Copyright
©2014 Baishideng Publishing Group Inc.
World J Stem Cells. Nov 26, 2014; 6(5): 526-539
Published online Nov 26, 2014. doi: 10.4252/wjsc.v6.i5.526
Published online Nov 26, 2014. doi: 10.4252/wjsc.v6.i5.526
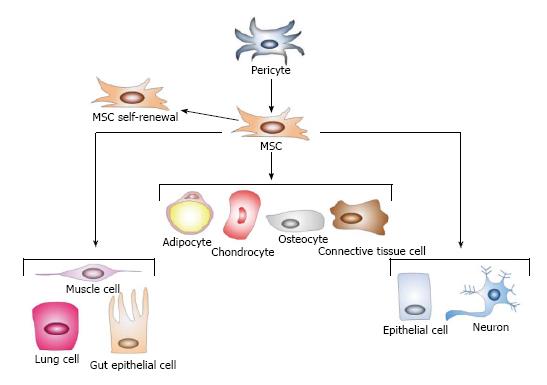
Figure 1 Basic properties of mesenchymal stem cells.
Mesenchymal stem cells (MSCs) are a heterogeneous population of stromal cells thought to be derived from pericytes. These cells are defined by self-renewal and the ability to differentiate into the mesodermal cells (solid lines): adipocytes, chondrocytes, osteocytes, and connective tissue cells. Though controversial (dotted lines), they may also transdifferentiate into cells of the endoderm (lung, muscle, and gut epithelial cells) and of the ectoderm (neurons and epithelial cells). Adapted from ref [22].
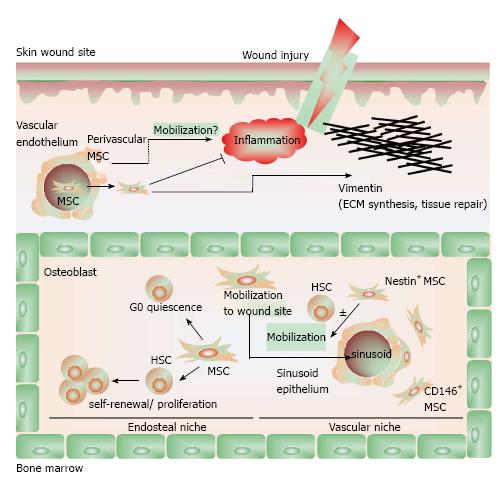
Figure 2 The biology of mesenchymal stem cells.
In the bone marrow, mesenchymal stem cells (MSCs) aid in constructing the endosteal niche and regulate the homeostasis of HSCs. MSCs maintain HSCs in a state of quiescence defined by self-renewal and proliferation without differentiation. CD146+ MSCs in the vascular niche also maintain HSC homeostasis and, along with Nestin+ MSCs, regulate the mobilization of HSC into the vascular system. In response to inflammatory cues and chemokine gradients, MSCs mobilize out of the bone marrow and to peripheral sites of injury, where they suppress inflammation to facilitate wound healing. MSCs contribute to tissue reconstruction with the production and deposition of vimentin. In is incompletely understood whether perivascular MSCs may also migrate to sites of injury to contribute to wound healing. Adapted from ref [22].
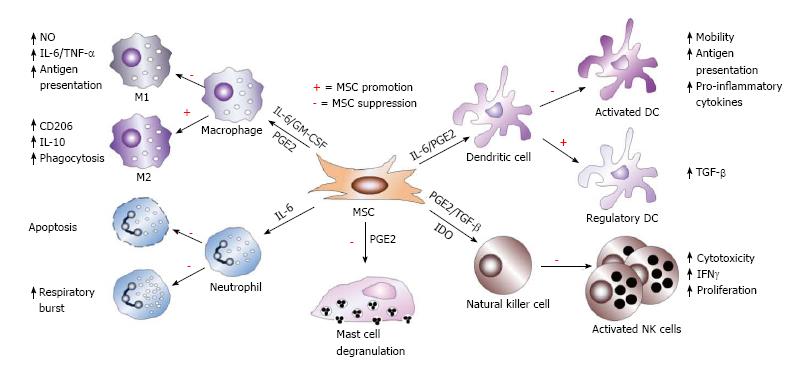
Figure 3 Mesenchymal stem cell immunosuppression of innate immune cells.
Mesenchymal stem cells (MSCs) utilize diverse molecular mechanisms to suppress innate immune cells. MSCs suppress macrophage polarization to M1, though favors M2 polarization. MSCs inhibit mast cell degranulation of histamine-containing granules and inhibit NK cell and DC activation, differentiation, and effector functions. MSC-derived PGE2 contributes to all of these effects. MSC-produced IL-6 suppresses neutrophil apoptosis and respiratory burst and also contributes to inhibition of DC function. In the presence of IL-6 and GM-CSF, MSCs also affect macrophage function, while TGF-β and IDO suppress NK cell function. In addition, MSCs also favor the generation of regulatory DCs.
