Published online Jun 26, 2018. doi: 10.4252/wjsc.v10.i6.66
Peer-review started: March 28, 2018
First decision: April 16, 2018
Revised: April 19, 2018
Accepted: May 9, 2018
Article in press: May 10, 2018
Published online: June 26, 2018
Processing time: 90 Days and 14 Hours
A simple overview of daily orthodontic practice involves use of brackets, wires and elastomeric modules. However, investigating the underlying effect of orthodontic forces shows various molecular and cellular changes. Also, orthodontics is in close relation with dentofacial orthopedics which involves bone regeneration. In this review current and future applications of stem cells (SCs) in orthodontics and dentofacial orthopedics have been discussed. For craniofacial anomalies, SCs have been applied to regenerate hard tissue (such as treatment of alveolar cleft) and soft tissue (such as treatment of hemifacial macrosomia). Several attempts have been done to reconstruct impaired temporomandibular joint. Also, SCs with or without bone scaffolds and growth factors have been used to regenerate bone following distraction osteogenesis of mandibular bone or maxillary expansion. Current evidence shows that SCs also have potential to be used to regenerate infrabony alveolar defects and move the teeth into regenerated areas. Future application of SCs in orthodontics could involve accelerating tooth movement, regenerating resorbed roots and expanding tooth movement limitations. However, evidence supporting these roles is weak and further studies are required to evaluate the possibility of these ideas.
Core tip: Stem cell therapy has multiple applications in the field of orthodontics and dentofacial orthopedics. Recent researches have demonstrated advantageous use of stem cells (SCs) for correction of craniofacial anomalies, rapid consolidation phase of distraction osteogenesis, reconstruction of temporomandibular joint and stability of palatal expansion. SCs also could be used to regenerate infrabony alveolar defects and move the teeth into regenerated areas. Future application of SCs in orthodontics could involve accelerating tooth movement, regenerating resorbed roots and expanding tooth movement limitations.
- Citation: Safari S, Mahdian A, Motamedian SR. Applications of stem cells in orthodontics and dentofacial orthopedics: Current trends and future perspectives. World J Stem Cells 2018; 10(6): 66-77
- URL: https://www.wjgnet.com/1948-0210/full/v10/i6/66.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v10.i6.66
Orthodontics involves treatment of dental malocclusions and correction of dentofacial deformities. The aim of orthodontic treatment is to achieve facial aesthetics and improve oral health related quality of life[1,2]. The prevalence of dental malocclusion varies in different communities and have been reported to be 22.5% to 93%[3-6]. Orthodontic treatment of malocclusions has several shortcomings such as prolonged treatment time, apical root resorption, tooth movement limited to alveolar bone and difficulties to overcome periodontal defects.
Although facial anomalies and jaw base deformities are less frequent compared to simple dental malocclusions, they are more burdensome[7]. About 5% of orthodontic patients could be considered as handicapped and need multidisciplinary treatments[8]. Current treatment modalities of craniofacial deformities can reduce the severity of these deformities but their final aesthetic outcomes are still not pleasing.
Stem cells (SCs) are self-renewal cells that could differentiate toward various cells under suitable conditions[9]. Various sources for harvesting SCs have been introduced such as muscle, dermis, bone marrow, adipose tissue, periosteum, blood, umbilical cord, synovial membrane and teeth[10,11]. Among these sources, some are easily accessible in orthodontics. As extraction of primary teeth or permanent premolar or wisdom teeth is common interventions in orthodontic treatment of malocclusions, SCs sources from the teeth could be gained without extra morbidity. Several studies have revealed differentiation and proliferation potential of mesenchymal stem cells (MSCs) obtained from dental pulp, periodontal ligament or human exfoliated deciduous teeth[12-15].
Nowadays, MSCs could be considered as “research trends” in the field of biology and medicine and their application in regenerative medicine is growing. Some modalities involve direct plantation of MSCs into the defect site while others use proper scaffolds to support the cells. In bone tissue engineering, MSCs are carried by an osteoconductive scaffold and differentiated toward osteogenic cells using osteoinductive growth factors[16]. Several types of scaffolds and growth factors have been used for regeneration of craniofacial bone defects including orthodontic related bone defects[17-19]. The aim of the current study was to review applications of SCs in treatment of dentofacial defects and deformities and to propose possible advantages of SC therapy in enhancing orthodontic treatments.
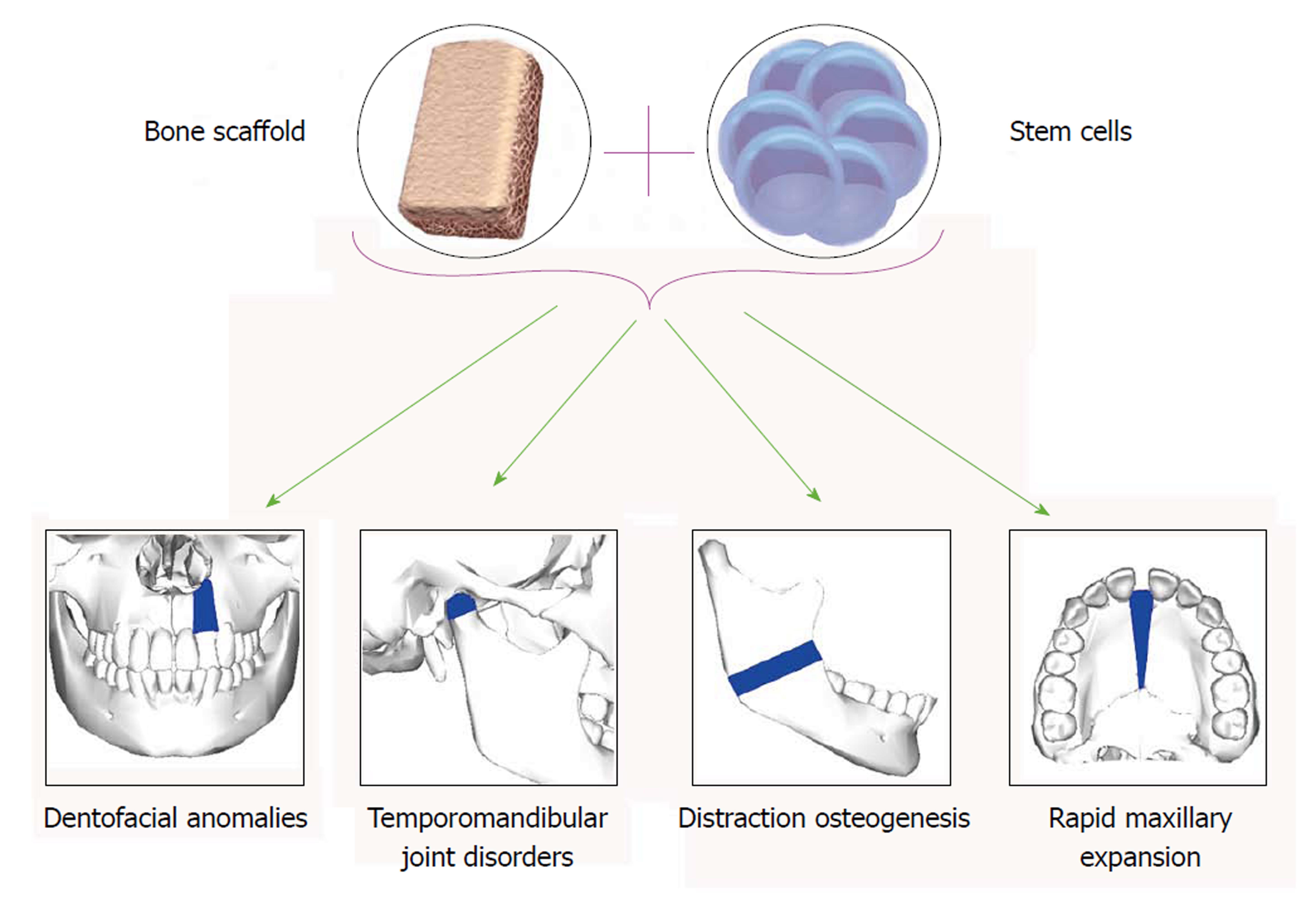
To evaluate the uses of SCs in dentofacial orthopedics, application of SCs in treatment of dentofacial anomalies and temporomandibular joint (TMJ) disorders as well as their possible role in distraction osteogenesis (DO) and maxillary expansion have been discussed (Figure 1).
