Copyright
©2013 Baishideng Publishing Group Co.
World J Stem Cells. Apr 26, 2013; 5(2): 43-52
Published online Apr 26, 2013. doi: 10.4252/wjsc.v5.i2.43
Published online Apr 26, 2013. doi: 10.4252/wjsc.v5.i2.43
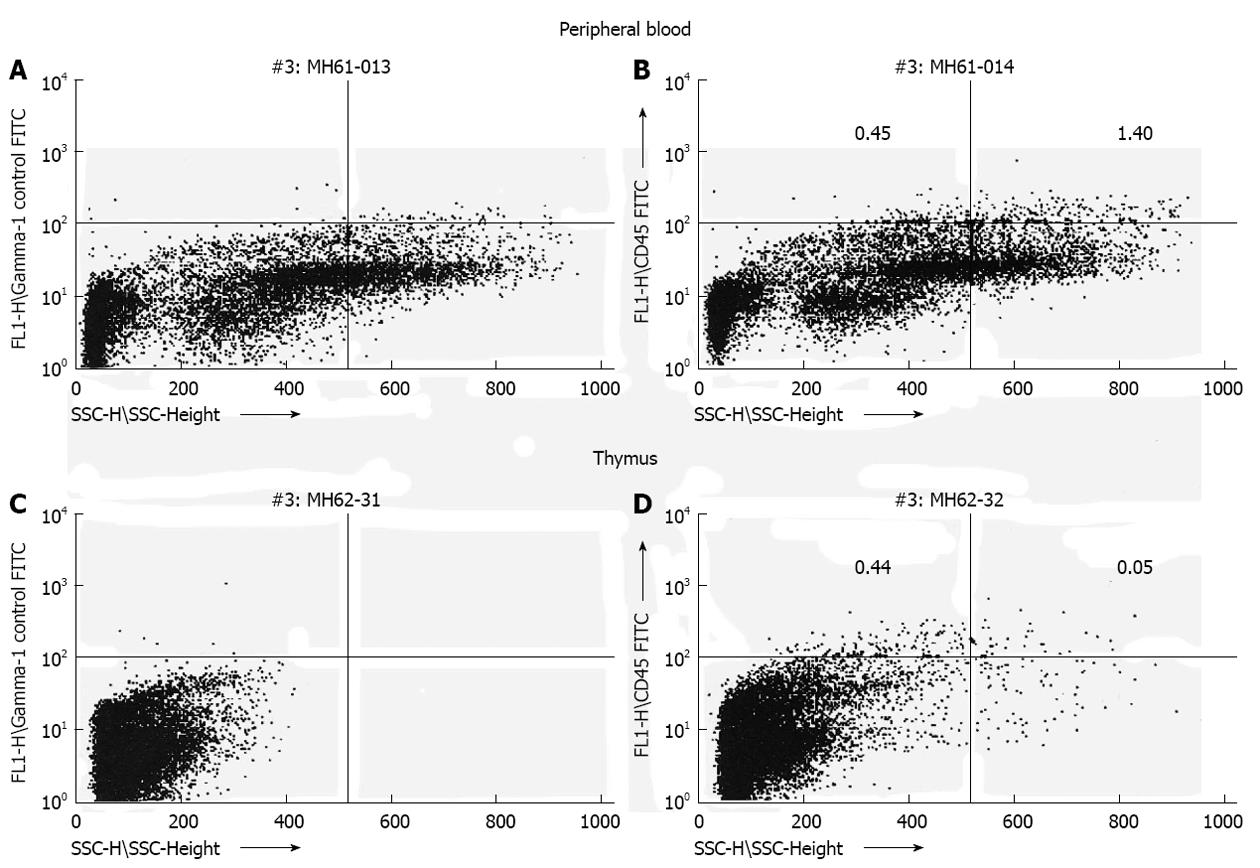
Figure 1 Transplantation of human hematopoietic stem cell (CD34+) during the murine engraftment window results in bi-lineage expression and relevant cell migration of the differentiated human progeny 6 mo after transplantation.
Human CD45 cells exhibit different side scatter characteristics (low side scatter: lymphocytes; high side scatter: granulocytes) dependent on organ tested (A, C: Control mouse; B, D: Experimental mouse)[23].
