li TF, Cheng SD, Shi Q, Harris SE, Chen D, Wang YJ. Osthole stimulates osteoblast differentiation and bone formation by activation of beta-catenin-BMP signaling. J Bone Miner Res. 2010;25:1234-1245. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 98] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 44. | Dai J, Li Y, Zhou H, Chen J, Chen M, Xiao Z. Genistein promotion of osteogenic differentiation through BMP2/SMAD5/RUNX2 signaling. Int J Biol Sci. 2013;9:1089-1098. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 62] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 45. | Arjmandi BH, Alekel L, Hollis BW, Amin D, Stacewicz-Sapuntzakis M, Guo P, Kukreja SC. Dietary soybean protein prevents bone loss in an ovariectomized rat model of osteoporosis. J Nutr. 1996;126:161-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 369] [Cited by in RCA: 312] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 46. | Marini H, Minutoli L, Polito F, Bitto A, Altavilla D, Atteritano M, Gaudio A, Mazzaferro S, Frisina A, Frisina N, Lubrano C, Bonaiuto M, D'Anna R, Cannata ML, Corrado F, Adamo EB, Wilson S, Squadrito F. Effects of the phytoestrogen genistein on bone metabolism in osteopenic postmenopausal women: a randomized trial. Ann Intern Med. 2007;146:839-847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 213] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 47. | Zhao XL, Chen JJ, Zhang GN, Wang YC, Si SY, Chen LF, Wang Z. Small molecule T63 suppresses osteoporosis by modulating osteoblast differentiation via BMP and WNT signaling pathways. Sci Rep. 2017;7:10397. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 48. | Bhanot P, Brink M, Samos CH, Hsieh JC, Wang Y, Macke JP, Andrew D, Nathans J, Nusse R. A new member of the frizzled family from Drosophila functions as a Wingless receptor. Nature. 1996;382:225-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1116] [Cited by in RCA: 1118] [Article Influence: 38.6] [Reference Citation Analysis (0)] |
| 49. | Mao B, Wu W, Li Y, Hoppe D, Stannek P, Glinka A, Niehrs C. LDL-receptor-related protein 6 is a receptor for Dickkopf proteins. Nature. 2001;411:321-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 803] [Cited by in RCA: 847] [Article Influence: 35.3] [Reference Citation Analysis (0)] |
| 50. | Eastman Q, Grosschedl R. Regulation of LEF-1/TCF transcription factors by Wnt and other signals. Curr Opin Cell Biol. 1999;11:233-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 414] [Cited by in RCA: 430] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 51. | Bennett CN, Longo KA, Wright WS, Suva LJ, Lane TF, Hankenson KD, MacDougald OA. Regulation of osteoblastogenesis and bone mass by Wnt10b. Proc Natl Acad Sci USA. 2005;102:3324-3329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 644] [Cited by in RCA: 680] [Article Influence: 34.0] [Reference Citation Analysis (0)] |
| 52. | Kang S, Bennett CN, Gerin I, Rapp LA, Hankenson KD, Macdougald OA. Wnt signaling stimulates osteoblastogenesis of mesenchymal precursors by suppressing CCAAT/enhancer-binding protein alpha and peroxisome proliferator-activated receptor gamma. J Biol Chem. 2007;282:14515-14524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 311] [Cited by in RCA: 333] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 53. | Zhang H, Li H. Tricin enhances osteoblastogenesis through the regulation of Wnt/β-catenin signaling in human mesenchymal stem cells. Mech Dev. 2018;152:38-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 54. | Zhang G, Li C, Niu Y, Yu Q, Chen Y, Liu E. Osteoprotective Effect of Radix Scutellariae in Female Hindlimb-Suspended Sprague-Dawley Rats and the Osteogenic Differentiation Effect of Its Major Constituent. Molecules. 2017;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 55. | Guo AJ, Choi RC, Cheung AW, Chen VP, Xu SL, Dong TT, Chen JJ, Tsim KW. Baicalin, a flavone, induces the differentiation of cultured osteoblasts: an action via the Wnt/beta-catenin signaling pathway. J Biol Chem. 2011;286:27882-27893. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 98] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 56. | Li G, Zhang XA, Zhang JF, Chan CY, Yew DT, He ML, Lin MC, Leung PC, Kung HF. Ethanol extract of Fructus Ligustri Lucidi promotes osteogenesis of mesenchymal stem cells. Phytother Res. 2010;24:571-576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 37] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 57. | Tyagi AM, Gautam AK, Kumar A, Srivastava K, Bhargavan B, Trivedi R, Saravanan S, Yadav DK, Singh N, Pollet C, Brazier M, Mentaverri R, Maurya R, Chattopadhyay N, Goel A, Singh D. Medicarpin inhibits osteoclastogenesis and has nonestrogenic bone conserving effect in ovariectomized mice. Mol Cell Endocrinol. 