Craniofacial deformities such as congenital and developmental malformation and those resulting from trauma, tumor resection and nonunion of fractures, are common clinical problems in craniofacial surgery, which are difficult to remedy. Current surgical techniques in various combinations, autogenous, allogeneic, and prosthetic materials have been used to achieve bone and soft tissue reconstruction[20]. These approaches have several complications such as insufficient autogenous resources, donor site morbidity, contour irregularities, postoperative pain, additional cost, long surgical time and postsurgical reabsorption, disease transmission, major histoincompatibility, graft versus-host disease (GVHD), immunosuppression, unpredictable outcome for tissue formation and infection of foreign material[21-24]. In order to overcome these complications, stem cell-based tissue regeneration offers a promising approach to provide an advanced and reliable therapeutic strategy for craniofacial tissue reconstruction[25]. In the current review, regenerative approaches for two types of craniofacial anomalies are presented; cleft lip and palate (CLP) (for hard tissue regeneration) and hemifacial microsomia (HFM) (for soft tissue regeneration).
CLP is one of the most prevalent congenital anomalies which results from fusion failure of nasal process and oropalatal shelves. The prevalence of this malformation is 0.36-0.83 in 1000 live-born infants[26]. Alveolar bone defect, problem in swallowing and pronunciation, facial deformity, missing teeth, and maxillary deformity can be seen in CLP patients[27]. Repair of the malformed alveolar bone is critical for oronasal fistula closure, maxilla unification, tooth eruption, and support of the alar base[28,29]. The gold standard treatment for alveolar reconstruction in CLP patients is autogenous cancellous bone grafts[30] since they are immunologically inert and potential suppliers of cells with osteoconductive and osteoinductive properties[31,32]. The commonest site for acquiring autogenous bone for grafting is the anterior iliac crest[33]. An overall success rate for iliac crest bone grafting to the alveolar cleft with respect to bone resorption is 88%[34]. With the advent of tissue engineering techniques, alternatives to the traditional iliac crest bone grafting techniques are available. MSCs have been shown to have the ability to form new bone when transplanted[35].
Some case reports and case series studies reported results of MSCs usage to regenerate alveolar cleft[36]. Composite scaffold of demineralized bone mineral and calcium phosphate loaded with MSCs showed 34.5% regenerated bone in the cleft area in one case and in the other there was 25.6% presentation of bone integrity[37]. About 50% fill of the bone defect was measured after placement of the scaffold, growth factor and MSCs in cleft area[38], whereas 79.1% bone regeneration has been reported in the another study[22]. Autogenous osteoblasts cultured on demineralized bone matrix showed more reduction in defect size in comparison to control group[39]. About 90% defect correction of soft palate defect has been reported 14 d after injection of autologous MSCs[40]. Biomaterial seeded with autogenous osteogenic cells into the alveolar cleft resulted in spontaneously eruption of canine in its proper place after eighteen months[41]. Poly-L-lactic acid with osteogenically differentiated fat-derived stem cells showed substantial bone regeneration in palatal defect[42]. The mean pain score, including both intensity and pain frequency and donor site morbidity was greatest at all-time points in traditional iliac crest bone graft and least at all-time points in tissue engineering[31].
Thus, it can be concluded that SCs seem to possess favorable potential for bone regeneration in oral and maxillofacial region and use of them in alveolar defect repair, reduce defect size by bone formation[37,39,42], have less postoperative morbidity compared to autogenous bone grafting[31] and help the teeth in the defect area to erupt in their proper position[41].
HFM is a rare, multi-systemic congenital disease. It is considered to be the product of unilateral abnormal morphogenesis of the first and second pharyngeal arches. HFM is a frequently encountered form of congenital facial malformation, ranking second only to cleft lip and palate[43]. The fundamental features of HFM include unilateral hypoplasia of the craniofacial skeleton and its overlying soft tissue[44]. Autologous fat grafting is considered to reconstruct soft tissue defect in the treatment of congenital malformations as well as post-traumatic malformations[45]. To overcome problems associated with fat grafting, such as unpredictable clinical results and a low rate of graft survival, many innovative efforts and refinements of surgical techniques have been reported[46]. Use of adipose derived stromal cells (ASCs) for tissue regeneration has attracted attention recently.
Patients with HFM which have been grafted with supplementation of ASCs Showed 88% of fat volume surviving after 6 mo in comparison to control group which was 54%[46]. Also, residual graft volumes of ASCs enriched grafts was significantly higher in comparison to control group[45].
Studies are ongoing, and as results are reported, it will be crucial to evaluate the long term outcome of such procedures. The current evidence suggests that use of ASCs for soft tissue reconstruction may enhance angiogenesis[47], improve the survival of grafts[45,46] and thus reduce atrophy[47].
The temporomandibular joint (TMJ) is comprised of both osseous and cartilaginous structures. It is enclosed in a capsule that is lubricated with synovial fluid and serves as an important growth site during postnatal development with two articular surfaces that can adapt to changing environment conditions[48,49]. The mandibular condyle grows by proliferation of the progenitor/SCs that differentiate into chondrocytes[49,50] leading to formation and increase of cartilage matrix, which will be replaced with lamellar trabecular bone[51]. As SCs possess the ability to differentiate into chondrogenic and osteogenic cells, they could be used for both maintenance of mandible in new position and repair of TMJ lesions.
Forward positioning of mandible, for example in functional therapy, leads to increase in the number of mesenchymal cells (stem/progenitor cells) in the temporal fossa, which resulted in new cortical bone formation[52]. Thus, the question arises as to whether the injection of SCs into articular space accelerates bone formation in the temporal fossa? This issue requires Further targeted researches.
TMJ is prone to injuries, tumors, osteoarthritis, rheumatoid arthritis and congenital anomalies. Approximately 10 million individuals in the United States have been affected by temporomandibular disorders (TMD)[53]. TMD manifest as pain, myalgia, headaches, and structural destruction, collectively known as degenerative joint disease[54]. The primary methods used to reconstruct the TMJ includes autogenous bone grafting such as harvesting from the rib, or the use of alloplastic materials, with neither being ideally suited for the task and sometimes leading to unwanted adverse effects. The major and final option for those patients with advanced degenerative diseases is surgical replacement of the mandibular condyle[55]. These approaches have complications such as immunorejection, infection, implant wear, dislocation, suboptimal biocompatibility, donor site limitation and morbidity, and potential pathogen transmission[56,57]. To overcome these disadvantages, strategies have been found to engineer osteochondral tissue, such as that found in the TMJ, will produce tissue that is both biologically and mechanically functional used. Recently, these cells have attracted much interest to joint reconstruction.
Engineering a TMJ-like osteochondral graft has been studied in several studies. The culture of human umbilical cord matrix (HUCM) SCs in growth medium containing chondrogenic factors, showed the HUCM SCs can outperform the TMJ condylar cartilage cells[58]. Rat bone marrow MSCs which encapsulated in poly (ethylene glycol)-based hydrogel molded into the shape of a cadaver human mandibular condyle, demonstrated two stratified layers of histogenesis of cartilaginous and osseous phenotypes[59,60]. Porcine MSCs which had been cultured in osteogenic induction medium and were seeded onto a poly DL-lactic–co-glycolic acid scaffold, formed the construct had a shape that closely resembled to the model condyle and it’s radiodensity was between that of the normal condyle and that of control scaffolds[61].
Because of fibrocartilaginous structure of disk, there has been little success in the manufacture of synthetic TMJ discs rather than bone and cartilage and attention has turned to tissue engineering to reconstruct the disc[62]. In one study, Combination of polylactide acid discs with adipose tissue stem cell demonstrated the potential to development a tissue-engineered TMJ disc[63].
While animal studies are in progress to replicate bone the osteochondral interface to engineer TMJ, yet no clinical trials on humans have been done. These data revealed possibility of application of SCs in combination with different scaffolds as a promising approach to regenerate osteochondral tissues of TMJ and ultimately the joint disk.
DO which is regarded as “endogenous bone tissue engineering” has been widely applied in orthopedic surgery for correction of limb length and also in the treatment of many craniofacial deformities[64]. DO is done by creating a corticotomy, placing a rigid distractor across the cut bone and gradually activating the device[65]. The mechanism of osteogenesis and gap repair initiated by an immediate inflammatory response that leads to the recruitment of MSCs and subsequent differentiation into chondrocytes that produce cartilage and osteoblasts which form bone[66]. Despite its great advantages, long treatment periods and fibrous union or even non-union of bone are possible major draw backs impeding its widespread clinical application[67,68].
Efforts have been made to accelerate osteogenesis in the distraction Gap, shorten the consolidation period and reduce complications such as the development of nonunion, infection, or fracture.
Recently, because of the role of MSCs in osteogenesis, many researchers have successfully documented the ability of SCs on promoting bone formation and shortening the consolidation period during DO. For this purpose various sources of SCs such as human exfoliated deciduous teeth (SHED)[69], bone marrow[70-77] and adipose tissue[78-80] have been used in studies. In some studies, alone MSCs[71,79,81,82], in the others, gene transferred MSCs[72,76-78,83] and factors[75,84,85] have been used to enhance bone regeneration following distraction osteogenesis. The modifications such as use of scaffolds[75], demineralized bone matrix[74] and Platelet-rich Plasma[73] have been done in some studies.