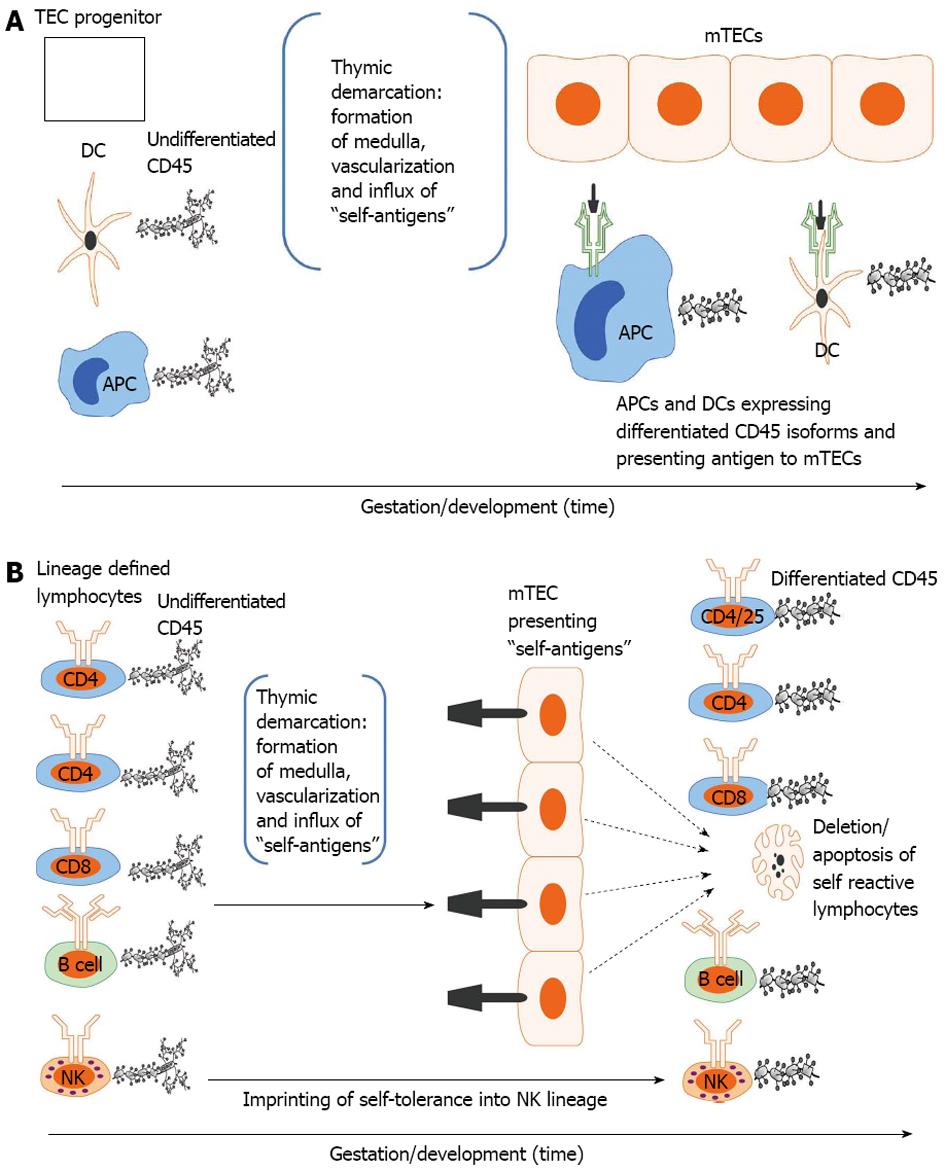
Figure 2 Developmental acquisition of self-tolerance: Antigen exposure model.