2010;325:101-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 59] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 58. | Dixit M, Raghuvanshi A, Gupta CP, Kureel J, Mansoori MN, Shukla P, John AA, Singh K, Purohit D, Awasthi P, Singh D, Goel A. Medicarpin, a Natural Pterocarpan, Heals Cortical Bone Defect by Activation of Notch and Wnt Canonical Signaling Pathways. PLoS One. 2015;10:e0144541. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 59. | Wang F, Yin P, Lu Y, Zhou Z, Jiang C, Liu Y, Yu X. Cordycepin prevents oxidative stress-induced inhibition of osteogenesis. Oncotarget. 2015;6:35496-35508. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 41] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 60. | Shin SY, Kim CG, Jho EH, Rho MS, Kim YS, Kim YH, Lee YH. Hydrogen peroxide negatively modulates Wnt signaling through downregulation of beta-catenin. Cancer Lett. 2004;212:225-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 82] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 61. | Chen YX, Zhu DY, Xu ZL, Yin JH, Yu XW, Mei J, Gao YS, Zhang CQ. The Protective Effect of Cordycepin On Alcohol-Induced Osteonecrosis of the Femoral Head. Cell Physiol Biochem. 2017;42:2391-2403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 62. | Driver J, Weber CE, Callaci JJ, Kothari AN, Zapf MA, Roper PM, Borys D, Franzen CA, Gupta GN, Wai PY, Zhang J, Denning MF, Kuo PC, Mi Z. Alcohol inhibits osteopontin-dependent transforming growth factor-β1 expression in human mesenchymal stem cells. J Biol Chem. 2015;290:9959-9973. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 63. | Gwak J, Hwang SG, Park HS, Choi SR, Park SH, Kim H, Ha NC, Bae SJ, Han JK, Kim DE, Cho JW, Oh S. Small molecule-based disruption of the Axin/β-catenin protein complex regulates mesenchymal stem cell differentiation. Cell Res. 2012;22:237-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 110] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 64. | Wu Z, Zhang J, Gu X, Zhang X, Shi S, Liu C. Effects of the extract of Ginkgo biloba on the differentiation of bone marrow mesenchymal stem cells in vitro. Am J Transl Res. 2016;8:3032-3040. [PubMed] |
| 65. | Gu Q, Chen C, Zhang Z, Wu Z, Fan X, Zhang Z, Di W, Shi L. Ginkgo biloba extract promotes osteogenic differentiation of human bone marrow mesenchymal stem cells in a pathway involving Wnt/β-catenin signaling. Pharmacol Res. 2015;97:70-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 42] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 66. | Hori K, Sen A, Artavanis-Tsakonas S. Notch signaling at a glance. J Cell Sci. 2013;126:2135-2140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 327] [Cited by in RCA: 390] [Article Influence: 32.5] [Reference Citation Analysis (0)] |
| 67. | Ugarte F, Ryser M, Thieme S, Fierro FA, Navratiel K, Bornhäuser M, Brenner S. Notch signaling enhances osteogenic differentiation while inhibiting adipogenesis in primary human bone marrow stromal cells. Exp Hematol. 2009;37:867-875. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 98] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 68. | Yang Z, Huang JH, Liu SF, Zhao YJ, Shen ZY, Wang YJ, Bian Q. The osteoprotective effect of psoralen in ovariectomy-induced osteoporotic rats via stimulating the osteoblastic differentiation from bone mesenchymal stem cells. Menopause. 2012;19:1156-1164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 69. | Bian Q, Liu SF, Huang JH, Yang Z, Tang DZ, Zhou Q, Ning Y, Zhao YJ, Lu S, Shen ZY, Wang YJ. Oleanolic acid exerts an osteoprotective effect in ovariectomy-induced osteoporotic rats and stimulates the osteoblastic differentiation of bone mesenchymal stem cells in vitro. Menopause. 2012;19:225-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 37] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 70. | Nakae J, Kido Y, Accili D. Distinct and overlapping functions of insulin and IGF-I receptors. Endocr Rev. 2001;22:818-835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 303] [Cited by in RCA: 341] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 71. | Giustina A, Mazziotti G, Canalis E. Growth hormone, insulin-like growth factors, and the skeleton. Endocr Rev. 2008;29:535-559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 618] [Cited by in RCA: 599] [Article Influence: 35.2] [Reference Citation Analysis (0)] |
