Copyright
©The Author(s) 2015.
World J Stem Cells. May 26, 2015; 7(4): 669-680
Published online May 26, 2015. doi: 10.4252/wjsc.v7.i4.669
Published online May 26, 2015. doi: 10.4252/wjsc.v7.i4.669
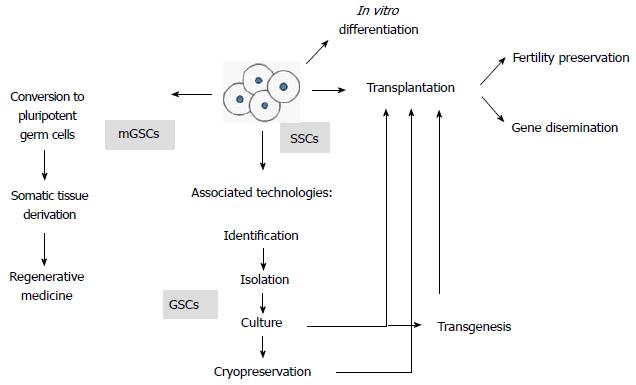
Figure 1 Overview of biotechnologies involving spermatogonial stem cells.
Fertility preservation includes fertility restoration in oncological patients after therapeutic agents are administered that produce germ cell damage, strategies for infertile male patients and reproduction of endangered species. Gene dissemination represents the possibility to use several low-genetics animals to disseminate elite genes in animal production operations. Culture involves maintenance, survival, expansion and possibly genetic manipulations of spermatogonial stem cells (SSCs). Germ stem cells (GSCs), that is, SSCs that self-renew in vitro, therefore proliferating and expanding. Multipotent germ stem cells (mGSCs), which spontaneously appear in culture from within the SSC population, showing pluripotency properties[2].
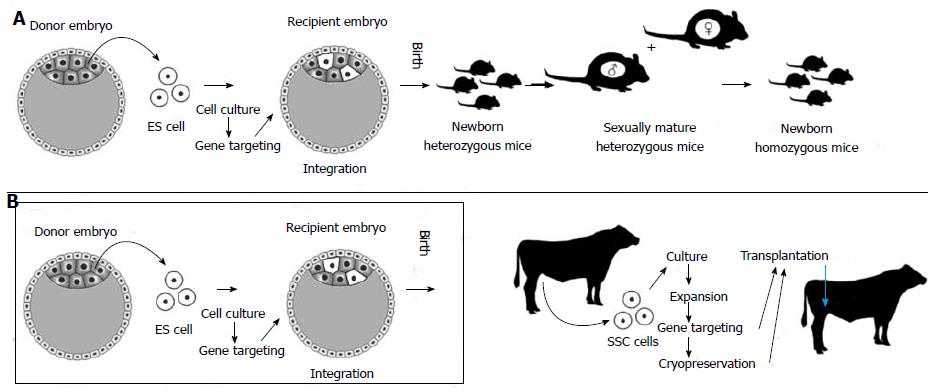
Figure 2 Alternative ways to exploit stem cells to produce transgenic animals.
A: Standard method (well developed in the mouse): Embryonic stem cells (ES cells) are obtained from a donor mouse blastocyst. These cells are cultured and genetically modified (usually, DNA sequences are inserted on a gene of interest to disrupt its expression or gene knockout). ES cells are injected into another mouse blastocyst so that they can integrate into the embryo (chimera formation) with the hope that some of them will contribute to the male germline. In this case, donor germline cells will undergo homologous recombination (meiosis) in the testis after animals are born and develop towards puberty. The male animals will be heterozygous for the mutated gene and when crossed with their (also heterozygous) sisters, offspring will be obtained homozygous for the transgene; B: Transgenesis technique involving spermatogonial stem cells (SSCs), particularly useful for farm animals, for which ES cell technologies are not feasible. SSCs from postnatal animals can be expanded in culture, subject to gene targeting and transplanted to a recipient foreign testis. This way, steps used with ES cell technologies become redundant (Figure 2B, inside the box). With the advent of spermatogenesis in vitro, even the SSC transplantation procedure will not be necessary. Cryopreservation techniques for SSCs are already available.
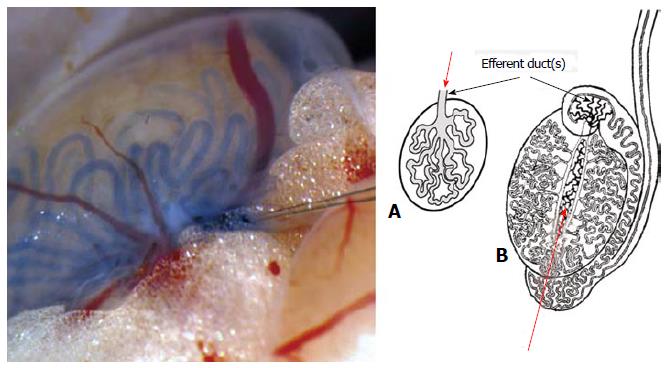
Figure 3 Species differences on anatomical points for spermatogonial stem cell injection for transplantation.
The transplantation procedure for spermatogonial stem cells (SSCs) is simple in the mouse. In this species, (A) there is a single efferent duct emerging from the rete testis, which is easy to cannulate to inject SSCs (arrow). In most farm animals and men (B) several efferent ducts emerge from the testis, a reason why transplantation of SSCs is often done by injection of SSCs into the rete testis (arrow) with ultrasound guidance.
- Citation: Aponte PM. Spermatogonial stem cells: Current biotechnological advances in reproduction and regenerative medicine. World J Stem Cells 2015; 7(4): 669-680
- URL: https://www.wjgnet.com/1948-0210/full/v7/i4/669.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v7.i4.669