The injection of MSCs 1 d before onset of distraction resulted in increase in new bone volume in the distracted callus and the bone mineral density (BMD)[81], MSCs injection after distraction was complete showed higher radiodensity of the distraction zone and grater histologically callus, new bone volume and thickness of the new trabeculae[71] and doing this intervention on the first day of consolidation resulted in greater biomechanical strength and increase in total and compact bone ratio in regenerate bone[82]. The injection of SHED during osteotomy period showed higher percentage of newly formed bone after 2, 4, and 6 wk[69]. One study revealed that callus density, the ossification rate, quality of newly formed bone and the number of active cells in bone formation were higher in group which osteoblast-differentiated stem cell were injected to distraction site compared to control group and stem cell group[79]. Addition of MSCs sheet fragments yielded significant increases in bony union, more intensive bone formation on histomorphometric analysis and higher peak load on biomechanical testing[70]. MSCs transfected with bFGF showed excellent bone formation and higher BMD and bone mineral content (BMC) in the distracted callus[76]. The use of MSCs osteogenic differentiation using FGF-2 and confirm cell integration with a gelatin-based Gelfoam scaffold, demonstrated less interfragmentary mobility, more advanced gap obliteration, higher mineral content and faster mineral apposition[75]. One study suggested that gene therapy using rhRunx2-modified ASCs promoted new bone formation during osteoporotic mandibular DO[78]. Application of ASCs transfected with pEGFP-OSX showed the highest BMD, thickness of new trabecula (TNT), and the volumes of the newly generated cortical bone (NBV1) and the cancellous bone (NBV2) in the distraction zones[78]. Excellent bone formation and highest BMD, TNT and NBV in the distraction zones was observed in groups that MSCs transfected with OSX[72]. The injection of MSCs transfected with Bone Morphogenic Protein (BMP) showed greater bone formation and earlier mineralization in the distracted callus[77], more mature medullary cavity[83], better bone quality and higher trabecular parameters (trabecular thickness, trabecular number, volumetric bone mineral density at tissue, and bone volume fraction) at the second and fourth weeks of the consolidation period[86] and acceleration of osteogenesis[87]. The use of stromal cell-derived factor-1 (SDF-1) facilitated migration of MSCs into osteogenesis site[84]. The addition of MSCs transfected with recombinant plasmids pIRES-hBMP2-hVEGF165 at the beginning of distraction is more ideal than the start of latency period[85].
These data shows that SCs from Various sources, alone or in combination of genes and factors, in different phases of treatment can lead to an increase in new bone volume and quality[69,71,72,77,78,81,86], bone mineral density[71,72,76,78,81], trabecular thickness[71,78,86], biomechanical strength[70,82].
Maxillary constriction can be associated with several problems that include occlusal disharmony and esthetics as well as such functional difficulties as narrowing of the pharyngeal airway, increased nasal resistance, and alterations in tongue posture, resulting in retroglossal airway narrowing and mouth breathing[88-90]. Maxillary constriction can be corrected with slow orthodontic expansion, rapid maxillary expansion (RME), surgically assisted rapid palatal expansion or a two-segmented Le Fort I-type osteotomy with expansion[91]. RME is indicated in patients younger than 12 years, who have lateral discrepancies involving several teeth, whether the constriction is skeletal, dental or a combination of both[92]. It is an effective orthopedic procedure to open the midpalatal suture, providing appropriate and stable maxillary width increase and re-stablish balance between the width of the jaws[93,94].
RME is similar to DO histologically. During RME, a gap in the midpalatal suture is created which is filled with blood and granulated tissue and followed by active bone formation. The expanded arch width relapses unless followed by an appropriate retention period. Therefore, providing a strategy to accelerate bone formation in the midpalatal suture might shorten treatment and retention period, achieve stability and prevent relapse. Because of the ability of SCs to differentiate into osteogenic cells, injection of SCs seems to have the ability to accelerate the process of bone formation. This was studies in one study by Ekizer et al[95]. In their animal study, local injection of MSCs into intermaxillary suture after force application resulted in increased new bone formation in the suture by increasing the number of osteoblasts and new vessel formation[95]. Thus, locally applied MCSs to the expanded maxilla might be a useful and practical treatment strategy to accelerate new bone formation in midpalatal suture and to shorten the treatment and retention period for patients undergoing orthopedic maxillary expansion.
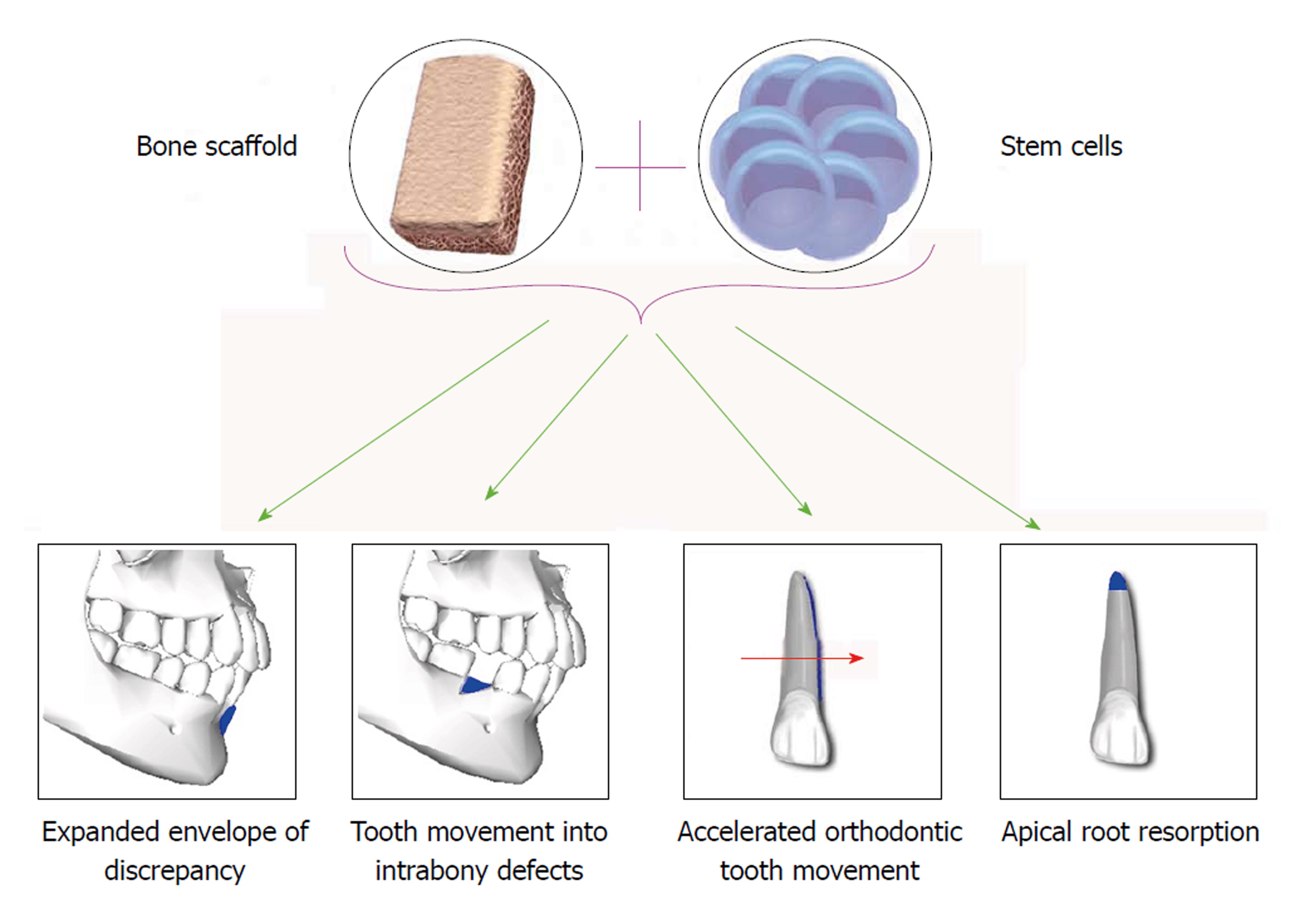
To evaluate the uses of SCs in orthodontics, current evidence regarding application of SCs in expanding the limitations of orthodontic tooth movement (OTM), tooth movement into periodontal defects, accelerating OTM and treatment of external root resorption (ERR) have been reviewed (Figure 2).