| 72. | Liu JP, Baker J, Perkins AS, Robertson EJ, Efstratiadis A. Mice carrying null mutations of the genes encoding insulin-like growth factor I (Igf-1) and type 1 IGF receptor (Igf1r). Cell. 1993;75:59-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 285] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 73. | Zhang M, Xuan S, Bouxsein ML, von Stechow D, Akeno N, Faugere MC, Malluche H, Zhao G, Rosen CJ, Efstratiadis A, Clemens TL. Osteoblast-specific knockout of the insulin-like growth factor (IGF) receptor gene reveals an essential role of IGF signaling in bone matrix mineralization. J Biol Chem. 2002;277:44005-44012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 549] [Cited by in RCA: 542] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 74. | Fujita T, Azuma Y, Fukuyama R, Hattori Y, Yoshida C, Koida M, Ogita K, Komori T. Runx2 induces osteoblast and chondrocyte differentiation and enhances their migration by coupling with PI3K-Akt signaling. J Cell Biol. 2004;166:85-95. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 319] [Cited by in RCA: 354] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 75. | Ghosh-Choudhury N, Abboud SL, Nishimura R, Celeste A, Mahimainathan L, Choudhury GG. Requirement of BMP-2-induced phosphatidylinositol 3-kinase and Akt serine/threonine kinase in osteoblast differentiation and Smad-dependent BMP-2 gene transcription. J Biol Chem. 2002;277:33361-33368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 249] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 76. | Liang D, Ren H, Qiu T, Shen G, Xie B, Wei Q, Yao Z, Tang J, Zhang Z, Jiang X. Extracts from plastrum testudinis reverse glucocorticoid-induced spinal osteoporosis of rats via targeting osteoblastic and osteoclastic markers. Biomed Pharmacother. 2016;82:151-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 77. | Shen GY, Ren H, Huang JJ, Zhang ZD, Zhao WH, Yu X, Shang Q, Qiu T, Zhang YZ, Tang JJ, Liang, Yang ZD, Jiang XB. Plastrum Testudinis Extracts Promote BMSC Proliferation and Osteogenic Differentiation by Regulating Let-7f-5p and the TNFR2/PI3K/AKT Signaling Pathway. Cell Physiol Biochem. 2018;47:2307-2318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 35] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 78. | Zhai Y, Wang Q, Li Y, Cui J, Feng K, Kong X, Xian CJ. The higher osteoprotective activity of psoralidin in vivo than coumestrol is attributed by its presence of an isopentenyl group and through activated PI3K/Akt axis. Biomed Pharmacother. 2018;102:1015-1024. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 79. | Kim EK, Lim S, Park JM, Seo JK, Kim JH, Kim KT, Ryu SH, Suh PG. Human mesenchymal stem cell differentiation to the osteogenic or adipogenic lineage is regulated by AMP-activated protein kinase. J Cell Physiol. 2012;227:1680-1687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 81] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 80. | Wu JB, Fong YC, Tsai HY, Chen YF, Tsuzuki M, Tang CH. Naringin-induced bone morphogenetic protein-2 expression via PI3K, Akt, c-Fos/c-Jun and AP-1 pathway in osteoblasts. Eur J Pharmacol. 2008;588:333-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 105] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 81. | Peng-Zhang, Dai KR, Yan SG, Yan WQ, Chao-Zhang, Chen DQ, Bo-Xu, Xu ZW. Effects of naringin on the proliferation and osteogenic differentiation of human bone mesenchymal stem cell. Eur J Pharmacol. 2009;607:1-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 112] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 82. | Wang D, Ma W, Wang F, Dong J, Wang D, Sun B, Wang B. Stimulation of Wnt/β-Catenin Signaling to Improve Bone Development by Naringin via Interacting with AMPK and Akt. Cell Physiol Biochem. 2015;36:1563-1576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 46] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 83. | Zhang W, Liu HT. Mapk signal pathways in the regulation of cell proliferation in mammalian cells. Cell Res. 2002;12:9-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1583] [Cited by in RCA: 2010] [Article Influence: 87.4] [Reference Citation Analysis (0)] |