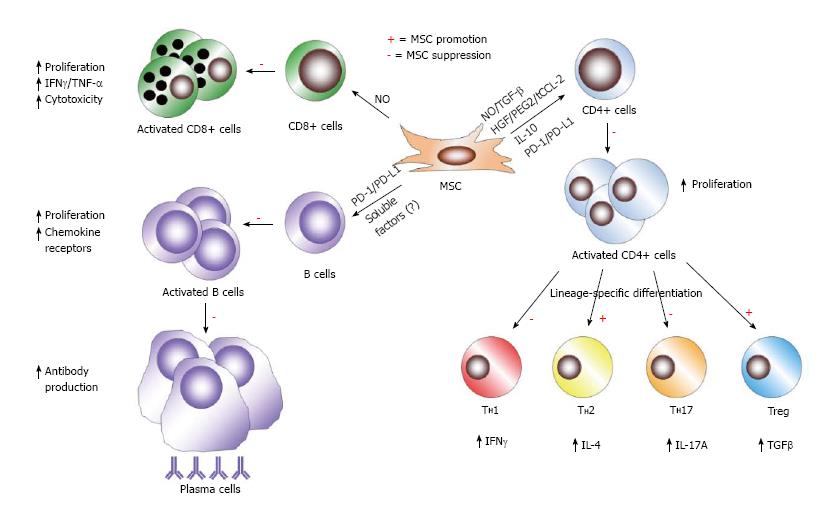
Figure 4 Mesenchymal stem cell immunosuppression of adaptive immune cells.
In the context of B cells, mesenchymal stem cells (MSCs) inhibit various facets of B cells activity, including activation, proliferation, chemokine receptor expression, and differentiation to becoming antibody-secreting plasma cells. Unknown soluble factors and PD-1/PD-L1 ligation mediate these effects of MSCs on B cells. MSC have been shown to induce NO in response to inflammatory cytokine detection to suppress CD8+ T cell proliferation, cytokine production, and cytotoxicity. In response to activation in specific cytokine milieus, CD4+ T cells can differentiate into numerous effector populations. MSCs produce soluble factors (NO, TGF-B, HGF, PGE2, truncated CCL-2, and IL-10) and membrane-bound molecules (PD-1 ligation) to achieve suppression of CD4+ T cell proliferation and the polarization of CD4+ T cells towards TH1 and TH17 cells. MSCs favor the development of TH2 and anti-inflammatory Treg populations.
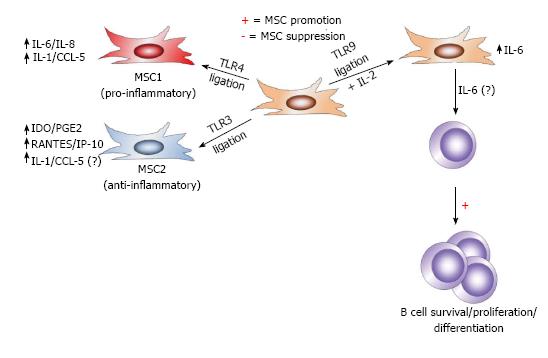
Figure 5 Differential toll-like receptor stimulation affects mesenchymal stem cell immune-modulation.
Mesenchymal stem cells (MSCs) are situated throughout the body as sentinels in virtually all organs and the perivasculature and are equipped with pattern-recognition receptors, including Toll-like receptorS (TLRS), to detect DAMPs from dying cells and PAMPs from pathogens. In response to TLR3 signaling, MSCs maintain an anti-inflammatory MSC2 phenotype, marked by induction of IDO, PGE2, RANTES, and IP-10 (in addition to IL-1 and CCL-5). However, in response to signaling through TLR3, MSC adopt the pro-inflammatory MSC1 phenotype and up-regulate IL-6 and IL-8, in addition to IL-1 and CCL-5. In the presence of IL-2 in combination with TLR9 signaling, MSCs have been shown to also produce IL-6, which promotes B cells survival, proliferation, and differentiation, though MSC-derived IL-6 has not been demonstrated to directly exert these effects on B cells.