The extent of OTM is limited by several factors including the anatomy of the alveolar bone, pressures exerted by soft tissues, periodontal tissue attachment levels, neuromuscular forces and lip–tooth relationships[96,97]. The anteroposterior, vertical, and transverse millimetric range of treatment possibilities in orthodontics can be expressed as an “envelope of discrepancy”[98] Gingival recession occurs secondarily to an alveolar bone dehiscence, if overlying tissues are stressed during OTM beyond this envelope. Sites in which the buccal or lingual bone cortex and covering gingival tissue are thin, such as lower incisors in patients with a prominent chin and compensation in the form of lingual tipping of these teeth are at particular risk of bone defects like fenestrations and dehiscence[99,100].
SCs have the potential to generate different tissues, including bone, thereby stem cell therapy is a promising approach to alveolar bone regeneration[101]. Some researches have applied stem cell therapy in case of bone ridge augmentation in humans and mainly used bone marrow cells[102-104]. The outcome of alveolar bone regeneration showed a tendency to enhance bone formation[105]. Hence, bone regeneration methods using SCs might provide an approach for expanding limitations of envelope of discrepancy.
As a hypothesis, relying on the results of alveolar bone augmentation studies, it might be possible with the aid of stem cell based osteogenesis to horizontally augment the ridge in order to extend the tooth movement extent and to overcome some anatomical boundaries.
Periodontal complications are one of the most actual side effects linked to the orthodontics. It can be found in various forms, from gingivitis to periodontitis, dehiscence, fenestrations, interdental fold, gingival recession or overgrowth, black triangles[100]. Periodontal regeneration has been defined as the formation of new cementum, alveolar bone, and a functional periodontal ligament on a previously diseased root surface[106]. The current treatment approaches include the use of surgery, guided tissue regeneration (GTR), bone fillers and growth factors and application of bioactive molecules to induce regeneration[107,108]. Based on the differential potential capability of SCs and their ability of renewal via mitosis[109], they have the quality to regenerate damaged tissues, hence they can be used for regeneration of periodontium.
Periodontal defects could be a challenging situation both pre and post orthodontic treatment. On one hand, because of the increasing number of adult patients seeking orthodontic treatment, encountering the periodontally involved patients may be a potential problem for every practitioner. It has been suggested that, by moving the teeth into infrabony defects, we can achieve the regeneration of the attachment apparatus[110]. Accordingly with the combination of periodontal regeneration treatments such as GTR and OTM, it might be possible to reduce infrabony defect and upgrade periodontal health[111]. On the other hand, periodontal defects such as fenestration, dehiscence and attachment loss are among common complications of orthodontic treatments[112].
Several reports on application of SCs for regeneration of periodontal tissues have been published. In a study, induced pluripotent SCs have been implanted into a mouse periodontal fenestration defect model with a silk fibroin scaffold in combination with enamel matrix derivative gel. As a result, higher rate of cementum and alveolar bone formation was observed[113]. Also, it has been shown that the bone marrow derived mesenchymal stem cells (BM-MSC)-treated wounds exhibited significantly accelerated wound closure, with increased re-epithelialization, cellularity, and angiogenesis[114]. In another study conditioned medium (CM) obtained from PDLSCs were transplanted into a rat periodontal defect model and consequently PDLSC-CM enhanced periodontal regeneration by suppressing the inflammatory response via TNF-α production[115]. Incubation of induced PDLSCs with dentin non collagenous proteins in vivo revealed that cementum-like tissues formed along the chemical-conditioned root dentin surface, enhanced alkaline phosphatase (ALP) activity, increased matrix mineralization, and upregulated expression of mineralization-associated genes[116]. One study has revealed that autologous PDLSCs obtained from extracted teeth of the miniature pigs which were transplanted into the surgically created periodontal defect areas were capable of regenerating periodontal tissues, leading to a favorable treatment for periodontitis[117]. PDLSCs were delivered onto suitable collagen sponges and implanted into periodontal defects of immunodeficient nude rats in an in vivo study, as a result reformation of periodontal ligament-like tissue, collagen fibers, and elements of bone was observed[118]. In another in vivo study, PDLSCs sheet were transferred to a miniature pig periodontitis model. Significant periodontal tissue regeneration was achieved in both the autologous and the allogeneic PDLSCs transplantation[119]. Using amniotic membrane for transferring PDLSCs for periodontal regeneration in a rat periodontal model as a new method of transplantation is also being suggested in a study[120].
According to aforesaid studies, human adult PDLSCs are capable of regenerating elements of bone and collagen, since the periodontitis is a chronic disease, it may benefit from such stem cell based therapies[114,117-119]. Thus the use of PDLSC transplantation in periodontal therapies can reduce treatment time and better outcomes followed by patient comfort, however, due to complex structure of periodontium, regeneration is a feasible and yet complicated procedure and may need pluripotent SCs and more investigations.
OTM is achieved by the remodeling of periodontal ligament (PDL) and alveolar bone in response to mechanical loading[121,122]. The initiating inflammatory event at compression sites is caused by constriction of the PDL microvasculature, resulting in a focal necrosis, followed by recruiting of osteoclasts from the adjacent marrow spaces[123]. These osteoclasts are mostly derived from hematopoietic SCs[124]. Hence, SCs could be used to accelerate OTM by providing progenitor cells.
The development of new methods to accelerate OTM has been sought by clinicians as a way to shorten treatment times, reduce adverse effects such as pain, discomfort, dental caries, and periodontal diseases, and minimize iatrogenic damages such as root resorption and the subsequent development of non-vital teeth[125]. There are surgical methods like surgically-facilitated orthodontic therapy or corticotomy[126], periodontally accelerated osteogenic orthodontics[127] and some nonsurgical procedures such as systemic/local administration of chemical substances like epidermal growth factor, parathyroid hormone, 1,25-dihydroxyvitamin d 3, osteocalcin and prostaglandins, resonance vibration, static or pulsed magnetic field, low-intensity laser irradiation therapy[128].
In a study, increased PDL progenitor cells with suppressed expression of type I collagen (Col-I) were observed during orthodontic force application, whilst after force withdrawal they increase in Col-I expression, which suggests that PDLSCs are able to respond to orthodontic mechanical forces with suppressed collagen expression[129]. This ability of SCs could be used to accelerate OTM in response to orthodontic forces. When orthodontic force is applied, tooth movement is hindered until the necrosis is removed, leading to the clinical manifestation of a delay period. Hypothetically, transplantation of SCs in pressure sites may speed up the process, resulting in accelerated OTM.
ERR is a common and unfavorable side effect of orthodontic treatment[130,131], which any specialist may encounter. Many factors seems to be involved in ERR such as genetics, individual biological variability, age, sex, and orthodontic forces and treatment duration[132,133]. Orthodontic forces yet seem to be the main etiologic factors. ERR may lead to loss of tooth structure such as cementum and in more advanced stages, dentin, however no specific treatment has been introduced so far. One possible treatment modality could be regeneration of resorbed roots by application SCs and tissue engineering.
In severe cases ERR may cause poor prognosis of tooth, resulting in tooth loss. Regeneration of these lesions increases the longevity of tooth and may play an important role in facilitating the treatment. In a study designed to induce de novo cementum formation by SC therapy, MSCs driven from periodontal ligament in in vivo transplantation were able to form cellular cementum-like hard tissue containing embedded osteocalcin-positive cells[134]. According to studies in which the whole tooth structure has been bioengineered and transplanted into Rodent[135,136] and beagle dogs[137] models, it might be possible to regenerate the damaged tooth structure such as dentin and cementum and in the future to achieve a bioengineered functional human tooth structure.
Although it seems that there is a long way until regeneration of the teeth materials, cementogenesis and regeneration of dental structures through stem cell based therapies could be anticipated.
The current review showed application of SCs alone or in conjugation with bone scaffold or growth factors in surgical correction of dentofacial deformities, TMJ defects, and alveolar bone lesions. Recent studies show that SCs could improve treatment results and reduce treatment duration. Use of SCs is associated with accelerated healing and less morbidity compared to current surgical approached. Also, SCs could be used in DO surgeries and RME to increase consolidation rate and reduce relapse.
The contemporary evidence reveals feasibility of use of SCs for accelerating OTM, regenerating resorbed roots, expanding limitations of OTM while preserving periodontal health. In addition, SCs could be used for regeneration of periodontal tissues both pre and post OTM. In vivo studies are required to assess the possibility of such interventions.