| 84. | Salasznyk RM, Klees RF, Hughlock MK, Plopper GE. ERK signaling pathways regulate the osteogenic differentiation of human mesenchymal stem cells on collagen I and vitronectin. Cell Commun Adhes. 2004;11:137-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 125] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 85. | Liu Y, Zuo H, Liu X, Xiong J, Pei X. The antiosteoporosis effect of icariin in ovariectomized rats: a systematic review and meta-analysis. Cell Mol Biol (Noisy-le-grand). 2017;63:124-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 86. | Zhang S, Feng P, Mo G, Li D, Li Y, Mo L, Yang Z, Liang. Icariin influences adipogenic differentiation of stem cells affected by osteoblast-osteoclast co-culture and clinical research adipogenic. Biomed Pharmacother. 2017;88:436-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 87. | Fan JJ, Cao LG, Wu T, Wang DX, Jin D, Jiang S, Zhang ZY, Bi L, Pei GX. The dose-effect of icariin on the proliferation and osteogenic differentiation of human bone mesenchymal stem cells. Molecules. 2011;16:10123-10133. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 83] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 88. | Wu Y, Xia L, Zhou Y, Xu Y, Jiang X. Icariin induces osteogenic differentiation of bone mesenchymal stem cells in a MAPK-dependent manner. Cell Prolif. 2015;48:375-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 99] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 89. | Zhao J, Ohba S, Shinkai M, Chung UI, Nagamune T. Icariin induces osteogenic differentiation in vitro in a BMP- and Runx2-dependent manner. Biochem Biophys Res Commun. 2008;369:444-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 139] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 90. | Huang J-m, Bao Y, Xiang W, Jing X-z, Guo J-c, Yao X-d, Wang R, Guo F-j. Icariin regulates the bidirectional differentiation of bone marrow mesenchymal stem cells through canonical wnt signaling pathway. Evid Based Complement Alternat Med. 2017;2017:8085325. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 91. | Zhai YK, Guo XY, Ge BF, Zhen P, Ma XN, Zhou J, Ma HP, Xian CJ, Chen KM. Icariin stimulates the osteogenic differentiation of rat bone marrow stromal cells via activating the PI3K-AKT-eNOS-NO-cGMP-PKG. Bone. 2014;66:189-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 93] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 92. | Cao H, Zhang Y, Qian W, Guo XP, Sun C, Zhang L, Cheng XH. Effect of icariin on fracture healing in an ovariectomized rat model of osteoporosis. Exp Ther Med. 2017;13:2399-2404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 93. | Zhang G, Qin L, Shi Y. Epimedium-derived phytoestrogen flavonoids exert beneficial effect on preventing bone loss in late postmenopausal women: a 24-month randomized, double-blind and placebo-controlled trial. J Bone Miner Res. 2007;22:1072-1079. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 202] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 94. | Liu S, Huang J, Wang J, Zhao Y, Lu S, Wang Y, Bian Q. Er-Xian Decoction Stimulates Osteoblastic Differentiation of Bone Mesenchymal Stem Cells in Ovariectomized Mice and Its Gene Profile Analysis. Stem Cells Int. 2016;2016:4079210. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 95. | Li JY, Jia YS, Chai LM, Mu XH, Ma S, Xu L, Wei X. Effects of Chinese herbal formula Erxian decoction for treating osteoporosis: a systematic review. Clin Interv Aging. 2017;12:45-53. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 96. | Dai Z, Li Y, Quarles LD, Song T, Pan W, Zhou H, Xiao Z. Resveratrol enhances proliferation and osteoblastic differentiation in human mesenchymal stem cells via ER-dependent ERK1/2 activation. Phytomedicine. 2007;14:806-814. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 186] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 97. | Tseng PC, Hou SM, Chen RJ, Peng HW, Hsieh CF, Kuo ML, Yen ML. Resveratrol promotes osteogenesis of human mesenchymal stem cells by upregulating RUNX2 gene expression via the SIRT1/FOXO3A axis. J Bone Miner Res. 2011;26:2552-2563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 238] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 98. | Shakibaei M, Shayan P, Busch F, Aldinger C, Buhrmann C, Lueders C, Mobasheri A. Resveratrol mediated modulation of Sirt-1/Runx2 promotes osteogenic differentiation of mesenchymal stem cells: potential role of Runx2 deacetylation. PLoS One. 2012;7:e35712. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 149] [Cited by in RCA: 180] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 99. | Ornstrup MJ, Harsløf T, Kjær TN, Langdahl BL, Pedersen SB. Resveratrol increases bone mineral density and bone alkaline phosphatase in obese men: a randomized placebo-controlled trial. J Clin Endocrinol Metab. 2014;99:4720-4729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 109] [Article Influence: 9.9] [Reference Citation Analysis (1)] |