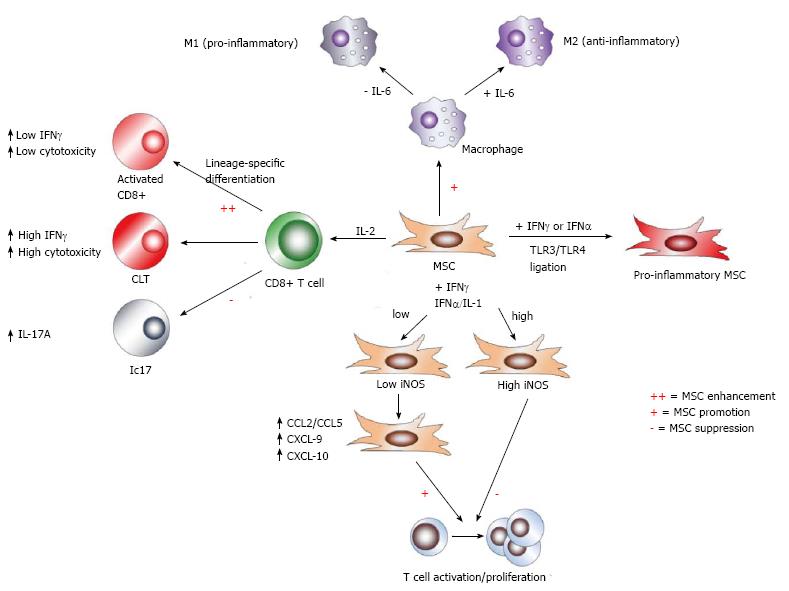
Figure 6 Effects of cytokine milieu on mesenchymal stem cell immune-modulation.
Mesenchymal stem cell (MSC) modulation of immune responses is strongly affected by the makeup of cytokine milieus. Toll-like receptor (TLR) ligation in conjunction with interferon signaling drives MSCs down a pro-inflammatory route. While high concentrations of the pro-inflammatory cytokines IFNγ and either tumor necrosis factor-α (TNF-α) or IL-1 have been shown to induce iNOS and NO in MSCs to mediate suppression of T cell proliferation, low concentrations of these factors fail to fully induce iNOS, and instead enhance T cell proliferation, presumably via cytokine-induced chemokines. Furthermore, MSCs differentially affect the polarization of effector CD8+ T cell subsets: through enhanced early IL-2 expression induced by MSCs, activated CD8+ T cells exhibit increased IFNγ expression and cytotoxicity, while fully differentiated cytotoxic T lymphocytes (CTLs) are largely unaffected by MSC action. In contrast, MSCs potently suppress Tc17 development. Moreover, IL-6 signaling acts as a switch for MSC immune-modulation of macrophages. In the presence of IL-6, MSCs retain promotion of M2, but favor M1 polarization in the absence of this cytokine.
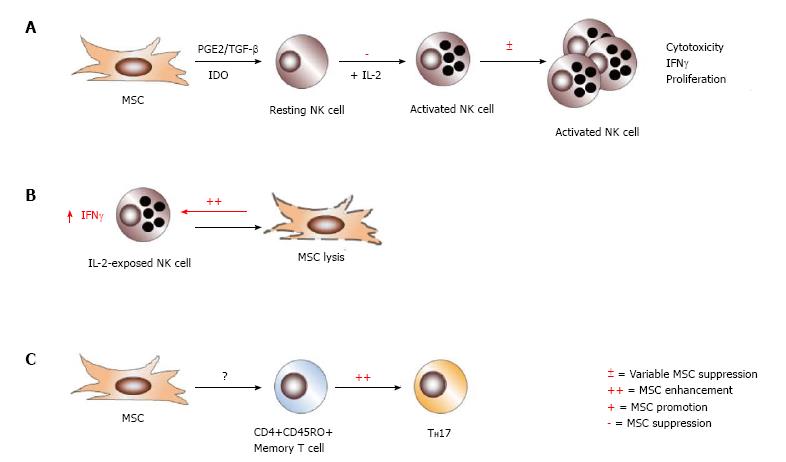
Figure 7 Effects of immune cell activation state on mesenchymal stem cell immune-modulation.
The differentiation state of immune cells can render them susceptible or refractory to mesenchymal stem cell (MSC) action. Though MSCs efficiently inhibit the activation and downstream cytotoxicity of resting NK cells, they exert variable suppression on IL-2-activated NK cells, which is partially ratio dependent (A). MSCs themselves may become targets of activated NK cells for lysis, and enhance NK cell production of IFNγ in the process (B). Interestingly, MSCs promote TH17 differentiation from CD4+CD45RO+ memory T cells, but no other CD4+ or CD8+ T cell population (C).
- Citation: Glenn JD, Whartenby KA. Mesenchymal stem cells: Emerging mechanisms of immunomodulation and therapy. World J Stem Cells 2014; 6(5): 526-539
- URL: https://www.wjgnet.com/1948-0210/full/v6/i5/526.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v6.i5.526