Manuscript source: Invited manuscript
Specialty type: Cell and tissue engineering
Country of origin: Iran
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Chivu-Economescu M S- Editor: Ji FF L- Editor: A E- Editor: Tan WW
| 1. | Kiyak HA. Does orthodontic treatment affect patients’ quality of life? J Dent Educ. 2008;72:886-894. [PubMed] |
| 2. | Silvola AS, Varimo M, Tolvanen M, Rusanen J, Lahti S, Pirttiniemi P. Dental esthetics and quality of life in adults with severe malocclusion before and after treatment. Angle Orthod. 2014;84:594-599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 53] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 3. | Silva RG, Kang DS. Prevalence of malocclusion among Latino adolescents. Am J Orthod Dentofacial Orthop. 2001;119:313-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 57] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 4. | Tausche E, Luck O, Harzer W. Prevalence of malocclusions in the early mixed dentition and orthodontic treatment need. Eur J Orthod. 2004;26:237-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 152] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 5. | Krooks L, Pirttiniemi P, Kanavakis G, Lähdesmäki R. Prevalence of malocclusion traits and orthodontic treatment in a Finnish adult population. Acta Odontol Scand. 2016;74:362-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 6. | Akbari M, Lankarani KB, Honarvar B, Tabrizi R, Mirhadi H, Moosazadeh M. Prevalence of malocclusion among Iranian children: A systematic review and meta-analysis. Dent Res J (Isfahan). 2016;13:387-395. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 37] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 7. | Salzmann JA. Editorial: Seriously handicapping orthodontic conditions. Am J Orthod. 1976;70:329-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 8. | Kelly JE, Sanchez M, Van Kirk LE. An Assessment of the Occlusion of the Teeth of Children 6-11Years, United States. Vital Health Stat 11. 1973;1-60. [PubMed] |
| 9. | Khojasteh A, Motamedian SR. Mesenchymal Stem Cell Therapy for Treatment of Craniofacial Bone Defects: 10 Years of Experience. Reg Reconst Restor. 2016;1:1-7. [DOI] [Full Text] |
| 10. | Mafi R, Hindocha S, Mafi P, Griffin M, Khan WS. Sources of adult mesenchymal stem cells applicable for musculoskeletal applications - a systematic review of the literature. Open Orthop J. 2011;5 Suppl 2:242-248. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 92] [Cited by in RCA: 94] [Article Influence: 6.7] [Reference Citation Analysis (1)] |
| 11. | Hass R, Kasper C, Böhm S, Jacobs R. Different populations and sources of human mesenchymal stem cells (MSC): A comparison of adult and neonatal tissue-derived MSC. Cell Commun Signal. 2011;9:12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1330] [Cited by in RCA: 1242] [Article Influence: 88.7] [Reference Citation Analysis (0)] |
| 12. | Motamedian SR, Tabatabaei FS, Akhlaghi F, Torshabi M, Gholamin P, Khojasteh A. Response of Dental Pulp Stem Cells to Synthetic, Allograft, and Xenograft Bone Scaffolds. Int J Periodontics Restorative Dent. 2017;37:49-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 13. | Gay IC, Chen S, MacDougall M. Isolation and characterization of multipotent human periodontal ligament stem cells. Orthod Craniofac Res. 2007;10:149-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 277] [Cited by in RCA: 298] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 14. | Khojasteh A, Motamedian SR, Rad MR, Shahriari MH, Nadjmi N. Polymeric vs hydroxyapatite-based scaffolds on dental pulp stem cell proliferation and differentiation. World J Stem Cells. 2015;7:1215-1221. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 15. | Miura M, Gronthos S, Zhao M, Lu B, Fisher LW, Robey PG, Shi S. SHED: stem cells from human exfoliated deciduous teeth. Proc Natl Acad Sci USA. 2003;100:5807-5812. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1922] [Cited by in RCA: 1984] [Article Influence: 90.2] [Reference Citation Analysis (0)] |
| 16. | Motamedian SR, Iranparvar P, Nahvi G, Khojasteh A. Bone Tissue Engineering: A Literature Review. Regen Reconst Restor. 2016;1:103-120. |
| 17. | Motamedian SR, Hosseinpour S, Ahsaie MG, Khojasteh A. Smart scaffolds in bone tissue engineering: A systematic review of literature. World J Stem Cells. 2015;7:657-668. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 130] [Cited by in RCA: 88] [Article Influence: 8.8] [Reference Citation Analysis (2)] |
| 18. | Jafari M, Paknejad Z, Rad MR, Motamedian SR, Eghbal MJ, Nadjmi N, Khojasteh A. Polymeric scaffolds in tissue engineering: a literature review. J Biomed Mater Res B Appl Biomater. 2017;105:431-459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 172] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 19. | Hosseinpour S, Ghazizadeh Ahsaie M, Rezai Rad M, Baghani MT, Motamedian SR, Khojasteh A. Application of selected scaffolds for bone tissue engineering: a systematic review. Oral Maxillofac Surg. 2017;21:109-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 55] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 20. | Cowan CM, Shi YY, Aalami OO, Chou YF, Mari C, Thomas R, Quarto N, Contag CH, Wu B, Longaker MT. Adipose-derived adult stromal cells heal critical-size mouse calvarial defects. Nat Biotechnol. 2004;22:560-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 725] [Cited by in RCA: 686] [Article Influence: 32.7] [Reference Citation Analysis (0)] |
| 21. | Warren SM, Fong KD, Chen CM, Loboa EG, Cowan CM, Lorenz HP, Longaker MT. Tools and techniques for craniofacial tissue engineering. Tissue Eng. 2003;9:187-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 38] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 22. | Hibi H, Yamada Y, Ueda M, Endo Y. Alveolar cleft osteoplasty using tissue-engineered osteogenic material. Int J Oral Maxillofac Surg. 2006;35:551-555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 116] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 23. | Younger EM, Chapman MW. Morbidity at bone graft donor sites. J Orthop Trauma. 1989;3:192-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1434] [Cited by in RCA: 1308] [Article Influence: 36.3] [Reference Citation Analysis (0)] |
| 24. | Bayerlein T, Proff P, Heinrich A, Kaduk W, Hosten N, Gedrange T. Evaluation of bone availability in the cleft area following secondary osteoplasty. J Craniomaxillofac Surg. 2006;34 Suppl 2:57-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 25. | Miura M, Miura Y, Sonoyama W, Yamaza T, Gronthos S, Shi S. Bone marrow-derived mesenchymal stem cells for regenerative medicine in craniofacial region. Oral Dis. 2006;12:514-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 57] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 26. | Luaces-Rey R, Arenaz-Búa J, Lopez-Cedrún-Cembranos JL, Herrero-Patiño S, Sironvalle-Soliva S, Iglesias-Candal E, Pombo-Castro M. Is PRP useful in alveolar cleft reconstruction? Platelet-rich plasma in secondary alveoloplasty. Med Oral Patol Oral Cir Bucal. 2010;15:e619-e623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 27. | Le BT, Woo I. Alveolar cleft repair in adults using guided bone regeneration with mineralized allograft for dental implant site development: a report of 2 cases. J Oral Maxillofac Surg. 2009;67:1716-1722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 28. | Brito LA, Paranaiba LM, Bassi CF, Masotti C, Malcher C, Schlesinger D, Rocha KM, Cruz LA, Bárbara LK, Alonso N. Region 8q24 is a susceptibility locus for nonsyndromic oral clefting in Brazil. Birth Defects Res A Clin Mol Teratol. 2012;94:464-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 29. | Ramalingam M, Haidar Z, Ramakrishna S, Kobayashi H, Haikel y. Integrated Biomaterials in Tissue Engineering. Wiley. 2012;183-234. [DOI] [Full Text] |
| 30. | Nwoku AL, Al Atel A, Al Shlash S, Oluyadi BA, Ismail S. Retrospective analysis of secondary alveolar cleft grafts using iliac of chin bone. J Craniofac Surg. 2005;16:864-868. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 31. | Gimbel M, Ashley RK, Sisodia M, Gabbay JS, Wasson KL, Heller J, Wilson L, Kawamoto HK, Bradley JP. Repair of alveolar cleft defects: reduced morbidity with bone marrow stem cells in a resorbable matrix. J Craniofac Surg. 2007;18:895-901. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 91] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 32. | Ishaug-Riley SL, Crane GM, Gurlek A, Miller MJ, Yasko AW, Yaszemski MJ, Mikos AG. Ectopic bone formation by marrow stromal osteoblast transplantation using poly(DL-lactic-co-glycolic acid) foams implanted into the rat mesentery. J Biomed Mater Res. 1997;36:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 33. | Eufinger H, Leppänen H. Iliac crest donor site morbidity following open and closed methods of bone harvest for alveolar cleft osteoplasty. J Craniomaxillofac Surg. 2000;28:31-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 92] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 34. | Schultze-Mosgau S, Nkenke E, Schlegel AK, Hirschfelder U, Wiltfang J. Analysis of bone resorption after secondary alveolar cleft bone grafts before and after canine eruption in connection with orthodontic gap closure or prosthodontic treatment. J Oral Maxillofac Surg. 2003;61:1245-1248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 73] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 35. | Horswell BB, Henderson JM. Secondary osteoplasty of the alveolar cleft defect. J Oral Maxillofac Surg. 2003;61:1082-1090. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 58] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 36. | Khojasteh A, Kheiri L, Motamedian SR, Nadjmi N. Regenerative medicine in the treatment of alveolar cleft defect: A systematic review of the literature. J Craniomaxillofac Surg. 2015;43:1608-1613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 46] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 37. | Behnia H, Khojasteh A, Soleimani M, Tehranchi A, Khoshzaban A, Keshel SH, Atashi R. Secondary repair of alveolar clefts using human mesenchymal stem cells. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;108:e1-e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 79] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 38. | Behnia H, Khojasteh A, Soleimani M, Tehranchi A, Atashi A. Repair of alveolar cleft defect with mesenchymal stem cells and platelet derived growth factors: a preliminary report. J Craniomaxillofac Surg. 2012;40:2-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 116] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 39. | Pradel W, Lauer G. Tissue-engineered bone grafts for osteoplasty in patients with cleft alveolus. Ann Anat. 2012;194:545-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 47] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 40. | Carstanjen B, Desbois C, Hekmati M, Behr L. Successful engraftment of cultured autologous mesenchymal stem cells in a surgically repaired soft palate defect in an adult horse. Can J Vet Res. 2006;70:143-147. [PubMed] |
| 41. | Pradel W, Tausche E, Gollogly J, Lauer G. Spontaneous tooth eruption after alveolar cleft osteoplasty using tissue-engineered bone: a case report. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;105:440-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 29] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 42. | Conejero JA, Lee JA, Parrett BM, Terry M, Wear-Maggitti K, Grant RT, Breitbart AS. Repair of palatal bone defects using osteogenically differentiated fat-derived stem cells. Plast Reconstr Surg. 2006;117:857-863. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 68] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 43. | Cohen N, Cohen E, Gaiero A, Zecca S, Fichera G, Baldi F, Giordanetto JF, Mercier JM, Cohen A. Maxillofacial features and systemic malformations in expanded spectrum Hemifacial Microsomia. Am J Med Genet A. 2017;173:1208-1218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 44. | Gougoutas AJ, Singh DJ, Low DW, Bartlett SP. Hemifacial microsomia: clinical features and pictographic representations of the OMENS classification system. Plast Reconstr Surg. 2007;120:112e-120e. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 83] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 45. | Kølle SF, Fischer-Nielsen A, Mathiasen AB, Elberg JJ, Oliveri RS, Glovinski PV, Kastrup J, Kirchhoff M, Rasmussen BS, Talman ML. Enrichment of autologous fat grafts with ex-vivo expanded adipose tissue-derived stem cells for graft survival: a randomised placebo-controlled trial. Lancet. 2013;382:1113-1120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 401] [Cited by in RCA: 427] [Article Influence: 35.6] [Reference Citation Analysis (0)] |
| 46. | Tanikawa DY, Aguena M, Bueno DF, Passos-Bueno MR, Alonso N. Fat grafts supplemented with adipose-derived stromal cells in the rehabilitation of patients with craniofacial microsomia. Plast Reconstr Surg. 2013;132:141-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 104] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 47. | Tabit CJ, Slack GC, Fan K, Wan DC, Bradley JP. Fat grafting versus adipose-derived stem cell therapy: distinguishing indications, techniques, and outcomes. Aesthetic Plast Surg. 2012;36:704-713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 78] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 48. | Roberts WE, Huja S, Roberts JA, editors . Bone modeling: biomechanics, molecular mechanisms, and clinical perspectives. Semin Orthod. 2004;123-161. |
| 49. | Carlson DS. Biological rationale for early treatment of dentofacial deformities. Am J Orthod Dentofacial Orthop. 2002;121:554-558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 50. | Rabie AB, She TT, Hägg U. Functional appliance therapy accelerates and enhances condylar growth. Am J Orthod Dentofacial Orthop. 2003;123:40-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 103] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 51. | St-Jacques B, Hammerschmidt M, McMahon AP. Indian hedgehog signaling regulates proliferation and differentiation of chondrocytes and is essential for bone formation. Genes Dev. 1999;13:2072-2086. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1290] [Cited by in RCA: 1299] [Article Influence: 50.0] [Reference Citation Analysis (0)] |
| 52. | Rabie AB, Wong L, Hägg U. Correlation of replicating cells and osteogenesis in the glenoid fossa during stepwise advancement. Am J Orthod Dentofacial Orthop. 2003;123:521-526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 53. | LeResche L. Epidemiology of temporomandibular disorders: implications for the investigation of etiologic factors. Crit Rev Oral Biol Med. 1997;8:291-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 584] [Cited by in RCA: 589] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 54. | Okeson JP. The American Academy of Orofacial Pain: Orofacial Pain Guidelines for assessment, diagnosis, and management. Quintessence Publishing Co. Inc: Chicago 1996; 113-84. |
| 55. | Ta LE, Phero JC, Pillemer SR, Hale-Donze H, McCartney-Francis N, Kingman A, Max MB, Gordon SM, Wahl SM, Dionne RA. Clinical evaluation of patients with temporomandibular joint implants. J Oral Maxillofac Surg. 2002;60:1389-1399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 56. | Jacobs JJ, Gilbert JL, Urban RM. Corrosion of metal orthopaedic implants. J Bone Joint Surg Am. 1998;80:268-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 714] [Cited by in RCA: 474] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 57. | Shanti RM, Li WJ, Nesti LJ, Wang X, Tuan RS. Adult mesenchymal stem cells: biological properties, characteristics, and applications in maxillofacial surgery. J Oral Maxillofac Surg. 2007;65:1640-1647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 52] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 58. | Bailey MM, Wang L, Bode CJ, Mitchell KE, Detamore MS. A comparison of human umbilical cord matrix stem cells and temporomandibular joint condylar chondrocytes for tissue engineering temporomandibular joint condylar cartilage. Tissue Eng. 2007;13:2003-2010. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 104] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 59. | Alhadlaq A, Mao JJ. Tissue-engineered neogenesis of human-shaped mandibular condyle from rat mesenchymal stem cells. J Dent Res. 2003;82:951-956. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 123] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 60. | Alhadlaq A, Elisseeff JH, Hong L, Williams CG, Caplan AI, Sharma B, Kopher RA, Tomkoria S, Lennon DP, Lopez A. Adult stem cell driven genesis of human-shaped articular condyle. Ann Biomed Eng. 2004;32:911-923. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 119] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 61. | Abukawa H, Terai H, Hannouche D, Vacanti JP, Kaban LB, Troulis MJ. Formation of a mandibular condyle in vitro by tissue engineering. J Oral Maxillofac Surg. 2003;61:94-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 82] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 62. | Allen KD, Athanasiou KA. Tissue Engineering of the TMJ disc: a review. Tissue Eng. 2006;12:1183-1196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 54] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 63. | Mäenpää K, Ellä V, Mauno J, Kellomäki M, Suuronen R, Ylikomi T, Miettinen S. Use of adipose stem cells and polylactide discs for tissue engineering of the temporomandibular joint disc. J R Soc Interface. 2010;7:177-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 33] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 64. | McCarthy JG, Stelnicki EJ, Mehrara BJ, Longaker MT. Distraction osteogenesis of the craniofacial skeleton. Plast Reconstr Surg. 2001;107:1812-1827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 197] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 65. | Lawler ME, Tayebaty FT, Williams WB, Troulis MJ, Kaban LB. Histomorphometric analysis of the porcine mandibular distraction wound. J Oral Maxillofac Surg. 2010;68:1543-1554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 66. | Ai-Aql ZS, Alagl AS, Graves DT, Gerstenfeld LC, Einhorn TA. Molecular mechanisms controlling bone formation during fracture healing and distraction osteogenesis. J Dent Res. 2008;87:107-118. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 521] [Cited by in RCA: 492] [Article Influence: 28.9] [Reference Citation Analysis (0)] |