| 100. | Joe Y, Zheng M, Kim HJ, Kim S, Uddin MJ, Park C, Ryu DG, Kang SS, Ryoo S, Ryter SW, Chang KC, Chung HT. Salvianolic acid B exerts vasoprotective effects through the modulation of heme oxygenase-1 and arginase activities. J Pharmacol Exp Ther. 2012;341:850-858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 63] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 101. | Cui L, Li T, Liu Y, Zhou L, Li P, Xu B, Huang L, Chen Y, Liu Y, Tian X, Jee WS, Wu T. Salvianolic acid B prevents bone loss in prednisone-treated rats through stimulation of osteogenesis and bone marrow angiogenesis. PLoS One. 2012;7:e34647. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 108] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 102. | Xu D, Xu L, Zhou C, Lee WY, Wu T, Cui L, Li G. Salvianolic acid B promotes osteogenesis of human mesenchymal stem cells through activating ERK signaling pathway. Int J Biochem Cell Biol. 2014;51:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 76] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 103. | Rosen ED, MacDougald OA. Adipocyte differentiation from the inside out. Nat Rev Mol Cell Biol. 2006;7:885-896. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1830] [Cited by in RCA: 1999] [Article Influence: 111.1] [Reference Citation Analysis (0)] |
| 104. | Rosen ED, Walkey CJ, Puigserver P, Spiegelman BM. Transcriptional regulation of adipogenesis. Genes Dev. 2000;14:1293-1307. [PubMed] |
| 105. | Halvorsen YD, Bond A, Sen A, Franklin DM, Lea-Currie YR, Sujkowski D, Ellis PN, Wilkison WO, Gimble JM. Thiazolidinediones and glucocorticoids synergistically induce differentiation of human adipose tissue stromal cells: biochemical, cellular, and molecular analysis. Metabolism. 2001;50:407-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 161] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 106. | Cao Z, Umek RM, McKnight SL. Regulated expression of three C/EBP isoforms during adipose conversion of 3T3-L1 cells. Genes Dev. 1991;5:1538-1552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1215] [Cited by in RCA: 1350] [Article Influence: 39.7] [Reference Citation Analysis (0)] |
| 107. | van der Horst G, Farih-Sips H, Löwik CW, Karperien M. Hedgehog stimulates only osteoblastic differentiation of undifferentiated KS483 cells. Bone. 2003;33:899-910. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 79] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 108. | Fleury A, Hoch L, Martinez MC, Faure H, Taddei M, Petricci E, Manetti F, Girard N, Mann A, Jacques C, Larghero J, Ruat M, Andriantsitohaina R, Le Lay S. Hedgehog associated to microparticles inhibits adipocyte differentiation via a non-canonical pathway. Sci Rep. 2016;6:23479. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 109. | Ross SE, Hemati N, Longo KA, Bennett CN, Lucas PC, Erickson RL, MacDougald OA. Inhibition of adipogenesis by Wnt signaling. Science. 2000;289:950-953. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1436] [Cited by in RCA: 1503] [Article Influence: 60.1] [Reference Citation Analysis (0)] |
| 110. | Topol L, Jiang X, Choi H, Garrett-Beal L, Carolan PJ, Yang Y. Wnt-5a inhibits the canonical Wnt pathway by promoting GSK-3-independent beta-catenin degradation. J Cell Biol. 2003;162:899-908. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 573] [Cited by in RCA: 592] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 111. | Christodoulides C, Lagathu C, Sethi JK, Vidal-Puig A. Adipogenesis and WNT signalling. Trends Endocrinol Metab. 2009;20:16-24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 481] [Cited by in RCA: 455] [Article Influence: 28.4] [Reference Citation Analysis (0)] |