A: Thymic epithelial (TEC) progenitors mature in intimate contact with dendritic cells (DCs) and likely other antigen presenting cells (APCs) thus forming the thymic medulla (i.e., thymic demarcation). With vascularization, self-antigens (i.e., antigens present during the finite tolerance window) from the periphery are processed and presented to medullary thymic epithelial cells (mTECs). The developing mTEC matrix is only receptive to antigen presentation during the tolerance window (time dependence) and subsequently exhibits on its surface the self-antigen repertoire (see 3. Ramifications: Dissection of immune tolerance); B: All lymphoid compartments [T, B and natural killer (NK) cell] enter the thymus expressing undifferentiated CD45 but are lineage defined. They then migrate through the thymus encountering mTECs expressing the self-antigen repertoire. CD4, CD8 and B cells reactive to self-antigens are deleted. CD4/CD25 cells reactive to self-antigens are rendered suppressive (Treg) in Hassal’s corpuscles. NK cells are imprinted in an undefined fashion (likely based on major histocompatibility complex exposure) to become tolerant to self. All differentiated lymphocytes (i.e., cells that have undergone CD45 isoform maturation and are not self-reactive or are tolerogenic (i.e.,Treg) then migrate to the spleen or other secondary lymphoid organs (see 3. Ramifications: Dissection of self-tolerance)[6,21,31-37,42].
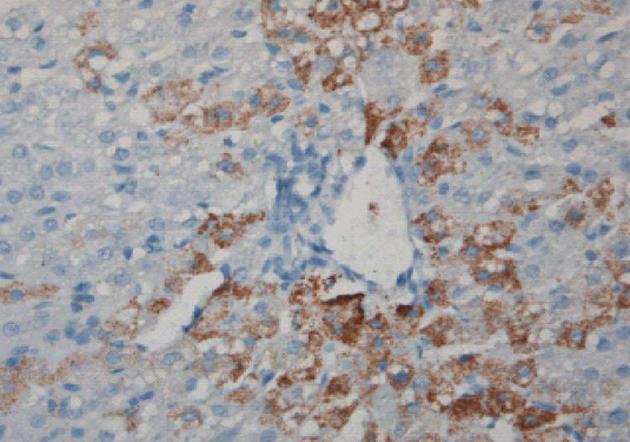
Figure 3 Induction of sheep tolerance to human hepatocytes by transplantation of human hematopoietic stem cells during the temporal engraftment window (note absence of sheep round cell infiltration).
Sheep liver stained for human specific antibody 11 mo following transplantation[49].
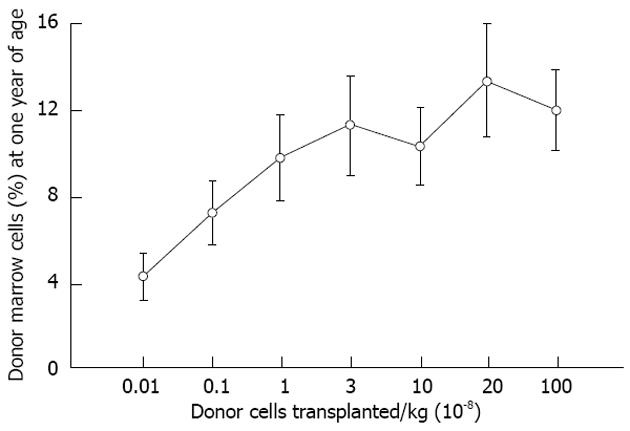
Figure 4 Levels of allogeneic engraftment plateau with transplantation of log-fold increases in donor cells in sheep.
This is most consistent with a model of saturation kinetics with engraftment limited by available receptive sites. An identical curve is seen in the xenogeneic human-sheep model[2].
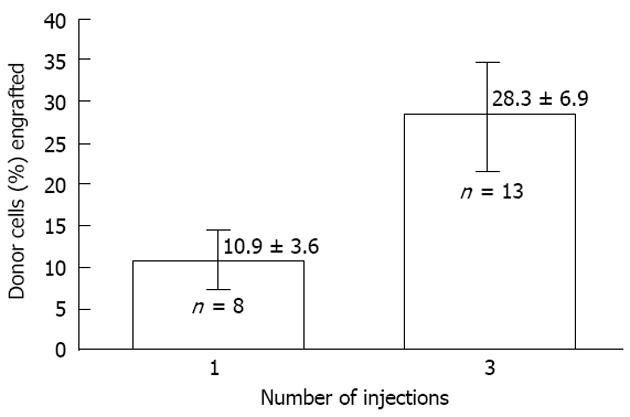
Figure 5 The effect of dividing cell dose on engraftment in sheep.
Transplantation of the same total number of cells as a single injection vs a series of three injections given 1 wk apart is shown. The engraftment with divided doses is significantly higher supporting the concept that engraftment is limited at any particular time by the number of available receptive sites[2].