| 67. | McCarthy JG, Katzen JT, Hopper R, Grayson BH. The first decade of mandibular distraction: lessons we have learned. Plast Reconstr Surg. 2002;110:1704-1713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 102] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 68. | Li G. New developments and insights learned from distraction osteogenesis. C. urr Orthop Pract. 2004;15:325-330. [RCA] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 69. | Alkaisi A, Ismail AR, Mutum SS, Ahmad ZA, Masudi S, Abd Razak NH. Transplantation of human dental pulp stem cells: enhance bone consolidation in mandibular distraction osteogenesis. J Oral Maxillofac Surg. 2013;71:1758.e1-1758.13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 70. | Ma D, Ren L, Yao H, Tian W, Chen F, Zhang J, Liu Y, Mao T. Locally injection of cell sheet fragments enhances new bone formation in mandibular distraction osteogenesis: a rabbit model. J Orthop Res. 2013;31:1082-1088. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 71. | Qi M, Hu J, Zou S, Zhou H, Han L. Mandibular distraction osteogenesis enhanced by bone marrow mesenchymal stem cells in rats. J Craniomaxillofac Surg. 2006;34:283-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 38] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 72. | Lai QG, Yuan KF, Xu X, Li DR, Li GJ, Wei FL, Yang ZJ, Luo SL, Tang XP, Li S. Transcription factor osterix modified bone marrow mesenchymal stem cells enhance callus formation during distraction osteogenesis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2011;111:412-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 28] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 73. | Lee DH, Ryu KJ, Kim JW, Kang KC, Choi YR. Bone marrow aspirate concentrate and platelet-rich plasma enhanced bone healing in distraction osteogenesis of the tibia. Clin Orthop Relat Res. 2014;472:3789-3797. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 64] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 74. | Hatzokos I, Stavridis SI, Iosifidou E, Karataglis D, Christodoulou A. Autologous bone marrow grafting combined with demineralized bone matrix improves consolidation of docking site after distraction osteogenesis. J Bone Joint Surg Am. 2011;93:671-678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 56] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 75. | Sun Z, Tee BC, Kennedy KS, Kennedy PM, Kim DG, Mallery SR, Fields HW. Scaffold-based delivery of autologous mesenchymal stem cells for mandibular distraction osteogenesis: preliminary studies in a porcine model. PLoS One. 2013;8:e74672. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 76. | Jiang X, Zou S, Ye B, Zhu S, Liu Y, Hu J. bFGF-Modified BMMSCs enhance bone regeneration following distraction osteogenesis in rabbits. Bone. 2010;46:1156-1161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 58] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 77. | Hu J, Qi MC, Zou SJ, Li JH, Luo E. Callus formation enhanced by BMP-7 ex vivo gene therapy during distraction osteogenesis in rats. J Orthop Res. 2007;25:241-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 39] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 78. | Lai QG, Sun SL, Zhou XH, Zhang CP, Yuan KF, Yang ZJ, Luo SL, Tang XP, Ci JB. Adipose-derived stem cells transfected with pEGFP-OSX enhance bone formation during distraction osteogenesis. J Zhejiang Univ Sci B. 2014;15:482-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 79. | Sunay O, Can G, Cakir Z, Denek Z, Kozanoglu I, Erbil G, Yilmaz M, Baran Y. Autologous rabbit adipose tissue-derived mesenchymal stromal cells for the treatment of bone injuries with distraction osteogenesis. Cytotherapy. 2013;15:690-702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 80. | Sun JJ, Zheng XH, Wang LY, Liu L, Jing W, Lin YF, Tian W, Tang W, Long J. New bone formation enhanced by ADSCs overexpressing hRunx2 during mandibular distraction osteogenesis in osteoporotic rabbits. J Orthop Res. 2014;32:709-720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 81. | Kim IS, Cho TH, Lee ZH, Hwang SJ. Bone regeneration by transplantation of human mesenchymal stromal cells in a rabbit mandibular distraction osteogenesis model. Tissue Eng Part A. 2013;19:66-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 82. | Aykan A, Ozturk S, Sahin I, Gurses S, Ural AU, Oren NC, Isik S. Biomechanical analysis of the effect of mesenchymal stem cells on mandibular distraction osteogenesis. J Craniofac Surg. 2013;24:e169-e175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 83. | Zhang WB, Zheng LW, Chua DT, Cheung LK. Treatment of irradiated mandibles with mesenchymal stem cells transfected with bone morphogenetic protein 2/7. J Oral Maxillofac Surg. 2012;70:1711-1716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 84. | Cao J, Wang L, Du ZJ, Liu P, Zhang YB, Sui JF, Liu YP, Lei DL. Recruitment of exogenous mesenchymal stem cells in mandibular distraction osteogenesis by the stromal cell-derived factor-1/chemokine receptor-4 pathway in rats. Br J Oral Maxillofac Surg. 2013;51:937-941. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 85. | Wu G, Hu C, He X, Yin K, Lan Y, Zhou B, Li S, Guo L. Effect of gene transfecting at different times on mandibular distraction osteogenesis. J Craniofac Surg. 2013;24:232-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 86. | Long J, Li P, Du HM, Liu L, Zheng XH, Lin YF, Wang H, Jing W, Tang W, Chen WH. Effects of bone morphogenetic protein 2 gene therapy on new bone formation during mandibular distraction osteogenesis at rapid rate in rabbits. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2011;112:50-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 87. | Castro-Govea Y, Cervantes-Kardasch VH, Borrego-Soto G, Martínez-Rodríguez HG, Espinoza-Juarez M, Romero-Díaz V, Marino-Martínez IA, Robles-Zamora A, Álvarez-Lozano E, Padilla-Rivas GR. Human bone morphogenetic protein 2-transduced mesenchymal stem cells improve bone regeneration in a model of mandible distraction surgery. J Craniofac Surg. 2012;23:392-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 38] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 88. | Vidya V, Sumathi F. Rapid maxillary expansion as a standard treatment for obstructive sleep apnea syndrome: a systematic review. J Dental Med Sci. 2015;14:51-55. |
| 89. | Aloufi F, Preston CB, Zawawi KH. Changes in the upper and lower pharyngeal airway spaces associated with rapid maxillary expansion. ISRN Dent. 2012;2012:290964. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 90. | De Rossi M, De Rossi A, Hallak JE, Vitti M, Regalo SC. Electromyographic evaluation in children having rapid maxillary expansion. Am J Orthod Dentofacial Orthop. 2009;136:355-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 28] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 91. | Mommaerts MY. Transpalatal distraction as a method of maxillary expansion. Br J Oral Maxillofac Surg. 1999;37:268-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 183] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 92. | Bishara SE, Staley RN. Maxillary expansion: clinical implications. Am J Orthod Dentofacial Orthop. 1987;91:3-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 290] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 93. | Lagravere MO, Major PW, Flores-Mir C. Long-term dental arch changes after rapid maxillary expansion treatment: a systematic review. Angle Orthod. 2005;75:155-161. [PubMed] |
| 94. | Baratieri C, Alves M Jr, de Souza MM, de Souza Araújo MT, Maia LC. Does rapid maxillary expansion have long-term effects on airway dimensions and breathing? Am J Orthod Dentofacial Orthop. 2011;140:146-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 85] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 95. | Ekizer A, Yalvac ME, Uysal T, Sonmez MF, Sahin F. Bone marrow mesenchymal stem cells enhance bone formation in orthodontically expanded maxillae in rats. Angle Orthod. 2015;85:394-399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 96. | Ackerman JL, Proffit WR. Soft tissue limitations in orthodontics: treatment planning guidelines. Angle Orthod. 1997;67:327-336. [PubMed] |
| 97. | Proffit WR. Equilibrium theory revisited: factors influencing position of the teeth. Angle Orthod. 1978;48:175-186. [PubMed] |
| 98. | Proffit WR, Fields H, Sarver D, Ackerman J. Contemporary Orthodontics. 5th ed. Mosbey Philadelphia. 2012;691. |
| 99. | Proffit WR. Special considerations in treatment for adults. Contemporary orthodontics: Mosby, St Louis 2007; 635-685. |
| 100. | Preoteasa CT, Ionescu E, Preoteasa E. Risks and complications associated with orthodontic treatment. Orthodontics-Basic Aspects and Clinical Consideration. InTech. 2012;31-35. |