| 112. | Christodoulides C, Laudes M, Cawthorn WP, Schinner S, Soos M, O'Rahilly S, Sethi JK, Vidal-Puig A. The Wnt antagonist Dickkopf-1 and its receptors are coordinately regulated during early human adipogenesis. J Cell Sci. 2006;119:2613-2620. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 132] [Cited by in RCA: 127] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 113. | Lee J, Lee J, Jung E, Hwang W, Kim YS, Park D. Isorhamnetin-induced anti-adipogenesis is mediated by stabilization of beta-catenin protein. Life Sci. 2010;86:416-423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 36] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 114. | Jang WS, Seo CR, Jang HH, Song NJ, Kim JK, Ahn JY, Han J, Seo WD, Lee YM, Park KW. Black rice (Oryza sativa L.) extracts induce osteoblast differentiation and protect against bone loss in ovariectomized rats. Food Funct. 2015;6:265-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 115. | Miki H, Yamauchi T, Suzuki R, Komeda K, Tsuchida A, Kubota N, Terauchi Y, Kamon J, Kaburagi Y, Matsui J, Akanuma Y, Nagai R, Kimura S, Tobe K, Kadowaki T. Essential role of insulin receptor substrate 1 (IRS-1) and IRS-2 in adipocyte differentiation. Mol Cell Biol. 2001;21:2521-2532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 165] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 116. | Lee PL, Jung SM, Guertin DA. The Complex Roles of Mechanistic Target of Rapamycin in Adipocytes and Beyond. Trends Endocrinol Metab. 2017;28:319-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 54] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 117. | Yu W, Chen Z, Zhang J, Zhang L, Ke H, Huang L, Peng Y, Zhang X, Li S, Lahn BT, Xiang AP. Critical role of phosphoinositide 3-kinase cascade in adipogenesis of human mesenchymal stem cells. Mol Cell Biochem. 2008;310:11-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 108] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 118. | Jin J, Wang L, Wang XK, Lai PL, Huang MJ, Jin DD, Zhong ZM, Chen JT, Bai XC. Risedronate inhibits bone marrow mesenchymal stem cell adipogenesis and switches RANKL/OPG ratio to impair osteoclast differentiation. J Surg Res. 2013;180:e21-e29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 119. | Lee WJ, Kim M, Park HS, Kim HS, Jeon MJ, Oh KS, Koh EH, Won JC, Kim MS, Oh GT, Yoon M, Lee KU, Park JY. AMPK activation increases fatty acid oxidation in skeletal muscle by activating PPARalpha and PGC-1. Biochem Biophys Res Commun. 2006;340:291-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 287] [Cited by in RCA: 313] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 120. | Huang PI, Chen YC, Chen LH, Juan CC, Ku HH, Wang ST, Chiou SH, Chiou GY, Chi CW, Hsu CC, Lee HC, Chen LK, Kao CL. PGC-1α mediates differentiation of mesenchymal stem cells to brown adipose cells. J Atheroscler Thromb. 2011;18:966-980. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 42] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 121. | Imran KM, Yoon D, Kim YS. A pivotal role of AMPK signaling in medicarpin-mediated formation of brown and beige. Biofactors. 2018;44:168-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 122. | Chen SC, Brooks R, Houskeeper J, Bremner SK, Dunlop J, Viollet B, Logan PJ, Salt IP, Ahmed SF, Yarwood SJ. Metformin suppresses adipogenesis through both AMP-activated protein kinase (AMPK)-dependent and AMPK-independent mechanisms. Mol Cell Endocrinol. 2017;440:57-68. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 106] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 123. | Peltz L, Gomez J, Marquez M, Alencastro F, Atashpanjeh N, Quang T, Bach T, Zhao Y. Resveratrol exerts dosage and duration dependent effect on human mesenchymal stem cell development. PLoS One. 2012;7:e37162. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 80] [Cited by in RCA: 97] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 124. | Wang S, Liang X, Yang Q, Fu X, Rogers CJ, Zhu M, Rodgers BD, Jiang Q, Dodson MV, Du M. Resveratrol induces brown-like adipocyte formation in white fat through activation of AMP-activated protein kinase (AMPK) α1. Int J Obes (Lond). 2015;39:967-976. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 207] [Cited by in RCA: 235] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 125. | Han YH, Kee JY, Park J, Kim HL, Jeong MY, Kim DS, Jeon YD, Jung Y, Youn DH, Kang J, So HS, Park R, Lee JH, Shin S, Kim SJ, Um JY, Hong SH. Arctigenin Inhibits Adipogenesis by Inducing AMPK Activation and Reduces Weight Gain in High-Fat Diet-Induced Obese Mice. J Cell Biochem. 2016;117:2067-2077. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 126. | Jeong MY, Park J, Youn DH, Jung Y, Kang J, Lim S, Kang MW, Kim HL, So HS, Park R, Hong SH, Um JY. Albiflorin ameliorates obesity by inducing thermogenic genes via AMPK and PI3K/AKT in vivo and in vitro. Metabolism. 2017;73:85-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 38] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 127. | Kim HL, Park J, Jung Y, Ahn KS, Um JY. Platycodin D, a novel activator of AMP-activated protein kinase, attenuates obesity in db/db mice via regulation of adipogenesis and thermogenesis. Phytomedicine. 