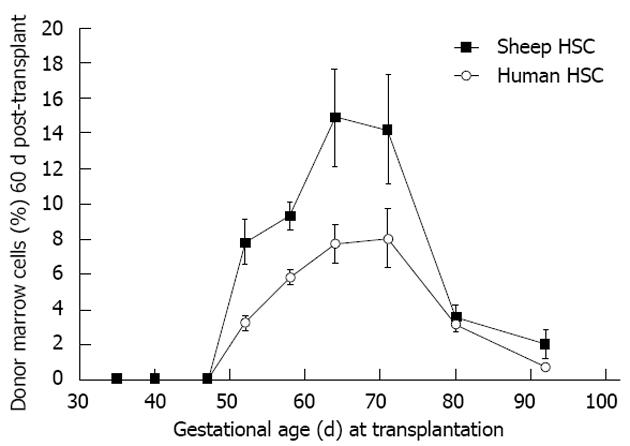
Figure 6 Allo- or xenogeneic stem cell bone marrow engraftment in sheep following transplantation during the temporal window when self non-self discrimination occurs (note parallel kinetics).
Engraftment receptivity is gestational age-dependent[6].
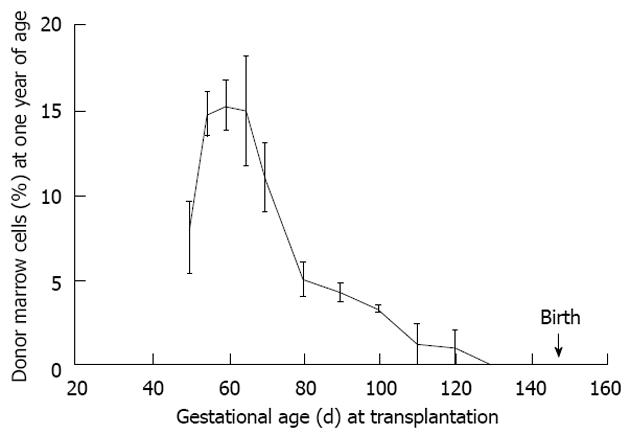
Figure 7 Maximal donor cell engraftment in sheep occurs during the mid-to-later stages of the tolerance induction period.
This period follows thymic demarcation and formation of the osteoblastic niche. Further, engraftment is durable as the engraftment kinetics are similar to that noted at 60 d post transplant supporting our contention that the graft is viewed by the recipient as self (see Figure 6)[2].
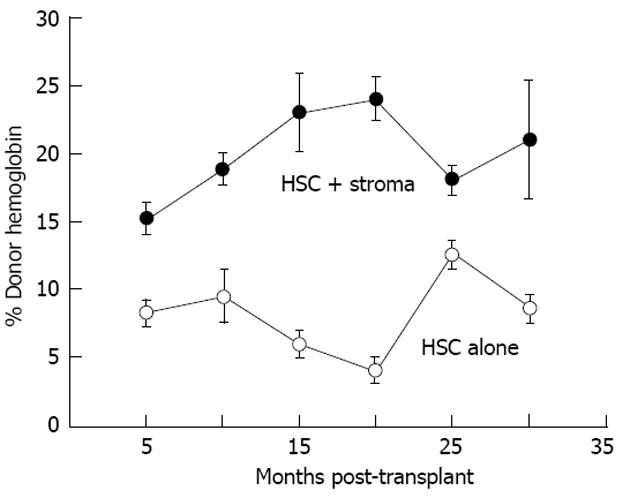
Figure 8 Co-transplantation with autologous adult sheep stromal cells improves allogeneic donor hematopoietic stem cell expression.
Donor expression (circulating hemoglobin) persists more that 30 mo after transplantation. Donor hemoglobin levels in the peripheral blood of sheep co-transplanted with autologous adult bone marrow stroma (n = 4) are significantly higher (P < 0.01) than those transplanted with adult T-depleted bone marrow mononuclear cells alone (n = 3)[67]. HSC: Human stem cell.
- Citation: Pixley JS, Zanjani ED. In utero transplantation: Disparate ramifications. World J Stem Cells 2013; 5(2): 43-52
- URL: https://www.wjgnet.com/1948-0210/full/v5/i2/43.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v5.i2.43