| 101. | Black CR, Goriainov V, Gibbs D, Kanczler J, Tare RS, Oreffo RO. Bone Tissue Engineering. Curr Mol Biol Rep. 2015;1:132-140. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 194] [Cited by in RCA: 151] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 102. | Rickert D, Sauerbier S, Nagursky H, Menne D, Vissink A, Raghoebar GM. Maxillary sinus floor elevation with bovine bone mineral combined with either autogenous bone or autogenous stem cells: a prospective randomized clinical trial. Clin Oral Implants Res. 2011;22:251-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 86] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 103. | Payer M, Lohberger B, Strunk D, Reich KM, Acham S, Jakse N. Effects of directly autotransplanted tibial bone marrow aspirates on bone regeneration and osseointegration of dental implants. Clin Oral Implants Res. 2014;25:468-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 104. | Yamada Y, Nakamura S, Ueda M, Ito K. Osteotome technique with injectable tissue-engineered bone and simultaneous implant placement by cell therapy. Clin Oral Implants Res. 2013;24:468-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 105. | Miguita L, Mantesso A, Pannuti CM, Deboni MCZ. Can stem cells enhance bone formation in the human edentulous alveolar ridge? A systematic review and meta-analysis. Cell Tissue Bank. 2017;18:217-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 106. | Reynolds MA, Aichelmann-Reidy ME, Branch-Mays GL, Gunsolley JC. The efficacy of bone replacement grafts in the treatment of periodontal osseous defects. A systematic review. Ann Periodontol. 2003;8:227-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 254] [Cited by in RCA: 238] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 107. | Reynolds MA, Kao RT, Camargo PM, Caton JG, Clem DS, Fiorellini JP, Geisinger ML, Mills MP, Nares S, Nevins ML. Periodontal regeneration - intrabony defects: a consensus report from the AAP Regeneration Workshop. J Periodontol. 2015;86:S105-S107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 128] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 108. | Khojasteh A, Kheiri L, Motamedian SR, Khoshkam V. Guided Bone Regeneration for the Reconstruction of Alveolar Bone Defects. Ann Maxillofac Surg. 2017;7:263-277. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 55] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 109. | Bianco P, Robey PG, Saggio I, Riminucci M. “Mesenchymal” stem cells in human bone marrow (skeletal stem cells): a critical discussion of their nature, identity, and significance in incurable skeletal disease. Hum Gene Ther. 2010;21:1057-1066. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 139] [Cited by in RCA: 122] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 110. | Geraci TF. Orthodontic movement of teeth into artificially produced infrabony defects in the rhesus monkey. A histological report. J Periodontol. 1973;44:116. |
| 111. | Ghezzi C, Masiero S, Silvestri M, Zanotti G, Rasperini G. Orthodontic treatment of periodontally involved teeth after tissue regeneration. Int J Periodontics Restorative Dent. 2008;28:559-567. [PubMed] |
| 112. | Trossello VK, Gianelly AA. Orthodontic treatment and periodontal status. J Periodontol. 1979;50:665-671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 67] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 113. | Duan X, Tu Q, Zhang J, Ye J, Sommer C, Mostoslavsky G, Kaplan D, Yang P, Chen J. Application of induced pluripotent stem (iPS) cells in periodontal tissue regeneration. J Cell Physiol. 2011;226:150-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 139] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 114. | Wu Y, Chen L, Scott PG, Tredget EE. Mesenchymal stem cells enhance wound healing through differentiation and angiogenesis. Stem Cells. 2007;25:2648-2659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1223] [Cited by in RCA: 1195] [Article Influence: 66.4] [Reference Citation Analysis (0)] |
| 115. | Nagata M, Iwasaki K, Akazawa K, Komaki M, Yokoyama N, Izumi Y, Morita I. Conditioned Medium from Periodontal Ligament Stem Cells Enhances Periodontal Regeneration. Tissue Eng Part A. 2017;23:367-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 128] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 116. | Ma Z, Li S, Song Y, Tang L, Ma D, Liu B, Jin Y. The biological effect of dentin noncollagenous proteins (DNCPs) on the human periodontal ligament stem cells (HPDLSCs) in vitro and in vivo. Tissue Eng Part A. 2008;14:2059-2068. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 35] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 117. | Liu Y, Zheng Y, Ding G, Fang D, Zhang C, Bartold PM, Gronthos S, Shi S, Wang S. Periodontal ligament stem cell-mediated treatment for periodontitis in miniature swine. Stem Cells. 2008;26:1065-1073. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 470] [Cited by in RCA: 441] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 118. | Grimm WD, Dannan A, Becher S, Gassmann G, Arnold W, Varga G, Dittmar T. The ability of human periodontium-derived stem cells to regenerate periodontal tissues: a preliminary in vivo investigation. Int J Periodontics Restorative Dent. 2011;31:e94-e101. [PubMed] |
| 119. | Ding G, Liu Y, Wang W, Wei F, Liu D, Fan Z, An Y, Zhang C, Wang S. Allogeneic periodontal ligament stem cell therapy for periodontitis in swine. Stem Cells. 2010;28:1829-1838. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 263] [Cited by in RCA: 304] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 120. | Iwasaki K, Komaki M, Yokoyama N, Tanaka Y, Taki A, Honda I, Kimura Y, Takeda M, Akazawa K, Oda S. Periodontal regeneration using periodontal ligament stem cell-transferred amnion. Tissue Eng Part A. 2014;20:693-704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 121. | Masella RS, Meister M. Current concepts in the biology of orthodontic tooth movement. Am J Orthod Dentofacial Orthop. 2006;129:458-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 188] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 122. | Meikle MC. The tissue, cellular, and molecular regulation of orthodontic tooth movement: 100 years after Carl Sandstedt. Eur J Orthod. 2006;28:221-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 333] [Cited by in RCA: 344] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 123. | Rody WJ Jr, King GJ, Gu G. Osteoclast recruitment to sites of compression in orthodontic tooth movement. Am J Orthod Dentofacial Orthop. 2001;120:477-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 90] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 124. | Miyamoto T, Suda T. Differentiation and function of osteoclasts. Keio J Med. 2003;52:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 110] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 125. | Zainal Ariffin SH, Yamamoto Z, Zainol Abidin IZ, Megat Abdul Wahab R, Zainal Ariffin Z. Cellular and molecular changes in orthodontic tooth movement. ScientificWorldJournal. 2011;11:1788-1803. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 96] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 126. | Suya H. Corticotomy in orthodontics. Mechanical and biological basics in orthodontic therapy. Heidelberg, Germany: Huthig Buch Verlag 1991; 207-226. |
| 127. | Wilcko WM, Wilcko T, Bouquot JE, Ferguson DJ. Rapid orthodontics with alveolar reshaping: two case reports of decrowding. Int J Periodontics Restorative Dent. 2001;21:9-19. [PubMed] |
| 128. | Almpani K, Kantarci A. Nonsurgical Methods for the Acceleration of the Orthodontic Tooth Movement. Front Oral Biol. 2016;18:80-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 129. | Feng L, Yang R, Liu D, Wang X, Song Y, Cao H, He D, Gan Y, Kou X, Zhou Y. PDL Progenitor-Mediated PDL Recovery Contributes to Orthodontic Relapse. J Dent Res. 2016;95:1049-1056. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 44] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 130. | Pizzo G, Licata ME, Guiglia R, Giuliana G. Root resorption and orthodontic treatment. Review of the literature. Minerva Stomatol. 2007;56:31-44. [PubMed] |
| 131. | Mohanty P, Prasad NK, Sahoo N, Kumar G, Mohanty D, Sah S. Reforming craniofacial orthodontics via stem cells. J Int Soc Prev Community Dent. 2015;5:13-18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 132. | Zahrowski J, Jeske A. Apical root resorption is associated with comprehensive orthodontic treatment but not clearly dependent on prior tooth characteristics or orthodontic techniques. J Am Dent Assoc. 2011;142:66-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 133. | Guo Y, He S, Gu T, Liu Y, Chen S. Genetic and clinical risk factors of root resorption associated with orthodontic treatment. Am J Orthod Dentofacial Orthop. 2016;150:283-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 39] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 134. | Shinagawa-Ohama R, Mochizuki M, Tamaki Y, Suda N, Nakahara T. Heterogeneous Human Periodontal Ligament-Committed Progenitor and Stem Cell Populations Exhibit a Unique Cementogenic Property Under In Vitro and In Vivo Conditions. Stem Cells Dev. 2017;26:632-645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 135. | Oshima M, Mizuno M, Imamura A, Ogawa M, Yasukawa M, Yamazaki H, Morita R, Ikeda E, Nakao K, Takano-Yamamoto T. Functional tooth regeneration using a bioengineered tooth unit as a mature organ replacement regenerative therapy. PLoS One. 2011;6:e21531. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 149] [Cited by in RCA: 138] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 136. | Ikeda E, Morita R, Nakao K, Ishida K, Nakamura T, Takano-Yamamoto T, Ogawa M, Mizuno M, Kasugai S, Tsuji T. Fully functional bioengineered tooth replacement as an organ replacement therapy. Proc Natl Acad Sci USA. 2009;106:13475-13480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 297] [Cited by in RCA: 276] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 137. | Ono M, Oshima M, Ogawa M, Sonoyama W, Hara ES, Oida Y, Shinkawa S, Nakajima R, Mine A, Hayano S. Practical whole-tooth restoration utilizing autologous bioengineered tooth germ transplantation in a postnatal canine model. Sci Rep. 2017;7:44522. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 50] [Article Influence: 6.3] [Reference Citation Analysis (0)] |