2019;52:254-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 62] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 128. | Vanella L, Kim DH, Asprinio D, Peterson SJ, Barbagallo I, Vanella A, Goldstein D, Ikehara S, Kappas A, Abraham NG. HO-1 expression increases mesenchymal stem cell-derived osteoblasts but decreases adipocyte lineage. Bone. 2010;46:236-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 104] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 129. | Zhou Y, Wang D, Zhu Q, Gao X, Yang S, Xu A, Wu D. Inhibitory effects of A-769662, a novel activator of AMP-activated protein kinase, on 3T3-L1 adipogenesis. Biol Pharm Bull. 2009;32:993-998. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 48] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 130. | Di Giacomo C, Vanella L, Sorrenti V, Santangelo R, Barbagallo I, Calabrese G, Genovese C, Mastrojeni S, Ragusa S, Acquaviva R. Effects of Tithonia diversifolia (Hemsl.) A. Gray extract on adipocyte differentiation of human mesenchymal stem cells. PLoS One. 2015;10:e0122320. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 131. | Sacerdoti D, Bolognesi M, Di Pascoli M, Gatta A, McGiff JC, Schwartzman ML, Abraham NG. Rat mesenteric arterial dilator response to 11,12-epoxyeicosatrienoic acid is mediated by activating heme oxygenase. Am J Physiol Heart Circ Physiol. 2006;291:H1999-H2002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 46] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 132. | Kim DH, Vanella L, Inoue K, Burgess A, Gotlinger K, Manthati VL, Koduru SR, Zeldin DC, Falck JR, Schwartzman ML, Abraham NG. Epoxyeicosatrienoic acid agonist regulates human mesenchymal stem cell-derived adipocytes through activation of HO-1-pAKT signaling and a decrease in PPARγ. Stem Cells Dev. 2010;19:1863-1873. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 92] [Cited by in RCA: 92] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 133. | Ohshima T, Koga H, Shimotohno K. Transcriptional activity of peroxisome proliferator-activated receptor gamma is modulated by SUMO-1 modification. J Biol Chem. 2004;279:29551-29557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 159] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 134. | Khanna-Gupta A. Sumoylation and the function of CCAAT enhancer binding protein alpha (C/EBP alpha). Blood Cells Mol Dis. 2008;41:77-81. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 135. | Fukuda I, Ito A, Hirai G, Nishimura S, Kawasaki H, Saitoh H, Kimura K, Sodeoka M, Yoshida M. Ginkgolic acid inhibits protein SUMOylation by blocking formation of the E1-SUMO intermediate. Chem Biol. 2009;16:133-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 235] [Cited by in RCA: 277] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 136. | Liu H, Li J, Lu D, Li J, Liu M, He Y, Williams BO, Li J, Yang T. Ginkgolic acid, a sumoylation inhibitor, promotes adipocyte commitment but suppresses adipocyte terminal differentiation of mouse bone marrow stromal cells. Sci Rep. 2018;8:2545. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 137. | Hauser AT, Robaa D, Jung M. Epigenetic small molecule modulators of histone and DNA methylation. Curr Opin Chem Biol. 2018;45:73-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 35] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 138. | Chen YS, Wu R, Yang X, Kou S, MacDougald OA, Yu L, Shi H, Xue B. Inhibiting DNA methylation switches adipogenesis to osteoblastogenesis by activating Wnt10a. Sci Rep. 2016;6:25283. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 46] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 139. | Lee JE, Park YK, Park S, Jang Y, Waring N, Dey A, Ozato K, Lai B, Peng W, Ge K. Brd4 binds to active enhancers to control cell identity gene induction in adipogenesis and myogenesis. Nat Commun. 2017;8:2217. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 112] [Cited by in RCA: 152] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 140. | Gjoksi B, Ghayor C, Bhattacharya I, Zenobi-Wong M, Weber FE. The bromodomain inhibitor N-methyl pyrrolidone reduced fat accumulation in an ovariectomized rat model. Clin Epigenetics. 2016;8:42. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 141. | Kawakami Y, Ishikawa T, Shimabara M, Tanda N, Enomoto-Iwamoto M, Iwamoto M, Kuwana T, Ueki A, Noji S, Nohno T. BMP signaling during bone pattern determination in the developing limb. Development. 1996;122:3557-3566. [PubMed] |
| 142. | Hill TP, Später D, Taketo MM, Birchmeier W, Hartmann C. Canonical Wnt/beta-catenin signaling prevents osteoblasts from differentiating into chondrocytes. Dev Cell. 2005;8:727-738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 837] [Cited by in RCA: 843] [Article Influence: 42.2] [Reference Citation Analysis (0)] |
| 143. | Reinhold MI, Kapadia RM, Liao Z, Naski MC. The Wnt-inducible transcription factor Twist1 inhibits chondrogenesis. J Biol Chem. 2006;281:1381-1388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 135] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 144. | Minina E, Kreschel C, Naski MC, Ornitz DM, Vortkamp A. Interaction of fgf, ihh/pthlh, and bmp signaling integrates chondrocyte proliferation and hypertrophic differentiation. Dev Cell. 2002;3:439-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 353] [Cited by in RCA: 328] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 145. | Warzecha J, Göttig S, Brüning C, Lindhorst E, Arabmothlagh M, Kurth A. Sonic hedgehog protein promotes proliferation and chondrogenic differentiation of bone marrow-derived mesenchymal stem cells in vitro. J Orthop Sci. 2006;11:491-496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 42] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 146. | Enomoto-Iwamoto M, Nakamura T, Aikawa T, Higuchi Y, Yuasa T, Yamaguchi A, Nohno T, Noji S, Matsuya T, Kurisu K, Koyama E, Pacifici M, Iwamoto M. Hedgehog proteins stimulate chondrogenic cell differentiation and cartilage formation. J Bone Miner Res. 2000;15:1659-1668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 75] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 147. | Taipale J, Beachy PA. The Hedgehog and Wnt signalling pathways in cancer. Nature. 2001;411:349-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1040] [Cited by in RCA: 1035] [Article Influence: 43.1] [Reference Citation Analysis (0)] |
| 148. | Tiet TD, Hopyan S, Nadesan P, Gokgoz N, Poon R, Lin AC, Yan T, Andrulis IL, Alman BA, Wunder JS. Constitutive hedgehog signaling in chondrosarcoma up-regulates tumor cell proliferation. Am J Pathol. 2006;168:321-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 115] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 149. | Li X, Wei G, Wang X, Liu DH, Deng RD, Li H, Zhou JH, Li YW, Zeng HP, Chen DF. Targeting of the Sonic Hedgehog pathway by atractylenolides promotes chondrogenic differentiation of mesenchymal stem cells. Biol Pharm Bull. 2012;35:1328-1335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 150. | Karlsson C, Lindahl A. Notch signaling in chondrogenesis. Int Rev Cell Mol Biol. 2009;275:65-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 151. | Cao Z, Liu C, Bai Y, Dou C, Li JM, Shi DW, Dong SW, Xiang Q. Inhibitory effect of dihydroartemisinin on chondrogenic and hypertrophic differentiation of mesenchymal stem cells. Am J Transl Res. 2017;9:2748-2759. [PubMed] |
| 152. | Lee SR, Choi E, Jeon SH, Zhi XY, Yu JS, Kim SH, Lee J, Park KM, Kim KH. Tirucallane Triterpenoids from the Stems and Stem Bark of Cornus walteri that Control Adipocyte and Osteoblast Differentiations. Molecules. 2018;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 153. | Kang HR, Yun HS, Lee TK, Lee S, Kim SH, Moon E, Park KM, Kim KH. Chemical Characterization of Novel Natural Products from the Roots of Asian Rice (Oryza sativa) that Control Adipocyte and Osteoblast Differentiation. J Agric Food Chem. 2018;66:2677-2684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 154. | Carpéné C, Pejenaute H, Del Moral R, Boulet N, Hijona E, Andrade F, Villanueva-Millán MJ, Aguirre L, Arbones-Mainar JM. The Dietary Antioxidant Piceatannol Inhibits Adipogenesis of Human Adipose Mesenchymal Stem Cells and Limits Glucose Transport and Lipogenic Activities in Adipocytes. Int J Mol Sci. 2018;19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 155. | Sawada Y, Sugimoto A, Osaki T, Okamoto Y. Ajuga decumbens stimulates mesenchymal stem cell differentiation and regenerates cartilage in a rabbit osteoarthritis model. Exp Ther Med. 2018;15:4080-4088. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 156. | Wu H, Yin Z, Wang L, Li F, Qiu Y. Honokiol improved chondrogenesis and suppressed inflammation in human umbilical cord derived mesenchymal stem cells via blocking nuclear factor-κB pathway. BMC Cell Biol. 2017;18:29. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |